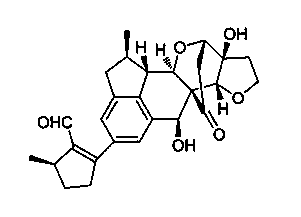
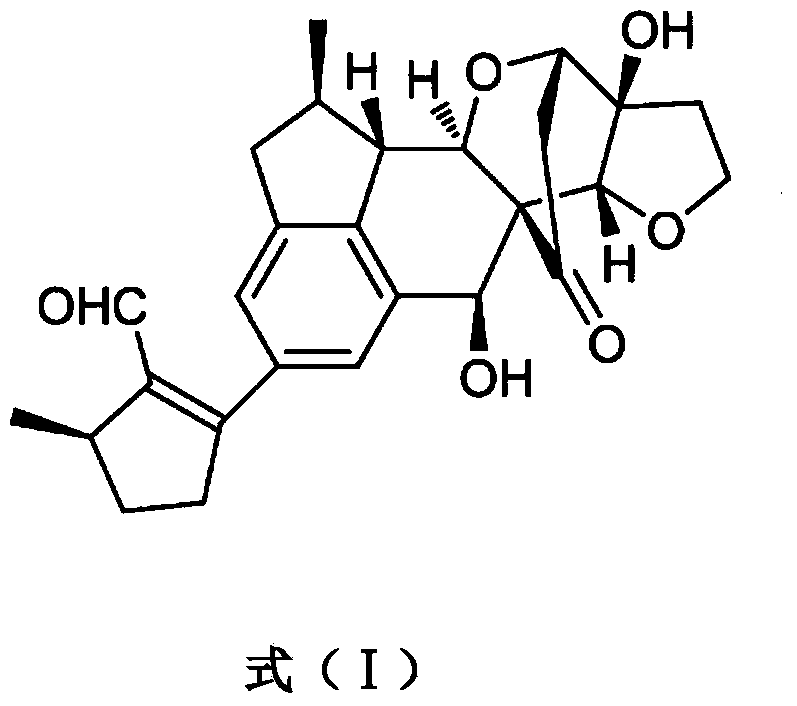
Application of Incarviatone A in drug for treating hemorrhagic fever with renal syndrome
A technology for renal syndrome and hemorrhagic fever, which can be applied to drug combinations, medical preparations containing active ingredients, and pharmaceutical formulations, etc., can solve problems such as uncertain effects, and achieve strong viral inhibitory activity and outstanding essence in hemorrhagic fever with renal syndrome. effects of sexuality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Embodiment 1: the preparation of compound Incarviatone A tablet involved in the present invention:
[0013] Take 20 grams of compound Incarviatone A, add 180 grams of conventional excipients for tablet preparation, mix well, and make 1000 tablets with a conventional tabletting machine.
Embodiment 2
[0014] Embodiment 2: the preparation of compound Incarviatone A capsules involved in the present invention:
[0015] Get 20 grams of compound Incarviatone A, add conventional excipients for preparing capsules such as 180 grams of starch, mix well, and pack into capsules to make 1000 tablets.
[0016] The following pharmacodynamic experiments will further illustrate its drug activity.
[0017] In order to better understand the essence of the present invention, the experimental results of Incarviatone A will be described below to illustrate its application in the field of pharmacy.
[0018] The specific experiment is as follows:
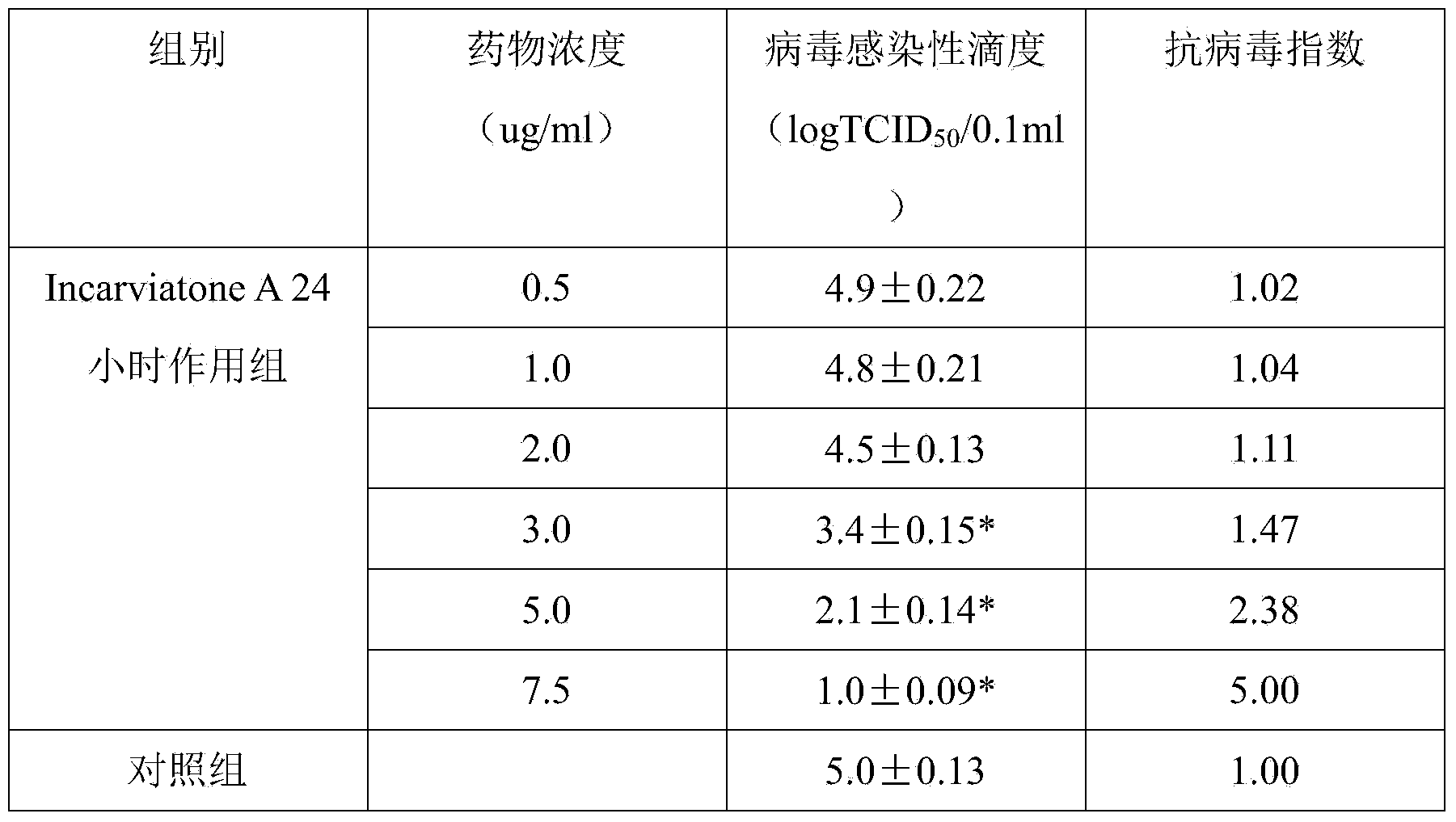
[0019] 1. Experimental study of Incarviatone A against hemorrhagic fever with renal syndrome virus
[0020] (1) In vitro inhibition experiment of hemorrhagic fever with renal syndrome virus
[0021] 1 material
[0022] (1) Virus strain: the international standard strain of hemorrhagic fever with renal syndrome virus 76-118, which is preserved by th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com