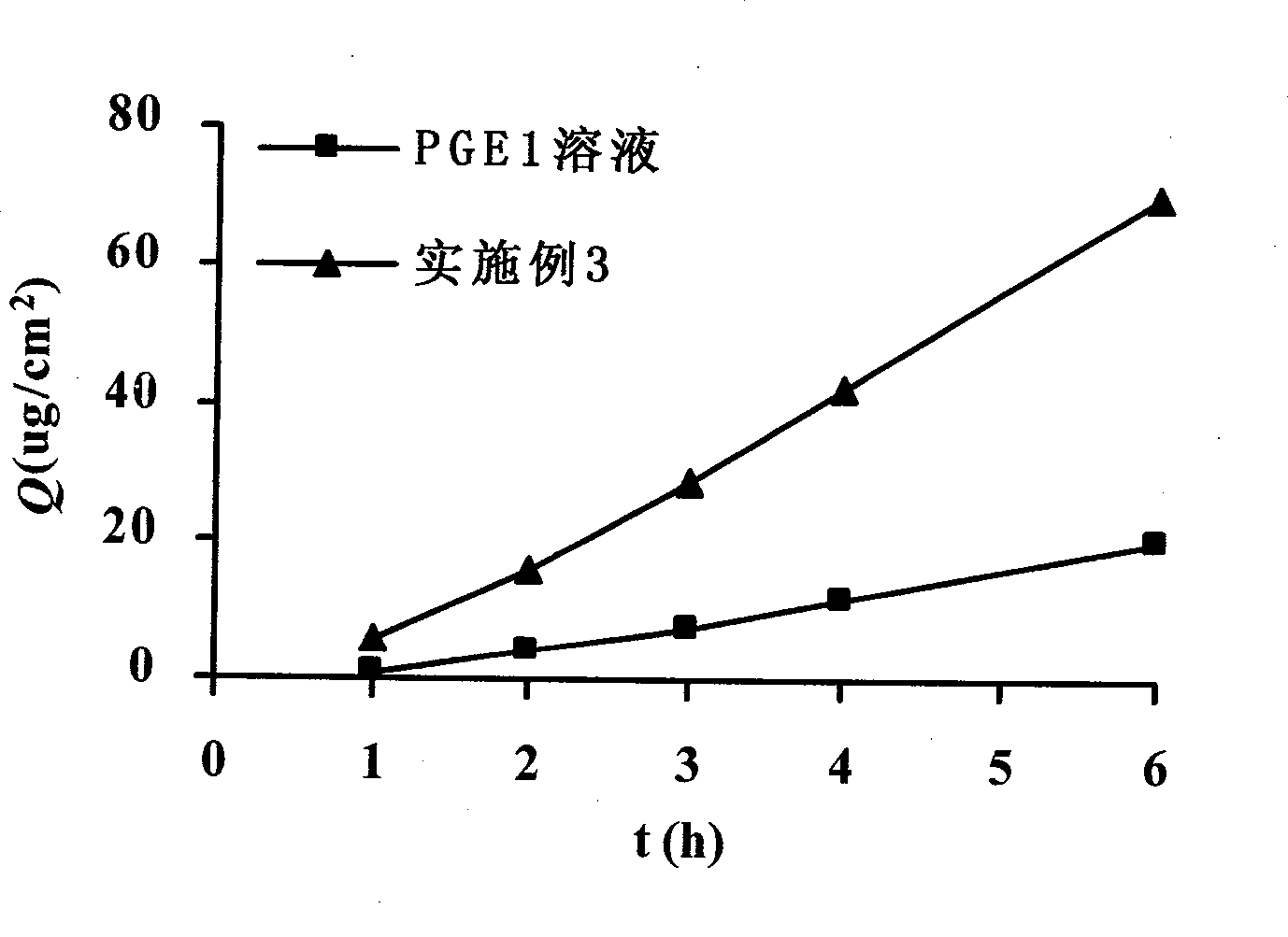
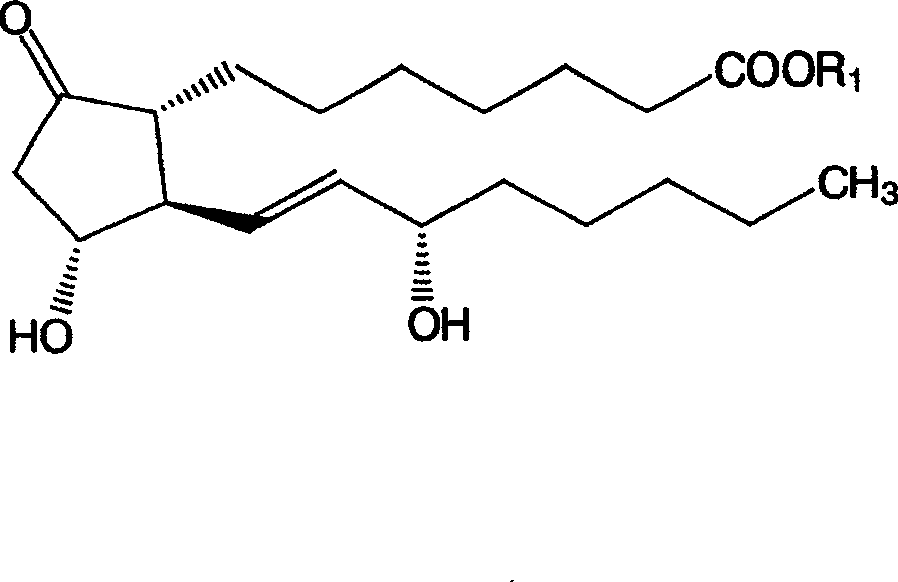
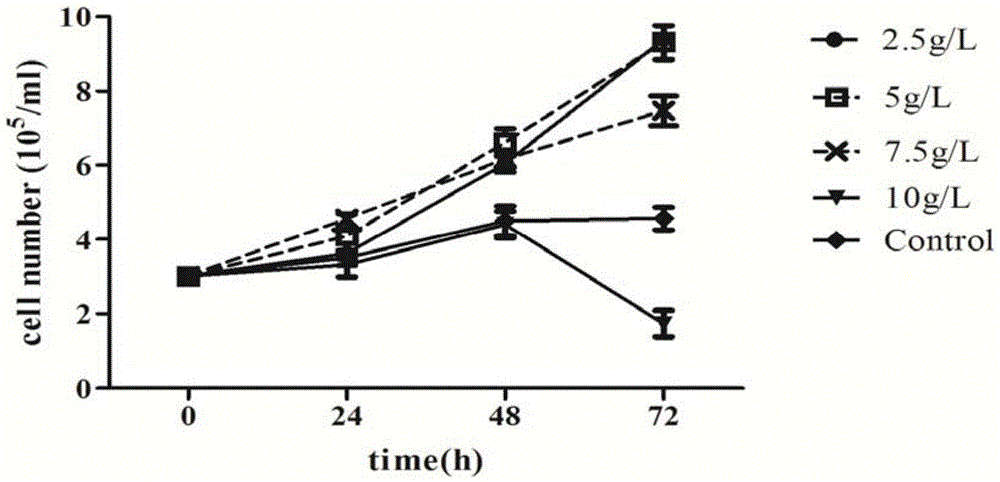
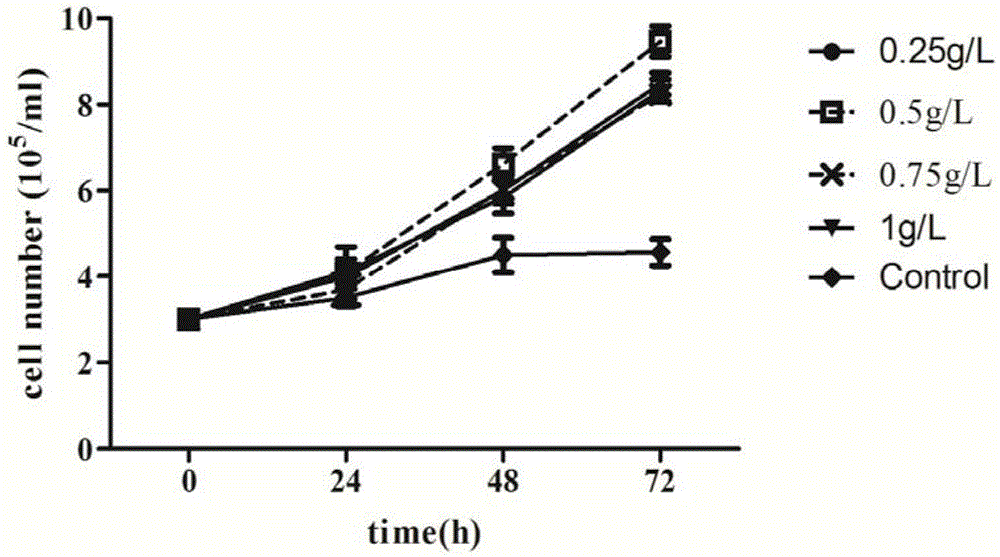
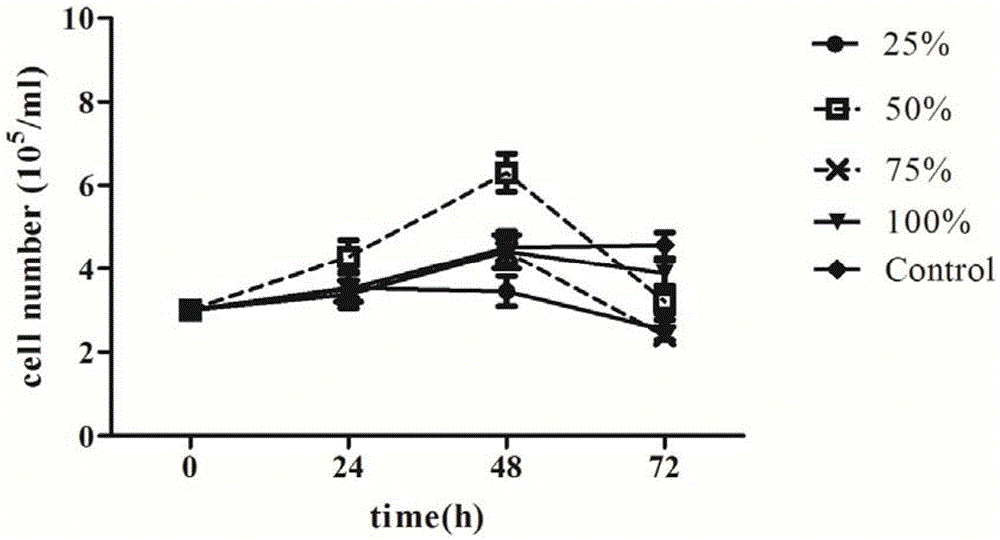
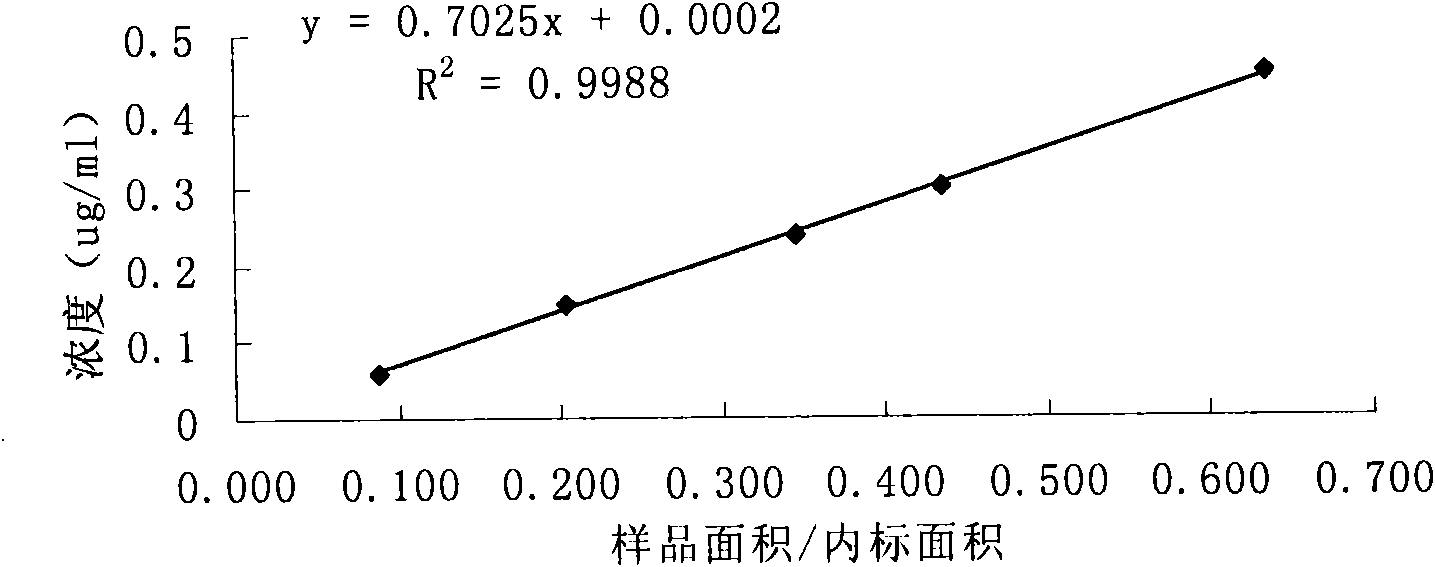
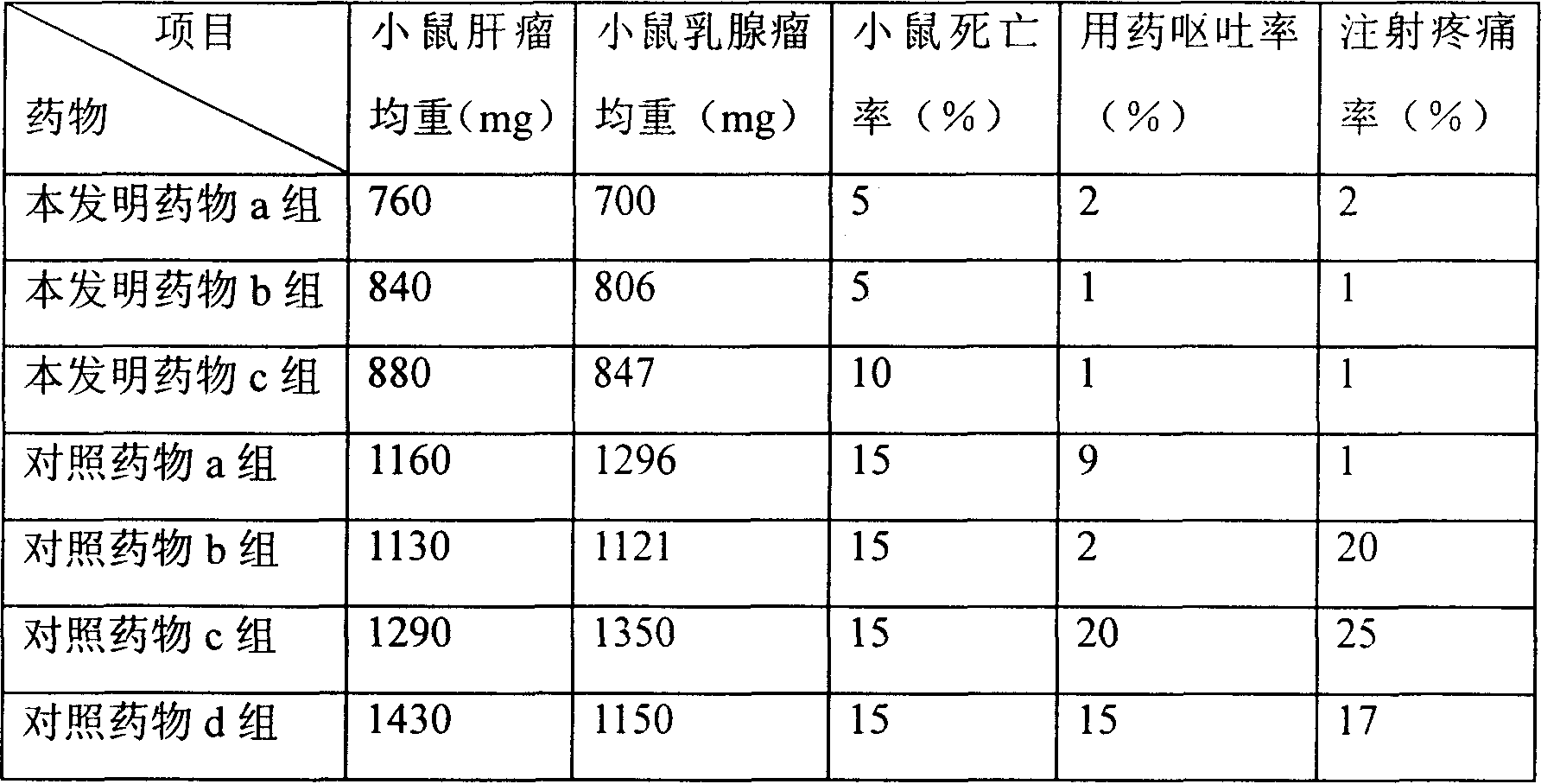
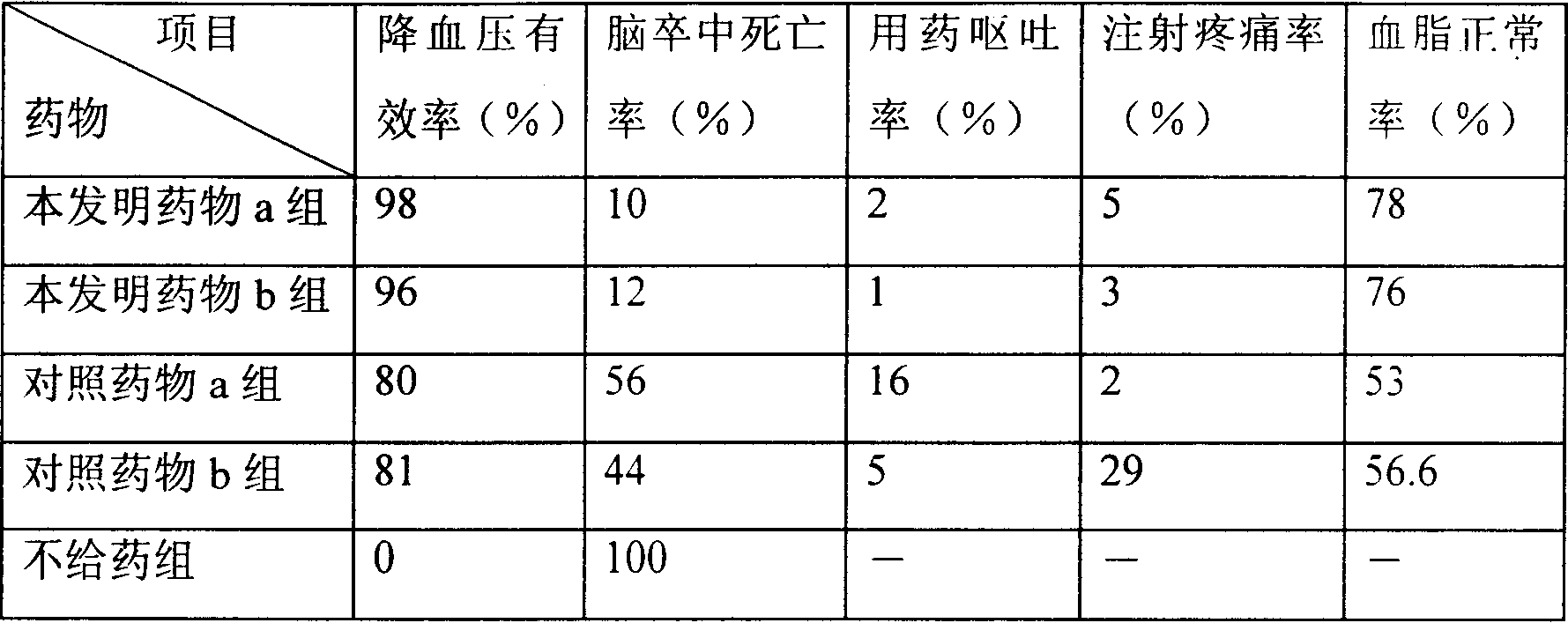
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
68 results about "Prostaglandin E1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
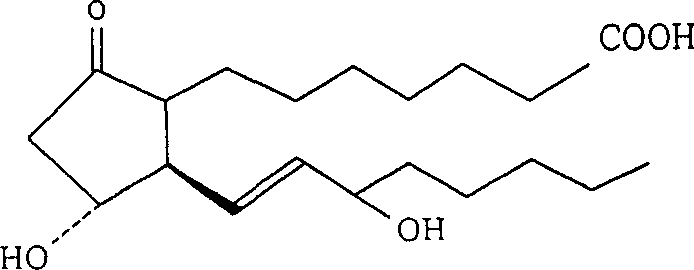
Prostaglandin E1 (PGE1), also known as alprostadil, is a naturally occurring prostaglandin which is used as a medication. In babies with congenital heart defects, it is used by slow injection into a vein to open the ductus arteriosus until surgery can be carried out. By injection into the penis or placement in the urethra, it is used to treat erectile dysfunction.
Drug preparations for treating sexual dysfunction
Topical gelled compositions comprising a drug which causes vasodilation, and optionally prostaglandin E1, dispersed within a polymer matrix, and methods of treating sexual dysfunction, including both male and female sexual dysfunction, using said compositions.
Owner:L A M PHARMA +1
Alpostadil freeze-dried emulsion and its preparing method
InactiveCN1562041AImprove stabilitySimple preparation processPowder deliveryOrganic active ingredientsEmulsionFreeze-drying
A freeze-dried emulsion of prostaglandin E1 is prepared from prostaglandin E1, the oil for injection, stabilizer, emulsifier, pH regulator, freeze-drying protector and the waer for injection. Its preparing process is also disclosed.
Owner:SHENYANG PHARMA UNIVERSITY
Nano emulsion injection of alprostadil and preparation method
InactiveCN1872072AImprove solubilityImprove stabilityOrganic active ingredientsEmulsion deliveryIsoprostaglandin E1Curative effect
A nano-emulsion injection of prostaglandin E1 contains the nano-emulsion particles of rostaglandin E1, the oil for injection, hydrophilic emulsifier, lipophilic emulsifier, isotonic agent, and stabilizer. Its preparing process and its quality control method are also disclosed.
Owner:广州中大创新药物研究与开发中心有限公司
Emulsion composition comprising prostaglandin e1
An emulsion composition includes prostaglandin E1 (PGE1), a phospholipid with a high purity and a non-proton-providing surfactant that improves stability of PGE1. Embodiments of the emulsion composition include an effective amount of PGE1, about 1% to about 30% (w / w) of a pharmaceutically acceptable oil as an oil base based on the weight of the emulsion composition, about 1% to about 30% (w / w) of a phospholipid with a high purity based on the weight of the oil base, about 1.6% to about 40% (w / w) of a non-proton-providing surfactant based on the weight of the oil base, and the balance of the emulsion composition being water.
Owner:TAIWAN LIPOSOME CO LTD +1
Prostaglandin E1 lipid microsphere injection with charge effect and preparation method thereof
InactiveCN101496787AClinical application safetyEnsure safetyOrganic active ingredientsDigestive systemChemical structureLipid formation
The invention relates to a method for preparing a prostaglandin E1 lipid microsphere injection of a charging non-homogeneous phase (comprising a water phase, an oil / water interfacial film phase and an oil phase) dispersion system, of which the surface of the lipid microsphere can be charged with positive electricity or negative electricity. The prostaglandin E1 is alprostadil, of which the chemical structure comprises a basic skeleton of 20-carbon fatty acid with a 5-carbon ring and two side chains, wherein one side chain is provided with a hydrophilic carboxylic acid group, so that the prostaglandin E1 has the characteristic of light surface activity action. By utilizing the characteristic, and according to the formula and the preparation process provided in the invention, the prostaglandin E1 has an unique drug-carrying mode in a solution of lipid microsphere with the non-homogeneous phase dispersion system, and the prepared lipid microsphere injection is fundamentally different from an alprostadil injection(Kaishi, and is prepared by adopting the technology of the Japanese business corporation LTT Bio-Pharma Co., Ltd. already sold in markets, and the difference lies in that the drug-carrying mode is completely different, the content of degradation products in the preparation such as impurities is more than 50 percent lower than that of in the Kaishi, so that the prostaglandin E1 lipid microsphere injection and the alprostadil injection are fundamentally different. The invention relates to a method for preparing the prostaglandin E1 lipid microsphere injection and the drug-carrying characteristics thereof in a three-phase system; in the formula, 0.0001 to 0.1 weight portion of prostaglandin E1 is used as a drug, the prostaglandin E1 is added with auxiliary materials for medical purpose to prepare the prostaglandin E1 lipid microsphere injection, and the auxiliary materials for medical purpose comprises the following materials in portion by weight: 5 to 20.
Owner:李淑斌
Controlled platelet activation to monitor therapy of ADP antagonists
ActiveUS7790362B2Elcosanoid active ingredientsMicrobiological testing/measurementMedicineIsoprostaglandin E1
A method is provided of determining whether an individual has reduced ability to form platelet thrombi. An ADP platelet activator and one or platelet inhibitors are provided. At least one of the platelet inhibitors is Prostaglandin E1 (PGE1). An alternate signal transduction pathway is produced. A final concentration of ADP is 2 to 35 μM and a final concentration of PGE1 is 2 to 30 nM, preferably 20 to 25 nM.
Owner:INSTR LAB
Prostaglandin microemulsion gel rubber preparation and method of producing the same
InactiveCN101427993AOrganic active ingredientsPharmaceutical delivery mechanismDiseaseGel preparation
The invention relates to prostaglandin microemulsion-gel preparation and a preparation method thereof. The preparation comprises prostaglandin E1 or the derivative thereof, oil, emulsifier, assistant for emulsifying agent, gel substrate and water, and also possibly comprises a permeation accelerator, a preparation stabilizer, an antioxidant and antiseptics. The preparation has the advantages of good percutaneous permeability, no stimulation to skin and mucous membrane, simple preparation technique and stable quality, the preparation can be used for treating diseases such as ulcer of limbs caused by erectile dysfunction and chronic arterial occlusion and rest pain of limbs caused by microcirculatory disturbance.
Owner:李淑斌
Serum-free culture medium used for full-suspension culture of MDCK (Madin Darby Canine Kidney) cells
The invention provides a serum-free culture medium used for full-suspension culture of MDCK (Madin Darby Canine Kidney) cells. The culture medium is prepared from a DMEM / F12 culture medium containing 5g / L of NaCl and the following components: sodium stannite, recombinant human epidermal growth factors, hydrocortisone, recombinant full-chain insulin, prostaglandin E1, human transferrin, thyroxine (T3), vitamin E, cholesterol, ethanol amine, beta-mercaptoethanol, Tween-80, Hypep1510, recombinant human serum albumin ACF and mannitol. With the adoption of the culture medium provided by the invention, the full-suspension culture of the MDCK cells can be carried out very well under the condition that serum is not added; after the MDCK cells are continuously cultured for 20 generations, the average proliferation concentration of the MDCK cells is 2.308*10<6> / mL, the average cell viability is 97.4 percent and the average doubling time is 34.48 hours, so that the serum-free culture medium provides technical supports for developing influenza vaccines of cell matrixes of mammals in China.
Owner:马忠仁 +2
Prostaglandin E1 liposome frozen dry powder injection and production technology thereof
InactiveCN1449759AImprove stabilityLess side effectsOrganic active ingredientsLiposomal deliveryCholesterolIsoprostaglandin E1
The present invention provides a prostaglandin E1 liposome fronen dry powder injection and its production process. It is mainly characterized by that it uses soya lectithin and cholesterol to cover high-purity prostaglandin E1 so as to make them into freeze-dried powder injection. Said process can greatly raise stability of the finished product in production, can prolong expiry date, and can reduce side reaction.
Owner:CHEM PHARMA FACTORY NANYANG PUKANG GROUP
Drug preparations for treating sexual dysfunction
Topical gelled compositions comprising a drug which causes vasodilation, and optionally prostaglandin E1, dispersed within a polymer matrix, and methods of treating sexual dysfunction, including both male and female sexual dysfunction, using said compositions.
Owner:GLYCOBIOSCI +1
Methods for measuring platelet reactivity of individuals treated with drug eluting stents
ActiveUS9506938B2Elcosanoid active ingredientsMicrobiological testing/measurementAdenosineAdenosine diphosphate
A method is provided for measuring inhibition of platelet reactivity in an individual treated with a drug-eluting stent (DES). First, a blood sample is obtained from an individual treated with a DES and a P2Y12 antagonist. The blood sample is then mixed with particles comprising an attached GPIIb / IIIa receptor ligand, adenosine diphosphate (ADP) and prostaglandin E1 (PGE1). The mixture is incubated under conditions suitable for agglutinating particles, and platelet-mediated agglutination is assessed in the mixture. The absence or reduction of agglutination indicates that the individual treated with a DES has reduced platelet reactivity. Also provided is a kit for measuring inhibition of platelet aggregation by a P2Y12 receptor antagonist that includes a GPIIb / IIIa receptor ligand immobilized on a particle, adenosine diphosphate (ADP), prostaglandin E1 (PGE1), an anticoagulant, and a buffer to maintain the anticoagulated blood in a condition suitable for platelet aggregation.
Owner:INSTR LAB
Drug preparations for treating sexual dysfunction
InactiveUS20030138494A1Powder deliveryOrganic active ingredientsSexual dysfunctionDrugs preparations
Topical gelled compositions comprising a drug which causes vasodilation, and optionally prostaglandin E1, dispersed within a polymer matrix, and, methods of treating sexual dysfunction, including both male and female sexual dysfunction, using said compositions.
Owner:L A M PHARMA
Prostaglandin E1 long-circulation fat microsphere preparation for intravenous injection and preparation method thereof
The invention relates to a formula and a preparation method of a prostaglandin E1 long-circulation fat microsphere for intravenous injection. The preparation comprises the following components: (1) a prostaglandin E1 medicine; (2) vegetable oil (dissolving oil diluted by a medicine); (3) an emulsion surfactant; (4) an emulsion preparation stabilizing agent, an emulsifying agent, a particle diameter control agent and a polyoxyalkylene phospholipid derivative increasing the half life of the fat microsphere in blood; (5) oleic acid or oleate which is used as the emulsion preparation stabilizing agent or the emulsifying agent; (6) an antioxidant; and (7) a complex compound (glycerol) controlling the cation concentration.
Owner:XIAN LIBANG PHARMA
Compound recipe formula containing kurarinone prostaglandin E1 and aspirin, its preparation method and application
InactiveCN1415301ANew formulaExact therapeutic effectSalicyclic acid active ingredientsDigestive systemIsoprostaglandin E1Freeze-drying
A compound medicine containing kurarinol, prostaglandic E1 and aspirin is prepared through including the kurarinol and prostaglandin E1 by 6-0-malto-beta-cyclodextrin, mixing, adding others, and preparing the freeze dried powder injection. It can be used for treating cancers, cardiovascular and cerebrovascular diseases and hepatitis. Its advantages are sure curative effect, and no toxic by-effect.
Owner:蔡海德
Preparation method of prostaglandin E1
ActiveCN101664390AHigh encapsulation efficiencyHigh affinityOrganic active ingredientsDigestive systemProstaglandin G2Microsphere
The invention relates to a preparation method of prostaglandin E1 which is prepared by the following materials: alprostadil, oil for injection, emulsifier, stabilizer, isotonic agent and water for injection. The method comprises the following steps: firstly dissolving medical materials and phospholipids into suitable solvent and then adding to oil phase after removing solvent. The product preparedby the method has an obvious improvement on entrapment rate and reduces generation of degradation products.
Owner:辽宁中海康生物制药股份有限公司
Sterilization technology of prostadil fatty emulsion and method of determination
InactiveCN1823786AHigh recovery rateMeet sterility requirementsOrganic active ingredientsMetabolism disorderEmulsionMicrowave
A bactericiding process for the fatty emulsion of prostaglandin E1 features that said fatty emulsion in the ampules is heated to 65-140 deg.C for 10s-5 min by microwaves at 915 or 2450 MH2. A method for measuring its main component PGE1 and impurity PGA1 includes such steps as freeze drying for demulsifying to obtain oily sample, extracting in organic solvent, and efficient liquid-phase chromatography at 204-214 nm in wavelength to measure PGE1 and PGA1.
Owner:SHENYANG WANJIA INST OF BIOLOGICAL TECH RES
Lavement preservation solution for organ transplantation
InactiveCN1415200APrevent edemaSuitable osmotic concentrationDead animal preservationSulfateMedicine
A lavation liquid for the organ to be transplated contains K, Na, Mg, Cl, phosphate, sulfate, prostaglandin E1, gossypose, and low-molecular dextran. It can elongate the storage time of organ and increase survival rate of transplanted organ.
Owner:陈静瑜
Method for controlling quality of alprostadil injection
InactiveCN101581702AComponent separationPreparing sample for investigationIsoprostaglandin E1Silica gel
The invention discloses a method for measuring prostaglandin E1 and / or prostaglandin A1 in alprostadil fat emulsion injection, which comprises the following steps: (1) ultrasonically processing the alprostadil fat emulsion injection, obtaining an alprostadil solution of which the fat phase is removed; and (2) performing high performance liquid chromatography measurement to the alprostadil solution of which the fat phase is removed, obtaining the content of the prostaglandin E1 and / or prostaglandin A1, wherein the conditions of the high performance liquid chromatography measurement are as follows: stationary phase: octadecyl ether-bonded monolithic silica is filling agent, mobile phase: the ratio of phosphate buffer to acetonitrile is equal to 1-6:1, and the detection wavelength is 278 nm. The method can accurately measure the alprostadil and the degradation products thereof in the alprostadil fat emulsion injection.
Owner:上海万特医药科技(集团)有限公司
Composition of liposome, and preparation method
InactiveCN1915222AInhibit transferRegulate immunityOrganic active ingredientsNervous disorderYolkDisease
A liposome composition used to prepare the medicines for treating tumor, cardiovascular and cerebrovascular disease, altitude ischemia and insomnia is prepared from the mixture of aspirin, prostaglandin E1, antioxidant, 2-hydroxypropyl-beta-dextrin and gamma-dextrin, the mixture of hydrogenated soybean lecithin and yolk lecithin, and the mixture of soybean sterol, polyethanediol-2000, VC and glycine. Its preparing process is also disclosed.
Owner:江苏仲德医药科技有限公司
Prostaglandin E1 lipid microsphere injection with charge effect and preparation method thereof
InactiveCN101496787BGood physiological compatibilitySolve the problem of high doseOrganic active ingredientsDigestive systemLipid formationChemical structure
Owner:李淑斌
Method for preparing prostaglandin E1 by using gene engineering cyclooxygenase-1 and gene engineering prostaglandin E synthetase-1
ActiveCN110951814AEasy to purifyEfficient use ofOxidoreductasesIsomerasesEscherichia coliProkaryotic expression
The invention discloses a method for preparing prostaglandin E1 by using gene engineering cyclooxygenase-1 and gene engineering prostaglandin E synthetase-1, and belongs to the field of bioengineering. The method comprises the following steps: expressing related enzymes required by prostaglandin E1 synthesis by a prokaryotic expression means, namely cyclooxygenase-1 (COX-1) and prostaglandin E synthetase-1 (mPGES-1); and reacting the enzymes with a substrate to synthesize the prostaglandin E1. By the method, a large amount of prostaglandin synthetase can be expressed, the prostaglandin E1 is synthesized, and the problem that tissue enzymes taken from living bodies are easy to pollute in industrial production can be solved. Meanwhile, the enzyme type expressed by a prokaryotic expression issingle, the concentration of the expressed enzyme is high, organic impurities in an escherichia coli system are few, and the impurities of a product after enzymatic reaction are few, so that the purification and utilization of the prostaglandin E1 are facilitated, and a new path is opened up for the artificial synthesis of the prostaglandin E1.
Owner:CHANGCHUN UNIV OF SCI & TECH
Methods and compositions for treating Raynaud's disease
In various embodiments, methods and compositions for treating Raynaud's disease and Raynaud's phenomenon are provided. Topical administration of a semisolid composition comprising a prostaglandin E1 compound provides the desired relief of the Raynaud's disease or Raynaud's phenomenon without the possible complications of systemic administration. The semisolid composition can be administered as needed, or in a regimen of several doses per day.
Owner:NEXMED HLDG INC
Stable fat emulsion containing prostaglandin e1
InactiveCN101743010AImprove stabilityActive ingredients will not decreaseOrganic active ingredientsSolution deliveryFat emulsionIsoprostaglandin E1
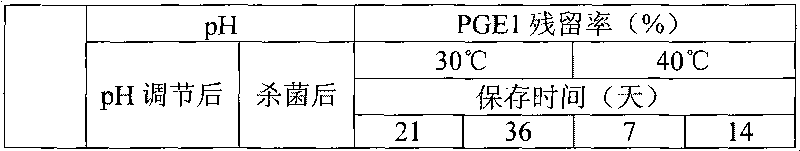
Disclosed is a fat emulsion containing a compound having prostaglandin E1 activity, which fat emulsion is hermetically sealed in a glass container and then heat sterilized. The residual amount of prostaglandin E1 activity after 16-month storage at 5 DEG C is not less than 65% but not more than 100% of the prostaglandin E1 activity at the beginning of storage.
Owner:MITSUBISHI TANABE PHARMA CORP +1
Compound prepn containing prostaglandin E1, prostaglandin A1 and prostaglandin B1 and its prepn process and use
InactiveCN1416815ANew formulaExact therapeutic effectOrganic active ingredientsNervous disorderSide effectIsoprostaglandin E1
The present invention discloses a compound injection in absolut ethyl alcohol or compound powdered injection preparation of prostaglandin E1, prostaglandin E1 potassium salt, prostaglandin E1 sodium salt or prostaglandin A1 and prostaglandin B1. The compound preparation is used in preventing and treating AIDS and abstaining from drugs. It has unique recipe, complementary components, determined curative effect and less toxic side effect.
Owner:蔡海德
Adhesion prevention and an endoscopic insufflation system therefor
InactiveUS7073512B2Effective preventionAvoid stickingOrganic active ingredientsInorganic active ingredientsWhite blood cellIsoprostaglandin E1
A method of treating or preventing adhesion formation during or following a surgical procedure comprising administering to a patient in need thereof at least one medicament selected from the group consisting of potassium channels; modulators of macrophage activation and leucocyte attraction through cytokines, or their inhibitors, antibodies or inhibitors blocking the effect of VEGF expression; prostaglandin E1; free radical scavengers, lipid peroxysomes; pregnatrienes; calcium antagonists; hypoxia; acidosis; MP; dopamine; and ATP-MgCl2, wherein the method prevents adhesion formation by preventing anoxemia.
Owner:SATURNUS
Composite preparations for curing schemic brain damage
InactiveCN101176735AReduce adverse reactionsGood curative effectOrganic active ingredientsNervous disorderIsoprostaglandin E1Clinical therapy
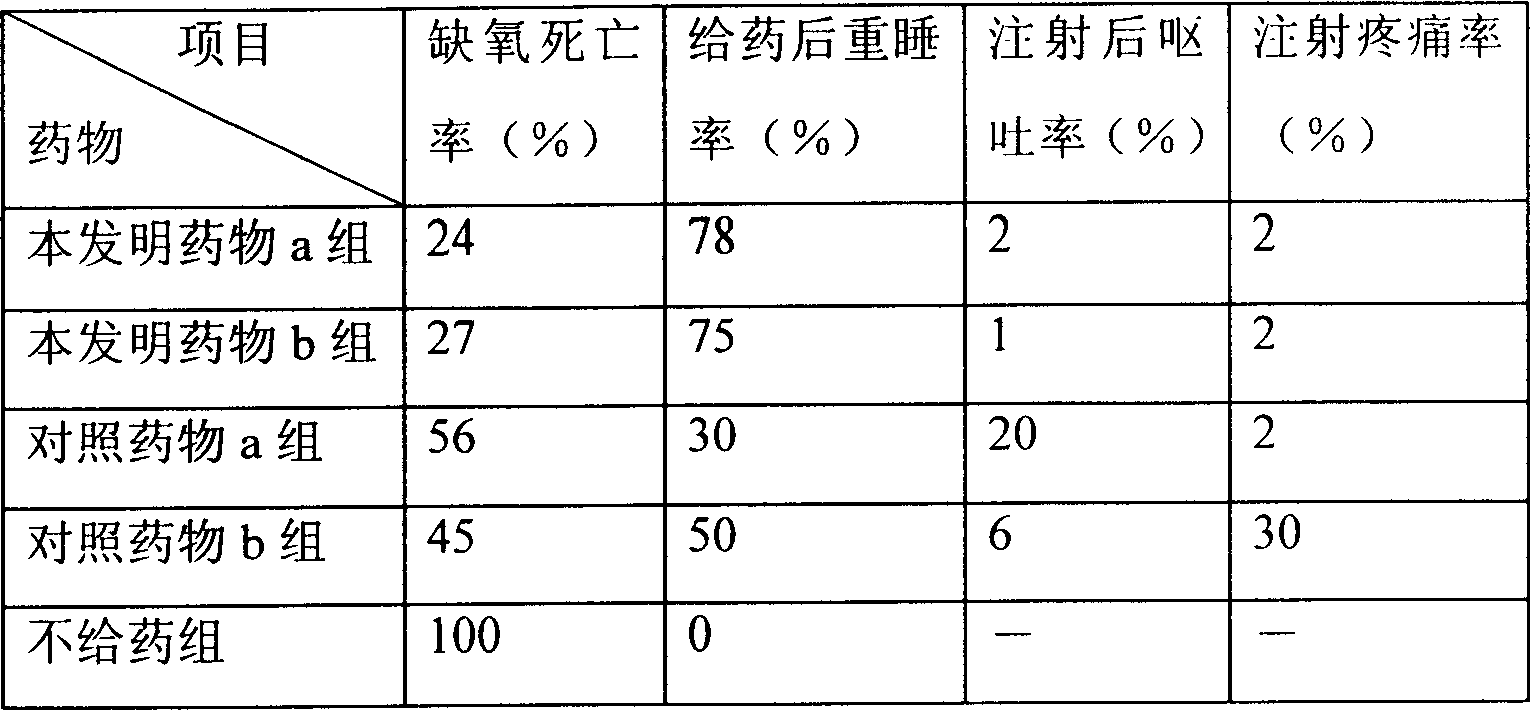
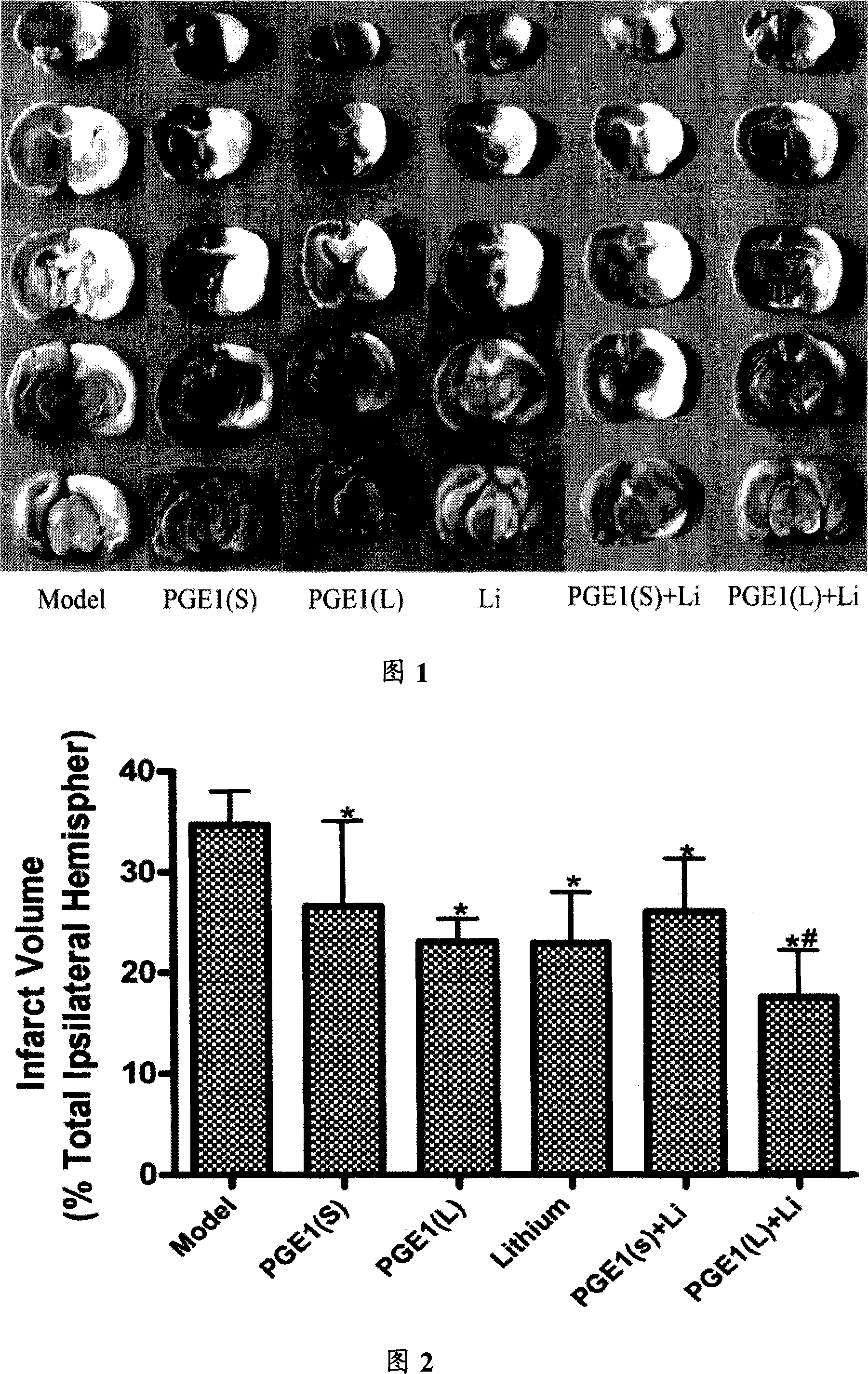
The invention relates to a compound preparation for treating the ischemic brain damage; wherein, the active component comprises prostaglandin E1 the lithium salt; the prostaglandin E1 and the lithium salt can be mixed into the intravenous injection or infusion mixture, and the prostaglandin E1 also can be made into the intravenous injection or infusion agent, meanwhile the lithium salt can be made into the subcutaneous injection. The invention has the advantages of enabling to be used on the people needing treatment or the experiment animal, inducing the formation of the heat shock protein, protects the neuron, increasingg the resistance of the cell hypoxia, and resisting the ischemic brain damage function through the synergistic interaction of the prostaglandin E1 and the lithium salt compound preparation. The invention also has an advantage that: the application dosage of two medicines in the prostaglandin E1 and the lithium salt compound preparation are both lower than the convention dosage of the two medicine in the clinic, so as to reduce the adverse reaction and improve the curative effect. The invention provides a new compound preparation for the ischemic cerebral apoplexy.
Owner:SUZHOU UNIV
Glycosides derivative of prostaglandin E1 and preparation method thereof
ActiveCN101671377AAdapt to treatment needsSugar derivativesSugar derivatives preparationHydrogenIsoprostaglandin E1
The invention discloses a derivative of prostaglandin, represented by the formula (I), the pharmaceutically acceptable salt and a preparation method thereof. In the formula, R1 is beta-D-glucosylsorbitol, beta-D-pyrane galactosyl, beta-D-pyrane xylosyl, alpha-L-pyrane rhamnoside, alpha-L-pyrane arabinose, alpha-D-pyrane mannose, alpha-D-fructofurano, beta-D-fructo ribosyl, beta-L-pyrane fucosyl, beta-D-glucosyl, beta-D-lactosyl, beta-D-cellobiose diglycosyl, and R2 is hydrogen or methyl. The invention provides a stable and long-acting derivative of prostaglandin E1 so as to adapt to treating requirement.
Owner:北京中海康医药科技发展有限公司
Method for preparing prostaglandin derivative
The invention relates to a method for preparing a compound with a structural formula (I). The compound with the formula (I) is a derivative of prostaglandin E1, and the chemical stability of the derivative is superior to that of the prostaglandin E1. The synthesis process disclosed by the invention is a complete synthesis method of the compound with the formula (I), and the method is simple and feasible.
Owner:沈阳万爱普利德医药科技有限公司
Medicine composition for treating tumor and cardiac and cerebral vascular diseases
InactiveCN1398592AAvoid monotonyGood curative effectOrganic active ingredientsAntineoplastic agentsSide effectClinical efficacy
The present invention is one kind of medicine composition for treating tumor and cardiac and cerebral vascular diseases and the composite medicine contains Prostaglandin E1, ribonucleic acid, aspirin or aspirin-DL-lysine and reductive glutathione. The medicine may be prepared into various forms, and has obvious clinical curative effect. The medicine composition can be used to treat both tumor and cardiac and cerebral vascular diseases and has less side effect and adverse reaction.
Owner:蔡海德
Composition of liposome, and preparation method
InactiveCN1915222BInhibit transferRegulate immunityOrganic active ingredientsNervous disorderDiseaseSterol
A liposome composition used to prepare the medicines for treating tumor, cardiovascular and cerebrovascular disease, altitude ischemia and insomnia is prepared from the mixture of aspirin, prostaglandin E1, antioxidant, 2-hydroxypropyl-beta-dextrin and gamma-dextrin, the mixture of hydrogenated soybean lecithin and yolk lecithin, and the mixture of soybean sterol, polyethanediol-2000, VC and glycine. Its preparing process is also disclosed.
Owner:江苏仲德医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com