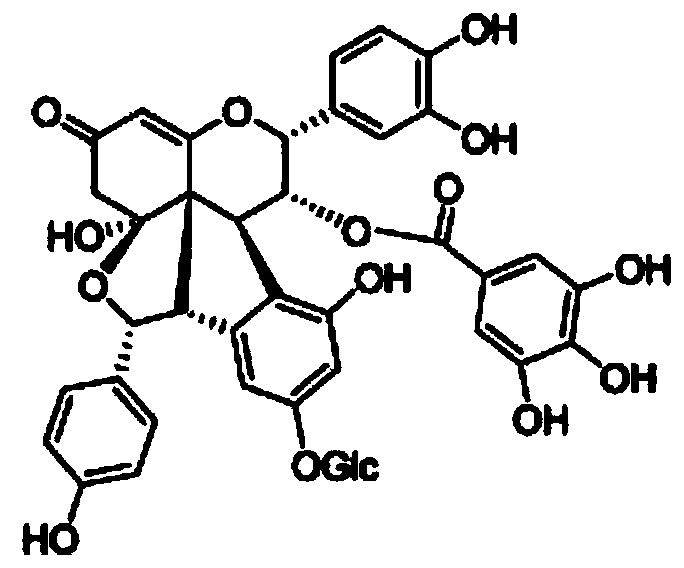
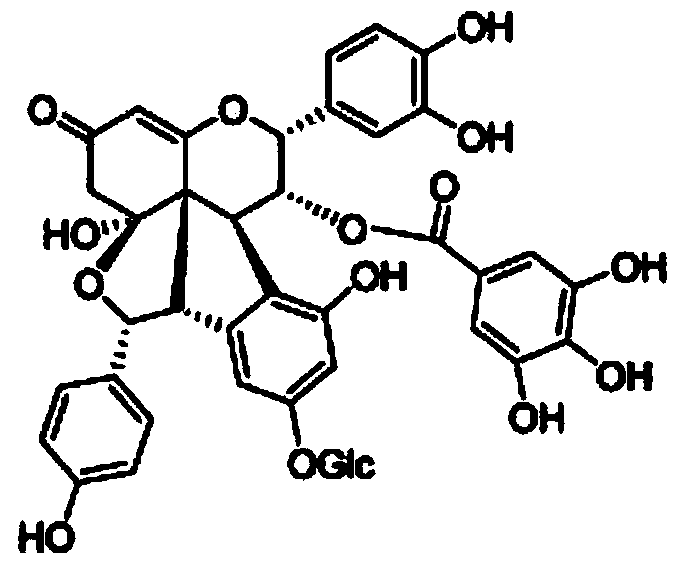
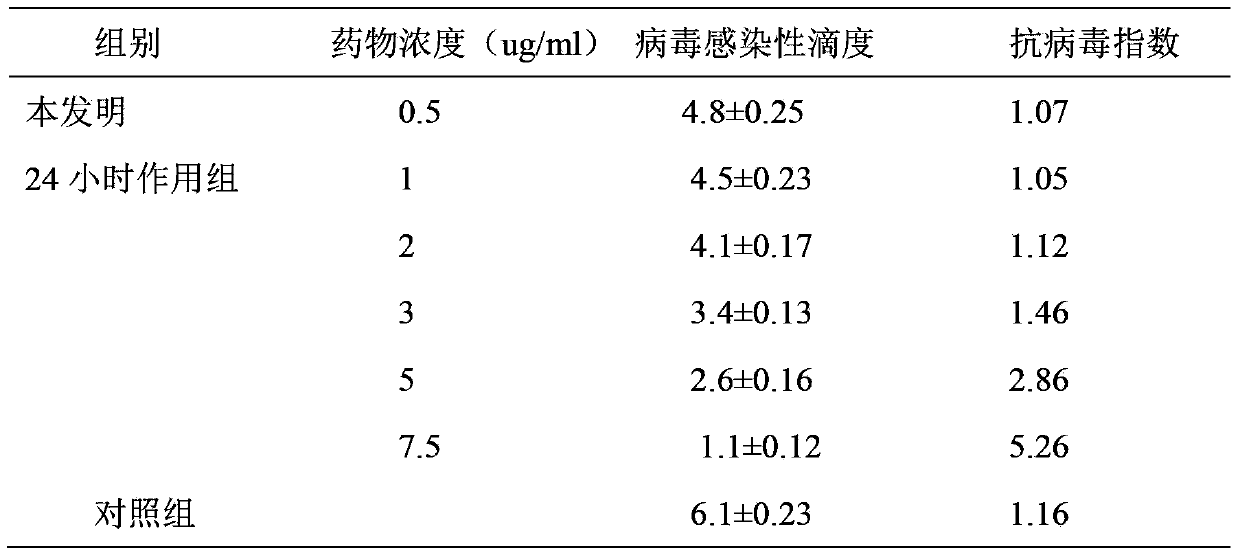
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
42 results about "Dengue hemorrhagic fever" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dengue (DENG-gey) fever is a mosquito-borne disease that occurs in tropical and subtropical areas of the world. Mild dengue fever causes a high fever, rash, and muscle and joint pain. A severe form of dengue fever, also called dengue hemorrhagic fever, can cause severe bleeding, a sudden drop in blood pressure (shock) and death.
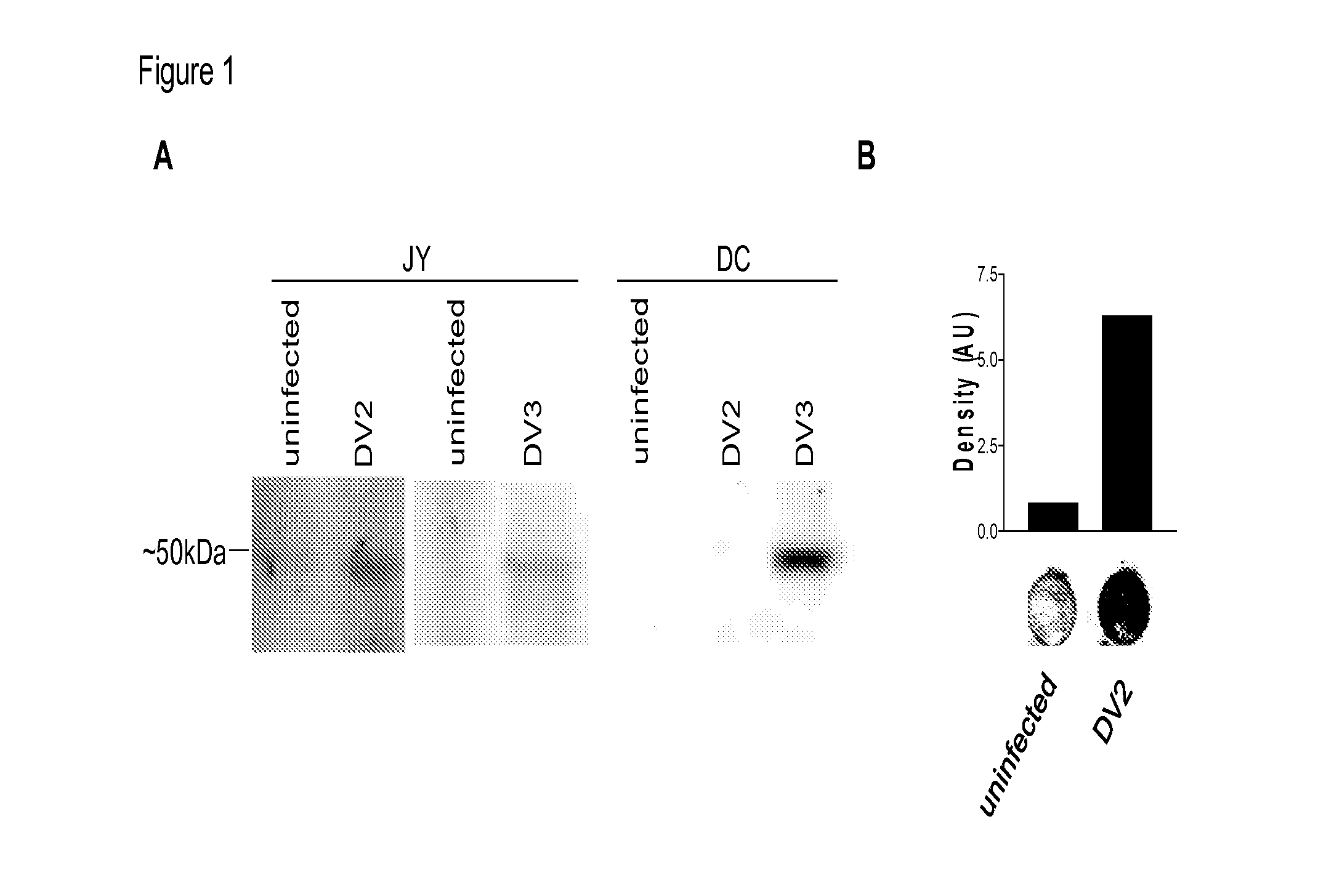
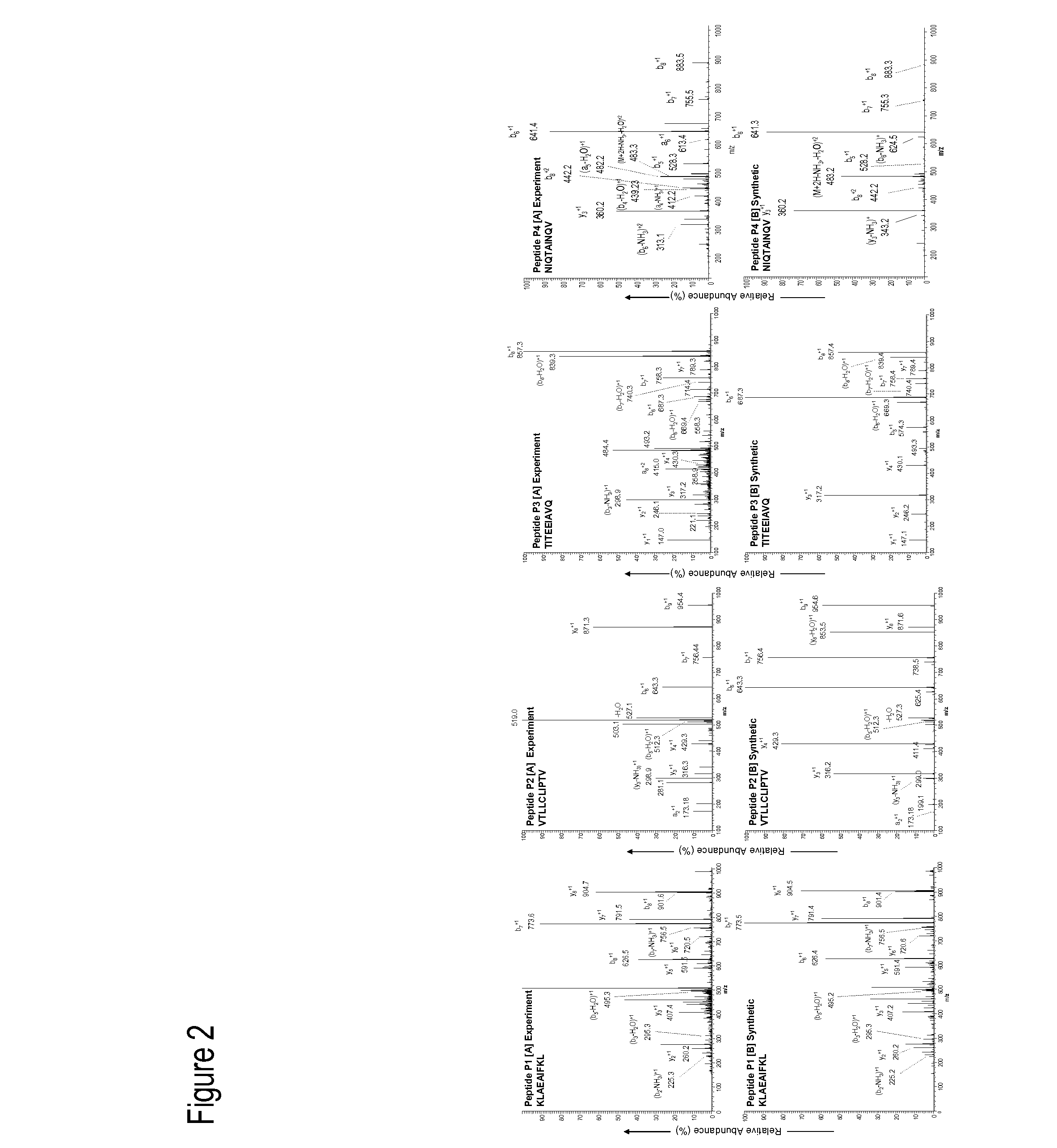
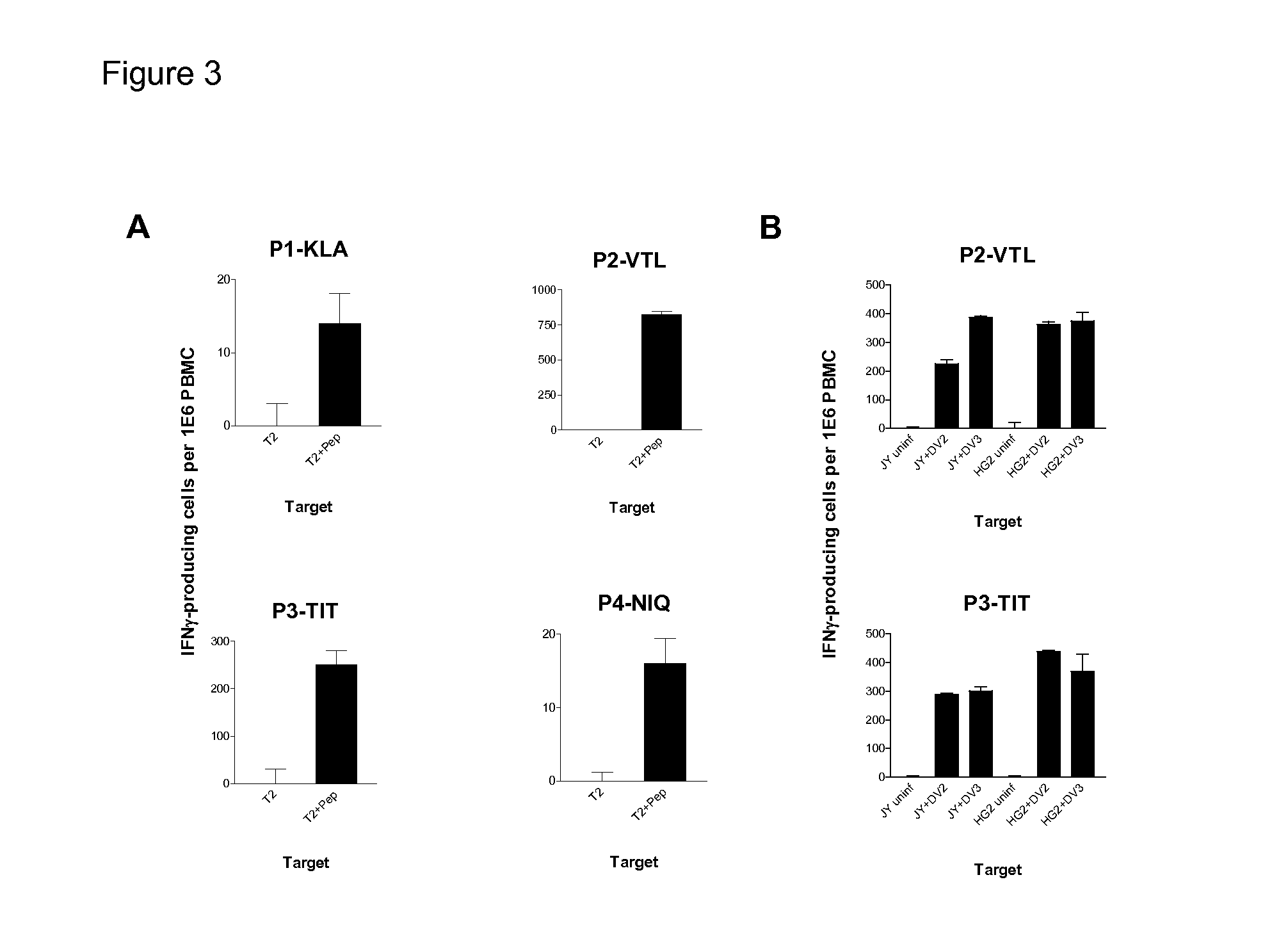
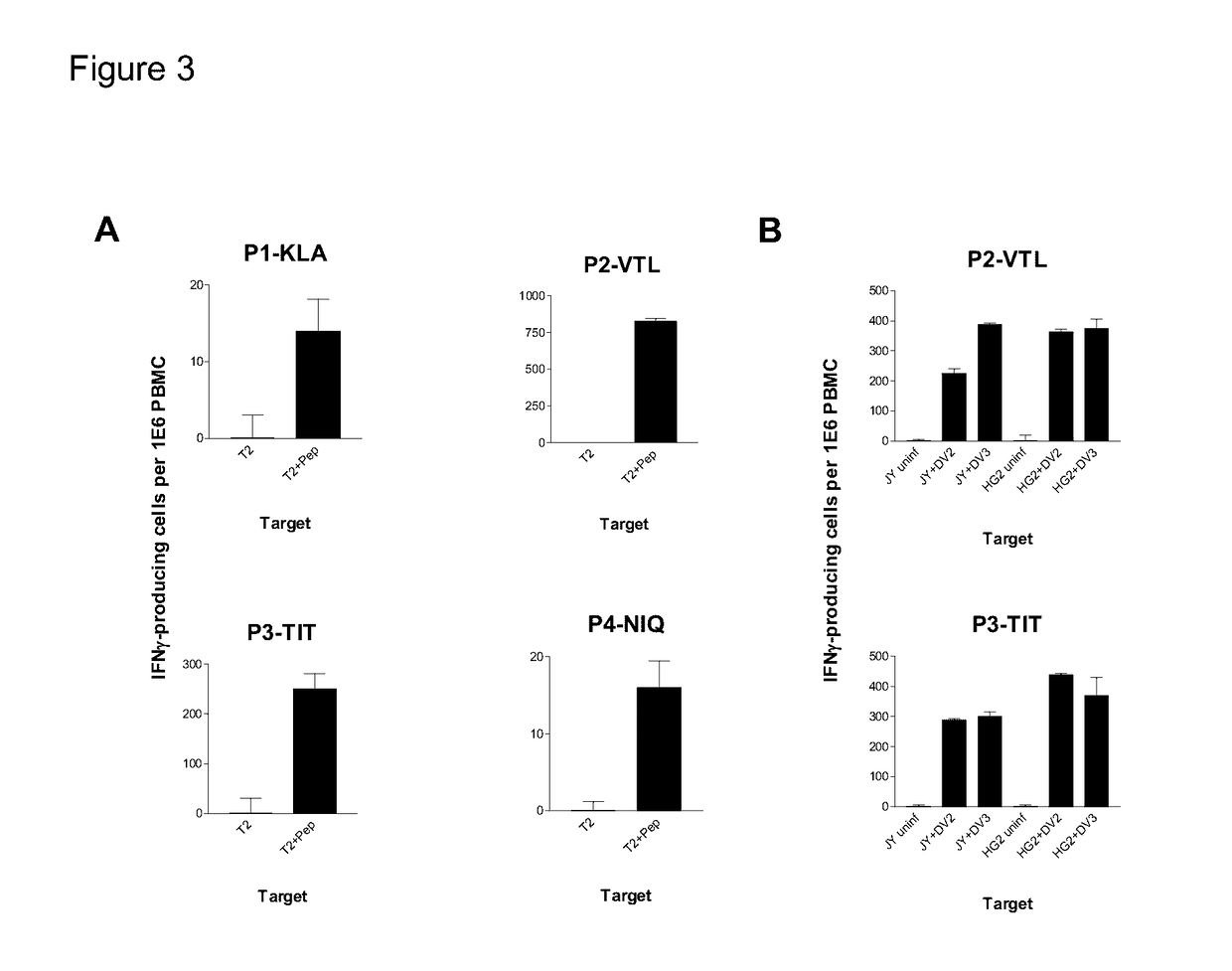
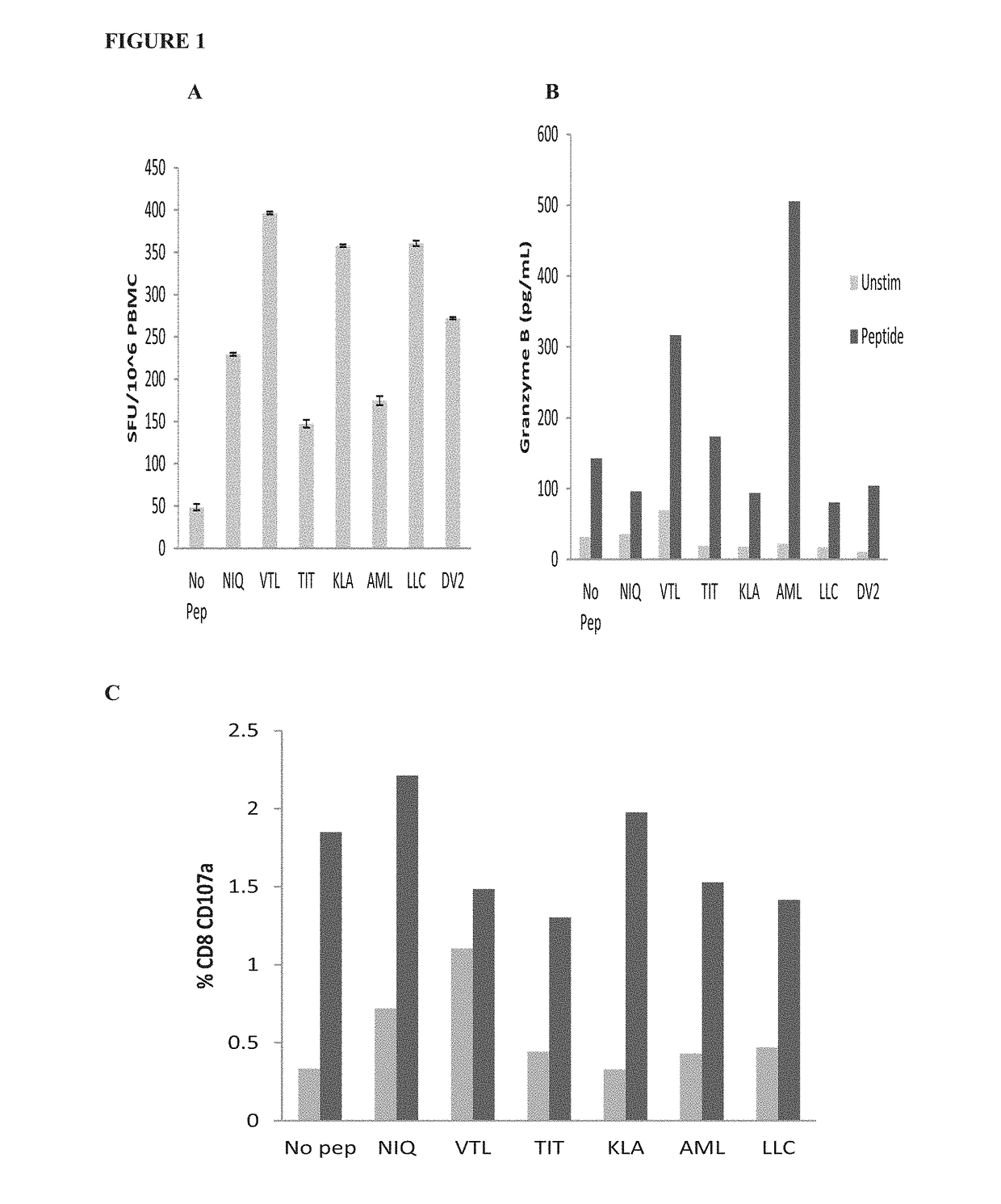
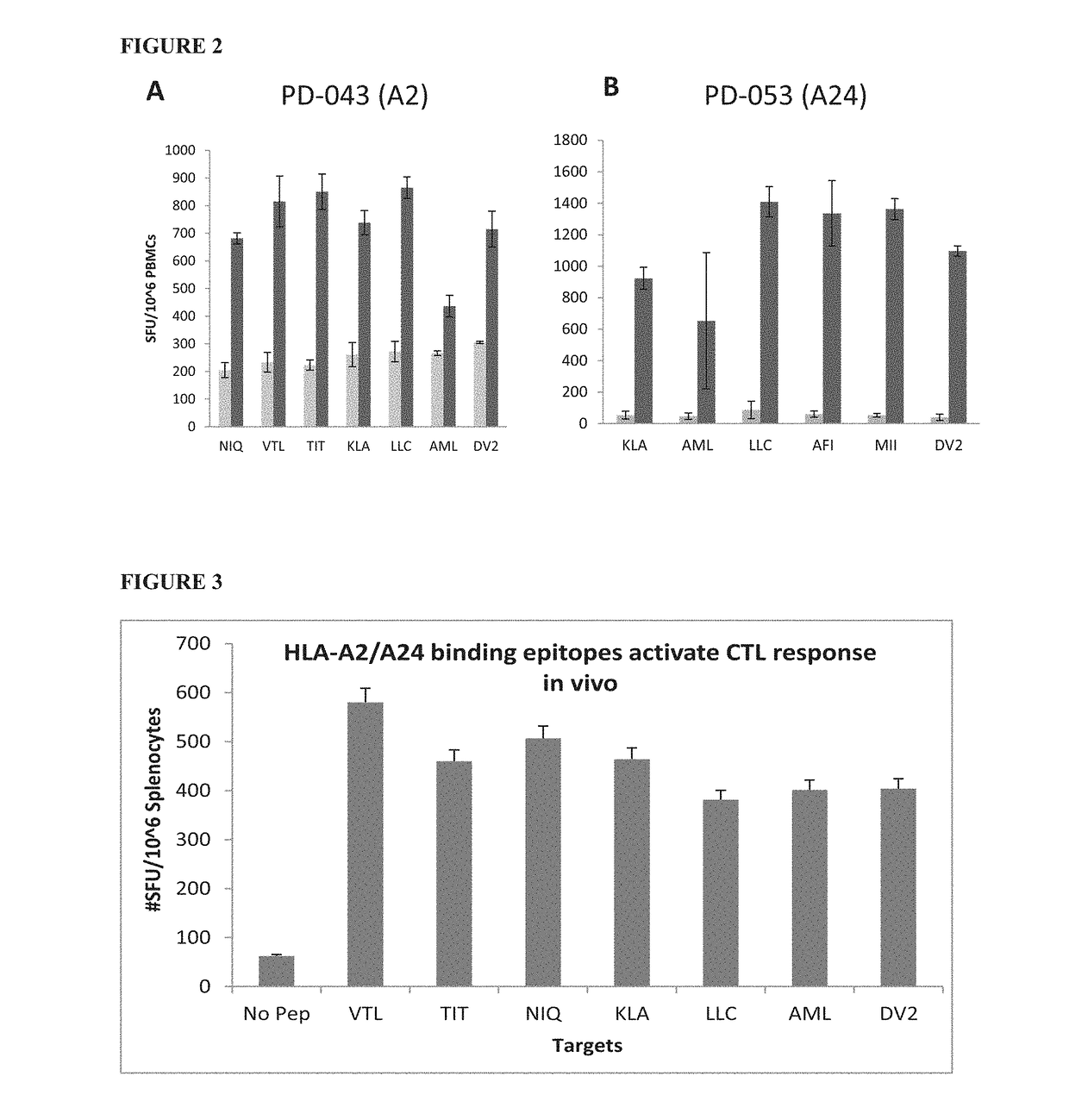
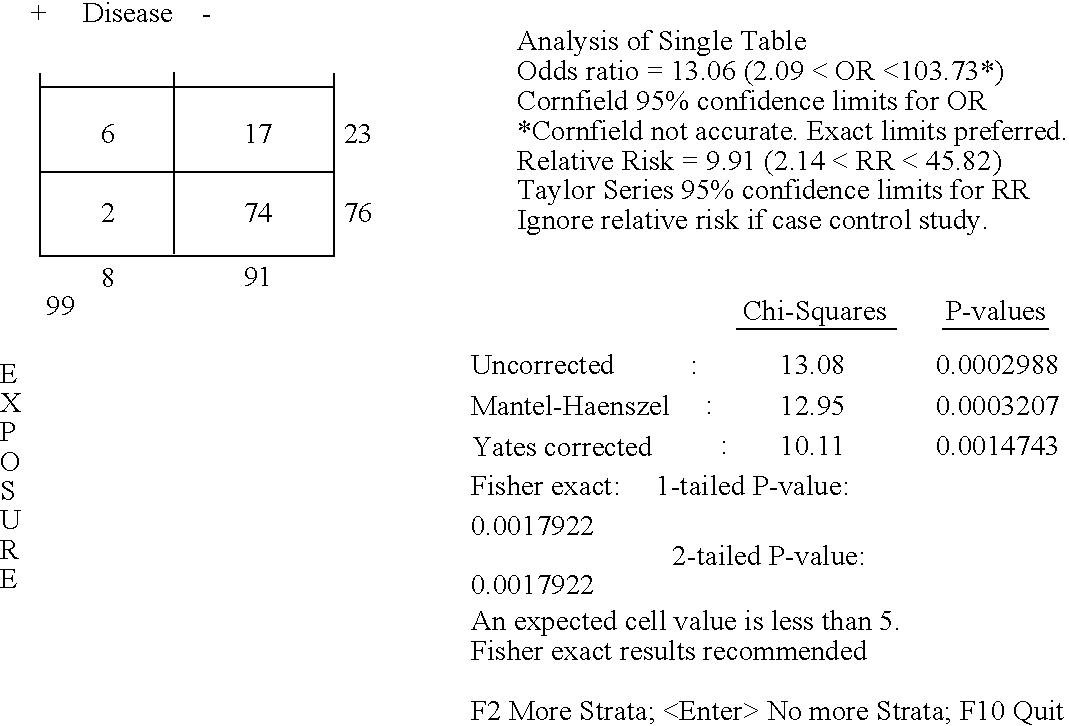
Cytotoxic T Lymphocyte Inducing Immunogens For Prevention Treatment and Diagnosis of Dengue Virus Infection
Dengue Fever (DF) and Dengue Hemorrhagic Fever (DHR) are significant global public health problems and understanding the overall immune response to infection will contribute to appropriate management of the disease and its potentially severe complications. Live attenuated and subunit vaccine candidates, which are under clinical evaluation, induce primarily an antibody response to the virus and minimal cross-reactive T cell responses. Currently, there are no available tools to assess protective T cell responses during infection or post vaccination. The present invention incorporates immunoproteomics to uncover novel HLA-A2 specific epitopes derived from Dengue Virus (DV)-infected cells. These epitopes are conserved with epitope-specific CTLs cross-reacting against all four DV serotypes. These epitopes have potential as new informational and diagnostic tools to characterize T cell immunity in Dengue virus (DV) infection, and serves as a universal vaccine candidate complementary to current vaccines.
Owner:EMERGEX VACCINES HLDG LTD
Detection method of hemorrhagic fever with renal syndrome IgM antibodies and reagent kit
ActiveCN103033616AReduce the impact of stabilityExtended shelf lifeMaterial analysisSerum igeIgm antibody
The invention relates to the technical field of biological detection, in particular to a detection method of hemorrhagic fever with renal syndrome IgM (immunoglobulin M) antibodies and a reagent kit. The detection method comprises the steps that hemorrhagic fever with renal syndrome antigens are fixed on a membrane; to-be-detected serum is added; the hemorrhagic fever with renal syndrome IgM antibodies in the to-be-detected serum are combined with the antigens fixed on the membrane; gold labeled operating fluid containing mouse anti-human IgM monoclonal antibodies is added; the mouse anti-human IgM monoclonal antibodies are combinec with the hemorrhagic fever with renal syndrome IgM antibodies in the to-be-detected serum; a red color is shown; scrubbing liquid is added finally; and the redundant gold labeled operating fluid and other impurities are washed out. The reagent kit has the characteristics of longer quality guarantee period, less cross reaction, better color rendering performance and the like.
Owner:山东康华生物医疗科技股份有限公司
Substituted quinoline-2-formaldehyde-phenylhydrazone derivative, and preparation method and application thereof
InactiveCN102731394AInhibition of replicationBlock life cycleOrganic chemistryAntiviralsQuinolineEnzyme inhibitor
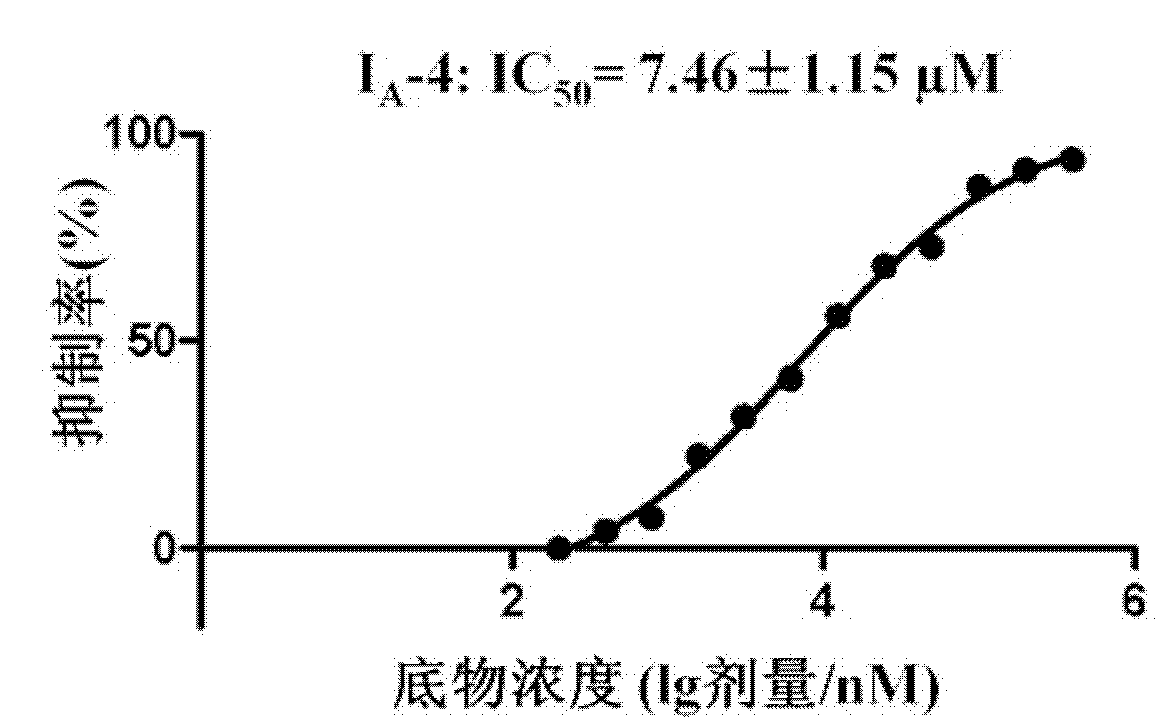
The present invention relates to a substituted-quinoline-2-formaldehyde-phenylhydrazone derivative and a preparation method and application thereof, and in particular relates to a novel 4-acyl-quinoline-2-formaldehyde-phenylhydrazone derivative, and a preparation method and application thereof to preparation of therapeutic drugs for dengue fever and dengue hemorrhagic fever caused by dengue virus. A structure of the compound is shown as a formula 1. The compound can be used as a protease inhibitor for a dengue virus NS2B-NS3, so as to block further proliferation of the dengue virus in a host.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
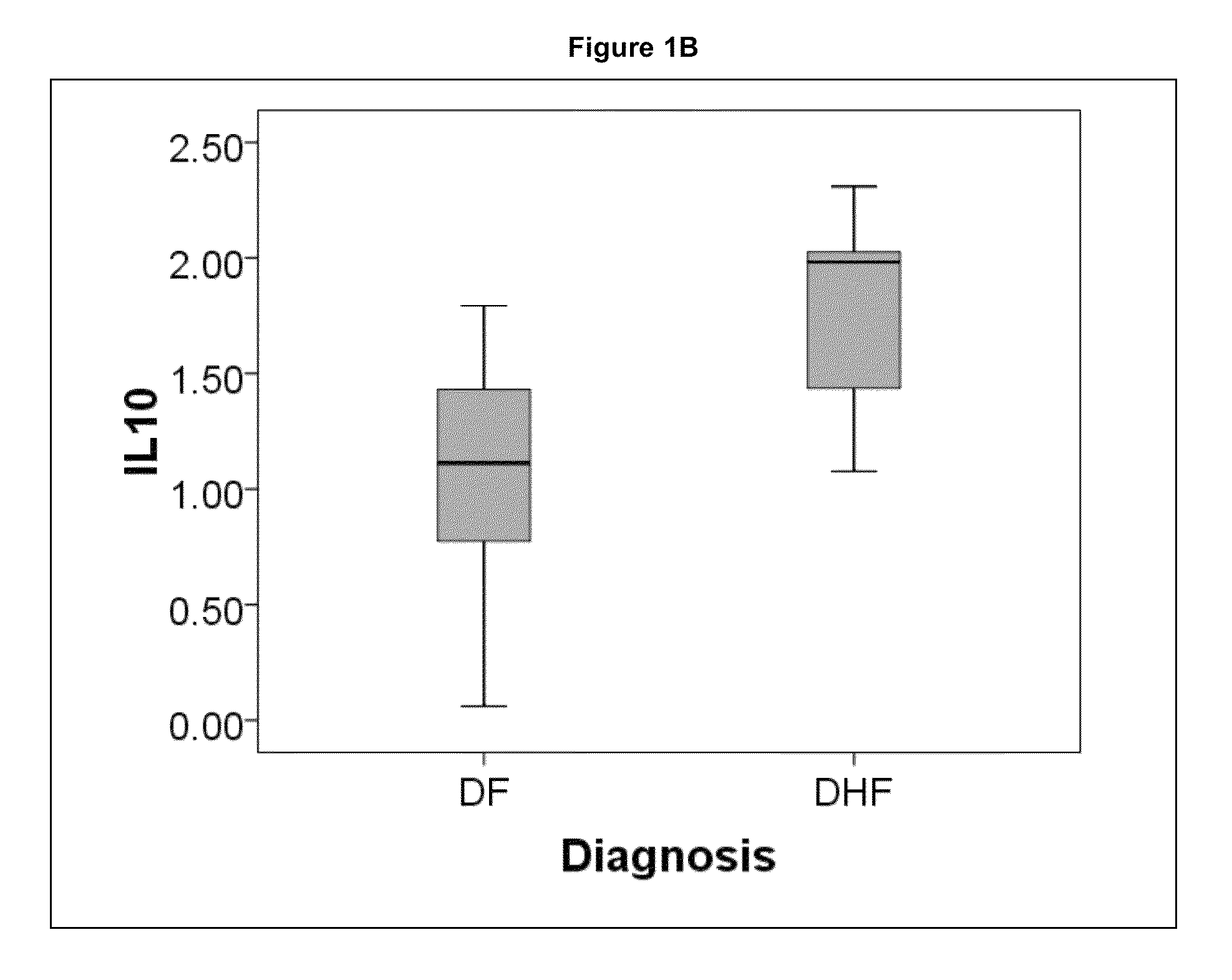
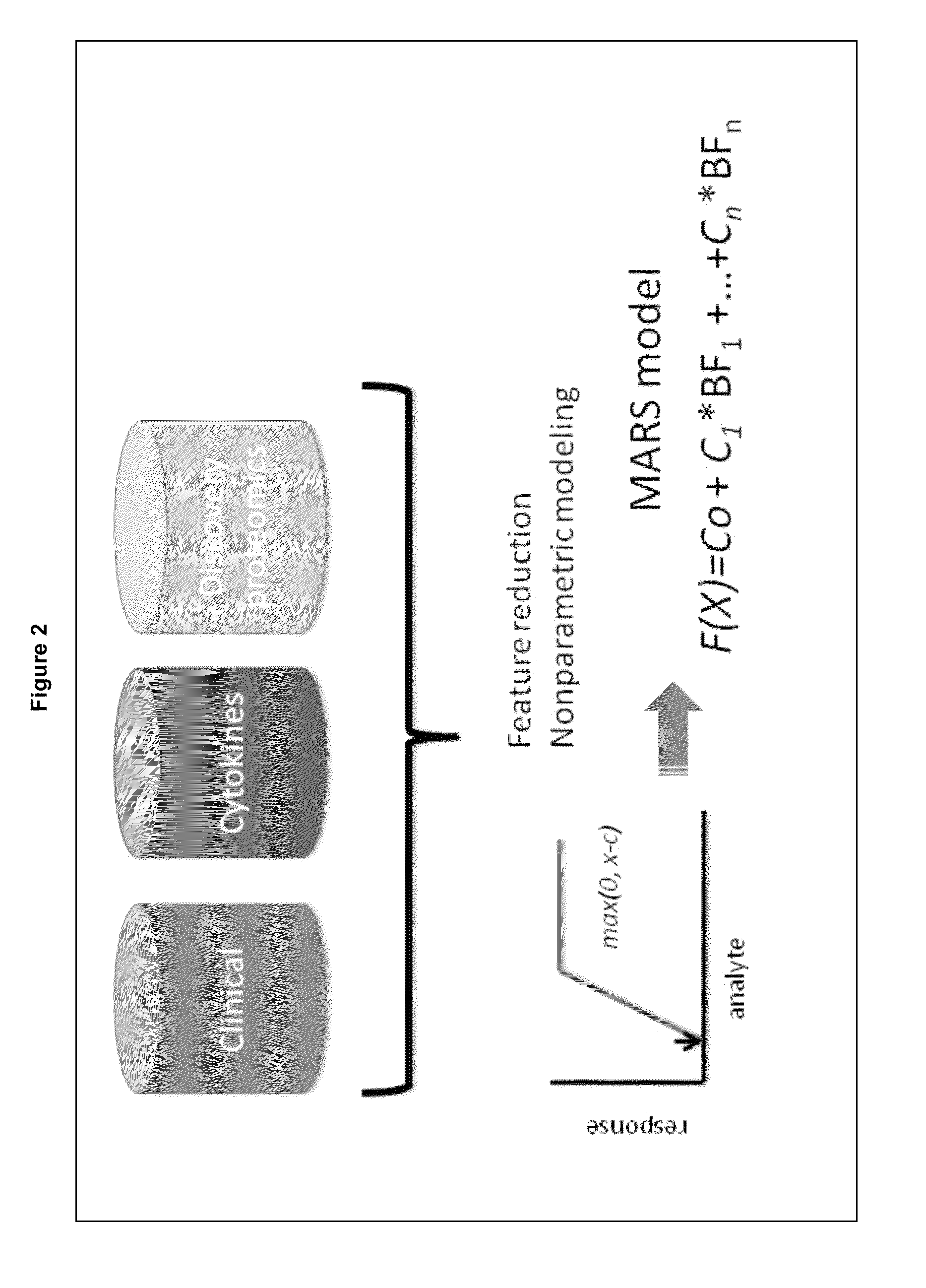
Methods and Biomarkers for the Detection of Dengue Hemorrhagic Fever
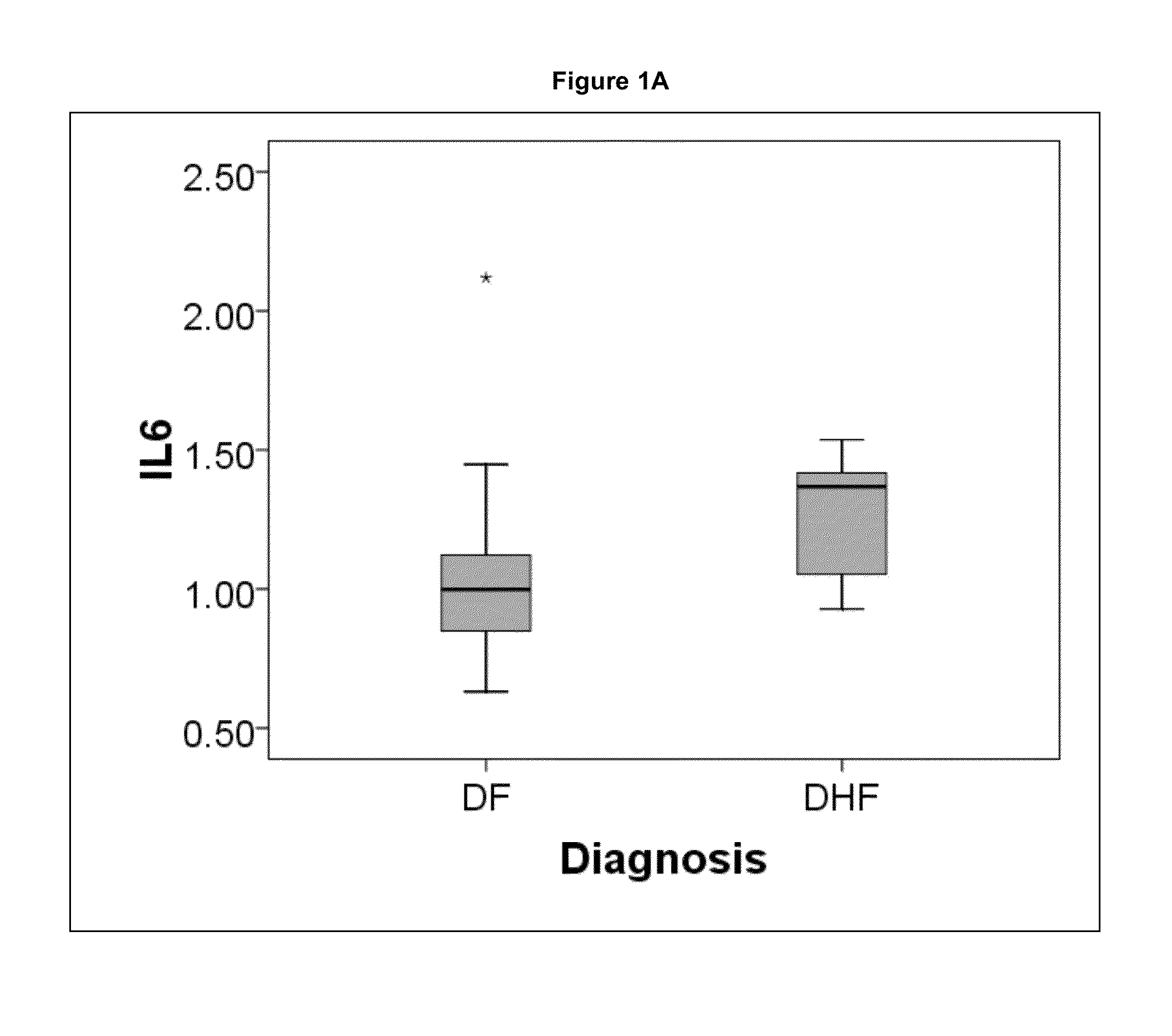
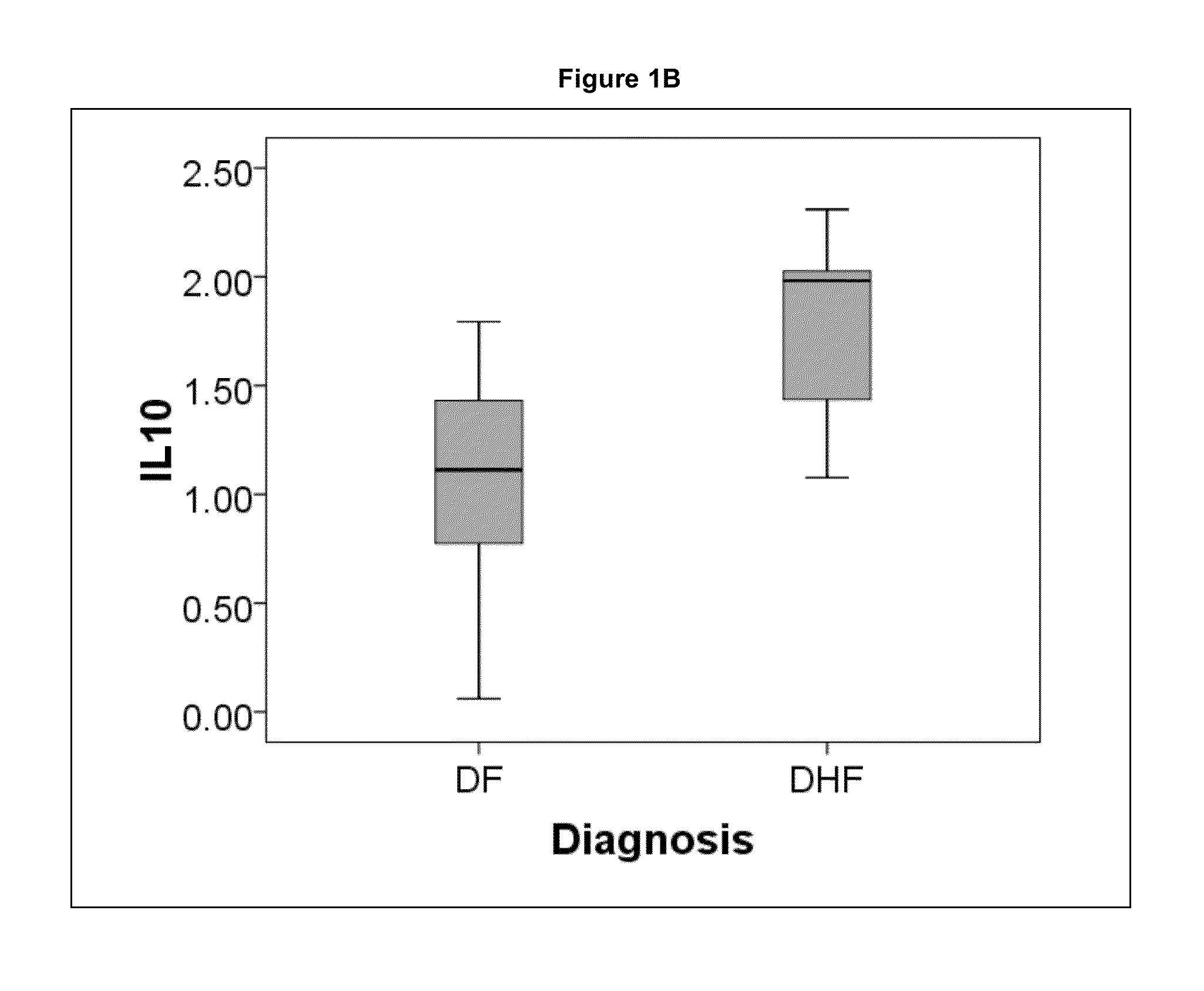
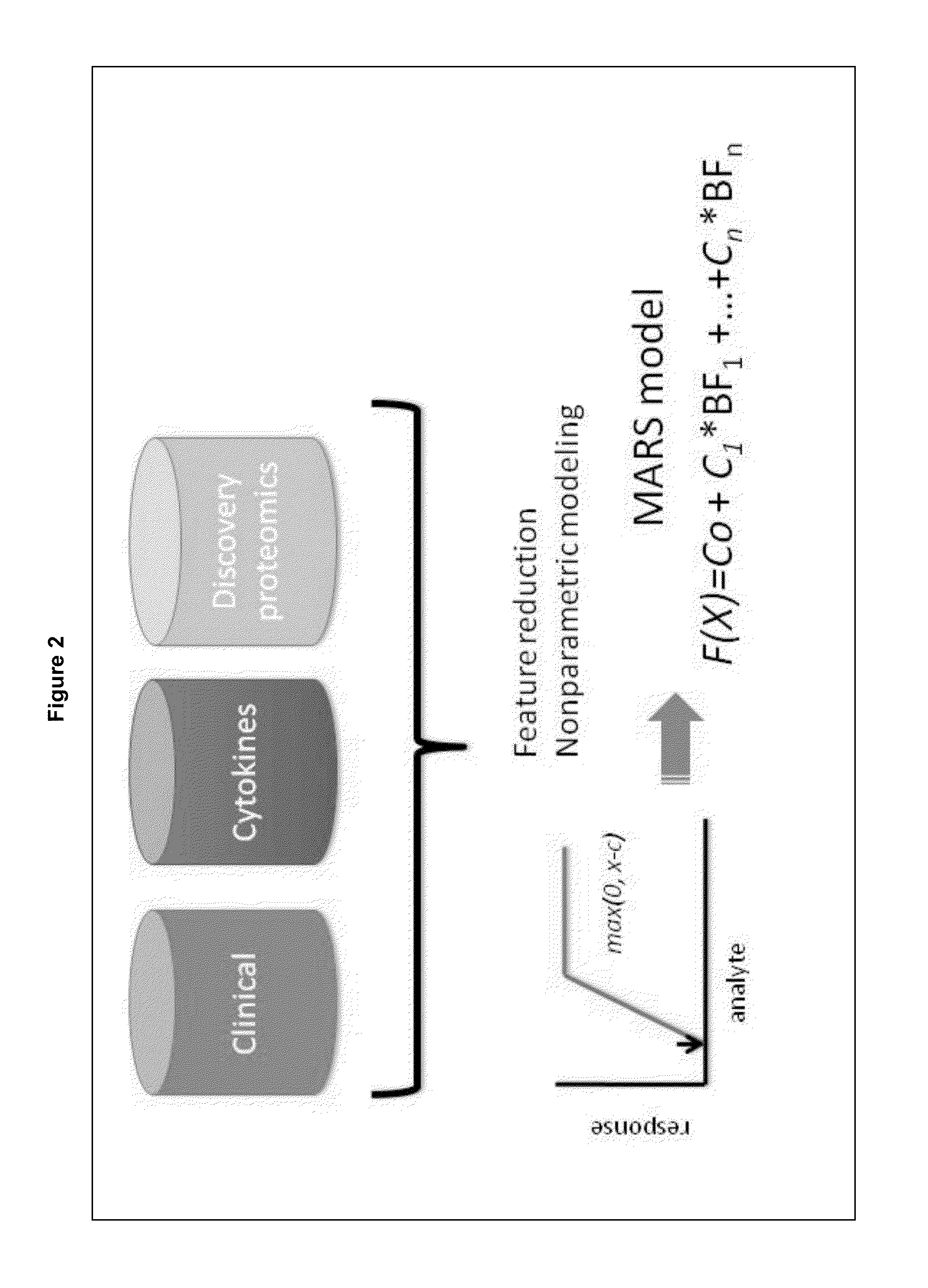
The present invention provides methods for detecting, analyzing, and identifying biomolecules used to identifying patient with dengue-like symptom who are at risk of DHF. The inventive method comprises detecting in a sample from a subject dengue infected patient one or more biomarkers selected from the group consisting of IL-10, fibrinogen, C4A, immunoglobulin, tropomyosin, and three isoforms of albumin, and which are used in a predictive MARS model to detect patients with risk of developing DHF.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Antiviral compositions and methods of their use
Novel compositions comprising viral fusion inhibition compounds and viral replication inhibition compounds as well as methods of their use are disclosed. The novel compositions are useful, inter alia, in the prevention, inhibition and / or treatment of Dengue Fever (DF) or Dengue Hemorrhagic Fever (DHF).
Owner:HUMANITAS TECH
Medicine for treating epidemic hemorrhagic fever
A Chinese medicine for treating epidemic hemorrhagic fever is prepared from 16 Chinese-medicinal materials including cinammon twigs, scutellaria root, prepared pinellia tuber, red peony root, etc. Its advantages include high cure rate (100%), and no complication and sequela.
Owner:薛凤荣
Methods and biomarkers for the detection of dengue hemorrhagic fever
The present invention provides methods for detecting, analyzing, and identifying biomolecules used to identifying patient with dengue-like symptom who are at risk of DHF. The inventive method comprises detecting in a sample from a subject dengue infected patient one or more biomarkers selected from the group consisting of IL-10, fibrinogen, C4A, immunoglobulin, tropomyosin, and three isoforms of albumin, and which are used in a predictive MARS model to detect patients with risk of developing DHF.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Cytotoxic T lymphocyte inducing immunogens for prevention treatment and diagnosis of dengue virus infection
Owner:EMERGEX VACCINES HLDG LTD
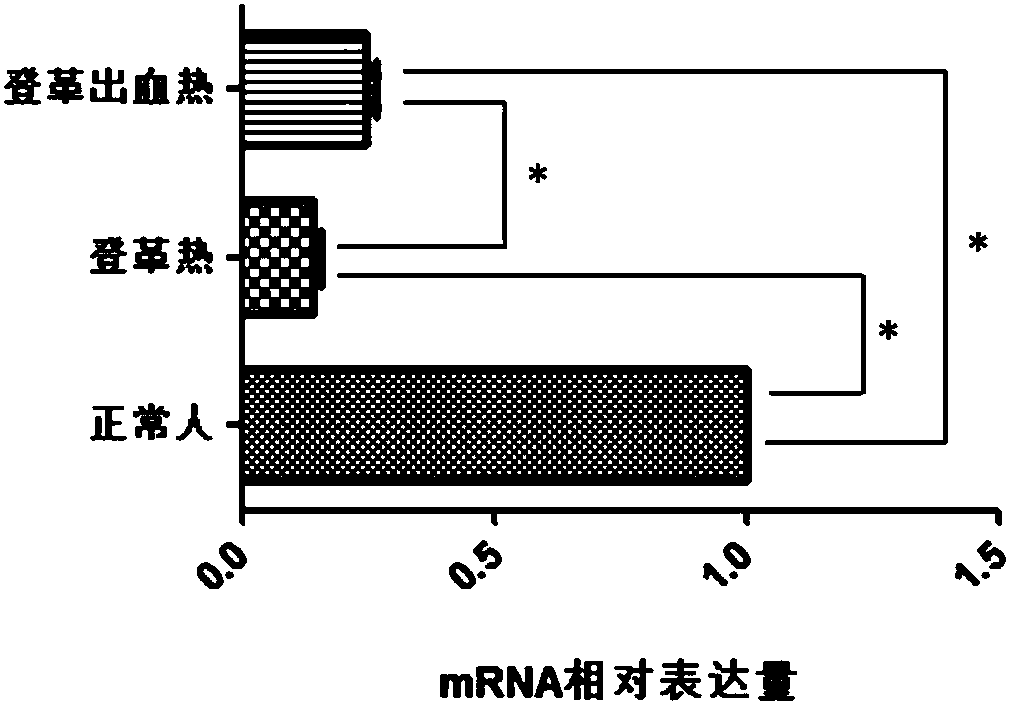
Differential marker for diagnosis of dengue fever and dengue hemorrhagic fever
InactiveCN108559775AIncrease sample sizeThe result is accurateMicrobiological testing/measurementBiological testingNon invasivePediatrics
The invention discloses the application of BRCA2 gene and the expression product in preparing a diagnostic product. The diagnostic product can be used for the diagnosis of dengue fever and dengue hemorrhagic fever. By detecting the level of the BRCA2 gene and the expression product, it is possible to determine the risk of dengue fever and dengue hemorrhagic fever in the subject or the status of dengue fever and dengue hemorrhagic fever. The diagnostic product uses the peripheral blood of a subject as a detection object, and can achieve the non-invasive, rapid, sensitive and accurate diagnosticresults.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
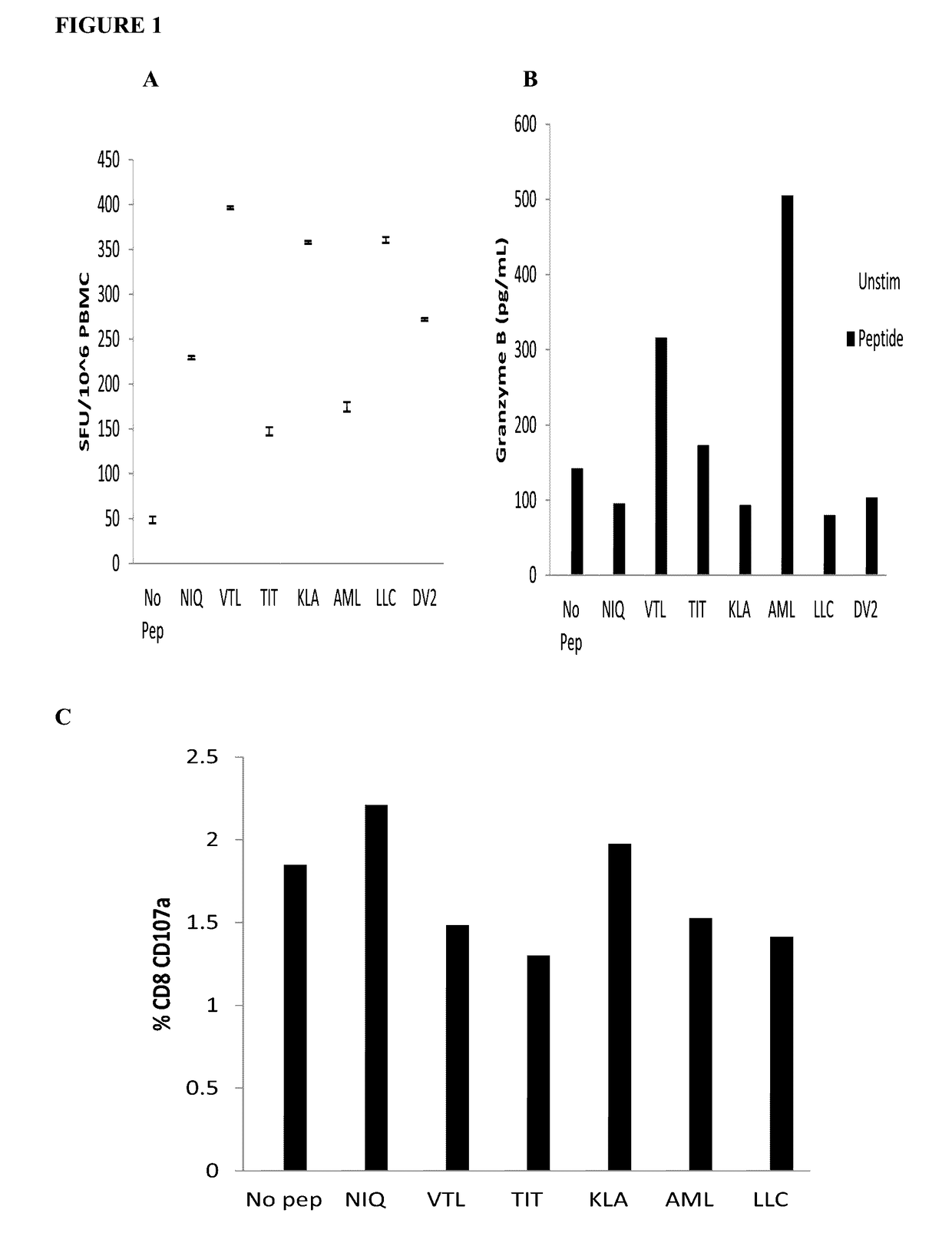
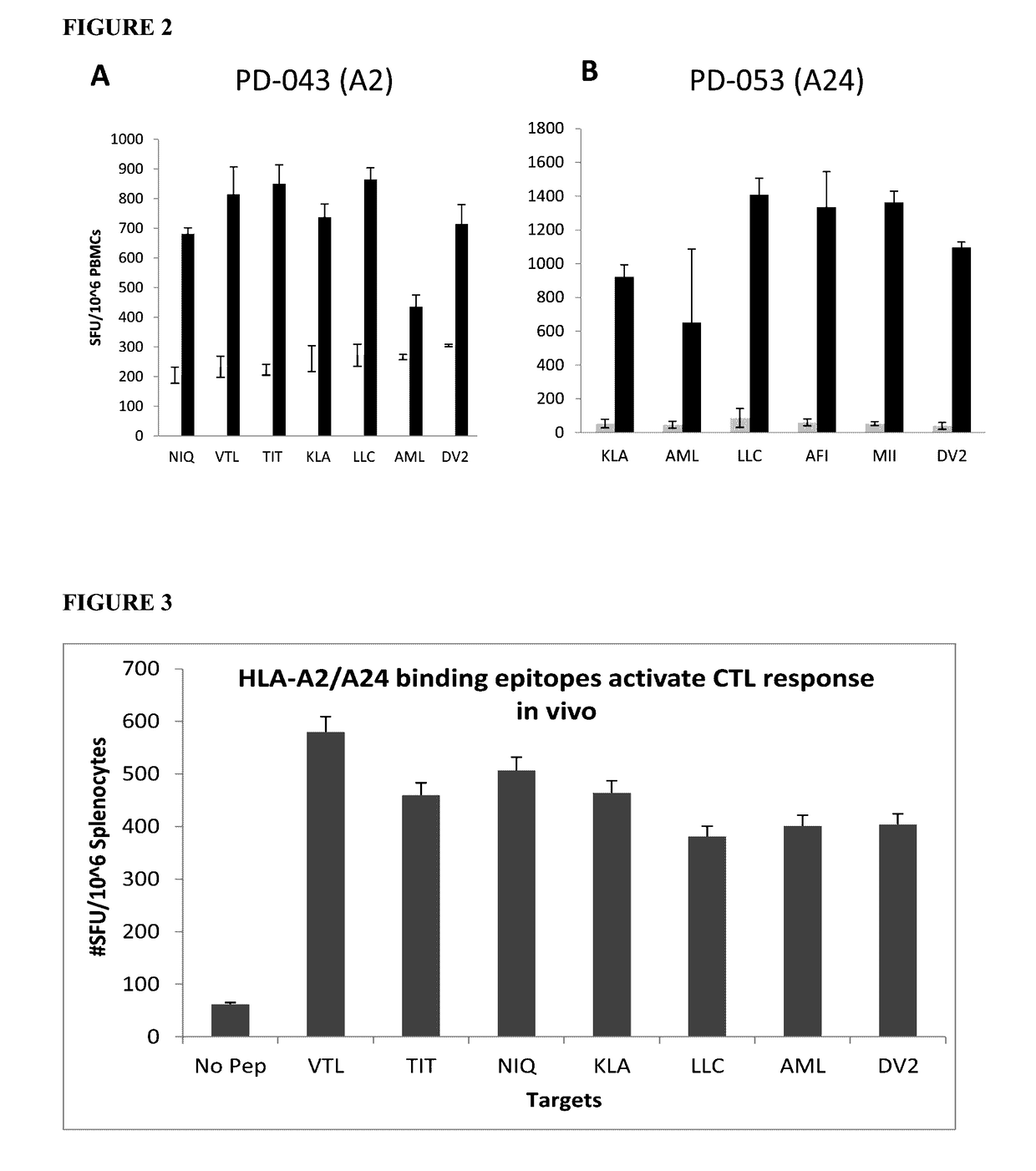
Dengue virus specific multiple HLA binding T cell epitopes for the use of universal vaccine development
Dengue Fever (DF) and Dengue Hemorrhagic Fever (DHF) are significant global public health problems and understanding the overall immune response to infection will contribute to appropriate management of the disease and its potentially severe complications. Live attenuated and subunit vaccine candidates, which are under clinical evaluation, induce primarily an antibody response to the virus and minimal cross-reactive T cell responses. Currently, there are no available tools to assess protective T cell responses during infection or post vaccination. Herein, we report novel, naturally processed and presented MHC class I restricted epitopes, a subset of which binds to and activates T cells in both an HLA-A2 and HLA-A24 restricted manner. We show that epitope specific T cells can be activated in vivo in transgenic mice and in vitro in seropositive and seronegative individuals and that these T cells are functional, recognizing peptide pulsed and dengue virus infected cells in a pro-inflammatory and cytotoxic manner. These epitopes have potential as new informational and diagnostic tools to characterize T cell immunity in Dengue virus (DV) infection, and may serve as a universal vaccine candidate complementary to current vaccines in trial.
Owner:EMERGEX VACCINES HLDG LTD
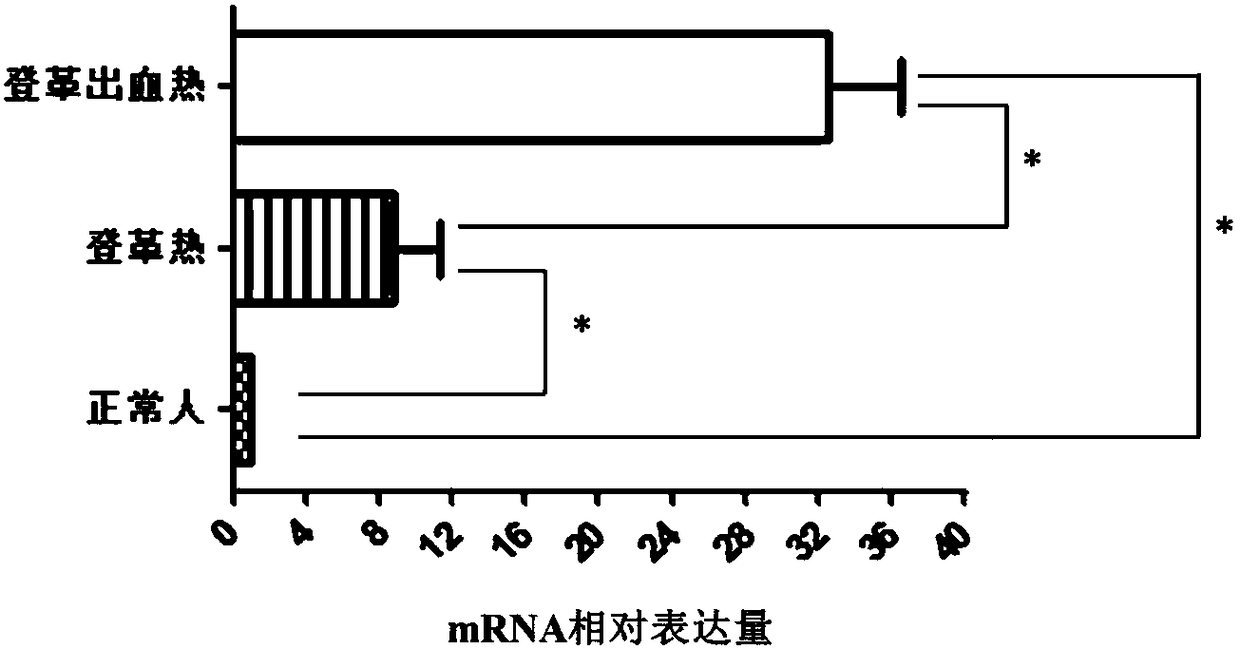
Molecular marker for distinguishing Dengue fever and Dengue hemorrhagic fever
InactiveCN108588281ALarge sample sizeThe result is accurateMicrobiological testing/measurementBiological material analysisPoor correlationBiology
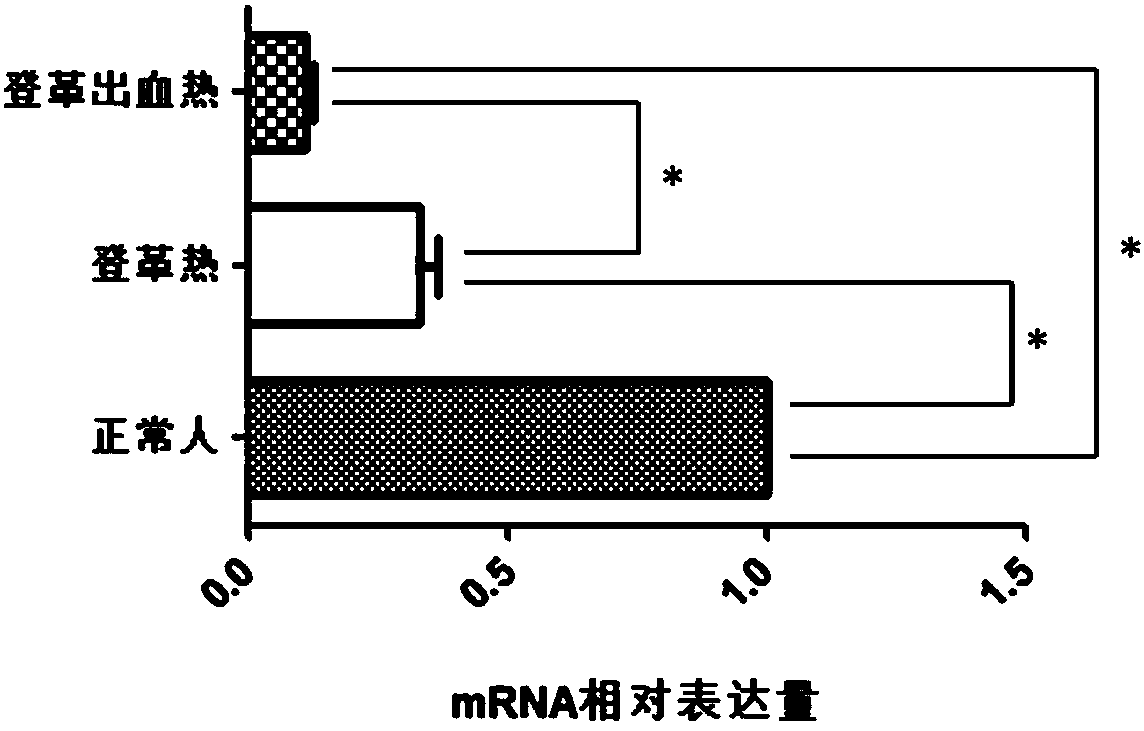
The invention discloses a CMIP gene capable of being used as a molecular marker for distinguishing Dengue fever and Dengue hemorrhagic fever. By a high-throughput sequencing and RT-PCR method, the CMIP gene has significant expression difference in blood of normal people and patients with Dengue fever and Dengue hemorrhagic fever, i.e., whether a subject suffers the Dengue fever or the Dengue hemorrhagic fever can be judged by detecting the expression condition of the CMIP gene in the blood. According to the correlation of the Dengue fever and the Dengue hemorrhagic fever, the invention develops a kit for diagnosing the Dengue fever and the Dengue hemorrhagic fever. The kit is used for performing the Dengue fever and Dengue hemorrhagic fever diagnosis by detecting the CMIP gene expression.The diagnosis kit can be used for early diagnosis of diseases and has wide application prospects in clinics.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
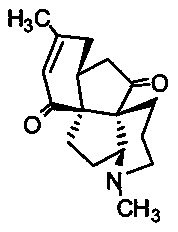
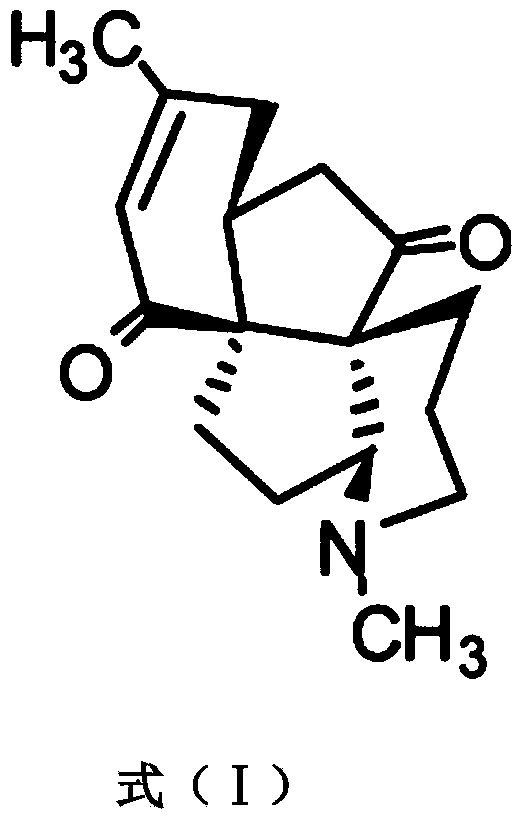
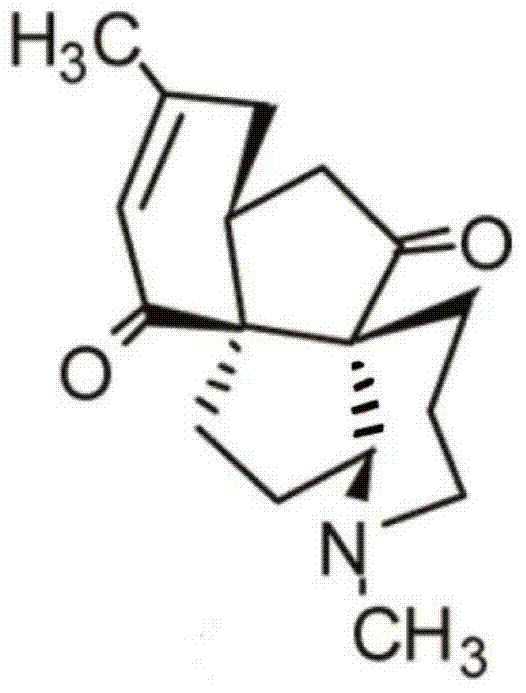
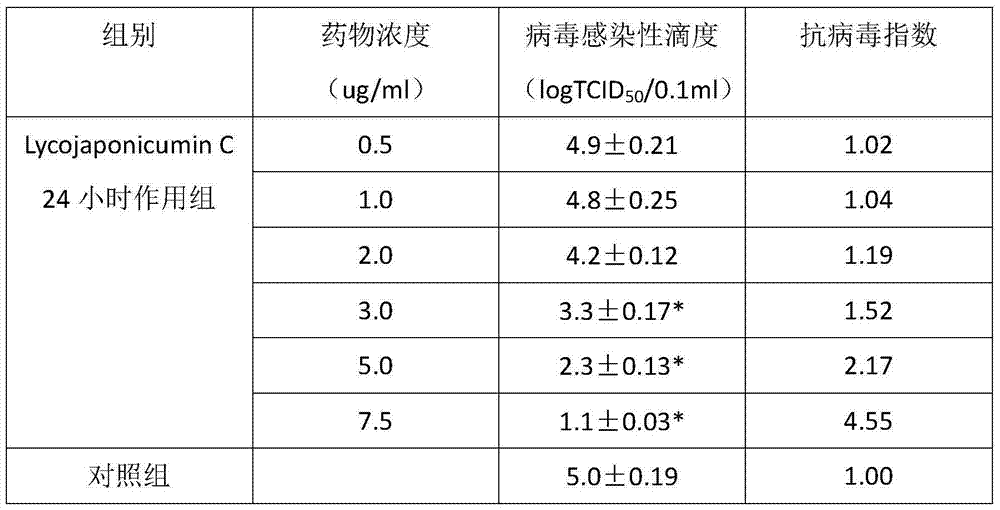
Application of Lycojaponicumin C in preparation of medicine for treating hemorrhagic fever with renal syndrome
ActiveCN103463073AStrong inhibitory activityHighlight substantive featuresOrganic active ingredientsAntiviralsDisease courseMortality rate
The invention discloses a new application of Lycojaponicumin C in preparation of a medicine for treating hemorrhagic fever with renal syndrome. Experimental researches of Lycojaponicumin C in-vitro and in-vivo inhibition on virus of hemorrhagic fever with renal syndrome indicate that Lycojaponicumin C has low toxicity to cells, can directly kill virus of hemorrhagic fever with renal syndrome, can remarkably inhibit multiplication of the virus, and can obviously protect shrewmouse infected by the virus so as to delay the disease time, prolong the course of disease and reduce the death rate. Therefore, the Lycojaponicumin C is an effective and safe medicine for treating hemorrhagic fever with renal syndrome.
Owner:山西明鼎医药科技有限公司
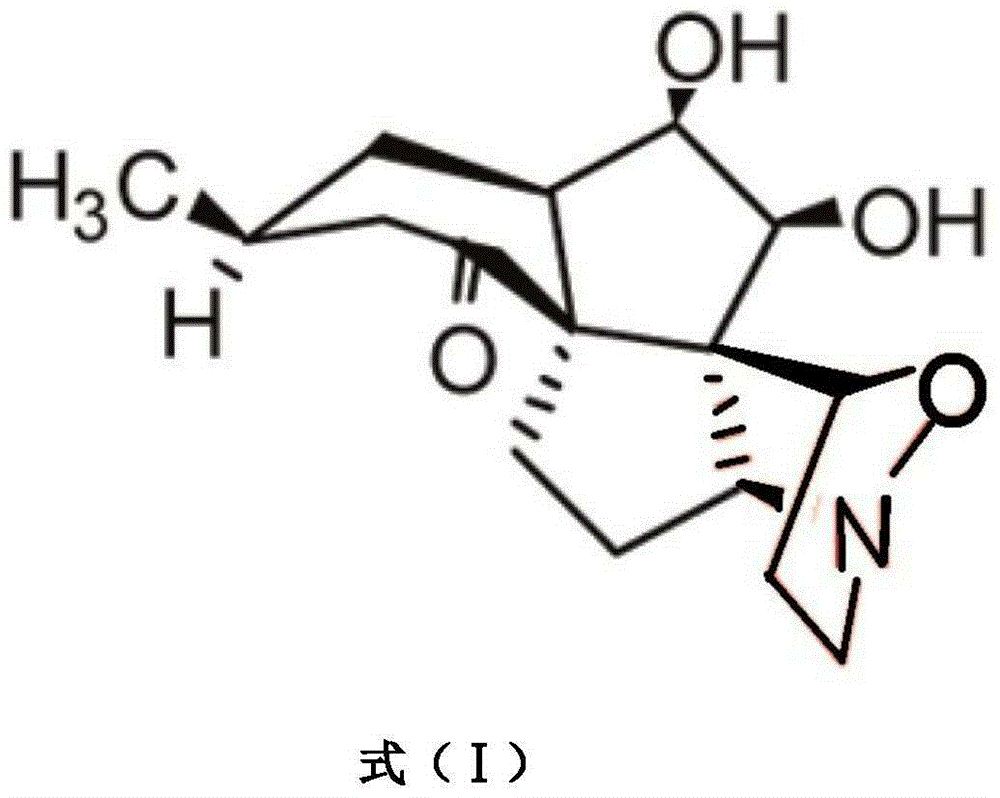
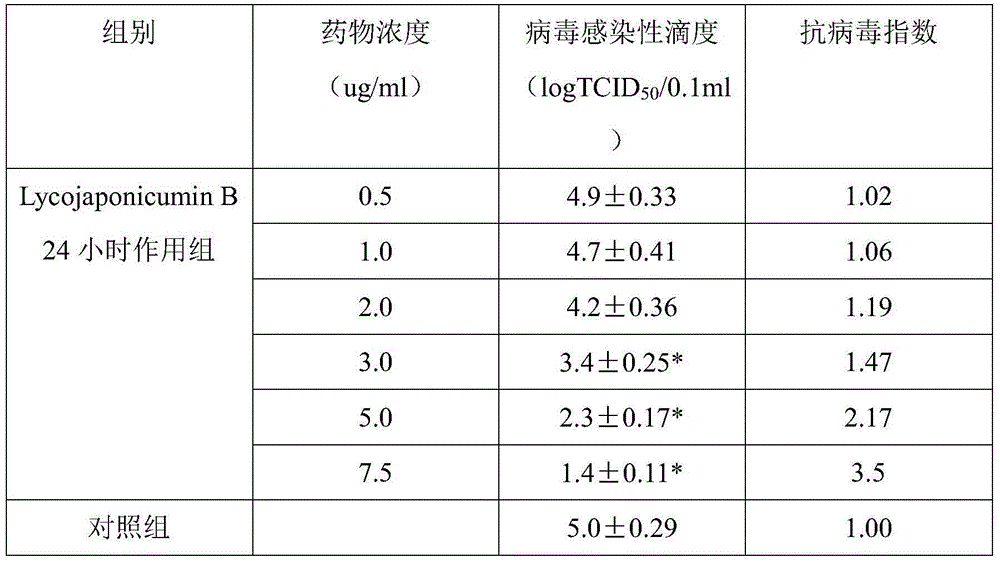
Application of lycojaponicumin B in preparation of medicine for treating hemorrhagic fever with renal syndrome
InactiveCN103463018BStrong inhibitory activityHighlight substantive featuresOrganic active ingredientsAntiviralsLycojaponicumin BMortality rate
The invention discloses a new application of Lycojaponicumin B in preparation of a medicine for treating hemorrhagic fever with renal syndrome. Experimental researches of the Lycojaponicumin B in-vitro and in-vivo inhibition on virus of hemorrhagic fever with renal syndrome indicate that the Lycojaponicumin B has low toxicity to cells, can directly kill the virus of hemorrhagic fever with renal syndrome, can remarkably inhibit multiplication of the virus, and can obviously protect shrewmouse infected by the virus so as to delay the disease time, prolong the course of disease and reduce the death rate. Therefore, the Lycojaponicumin B is an effective and safe medicine for treating hemorrhagic fever with renal syndrome.
Owner:付海柱
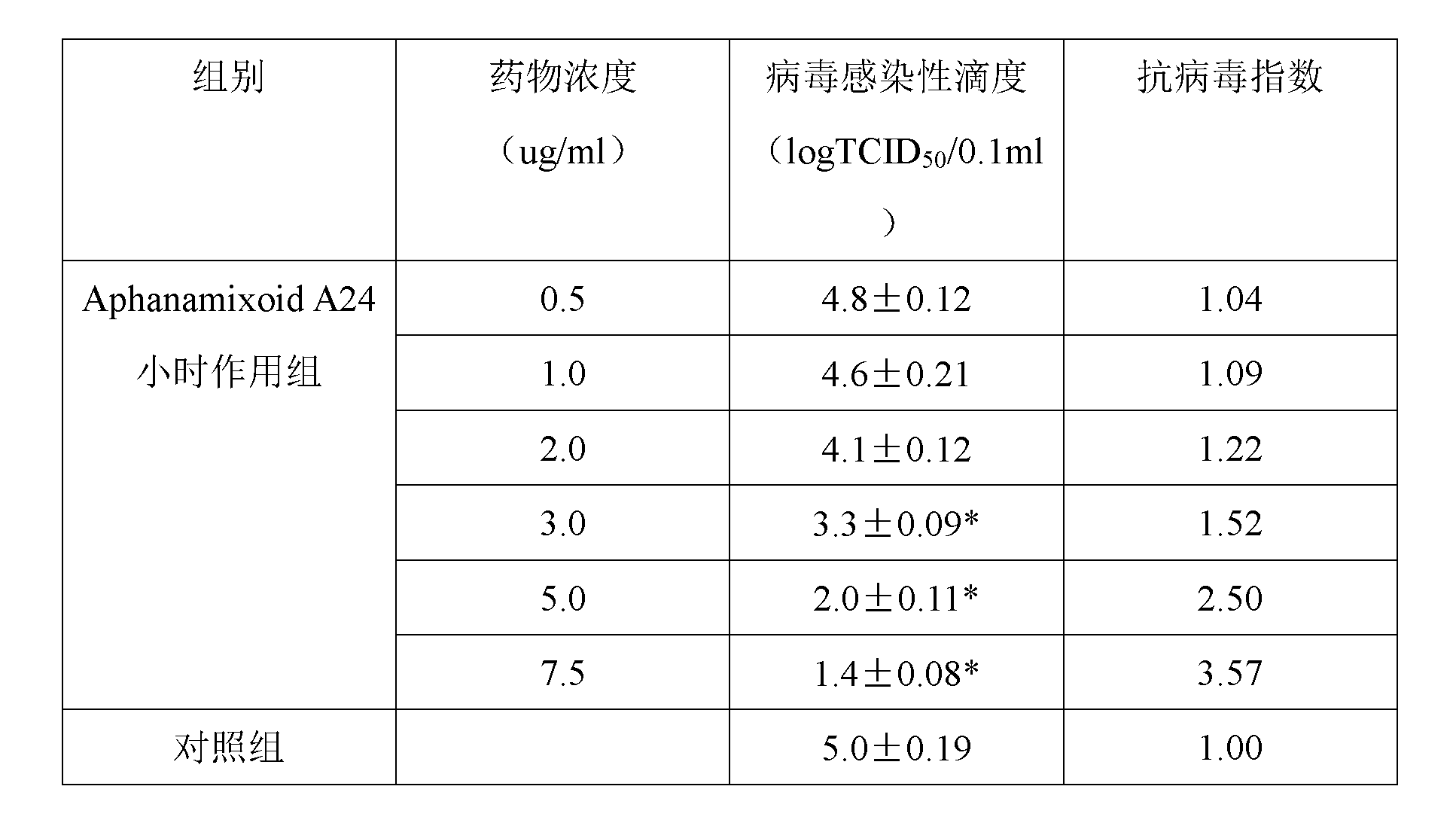
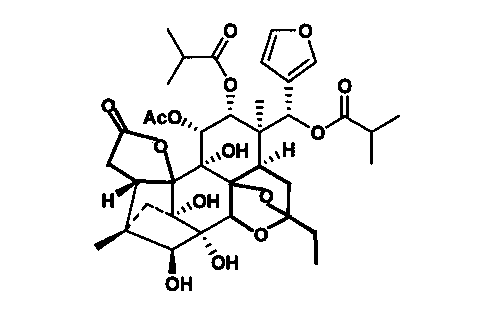
Application of aphanamixoid A in preparing medicament for treating hemorrhagic fever with renal syndrome
InactiveCN103006638AStrong inhibitory activityHighlight substantive featuresOrganic active ingredientsAntiviralsDisease courseMortality rate
The invention discloses an application of aphanamixoid A in preparing a medicament for treating hemorrhagic fever with renal syndrome. Researches of aphanamixoid A in virus in-vitro and in-vivo inhibition experiments of hemorrhagic fever with renal syndrome indicate that the aphanamixoid A has low toxicity to cells, can directly kill virus of hemorrhagic fever with renal syndrome, can remarkably inhibit multiplication of the virus, and can remarkably protect susliks infected by the virus, so that the attack time is postponed, the disease course is prolonged, and the death rate is reduced. Therefore, the aphanamixoid A is an effective and safe medicament for treating hemorrhagic fever with renal syndrome.
Owner:何晓涛
Application of Chukrasone B in medicine for treating hemorrhagic fever with renal syndrome
InactiveCN103371999AStrong inhibitory activityHighlight substantive featuresOrganic active ingredientsAntiviralsMortality rateIn vivo
The invention discloses a new application of Chukrasone B in preparation of a medicine for treating hemorrhagic fever with renal syndrome. Experiment researches on inhibiting hemorrhagic fever with renal syndrome virus by the Chukrasone B in vitro and in vivo show that the Chukrasone B has a low toxic effect on cells, has an effect of killing the hemorrhagic fever with renal syndrome viruses directly and can obviously inhibit the viruses from increasing, and the Chukrasone B has an obvious effect of protecting gophers infected by the virus, thus postponing the disease time, prolonging the course of disease and lowering the death rate. Therefore, the Chukrasone B is an effective and safe medicine for treating hemorrhagic fever with renal syndrome.
Owner:丁圣雨
Application of CIITA gene as biomarker for diagnosis of dengue and dengue hemorrhagic fever
InactiveCN108531655AIncrease sample sizeThe result is accurateMicrobiological testing/measurementMaterial analysisNormal peopleBiomarker (petroleum)
The invention discloses application of CIITA gene as a biomarker for diagnosis of dengue and dengue hemorrhagic fever. High-throughput screen is performed on genes having expression differences in normal people and patients with dengue and dengue hemorrhagic fever; large-sample reverse-transcription PCR (polymerase chain reaction) test proves that the content of CIITA gene in the blood of the patients with dengue and dengue hemorrhagic fever is lower than that in normal people. Therefore, the CIITA gene can serve as a biomarker for the diagnosis of dengue and dengue hemorrhagic fever. Researchresults herein provide a noninvasive method for the clinical early diagnosis of dengue and dengue hemorrhagic fever; the method is applicable to clinical popularization.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
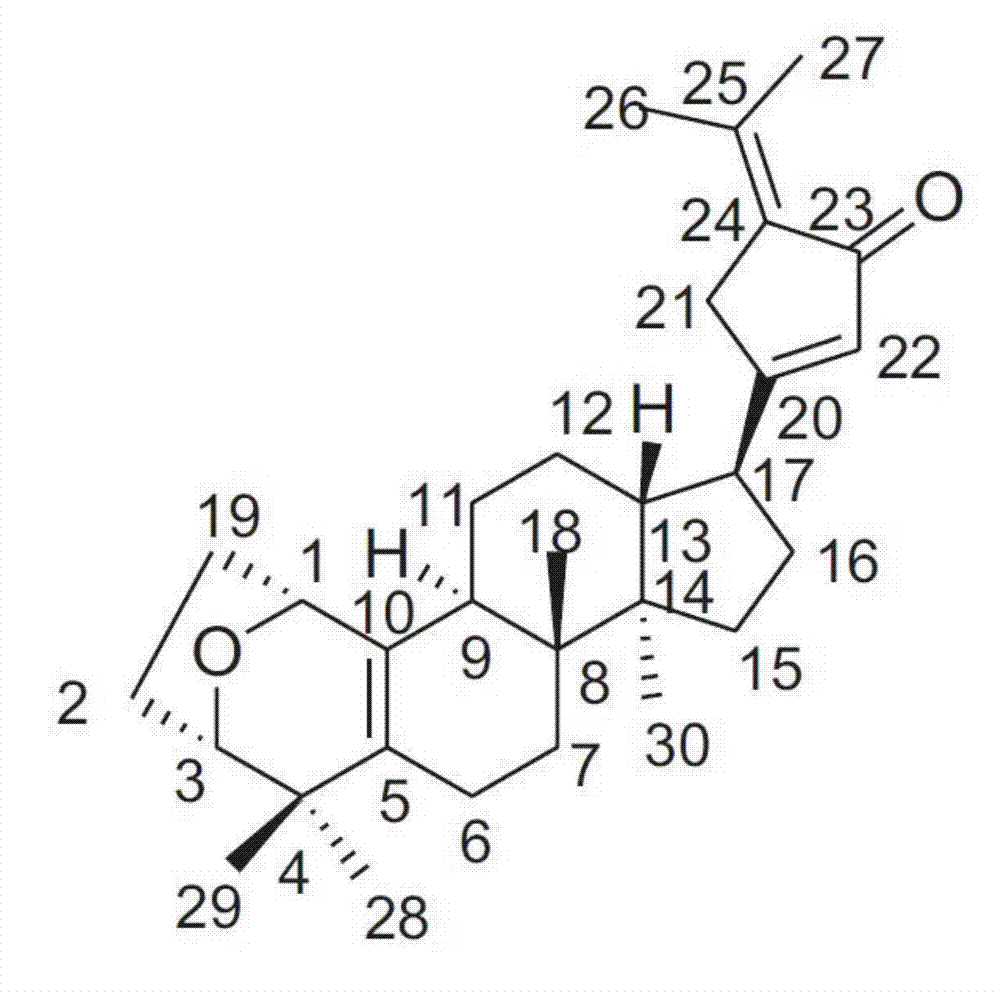
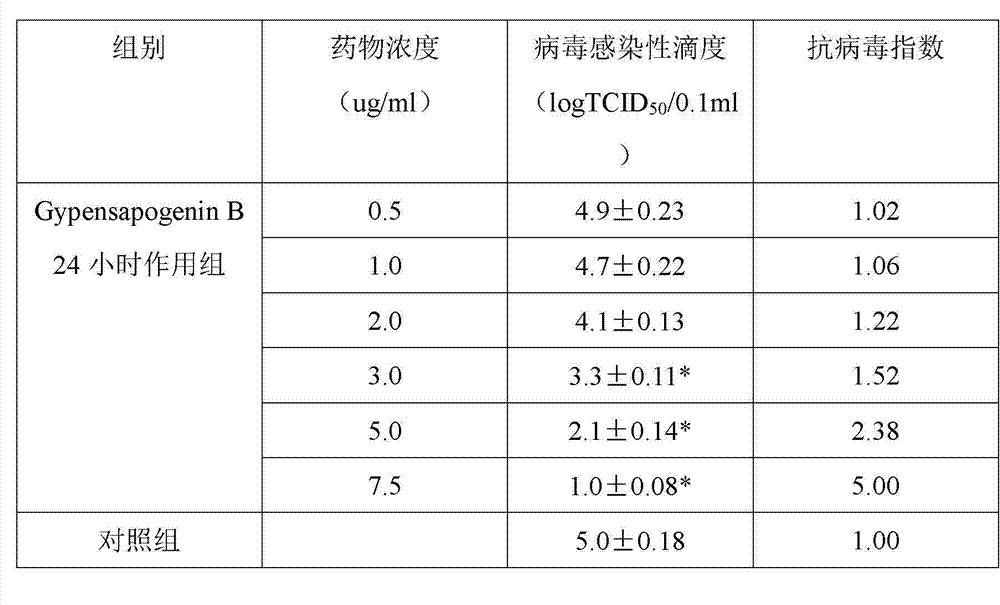
Application of Gypensapogenin B in medicine for treating hemorrhagic fever with renal syndrome
ActiveCN102861029AStrong inhibitory activityHighlight substantive featuresOrganic active ingredientsAntiviralsMortality rateIn vivo
The invention discloses new application of Gypensapogenin B in preparing a medicine for treating hemorrhagic fever with renal syndrome. The research on experiments of inhibiting hemorrhagic fever virus with renal syndrome by Gypensapogenin B in vivo and in vitro indicates that Gypensapogenin B has low toxic effect on cells, has direct killing effect on hemorrhagic fever virus with renal syndrome, and can obviously restrain the proliferation of the viruses. Gypensapogenin B has obvious protective function to mice infected by the viruses, and has the functions of putting off the attack time, prolonging the course of the disease and reducing the death rate. Therefore, Gypensapogenin B is an effective and safe medicine for treating hemorrhagic fever with renal syndrome.
Owner:启东市天汾电动工具技术创新中心
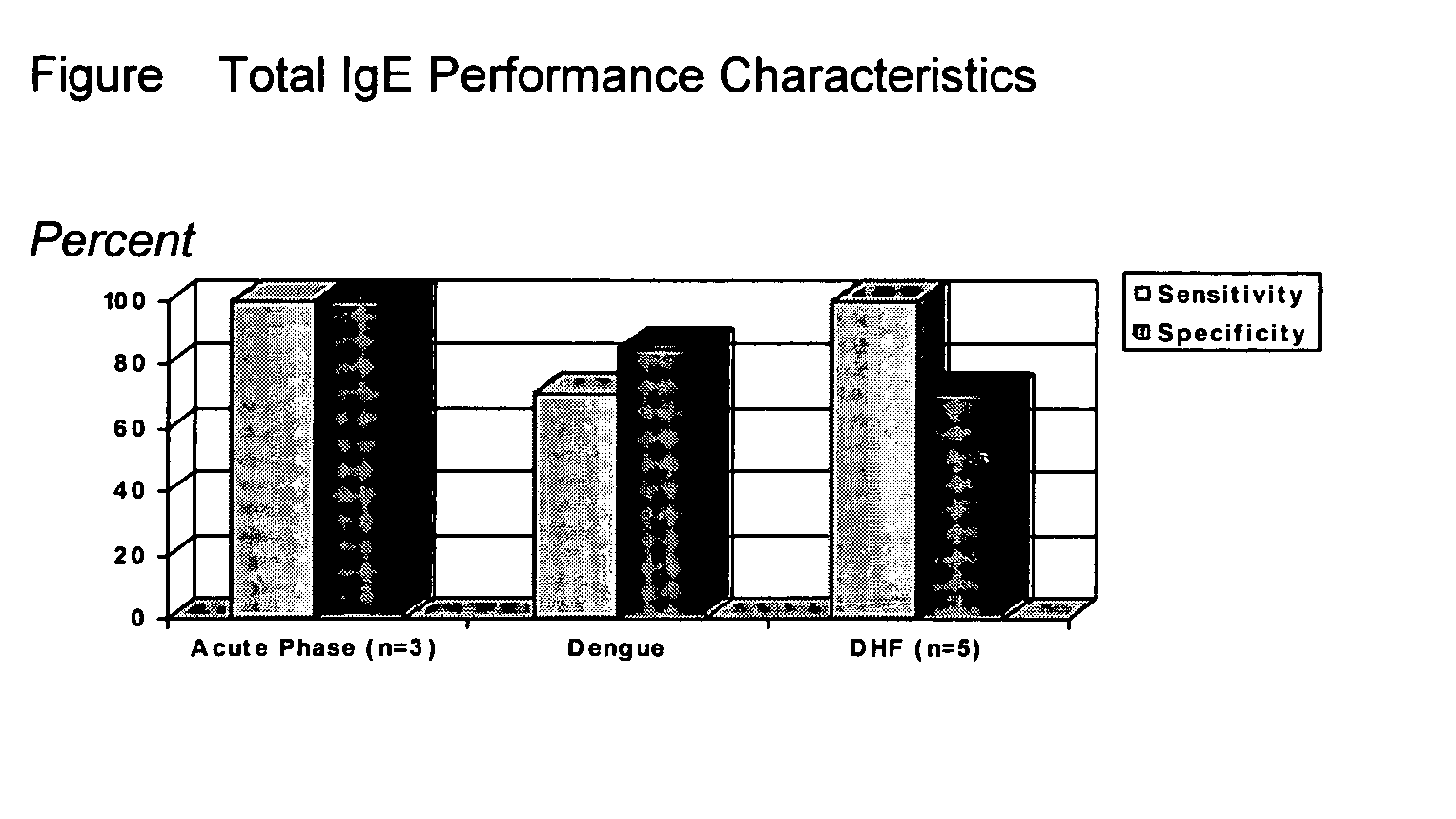
Diagnostic assays
InactiveUS20060099573A1Potent inflammatory reaction against pathogenMore efficientMicrobiological testing/measurementBiological testingMedicinePediatrics
Diagnostic assays using IgE for detecting HIV in a baby patient or a child patient that might be infected with HIV; distinguishing between classic dengue and dengue hemorrhagic fever in a patient that is believed to have either the classic or hemorrhagic version of dengue; and detecting cognitive impairment in a patient who is more than 40 years old.
Owner:MIGUEZ MARIA JOSE
Prediction model for incidence of hemorrhagic fever with renal syndrome
The invention discloses a prediction model for the incidence of hemorrhagic fever with renal syndrome, which belongs to the medical field. The steps are as follows: selecting a monitoring habitat, catching rats, calculating the poison index of rats, calculating the incidence of population, and building a prediction model. The problem that there is no prediction model for the incidence of hemorrhagic fever with renal syndrome in the prior art is solved. A method for accurately predicting the incidence regularity of hemorrhagic fever with renal syndrome is found by making direct use of a linearregression equation built based on the poison index of rats and the people's incidence of hemorrhagic fever with renal syndrome, and a scientific basis is provided for the prevention and control of hemorrhagic fever with renal syndrome.
Owner:李青
Dengue Virus Specific Multiple HLA Binding T Cell Epitopes For The Use Of Universal Vaccine Development
ActiveUS20170119869A1Reduce decreaseSsRNA viruses positive-senseViral antigen ingredientsMHC class ICytotoxicity
Dengue Fever (DF) and Dengue Hemorrhagic Fever (DHF) are significant global public health problems and understanding the overall immune response to infection will contribute to appropriate management of the disease and its potentially severe complications. Live attenuated and subunit vaccine candidates, which are under clinical evaluation, induce primarily an antibody response to the virus and minimal cross-reactive T cell responses. Currently, there are no available tools to assess protective T cell responses during infection or post vaccination. Herein, we report novel, naturally processed and presented MHC class I restricted epitopes, a subset of which binds to and activates T cells in both an HLA-A2 and HLA-A24 restricted manner. We show that epitope specific T cells can be activated in vivo in transgenic mice and in vitro in seropositive and seronegative individuals and that these T cells are functional, recognizing peptide pulsed and dengue virus infected cells in a pro-inflammatory and cytotoxic manner. These epitopes have potential as new informational and diagnostic tools to characterize T cell immunity in Dengue virus (DV) infection, and may serve as a universal vaccine candidate complementary to current vaccines in trial.
Owner:EMERGEX VACCINES HLDG LTD
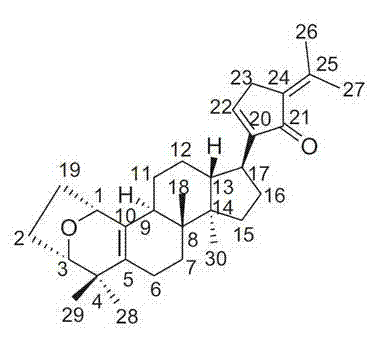
Application of Gypensapogenin A in medicine for treating hemorrhagic fever with renal syndrome
InactiveCN102885831AStrong inhibitory activityHighlight substantive featuresOrganic active ingredientsAntiviralsHamsterMortality rate
The invention discloses a new application of Gypensapogenin A in preparation for a medicine for treating hemorrhagic fever with renal syndrome. Experimental studies on the in-vitro inhibition and the in-vivo inhibition of Gypensapogenin A for hemorrhagic fever with renal syndrome show that Gypensapogenin A has a low toxic effect on cells, plays a role of directly killing the virus of hemorrhagic fever with renal syndrome, can obviously inhibit the multiplication of the virus, and plays an obvious role of protecting hamsters infected with the virus so as to delay the onset time, prolong the disease course and decrease the mortality. Thus, Gypensapogenin A is an effective and safe medicine for treating the hemorrhagic fever with the renal syndrome.
Owner:吴俊华
Application of lycojaponicumin C in preparation of medicine for treating hemorrhagic fever with renal syndrome
ActiveCN103463073BStrong inhibitory activityHighlight substantive featuresOrganic active ingredientsAntiviralsLycojaponicumin CVirus type
The invention discloses a new application of Lycojaponicumin C in preparation of a medicine for treating hemorrhagic fever with renal syndrome. Experimental researches of Lycojaponicumin C in-vitro and in-vivo inhibition on virus of hemorrhagic fever with renal syndrome indicate that Lycojaponicumin C has low toxicity to cells, can directly kill virus of hemorrhagic fever with renal syndrome, can remarkably inhibit multiplication of the virus, and can obviously protect shrewmouse infected by the virus so as to delay the disease time, prolong the course of disease and reduce the death rate. Therefore, the Lycojaponicumin C is an effective and safe medicine for treating hemorrhagic fever with renal syndrome.
Owner:山西明鼎医药科技有限公司
Blood marker for early diagnosis of dengue and dengue hemorrhagic fever
InactiveCN108342475AIncrease sample sizeThe result is accurateMicrobiological testing/measurementBiological testingAkt1 geneBlood marker
The invention discloses a gene marker for the early diagnosis of dengue and dengue hemorrhagic fever. The gene marker is an AKT1 gene. Whether a human subject suffers from the dengue and dengue hemorrhagic fever or whether the human subject has the risk of suffering from the dengue and dengue hemorrhagic fever is judged by detecting the levels of the AKT1 gene and an AKT1 protein in the blood of the human subject. A product for diagnosing the dengue and dengue hemorrhagic fever by detecting the content of the AKT1 gene or the AKT1 protein is developed according to the AKT1 gene and the AKT1 protein, and the diagnosis product can be popularized and used clinically.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
Novel use of Polyflavanostilbene A in preparation of drugs for treating hemorrhagic fever with renal syndrome
The invention discloses a novel use of Polyflavanostilbene A in preparation of drugs for treating hemorrhagic fever with renal syndrome. Hemorrhagic fever with renal syndrome virus in-vivo and in-vitro inhibition experiments prove that Polyflavanostilbene A has low cell toxicity, has effects of directly killing hemorrhagic fever with renal syndrome viruses and obviously inhibiting hemorrhagic fever with renal syndrome virus multiplication, has an obvious function of protecting a hamster infected by the hemorrhagic fever with renal syndrome viruses, delays disease time, prolongs a course of the disease and reduces a death rate. Therefore, Polyflavanostilbene A is an effective and safe drug for treating hemorrhagic fever with renal syndrome.
Owner:郭维 +4
Prediction model of annual incidence of hemorrhagic fever with renal syndrome
The present invention discloses a prediction model of an annual incidence of a hemorrhagic fever with renal syndrome, belonging to the medical field. The technical scheme of the prediction model comprises the steps of: selecting a monitoring point, determining a patient, calculating L, calculating S, calculating W, measuring and calculating a determination coefficient 0.8S, and establishing a prediction model. The problem is solved that there is no a prediction model of an annual incidence of the hemorrhagic fever with renal syndrome in the prior art. The L, the S and the W are directly employed to establish the prediction model to explore a rule of accurately predicting the disease number of hemorrhagic fever with renal syndrome so as to provide a scientific basis for prevention and control work of the hemorrhagic fever with renal syndrome.
Owner:李青
Detection method of hemorrhagic fever with renal syndrome IgM antibodies and reagent kit
ActiveCN103033616BReduce the impact of stabilityExtended shelf lifeMaterial analysisSerum igeIgm antibody
Owner:山东康华生物医疗科技股份有限公司
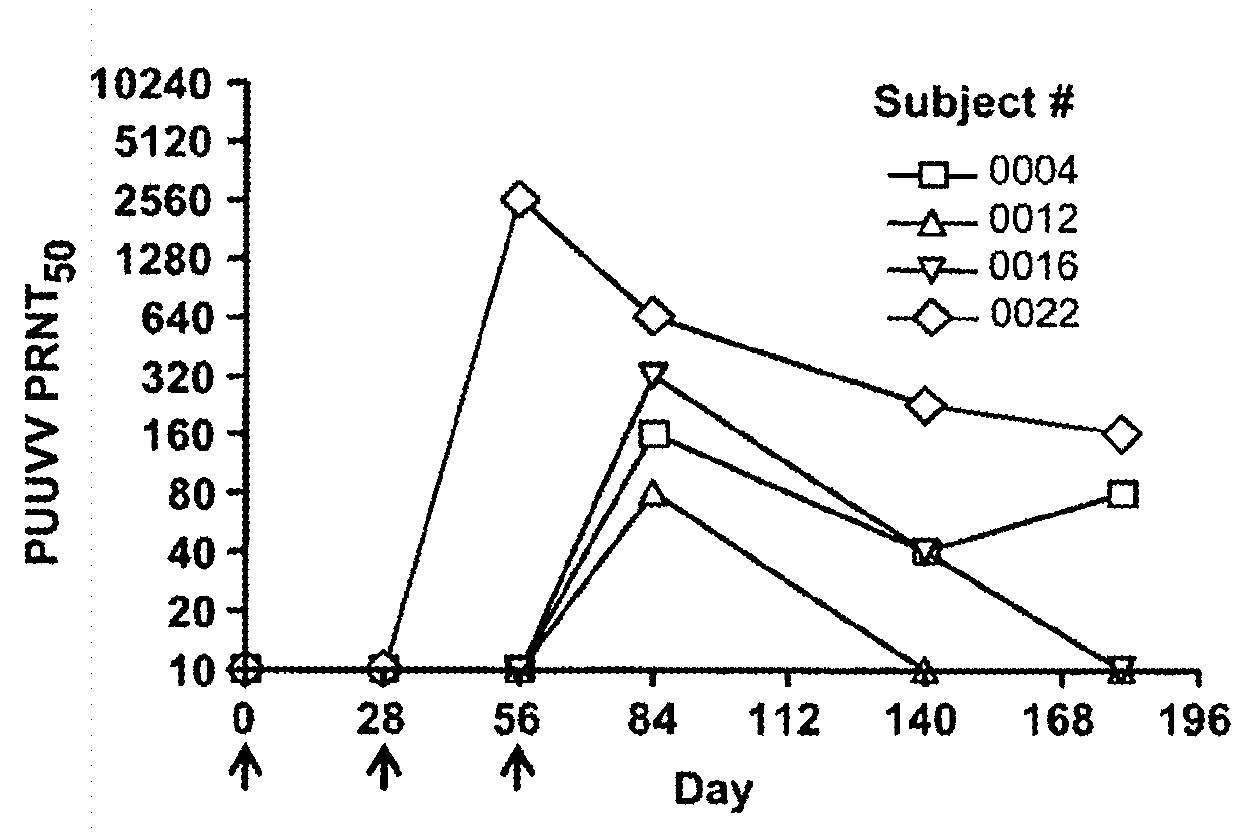
Gene optimized hantaan virus M segment DNA vaccine for hemorrhagic fever with renal syndrome
ActiveUS20160051659A1Reduce efficacyLow immunogenicityPowder deliveryVirusesOpen reading frameNucleotide
A synthetic, codon-optimized Hantaan virus (HTNV) full-length M gene open reading frame that consists of a unique nucleotide sequence encoding HTNV proteins. This synthetic gene was cloned into a plasmid to form the first optimized HTNV full-length M gene that elicits neutralizing antibodies in animals when delivered in combination with a similarly optimized Puumala virus (PUUV) DNA vaccine. The invention obviates the need for an extraneous gene sequence that was previously required for expression of the non-optimized HTNV gene. The synthetic gene is engineered into a molecular vaccine system to prevent hemorrhagic fever with renal syndrome (HFRS) caused by infection with HTNV, SEOV, or DOBV. Alternatively, it can be combined with the optimized PUUV DNA vaccine to protect against HFRS caused by any hantavirus.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
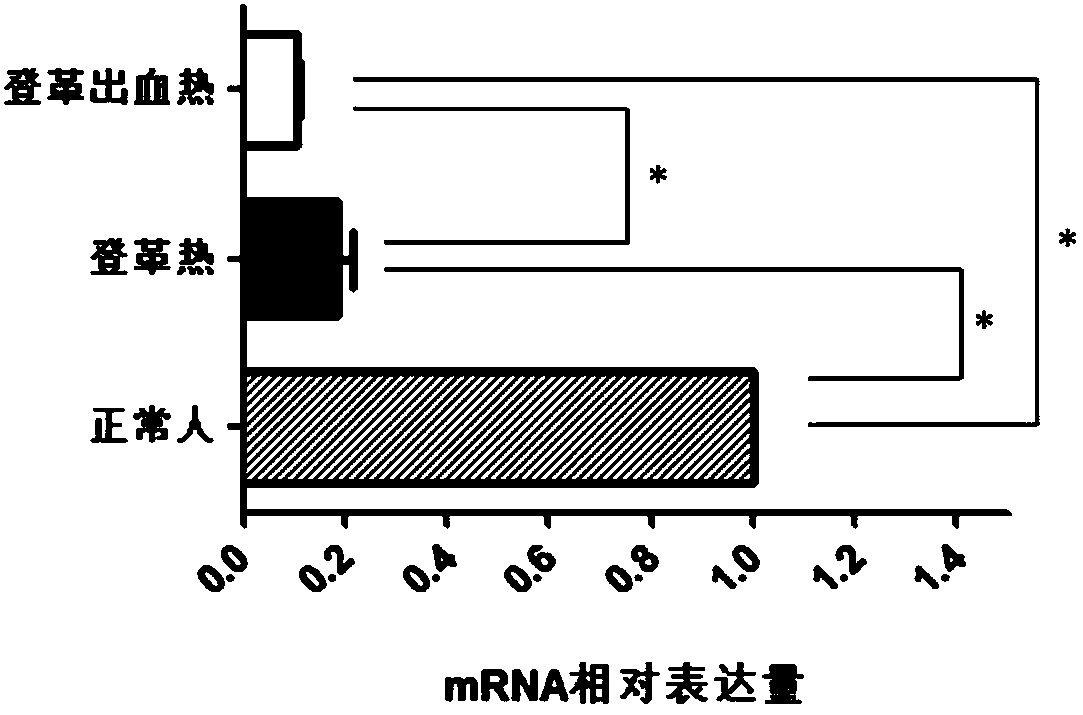
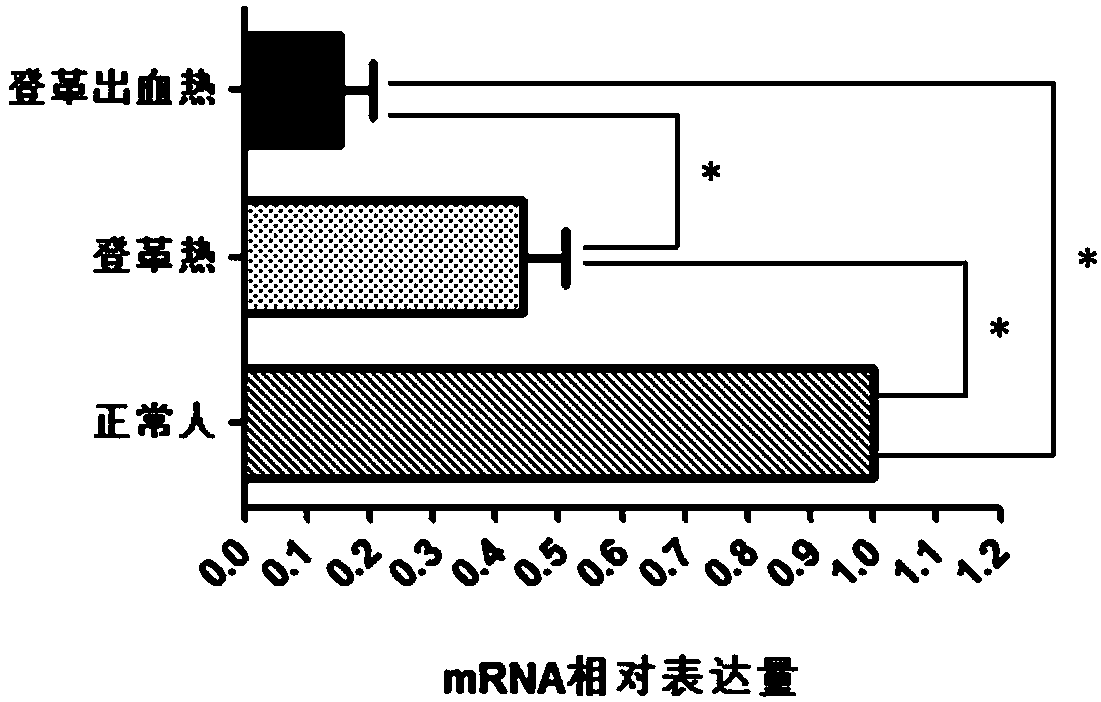
Gene diagnosis marker for dengue fever and dengue hemorrhagic fever
InactiveCN108588282ALarge sample sizeThe result is accurateMicrobiological testing/measurementBiological material analysisBiologyGene
The invention discloses a diagnosis tool for dengue fever and dengue hemorrhagic fever. The diagnosis tool fulfills the aim of diagnosis by detecting a CACNB1 gene in blood and an expression product thereof. According to the invention, researches prove that compared to normal people, mRNA expression levels of the CACNB1 gene in blood of patients suffering from the dengue fever and the dengue hemorrhagic fever are obviously reduced. According to correlation between the CACNB1 gene and the dengue fever and the dengue hemorrhagic fever, a kit for diagnosing the dengue fever and the dengue hemorrhagic fever can be prepared; the kit can be widely clinically applied.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
Dengue hemorrhagic fever virus II nucleic acid molecular characteristic standard sample and preparation method thereof
InactiveCN104388585AImprove uniformityImprove stabilityMicrobiological testing/measurementMicroorganism based processesFreeze-dryingQuality control
The invention discloses a method for preparing a dengue hemorrhagic fever virus II nucleic acid molecular characteristic standard sample. The dengue hemorrhagic fever virus II nucleic acid molecular characteristic standard sample is prepared according to the steps of synthesis of sequences, cloning of vectors, connection of target fragments and vectors, plasmid transformation, extraction and freezing dried storage of recombinant plasmids and the like. A standard sample that contains virus characteristic sequence information and is suitably used for analyzing the virus and the content thereof in a sample is prepared according to the method; the standard sample provided by the invention has good homogeneity, high stability and good purity, can be stored for long term, and is prepared according to the simple preparation method; a good standard sample is provided for detection research, medical research and the like of dengue hemorrhagic fever virus II type so as to achieve comparison of results from different laboratories and guarantee the quality control of laboratories; and a standard sample is also provided for fast and accurate detection of dengue hemorrhagic fever virus II type for inspection and quarantine institutions, import and export trade and the like.
Owner:薛芳
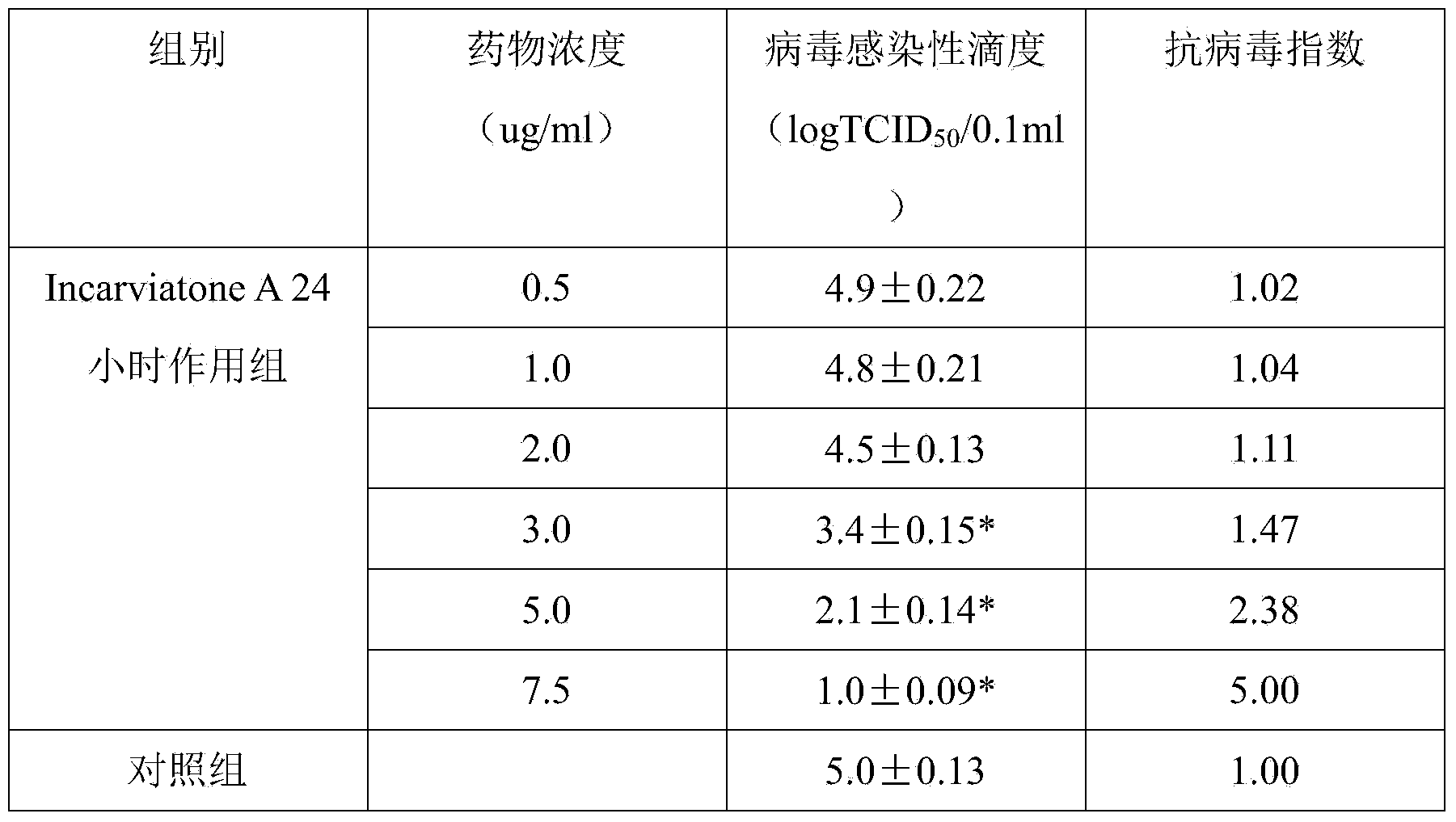
Application of Incarviatone A in drug for treating hemorrhagic fever with renal syndrome
InactiveCN103446093AStrong inhibitory activityHighlight substantive featuresOrganic active ingredientsAntiviralsDisease courseMortality rate
The invention discloses novel application of Incarviatone A in preparation of a drug for treating hemorrhagic fever with renal syndrome. In-vitro and in-vivo inhibition experimental study on the effect of the Incarviatone A to hemorrhagic fever virus with renal syndrome shows that the Incarviatone A has low toxic effect on cells, has the direct killing effect on the hemorrhagic fever virus with renal syndrome, can obviously inhabit viral multiplication, has obvious protecting effect on susliks infected with the virus and enables the disease time to be postponed, the disease course to be prolonged and the death rate to be reduced. Therefore, the Incarviatone A is an effective and safe drug for treating the hemorrhagic fever with renal syndrome.
Owner:李浩福
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com