Taxanes medicine preparation for intravenous injection and preparation method thereof
A technology of intravenous administration and taxanes, applied in the field of medicine, can solve problems such as hemolysis, unsatisfactory, and adverse reactions, and achieve good biocompatibility, high in vivo tolerance, and good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
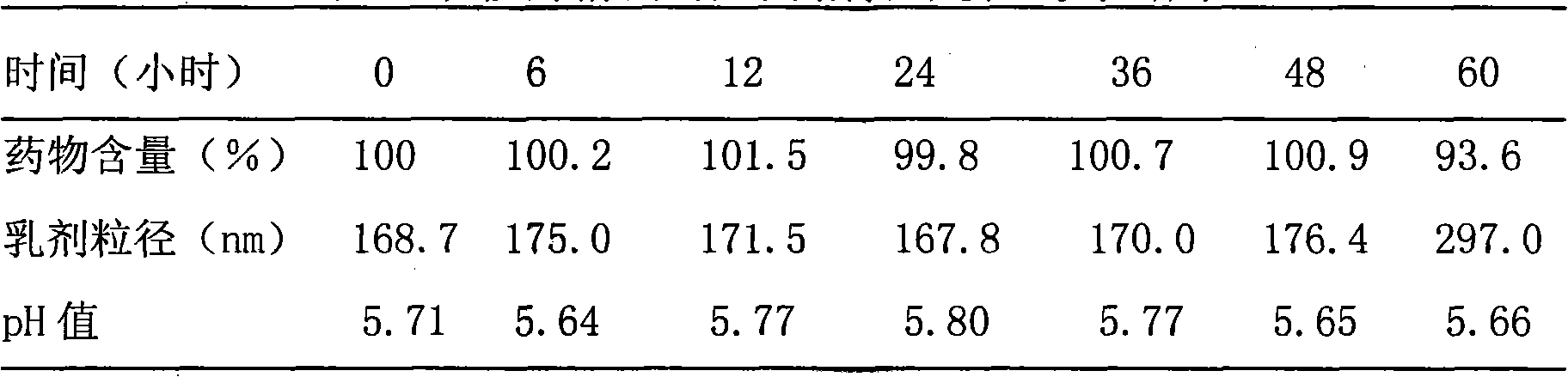
[0038] Example 1. Preparation of Paclitaxel Intravenous Administration Preparation
[0039] 1. Preparation of drug solution:
[0040] Take 2.5 grams of paclitaxel, add it to 100 ml of polyethylene glycol 400, stir and dissolve at 70°C, adjust the pH to 5.5 with hydrochloric acid and sodium bicarbonate, add 0.2 g of activated carbon for needles, and adsorb for 30 minutes at 25°C, then Filter through a 0.45 μm microporous membrane, aliquot, and sterilize by high-pressure steam at 115° C. for 30 minutes to obtain a drug solution.
[0041] 2. Preparation of emulsion:
[0042] (1) Preparation of the oil phase: take 200 grams of caprylic triglyceride for injection, heat it in a water bath to 70 ° C, add 12 grams of soybean lecithin for injection, stir to dissolve, add 0.5 g of tocopherol, stir and mix to obtain the oil phase ;
[0043] (2) Prepare the water phase: take 640 ml of water for injection, add 22.5 g of glycerin, 18810 g of poloxamer, stir at 70°C to dissolve, and obtai...
Embodiment 2
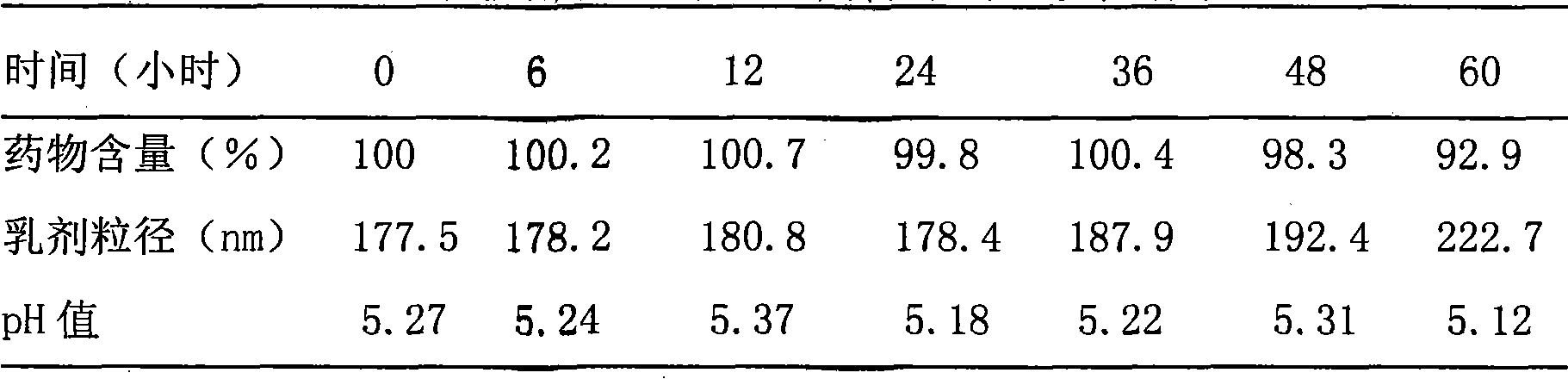
[0045] Example 2. Preparation of Docetaxel Intravenous Administration Preparation
[0046] 1. Preparation of drug solution:
[0047] Take 3.0 g of docetaxel, add it to 100 ml of polyethylene glycol 300, stir and dissolve at 70°C, adjust the pH to 6.0 with hydrochloric acid and sodium hydroxide, add 0.2 g of activated carbon for needles, and absorb at 25°C for 30 minutes, and then filtered through a 0.45 μm microporous membrane, dispensed, and sterilized by high-pressure steam at 115° C. for 30 minutes to obtain a drug solution.
[0048] 2. Preparation of emulsion:
[0049] (1) Prepare the oil phase: take 200 grams of soybean oil for injection, heat it to 70°C in a water bath, add 12 grams of soybean lecithin for injection, stir to dissolve, add 0.5 g of tocopherol, stir and mix to obtain the oil phase;
[0050] (2) Prepare the water phase: take 640 ml of water for injection, add 22.5 g of glycerin, 18810 g of poloxamer, stir at 70°C to dissolve, and obtain the water phase; ...
Embodiment 3
[0052] Example 3. Preparation of Paclitaxel Intravenous Administration Formulation
[0053] 1. Preparation of drug solution:
[0054] Take 8.0 grams of paclitaxel, add it to 100 ml of absolute ethanol, stir and dissolve it at 55 °C, adjust the pH to 4.5 with hydrochloric acid, add 4.5 g of activated carbon for needles, absorb at 45 °C for 60 minutes, and then use a 0.45 μm micropore Remove charcoal by membrane filtration, then sterilize by filtration through a 0.22 μm microporous membrane, and aseptically dispense to obtain the drug solution.
[0055] 2. Preparation of emulsion:
[0056] (1) Preparation of oil phase: Take 10 grams of elemene oil, 45 grams of capric monoglyceride, 58 grams of capric diglyceride, 47 grams of sunflower oil, and 20 grams of evening primrose oil, mix them, and heat them in a water bath to 75°C , add 65 grams of soybean lecithin for injection, 5 grams of glyceryl monooleate, 3 grams of cholic acid, stir to dissolve, add 3.0 grams of tocopherol, st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com