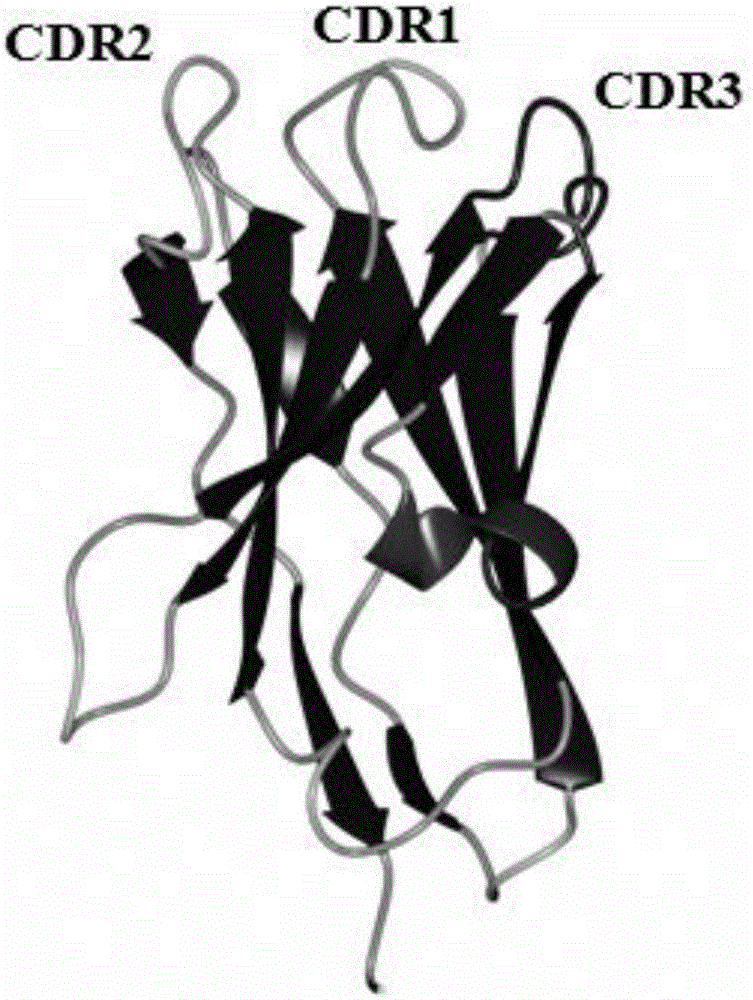Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
75 results about "Haemorrhagic fever" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Compositions for use in identification of viral hemorrhagic fever viruses
InactiveUS7312036B2Sugar derivativesMicrobiological testing/measurementOligonucleotide primersRapid identification
The present invention provides oligonucleotide primers, compositions, and kits containing the same for rapid identification of viruses that cause viral hemorrhagic fevers by amplification of a segment of viral nucleic acid followed by molecular mass analysis.
Owner:IBIS BIOSCI
Composition for widely treating viral diseases and application thereof
The invention relates to a composition for widely treating viral diseases. The composition contains the following raw materials, or extracts of the following raw materials, or derivatives of the extracts of the following raw materials in chemically-acceptable forms, wherein the raw materials include processed products prepared from the raw materials. The constituents of the composition are as follows: 100 parts of Baikal skullcap root, 50-150 parts of radix bupleuri and 50-150 parts of kudzu root; and according to specific circumstances, one or more selected from (but not limited to) the following raw materials can be added on this basis: radix isatidis, honeysuckle flower, Fructus Forsythiae, rhubarb, phellodendron bark, rhizoma anemarrhenae, Cyrtomium fortunei, gardenia, coptis, rhizoma cyperi, fritillaria, balloonflower and licorice root. The composition provided by the invention can be prepared into powder, liquid, paste, granules, capsules or other pharmaceutically-acceptable physical forms. The composition provided by the invention is used for preventing and treating avian influenza, epidemic hemorrhagic fever, epidemic parotitis, nameless hyperpyrexia, Newcastle diseases, swine fever, blue-ear diseases and other multiple human and animal viral diseases characterized by hemorrhage or body temperature rise, and reducing the degree of pathological damage to the body.
Owner:GUANGDONG ZIJIN ZHENGTIAN PHARMA
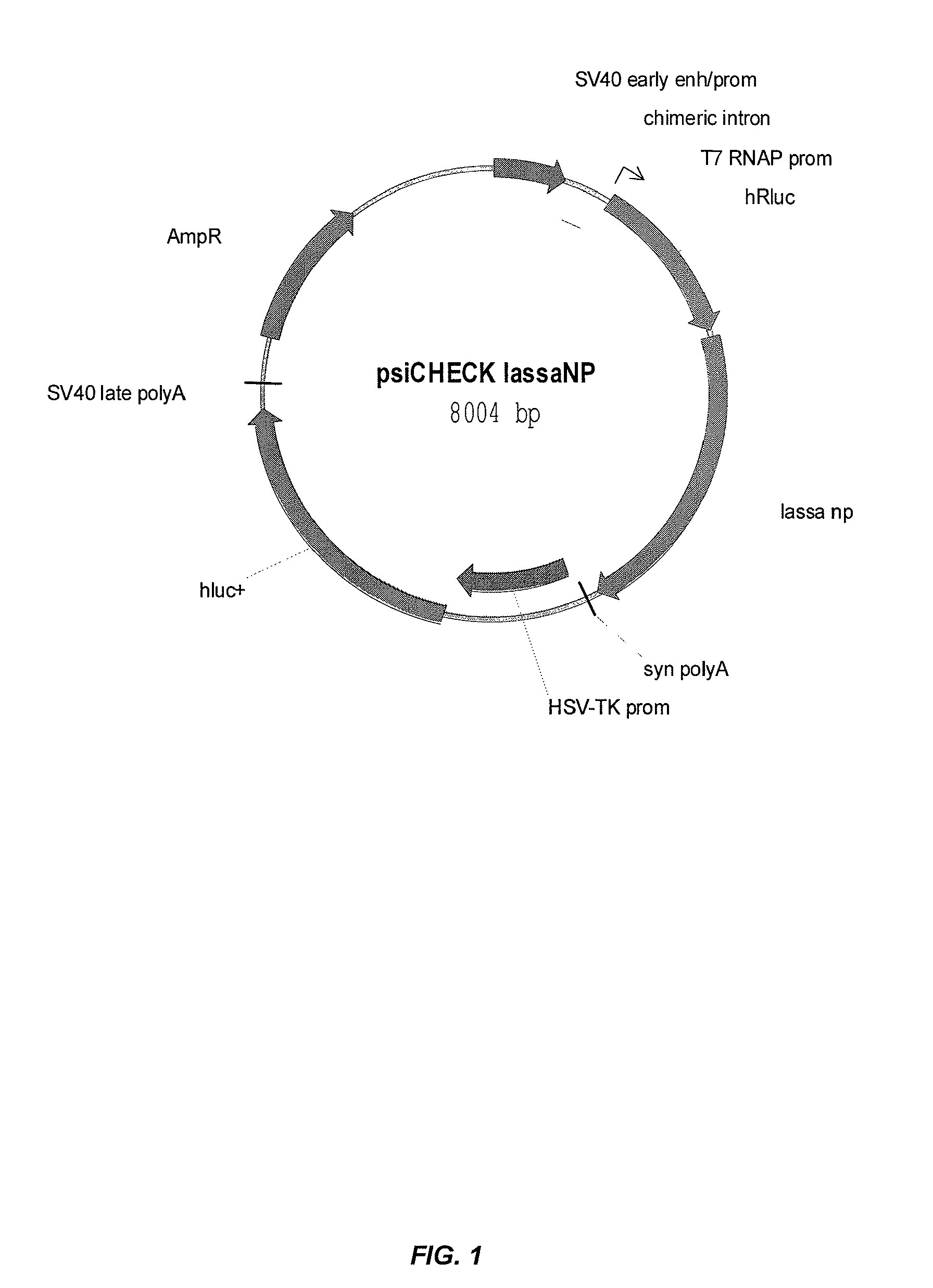
Compositions and methods for silencing genes involved in hemorrhagic fever
InactiveUS8455455B1Easy to useSugar derivativesMicrobiological testing/measurementGene expressionSilencing gene
The present invention provides compositions comprising therapeutic nucleic acids such as interfering RNA (e.g., dsRNA such as siRNA) that target Lassa virus (LASV) or tissue factor (TF) gene expression, lipid particles comprising one or more (e.g., a cocktail) of the therapeutic nucleic acids, methods of making the lipid particles, and methods of delivering and / or administering the lipid particles (e.g., for treating hemorraghic fever).
Owner:US ARMY MEDICAL RES MATERIEL COMMAND USAMRMC +1
Agents for the inhibition of virus replication through regulation of protein folding
The invention concerns agents for the treatment of acute and chronic infections with human and animal pathogenic viruses which assemble along the cell membrane and are released through budding on the surface of the cell. Hereunto count especially causative agents of infectious diseases such as AIDS, hepatitis, hemorrhagic fever, SARS, smallpox, measles, polio or the flu. The subjects of the invention are agents that contain inhibitors of the protein folding as active components. Hereunto count inhibitors of cellular folding enzymes (the enzymatic chaperones) as well as substances that disturb the folding of proteins through chemical chaperones. The following substance classes and their derivates belong thereunto: Geldanamycin, Deoxyspergualin, 4-PBA or Herbimycin A. Due to these agents the highly organised processes of the assembly and the proteolytical maturation of virus structure proteins is disturbed. As a result the release and production of infectious decendent viruses is prevented.
Owner:VIROLOGIK GMBH
Antiviral Drugs for Treatment of Arenavirus Infection
Compounds, methods and pharmaceutical compositions for treating viral infections, by administering certain compounds in therapeutically effective amounts are disclosed. Methods for preparing the compounds and methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment and prophylaxis of viral infections such as caused by the Arenavirus family such as Lassa fever, Argentine hemorrhagic fever, Bolivian hemorrhagic fever, and Venezuelan hemorrhagic fever.
Owner:KINETA FOUR LLC
RPA technology-based marburg virus detection kit and application thereof
InactiveCN106636469AAvoid spreading infectionEfficient amplificationMicrobiological testing/measurementMicroorganism based processesInfection transmissionQuarantine
The invention discloses an RPA technology-based marburg virus detection kit and an application thereof. An experiment proves that a target gene can be effectively amplified by an RPA primer and a probe of a marburg virus, the specificity reaches 100% and sensitivity is 1*10<2>copies / reaction; and the virus can reach the level equivalent to the sensitivity of fluorescent quantitative PCR, and has no cross reaction with a zaire ebola pseudovirus, a dengue virus, a hemorrhagic fever virus with renal syndrome and a Xinjiang hemorrhagic fever virus nucleic acid. The RPA isothermal amplification system is fast in reaction and wide in temperature range, effective amplification of a target gene can be achieved under the condition of 37-42 DEG C, the method can be used for fast field detection of an infectious nucleic acid of the marburg virus, and an available fast detection method is provided for field screening of a pathogen; and meanwhile, the marburg virus detection kit also has the important significance in prevention of infection transmission of the marburg virus in China and inspection and quarantine in affected areas and entry and exit ports.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Antihepatitis medicament, preparation method thereof and use thereof
ActiveCN101633683AEasy to slidePromote dissolutionOrganic active ingredientsDigestive systemSolubilityAllergic purpura
The invention relates to a glycyrrhizic acid derivative, preparation thereof and use thereof. The derivative has high water solubility and high storage stability and is suitable to be used in medicaments and health-care products for treating or preventing hepatitis, liver cirrhosis, hemorrhagic fever of liver damage and liver dysfunction syndrome, allergic purpura, psoriasis vulgaris, eczematousdermatitis and the like.
Owner:刘力
Gold-labeled test paper strip for quick diagnosis of hemorrhagic fever with renal syndrome
InactiveCN1800854AHigh sensitivityReduce fatality rateSugar derivativesPreparing sample for investigationAntigenDiagnosis methods
The invention discloses a recombination antigen for HFRS diagnosis and its rapid GIA diagnosis indicator paper opposite to shortened NP recombination antigen e112 of Hantaan virus by gene clone technique and simultaneous-detected IgM and IgG antigens in patient serum. The said test paper overcomes the limitation of current HFRS diagnosis method, benefit to early-stage diagnosis, reduces illness death rate, does not requires special device nor professional staff to meet more detection need, and cuts cost greatly.
Owner:FUJIAN CENT FOR DISEASE CONTROL & PREVENTION
Preparation method of rabbit hemorrhagic fever virus empty capsid antigen
ActiveCN102304529APromote safe productionReduce consumptionViral antigen ingredientsVirus peptidesAntigenBombyx mori
The invention relates to the field of genetic engineering and particularly relates to a preparation method of a rabbit hemorrhagic fever virus empty capsid antigen. The method provided by the invention comprises the following steps: 1) constructing a rhabdovirus transfer carrier containing a rabbit hemorrhagic fever virus capsid protein VP60 gene or an optimized gene, wherein codon optimization is performed according to the codon frequency of bombyx mori; 2) performing cotransfection on the constructed transfer expression carrier and DNA (deoxyribonucleic acid) of rhabdovirus so as to carry out homologous recombination or transposition to further obtain the recombinant rhabdovirus; 3) infecting the recombinant rhabdovirus with the host cells of an insect; and 4) culturing the infected host of the insect to express the corresponding rabbit hemorrhagic fever virus empty capsid antigen, and harvesting and purifying the expressed antigen. By adopting the method provided by the invention, the production cost of the rabbit hemorrhagic fever virus empty capsid antigen can be greatly reduced, and the method has a plurality of advantages of safety, high efficiency, low energy consumption, low cost and the like.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Horse anti-EBOV (Ebola virus) immune globulin F (ab')2 and preparation method thereof
InactiveCN105254755AImprove survival rateStable and efficient sourceSerum immunoglobulinsImmunoglobulins against virusesFreeze-dryingIon exchange
The invention provides horse anti-EBOV (Ebola virus) immune globulin F (ab')2 and a preparation method thereof. The preparation method comprises steps as follows: virus-like particles of a GP gene and a VP40 gene expressing EBOV-Z simultaneously are inactivated and purified and then are mixed with an immunologic adjuvant to be taken as immunogens, highly immunized plasma is obtained through repeated immunization of healthy horses, IgG protein is separated and purified, then the IgG protein is subjected to pepsase digestion, an enzyme-digested product is processed by a DEAE ion-exchange chromatographic column, an eluent is collected and subjected to desalination and freeze-drying, and the horse anti-EBOV immune globulin F (ab')2 is obtained. The horse anti-EBOV immune globulin F (ab')2 is an effective drug for preventing and treating Ebola hemorrhagic fever caused by EBOV and has remarkable curative effects for critical patients infected with the EBOV; the preparation method is controlled by adopting unique technological parameters and is suitable for industrial production, and the product is safe and reliable.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Rapid test paper bar for testing colloidal gold of antibody of epidemic hemorrhagic fever virus
InactiveCN1963516AQuick screeningSave manpower and material resourcesMaterial analysisAntigenGlass fiber
This invention provides one test bar, which comprises the following steps: a, covering antibody IgM linkage single clone antibody and anti-flu hemorrhagic fever virus reset antigen multiple clone antibody two NC fiber films; b, comprising glue gold label flu hemorrhagic fever virus glass fiber film; applying film analysis and glue gold label technique to test sample flu virus antibody.
Owner:BEIJING ZHUANGDI HAOHE BIOMEDICINE SCI & TECH
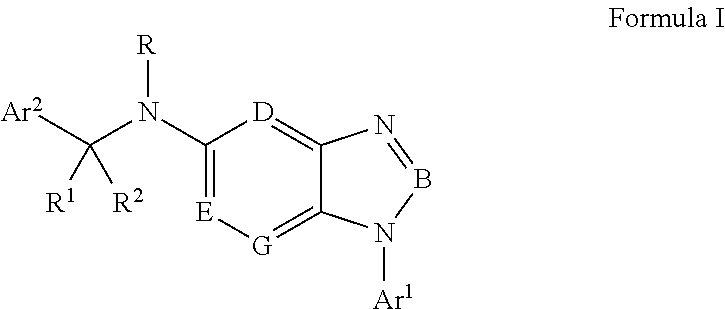
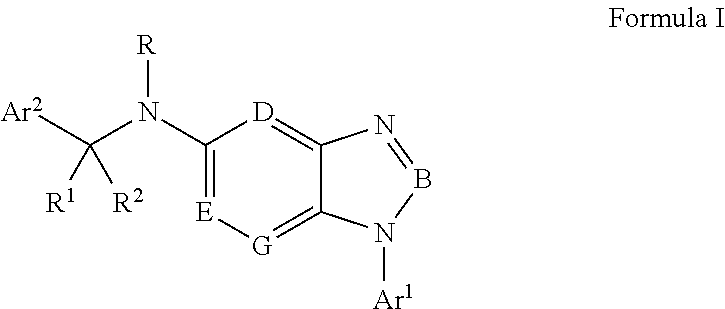
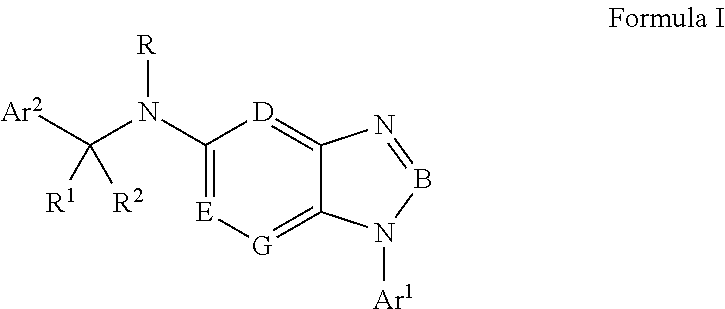
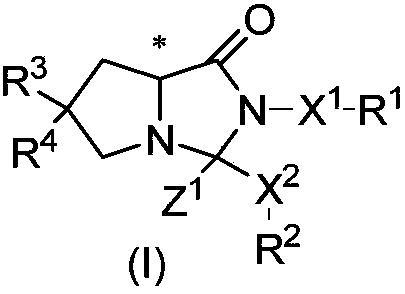
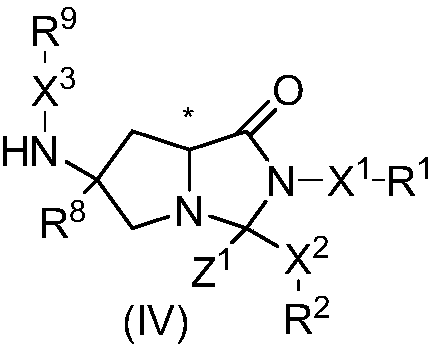
Cyclocompound of tetrahydropyrrole and dihydroimidazolone as well as preparation and pharmaceutical application thereof
The invention discloses a cyclocompound of tetrahydropyrrole and dihydroimidazolone as well as preparation and pharmaceutical application thereof. The cyclocompound is a compound shown in a formula (I), an isomer or pharmaceutically acceptable salt thereof. The compound, isomer or pharmaceutically acceptable salt thereof can be applied to preparation of medicines used for preventing or treating related diseases (such as dengue fever, dengue hemorrhagic fever, dengue shock syndrome, Zika, Chikungunya, Japanese encephalitis, yellow fever, hepatitis C and West Nile disease) caused by a dengue fever virus and related viruses. (The formula (I) is described in the specification).
Owner:NANJING TECH UNIV
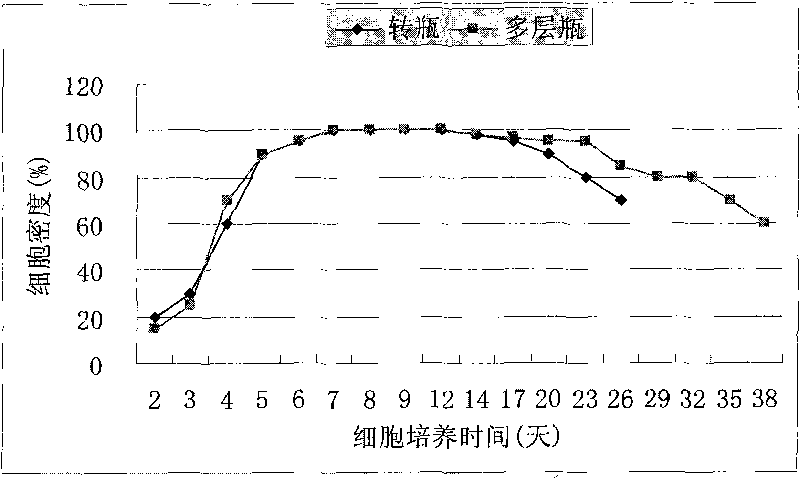
Method for preparing haemorrhagic fever vaccine by culturing primary cells in multilayer culture flask
ActiveCN101732709ALess cellsSmall footprintViral antigen ingredientsInactivation/attenuationCulture fluidPrimary cell
The invention relates to a method for preparing a haemorrhagic fever vaccine by culturing primary cells in a multilayer culture flask. The method comprises the following steps: anatomizing an animal to obtain the primary cells; inoculating half or one third cell of cell amount required by a spinner flask into the multilayer cell culture flask, adding proper culture solution in a hot house for culturing; and inoculating virus for culturing after the cell grows full of a single layer, obtaining virus culture solution, and preparing the vaccine after purification. The multilayer cell flask has the advantages of small amount of inoculated primary cells, large amount of the cultured cells, small occupied space, no need of a flask spinning machine and high yield.
Owner:ZHEJIANG PUKANG BIOTECH
SYBR Green I Real-time PCR method for quickly detecting and indentifying Hantaan virus infection
InactiveCN103436637AAccurate detectionStrong contrastMicrobiological testing/measurementFluorescence/phosphorescenceConserved sequenceHaemorrhagic fever
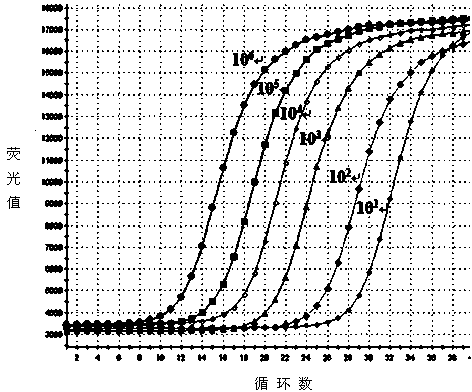
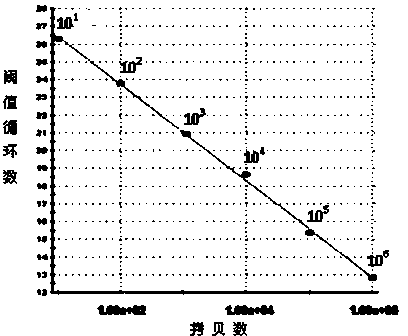
The invention discloses an SYBR Green I Real-time PCR (Real-time fluorescent quantitative polymerase chain reaction) method for quickly detecting and indentifying Hantaan virus infection. The method comprises the following steps: designing a pair of specific primers mainly according to a conserved sequence of an S gene in a GenBank database; using a positive plasmid subjected to 10-time gradient dilution as a standard product; optimizing a reaction condition; establishing the SYBR Green I Real-time PCR method used for detecting a Vero E6 cell, a mouse and a clinical patient, which are infected by Hantaan virus nucleic acids according to qRT-PCR specificity amplification, wherein a copy number of the detection sensitivity can reach 101, and sensitivity, repeatability and specificity of the Hantaan virus nucleic acids are detected. Compared with a conventional Hantaan virus detection method, the method has the advantages that types of detecting samples are rich, the application range is wide, the contrast is strong, operation steps are relatively few, convenience and quickness are realized, the sensitivity and the repeatability are high, the specificity is good, and a linear relationship is good, and can provide a strong experimental basis on clinical and laboratory detection of hemorrhagic fever with renal syndrome as well as studying of epidemiology.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Method of treatment of hemorrhagic disease using a factor VIIa/tissue factor inhibitor
ActiveUS20040266672A1Reduce incidence deathPrevent coagulationBiocidePeptide/protein ingredientsFactor VIIaNematode
The present invention provides methods of treating hemorrhagic fevers where selective inhibitors of fVIIa / TF are used as a treatment for hemorrhagic fevers and have therapeutic effects which include ameliorating and / or preventing coagulopathy and inflammatory responses. These inhibitors include certain proteins which are part of a family termed Nematode-Extracted Anticoagulant Proteins ("NAPs"). Other inhibitors include Tissue Factor Pathway Inhibitor ("TFPI") and TFPI analogs.
Owner:DENDREON PHARMA LLC
Recombinant interferon lambda 4 encoded cDNA sequence as well as preparation method and application thereof
InactiveCN106222178ARealize industrializationOptimizing the induction temperatureBacteriaPeptide/protein ingredientsEscherichia coliNucleotide
The invention relates to a recombinant interferon lambda 4 encoded cDNA sequence which can be efficiently expressed in a prokaryotic expression system. The nucleotide sequence of the recombinant interferon lambda 4 encoded cDNA sequence is as shown in SEQ ID NO:1. The invention further provides application of the cDNA sequence in preparing a medicine for treating and / or preventing hemorrhagic fever with renal syndrome caused by hantaan viruses. The invention provides a method and application of recombinant interferon lambda 4. Escherichia coli can be concerted and expression can be induced by using a prokaryotic expression vector containing human interferon lambda 4 encoded cDNA, the optimal induction temperature of a relatively large amount of dissoluble interferon lambda 4 is 18 DEG C, the bacterium shaking speed is 120rpm, the induction time is 4 hours, and the IPTG concentration is 0.1mM. By adopting the product prepared by using the preparation method, basis is made for study on biological activity and action mechanisms of human interferon lambda 4, and the product can be also applied to medicines for treating and / or preventing hemorrhagic fever with renal syndrome caused by hantaan viruses.
Owner:WUHAN UNIV
Therapeutic composition comprising annexin v
The present invention provides Annexin A5 for use in a prophylactic or therapeutic method of preventing, reducing, delaying the onset of, or delaying the progression of, direct viral damage to the vascular system and / or immune system, in a subject, wherein the viral infection is caused by a virus selected from the group consisting of (a) a virus capable of causing hemorrhagic fever (VHF), and (b) a virus that presents phosphatidylserine (PS) and mediates cell infection and / or internalisation through PS binding.
Owner:ANNEXIN PHARMA
Preparation method and application of double-effect vaccine for dengue fever
ActiveCN105601721AStop transmissionReduce bleedingSsRNA viruses positive-senseViral antigen ingredientsWAS PROTEINStructural protein
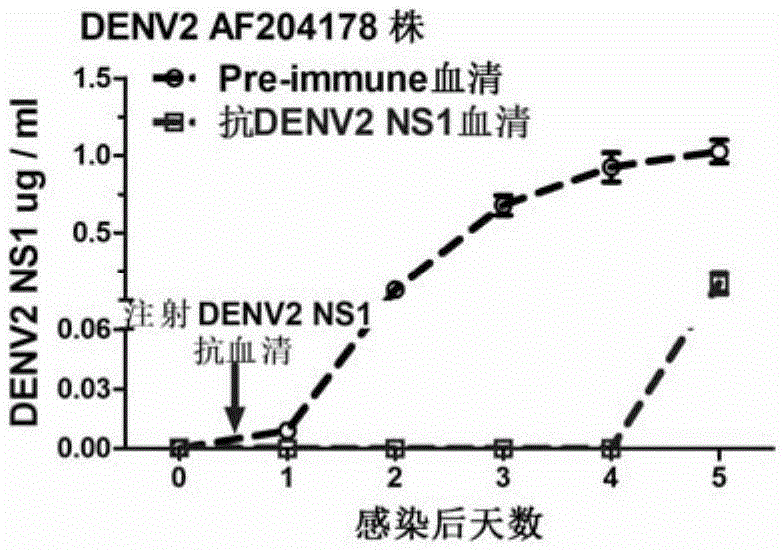
The invention discloses a preparation method and application of a double-effect vaccine for the dengue fever. The invention provides a protein which is protein a) or protein b, wherein protein a) is a protein composed of an amino acid sequence as shown in a sequence 4 in a sequence table, and protein b) is a protein which has same functions as protein a) and is derived from the protein a) by subjecting the sequence 4 in the sequence table to substitution and / or deletion and / or addition of one or more amino acid residues. Experiments in the invention prove that reconstructed dengue virus non-structural protein 1 (DENV delta NS1) can be used as the vaccine; and the double-effect vaccine can protect mankind or mammals from dengue haemorrhagic fever and block propagation of the dengue virus in nature via mosquitoes.
Owner:TSINGHUA UNIV
Detection type gene chip for detecting various infectious desease and use thereof
InactiveCN1195070CStrong specificityHigh sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesAntigenRickettsia
Owner:陶开华 +1
Method for preparing haemorrhagic fever vaccine by culturing primary cells in multilayer culture flask
ActiveCN101732709BLess cellsSmall footprintViral antigen ingredientsInactivation/attenuationCell massEngineering
The invention relates to a method for preparing a haemorrhagic fever vaccine by culturing primary cells in a multilayer culture flask. The method comprises the following steps: anatomizing an animal to obtain the primary cells; inoculating half or one third cell of cell amount required by a spinner flask into the multilayer cell culture flask, adding proper culture solution in a hot house for culturing; and inoculating virus for culturing after the cell grows full of a single layer, obtaining virus culture solution, and preparing the vaccine after purification. The multilayer cell flask has the advantages of small amount of inoculated primary cells, large amount of the cultured cells, small occupied space, no need of a flask spinning machine and high yield.
Owner:ZHEJIANG PUKANG BIOTECH
HTNV vaccine based on VSV vector as well as preparation method and application of HTNV vaccine
PendingCN113842454AReduce infectionImprove securitySsRNA viruses negative-senseViral antigen ingredientsNucleotideRecombinant vaccines
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Preparation method of fusion mutant of Ebola virus glycoprotein and matrix protein
ActiveCN107034225AEfficient research and developmentShort build cycleSsRNA viruses negative-senseViral antigen ingredientsViral glycoproteinMutant
The invention discloses a preparation method of a fusion mutant of Ebola virus glycoprotein and matrix protein by saccharomycetes. The preparation method includes steps of 1), introducing gene for encoding the fusion mutant of Ebola virus glycoprotein and matrix protein to a receptor yeast cell to obtain the recombined yeast cell for expressing the gene; 2), orderly cultivating and breaking the recombined yeast cell to obtain the fusion mutant of Ebola virus glycoprotein and matrix protein from the broken product. The fusion mutant of Ebola virus glycoprotein and matrix protein is a recombined protein obtained by fusing an Ebola virus glycoprotein core zone reserved with an Ebola virus glycoprotein receptor bond zone and a glycan cap zone to an N terminal of the Ebola virus matrix protein. The fusion mutant of Ebola virus glycoprotein and matrix protein has a polymer structure and has galactosylated modification; therefore, the fusion mutant is potential to be a vaccine for preventing Ebola hemorrhagic fever.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE +1
Polypeptide for typing hemorrhagic fever with renal syndrome caused by Hantaan virus and Seroul virus
InactiveCN107383176AAntibody positive rate is highHigh sensitivitySsRNA viruses negative-senseVirus peptidesSerum igeSeoul hantavirus
The present invention discloses a polypeptide for typing hemorrhagic fever with renal syndrome caused by Hantaan virus and Seroul virus, wherein the polypeptide is selected from a polypeptide having an amino acid sequence represented by SEQ ID NO.1-SEQ ID NO.8. The invention further discloses a kit used for typing hemorrhagic fever with renal syndrome caused by Hantaan virus and Seroul virus and prepared by using the polypeptide as the effective component. According to the present invention, with the polypeptide, the detection of the clinical typing of the HTNV hemorrhagic fever with renal syndrome and the SEOV hemorrhagic fever with renal syndrome can be effectively achieved; and compared to the Western blot, the method of the present invention has the following advantages that the positive rate of the antibody in the patient serum is high, and the detection sensitivity is high.
Owner:于学杰
Reagent kit capable of simultaneously detecting virulent viruses and bacteria and detecting method
PendingCN109852730ARNase resistantImprove stabilityMicrobiological testing/measurementMicroorganism based processesViral nucleic acidHemorrhagic fever virus
The invention provides a reagent kit capable of simultaneously detecting virulent viruses and bacteria and a detecting method. The reagent kit is mainly in accordance with 4 pathogenic microorganismsof ebola viruses, Sinkiang hemorrhagic fever viruses, Brucella and anthrax bacillus. The reagent kit comprises a specific primer probe combination for detecting the ebola viruses, the Sinkiang hemorrhagic fever viruses, the Brucella and the anthrax bacillus, a micro-fluidic solid-phase PCR chip on which a specificity probe is fixed, and an RNase-resistant high-stability positive quality control product consisting of virus-like particles containing viral nucleic acid and thalli containing plasmid carrying the specificity nucleic acid. According to the reagent kit, simultaneous detection of theebola viruses, the Sinkiang hemorrhagic fever viruses, the Brucella and the anthrax bacillus can be realized, and the reagent kit has the advantages of being high in detection flux, high in sensitivity, high in specificity, good in repeatability, short in detection time, low in detection cost, low in operation technique requirements, not liable to pollute and the like, and has great application prospects in the field of quick simultaneous detection of various pathogenic microorganisms.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
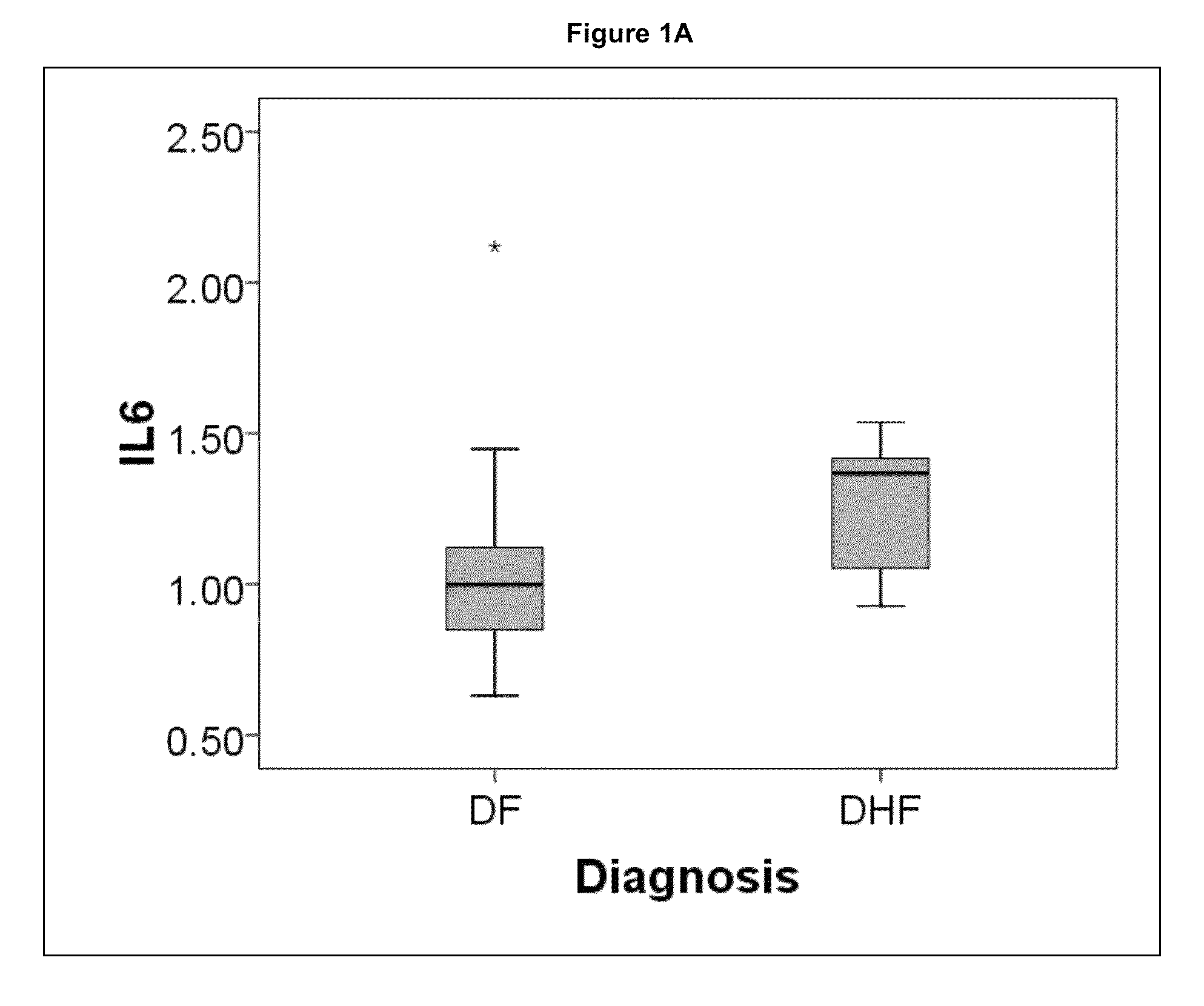
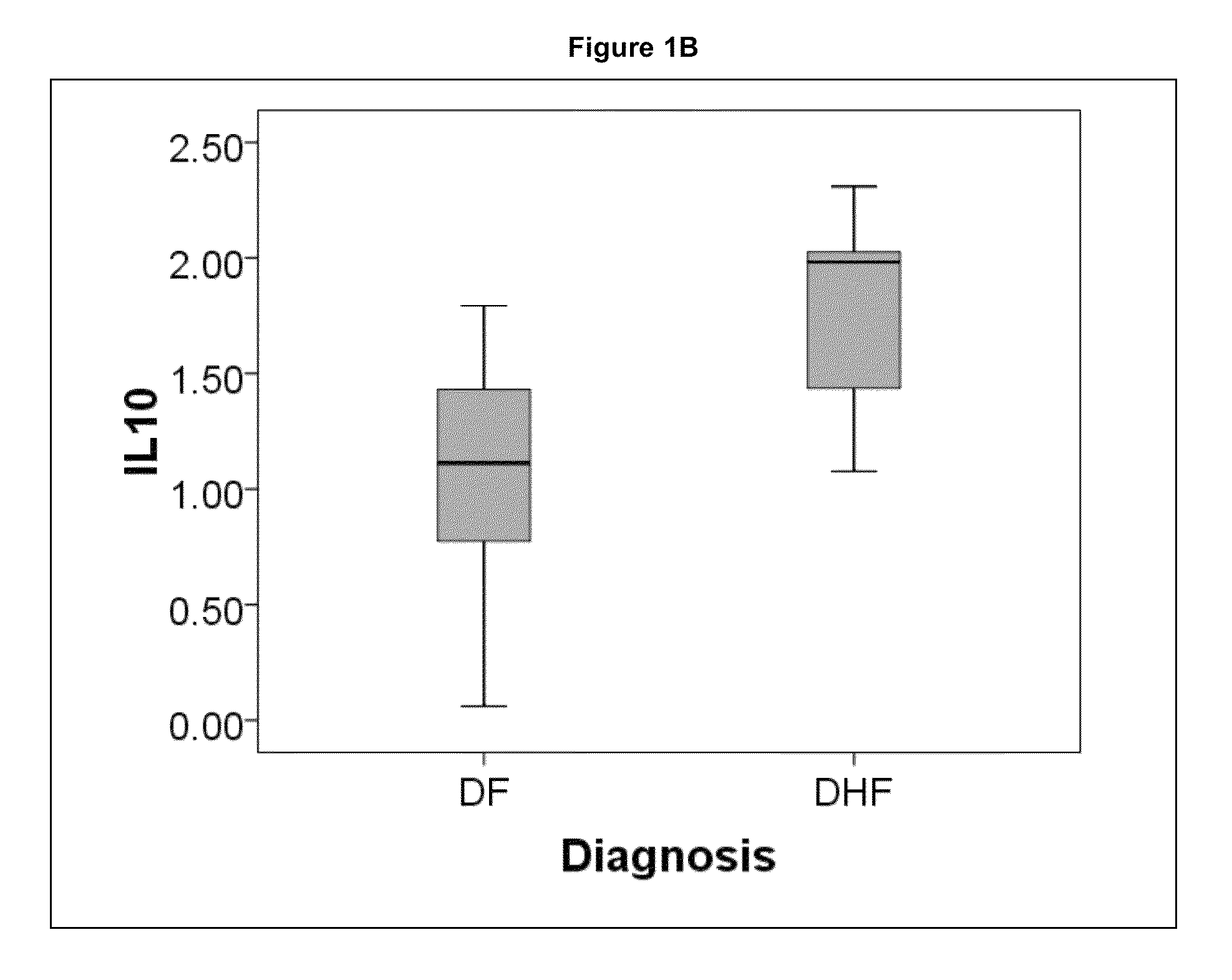
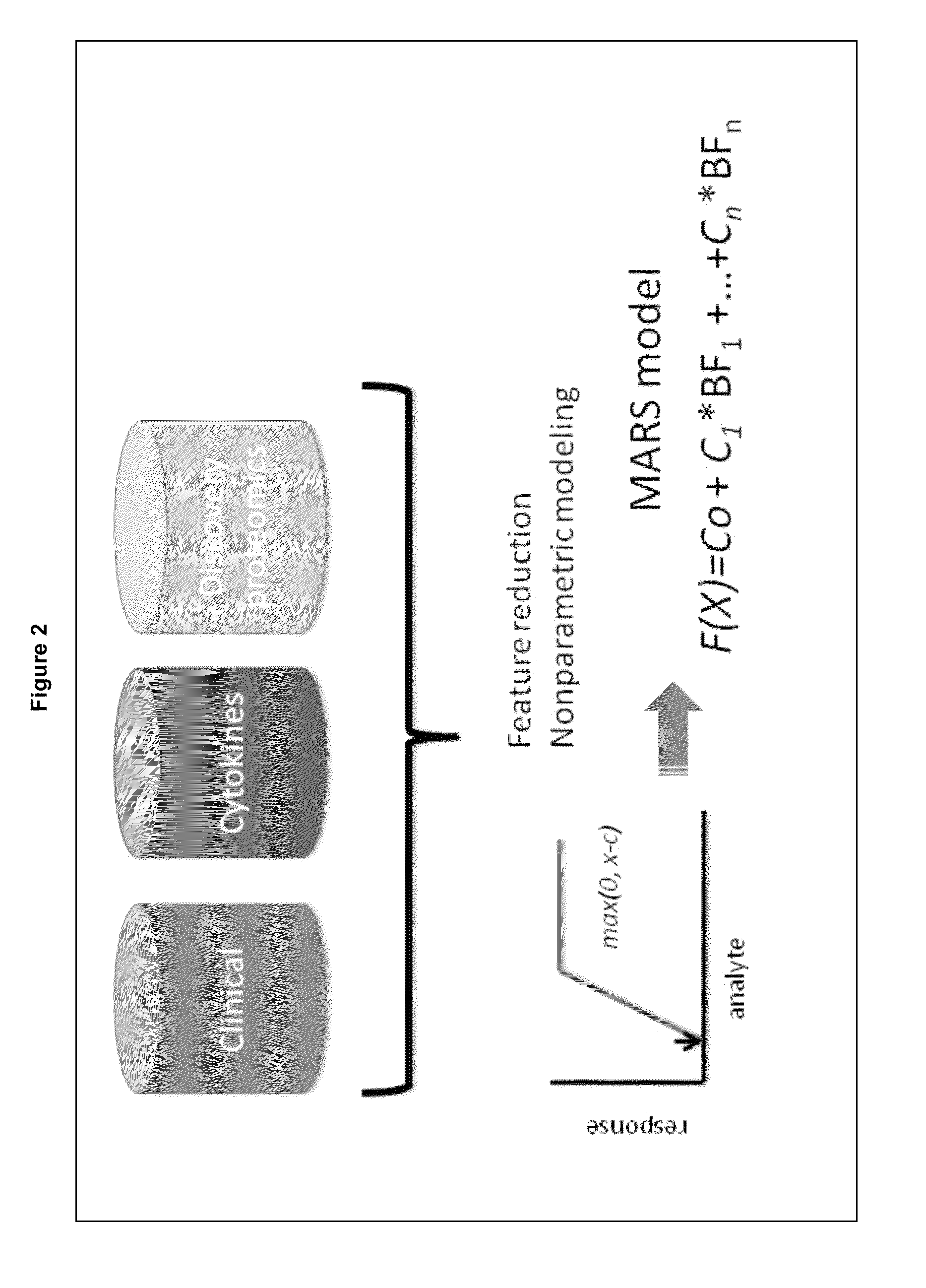
Methods and biomarkers for the detection of dengue hemorrhagic fever
The present invention provides methods for detecting, analyzing, and identifying biomolecules used to identifying patient with dengue-like symptom who are at risk of DHF. The inventive method comprises detecting in a sample from a subject dengue infected patient one or more biomarkers selected from the group consisting of IL-10, fibrinogen, C4A, immunoglobulin, tropomyosin, and three isoforms of albumin, and which are used in a predictive MARS model to detect patients with risk of developing DHF.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Single-domain antibody for neutralizing Xinjiang hemorrhagic fever virus
ActiveCN106188285AGood tissue permeabilityReduce manufacturing costAntibody mimetics/scaffoldsBiological material analysisProduction cycleSteric effects
The invention provides a single-domain antibody XHF4RC8 for neutralizing the Xinjiang hemorrhagic fever virus and application thereof in preparing medicine for preventing or treating Xinjiang hemorrhagic fever virus infection. The amino acid sequence of the single-domain antibody XHF4RC8 is shown as SEQ ID NO: 1, and the nucleotide sequence of a gene for coding XHF4RC8 is shown as SEQ ID NO: 2. The VH structural domain m0 of a human antibody IgG1 serves as the framework of the single-domain antibody. XHF4RC8 can be bound with the structural domain III of Xinjiang hemorrhagic fever virus envelope protein Gc and has the virus neutralizing effect. XHF4RC8 is small in molecular weight and has higher tissue penetration and higher capability for being bound with epitope with the steric effect. XHF4RC8 can be expressed in a prokaryotic expression system and is low in production cost and short in production cycle. XHF4RC8 has the potential function of treating the Xinjiang hemorrhagic fever virus clinically or can be used in combination with other antibodies to achieve an ideal treatment effect.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Replication-deficient modified vaccinia ankara (MVA) expressing marburg virus glycoprotein (GP) and matrix protein (VP40)
PendingUS20220152190A1Avoid infectionPrevents and ameliorates resultingSsRNA viruses negative-senseViral antigen ingredientsModified vaccinia AnkaraViral glycoprotein
The compositions and methods are described for generating an immune response to a hemorrhagic fever virus such as ebolavirus, Marburgvirus, or arenavirus. The compositions and methods described herein relate to a modified vaccinia Ankara (MVA) vector encoding one or more viral antigens for generating a protective immune response to a member of genus Ebolavirus (such as a member of species Zaire ebolavirus), a member of genus Marburgvirus (such as a member of species Marburg marburgvirus), or a member of genus Arenavirus (such as a member of species Lassa virus) in the subject to which the vector is administered. The compositions and methods of the present invention are useful both prophylactically and therapeutically and may be used to prevent and / or treat an infection caused by ebolavirus, Marburgvirus, or arenavirus.
Owner:GEOVAX INC
Traditional Chinese medicine composition for preventing and treating dengue virus and preparation method thereof
InactiveCN111743935AGood control effectGood curative effectAntiviralsUnknown materialsCurative effectHaemorrhagic fever
The invention is applicable to the technical field of medicines, and provides a traditional Chinese medicine composition for preventing and treating dengue virus and a preparation method thereof. Thetraditional Chinese medicine composition is prepared from plant-derived traditional Chinese medicine raw materials including an artemisia apiacea extract, a purple perilla extract and trogopterus dungthrough compatibility. The traditional Chinese medicine composition has a good prevention and treatment effect on dengue fever, dengue hemorrhagic fever and dengue heat shock syndrome caused by dengue virus infection, is long in drug effect time and good in curative effect, and particularly has a good inhibition effect on dengue fever type II viruses.
Owner:广东卫康有害生物防制有限公司
Ebolavirus pre-hairpin intermediate mimics and methods of use
Ebolavirus is a highly lethal filovirus that causes hemorrhagic fever in humans and non-human primates. With no approved treatments or preventatives, the development of an anti-ebolavirus therapy to protect against natural infections and potential weaponization is an urgent unmet global health need. The design, biophysical characterization, and validation of peptide mimics of the ebolavirus N-trimer (“N-trimer mimics”) are described herein.
Owner:UNIV OF UTAH RES FOUND +2
Dengue virus specific multiple HLA binding T cell epitopes for the use of universal vaccine development
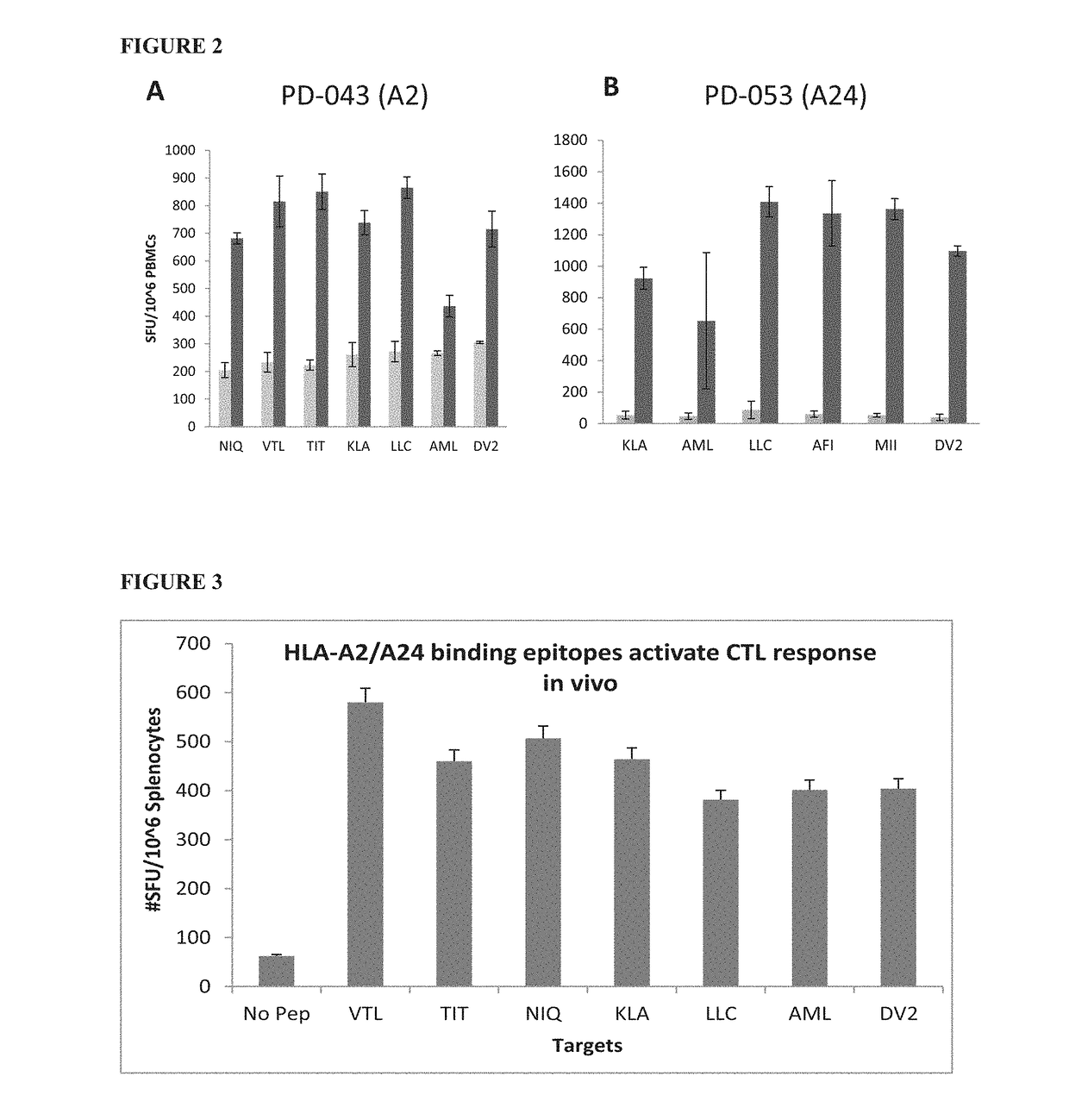
Dengue Fever (DF) and Dengue Hemorrhagic Fever (DHF) are significant global public health problems and understanding the overall immune response to infection will contribute to appropriate management of the disease and its potentially severe complications. Live attenuated and subunit vaccine candidates, which are under clinical evaluation, induce primarily an antibody response to the virus and minimal cross-reactive T cell responses. Currently, there are no available tools to assess protective T cell responses during infection or post vaccination. Herein, we report novel, naturally processed and presented MHC class I restricted epitopes, a subset of which binds to and activates T cells in both an HLA-A2 and HLA-A24 restricted manner. We show that epitope specific T cells can be activated in vivo in transgenic mice and in vitro in seropositive and seronegative individuals and that these T cells are functional, recognizing peptide pulsed and dengue virus infected cells in a pro-inflammatory and cytotoxic manner. These epitopes have potential as new informational and diagnostic tools to characterize T cell immunity in Dengue virus (DV) infection, and may serve as a universal vaccine candidate complementary to current vaccines in trial.
Owner:EMERGEX VACCINES HLDG LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com