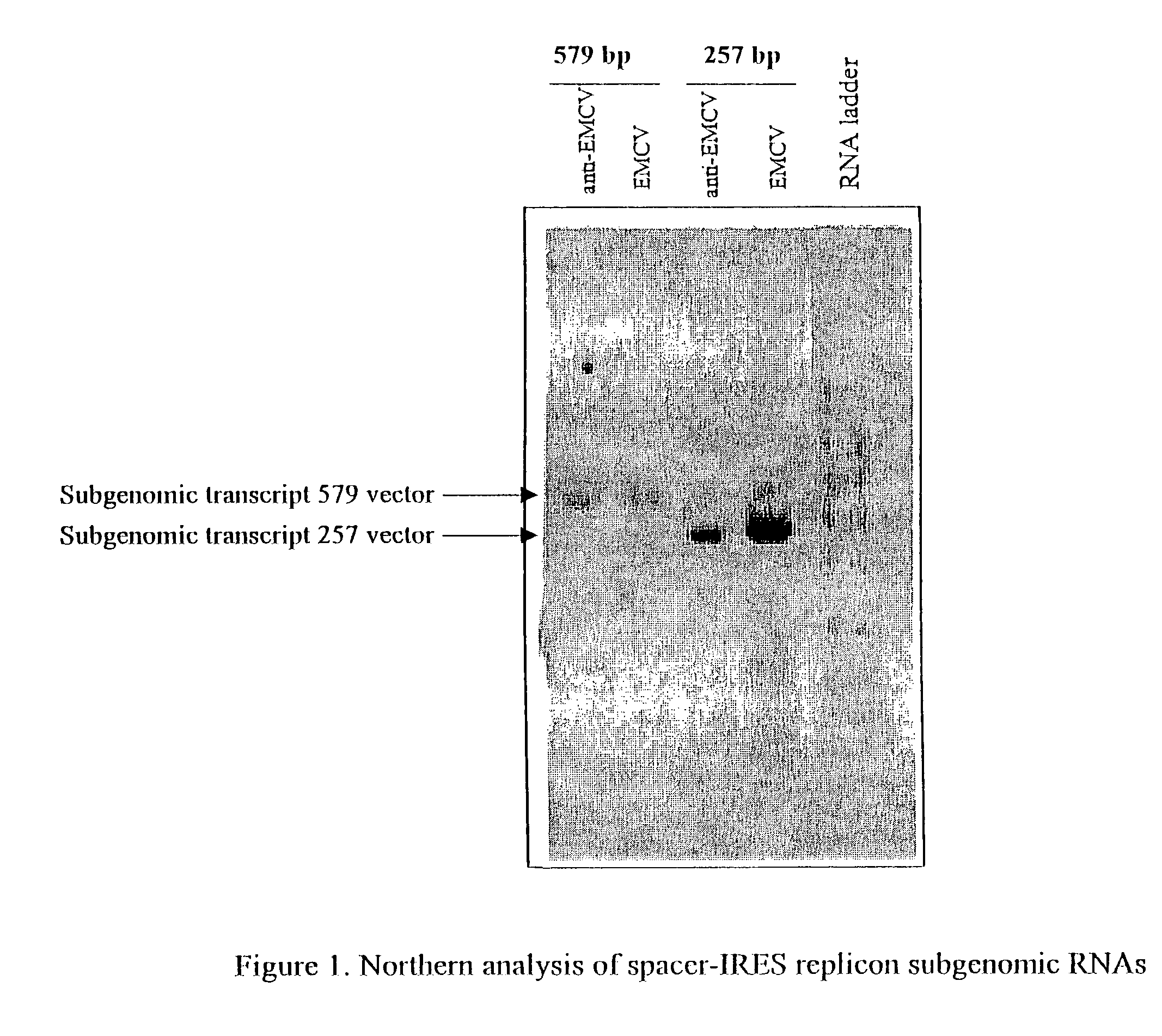
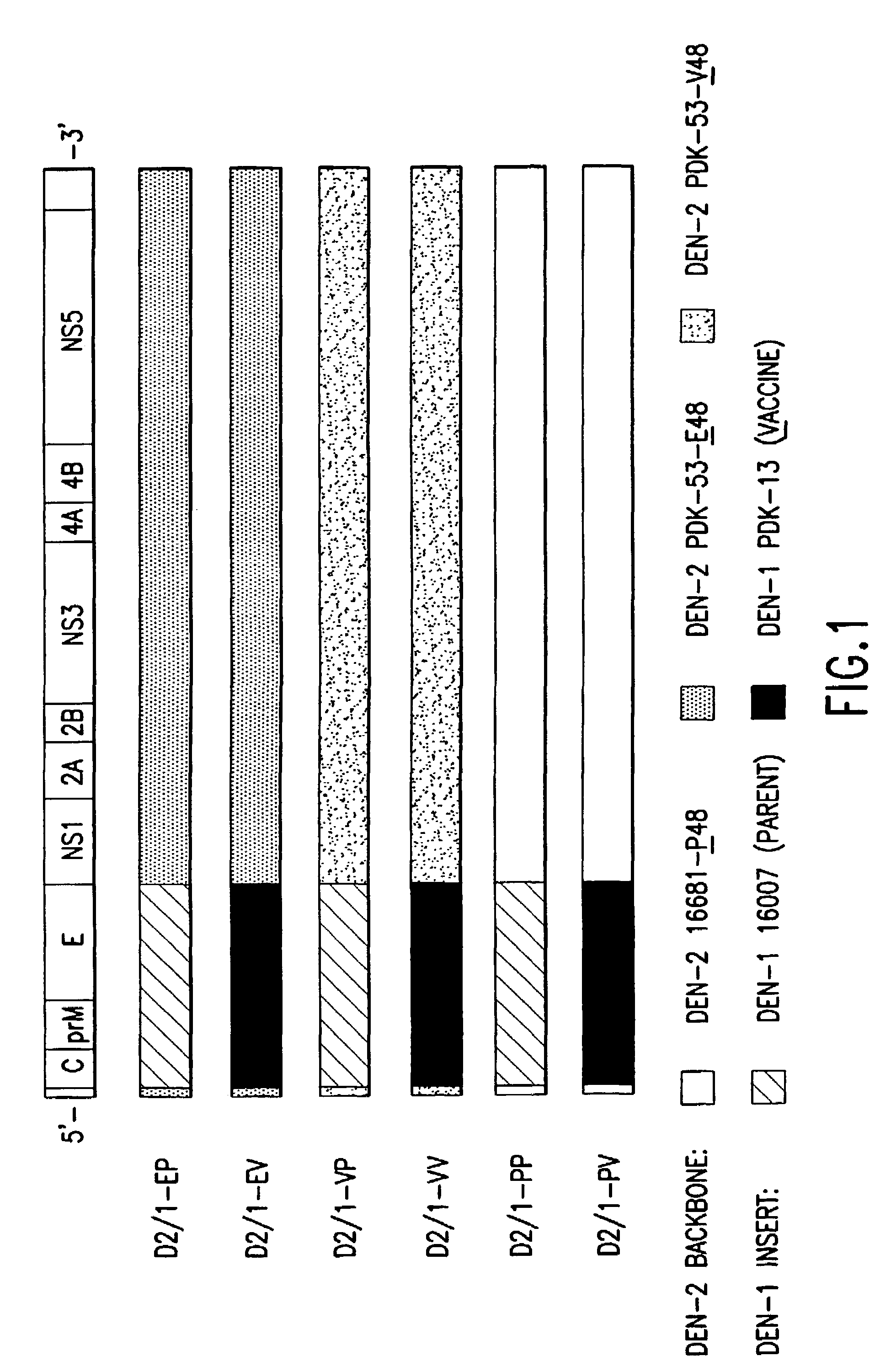
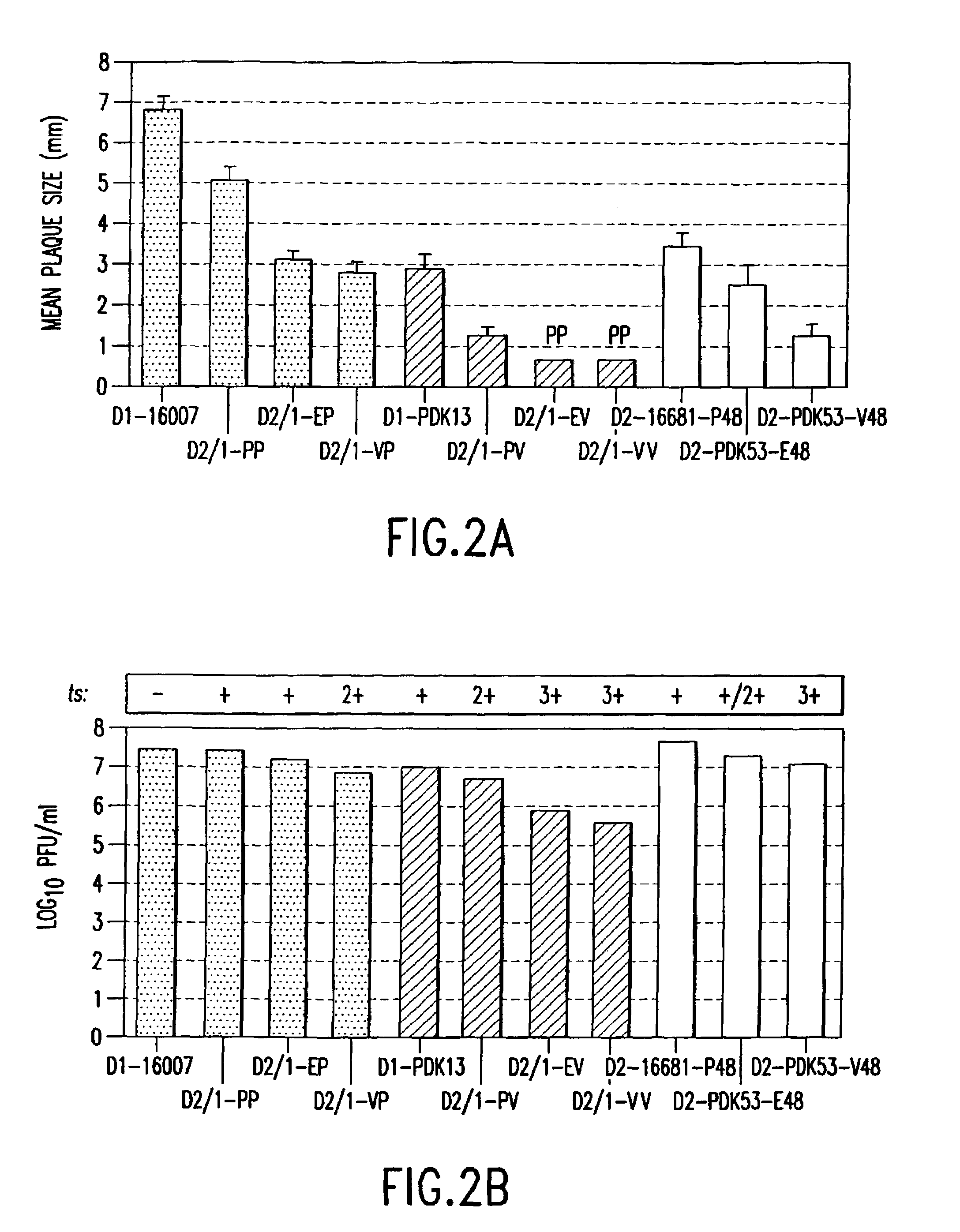
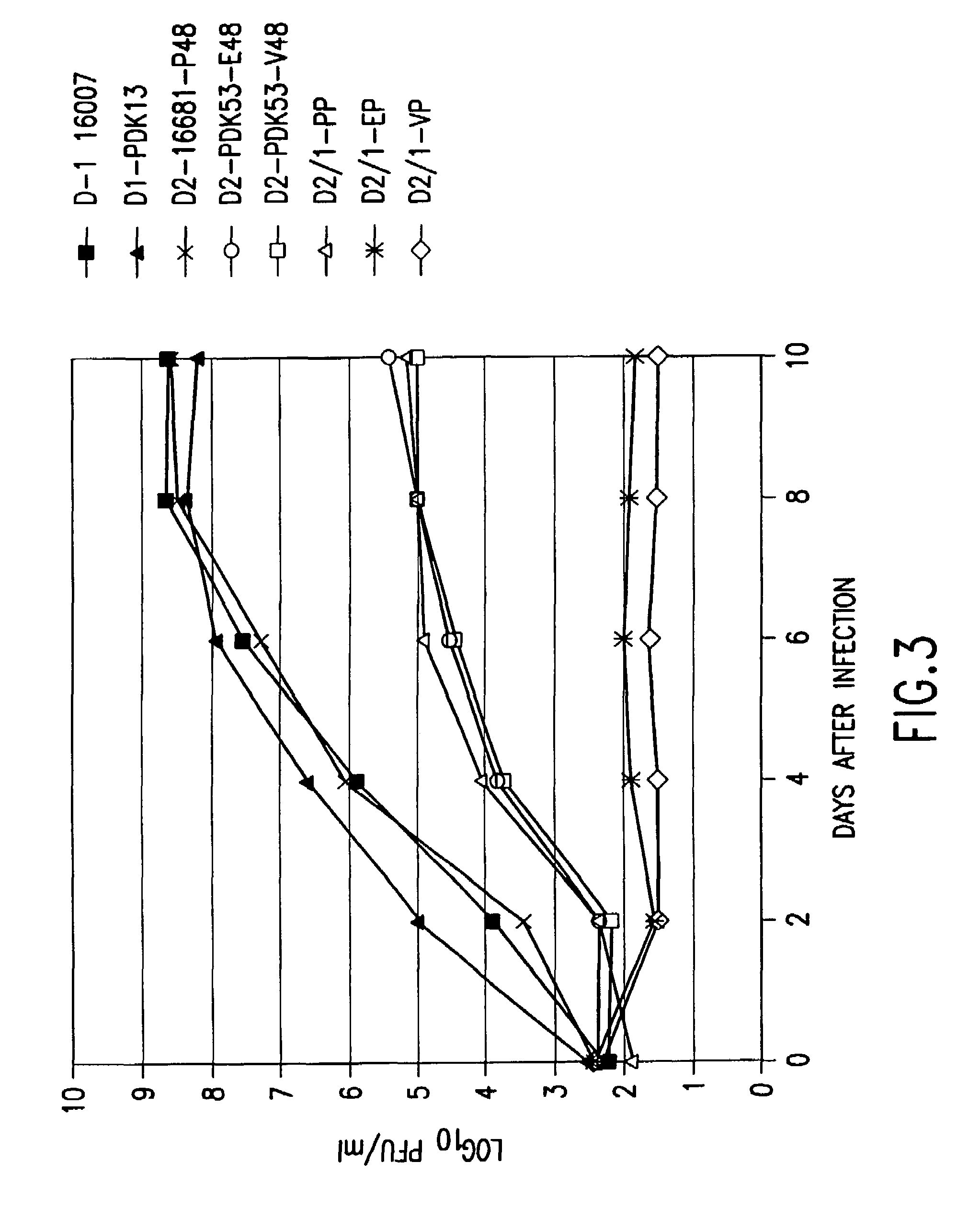
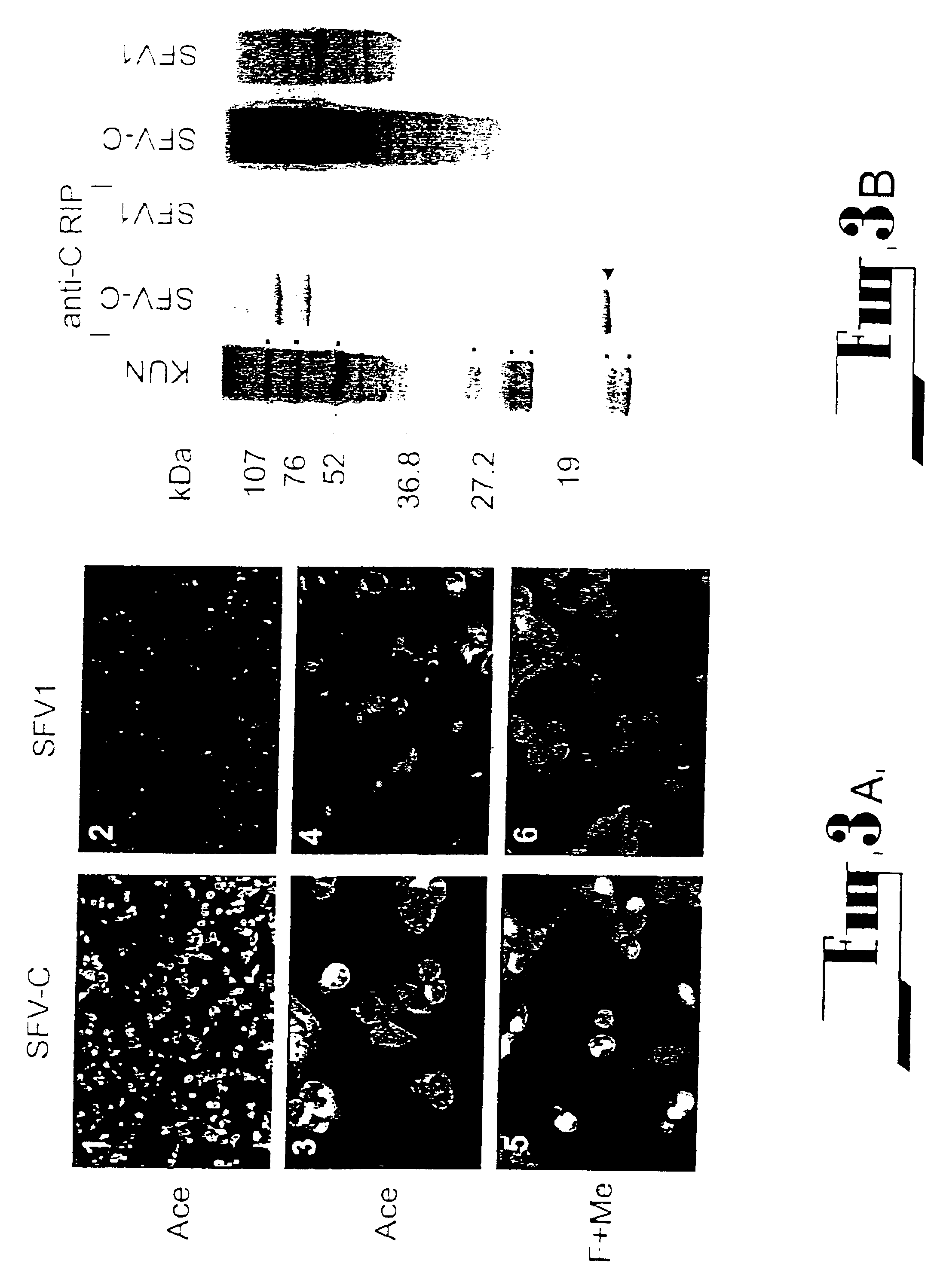
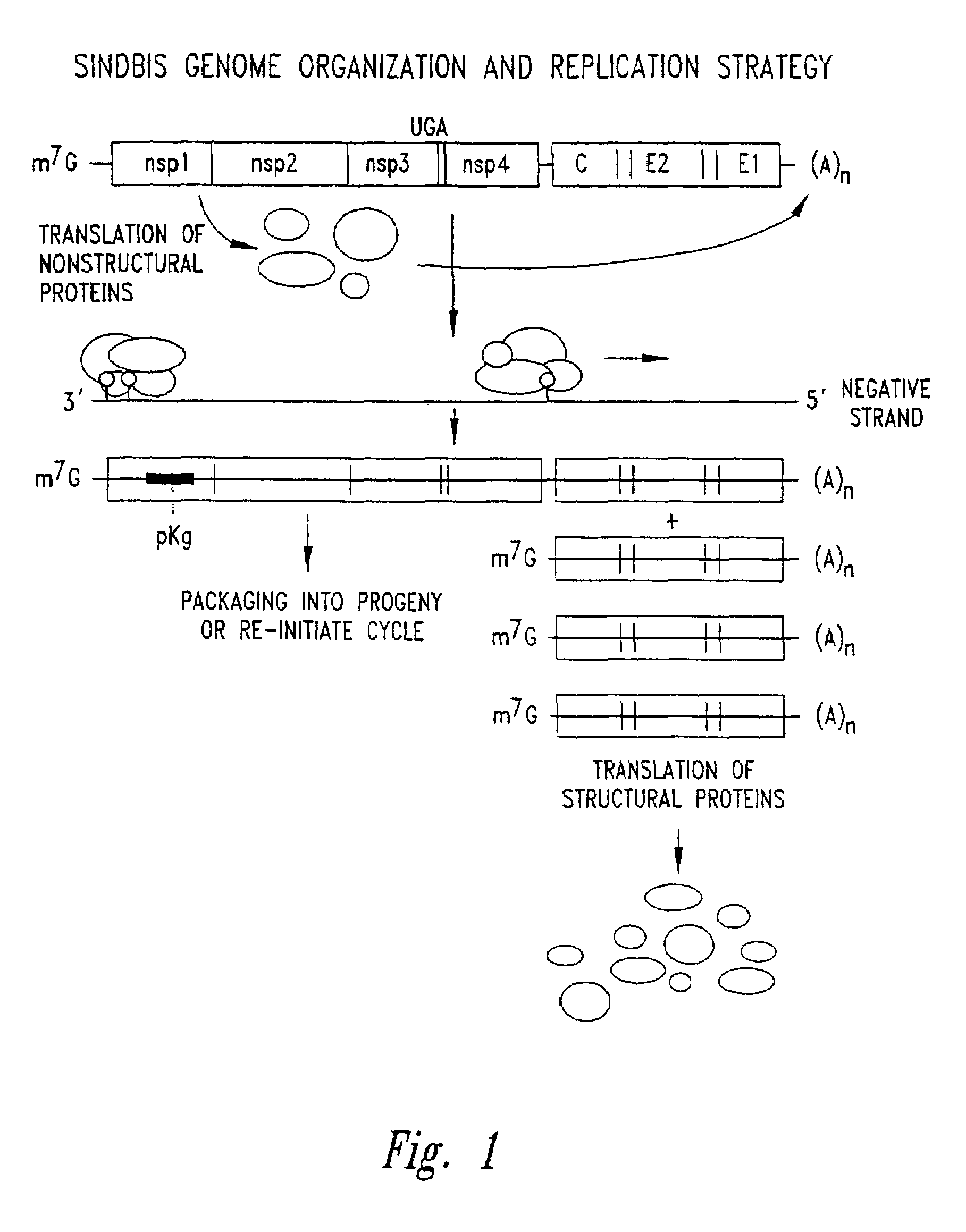
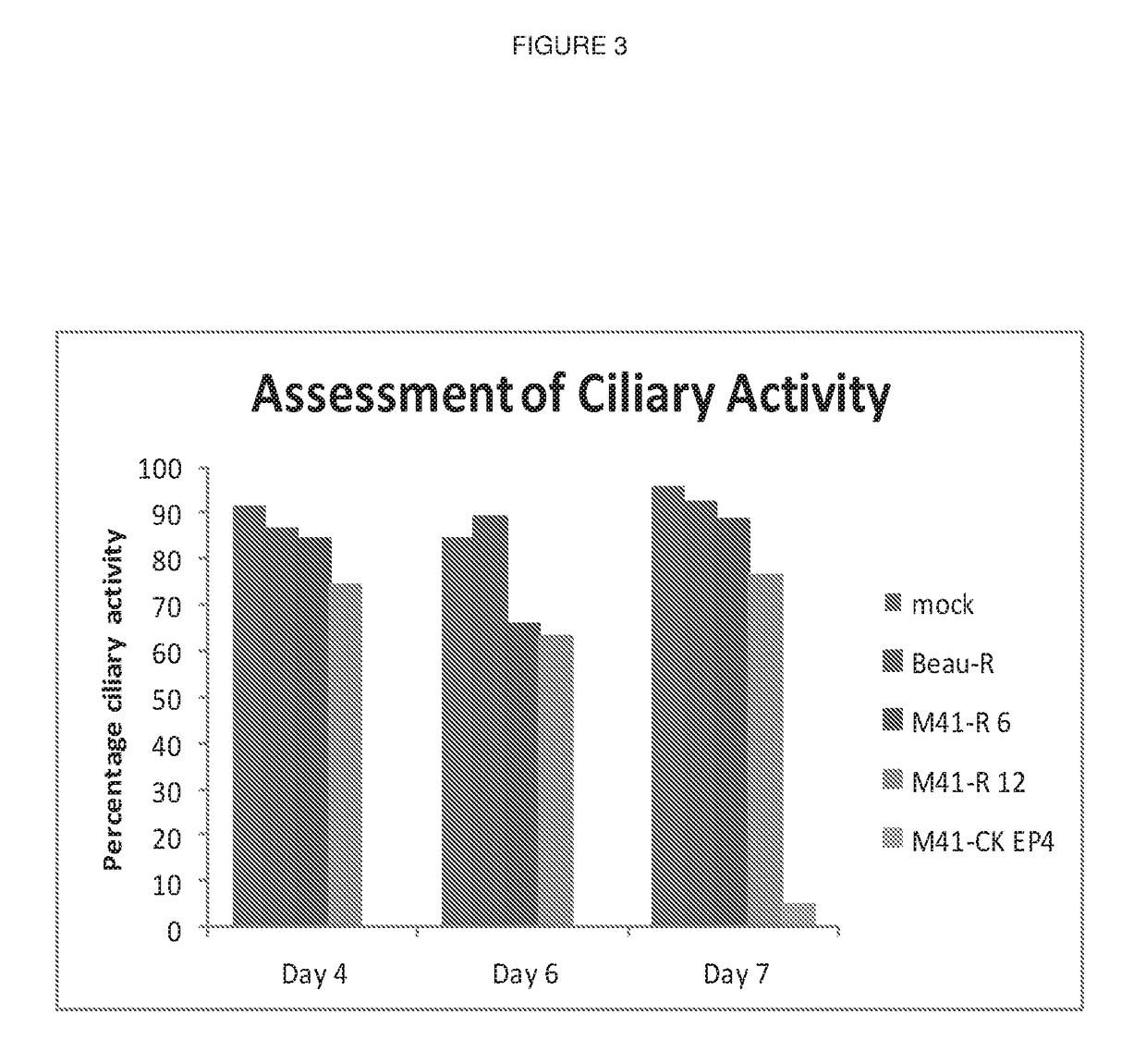
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
185 results about "Unstructured Proteins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Intrinsically unstructured protein. An intrinsically disordered protein (IDP) is a protein that lacks a fixed or ordered three-dimensional structure. IDPs cover a spectrum of states from fully unstructured to partially structured and include random coils, (pre-)molten globules, and large multi-domain proteins connected by flexible linkers.
Alphavirus replicons and helper constructs
Owner:ALPHAVAX INC
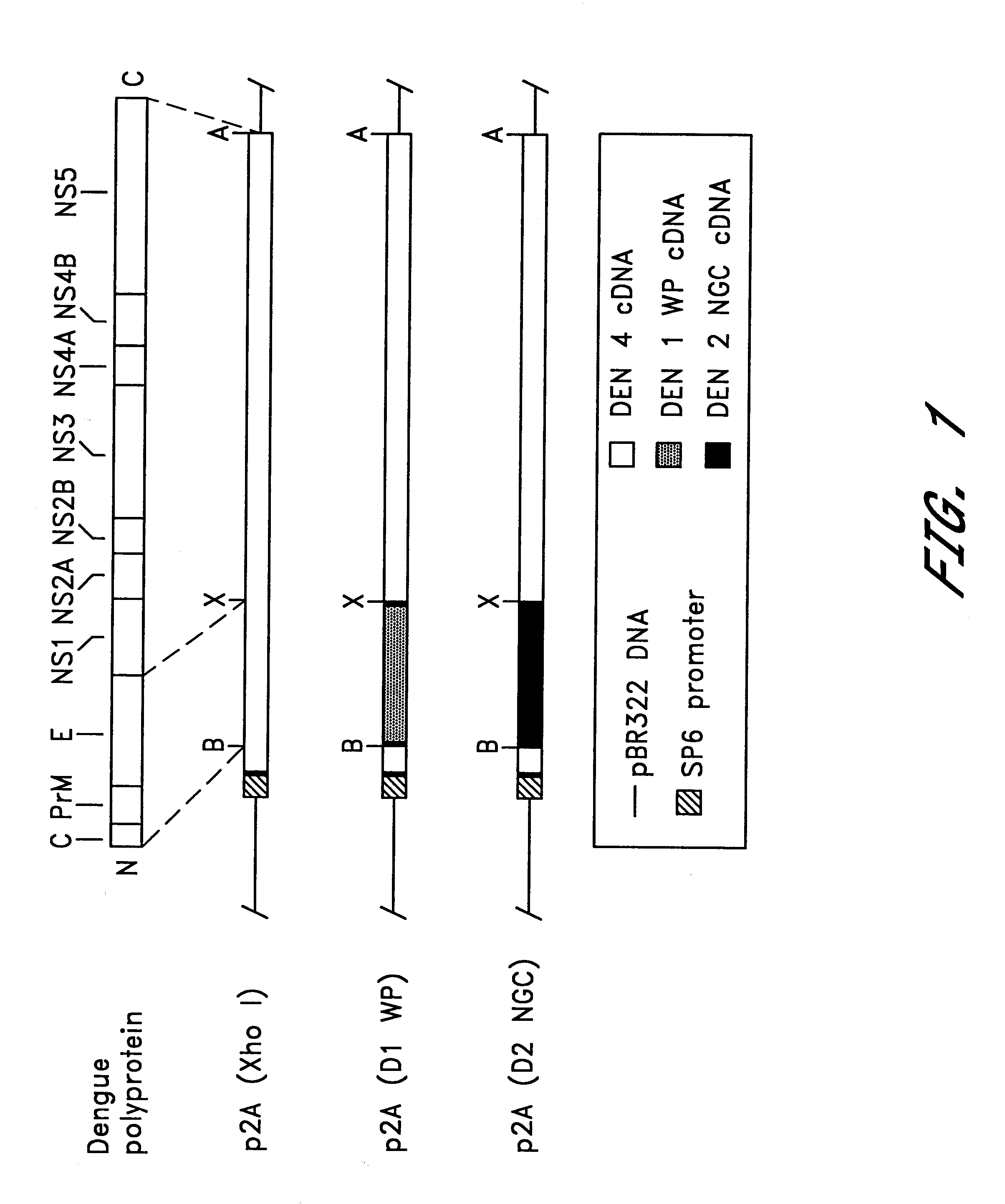
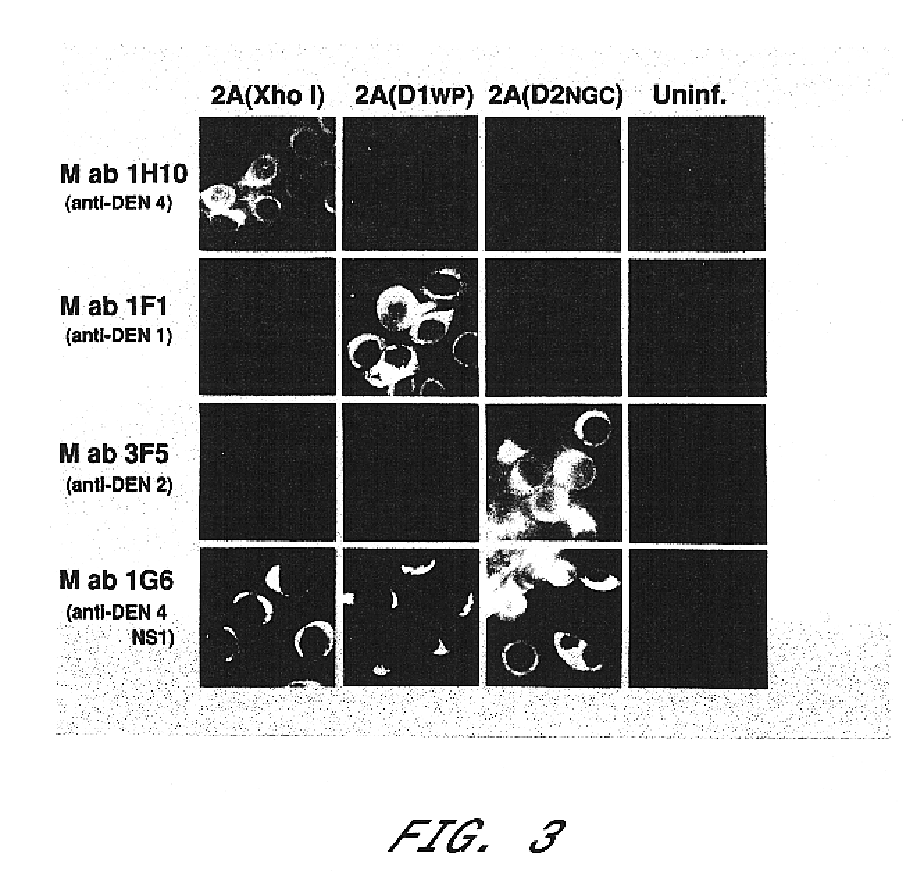
Avirulent, immunogenic flavivirus chimeras
InactiveUS7094411B2Minimize and inhibit infectionStable maintenanceOrganic active ingredientsVirusesViral diseaseAmino acid mutation
Chimeric flaviviruses that are avirulent and immunogenic are provided. The chimeric viruses are constructed to contain amino acid mutations in the nonstructural proteins of a flavivirus. Chimeric viruses containing the attenuation-mutated nonstructural genes of the virus are used as a backbone into which the structural protein genes of a second flavivirus strain are inserted. These chimeric viruses elicit pronounced immunogenicity yet lack the accompanying clinical symptoms of viral disease. The attenuated chimeric viruses are effective as immunogens or vaccines and may be combined in a pharmaceutical composition to confer simultaneous immunity against several strains of pathogenic flaviviruses.
Owner:MAHIDOL UNIV +2
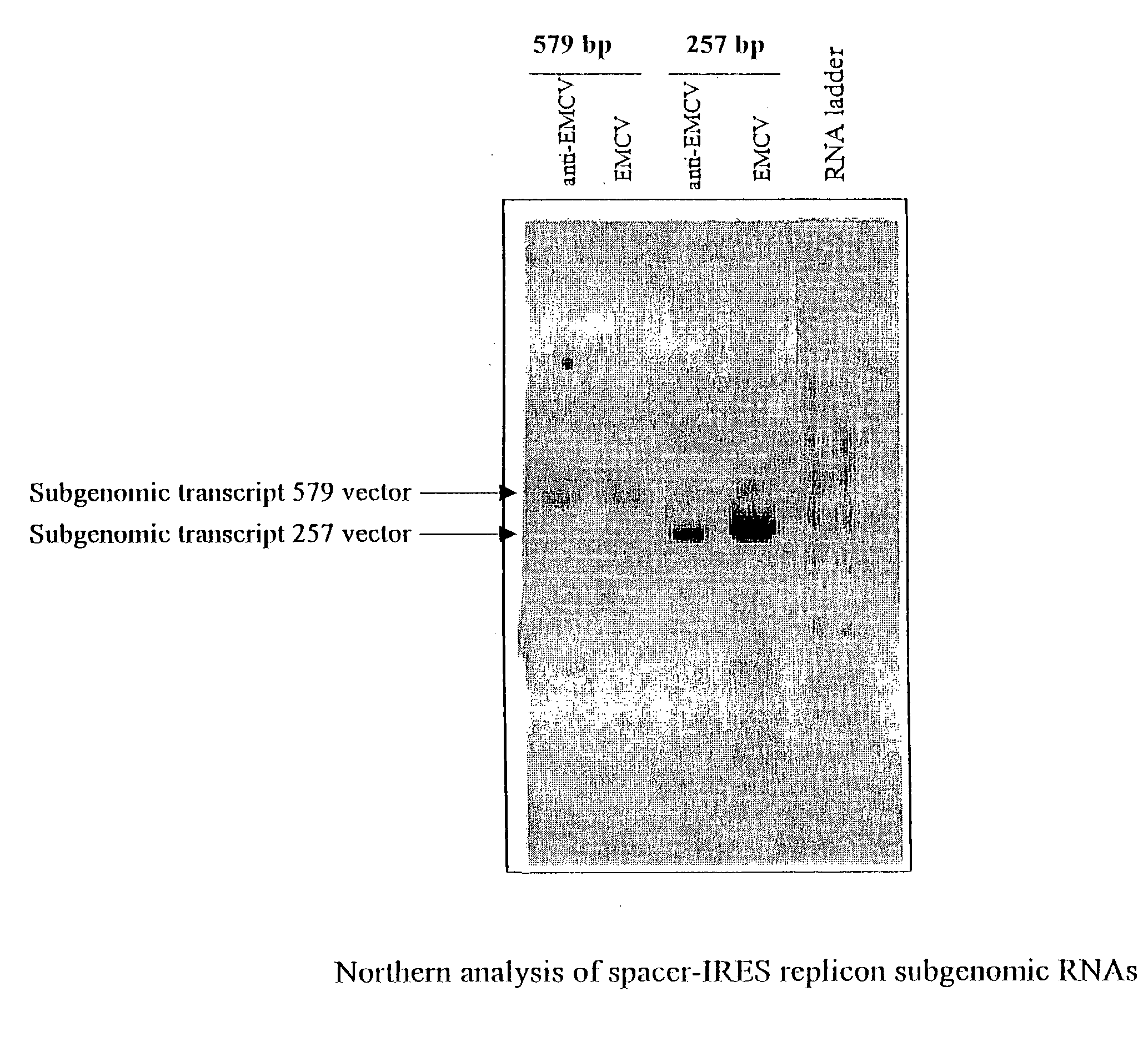
Alphavirus replicons and helper constructs
The present invention provides a recombinant nucleic acid comprising: a first nucleic acid sequence encoding a 5′ alphavirus replication recognition sequence; at least one second nucleic acid sequence encoding an alphavirus nonstructural protein; at least one alphavirus subgenomic promoter; at least one IRES element; at least one heterologous nucleic acid; and a third nucleic acid encoding a 3′ alphavirus replication recognition sequence. Further provided are methods of making alphavirus particles comprising a recombinant nucleic acid of this invention and methods of using the compositions of this invention. Also provided is a recombinant helper nucleic acid comprising: a first nucleic acid sequence encoding a 5′ alphavirus replication recognition sequence; an alphavirus subgenomic promoter; an IRES element; a second nucleic acid encoding an alphavirus structural protein; and a third nucleic acid encoding a 3′ alphavirus replication recognition sequence.
Owner:ALPHAVAX INC
Chimeric and/or growth-restricted flaviviruses
InactiveUS6676936B1BiocideSsRNA viruses positive-senseVirulent characteristicsJapanese B Encephalitis Virus
The invention includes a chimeric virus for use in a vaccine preparation having a genome comprising nucleic acid sequences encoding at least one structural protein from one flavivirus and nucleic acid sequences encoding nonstructural protein from another flavivirus. The genome preferably includes mutations within the viral genome that reduce virus virulence and in a particularly preferred embodiment these vaccines are directed to flaviviruses such as dengue virus, tick-borne encephalitis virus and Japanese encephalitis virus. The invention also includes a baculovirus having a recombinant dengue cDNA sequence which encodes: (1) dengue virus capsid protein, pre-matrix protein, envelope glycoprotein and NS1 and NS2a nonstructural proteins or (2) dengue envelope glycoprotein or (3) dengue non-structural proteins NS1 and NS2a. The invention further includes a baculovirus having a recombinant Japanese B encephalitis virus cDNA sequence which encodes the Japanese B encephalitis virus capsid protein, pre-matrix protein, envelope glycoprotein and non-structural proteins NS1 and NS2a. The invention further includes a vaccine and a method to produce that vaccine.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Monoclonal antibody blocking enzyme-linked immunosorbent assay (ELISA) kit and method for detecting nonstructural protein (NSP) antibody of foot-and-mouth disease virus (FMDV)
The invention discloses a monoclonal antibody blocking enzyme-linked immunosorbent assay (ELISA) kit and a method for detecting the nonstructural protein (NSP) antibody of a foot-and-mouth disease virus (FMDV) (FMD NSP B-ELISA); the kit comprises ELISA reaction plates, serum diluent, 25 times concentrated detergent, substrate solution, 100* concentrated ELISA detecting antibody, stop buffer, positive control serum and negative control serum; the ELISA reaction plates are two 96-pore high-affinity ELISA reaction plates, firstly 6* groups of amino acid monoclonal antibody or NSP 2C polyclonal antibody, and then FMDV 3ABC or 2C3AB NSP which is expressed by pronucleus and is provided with 6* groups of amino acid labels is captured through the monoclonal antibody or the polyclonal antibody; and compared with other similar kits, the method has higher coincidence rate and higher positive serum detection rate, and is applicable to detecting the serum of cattle, sheep, pigs and other susceptible animals.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Entire gene sequence of severe fever with thrombocytopenia syndrome virus (SFTSV) and application
ActiveCN102070704AStrong specificityPeptide/protein ingredientsGenetic material ingredientsPolymerase LStructural protein
The invention relates to a severe fever with thrombocytopenia syndrome virus (SFTSV), an entire gene sequence represented by Hubei isolate HB29, amino acid sequences of coding proteins and application. The entire gene sequence of the virus is subjected to homology analysis. The virus belongs to bunyaviridae and comprises three gene segments, namely, L, M and S which represent polymerase and glycoprotein (Gn and Gc), nucleoprotein (NP) and non-structural proteins (NSs) of the virus respectively, and the three segments are all positioned on the branch of phlebovirus but farther from other viruses of phlebovirus. The entire gene sequence and the coding proteins of the virus can be used for developing drugs, vaccines or diagnostic reagents for preventing and treating the epidemic diseases caused by the SFTSV.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Flavivirus expression and delivery system
InactiveUS6893866B1High expressionImprove translation efficiencySsRNA viruses positive-senseVectorsNucleotideStructural protein
The present invention provides a gene expression system comprising: a) a self-replicating expression vector of flavivirus origin which includes the flavivirus 5′ untranslated region (UTR), at least a portion of the 5′ coding region for flavivirus core protein, the nucleotide sequence coding for the flavivirus non-structural proteins, and the complete or most of the 3′-terminal sequence of the flavivrus 3′UTR, required for self-replication of flavivirus genomic material, which vector is adapted to receive at least a nucleotide sequence without disrupting its replication capabilities; and b) at least a second vector that is capable of expressing flavivirus structural protein(s) and any other proteins required for packaging of the self-replicating expression vector into flavivirus viral particles which vector is engineered to prevent recombination with the self-replicating vector when in its presence.
Owner:REPLIKUN BIOTECH
Method for assembling foot and mouth disease virus hollow capsid in insect with acidproof improvement
The present invention discloses a method for assembling foot-and-mouth disease virus empty capsids in insect cells via the alteration of acid-resistance. The method for assembling foot-and-mouth disease virus empty capsids in insect cells includes the following steps: (1) the altered P12A gene and the non-structural protein gene 3C of foot-and-mouth disease virus are introduced into bacteria via baculovirus vectors for recombination to produce recombinant rhabdovirus A; (2) the DNA of the recombinant rhabdovirus A is used to transfect the insect cells, so that the foot-and-mouth disease virus empty capsids are obtained. The method assembles the integral foot-and-mouth disease virus empty capsids in the insect cells for the first time, lays a foundation for the research and the development of gene-engineered subunit vaccines and novel diagnostic reagents.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Avirulent, immunogenic flavivirus chimeras
InactiveUS20060062803A1Inhibition effectPreserve immunogenicityBiocideOrganic active ingredientsViral diseaseFhit gene
Chimeric flaviviruses that are avirulent and immunogenic are provided. The chimeric viruses are constructed to contain amino acid mutations in the nonstructural proteins of a flavivirus. Chimeric viruses containing the attenuation-mutated nonstructural genes of the virus are used as a backbone into which the structural protein genes of a second flavivirus strain are inserted. These chimeric viruses elicit pronounced immunogenicity yet lack the accompanying clinical symptoms of viral disease. The attenuated chimeric viruses are effective as immunogens or vaccines and may be combined in a pharmaceutical composition to confer simultaneous immunity against several strains of pathogenic flaviviruses.
Owner:MAHIDOL UNIV +2
Large-scale preparation method for foot-and-mouth disease totivirus marked vaccine with high yield, high purity and high safety and product thereof
ActiveCN104491855AMark stableEnsure safetyMicroorganism based processesAntiviralsSucroseUltrafiltration
The invention discloses a large-scale preparation method for foot-and-mouth disease totivirus marked vaccine with high yield, high purity and high safety and a product thereof. The method comprises the following steps: a)collecting a virus solution; b)performing deep filtration on a membrane, performing ultrafiltration and performing enzymolysis on nuclease; c)purifying through a strong anion exchange adsorption bed or an adsorption film; d)depositing by PEG, extracting by chloroform-isoamyl aleohl; e)inactivating; F)performing density gradient centrifugation on an inactivation liquid through cane sugar and purifying; g)performing ultrafiltration dialysis and aseptic filtration; and h)reserving a stock solution or emulsifying. The provided foot-and-mouth disease totivirus marked vaccine antigen is uniform and complete foot-and-mouth virus particle, The vaccine is injected into body, so animal infection and immunization can be completely distinguished, does not contain foot-and-mouth disease virus non-structural protein and other virus particle, and does not contain animal-based foreign protein, polypeptide and oligopeptides, animal latent anaphylactic reaction, carcinogenesis and latent risk such as mad cow disease for causing animal infectious diseases due to vaccine injection can be effectively reduced, and the vaccine has no influence on animal food safety and trade.
Owner:吕宏亮 +2
Foot and mouth disease virus non-structural protein antibody enzyme-linked immunodetection kit
ActiveCN103864906AStrong specificityHigh sensitivitySsRNA viruses positive-senseVirus peptidesAntigen epitopeAntigen
The invention discloses a foot and mouth disease virus non-structural protein antibody enzyme-linked immunodetection kit. The kit comprises a foot and mouth disease virus non-structural protein 3B antigen epitope peptide-coated polymerase chain reaction plate and an enzyme-labeled antibody, wherein the foot and mouth disease virus non-structural protein 3B antigen epitope peptide is a polypeptide shown as a sequence 1 in a sequence table. The kit adopts a chemical synthesis non-structural protein 3B antigen peptide coated reaction plate and is small in antigen amount, high in sensitivity and high in specificity, and whether the foot and mouth disease virus infection exists can be efficiently detected. The kit is high in specificity, sensitive and high-efficiency and has good market prospects.
Owner:CHINA ANIMAL HUSBANDRY IND
Hybridoma cell line producing monoclonal antibody against foot-and-mouth disease virus, the monoclonal antibody therefrom, immunoassay reagent and kit, and immunoassay method
ActiveUS20110014639A1Useful for developmentImprove efficacyAnimal cellsMicrobiological testing/measurementMonoclonal antibody 14G2AStructural protein
Provided herein are a hybridoma cell line producing monoclonal antibody against foot-and-mouth disease virus (FMDV), the monoclonal antibody therefrom, reagent and kit for ELISA, and immunoassay method. The hybridoma cell line is produced by cell fusion of a parental cell and a myeloma cell line and has the same characteristics as the cell line whose strain designation is CmA40 and deposition number is ATCC (To be Provided). The parental cell is a splenocyte isolated from the spleen of a mouse immunized by an antigen derived from a 3ABC non-structural protein (NSP) of FMDV. The antigen used here is expressed by a prokaryotic cell. The monoclonal antibody produced by the hybridoma cell line can specifically recognize a 3ABC polypeptide and does not cross-react with an antiserum of swine vesicular disease virus.
Owner:NAT INST FOR ANIMAL HEALTH COUNCIL AGRI EXECUTIVE YUAN
Recombinant alphavirus-based vectors with reduced inhibition of cellular macromolecular synthesis
InactiveUS7811812B2Reduced and delayed and no inhibitionReduced and delayed and developmentFungiSsRNA viruses positive-senseWild typeInhibitory effect
Isolated nucleic acid molecules are disclosed, comprising an alphavirus nonstructural protein gene which, when operably incorporated into a recombinant alphavirus particle, eukaryotic layered vector initiation system, or RNA vector replicon, has a reduced level of vector-specific RNA synthesis, as compared to wild-type, and the same or greater level of proteins encoded by RNA transcribed from the viral junction region promoter, as compared to a wild-type recombinant alphavirus particle. Also disclosed are RNA vector replicons, alphavirus vector constructs, and eukaryotic layered vector initiation systems which contain the above-identified nucleic acid molecules.
Owner:CARDIOVENTION
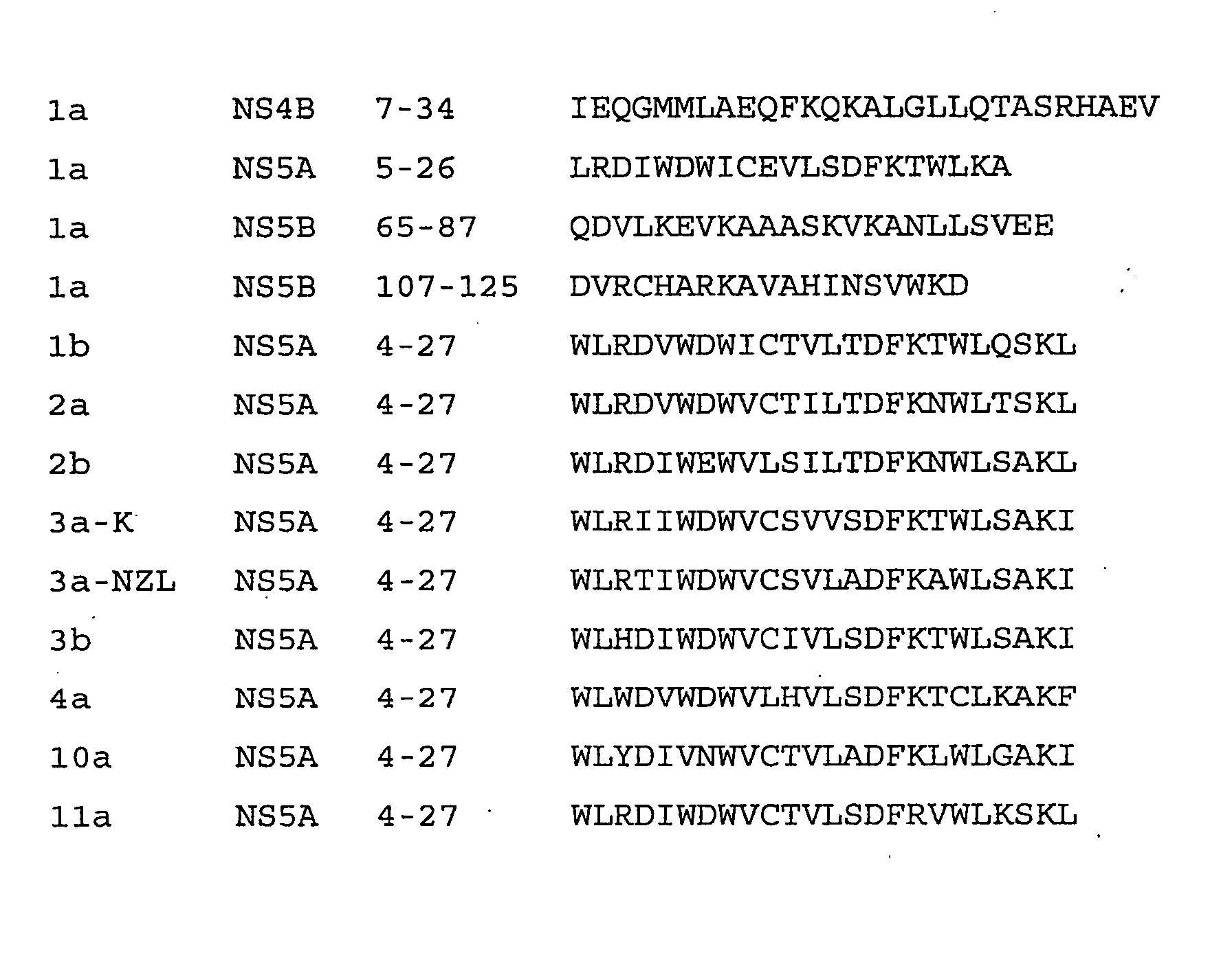
Agents for treatment of HCV and methods of use
InactiveUS7326536B2Interfere with abilityVirusesPeptide/protein ingredientsAmphipathic helixInfected cell
An amphipathic helix at the approximate N-terminus of Hepatitis C virus (HCV) nonstructural proteins mediates the association of these proteins with cytoplasmic membranes in infected cells. This association is essential for replication. Thus, assessing the ability of compounds or protocols to disrupt the association of such helices with cytoplasmic membranes permits identification of compounds and protocols which are useful in the treatment of HCV infection. Also useful in the invention are mimics, or function-disrupting ligands, of an amphipathic helix of the nonstructural proteins described herein and antibodies and fragments thereof immunoreactive with said helix.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Recombinant alphavirus-based vectors with reduced inhibition of cellular macro-molecular synthesis
InactiveUS20100330121A1Reduced and delayed and no inhibitionReduced delayed no developmentFungiSsRNA viruses positive-senseWild typeInhibitory effect
Isolated nucleic acid molecules are disclosed, comprising an alphavirus nonstructural protein gene which, when operably incorporated into a recombinant alphavirus particle, eukaryotic layered vector initiation system, or RNA vector replicon, has a reduced level of vector-specific RNA synthesis, as compared to wild-type, and the same or greater level of proteins encoded by RNA transcribed from the viral junction region promoter, as compared to a wild-type recombinant alphavirus particle. Also disclosed are RNA vector replicons, alphavirus vector constructs, and eukaryotic layered vector initiation systems which contain the above-identified nucleic acid molecules.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC +1
Virus-like particles for treatment of viral infections
The invention provides virus-like particles for treatment of viral infections based on the virus causing the infection. The virus-like particles comprise the virus recombinant proteins that form a capsid, recombinant virus membrane proteins attached to the capsid and vRNA packaged within said capsid. The vRNA is generated from a DNA sequence encoding a polypeptide capable of specifically binding to a constant region of a nonstructural protein of the virus that is essential for propagation of the virus.
Owner:BEN GURION UNIVERSITY OF THE NEGEV
Recombinant BHK cell line for stably expressing classical swine fever virus E0-E1-E2 protein, and applications of the same in preparation of vaccines and diagnosis reagents of classical swine fever
ActiveCN103751773AFully emulsifiedAvoid infectionAntiviralsBiological testingStructural proteinMicrobiological culture
The present invention discloses a recombinant cell line for stably expressing classical swine fever virus E0-E1-E2 protein, and applications of the recombinant cell line in preparation of vaccines and diagnosis reagents of classical swine fever, wherein the recombinant cell line is BCSFV-E012, is preserved in the China General Microbiological Culture Collection Center, and has the preservation number of CGMCC No.7720. In addition, the present invention further discloses an establishment method for the cell line for stably expressing classical swine fever virus E0-E1-E2 protein, and a method for preparing a classical swine fever prevention vaccine composition by using the cell line. The present invention further discloses applications of the E0-E1-E2 protein stably expressed by the recombinant cell line in preparation of classical swine fever prevention vaccines and diagnosis reagents. The classical swine fever vaccine prepared by using the recombinant cell line has characteristics of high safety, good immunization effect, easy mass production, less being susceptible to exogenous virus pollution or influence of antibodies, and no classical swine fever virus non-structural protein antibody production so as to identify the vaccinated animal and the virus infected animal.
Owner:HARBIN WEIKE BIOTECH DEV +1
Foot-and-mouth disease hybridoma cell line, monoclonal antibody, detection reagent and kit
ActiveCN102533663AHas the ability to identify specificityNo cross reactionImmunoglobulins against animals/humansVirus peptidesSwine vesicular diseaseStructural protein
The invention provides a hybridoma cell line and a monoclonal antibody thereof, an immune detection reagent containing the monoclonal antibody and a kit thereof, and an immune detection method. The hybridoma cell line is prepared by fusing parental cells and myeloma cells, and has the characteristics represented by the cell line with the preservation number of PTA-11304. The parental cell line is prepared by the following steps that: prokaryotic cells are adopted to express non-structural protein coded by a 3ABC gene fragment of foot-and-mouth disease virus, the non-structural protein is adopted as antigen to immunize mice, and spleen cells of the immunized mice are taken out to prepare the parental cell line. The monoclonal antibody produced from the hybridoma cell line can provide specific recognition for the 3ABC peptide of the foot-and-mouth disease virus, and do not generate the non-specific reaction with the swine vesicular disease antiserum.
Owner:IND TECH RES INST
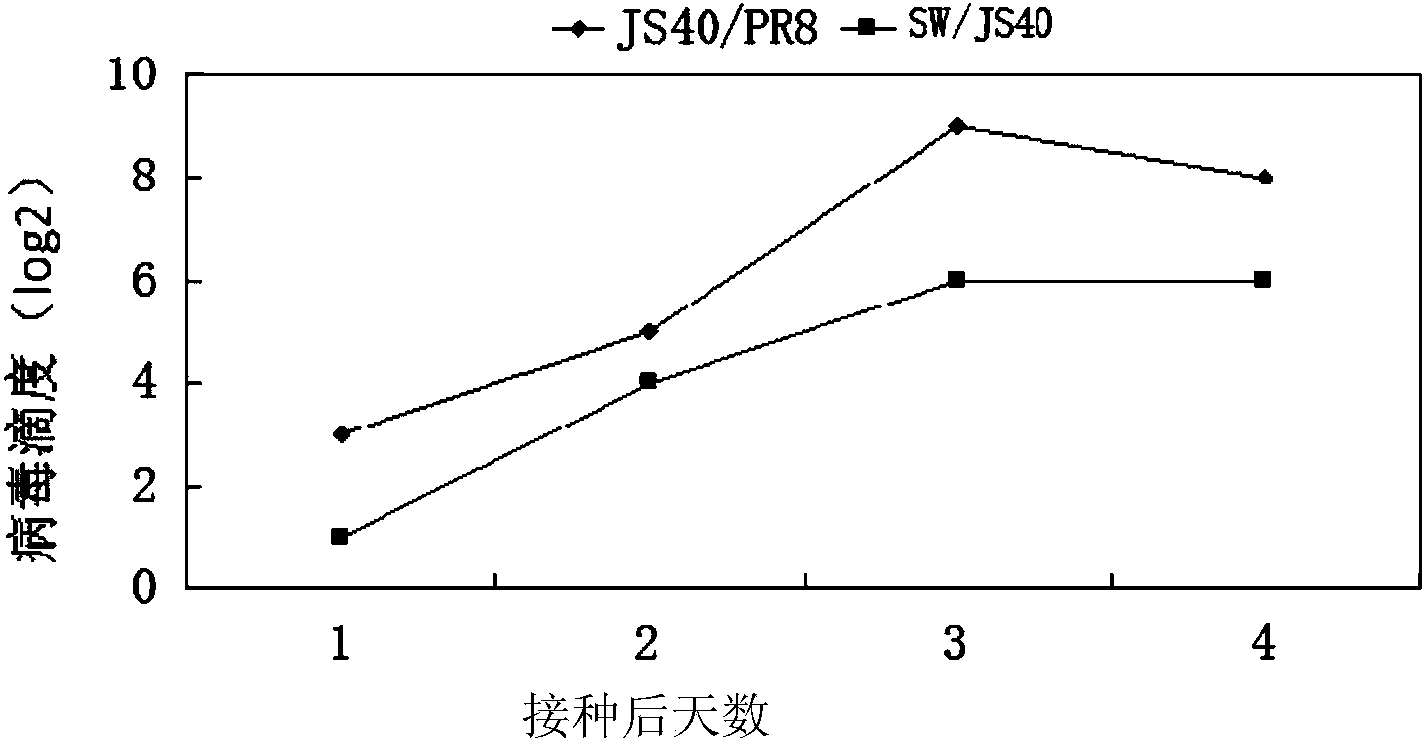
Recombinant homologous avian H1N1 influenza virus inactivated vaccine strain (JS40/PR8) as well as preparation method and application of inactivated vaccine strain
ActiveCN104232594AQuality improvementReduce manufacturing costMicroorganism based processesAntiviralsHemagglutininNeuraminidase
The invention discloses a recombinant homologous avian H1N1 influenza virus inactivated vaccine strain (JS40 / PR8) as well as a preparation method and an application of the inactivated vaccine strain. The recombinant avian H1N1 influenza virus inactivated vaccine strain takes A / swine / Jiangsu / 40 / 2011 (H1N1) as genetic donors of surface hemagglutinin (HA) and neuraminidase (NA), and influenza virus A / PR / 8 / 34(H1N1) as donors of six internal genes of a polymerase PB2 subunit, a polymerase PB1 subunit, a polymerase PA subunit, nucleoprotein (NP), a matrix protein (M) and non-structural protein (NS); the vaccine strain is obtained by natural recombination; and the preservation number of the vaccine strain is CCTCC NO:V201419. The invention also discloses a method for building the recombinant homologous avian H1N1 influenza virus inactivated vaccine strain, and an application of the homologous recombinant avian H1N1 influenza virus inactivated vaccine strain for preventing homologous avian H1N1 influenza of animals or people.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
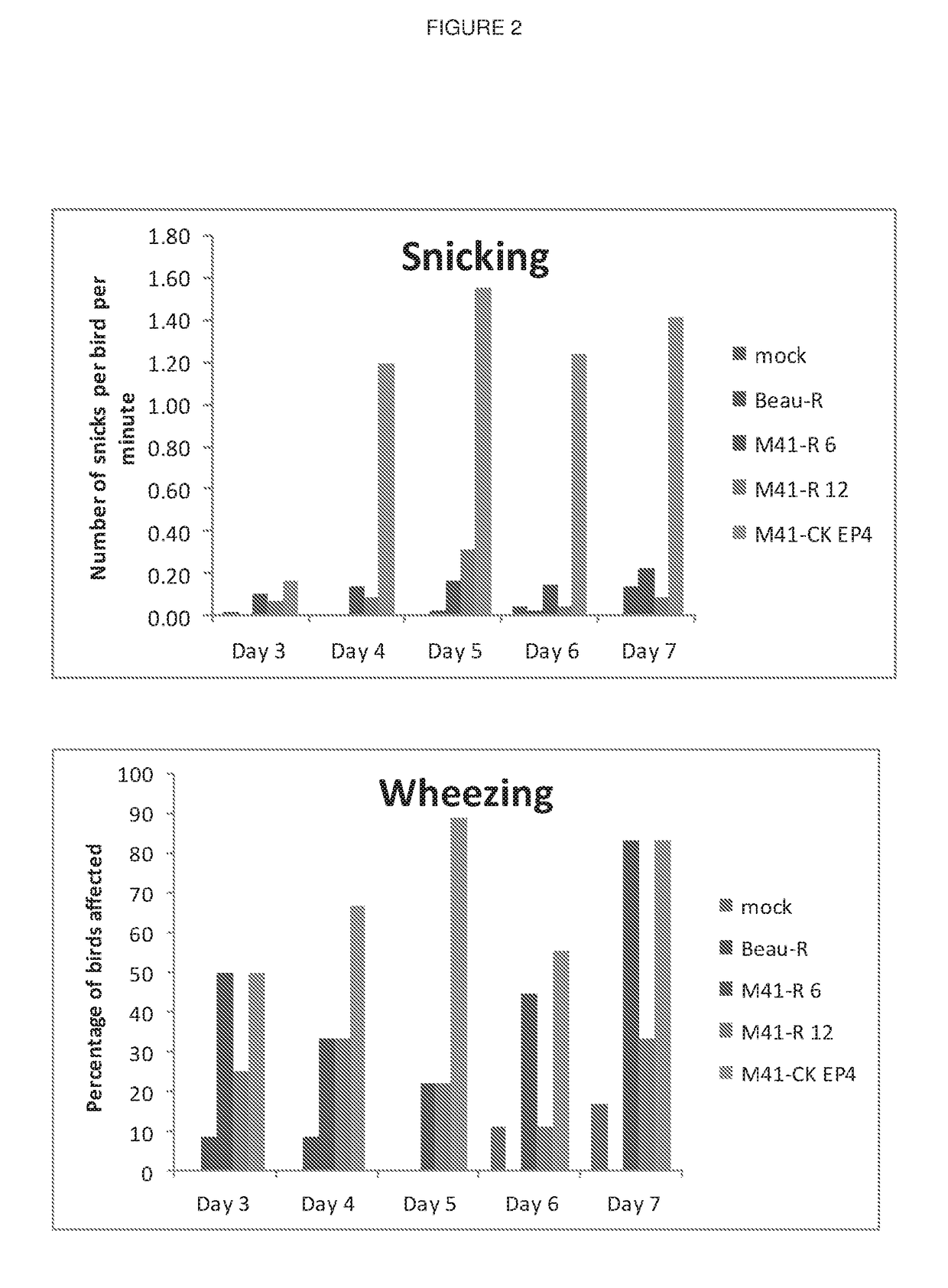
Coronavirus
ActiveUS10130701B2Reduced level of pathogenicityLower Level RequirementsSsRNA viruses positive-senseVirus peptidesDiseaseInfectious bronchitis
Owner:THE PIRBRIGHT INST
Agents for treatment of HCV and methods of use
InactiveUS20080125367A1Interfere with abilityCompound screeningBiocideInfected cellAmphipathic helix
An amphipathic helix at the approximate N-terminus of Hepatitis C virus (HCV) nonstructural proteins mediates the association of these proteins with cytoplasmic membranes in infected cells. This association is essential for replication. Thus, assessing the ability of compounds or protocols to disrupt the association of such helices with cytoplasmic membranes permits identification of compounds and protocols which are useful in the treatment of HCV infection. Also useful in the invention are mimics, or function-disrupting ligands, of an amphipathic helix of the nonstructural proteins described herein and antibodies and fragments thereof immunoreactive with said helix.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Detection reagent and detection method for PRRSV
ActiveCN102731615APerfect theoretical basisSsRNA viruses positive-senseMicrobiological testing/measurementOligonucleotide primersWild type
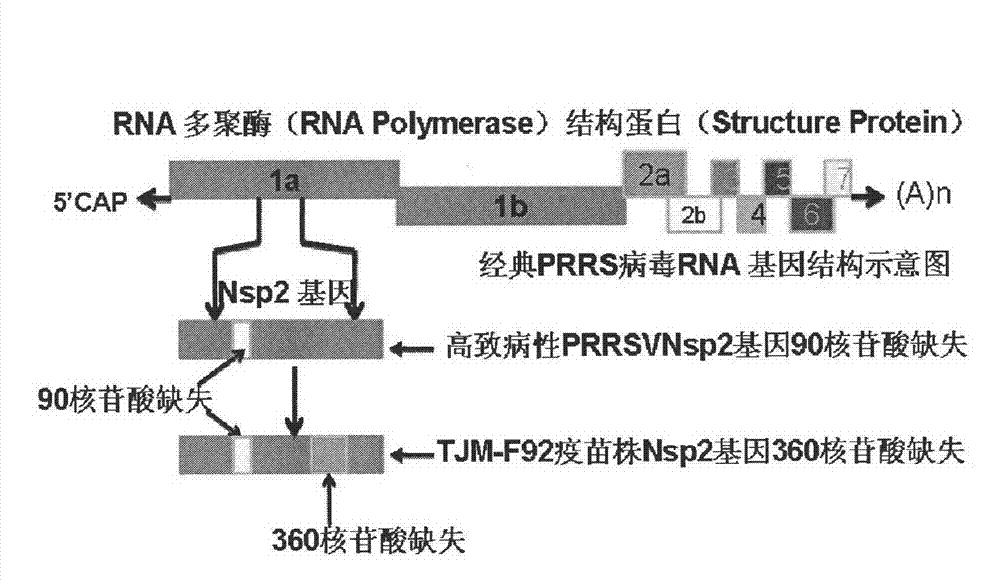
The invention relates to detection reagents and detection methods for porcine reproductive and respiratory syndrome virus (PRRSV). On the one hand, the invention provides immunogenic fragments in coded non-structural protein 2 (Nsp2), and in particular, the immunogenic fragments provided in the invention exist in Nsp2 protein of wild type PRRSV instead of in Nsp2 protein of attenuation PRRSV; on the other hand, the invention also provides oligonucleotide primers, and in particular, a to-be-amplified area of the oligonucleotide primers provided in the invention includes at least a part of the coding sequence of Nsp2 protein and the part of the coding sequence exists in Nsp2 coding sequence of wild type PRRSV instead of in Nsp2 coding sequence of attenuation PRRSV. The invention further provides the methods of using relevant detection reagents in detection and a method of using the above-mentioned methods to distinguish pigs immunized by attenuation PRRSV from pigs infected by wild type PRRSV.
Owner:华威特(江苏)生物制药有限公司
Recombinant flaviviral constructs and uses thereof
InactiveUS20130243809A1Successfully expressingHigh insertion capacitySsRNA viruses positive-senseViral antigen ingredientsStructural proteinViral replication
A recombinant viral construct for expressing an exogenous polypeptide in a cell and uses thereof are provided. The recombinant viral constructs are derived from Japanese encephalitis virus (JEV). The recombinant viral constructs encodes a fusion protein, which includes an exogenous (i.e., non-JEV) polypeptide and a JEV non-structural protein 1 (JEV NS1) or a segment thereof. Particularly, the exogenous polypeptide is inserted into the carboxyl-terminus of the JEV NS1, and the production of the recombinant fusion protein does not affect viral replication. Upon infection a cell with such recombinant viral constructs, JEV particles comprising limited multiplicative virions (LMV) may be produced. Each LMV comprises the as-described JEV replicon. The JEV particles are useful in eliciting an immune response to the exogenous polypeptide in a host and thereby confer the host with protective immunization against the exogenous polypeptide.
Owner:NAT DEFENSE MEDICAL CENT
Method for detecting foot and mouth disease (FMD) non-structural protein 3AB antibody by using dot immuno-gold filtration assay (DIGFA)
The invention discloses a method for detecting a foot and mouth disease (FMD) non-structural protein 3AB antibody by using dot immuno-gold filtration assay (DIGFA). The method specifically comprises the following steps of: a) synthesizing a foot and mouth disease(FMD) non-structural protein 3AB; b) preparing SPA colloidal gold; c) manufacturing a reaction kit; and d) applying the reaction kit as a carrier, placing antigen sites of the 3AB non-structural protein synthesized in the step a) on an NC membrane, sealing the NC membrane and adding a sample to be detected, and after washing the NC membrane, using the SPA prepared in the step b) as the colloidal gold to mark the protein for detecting the FMD non-structural protein 3AB antibody. The method has the characteristics of simple, convenient and fast operation, no need of special instrument, bright spot color, strong specificity, high sensitivity, easy judgment of results and preservable detected samples.
Owner:INSPECTION & QUARANTINE TECH CENT OF XIAMEN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
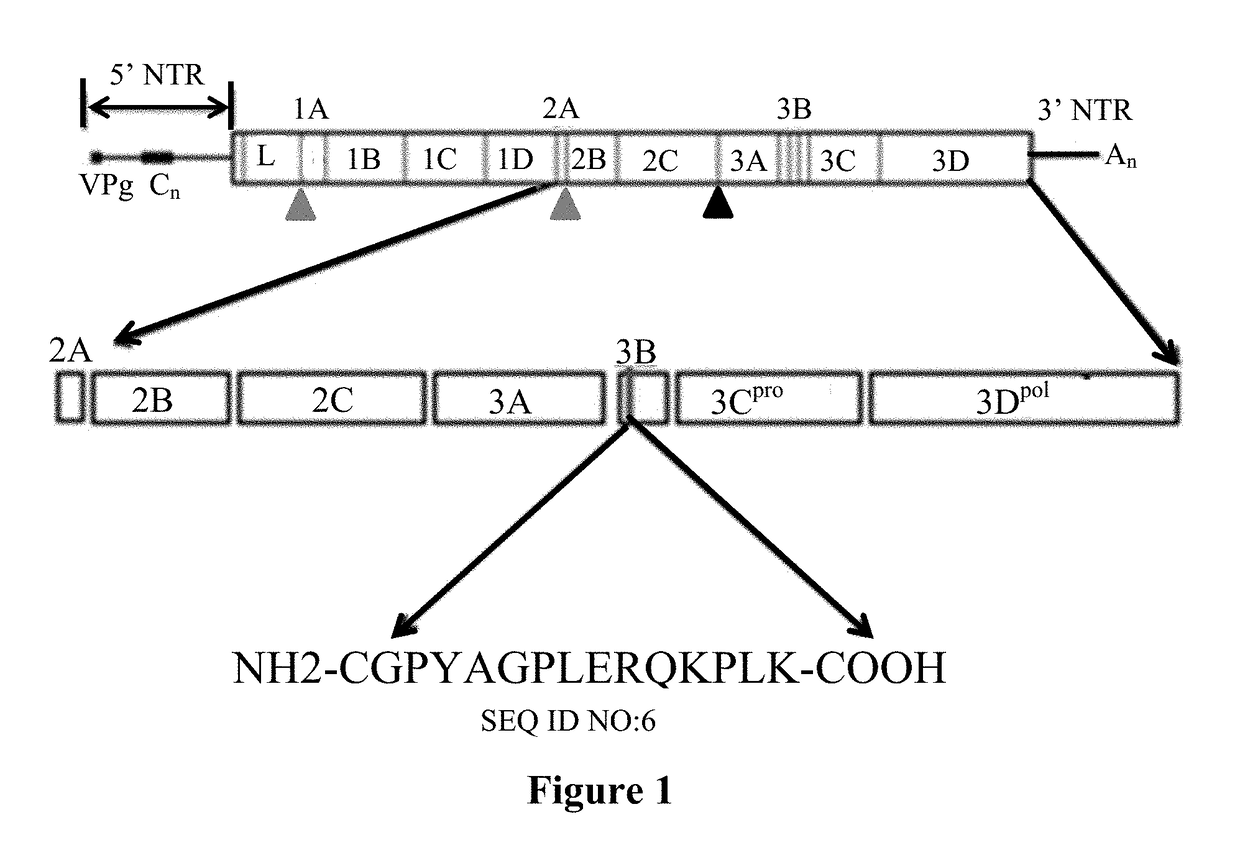
Foot-and-mouth disease marker vaccine strain with 3B protein dominant epitope deficiency and construction method and application thereof
ActiveCN107201346ASsRNA viruses positive-senseViral antigen ingredientsStructural proteinMarker vaccine
The invention provides a foot-and-mouth disease marker vaccine strain with 3B protein dominant epitope deficiency and a construction method and application thereof; 3B1 and 3B2 non-structural protein encoded amino acid sequences of the marker vaccine strain are shown as in SEQ ID No. 8. The vaccine strain constructed herein has mutation modifications (4AGP6-4TAA6) of 3B1 and 3B2 non-structural protein amino acids, wherein the mutations of the 3B1 and 3B2 protein amino acids enable a recombinant virus to be completely deprived of the ability to bind with monoclonal antibody 3B4B1 recognizing 3B1and 3B2 dominant epitopes, and the marked virus has the similar replicating ability as parent viruses. Therefore, the foot-and-mouth disease marker vaccine strain constructed herein is suitable for developing foot-and-mouth disease marker vaccines for distinguishing natural infections and vaccine immunity, and reliable technical support is provided for the construction of control, purification and infection-free zones in China.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Monoclonal antibody against novel epitopes of foot-and-mouth disease virus protein 3abc and uses thereof
ActiveUS20170218053A1SsRNA viruses positive-senseMicrobiological testing/measurementEpitopeElisa kit
This disclosure pertains to isolated antibodies or antigen binding fragments thereof that specifically bind to the 3ABC non-structural protein of Foot-and-Mouth Disease virus (FMDV), wherein the antibodies or antigen binding fragments thereof recognize the amino acid sequence of SEQ ID NO: 2, SEQ ID NO: 3, SEQ ID NO: 4, SEQ ID NO: 5, SEQ ID NO: 6 or SEQ ID NO: 12. Accordingly, this disclosure also pertains to polypeptides having an amino acid sequence selected from SEQ ID NO: 2, SEQ ID NO: 3, SEQ ID NO: 4, SEQ ID NO: 5 or SEQ ID NO: 12. Monoclonal antibody Mab 40C8 is also provided. The current disclosure also pertains to methods of detecting FMDV infection in an animal (including assays differentiating infected animals from vaccinated animals (DIVA)) and kits for performing the detection methods. Competitive ELISA kits comprising the antibody or antigen binding fragment thereof and immunoassay plates coated with the polypeptide comprising the amino acid sequence selected from SEQ ID NO: 2, SEQ ID NO: 3, SEQ ID NO: 4, SEQ ID NO: 5, SEQ ID NO: 6 and / or SEQ ID NO: 12 are also provided.
Owner:TEXAS A&M UNIVERSITY +2
Flavivirus replicon constructs for tumour therapy
InactiveCN101120086AOrganic active ingredientsSsRNA viruses positive-senseParanasal Sinus CarcinomaMelanoma
Owner:レプリカン バイオテク プロプライエタリー リミテッド
O type foot and mouth disease virus DNA vaccine and its preparing method
The present invention discloses type-One foot and mouth disease virus DNA vaccine and its preparation process, and is especially DNA vaccine including foot and mouth disease structure protein P1 gene and non-structure protein 2A, 2B and 3C gene as well as non-essential 3D gene and its preparation process.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
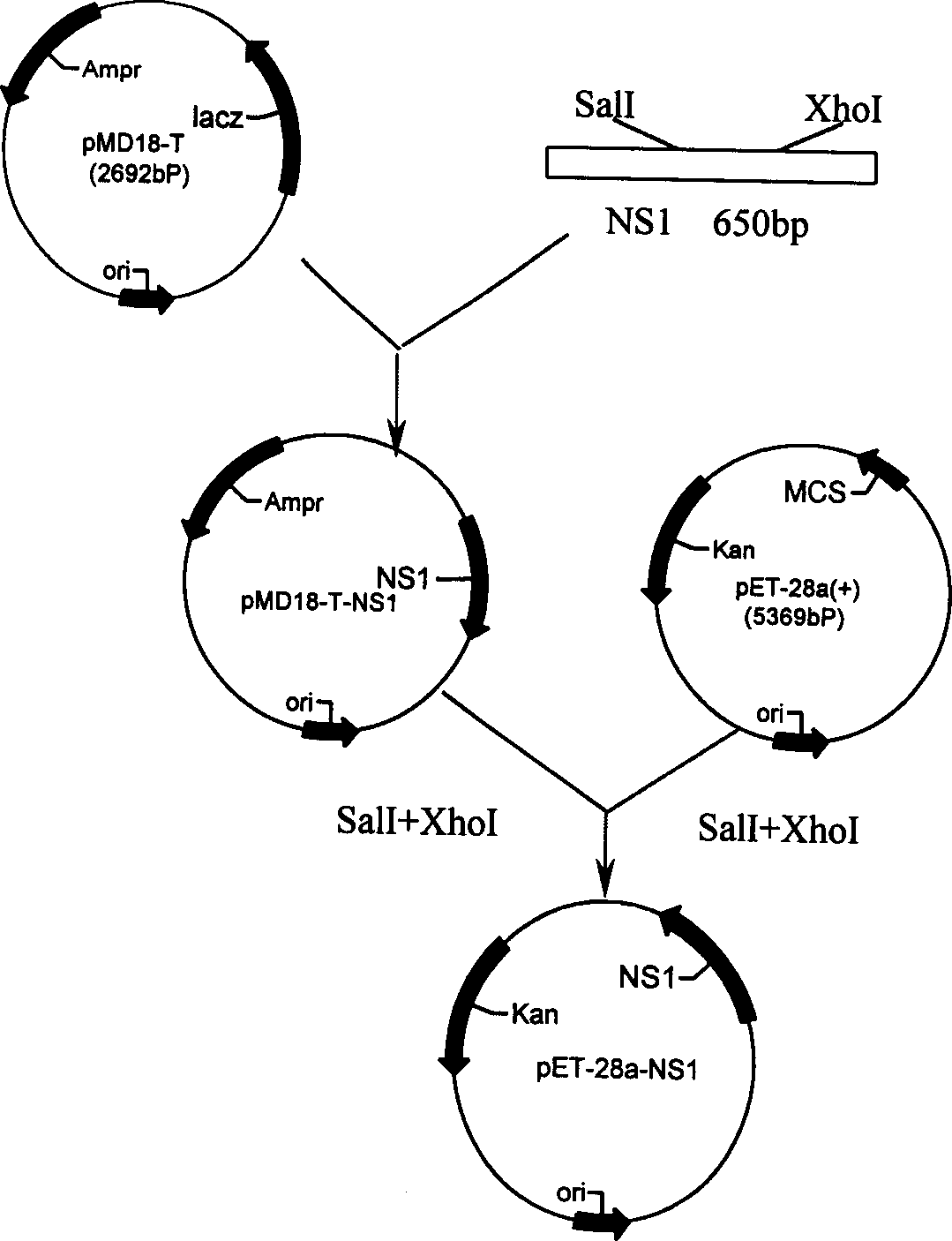
Non-structural protein gene NS1 of avian influenza virus and its preparation method and use
The invention discloses the cDNA sequences of the non-structural protein gene NS1 of avian influenza virus, its preparation process and use, wherein gene is employed for constructing prokaryotic expression vectors, the expression vectors are transformed into bacillus coli to obtain recombinant strains (Escherichia coli BL21 / pET-28a-NS1), the strains are utilized to express the non-structural protein NS1 of the avian influenza virus, the expression proteins are used to establish a differential diagnosis method through enzyme linked immunosorbent assay for differentiate vaccinum avian influenzae inactivatum immune chicken from infected chicken. The invention also relates to a differential diagnosis reagent kit and its application.
Owner:HUAZHONG AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com