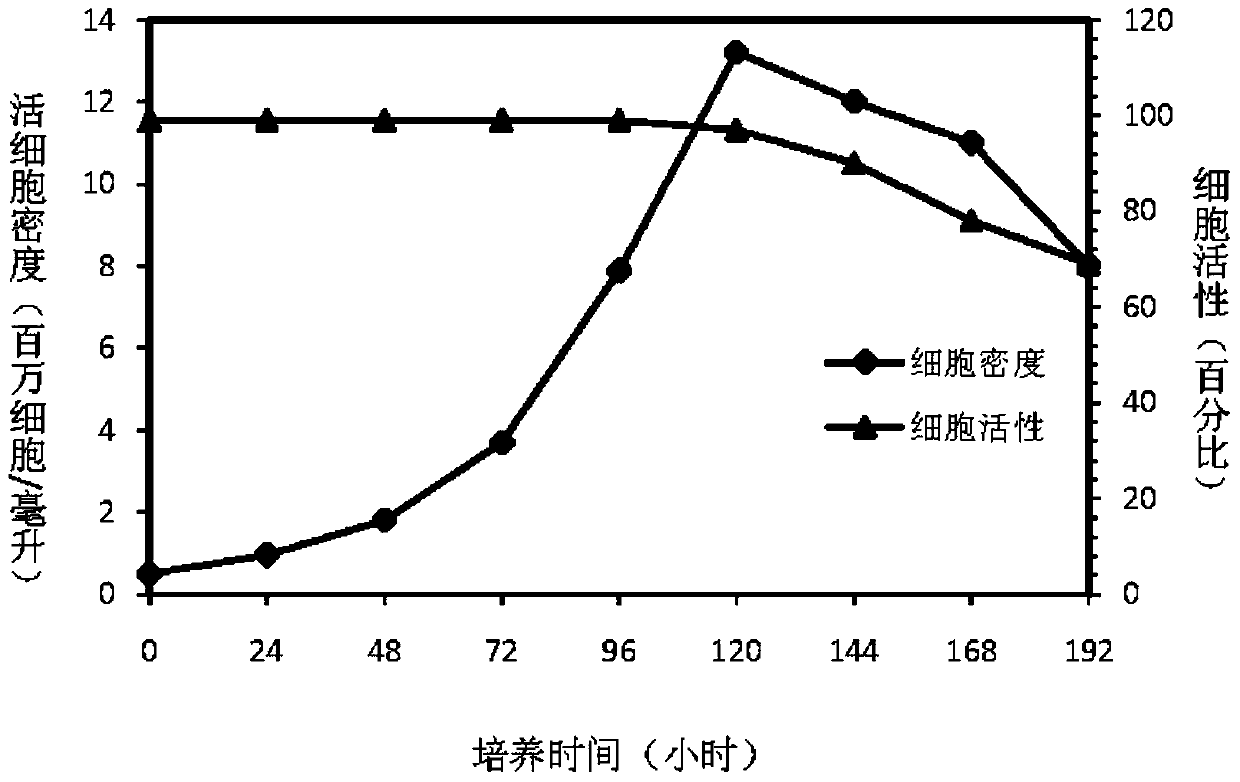
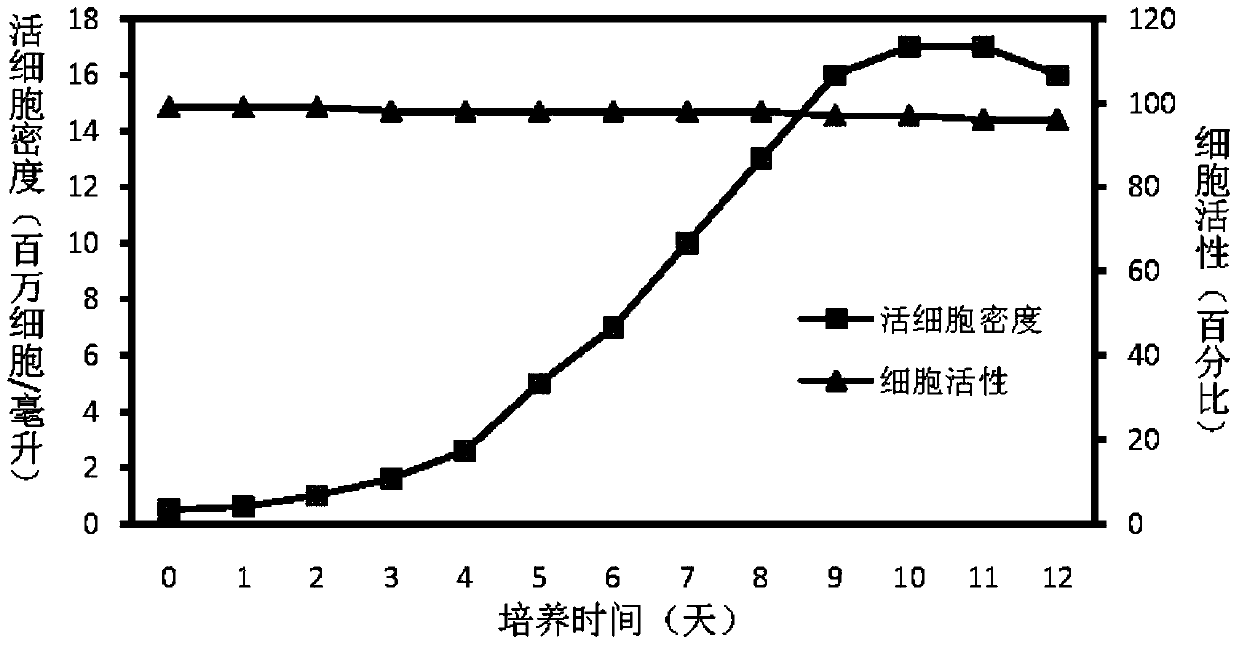
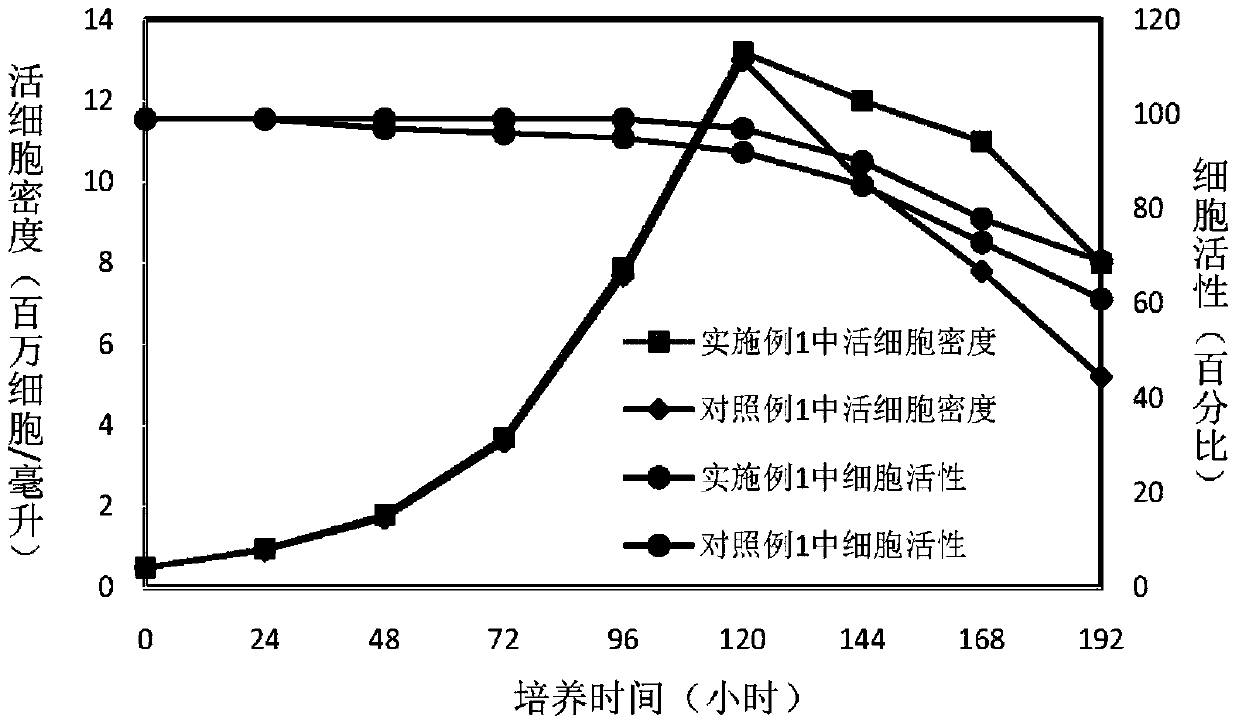
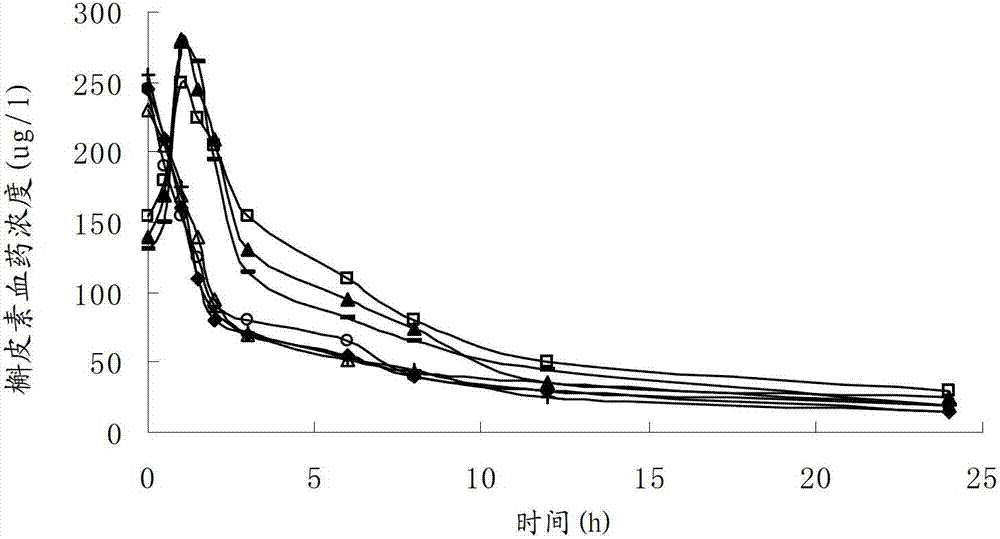
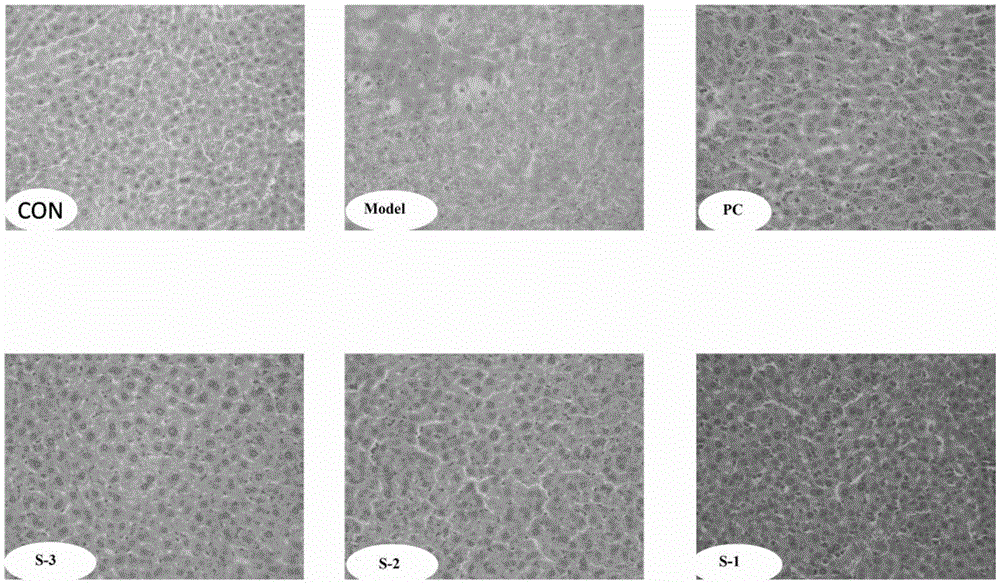
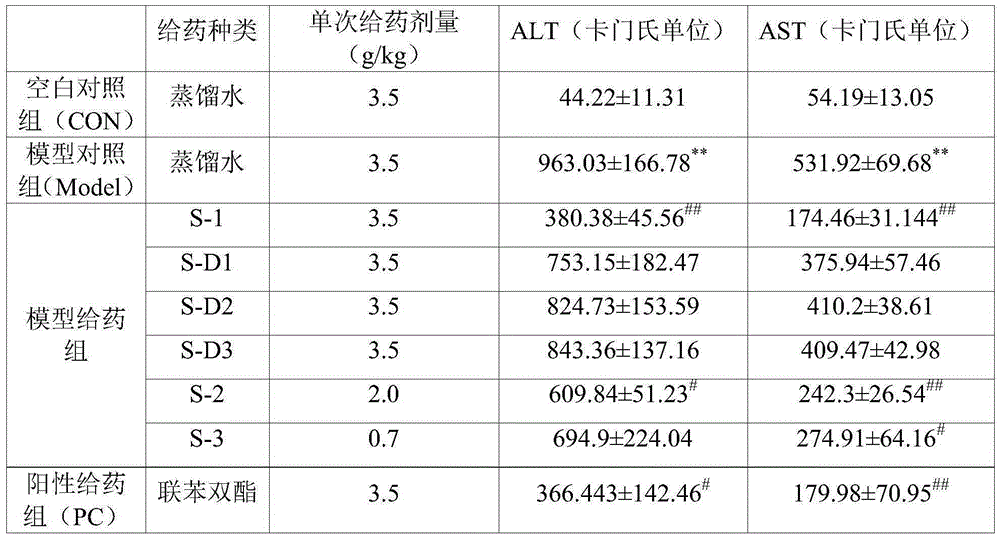
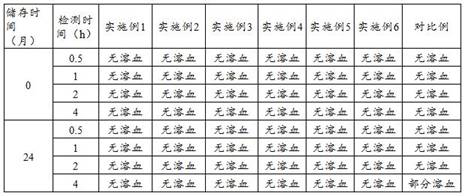
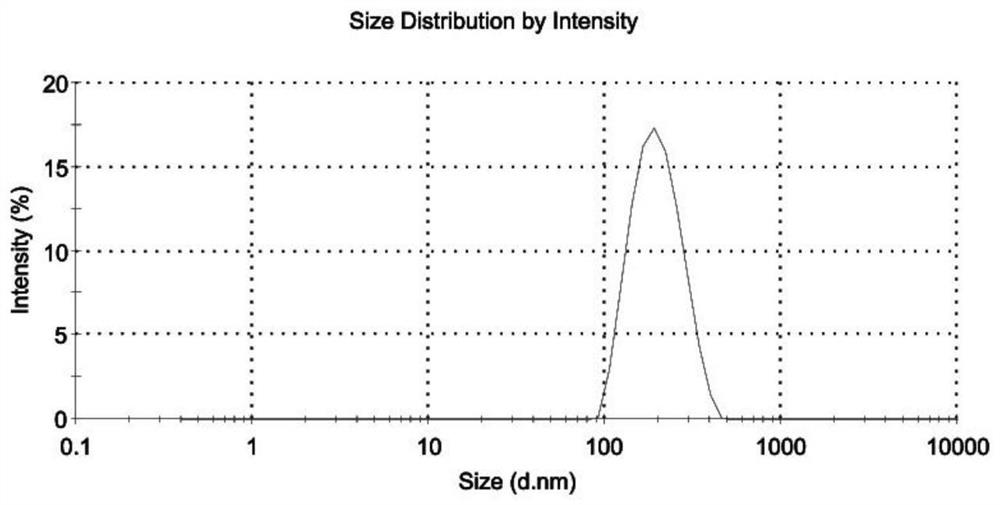
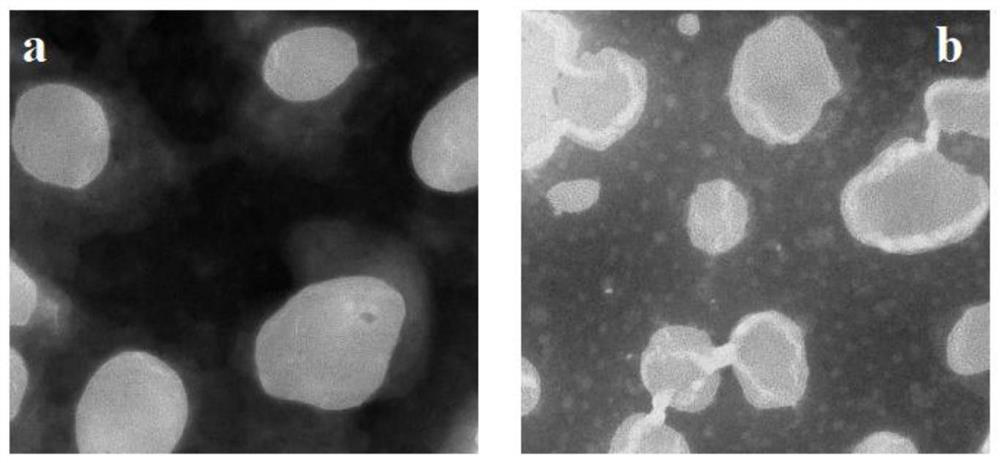
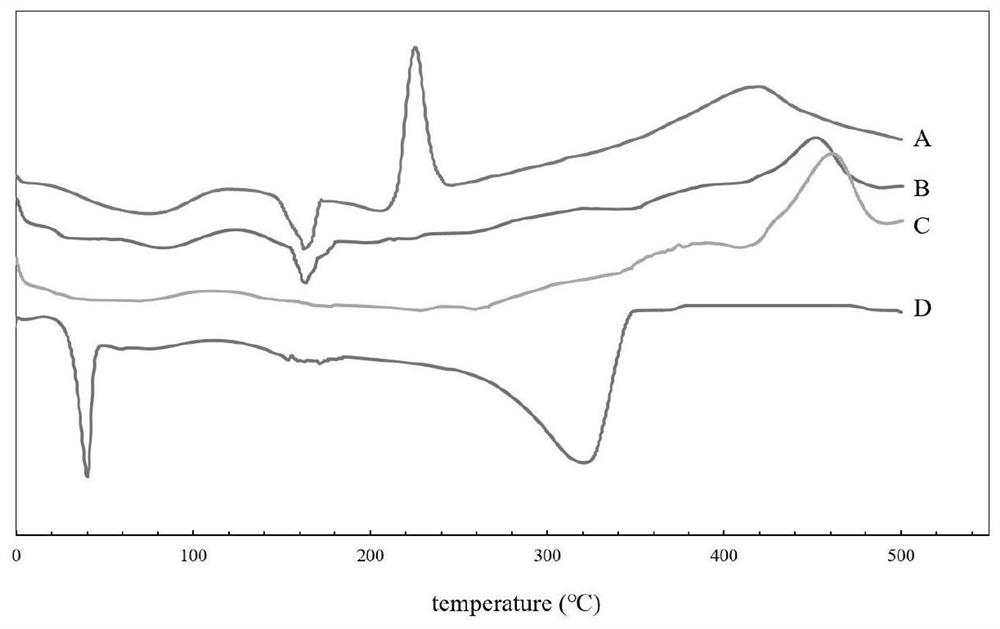
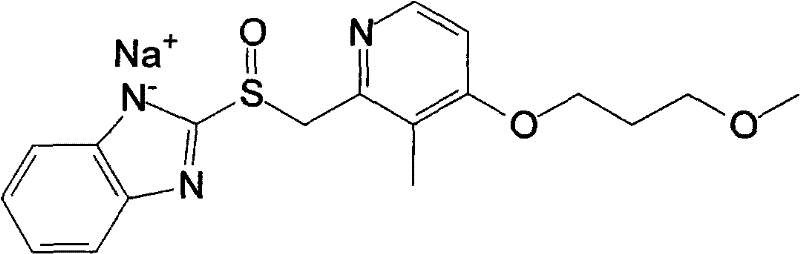
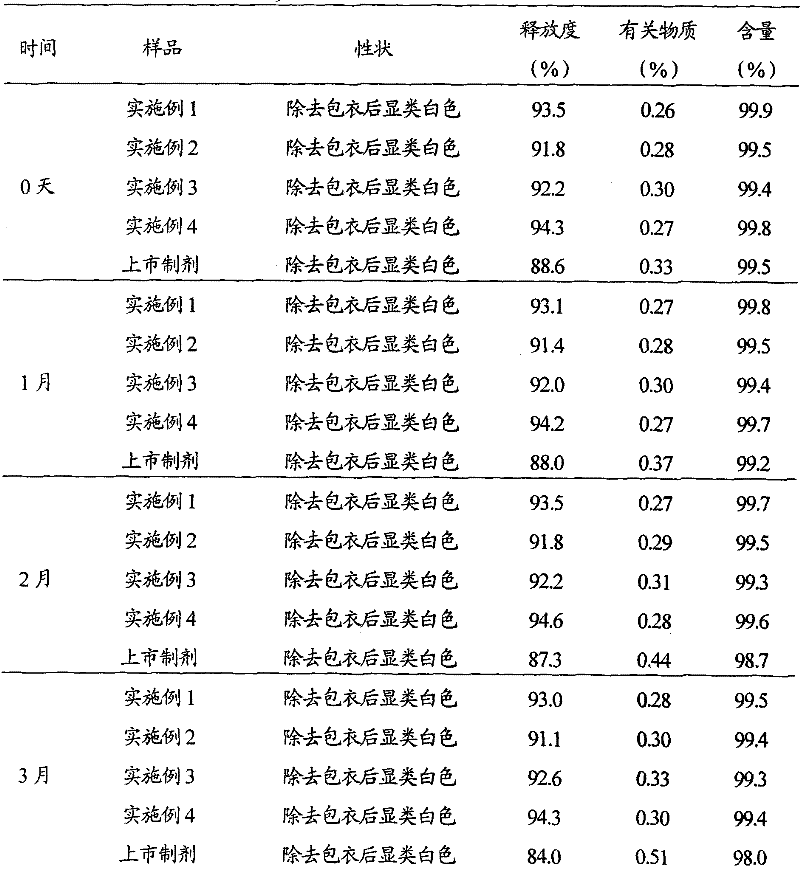
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Dioleoyl phosphatidylcholine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
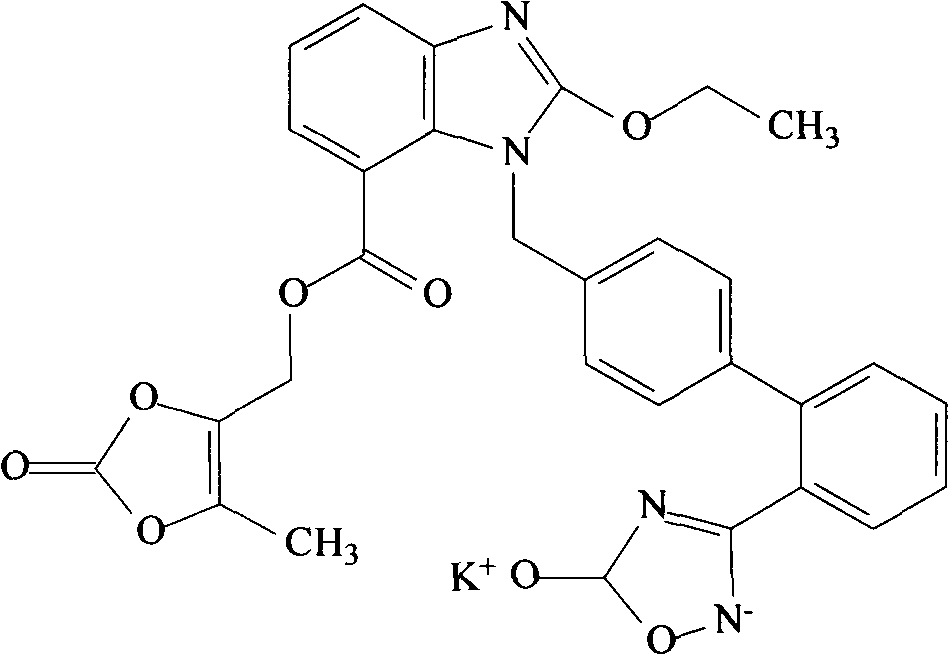
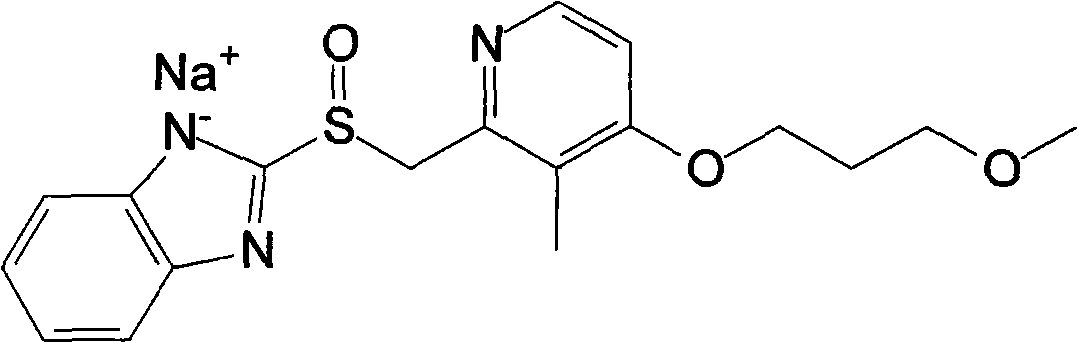
1,2-dioleoyl-sn-glycero-3-phosphocholine (1+) is a 1,2-diacyl-sn-glycero-3-phosphocholine in which the phosphatidyl acyl groups are both oleoyl. It is a conjugate acid of a 1,2-dioleoyl-sn-glycero-3-phosphocholine.
Cucurbitacin lipsome preparation method and formulation
InactiveCN1504191AHigh encapsulation efficiencyOrganic active ingredientsDigestive systemCucurbitacin BMedicine
The invention relates to a cucurbitacin liposome composition and its preparation, which has rather high encapsulation efficiency, and can be administered through vein, muscle, oral and nasal. The constituent percentage by weight of the composition are, cucurbitacin BE or cucurbitacin B 0.001-0.1%, phospholipids 0.1-10%, cholesterin 0-5%, the phospholipids can be lecithin, di-stearoyl phosphatidyl choline, di- palmityl phosphatidyl choline, di-oleoyl phosphatidyl choline, di- palmityl phosphatidyl ethanolamine, di-stearoyl phosphatidylglycerol. The preparation according to the invention can be prepared in the form of injection, oral liquid, syrup, drop and nasal spray
Owner:SHENYANG PHARMA UNIVERSITY
Lipoidosis of Chinese herbal medicine alkaloid and its preparation
InactiveCN1446534AHigh encapsulation efficiencyReduce leakageAmine active ingredientsLiposomal deliveryCholesterolMedicine
A Chinese-medicinal alkaloid lipoid in the form of injection, orally applied medicine, syrup, drops, inhalation, or exteriorlly applied ointment for suppressing tumor contains Chinese-medicinal alkaloid (0.1-2 wt.%), phosphatide (lecithin, distearyl phosphatidylglycerol, etc). (0.5-10 wt.%) and cholesterol (0-5 wt.%). Its advantages are high active concentration in blood, and high curative effect.
Owner:SHENYANG PHARMA UNIVERSITY
Insect cell serum-free culture medium and application thereof
ActiveCN104593316AImprove cultivation efficiencyThe components are simple and clearAnimal cellsFermentationLithium chlorideDiethylenetriamine
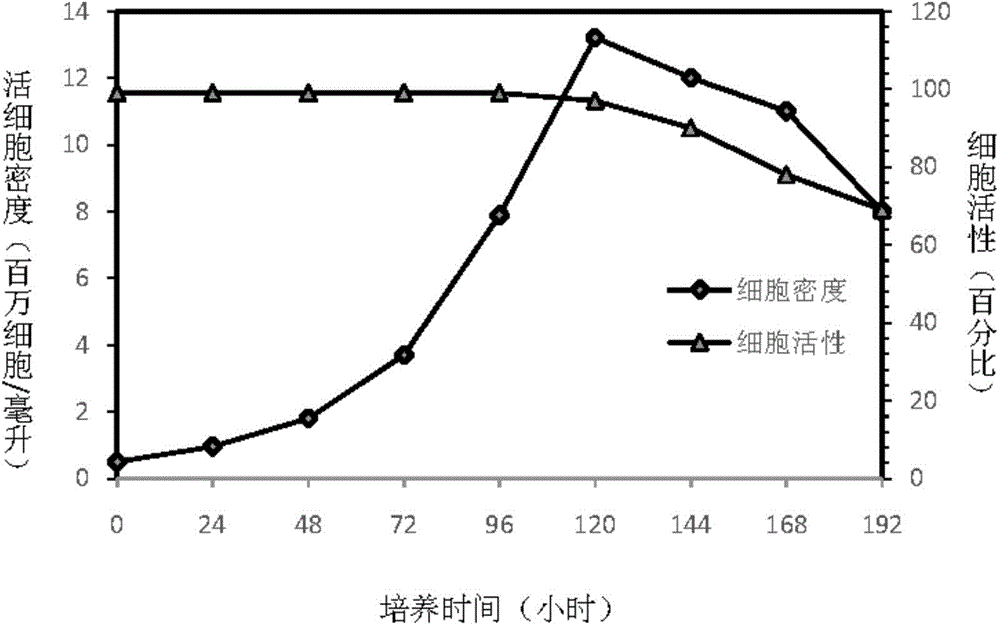
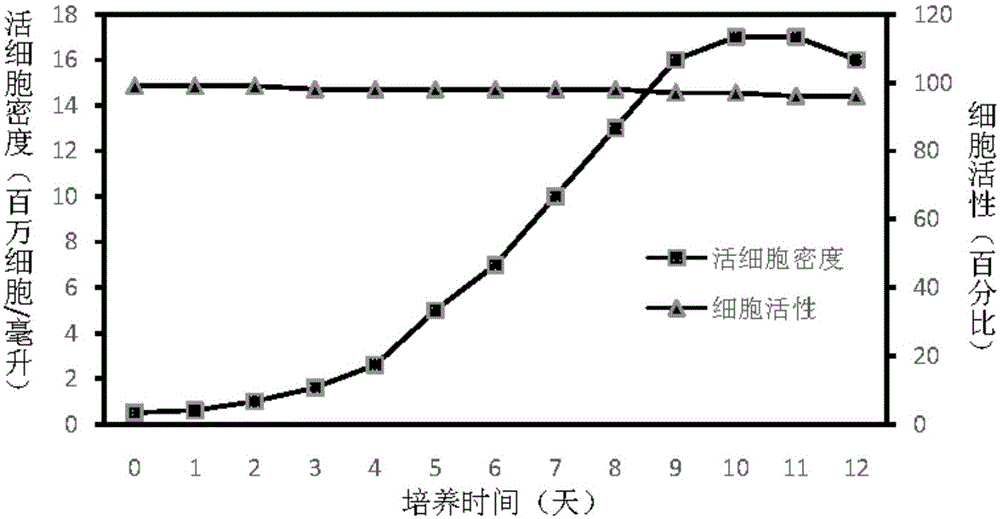
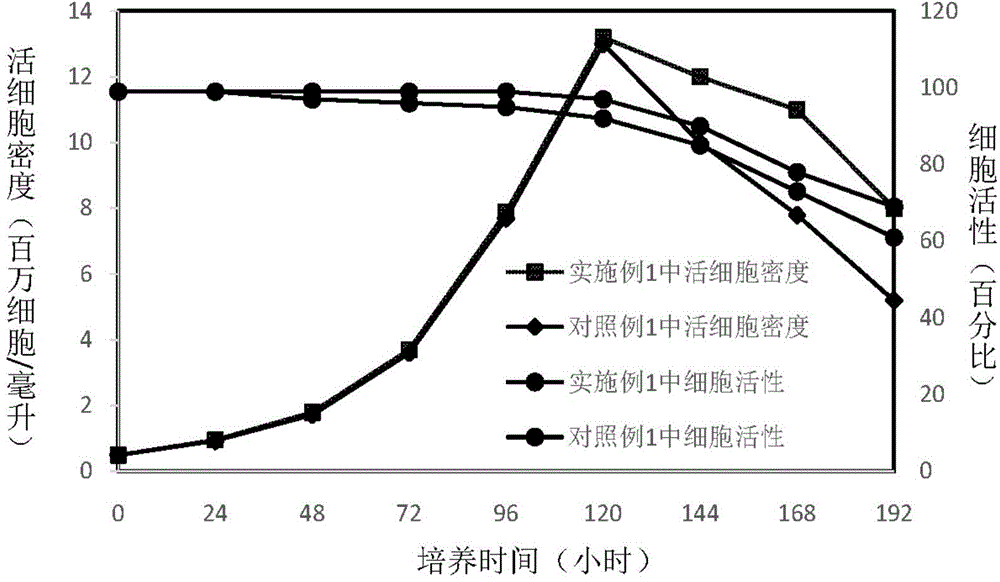
The invention discloses an insect cell serum-free culture medium and application thereof. The culture medium comprises amino acid, inorganic salt, vitamin and carbohydrate and further comprises 0.5-15mg / L of diethylenetriamine dioleoyl phosphatidylcholine and / or 0.1-10mg / L of distearoyl phosphatidyl choline; and particularly, further comprises 0.001-0.1mg / L of barium chloride dihydrate, 0.005-0.02mg / L of lithium chloride and 0.05-7mg / L of nickel chloride. The insect cell serum-free culture medium is simple and clear in components and low in cost and is easily prepared; by the insect cell serum-free culture medium, the insect cell culture efficiency and recombinant protein expression efficiency can be significantly increased and the large-scale culture and large-scale preparation of insect cells are well achieved.
Owner:苏州沃美生物有限公司
Liposomal Vaccine Adjuvants and Methods of Making and Using Same
ActiveUS20140341974A1Stable temperatureBacterial antigen ingredientsSnake antigen ingredientsLipid formationCholesterol
A vaccine adjuvant composition comprising: a lipid selected from the group consisting of: dipalmitoyl phosphatidicholine (DPPC), dipalmitoyl phosphatidylglycerol (DPPG), dioleoyl phosphatidylcholine (DOPC), and cholesterol and containing a positively or negatively charged lipid with associated / entrapped protein antigen.
Owner:ENGIMATA
Olmesartan liposome solid preparation
InactiveCN102138899AHigh encapsulation efficiencyImprove stabilityOrganic active ingredientsPill deliverySide effectSterol
The invention discloses an olmesartan liposome solid preparation prepared by the following raw and supplementary material components in parts by weight: 1 part of olmesartan, 2.5-14 parts of dioleoyl-phosphatidylcholine, 0.5-6 parts of cholesterol, 0.8-10 parts of sodium glycyl-cholate, 0.2-5 parts of soy sterol and 2-20 parts of pharmaceutically acceptable carriers or excipients. The liposome solid preparation provided by the invention has high entrapment rate, even grain size, long medicament retention time in blood circulation, simple equipment, easiness in operation, improved product quality of the preparation and lessened toxic and side effects and is suitable for industrial production.
Owner:HAINAN LINGKANG PHARMA CO LTD
Hypocrellin cationic liposome preparation and preparation method and application thereof
InactiveCN105477633AGood biocompatibilityEnhanced biophotodynamic activitySenses disorderPhotodynamic therapyCholesterolPhospholipid
The invention discloses a hypocrellin cationic liposome preparation and a preparation method and application thereof. The hypocrellin cationic liposome preparation is prepared from hypocrellin, cationic phospholipid, 1-2-dioleoyl phosphatidylcholine, cholesterol and octadecyl triphenylphosphonium bromide. The liposome is a novel hypocrellin liposome designed aiming at macular degeneration photodynamic therapy, the cationic liposome is prepared according to the film-ultrasonic wave dissolving technique, and due to wrapping of octadecyl triphenylphosphonium bromide molecules containing TPP (triphenylphosphine), targeting at new vessel endothelium of a lesion and selectively gathering at a target of endothelial cell mitochondria are realized. In addition, the liposome is technically simple and convenient in preparation and high in stability, biological photodynamic activity of the liposome is more than 2 times of that of parent hypocrellin, and the hypocrellin cationic liposome preparation is a highly potential medicine for macular degeneration photodynamic therapy.
Owner:FIRST HOSPITAL AFFILIATED TO GENERAL HOSPITAL OF PLA +1
Rabeprazole sodium liposome enteric-coated tablets
InactiveCN101966161AImprove stabilitySolve the problem of heat discolorationOrganic active ingredientsDigestive systemCure rateCholesterol
The invention discloses rabeprazole sodium liposome enteric-coated tablets and a preparation method and application thereof to treating gastroesophageal reflux disease (GERD). The rabeprazole sodium liposome enteric-coated tablets comprise rabeprazole sodium liposome solid, alkaline substances and other common auxiliary materials for a solid preparation, wherein the rabeprazole sodium liposome solid comprises the following components in part by weight: 10 to 20 parts of rabeprazole sodium, 40 to 90 parts of dioleoyl phosphatidylcholine, 15 to 50 parts of cholesterol and 8 to 30 parts of sodium glycocholate. The rabeprazole sodium liposome enteric-coated tablets have the advantages of improving stability, increasing dissolution, solving the problem of color change when being heated, and the like, and when the rabeprazole sodium liposome enteric-coated tablets are used for treating the GERD, the curative ratio is higher, the total effective rate is higher, and the clinical superiority is more obvious.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Compound soft capsule containing acanthopanax extract for strengthening brain and preparation method of compound soft capsule
ActiveCN103830294AEnhance memoryImprove sleepingNervous disorderUnknown materialsVegetable oilMedicine
The invention discloses a compound soft capsule containing an acanthopanax extract for strengthening the brain and a preparation method of the compound soft capsule. The method comprises the following steps: orderly dissolving the acanthopanax extract, mixed phospholipid and vegetable oil into an ethanol solution of poloxamer, removing ethanol to obtain content of the soft capsule; and encapsulating the content into a soft capsule shell, wherein the dosage of the mixed phospholipid is 90-240 parts by weight, the dosage of the vegetable oil is 140-300 parts by weight and the dosage of the poloxamer is 10-50 parts by weight relative to 100 parts of acanthopanax extract, the mixed phospholipid is prepared from an animal brain extract and adding ingredients, the weight ratio of the animal brain extract to the added ingredients is 1:(2-6) and the added ingredients comprise phosphatidylserine and / or dilinoleoyl phosphatidylcholine. The compound soft capsule for strengthening the brain, which is disclosed by the invention, can be effectively used for assisting improvement of memory, improvement of sleeping, and prevention of senile dementia, and has a good health-care effect.
Owner:侯文阁
Liposomal vaccine adjuvants and methods of making and using same
ActiveUS9603799B2Stable temperatureBacterial antigen ingredientsVertebrate antigen ingredientsMedicineCholesterol
Owner:ENGIMATA
Heat-and freeze-stable vaccines and methods of making and using same
InactiveUS20140271815A1Significant immunogenicAvoid damagePowder deliverySnake antigen ingredientsLipid formationCholesterol
A vaccine adjuvant composition comprising: a lipid selected from the group consisting of: Dipalmitoyl phosphatidlcholine (DPPC), Dipalmitoyl phosphatidylglycerol (DPPG), Dioleoyl phosphatidylcholine (DOPC), and cholesterol and containing a positively or negatively charged lipid with associated / entrapped protein antigen.
Owner:SORAYYA ARYO +2
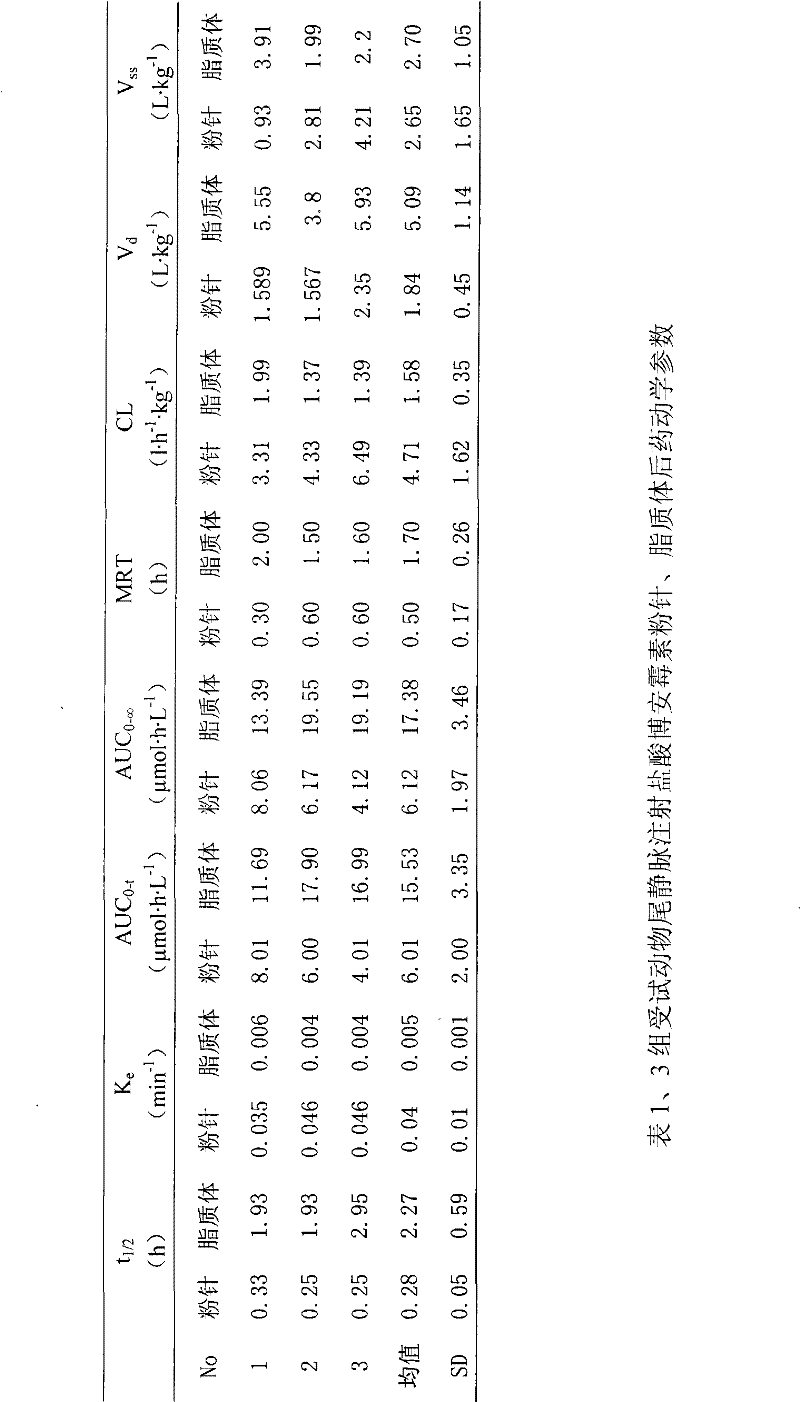
Hydrochloric acid boanmycin liposomes and preparation method thereof
InactiveCN101513390AHigh encapsulation efficiencyMeet usage habitsSaccharide peptide ingredientsPharmaceutical non-active ingredientsSide effectFreeze-drying
The invention relates to the field of pharmaceutical preparation, in particular to hydrochloric acid boanmycin liposomes and a preparation method thereof. The hydrochloric acid boanmycin liposome comprises the hydrochloric acid boanmycin liposomes, phospholipids, cholesterin, citric acid, and sodium citrate, gradient regulator which respectively have the following mass percent: 0.5-3.0%, 3-30%, 1-10%, 0.5-3%, and 1-5%. The phospholipids is lecithin, double stearoyl phosphatidyl choline (DSPC), dipalmitoyl phosphatidyl choline (DPPC), dioleoyl phosphatidyl choline (DOPC) and the like. The invention is prepared by adopting the PH gradient method. Compared with the prior art, the hydrochloric acid boanmycin liposomes of the invention can reduce dosage, toxic side effect on human body and production cost and has the advantages such as targeting at some specific organs. The hydrochloric acid boanmycin liposomes of the invention can be prepared into parenteral solution or further prepared into freeze-dried powder hydrochloric acid boanmycin liposomes by adding a supporting agent.
Owner:SHENYANG PHARMA UNIVERSITY
Drug composition for treating Aids and preparation method of drug composition
The invention discloses a drug composition for Aids dementia complex neuropsychiatric symptoms and a preparation method of the drug composition. The drug composition is prepared from Fructus Mori Albae, Stigma Croci, red psychotria leaves, Manyflower Solomonseal Rhizome, Spirogyra, aminothiopropionic acid and Dioleoyl Phosphatidylcholine in proportion. The drug composition can be prepared into various drug forms according to a conventional preparation technology. The effect on treating the Aids dementia complex neuropsychiatric symptoms is remarkable.
Owner:JINAN HAOYU QINGTIAN PHARMA TECH CO LTD
Soft capsule containing salvia miltiorrhiza extract and preparation method thereof
ActiveCN103830322AImprove chemical liver injuryImprove alcoholic liver damageMetabolism disorderDigestive systemSalvia miltiorrhizaActive component
The invention discloses a soft capsule containing a salvia miltiorrhiza extract and a preparation method thereof. The content of the soft capsule disclosed by the invention contains the salvia miltiorrhiza extract, medium-chain triglyceride, edible oil and phospholipid, wherein corresponding to 1 part by weight of the salvia miltiorrhiza extract, the content of medium-chain triglyceride is 40-600 parts by weight; the content of edible oil is 50-650 parts by weight; the content of phospholipid is 40-600 parts by weight; the content of unsaturated fatty acid in edible oil is not less than 70% by weight; the content of phosphatidylcholine in phospholipid is 20-80% by weight, and the content of dilinolein diplmitoyl phosphtidyl choline is 20-80% by weight. The preparation method of the soft capsule disclosed by the invention comprises the following steps of: mixing various components to obtain the content of the soft capsule; packaging the content in the shell of soft capsule. The soft capsule disclosed by the invention is capable of effectively improving chemical liver injury and alcoholic liver injury and reducing blood fat; furthermore, the active components, such as the salvia miltiorrhiza extract, phospholipid and the like, can exist stably for a long time.
Owner:侯文阁
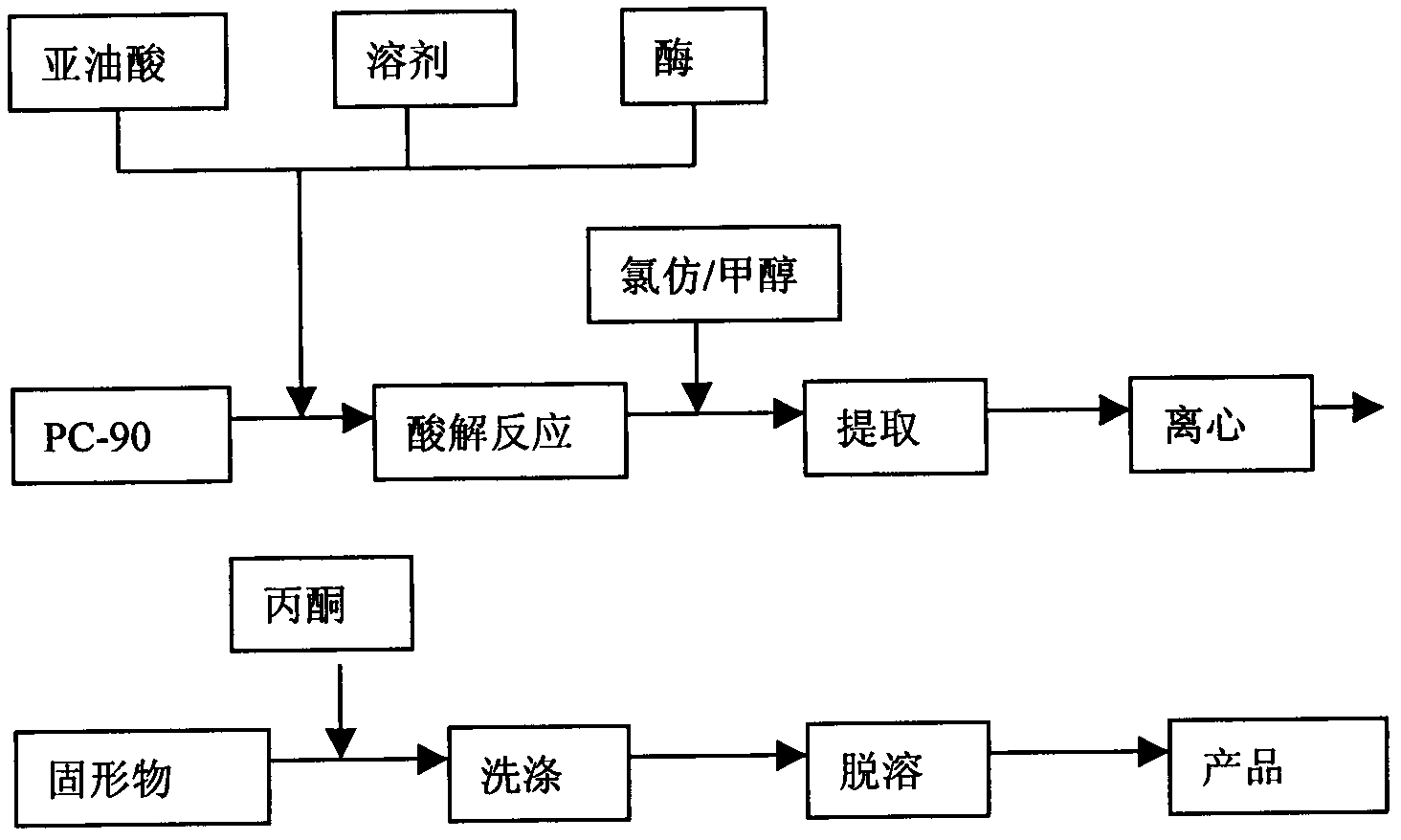
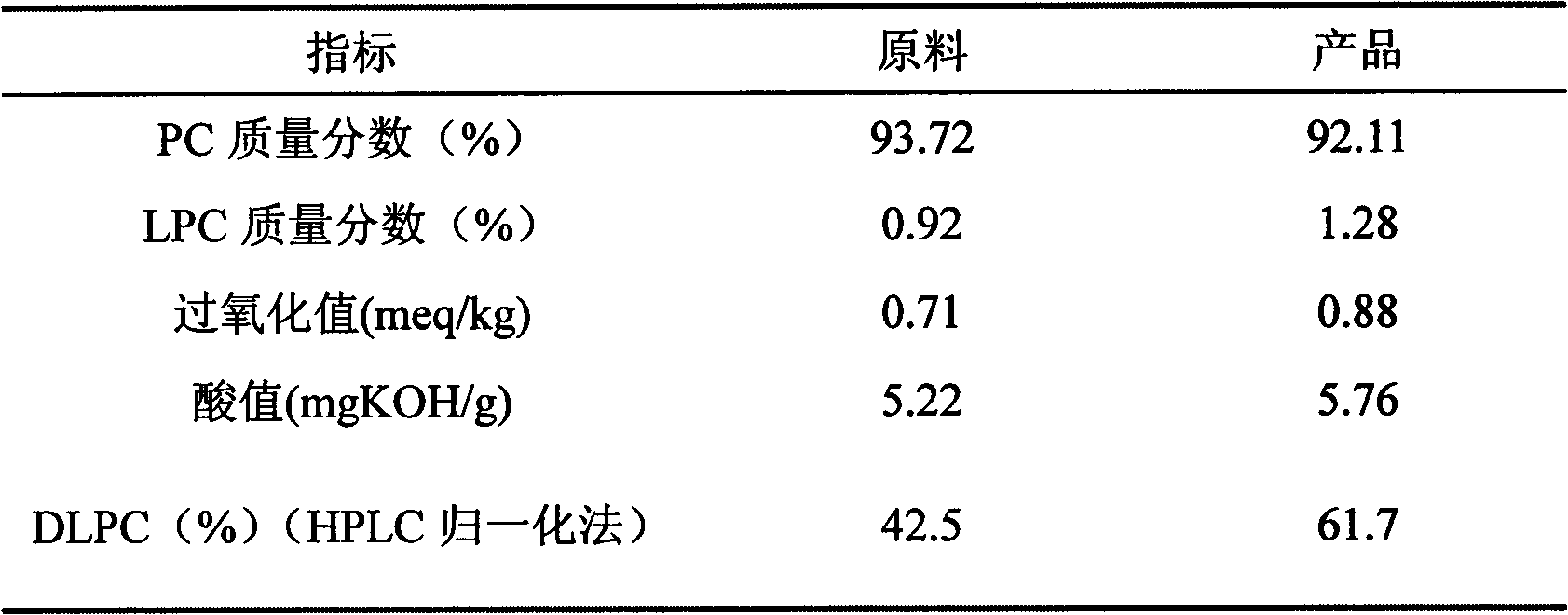
Method for improving dilinoleoyl phosphatidylcholine content with enzyme method
The invention relates to a method for improving dilinoleoyl phosphatidylcholine (DLPC) content with an enzyme method. The invention belongs to the technical field of phospholipid. According to the invention, soybean PC-90 is adopted as a raw material; the raw material, linoleic acid and immobilized lipase are added to a certain organic phase, and an enzymatic acidolysis reaction is carried out; after desolvation, a target product is extracted by using chloroform / methanol (2 / 1, V / V); immobilized lipase is recovered by centrifugation; desolvation is carried out, and acetone washing and desolvation are carried out, such that the product with a DLPC content higher than 60% (HPLC normalization method) is obtained.
Owner:JIANGNAN UNIV
PH-sensitive liposome as well as preparation method and application thereof
PendingCN114099698ADisinhibitionOvercome curative effectOrganic active ingredientsHeavy metal active ingredientsAdenosineCholesterol
The invention relates to the technical field of biological medicines, in particular to a pH-sensitive liposome as well as a preparation method and application thereof. The pH-sensitive liposome is prepared from the following medicines and a liposome carrier according to the following amount of substance: (2, 3-dioleoyl-propyl)-trimethyl ammonium-chloride salt, dioleoyl phosphatidylcholine, cholesterol succinic acid monoester and DSPE-PEGn-pep in a molar ratio of (2 to 5): (0.5 to 1.5): (0.5 to 1.5): (0.1 to 0.3), the total mass ratio of the ICD inducer to the (2, 3-dioleoyl-propyl)-trimethyl ammonium-chloride salt, the dioleoyl phosphatidylcholine and the cholesterol succinic acid monoester is (5-12): 100; the mass ratio of the adenosine pathway blocking agent to the ICD inducer is (0.5-2): (0.5-3). The ICD inducer and the adenosine pathway inhibitor are combined for use, so that the problems that the anti-tumor curative effect is poor and tumor immunity escapes when only the ICD inducer is used for treatment are solved, and in-vivo co-delivery of the ICD inducer and the adenosine pathway inhibitor is realized by utilizing the liposome carrier; the pH sensitive characteristic of the POL effectively ensures that the POL is kept stable in the blood circulation process.
Owner:SHANDONG UNIV QILU HOSPITAL
Hydrochloric acid boanmycin liposomes and preparation method thereof
InactiveCN101513390BHigh encapsulation efficiencyMeet usage habitsSaccharide peptide ingredientsPharmaceutical non-active ingredientsSide effectFreeze-drying
The invention relates to the field of pharmaceutical preparation, in particular to hydrochloric acid boanmycin liposomes and a preparation method thereof. The hydrochloric acid boanmycin liposome comprises the hydrochloric acid boanmycin liposomes, phospholipids, cholesterin, citric acid, and sodium citrate, gradient regulator which respectively have the following mass percent: 0.5-3.0%, 3-30%, 1-10%, 0.5-3%, and 1-5%. The phospholipids is lecithin, double stearoyl phosphatidyl choline (DSPC), dipalmitoyl phosphatidyl choline (DPPC), dioleoyl phosphatidyl choline (DOPC) and the like. The invention is prepared by adopting the PH gradient method. Compared with the prior art, the hydrochloric acid boanmycin liposomes of the invention can reduce dosage, toxic side effect on human body and production cost and has the advantages such as targeting at some specific organs. The hydrochloric acid boanmycin liposomes of the invention can be prepared into parenteral solution or further prepared into freeze-dried powder hydrochloric acid boanmycin liposomes by adding a supporting agent.
Owner:SHENYANG PHARMA UNIVERSITY
Olmesartan liposome solid preparation
The invention discloses an olmesartan liposome solid preparation prepared by the following raw and supplementary material components in parts by weight: 1 part of olmesartan, 2.5-14 parts of dioleoyl-phosphatidylcholine, 0.5-6 parts of cholesterol, 0.8-10 parts of sodium glycyl-cholate, 0.2-5 parts of soy sterol and 2-20 parts of pharmaceutically acceptable carriers or excipients. The liposome solid preparation provided by the invention has high entrapment rate, even grain size, long medicament retention time in blood circulation, simple equipment, easiness in operation, improved product quality of the preparation and lessened toxic and side effects and is suitable for industrial production.
Owner:HAINAN LINGKANG PHARMA CO LTD
Preparation method and application of self-assembled nano-drug liposome for resisting Alzheimer's disease
ActiveCN113262203AReliable preparation methodLow costNervous disorderKetone active ingredientsDiseaseCholesterol
The invention relates to a preparation method and application of self-assembled nano-drug liposome for resisting Alzheimer's disease, and can effectively solve the problems of low blood-brain barrier penetration rate and poor treatment effect of the traditional Alzheimer's disease treatment medicine. The preparation method comprises the following steps: dissolving a hydrophobic anti-inflammatory drug in absolute ethyl alcohol, adding into water, and violently stirring to obtain the self-assembled hydrophobic anti-inflammatory nano particle drug; and dissolving dioleoyl phosphatidylcholine, distearoyl phosphatidylcholine, cholesterol and T7 peptide-polyethylene glycol-phosphatidyl ethanol amine in trichloromethane to form a solution, adding the solution into trichloromethane, carrying out reduced pressure distillation to form a thin film, continuously carrying out rotary evaporation, adding the self-assembled hydrophobic anti-inflammatory nano particle drug, dispersing the thin film, and carrying out ultrasonic detection to obtain the self-assembled nano-drug liposome for resisting the Alzheimer's disease. The preparation method is stable, reliable and low in cost, plays a huge role in preparing the medicine for treating the Alzheimer's disease, enhances the effect of treating the Alzheimer's disease, and is an innovation of the medicine for treating the Alzheimer's disease.
Owner:ZHENGZHOU UNIV
Preparation method of polyene phosphatidylcholine
PendingCN113683637AQuality improvementSimple methodComponent separationGroup 5/15 element organic compoundsPolyene phosphatidylcholineSilica gel
The invention provides a preparation method of polyene phosphatidylcholine. Soybean phospholipid is used as a raw material, and the polyene phosphatidylcholine is prepared by silver nitrate chromatography silica gel column chromatography separation. The content of the main active ingredient 1,2-dilinoleyl phosphatidylcholine (DLPC) of the polyene phosphatidylcholine product prepared by the method disclosed by the invention is 60% or above, and the quality of the polyene phosphatidylcholine product is greatly improved.
Owner:海思科制药(眉山)有限公司
Method for preparing high-purity dilinoyl phosphatidylcholine through combination of enzymatic modification and reversed-phase column chromatography separation and product of high-purity dilinoyl phosphatidylcholine
ActiveCN113174412AImprove catalytic performanceShort reaction timeGroup 5/15 element organic compoundsPhosphatide foodstuff compositionsStationary phaseAlcohol
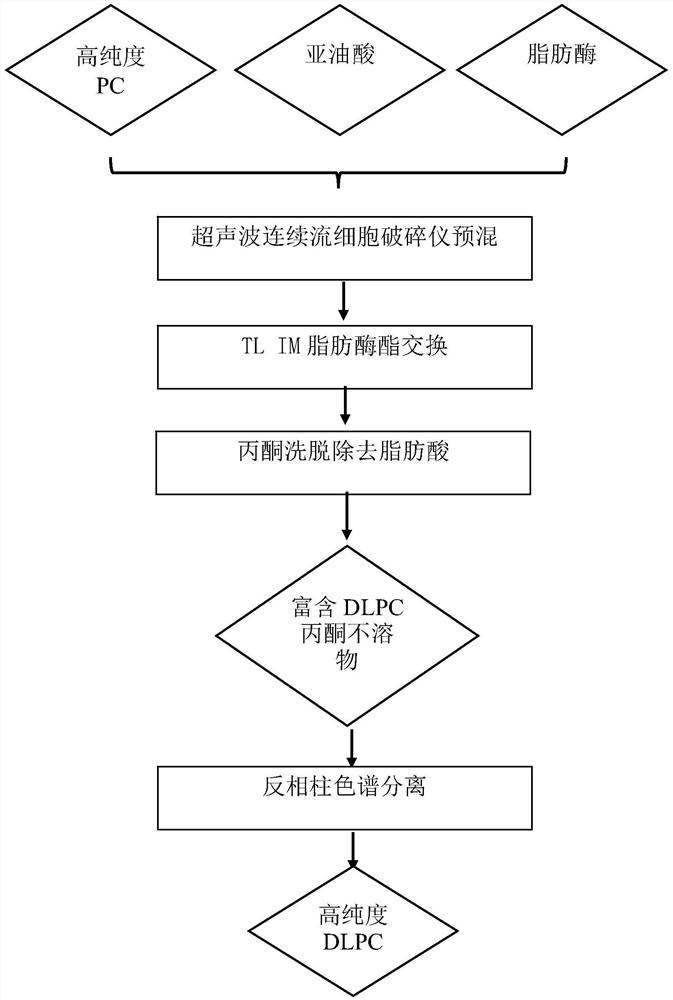
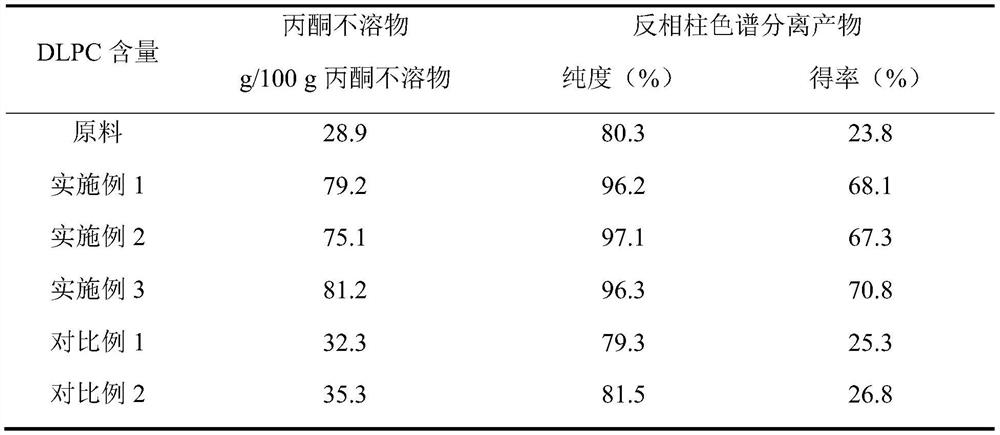
The invention belongs to the field of phospholipid processing, and particularly relates to a method for preparing high-purity dilinoyl phosphatidylcholine through combination of enzymatic modification and reversed-phase column chromatography separation and a product of the high-purity dilinoyl phosphatidylcholine. According to the method, high-purity phosphatidylcholine (PC) and linoleic acid are used as raw materials, an ultrasonic continuous flow cell crusher is adopted to pre-mix a substrate and lipase before lipase reaction, the reaction product of the system is subjected to acetone desolvation to obtain an acetone insoluble substance rich in dilinoyl phosphatidylcholine, the acetone insoluble substance is added to a chromatographic column with C18 as a stationary phase, and eluting is carried out with low-carbon alcohol or a low-carbon alcohol-water solution to obtain a DLPC product with the purity of more than 95% and the yield of more than 65%. The preparation method disclosed by the invention is mild in condition, efficient, simple and controllable in operation and easy to realize industrial production, and the dilinoyl phosphatidylcholine obtained by the method is high in purity and high in yield and can be an important source of high-purity dilinoyl phosphatidylcholine in the medicine and health care industry.
Owner:WUHAN POLYTECHNIC UNIVERSITY
Method for separating and purifying dilinoleoylphosphatidylcholine and dilinoleoylphosphatidylcholine product
ActiveCN112979694ADLPC content increasedEasy to recycleGroup 5/15 element organic compoundsPhosphatide foodstuff compositionsPhospholipinAlcohol
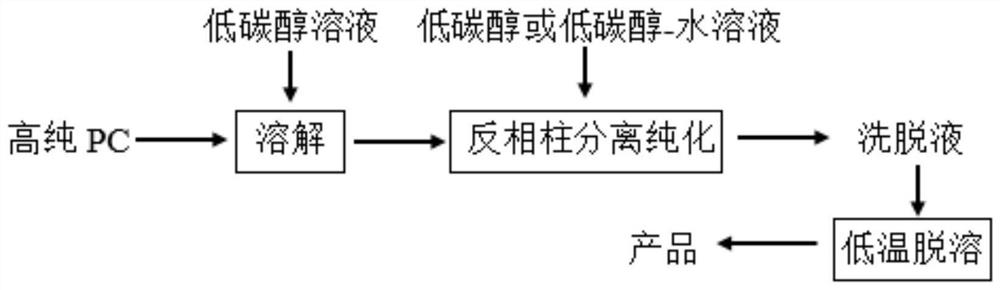
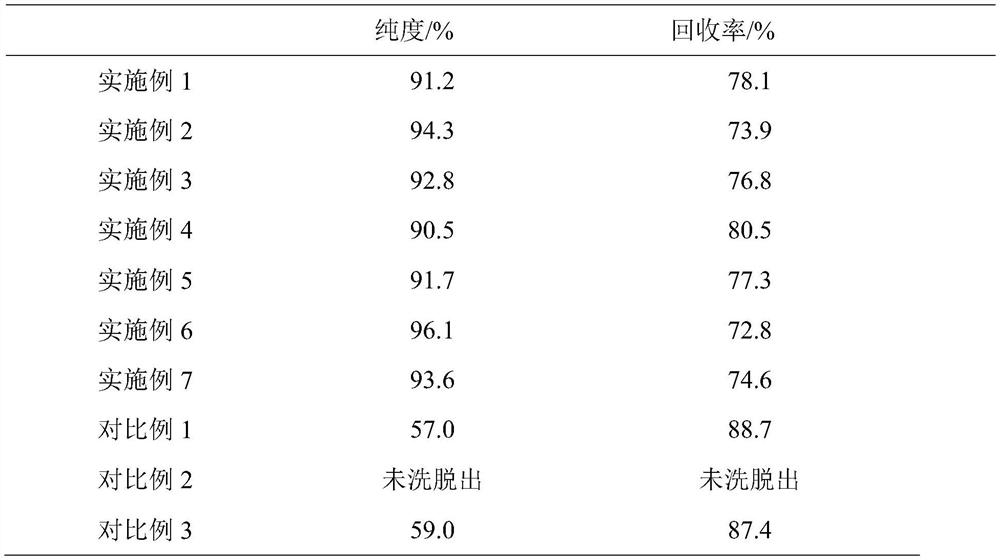
The invention belongs to the field of phospholipid processing, and particularly relates to a method for separating and purifying dilinoleoylphosphatidylcholine and a dilinoleoylphosphatidylcholine product. The method comprises the following steps: (1) preparation of a sample loading solution: dissolving high-purity phosphatidylcholine into low-carbon alcohol to obtain a sample solution; and (2) separation and purification: carrying out separation and purification on the sample solution by adopting a reversed-phase column system, then carrying out elution, collecting an eluent, and then carrying out low-fat desolvation on the eluent to obtain the dilinoleoylphosphatidylcholine product. According to the invention, the DLPC content is greatly increased, the purity is increased from 30% to 90% or above, and the recovery rate is higher than 70%; the low-carbon alcohol or the low-carbon alcohol water solution is used as the eluent, so that recycling is facilitated, and the process is green and environment-friendly; and the preparative chromatographic column is adopted for separation and purification, so that the separation capacity is high, repeatability is good, operation is easy and controllable, and industrial production is easy to achieve.
Owner:WUHAN POLYTECHNIC UNIVERSITY
Insect Cell Serum-Free Medium and Its Application
ActiveCN104593316BImprove cultivation efficiencyThe components are simple and clearAnimal cellsFermentationLithium chlorideDiethylenetriamine
The invention discloses an insect cell serum-free culture medium and application thereof. The culture medium comprises amino acid, inorganic salt, vitamin and carbohydrate and further comprises 0.5-15mg / L of diethylenetriamine dioleoyl phosphatidylcholine and / or 0.1-10mg / L of distearoyl phosphatidyl choline; and particularly, further comprises 0.001-0.1mg / L of barium chloride dihydrate, 0.005-0.02mg / L of lithium chloride and 0.05-7mg / L of nickel chloride. The insect cell serum-free culture medium is simple and clear in components and low in cost and is easily prepared; by the insect cell serum-free culture medium, the insect cell culture efficiency and recombinant protein expression efficiency can be significantly increased and the large-scale culture and large-scale preparation of insect cells are well achieved.
Owner:苏州沃美生物有限公司
Shuxuening lipidosome injection
InactiveCN103040752AQuality improvementImprove bioavailabilityPharmaceutical non-active ingredientsRespiratory disorderSodium metabisulfiteSide effect
The invention discloses a Shuxuening lipidosome injection. The Shuxuening lipidosome injection is mainly prepared by using a ginkgo biloba extract, dioleoylphosphocholine, octadecylamine, polyoxyethylene alkylamine, sodium metabisulfite and sodium chloride. Compared with the prior art, the Shuxuening lipidosome injection disclosed by the invention is suitable for large-scale industrial production and has the advantages that the stability and bioavailability of a preparation are greatly improved, product quality is improved and toxic and side effects are reduced.
Owner:海南路易丹尼生物科技有限公司
Non-aqueous liquid composition
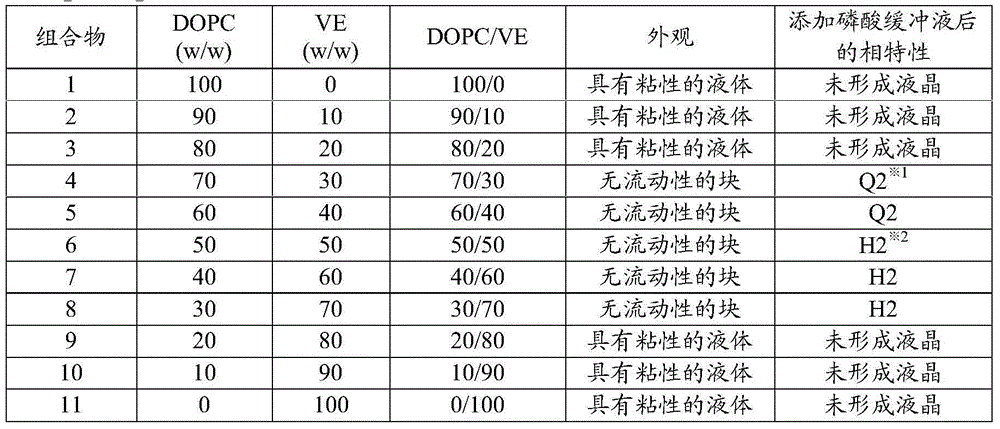
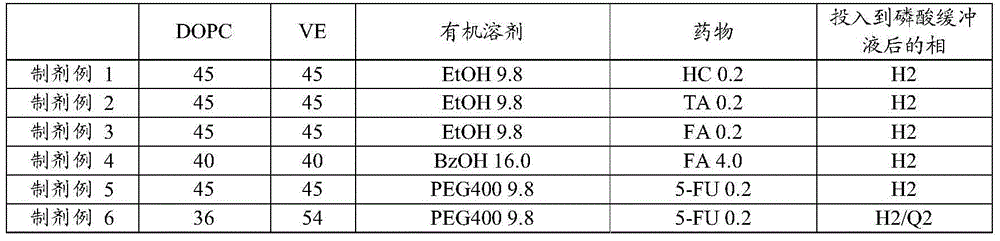
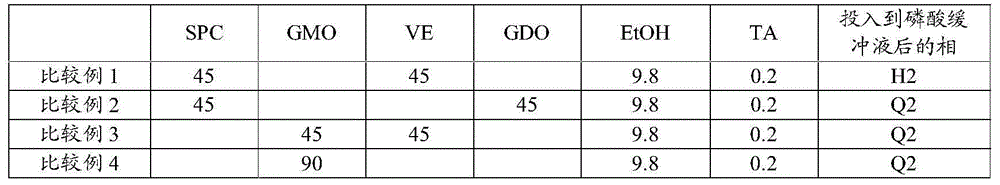
InactiveCN104080478AGood slow releaseOrganic active ingredientsSenses disorderOrganic solventVitreous Fluid
A drug-dissolved non-aqueous liquid composition comprising a drug, dioleylphosphatidylcholine, tocopherol and an organic solvent, wherein the ratio of the blend concentration of dioleylphosphatidylcholine to that of tocopherol falls within the range from 75 / 25 to 25 / 75, the blend concentration of dioleylphosphatidylcholine falls within the range from 15 to 85% (w / w) and the blend concentration of tocopherol falls within the range from 15 to 85% (w / w). The phase of the non-aqueous liquid composition can be changed into a non-lamellar liquid crystal upon the contact with water, a phosphate buffer, a body fluid, a tear fluid or a vitreous fluid.
Owner:SANTEN PHARMA CO LTD
A kind of soft capsule containing milk thistle extract and preparation method thereof
ActiveCN103830204BImprove chemical liver injuryImprove alcoholic liver damageOrganic active ingredientsMetabolism disorderActive componentPlanthopper
The invention discloses a soft capsule containing a silybum marianum extract and a preparation method of the soft capsule. The content of the soft capsule contains the following raw materials in parts by weight: 1 part of silybum marianum extract, 45-115 parts of medium chain triglycerides, 40-120 parts of edible oil and 140-300 parts of phospholipid relative to 1 part of silybum marianum extract; the unsaturated fatty acid content in the edible oil is not smaller than 70wt%; the content of phosphatidylcholine in the phospholipid is 20-80wt%; the content of the dilinoleoyl phosphatidylcholine is 20-80wt%. The preparation method of the soft capsule disclosed by the invention comprises the following steps: mixing the components to obtain the content of the soft capsule, and encapsulating the content into a soft capsule shell. By adopting the soft capsule disclosed by the invention, liver injury can be effectively improved, blood fat is reduced, and the active components such as the silybum marianum extract and the phospholipid in the soft capsule can stably exist for a long period of time.
Owner:侯文阁
A kind of cilostazol liposome solid formulation
ActiveCN112006992BHigh encapsulation efficiencyFacilitated releaseOrganic active ingredientsAntipyreticSodium acetateFreeze-drying
The invention discloses a cilostazol liposome solid preparation, which is prepared by the following method: first dissolving cilostazol in acetic acid, and then mixing dioleoylphosphatidylcholine and dimyristoylphosphatidylcholine , poloxamer P188, cholesterol succinate monoester to form a lipid film solution; then add acetic acid-sodium acetate buffer for dispersion and emulsification; then add lactose, sodium carboxymethyl starch, microcrystalline cellulose, magnesium stearate , finely dispersed after mixing; freeze-dried. The invention improves the drug loading capacity of cilostazol and the encapsulation rate of liposome of cilostazol, and at the same time, the preparation method is simpler, the technological process is shortened, and the raw materials in the production process are safe and non-toxic.
Owner:药大制药有限公司
Albumin-coated cabazitaxel cationic nano-lipid carrier and preparation method thereof
ActiveCN112716914AImprove solubilityExtension of timeOrganic active ingredientsMacromolecular non-active ingredientsCabazitaxelEther
The invention provides an albumin-coated cabazitaxel cationic nano-lipid carrier and a preparation method thereof. The albumin-coated cabazitaxel cationic nano-lipid carrier is prepared from the following raw materials in parts by weight: 5-10 parts of cabazitaxel, 20-170 parts of solid lipid material; 20-parts of liquid lipid material; 50-300 parts of fat-soluble emulsifiers; 100-600 parts of water-soluble emulsifiers; 4-15 parts of cationic material; and 10-100 parts of albumin material; the cationic material comprises at least one of didecyl dimethyl ammonium bromide, dioleoyl trimethyl ammonium propane, dioleoyl propyl trimethyl ammonium chloride, dioleoyl phosphatidylcholine ether and hexadecyl trimethyl ammonium bromide, and preferably, the cationic material is hexadecyl trimethyl ammonium bromide. The albumin-coated cabazitaxel cationic nano-lipid carrier provided by the invention is uniform in particle size, high in encapsulation efficiency and good in biocompatibility and biodegradability.
Owner:CHENGDU UNIV
Rabeprazole sodium liposome enteric-coated tablets
InactiveCN101966161BImprove stabilitySolve the problem of heat discolorationOrganic active ingredientsDigestive systemCholesterolDissolution
The invention discloses rabeprazole sodium liposome enteric-coated tablets and a preparation method and application thereof to treating gastroesophageal reflux disease (GERD). The rabeprazole sodium liposome enteric-coated tablets comprise rabeprazole sodium liposome solid, alkaline substances and other common auxiliary materials for a solid preparation, wherein the rabeprazole sodium liposome solid comprises the following components in part by weight: 10 to 20 parts of rabeprazole sodium, 40 to 90 parts of dioleoyl phosphatidylcholine, 15 to 50 parts of cholesterol and 8 to 30 parts of sodium glycocholate. The rabeprazole sodium liposome enteric-coated tablets have the advantages of improving stability, increasing dissolution, solving the problem of color change when being heated, and the like, and when the rabeprazole sodium liposome enteric-coated tablets are used for treating the GERD, the curative ratio is higher, the total effective rate is higher, and the clinical superiority is more obvious.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
A kind of soft capsule containing salvia miltiorrhiza extract and preparation method thereof
ActiveCN103830322BImprove chemical liver injuryImprove alcoholic liver damageMetabolism disorderDigestive systemSalvia miltiorrhizaActive component
Owner:侯文阁
Shuxuening lipidosome injection
InactiveCN103040752BQuality improvementImprove bioavailabilityPharmaceutical non-active ingredientsRespiratory disorderSodium metabisulfiteSide effect
The invention discloses a Shuxuening lipidosome injection. The Shuxuening lipidosome injection is mainly prepared by using a ginkgo biloba extract, dioleoylphosphocholine, octadecylamine, polyoxyethylene alkylamine, sodium metabisulfite and sodium chloride. Compared with the prior art, the Shuxuening lipidosome injection disclosed by the invention is suitable for large-scale industrial production and has the advantages that the stability and bioavailability of a preparation are greatly improved, product quality is improved and toxic and side effects are reduced.
Owner:海南路易丹尼生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com