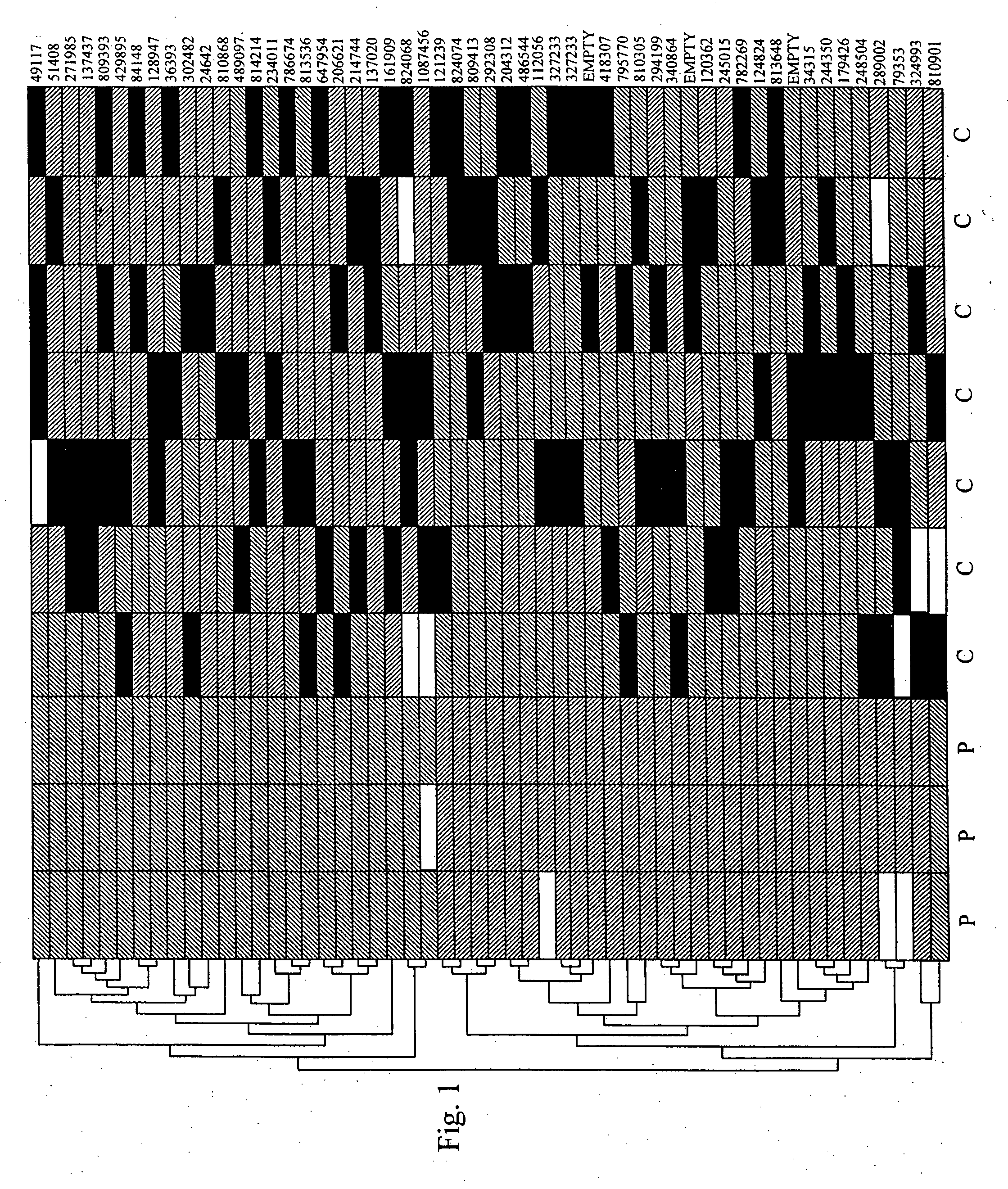
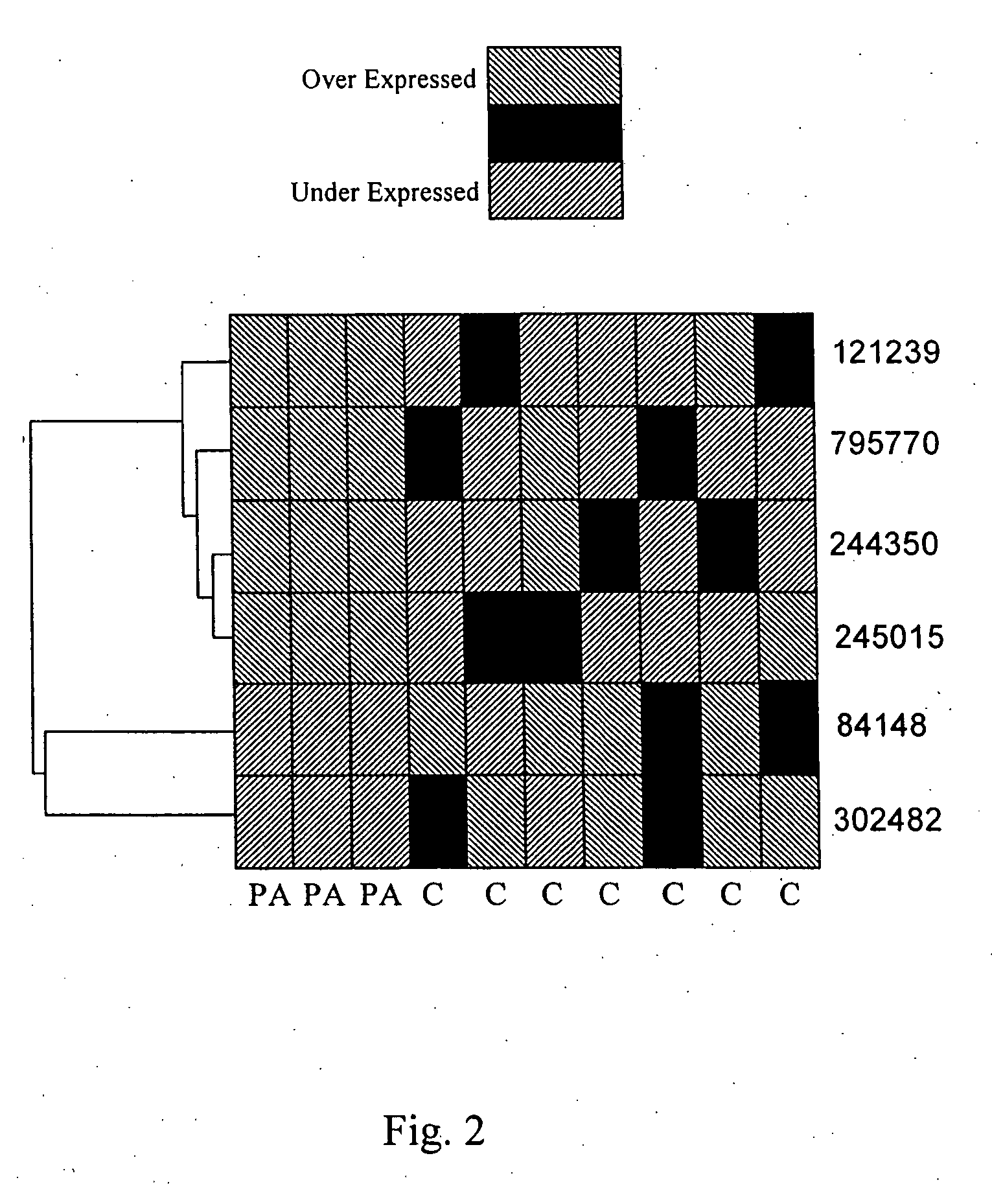
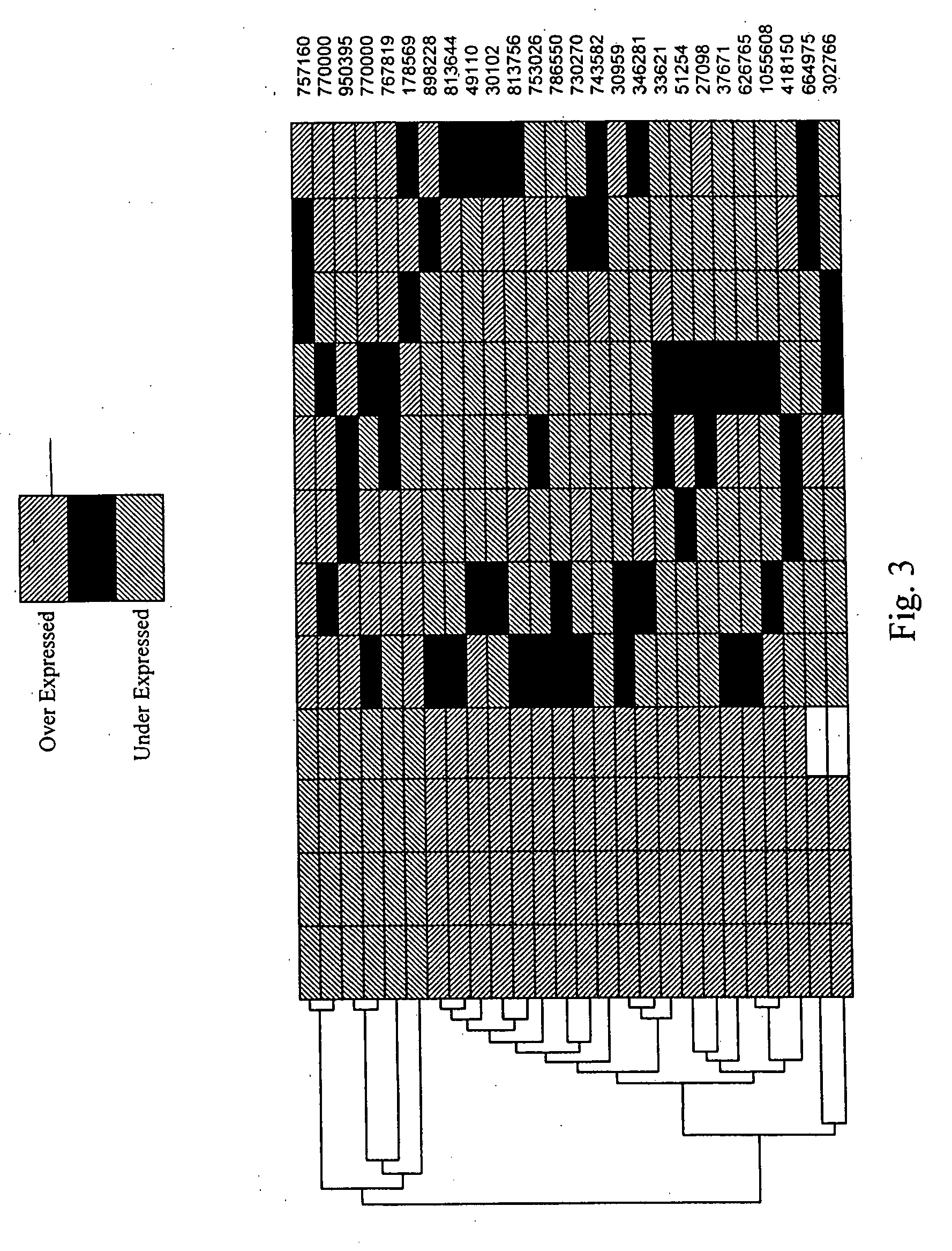
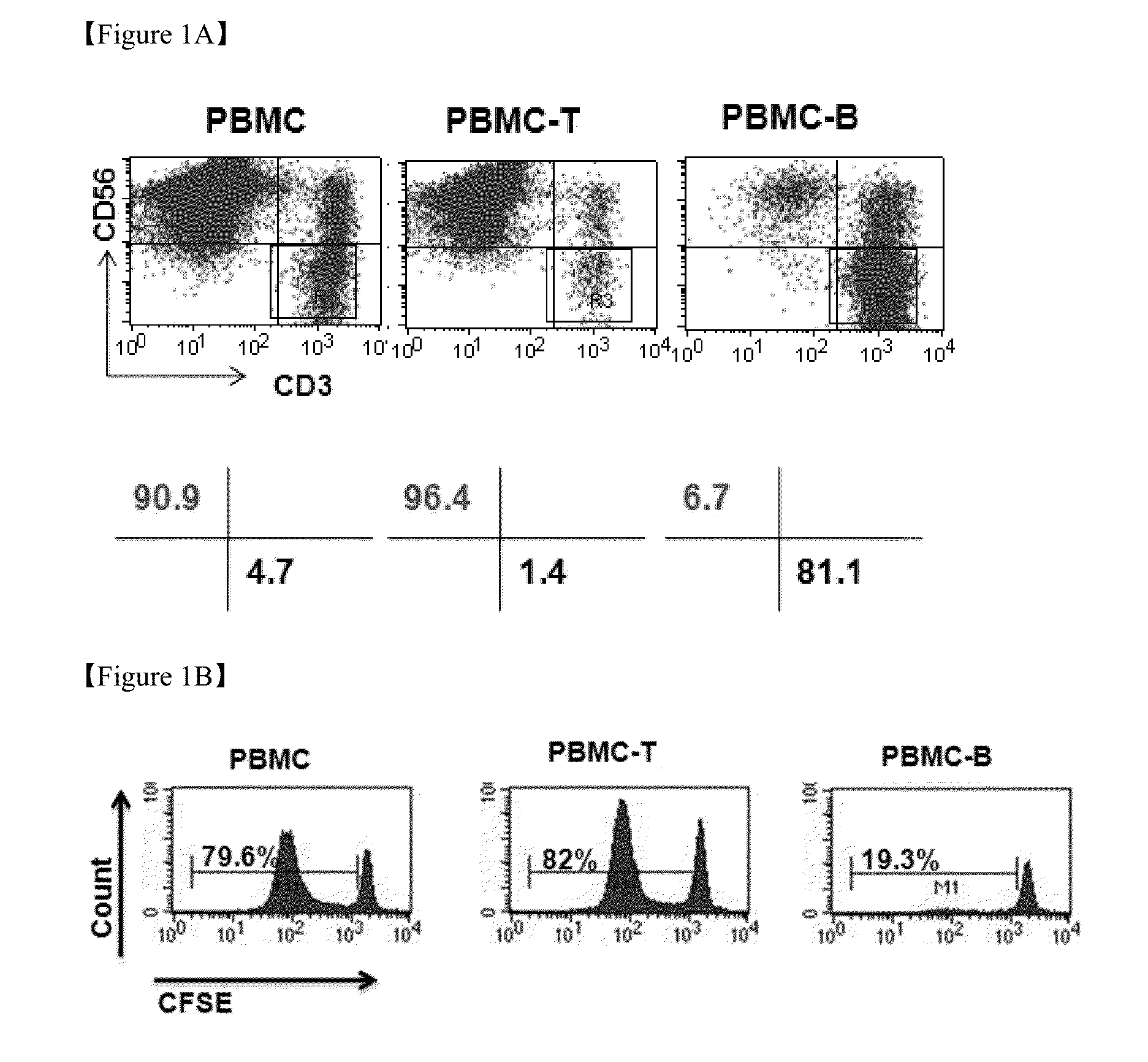
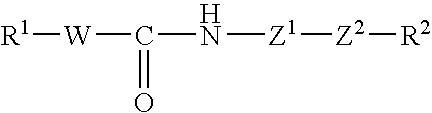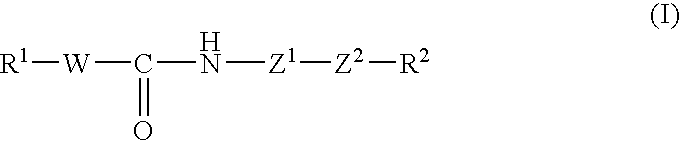Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
189 results about "Peripheral blood monocyte" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
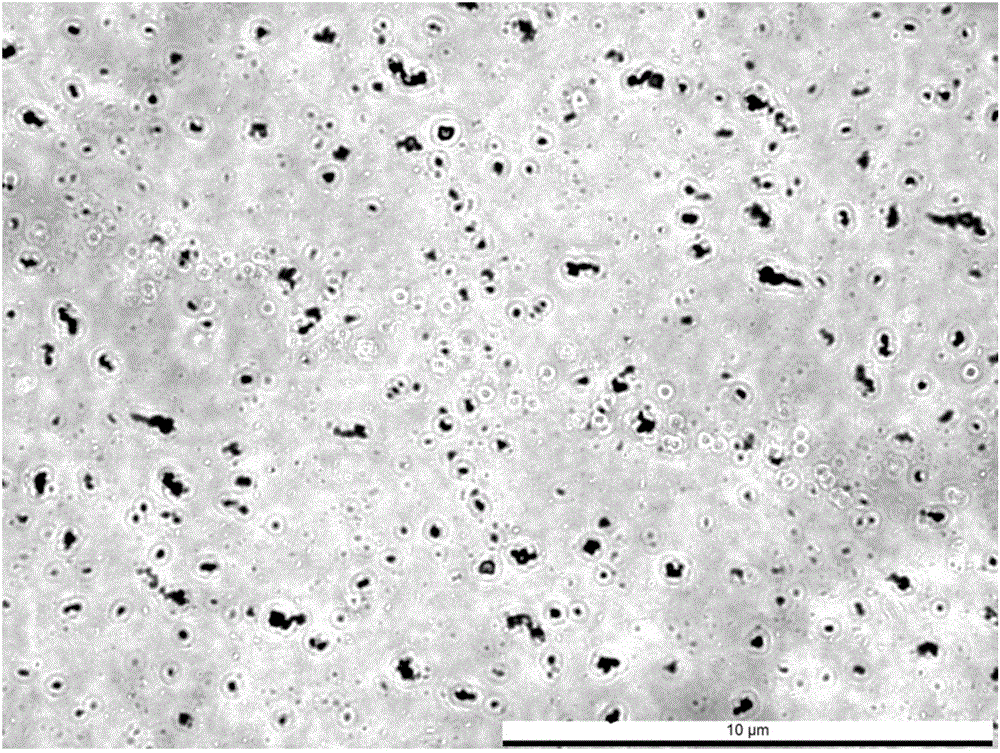
Monocyte under a light microscope (40x) from a peripheral blood smear surrounded by red blood cells. Monocytes are a type of white blood cell, part of the human body's immune system. They are usually identified in stained smears by their large two-lobed nucleus.
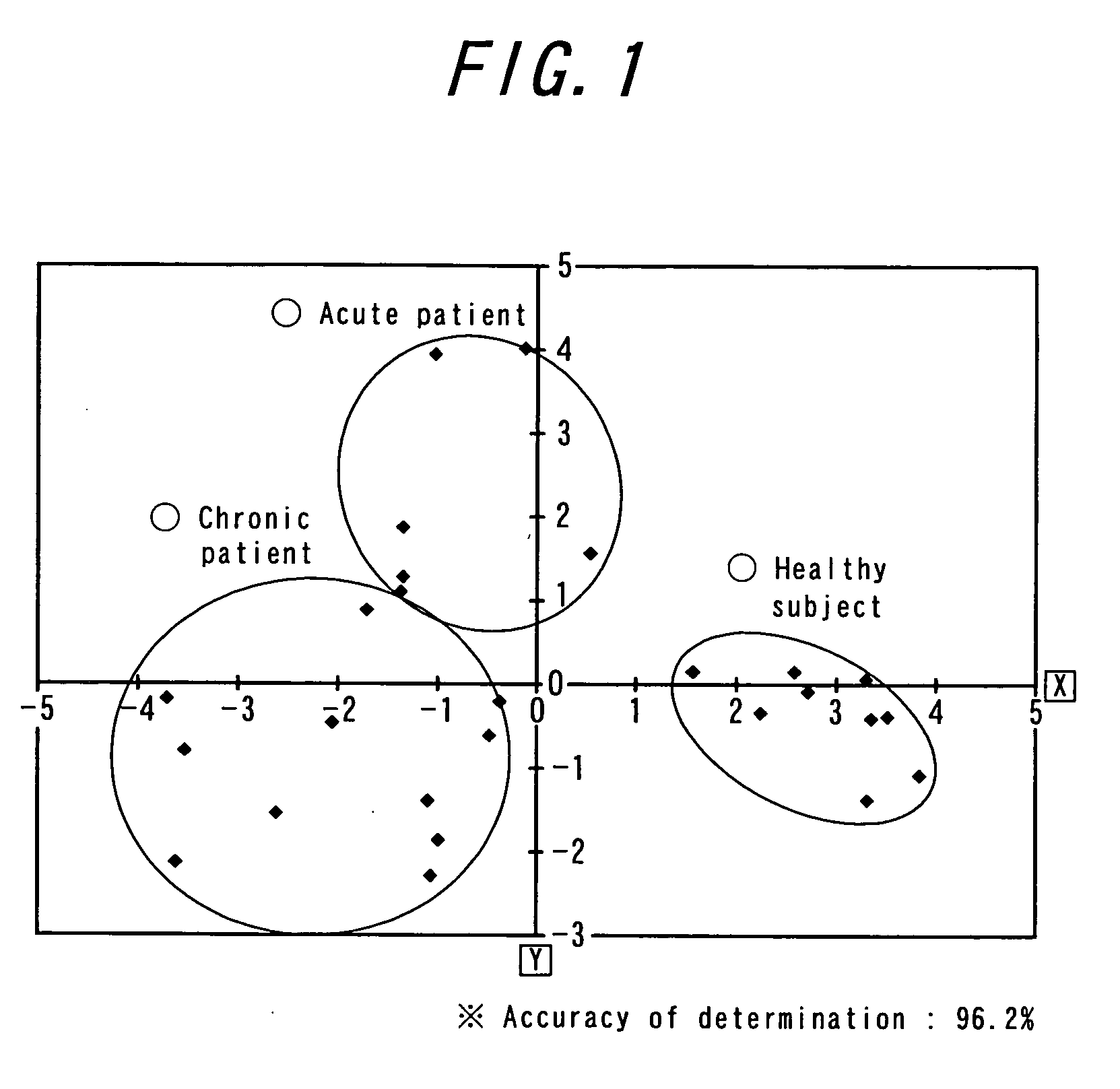
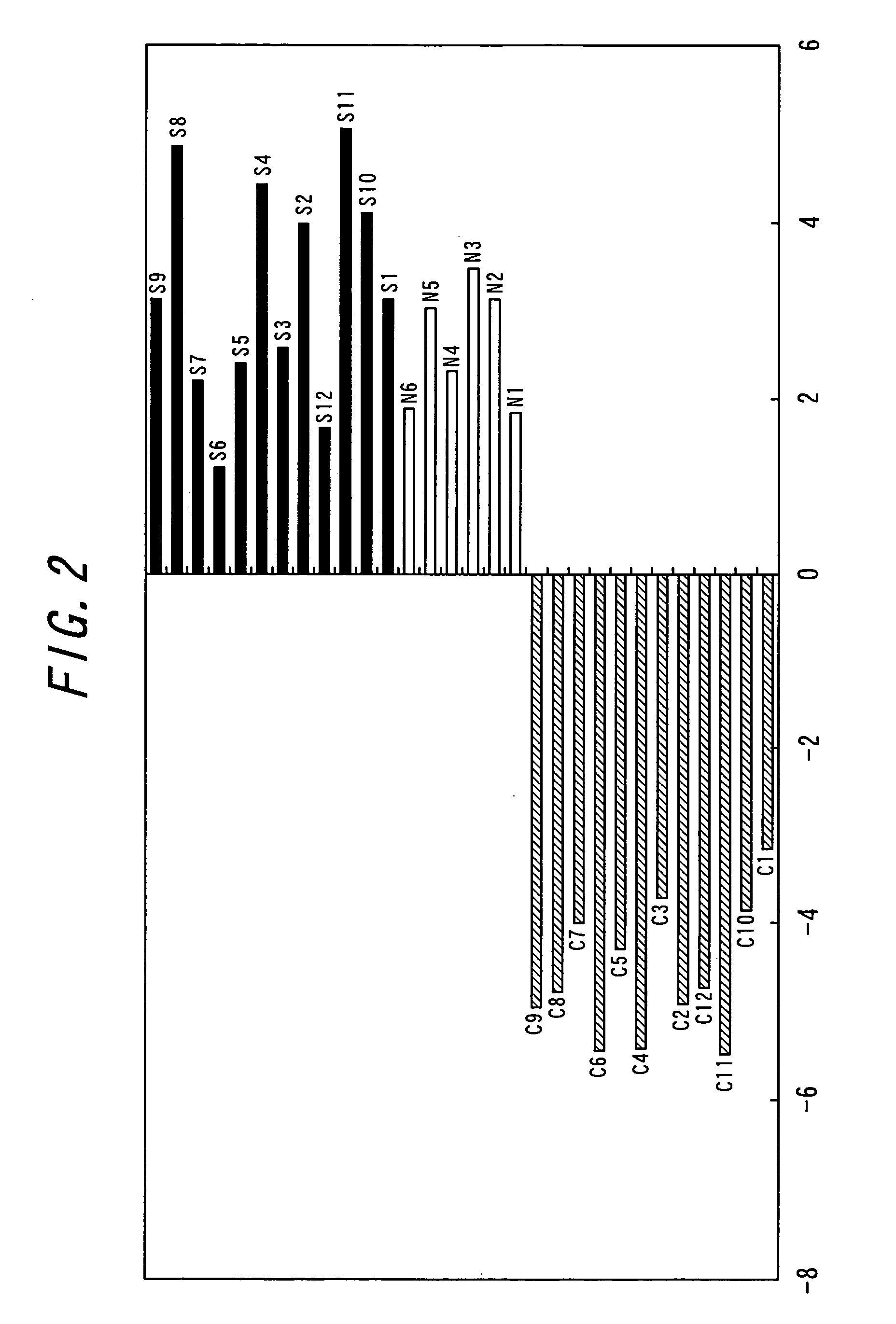
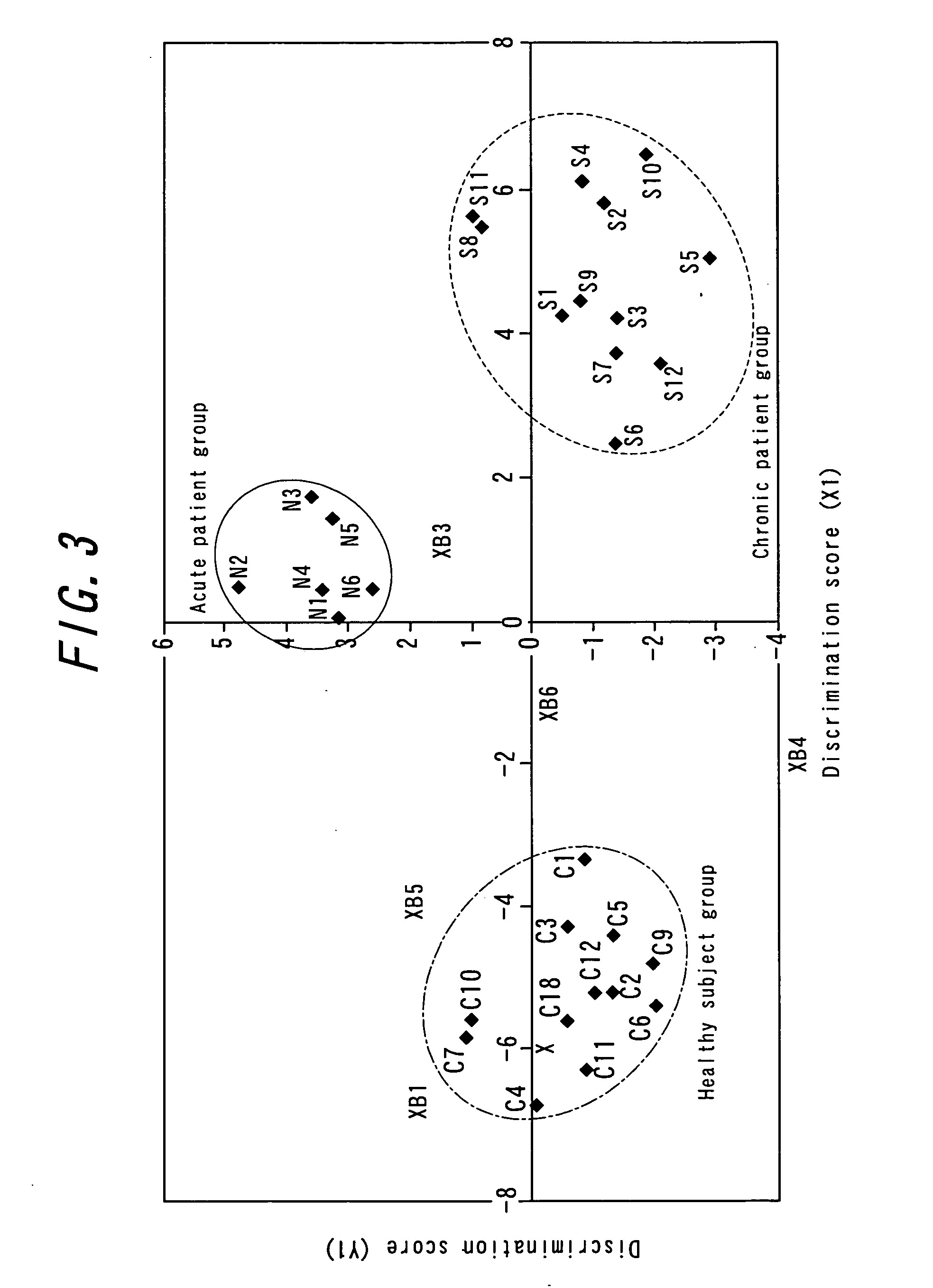
Method for the early detection of breast cancer, lung cancer, pancreatic cancer and colon polyps, growths and cancers as well as other gastrointestinal disease conditions and the preoperative and postoperative monitoring of transplanted organs from the donor and in the recipient and their associated conditions related and unrelated to the organ transplantation
ActiveUS20060088876A1Early diagnosisMicrobiological testing/measurementDisease diagnosisLung cancerDisease cause
A method for the early diagnosis of breast, lung, pancreatic and colon growths and cancers as well as conditions associated with donor and recipient organ transplants, both before and after transplantation to identify and allow treatment of possible transplanted organ rejection and other disease conditions related and unrelated to the transplantation, compares the gene expression patterns from a patient's peripheral blood monocytes-lymphocyte's gene system with either the similar gene expression patterns of a normal person, or with the similar gene expression patterns of a person known to have the condition being screened for. Differences between the patient's gene expression patterns for particular genes and the normal patterns indicates the presence of the condition with the number of differences indicating the probability of the condition. Similarities between the patient's gene expression patterns for those particular genes and the patterns of a person known to have the condition indicates the presence of the condition with the number of similarities indicating the probability of the condition. For example, particular genes for use in identifying pancreatic cancer are disclosed.
Owner:BAUER A ROBERT
Method for the early detection of pancreatic cancer and other gastrointestinal disease conditions
InactiveUS20080248484A1Early diagnosisSpecific and focused early diagnosisMicrobiological testing/measurementOrgan systemNeoplasm
The present invention uses peripheral blood monocyte-lymphocyte for the early diagnosis of pancreatic cancer, as well as other conditions of the pancreas and other organs. The peripheral blood lymphocytes recognize the new neoplasm in the pancreas, as well as disease processes in other organ systems. The evaluation of this specific recognition of the disease process by the peripheral blood monocyte-lymphocyte through gene microarray expression patterns constitute a successful method for the early detection of pancreatic cancer and other organ disease processes. This document describes the process used in this method of early diagnosis.
Owner:BAUER A ROBERT
Use of cell adhesion inhibitor for the mobilization of antigen presenting cells and immune cells in a cell mixture (AIM) from the peripheral blood and methods of use
Disclosed is a method to recover an antigen presenting cells (APCs) and immune cells rich mixture (AIM) from peripheral blood mononuclear cells (PBMC) mobilized with one or more cell adhesion inhibitors for the preparation of an AIM vaccine or an AIM adoptive immunotherapy preparation. In addition, AIM mobilization can be enhanced by priming, simultaneously or in sequence, one or more of a combination of different chemical compounds, cytokines, hormones, growth factors, etc. The interaction of chemokines and chemokine receptors enable tumor cells attachment or in close proximity to antigen presenting cells and immune cells which possess similar receptors in a micro niche environment. Severing the chemokine / chemokine receptor linkage by a cell adhesion inhibitor will release these specifically primed cell mixtures into the peripheral blood. The collection of these cells from the peripheral blood has never been described and is the basis of this invention. AIM cells can either be used alone or better still, be induced into more target specific preparations with additions, modifications and incubation, pre or post cell adhesion inhibitors mobilization, with vaccines, different target specific antigens, peptides, chemotherapeutic agents, oncolytic viral therapeutic agents, cytokines, co-stimulatory molecules, anti-regulatory T cell therapeutic agents, anti-CTLA4, anti-PD1 molecules and other methodologies of immunological enhancement known to the art. The AIM vaccine or AIM adoptive immunotherapy preparation can then be used, but not limited to, the treatment of cancer and other diseases.
Owner:YEUNG ALEX WAH HIN +1
Method for the induction and expansion of natural killer cells derived from peripheral blood mononuclear cells
ActiveUS20150152387A1Improve efficiencyImprove efficacyBiocideMammal material medical ingredientsNatural Killer Cell FunctionNatural killer cell
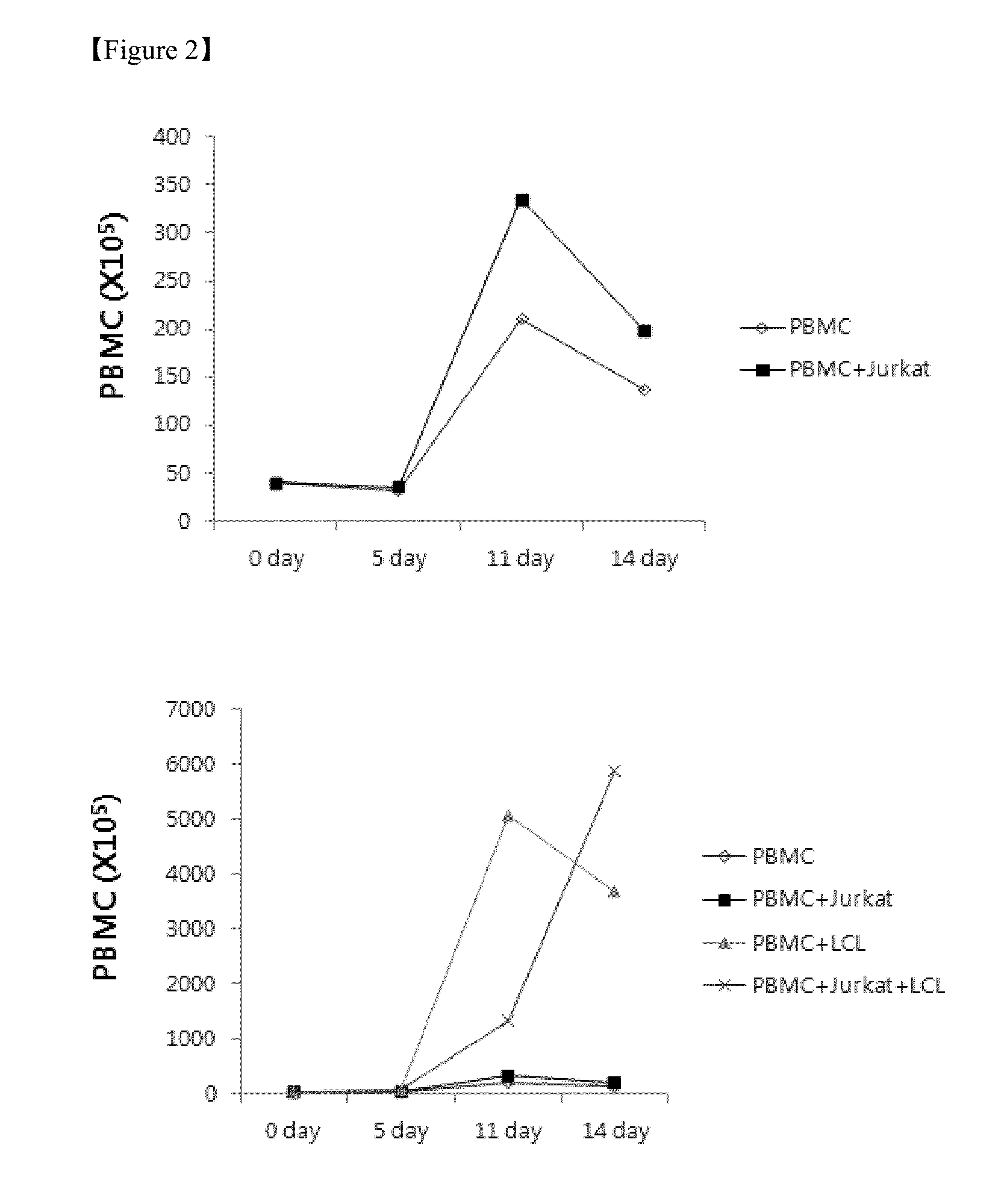
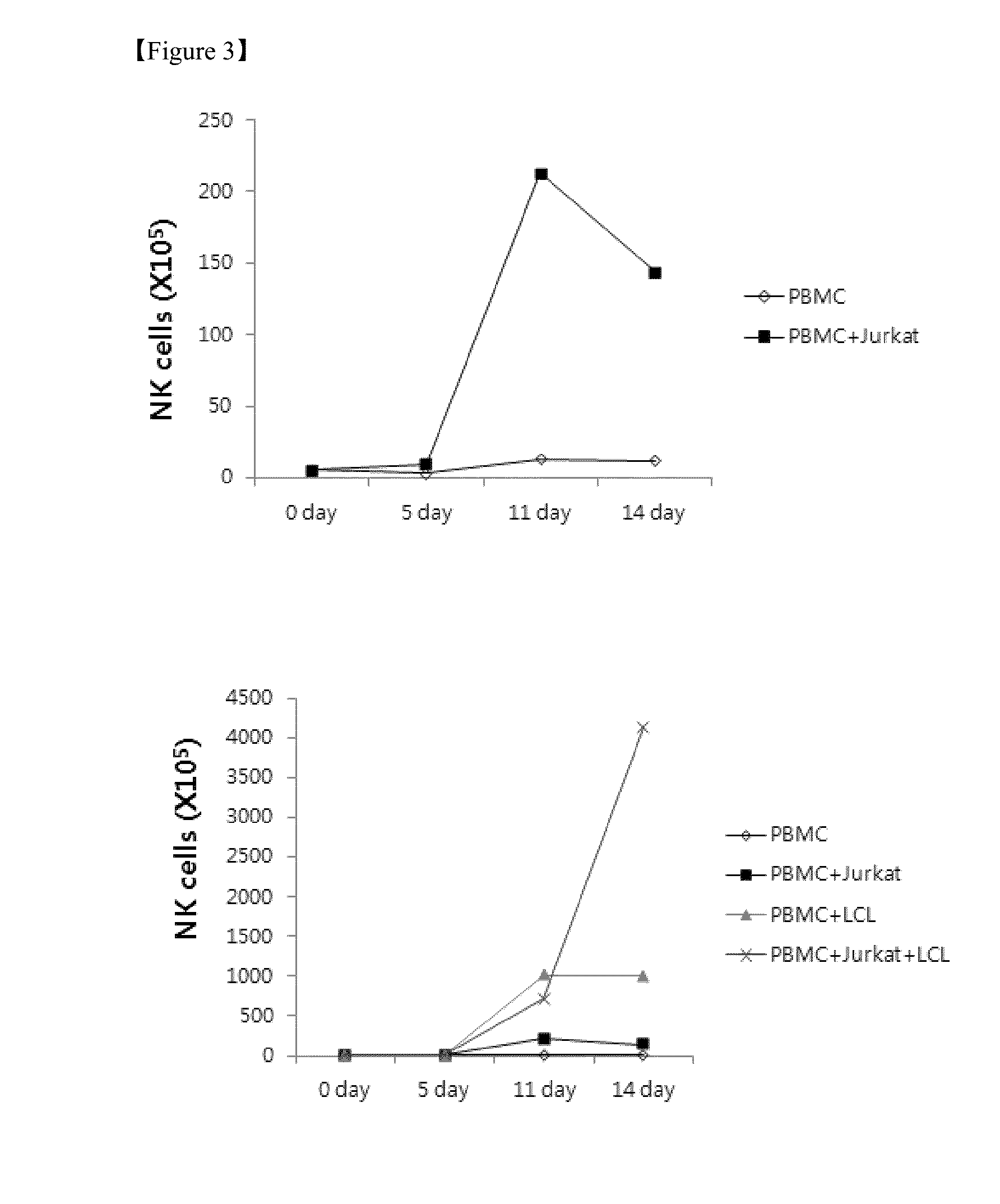
The present invention relates to a method for inducing and expanding natural killer cells derived from peripheral blood mononuclear cells, which comprises co-culturing, as feeder cells, irradiated Jurkat cells and irradiated Epstein-Barr virus transformed lymphocyte continuous line (EBV-LCL) cells in the presence of cytokines, along with peripheral blood mononuclear cells. According to the present invention, a large quantity of natural killer cells can be induced and proliferated from a small quantity of peripheral blood mononuclear cells even without the use of high-cost equipment or various kinds of expensive cytokines, thereby making it possible to significantly improve the efficiency and efficacy of the prevention and treatment of cancer using the natural killer cells.
Owner:NKMAX CO LTD
Treatment and prevention of immunodeficiency virus infection by administration of non-pyrogenic derivatives of lipid A
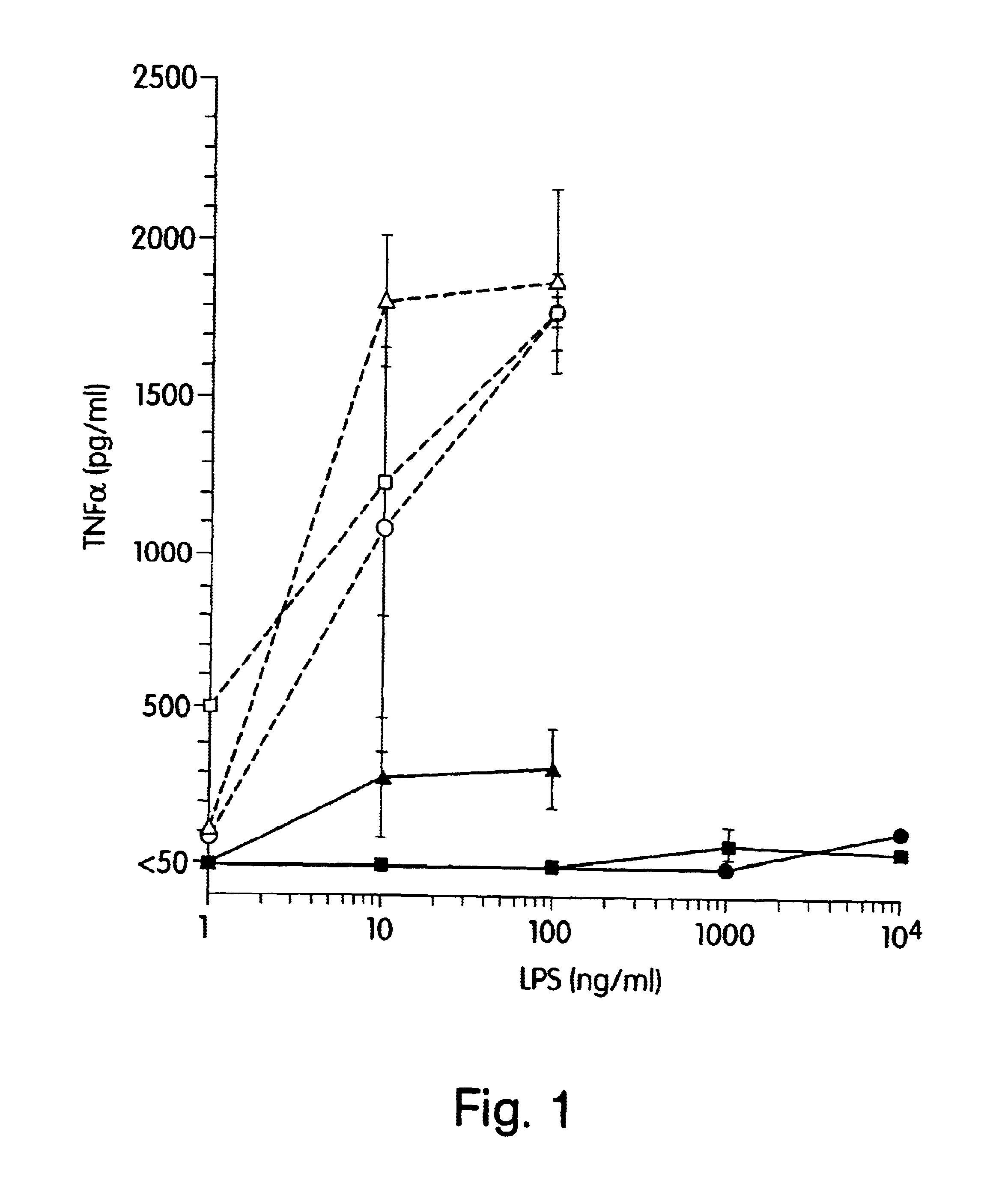
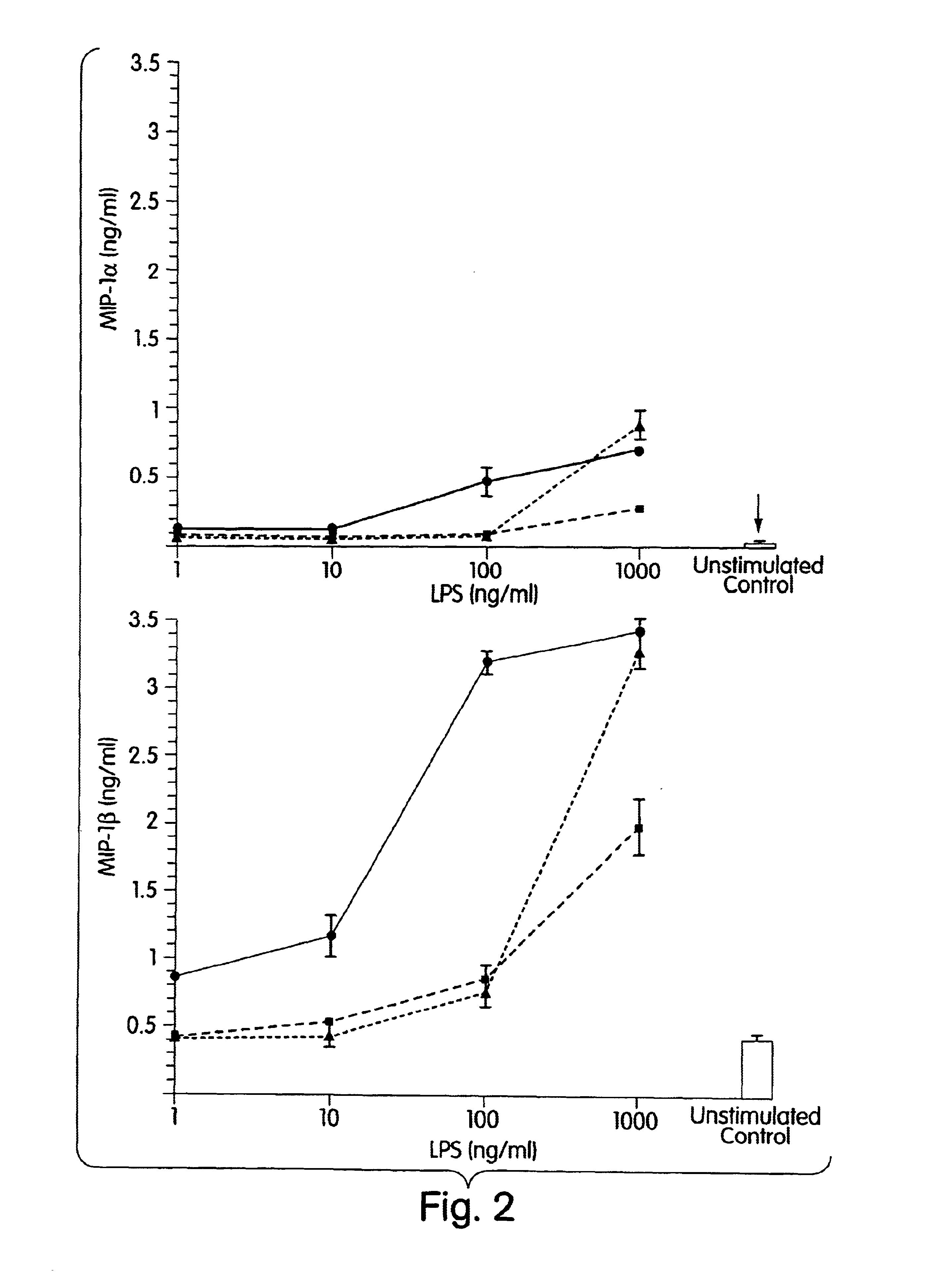
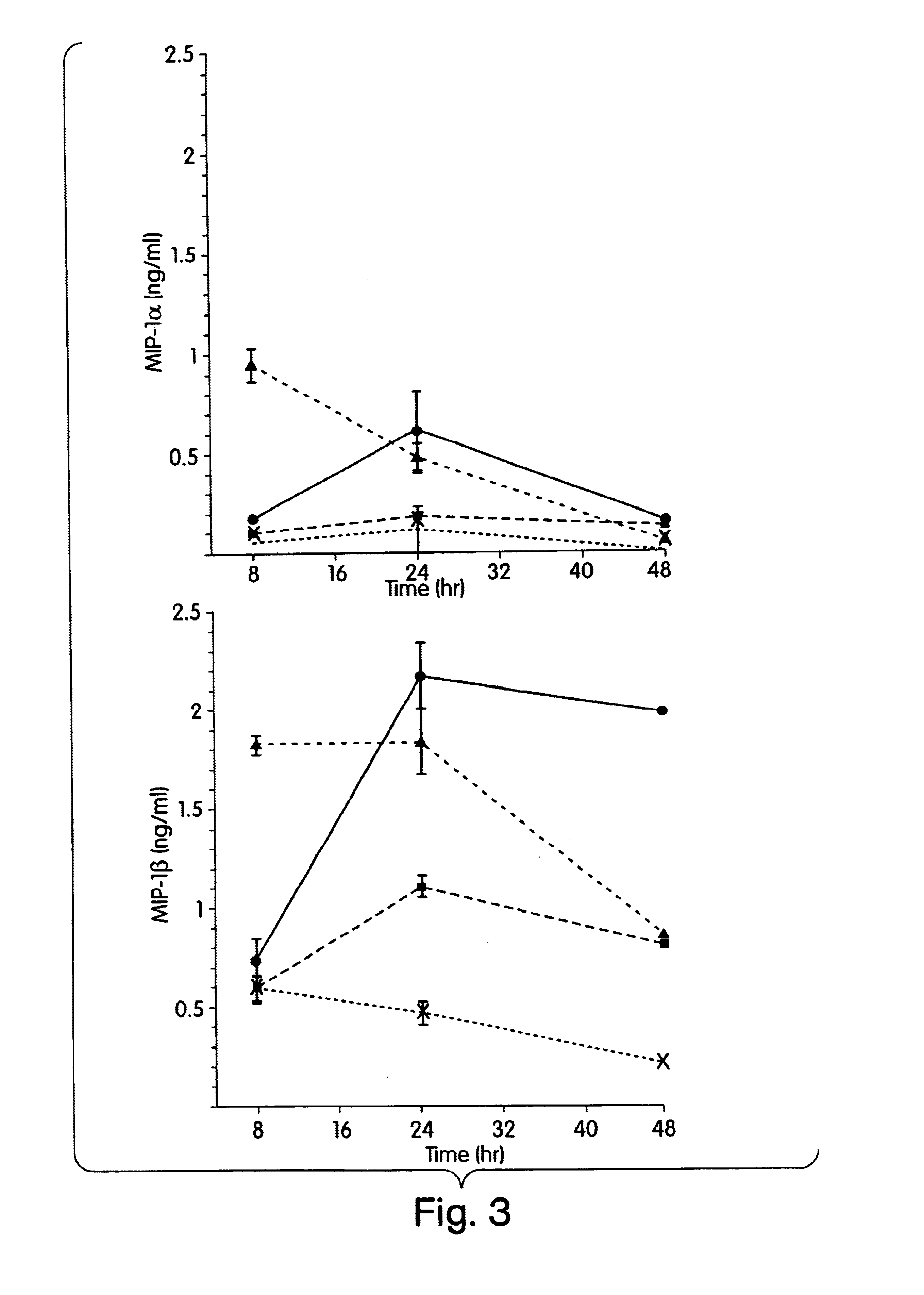
The present inventors have found that certain preparations containing LPS and / or lipid A variants, derivatives, and / or analogs demonstrate non-pyrogenic properties and exhibit anti-viral activities. In particular, non-pyrogenic preparations of LPS, lipid A, LPS antagonists and lipid A antagonists, and derivatives thereof induce β chemokine secretion, such as MIP-1β, but not proinflammatory cytokines, such as TNFα, IL-1β and IL-6. Non-pyrogenic preparations of the invention have been demonstrated by the Applicant to suppress HIV replication in human peripheral blood monocytes, as described by way of example herein. The present invention provides preparations of LPS or lipid A variants, analogs and derivatives of decreased or absent pyrogenicity which can be used as therapeutics for the treatment or prevention of immunodeficiency virus infection and its consequences.
Owner:UNIV OF MARYLAND BIOTECH INST
Method for mapping and eliminating T cell epitopes
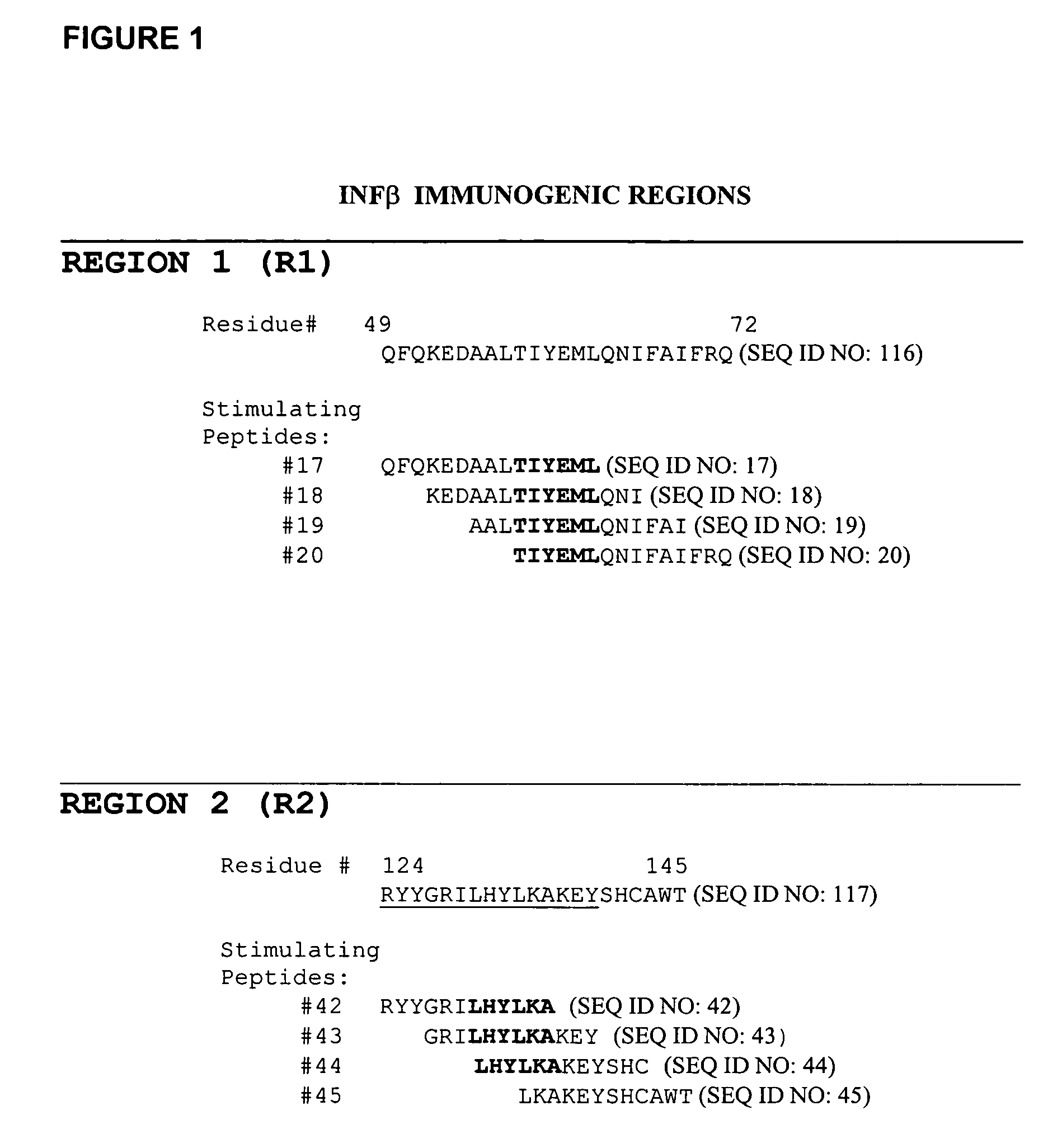
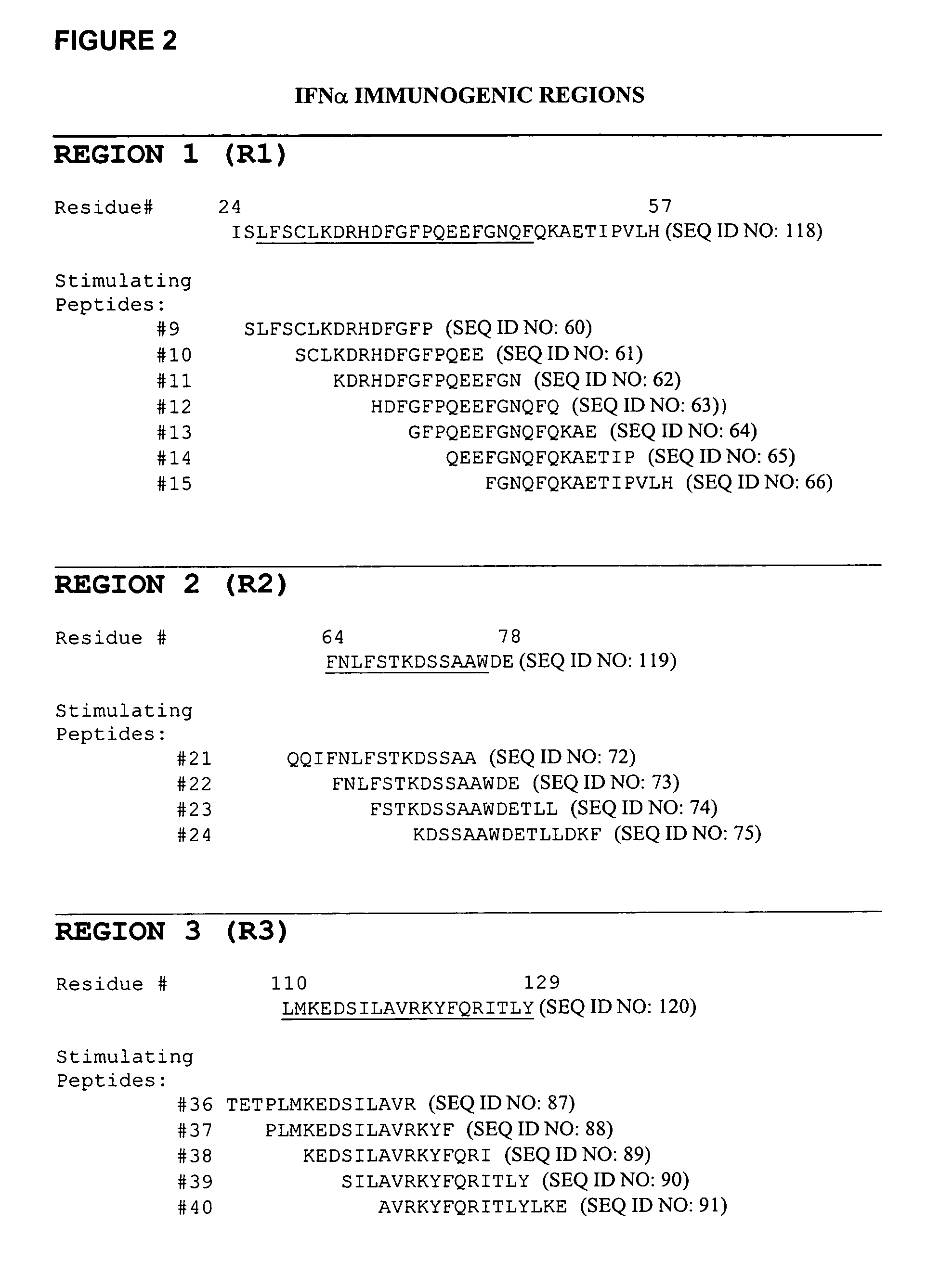
The invention provides methods for the identification of immunogenic regions within the amino acid residue sequence of a polypeptide, such as a therapeutic protein or a fragment thereof. The method comprises the steps of: (i) culturing, in vitro, an aliquot of peripheral blood monocyte cells (PBMC) isolated from a donor in the presence of a peptide for a period of up to about 7 days, the amino acid residue sequence of the peptide being identical to at least a portion of the amino acid residue sequence of the polypeptide of interest, the peptide being selected from a library of peptides, the amino acid residue sequences of the individual peptides of the library collectively encompassing the entire amino acid residue sequence of the polypeptide of interest; culturing the T cell aliquot from step (i) for an additional period of up to about 3 days in the presence of a T cell proliferation-stimulating cytokine to expand the number of T cells therein; (iii) culturing the T cell aliquot from step (ii) for a period of about 4 days in the presence of autologous irradiated PBMC from the same donor and in the presence of an additional amount of the peptide sufficient to re-prime the T cells within the PBMC with the peptide; (iv) determining the level of T cell proliferation of the re-primed T cells relative to an established baseline control level of proliferation; and (v) repeating steps (i) through (iv) with each peptide of the library of peptides to thereby identify at least one immunogenic region within the amino acid residue sequence of the polypeptide of interest.
Owner:MERCK PATENT GMBH
Compounds of therapeutic value in the treatment of multiple sclerosis and other diseases wherein foamy cells are involved in the disease etiology
InactiveUS7524820B1Requirement reductionLow costAntibacterial agentsPeptide/protein ingredientsDisease etiologyMedicine
The invention provides a method for assessing or determining activity of a test compound on modulation of gene product levels comprising culturing cells, contacting at least one of the cultured cells with a lipid-rich fraction, contacting at least one of the cultured cells with said test compound, determining the presence of a gene product of at least one cell of the cultured cells, and optionally determining the presence of the gene product of at least one cultured cell not contacted with said test compound. To assess human conditions most fully, it is preferred that the cell is of human origin, for example a peripheral blood monocyte taken from a healthy donor.
Owner:BIOTEMPT
Methods for manufacturing t cells
ActiveUS20190247433A1Shorten the resting timeGenetically modified cellsGenetic material ingredientsVirus typeCD28
The disclosure relates to methods of manufacturing T cells for adoptive immunotherapy. The disclosure further provides for methods of genetically transducing T cells, methods of using T cells, and T cell populations thereof. In an aspect, the disclosure provides for methods of thawing frozen peripheral blood mononuclear cells (PBMC), resting the thawed PBMC, activating the T cell in the cultured PBMC with an anti-CD3 antibody and an anti-CD28 antibody immobilized on a solid phase, transducing the activated T cell with a viral vector, expanding the transduced T cell, and obtaining expanded T cells.
Owner:IMMATICS US INC
Method to diagnose or screen for inflammatory diseases
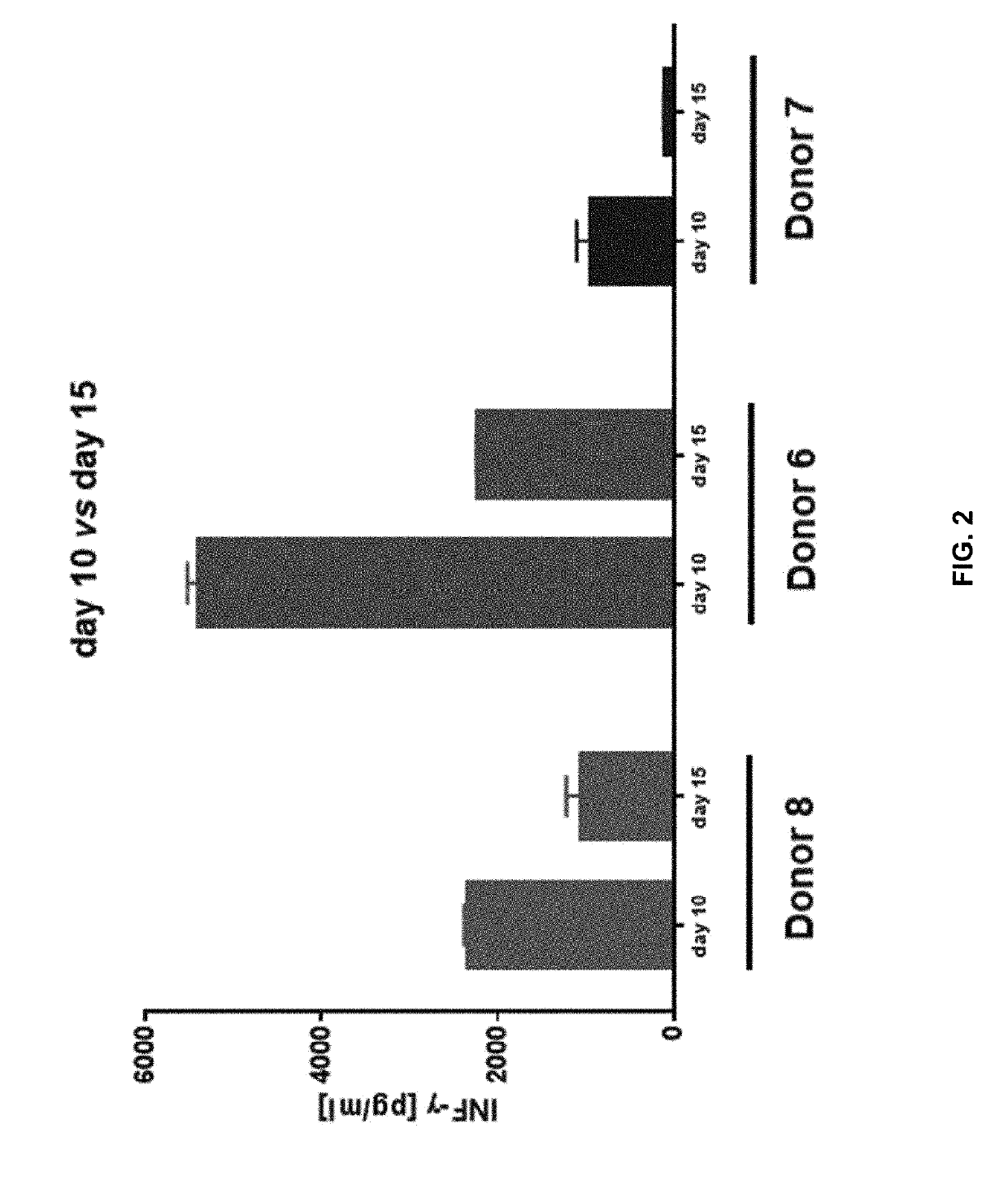
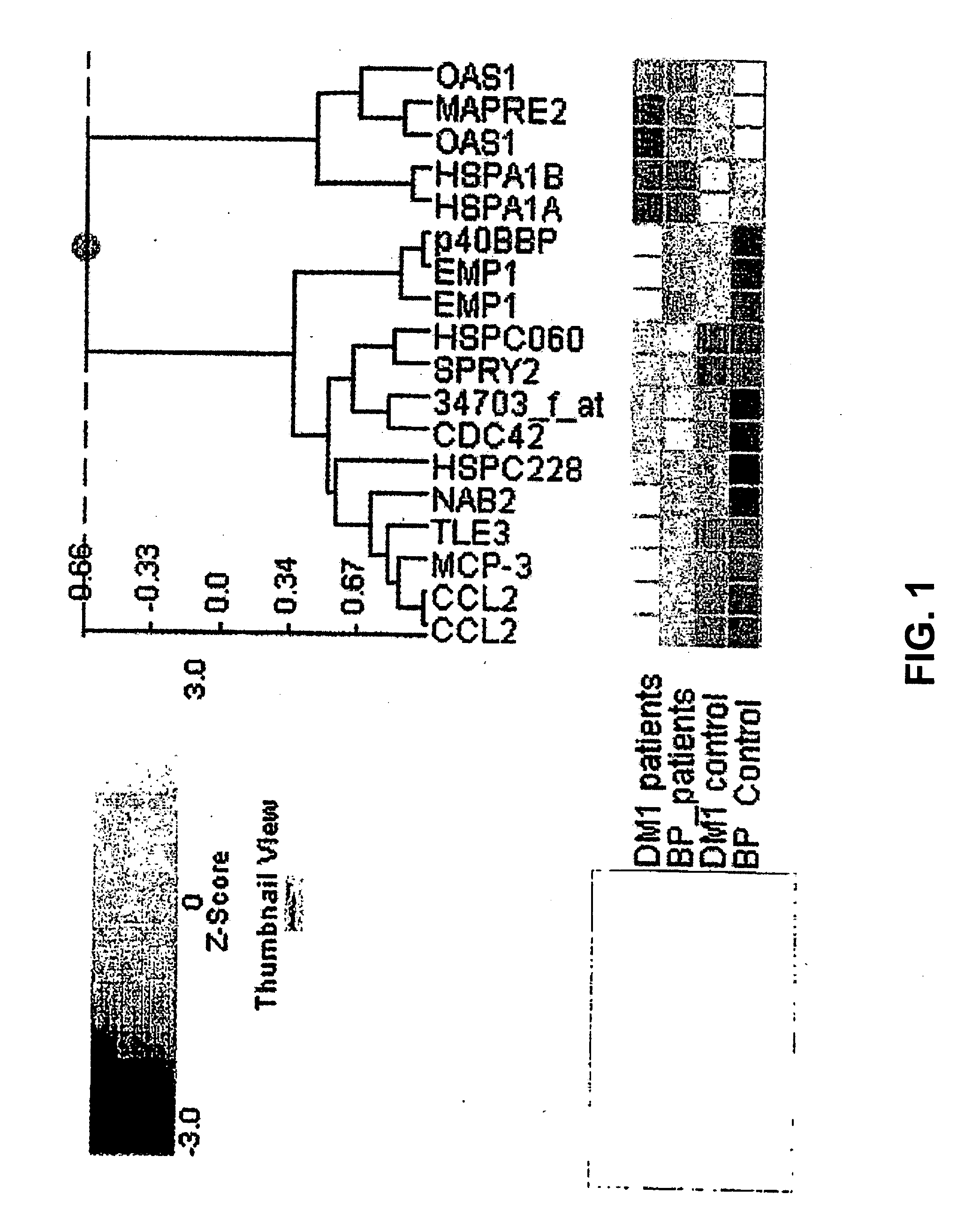
The invention relates to the field of medical diagnostics. More specifically, the invention relates to methods to diagnose or screen for inflammatory conditions or disease, including auto-inflammatory disease and affective disorder, in a subject, preferably a human subject, by assaying for a marker for an inflammatory disease. Provided is a method to diagnose, screen for or predict the development of an affective disorder (AD), preferably bipolar disorder (BP), in a subject, the method comprising determining the level of at least one, preferably at least two, more preferably at least three, most preferred at least four, AD-specific gene product(s) in a biological sample isolated from the subject, preferably peripheral blood monocytes, wherein the gene is selected from the group comprising ATF3, phosphodiesterase 4 B, CXCL2, BCL2-related protein A2, Dual specificity phosphatase 2, TNFα-induced protein 3 / A20, BTEB1 CXCL3, Chemokine CCL-3 like, CCL-4, CCL20, CX2CR1, Amphiregulin, Thrombomodulin, Heparin-binding EGF-like growth factor, DNA-damaged inducible transcript, V28 chemokine-like receptor, TRAIL. MAPK6, B4BP4, PBEF1, Thrombospondin 1, MAFF, HSP70, CCL2, MCP-3, CCR2, CX3CR1, DOK1, HBB, G-gamma globin, THBD, PHLDA1, DTR and GNLY.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Gene engineering cell and application thereof in NK (Nature Killer) cell proliferation
InactiveCN102911918AStrong cytotoxicityHigh purityBlood/immune system cellsForeign genetic material cellsHigh cellCancer cell
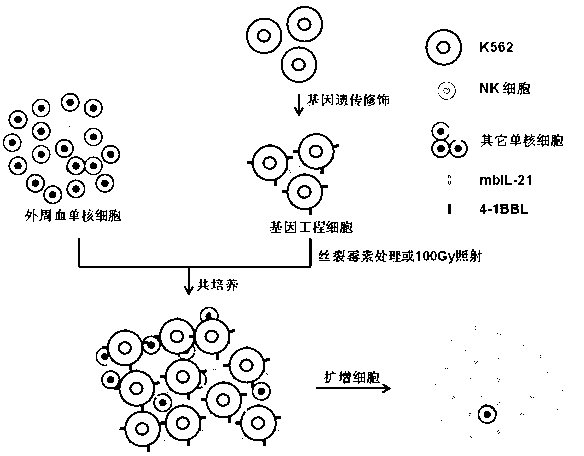
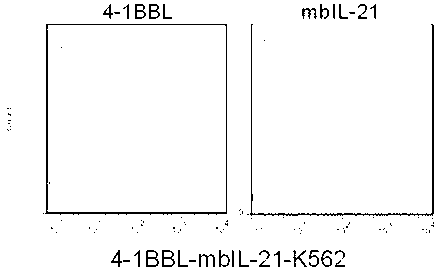
The invention discloses a gene engineering cell and application thereof in NK (Nature Killer) cell proliferation, and provides an in vitro proliferation method of an NK cell with high efficiency and high cytotoxicity. A 4-1BB ligand (4-1BBL) and a membrane fixed interleukin 21 (mbIl-21) are stably expressed on the surface of a cell membrane by adopting a genetic engineering technology for constructing 4-1BBL-mbIl-21-gene engineering cells (4-1BBL-mbIl-21-Gene Engineering Cells, 4-1BBL-mbIl-21-GEC), such as 4-1BBL-mbIl-21-K562 cells, to be used as feeder cells for proliferation, and the NK cells are directly proliferated from peripheral blood monouclear cells, thus the operation is simpler and easier. Studies such as cell counting, flow cytometry and cytotoxicity tests indicate that high cell proliferation times, high NK cell purity, strong cytotoxicity and remarkable cancer cell killer effect are achieved.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Method for diagnosing lung cancers using gene expression profiles in peripheral blood mononuclear cells
ActiveUS8476420B2Bioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseReference genes
Owner:THE WISTAR INST OF ANATOMY & BIOLOGY
Preparation method and application of a high-throughput fully human antibody
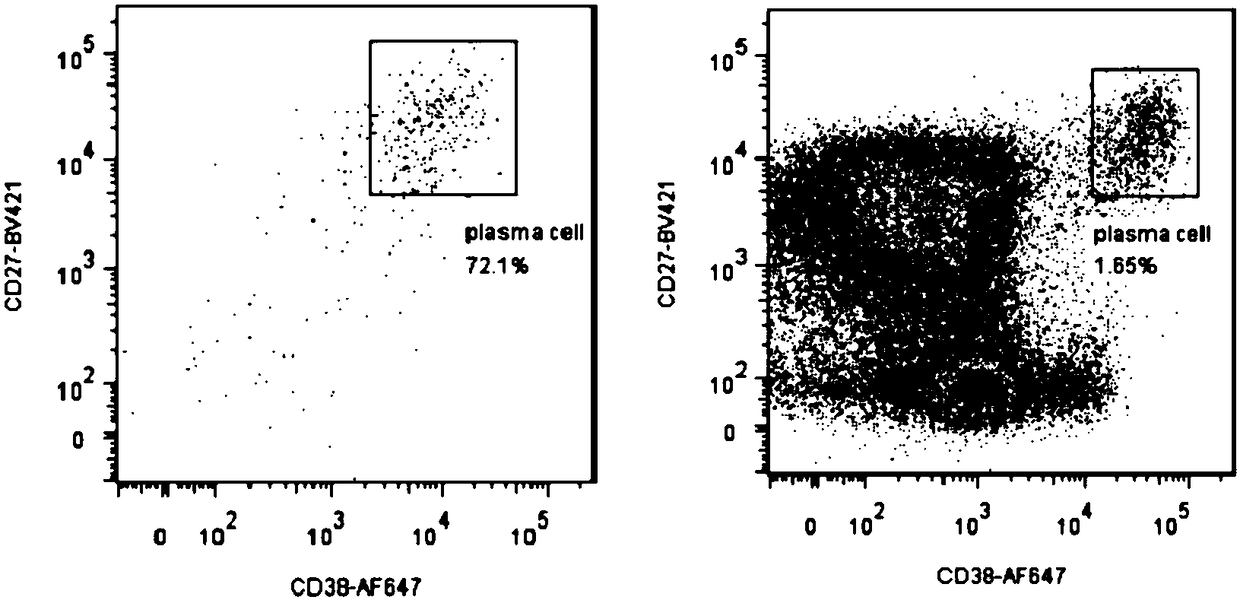
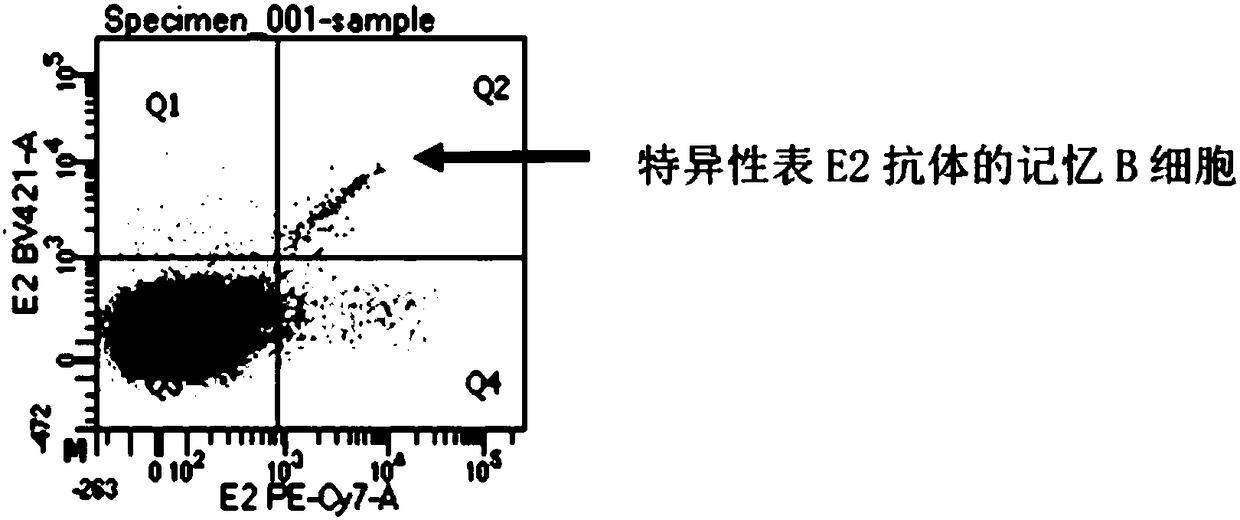
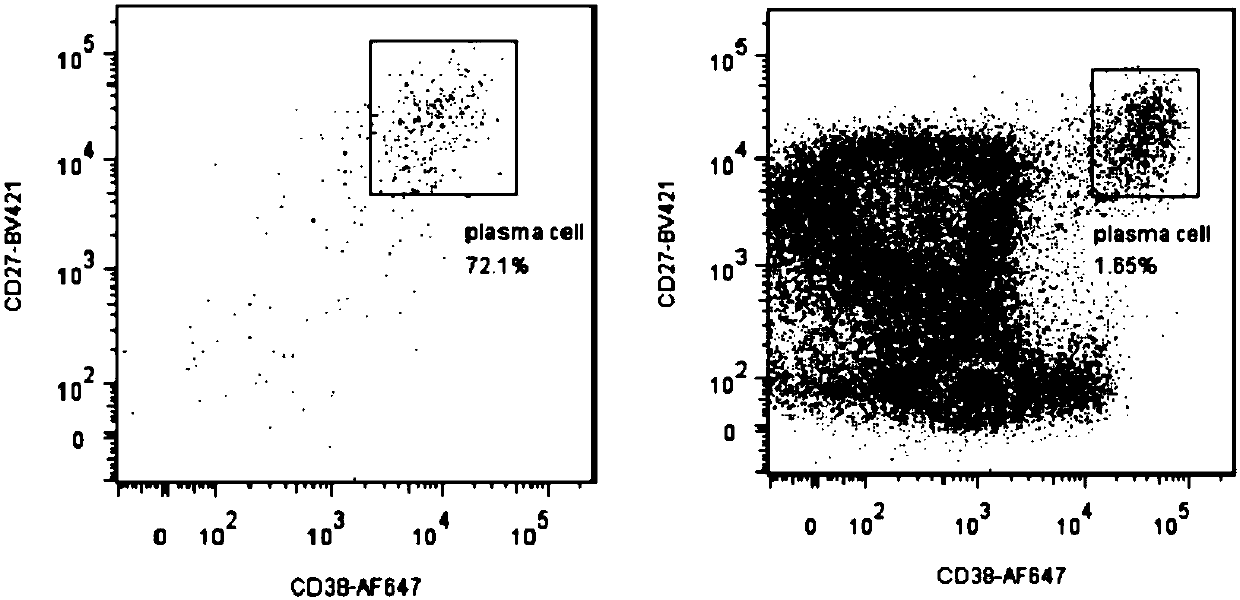
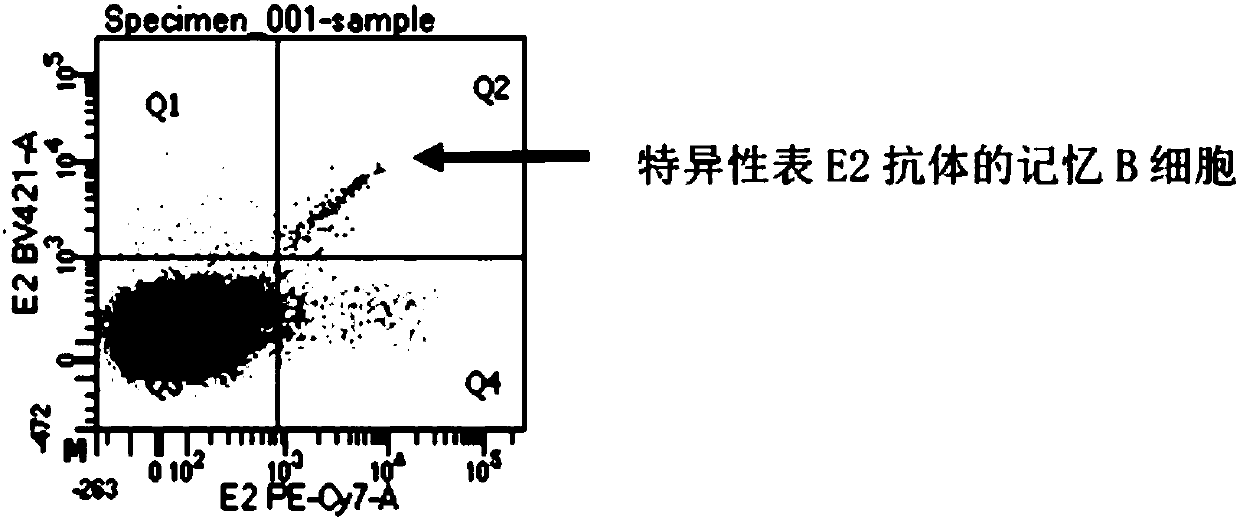
ActiveCN107760690BHigh affinityHigh potencyMicrobiological testing/measurementImmunoglobulinsPlasma cellHumanized antibody
The invention relates to gene engineering and antibody preparation and specifically discloses a preparation method and application of a high-throughput fully-humanized antibody. The preparation methodcomprises the following steps: separating peripheral blood monouclear cells; separating single cells of plasma cells or antigen-specificity memory B blymphocytes; amplifying the heavy-chain and light-chain gene variable regions of single B cell antibody by use of a primer provided by the inventor; establishing an expression system containing antibody heavy-chain and light-chain genes by use of alinear expression system containing a heavy-chain fragment or light-chain fragment constant area; finally, separating and purifying a fully humanized monoclonal antibody. By adopting the primer combination provided by the invention to amplify the genes in the antibody heavy-chain and light-chain variable areas, natural pairing of the light-chain and heavy-chain variable areas can be maintained, and the preparation method has the advantages of high gene diversity, high titer, full humanization, high antibody affinity, strong specificity, no heterologous serum reaction, no risk of propagating other infectious diseases, etc.
Owner:ZHUHAI TRINOMAB BIOTECHNOLOGY CO LTD
Method for detection of inflammatory processes
InactiveUS20040023246A1Reliable methodReduce handlingMicrobiological testing/measurementRecombinant DNA-technologyHigh probabilityHealthy individuals
The present invention is based on the surprising observation, that a high degree of expression of certain distinct Genes in peripheral blood mononuclear cells is associated with in inflammatory process with a higher probability than it is the case for other Genes. Therefore, the present invention provides a method for diagnosis of an inflammatory process comprising monitoring expression levels of 4-6 selected targets in peripheral blood mononuclear cells as compared to expression levels of said selected targets in peripheral blood mononuclear cells in healthy individuals, characterized in that over-expression or repression of the majority of said selected Genes is indicative for an inflammatory process.
Owner:ROCHE DIAGNOSTICS GMBH
Methods for Stimulating an Immune Response Using Bacterial Antigen Delivery System
InactiveUS20090324651A1Useful in treatment of cancerBiocideBacteriaAbnormal tissue growthTumor antigen
The invention relates to the use of the type III secretion system of bacteria to stimulate immune responses against tumor antigen(s) for treating antigen-loss variant tumors. Methods are provided for stimulating and / or increasing an immune response against tumor antigens. The invention also relates to the preparation of antigen presenting cells from peripheral blood mononuclear cells using bacteria having a type III secretion system.
Owner:YALE UNIV +1
Preparation method and application of high-throughput fully-humanized antibody
ActiveCN107760690AHigh affinityHigh potencyMicrobiological testing/measurementImmunoglobulinsDiseaseAntibody Affinities
The invention relates to gene engineering and antibody preparation and specifically discloses a preparation method and application of a high-throughput fully-humanized antibody. The preparation methodcomprises the following steps: separating peripheral blood monouclear cells; separating single cells of plasma cells or antigen-specificity memory B blymphocytes; amplifying the heavy-chain and light-chain gene variable regions of single B cell antibody by use of a primer provided by the inventor; establishing an expression system containing antibody heavy-chain and light-chain genes by use of alinear expression system containing a heavy-chain fragment or light-chain fragment constant area; finally, separating and purifying a fully humanized monoclonal antibody. By adopting the primer combination provided by the invention to amplify the genes in the antibody heavy-chain and light-chain variable areas, natural pairing of the light-chain and heavy-chain variable areas can be maintained, and the preparation method has the advantages of high gene diversity, high titer, full humanization, high antibody affinity, strong specificity, no heterologous serum reaction, no risk of propagating other infectious diseases, etc.
Owner:ZHUHAI TRINOMAB BIOTECHNOLOGY CO LTD
Method for early detection of various cancers and gastrointestinal disease and monitoring of transplanted organs
InactiveUS20080280282A1Early diagnosisMicrobiological testing/measurementDead animal preservationReceptor for activated C kinase 1Disease cause
A method for the early diagnosis of breast, lung, pancreatic and colon growths and cancers as well as conditions associated with donor and recipient organ transplants, both before and after transplantation to identify and allow treatment of possible transplanted organ rejection and other disease conditions related and unrelated to the transplantation, compares the gene expression patterns from a patient's peripheral blood monocytes-lymphocyte's gene system with either the similar gene expression patterns of a normal person, or with the similar gene expression patterns of a person known to have the condition being screened for. Differences between the patient's gene expression patterns for particular genes and the normal patterns indicates the presence of the condition with the number of differences indicating the probability of the condition. Similarities between the patient's gene expression patterns for those particular genes and the patterns of a person known to have the condition indicates the presence of the condition with the number of similarities indicating the probability of the condition. Portions of the method may be performed with the use of a microfluidic machine.
Owner:BAUER JR A ROBERT
Method of diagnosing integration dysfunction syndrome using blood
InactiveUS20060099593A1Reliable methodForcing riskBioreactor/fermenter combinationsBiological substance pretreatmentsClinical psychologyHealthy subjects
An object of the present invention is. to provide an objective method for diagnosis of schizophrenia using gene expression in mononuclear cells of peripheral blood as an index, and this invention provide a method for diagnosing whether a test subject suffers from schizophrenia or not. The method according to this invention is a method for diagnosing whether a test subject suffers from schizophrenia or not, the method comprising the steps of; obtaining mononuclear cells in blood containing nucleic acid from said subject, measuring the content of at least one nucleic acid selected from the group consisting of nucleic acid(s) (containing its fragment and a nucleic acid complementary to the nucleic acid) defining gene(s) exhibiting altered expression by occurrence of schizophrenia or nucleic acid(s) (containing its fragment and a nucleic acid complementary to the nucleic acid) defining gene(s) exhibiting altered expression by progression of schizophrenia in said mononuclear cells, and determining alteration of the quantified level(s) of the gene(s) in said test subject is statistically significant in comparison with the quantified level(s) of said nucleic acid(s) defining gene(s) exhibiting altered expression by occurrence of schizophrenia or said nucleic acid(s) defining gene(s) exhibiting altered expression by progression of schizophrenia in healthy subjects or schizophrenic patients, thereby diagnosing whether said subject is suffering from schizophrenia or not.
Owner:JAPAN SCI & TECH CORP +1
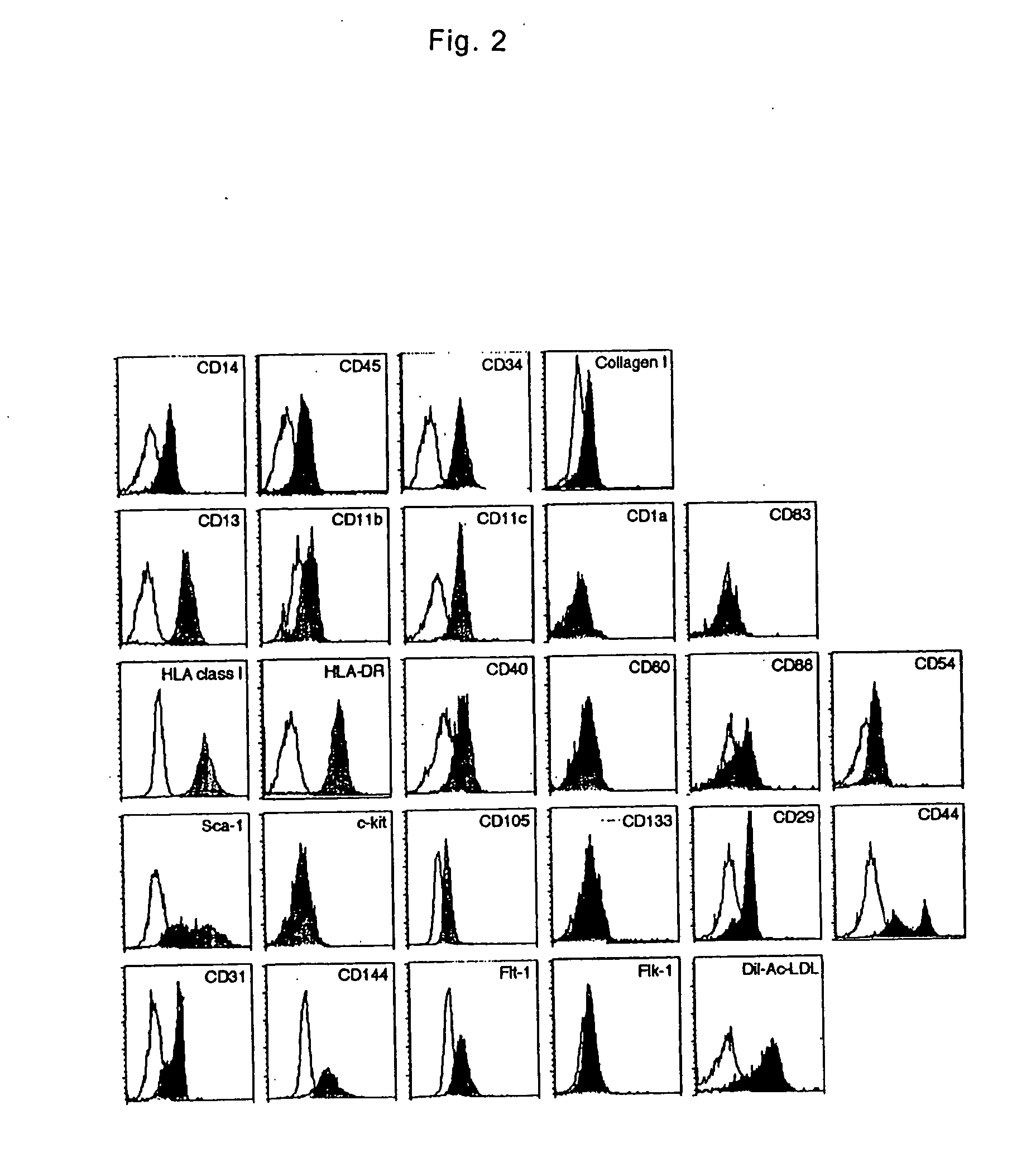
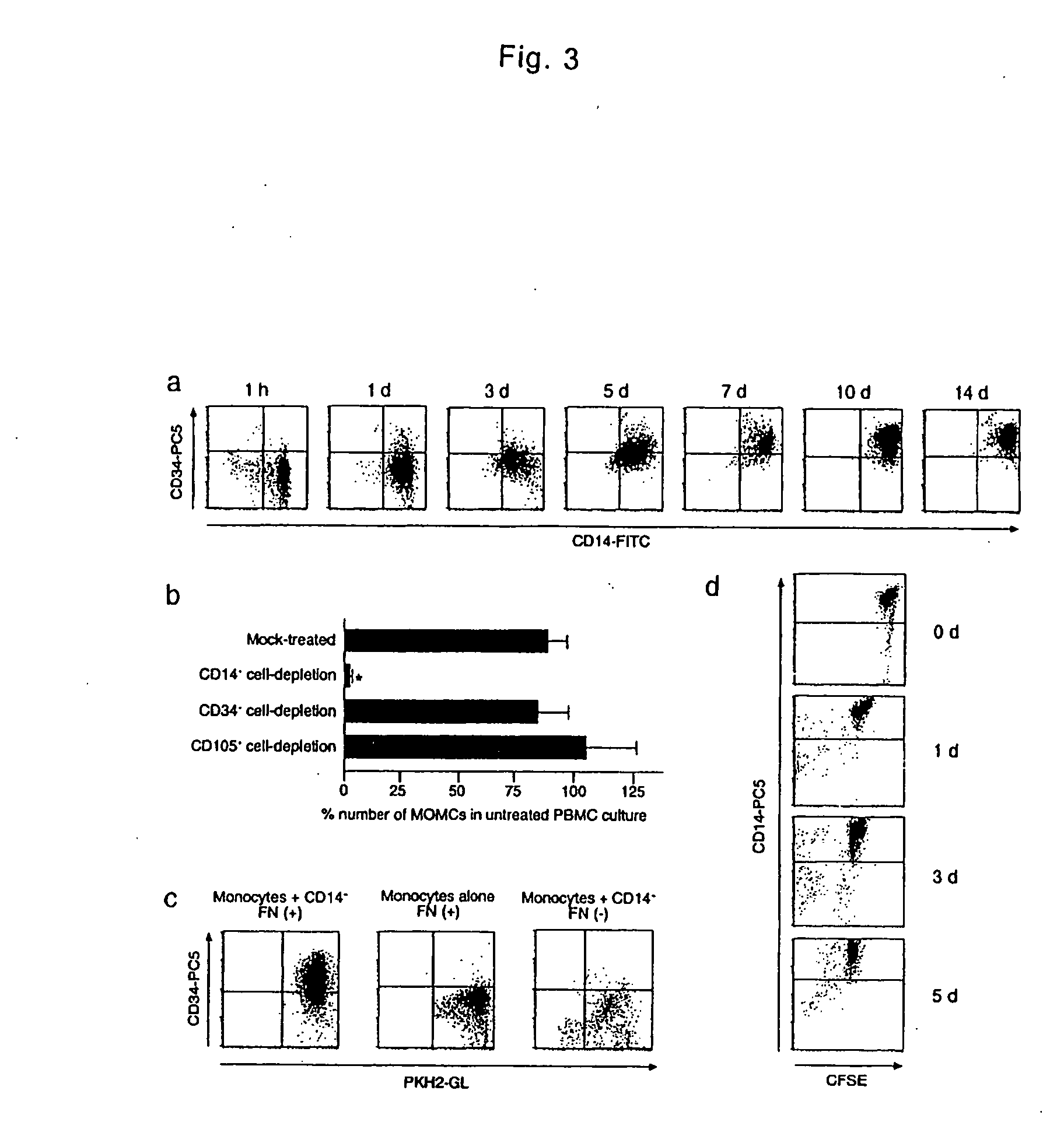
Monocyte-origin multipotent cell momc
The present invention is to provide a multipotent cell wherein the sufficient amount necessary can be stably and conveniently supplied with a minimum invasion, that will not cause rejection at the time of cell transplantation, that has a potential to differentiate into various cells such as mesenchymal cells including bone, cartilage, skeletal muscle and fat, endothelial cells, myocardial cells, neurons, mesenchymal cells, myocardial cells, endothelial cells, neurons induced to differentiate from the multipotent cell, and a therapeutic agent / treating method comprising these as active ingredient. Peripheral blood mononuclear cells (PMBC) are cultured on fibronectin-coated plastic plates for 7 to 10 days. The generating cell population with a fibroblast-like morphology is derived from circulating CD14+ monocyte, with a unique phenotype of CD14+CD45+CD34+ type I collagen+. These cells have a potential to differentiate into mesenchymal cells including bone, cartilage, skeletal muscle and fat, endothelial cells, myocardial cells, and neurons under particular culture conditions.
Owner:KEIO UNIV
Immune-modulating peptide
InactiveUS20070219139A1Inhibition of secretionPromote secretionBiocideNervous disorderExtracellular signalPhosphorylation
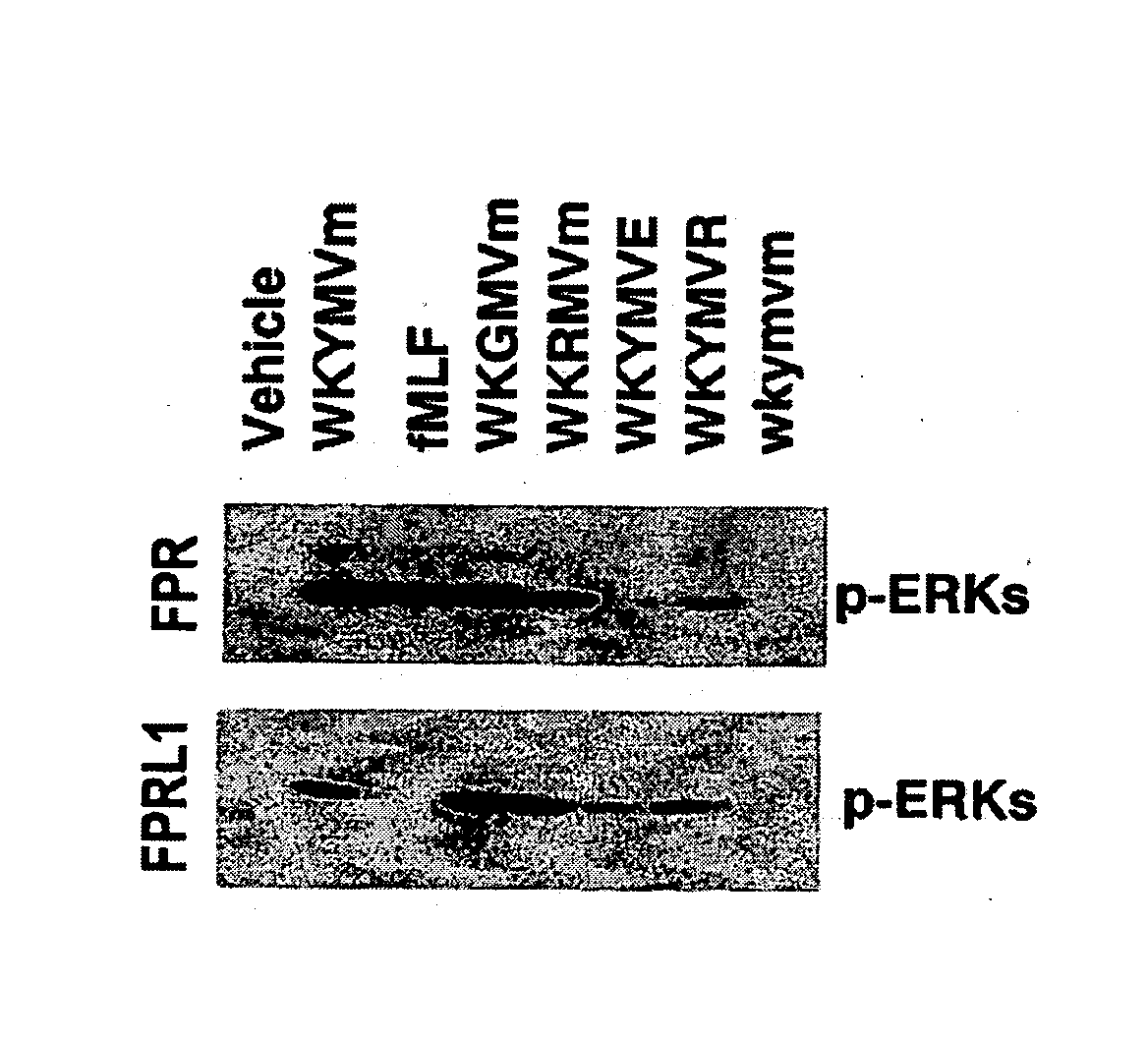
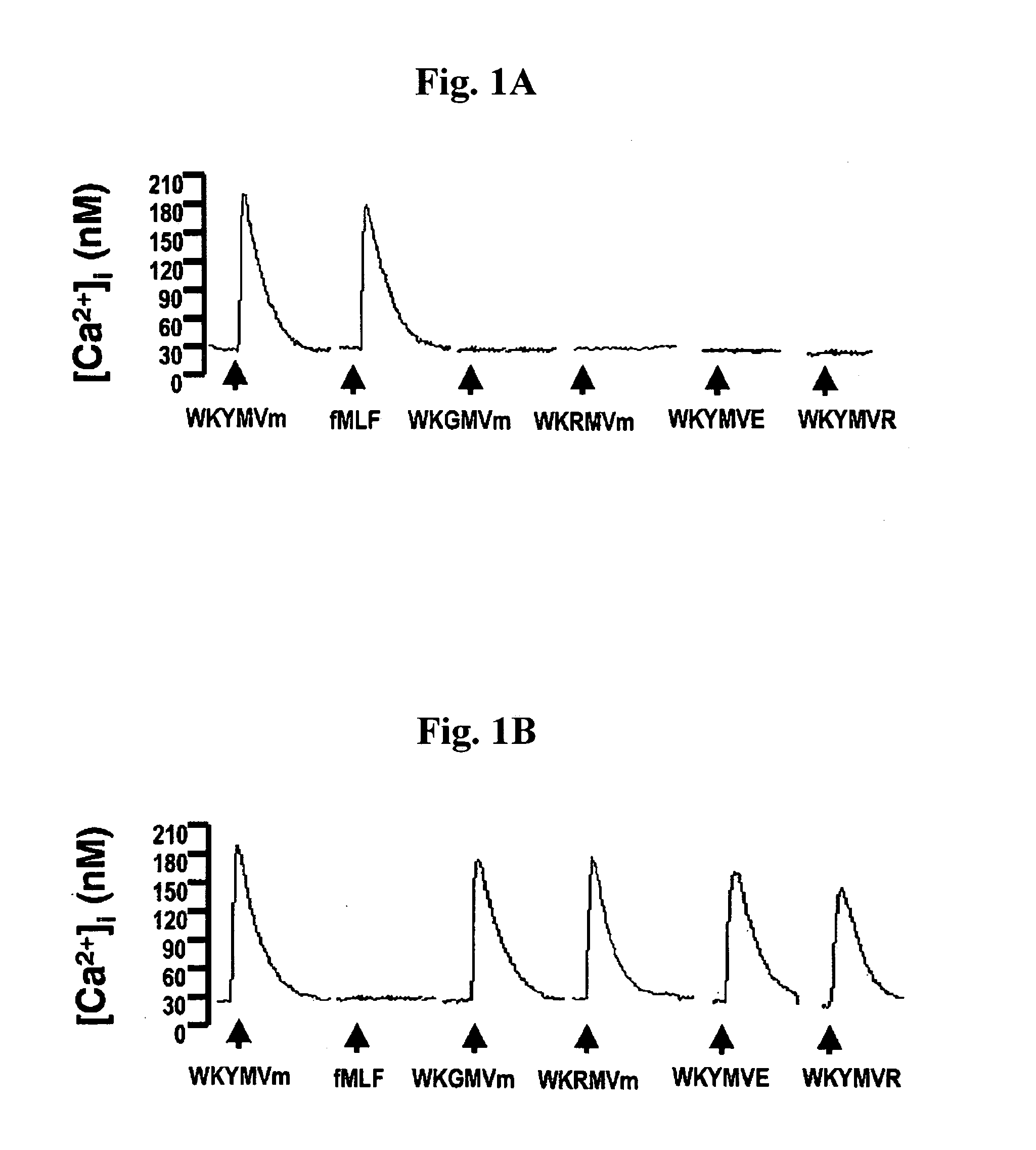
Disclosed are peptides having SEQ ID NOs: 1 to 24 that induce superoxide generation by human monocytes or neutrophils; that induce an intracellular calcium increase by human peripheral blood monocytes or neutrophils; binds to formyl peptide receptor or formyl peptide receptor-like 1; that induce chemotactic migration of human monocytes or neutrophils in vitro; that induce degranulation in formyl peptide receptor expressing cells or formyl peptide receptor-like 1 expressing cells; that stimulate extracellular signal regulated protein kinase phosphorylation via activation of formyl peptide receptor or formyl peptide receptor-like 1; or that stimulate Akt phosphorylation via activation of formyl peptide receptor or formyl peptide receptor-like 1.
Owner:POSTECH ACAD IND FOUND
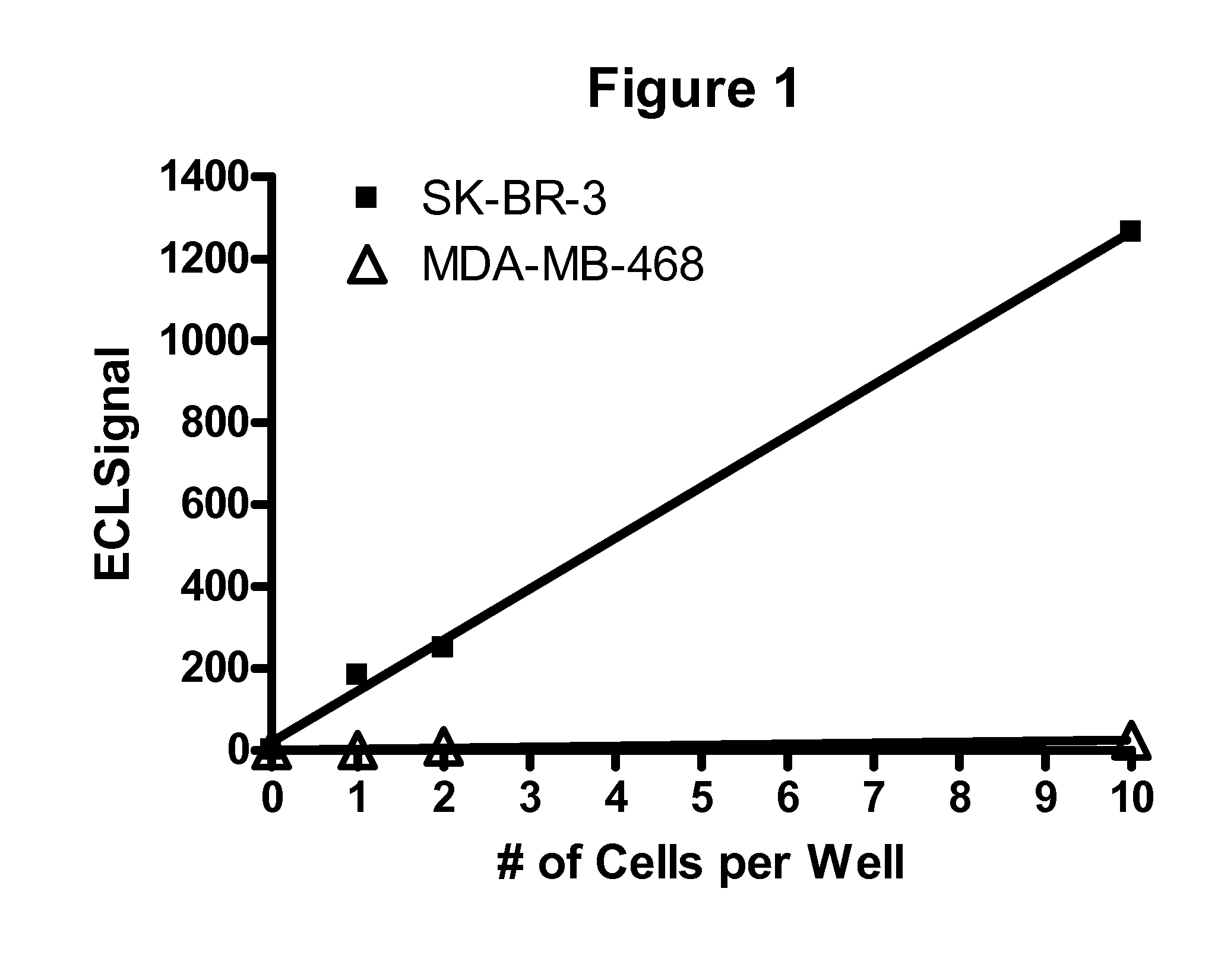
Detection of elevated levels of her-2/neu protein from non-isolated circulating cancer cells and treatment
InactiveUS20100120072A1Delay in testingRapidity of assayDisease diagnosisAntineoplastic agentsAnticarcinogenCirculating cancer cell
The expression of Her-2 / neu protein on circulating cancer cells in a sample of blood or peripheral blood mononuclear cells (PBMCs) is detected by performing a sensitive Her-2 / neu immunoassay. There is no need to isolate the cancer cells before performing the immunoassay. A positive result indicates the expression of Her-2 / neu on cancer cells in the blood sample. This method can be used to identify cancer patients who are likely to benefit from treatment with an anticancer agent that targets Her-2 / neu, such as trastuzumab (HERCEPTIN), lapatinib, CP-724,714, NKI-272, and BMS-599626
Owner:WELLSTAT BIOLOGICS CORP
Peptide-mediated gene transfer
InactiveUSRE39220E1Improve efficiencyProlong lifePeptide/protein ingredientsPeptide sourcesHinge regionPrimary cell
A methodology that allows for highly efficient transfer and stable integration of DNA into both established eukaryotic cell lines and primary cells, including non-dividing cells such as human peripheral blood monocytes and macrophages, entails the use of a synthetic polypeptide comprised of a peptide domain which corresponds to a nuclear localization signal sequence and a DNA binding domain which is rich in basic amino acids, separated by a hinge region of neutral acid which prevents stearic interference between the two domains.A synthetic polypeptide that allows for highly efficient transfer of DNA into eukaryotic cells, including, for example, non-dividing cells such as human peripheral blood monocytes and macrophages.<?insert-end id="INS-S-00001" ?>
Owner:GENETIC APPL
Method for preparing natural killer cells using irradiated pbmcs, and Anti-cancer cell therapeutic agent comprising the nk cells
InactiveUS20180155690A1High purityCell dissociation methodsMammal material medical ingredientsCancer cellCD16
Provided is a method for preparing natural killer cell with high efficiency using irradiated peripheral blood mononuclear cells, more particularly to a method for proliferating highly activated NK cells using a combination of irradiated peripheral blood mononuclear cells (PBMCs) and a CD16 antibody and an anti-cancer cell therapeutic composition containing the natural killer cells (NK cells) prepared thereby. Further provided is a method for large-scale proliferation of activated NK cells with high efficiency using a combination of irradiated peripheral blood mononuclear cells (PBMCs) and a CD16 antibody without the use of cancer cells or genetically modified feeder cells having safety issues as feeder cells. The highly purified and highly cytotoxic NK cells proliferated in large quantities can be used as an active ingredient of a cancer immunotherapeutic composition.
Owner:KOREA INST OF RADIOLOGICAL & MEDICAL SCI
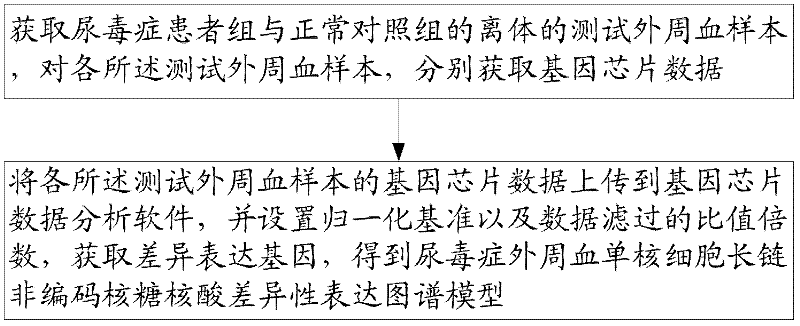
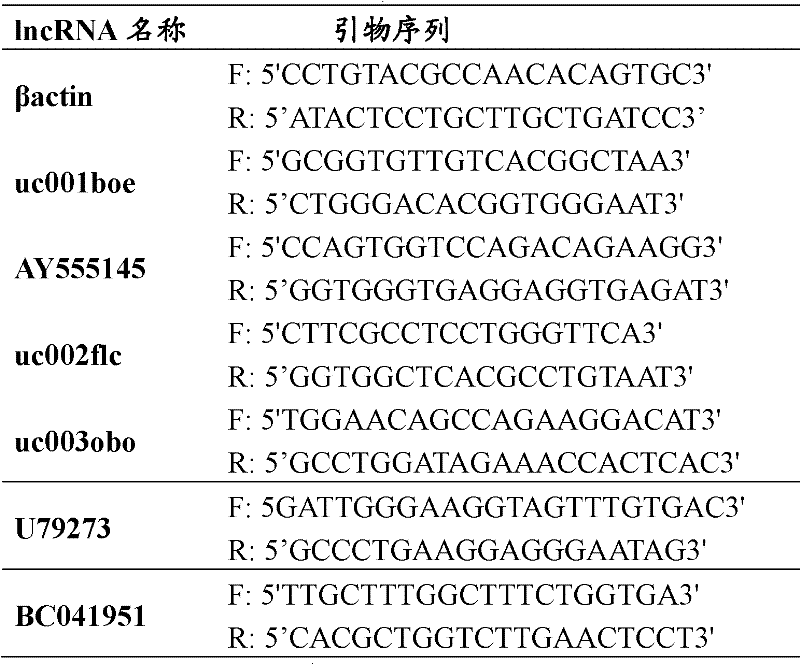
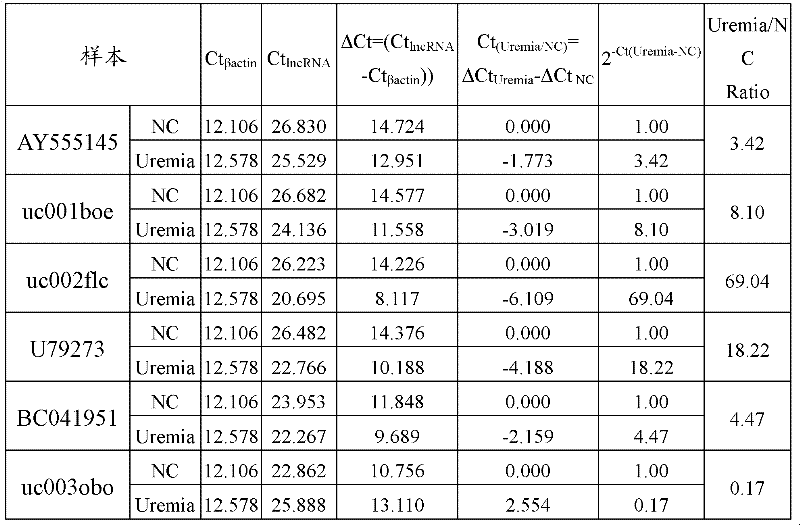
Differential expression map model of long-chain non-coding RNA in uremia and its construction method
The invention relates to a construction method of a uremia peripheral blood mononuclear cell long-chain non-coding ribonucleic acid difference expression spectrum model, which comprises the following steps of: A1, obtaining the in vitro test peripheral blood samples of the uremia patient group and the normal control group and respectively obtaining gene chip data for each test peripheral blood sample; and A2, uploading the gene chip data of each test peripheral blood sample to gene chip data analysis software, setting the normalized standard and data filtering ratio times, obtaining the difference expression gene and obtaining the uremia peripheral blood mononuclear cell long-chain non-coding ribonucleic acid difference expression spectrum model. Through the scheme, the information as themiddle results is provided for the study on the uremia, and in addition, a novel idea is provided for further disclosing the pathological mechanism of the uremia. In addition, the invention also relates to the uremia peripheral blood mononuclear cell long-chain non-coding ribonucleic acid difference expression spectrum model.
Owner:中国人民解放军联勤保障部队第九二四医院
Method for rapidly and efficiently separating individual antigen specific cell B
ActiveCN106350485AReduce the impactQuality improvementCell dissociation methodsBlood/immune system cellsMagnetic beadCell separation
The invention discloses a method for rapidly and efficiently separating an individual antigen specific cell B. The method specifically comprises the following steps: step I, separating peripheral blood whole blood of an antigen-infected person or animal to obtain peripheral blood mononuclear cell suspension; step II, preparing activated immunomagnetic beads of a coupling antigen ligand, wherein the ligand is an artificial antigen of a corresponding antigen; step III, removing a non-specific adsorption cell in the peripheral blood mononuclear cell suspension obtained in step I by using un-coupled un-activated magnetic beads, and separating a mixture obtained in step III by using the activated immunomagnetic beads of the coupling antigen obtained in the step II to obtain the individual antigen specific cell B. The scheme provides a universal method which does not depend on myeloma cell and is low in operation complexity and low in cost and obtains the specific cell B corresponding to the individual antigen, so that the single-cell separation effect is better.
Owner:HANGZHOU BIOLYNX TECH CO LTD
Methods for inducing systemic immune responses to cancer
InactiveUS20140205609A1Enhance all ongoing immune responseLow in DOCancer antigen ingredientsWhole bodyAntigen stimulation
Pharmaceutical compositions comprising molecules produced by antigen-stimulated peripheral blood mononuclear cells (PBMCs) that induce systemic immune responses to cancers are encompassed herein, as are methods for making and administering such molecules and pharmaceutical compositions comprising same. Also encompassed herein are mixtures of molecules produced by antigen-stimulated PBMCs and compositions thereof for use in treating cancer. Methods for stimulating a systemic immune response in a subject afflicted with a cancer are also envisioned, whereby molecules produced by antigen-stimulated PBMCs or supernatants comprising same are administered to the subject, wherein the molecules stimulate an immune response to the cancer in the subject.
Owner:NEW YORK UNIV
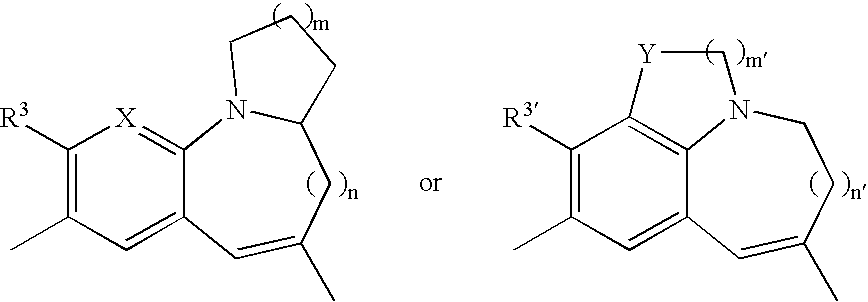
Tricyclic compound, process for producing the same, and use
A compound of the formula: wherein R1 is a 5- or 6-membered ring; Z1 is a 5- or 6-membered aromatic ring; Z2 is a group -Z2a-W2-Z2b-, wherein Z2a and Z2b are each O, S(O)q (wherein q is 0, 1 or 2), an imino group, or a bond; and W2 is an alkylene chain; W is a group represented by wherein R3 and R3′ are each a hydrogen atom, a lower alkyl group, or a lower alkoxy group; X is CH or N; n and n′ are each an integer of 0 or 1 to 4; m and m′ are each 1 or 2; Y is O, S(O)p (wherein p is 0, 1 or 2), CH2 or NR4 (wherein R4 is a hydrogen atom, a lower alkyl group, or a lower acyl group); and R2 is (1) an amino group, in which the nitrogen atom may be converted to a quaternary ammonium or an oxide, or (2) a nitrogen-containing heterocyclic group which may contain a sulfur atom or an oxygen atom as the ring-constituting atom, in which the nitrogen atom may be converted to a quaternary ammonium or an oxide; or a salt thereof. The compound exhibits excellent CCR antagonist activity against CCR5, and is useful as a prophylactic and / or therapeutic agent for HIV infection in human peripheral blood mononuclear cells, especially for AIDS.
Owner:SHIRAISHI MITSURU +4
Method for the early detection of breast cancer, lung cancer, pancreatic cancer and colon polyps, growths and cancers as well as other gastrointestinal disease conditions and the preoperative and postoperative monitoring of transplanted organs from the donor and in the recipient and their associated conditions related and unrelated to the organ transplantation
ActiveUS7820382B2Early diagnosisMicrobiological testing/measurementDisease diagnosisEarly breast cancerLung cancer
A method for the early diagnosis of breast, lung, pancreatic and colon growths and cancers as well as conditions associated with donor and recipient organ transplants, both before and after transplantation to identify and allow treatment of possible transplanted organ rejection and other disease conditions related and unrelated to the transplantation, compares the gene expression patterns from a patient's peripheral blood monocytes-lymphocyte's gene system with either the similar gene expression patterns of a normal person, or with the similar gene expression patterns of a person known to have the condition being screened for. Differences between the patient's gene expression patterns for particular genes and the normal patterns indicates the presence of the condition with the number of differences indicating the probability of the condition. Similarities between the patient's gene expression patterns for those particular genes and the patterns of a person known to have the condition indicates the presence of the condition with the number of similarities indicating the probability of the condition. For example, particular genes for use in identifying pancreatic cancer are disclosed.
Owner:BAUER A ROBERT
METHOD FOR THE SIMULTANEOUS INDUCTION OF CTL AND gamma-delta T CELL
ActiveUS20120107292A1Good treatment effectEfficient processBiocideMammal material medical ingredientsDiseaseDelta cell
Disclosed are: a method for culture of disease antigen specific CTLs and γδT cells in one culture step conveniently and efficiently; and a pharmaceutical agent and a therapeutic / prophylactic method both of which use a cell produced by the method. Blood is collected and peripheral blood mononuclear cells are separated from the blood. Aminobisphosphonate and a disease antigen are added to the peripheral blood mononuclear cells at the beginning of culture, and the cell culture is carried out for a predetermined period to proliferate / induce disease antigen specific CTLs and γδT cells simultaneously until the numbers of the cells reach values that are effective for the treatment of a disease. The CTLs and the γδT cells thus produced are used for the treatment.
Owner:MEDINET CO LTD
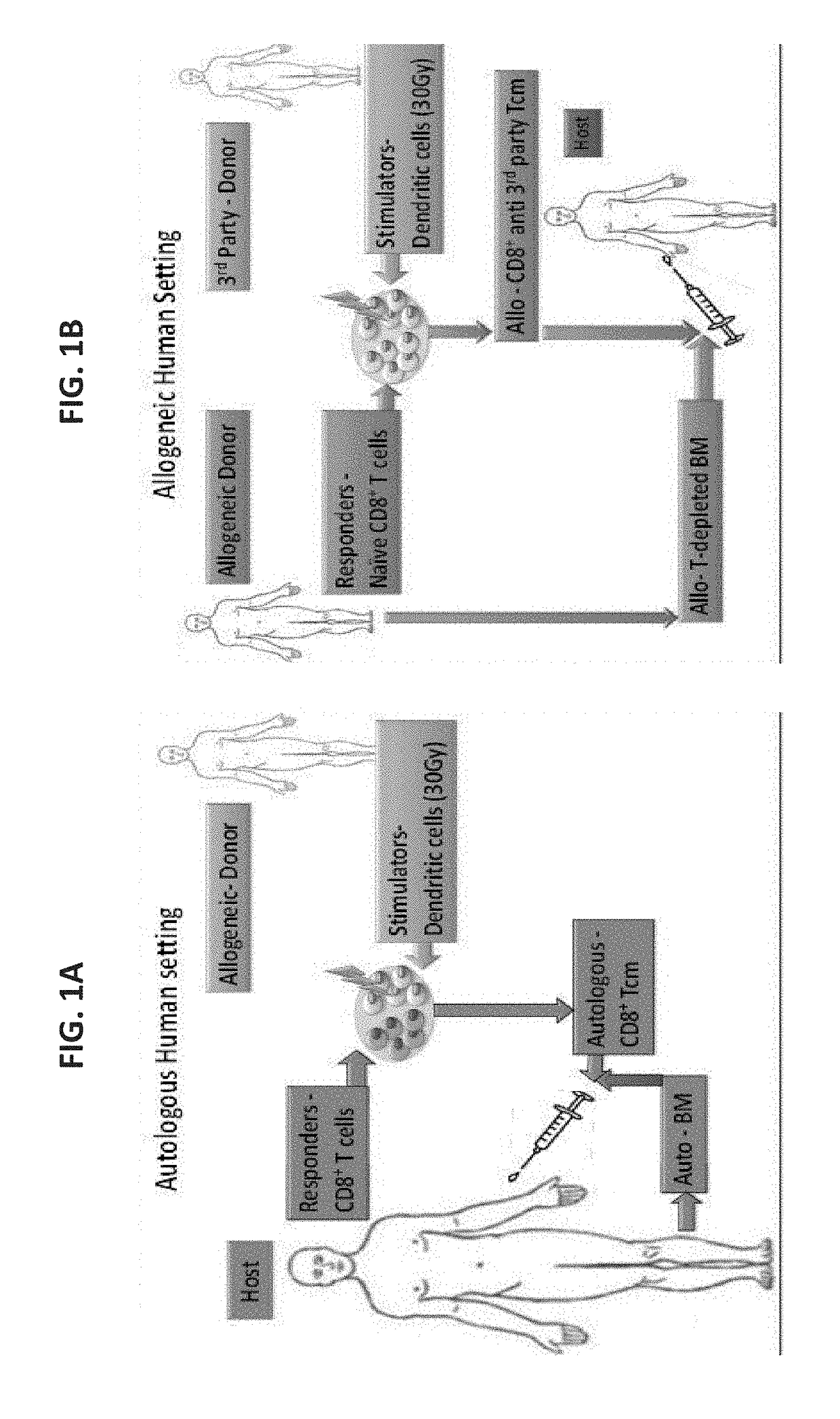
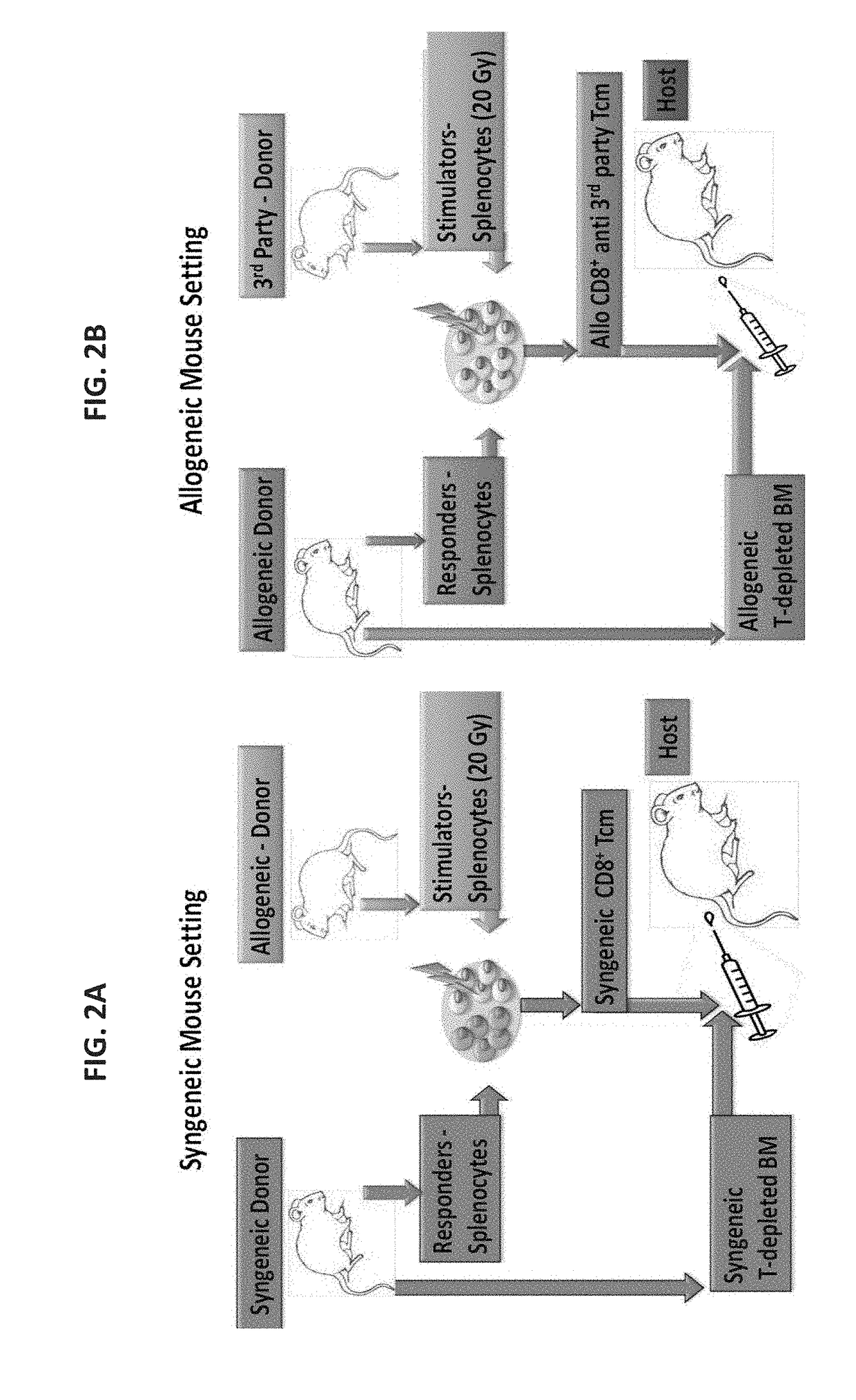
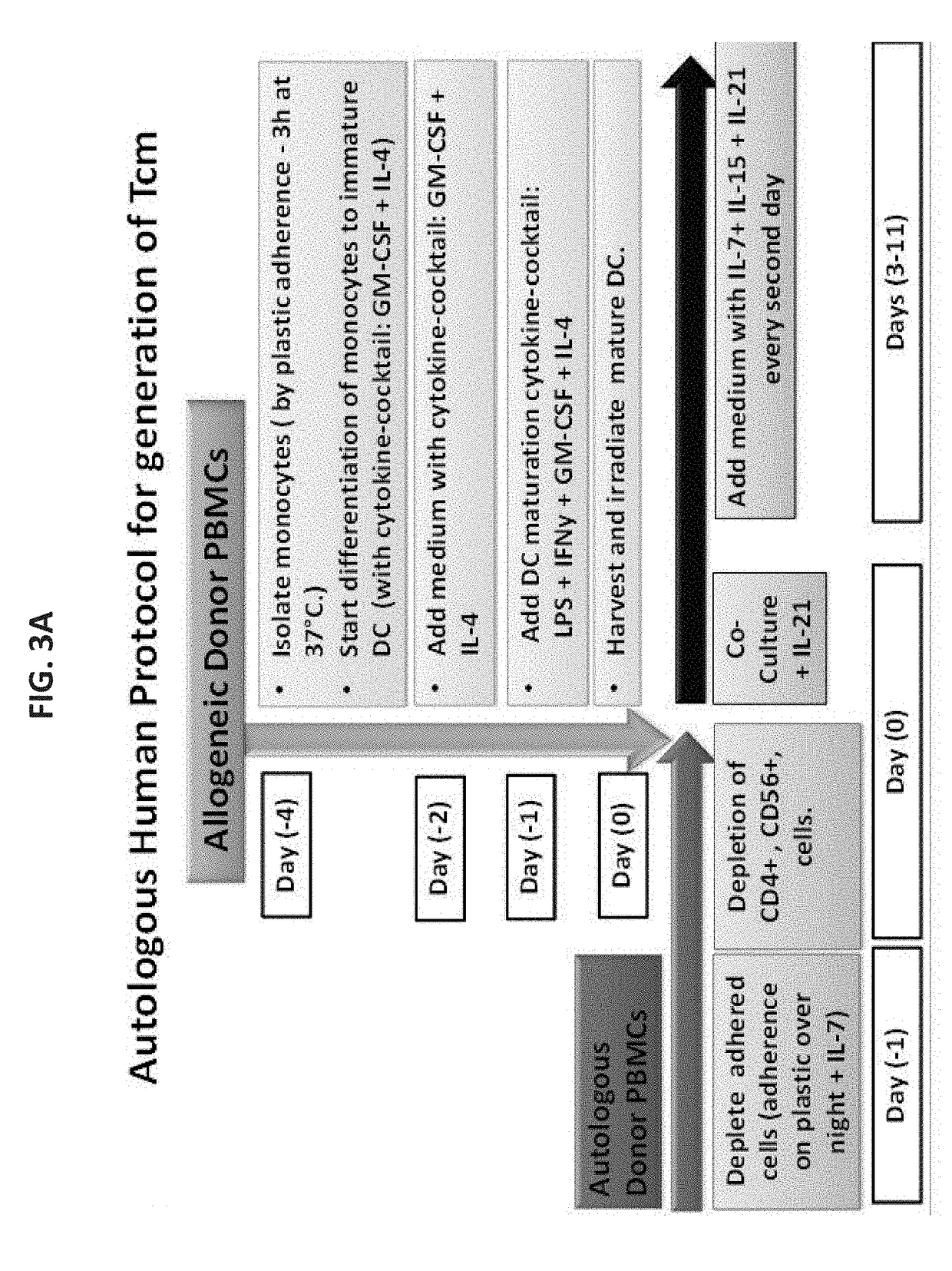
Anti third party central memory t cells, methods of producing same and use of same in transplantation and disease treatment
A method of generating an isolated population of cells comprising anti-third party cells having a central memory T-lymphocyte (Tcm) phenotype, the cells being tolerance-inducing cells and / or endowed with anti-disease activity, and capable of homing to the lymph nodes following transplantation is disclosed. The method comprising: (a) contacting peripheral blood mononuclear cells (PBMC) with a third party antigen or antigens in the presence of IL-21 so as to allow enrichment of antigen reactive cells; and (b) culturing the cells resulting from step (a) in the presence of IL-21, IL-15 and IL-7 in an antigen free environment so as to allow proliferation of cells comprising the central memory T-lymphocyte (Tcm) phenotype.
Owner:YEDA RES & DEV CO LTD
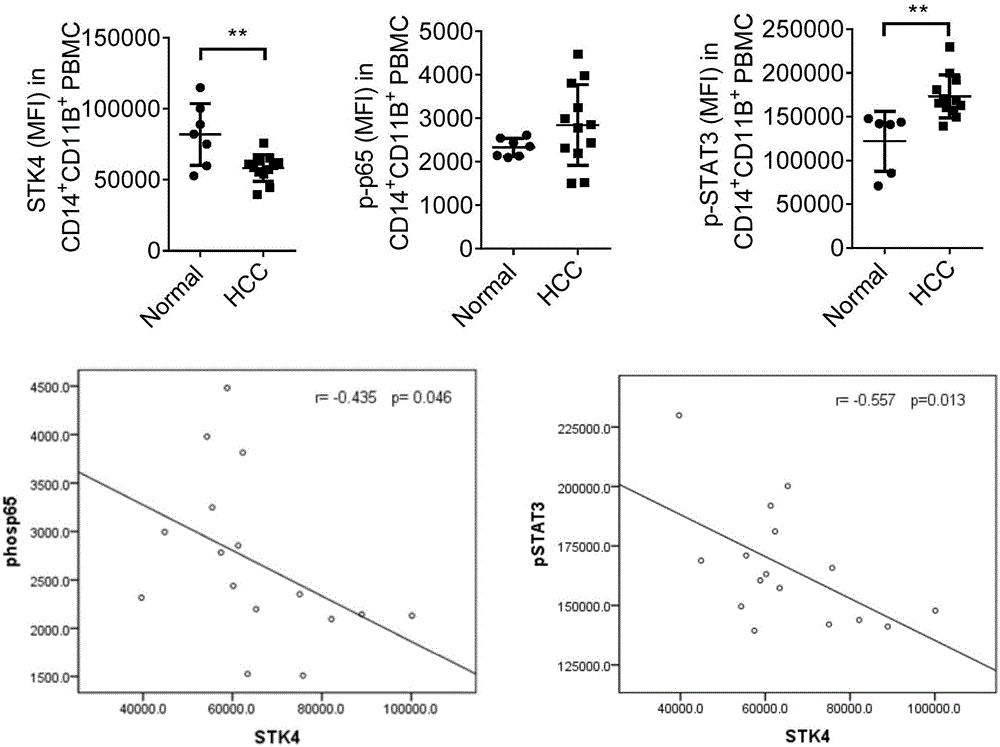
Marker for diagnosis of inflammation-associated HCC and application thereof
The invention relates to a combined diagnostic marker for inflammation-associated HCC and an application thereof. Combined application of an STK4 protein in a peripheral blood monocyte or plasma, phosphorylated p65 (p-p65) and phosphorylated STAT3 (p-STAT3) in the peripheral blood monocyte and an IL-6 protein in the plasma as the marker for inflammation-associated HCC is provided for the first time, and a theoretical basis and a simpler detection method are provided for fast and early diagnosis and monitoring of HCC.
Owner:CENT FOR EXCELLENCE IN MOLECULAR CELL SCI CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com