Preparation method and application of high-throughput fully-humanized antibody
A fully human antibody, high-throughput technology, applied in botanical equipment and methods, biochemical equipment and methods, applications, etc., can solve problems such as poor affinity, inability to ensure identical antibodies, and good cell accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Example 1 Sample Collection and PBMC Separation
[0118] According to different experimental purposes, through literature research, understand the pathogen information, pathogenesis, disease progression process, body immune response, main antigen structure and neutralizing epitope that cause these infectious diseases, autoimmune diseases or tumors and the development of corresponding treatments and vaccines. Then 5-10ml of blood at the time point with the highest neutralizing antibody titer was drawn for PBMC separation.
[0119] PBMC separation and cryopreservation: Freshly obtained blood samples were centrifuged at 2400 rpm for 7 minutes at room temperature, and another centrifuge tube was added to an equal volume of Ficoll (GE) for use; after centrifugation, the upper layer plasma was transferred to a new centrifuge tube for storage at -80°C, the middle layer Take the leukocytes into another centrifuge tube, add an equal volume of 1640 medium (Gibco) containing 5% F...
Embodiment 2
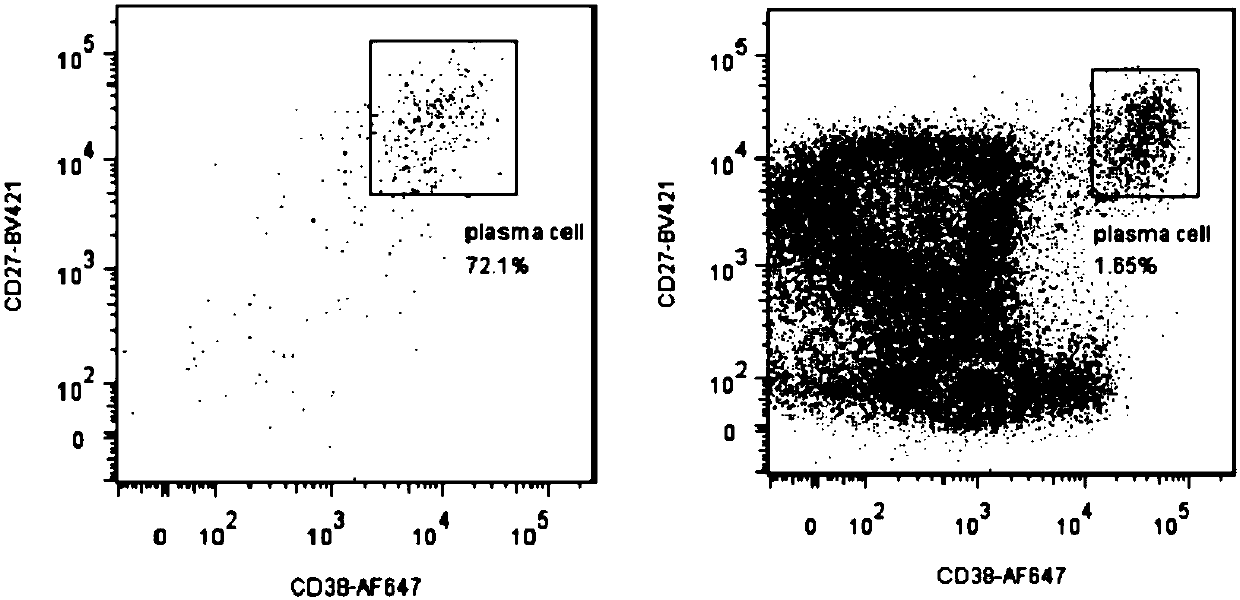
[0121] 1. Sorting plasma cells
[0122] In view of the significant increase in antigen-specific plasma cells after immunization, flow cytometry will be used to sort individual plasma cells for samples with clear immunization and appropriate sampling time. PBMC staining: mark the flow tube according to the staining scheme, and set the single-staining positive control, negative control and Isotype control; the plasma cell staining scheme is:
[0123] Combination 1: AqVD-Amycan- / CD19-BV605+ / CD3 / 14 / 16 / 235a-PE-Cy5- / CD20-PerCP- / IgD-PE- / CD138-FITC- / CD38-AF647+ / CD27-BV421+;
[0124] Combination 2: AqVD-Amycan- / CD19-PE+ / CD3 / 14 / 16 / 235a-PE-Cy5- / CD20-PerCP- / IgD-BB515- / CD138-PE-CF594- / CD38-AF647+ / CD27-BV421+;
[0125] Combination 3: AqVD-Amycan- / CD19-PerCP+ / CD3 / 14 / 16 / 235a-PE-Cy5- / CD20-PE-Cy7- / IgD-PE- / CD138-FITC- / CD38-BV605+ / CD27-BV421+.
[0126] Plasma cell sorting as figure 1 shown.
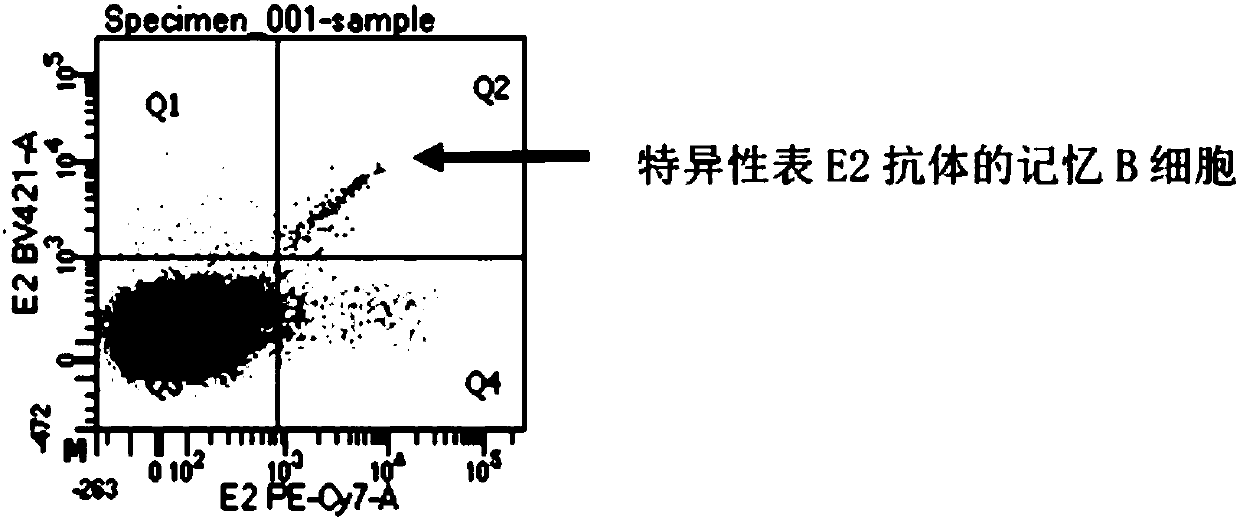
[0127] 2. Sorting memory B lymphocytes
[0128] Since the plasma cells will return to the basic leve...
Embodiment 3
[0134] Example 3 Single B Lymphocyte Gene Amplification
[0135] The isolated single peripheral nuclear cells were subjected to cloning of variable region genes of immunoglobulin heavy and light chains.
[0136] 1. Reverse transcription to synthesize cDNA:
[0137] Synthesize cDNA in a 20 μl reaction system in a 96-well PCR plate, 50ng / μl Random hexamer Primers, 1.5μl 25mMdNTPs, and 50U Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA), and react on a PCR machine at 65°C 1 hour;
[0138] 2. Nested PCR amplification of antibody variable region genes:
[0139] Use the nested PCR primers provided by the present invention to carry out antibody variable region genes. Synthesize IgH, Igλ, and Igκ variable region genes in a 96-well plate with 50 μl reaction system for PCR reaction. The first round of PCR: 5 μl RT reaction product in 50 μl system, 5 units of HotStarTaq Plus enzyme (QIAGEN), 0.2 mM dNTPs, and 0.5μM IgH (VH1.1-VH7.1), Igκ (VK0.1-VK0.5) or Igλ (VL1.1-V...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com