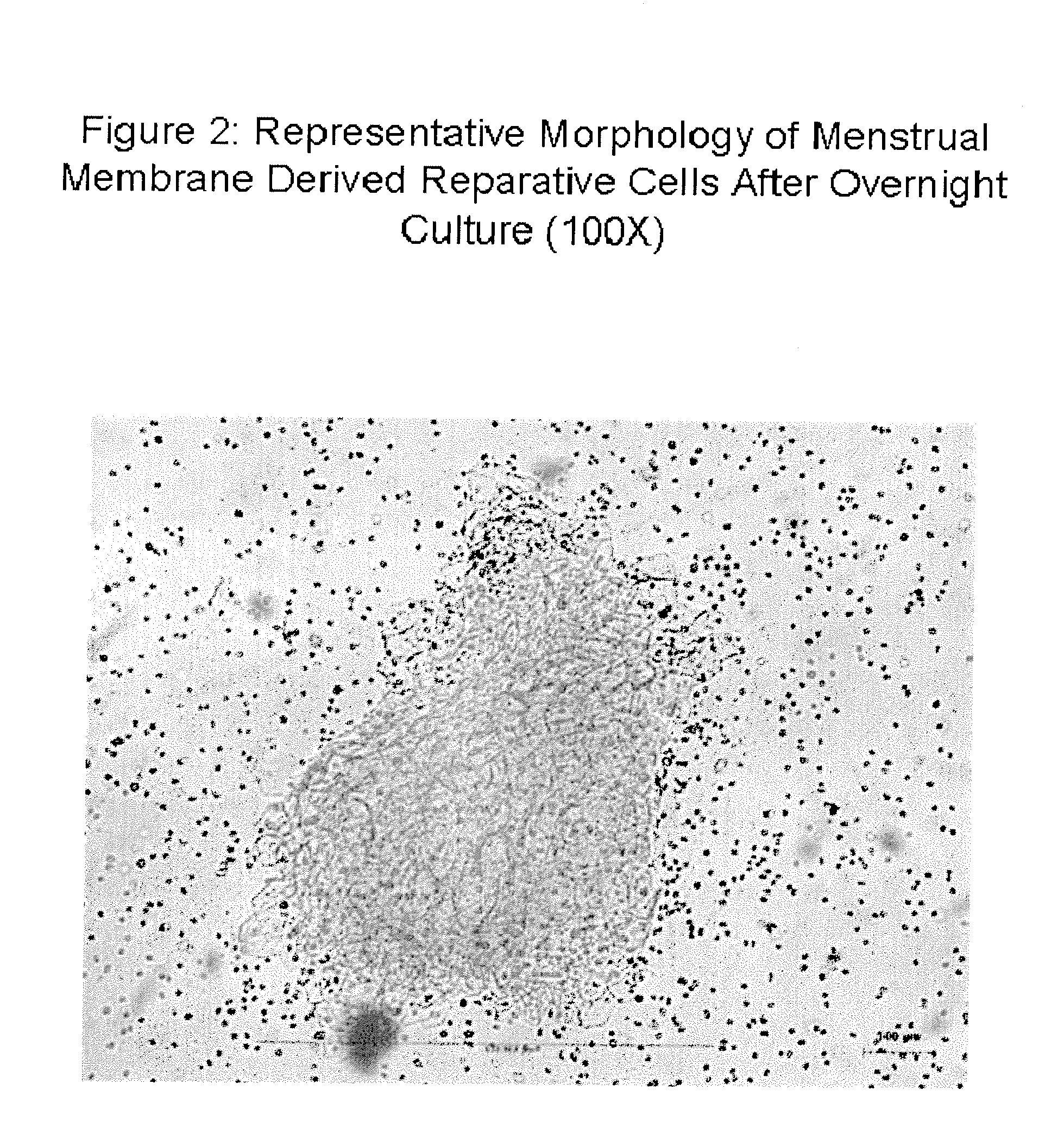
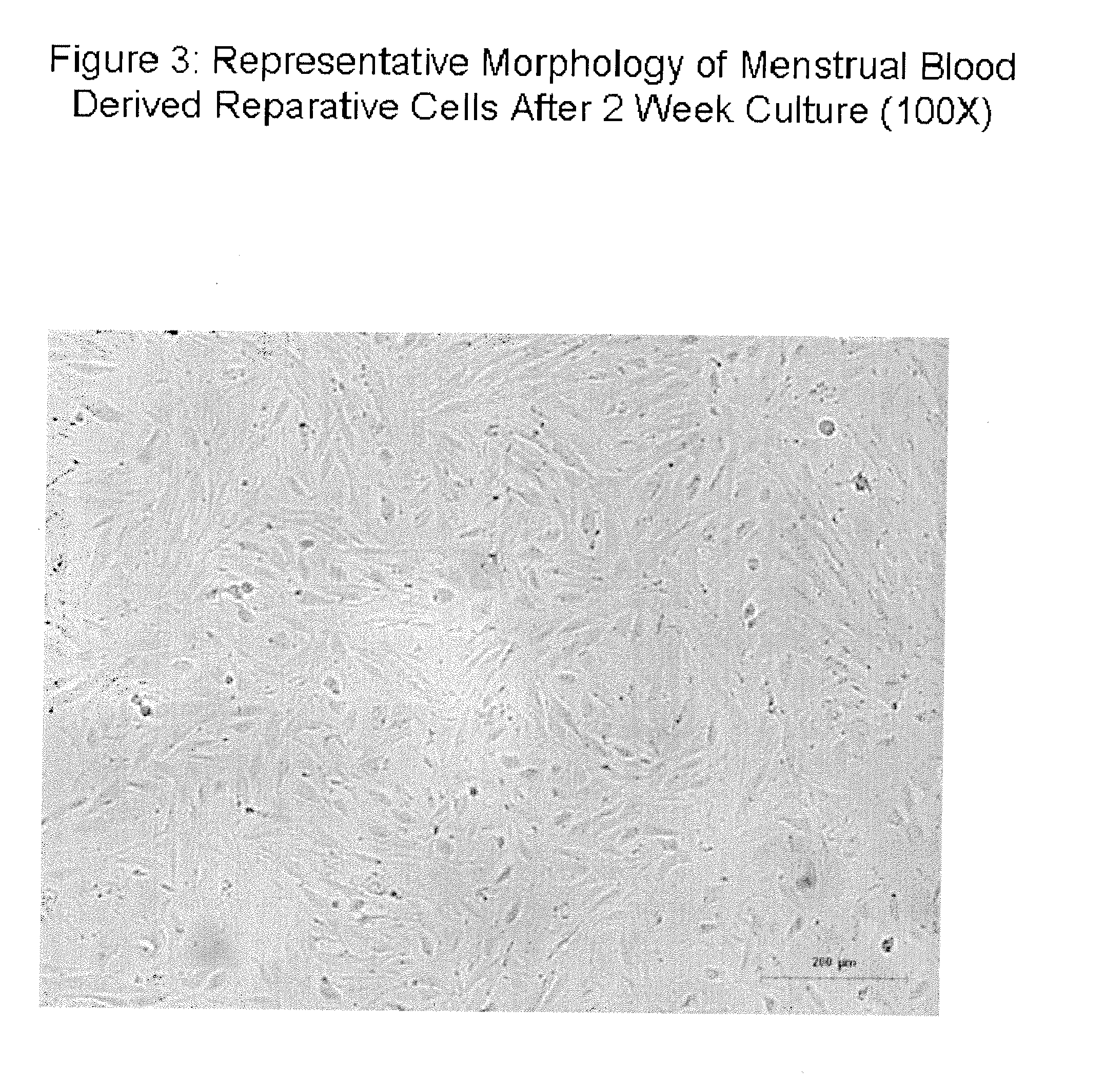
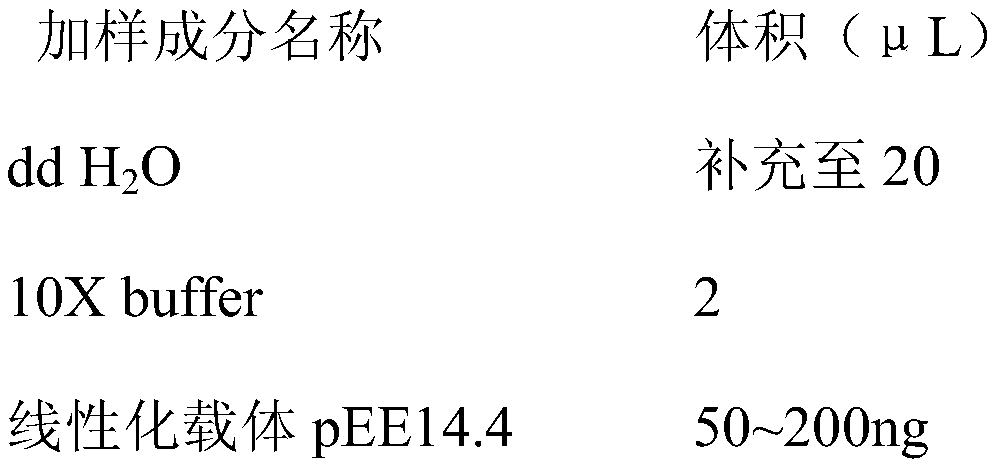
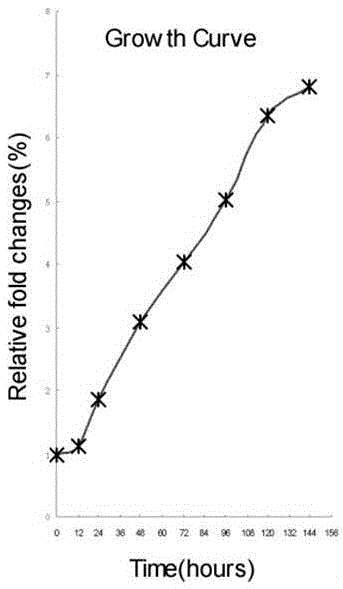
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
848results about "Genital tract cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
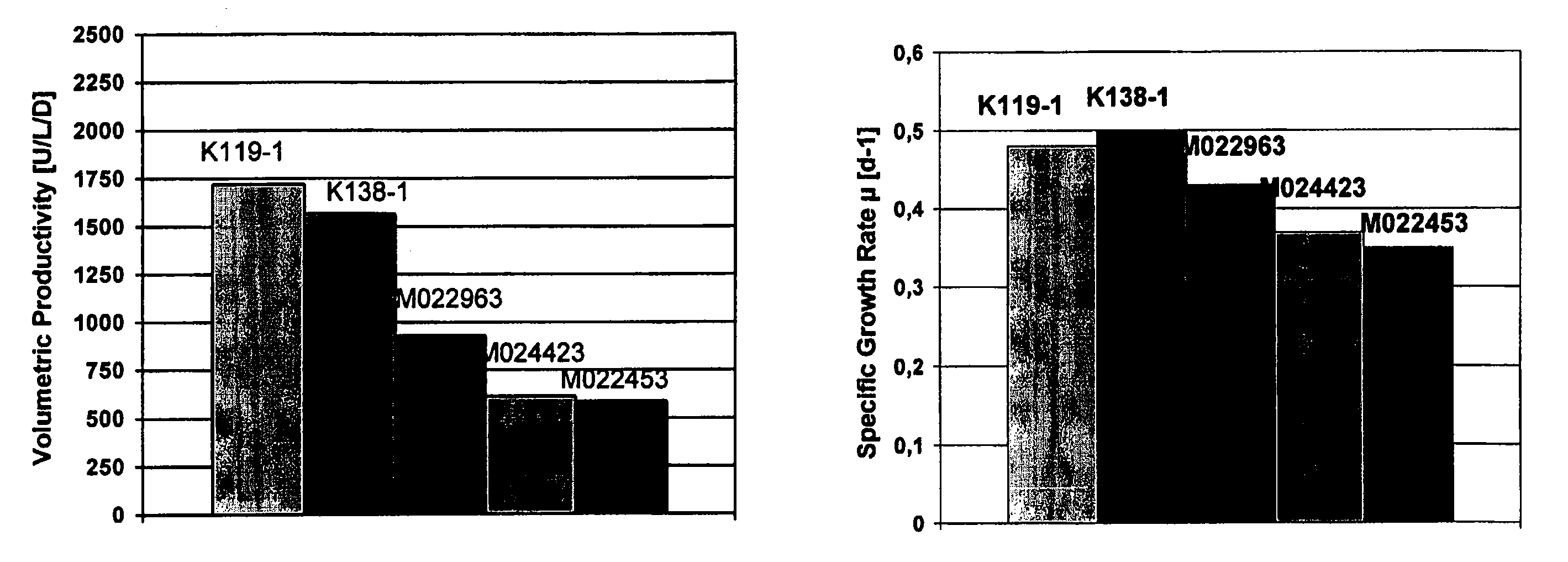
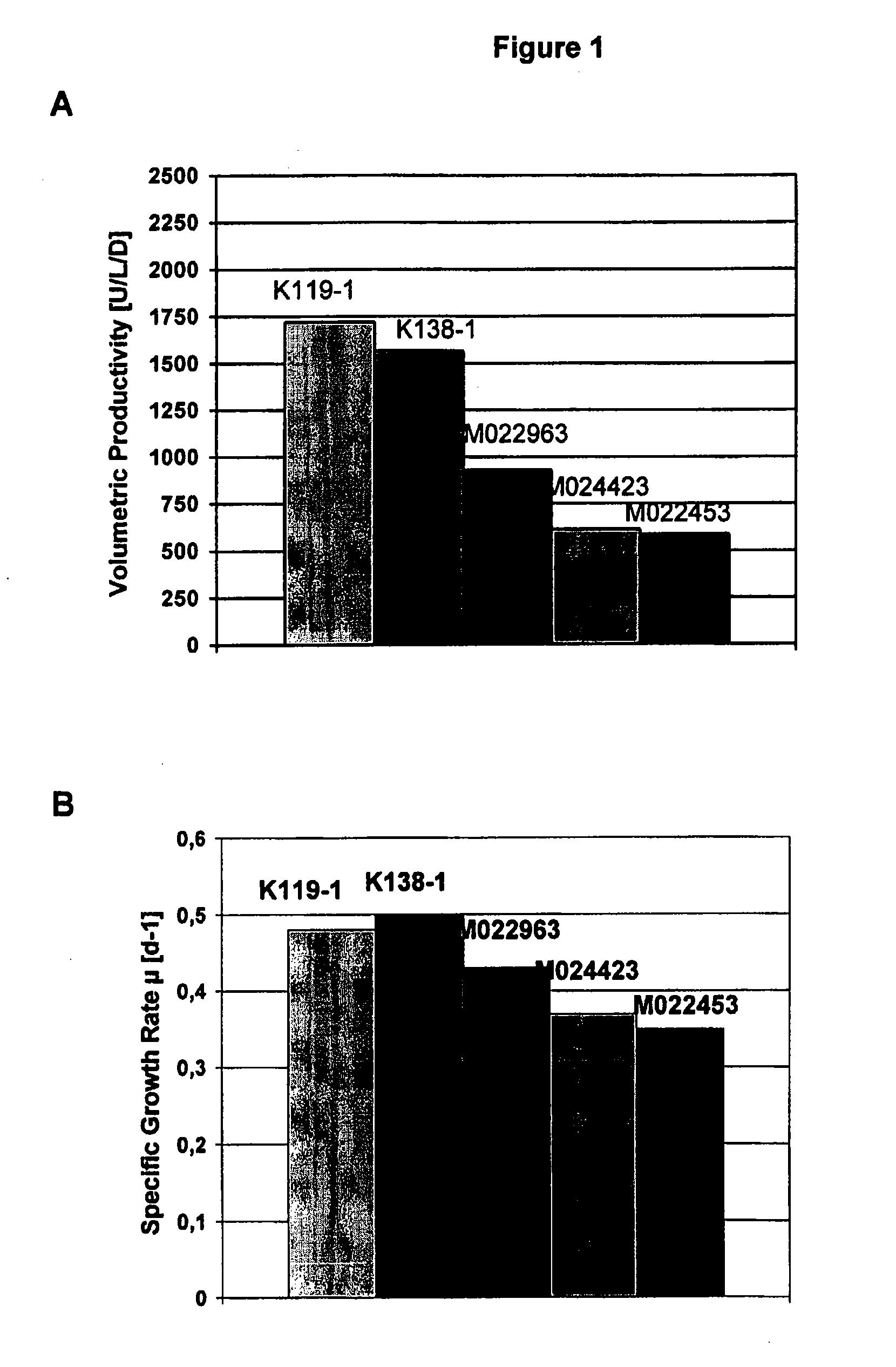
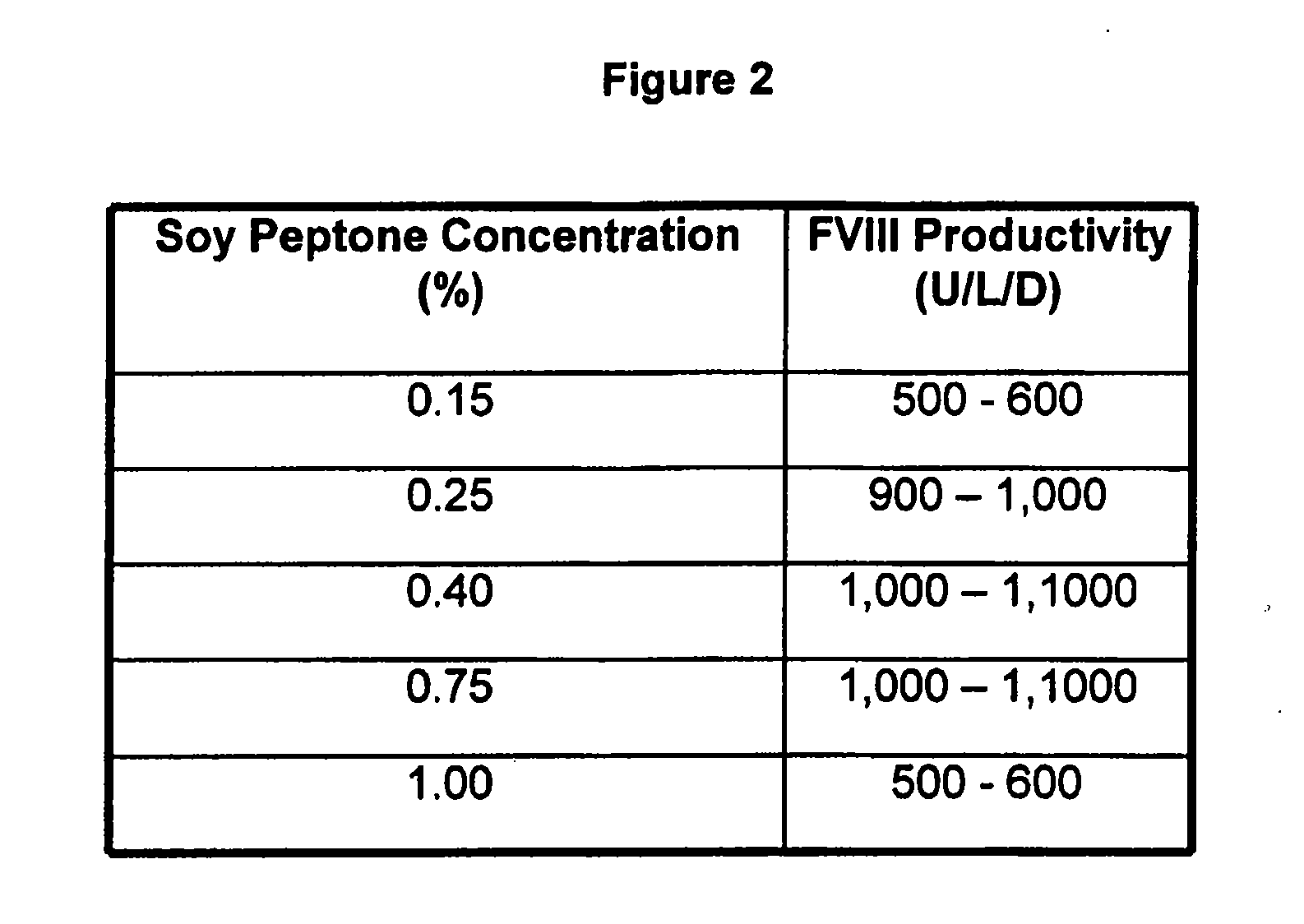
Animal protein-free media for cultivation of cells
InactiveUS20080009040A1Efficient expressionGuaranteed efficient growthMicroorganismsCulture processHydrolysateCell culture media
The present invention relates to animal protein-free cell culture media comprising polyamines and a plant- and / or yeast-derived hydrolysate. The invention also relates to animal protein-free culturing processes, wherein cells can be cultivated, propagated and passaged without adding supplementary animal proteins in the culture medium. These processes are useful in cultivating cells, such as recombinant cells or cells infected with a virus, and for producing biological products by cell culture processes.
Owner:BAXTER INT INC +1
Endometrial stem cells and methods of making and using same
InactiveUS20090053182A1Rapid rate of cellular divisionBiocidePeptide/protein ingredientsCell lineageEndometrium
The invention provides pluripotent stem cells and methods for making and using pluripotent stem cells. Pluripotent stem cells, among other things, can differentiate into various cell lineages in vitro, ex vivo and in vivo. Pluripotent stem cells, among other things, can also be used to produce conditioned medium.
Owner:MEDISTEM LAB
Methods for the culture of human embryonic stem cells on human feeder cells
InactiveUS7432104B2Artificial cell constructsMammal material medical ingredientsBone Marrow Stromal CellCell culture media
Methods and cell culture medium for the generation of human pluripotent embryonic stem cells are disclosed. Human embryonic stem cells are cultured with human granulosa feeder cells, muscle cells, Fallopian ductal epithelial cells, bone marrow stromal cells, and skin fibroblasts and the embryonic stem cells maintain their pluripotent phenotype. The human pluripotent embryonic stem cells can be cultured without feeder cells, and in the presence of supplemental growth factors. The human pluripotent embryonic stem cells can be alternatively cultured with conditioned medium obtained from a cell culture capable of maintaining human embryonic stem cells in a pluripotent state, wherein the cell culture is a human granulosa cell culture.
Owner:VIACYTE INC
Alternative compositions and methods for the culture of stem cells
InactiveUS20050037488A1Artificial cell constructsMammal material medical ingredientsBone Marrow Stromal CellCell culture media
Methods and cell culture medium for the generation of human pluripotent embryonic stem cells are disclosed. Human embryonic stem cells are cultured with human granulosa feeder cells, muscle cells, Fallopian ductal epithelial cells, bone marrow stromal cells, and skin fibroblasts and the embryonic stem cells maintain their pluripotent phenotype. The human pluripotent embryonic stem cells can be cultured without feeder cells, and in the presence of supplemental growth factors. The human pluripotent embryonic stem cells can be alternatively cultured with conditioned medium obtained from a cell culture capable of maintaining human embryonic stem cells in a pluripotent state, wherein the cell culture is a human granulosa cell culture.
Owner:VIACYTE INC
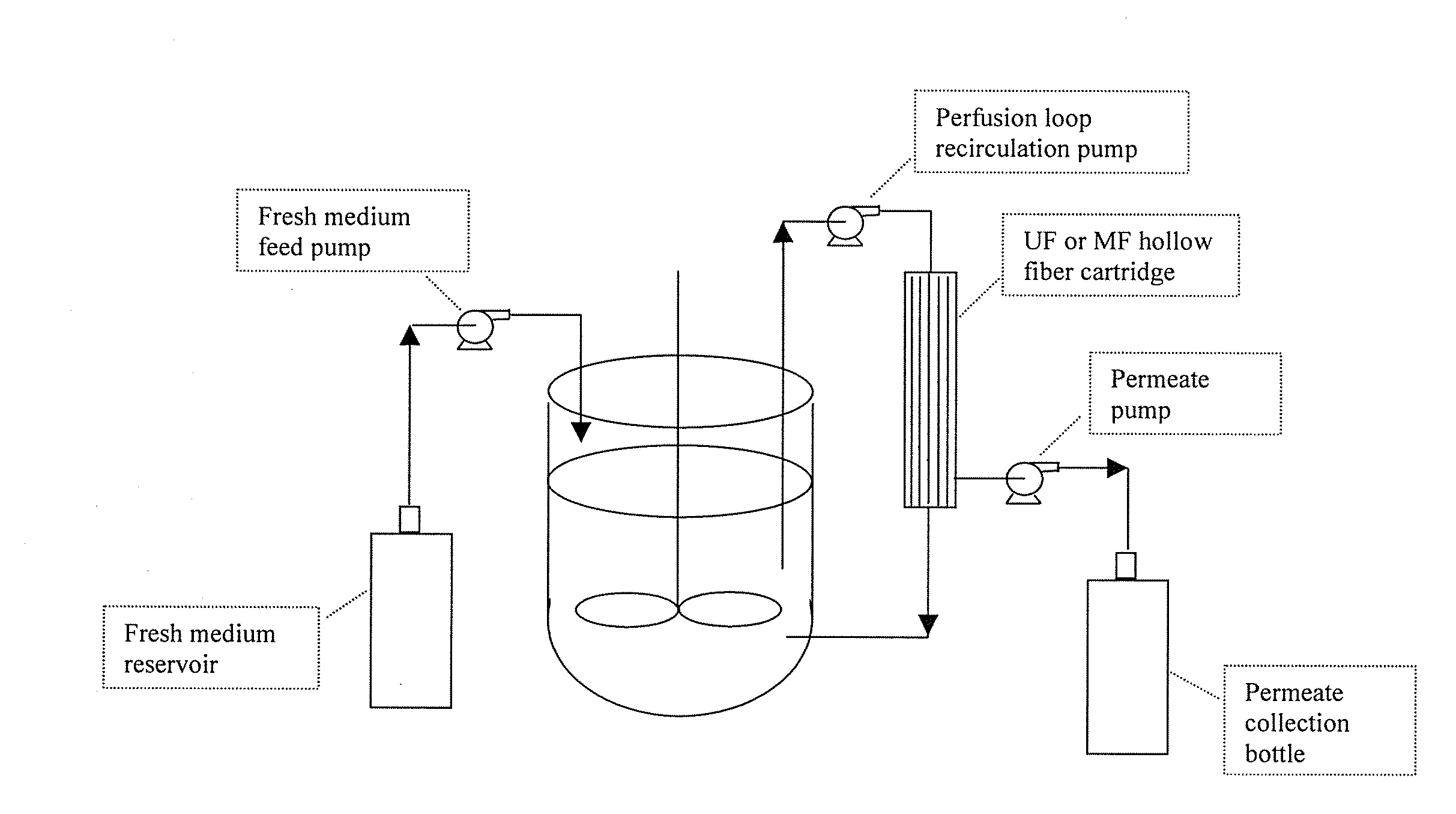
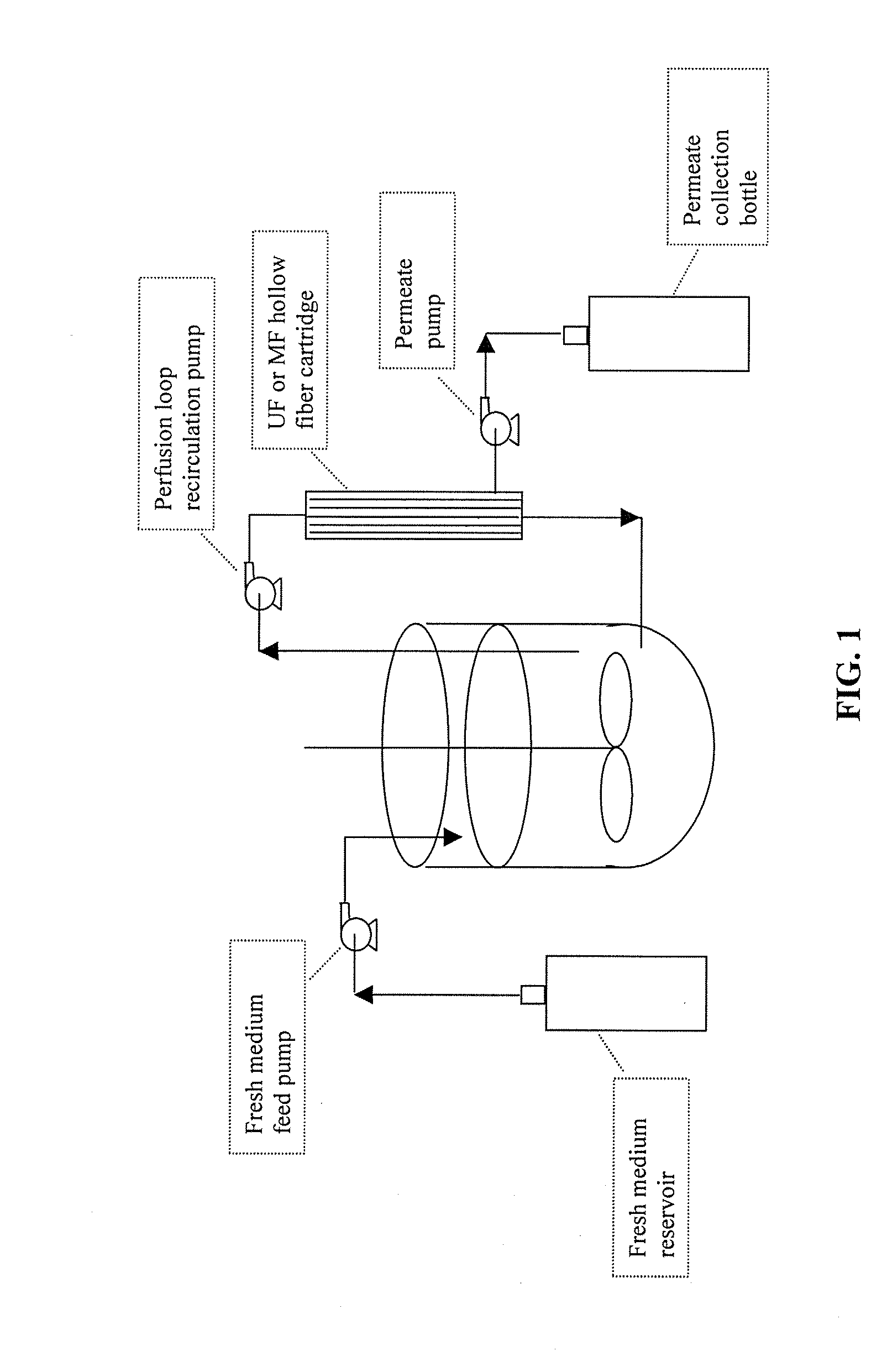
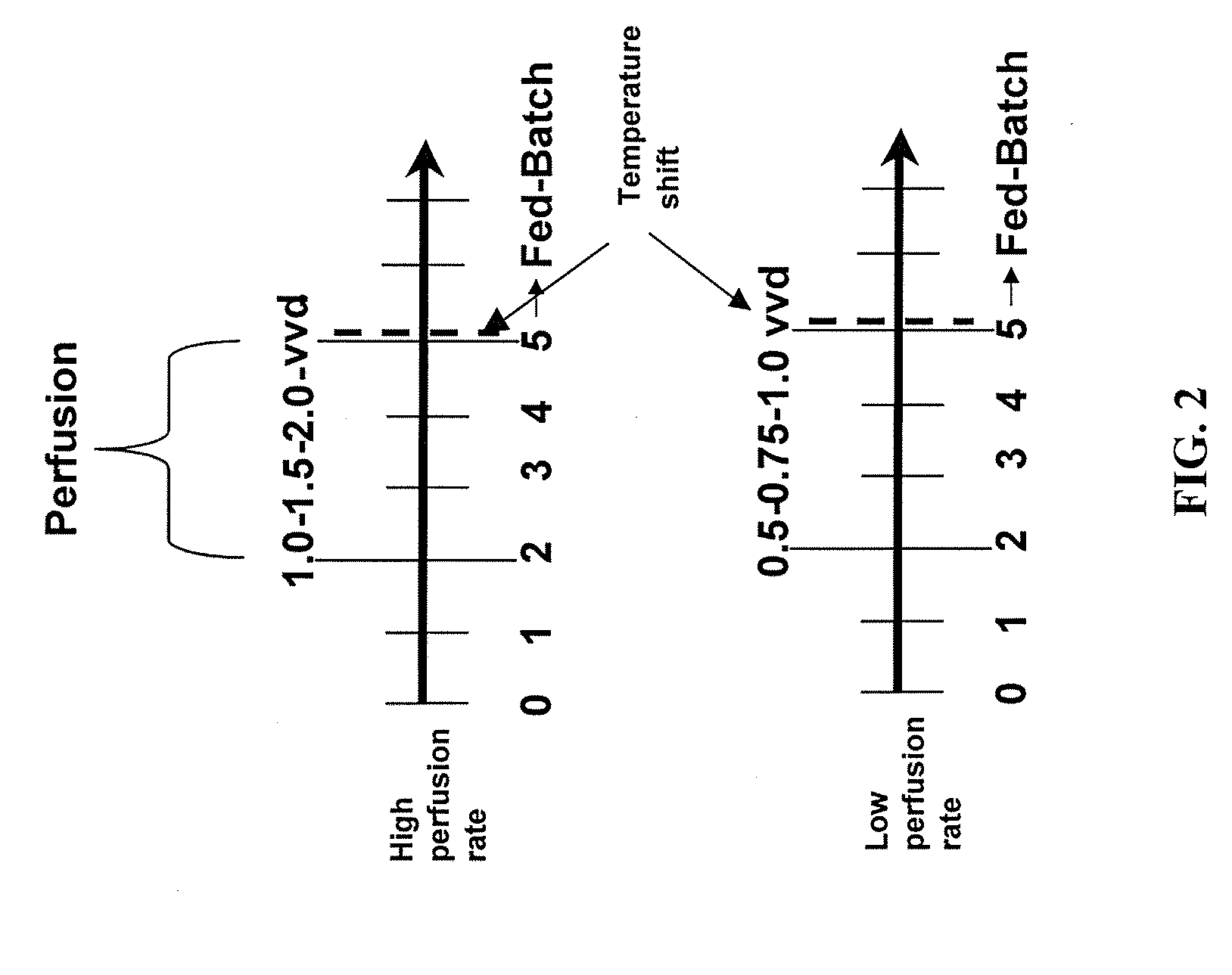
Use of perfusion to enhance production of fed-batch cell culture in bioreactors
InactiveUS20090042253A1Bioreactor/fermenter combinationsBiological substance pretreatmentsFiltrationFeed pump
The invention relates to methods of improving protein production, e.g., large-scale commercial protein production, e.g., antibody production, utilizing a modified fed-batch cell culture method comprising a cell growth phase and a polypeptide production phase. The modified fed-batch cell culture method combines both cell culture perfusion and fed-batch methods to achieve higher titers of polypeptide products. Because the modified fed-batch cell culture method of the invention produces higher polypeptide product titers than fed-batch culture alone, it will substantially improve commercial-scale protein production. The invention also relates to a perfusion bioreactor apparatus comprising a fresh medium reservoir connected to a bioreactor by a feed pump, a recirculation loop connected to the bioreactor, wherein the recirculation loop comprises a filtration device, e.g., ultrafiltration or microfiltration, and a permeate pump connecting the filtration device to a permeate collection container.
Owner:WYETH LLC
Methods of preparing transplantable product for treatment of skin defects
InactiveUS20060228339A1Facilitated DiffusionDelaying their differentiationBiocideEpidermal cells/skin cellsStromal cellMembrane configuration
A method for preparing a tissue culture insert that is used for constructing a transplantable graft of an engineered tissue equivalent comprising living main functional cells, stromal cells and tissue matrix on / in a biological supporting membrane for treatment of body tissue defects.
Owner:WANG ZHENG PIN
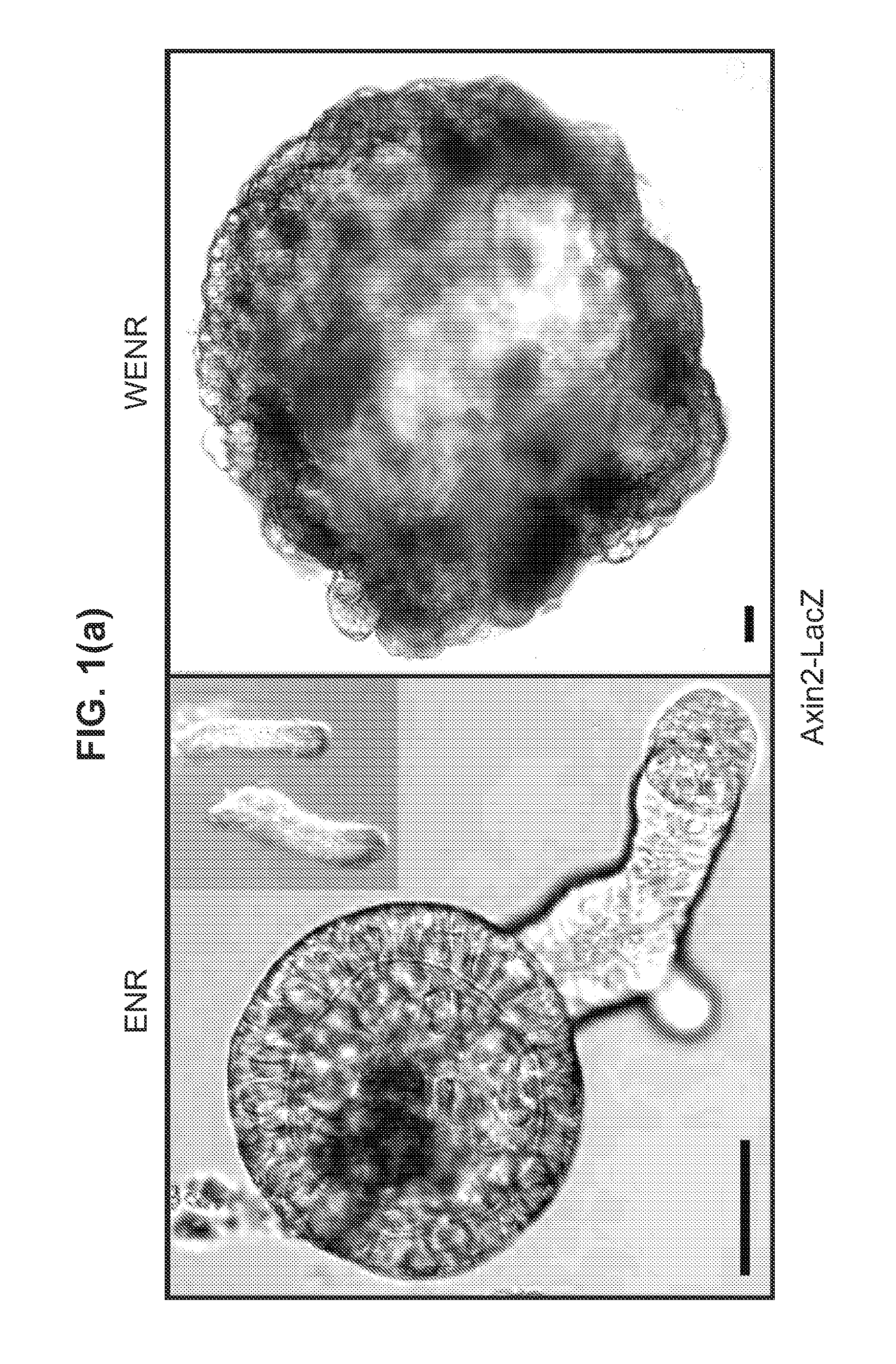
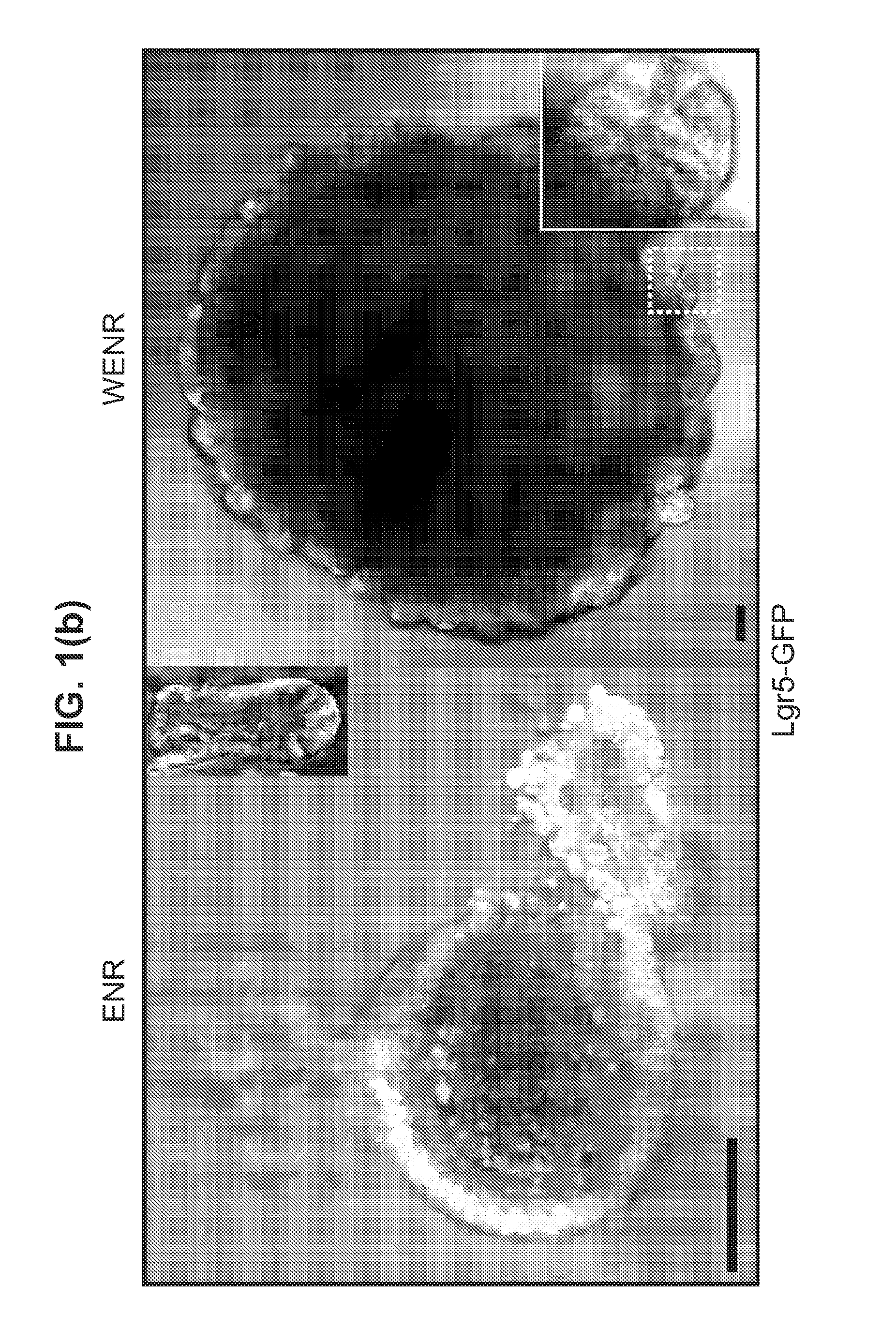
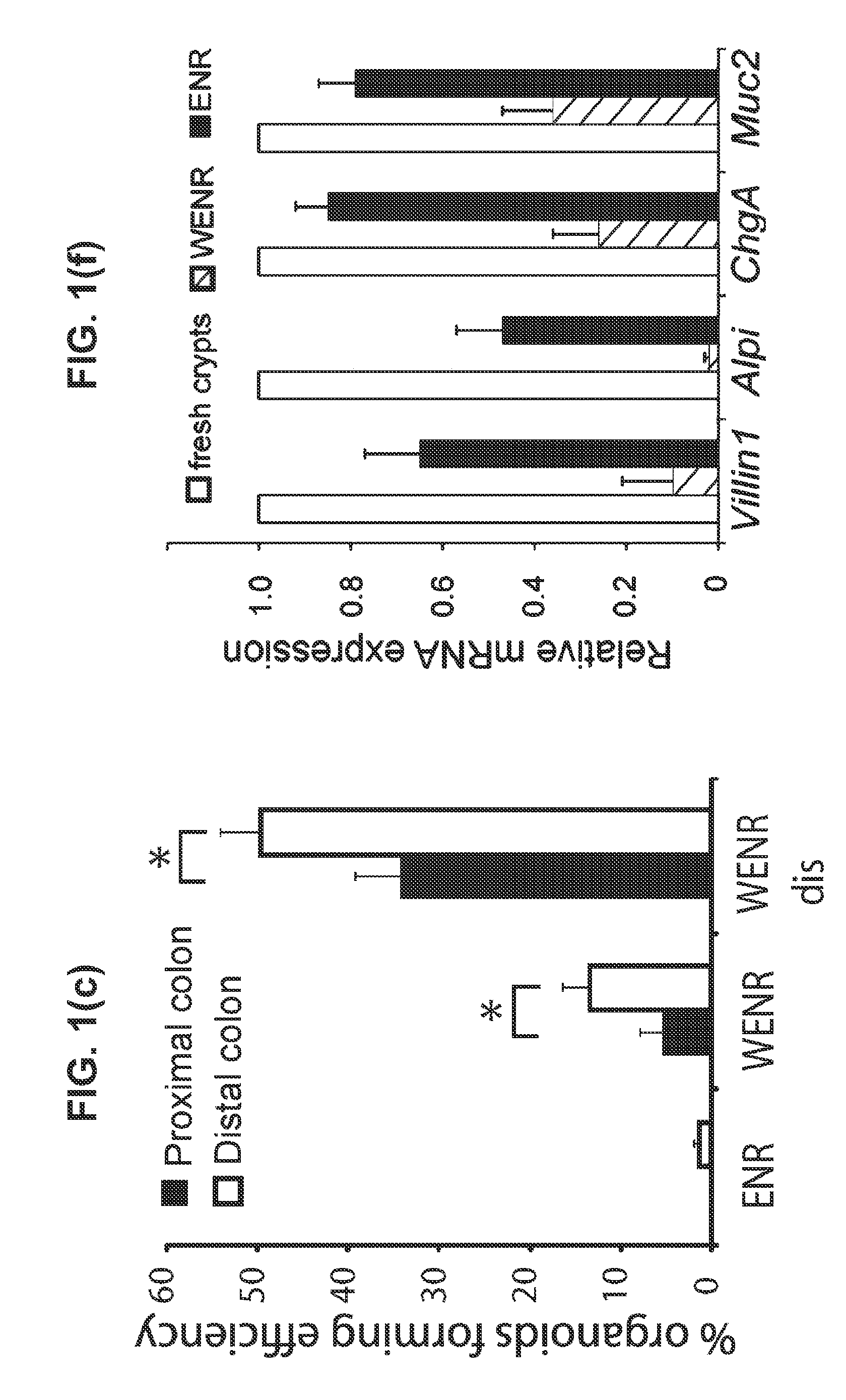
Culture media for stem cells
PendingUS20140243227A1Slow proliferationIncrease surface areaBioreactor/fermenter combinationsBiological substance pretreatmentsStem cell cultureBiology
Culture media and methods for expanding and differentiating populations of stem cells and for obtaining organoids. Expanded cell populations and organoids obtainable by methods of the invention and their use in drug screening, toxicity assays and regenerative medicine.
Owner:KONINK NEDERLANDSE AKADE VAN WETENSCHAPPEN
Mammalian cell culture processes for protein production
ActiveUS20110081679A1High densityReduce aggregationPolypeptide with localisation/targeting motifAntibody mimetics/scaffoldsBiotechnologyGlucocorticoid
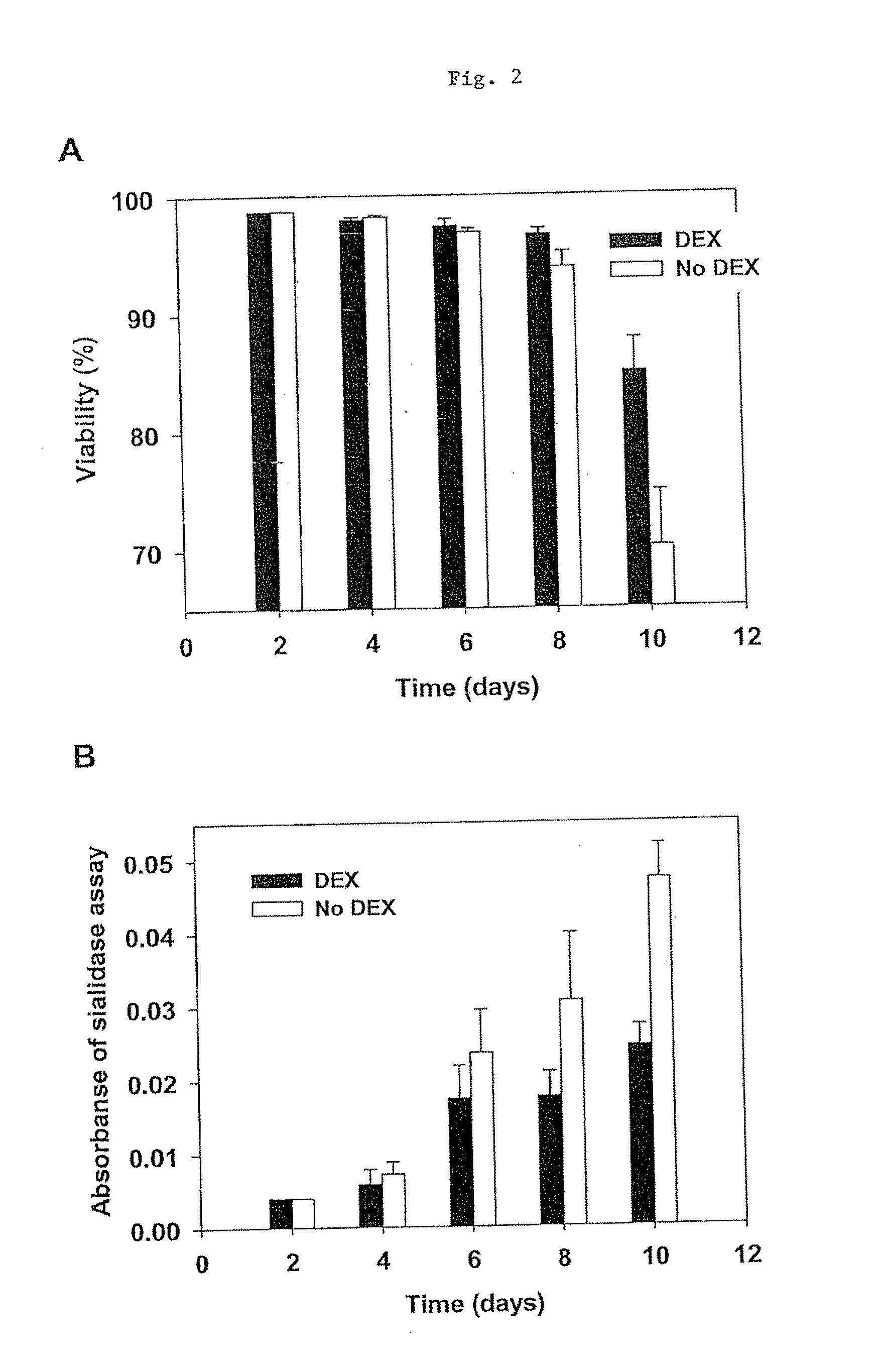
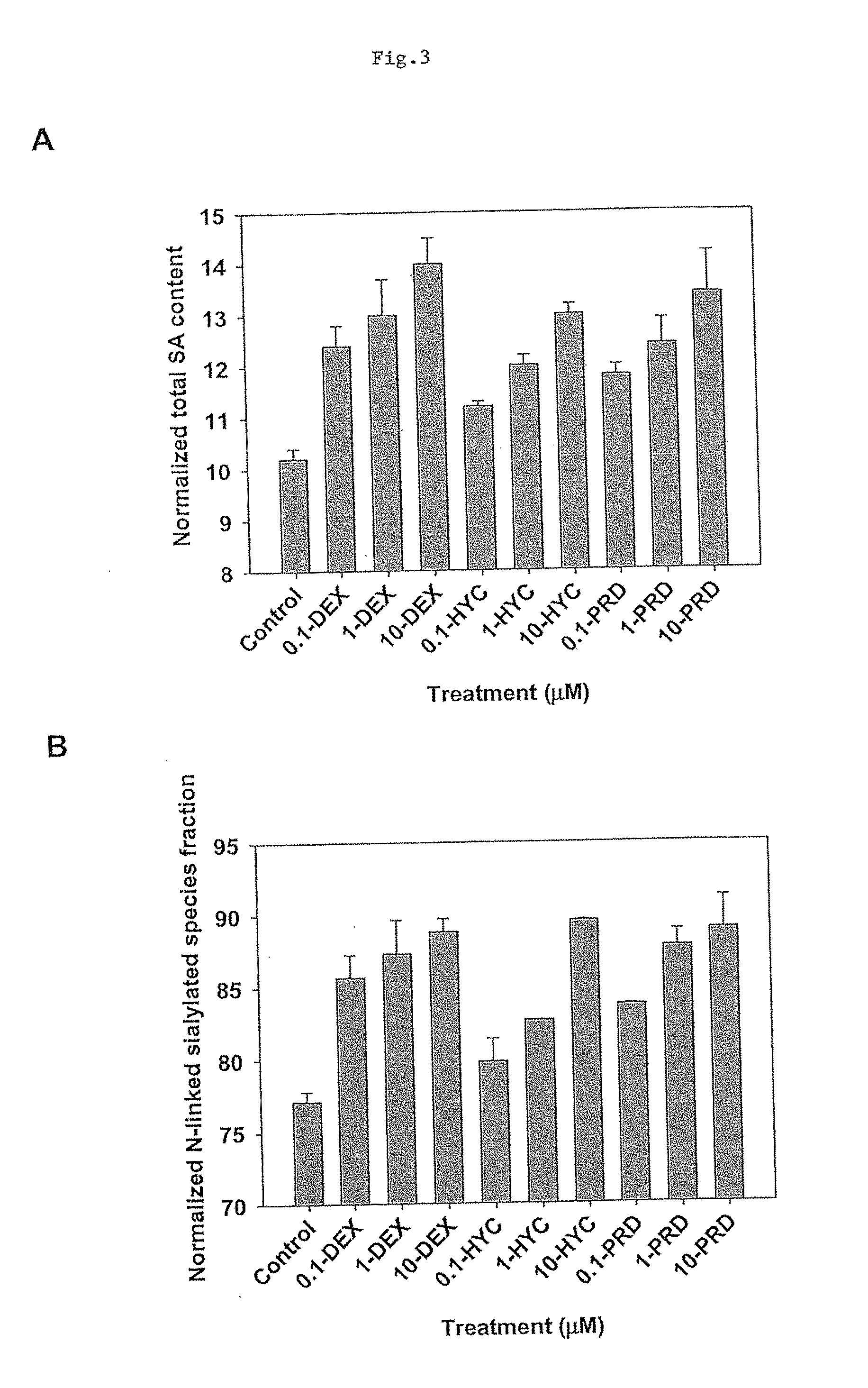
The present invention describes methods and processes for the production of proteins, particularly glycoproteins, by animal cell or mammalian cell culture, preferably, but not limited to, fed-batch cell cultures. In one aspect, the methods comprise the addition of glucocorticoid compound during the culturing period. The addition of glucocorticoid compound sustain a high viability of the cultured cells, and can yield an increased end titer of protein product, and a high quality of protein product, as determined, e.g., by sialic acid content of the produced protein.
Owner:BRISTOL MYERS SQUIBB CO
Three dimensional vaginal tissue model containing immune cells
InactiveUS6943021B2Improve survivabilityInduced proliferationBiocideEpidermal cells/skin cellsSerum free mediaAir liquid interface
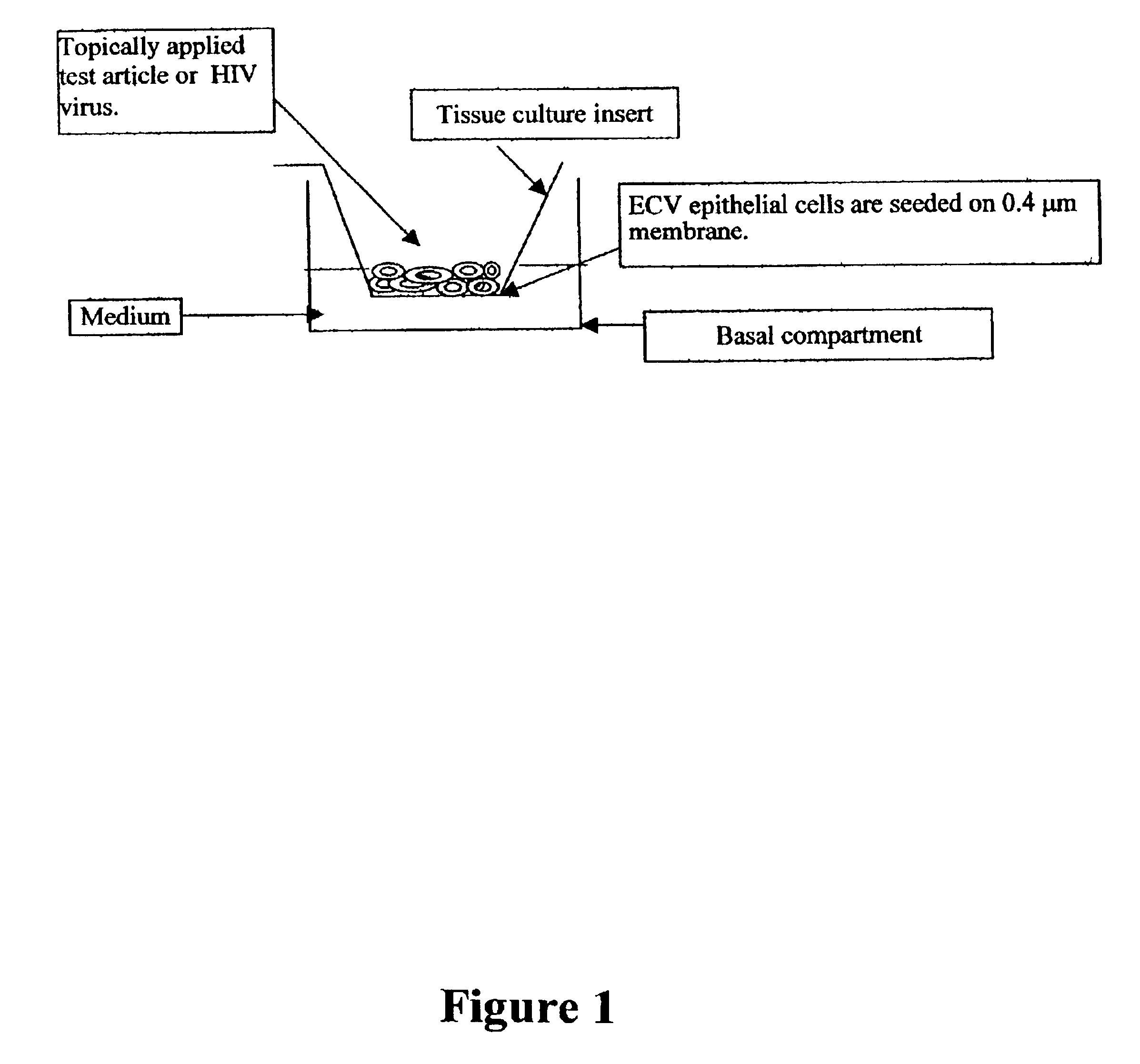
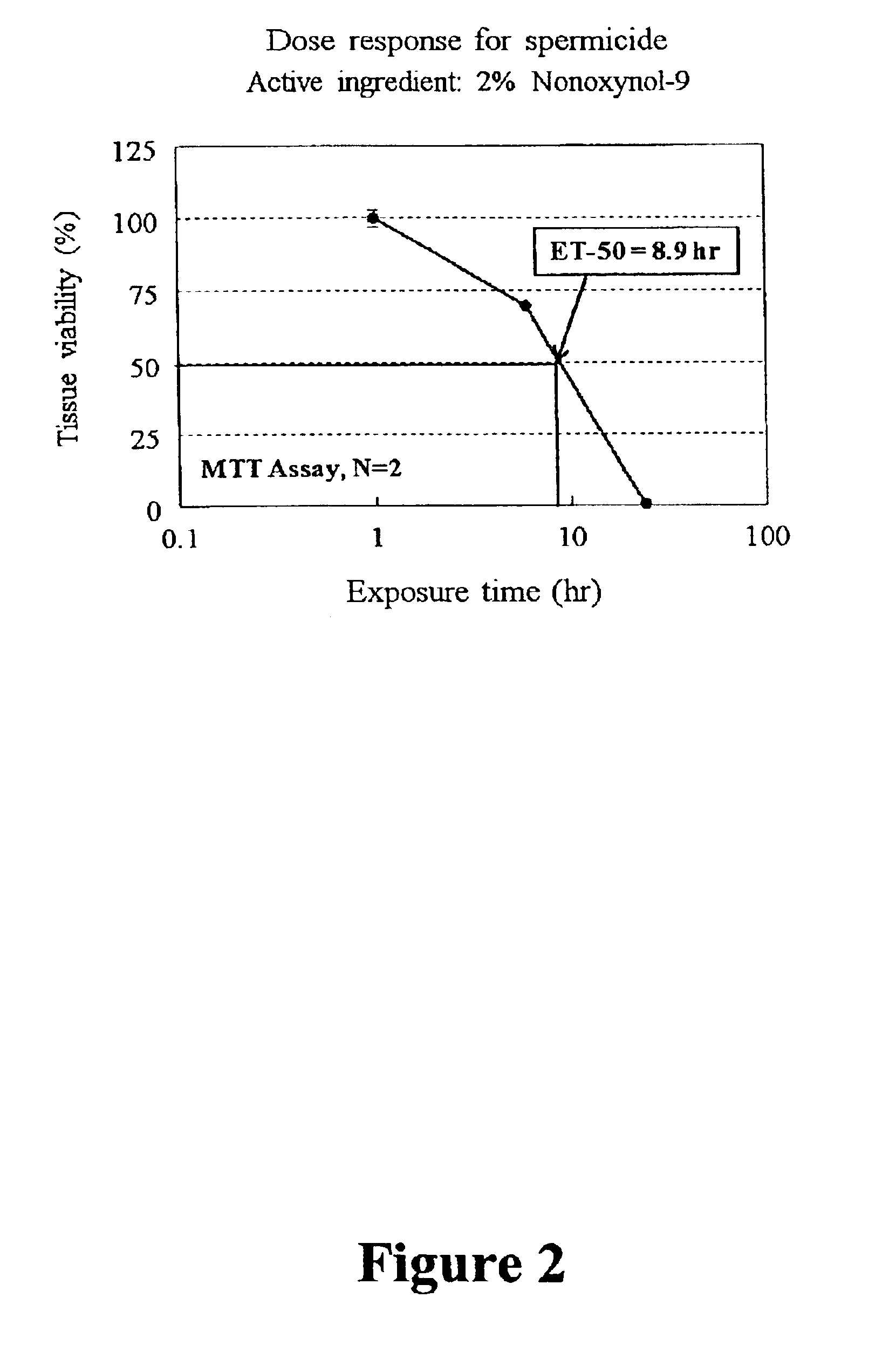
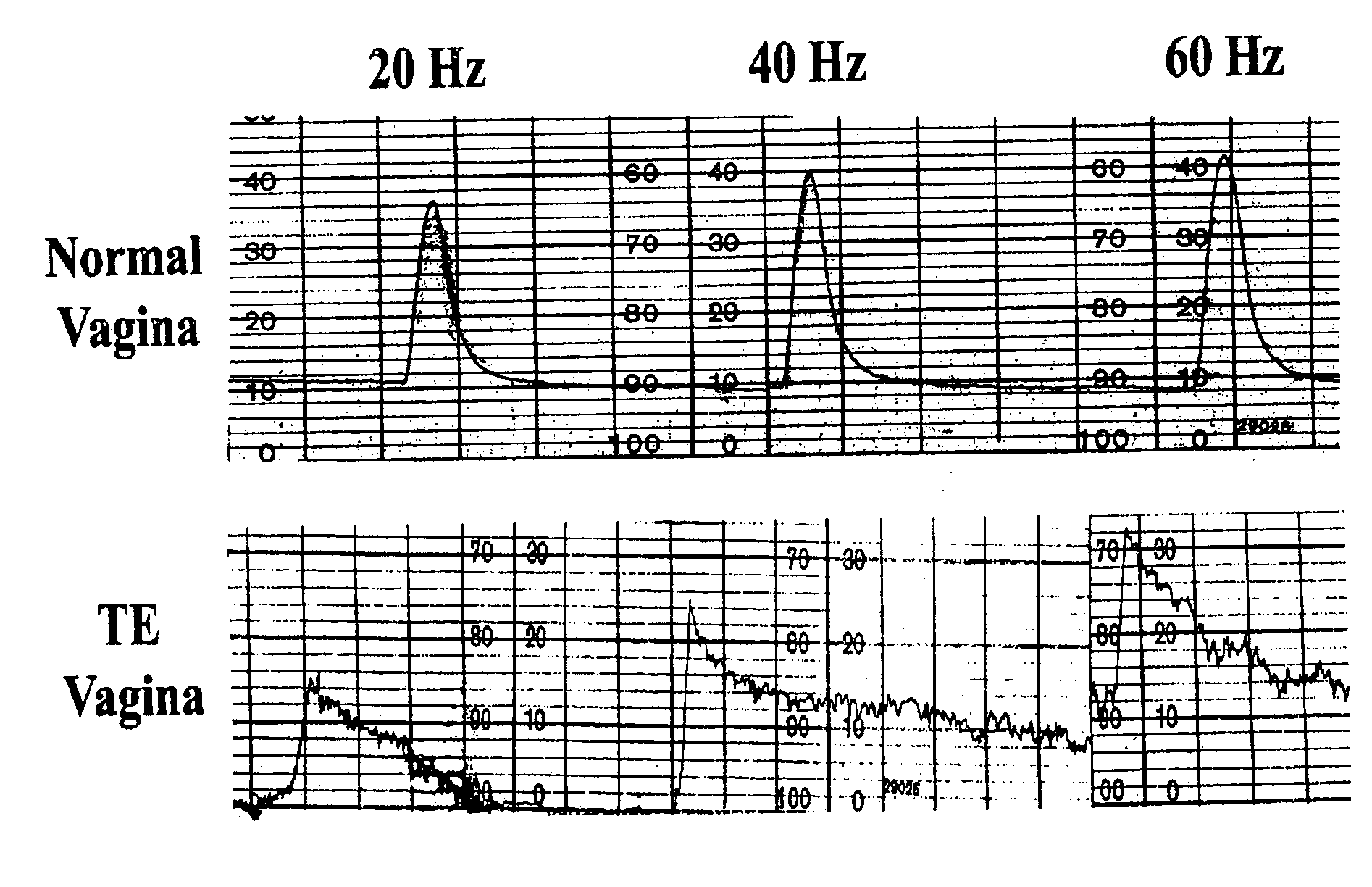
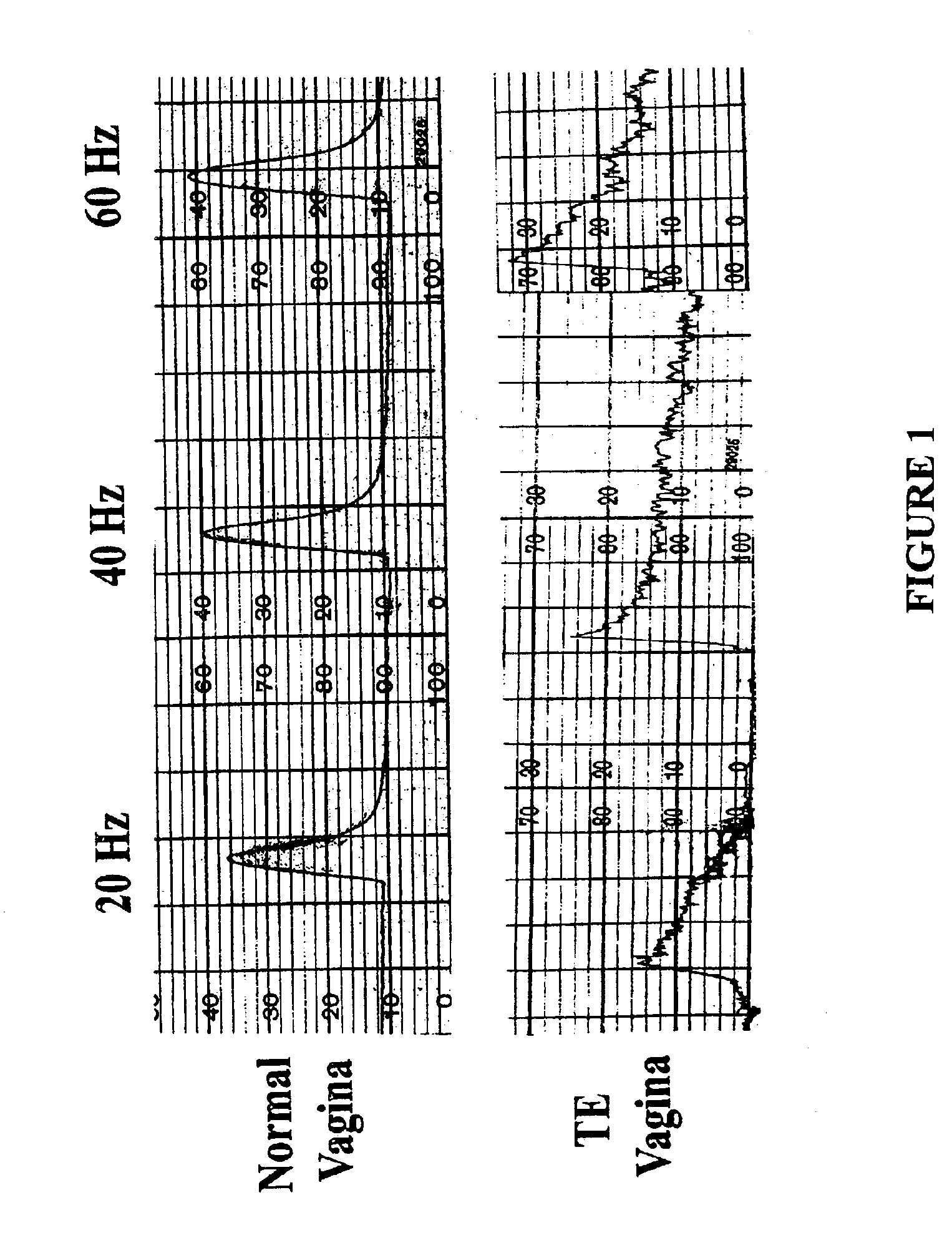
Disclosed is a cervico-vaginal tissue equivalent comprised of vaginal epithelial cells and immune cells, cultured at the air-liquid interface. The tissue equivalent is capable of being infected with a sexually transmitted pathogen such as a virus (e.g., HIV), a bacteria, a helminthic parasite, or a fungus. The tissue equivalent is also capable of undergoing an allergic-type reaction or an irritant-type reaction. The tissue equivalent is characterized as having nucleated basal layer cells and nucleated suprabasal layer cells, and further as having cell layers external to the suprabasal layer progressively increasing in glycogen content and progressively decreasing in nuclei content. Immune cells of the tissue equivalent are primarily located in the basal and suprabasal layers. Also disclosed are methods for producing the tissue equivalent. The methods involve providing vaginal epithelial cells and immune cells, seeding the cells onto a porous support, and co culturing the seeded cells at the air-liquid interface under conditions appropriate for differentiation. One such method disclosed is for generation of the tissue equivalent in serum free medium. Specific cells from which the tissue equivalent is generated, and also specific preferred components of the medium in which the tissue equivalent is generated are provided. Also disclosed is a cervico-vaginal tissue equivalent produced by the methods disclosed herein.
Owner:MATTEK CORP
Culture media for stem cells
Culture media and methods for expanding and differentiating populations of stem cells and for obtaining organoids. Expanded cell populations and organoids obtainable by methods of the invention and their use in drug screening, toxicity assays and regenerative medicine.
Owner:KONINK NEDERLANDSE AKADE VAN WETENSCHAPPEN
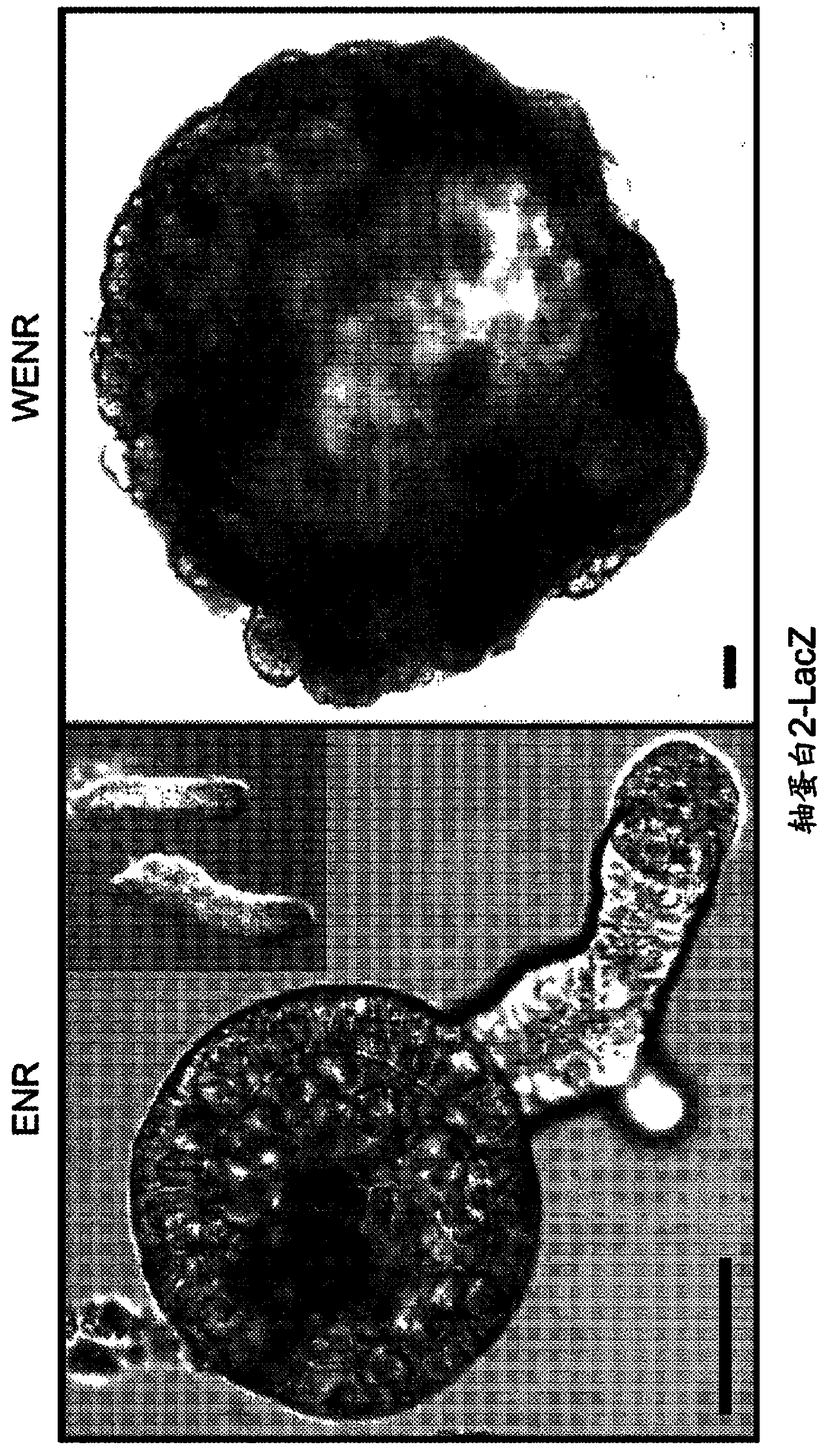
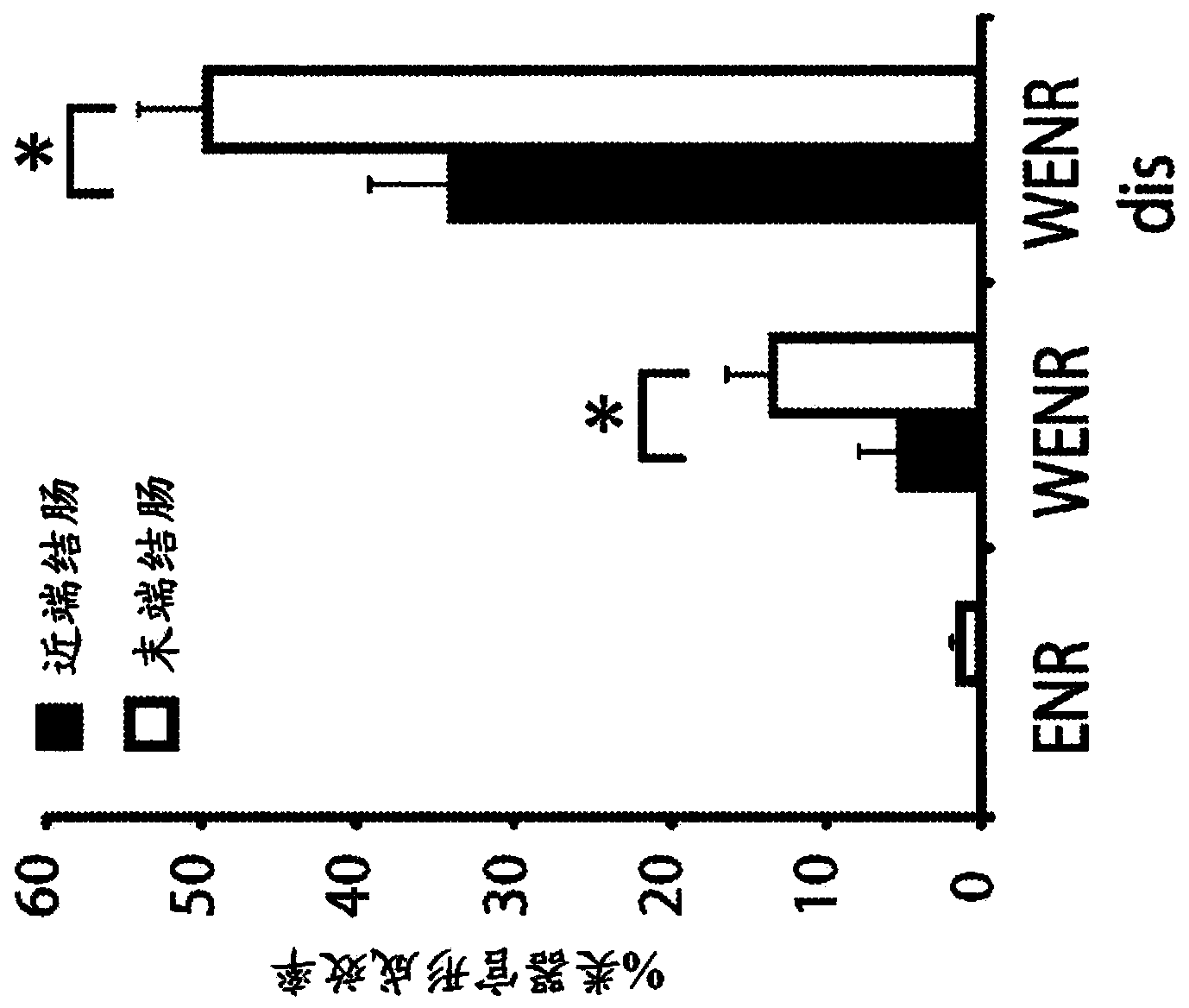
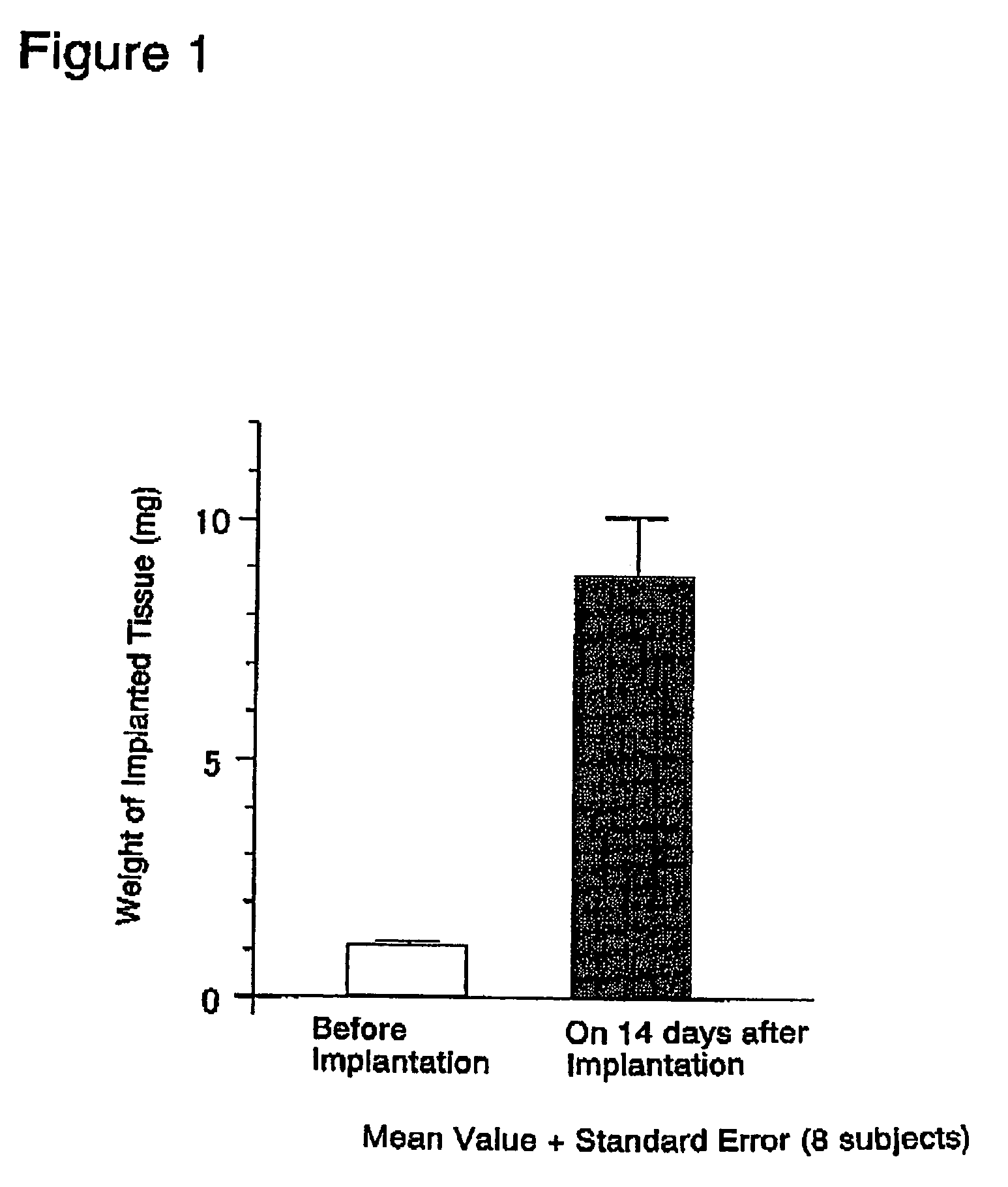
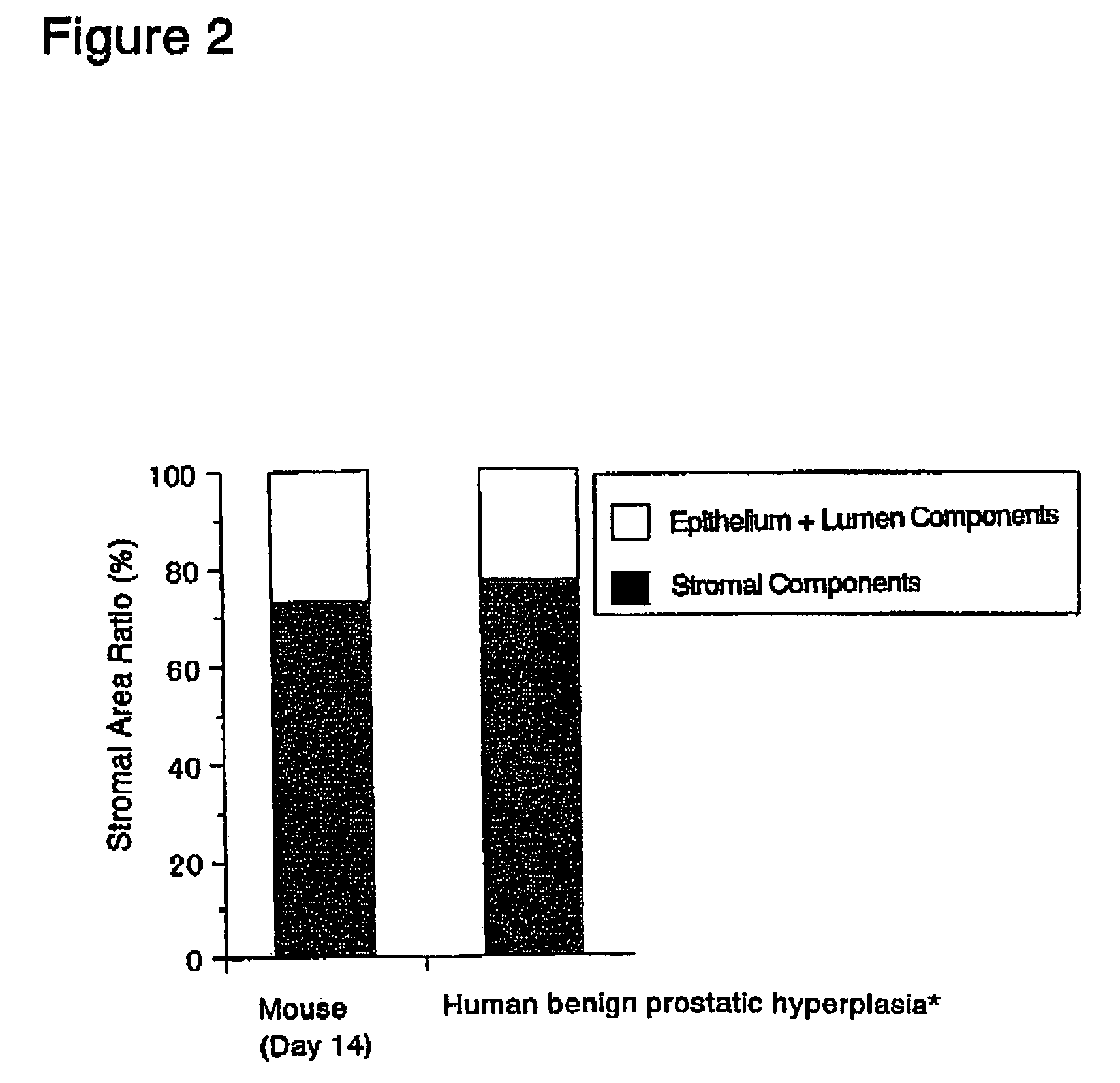
Interstitial prostatism model animal
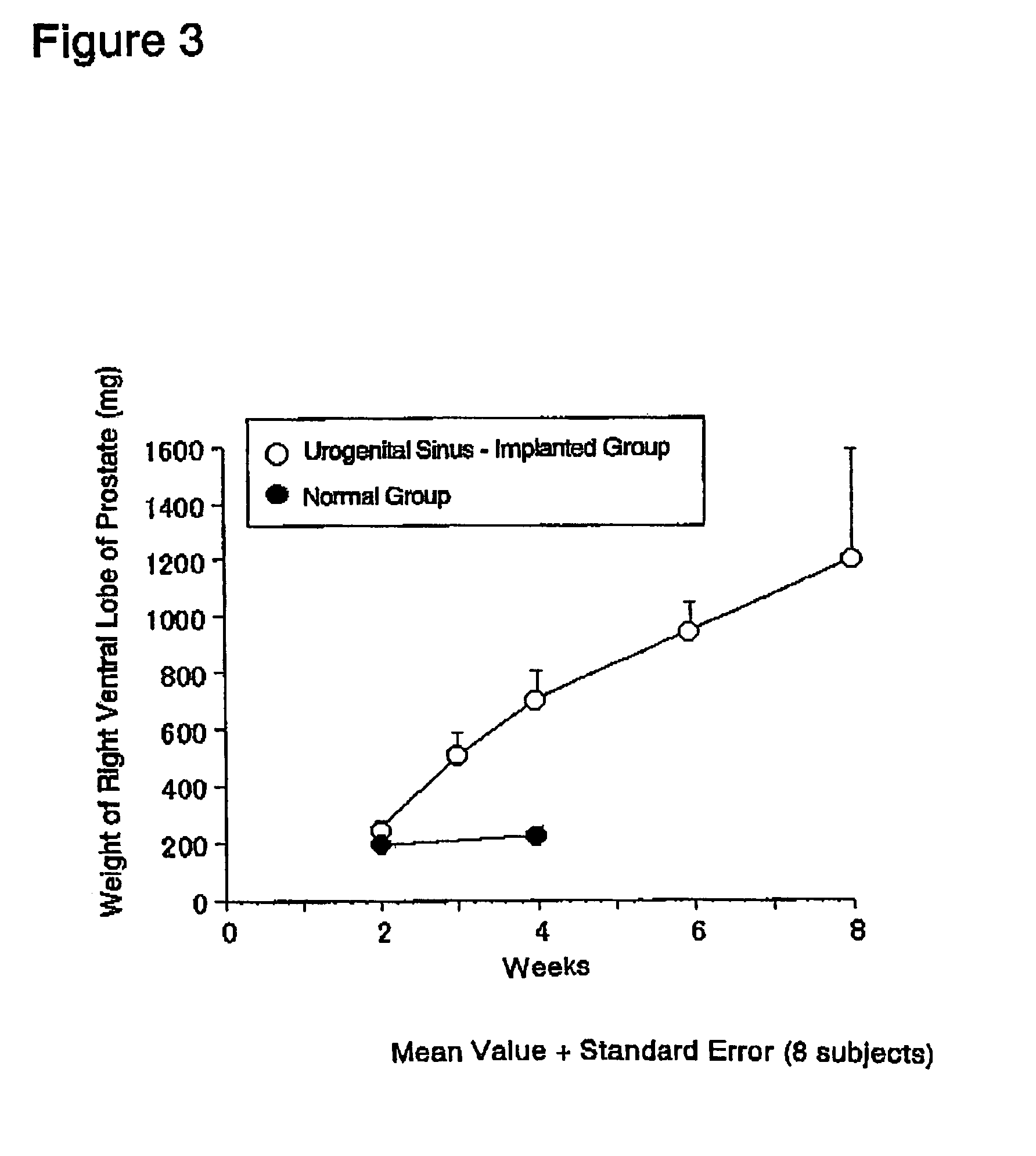
InactiveUS7417175B2Evaluate effectReduces stromal area ratio and weightArtificial cell constructsDisease diagnosisTreatment effectProstatism
The present invention provides an animal model for prostatic stromal hyperplasia, and a method for screening for a substance effective for preventing / treating human benign prostatic hyperplasia using the animal model. The animal model for prostatic stromal hyperplasia is produced by implanting the fetal urogenital sinus of a non-human animal under the skin or beneath the prostatic capsule of a non-human animal belonging to the species of the same as or different from the animal. A substance effective for preventing / treating human benign prostatic hyperplasia can be screened by administering a test substance to the animal model and measuring the preventive or therapeutic effect of the test substance upon the implanted tissue (fetal urogenital sinus or tissue derived therefrom).
Owner:TAIHO PHARMA CO LTD
Animal protein-free media for cultivation of cells
InactiveUS20060094104A1Efficient expressionGuaranteed efficient growthMicroorganismsCulture processBiotechnologyHydrolysate
The present invention relates to animal protein-free cell culture media comprising polyamines and a plant and / or yeast-derived hydrolysate. The invention also relates to animal protein-free culturing processes, wherein cells can be cultivated, propagated and passaged without adding supplementary animal proteins in the culture medium. These processes are useful in cultivating cells, such as recombinant cells or cells infected with a virus, and for producing biological products by cell culture processes.
Owner:BAXTER INT INC +1
Tissue engineered uterus
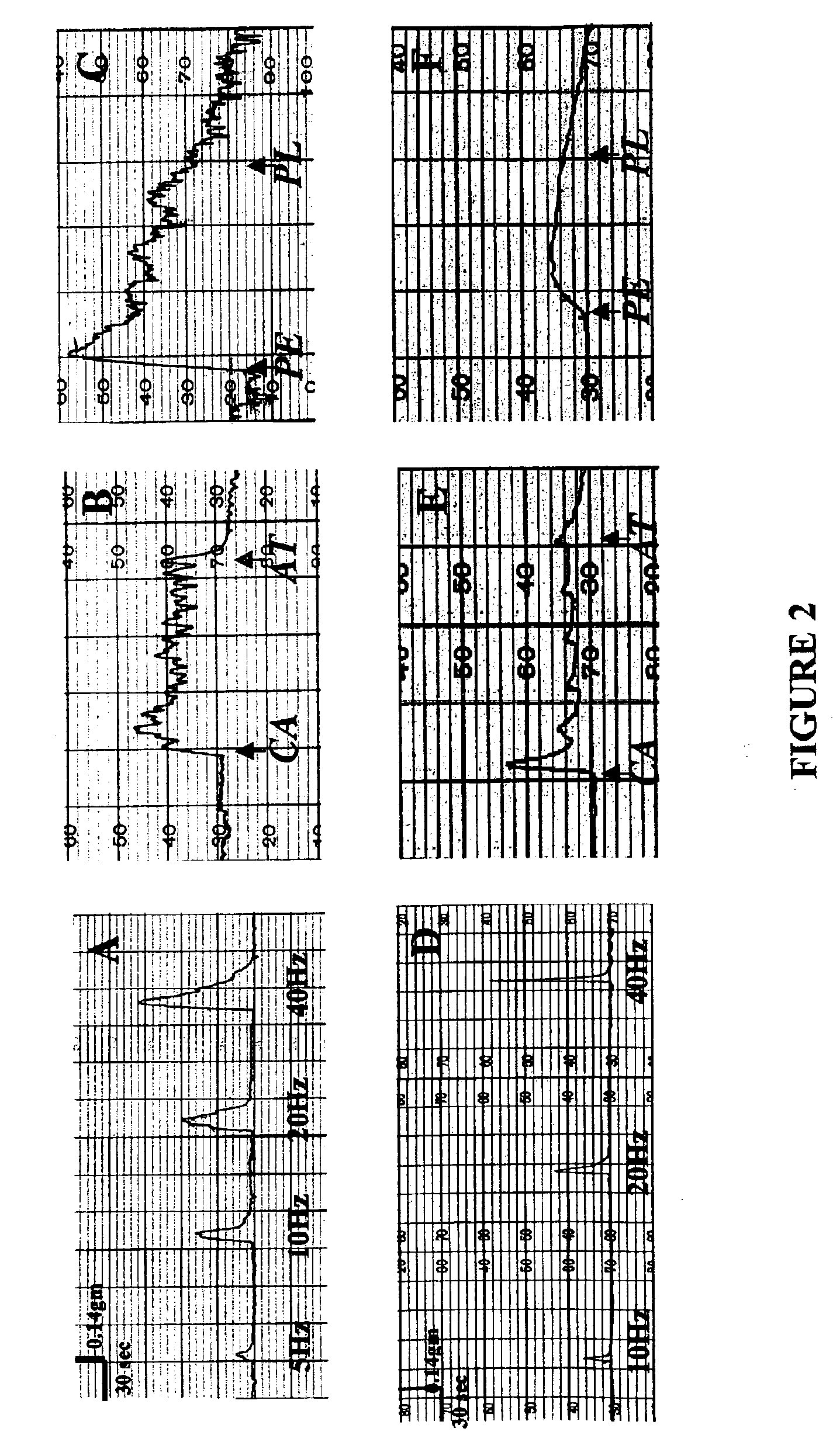
The invention is directed to compositions and methods for reconstructing artificial female reproductive organs. The constructs and methods of the invention can be used for ameliorating congenital malformations and disorders of female reproductive tract using tissue engineered female reproductive organs, such as the uterus, vagina, cervix, and fallopian tubes. These tissue engineered female reproductive organs can be generated by perfusing cultured cell populations derived from cells of the female reproductive tissues, such as uterine, vaginal, cervical, fallopian tube epithelial cells as well as smooth muscle cells.
Owner:ATALA ANTHONY J
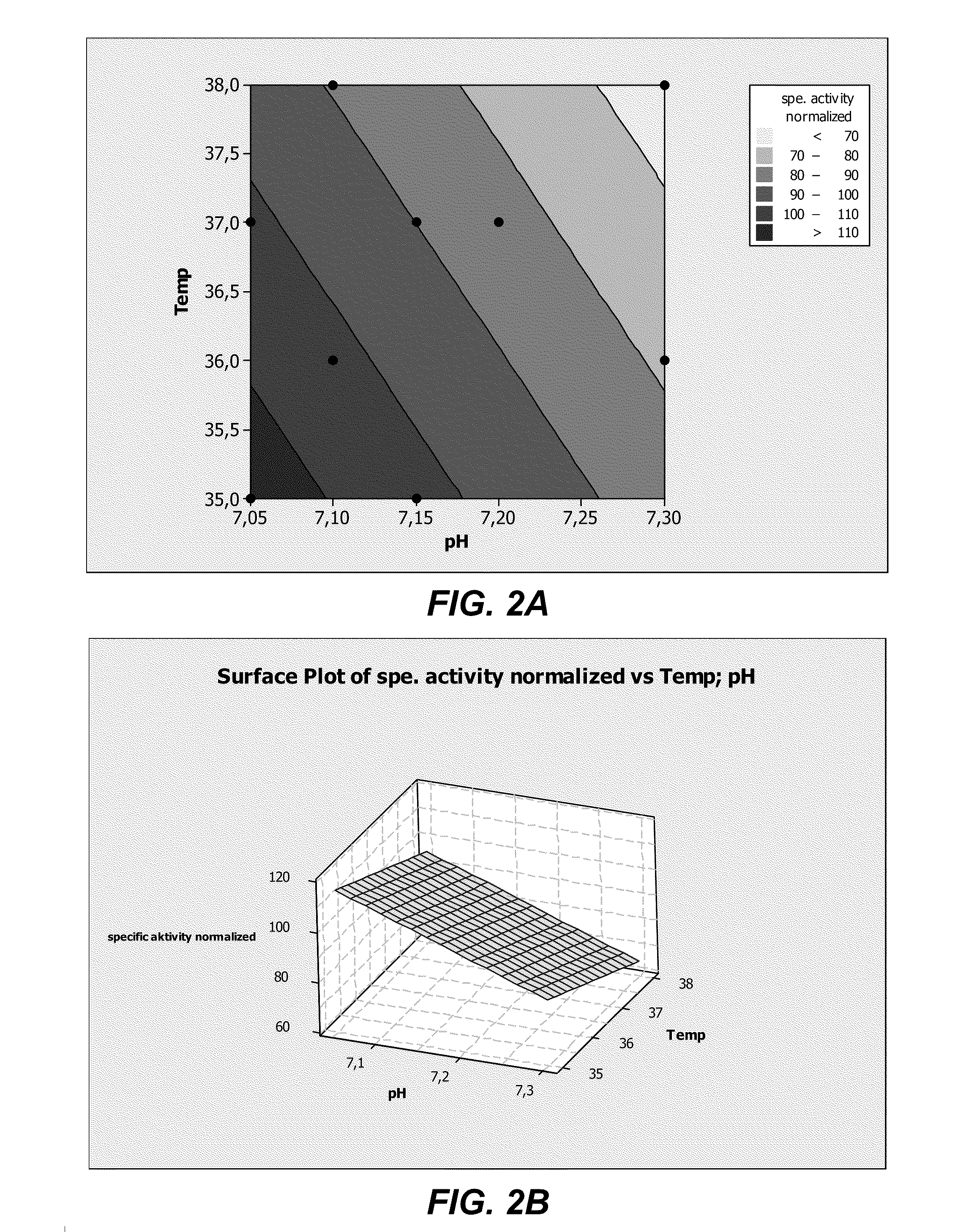
Cell Culture Medium For ADAMTS Protein Expression
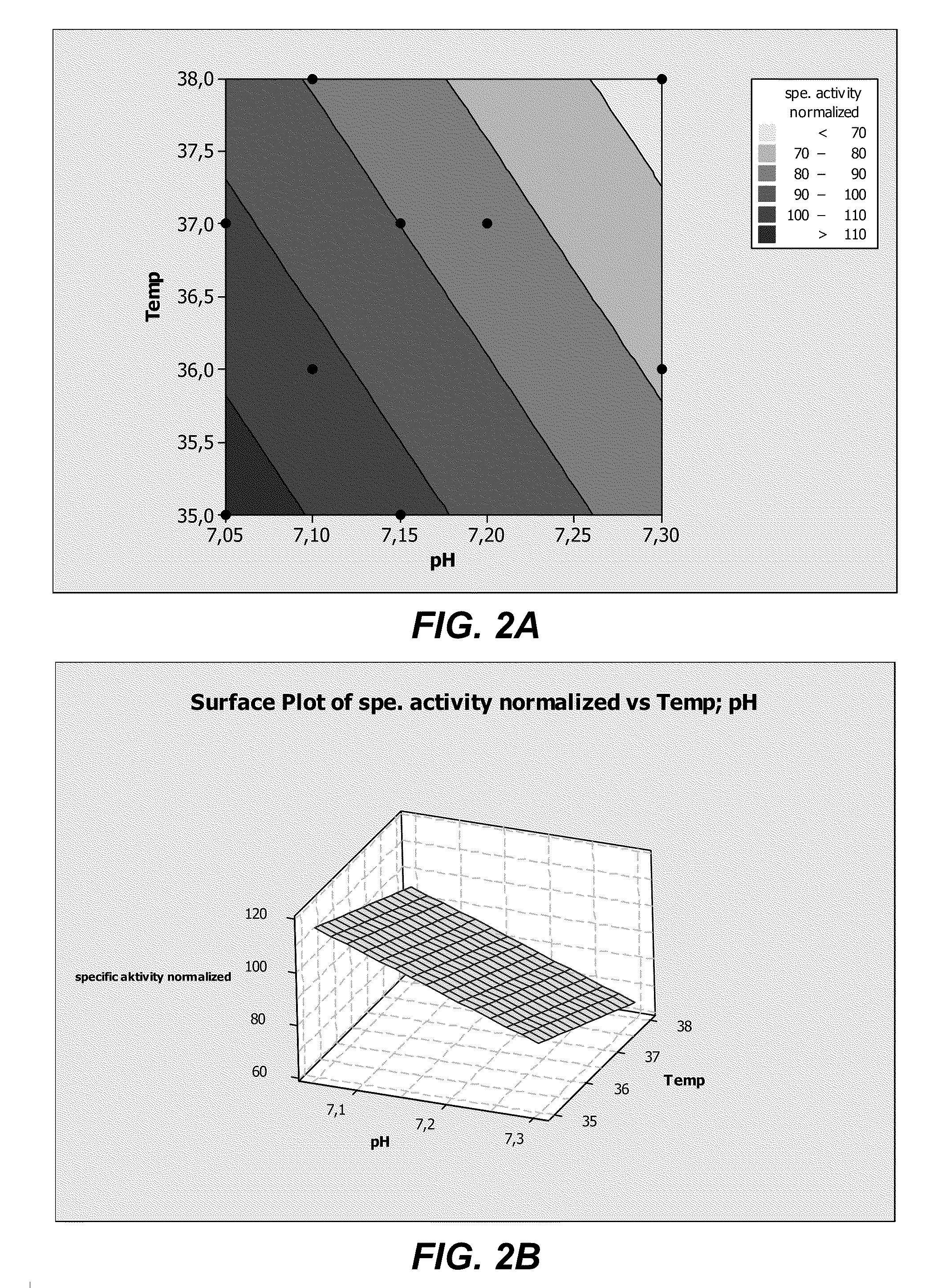
ActiveUS20110086413A1Improve expression levelHigh activitySugar derivativesGenetically modified cellsProtein compositionADAMTS Proteins
The present invention provides culture mediums that are useful for the expression of ADAMTS proteins, such as ADAMTS13. Methods for the expression and purification of ADAMTS proteins are also provided. In some embodiments, the mediums and methods of the invention are useful for the expression of ADAMTS proteins having high specific activities. Also provided are ADAMTS, e.g., ADAMTS13, protein compositions with high specific activities, which are expressed and purified according to the methods provided herein.
Owner:TAKEDA PHARMA CO LTD
Endometrial stem cells and methods of making and using same
InactiveUS20130156726A1Rapid rate of cellular divisionBiocidePeptide/protein ingredientsCell lineageEx vivo
The invention provides pluripotent stem cells and methods for making and using pluripotent stem cells. Pluripotent stem cells, among other things, can differentiate into various cell lineages in vitro, ex vivo and in vivo. Pluripotent stem cells, among other things, can also be used to produce conditioned medium.
Owner:XON CELLS
Methods for expressing ADAMTS proteins in cell culture medium supplemented with zinc
ActiveUS8313926B2Improve expression levelHigh activitySugar derivativesGenetically modified cellsProtein compositionCell culture media
The present invention provides culture mediums that are useful for the expression of ADAMTS proteins, such as ADAMTS13. Methods for the expression and purification of ADAMTS proteins are also provided. In some embodiments, the mediums and methods of the invention are useful for the expression of ADAMTS proteins having high specific activities. Also provided are ADAMTS, e.g., ADAMTS13, protein compositions with high specific activities, which are expressed and purified according to the methods provided herein.
Owner:TAKEDA PHARMA CO LTD
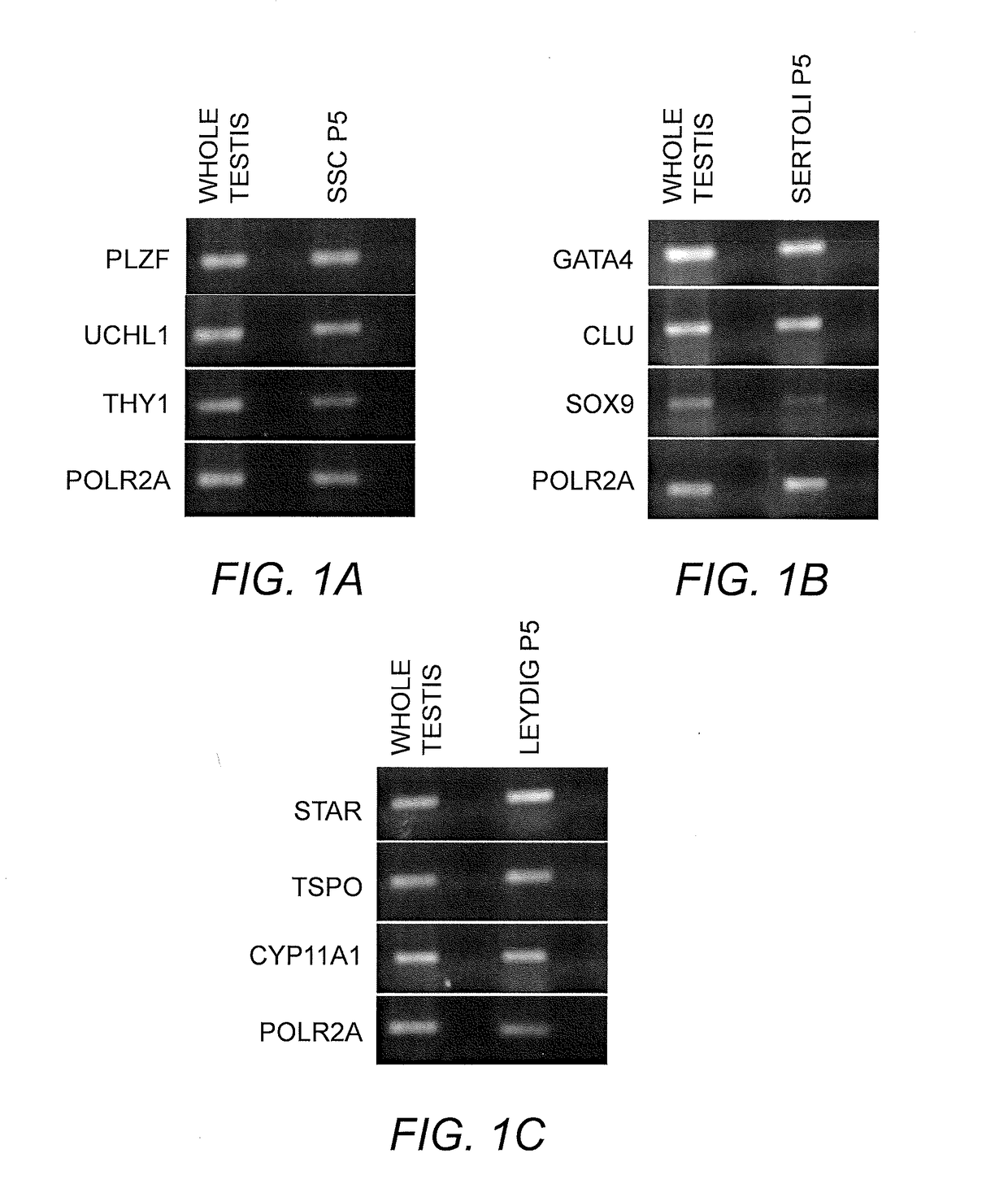
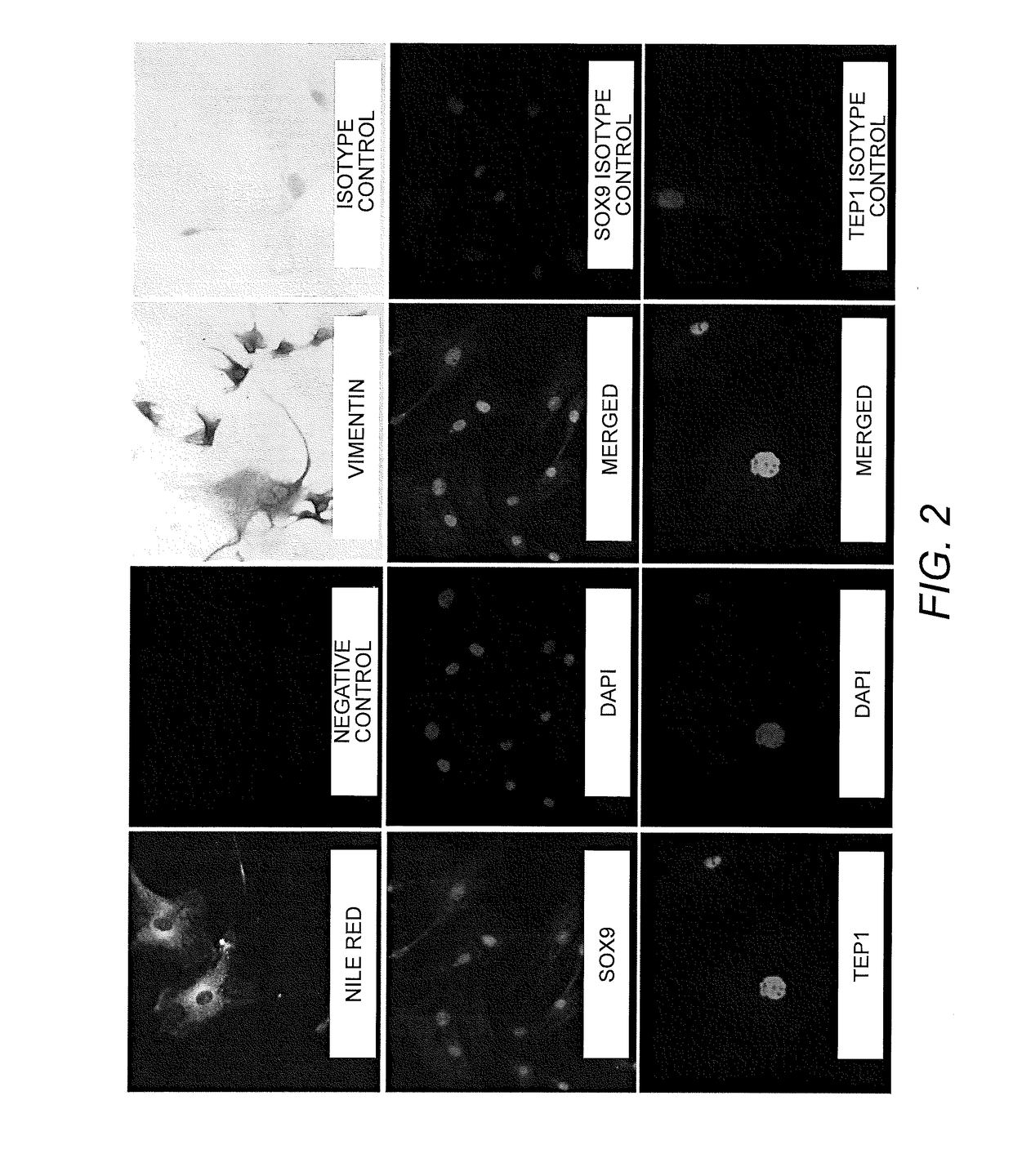
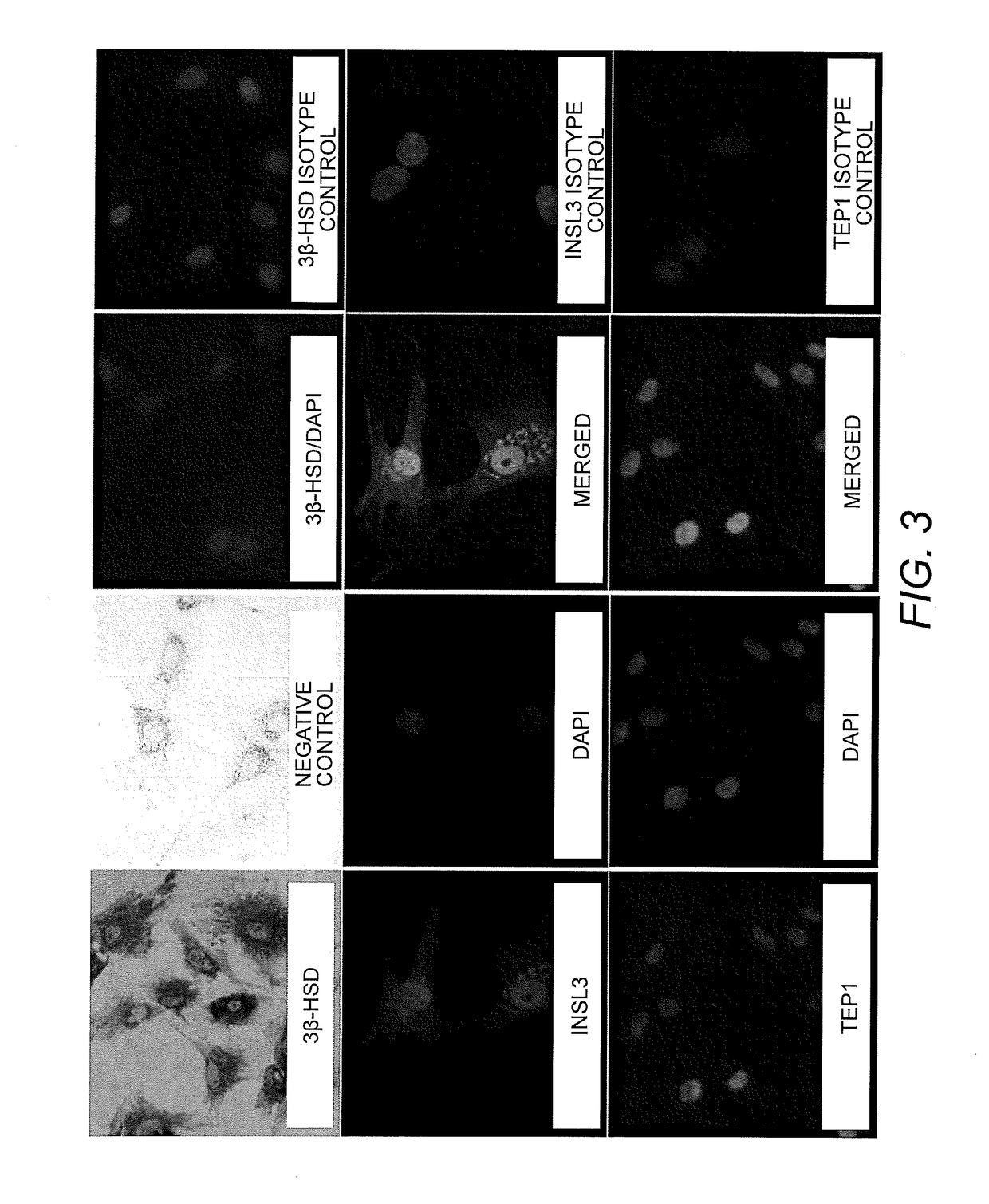
Isolation, characterization and differentiation of in vitro adult human germ line stem cells
A method of in vitro maturation of adult human germ line cells in an artificial biological environment, which entails:a) isolating human spermatogonial stem cells (SSCs), and optionally purifying the same; andb) co-culturing the isolated and optionally purified SSCs with a suitably adjusted Sertoli cell environment to obtain haploid germ cells.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
CHO (Chinese hamster ovary) cell strain with high-efficiency expression of CD2V protein of African swine fever (ASF)
ActiveCN110078801AHigh expressionEasy to purifyVirus peptidesMicroorganism based processesAfrican swine feverChinese hamster
The invention provides CD2V protein of African swine fever (ASF) capable of being expressed with high-efficiency in a CHO (Chinese hamster ovary) cell strain. The amino acid sequence of the CD2V protein is shown in SEQ ID NO:4; a recombinant plasmid constructed by the invention is used for expressing the CD2V protein of an African swine fever virus in CHO cells; the invention further provides a recombinant CHO cell strain prepared by transfecting the CHO cells through the recombinant plasmid, the recombinant CHO cell strain can be used to prepare the CD2V protein, and the prepared protein canbe used for differential diagnosis of the African swine fever. According to the cell strain with the expression of the CD2V protein of the African swine fever, the expression quantity is high, purification is easy, the cell strain can be used for the differential diagnosis, and a solid foundation is laid for the production of subunit vaccines and diagnostic reagents of the African swine fever.
Owner:YEBIO BIOENG OF QINGDAO
Compositions and methods for enhancing bioenergetic status in female germ cells
ActiveUS20130059384A1Quality improvementImprove mitochondrial functionSugar derivativesHydroxy compound active ingredientsIsolated mitochondriaPhysiology
Owner:THE GENERAL HOSPITAL CORP +1
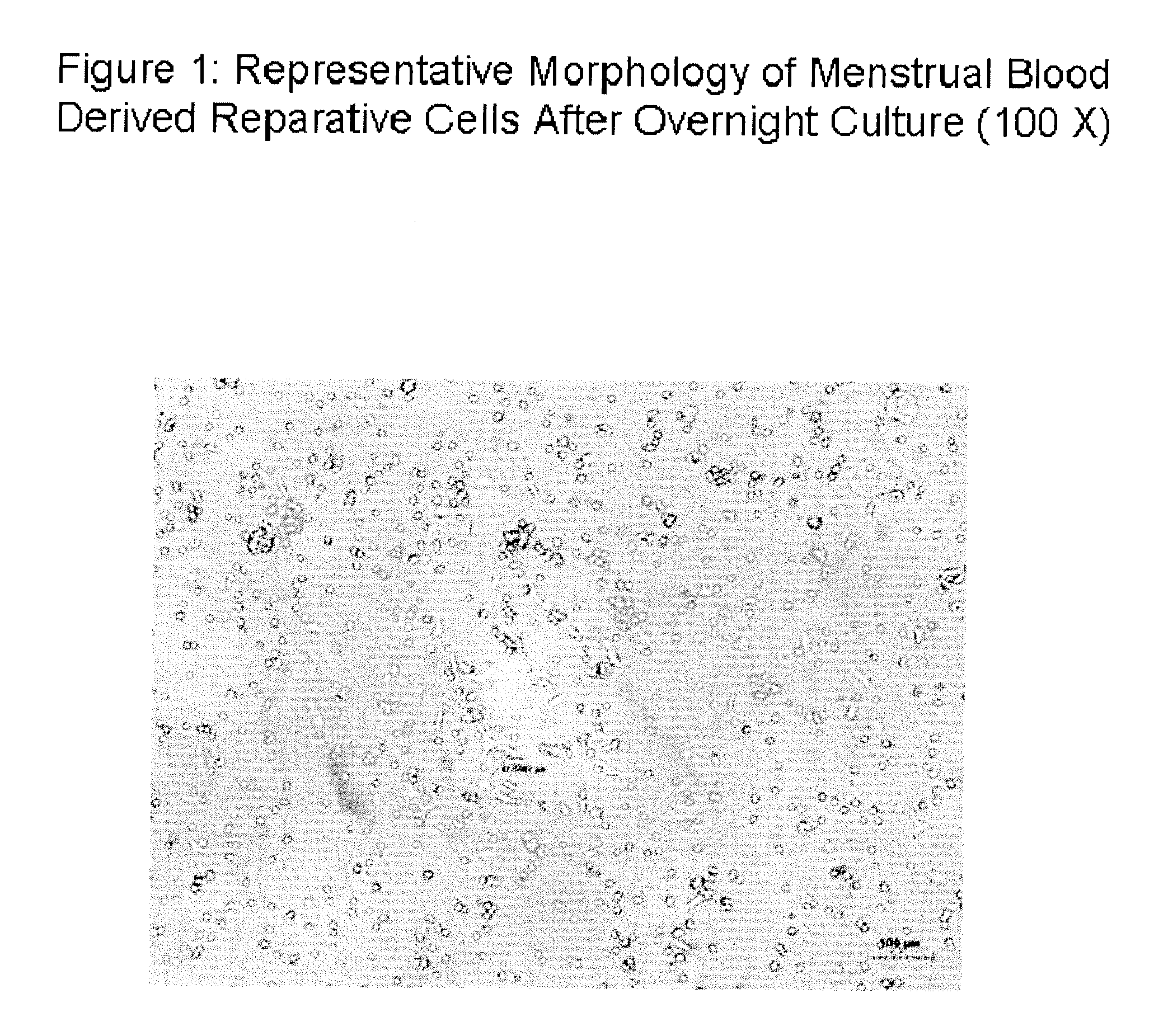
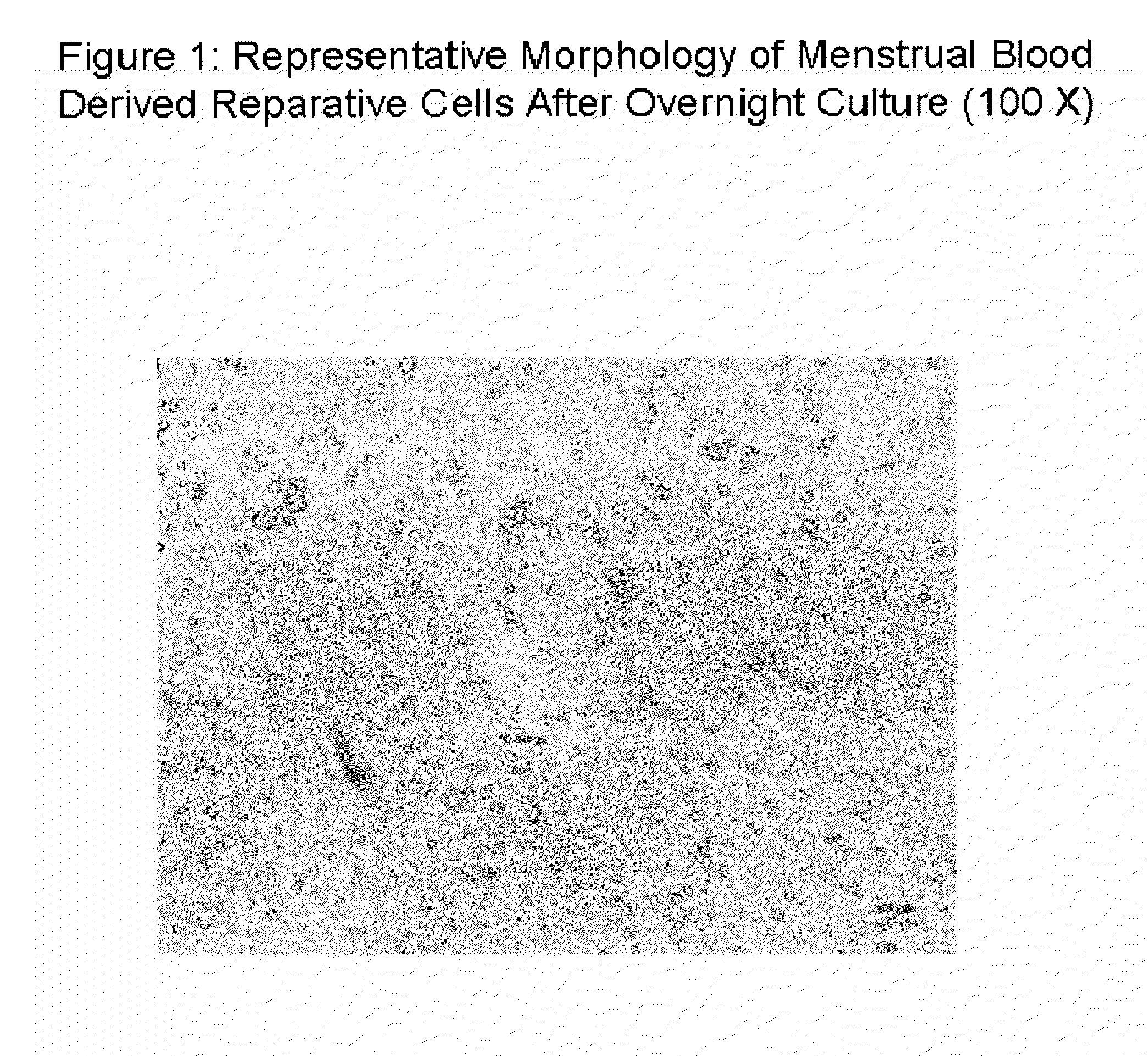
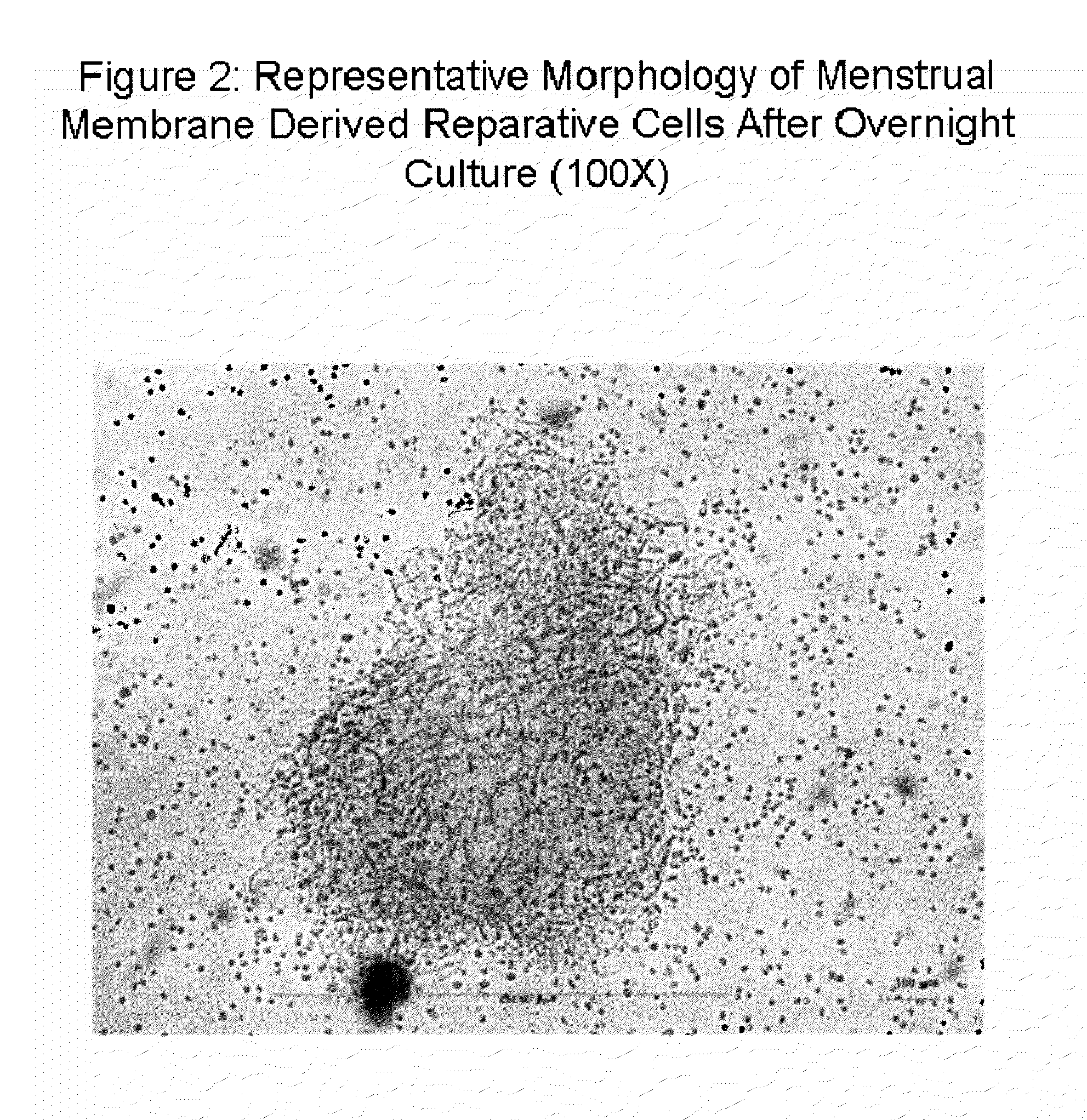
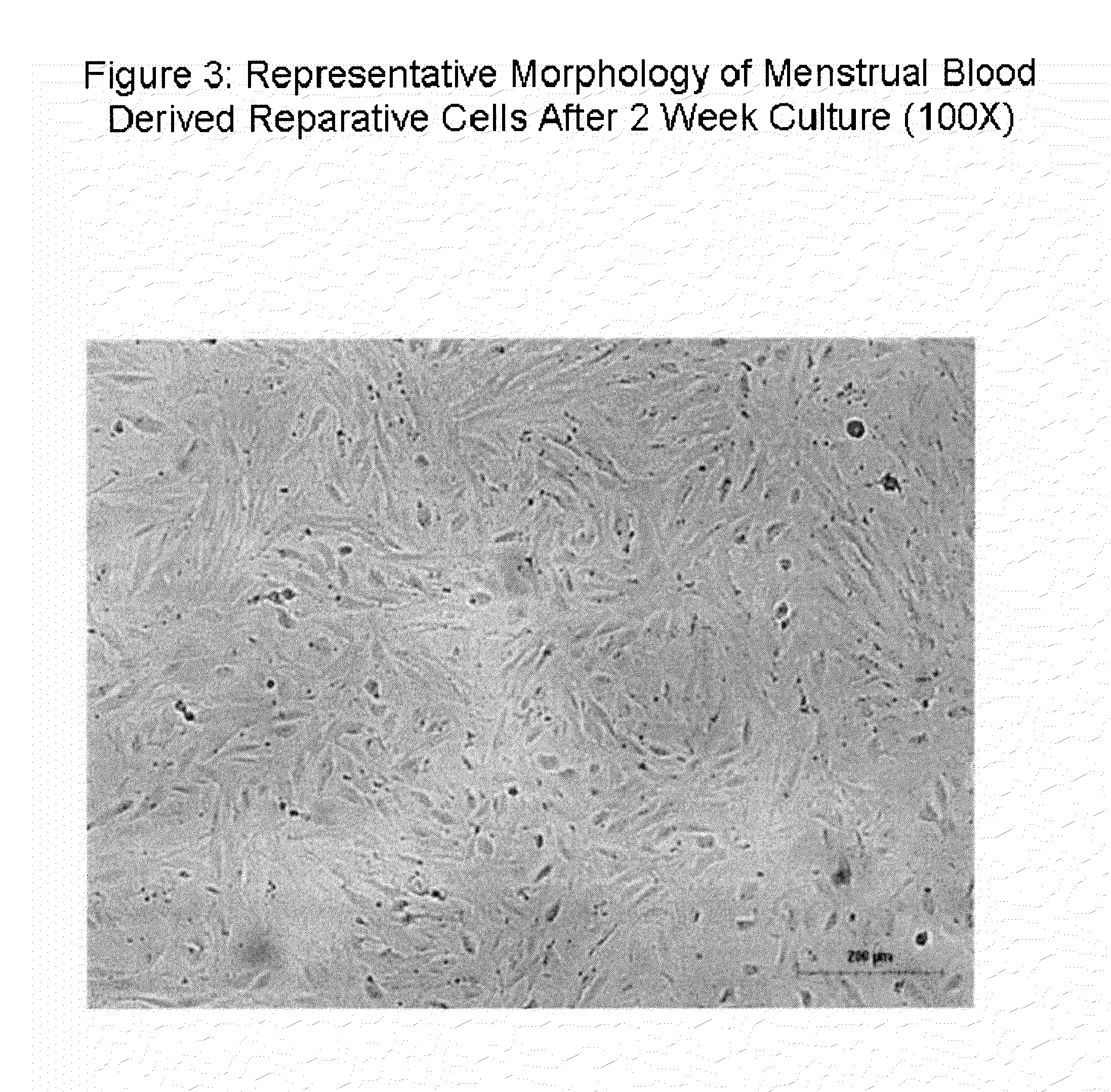
Stem cell culture medium and method for culturing endometrium stem cells
InactiveCN105586308ALess prone to agingLess prone to degradationCulture processDead animal preservationSimple componentTrypsinization
The invention provides a stem cell culture medium and a method for culturing endometrium stem cells by using the stem cell culture medium. The method comprises the following steps: separately collecting menstrual blood and endometrium tissues, respectively culturing the menstrual blood and endometrium tissues in the stem cell culture medium provided by the invention to respectively obtain menstrual blood adherent cells and endometrium adherent cells, culturing the menstrual blood adherent cells and endometrium adherent cells in a cell culture bottle, collecting the adherent cells by trypsinization, inoculating the adherent cells in a cell coculture dish, and culturing the adherent cells in the stem cell culture medium provided by the invention. The stem cell culture medium has the advantages of simple components, fewer added components and lower cost. After more than 20 generations of in-vitro culture, the cells can not easily have the phenomenon of aging or degeneration, and can maintain the activity and stem property of the stem cells for a long time. The stem cell culture method is simple and effective, the cell proliferation efficiency is high, and the in-vitro culture doubling time is only 20 hours or so. The cells can be stably amplified by 50 generations.
Owner:HANGZHOU S EVANS BIOSCI LTD
Cell lines and methods for producing proteins
The present invention relates to the isolation and enrichment of avian oviduct tumor cells to obtain sustainable cell lines that can be passaged multiple times in cell culture and can be used for long-term production of heterologous polypeptides at higher yields. The methods of the instant invention involve the manipulation and / or propagation of oviduct tumor cells derived from either wild-type or transgenic avians.
Owner:SYNAGEVA BIOPHARMA CORP
Methods for determining developmental stage of human cumulus cells
ActiveUS20140134632A1Improve scalabilityImprove pregnancy rateMicrobiological testing/measurementCell culture supports/coatingDevelopmental stageBiology
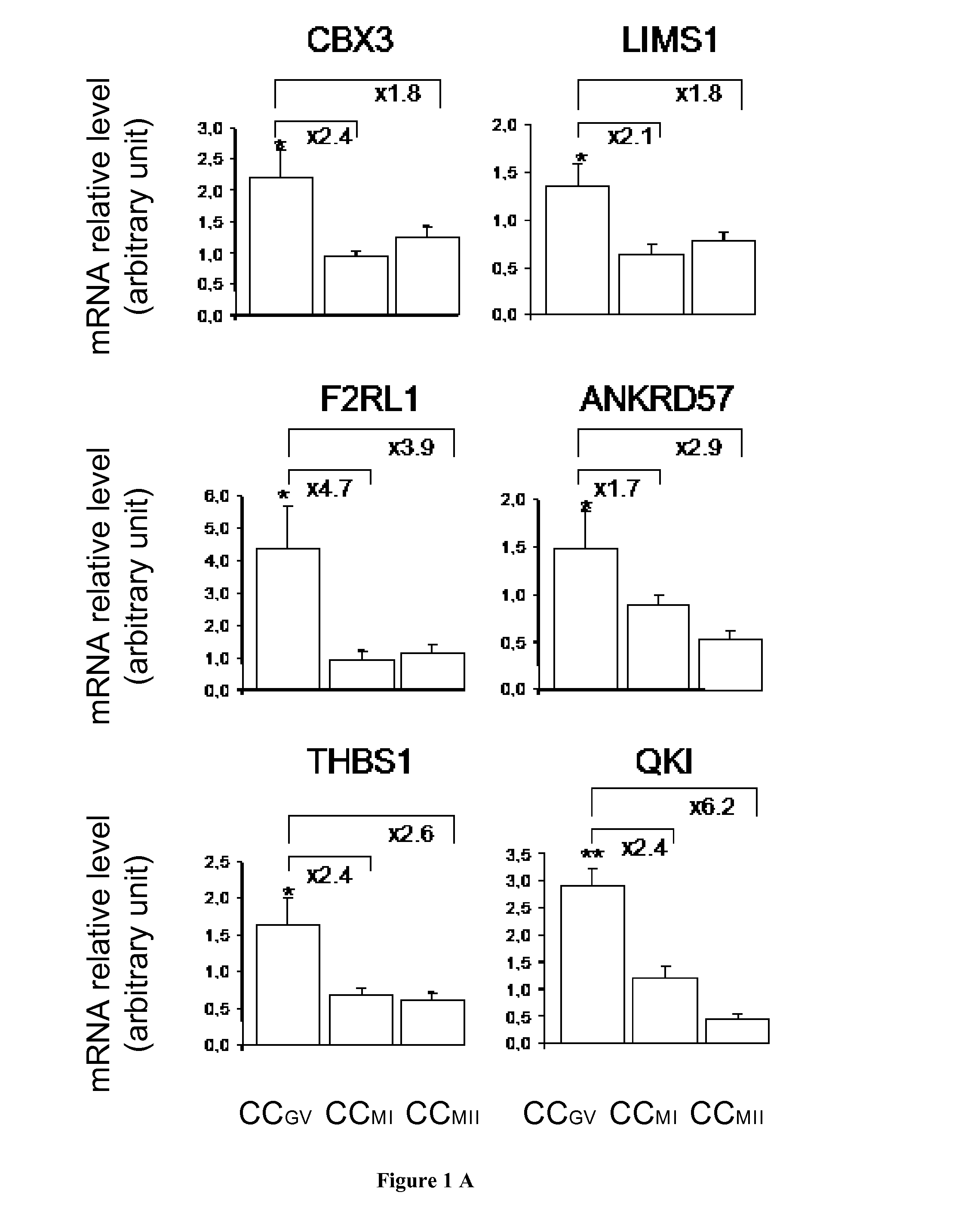
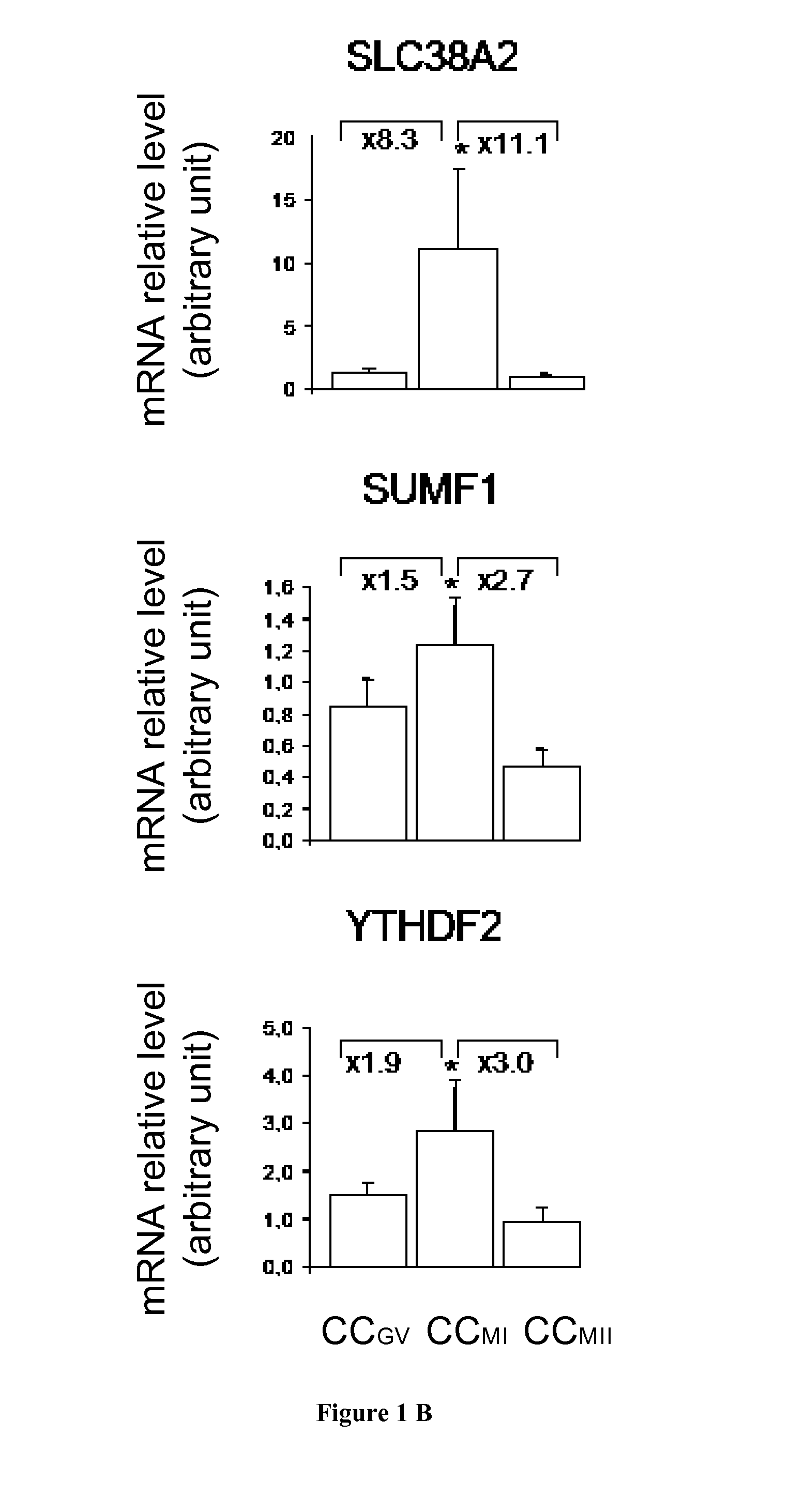
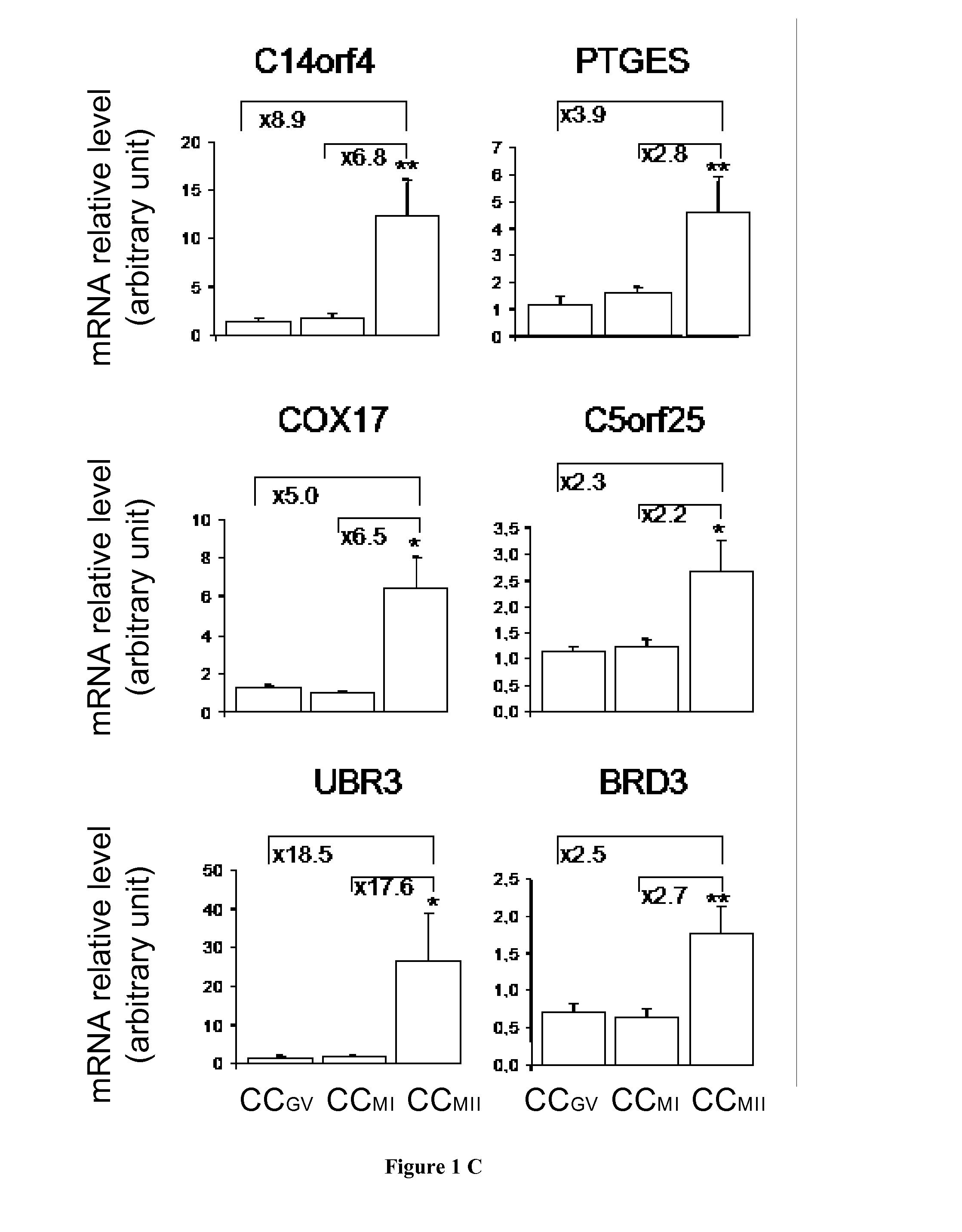
The present invention relates generally to the fields of reproductive medicine. More specifically, the present invention relates to a method determining the developmental stage of human cumulus cells issues from MII oocyte.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
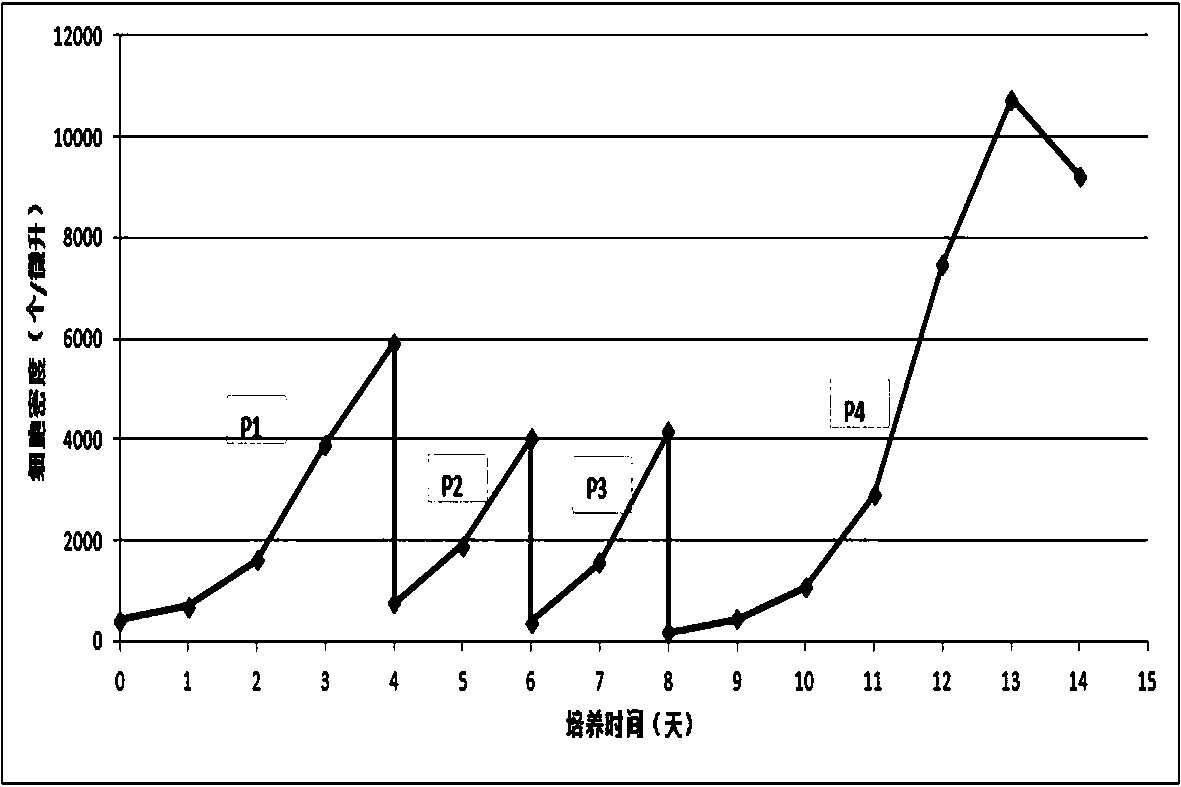
Method for culturing avian spermatogonial stem cells and avian spermatogonial stem cells prepared thereby
InactiveUS20070061910A1Improve reliabilityArtificially induced pluripotent cellsCell culture active agentsTesticleCell growth
Owner:SEOUL NAT UNIV R&DB FOUND +1
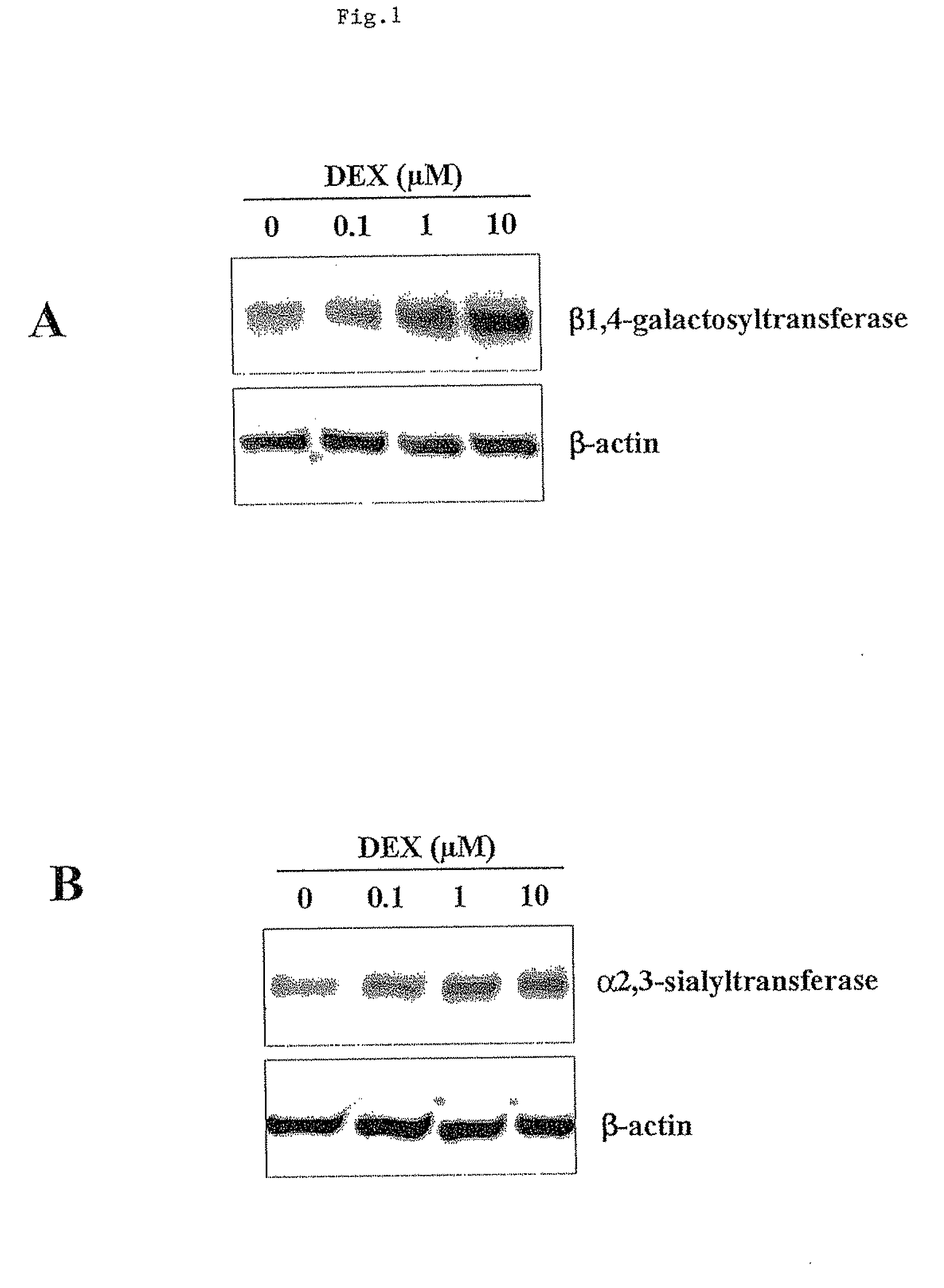
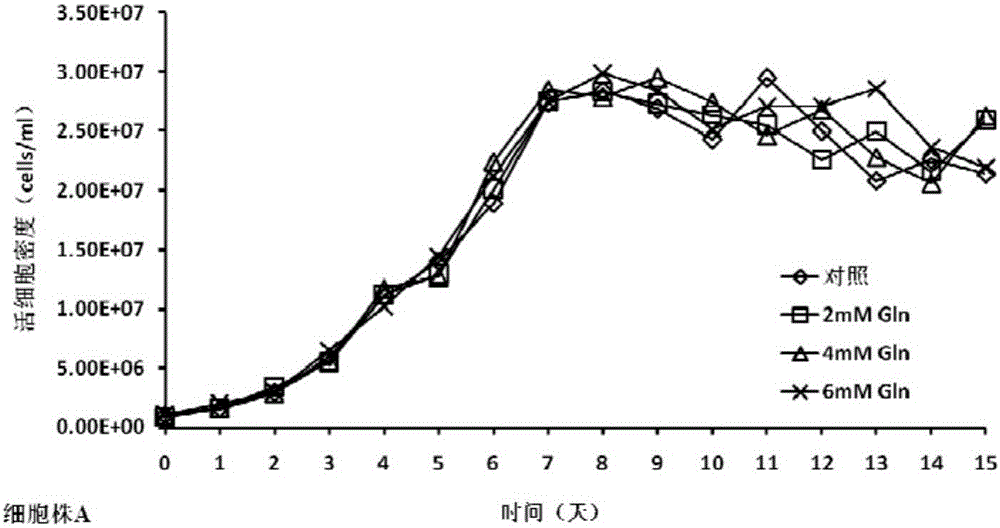
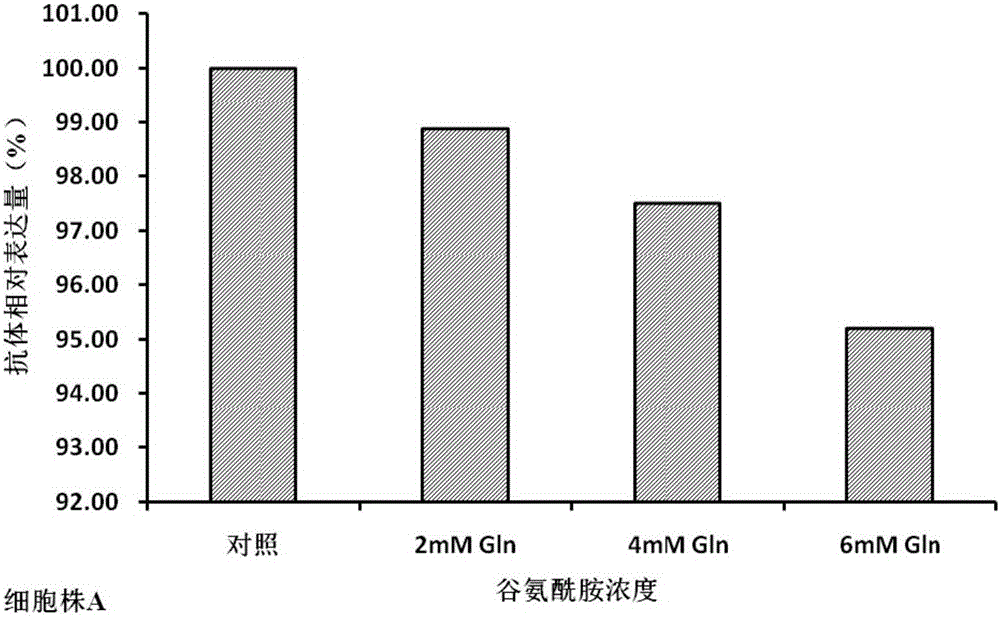
Cell culture method for reducing acidic peak content of antibody and improving glycoform of antibody
InactiveCN105779394AOptimizing Glycosylation LevelsGuaranteed efficacyCulture processImmunoglobulinsAntibody expressionEconomic benefits
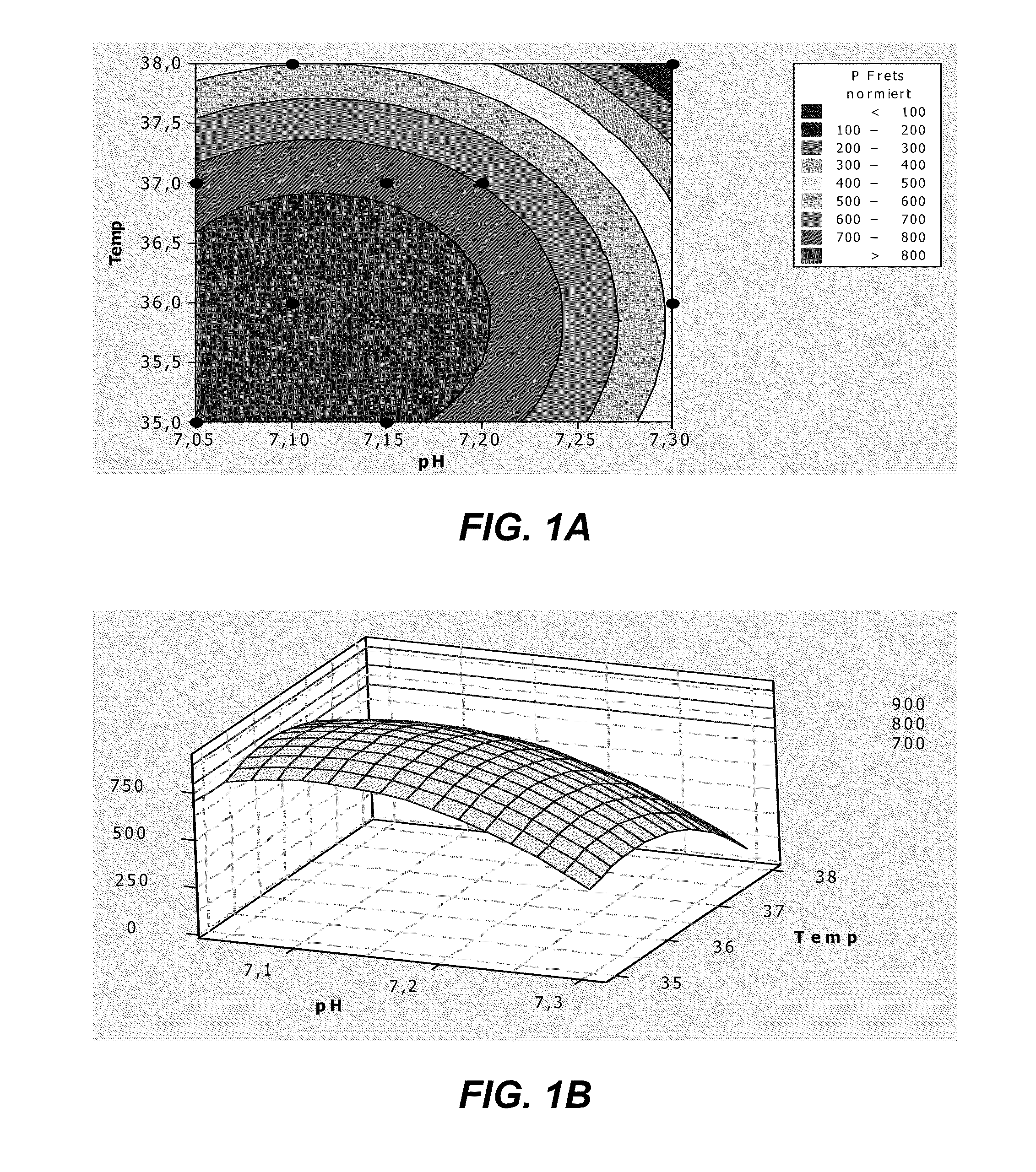
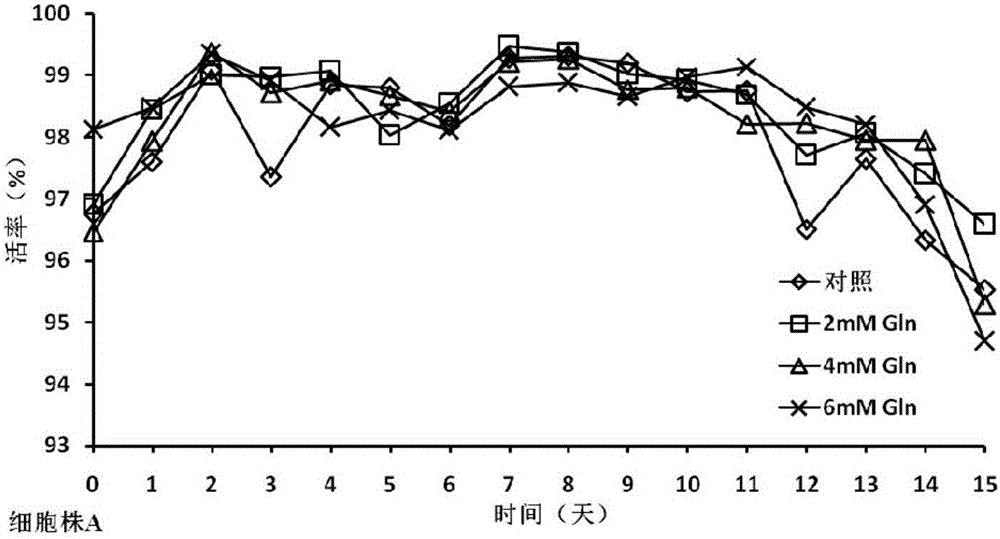
The invention discloses a cell culture method for reducing acidic peak content of an antibody and improving glycoform of the antibody. In the method, GS-CHO cells are used as an expression system, glutamine is added into a basic medium for antibody-expressed cell strain culture. After glutamine is added to the basic medium, it is possible to effectively reduce acidic peak content of the antibody and improve glycosylation level, thus modifying the activity and efficacy of antibody drugs and improving the quality of the antibody so that the quality is close to that of a standard product; the method is adapted to the development and study and later large-scale production of new antibody drugs and has a promising application prospect and good economic benefit.
Owner:SUNSHINE LAKE PHARM CO LTD
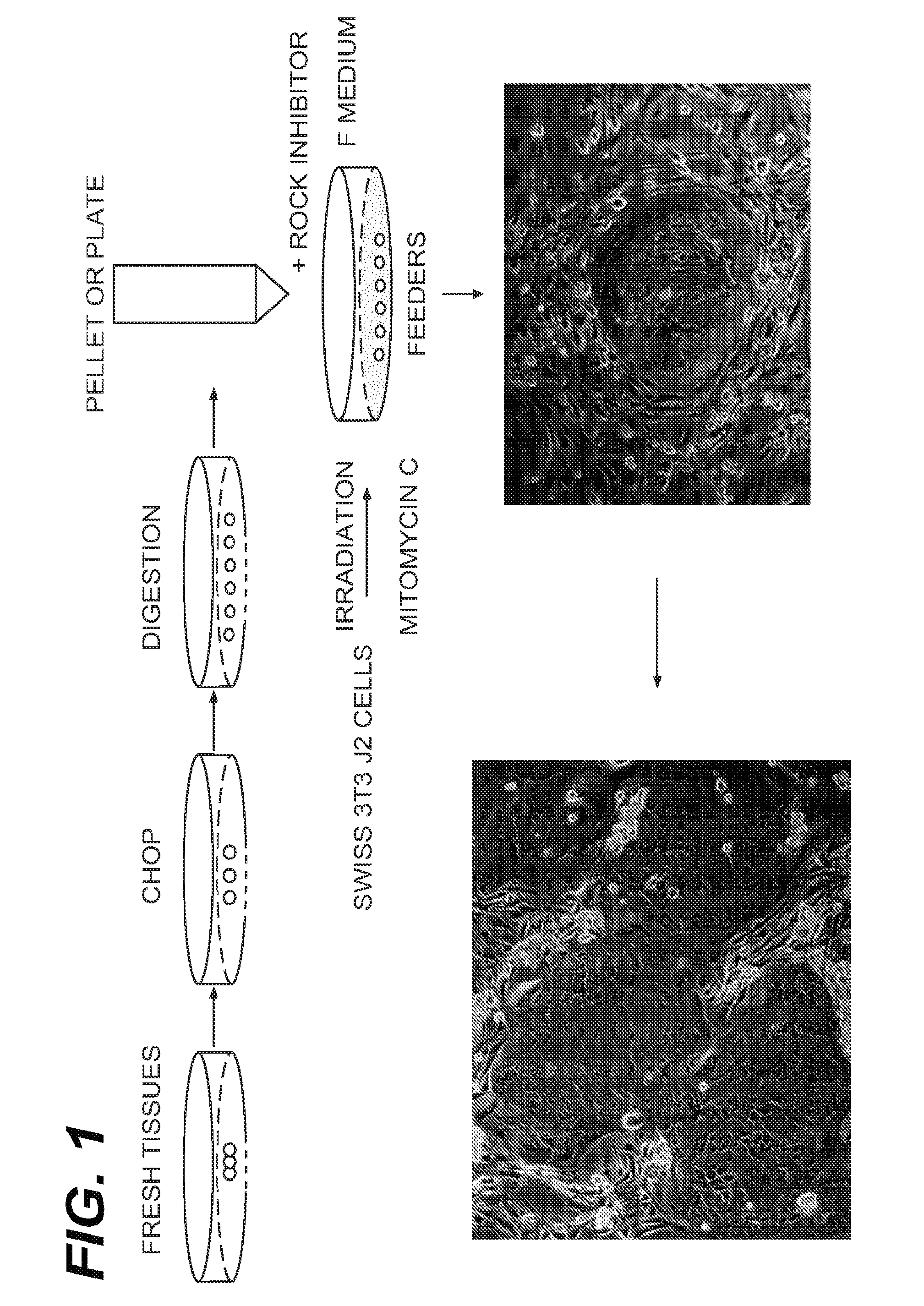
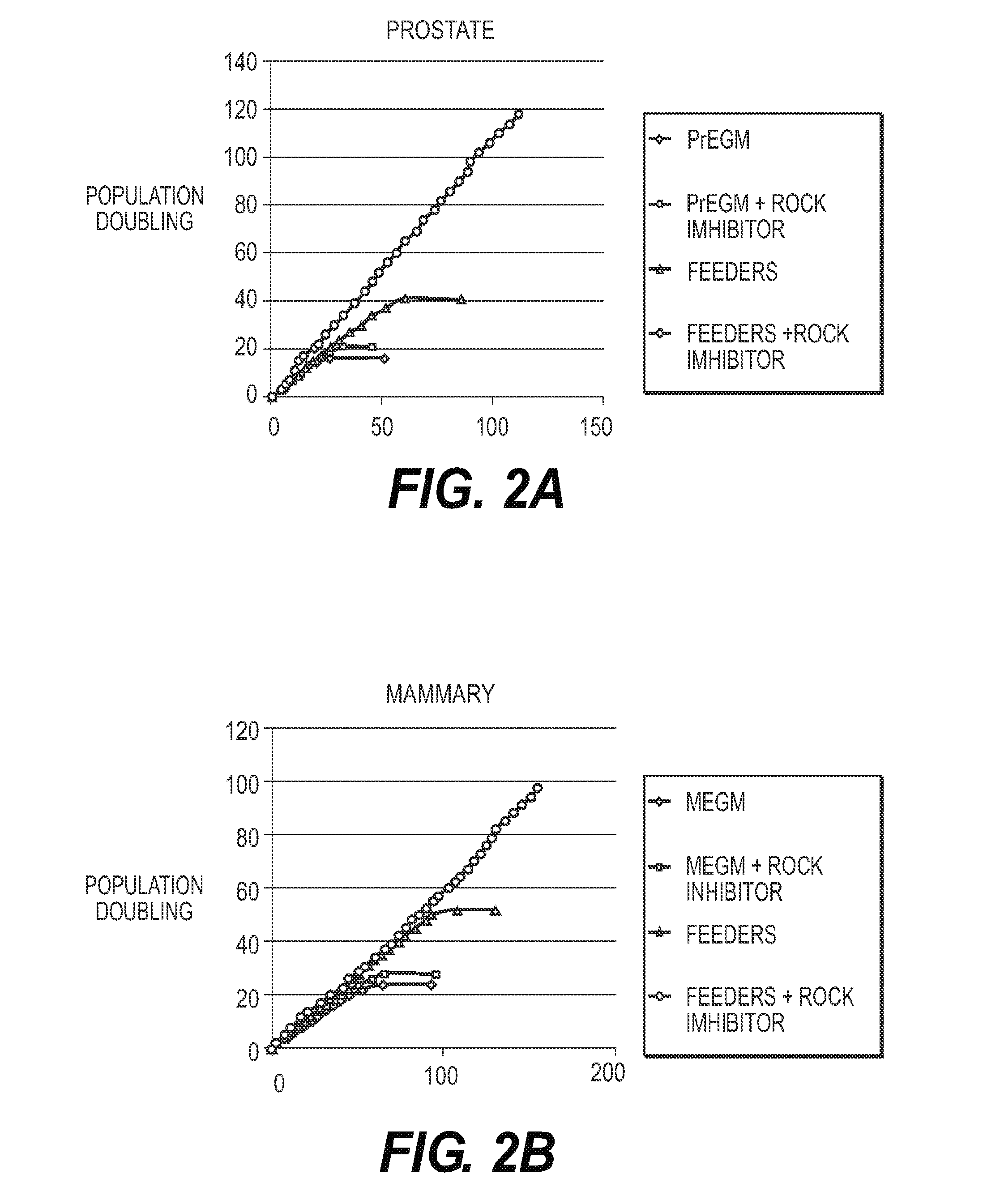
Immortalization of Epithelial Cells and Methods of Use
The present invention is directed towards methods of culturing non-keratinocyte epithelial cells, with the methods comprising culturing non-keratinocyte epithelial cells in the presence of feeder cells and a calcium-containing medium while inhibiting the activity of Rho kinase (ROCK) in the feeder cell, the non-keratinocyte epithelial cells or both during culturing.
Owner:GEORGETOWN UNIV
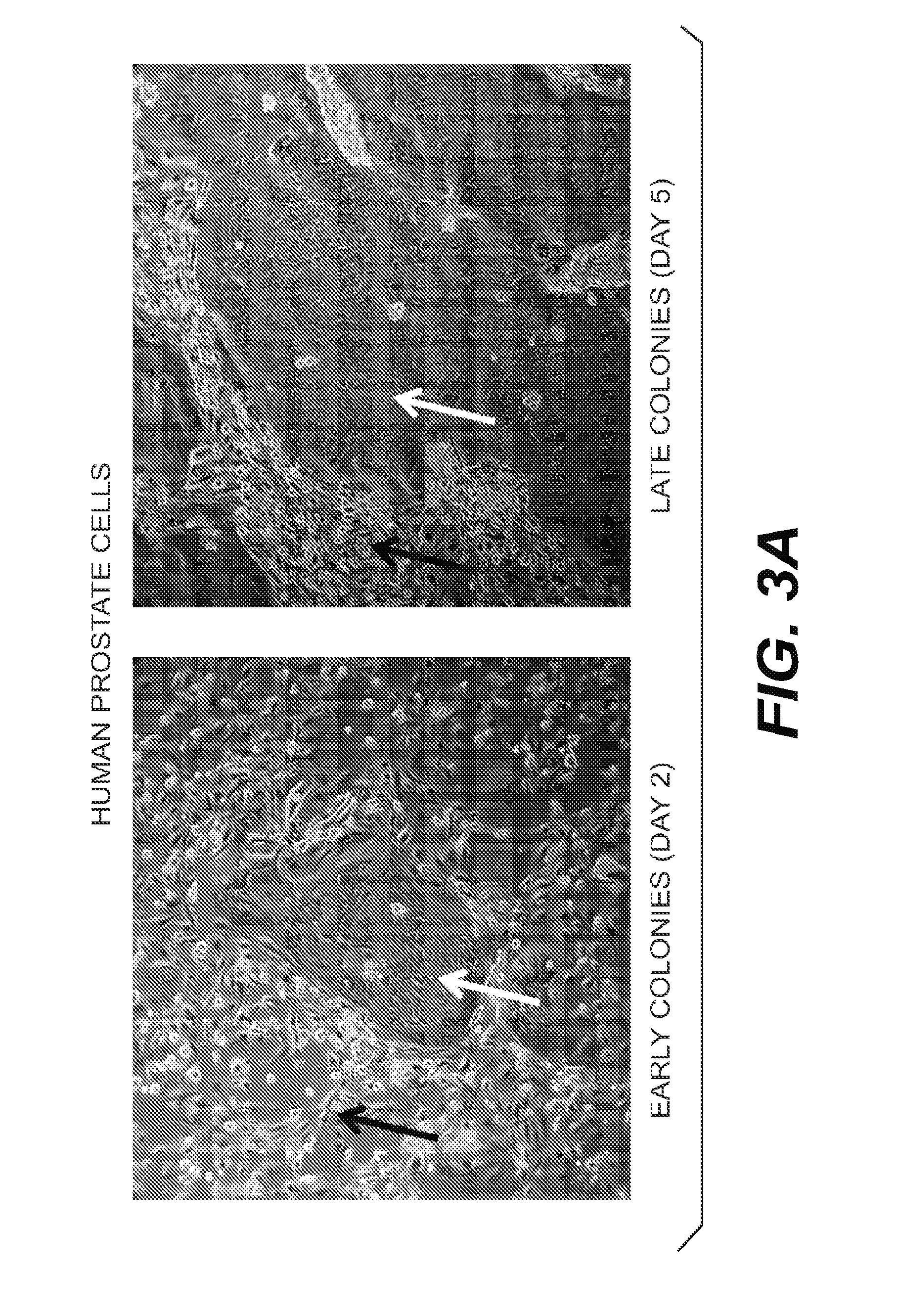
Seed Train Processes and Uses Thereof
ActiveUS20150353896A1Less complexReduce in quantityGenetically modified cellsCulture processBiotechnology
Owner:GENZYME CORP
Production of a biological factor and creation of an immunologically privileged environment using genetically altered Sertoli cells
Owner:SERTOLI TECH
Method of producing in vitro testicular constructs and uses thereof
A cell composition composed of spermatogonial stem cells, Sertoli cells, Leydig cells and optionally peritubular cells, is provided, as is a culture composition, artificial testicular construct, hydrogel composition, and device containing the same. A method for using the device as a physiologically relevant in vitro model of human testicular function to screen compounds for pharmacological or toxicological activity is also provided.
Owner:WAKE FOREST UNIV HEALTH SCI INC
CHO (Chinese hamster ovary) cell serum-free medium supporting high expression of product
ActiveCN109337861APromote generationHigh densityCulture processArtificial cell constructsSerum free mediaHydrolysate
The invention discloses a CHO (Chinese hamster ovary) cell serum-free medium. The culture medium comprises main components such as amino acids, inorganic salt components, vitamins, trace elements, yeast hydrolysates, albumin and the like. The CHO cell serum-free medium disclosed by the invention does not contain any serum components and animal-derived components, and is increased in affinity compared with that of other serum-free mediums. Cells can be directly inoculated into the serum-free medium from a serum culture medium or other serum-free mediums and grow normally, thus eliminating a cumbersome domestication process. By adopting the CHO cell serum-free medium, the growth density and the viability of CHO cells are improved, the tolerance of the cells therein is increased, the growth platform maintenance time is prolonged, most importantly, the ability of CHO engineering cells to express foreign proteins in the CHO cell serum-free medium is improved, and the product yield is greatly enhanced.
Owner:YOCON BIOLOGY TECH CO
Construction method and application of human normal vaginal epithelium 3D (Three Dimensional) differentiation culture model
The invention belongs to the field of biomedicines and discloses a construction method and application of a human normal vaginal epithelium 3D (Three Dimensional) differentiation culture model. A 2D (Two Dimensional) growth culture medium is provided and normal vaginal epithelial cells separated and cultured from normal tissues beside female vaginal cancer are obtained; any exogenous gene is not introduced and the normal vaginal epithelial cells have a physiological function of normal differentiation. The method for constructing the human normal differentiation vaginal epithelium 3D model comprises the following steps: re-suspending single cells by the 2D culture medium and inoculating the single cells into a gas-liquid culture device; replacing the growth culture medium with a differentiation culture medium and culturing for 14 to 21 days; after completely differentiating 3D gas-liquid culture of the human vaginal epithelial cells, inoculating HSV-2 (Herpes Simplex Virus-2) virus liquid to obtain an HSV-2 virus infection 3D model. The two types of 3D models can be used for physiological studies and drug toxicity safety evaluation of human normal reproductive tract epithelium, researches of pathogenic mechanisms of HSV-2 virus infected diseases and sexual transmission pathogen infected diseases and researches and development of anti-viruses medicines.
Owner:深圳涌泰生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com