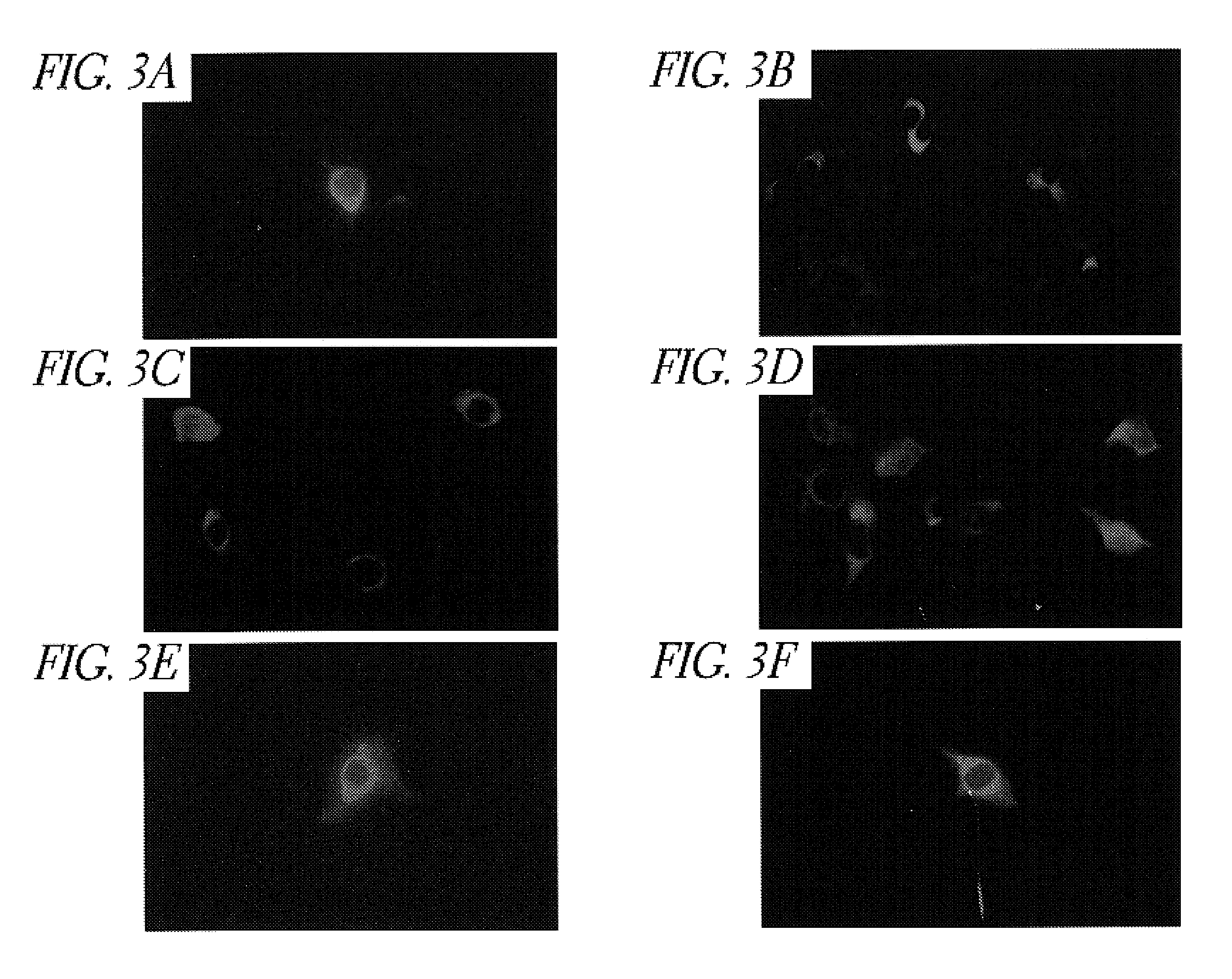
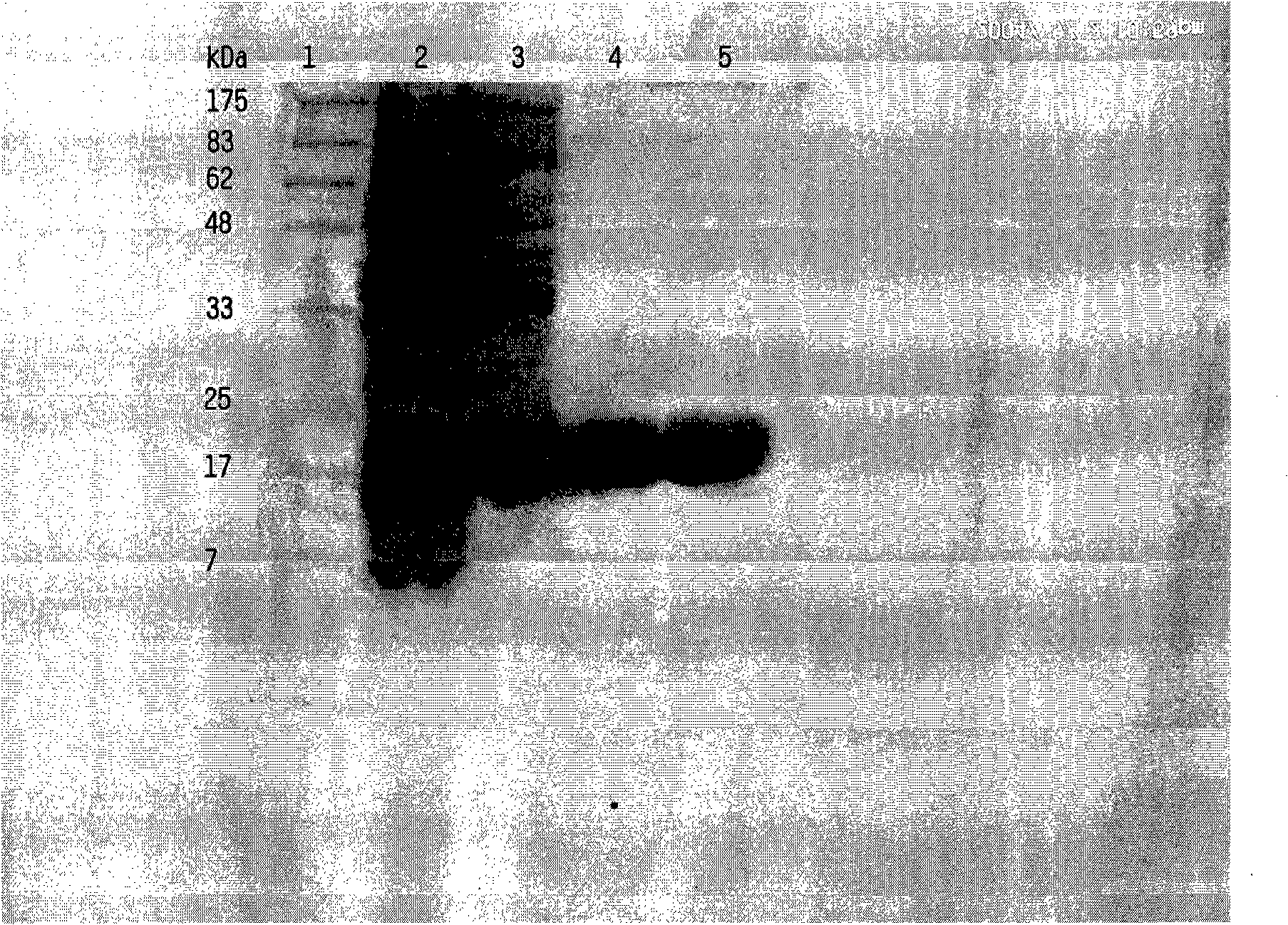Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
111 results about "Densovirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A genus of PARVOVIRIDAE, subfamily DENSOVIRINAE, comprising helper-independent viruses containing only two species. Junonia coenia densovirus is the type species.
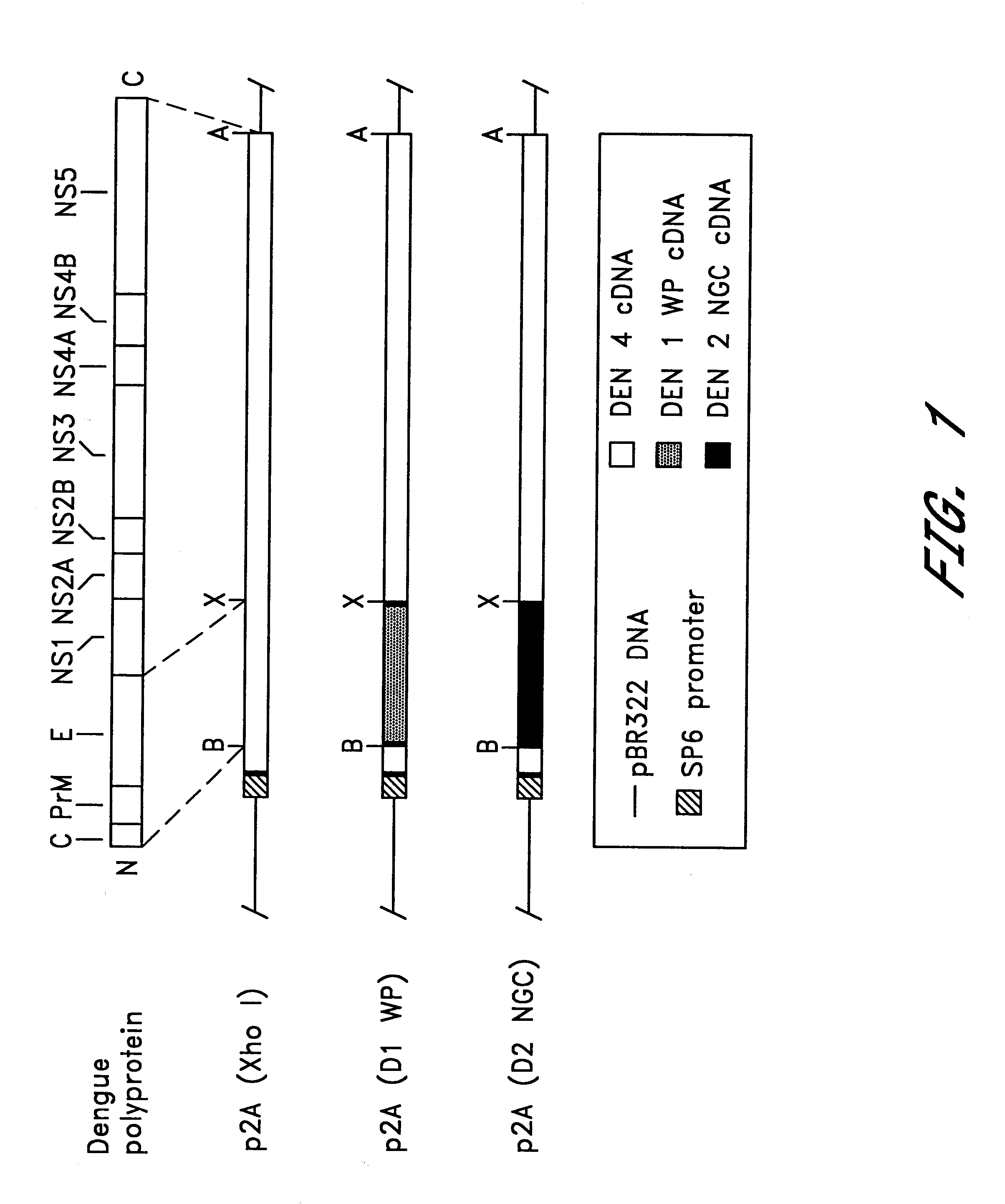
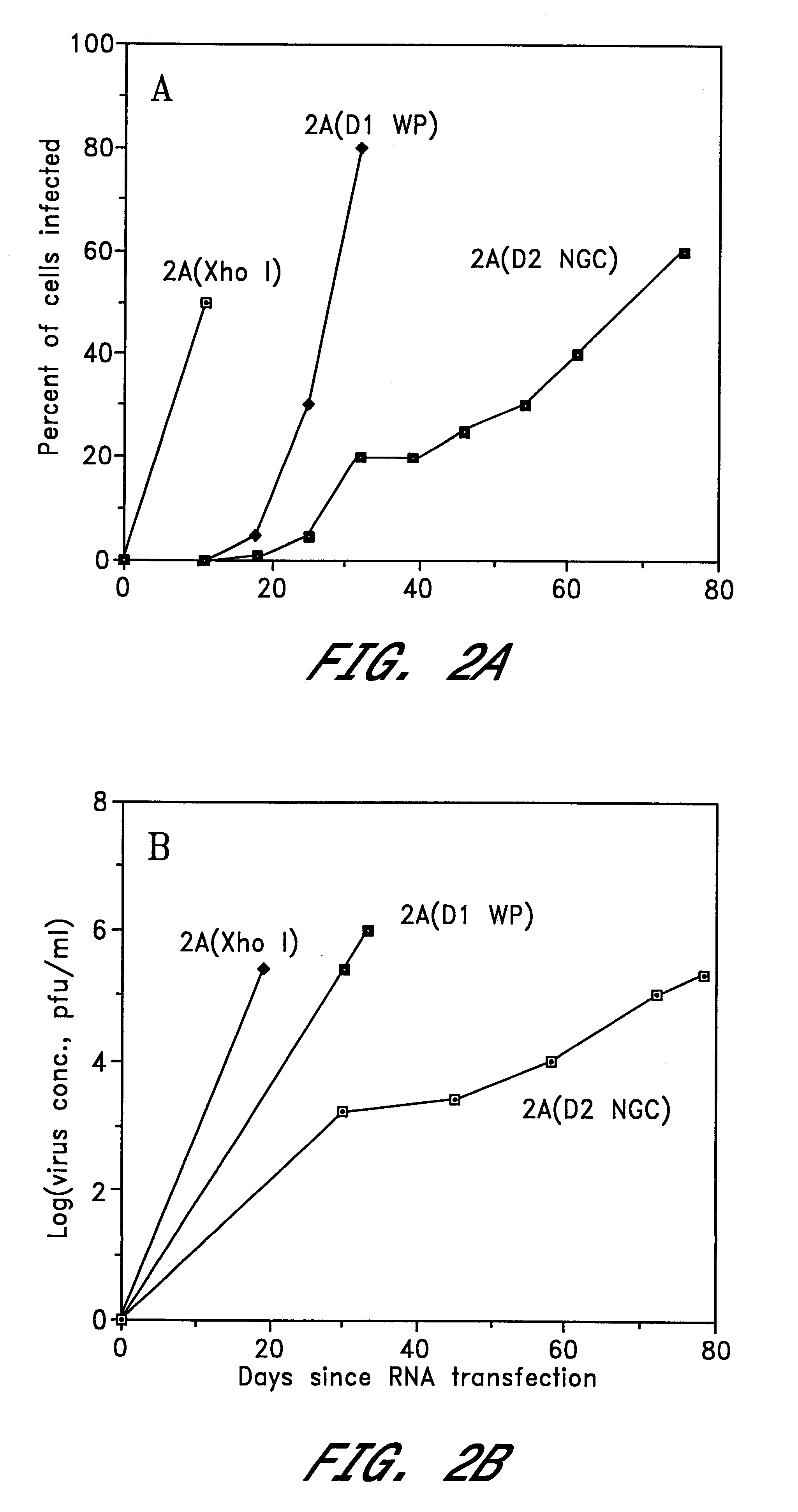
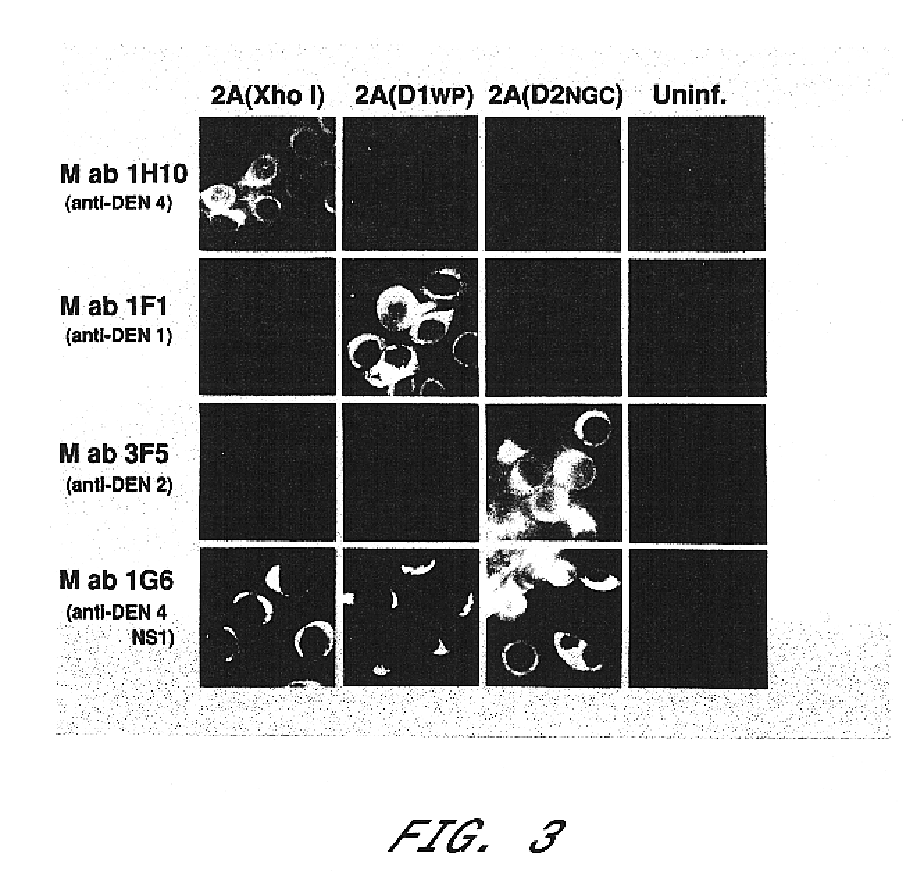
Chimeric and/or growth-restricted flaviviruses
InactiveUS6676936B1BiocideSsRNA viruses positive-senseVirulent characteristicsJapanese B Encephalitis Virus
The invention includes a chimeric virus for use in a vaccine preparation having a genome comprising nucleic acid sequences encoding at least one structural protein from one flavivirus and nucleic acid sequences encoding nonstructural protein from another flavivirus. The genome preferably includes mutations within the viral genome that reduce virus virulence and in a particularly preferred embodiment these vaccines are directed to flaviviruses such as dengue virus, tick-borne encephalitis virus and Japanese encephalitis virus. The invention also includes a baculovirus having a recombinant dengue cDNA sequence which encodes: (1) dengue virus capsid protein, pre-matrix protein, envelope glycoprotein and NS1 and NS2a nonstructural proteins or (2) dengue envelope glycoprotein or (3) dengue non-structural proteins NS1 and NS2a. The invention further includes a baculovirus having a recombinant Japanese B encephalitis virus cDNA sequence which encodes the Japanese B encephalitis virus capsid protein, pre-matrix protein, envelope glycoprotein and non-structural proteins NS1 and NS2a. The invention further includes a vaccine and a method to produce that vaccine.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Novel vaccines against multiple subtypes of dengue virus
ActiveUS20100291144A1Elicit immune responseSsRNA viruses positive-senseViral antigen ingredientsAntigenMammal
An aspect of the present invention is related to nucleic acid constructs capable of expressing a polypeptide that elicits an immune response in a mammal against more than one subtype of dengue virus, and methods of use thereof. Additionally, there are DNA plasmid vaccines capable of generating in a mammal an immune response against a plurality of dengue virus subtypes, comprising a DNA plasmid and a pharmaceutically acceptable excipient, and methods of use thereof. The DNA plasmid is capable of expressing a consensus dengue antigen in a cell of the mammal in a quantity effective to elicit an immune response in the mammal.
Owner:VGX PHARMA
Anti-dengue virus antibodies
ActiveUS8637035B2Viral antigen ingredientsMicrobiological testing/measurementDengue virus antibodyAntigen Binding Fragment
Provided herein are monoclonal antibodies specific to dengue virus as well as their antigen-binding fragments, and functional variants. Also disclosed are uses thereof for treating or diagnosing dengue virus infection.
Owner:ACAD SINIC
Immunologic diagnosis kit for detecting type II dengue virus NS1 antigen
ActiveCN101226196AAccurate detectionQuick checkMaterial analysisAgainst vector-borne diseasesSerotypeElisa test
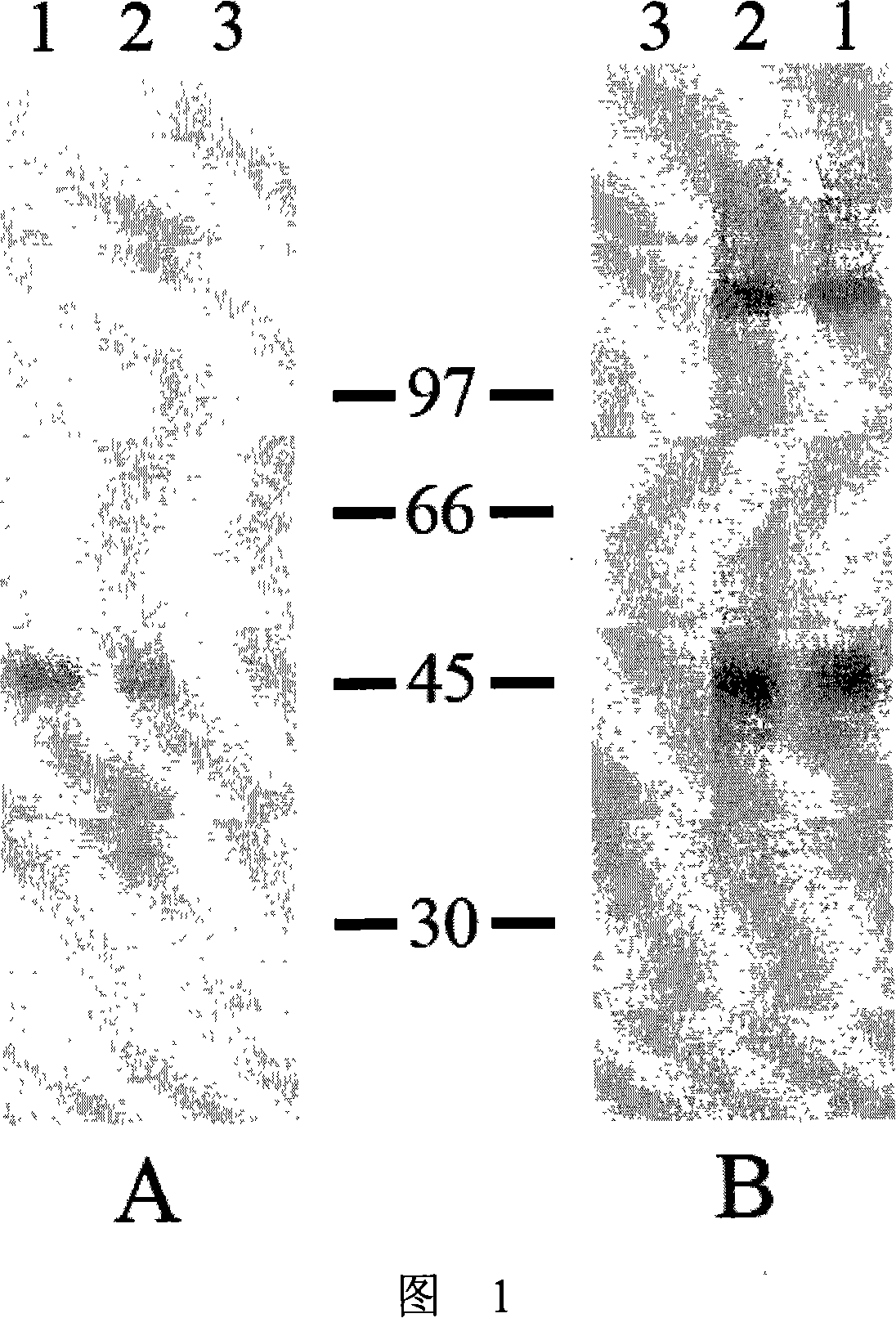
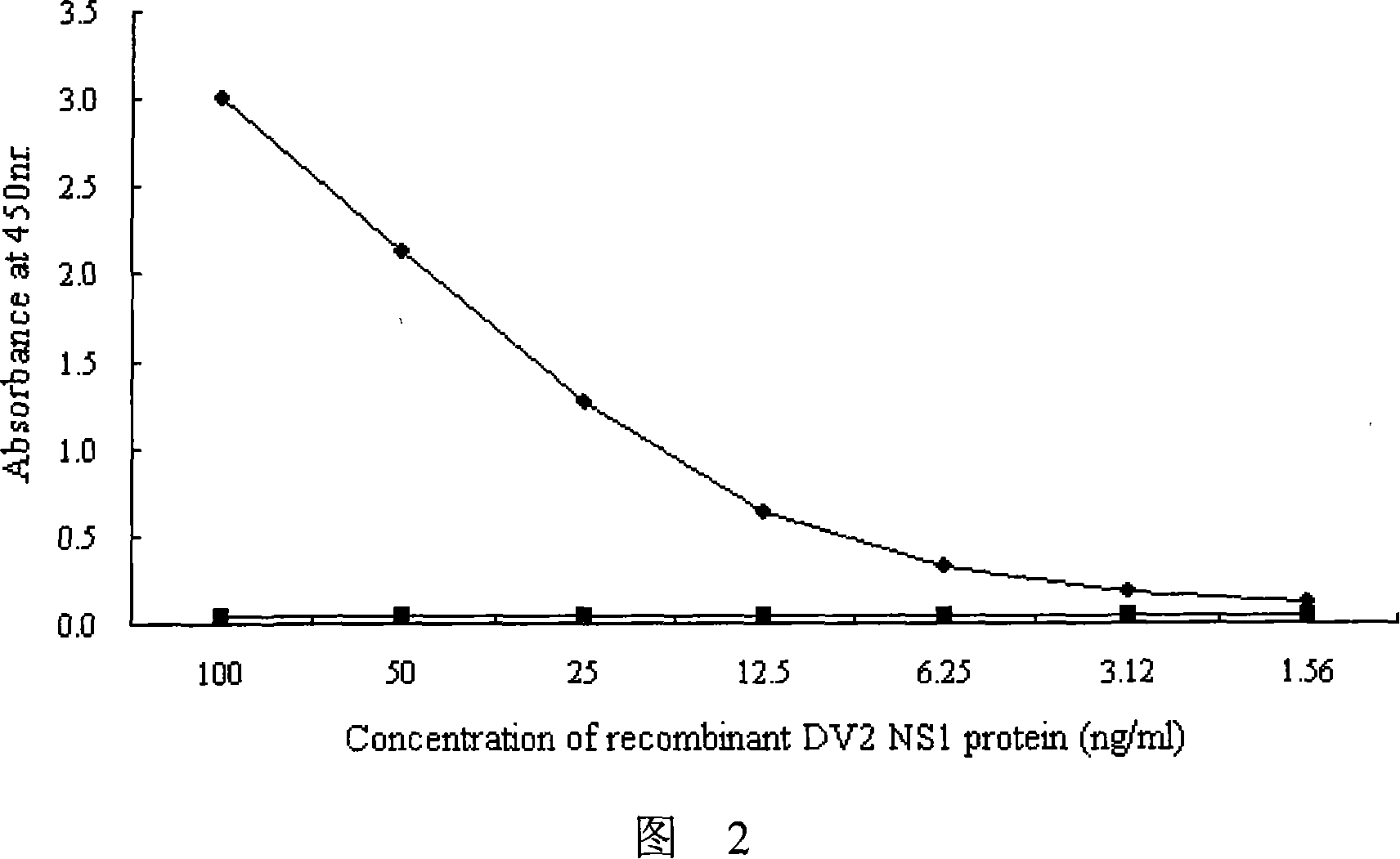
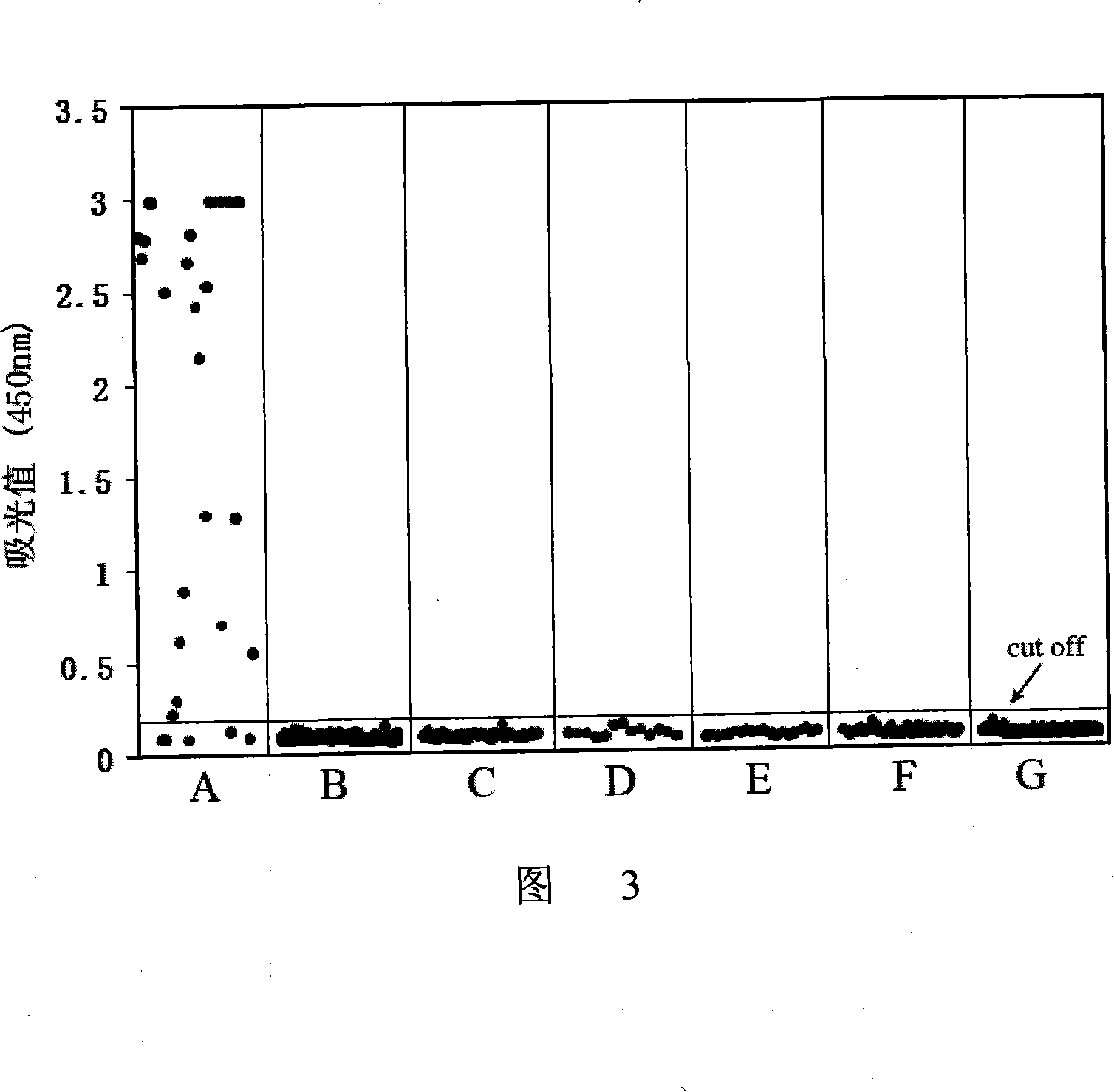
The invention provides an immunity diagnosis test kit for detecting II-type dengue virus antigen, which comprises a porous reaction plate covering monoclonal antibody DV2-M6, a sample treatment liquid, a monoclonal antibody DV2-M15 marked with a label, a positive contrast, a negative contrast, a concentration washing liquid, a develop liquid and a termination liquid, wherein the monoclonal antibodies DV2-M6 and DV2-M14 of the test kit can be specifically combined with NS1 protein of II-type dengue virus, without cross reaction with other three kinds of serotype dengue viruses NS1 and respectively combined with different antigen points of NS1, while the check sensitivity of NS1 protein of II-type dengue virus can reach 3ng / ml and the check sensitivity of culture supernatant of II-type dengue virus infection cell is 8 power of Pan-E dengue early elisa test kit, thereby improving the sensitivity of clinical serum sample check.
Owner:SOUTHERN MEDICAL UNIVERSITY
Monoclonal antibodies that bind or neutralize dengue virus
The present invention relates to monoclonal antibodies that bind or neutralize dengue type 1, 2, 3, and / or 4 virus. The invention provides such antibodies, fragments of such antibodies retaining dengue virus-binding ability, fully human or humanized antibodies retaining dengue virus-binding ability, and pharmaceutical compositions including such antibodies. The invention further provides for isolated nucleic acids encoding the antibodies of the invention and host cells transformed therewith. Additionally, the invention provides for prophylactic, therapeutic, and diagnostic methods employing the antibodies and nucleic acids of the invention.
Owner:HEALTH & HUMAN SERVICES THE GOVERMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC DEPT OF
Anti-Dengue Virus NS1 Protein Monoclonal Antibodies
ActiveUS20170233460A1Improve developmentUseful in therapyImmunoglobulins against virusesAntibody ingredientsProtein.monoclonalSpecific detection
The present invention provides matched antibody pairs for the specific detection of one or more of the four dengue virus serotypes in a biological sample that may contain one or more of such dengue virus serotypes. Each matched antibody pair is capable of detecting not more than one serotype of dengue virus NS1 protein that may be present in the sample and will not cross react with other serotypes that may be present in the sample. Multiple matched pairs may be used to detect one or more dengue virus serotypes that may be present in a sample. Such matched pair antibodies, facilitate the development of confirmatory in vitro diagnostic tests such as sandwich immunoassays, that detect and distinguish the presence of one or more dengue virus serotypes in a biological sample, preferably a sample derived from human subject. The invention also provides kits comprising the matched antibody pairs of the invention and methods for using the kits for immunoassays for the specific detection of one or more serotypes of dengue virus in a patient population. The present invention also provides monoclonal antibodies specific for the NS1 protein of dengue virus and therapeutic compositions and methods for treating dengue virus infection.
Owner:THE FOOD & DRUG ADMINISTATION +1
Effective Cas13a-based anti-dengue virus nucleic acid target and application thereof
ActiveCN108715849ALower resistanceImprove efficiencyOrganic active ingredientsSsRNA viruses positive-senseAnti virusGene targets
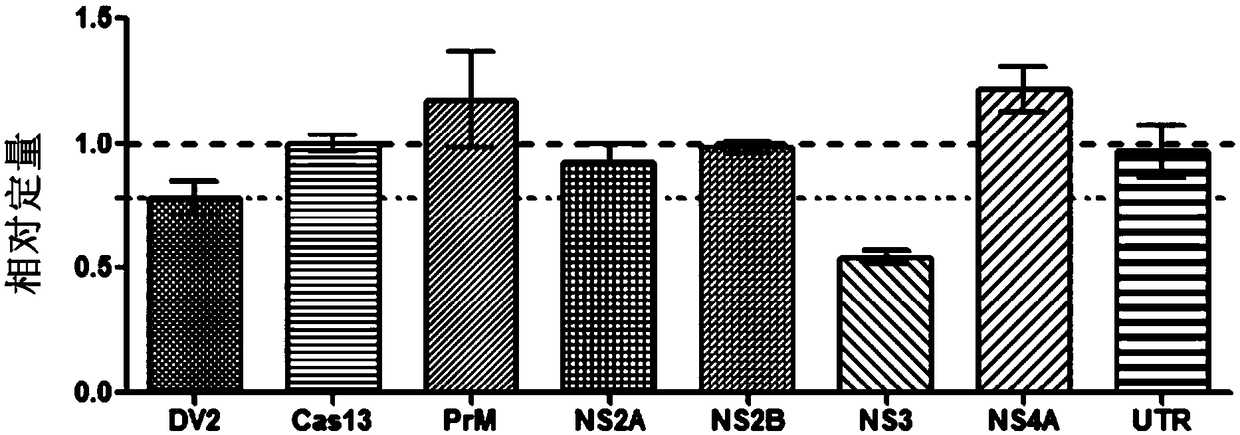
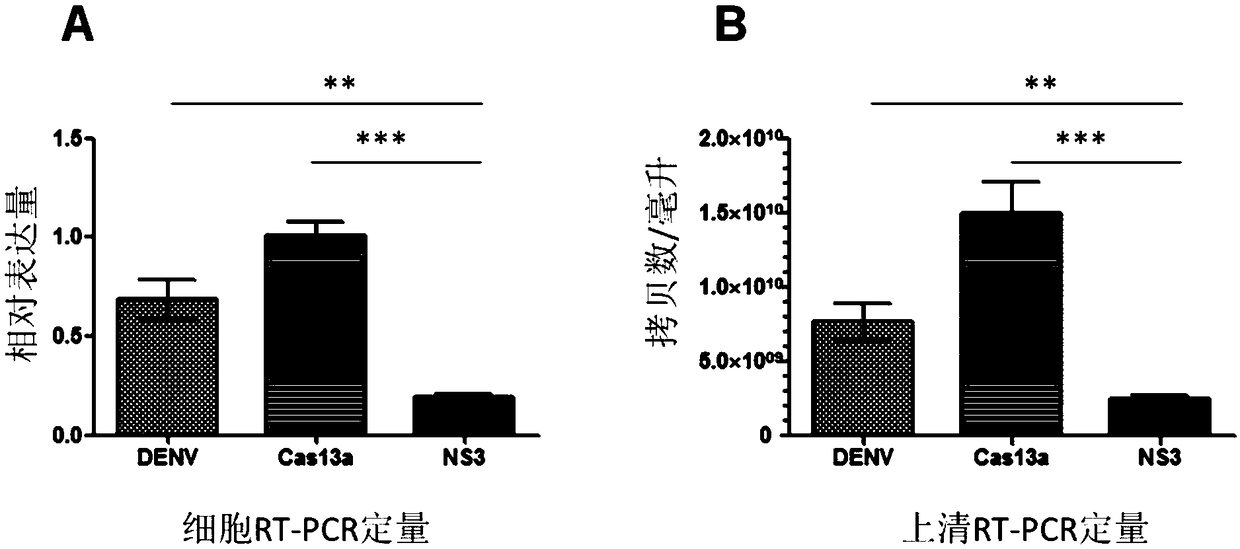
The invention discloses an effective Cas13a-based anti-dengue virus nucleic acid target and application thereof. The invention provides a CRISPR-Cas13a system for inhibiting dengue virus, wherein theCRISPR-Cas13a system comprises a Cas13a protein and a crRNA corresponding to an NS3 gene target of the dengue virus, or a complex formed by the Cas13a protein and crRNA; and NS3 gene targets of the dengue virus are 4657th-4685th nucleotide sequences of the NS3 gene. In the invention, a novel anti-dengue virus method is found, is different from a traditional anti-virus method, directly targets a viral nucleic acid, specifically degrades a target gene, and makes the virus lose the replication ability; therefore, the effective Cas13a-based anti-dengue virus nucleic acid target has characteristicsof high efficiency, high specificity and programmability, is not easy to produce drug resistance generated by traditional antiviral drugs, and may become a novel antiviral drug in the future.
Owner:ACADEMY OF MILITARY MEDICAL SCI +1
Dengue virus IgG antibody ELISA diagnostic kit
InactiveCN101308137AAvoid cross reactionIncreased sensitivityBiological testingAgainst vector-borne diseasesAntigenPositive control
The invention relates to a dengue virus antibody enzyme-linked immunity diagnostic kit. The kit includes an enzyme labeling board, a negative control serum, a positive control serum, an enzyme marker, a sample diluent, a concentrated washing liquid, a substrate chromogenic liquid, a stop solution and a sealing plate glue, wherein the enzyme marker contains a goat anti-human IgG-HRP; an envelope antigen on the enzyme labeling board includes: a recombinant dengue virus type 1 envelope protein specific antigen with the amino acid sequence of SEQ ID No.1; a recombinant dengue virus type 2 envelope protein specific antigen with the amino acid sequence of SEQ ID No.3; a recombinant dengue virus type 3 envelope protein specific antigen with the amino acid sequence of SEQ ID No.5; and a recombinant dengue virus type 4 envelope protein specific antigen with the amino acid sequences of SEQ ID No .7. Early diagnoses can be made to patients infected with dengue fever for the second time or more than two times and serum epidemiological investigation to healthy people can be conducted through detecting dengue virus IgG antibodies in serum. Final diagnoses can be made to patients infected with dengue fever for the first time.
Owner:广东省疾病预防控制中心
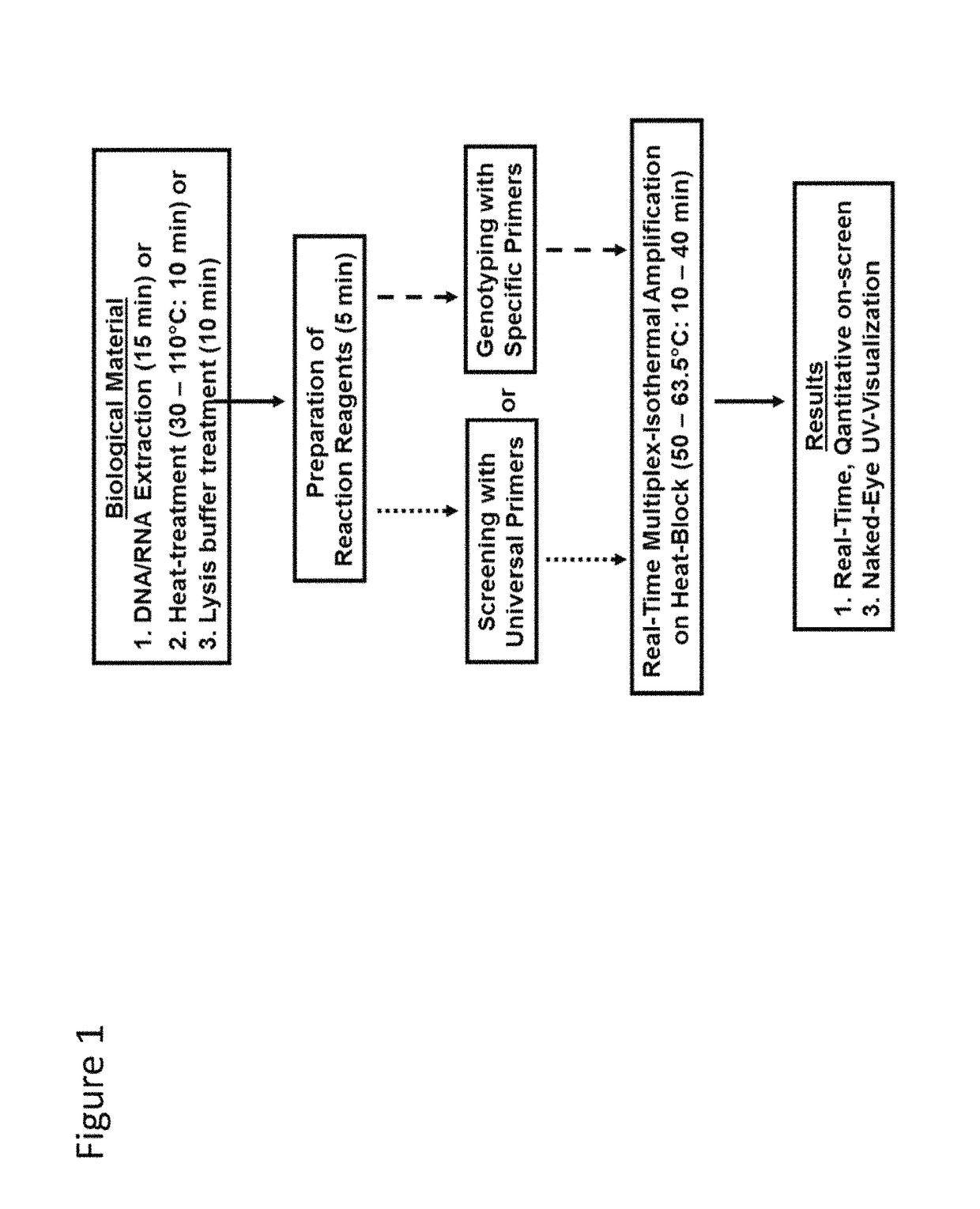
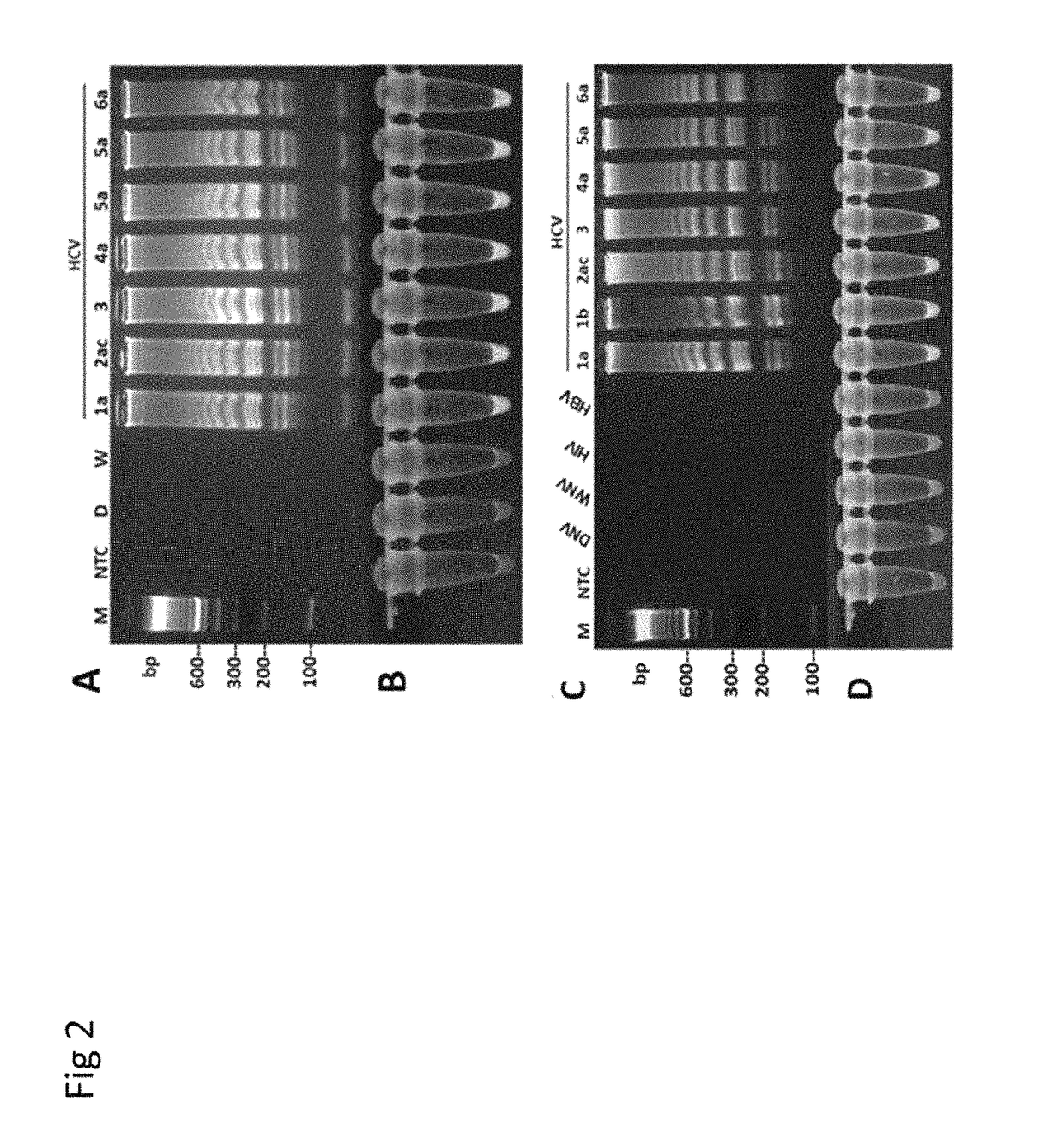
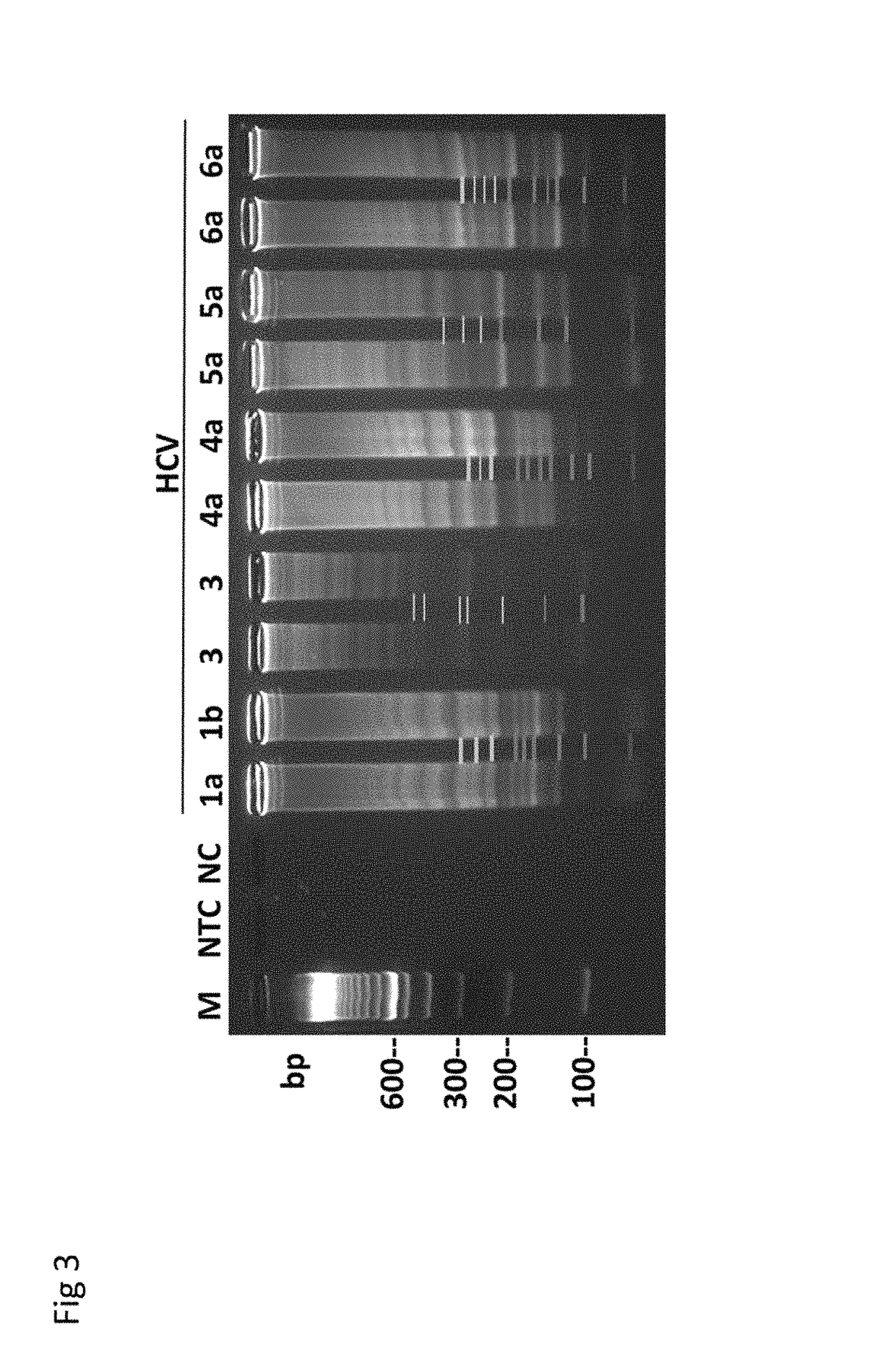
Methods for real-time multiplex isothermal detection and identification of bacterial, viral, and protozoan nucleic acids
ActiveUS10072309B1Flexible processFacilitate strand displacementMicrobiological testing/measurementAgainst vector-borne diseasesProtozoaFluorescence
Herein disclosed are rapid real-time isothermal multiplex methods of detecting, identifying and quantifying bacterial, viral, and protozoan nucleic acids in a sample. These include contacting the sample with two or more sets of pathogen-specific reverse transcription loop-mediated isothermal amplification primers and novel oligofluorophores specific for the target bacterial, viral, and parasitic nucleic acids of interest such as human immunodeficiency virus, Ebola virus, Marburg virus, Yellow fever virus, hepatitis-B virus, Lassa fever virus, Plasmodium, hepatitis-C virus, hepatitis-E virus, dengue virus, Chikungunya virus, Japanese Encephalitis virus, Middle Eastern Respiratory Syndrome Corona virus, Mycobacterium, West Nile virus, Cytomegalovirus, Parvovirus, Leishmania, Trypanosoma, and Zika virus nucleic acids, under conditions sufficient to produce detectable real-time amplification signals in about 10 to 40 minutes. The amplification signals are produced by pathogen-specific fluorogenic labels included in one or more of the primers. Also, novel reaction and sample lysis buffers, primers, and kits for rapid multiplex detection, quantification, and identification of bacterial, viral, and protozoan nucleic acids by real-time isothermal amplification are herein disclosed.
Owner:NYAN DOUGBEH CHRIS
Development of dengue virus vaccine components
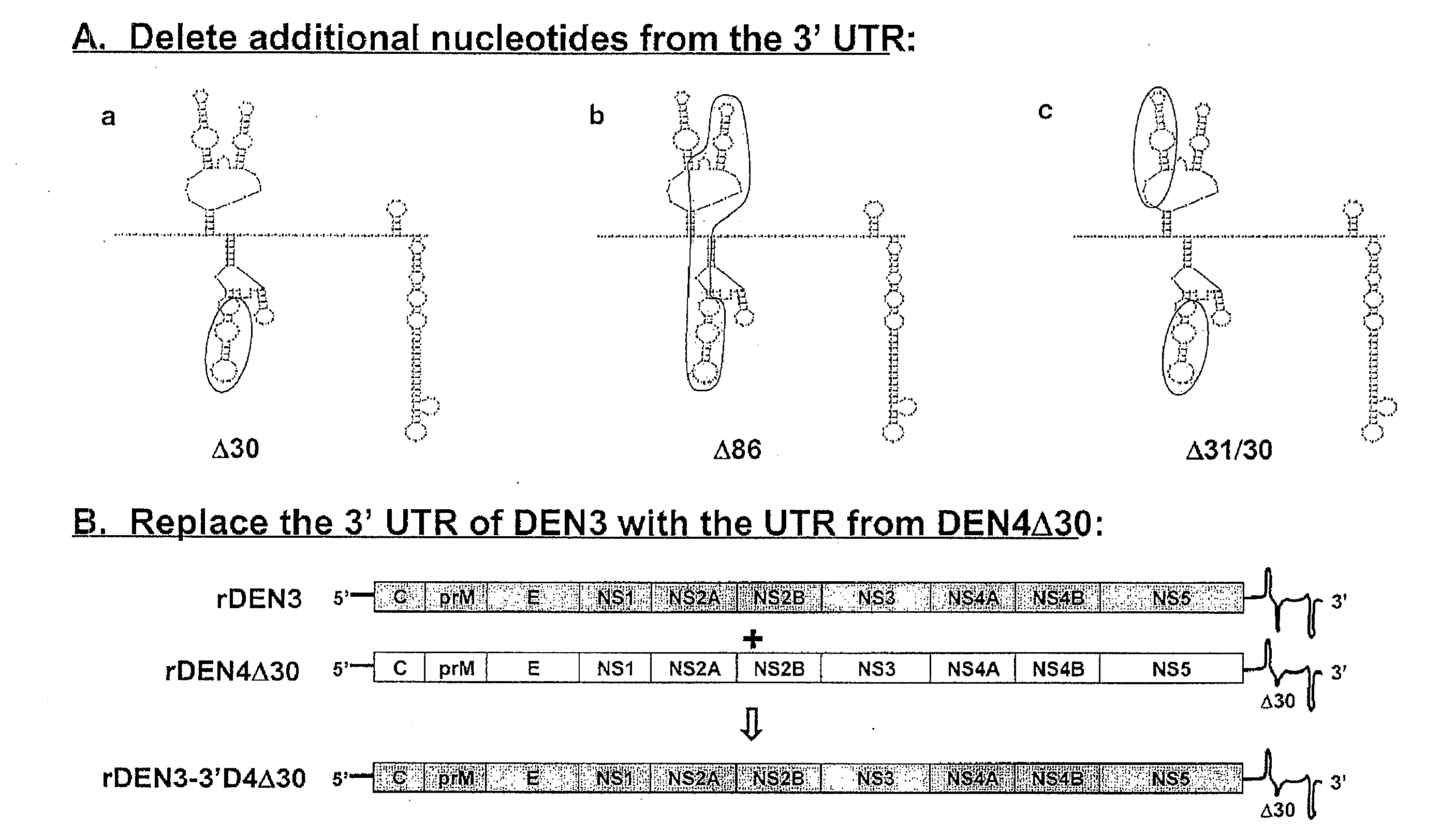
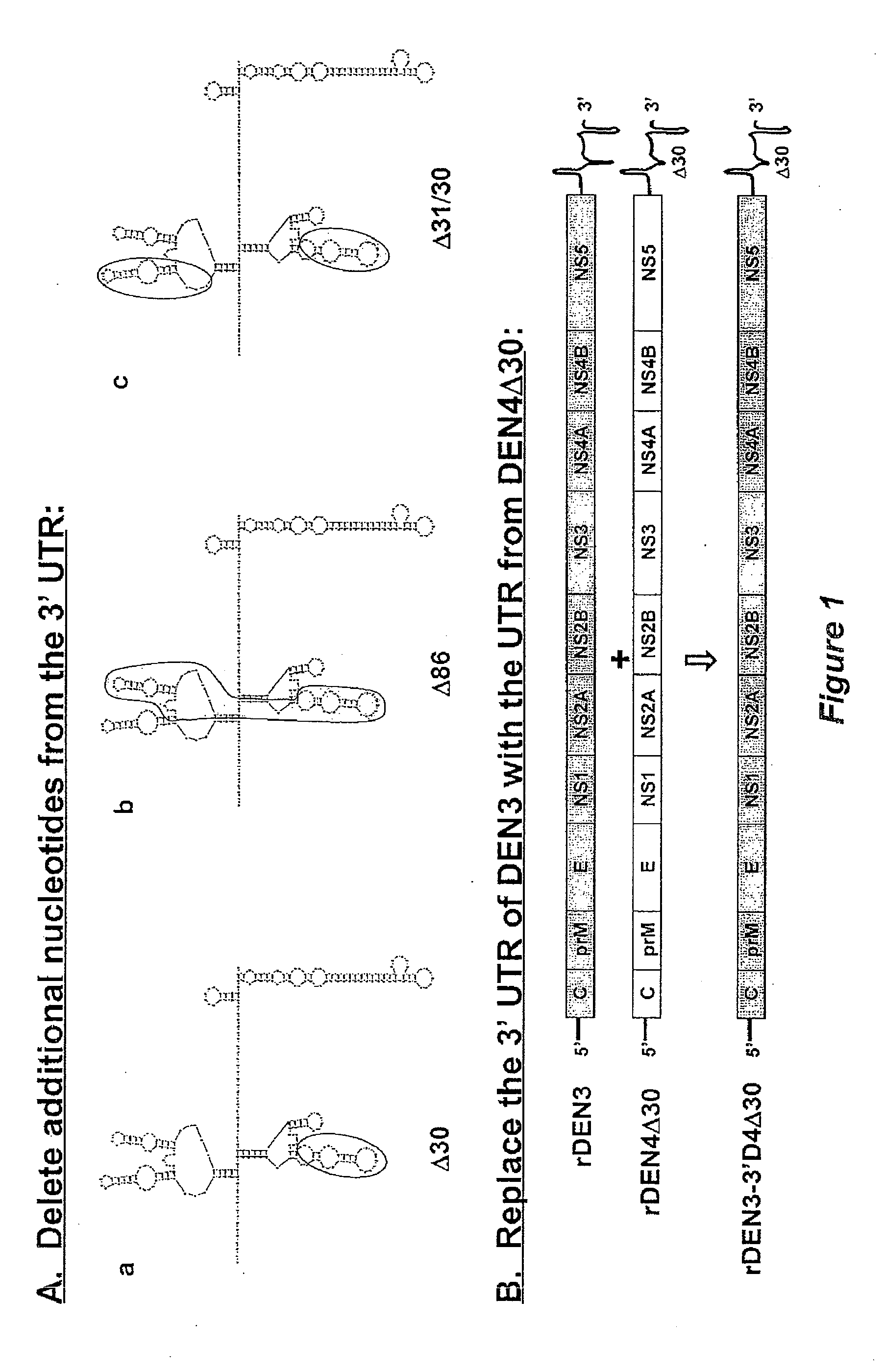
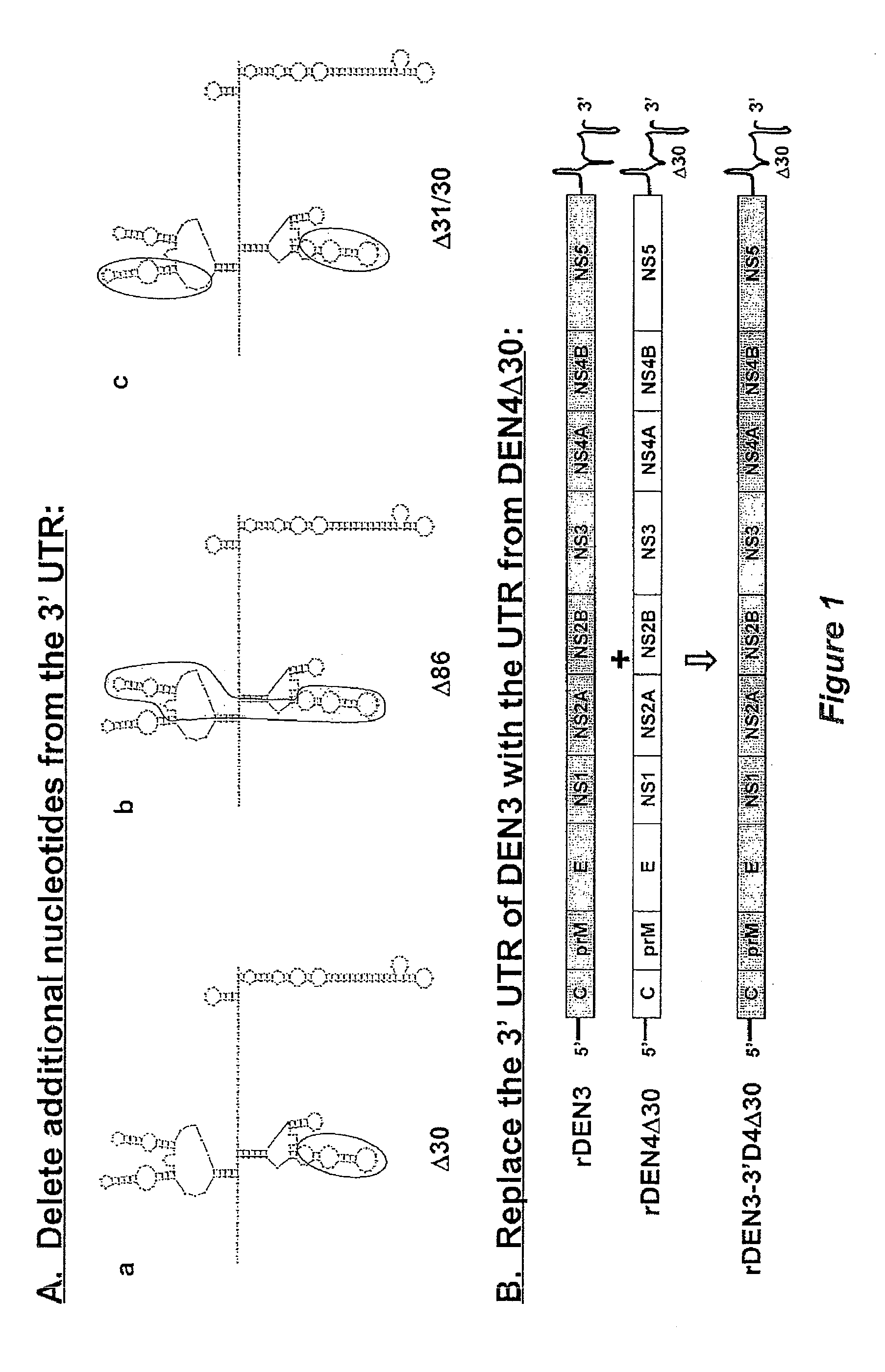
The invention is related to a dengue virus or chimeric dengue virus that contains a mutation in the 3′ untranslated region (3′-UTR) comprising a Δ30 mutation that removes the TL-2 homologous structure in each of the dengue virus serotypes 1, 2, 3, and 4, and nucleotides additional to the Δ30 mutation deleted from the 3′-UTR that removes sequence in the 5′ direction as far as the 5′ boundary of the TL-3 homologous structure in each of the dengue virus serotypes 1, 2, 3, and 4, or a replacement of the 3′-UTR of a dengue virus of a first serotype with the 3′-UTR of a dengue virus of a second serotype, optionally containing the Δ30 mutation and nucleotides additional to the Δ30 mutation deleted from the 3′-UTR; and immunogenic compositions, methods of inducing an immune response, and methods of producing a dengue virus or chimeric dengue virus.
Owner:UNITED STATES OF AMERICA
RPA technology-based marburg virus detection kit and application thereof
InactiveCN106636469AAvoid spreading infectionEfficient amplificationMicrobiological testing/measurementMicroorganism based processesInfection transmissionQuarantine
The invention discloses an RPA technology-based marburg virus detection kit and an application thereof. An experiment proves that a target gene can be effectively amplified by an RPA primer and a probe of a marburg virus, the specificity reaches 100% and sensitivity is 1*10<2>copies / reaction; and the virus can reach the level equivalent to the sensitivity of fluorescent quantitative PCR, and has no cross reaction with a zaire ebola pseudovirus, a dengue virus, a hemorrhagic fever virus with renal syndrome and a Xinjiang hemorrhagic fever virus nucleic acid. The RPA isothermal amplification system is fast in reaction and wide in temperature range, effective amplification of a target gene can be achieved under the condition of 37-42 DEG C, the method can be used for fast field detection of an infectious nucleic acid of the marburg virus, and an available fast detection method is provided for field screening of a pathogen; and meanwhile, the marburg virus detection kit also has the important significance in prevention of infection transmission of the marburg virus in China and inspection and quarantine in affected areas and entry and exit ports.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Dengue virus rapid classification identification detection kit
InactiveCN104630388AEasy to operateShort timeMicrobiological testing/measurementMicroorganism based processesFluorescenceEnzyme system
The invention relates to a dengue virus rapid classification identification detection kit, and in particular relates to a detection kit used for identifying four serum types of the dengue virus by using the multiple real-time fluorescent rapid polymerase chain reaction technology, namely, I type, II type, III type and IV type. The kit mainly comprises an amplification reaction buffer liquid, a primer probe reaction liquid, an amplification reaction enzyme system, a negative quality control product, a positive quality control product, and a packing box used for packaging the reagent bottles or tubes in a separation and centralized package mode. The kit adopts the multiple fluorescent channel respective detection mode, the Taq enzyme having rapid polymerization and the one-step real time rapid PCR reaction mode for exactly detecting whether the sample has the dengue virus nucleic acid and identifying the four serum types of the dengue virus; the dengue virus rapid classification identification detection kit is widely used for the early differential diagnosis, epidemic prevention and control, inspection and quarantine and other fields for the dengue, especially the severe dengue fever and mixed infection dengue virus.
Owner:GUANGZHOU EIGHTH PEOPLES HOSPITAL +1
Development of dengue virus vaccine components
The invention is related to a dengue virus or chimeric dengue virus that contains a mutation in the 3′ untranslated region (3′-UTR) comprising a Δ30 mutation that removes the TL-2 homologous structure in each of the dengue virus serotypes 1, 2, 3, and 4, and nucleotides additional to the Δ30 mutation deleted from the 3′-UTR that removes sequence in the 5′ direction as far as the 5′ boundary of the TL-3 homologous structure in each of the dengue virus serotypes 1, 2, 3, and 4, or a replacement of the 3′-UTR of a dengue virus of a first serotype with the 3′-UTR of a dengue virus of a second serotype, optionally containing the Δ30 mutation and nucleotides additional to the Δ30 mutation deleted from the 3′-UTR; and immunogenic compositions, methods of inducing an immune response, and methods of producing a dengue virus or chimeric dengue virus.
Owner:UNITED STATES OF AMERICA
Dengue virus IgM antibody ELISA diagnostic kit
InactiveCN101308138AIncreased sensitivitySimple methodBiological testingAgainst vector-borne diseasesPositive controlIgm antibody
The invention relates to a dengue virus antibody enzyme-linked immunity diagnostic kit. The kit includes an enzyme labeling board, a negative control serum, a positive control serum, an enzyme marker, a sample diluent, a concentrated washing liquid, a substrate chromogenic liquid, a stop solution and a sealing plate glue, wherein the enzyme marker contains a goat anti-human IgM-HRP; an envelope antigen on the enzyme labeling board includes: a recombinant dengue virus type 1 envelope protein specific antigen with the amino acid sequence of SEQ ID No.1; a recombinant dengue virus type 2 envelope protein specific antigen with the amino acid sequence of SEQ ID No.3; a recombinant dengue virus type 3 envelope protein specific antigen with the amino acid sequence of SEQ ID No.5; and a recombinant dengue virus type 4 envelope protein specific antigen with the amino acid sequences of SEQ ID No .7. An early diagnosis can be made to patients infected with dengue fever for the first time through detecting dengue virus IgM antibodies in serum.
Owner:广东省疾病预防控制中心
Japanese encephalitis/dengue chimeric virus and application thereof
InactiveCN101560520AStable passageViral antigen ingredientsAntiviralsJapanese encephalitisWestern blot
The invention provides a Japanese encephalitis / dengue chimeric virus, which is obtained by replacing a prM / E gene in Japanese encephalitis cDNA clone with the prM / E gene of dengue virus type II. IFA and Western blot confirm that the chimeric virus can express E protein of a dengue virus and can stabilize passage in C6 / 36 cells. The invention lays a foundation for developing a dengue virus vaccine. The chimeric virus can be taken as a vaccine to immunize animals so as to enable the animals to obtain immune protection against the dengue virus.
Owner:中国疾病预防控制中心病毒病预防控制所
Rapid PCR (Polymerase Chain Reaction) testing method of silkworm densovirus BmDNV
InactiveCN102134612AMeeting the Needs of Molecular DiagnosticsEfficient extractionMicrobiological testing/measurementPcr methodMicrobiology
The invention discloses a rapid PCR ((Polymerase Chain Reaction) testing method of a silkworm densovirus BmDNV, which comprises the steps of: rapidly extracting a template DNA of the silkworm densovirus BmDNV by adopting a boiling precipitation method, and designing a specifc primer to test the silkworm densovirus BmDNV through the PCR method. The rapid PCR testing method of the silkworm densovirus BmDNV can be used for effectively extracting the template DNA of the silkworm densovirus BmDNV and testing the silkworm densovirus BmDNV rapidly and accurately, is applicable to small-amount sample operation, and is simple to operate; the DNA sample prepared by using the method can meet the requirement of the PCR method on molecular diagnosis of the silkworm densovirus BmDNV; and the rapid PCR testing method of the silkworm densovirus BmDNV lays the foundation and provides technical reservation for early diagnosis and prediction check of BmDNV by applying a PCR technology.
Owner:SOUTH CHINA AGRI UNIV
Cytotoxic T Lymphocyte Inducing Immunogens For Prevention Treatment and Diagnosis of Dengue Virus Infection
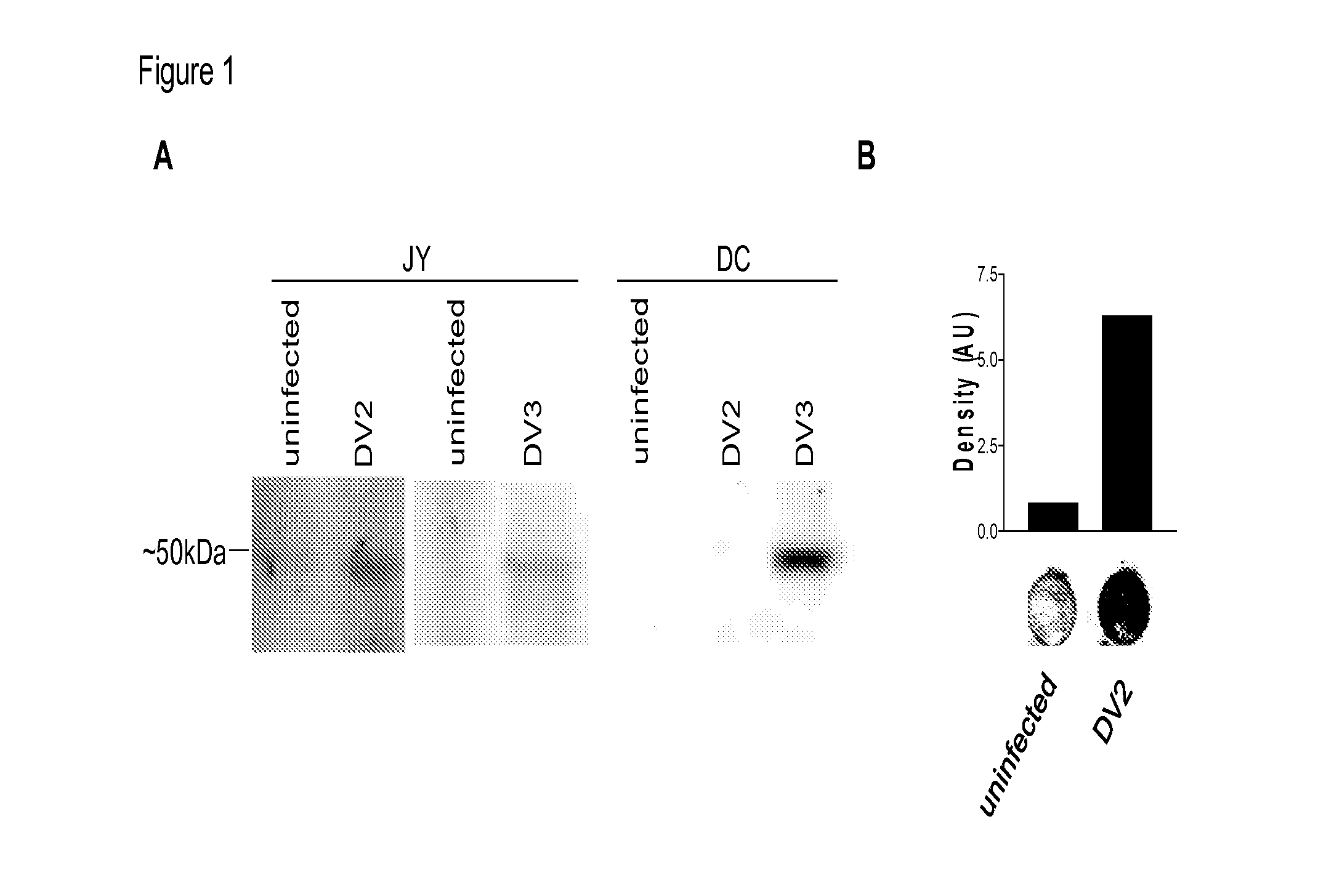
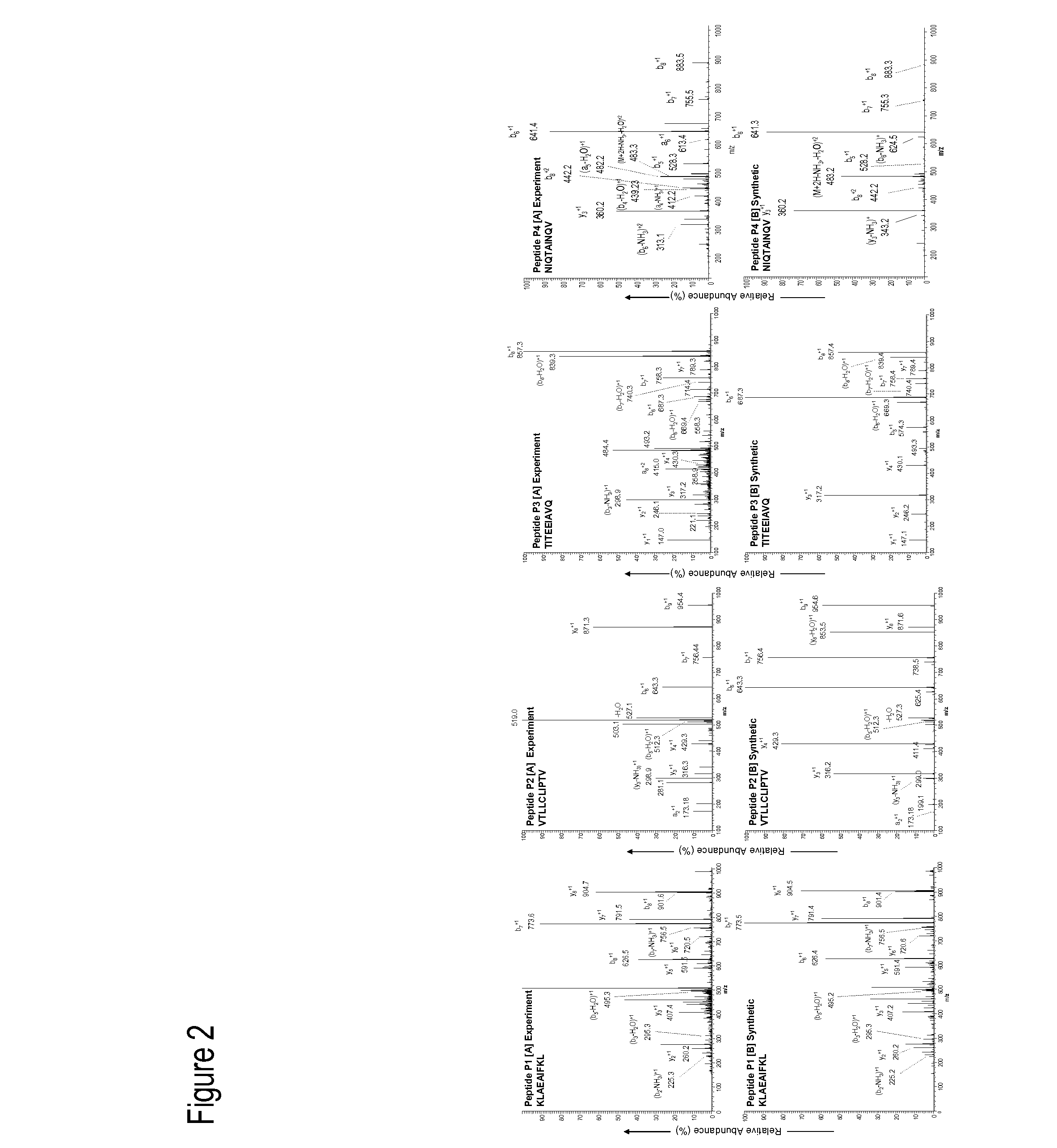
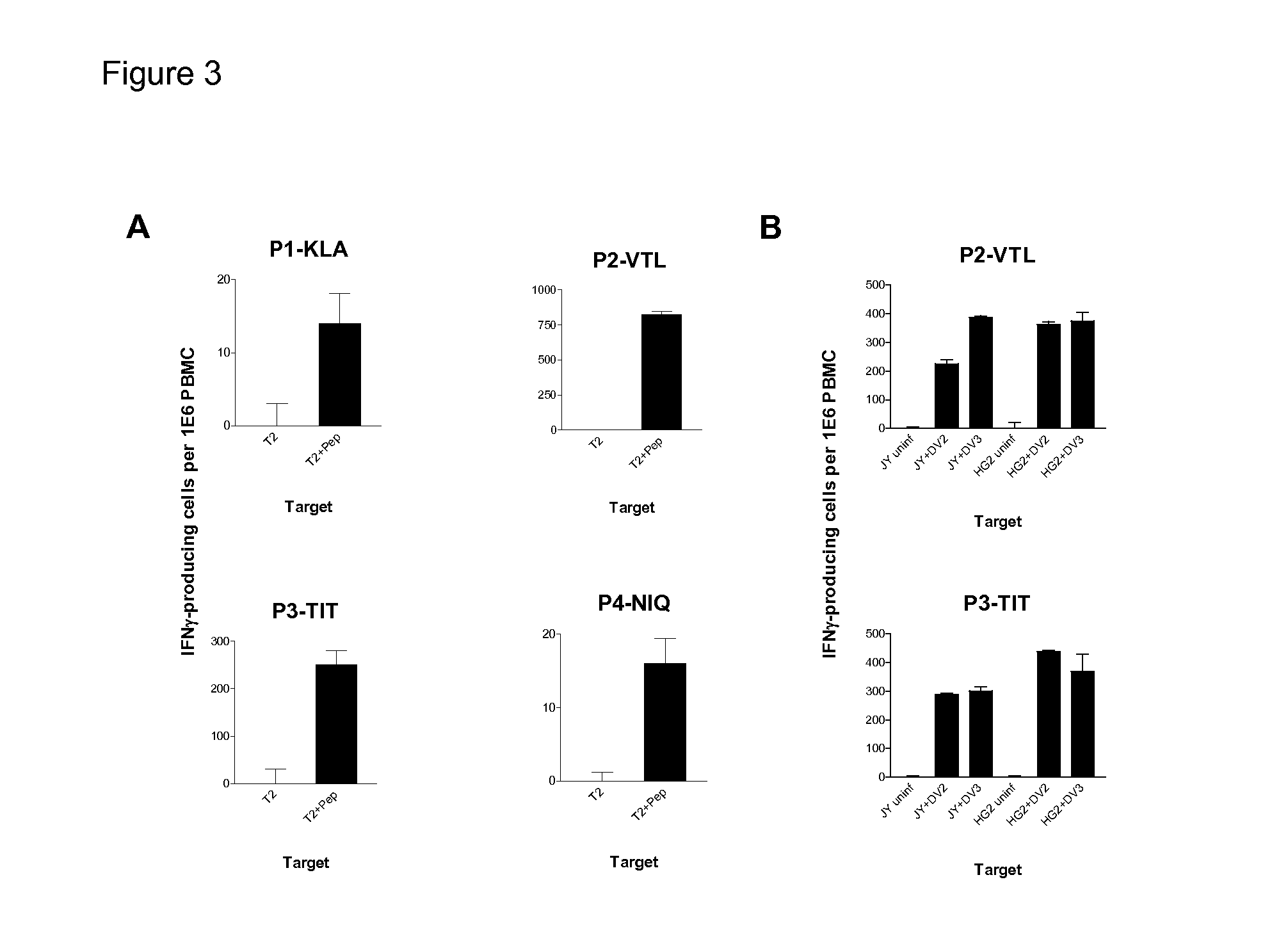
Dengue Fever (DF) and Dengue Hemorrhagic Fever (DHR) are significant global public health problems and understanding the overall immune response to infection will contribute to appropriate management of the disease and its potentially severe complications. Live attenuated and subunit vaccine candidates, which are under clinical evaluation, induce primarily an antibody response to the virus and minimal cross-reactive T cell responses. Currently, there are no available tools to assess protective T cell responses during infection or post vaccination. The present invention incorporates immunoproteomics to uncover novel HLA-A2 specific epitopes derived from Dengue Virus (DV)-infected cells. These epitopes are conserved with epitope-specific CTLs cross-reacting against all four DV serotypes. These epitopes have potential as new informational and diagnostic tools to characterize T cell immunity in Dengue virus (DV) infection, and serves as a universal vaccine candidate complementary to current vaccines.
Owner:EMERGEX VACCINES HLDG LTD
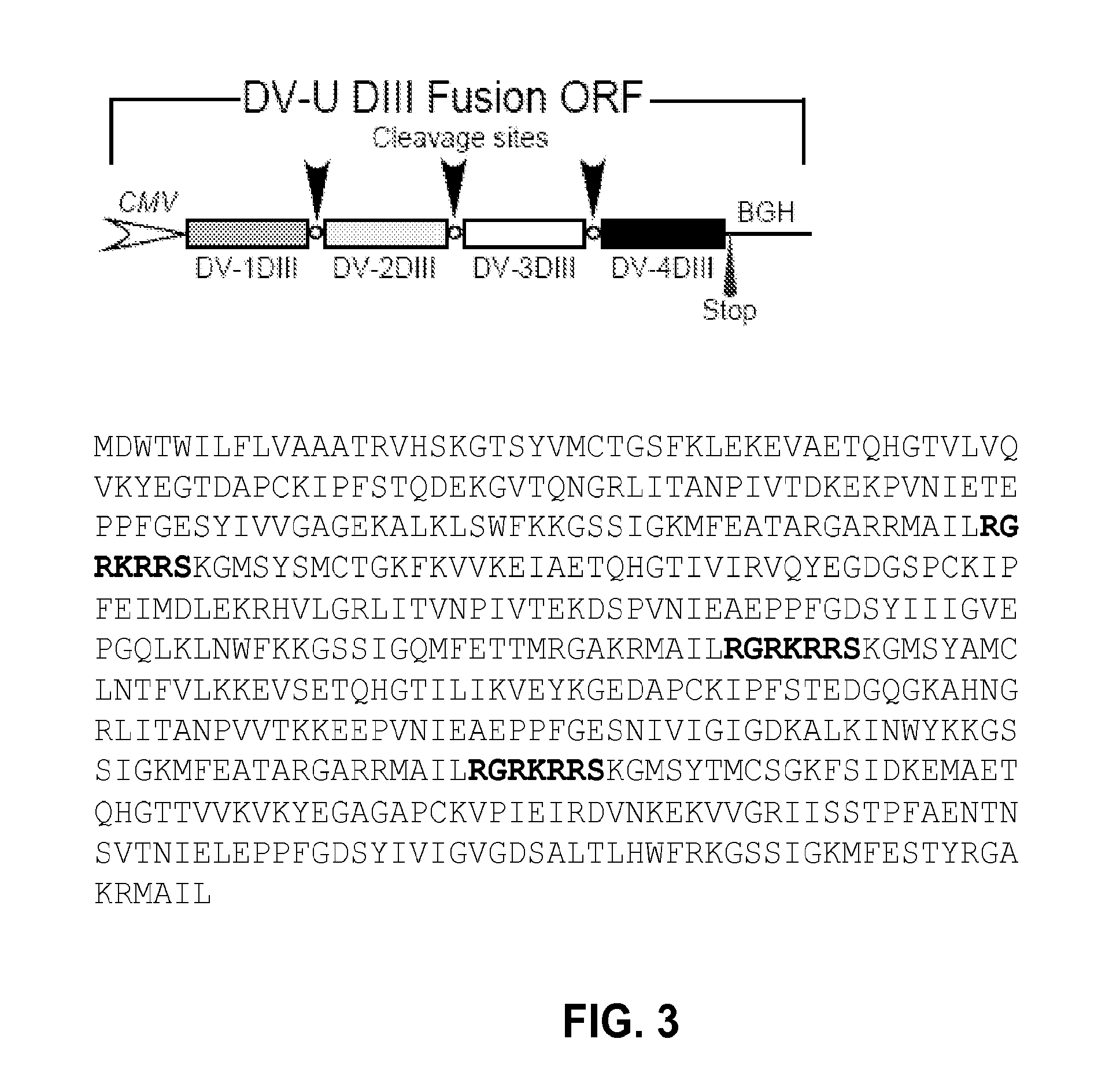
Subgenomic replicons of the flavivirus dengue
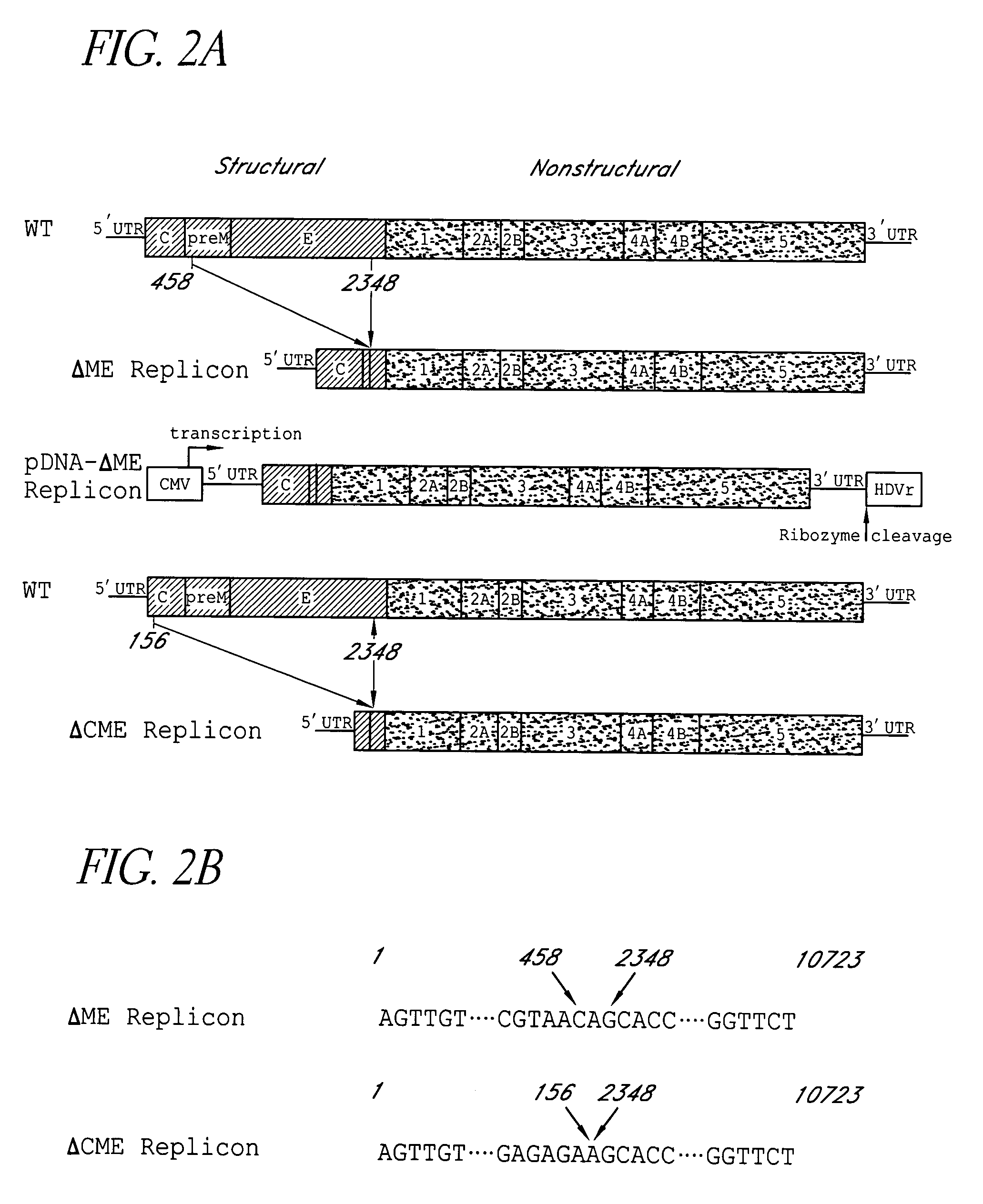
The present invention discloses the construction of dengue virus subgenomic replicons containing large deletions in the structural region (C-preM-E) of the genome, which replicons are useful as vaccines to protect against dengue virus infection.
Owner:DEPT OF HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US SEC
Hybridoma cells and monoclonal antibody capable of secreting II type dengue virus NS1 monoclonal antibody and application
ActiveCN104357401AHigh secretion yieldIncreased sensitivityImmunoglobulins against virusesTissue cultureAntigenNanoparticle
The invention relates to an II type dengue virus test paper which is high in sensitivity and specificity. The test paper uses the II type dengue virus NS1 monoclonal antibody secreted by the hybridoma cells for indirectly marking when markers are large-sized nano particles, so as to reduce the usage amount of labelled antigen, and improve the sensitivity while improving the specificity. The invention, in addition, further relates to hybridoma cells and a monoclonal antibody which are capable of secreting an II type dengue virus NS1 monoclonal antibody and the application. The two hybridoma cells are preserved in the preservation center of Wuhan University, Luojiashan street, Wuchang district, Wuhan City, Hubei province, and the preservation numbers are respectively CCTCC No: C2014183 and CCTCC No: C2014182.
Owner:FAPON BIOTECH INC +1
Dengue virus serology early diagnosis reagent
InactiveCN101962411AImprove featuresHigh sensitivityMicroorganism based processesFermentationSerodiagnosesSerological assay
The invention provides a dengue virus serology early diagnosis reagent, in particular, EIII structural domains of dengue viruses 1, 2, 3 and 4 are serially connected to obtain a recombinant fusion protein rEIII used for serodiagnosis of the dengue virus infection. In addition, the invention also provides an early diagnosis agent kit suitable for the dengue virus infection. The invention provides a new idea for the serodiagnosis of the dengue virus infection. The dengue virus infection can be effectively diagnosed by using the diagnosis reagent, especially, the Mac-ELISA (Enzyme Linked Immunosorbent Assay) diagnosis agent kit can be effectively used for the early serodiagnosis of the dengue virus infection. The diagnosis reagent has the advantages of better specificity and sensitivity and simple operation, and is suitable for being widely popularized and used.
Owner:中国疾病预防控制中心病毒病预防控制所
Primer and probe sequence for detecting dengue virus II type nucleic acid fragment
InactiveCN101240349AMicrobiological testing/measurementAgainst vector-borne diseasesAgricultural scienceDensovirus
The invention is a PCR amplifying primer and probe sequence for dengue virus II nucleotide fragment. The primer sequence includes a primer pair composed of upstream primer Den II pf having sequence TTCATGGCCCTGGTGGC and downstream primer Den II pr having sequence TGATTTTTTRATTGTTCCCCATCT. The probe Den II pb sequence is CGTTTCCTAACAATCCCACCAACAGC.
Owner:TAITAI GENOMICS +1
Immunodiagnosis kit for detecting III dengue virus NS1 antigen and application thereof
InactiveCN101576560ALow costSimple process requirementsMaterial analysisAgainst vector-borne diseasesPositive controlEarly type
The invention discloses an immunodiagnosis kit for detecting III dengue virus NS1 antigen and the application of the kit to the detection of the III dengue virus NS1 antigen. The immunodiagnosis kit for detecting III dengue virus NS1 antigen provided by the invention comprises a microplate coated by peridium monoclonal antibody 1D14A2A10, monoclonal antibody 5D32A17 marked by biotin, positive control, negative control, concentration wash solution, colored solution and stopping solution. The kit can specifically detect that NS1 protein of the III dengue virus and the other three types of serotype dengue virus NS1 have no cross reaction and combines the different antigen sites of the NS1. The immunodiagnosis kit for detecting III dengue virus NS1 antigen can rapidly detect large amounts of samples at the same time, has the characteristics of high specificity, high sensitivity, and the like and important clinical application value, and can rapidly and correctly perform early typing diagnosis to the patients with the dengue virus infection.
Owner:SOUTHERN MEDICAL UNIVERSITY
Preparation and application of hemorrhagic fever associated pathogen identifying gene chip
ActiveCN105087824APracticalShort detection cycleNucleotide librariesMicrobiological testing/measurementOligonucleotide chipOligonucleotide
The invention relates to a hemorrhagic fever associated pathogen identifying gene chip; the preparation method comprises preparation of a specific primer, preparation of a pathogen specific oligonucleotide probe, preparation of an oligonucleotide chip, establishment of an RT-PCR (reverse transcription-polymerase chain reaction) system and establishment of a hybrid system and a signal detection method. The gene chip prepared by the invention can be used for simultaneously identifying 16 hemorrhagic fever associated pathogen microorganisms, including Zaire Ebola virus, Sudan Ebola virus, marburg virus, lassa virus, junin virus, Machupo virus, rift valley fever virus, Crimea-Congo hemorrhagic fever virus, plasmodium, hantaan virus, SFTS (severe fever with thrombocytopenia syndrome) virus, dengue virus, yellow fever virus, Chikungunya virus, influenza A virus and influenza B virus. The gene chip has the characteristics of being rapid and accurate, high in throughput and high in sensitivity; and a new technological means is offered for the diagnosis of hemorrhagic fever pathogen, health supervision and the control and prevention of infectious diseases.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION +1
Preparation method and application of virus-like particles of dengue viruses
InactiveCN101948850AViral antigen ingredientsInactivation/attenuationImmune effectsVirus-like particle
The invention relates to a preparation method and application of virus-like particles (VLPs) of dengue viruses. The preparation method comprises the steps of: respectively modifying four types of dengue viruses (DENV) prM-E gene elements by means of a fusing PCR (Polymerase Chain Reaction) technology, then respectively cloning the four types of modified dengue viruses prM-E gene elements into a eukaryon system expression vector, respectively transfecting a mammalian cell with the recombinant expression vectors, and secreting the mammalian cells to express the virus-like particles of the dengue viruses. In addition, the invention lays a foundation for the development of a dengue viruses VLPs polyvaccine by carrying out preliminary study on the immune effects of DENV-1 VLPs and DENV-2 VLPs.
Owner:中国疾病预防控制中心病毒病预防控制所
Flavivirus antigens
Owner:ALTRAVAX
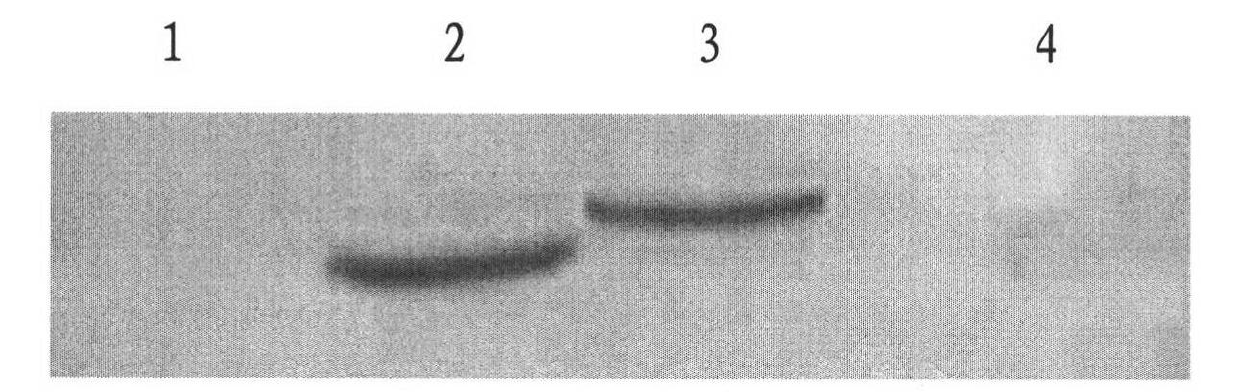
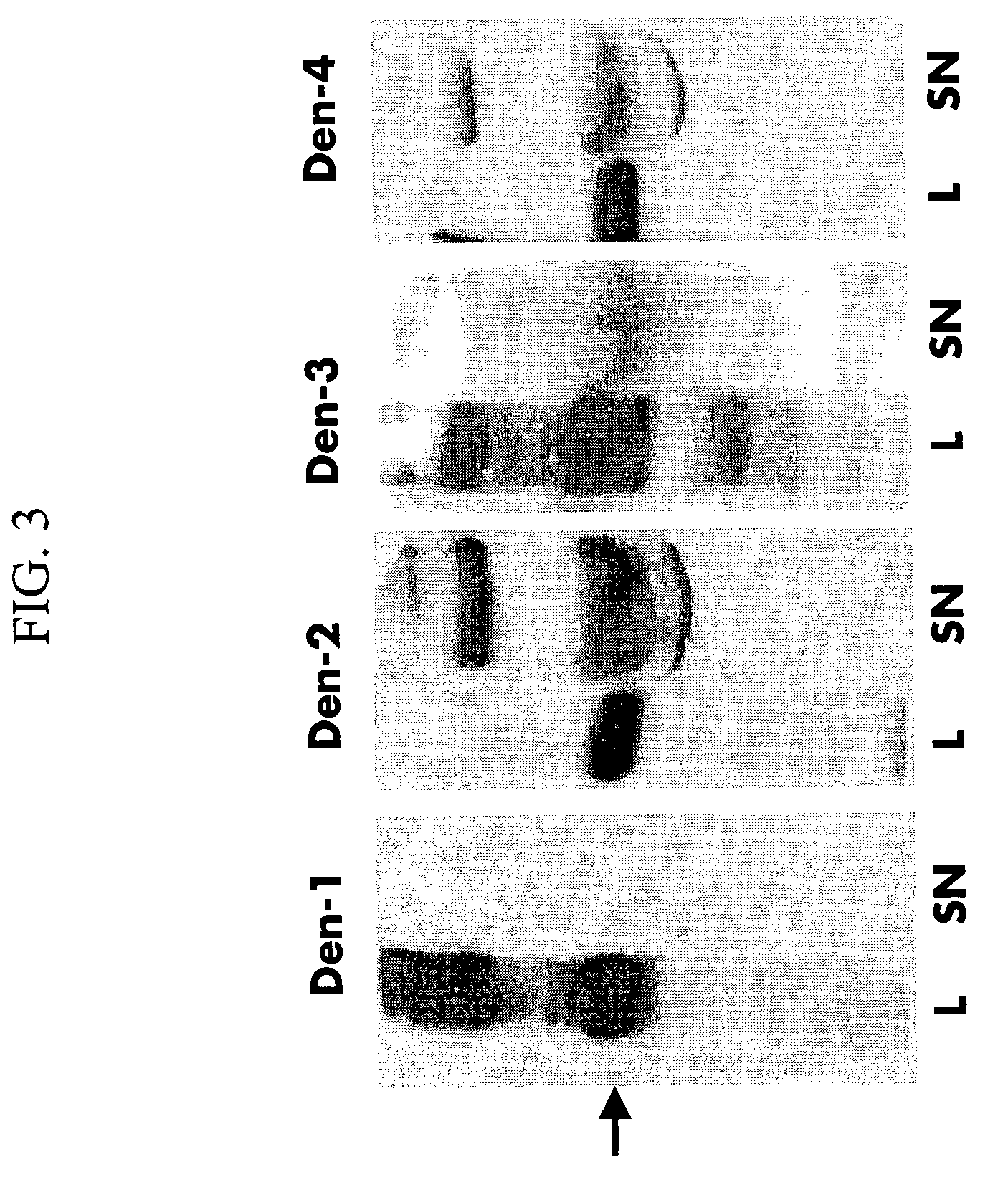
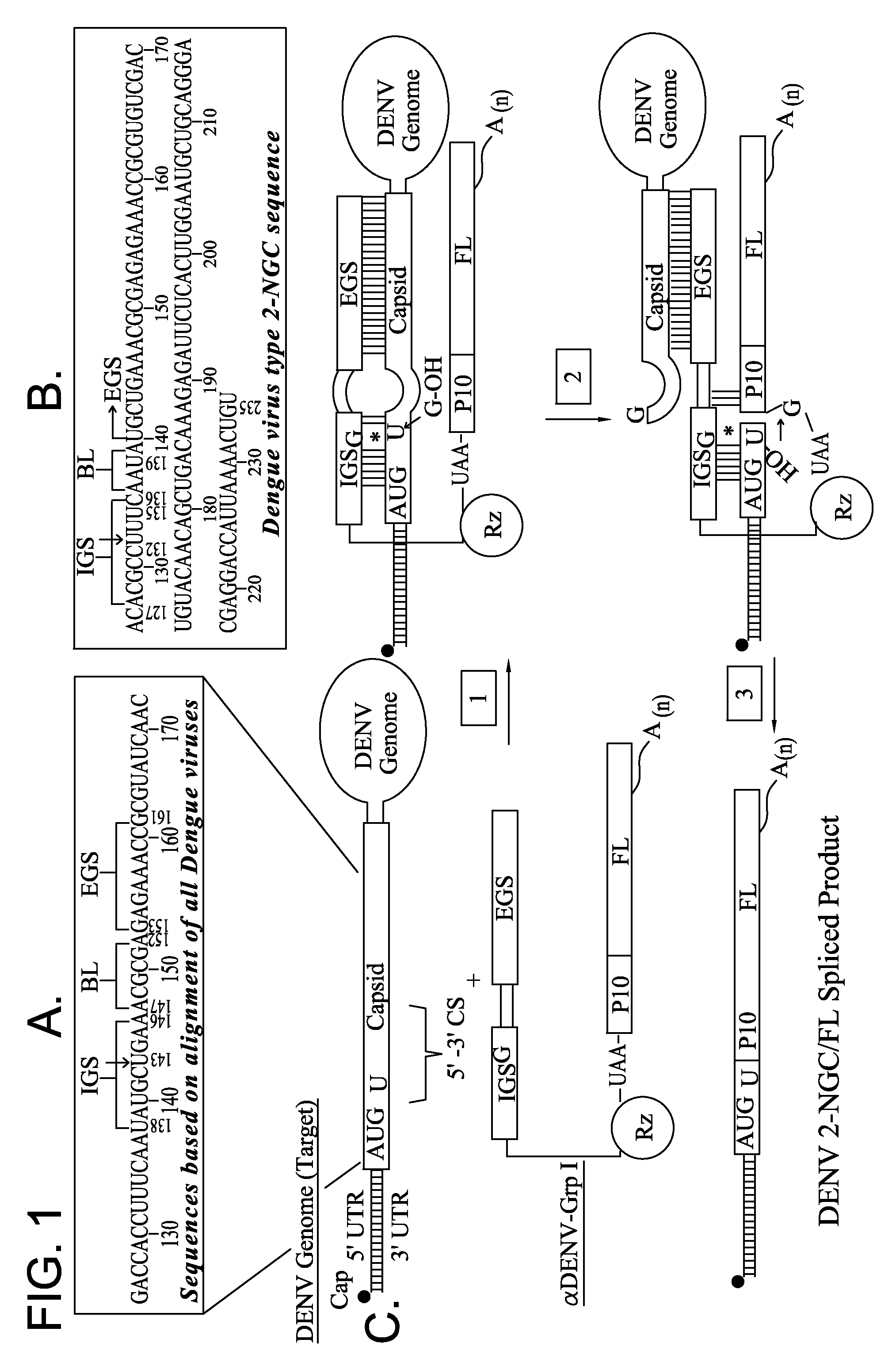
Ribozyme Effector Gene in Dengue Fever Transmission and Disease Control
InactiveUS20120278913A1Level of activation were somewhat lowLower levelAnimal cellsSsRNA viruses positive-senseDiseaseIntein
Disclosed are anto-DENV ribozyme based methods and compositions useful in the inhibition and control of all Dengue fever serotypes (designated DENV 1 through 4). A group of anti-DENV Group 1 trans-splicing introns (αDENV-GrpIa) are presented that target DENV-2 NGC genomes in situ. Methods for specifically targeting a highly conserved 5′-3′ cyclization sequence (CS) region that is common to all serotypes of the DENV are provided. The anti-DENV Group 1 trans-splicing introns (αDENV-GrpIa) specifically target two different uracil bases on the positive sense genomic strand. The invention provides an RNA based approach for transgeneic suppression of DENV in transformed mosquitoes using a group of specifically designed introns that trans-splice a new RNA sequence downstream of a targeted site. The aDENV-GrpIs target DENV infected genomes and thus provide a method for inhibiting the spread of Dengue fever. An αDENV-GrpI 9v1 is presented that is designed to be active against all forms of Dengue virus, and to effectively target the DENV-2 NGC genome in a sequence specific manner
Owner:UNIV OF NOTRE DAME DU LAC
Dengue Reporter Virus and Methods of Making and Using the Same
The present invention relates to the production and uses of Dengue virus replicons and Dengue reporter virus particles. The present invention relates to methods of identifying inhibitors of Dengue virus infection, inhibitors of Dengue virus replication, and inhibitors of Dengue virus assembly.
Owner:INTEGRAL MOLECULAR
Antibody moleules to dengue virus and uses thereof
Antibody molecules that specifically bind to dengue virus are disclosed. In certain embodiments, the antibody molecule bind to dengue virus serotypes DV-1, DV-2, DV-3, and DV-4. The antibody molecules can be used to treat, prevent, and / or diagnose dengue virus.
Owner:VISTERRA
Primer and probe sequence for detecting dengue virus 3 nucleotides fragment
InactiveCN101139638AMicrobiological testing/measurementAgainst vector-borne diseasesAgricultural scienceDensovirus
The invention provides a PCR expansion primer and probe sequence for a dengue virus type III nucleotide section. The primer sequence comprises a primer pair comprising an upstream primer DenIIIpf of sequence GGAAAACCGTCTATCAATATGCTG and a downstream primer Den IIIpr of sequence TCCTCTTGAGAATCTCTTCGCC. The probe DenIIIpb is of sequence CGCGTGAGAAACCGTGTGTCAACTG.
Owner:TAITAI GENOMICS +1
Method for detection and typing of dengue virus, special chip and kit
InactiveCN102286636AStrong specificityFor long-term storageMicrobiological testing/measurementAgainst vector-borne diseasesClassification methodsTrue positive rate
The invention discloses a dengue virus detection and parting method, a special chip and a kit. The kit comprises a biological chip and target segment primer pair compositions: primer pairs SEQ ID NO:6 and SEQ ID NO:7; primer pairs SEQ ID NO:8 and SEQ ID NO:9; primer pairs SEQ ID NO:10 and SEQ ID NO:11; primer pairs SEQ ID NO:12 and SEQ ID NO:13; and primer pairs SEQ ID NO:14 and SEQ ID NO:15, wherein the target segment primer pair compositions are hybridized with probes in amplification samples. The kit can detect dengue viruses and can carry out parting identification on the dengue viruses, the dengue viruses can be classified and directly identified into dengue viruses, first dengue viruses, second dengue viruses, third dengue viruses and fourth dengue viruses, the dengue virus classification experiment is carried out on the basis of the method, a better effect is obtained, the true positive rate reaches 100 percent, and the true negative rate reaches 100 percent. The accuracy rate of the four-type dengue virus identification reaches 100 percent.
Owner:SHENZHEN ACAD OF INSPECTION & QUARANTINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com