A kind of cilostazol liposome solid formulation
A technology of cilostazol lipid and cilostazol, which is applied in the field of pharmacy, can solve the problems of low drug loading, large toxic and side effects, and low encapsulation efficiency, and achieve reduction of toxic and side effects, simplified preparation method, and high encapsulation efficiency. Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
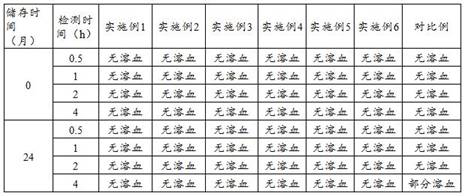
Examples
Embodiment 1
[0030] Raw materials and their quality: cilostazol 14g, dioleoylphosphatidylcholine 10g, dimyristoylphosphatidylcholine 8g, poloxamer P188 3g, cholesterol succinate monoester 5g, lactose 16g, carboxymethyl 0.5 g of sodium starch glycolate, 1 g of microcrystalline cellulose, 0.5 g of magnesium stearate, 3.5 g of acetic acid, and 86 g of acetic acid-sodium acetate buffer solution with a pH value of 6 to 6.5;
[0031] Preparation:
[0032] (1) Add the above-mentioned parts by mass of acetic acid into a pear-shaped bottle, place it in an ice-water bath at 8-12°C and stir at a stirring rate of 150rpm-200rpm, add the above-mentioned parts by mass of cilostazol to dissolve, and then add the above-mentioned parts by mass of cilostazol Dioleoylphosphatidylcholine, dimyristoylphosphatidylcholine, poloxamer P188, cholesterol succinic acid monoester, continue stirring to form a uniform lipid film solution;
[0033] (2) Add the above mass parts of acetic acid-sodium acetate buffer solutio...
Embodiment 2
[0038]Raw materials and their quality: cilostazol 14g, dioleoylphosphatidylcholine 18g, dimyristoylphosphatidylcholine 14g, poloxamer P188 6g, cholesterol succinate monoester 8g, lactose 23g, carboxymethyl 0.9 g of sodium starch glycolate, 3 g of microcrystalline cellulose, 1.3 g of magnesium stearate, 3.5 g of acetic acid, and 86 g of acetic acid-sodium acetate buffer solution with a pH value of 6 to 6.5;
[0039] Preparation:
[0040] (1) Add the above-mentioned parts by mass of acetic acid into a pear-shaped bottle, place it in an ice-water bath at 8-12°C and stir at a stirring rate of 150rpm-200rpm, add the above-mentioned parts by mass of cilostazol to dissolve, and then add the above-mentioned parts by mass of cilostazol Dioleoylphosphatidylcholine, dimyristoylphosphatidylcholine, poloxamer P188, cholesterol succinic acid monoester, continue stirring to form a uniform lipid film solution;
[0041] (2) Add the above mass parts of acetic acid-sodium acetate buffer solutio...
Embodiment 3
[0046] Raw materials and their quality: cilostazol 14g, dioleoylphosphatidylcholine 27g, dimyristoylphosphatidylcholine 22g, poloxamer P188 10g, cholesterol succinate monoester 12g, lactose 32g, carboxymethyl 15g of sodium starch glycolate, 8g of microcrystalline cellulose, 2g of magnesium stearate, 3.5g of acetic acid, and 86g of acetic acid-sodium acetate buffer solution with a pH value of 6~6.5;
[0047] Preparation:
[0048] (1) Add the above-mentioned parts by mass of acetic acid into a pear-shaped bottle, place it in an ice-water bath at 8-12°C and stir at a stirring rate of 150rpm-200rpm, add the above-mentioned parts by mass of cilostazol to dissolve, and then add the above-mentioned parts by mass of cilostazol Dioleoylphosphatidylcholine, dimyristoylphosphatidylcholine, poloxamer P188, cholesterol succinic acid monoester, continue stirring to form a uniform lipid film solution;
[0049] (2) Add the above mass parts of acetic acid-sodium acetate buffer solution to the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com