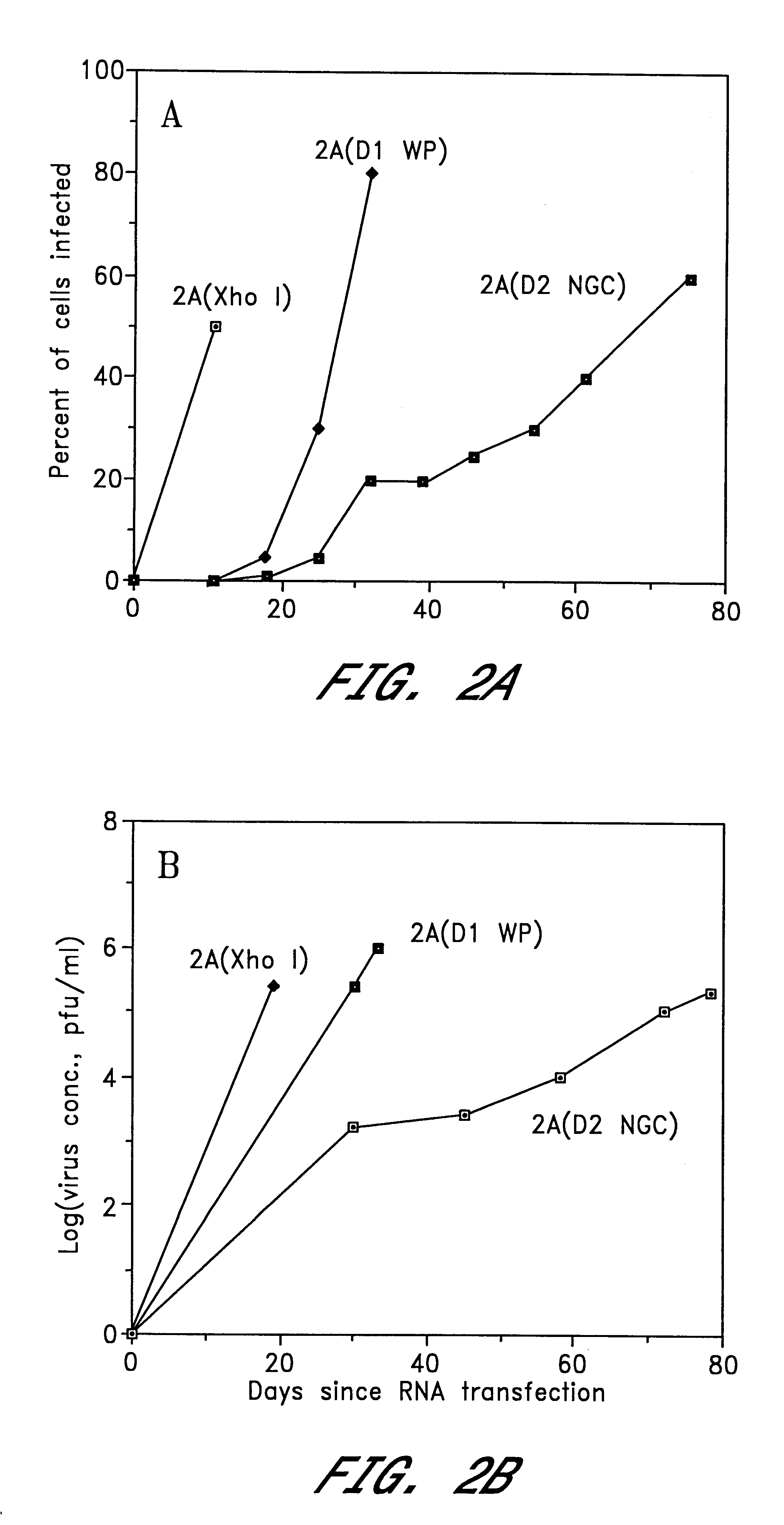
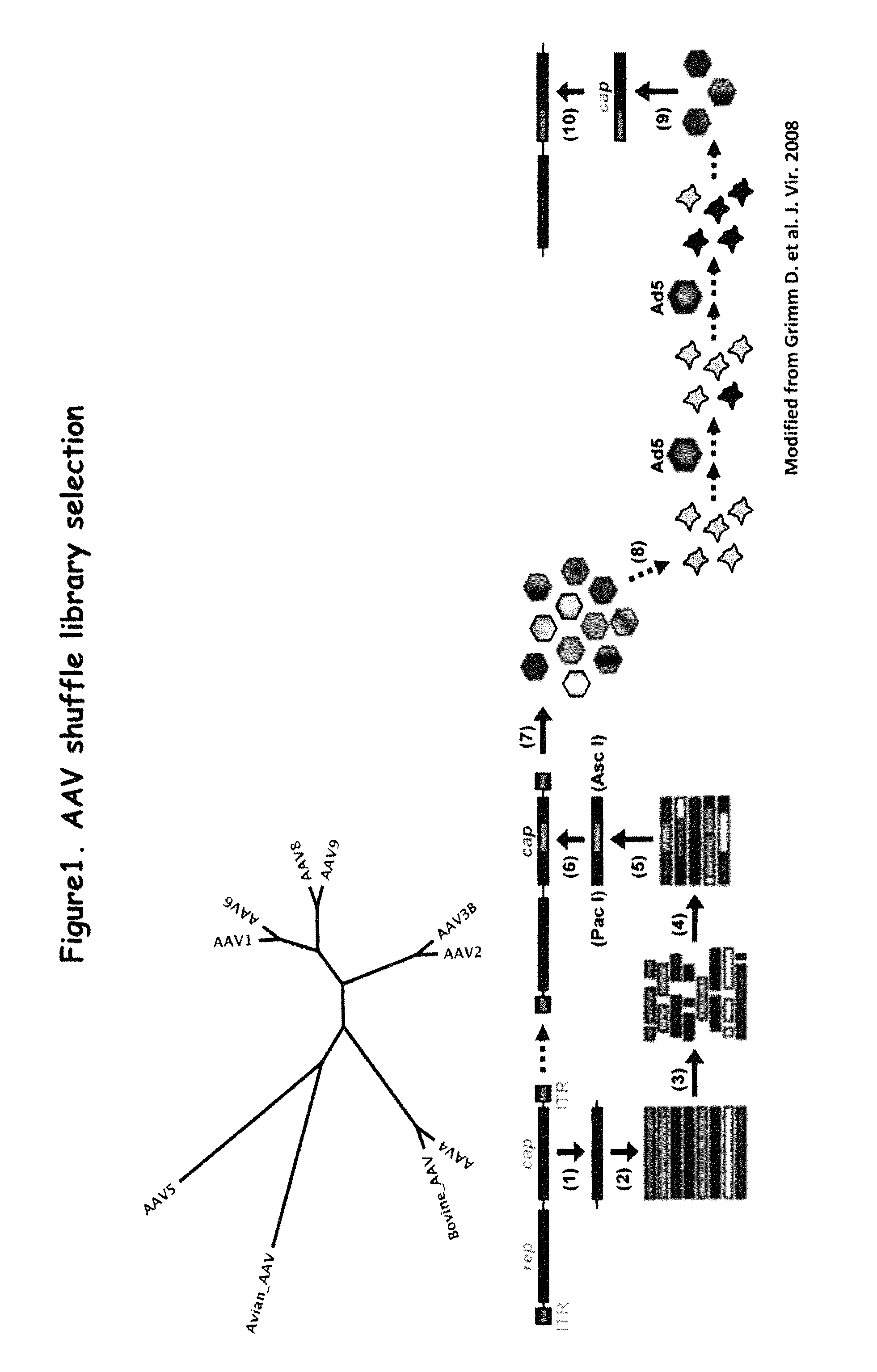
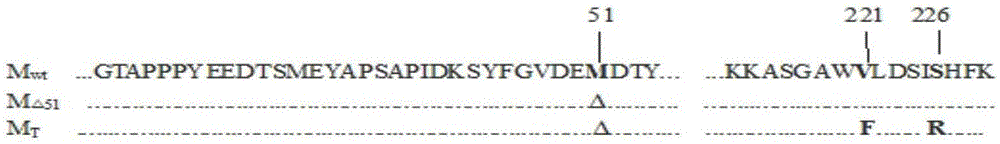Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
88 results about "Viral matrix protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Viral matrix proteins are structural proteins linking the viral envelope with the virus core. They play a crucial role in virus assembly, and interact with the RNP complex as well as with the viral membrane. They are found in many enveloped viruses including paramyxoviruses, orthomyxoviruses, herpesviruses, retroviruses, filoviruses and other groups.
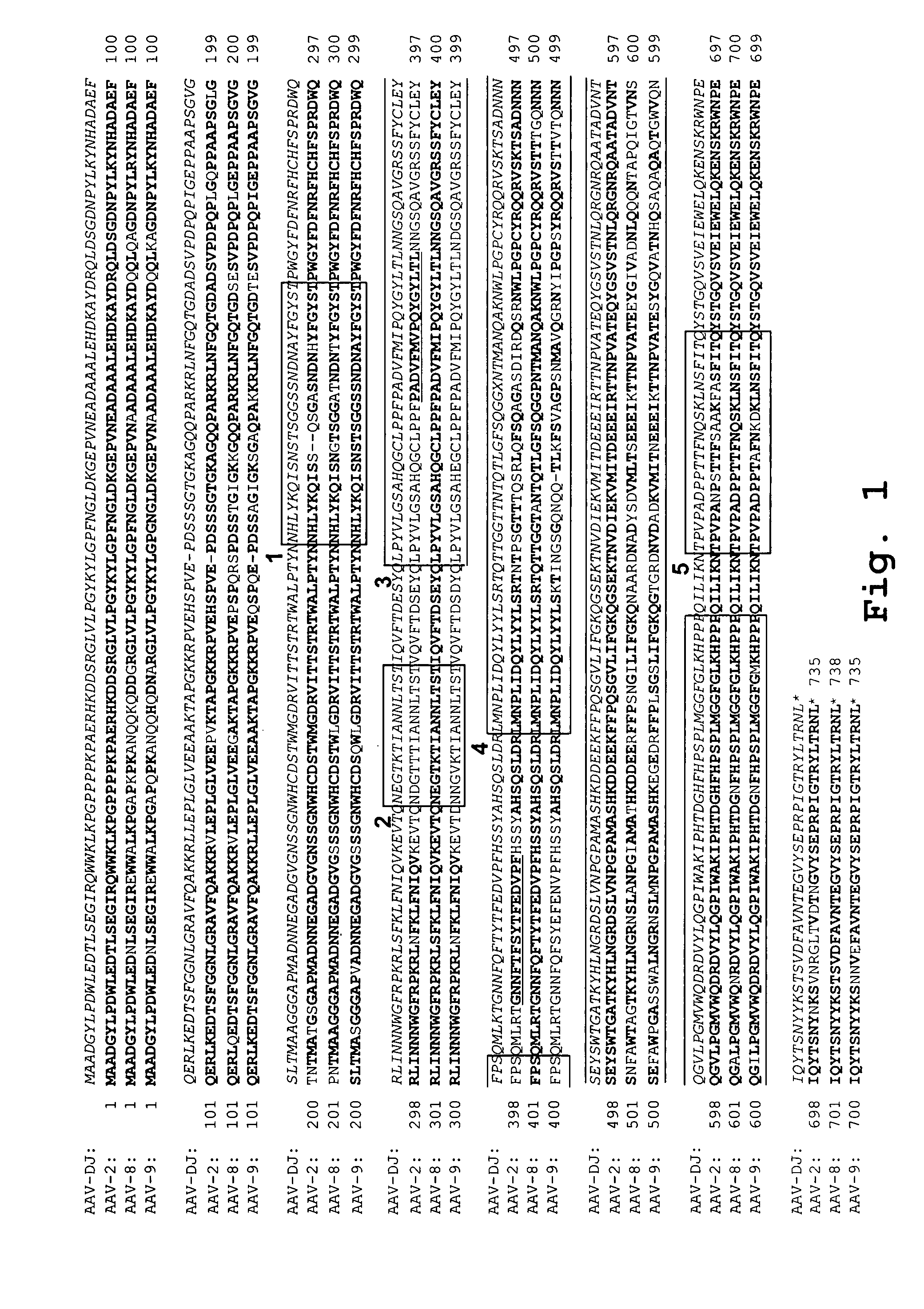
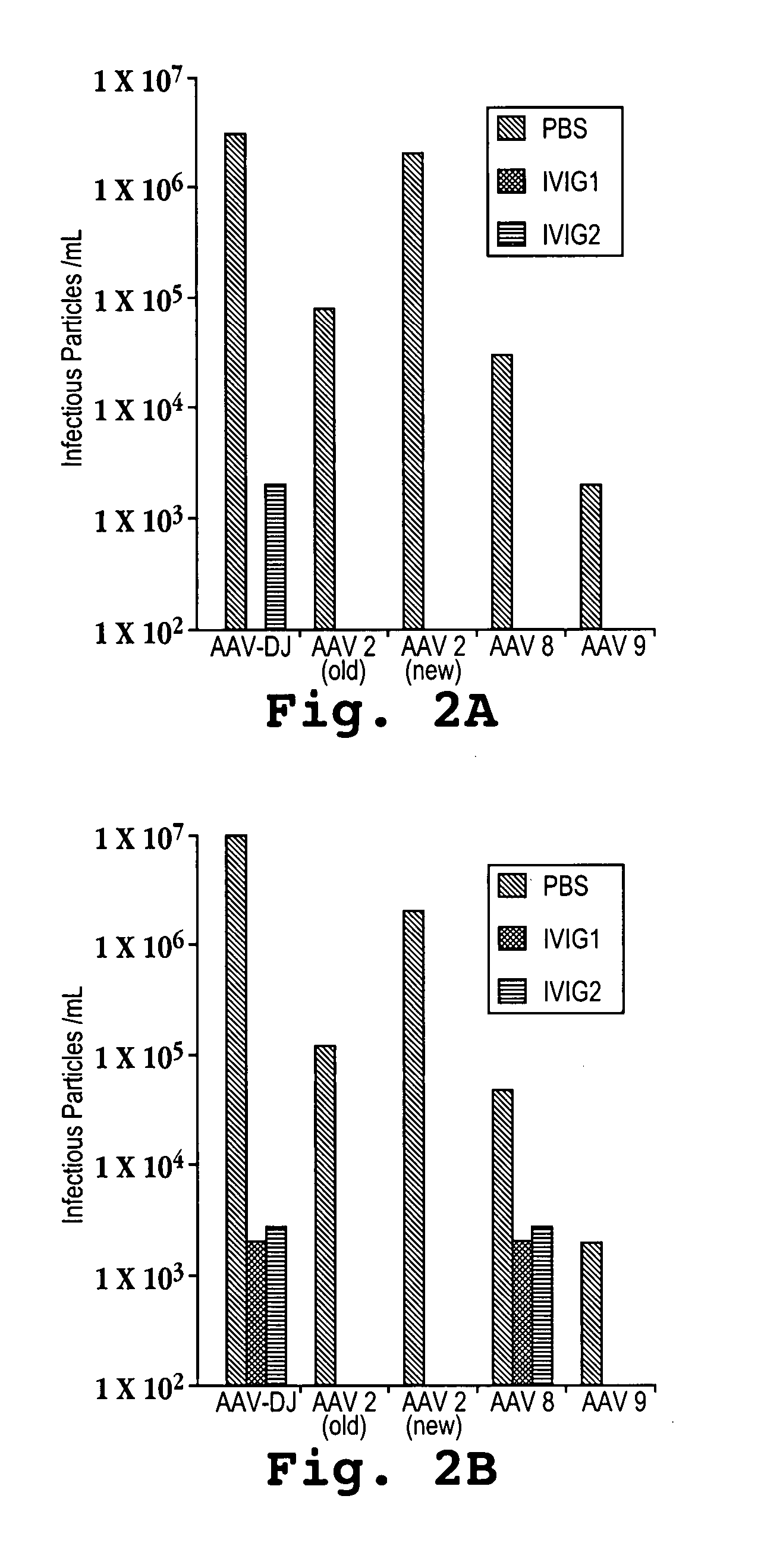
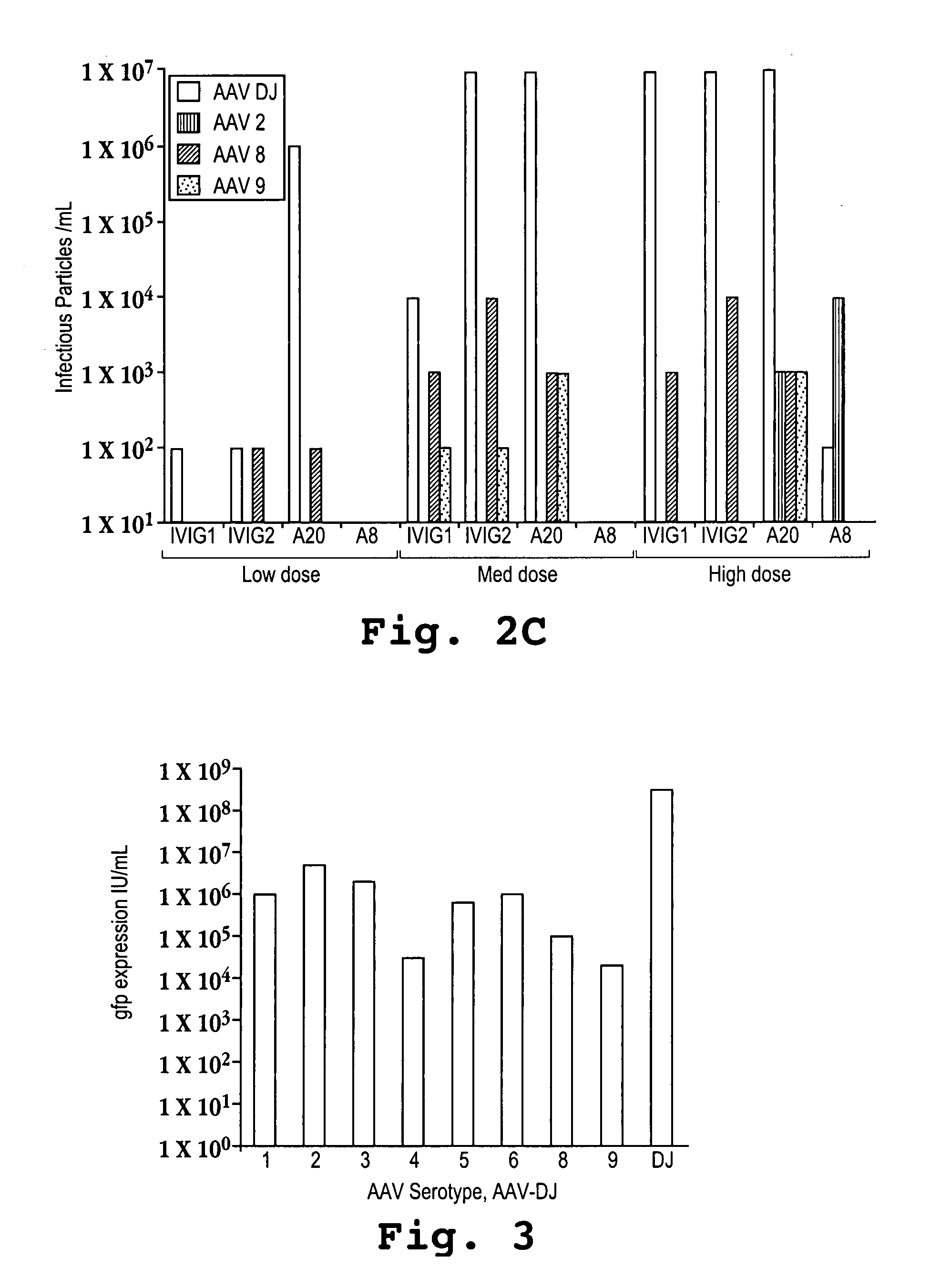
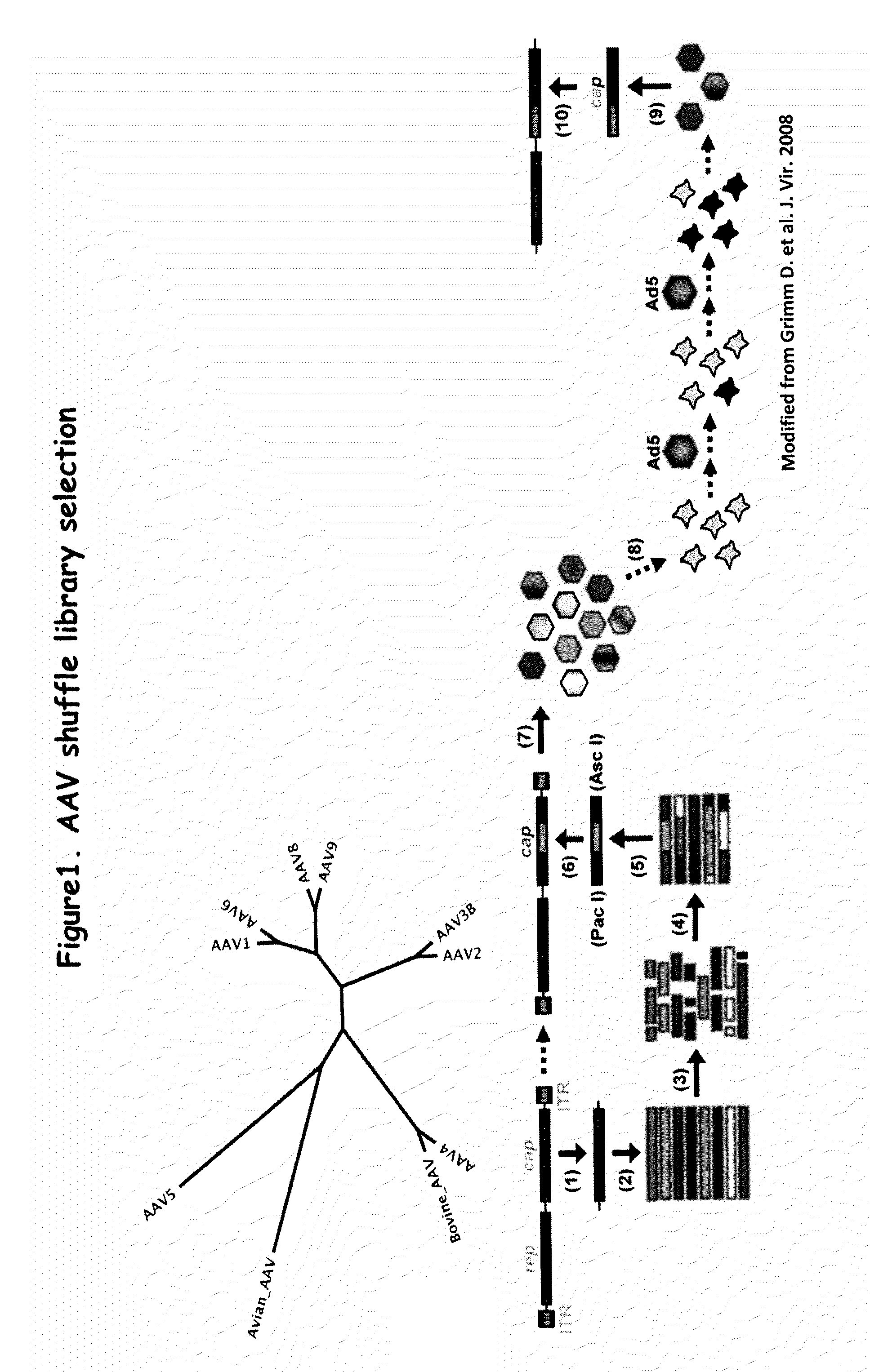
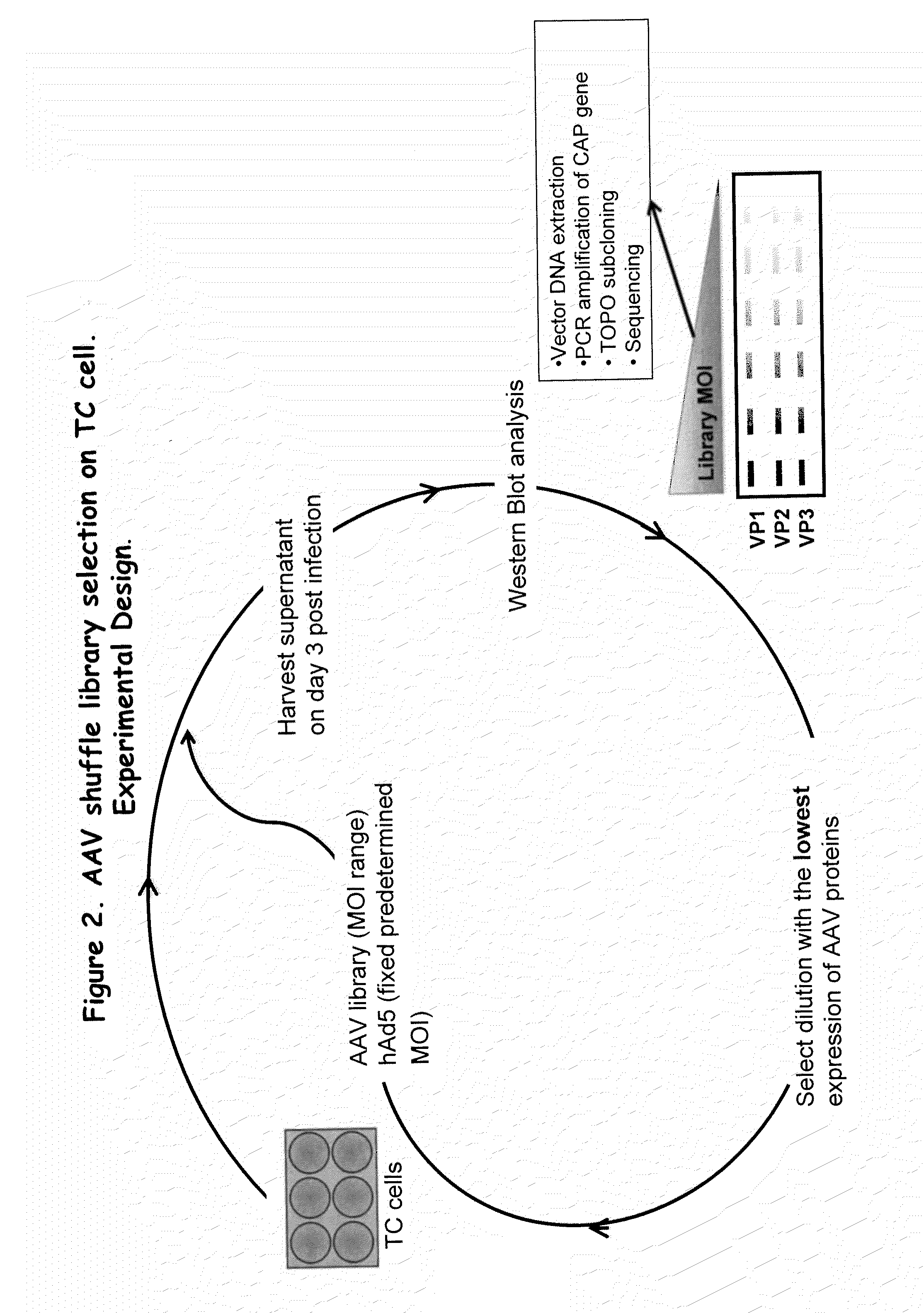
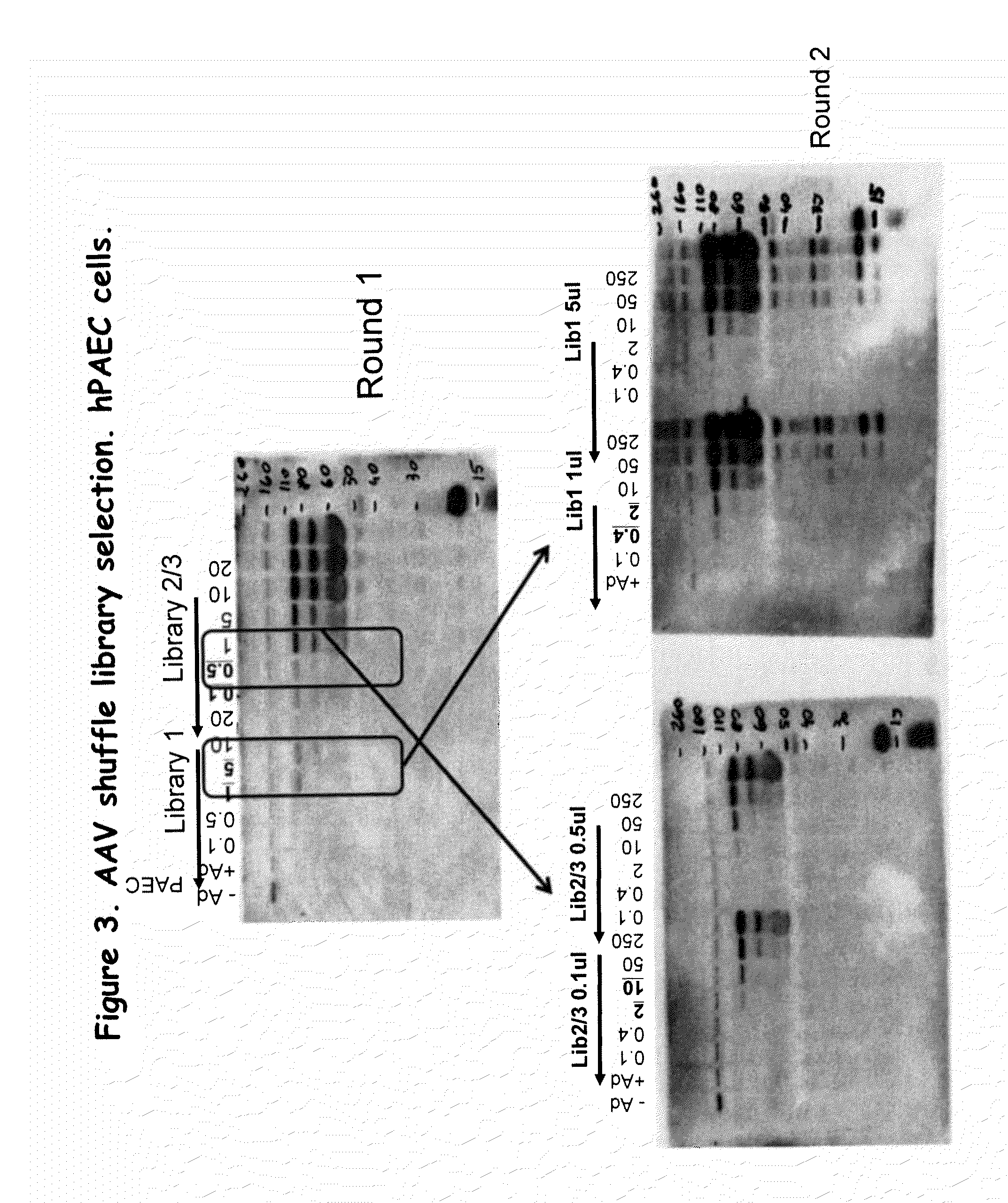
AAV capsid library and AAV capsid proteins
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
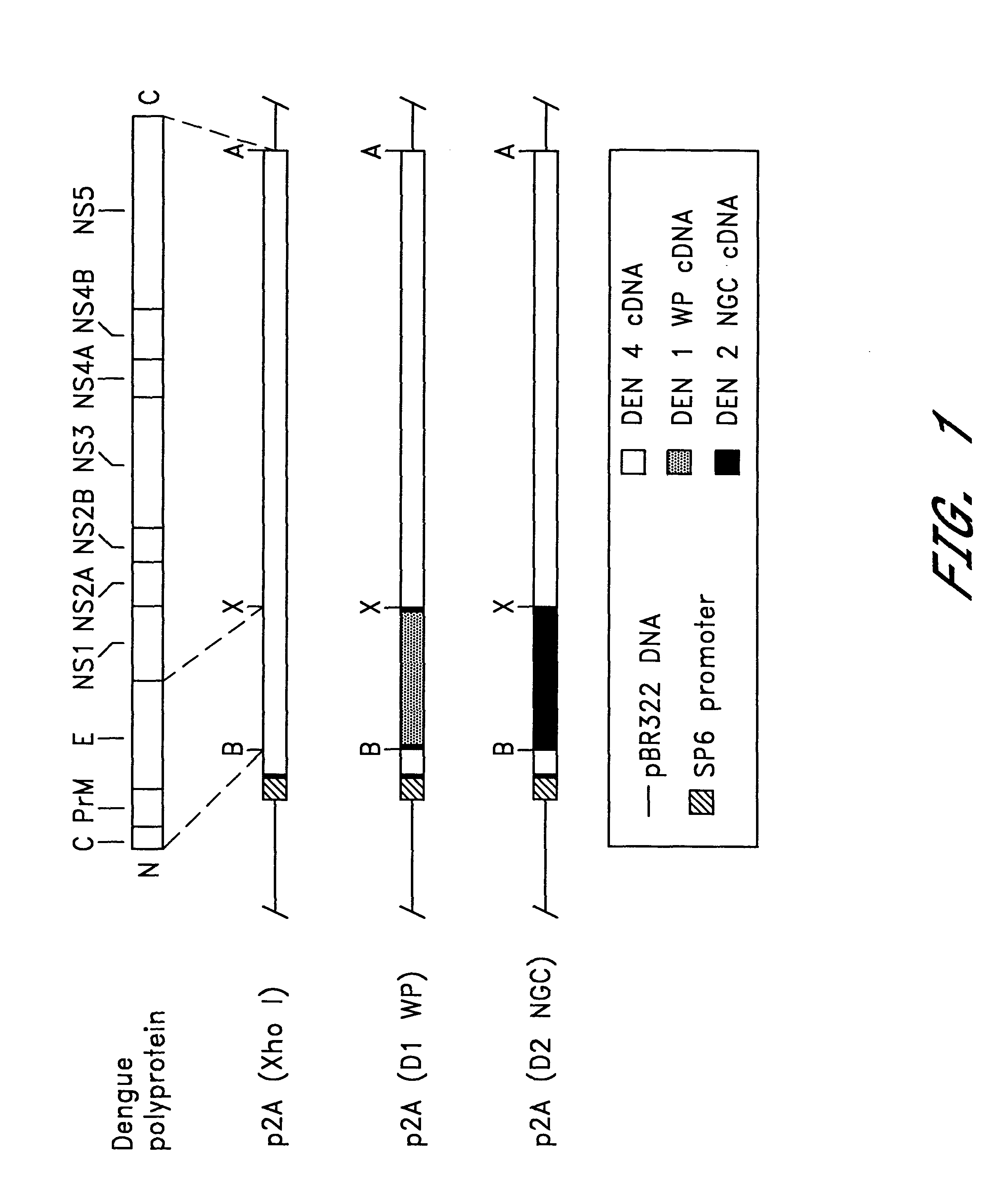
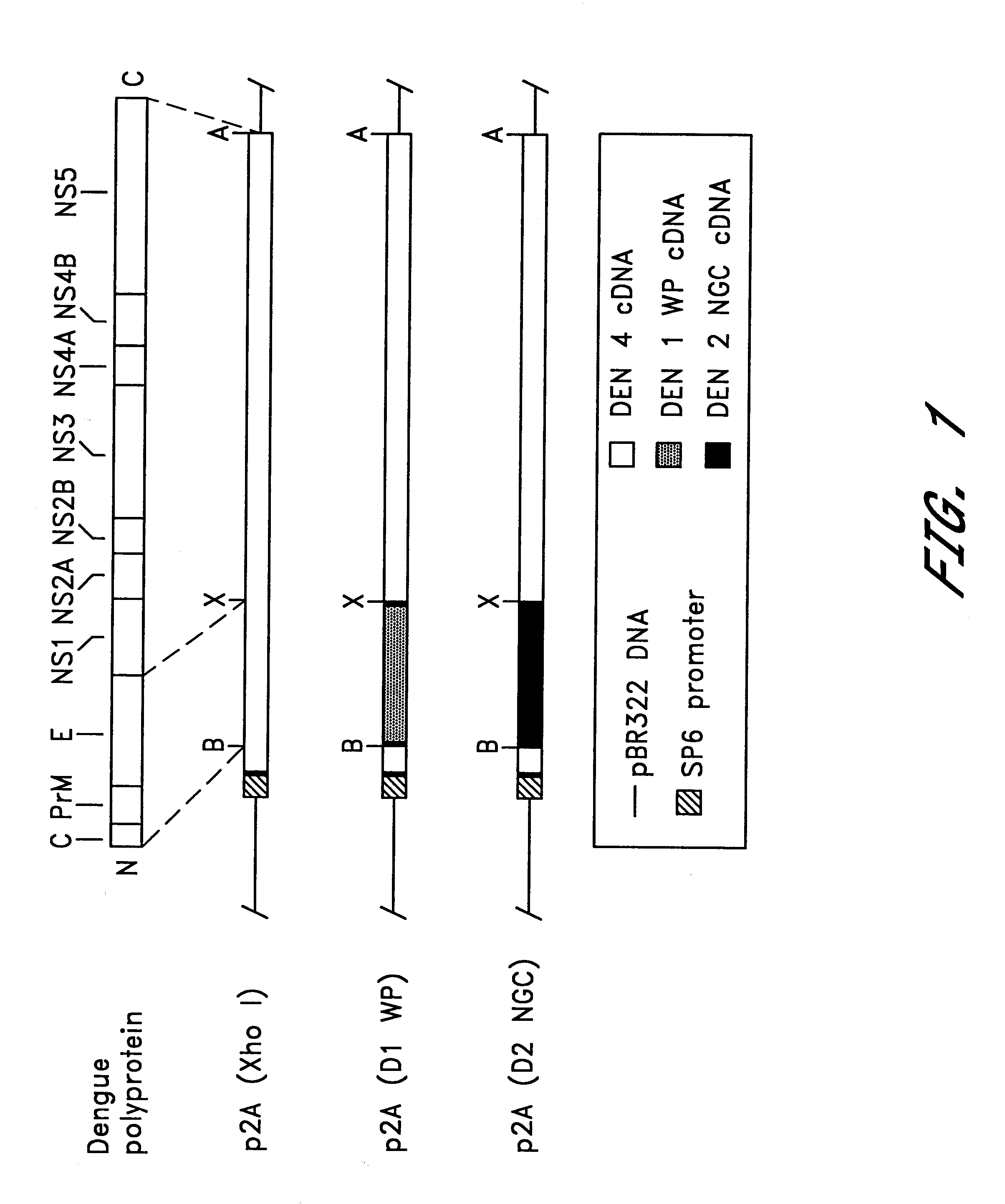
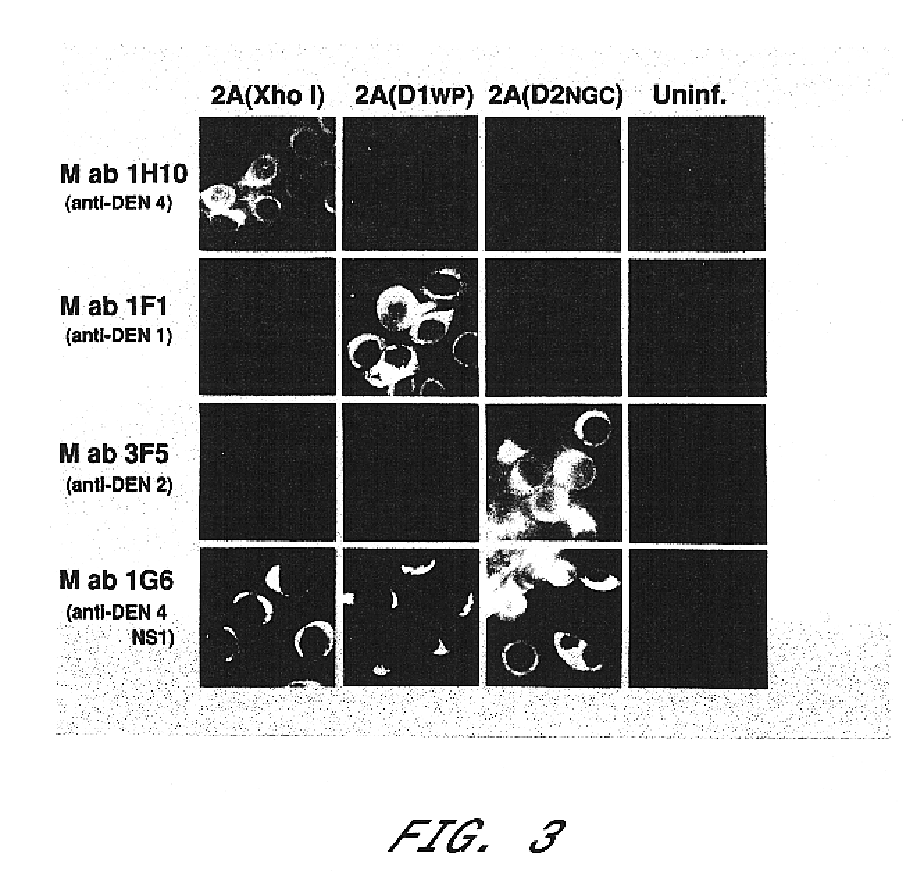
Chimeric and/or growth-restricted flaviviruses
InactiveUS6184024B2BiocideSsRNA viruses positive-senseViral matrix proteinJapanese encephalitis viruses
The invention includes a chimeric virus for use in a vaccine preparation having a genome comprising nucleic acid sequences encoding at least one structural protein from one flavivirus and nucleic acid sequences encoding nonstructural protein from another flavivirus. The genome preferably includes mutations within the viral genome that reduce virus virulence and in a particularly preferred embodiment these vaccines are directed to flaviviruses such as dengue virus, tick-borne encephalitis virus and Japanese encephalitis virus. The invention also includes a baculovirus having a recombinant dengue cDNA sequence which encodes: (1) dengue virus capsid protein, pre-matrix protein, envelope glycoprotein and NS1 and NS2a nonstructural proteins or (2) dengue envelope glycoprotein or (3) dengue non-structural proteins NS1 and NS2a. The invention further includes a baculovirus having a recombinant Japanese B encephalitis virus cDNA sequence which encodes the Japanese B encephalitis virus capsid protein, pre-matrix protein, envelope glycoprotein and non-structural proteins NS1 and NS2a. The invention further includes a vaccine and a method to produce that vaccine.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
AAV capsid proteins for nucleic acid transfer
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Virus vectors for highly efficient transgene delivery
ActiveUS20140336245A1Enhanced transductionPeptide/protein ingredientsGenetic material ingredientsGene transferTransgene
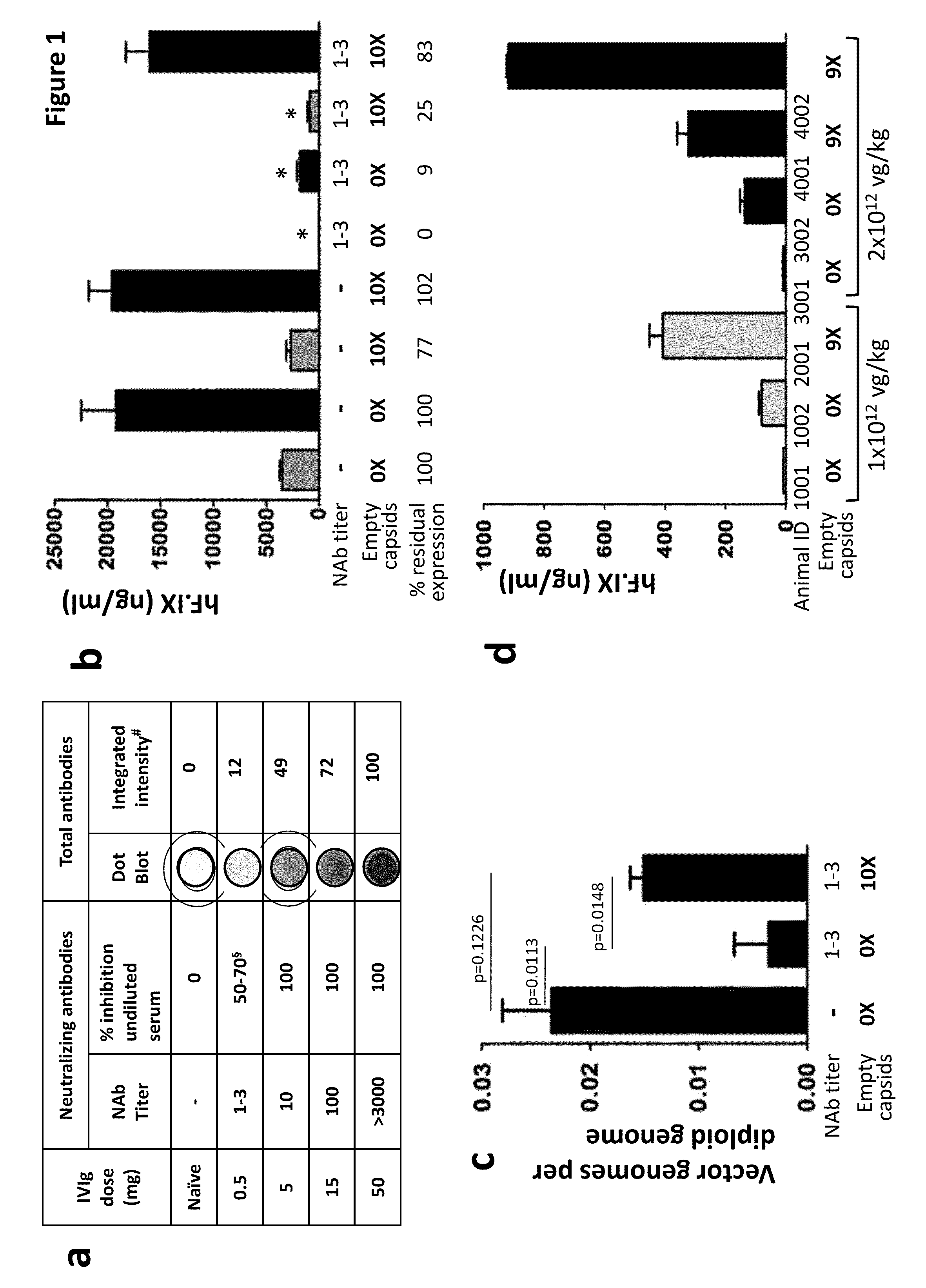
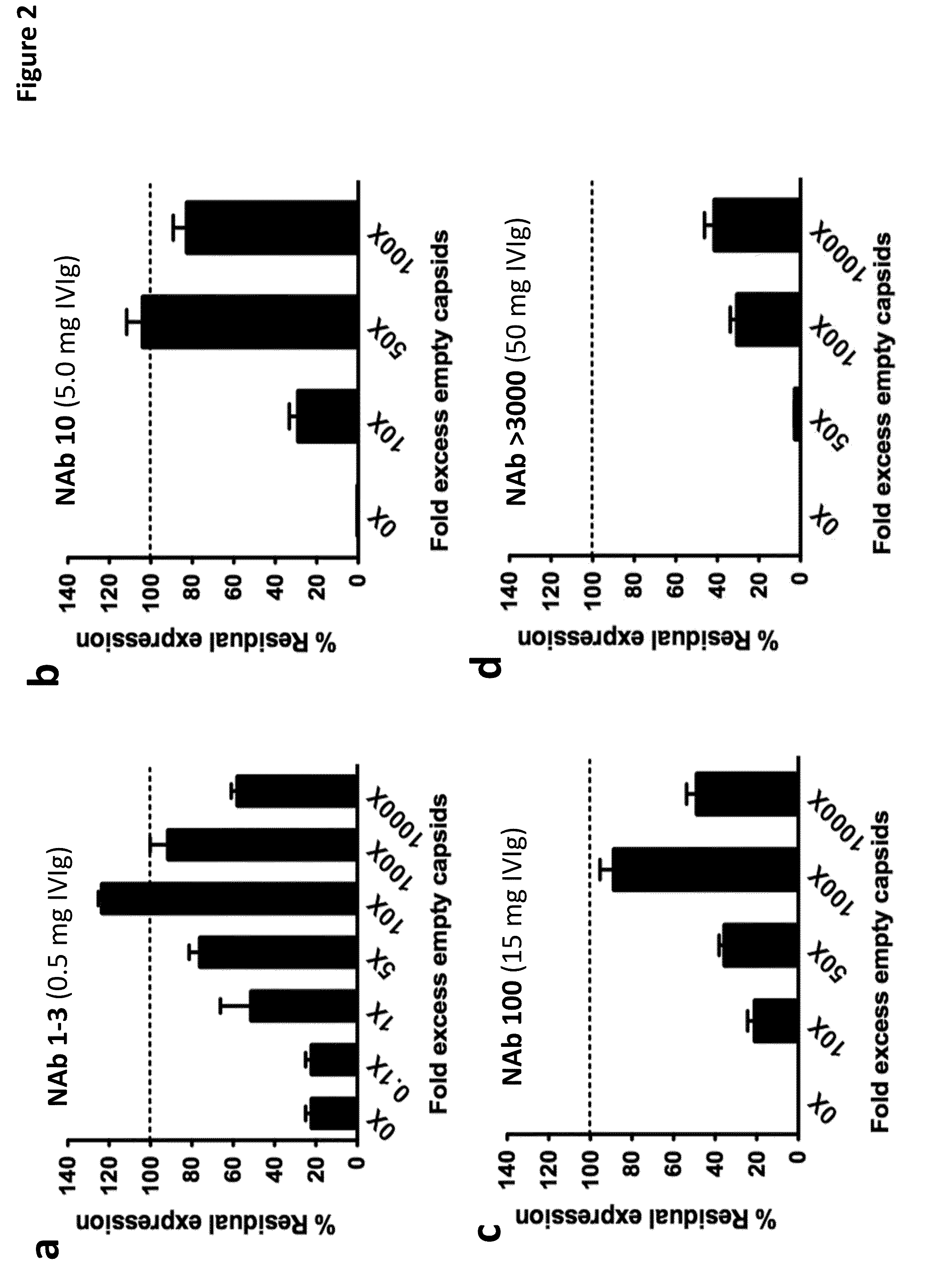
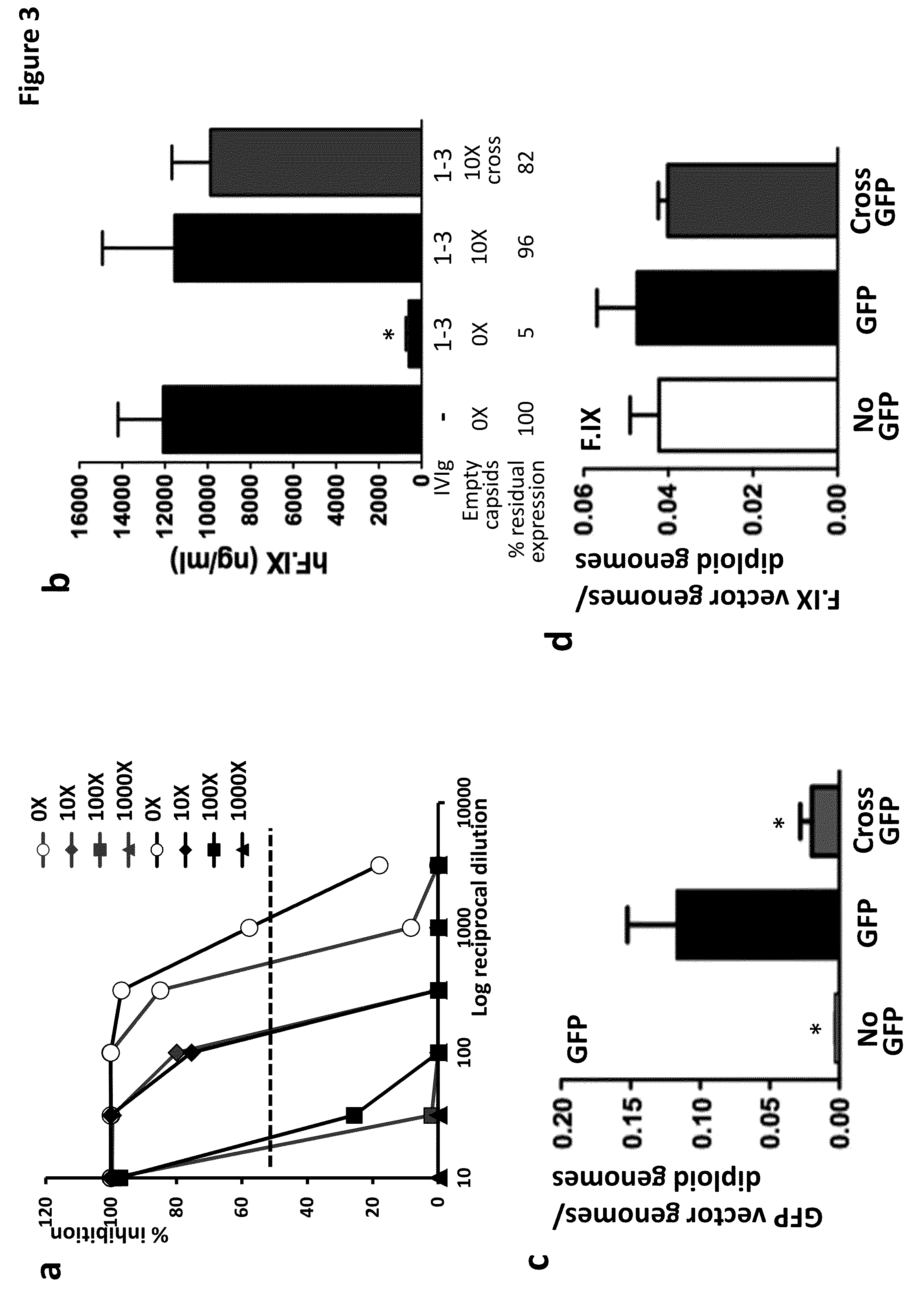
The invention provides viral vector formulations and methods of uses thereof for delivery of transgenes or therapeutic nucleic acids to human subjects. The formulations include a vector and suitable amounts of empty capsids, viral genome-containing capsids, or viral capsid proteins which are optionally chemically or structurally modified and which bind to neutralizing anti-AAV antibodies thereby reducing or preventing antibody-mediated clearance of the vector, but still allowing the genome-containing (therapeutic) vector to transduce target cells and achieve therapeutic gene transfer.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
Aav capsid proteins for nucleic acid transfer
ActiveUS20130059732A1Wide host rangeSufficient infection levelVirus peptidesDirected macromolecular evolutionCapsidBiology
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Influenza vaccines
InactiveUS20080299151A1Less protectionBroad and efficient protective immunitySsRNA viruses negative-senseOrganic active ingredientsHemagglutininMammal
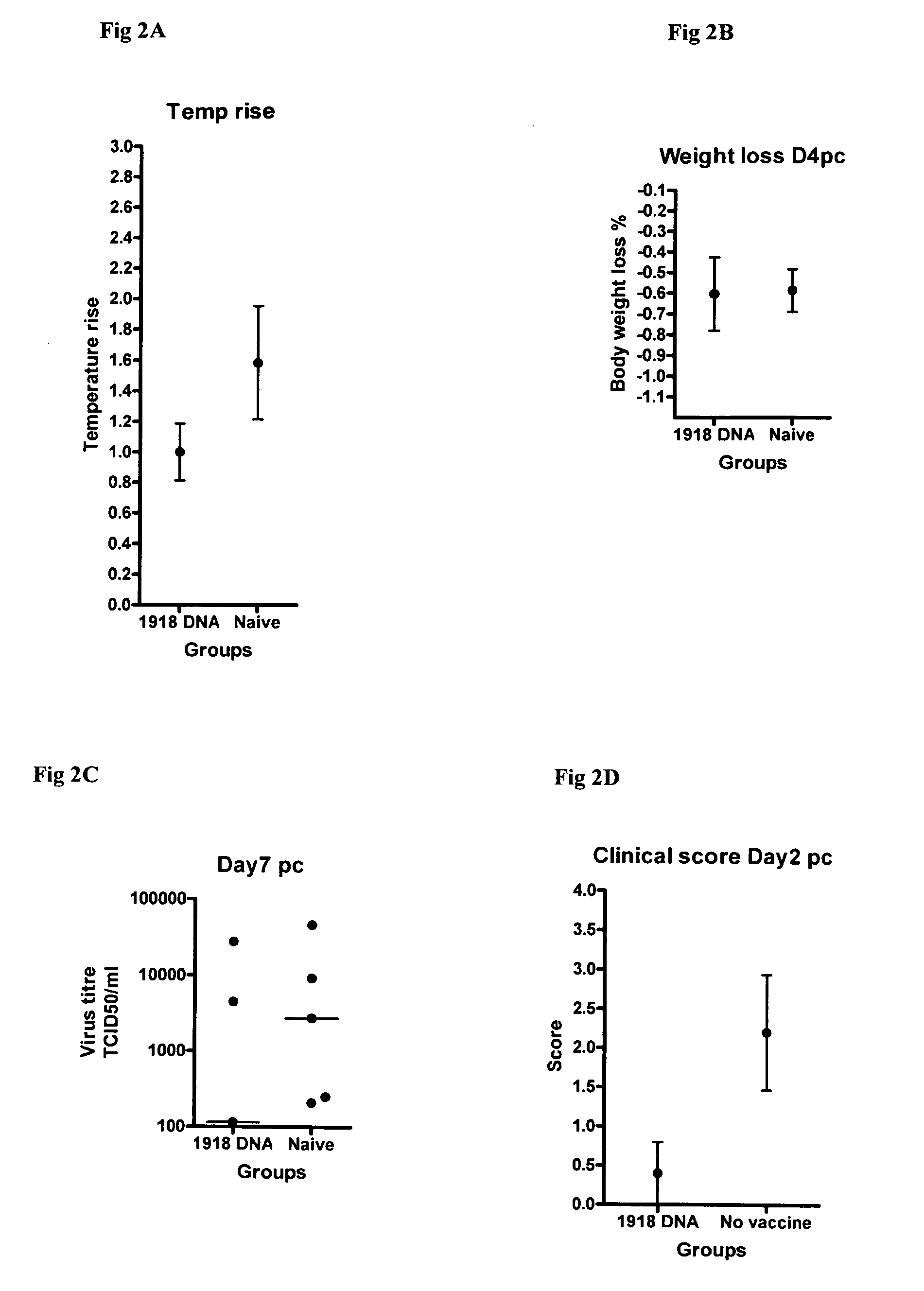
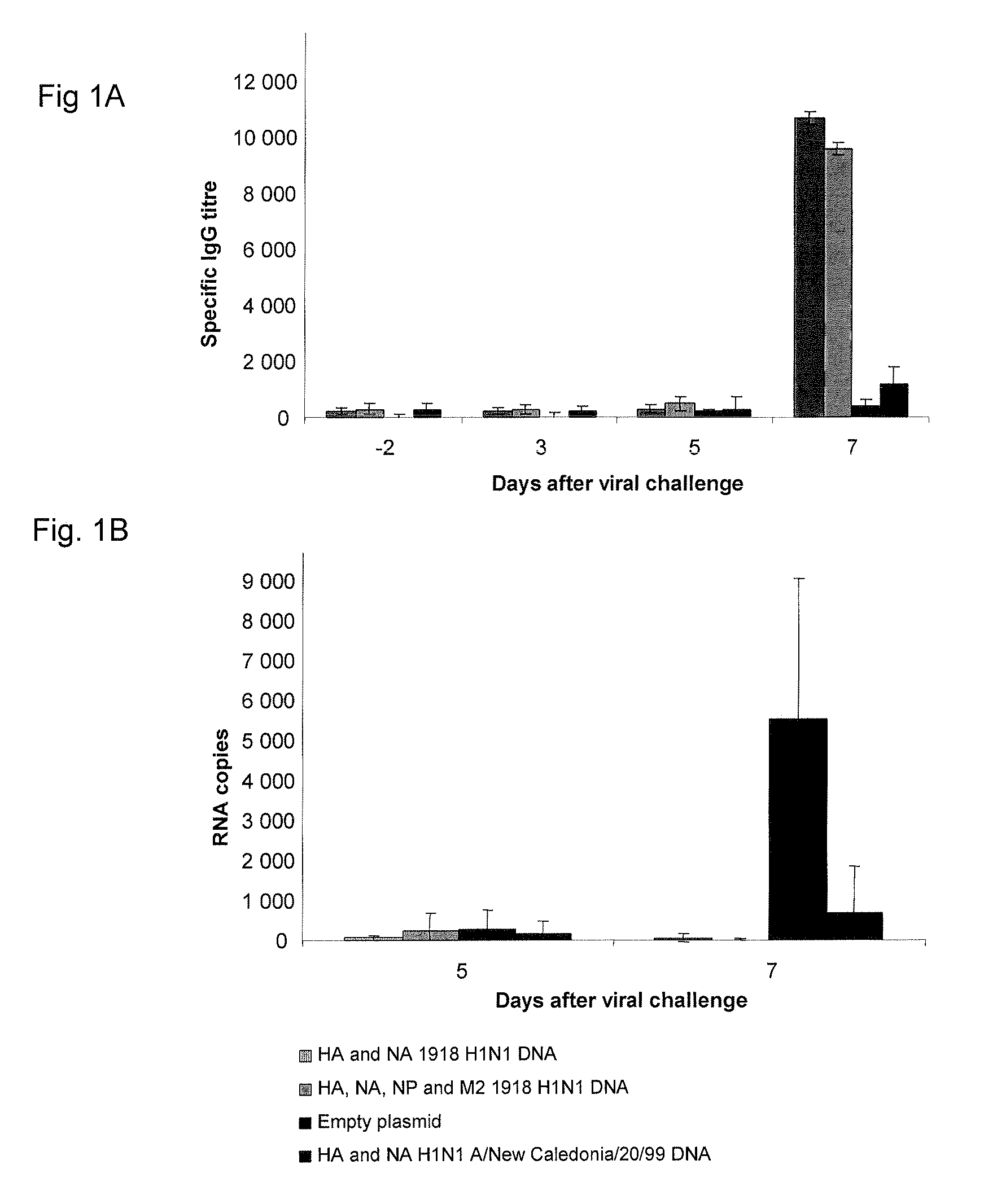
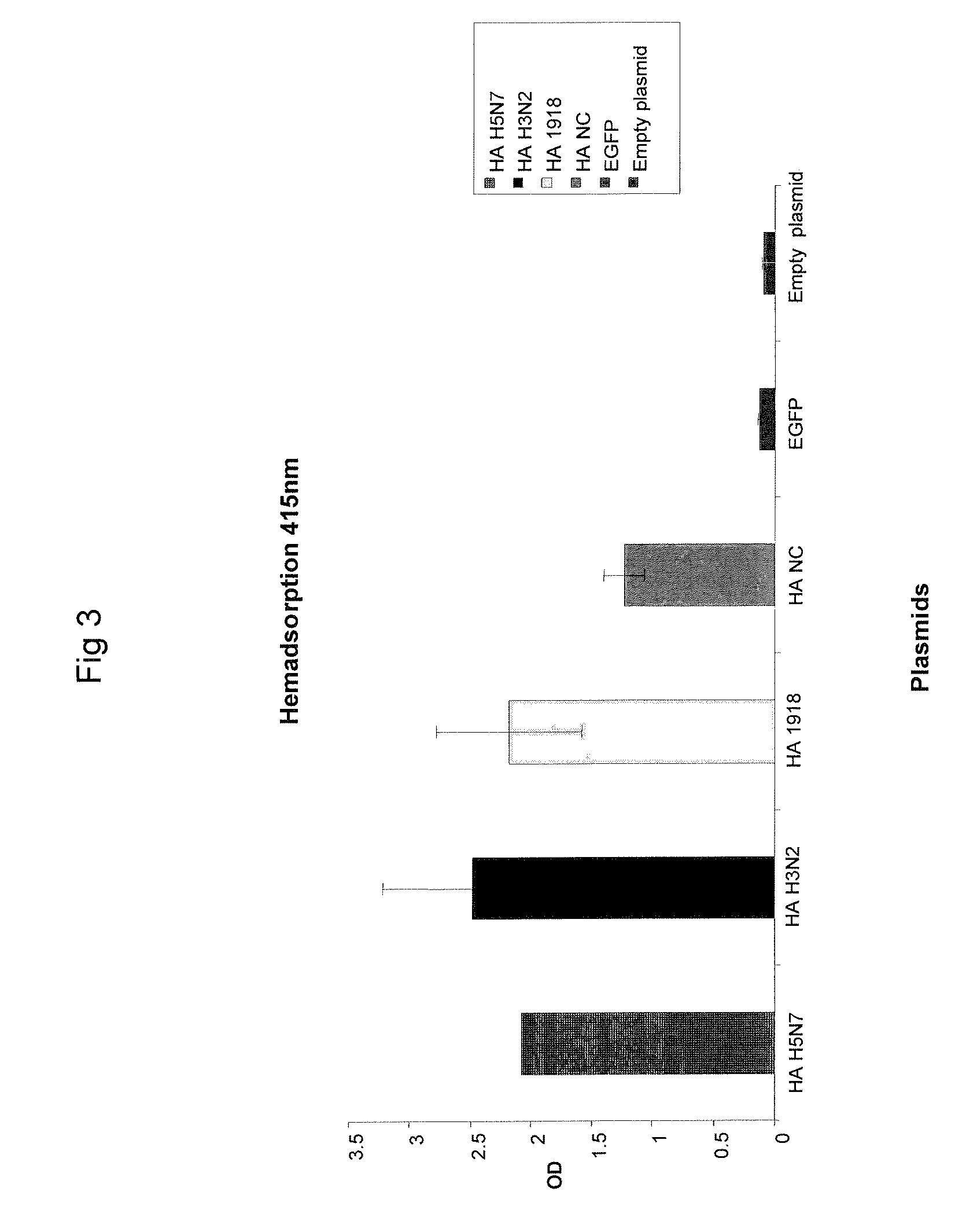
Described herein are vaccines and the use of naked DNA and / or RNA encoding hemagglutinin (HA) from pandemic influenza, e.g., the 1918 H1N1 and / or the 1957 H2N2 and / or the 1968 H3N2 influenza A virus, as a vaccine component against present day and coming H1, H2, H3, H5, N1, N2 containing influenza A infections in humans and swine optionally with the naked DNA and / or RNA encoding Neuraminidase (NA) and / or matrix protein (M) and / or the nucleoprotein (NP) from pandemic influenza virus included. If the vaccine components are used as DNA or RNA vaccines with or without the corresponding protein, the codons can optionally be “humanized” using preferred codons from highly expressed mammalian genes and the administration of this DNA vaccine can be by saline or buffered saline injection of naked DNA or RNA, or injection of DNA plasmid or linear gene expressing DNA fragments coupled to particles. Addition of the matrix protein (M) and / or the nucleoprotein (NP) from the 1918 influenza strain is also disclosed.
Owner:STATENS SERUM INST
Aav capsid proteins for nucleic acid transfer
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Chimeric and/or growth-restricted flaviviruses
InactiveUS6676936B1BiocideSsRNA viruses positive-senseVirulent characteristicsJapanese B Encephalitis Virus
The invention includes a chimeric virus for use in a vaccine preparation having a genome comprising nucleic acid sequences encoding at least one structural protein from one flavivirus and nucleic acid sequences encoding nonstructural protein from another flavivirus. The genome preferably includes mutations within the viral genome that reduce virus virulence and in a particularly preferred embodiment these vaccines are directed to flaviviruses such as dengue virus, tick-borne encephalitis virus and Japanese encephalitis virus. The invention also includes a baculovirus having a recombinant dengue cDNA sequence which encodes: (1) dengue virus capsid protein, pre-matrix protein, envelope glycoprotein and NS1 and NS2a nonstructural proteins or (2) dengue envelope glycoprotein or (3) dengue non-structural proteins NS1 and NS2a. The invention further includes a baculovirus having a recombinant Japanese B encephalitis virus cDNA sequence which encodes the Japanese B encephalitis virus capsid protein, pre-matrix protein, envelope glycoprotein and non-structural proteins NS1 and NS2a. The invention further includes a vaccine and a method to produce that vaccine.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
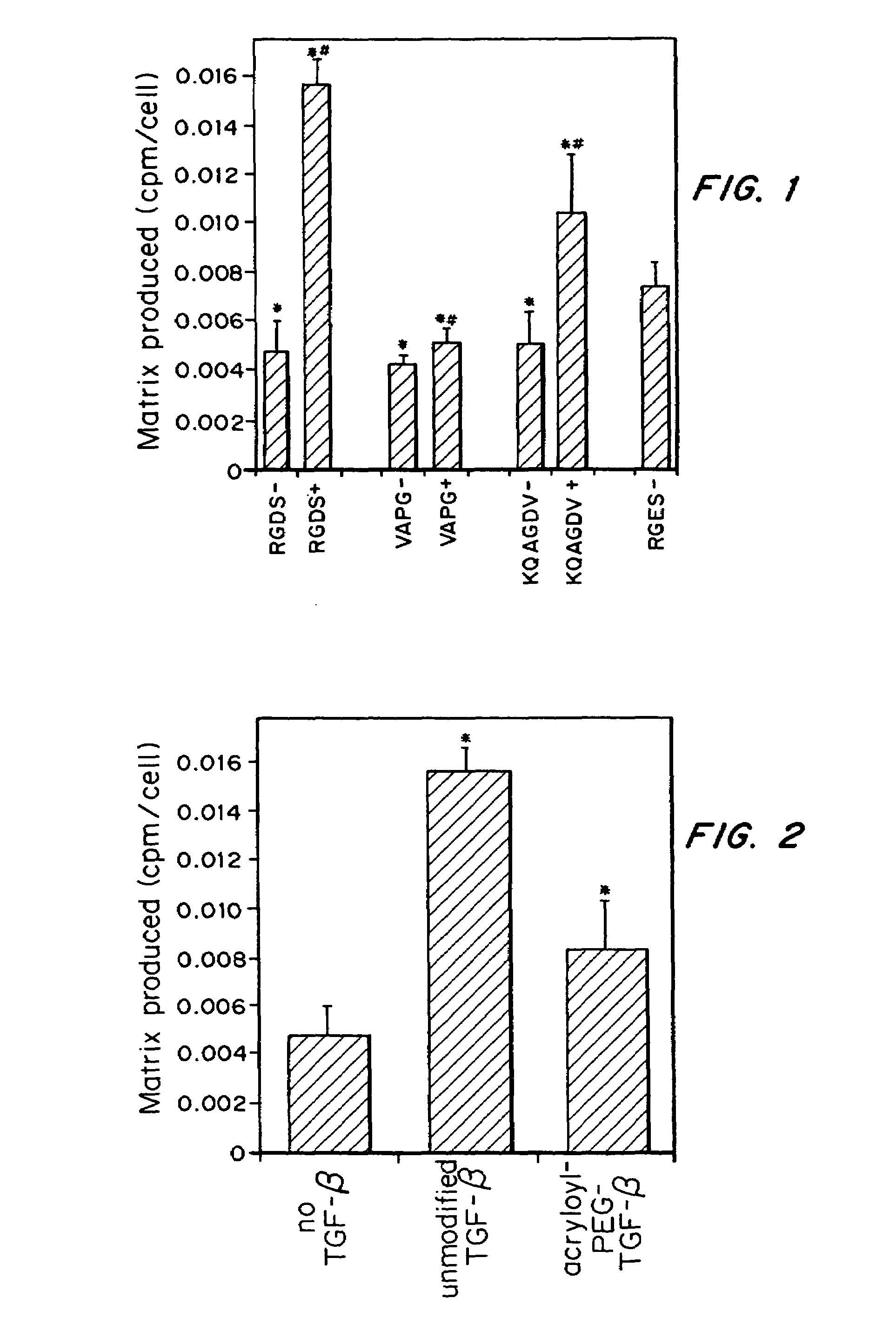
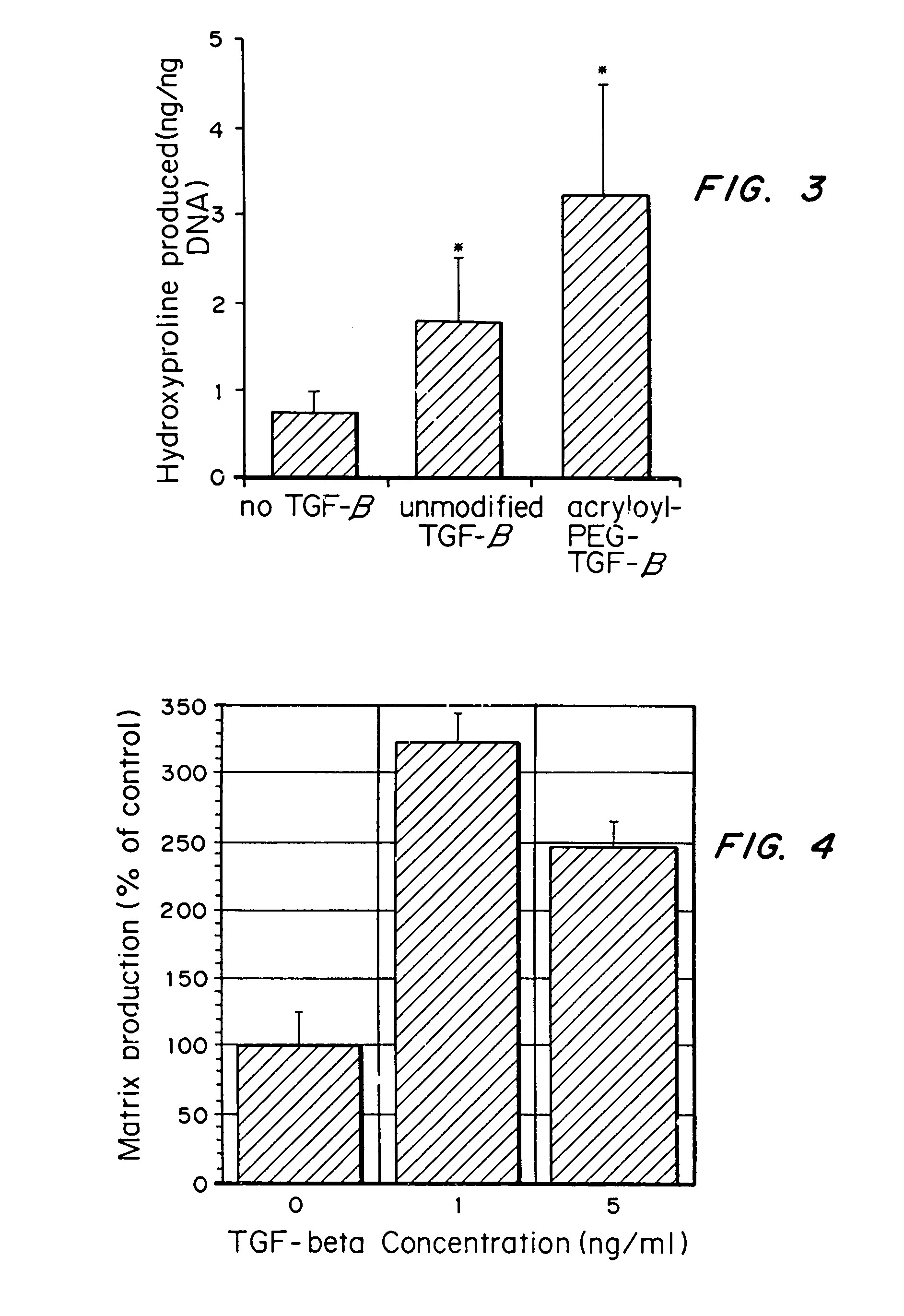
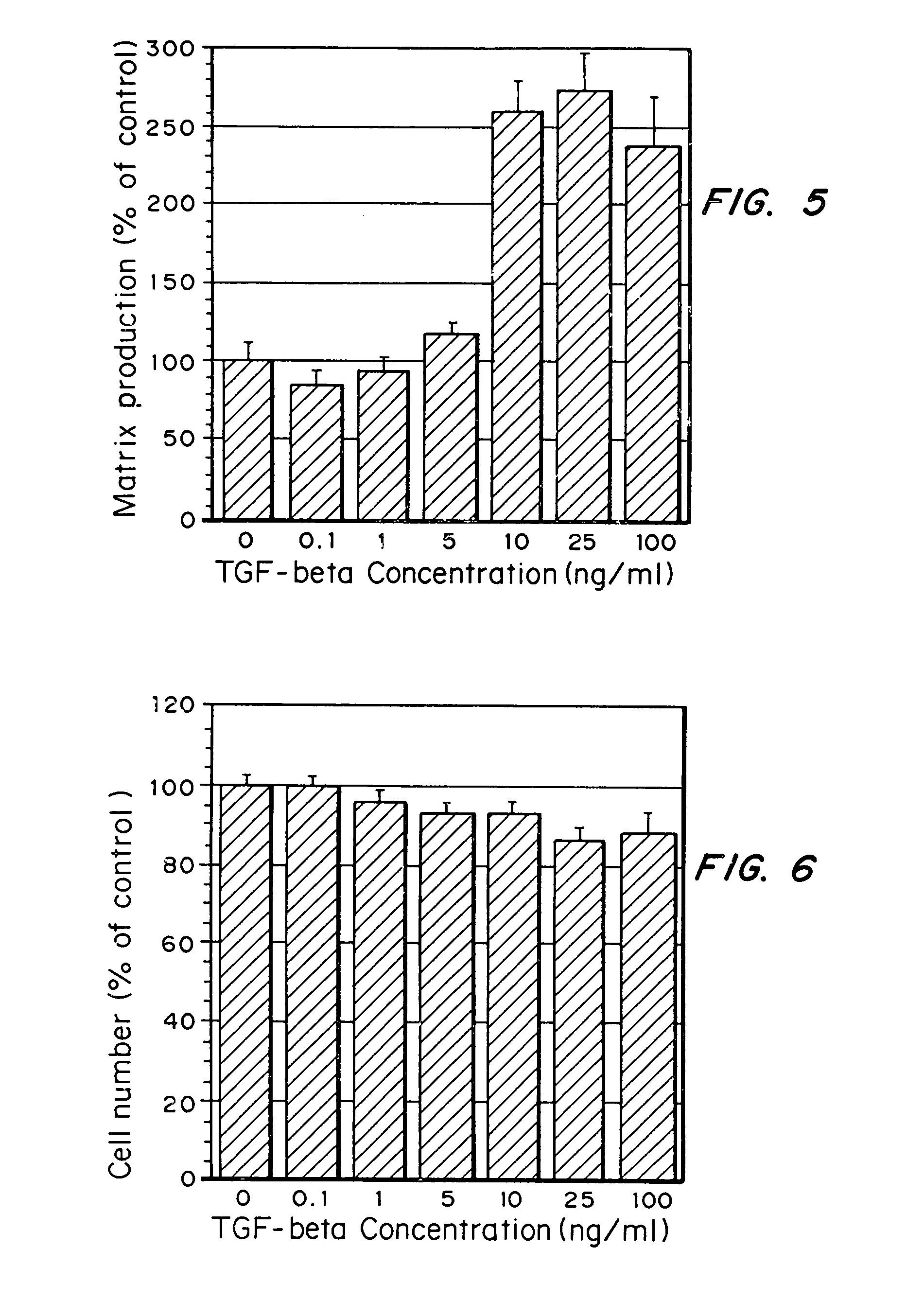
Tissue engineering scaffolds promoting matrix protein production
InactiveUS7635592B2Increase ECM productionIncrease gene expressionSkeletal disorderVertebrate cellsCell adhesionCell-Extracellular Matrix
Matrix-enhancing molecules, such as TGF-β, are conjugated to or immobilized on scaffolds to increase ECM production by cells for tissue engineering, tissue regeneration and wound healing applications. The matrix-enhancing molecule is conjugated to a tether, such as polyethylene glycol (PEG) monoacrylate, for attachment to a tissue engineering or cell growth scaffold. The matrix-enhancing molecule retains activity after attachment to the scaffold, and causes cells growing in or on the scaffold to increase extracellular matrix (ECM) production, without substantially increasing proliferation of the cells, even when the scaffold additionally contains cell adhesion ligands. The increased ECM produced by the cells aids in maintaining the integrity of the scaffold, particularly when the scaffold is degradable, either by hydrolysis or by enzymatic degradation.
Owner:RICE UNIV
Coxsackievirus B4 expression vectors and uses thereof
InactiveUS7270997B2Prevents and inhibits disease progressionSsRNA viruses positive-senseVirus peptidesHeterologousCoxsackieviruses B
Disclosed is a recombinant attenuated coxsackievirus B4 virion which is engineered to contain a heterologous nucleic acid within the open reading frame of its genome, wherein the heterologous nucleic acid encodes a heterologous polypeptide which is expressed by the virion. Specific examples of attenuated coxsackievirus B4 virions suitable for use in the present invention are CB4-P and JVB. In one embodiment the heterologous nucleic acid is inserted into the P1 region of the genome such that the heterologous polypeptide is expressed as a fusion of a viral capsid protein. Methods of use of the recombinant attenuated coxsackievirus B4 virion include inducing an immune response in an individual to the heterologous polypeptide contained therein.
Owner:RAMSINGH ARLENE I
Engineered extracellular matrix preparation method
ActiveCN1800372AWith strengthRepair deep skin defectsAnimal cellsProsthesisFiberCell-Extracellular Matrix
The invention relates to a method for preparing for organization project cell epimatrix, which adopts animal stromatin and desmocyte as raw material. It comprises: preparing for the culture liquid, extracting the stromatin, preparing for the cell-stromatin culture material, preparing for the cell epimatrix, dispelling the cell by freezing, and sterilization sanitizing packaging material and so on.
Owner:SHAANXI BOYU REGENERATIVE MEDICINE CO LTD
Novel vaccines against multiple subtypes of influenza virus
ActiveUS20090169505A1Elicit immune responseSsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininMammal
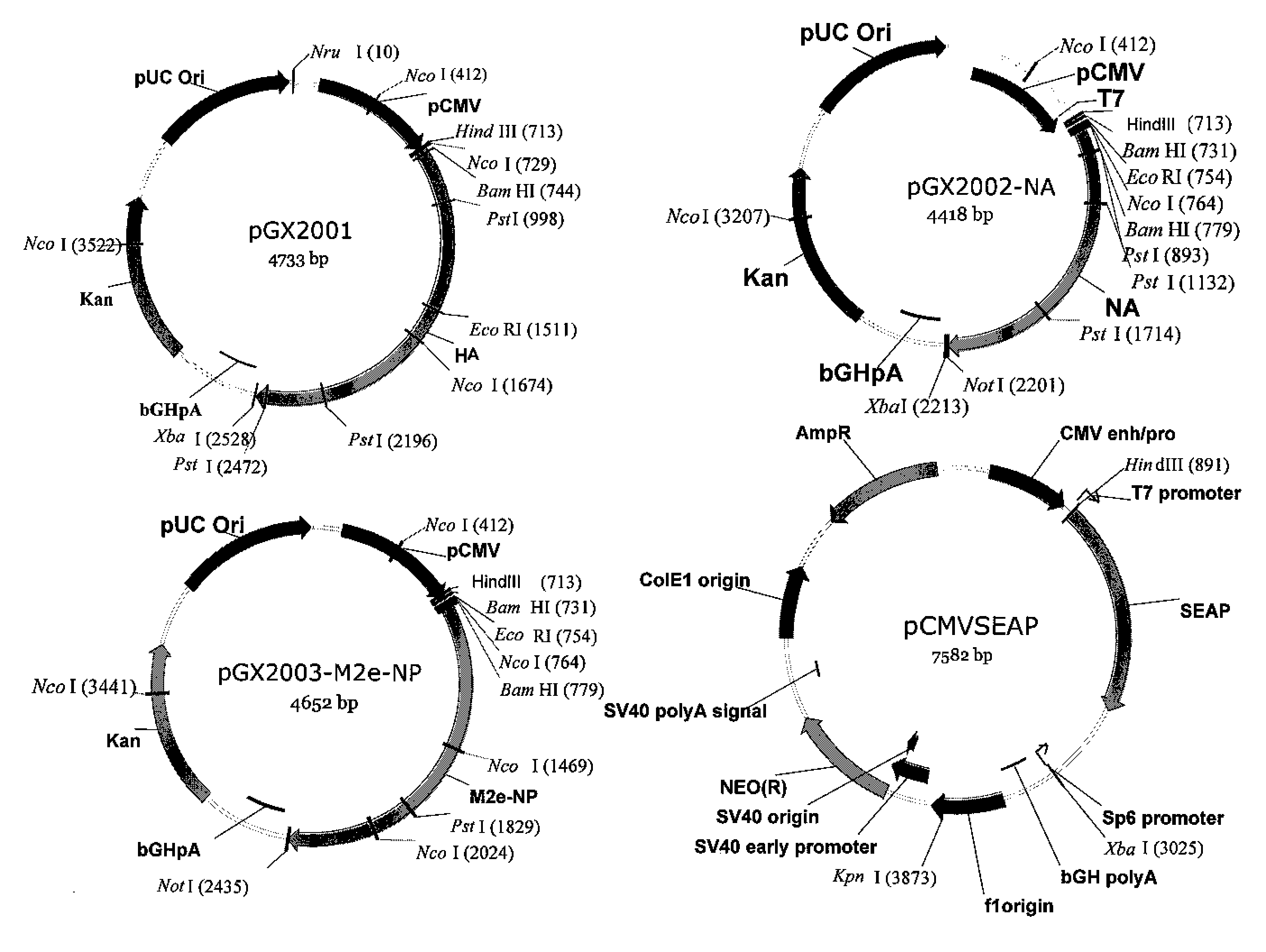
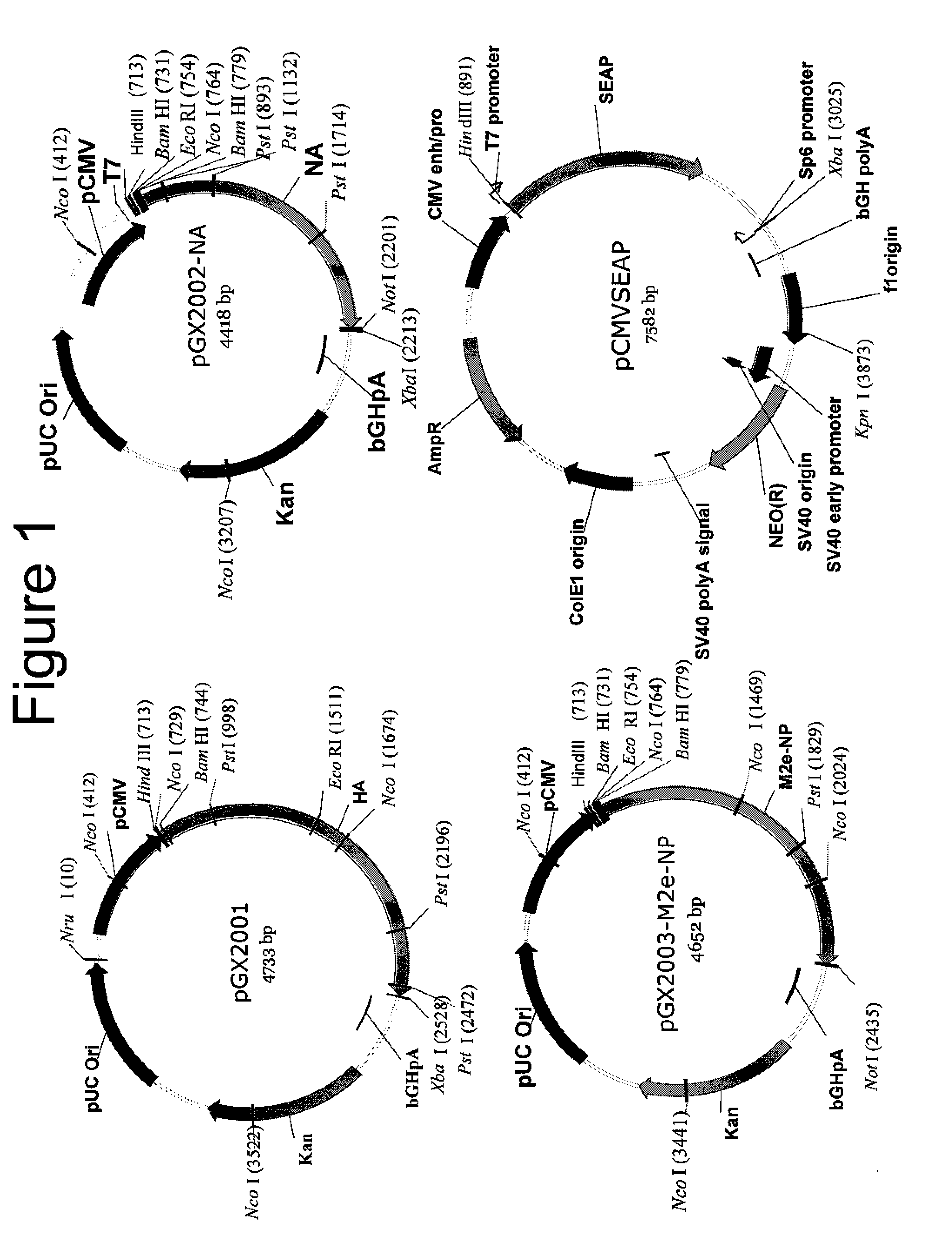
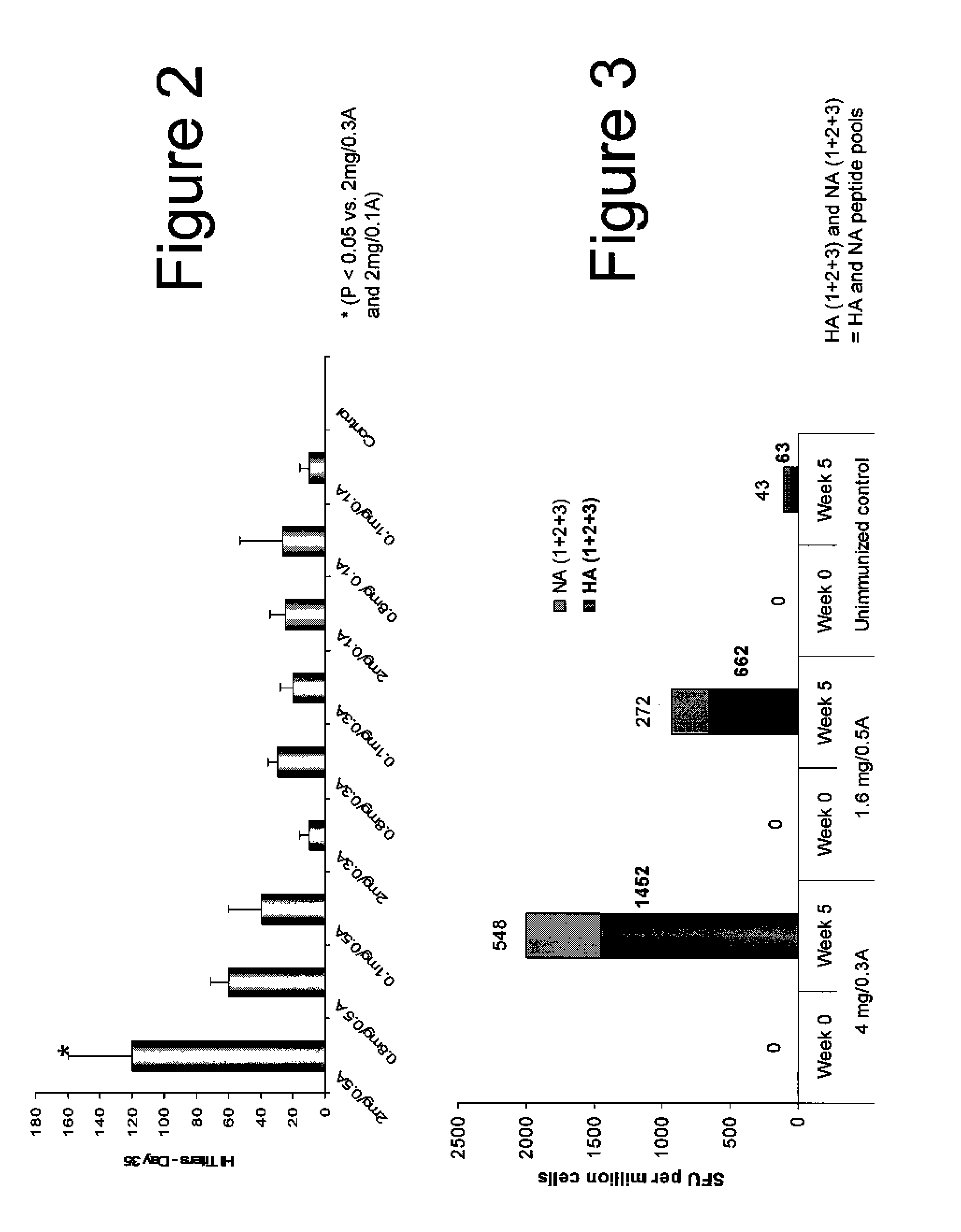
An aspect of the present invention is directed towards DNA plasmid vaccines capable of generating in a mammal an immune response against a plurality of influenza virus subtypes, comprising a DNA plasmid and a pharmaceutically acceptable excipient. The DNA plasmid is capable of expressing a consensus influenza antigen in a cell of the mammal in a quantity effective to elicit an immune response in the mammal, wherein the consensus influenza antigen comprises consensus hemagglutinin (HA), neuraminidase (NA), matrix protein, nucleoprotein, M2 ectodomain-nucleo-protein (M2e-NP), or a combination thereof. Preferably the consensus influenza antigen comprises HA, NA, M2e-NP, or a combination thereof. The DNA plasmid comprises a promoter operably linked to a coding sequence that encodes the consensus influenza antigen. Additionally, an aspect of the present invention includes methods of eliciting an immune response against a plurality of influenza virus subtypes in a mammal using the DNA plasmid vaccines provided.
Owner:VGX PHARMA +1
Peptides Modified with Triterpenoids and Small Organic Molecules: Synthesis and use in Cosmeceuticals
ActiveUS20100034758A1Reduce wrinklesReduce appearanceCosmetic preparationsPeptide/protein ingredientsWrinkle skinAdditive ingredient
The present invention relates to the Synthesis of Triterpenoid peptides and mechanism of action for Anti ageing and skin care. The present invention is directed towards anti-aging skin care compositions comprising peptides which are made by linking herbal actives to a pentapeptide for enhanced anti ageing activity by regenerating the dermal matrix. In detail, the present invention relates to the synthesis of Triterpenoid peptides, providing an enhanced and synergistic activity for reducing the consequences of ageing such as appearance of fine expression lines and wrinkles on the skin by cosmetic modes of application. The Triterpenoid peptides of the present invention with its novel dual action mode can be used for skin ageing & collagen insufficiency. Its Triterpenoid group acts by preventing oxidation and excess activity of serine proteases like elastase and collagenase that result in wrinkling of skin. With added peptides which boost the collagen and other matrix protein, Triterpenoid peptides provide a complete protection against pre mature ageing and functions as a best anti ageing ingredient.
Owner:SAMI LABS LTD
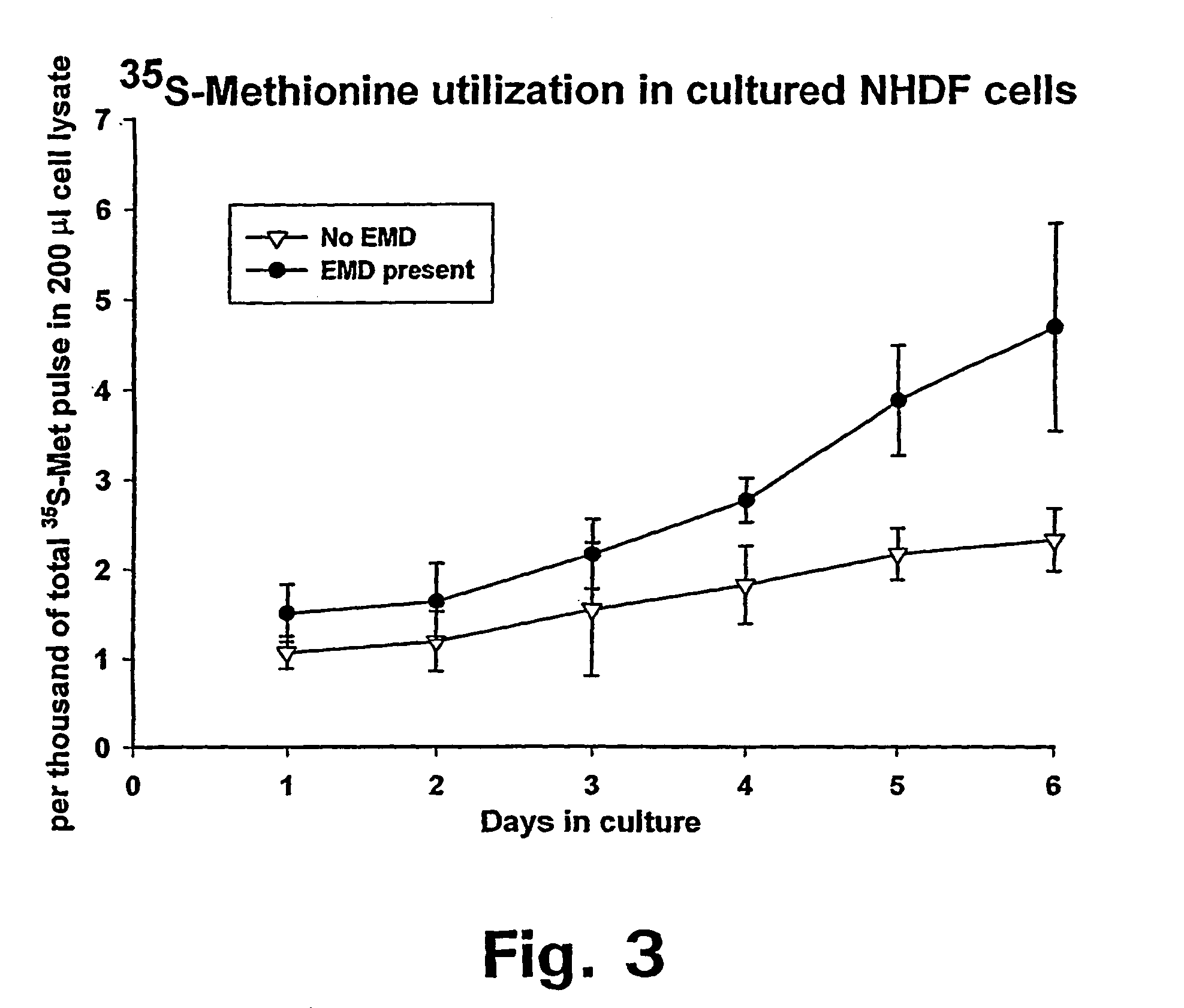
Matrix protein compositions for guided connective tissue growth
InactiveUS7033611B2Relieve painHigh tensile strengthPeptide/protein ingredientsUnknown materialsAbnormal tissue growthProtein composition
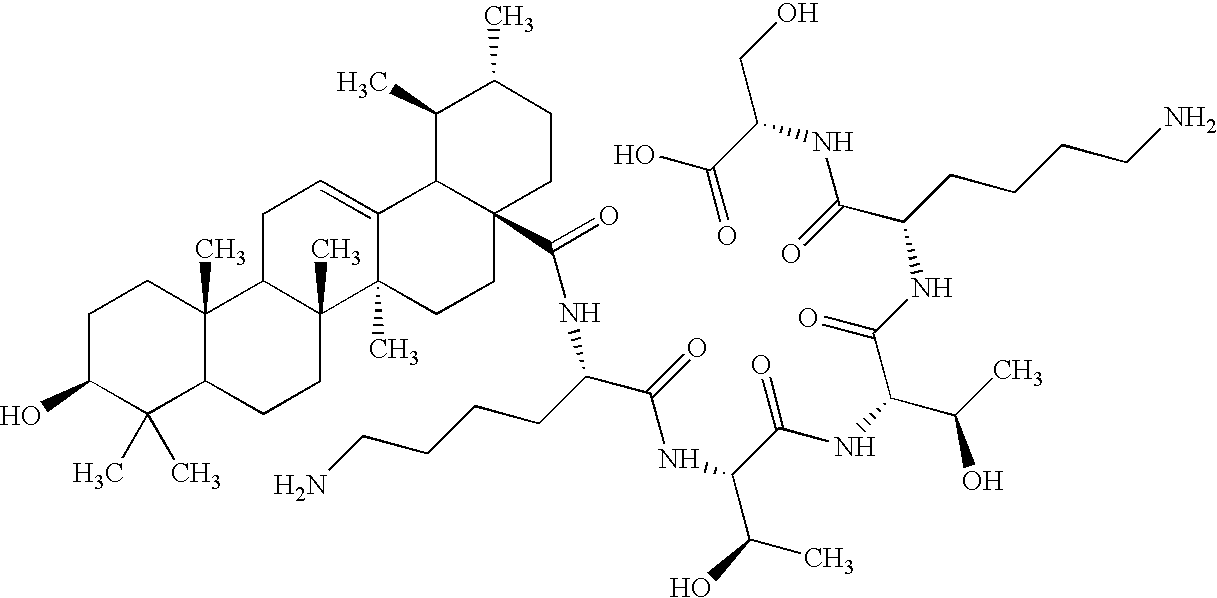
The present invention relates to the use of enamel matrix, enamel matrix derivatives and / or enamel matrix proteins as therapeutic and / or cosmetic agents. These substances are used for the manufacture of a pharmaceutical and / or cosmetic composition for actively inducing, guiding and / or stimulating connective tissue growth and thus to prevent connective tissue scarring and / or contraction in a wound cavity and / or tissue defect that is characterized by a substantial loss of tissue. The invention comprises, in particular, the use of active enamel substances for guided connective soft tissue growth and resistance to contraction in deep cavity shaped wounds following loss or removal of significant volumes of tissue, such as e.g., after surgical removal of a tumor and especially in combination with radiation therapy.
Owner:INSTITUT STRAUMANN AG +1
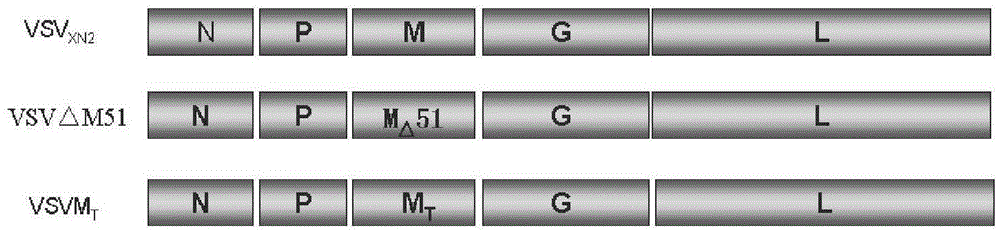
Building and application of M protein three-amino acid site-mutated vesicular stomatitis virus (VSV) carrier for pigs
InactiveCN105087645AComprehensive immune responseReduce pathogenicityAntiviralsAntibody medical ingredientsGlycineArginine
The invention provides building and application of an M protein three-amino acid site-mutated vesicular stomatitis virus (VSV) carrier for pigs. The VSV carrier is matrix protein (M) three-amino acid site-mutated recombinant virus VSV-MT, the 51st-site methionine of the virus M protein is knocked out, the 221st-site valine of the virus M protein is mutated into phenylalanine, and the 226th-site glycine of the virus M protein is mutated into arginine. The VSV carrier is extremely low in pathogenicity, high in safety, capable of effectively stimulate a body to produce protective immune response, applicable to vaccine or vaccine carrier preparation, and promising in application prospect.
Owner:SHANGHAI JIAO TONG UNIV
Novel Drug-Eluting Coronary Artery Stent Coated With Anti-Platelet-Derived Growth Factor Antibodies Overlaying Extracellular Matrix Proteins With an Outer Coating of Anti-Inflammatory (Calcineurin Inhibitor) and/or Anti-Proliferatives
ActiveUS20090157173A1Prevent acute thrombosisPromote growthStentsSurgeryCell-Extracellular MatrixPercent Diameter Stenosis
The present invention relates to a combination of agents, including an anti-proliferative agent, an anti-inflammatory agent, an anti-growth factor, and an extracellular matrix (ECM) molecule coated on a stent to prevent acute and subacute thrombosis, enhance endothelial in-growth, and prevent neointimal hyperplasia, and / or suppress neovascularization, and thereby reduce restenosis rates for drug eluting stents. The present invention also relates to methods of using such multiple drug eluting stents for the treatment of heart disease and other vascular conditions.
Owner:BJORK JR ROBERT L
Virus like particles comprising target proteins fused to plant viral coat proteins
InactiveUS20120219579A1SsRNA viruses negative-senseSsRNA viruses positive-senseProtein targetPlant virus
Virus like particles comprising a fusion protein and substantially free of nucleic acid, wherein the fusion protein comprises a plant viral coat protein and a target protein, are provided. Immunogenic compositions comprising the virus like particles can be administered to subjects to induce protective immune responses in the subjects. Methods of producing the virus like particles are also provided.
Owner:FRAUNHOFER USA
Method for preparing low-immunogenicity pig dermal support
The invention discloses a method for preparing low-immunogenicity pig dermal support. The method comprise the following steps of: (1) preparing double fault derma; (2) performing acellular treatment; (3) performing matrix metalloproteinase-7 treatment; and (4) performing vacuum freeze drying treatment. The low-immunogenicity pig dermal support is manufactured by using pig skin as a raw material, aiming at maintaining low-immunogenicity ingredients of pig dermal collagenous fiber and the like and natural three-dimensional structures based on the low-immunogenicity ingredients, removing (eliminating) high-immunogenicity ingredients such as cell and stromatin (such as laminin and fibronectin), and using means of skin material taking and treatment improvement, acellular method optimization, dematrix protein technique establishment, cross-linking agent optimization, vacuum freeze drying technique and the like. According to the method, the purposes of reducing the pig dermal immunogenicity to the level which ensures that the human body can be tolerated and synchronously maintaining the natural three-dimensional structure of the pig skin so as to guide 'ordered' regeneration and reconstruction of human derma in an orginzation mode of in-situ construction organization engineering are reached; and the problems or the prior art are solved.
Owner:武岩
Rabies vaccine for human beings
InactiveCN102526722AGood immune effectImprove securityAntiviralsDepsipeptidesVirus ProteinTetanus toxoids
The invention provides a rabies vaccine for human beings, which contains an outer membrane fragment of a split rabies virus particle and tetanus toxoid; the outer membrane fragment comprises furcella and matrix protein, wherein each dosage of rabies vaccine for human beings contains 10-150 micrograms of virus protein; and the dosage of the tetanus toxoid is 3-10 Lf / dosage. Compared with the existing virus purification stock solution, the rabies vaccine for human beings contains less other proteins, has higher immunogenicity and effect, and is more secure for use.
Owner:SHANGHAI ZERUN BIOTECHNOLOGY CO LTD
Influenza vaccines
ActiveUS20100160421A1Less protectionBroad and efficient immunitySsRNA viruses negative-senseOrganic active ingredientsHemagglutininSaline injection
Described herein are vaccines and the use of naked DNA and / or RNA encoding hemagglutinin (HA) from pandemic influenza, e.g., the 1918 H1N1 and / or the 1957 H2N2 and / or the 1968 H3N2 influenza A virus, as a vaccine component against present day and coming H1, H2, H3, H5, N1, N2 containing influenza A infections in humans and swine optionally with the naked DNA and / or RNA encoding Neuraminidase (NA) and / or matrix protein (M) and / or the nucleoprotein (NP) from pandemic influenza virus included. If the vaccine components are used as DNA or RNA vaccines with or without the corresponding protein, the codons can optionally be “humanized” using preferred codons from highly expressed mammalian genes and the administration of this DNA vaccine can be by saline or buffered saline injection of naked DNA or RNA, or injection of DNA plasmid or linear gene expressing DNA fragments coupled to particles. Addition of the matrix protein (M) and / or the nucleoprotein (NP) from the 1918 influenza strain is also disclosed.
Owner:STATENS SERUM INST
Preparation of rabies vaccine for human being
InactiveCN103182081AGood immune effectImprove securityBacterial antigen ingredientsAntiviralsRabiesAdjuvant
The invention provides rabies vaccine for human being. The vaccine comprises an outer membrane segment of cracked rabies complete viral particles and tetanus toxoid, and the outer membrane segment comprises spike and matrix protein. Each dose of the rabies vaccine comprises 10 to 100 micrograms of viral protein; the dosage of the tetanus toxoid is 2 Lf / dose to 10 Lf / dose; and the dosage of aluminum phosphate adjuvant is 0.5mg / dose. Compared with the existing virus purified stock solution, the rabies vaccine for human being has the advantages that the content of impurity protein is greatly reduced, the immunogenicity and the potency are high, and the rabies vaccine is safer to use.
Owner:SHANGHAI ZERUN BIOTECHNOLOGY CO LTD
Recombinant homologous avian H1N1 influenza virus inactivated vaccine strain (JS40/PR8) as well as preparation method and application of inactivated vaccine strain
ActiveCN104232594AQuality improvementReduce manufacturing costMicroorganism based processesAntiviralsHemagglutininNeuraminidase
The invention discloses a recombinant homologous avian H1N1 influenza virus inactivated vaccine strain (JS40 / PR8) as well as a preparation method and an application of the inactivated vaccine strain. The recombinant avian H1N1 influenza virus inactivated vaccine strain takes A / swine / Jiangsu / 40 / 2011 (H1N1) as genetic donors of surface hemagglutinin (HA) and neuraminidase (NA), and influenza virus A / PR / 8 / 34(H1N1) as donors of six internal genes of a polymerase PB2 subunit, a polymerase PB1 subunit, a polymerase PA subunit, nucleoprotein (NP), a matrix protein (M) and non-structural protein (NS); the vaccine strain is obtained by natural recombination; and the preservation number of the vaccine strain is CCTCC NO:V201419. The invention also discloses a method for building the recombinant homologous avian H1N1 influenza virus inactivated vaccine strain, and an application of the homologous recombinant avian H1N1 influenza virus inactivated vaccine strain for preventing homologous avian H1N1 influenza of animals or people.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Recombinant mutants of rhabdovirus and methods of use thereof
The present invention relates to recombinant Rhabdoviridae, isolated nucleic acids, vectors, cells and compositions comprising same. The recombinant Rhabdoviridae, isolated nucleic acids, vectors, cells and compositions express Rhabdoviral proteins including a mutated matrix protein (M) and / or a mutated glycoprotein (G), in addition to expression of at least one foreign nucleic acid. The present invention also relates to methods of use thereof, including their use in vivo, in anti-cancer applications, such as in the treatment of gliomas. The recombinant Rhabdoviridae of the present invention are also useful in gene therapy and vaccine applications.
Owner:UNIV OF TENNESSEE RES FOUND
Peptide fragments for inducing synthesis of extracellular matrix proteins
ActiveUS20070299015A1Simple and cost-effective to produceGood for healthCosmetic preparationsToilet preparationsGlycineCell-Extracellular Matrix
Short biologically active tetrapeptides are disclosed that are comprised of the sequences GxxG and PxxP where G (glycine) and P (proline) are maintained and x is a variable amino acid. The peptides can be used singly or in combination to stimulate production of extracellular matrix proteins in skin. A rapid, low-cost method of producing heterogenous formulations of tetrapeptides is disclosed.
Owner:HELIX BIOMEDIX INC
Composition and preparing method and beauty preparation thereof
InactiveCN105030645AImprove wrinkle removal functionPromote generationCosmetic preparationsToilet preparationsWrinkle skinHigh concentration
The invention provides a composition and a preparing method and beauty preparation thereof. The composition comprises 30-70 volume parts of cell growth factor solutions, 30-70 volume parts of high-concentration blood platelet plasma, and adipose-derived stem cells with the concentration of 0.1*10<6>-2*10<6> / mL. Compared with the prior art, the composition is used for the beauty preparation, and the cell growth factor is a high-activity factor capable of promoting cell growth and promoting angiogenesis, wound healing and tissue damage repairing; the high-concentration blood platelet plasma is beneficial for binding cells and cell surface receptors and improving release of extracellular matrix protein and has a remarkable effect on removing wrinkles and relieving scars and stains; the adipose-derived stem cells can secrete various activity factors with remarkable biological functions, and the three substances interact to improve the wrinkle removal function of the beauty preparation.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
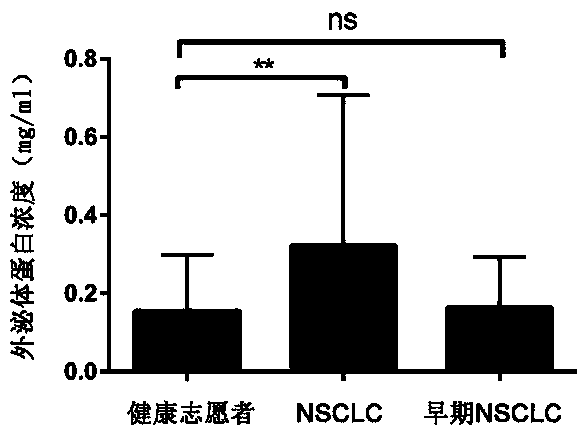
Kit for diagnosis of early non-small cell lung cancer
ActiveCN108802389AEasy extractionEasy to prepareMaterial analysisExtracellular matrix protein 1WAS PROTEIN
The invention provides a kit for diagnosis of early non-small cell lung cancer. A marker of the kit is protein of serum exosomes, and the exosome protein is exosome human fetuin A, namely AHSG or / andexosome extracellular matrix protein-1, namely ECM-1. The content of the marker in a sample is detected, and if the content of the marker in the sample is greater than the critical value range thereof, a tester can preliminarily judge that the sample has larger risk of non-small cell lung cancer. The marker in the kit is simple to extract, the preparation is convenient and rapid, the detection process is simple, and the kit has a good diagnostic effect on early non-small cell lung cancer.
Owner:郭伟
Swine fever virus chimeric type virus-like particle, preparation method, application thereof and vaccine
InactiveCN109535233AImprove immunityImprove functionalitySsRNA viruses positive-senseVirus peptidesSwine Fever VirusViral Vaccine
The present invention provides a swine fever virus chimeric type virus-like particle, a preparation method thereof, an application thereof and a vaccine, and belongs to the technical fields of bioengineering and virus vaccines. The swine fever virus chimeric type virus-like particle selects a Gag precursor protein of retroviruses as a matrix protein and swine fever virus envelope proteins E0 and E2 as surface membrane proteins to self-assemble into the ideal and functional virus-like particle, weaknesses of a subunit protein vaccine are overcome, and immunogenicity, safety and stability of thevaccine are effectively improved. The swine fever virus chimeric type virus-like particle can be prepared in large quantities by constructing expression vectors to transfect cells. The swine fever virus chimeric type virus-like particle can be applied to prepare the vaccine for preventing swine fever epidemic diseases, a swine fever virus antibody detection preparation or a swine fever virus epidemic disease monitoring preparation, and has a relatively good practical application value.
Owner:NOVARTIS BIOTECH WUHAN +1
Antibody against rgd in amino acid sequence of extracellular matrix protein and production method and use of the same
ActiveUS20110065899A1Inhibit bindingAntibacterial agentsAntipyreticCell-Extracellular MatrixOsteopoikilosis
The present invention provides a monoclonal antibody which specifically recognizes RGD in the amino acid sequence of extracellular matrix proteins of a human and a mouse. By specifically inhibiting the RGD sequence-mediated adhesion, exertion of efficient effects on diseases such as inflammation, cancer, infectious disease, autoimmune diseases and osteoporosis and reduction in adverse effects can be expected at the same time. Therefore, better treatment methods can be provided to these diseases.
Owner:GENE TECH SCI CO LTD
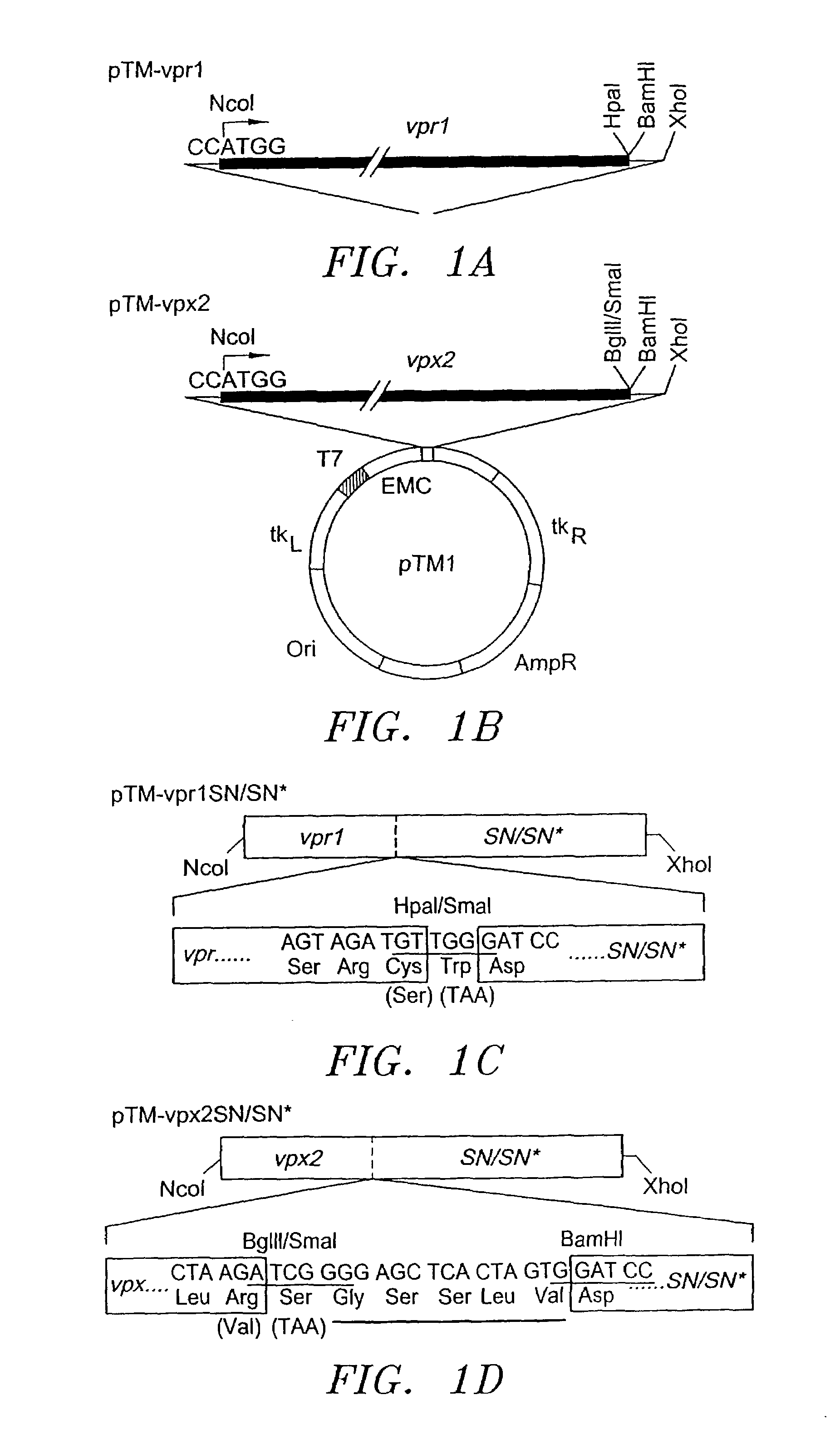
Fusion protein delivery system and uses thereof
The present invention provides a composition of matter, comprising: DNA encoding a viral Vpx protein fused to DNA encoding a protein. In another embodiment of the present invention, there is provided a composition of matter, comprising: DNA encoding a viral Vpr protein fused to DNA encoding a protein. The present invention further provides DNA, vectors and methods for expressing a lentiviral pol gene in trans, independent of the lentiviral gag-pol. A gene transduction element is optionally delivered to a lentiviral vector according to the present invention. Also provided are various methods of delivering a virus inhibitory molecule to a target in an animal. Further provided is a pharmaceutical composition.
Owner:UAB RES FOUND
Monoclonal antibody of influenza virus matrix protein M1 and application of monoclonal antibody
ActiveCN110627901AHigh sensitivityHigh application valueBiological material analysisImmunoglobulins against virusesImmunoprecipitationIndirect immunofluorescence
The invention relates to the biology field, in particular to a monoclonal antibody of influenza virus matrix protein M1 and application of the monoclonal antibody. The monoclonal antibody of influenzavirus matrix protein comprises a heavy chain variable region and a light chain variable region, amino acid sequences of the CDR1, CDR2 and CDR3 of the heavy chain variable region are shown in SEQ IDNO.1-3 separately; and amino acid sequences of the CDR1, CDR2 and CDR3 of the light chain variable region are shown in SEQ ID NO.4-6 separately. The monoclonal antibody can specifically recognize andbind multiple subtypes of influenza virus matrix protein M1, has broad spectrum applicability among influenza virus subtypes, has higher specificity and sensitivity, can be well applied to western blotting experiments, indirect immunofluorescence experiments, immunoprecipitation technologies and the like and has higher application values.
Owner:CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com