Hydrochloric acid boanmycin liposomes and preparation method thereof
A technology of boanmycin hydrochloride fat and boanmycin hydrochloride, which is applied in the field of boanmycin hydrochloride liposome and its preparation, can solve the problems affecting the behavior in the body, affecting the therapeutic effect and toxicity of encapsulated drugs, etc. Achieve the effects of reduced cost of freeze-drying, reduced dosage, and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of Boanmycin hydrochloride liposome preparation
[0029] Boanmycin Hydrochloride 4.0g
[0030] Egg yolk lecithin 24.2g
[0031] Cholesterol 8.2g
[0032] Vitamin E 0.1g
[0033] Citric acid 4.5g
[0034] Sodium citrate 4.8g
[0036] The rest is water for injection
[0037] Total 800g
[0038] Preparation Process:
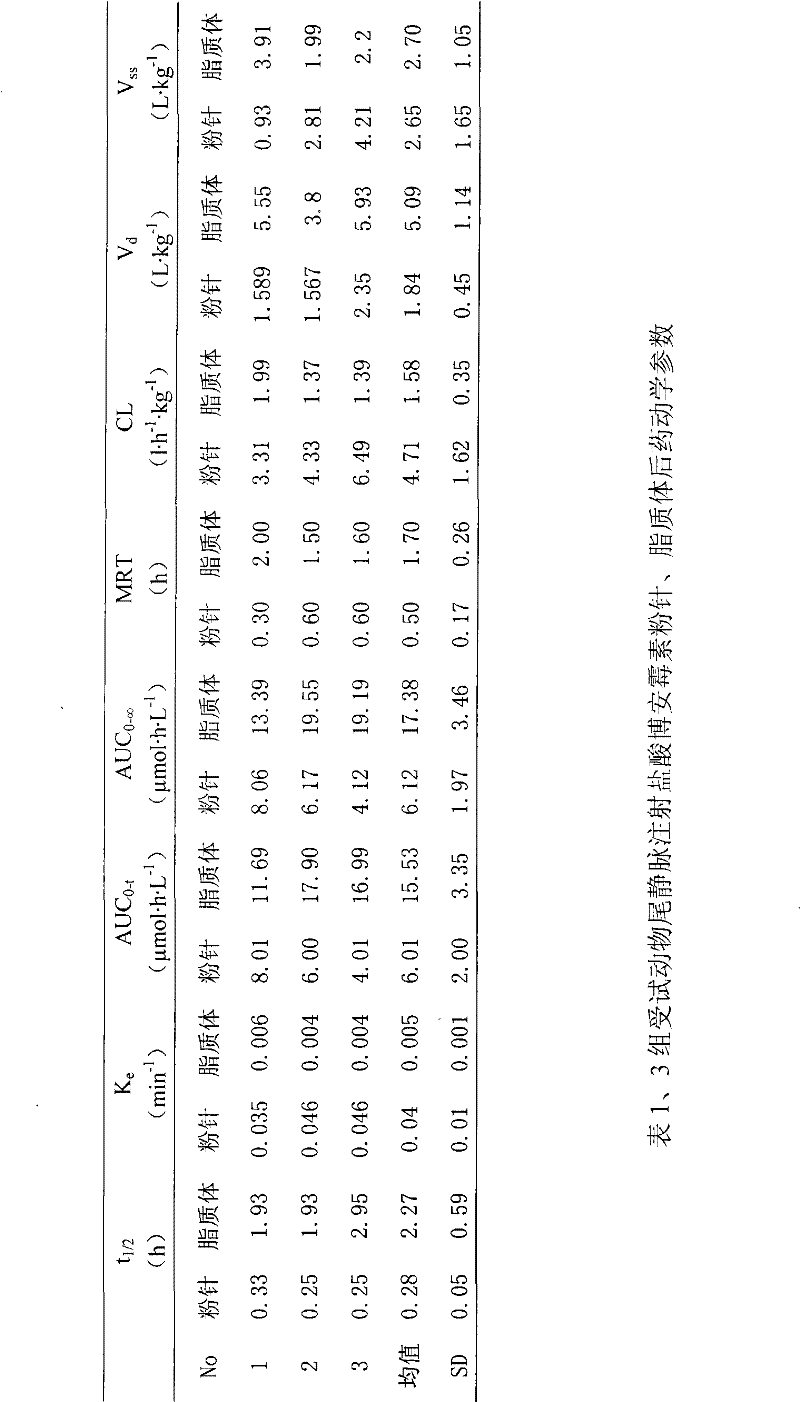
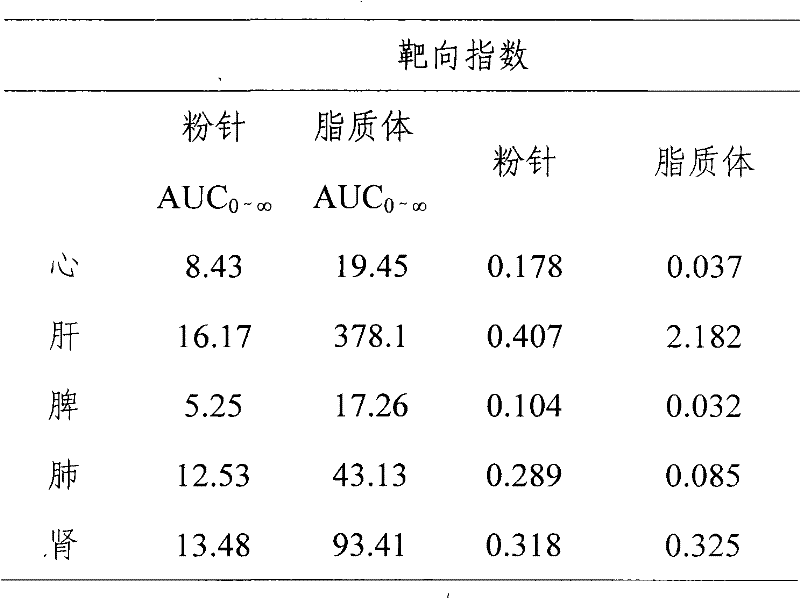
[0039] Weigh the prescribed amount of phospholipids and cholesterol and disperse them into an appropriate amount of pH4.0 citric acid-sodium citrate buffer solution, stir at 30°C until uniformly dispersed; add the above solution into a high-pressure micro-jet, and repeat at 200 atmospheres Operate 10 times to get a translucent solution; take 2ml of liposome solution, and use calcium carbonate solution to adjust the pH value of the solution to 9.0; add 4.0g of Boanmycin hydrochloride to the above solution, and place it in a water bath at 59-61°C Incubate in medium for 6...
Embodiment 2
[0041] Embodiment 2: the preparation of Boanmycin hydrochloride liposome freeze-dried preparation
[0042] Boanmycin Hydrochloride 8.3g
[0043] Soy Lecithin 120.2g
[0044] Cholesterol 40.5g
[0045]Vitamin E 0.2g
[0046] Citric acid 12.5g
[0047] Sodium citrate 12.8g
[0048] Lactose 160.6g
[0049] Sodium bicarbonate 24.2g
[0050] The rest is water for injection
[0051] Total 800g
[0052] Preparation Process:
[0053] Weigh the prescribed amount of phospholipids and cholesterol and disperse them into an appropriate amount of pH4.0 citric acid-sodium citrate buffer solution, stir at 50°C until uniformly dispersed; add the above solution into a high-pressure micro-jet, and repeat at 400 atmospheres After 25 operations, a translucent solution was obtained; lactose was added to 2ml of the above solution, and freeze-dried to obtain a white freeze-dried powder. Rehydrate the liposome freeze-dried powder with 2ml of normal saline for injection, a...
Embodiment 3
[0055] Embodiment 3: the preparation of boanmycin hydrochloride liposome freeze-dried preparation
[0056] Boanmycin Hydrochloride 24.6g
[0057] Distearoylphosphatidylcholine 240.2g
[0058] Cholesterol 80.2g
[0059] Vitamin E 0.2g
[0060] Trehalose 120.6g
[0061] Lactose 120.5g
[0062] Citric acid 24.2g
[0063] Sodium citrate 24.1g
[0064] Anhydrous sodium carbonate 40.8g
[0065] The rest is water for injection
[0066] Total 800g
[0067] Preparation Process:
[0068] Weigh the prescribed amount of phospholipids and cholesterol and disperse them into an appropriate amount of pH 4.0 citric acid-sodium citrate buffer solution, stir at 70°C until uniformly dispersed; add the above solution into a high-pressure microjet, and repeat at 800 atmospheres Operate 30 times to obtain a translucent solution; add lactose and trehalose to 2ml of the above solution, and spray dry to obtain a white freeze-dried powder. Rehydrate the liposome lyo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| encapsulation rate | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com