Compounds based on ibuprofen, preparation methods, uses and pharmaceutical preparation thereof
A technology for pharmaceutical preparations and compounds is applied in the application field of preparing non-steroidal anti-inflammatory drugs, and can solve the problems of reduced medicinal effect, limited sterilization conditions and effects of injections, and large changes in the structure of ibuprofen, and the like, To achieve the effect of rapid onset and long drug action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097]Add 10.3g (0.05mol) of ibuprofen and 8g of potassium bicarbonate to a 250mL three-necked flask, add 110mL of acetone while stirring, add 13.4g (0.08mol) of 1-bromoethyl acetate dropwise at room temperature, and continue to The mixture was stirred and reacted for 5 h, diluted by adding 200 mL of ethyl acetate, the reaction solution was transferred to a separatory funnel, washed with a 3% by weight aqueous solution of sodium carbonate (2×100 mL), the organic layer was separated, dried over anhydrous sodium sulfate, Remove the desiccant by filtration, add activated carbon to reflux for decolorization for 20 minutes, remove the activated carbon by filtration, concentrate the filtrate under normal pressure until no liquid distills out, distill the residue under reduced pressure, collect fractions at 164-166°C / 2mmHg, and obtain 12.6 g of colorless liquid. The colorless liquid is the target product ibuprofen-1-acetoxyethyl ester, and the yield relative to the raw material ibupro...
Embodiment 2
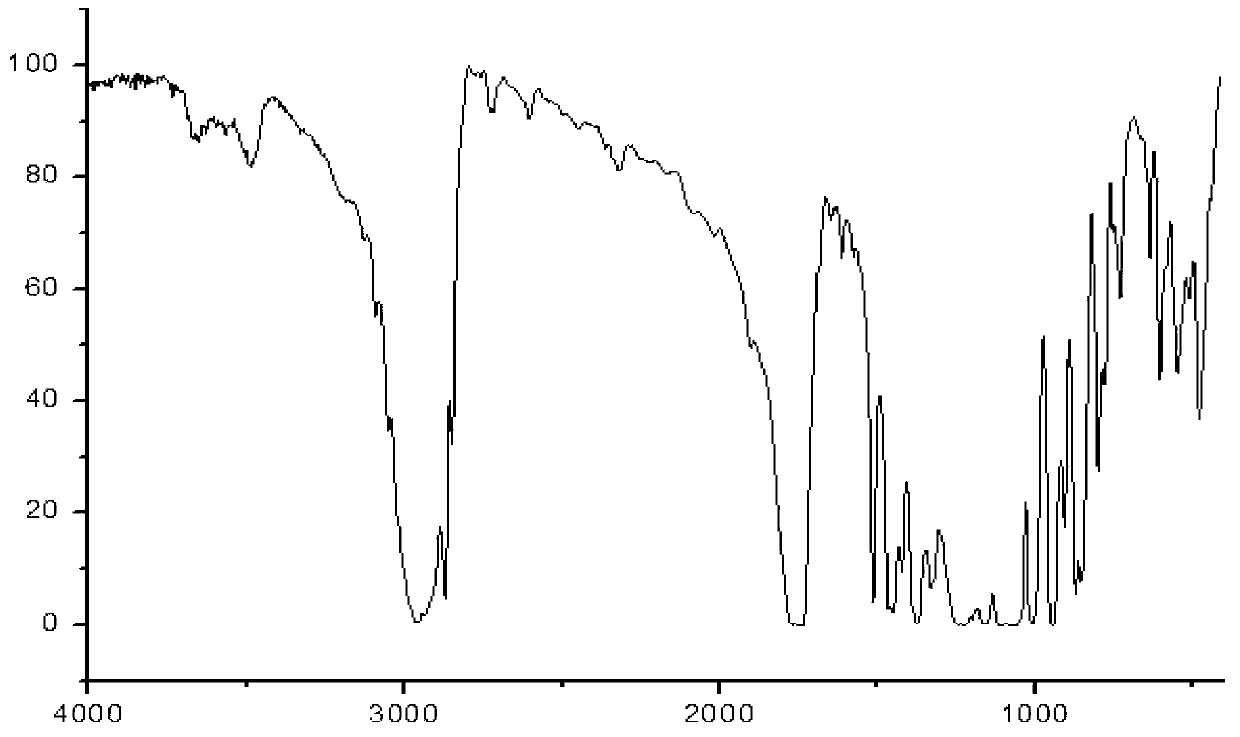
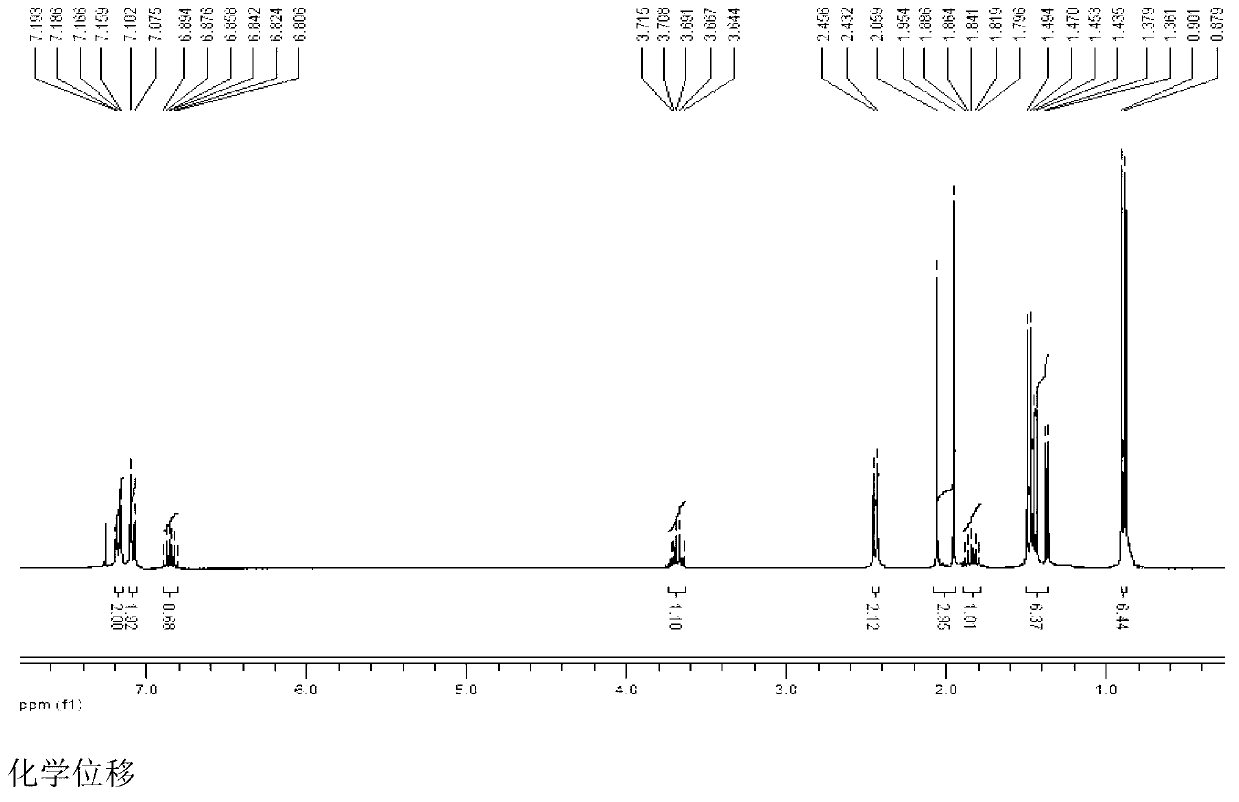
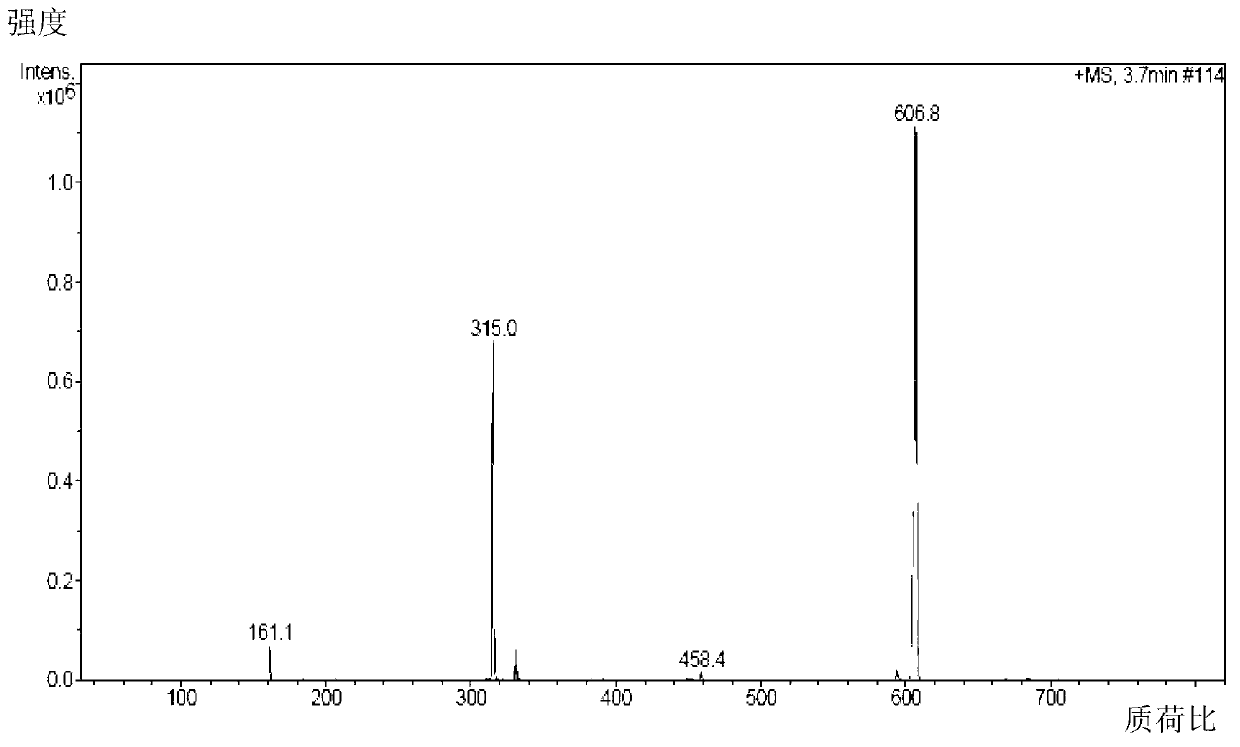
[0103] Add 103g (0.5mol) of ibuprofen and 100g of potassium bicarbonate to a 2500mL three-necked bottle, add 1000mL of acetone while stirring, add 134g (0.8mol) of 1-bromoethyl acetate dropwise at room temperature, and continue to store at 40°C. Stir the reaction for 3h, add 2000mL ethyl acetate to dilute, transfer the reaction solution into a separatory funnel, wash with a sodium carbonate solution with a concentration of 3% by weight (2×800mL), separate the organic layer, dry over anhydrous sodium sulfate, and filter Remove the desiccant, add activated carbon to reflux for decolorization for 20min, remove the activated carbon by filtration, concentrate the filtrate under normal pressure until no liquid is distilled out, distill the residue under reduced pressure, collect 164~166℃ / 2mmHg fractions, and obtain 130g of colorless liquid, which is subjected to IR, 1 HNMR and MS (ESI) spectrograms confirmed that the colorless liquid was the target product ibuprofen-1-acetoxyethyl es...
Embodiment 3
[0105] Add 2060g (10mol) of ibuprofen and 240g of potassium bicarbonate to a 5L three-necked flask, add 1L of acetone while stirring, add 2345g (14mol) of 1-bromoethyl acetate dropwise at room temperature, and continue to stir the reaction at 25°C 3h, add 1L ethyl acetate to dilute, transfer the reaction solution into a separatory funnel, wash with 3% by weight sodium carbonate solution (2×5000mL), separate the organic layer, dry over anhydrous sodium sulfate, filter to remove the dry Add activated carbon to reflux for decolorization for 20min, remove the activated carbon by filtration, concentrate the filtrate under normal pressure until no liquid is distilled out, distill the residue under reduced pressure, collect 164~166℃ / 2mmHg fractions, and obtain 2642g of colorless liquid, which is tested by IR, 1 HNMR and MS (ESI) spectrograms confirmed that the colorless liquid was the target product ibuprofen-1-acetoxyethyl ester, and the yield relative to the raw material ibuprofen w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com