Method for constructing human peripheral blood immune cell bank
An immune cell bank and human peripheral blood technology, applied in the field of cell bank construction, can solve the problems of unsatisfactory survival rate of mononuclear cells, high price of serum-free cryopreservation solution, and unsuitable cryopreservation conditions. Low cost, high activity, easy to use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
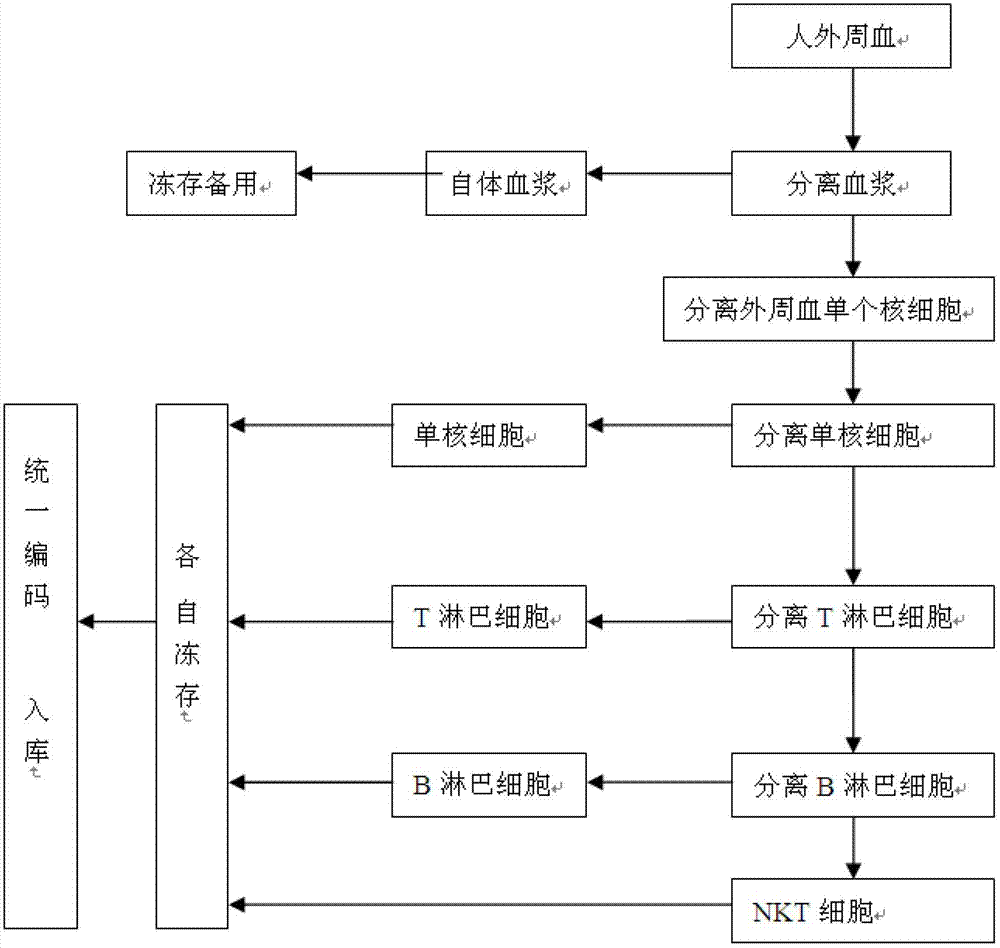
[0023] Example 1 Construction method of human peripheral blood immune cell bank
[0024] (1) Collect human peripheral blood: perform a physical examination on the donor, and check it according to the blood donation standard, and the blood will be drawn after two days if the standard is met;
[0025] (2) Separate autologous plasma: transfer the extracted peripheral blood to a centrifuge tube, in a centrifuge at 1500 rpm / centrifuge for 10 minutes, take the supernatant, inactivate at 56°C for 30 minutes, and set it at -20°C after completion Store frozen for later use.
[0026] (3) Separate peripheral blood mononuclear cells: Dilute the precipitate after removing autologous plasma in the above steps by volume with normal saline or PBS 1 times, and then add 0.5 times the volume of 4wt% hydroxyethyl starch solution , After mixing, let stand for 30 minutes, separate the supernatant, and add the supernatant to an equal volume of the lymphocyte separation liquid surface, 3000 rpm / separatio...
Embodiment 2
[0032] Example 2 Method for constructing human peripheral blood immune cell bank
[0033] (1) Collect human peripheral blood: perform a physical examination on the donor, and check it in accordance with the blood donation standard. The blood will be drawn after two days if the standard is met;
[0034] (2) Separate autologous plasma: transfer the extracted peripheral blood to a centrifuge tube, separate the heart in a centrifuge at 2500 rpm for 5 minutes, take the supernatant, inactivate at 56°C for 30 minutes, and set it at -20°C after completion Store frozen for later use.
[0035] (3) Separate peripheral blood mononuclear cells: Dilute the precipitate after removing autologous plasma from the above steps by volume with normal saline or PBS 1 times, and then add 1 times the volume of 3wt% hydroxyethyl starch solution , After mixing, let stand for 40 minutes, separate the supernatant, and add the supernatant to an equal volume of the lymphocyte separation liquid surface, 4000 rpm / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com