Animal protein-free media for cultivation of cells
a technology of protein-free culture media and animal protein, which is applied in the field of animal protein-free cell culture media, can solve the problems of bse contamination of all serum-derived products, risk of contamination with mycoplasma, and all serum-derived products can be contaminated by unknown constituents, so as to increase the protein expression per cell, promote cell growth, and increase the yield of desired products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1 (
BAV-medium)
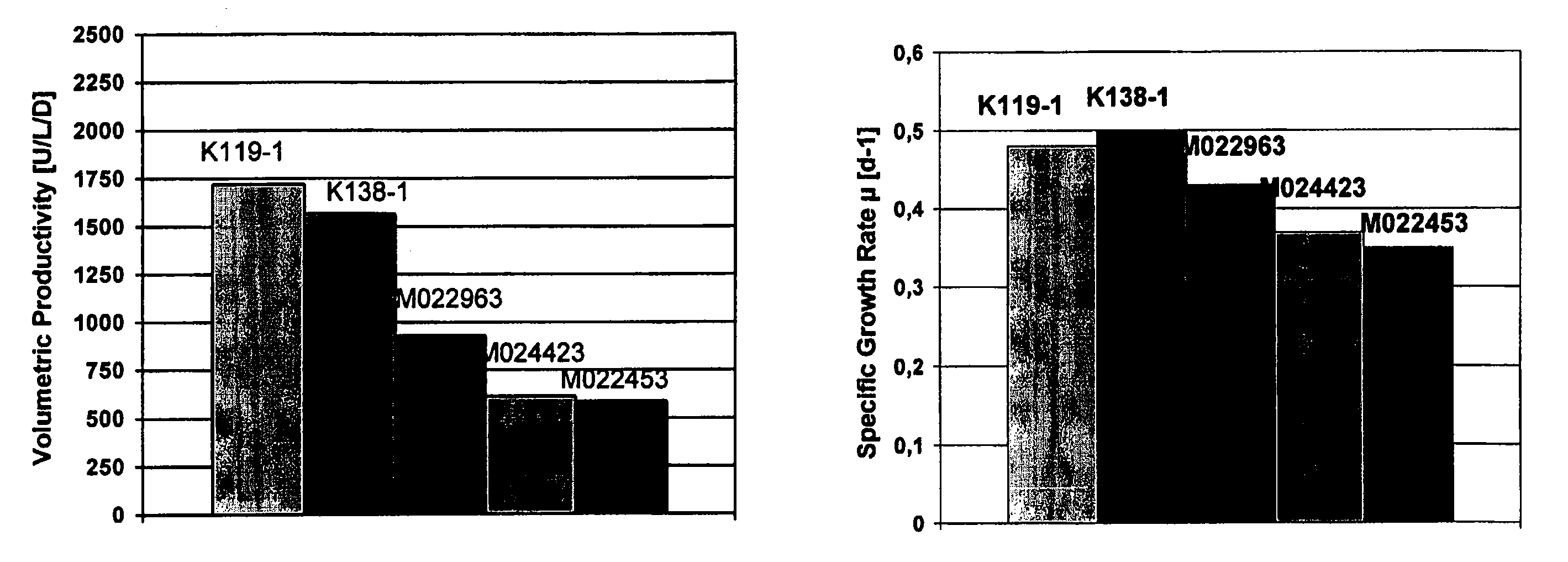
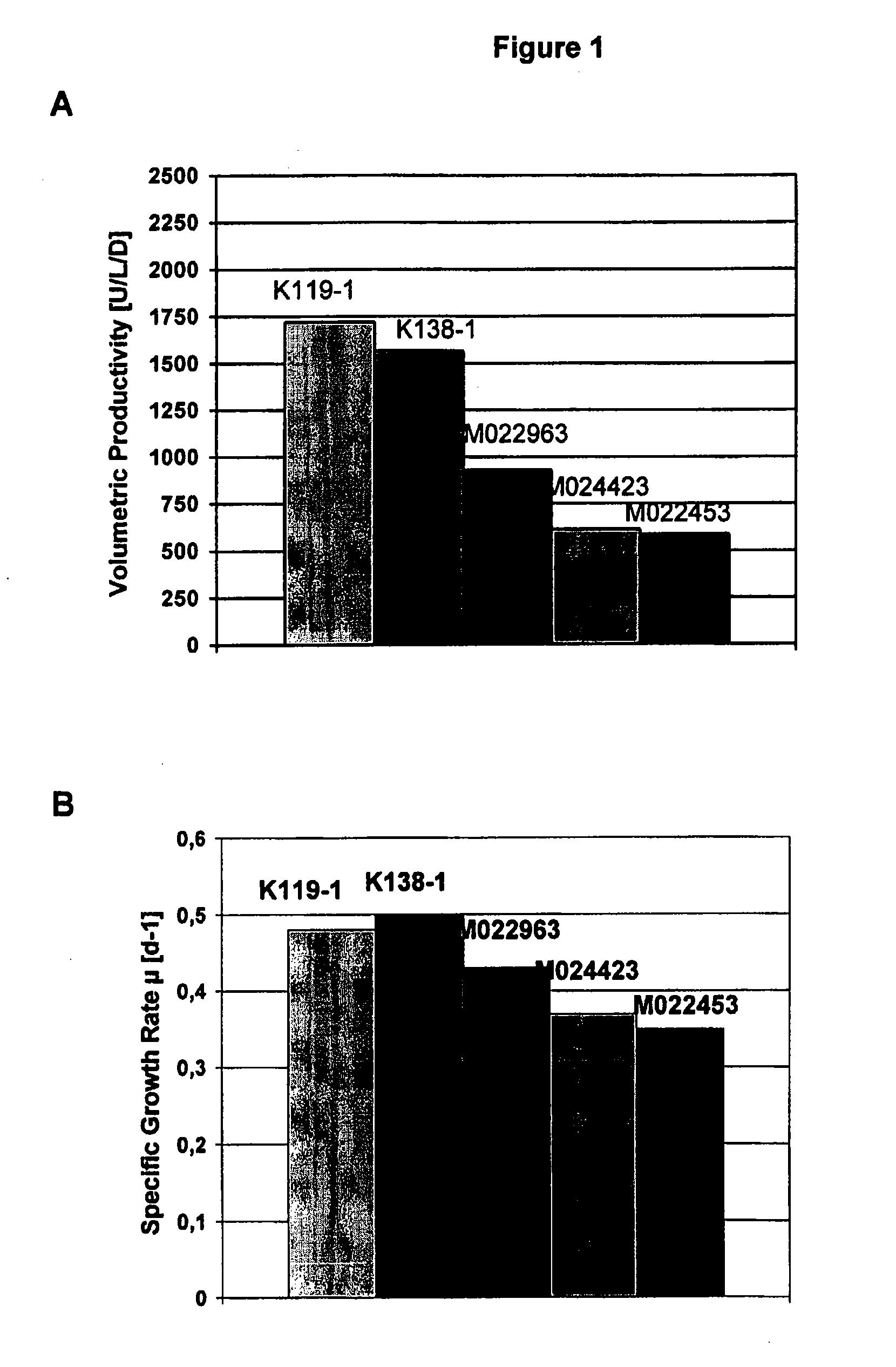
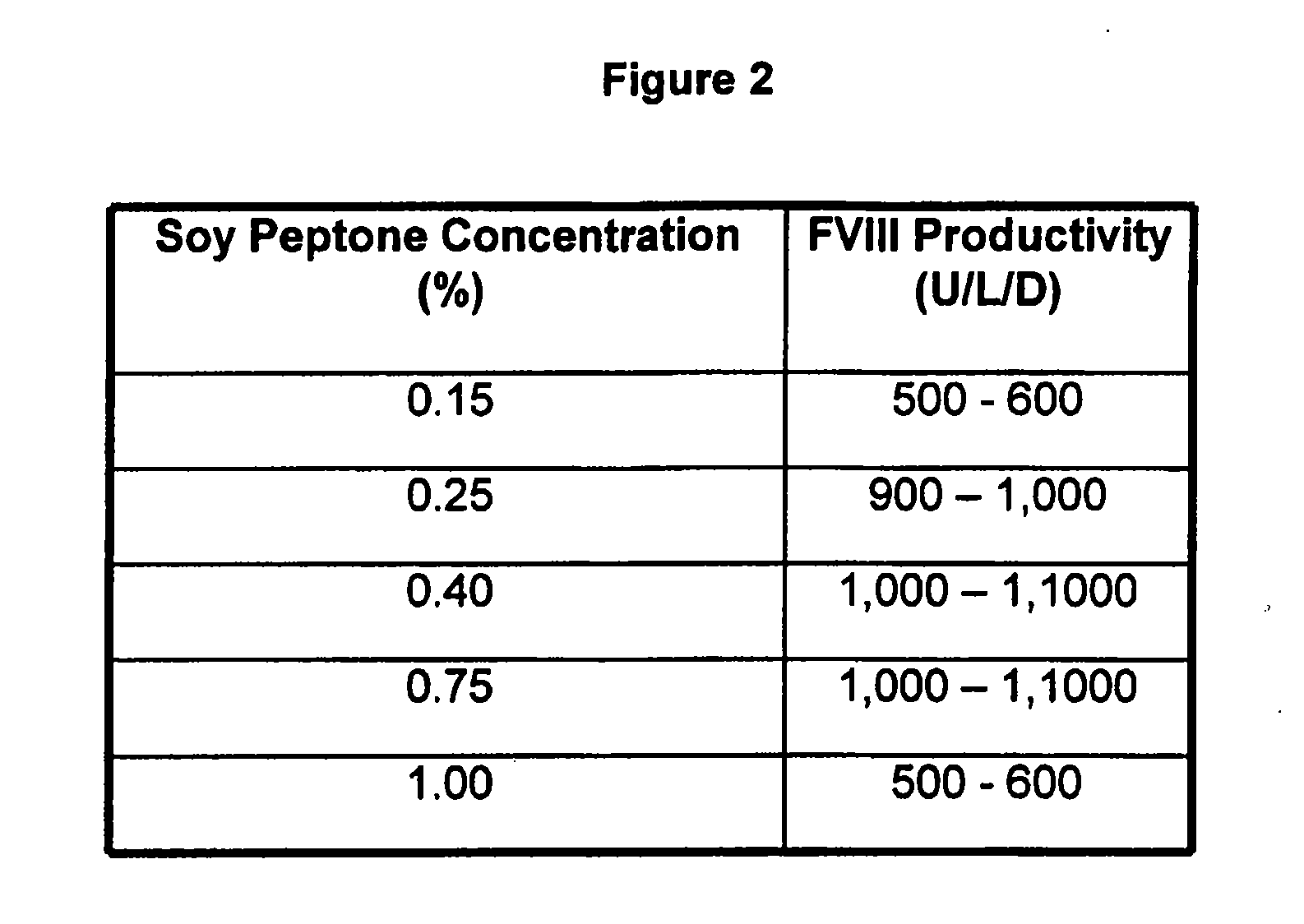
[0071] Animal protein free medium was prepared with basal DMEM / HAM's F12 (1:1) medium supplemented with inorganic salts, amino acids, vitamins and other components (Life technologies, 32500 Powder). Also added were L-glutamine (600 mg / L), ascorbic acid (20 μM), ethanol amine (25 μM), Synperonic® (SERVA) (0.25 g / L), sodium selenite (50 nM). Additionally, essential amino acids were supplemented to the cell culture medium. Further, varying concentrations of soy hydrolysate (Quest Technologies, NY or DMV Intl., NY) in the range of 0.0-1.0% and varying concentrations of polyamines (0-10 mg / L) were added (FIG. 1-9)
example 2
[0072] Cell cultures of recombinant mammalian cells (e.g. CHO-cells stably expressing Factor VIII=GD8 / 6-cells) were grown in suspension in a chemostat culture in 10 l bioreactors. The culture conditions of 37° C., oxygen saturation 20% and pH 7.0 to 7.1 were kept constant. The cultures were supplied with a constant feed of BAV-medium as defined in Example 1 additionally supplemented with soy hydrolysates in the range of 0.1-1.0% and / or addition of putrescine.2HCl in the range of 0-1 mg / L (cf. FIG. 1-5).
[0073] Small scale experiments with GD8 / 6 cells in suspension culture were carried in Techne spinner flasks at 200 ml working volume in batch refeed mode at 37° C., without pH and pO2 control. The cultures were supplied with BAV-medium as defined in Example 1 without supplementation of soy hydrolysate and polyamines, or supplemented with soy hydrolysate in the range of 0.1-0.4% and / or putrescine.2HCl, ornithine.HCl, spermine.4HCl in the range of 0-18 mg / L (equivalent to 0-10 mg / L of ...
example 4
[0075] The activity of Factor VIII (FVIII) (cf. FIGS. 1 to 5 and 9) was measured by a chromogenic assay (Chromogenic, Sweden). The activity of erythropoeitin (cf. FIG. 8) and the monoclonal antibody titer (cf. FIG. 7) were measured by ELISA test systems.
[0076] The volumetric productivity is calculated from the amount of activity units or antigen titers yielded per liter reactor volume per day (U / L / d or mg / L / d) in the respective production systems.
[0077] The cell specific productivity is defined as the specific amount of produced protein (U or μg) per number of cells per day (cf. FIGS. 7 and 9) or as the specific amount of produced protein (U) produced per amount of D-glucose consumed by the cells (cf. FIG. 8).
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com