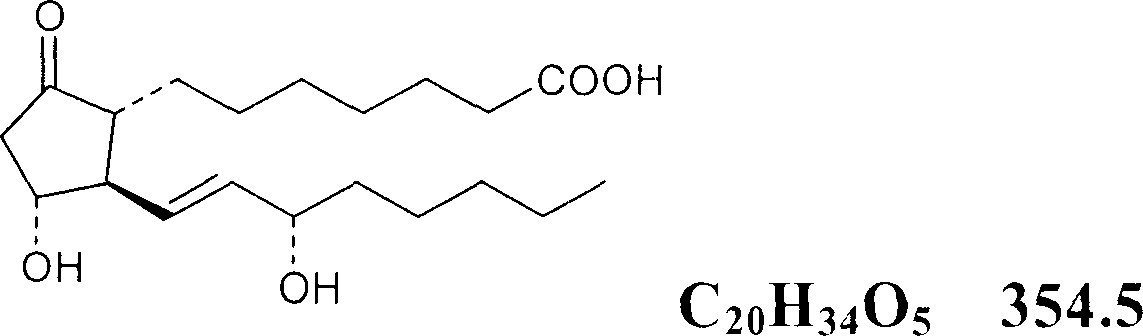
Nose taking powder of prostaglandin E
A preparation and transnasal technology, which is applied in the field of prostaglandin E1 nasal administration preparations, can solve the problems of injections such as obvious vascular irritation, patient pain, and clinical application limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 PGE 1 Preparation of -α-CD inclusion complex
[0018] Weigh PGE 1 Dissolve 50mg in 1ml of absolute ethanol, dissolve α-CD 686mg in 10ml of distilled water, then mix the two liquids, after ultrasonic treatment for 10 minutes, freeze-dry in a freeze dryer for 24 hours, and place the dried product in a vacuum dryer Available in 48 hours. The moisture content of the finally obtained freeze-dried product should be controlled below 1%. PGE can be determined by differential thermal analysis, X-ray powder diffraction and nuclear magnetic resonance 1 Formed an inclusion complex with α-CD.
Embodiment 2
[0019] Example 2 PGE 1 - Preparation of HP-β-CD inclusion complex
[0020] Weigh PGE 1 50mg, dissolved in 1ml of absolute ethanol, 1.9g of HP-β-CD (substitution degree = 5.0) was dissolved in 10ml of distilled water, then the two liquids were mixed, ultrasonicated for 5 minutes, and placed in a freeze dryer for 24 hours. The resulting dried product was placed in a vacuum desiccator for 48 hours. The moisture content of the resulting lyophilizate should be less than 1%.
Embodiment 3
[0021] Example 3 PGE 1 - Preparation of β-CD inclusion complex
[0022] Weigh PGE 1 Dissolve 50mg in 1ml of absolute ethanol, dissolve β-CD 1.6g in 100ml of distilled water, then mix the two liquids, after ultrasonic treatment for 5 minutes, freeze-dry in a freeze dryer for 24 hours, and place the dried product in a vacuum dryer Leave it for 48 hours. The moisture content of the resulting lyophilizate should be less than 1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com