Human Anti-OPGL Neutralizing Antibodies As Selective OPGL Pathway Inhibitors
a technology of opgl pathway inhibitors and neutralizing antibodies, which is applied in the field of antibodies, can solve the problems of reduced bone mass and strength, slow or incomplete repair of broken bones, and increased risk of fractures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Human Monoclonal Antibodies Against OPGL
Transgenic HuMab Mice
[0231]Fully human monoclonal antibodies to OPGL were prepared using HCo7, HCo12, and HCo7+HCo12 strains of transgenic mice, each of which expresses human antibody genes. In each of these mouse strains, the endogenous mouse kappa light chain gene has been homozygously disrupted (as described in Chen et al., 1993, EMBO J. 12: 811-820) and the endogenous mouse heavy chain gene has been homozygously disrupted as described in Example 1 of PCT Publication WO 01 / 09187 (incorporated by reference). Each of these mouse strains carries a human kappa light chain transgene, KCo5 (as described in Fishwild et al., 1996, Nature Biotechnology 14: 845-851). The HCo7 strain carries the HCo7 human heavy chain transgene as described in U.S. Pat. Nos. 5,545,806; 5,625,825; and 5,545,807 (incorporated by reference). The HCo12 strain carries the HCo12 human heavy chain transgene as described in Example 2 of PCT Publication WO 01 / 091...
example 2
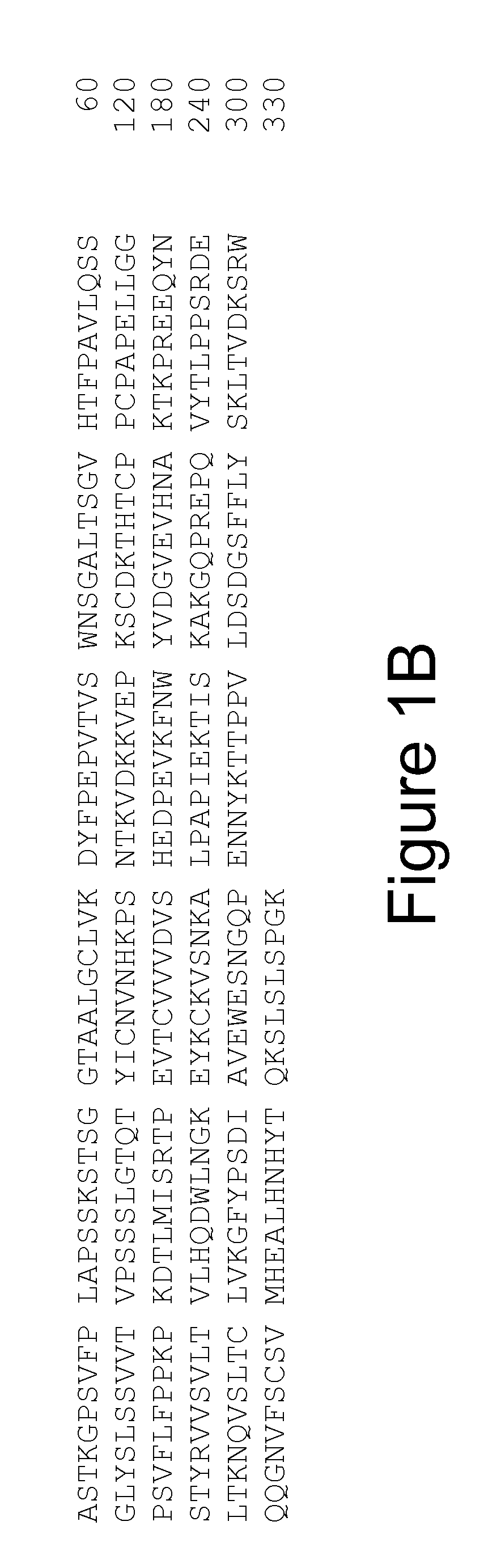
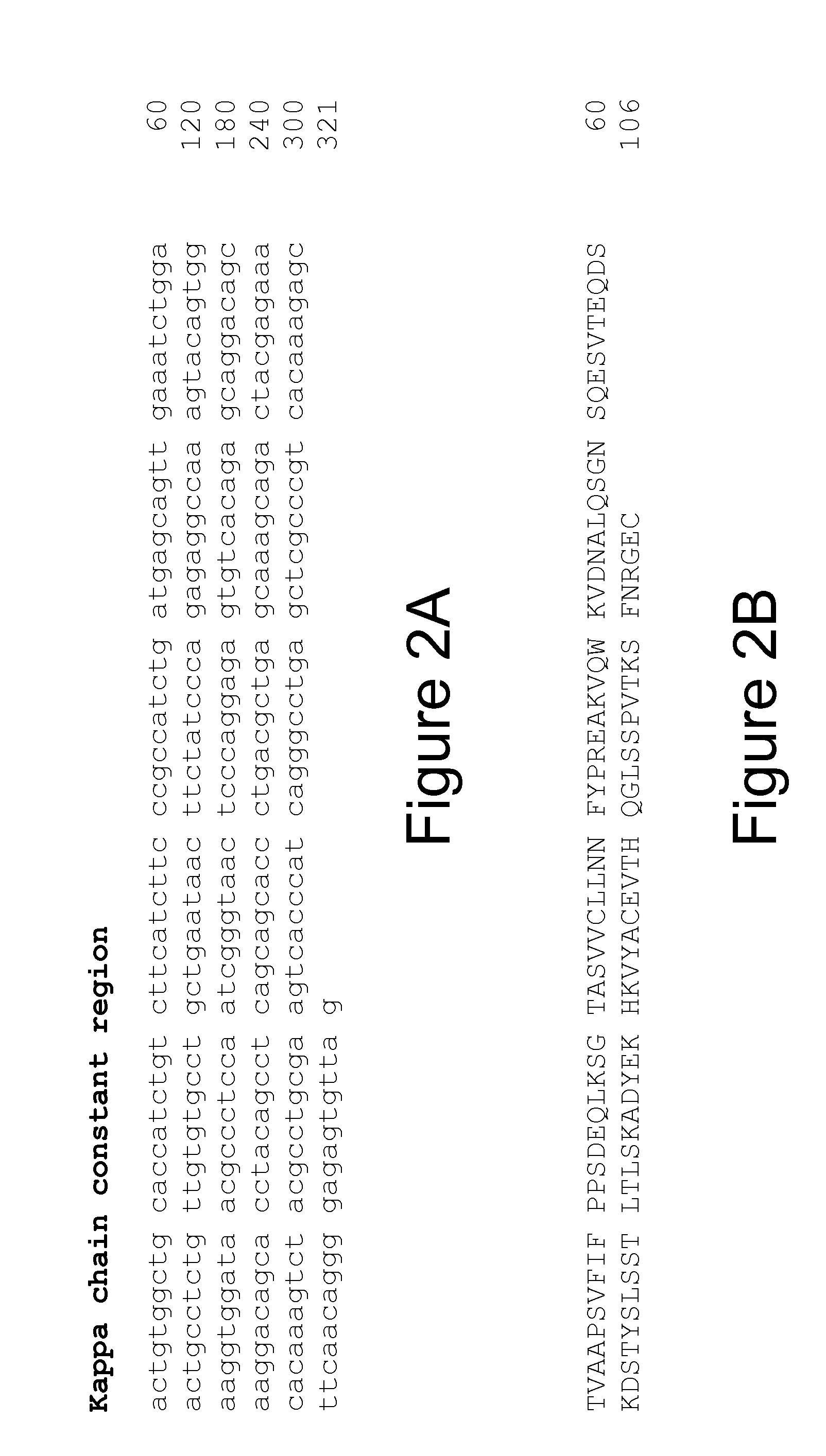
Cloning the 9H7 Anti-OPGL Heavy and Light Chains
[0241]Cloning of the 9H7 anti-OPGL MAb light chain
[0242]Three anti-OPGL hybridoma light chain cDNAs (9H7, 16E1 and 18B2) were cloned into pDSR19 mammalian cell expression vector. The construction of a plasmid encoding the 9H7 kappa light chain is explicitly described; cloning of the other light chain species was performed using similar procedures. The anti-OPGL-9H7 kappa light chain variable region was obtained using polymerase chain reaction (PCR) amplification methods from first strand cDNA prepared from hybridoma 9H7 total RNA. First strand cDNA was prepared from 9H7 total RNA using a random primer with an extended 5′-adapter (5′-GGCCGGATAGGCCTCACNNNNNNT-3′, SEQ ID NO: 53) and the materials and methods provided by the Gibco SuperScript II™ Preamplification System for First Strand cDNA Synthesis kit (Catalogue No. 18089-011). The oligonucleotides below were used for the PCR:
5′ GeneRacer™ (Invitrogen) primer(SEQ ID NO: 54):5′-GGA CAC ...
example 3
9H7 Anti-OPGL MAb Expression in CHO Cells
[0251]Recombinant anti-OPGL antibodies are produced by Chinese hamster ovary cells, specifically CHO AM-1 / D, as disclosed in U.S. Pat. No. 6,210,924 (incorporated by reference). DNA sequences encoding the complete heavy or light chains of each anti-OPGL antibody of the invention are cloned into expression vectors such as those described above. CHO AM-1 / D cells are co-transfected with an expression vector capable of expressing a complete heavy chain and an expression vector expressing the complete light chain of the appropriate anti-OPGL antibody. For example, to generate the 22B3 antibody, cells are co-transfected with a vector capable of expressing a complete heavy chain comprising the amino acid sequence as set forth in SEQ ID NO: 30 and a vector capable of expressing a complete light chain comprising the amino acid sequence set forth in SEQ ID NO: 32. To generate the 2E11 antibody, cells are co-transfected with a vector capable of expressi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dissociation constant | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com