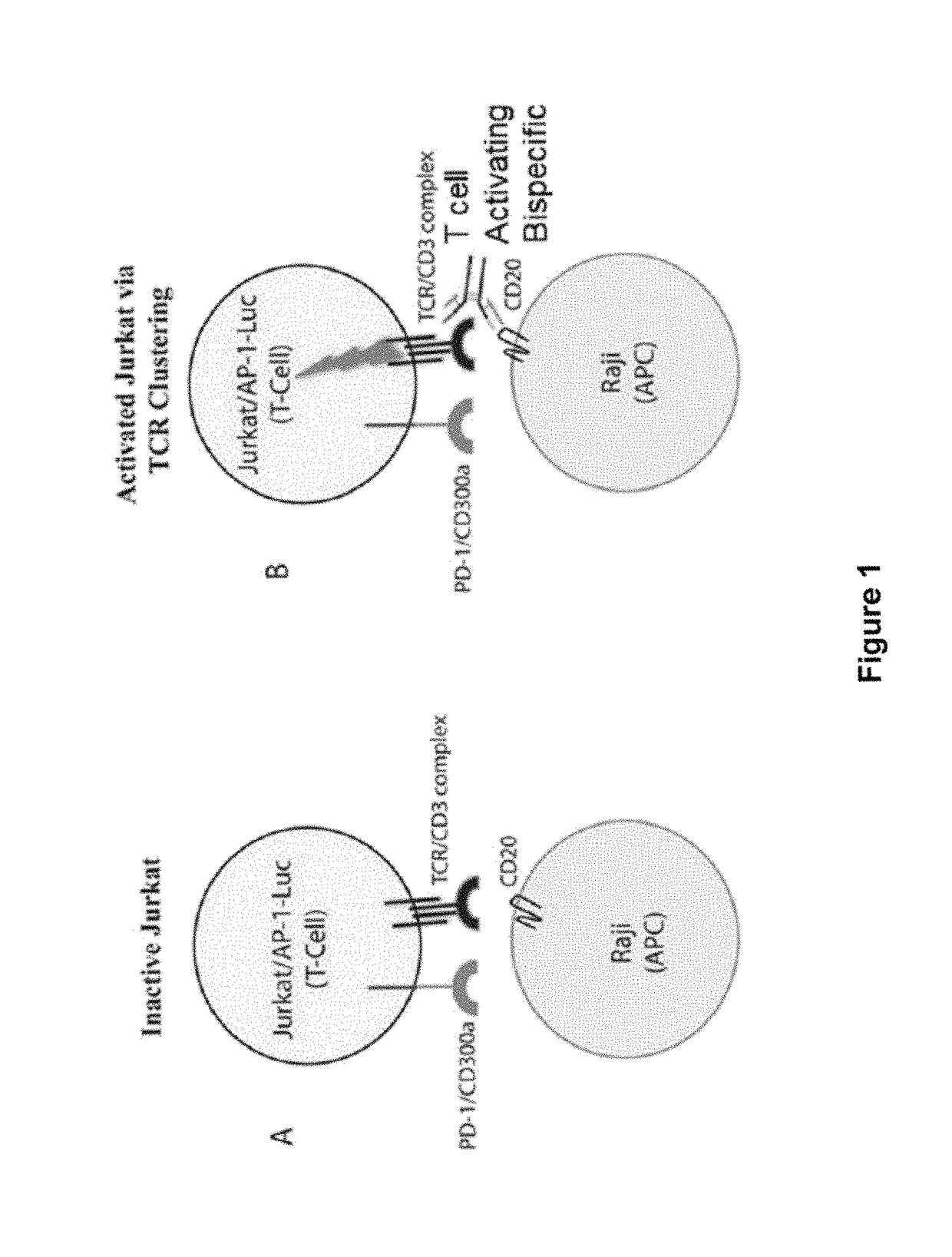
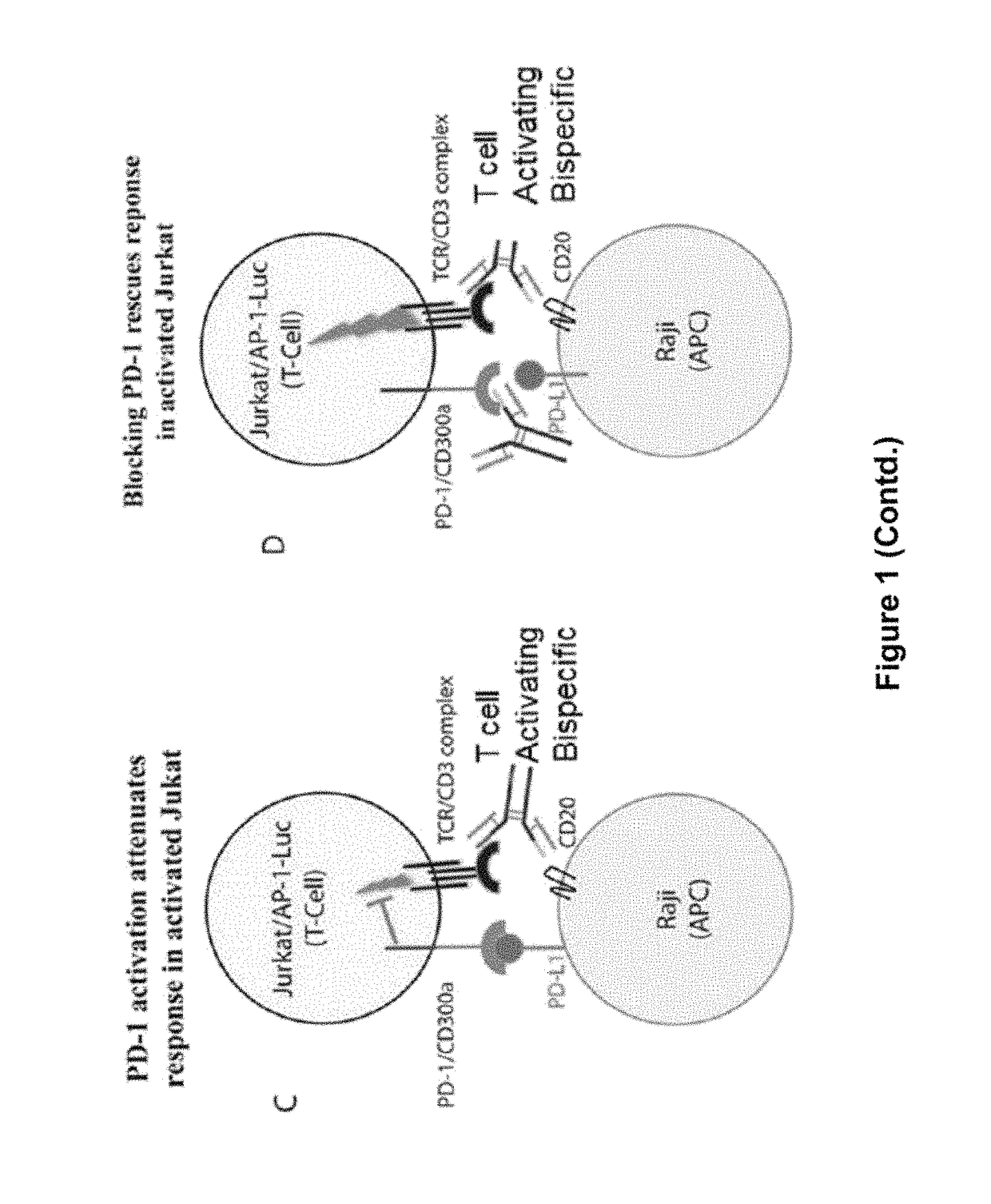
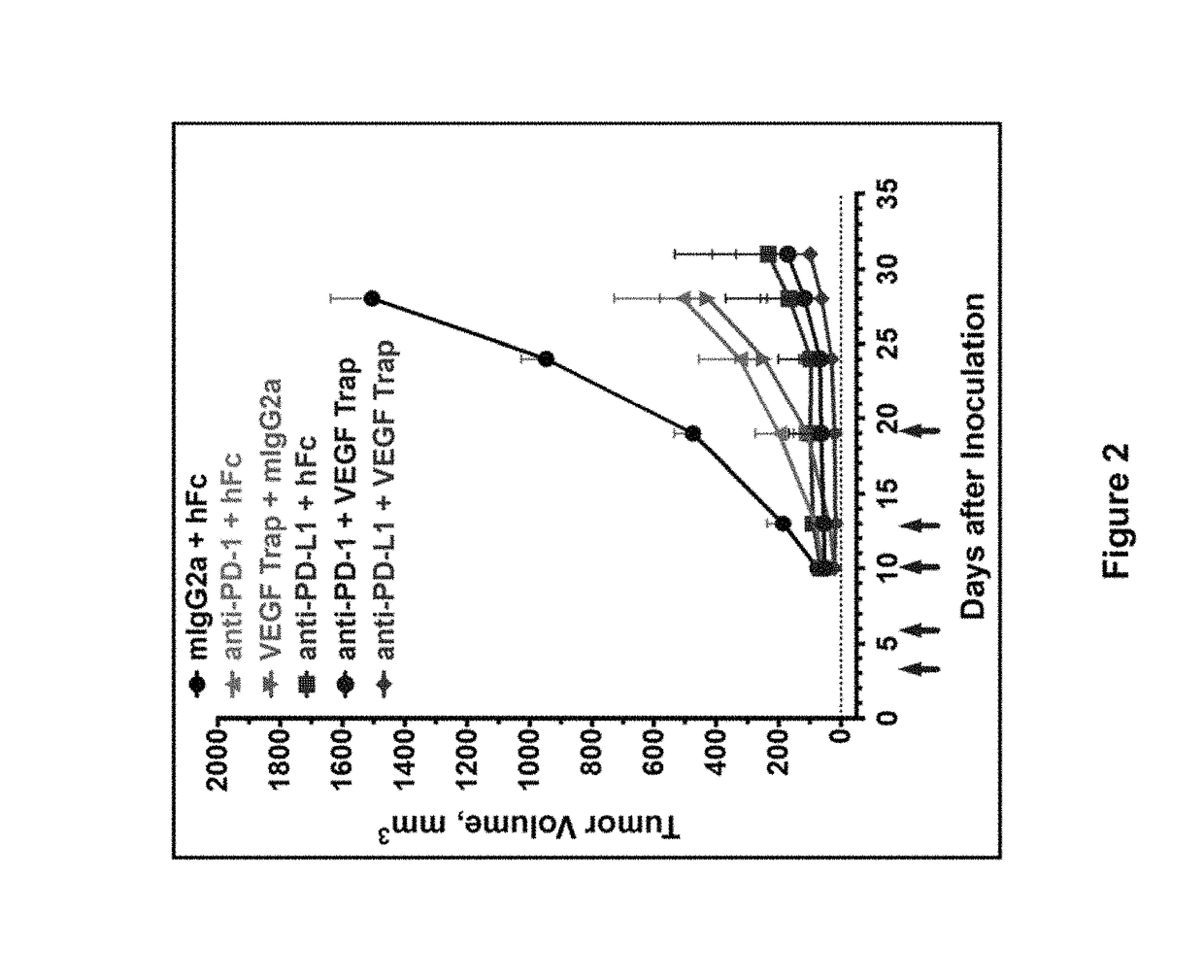
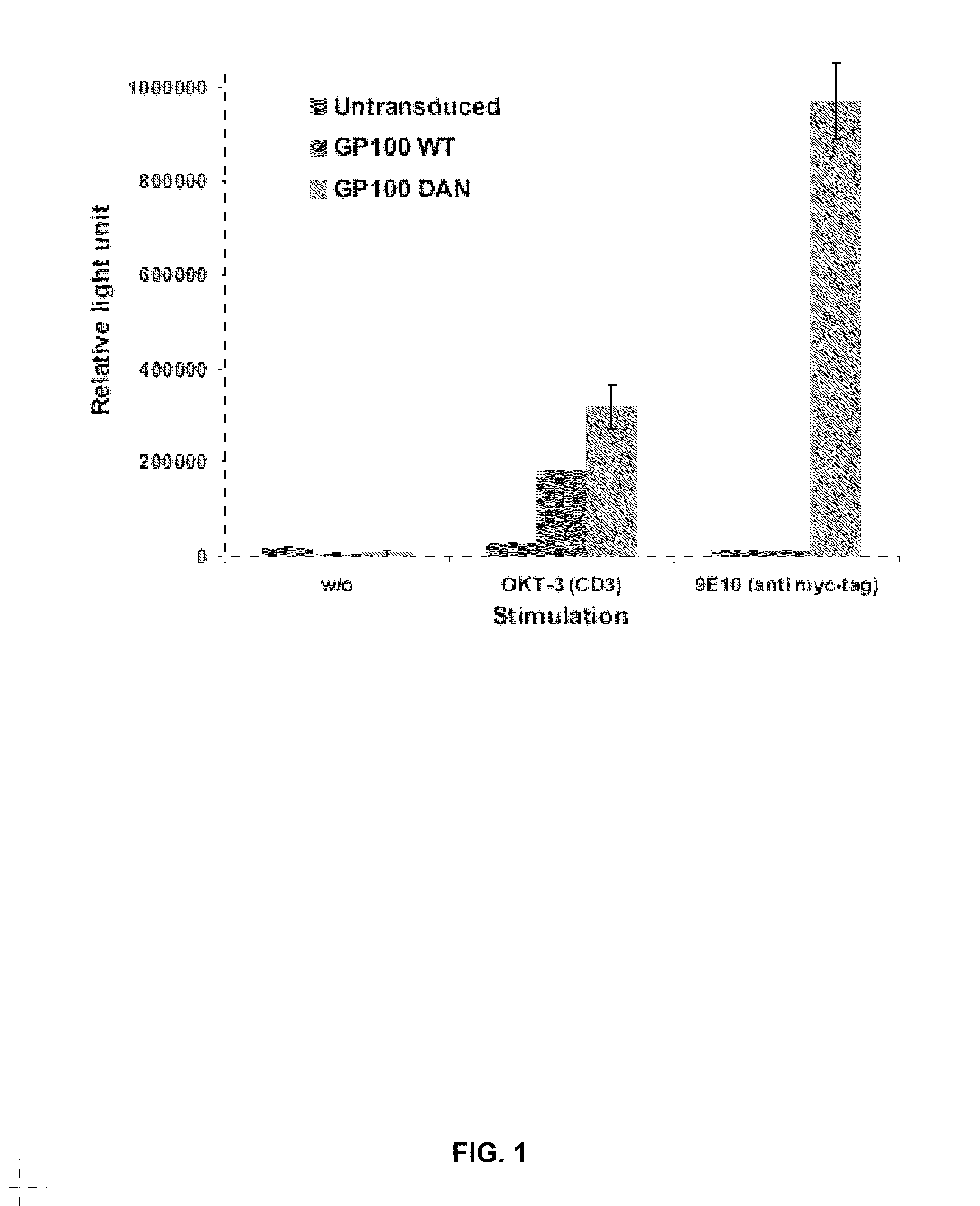
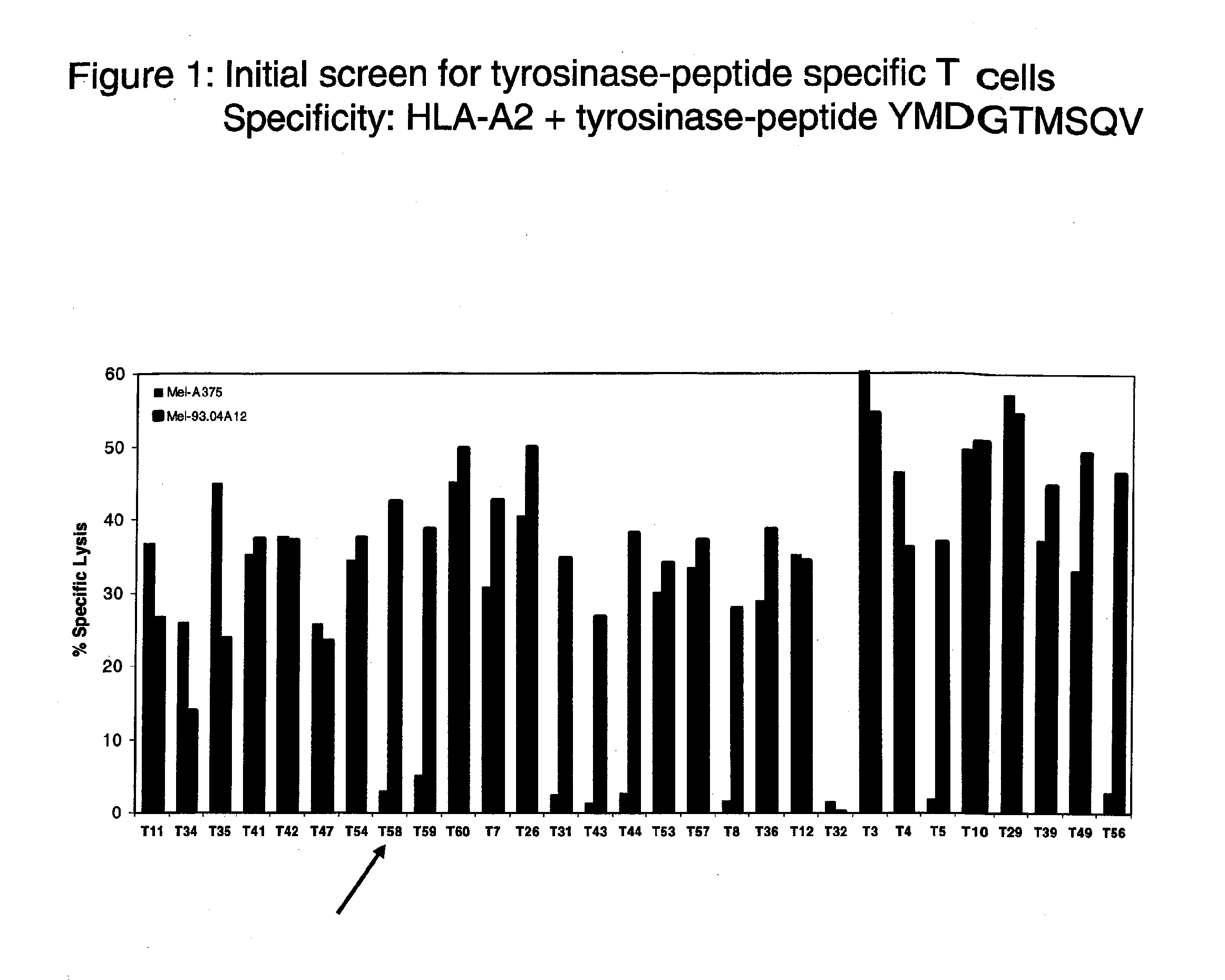
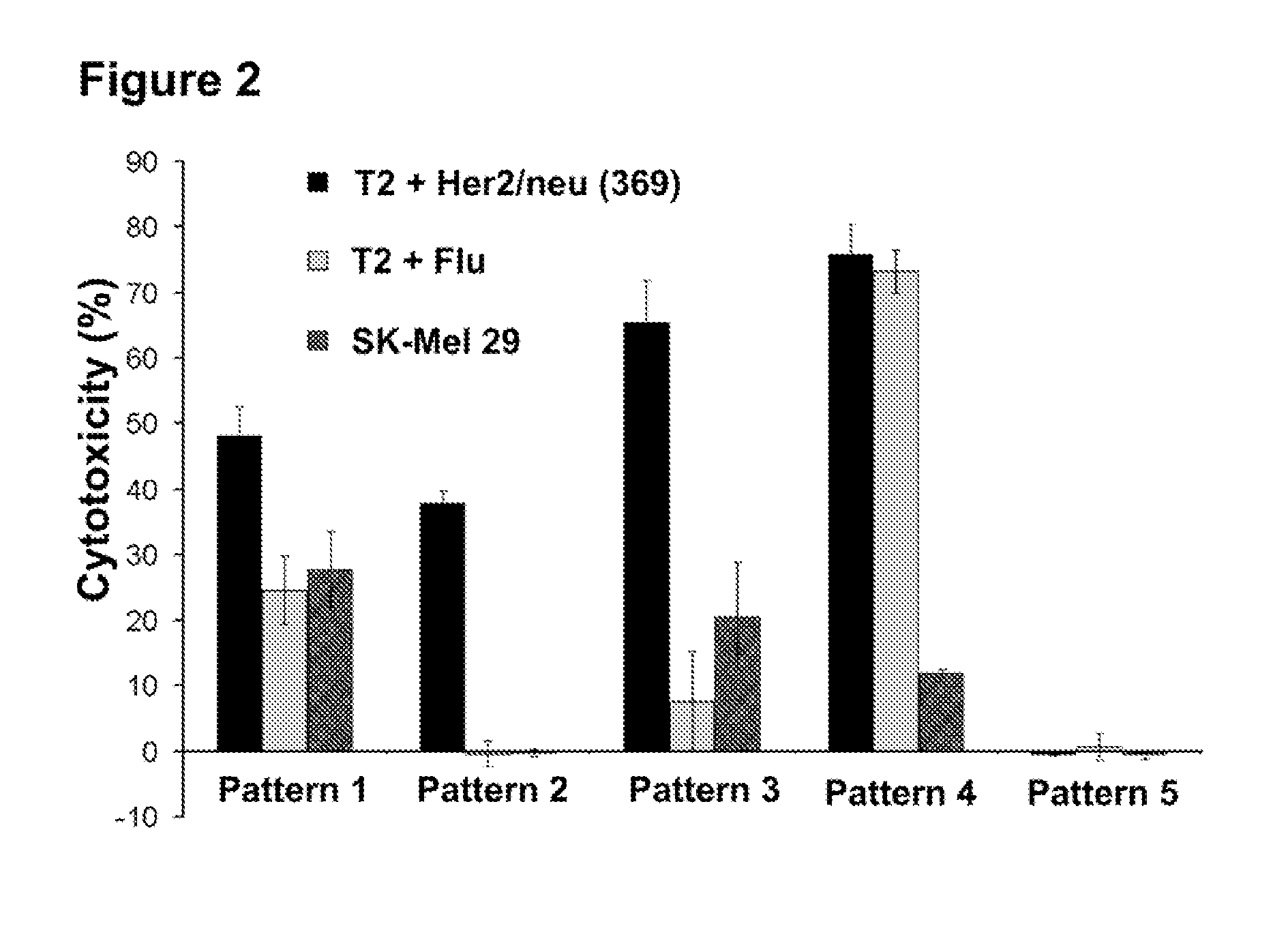
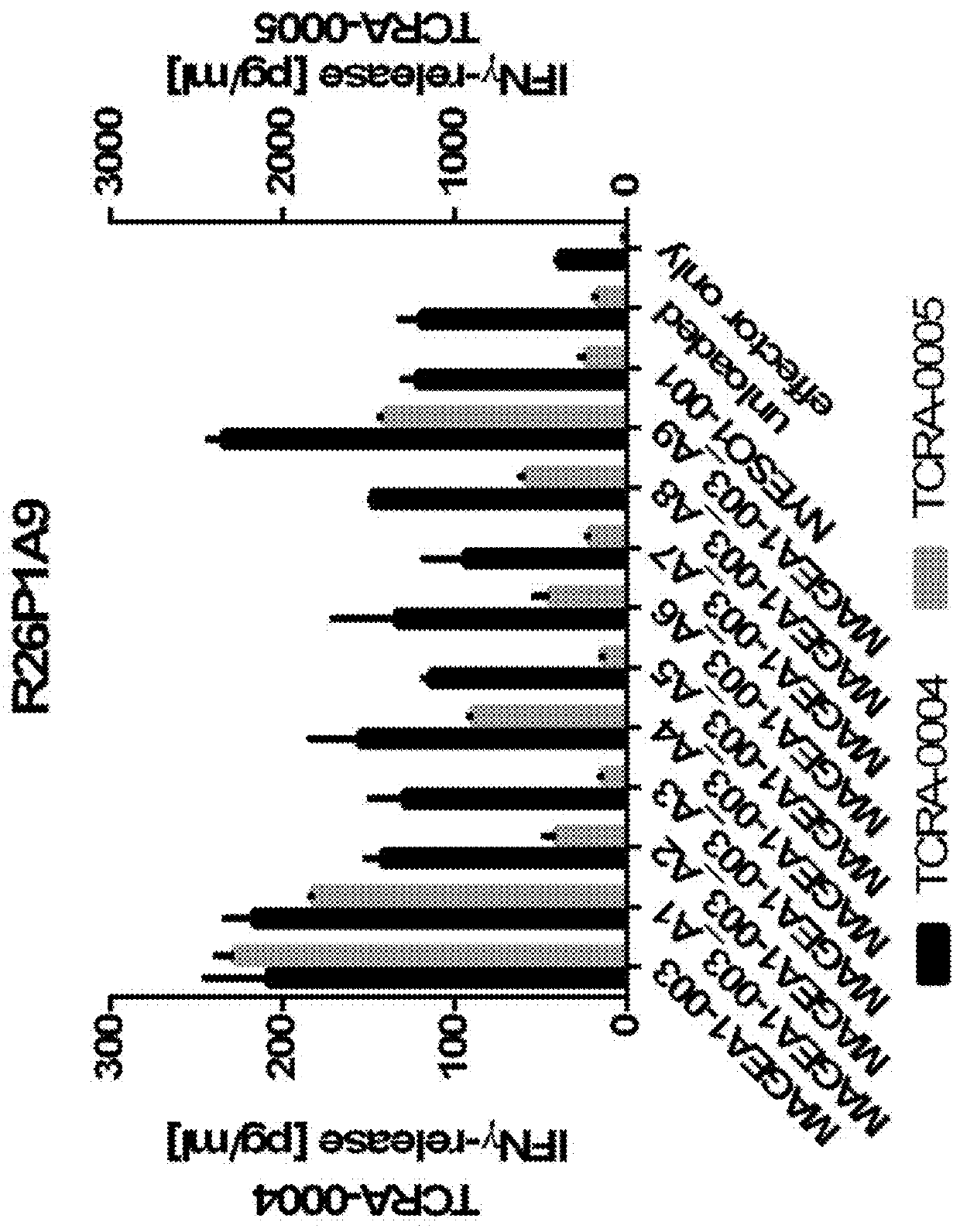
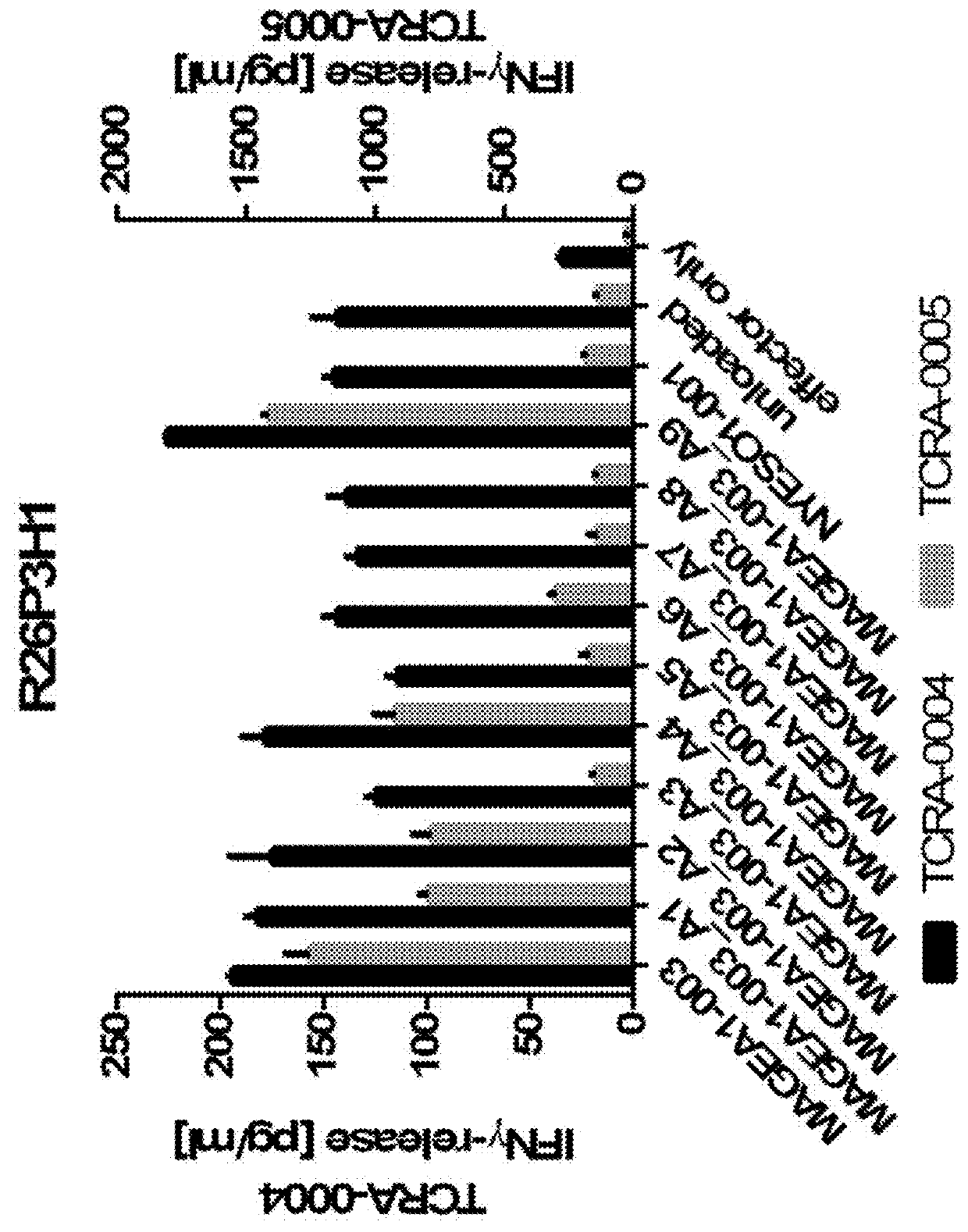
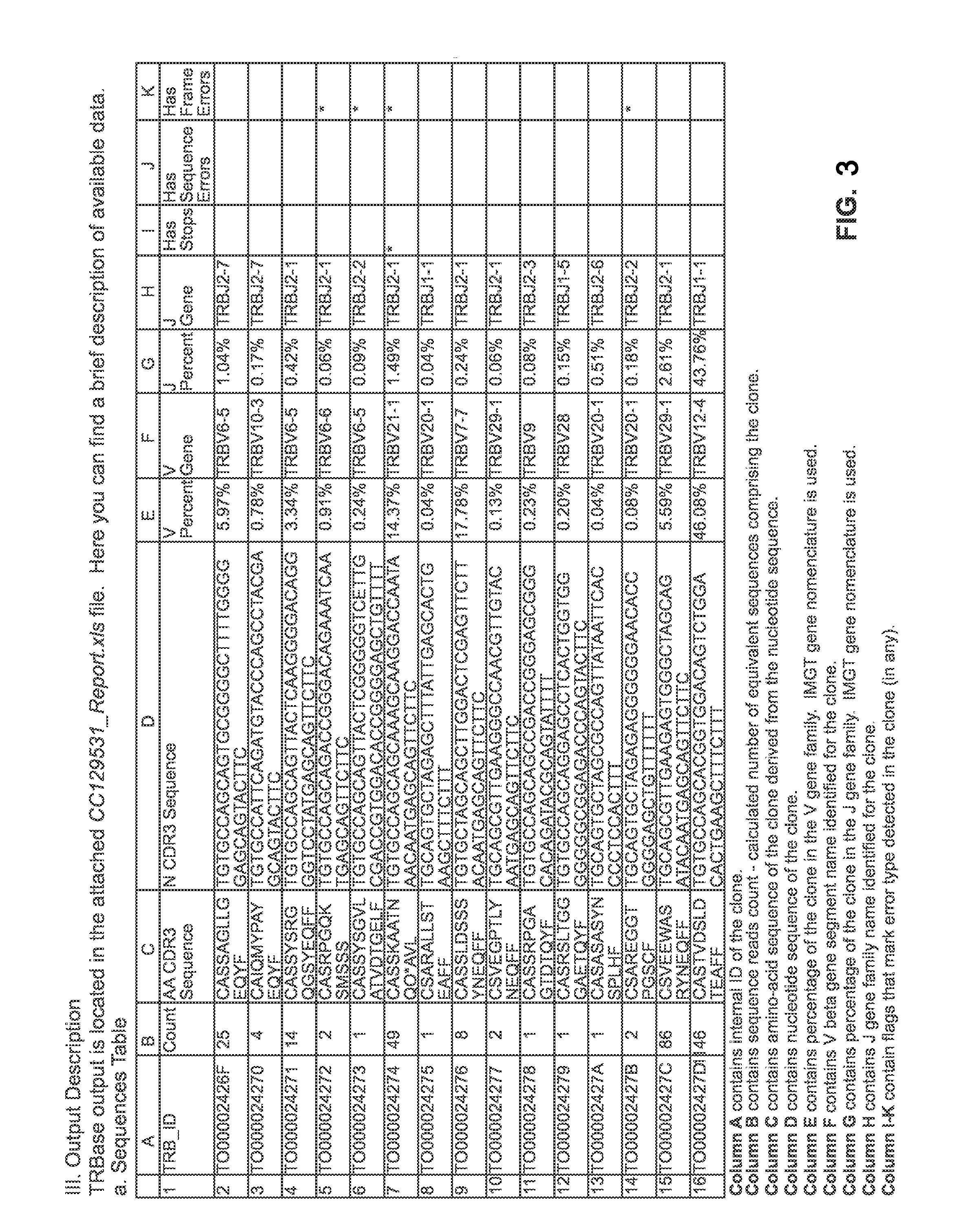
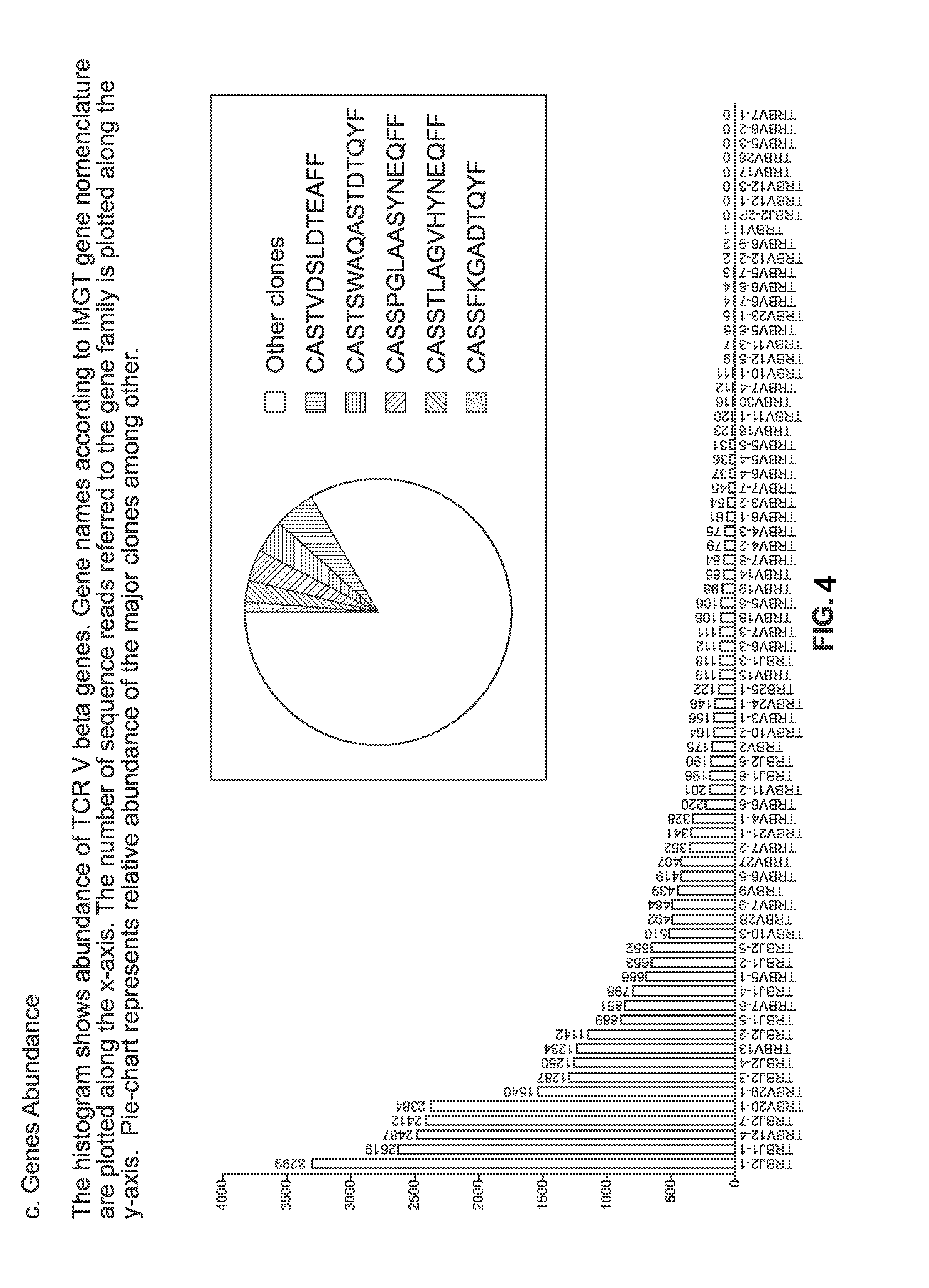
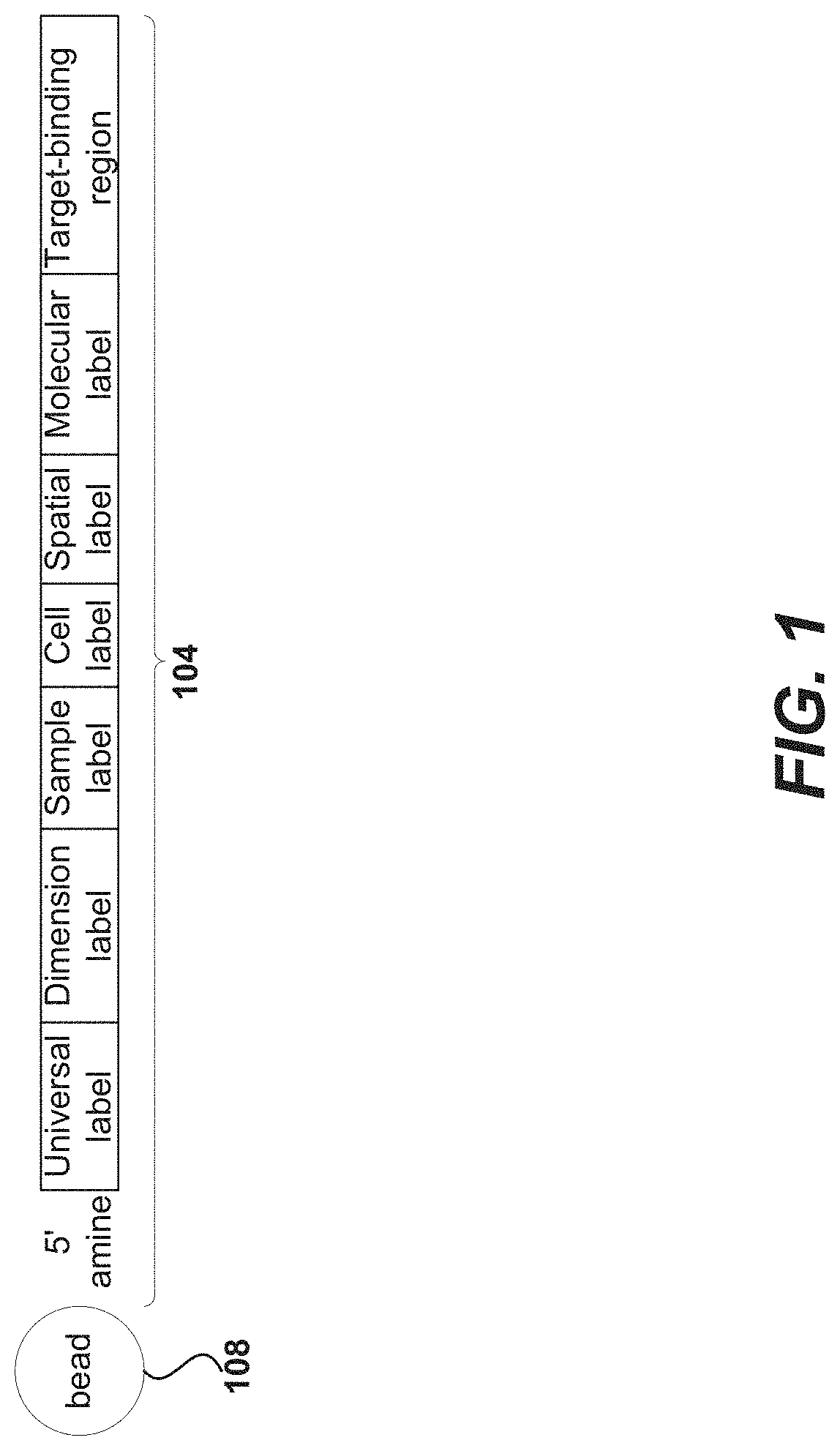
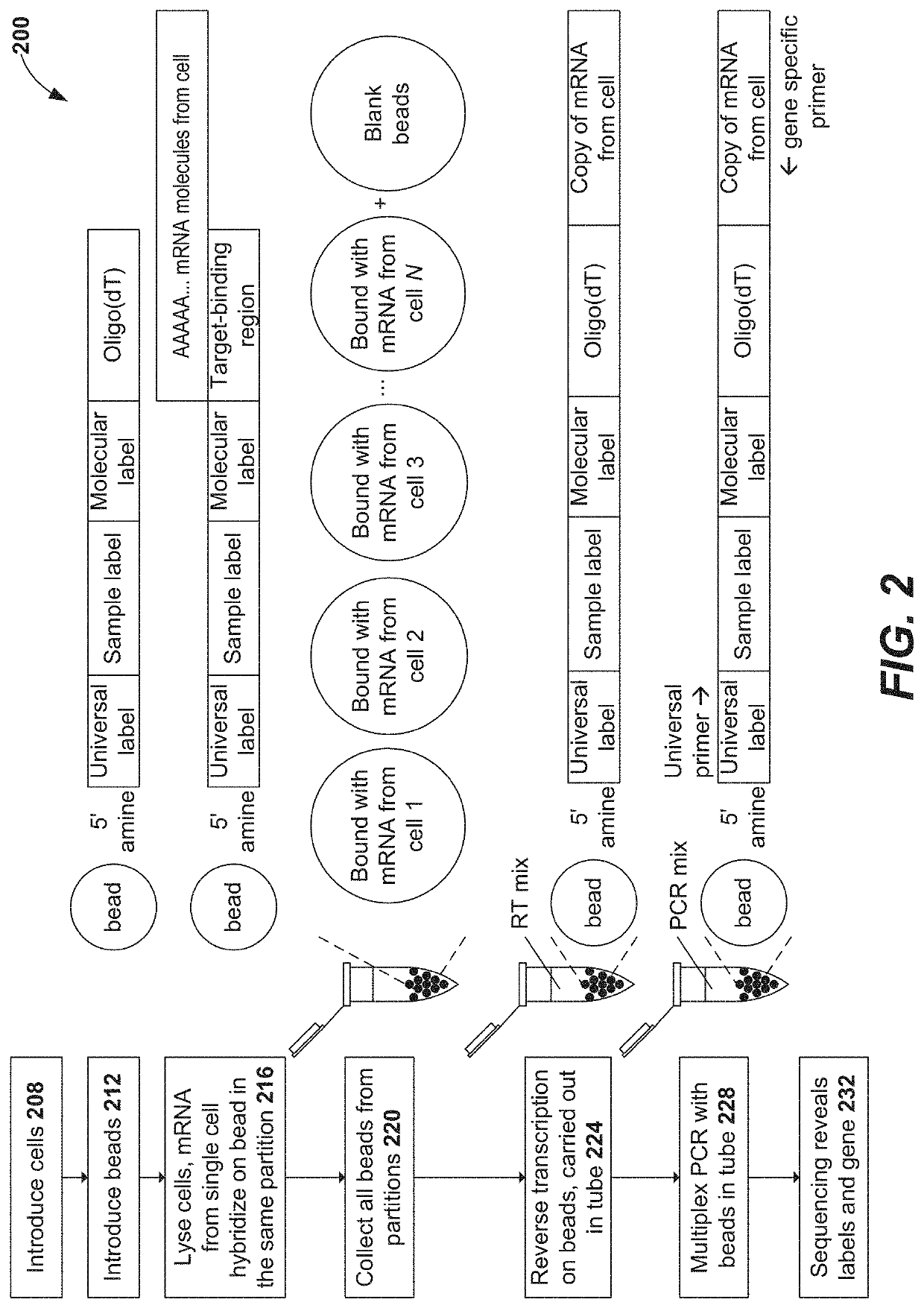
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
120 results about "B-cell receptor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
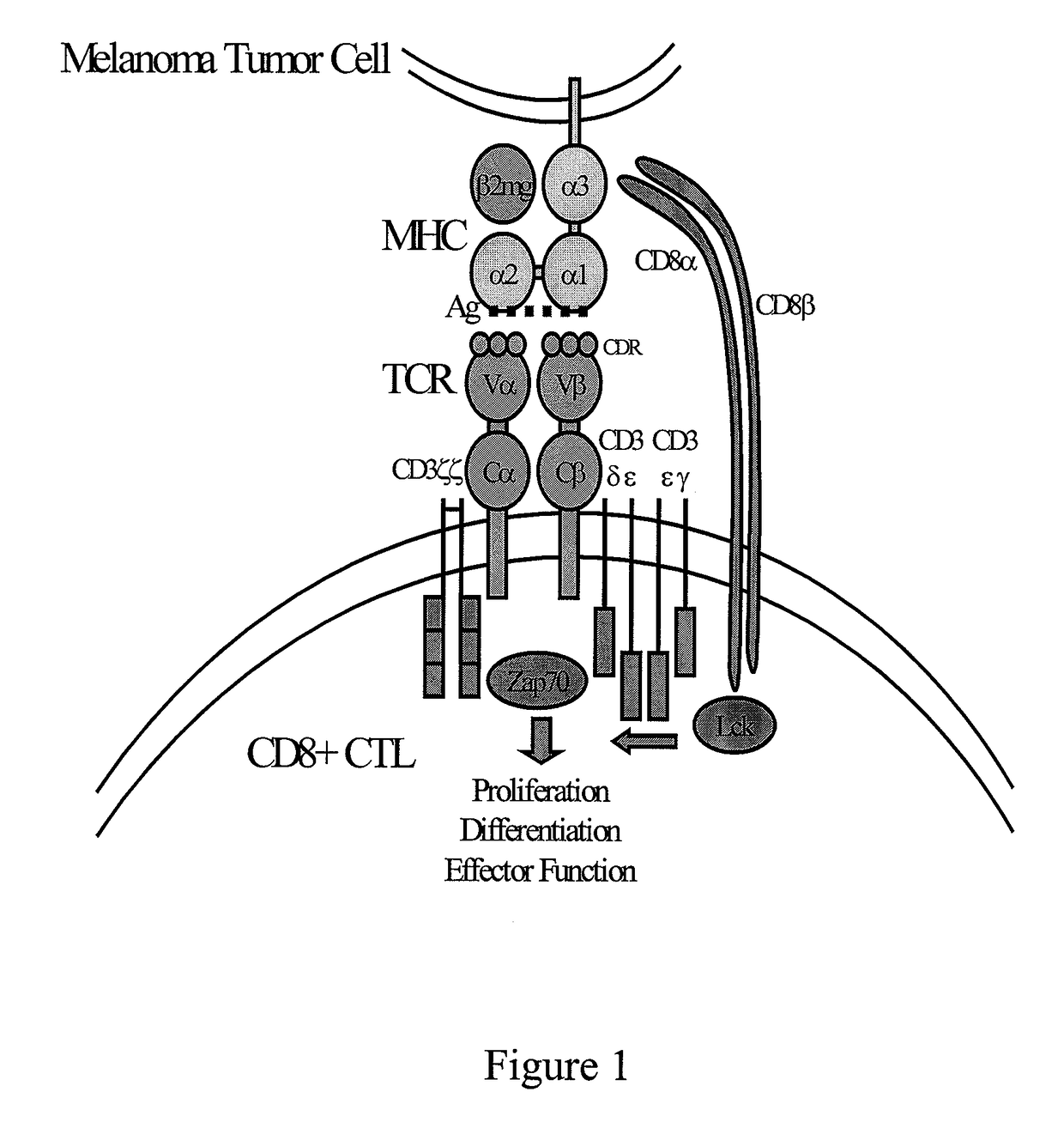
The B-cell receptor (BCR) is composed of immunoglobulin molecules that form a type 1 transmembrane receptor protein usually located on the outer surface of a lymphocyte type known as B cells. Through biochemical signaling and by physically acquiring antigens from the immune synapses, the BCR controls the activation of B-cell. B cells are able to gather and grab antigens by engaging biochemical modules for receptor clustering, cell spreading, generation of pulling forces, and receptor transport, which eventually culminates in endocytosis and antigen presentation. B-cells’ mechanical activity adheres to a pattern of negative and positive feedbacks that regulate the quantity of removed antigen by manipulating the dynamic of BCR-antigen bonds directly. Particularly, grouping and spreading increase the relation of antigen with BCR, thereby proving sensitivity and amplification. On the other hand, pulling forces delinks the antigen from the BCR, thus testing the quality of antigen binding.
CE7-specific redirected immune cells
Genetically engineered, CE7-specific redirected immune cells expressing a cell surface protein having an extracellular domain comprising a receptor which is specific for CE7, an intracellular signaling domain, and a transmembrane domain, and methods of use for such cells for cellular immunotherapy of CE7+ neuroblastoma are disclosed. In one embodiment, the immune cell is a T cell and the cell surface protein is a single chain FvFc:ζ receptor where Fv designates the VH and VL chains of a single chain monoclonal antibody to CE7 linked by peptide, Fc represents a hinge —CH2—CH3 region of a human IgG1, and ζ represents the intracellular signaling domain of the zeta chain of human CD3. DNA constructs encoding a chimeric T-cell receptor and a method of making a redirected T cell expressing a chimeric T cell receptor by electroporation using naked DNA encoding the receptor are also disclosed.
Owner:CITY OF HOPE
Human Antibodies to PD-1
ActiveUS20150203579A1Rescue T-cell signalingInhibit tumor growthNervous disorderAntipyreticFc(alpha) receptorDisease
The present invention provides antibodies that bind to the T-cell co-inhibitor programmed death-1 (PD-1) protein, and methods of use. In various embodiments of the invention, the antibodies are fully human antibodies that bind to PD-1. In certain embodiments, the present invention provides multi-specific antigen-binding molecules comprising a first binding specificity that binds to PD-1 and a second binding specificity that binds to an autoimmune tissue antigen, another T-cell co-inhibitor, an Fc receptor, or a T-cell receptor. In some embodiments, the antibodies of the invention are useful for inhibiting or neutralizing PD-1 activity, thus providing a means of treating a disease or disorder such as cancer or a chronic viral infection. In other embodiments, the antibodies are useful for enhancing or stimulating PD-1 activity, thus providing a means of treating, for example, an autoimmune disease or disorder.
Owner:REGENERON PHARM INC
Method for linking sequences of interest
ActiveUS7749697B2Efficient methodAntibacterial agentsSugar derivativesNucleotideNucleotide sequencing
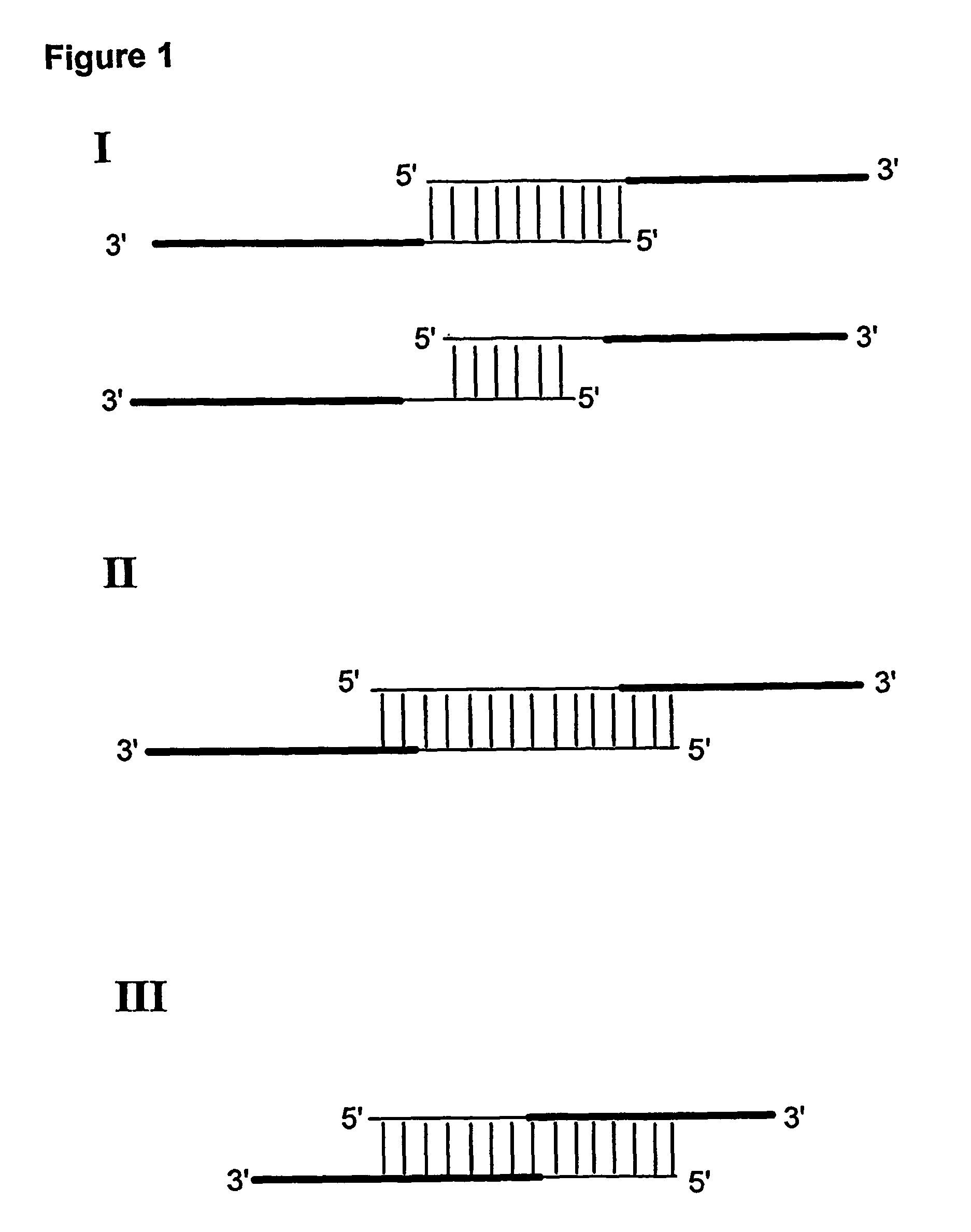
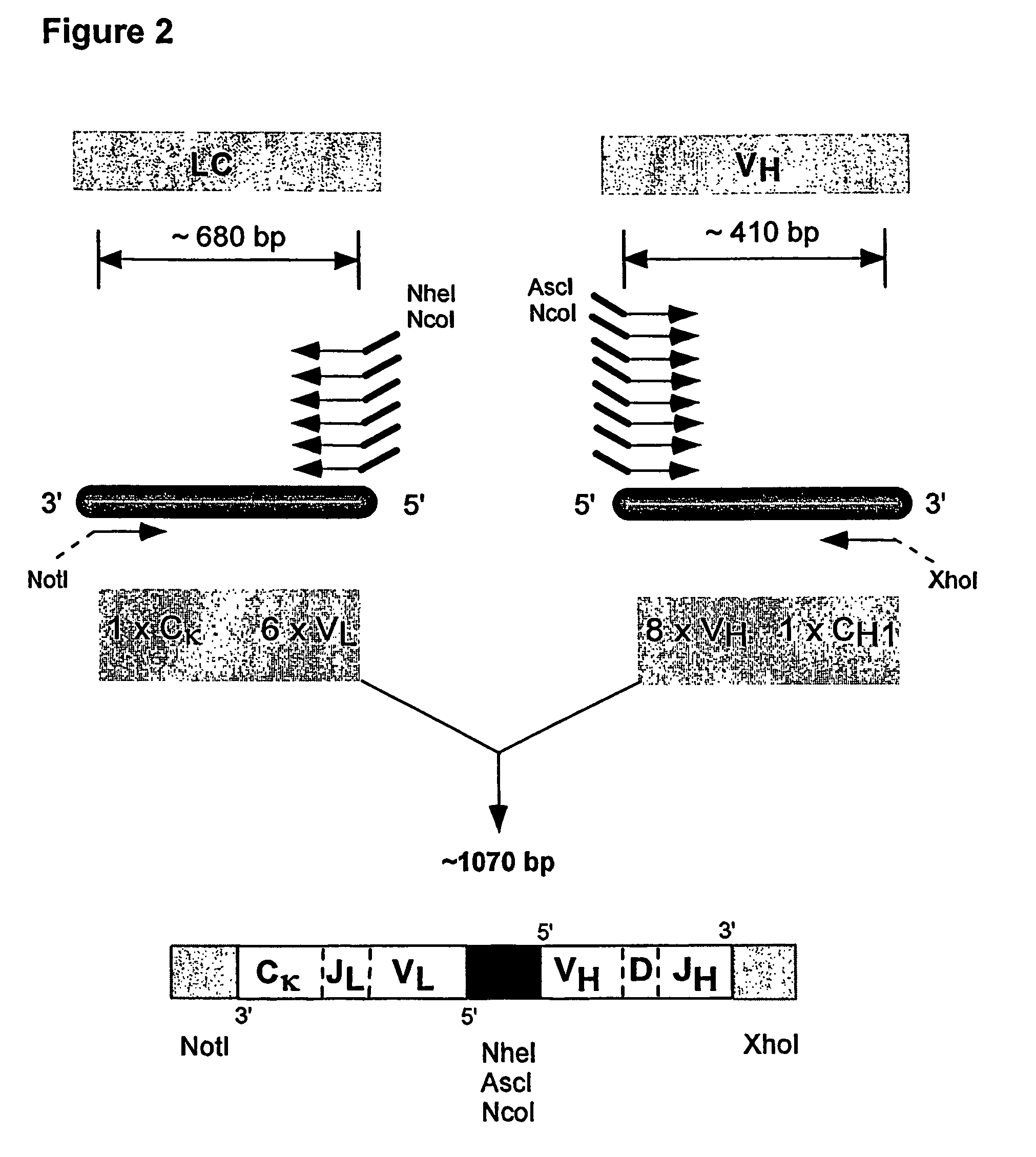
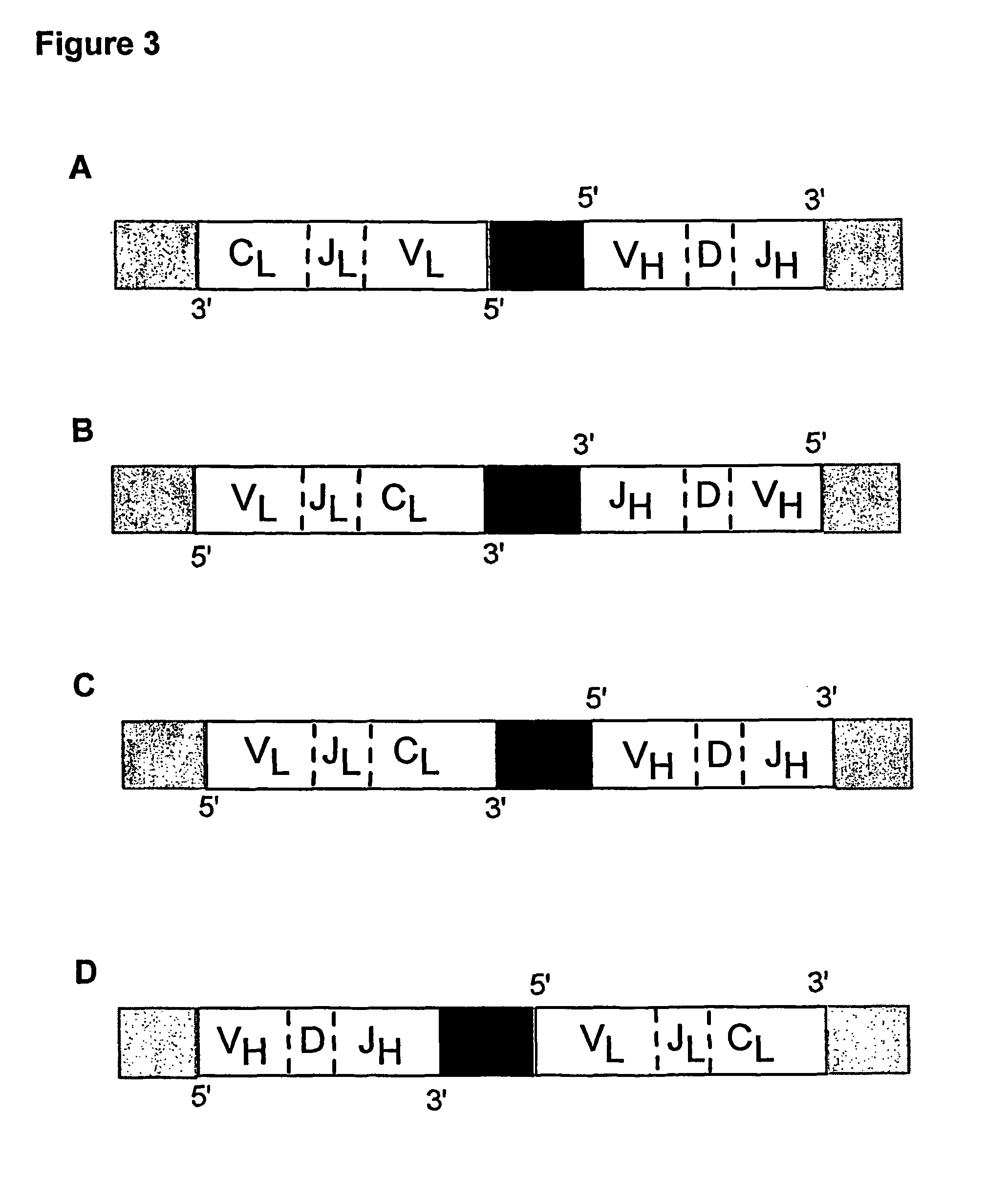
Multiplex overlap-extension RT-PCR provides an efficient method of linking two or more nucleotide sequences encoding for domains or subunits of a heteromeric protein, in a single reaction. Especially, the linkage of variable region encoding sequences from e.g. immunoglobulins, T cell receptors or B cell receptors is eased with the method of the present invention. This allows for a more efficient way of generating libraries of variable region encoding sequences. The capability to perform the multiplex overlap-extension RT-PCR using template derived from an isolated single cell enables the generation of cognate pair libraries in a high-throughput format.
Owner:LES LAB SERVIER
Sequence analysis of complex amplicons
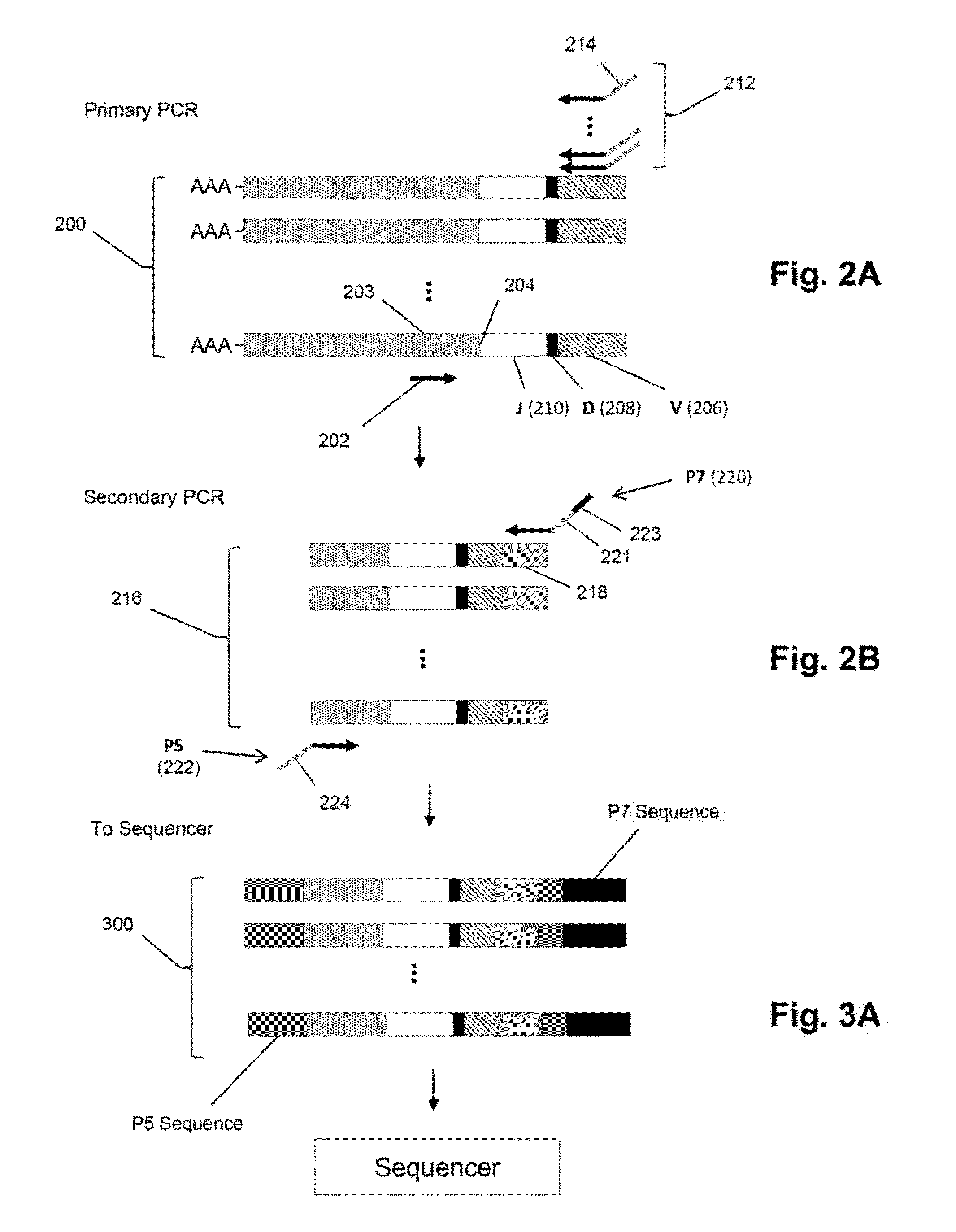
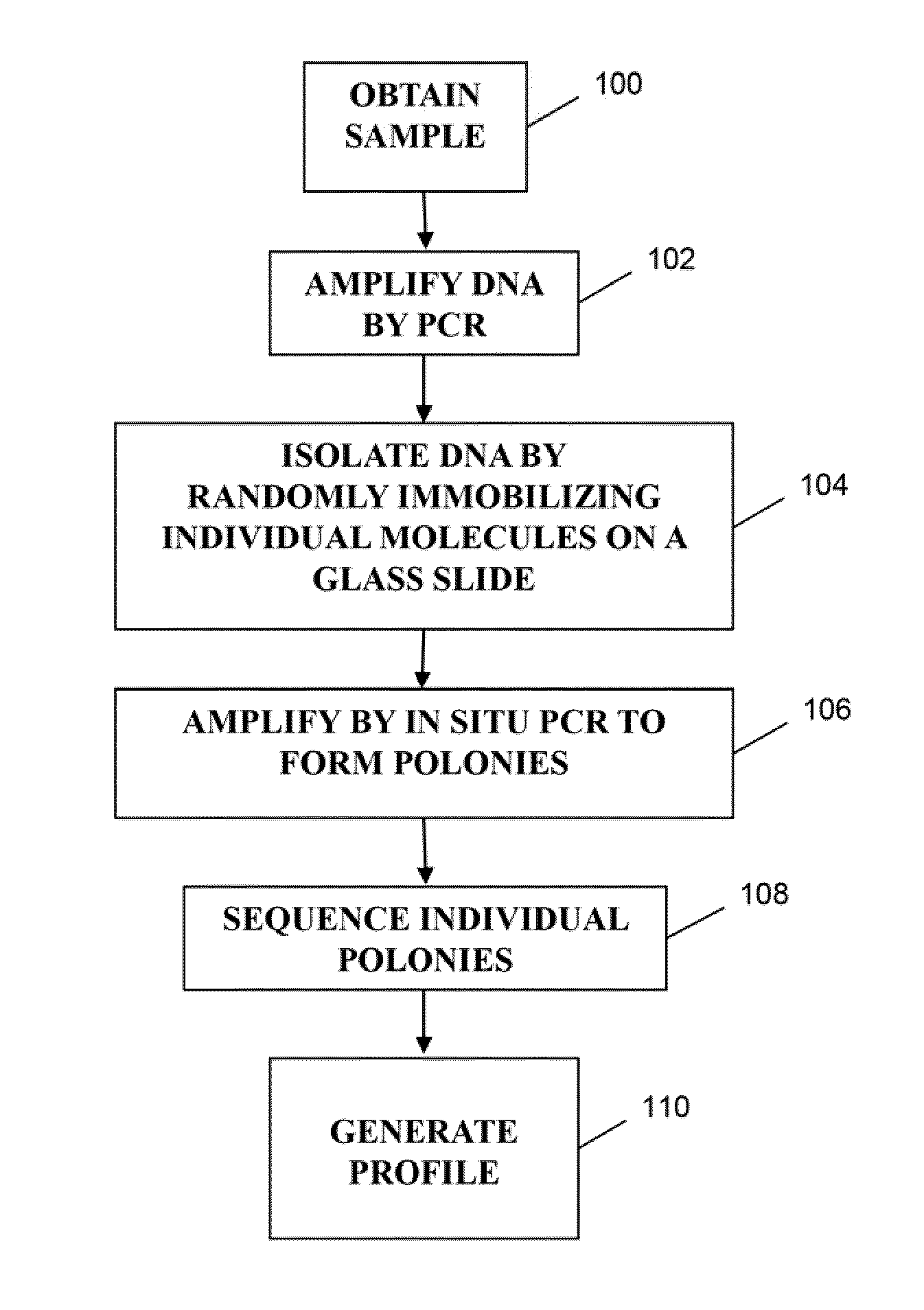
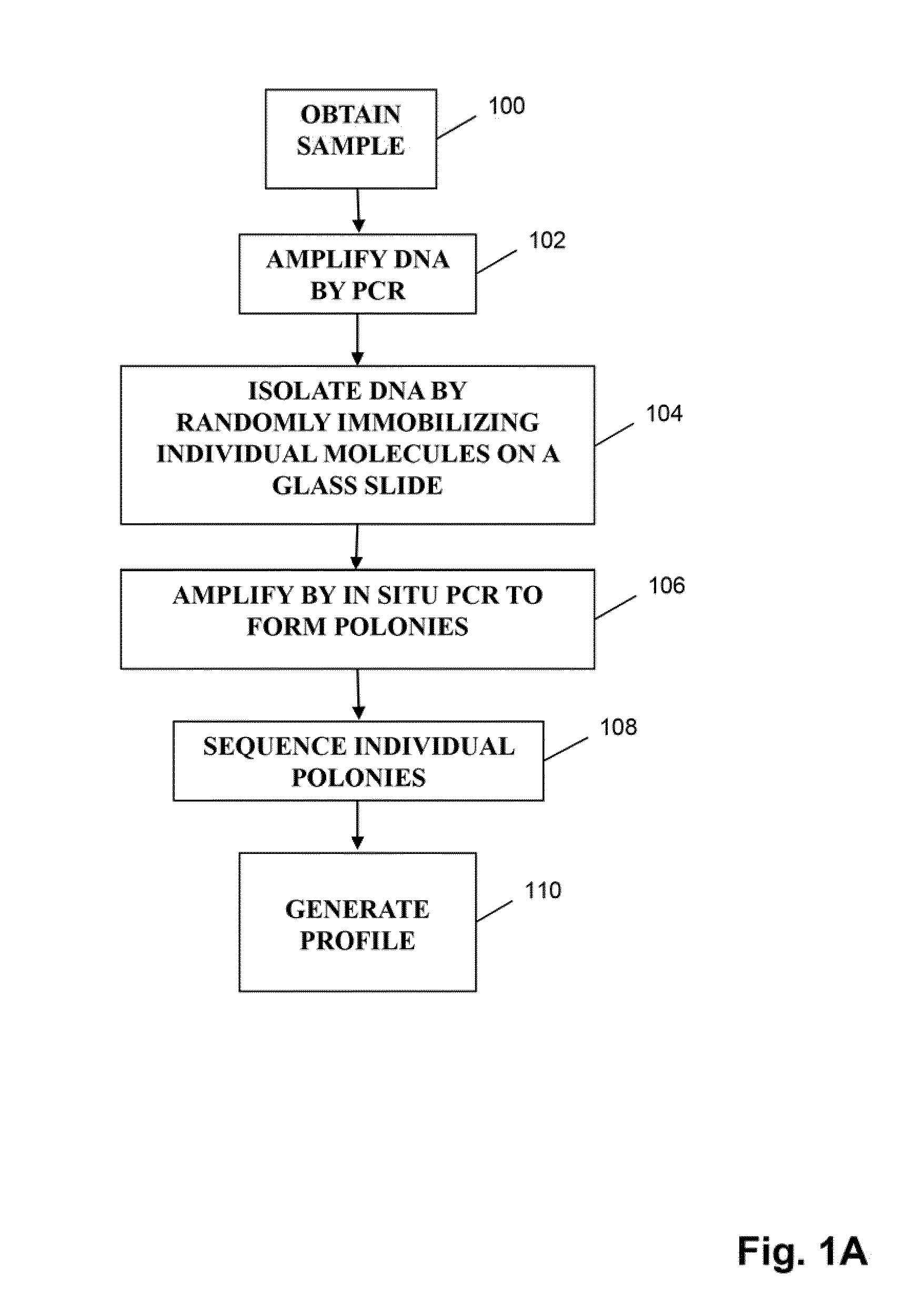
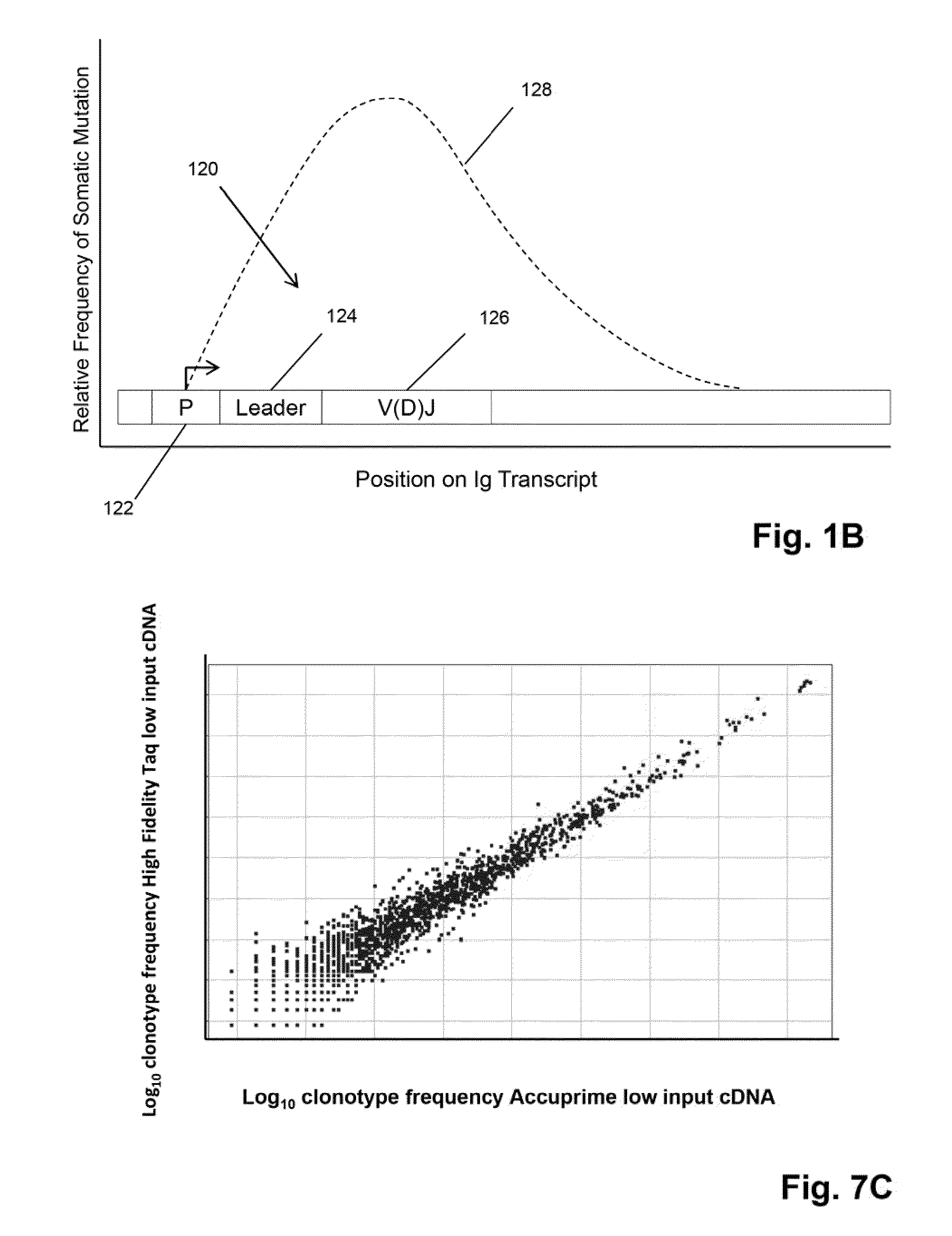
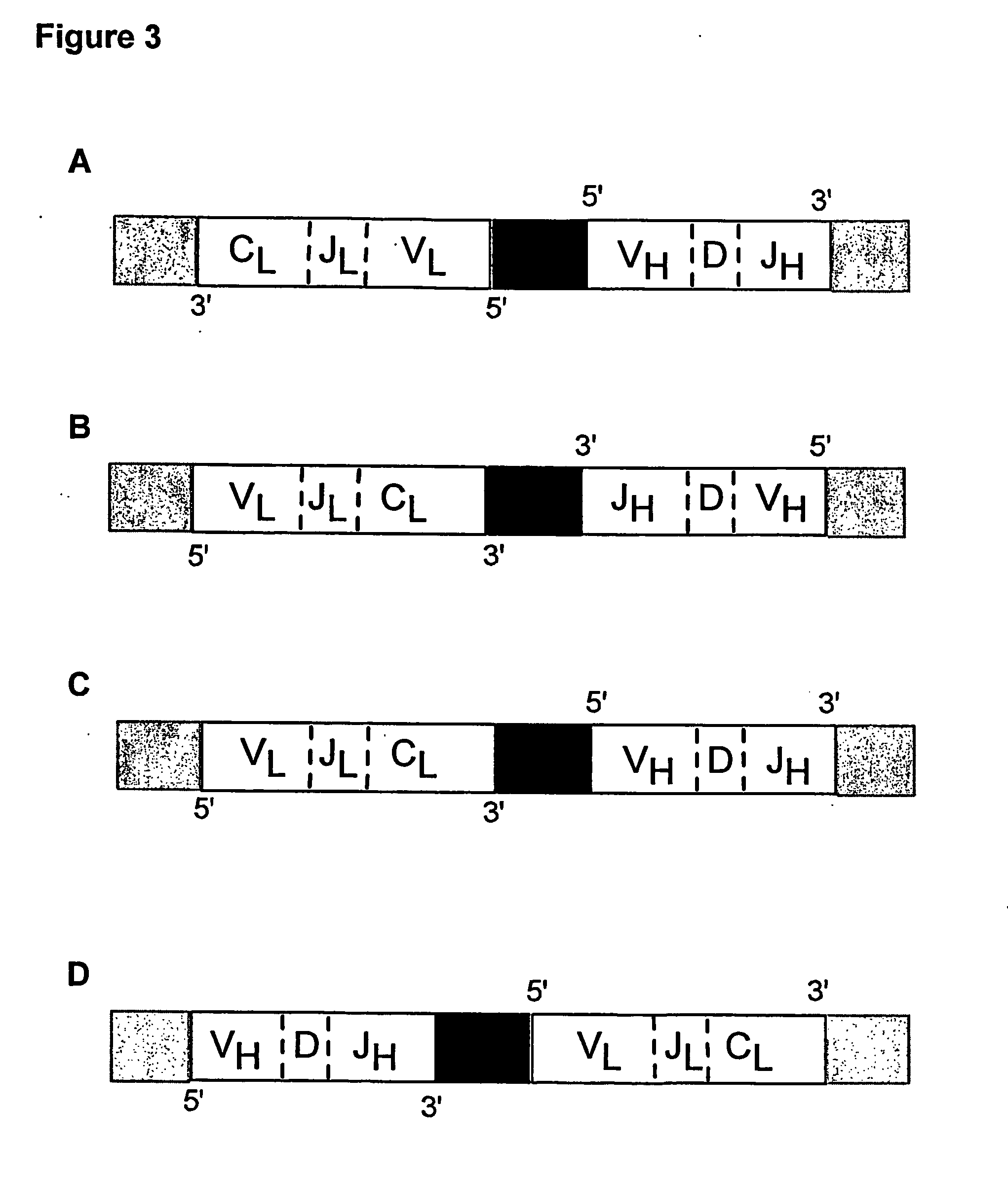
ActiveUS20140315725A1Microbiological testing/measurementLibrary member identificationSequence analysisB-cell receptor
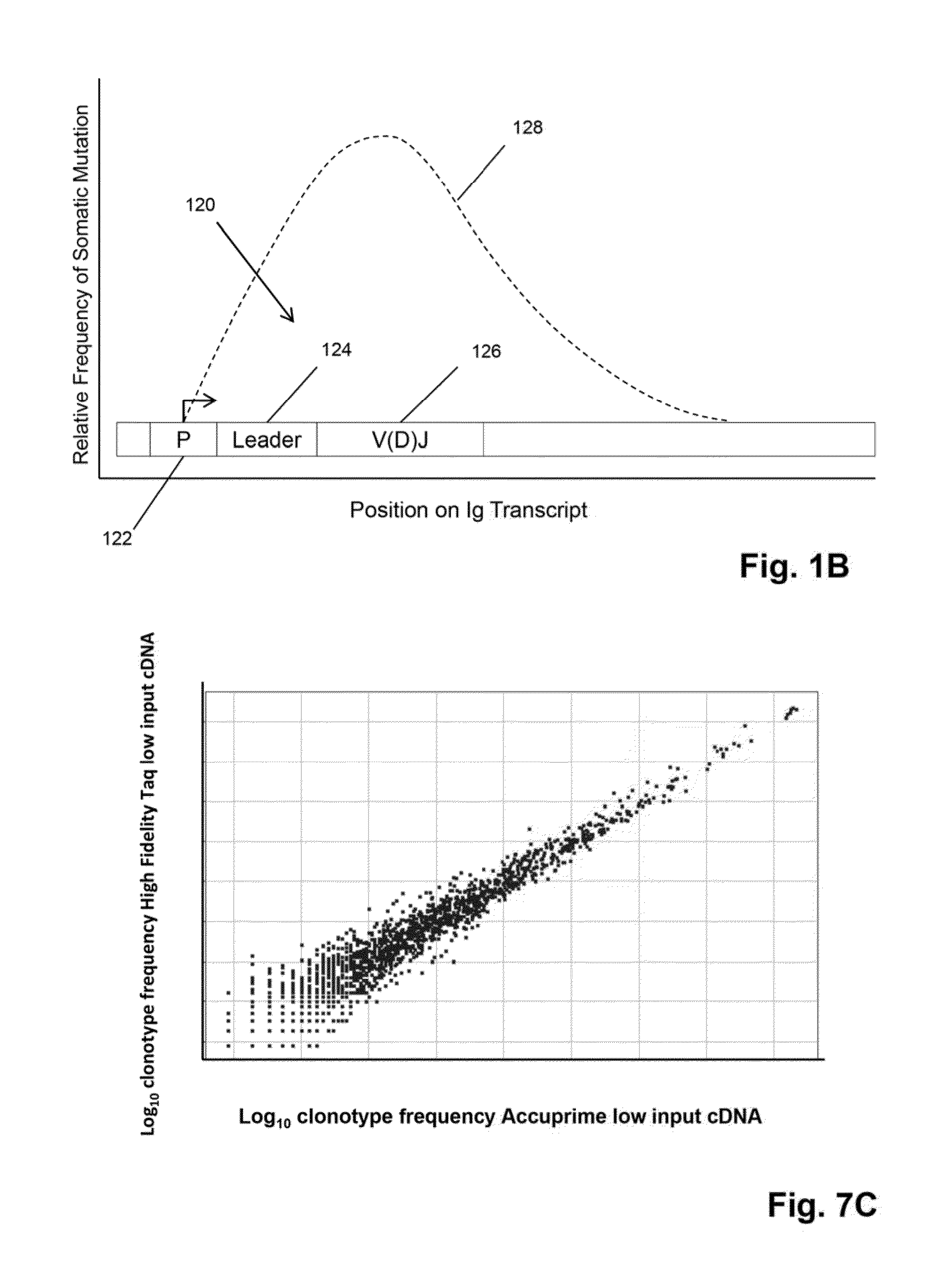
The invention is directed to methods of generating sequence profiles of populations of nucleic acids, whose member nucleic acids contain regions of high variability, such as populations of nucleic acids encoding T cell receptors or B cell receptors. In one aspect, the invention provides pluralities of sets of primers for generating nested sets of templates from nucleic acids in such populations, thereby insuring the production of at least one template from which sequence reads are generated, despite such variability, or despite limited lengths or quality of sequence reads. In another aspect, members of such populations are bidirectionally sequenced so that further sequence information is obtained by analyzing overlapping sequence reads in the zones of highest variability.
Owner:ADAPTIVE BIOTECH
Methods of monitoring conditions by sequence analysis
ActiveUS20110207135A1Low quality scoreMicrobiological testing/measurementICT adaptationSequence analysisGenetics
The invention is directed to methods of generating sequence profiles of populations of nucleic acids, whose member nucleic acids contain regions of high variability, such as populations of nucleic acids encoding T cell receptors or B cell receptors. In one aspect, the invention provides pluralities of sets of primers for generating nested sets of templates from nucleic acids in such populations, thereby insuring the production of at least one template from which sequence reads are generated, despite such variability, or dispite limited lenghs or quality of sequence reads. In another aspect, members of such populations are bidirectionally sequenced so that further sequence information is obtained by analyzing overlapping sequence reads in the zones of highest variability.
Owner:ADAPTIVE BIOTECH
Human antibodies to PD-1
ActiveUS9987500B2Rescues T-cell signalingInhibit tumor growthNervous disorderAntipyreticFc(alpha) receptorDisease
The present invention provides antibodies that bind to the T-cell co-inhibitor programmed death-1 (PD-1) protein, and methods of use. In various embodiments of the invention, the antibodies are fully human antibodies that bind to PD-1. In certain embodiments, the present invention provides multi-specific antigen-binding molecules comprising a first binding specificity that binds to PD-1 and a second binding specificity that binds to an autoimmune tissue antigen, another T-cell co-inhibitor, an Fc receptor, or a T-cell receptor. In some embodiments, the antibodies of the invention are useful for inhibiting or neutralizing PD-1 activity, thus providing a means of treating a disease or disorder such as cancer or a chronic viral infection. In other embodiments, the antibodies are useful for enhancing or stimulating PD-1 activity, thus providing a means of treating, for example, an autoimmune disease or disorder.
Owner:REGENERON PHARM INC
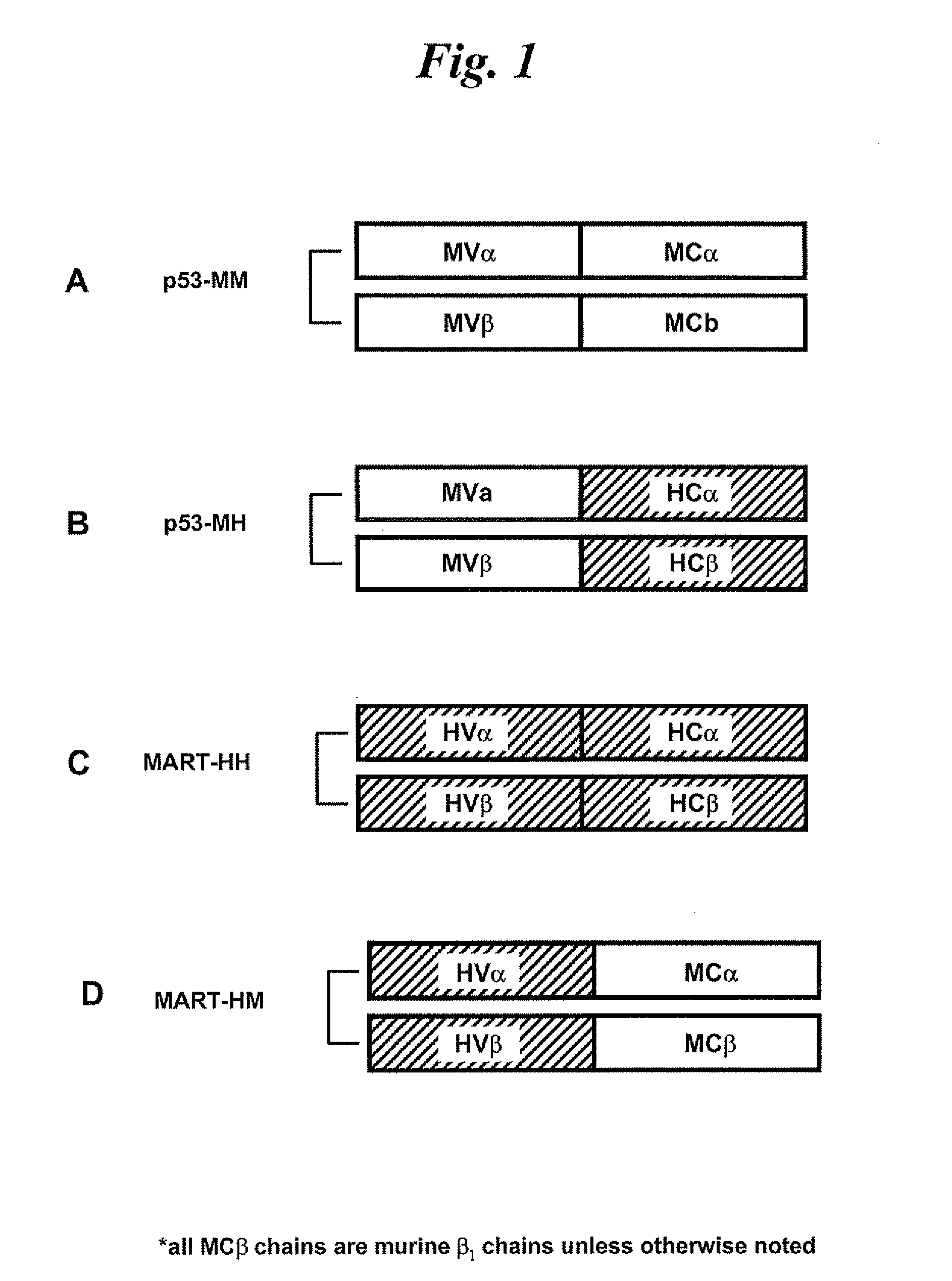
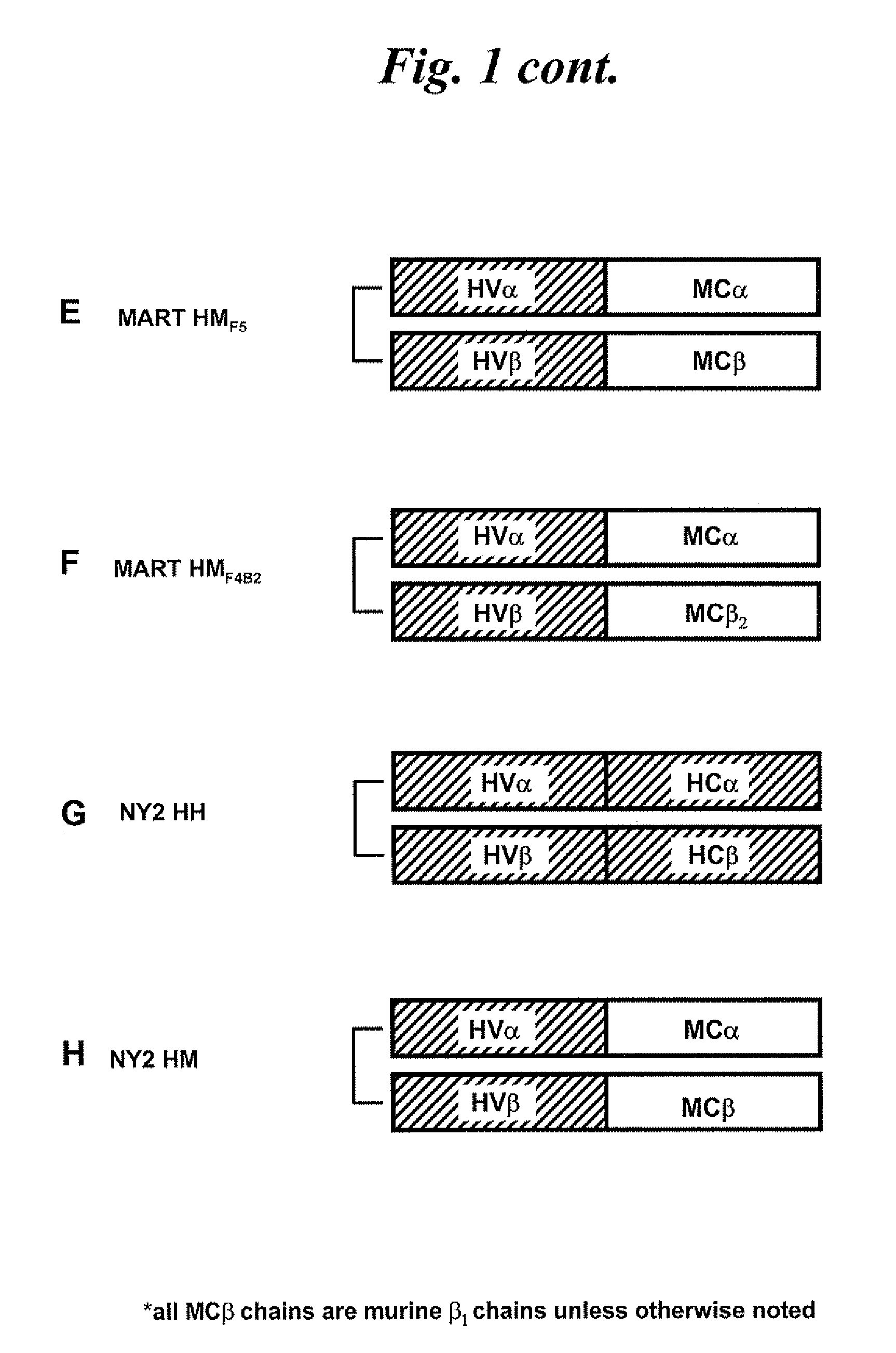
Compositions comprising t cell receptors and methods of use thereof
InactiveUS20090053184A1High affinityEfficient killingOrganic active ingredientsBiocideMelanomaBiology
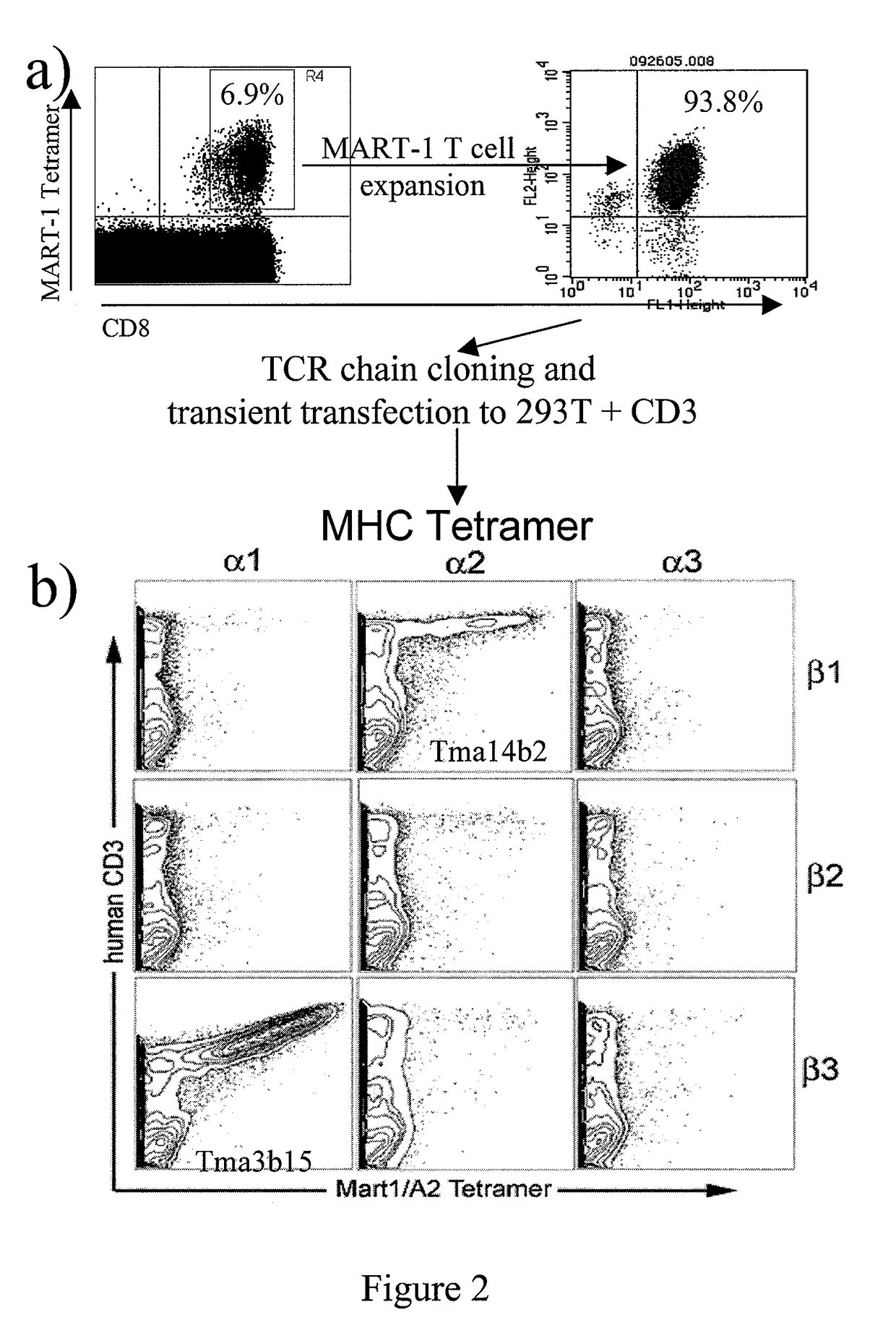
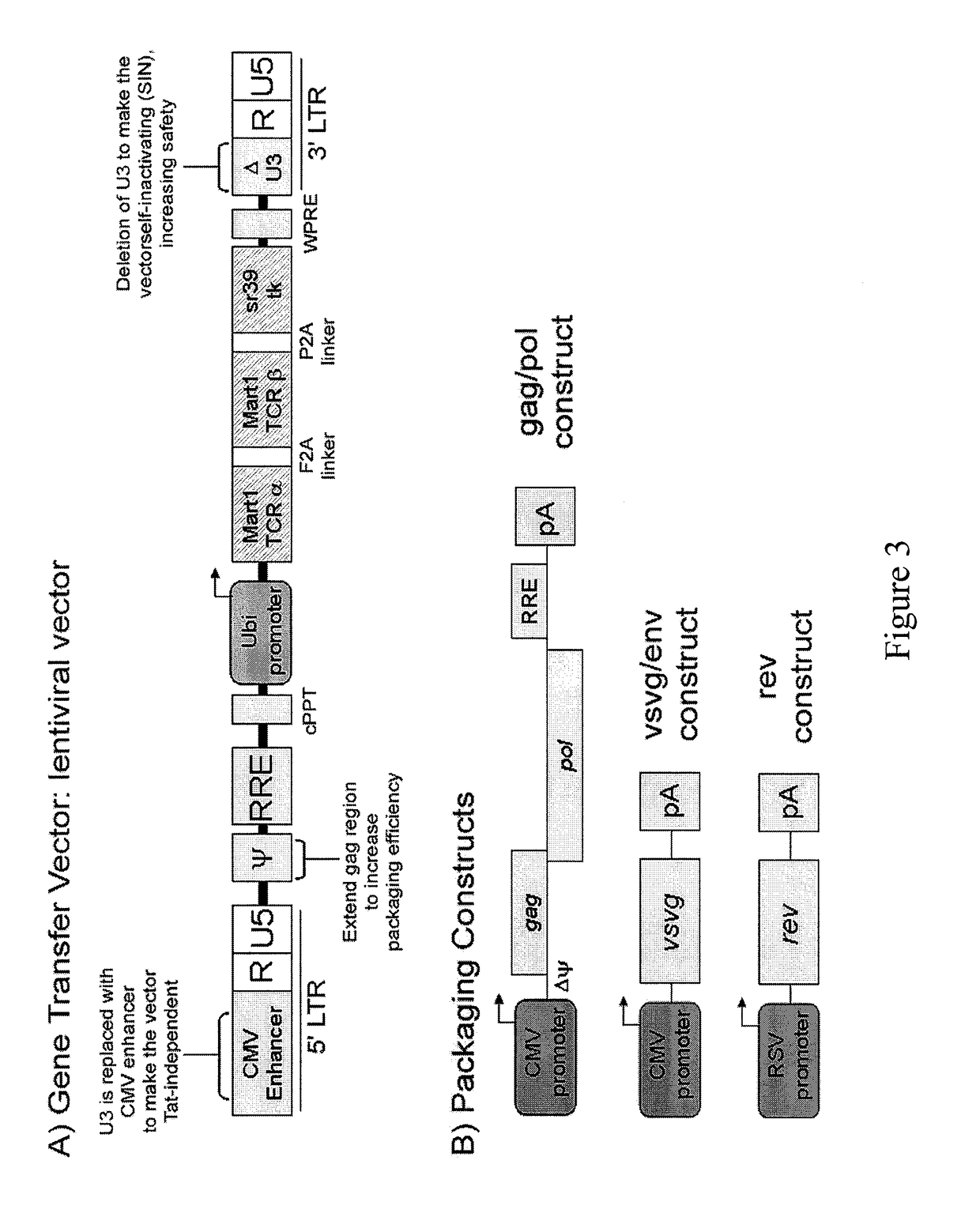
Nucleic acids encoding antitumor TCRs recognizing MART-1, NY-ESO-1, and melanoma gp100 peptides; vectors and cells comprising the same; and methods of using the foregoing.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
Multiple target t cell receptor
InactiveUS20110189141A1Convenient treatmentHigh affinityBiocideOrganic active ingredientsDiseaseWilms' tumor
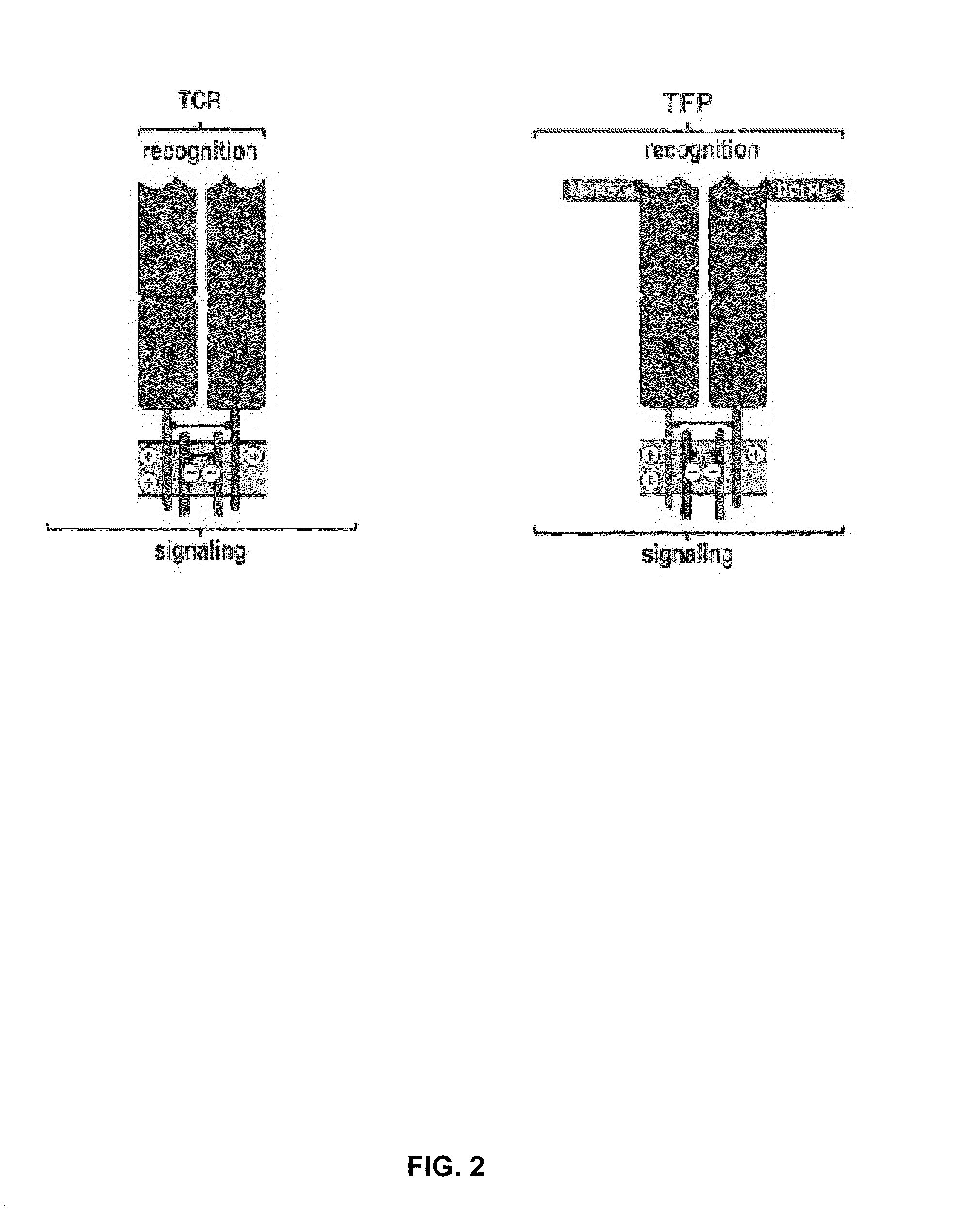
The present invention is directed to a functional T cell receptor (TCR) fusion protein (TFP) recognizing and binding to at least one MHC-presented epitope, and containing at least one amino acid sequence recognizing and binding an antigen. The present invention is further directed to an isolated nucleic acid molecule encoding the same, a T cell expressing said TFP, and a pharmaceutical composition for use in the treatment of diseases involving malignant cells expressing said tumor-associated antigen.
Owner:MAX DELBRUECK CENT FUER MOLEKULARE MEDIZIN
Method for linking sequences of interest
Multiplex overlap-extension RT-PCR provides an efficient method of linking two or more nucleotide sequences encoding for domains or subunits of a heteromeric protein, in a single reaction. Especially, the linkage of variable region encoding sequences from e.g. immunoglobulins, T cell receptors or B cell receptors is eased with the method of the present invention. This allows for a more efficient way of generating libraries of variable region encoding sequences. The capability to perform the multiplex overlap-extension RT-PCR using template derived from an isolated single cell enables the generation of cognate pair libraries in a high-throughput format.
Owner:LES LAB SERVIER
Quantifying and profiling antibody and t cell receptor gene expression
InactiveUS20070161001A1Microbiological testing/measurementImmunoglobulinsNucleotideT-Cell Receptor Gene
A method of sequencing a population of polynucleotides encoding antibodies or T-cell receptors. Also provided are a method of quantifying an expression of a population of polynucleotides encoding antibodies or T-cell receptors as well as an oligonucleotide library for sequencing by hybridization of polynucleotides encoding variable regions of antibodies or T cell receptors.
Owner:LESHKOWITZ DENA
Methods for engineering t-cell receptors
InactiveUS20130040836A1Increased serum half-lifePeptide librariesImmunoglobulin superfamilyEpitopeB-cell receptor
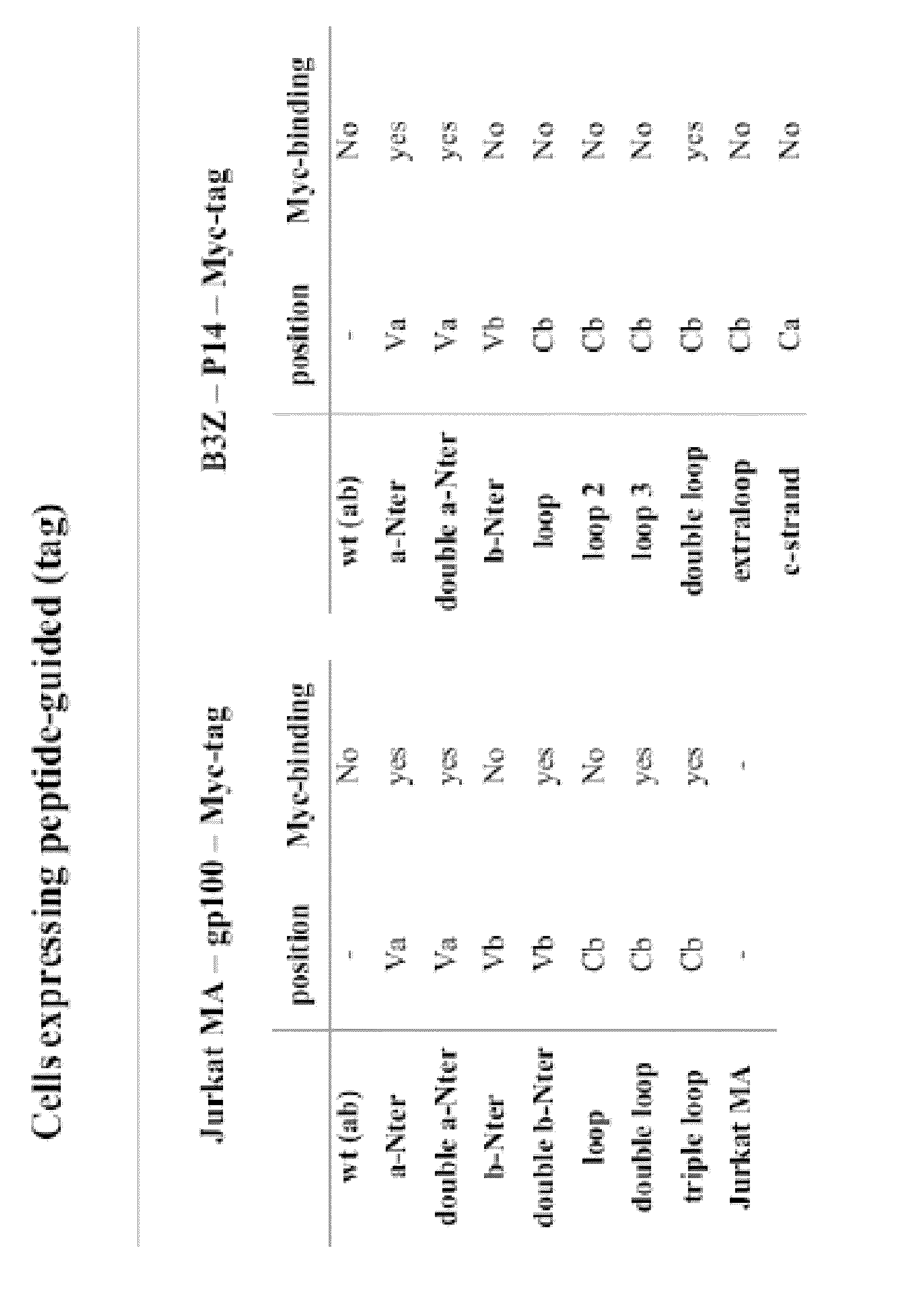
The present invention provides a method for engineering a T-cell receptor domain polypeptide comprising at least one modification in a structural loop region of the T-cell receptor domain polypeptide and determining the binding of the T-cell receptor domain polypeptide to an epitope of an antigen, wherein the unmodified T-cell receptor domain polypeptide does not significantly bind to said epitope. The present invention also covers modified T cell receptor domain polypeptides, their use and libraries containing the modified T cell receptor domain polypeptides.
Owner:F STAR BIOTECHNOLOGISCHE FORSCHUNGS & ENTWICKLUNGS GMBH
Sequence analysis of complex amplicons
The invention is directed to methods of generating sequence profiles of populations of nucleic acids, whose member nucleic acids contain regions of high variability, such as populations of nucleic acids encoding T cell receptors or B cell receptors. In one aspect, the invention provides pluralities of sets of primers for generating nested sets of templates from nucleic acids in such populations, thereby insuring the production of at least one template from which sequence reads are generated, despite such variability, or dispite limited lengths or quality of sequence reads. In another aspect, members of such populations are bidirectionally sequenced so that further sequence information is obtained by analyzing overlapping sequence reads in the zones of highest variability.
Owner:ADAPTIVE BIOTECH
Chimeric t cell receptors and related materials and methods of use
InactiveUS20090304657A1Good biological propertiesGreat T cell responseBiocideAntibody mimetics/scaffoldsDiseaseDrug biological activity
The invention provides a chimeric T cell receptor (TCR) comprising a variable region of a human TCR and a constant region comprising at least an extracellular domain of a constant region of a non-human TCR, as well as functional variants thereof. The invention also provides polypeptides and proteins related to the inventive TCRs, as well as nucleic acids encoding the TCRs, polypeptides, or proteins, recombinant expression vectors, and host cells. Further provided are pharmaceutical compositions related to the inventive TCRs and methods of preventing or treating a disease, e.g., an infectious disease, cancer, in a host, methods of detecting a diseased cell in a host, and methods of improving the biological activity of a TCR.
Owner:UNITED STATES OF AMERICA
Methods of isolating t cell receptors having antigenic specificity for a cancer-specific mutation
Owner:UNITED STATES OF AMERICA
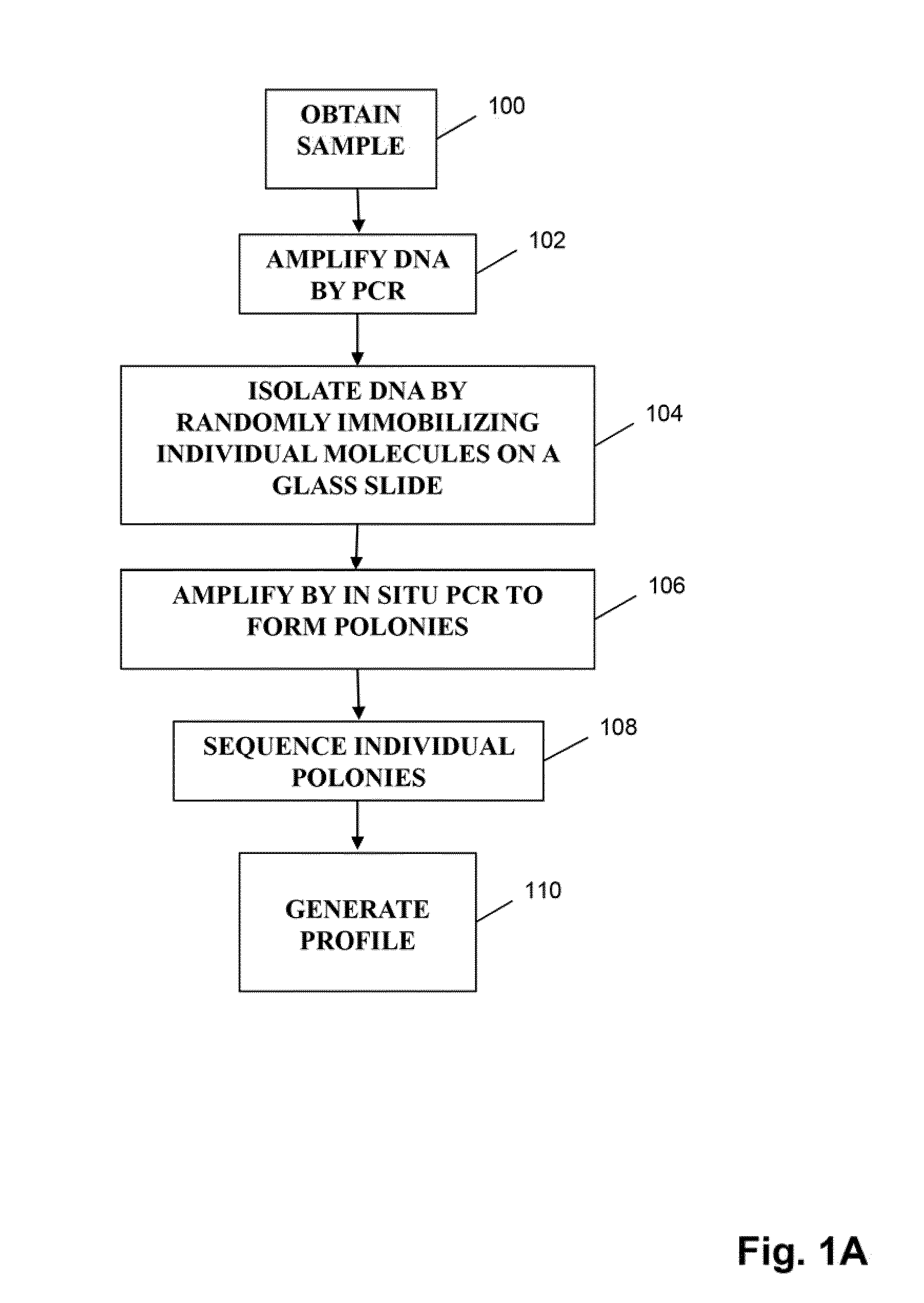
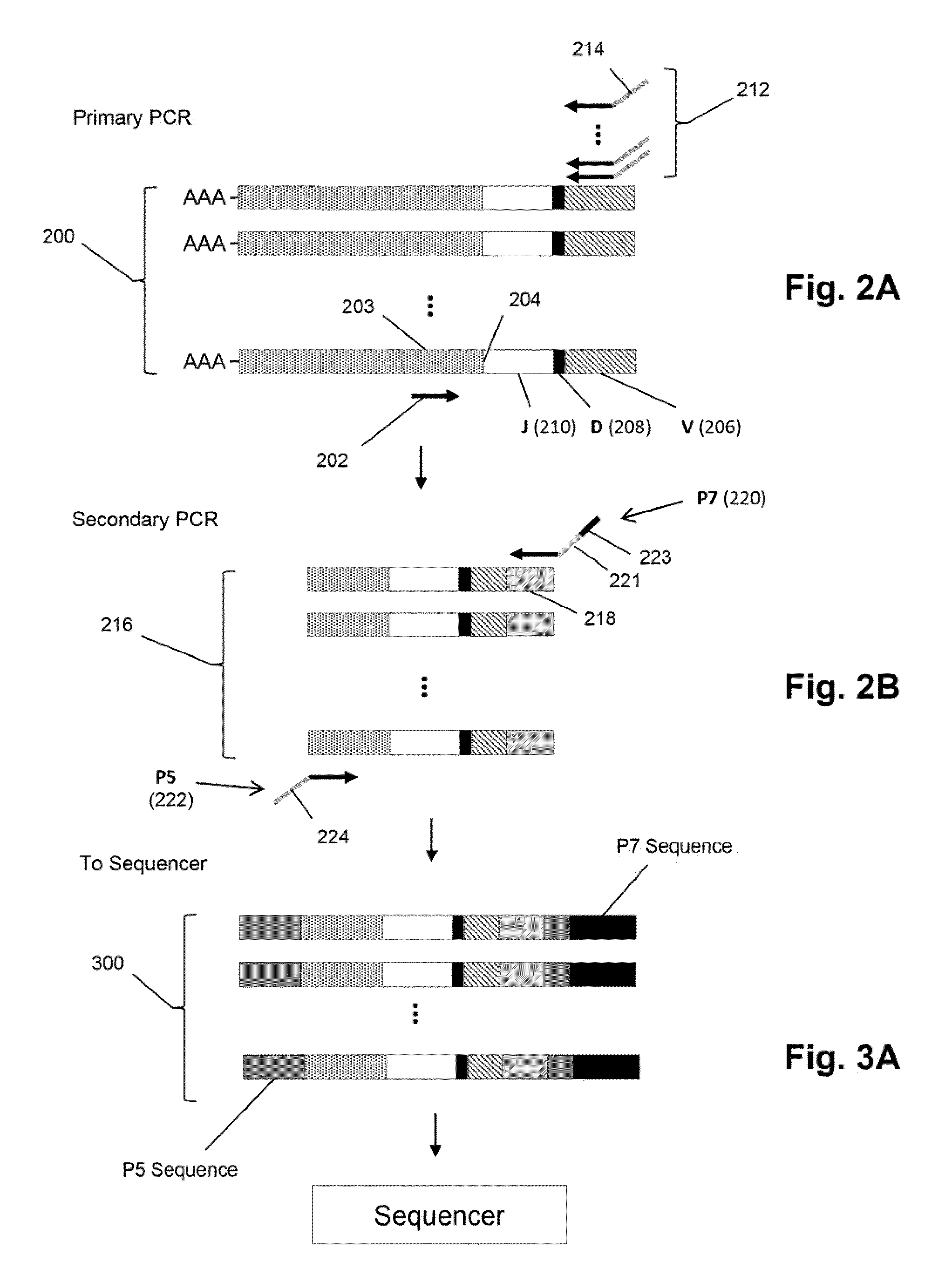
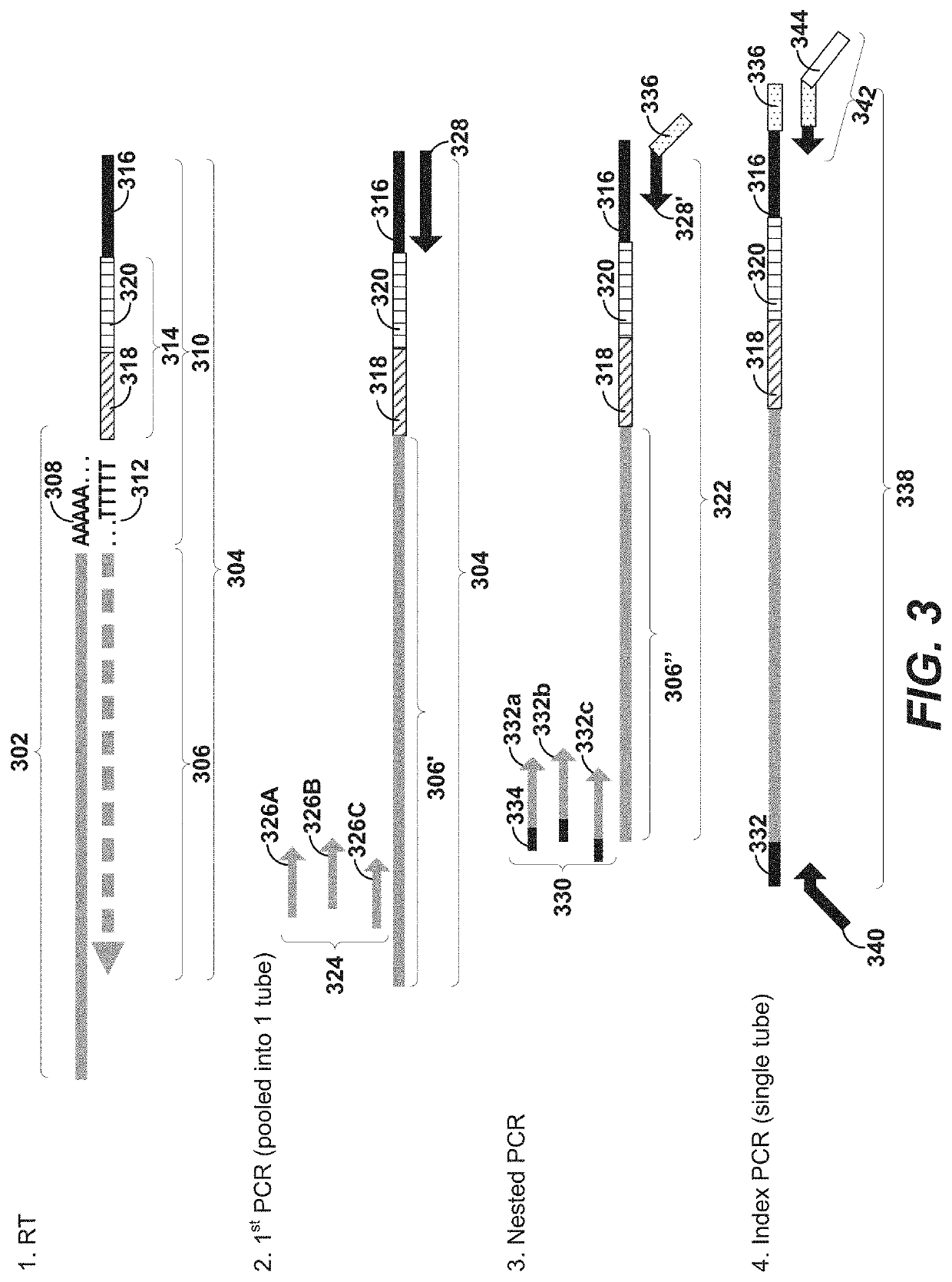
Method for carrying out high-throughput sequencing on TCR (T cell receptor) or BCR (B cell receptor) and method for correcting multiplex PCR (polymerase chain reaction) primer deviation by utilizing tag sequences
InactiveCN103710454AFully functionalReduce sequencing errorsMicrobiological testing/measurementDNA/RNA fragmentationV regionPcr ctpp
The invention provides a method for carrying out high-throughput sequencing on a TCR (T cell receptor) or a BCR (B cell receptor). The method is characterized by designing upstream primers according to gene features of a V region of the TCR or the BCR and designing downstream primers according to gene features of a C region or a J region of the TCR or the BCR and obtaining sequences of the of the TCR or the BCR in combination with the multiplex PCR (polymerase chain reaction) technology and high-throughput sequencing, thus analyzing the rearrangement information of the TCR or the BCR. Compared with 25-30 cycles of existing multiplex PCR, two cycles of the multiplex PCR technology provided by the invention can conduce to greatly reducing the sequencing errors caused by primer amplification preference. Besides, the invention also provides a method for correcting multiplex PCR (polymerase chain reaction) primer deviation by utilizing DNA (deoxyribonucleic acid) tag sequences, thus further reducing the sequencing errors caused by primer amplification preference and intrinsic sequencing errors of high-throughput sequencing.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA +1
Antibody having a T-cell receptor-like specificity, yet higher affinity, and the use of same in the detection and treatment of cancer, viral infection and autoimmune disease
InactiveUS6992176B2Antibody mimetics/scaffoldsMicrobiological testing/measurementDiseaseAutoimmune disease
An isolated molecule which comprises an antibody specifically bindable with a binding affinity below 20 nanomolar, preferably below 10 nanomolar, to a human major histocompatibility complex (MHC) class I being complexed with a HLA-restricted antigen and optionally further comprises an identifiable or therapeutic moiety conjugated to the antibody.
Owner:TECHNION RES & DEV FOUND LTD
High affinity t cell receptor and use thereof
ActiveUS20110280889A1High affinityEffective treatmentPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseT cell
The present invention is directed to a high affinity T cell receptor (TCR) against a tumor-associated antigen, an isolated nucleic acid molecule encoding same, a T cell expressing said TCR, and a pharmaceutical composition for use in the treatment of diseases involving malignant cells expressing said tumor-associated antigen.
Owner:MAX DELBRUECK CENT FUER MOLEKULARE MEDIZIN
Her2/neu specific t cell receptors
InactiveUS20110280894A1Avoid disadvantagesBiocidePeptide/protein ingredientsMalignancyB-cell receptor
The present invention is directed to T cell receptors (TCR) recognizing antigenic peptides derived from Her2 / neu, in particular peptide 369, and being capable of inducing peptide specific killing of a target cell overexpressing HER2 / neu. The present invention is further directed to an antigen specific T cell, comprising said TCR, to a nucleic acid coding for said TCR and to the use of the antigen specific T cells for the manufacture of a medicament for the treatment of malignancies characterized by overexpression of HER2 / neu. The present invention is further disclosing a method of generating antigen specific T cells.
Owner:HELMHOLTZ ZENT MUNCHEN DEUTES FORSCHUNGSZENT FUR GESUNDHEIT & UMWELT
Novel t cell receptors and immune therapy using the same
ActiveUS20180161396A1Immunoglobulin superfamilyTumor rejection antigen precursorsDiseaseImmune therapy
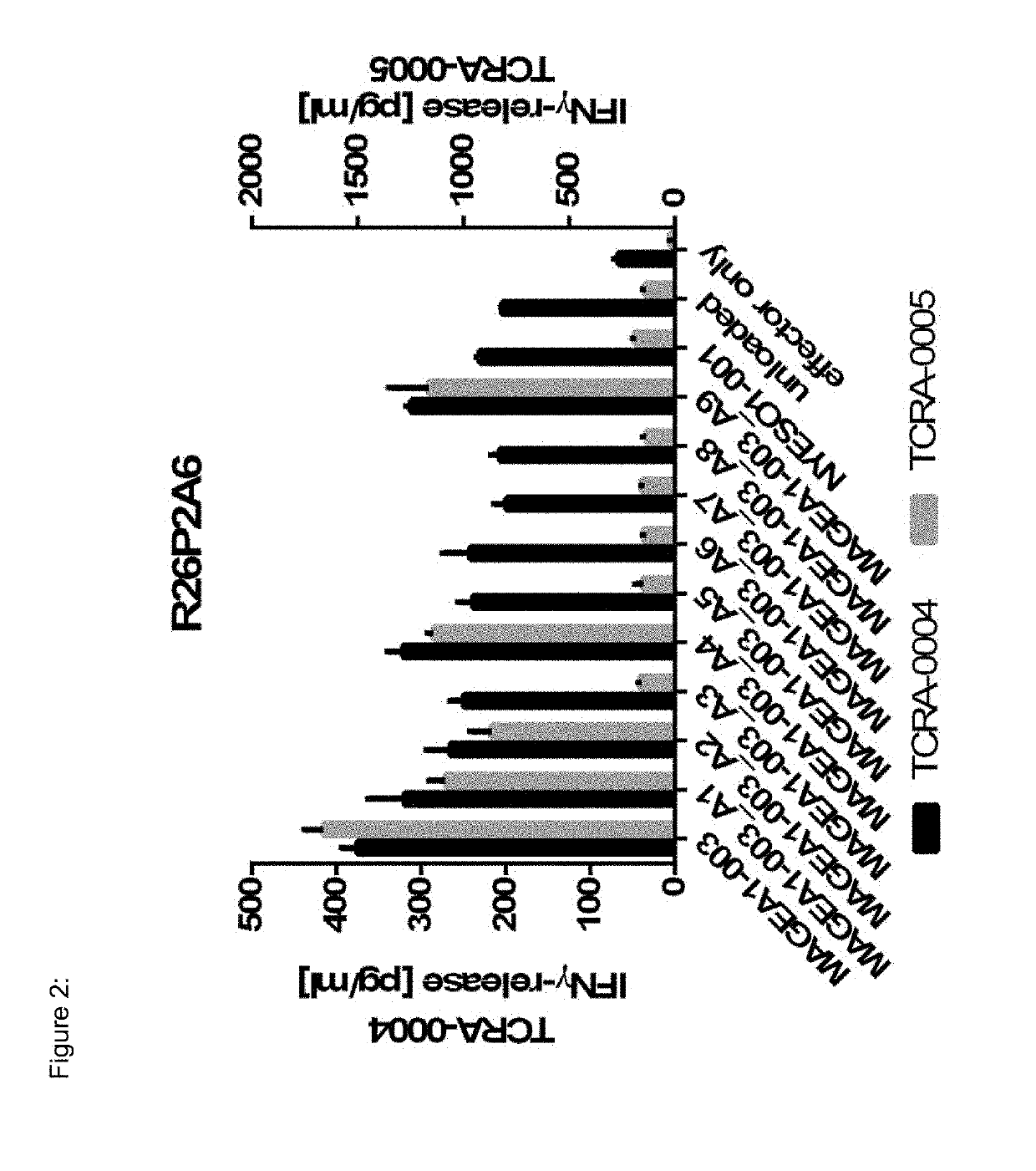
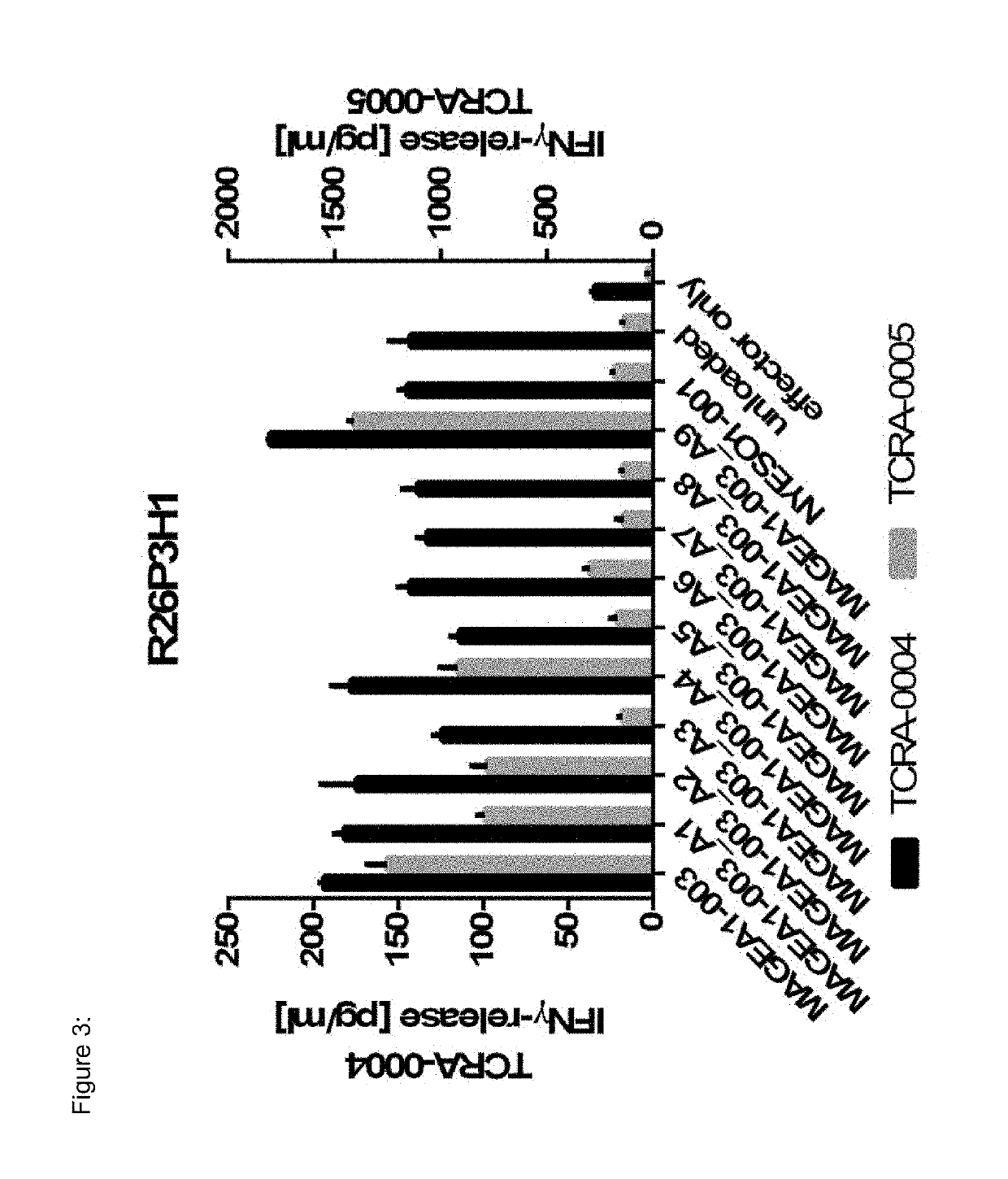
The present invention pertains to antigen recognizing constructs against tumor associated antigens (MAGEA1). The invention in particular provides novel T cell receptor (TCR) based molecules which are selective and specific for the tumor expressed antigen of the invention. The TCR of the invention, and TAA binding fragments derived therefrom, are of use for the diagnosis, treatment and prevention of TAA expressing cancerous diseases. Further provided are nucleic acids encoding the antigen recognizing constructs of the invention, vectors comprising these nucleic acids, recombinant cells expressing the antigen recognizing constructs and pharmaceutical compositions comprising the compounds of the invention.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Novel t cell receptors and immune therapy using the same
ActiveUS20190321478A1Tumor rejection antigen precursorsPeptide/protein ingredientsDiseaseImmune therapy
The present invention pertains to antigen recognizing constructs against tumor associated antigens (MAGEA1). The invention in particular provides novel T cell receptor (TCR) based molecules which are selective and specific for the tumor expressed antigen of the invention. The TCR of the invention, and TAA binding fragments derived therefrom, are of use for the diagnosis, treatment and prevention of TAA expressing cancerous diseases. Further provided are nucleic acids encoding the antigen recognizing constructs of the invention, vectors comprising these nucleic acids, recombinant cells expressing the antigen recognizing constructs and pharmaceutical compositions comprising the compounds of the invention.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
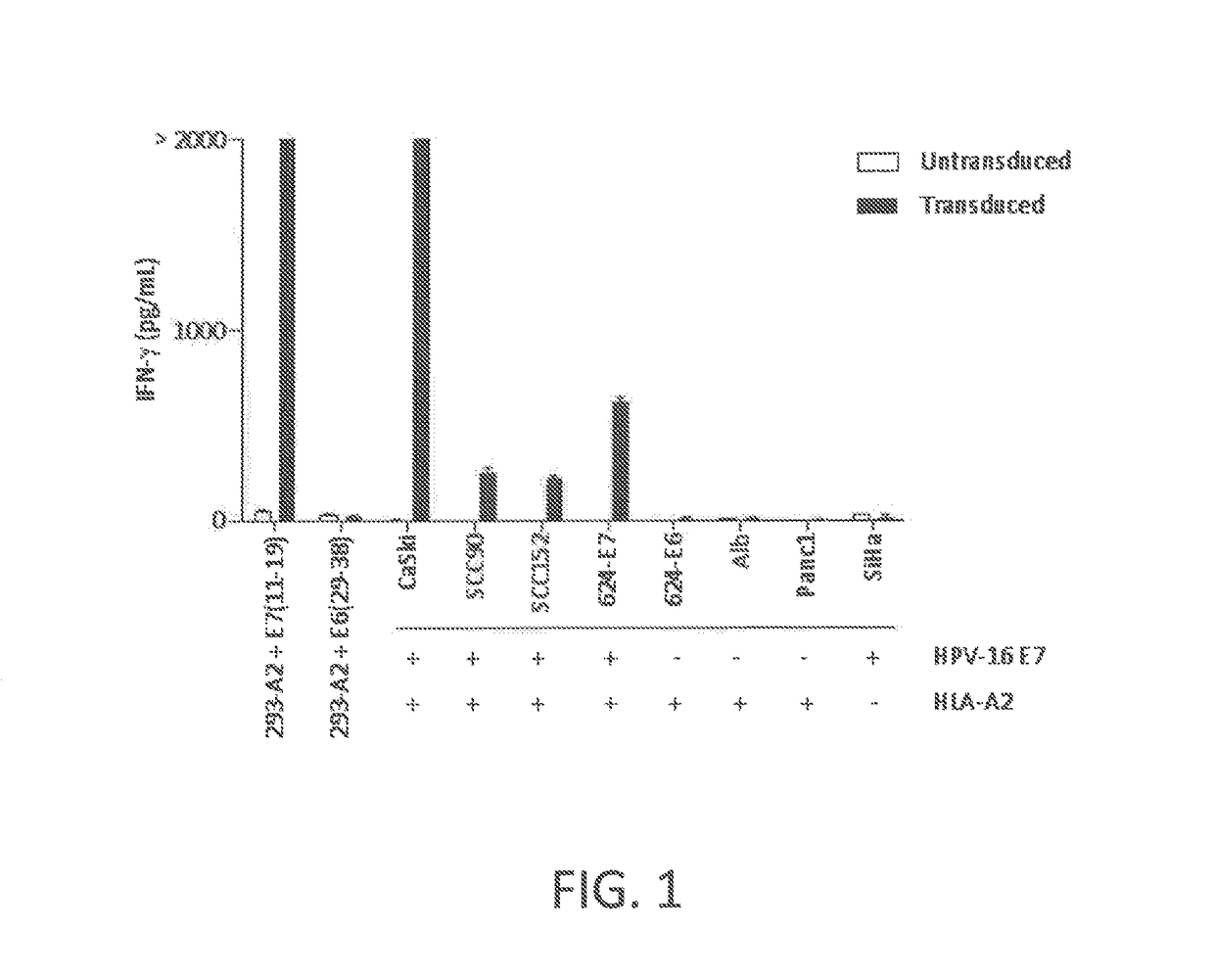
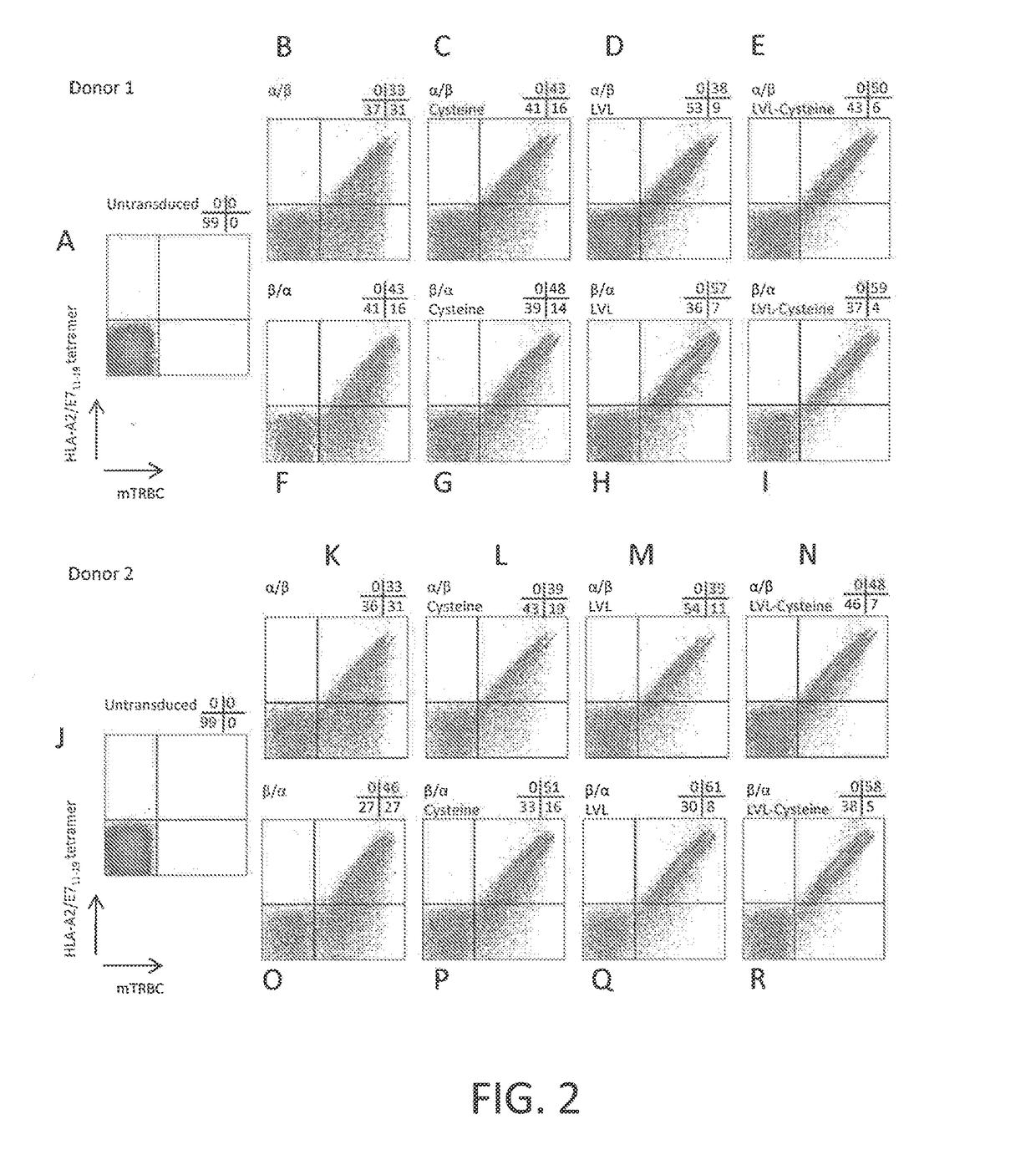
Anti-human papillomavirus 16 e7 t cell receptors
ActiveUS20170145070A1Minimize ToxicityImprove abilitiesPeptide/protein ingredientsAntibody mimetics/scaffoldsEpitopeHuman papillomavirus
Disclosed is a synthetic T cell receptor (TCR) having antigenic specificity for an HLA-A2-restricted epitope of human papillomavirus (HPV) 16 E7, E711-19. Related polypeptides and proteins, as well as related nucleic acids, recombinant expression vectors, host cells, and populations of cells are also provided. Antibodies, or an antigen binding portion thereof, and pharmaceutical compositions relating to the TCRs of the invention are also provided. Also disclosed are methods of detecting the presence of a condition in a mammal and methods of treating or preventing a condition in a mammal, wherein the condition is cancer, HPV 16 infection, or HPV-positive premalignancy.
Owner:UNITED STATES OF AMERICA
Anti-human papillomavirus 16 e6 t cell receptors
ActiveUS20160152681A1Highly avid recognitionMinimize destructionPeptide/protein ingredientsAntibody mimetics/scaffoldsEpitopeHuman papillomavirus
Disclosed is a T cell receptor (TCR) having antigenic specificity for an HLA-A2-restricted epitope of human papillomavirus (HPV) 16 E6, E629-38. Related polypeptides and proteins, as well as related nucleic acids, recombinant expression vectors, host cells, and populations of cells are also provided. Antibodies, or an antigen binding portion thereof, and pharmaceutical compositions relating to the TCRs of the invention are also provided. Also disclosed are methods of detecting the presence of a condition in a mammal and methods of treating or preventing a condition in a mammal, wherein the condition is cancer, HPV 16 infection, or HPV-positive premalignancy.
Owner:UNITED STATES OF AMERICA
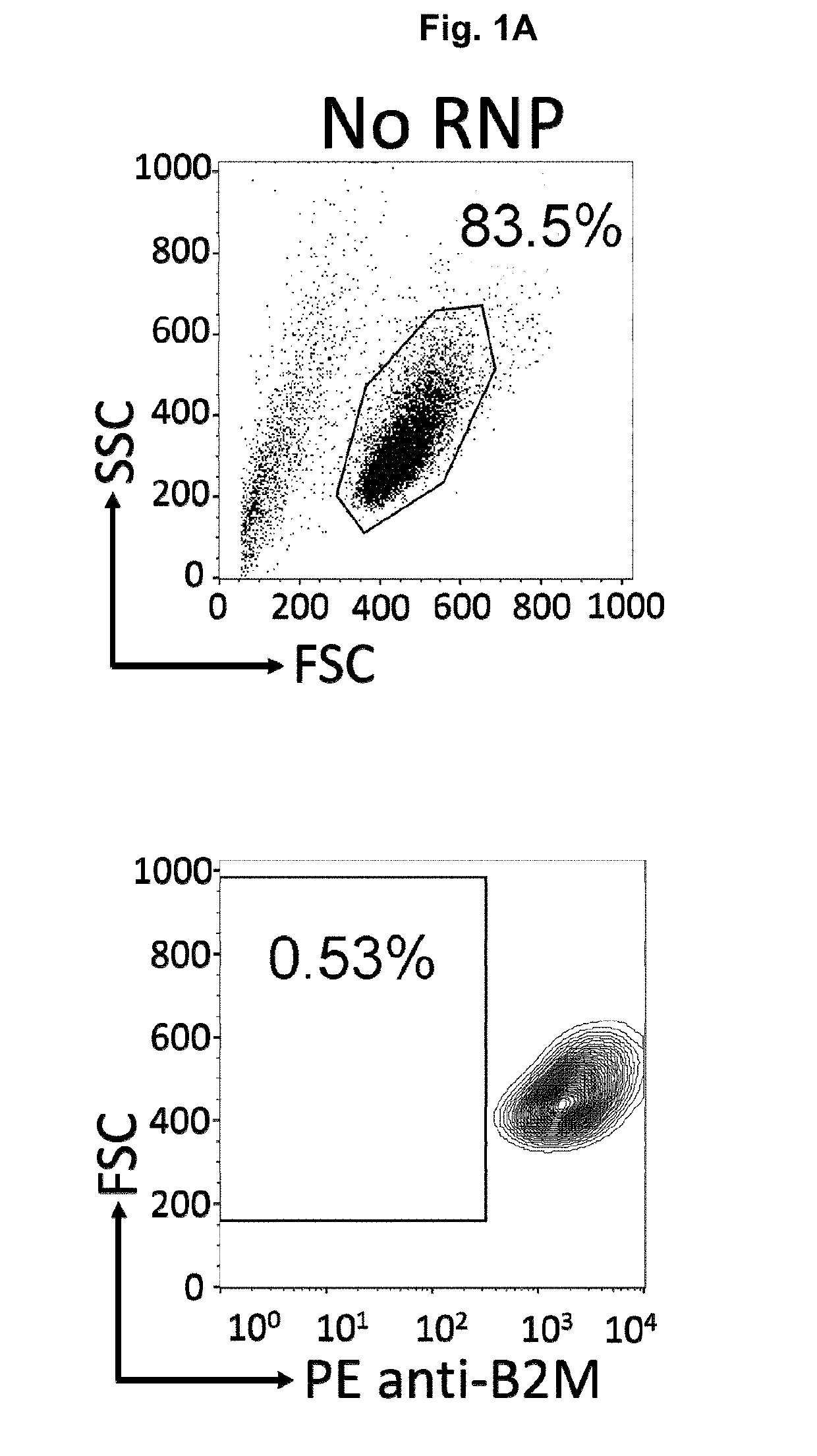
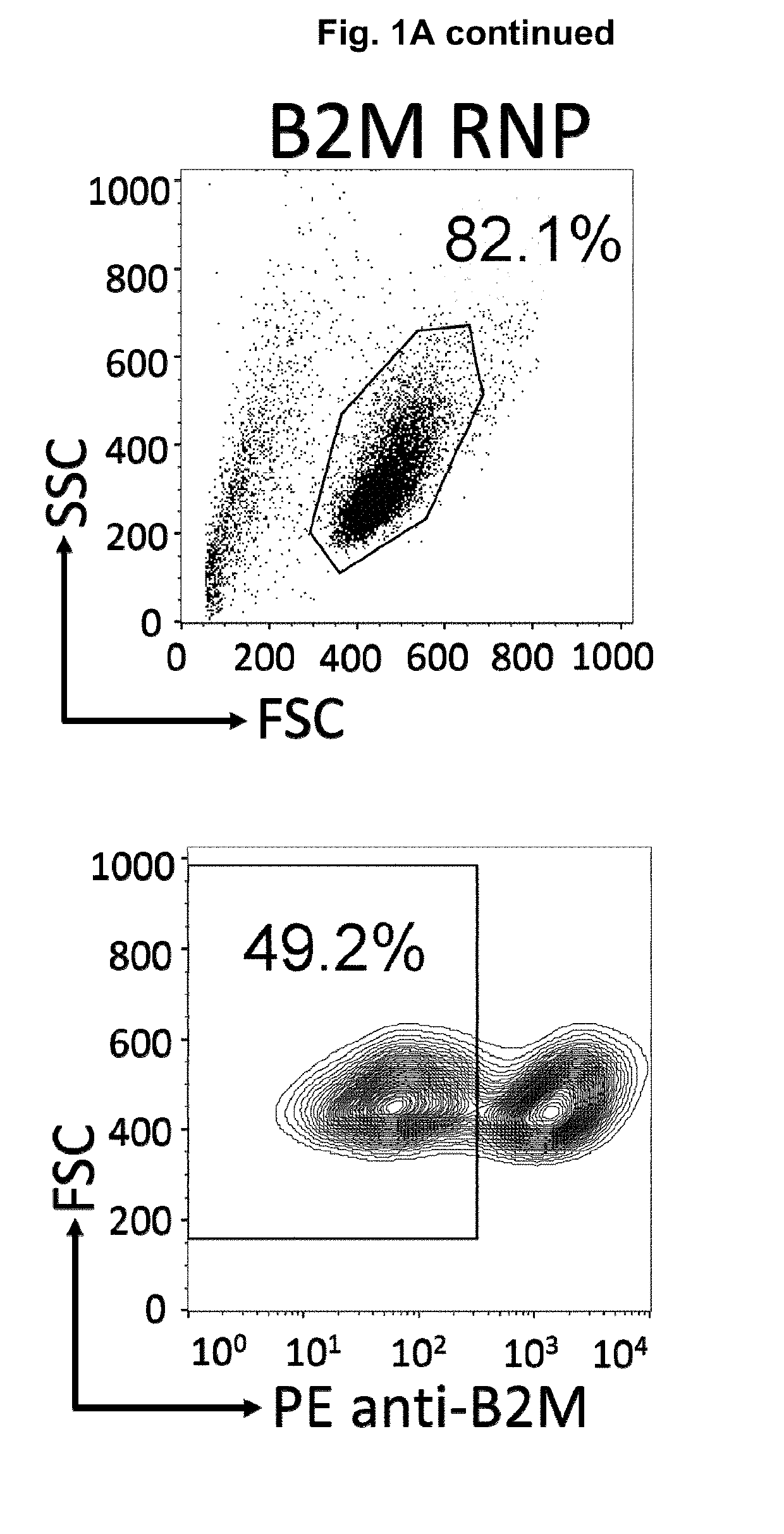
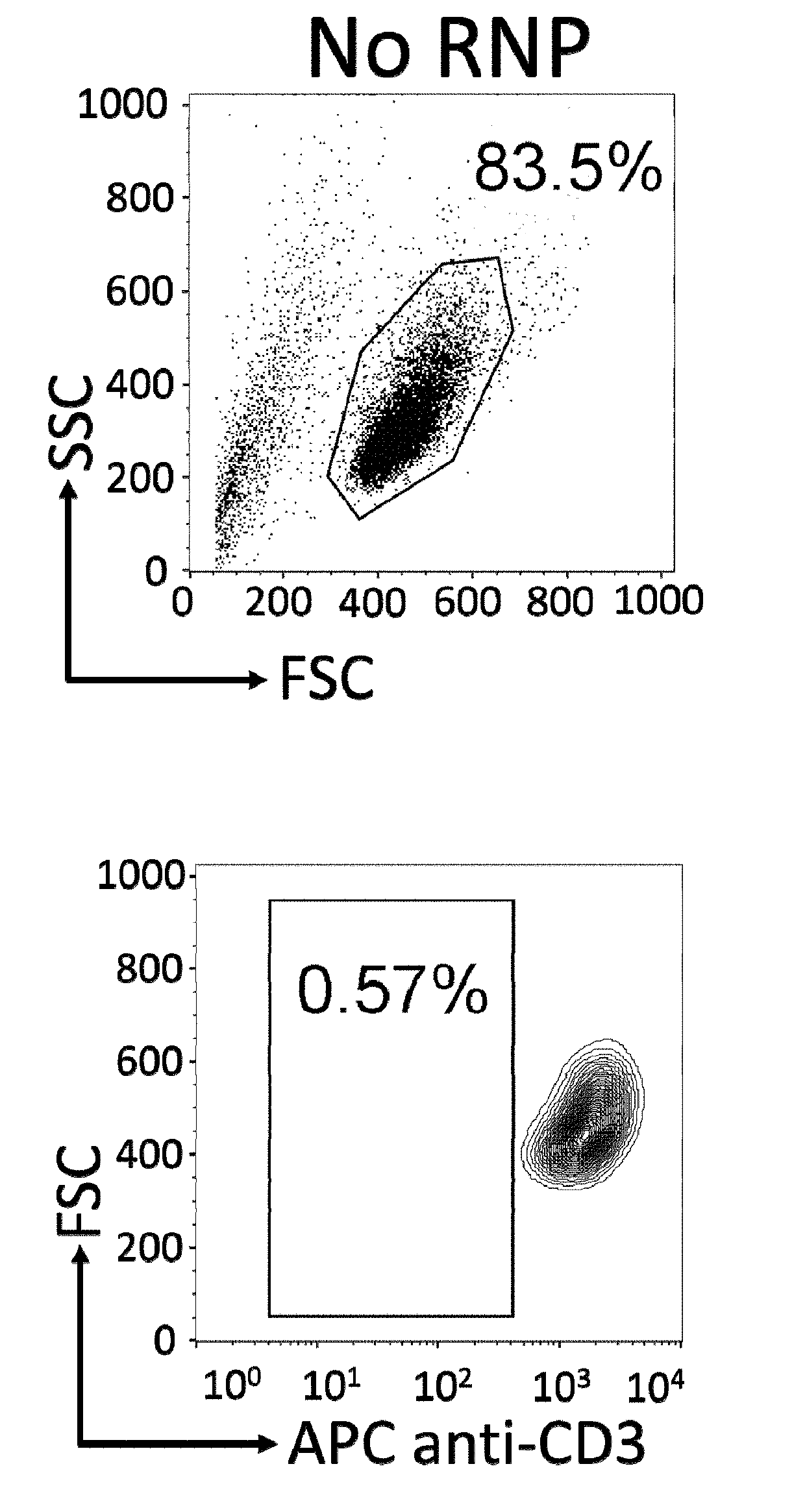
Immortalized car-t cells genetically modified to elminate t-cell receptor and beta 2-microglobulin expression
InactiveUS20190175651A1None is suitable for purposeTumor rejection antigen precursorsGenetic material ingredientsT cellAuto immune disease
The present invention pertains to engineered immortalized T-cell lines, method for their preparation and their use as medicament, particularly for immunotherapy. The engineered immortalized T-cell lines of the invention are characterized in that the expression of endogenous T-cell receptors (TCRs) and beta 2-microglobulin (B2M) is inhibited, e.g., by using an endonuclease able to selectively inactivate the TCR and B2M genes in order to render the immortalized T-cells non-alloreactive. In addition, expression of immunosuppressive polypeptide can be performed on those engineered immortalized T-cells in order to prolong the survival of these T-cells in host organisms. Such engineered immortalized T-cells are particularly suitable for allogeneic transplantations, especially because it reduces both the risk of rejection by the host's immune system and the risk of developing graft versus host disease. The invention opens the way to standard and affordable adoptive immunotherapy strategies using immortalized T-cells for treating cancer, infections and auto-immune diseases.
Owner:JANSSEN BIOTECH INC
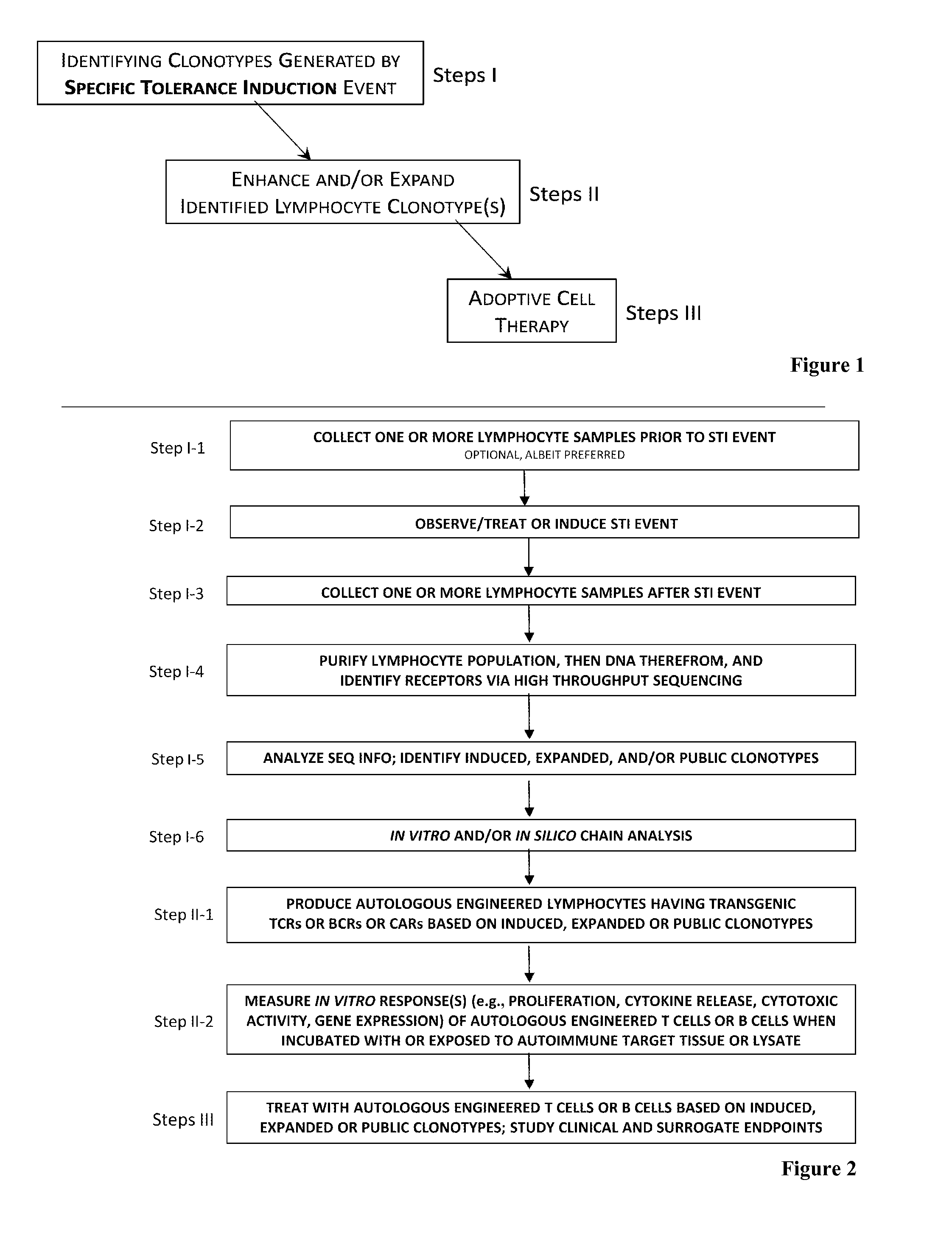
Adoptive cell therapy with specific regulatory lymphocytes
InactiveUS20140356318A1BiocidePeptide/protein ingredientsTolerance inductionAdoptive cellular therapy
The present invention comprises a method of treatment of an autoimmune disease involving a specific tolerance induction (“STI”) event, wherein the method includes: collecting a first sample from the patient prior to the STI event; detecting an STI event or performing a procedure that correlates in time to an STI event; collecting a second sample from the patient after the STI event; preparing lymphocytes from the first and second samples; preparing and sequencing DNA or cDNA from the prepared lymphocytes; identifying sequences of prevalent T or B cell receptors (“prevalent receptor sequences”) among the lymphocytes of the second sample; selecting a regulatory lymphocyte that carries at least one prevalent receptor sequence, which selected regulatory lymphocyte (i) expresses at least one prevalent receptor sequence or (ii) is generated from an autologous or allogeneic naïve lymphocyte, which naïve lymphocyte is engineered and induced to become a regulatory lymphocyte that expresses at least one prevalent receptor sequence; culturing the selected regulatory lymphocyte, thereby generating daughter cells of said regulatory lymphocyte; and administering the daughter cells to the patient.
Owner:BARKEN ISRAEL
Method of making a vaccine
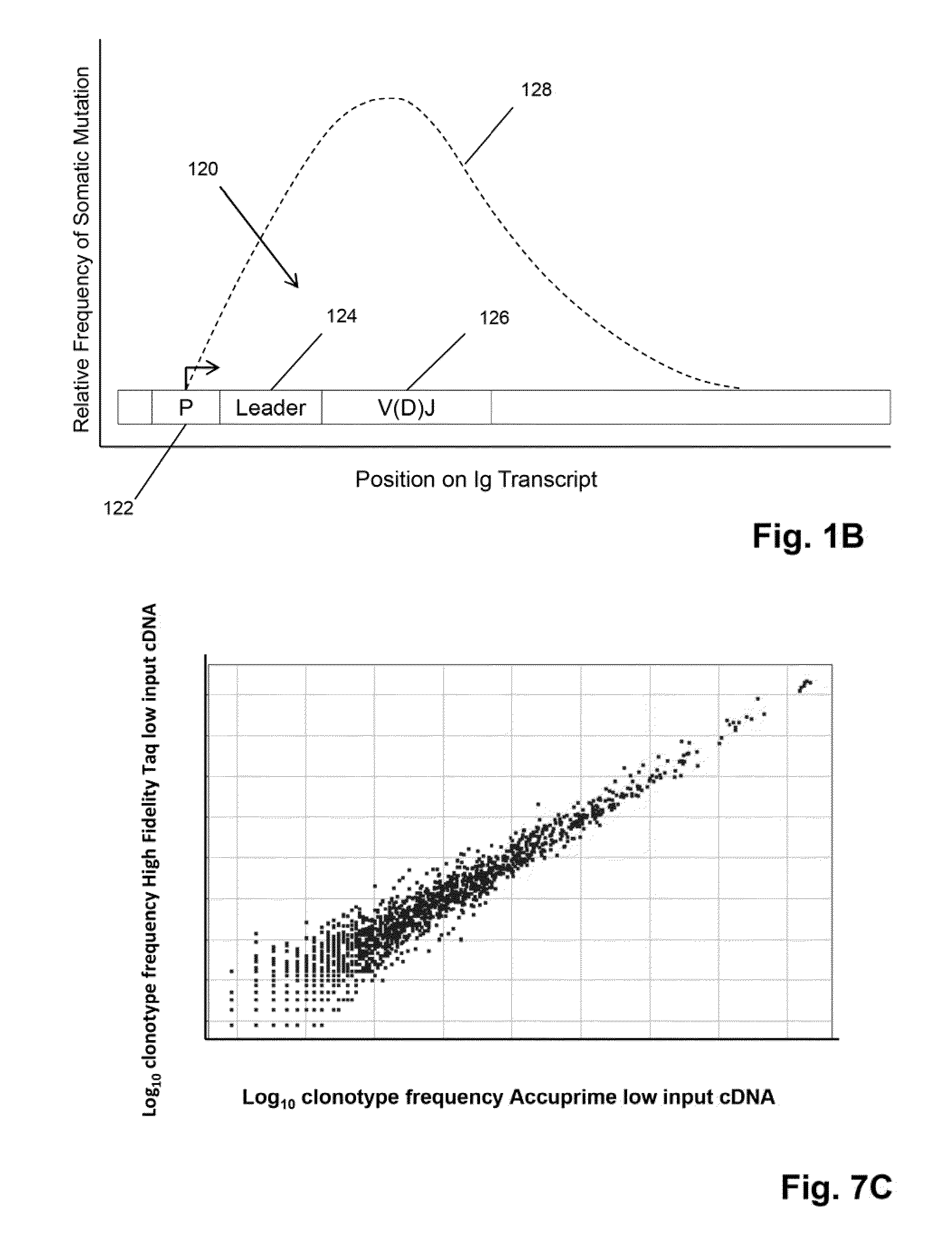
The present invention provides a vaccine and method for making same which is effective to elicit a desired antibody against a target antigen comprising a primary immunogen and a secondary immunogen, wherein the primary immunogen is effective to elicit B cell receptors (BCRs) that are on the maturational pathway of the desired antibody and have an intermediate degree of somatic mutational diversity, and the secondary immunogen comprises an epitope of the desired target antibody and is effective to further diversify the BCRs sufficient to form mature BCRs having the identical or substantially identical sequence as the desired antibody.
Owner:UNITED STATES OF AMERICA +1
Primers for immune repertoire profiling
ActiveUS20210355484A1Immunoglobulin superfamilyMicrobiological testing/measurementSingle cell transcriptomeNucleotide
Disclosed herein include systems, methods, compositions, and kits for immune repertoire profiling. There are provided, in some embodiments, primer panels enabling the determination of the nucleotide sequence of the complete variable region of nucleic acids encoding mouse B cell receptor (BCR) and T cell receptor (TCR) polypeptides. In some embodiments, the method comprises single cell transcriptomic analysis.
Owner:BECTON DICKINSON & CO
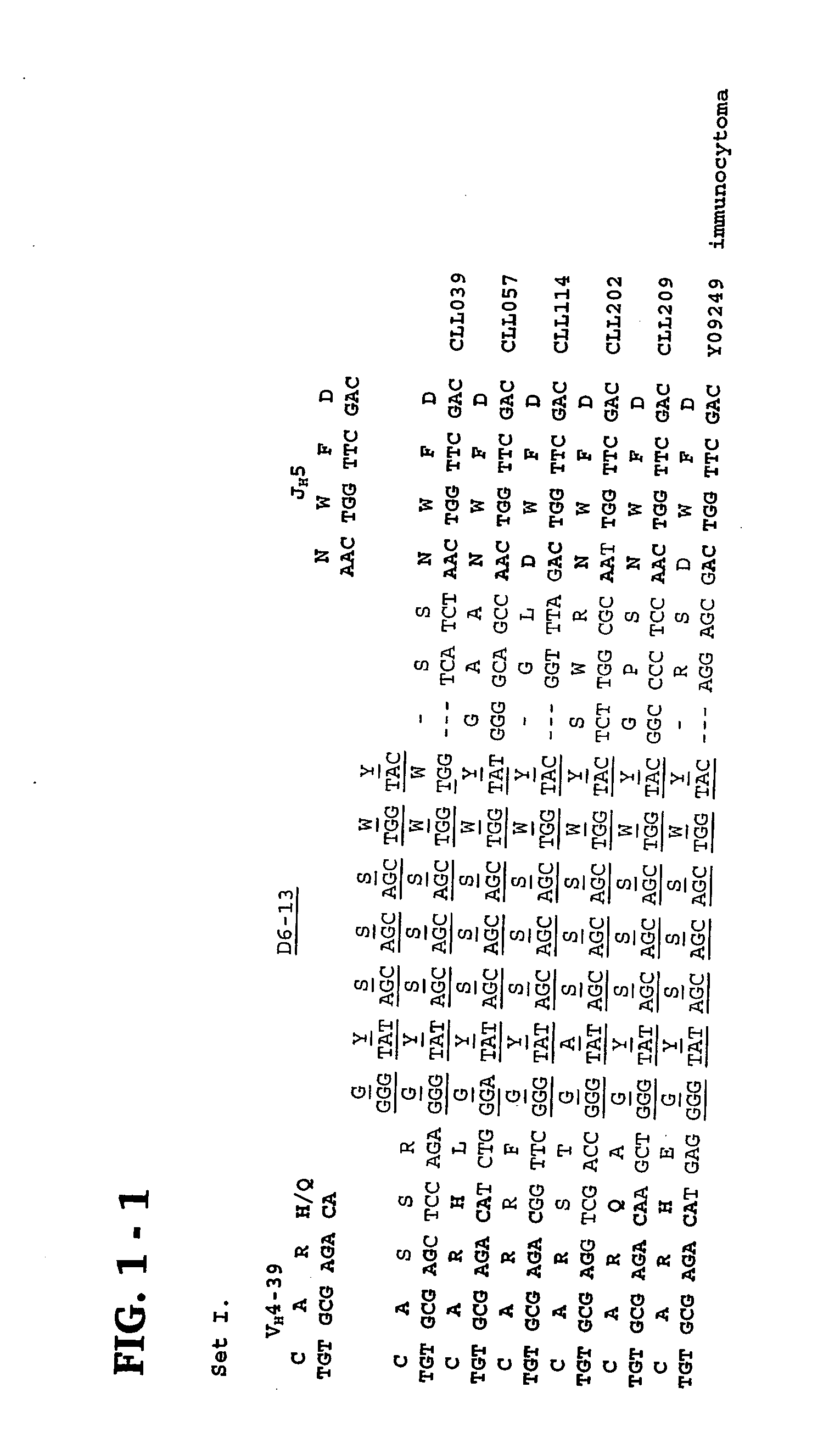
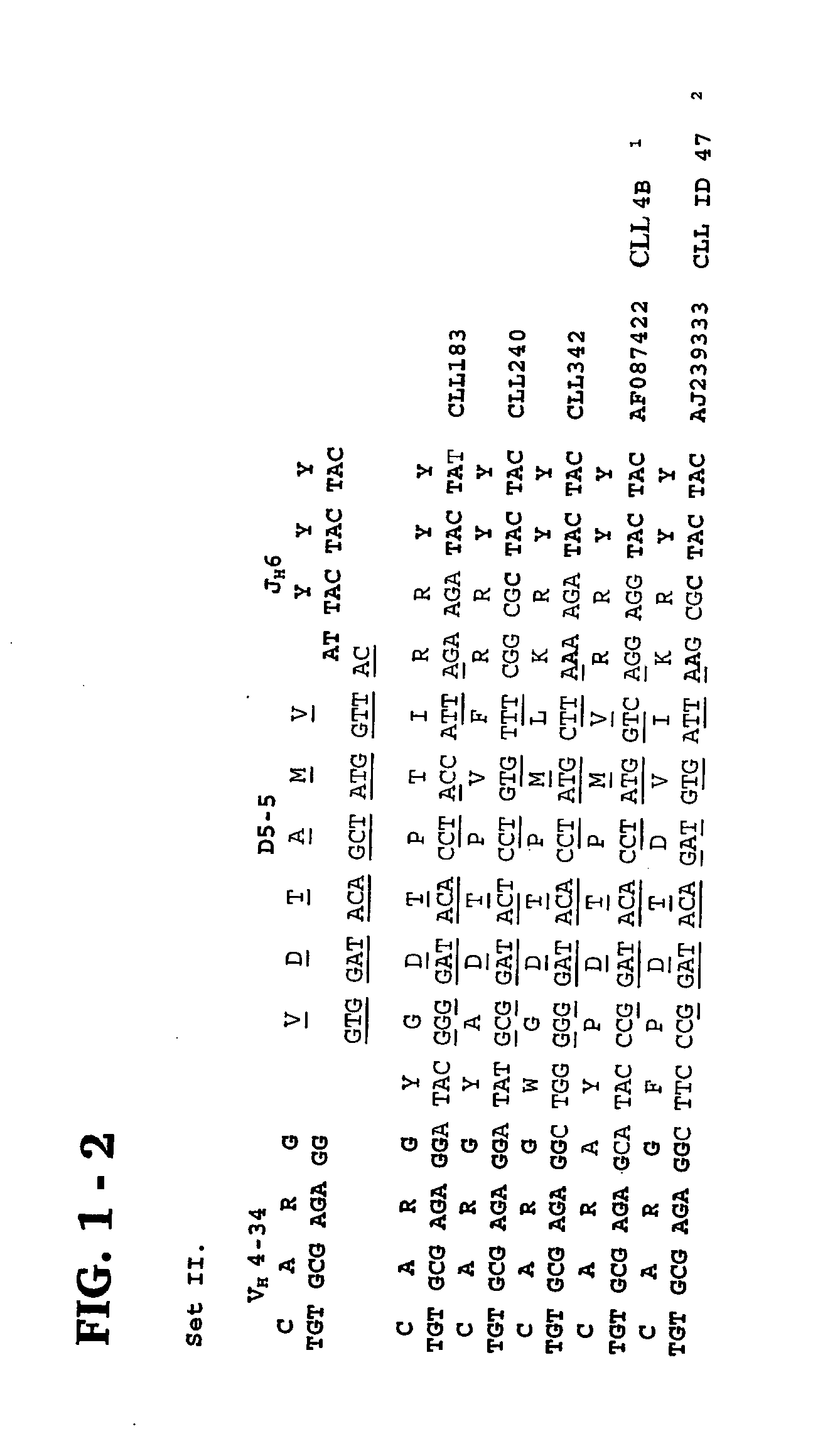
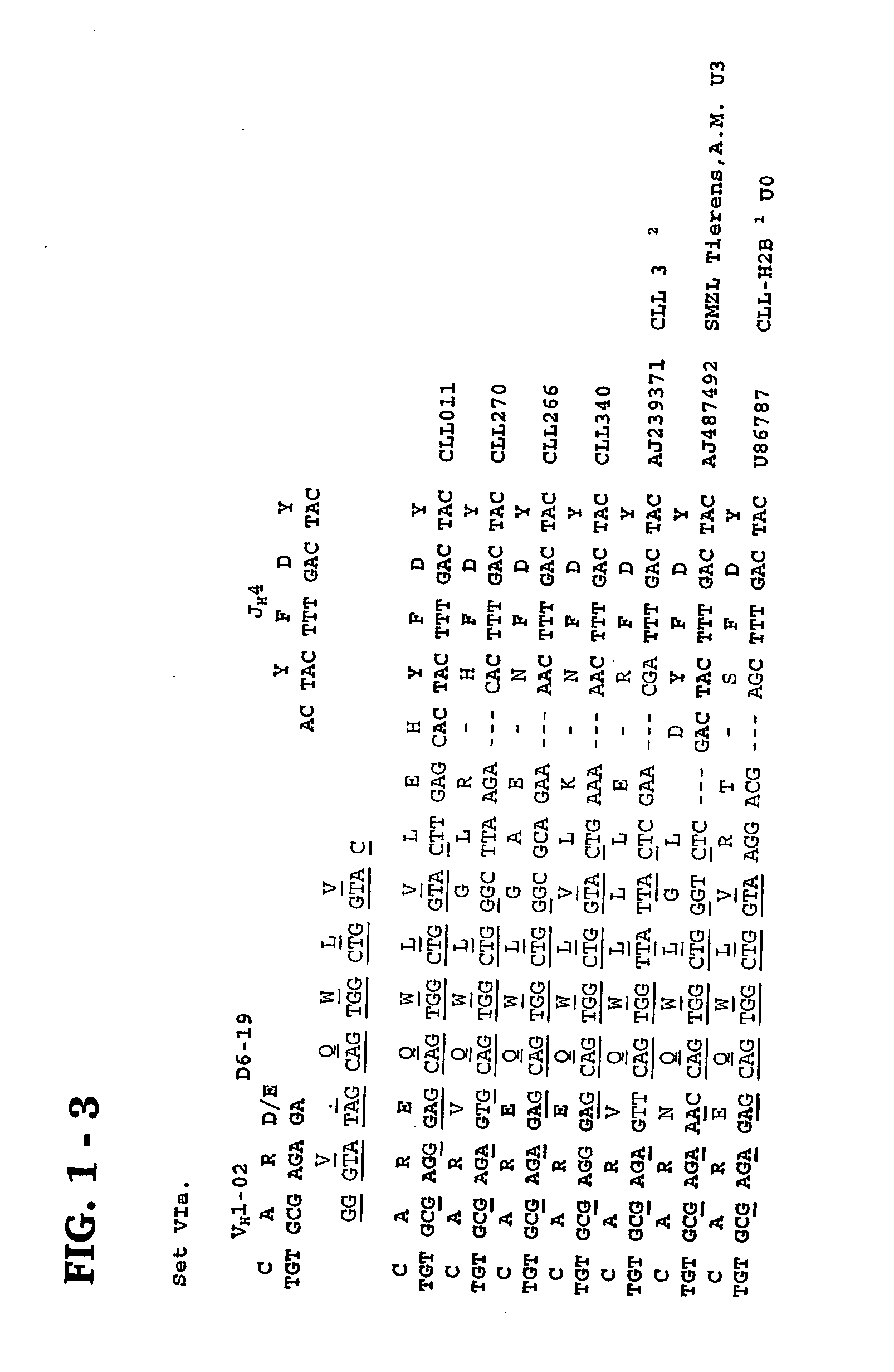
Methods and compositions for diagnosis and treatment of b cell chronic lymphocytic leukemia
Provided are isolated and purified preparations of a combination of a light chain antibody gene and a heavy chain antibody gene, where the light chain and heavy chain antibody genes are the same among more than one patient with B cell chronic lymphocytic leukemia (B-CLL). Vectors comprising those genes and cells comprising those vectors are also provided, as are isolated and purified antibodies encoded by the antibody genes. Anti-idiotype antibodies, peptides, and aptamers that bind to the antigen-binding region of an antibody encoded by the antibody genes are additionally provided, as are multimeric molecules comprising multiple binding sites that bind to the antigen-binding region of an antibody encoded by the antibody genes. Methods of determining whether a patient with B cell chronic lymphocytic leukemia (B-CLL) has a form of B-CLL that is susceptible to treatment directed to eliminating idiotype specific B cell receptor-bearing B-CLL cells are also provided, as are methods of following the progression of treatment of B-CLL in the patient. Additionally, methods of treating a patient having B-CLL are provided, as are methods of identifying a therapeutic agent for B-CLL.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Yeast cell surface display of proteins and uses thereof
InactiveUS20090280560A1Effectively mimickedHigh affinityFungiSaccharide peptide ingredientsSurface displayAgglutinin-B
The present invention provides a genetic method for tethering polypeptides to the yeast cell wall in a form accessible for binding to macromolecules. Combining this method with fluorescence-activated cell sorting provides a means of selecting proteins with increased or decreased affinity for another molecule, altered specificity, or conditional binding. Also provided is a method for genetic fusion of the N terminus of a polypeptide of interest to the C-terminus of the yeast Aga2p cell wall protein. The outer wall of each yeast cell can display approximately 104 protein agglutinins. The native agglutinins serve as specific adhesion contacts to fuse yeast cells of opposite mating type during mating. In effect, yeast has evolved a platform for protein-protein binding without steric hindrance from cell wall components. As one embodiment, attaching an scFv antibody fragment to the Aga2p agglutinin effectively mimics the cell surface display of antibodies by B cells in the immune system for affinity maturation in vivo. As another embodiment, T cell receptor mutants can be isolated by this method that are efficiently displayed on the yeast cell surface, providing a means of altering T cell receptor binding affinity and specificity by library screening.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com