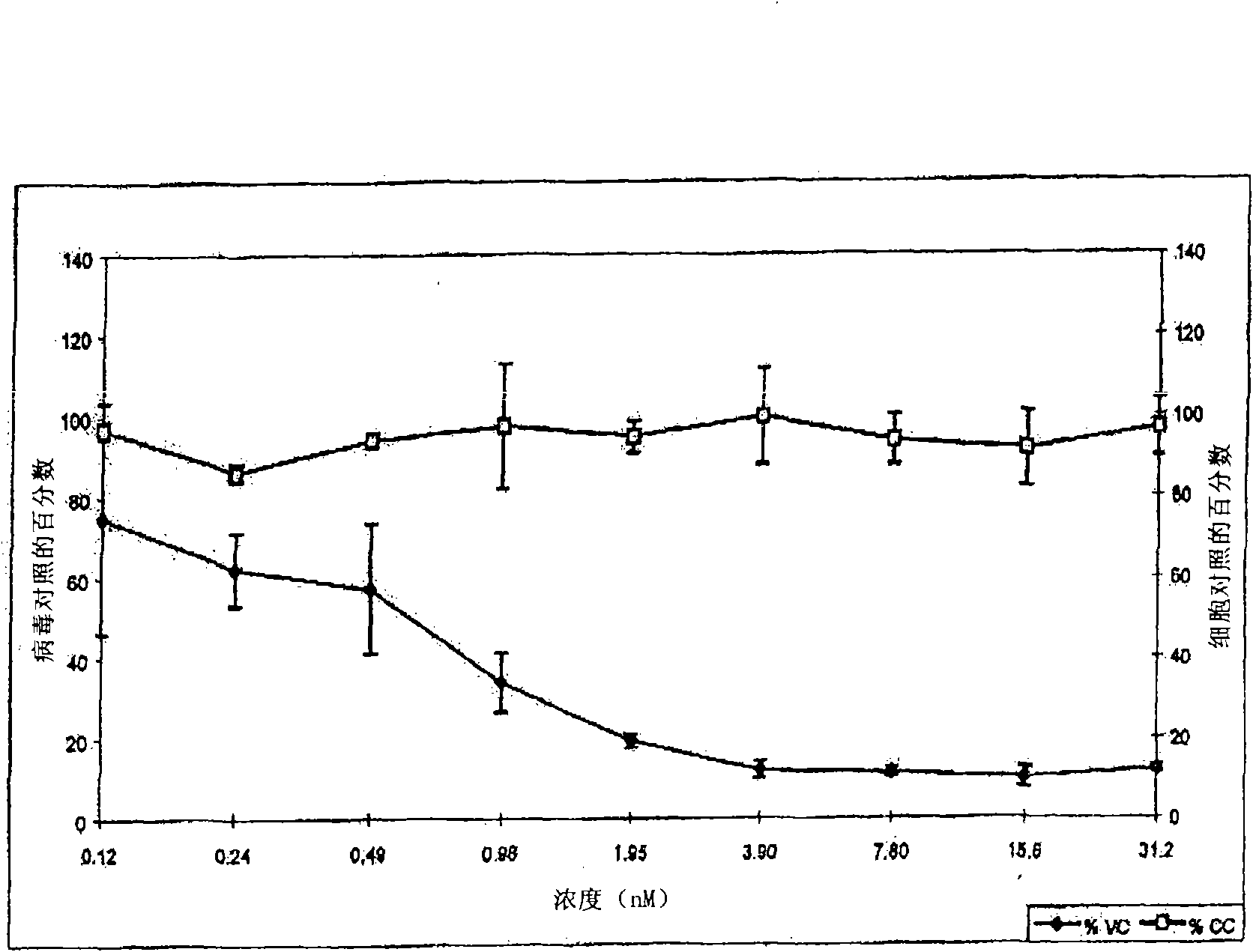
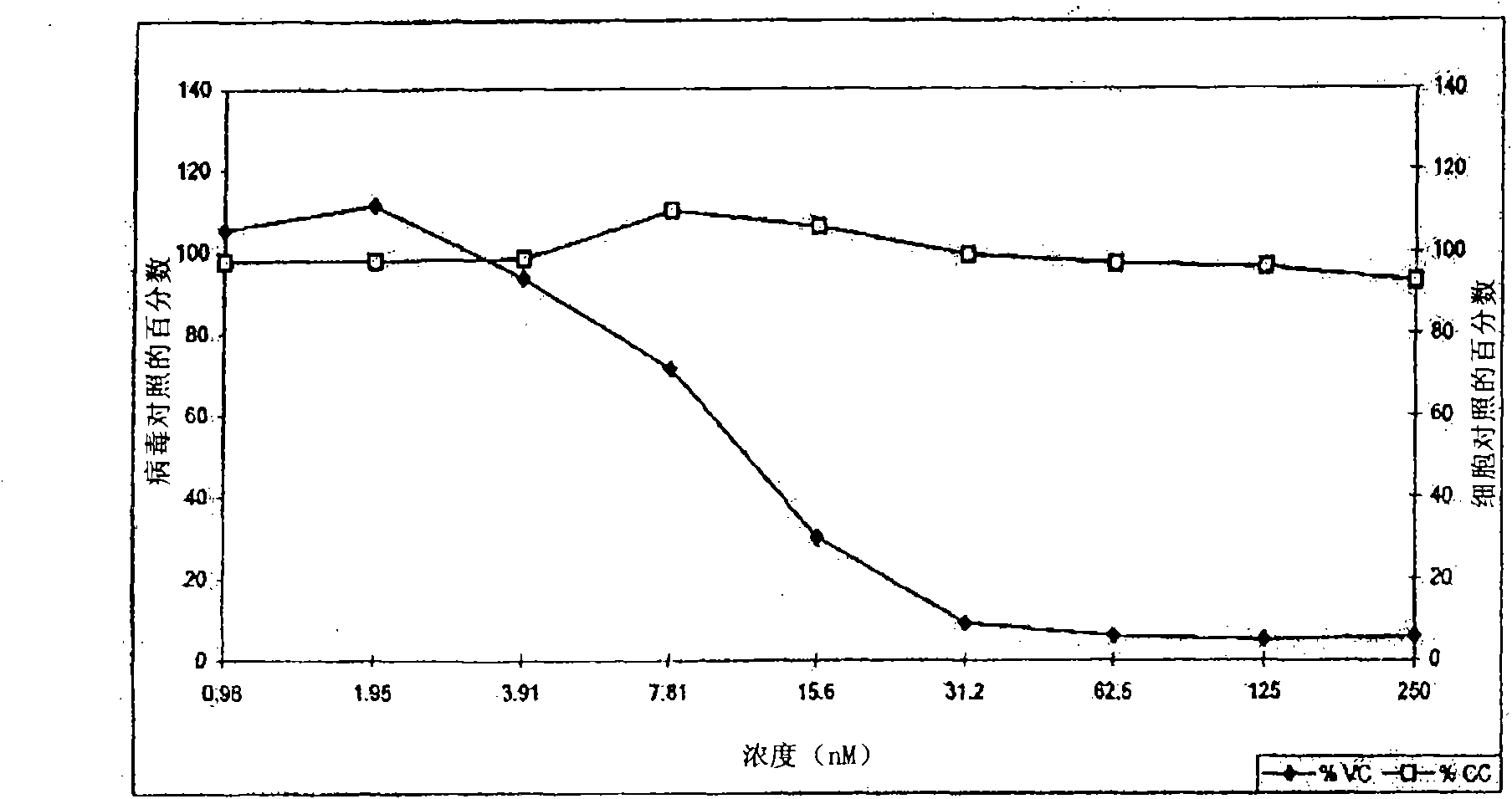
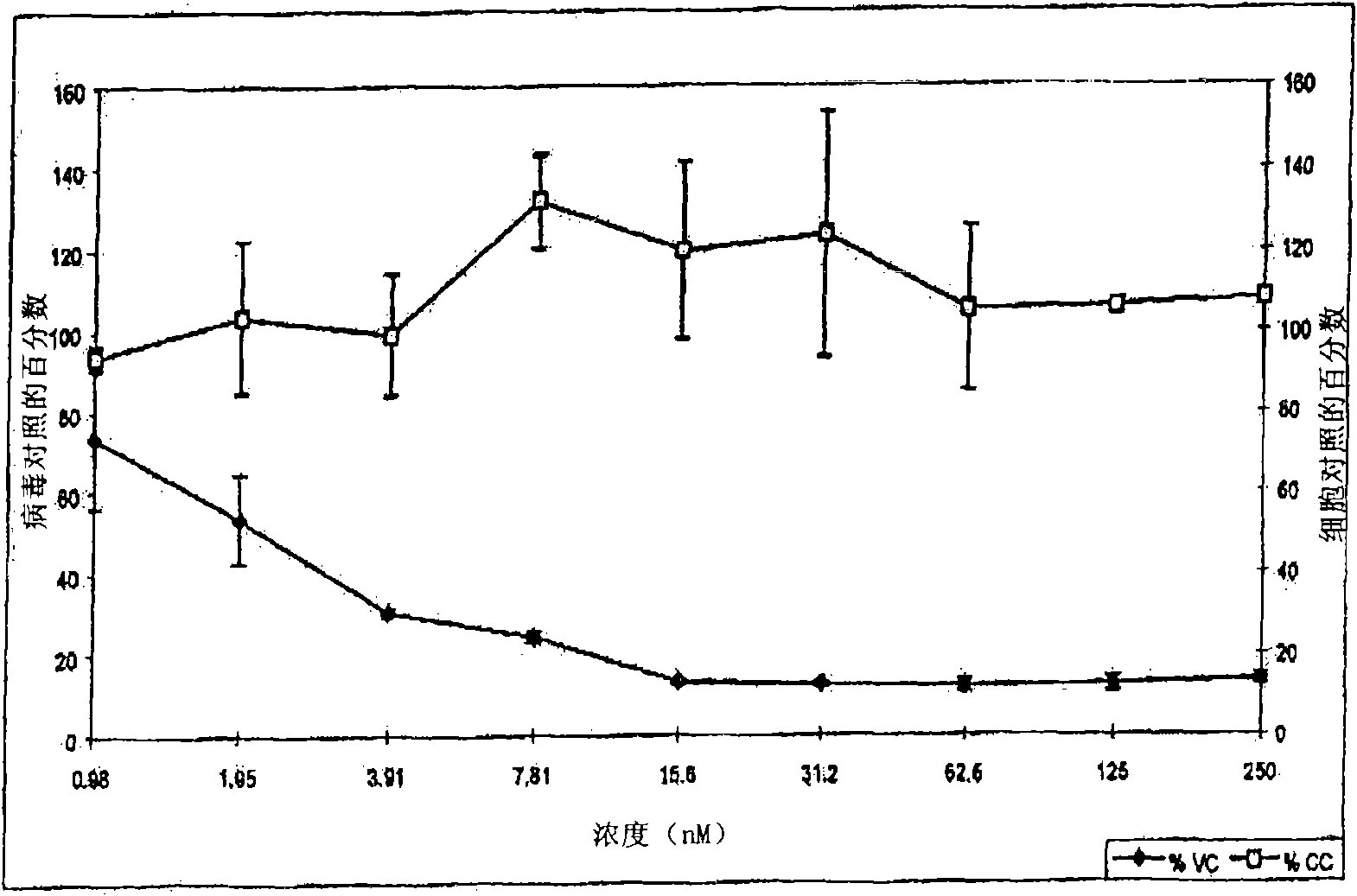
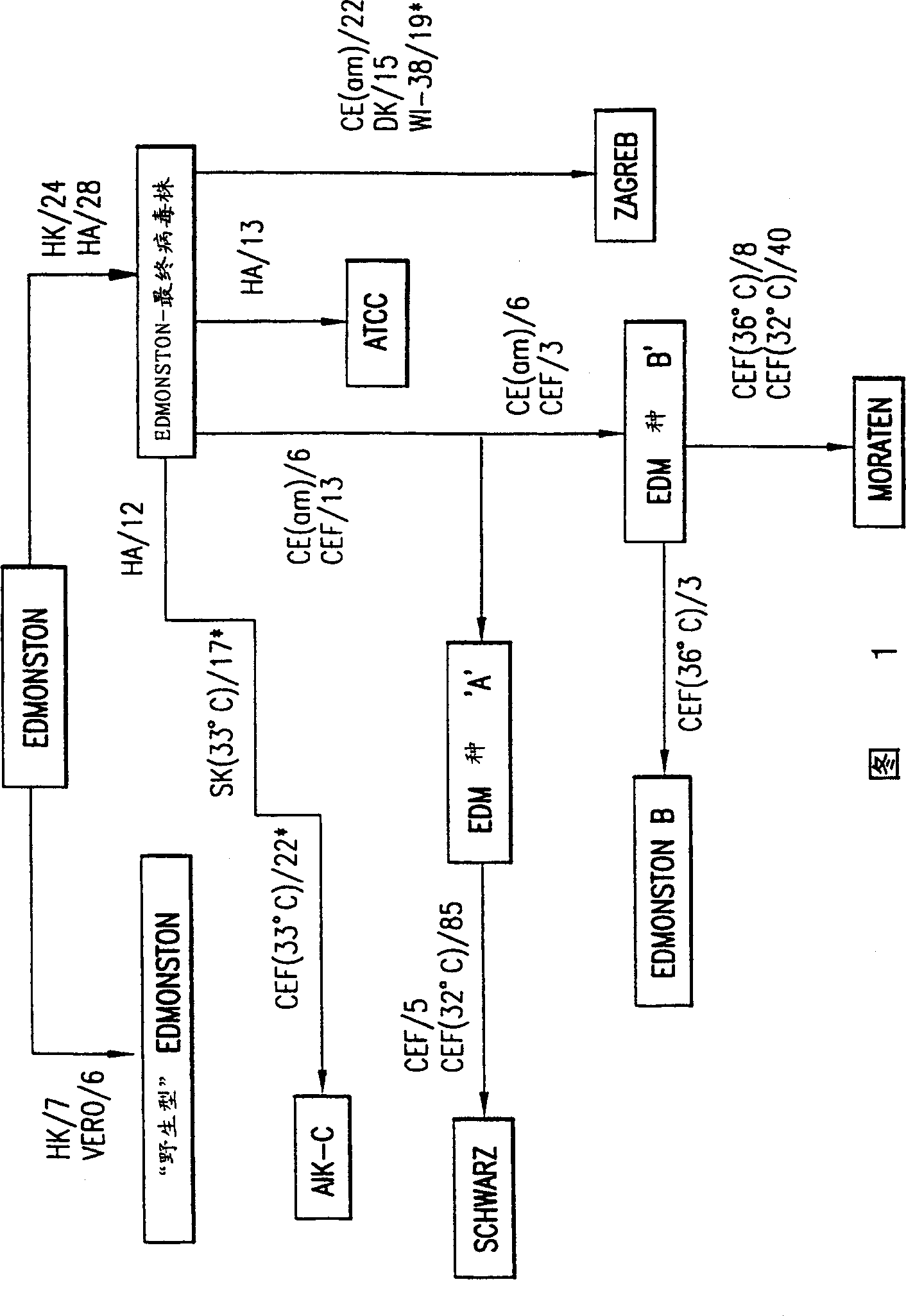
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
63 results about "Measles virus IgG" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Long lasting fusion peptide inhibitors for hiv infection
InactiveUS20050065075A1Prolong half-life in vivoBiocidePeptide/protein ingredientsImmunodeficiency virusHuman Parainfluenza Virus
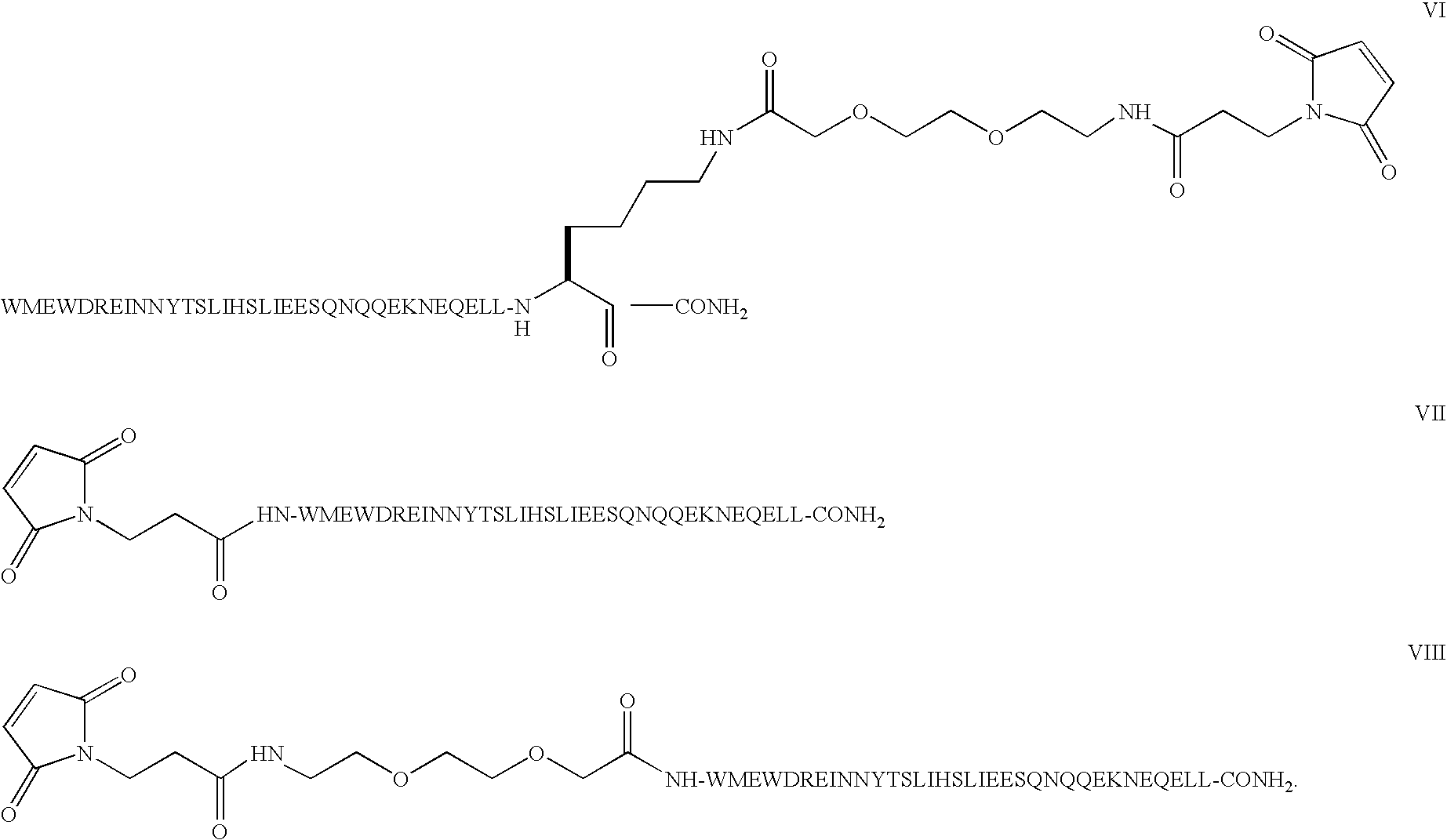
The present invention relates to C34 peptide derivatives that are inhibitors of viral infection and / or exhibit antifusogenic properties. In particular, this invention relates to C34 derivatives having inhibiting activity against human immunodeficiency virus (HIV), respiratory syncytial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) with long duration of action for the treatment of the respective viral infections.
Owner:CONJUCHEM
Long lasting fusion peptide inhibitors for HIV infection
InactiveUS7741453B2Prolong half-life in vivoPeptide/protein ingredientsAntibody mimetics/scaffoldsImmunodeficiency virusHuman Parainfluenza Virus
The present invention relates to C34 peptide derivatives that are inhibitors of viral infection and / or exhibit antifusogenic properties. In particular, this invention relates to C34 derivatives having inhibiting activity against human immunodeficiency virus (HIV), respiratory syncytial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) with long duration of action for the treatment of the respective viral infections.
Owner:CONJUCHEM
Immunization Compositions and Methods
ActiveUS20110189226A1SsRNA viruses negative-senseBacterial antigen ingredientsSerotypeNeutralizing antibody
The present invention provides methods and compositions to induce neutralizing antibodies in mammals to serotypes of dengue virus, measles virus, mumps virus, rubella and / or VZV virus.
Owner:SANOFI PASTEUR SA
Multiple real-time quantitative PCR primer, probe and detection method for identifying viral pathogens relevant to fever with eruption syndrome as infection diseases
ActiveCN102140543ADetection ExpressImprove efficiencyMicrobiological testing/measurementFluorescence/phosphorescenceChickenpoxHerpes zoster virus
The invention discloses multiple real-time quantitative PCR primer, probe and a detection method for identifying viral pathogens relevant to fevers with eruption syndromes as infection diseases, which is used for carrying out multiple real-time fluorescent quantitative PCR detection on varicella-herpes zoster viruses, human small DNA (Deoxyribonucleic Acid) viruses B19, enteroviruses (enteroviruses 71 type and coxsackie viruses A16 type), dengue viruses, rubella viruses and measles viruses. The invention can simultaneously carry out qualitative or quantitative detection on eight kinds of human viruses in various types of samples by multiple double-tubes PCR. The detection method has the advantages of simple operation, short time consumption, high sensitivity and strong specificity, is suitable for field detection, early diagnosis, epidemics detection and research and the like, and takes the actions of assistance and identification diagnosis on the fevers with eruption syndromes.
Owner:SUN YAT SEN UNIV
cDNA corresponding to the antigenome of nonsegmented negative strand RNA viruses and process for the production of such viruses
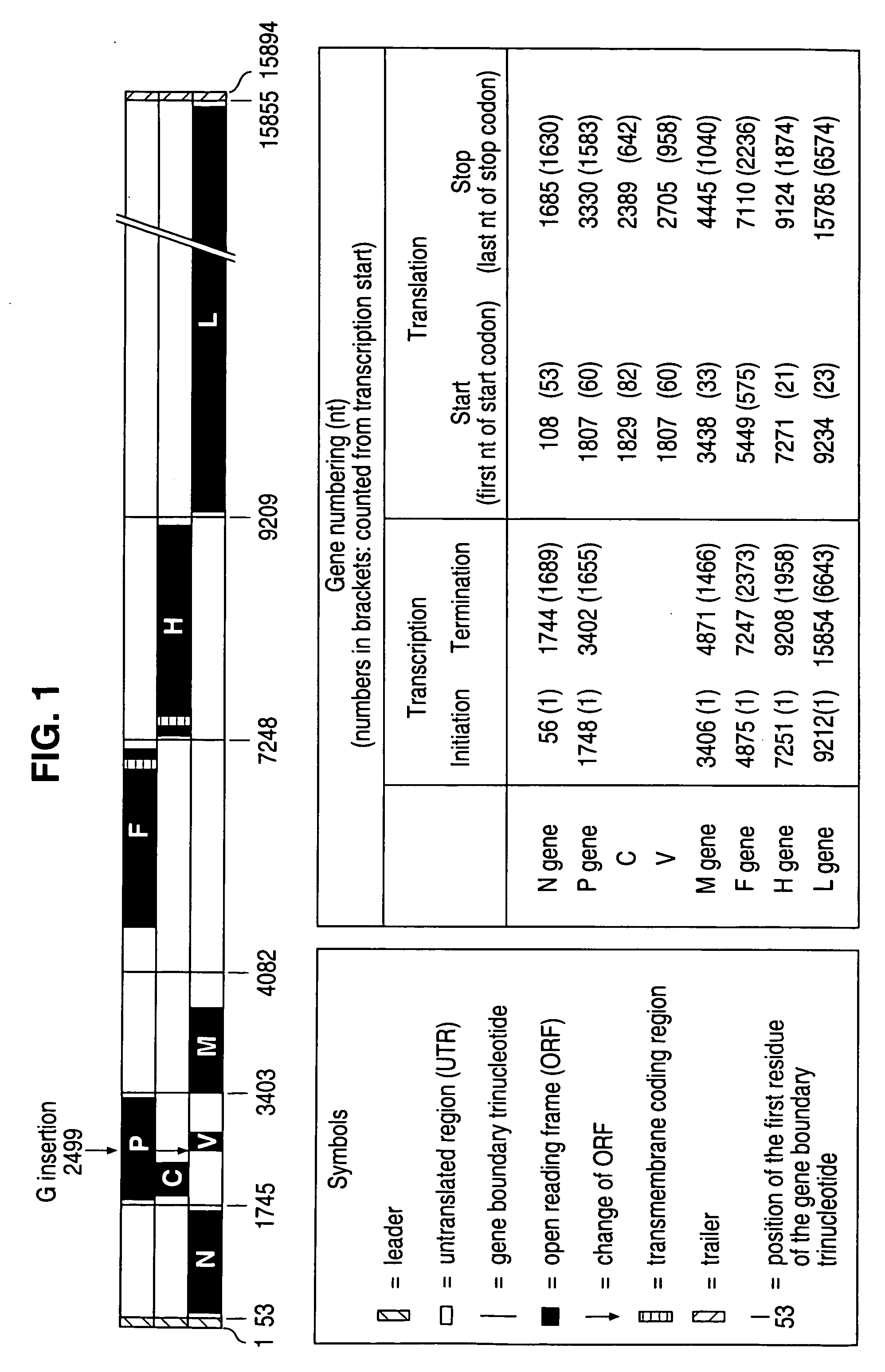
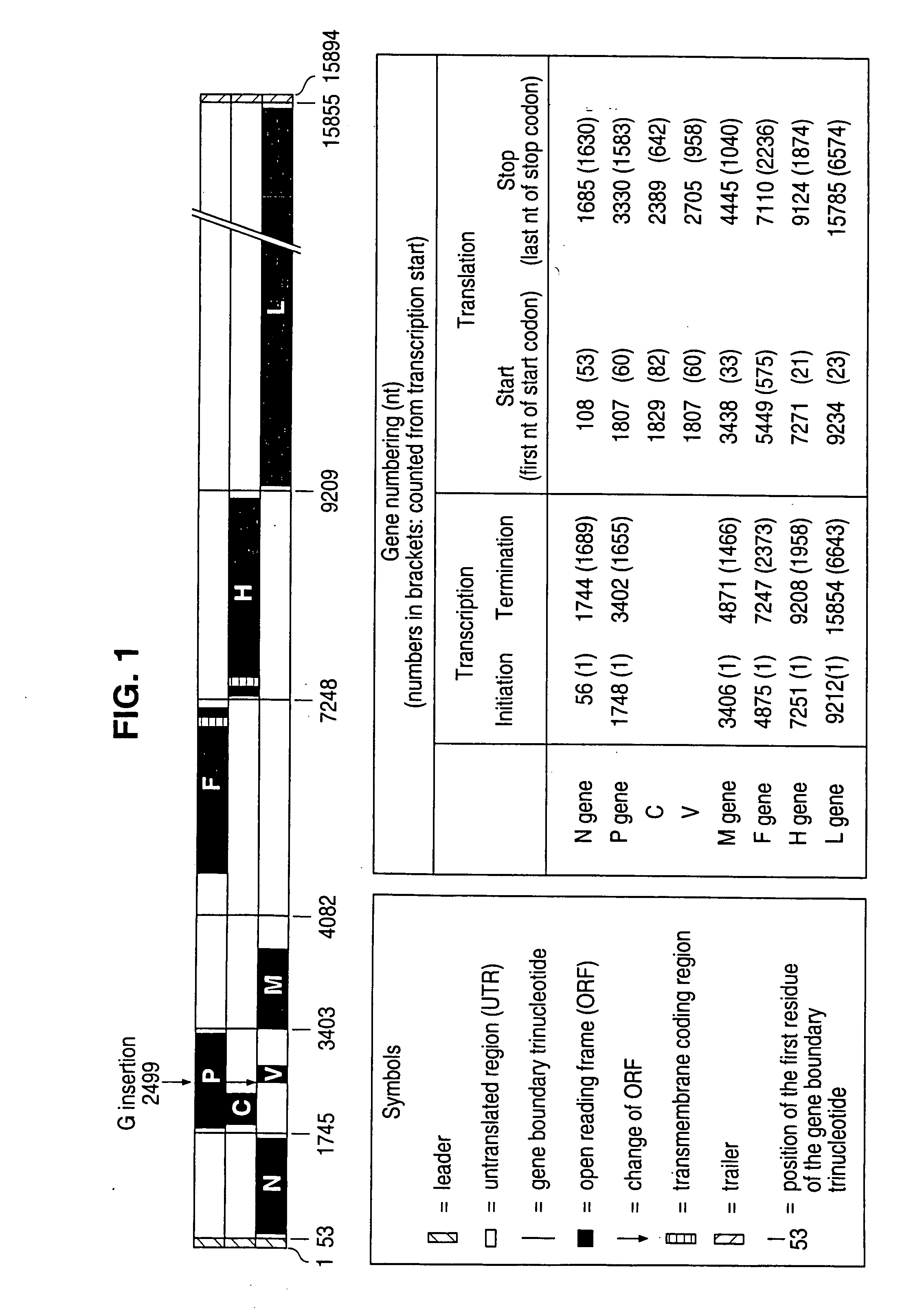
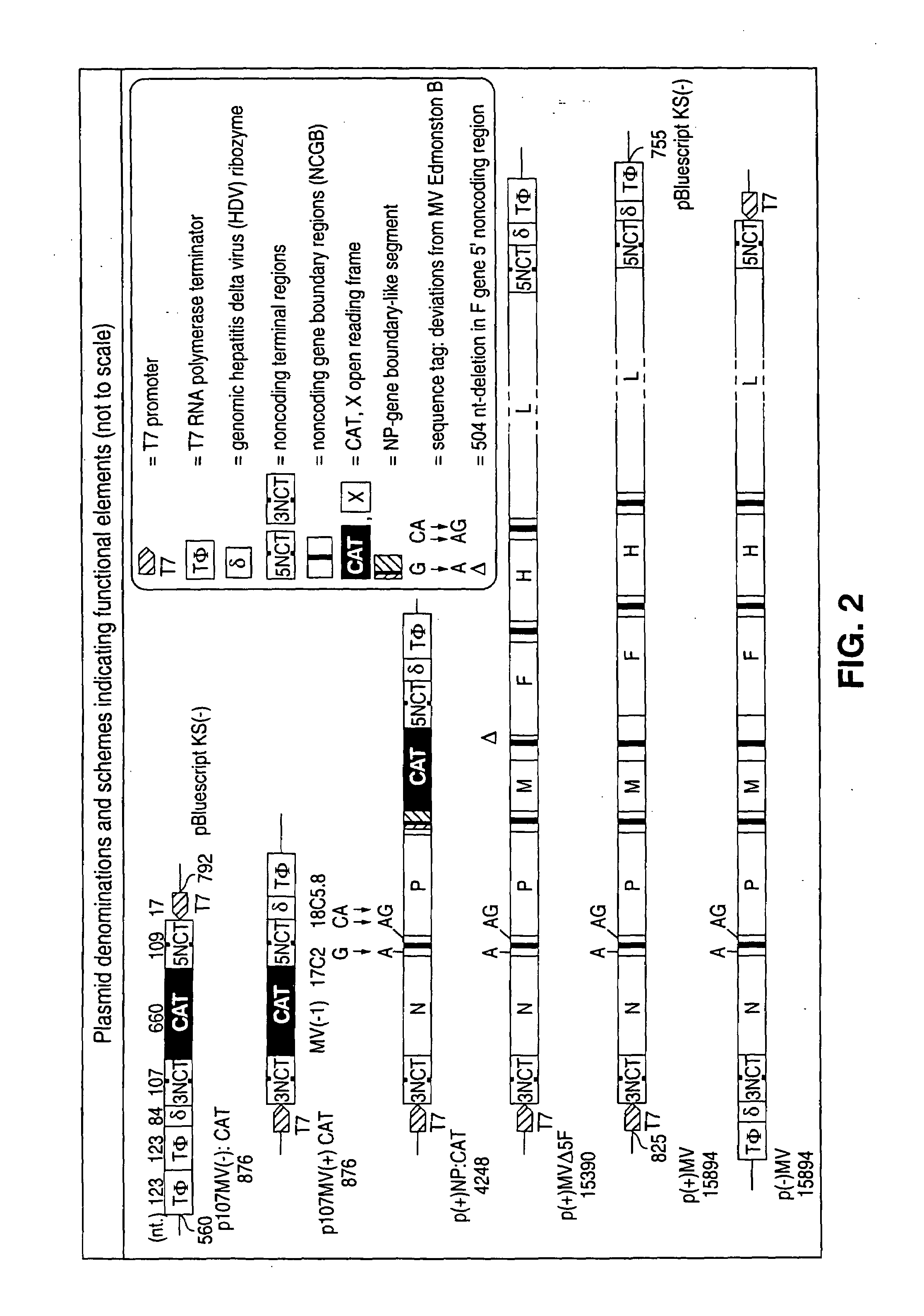
The present invention relates, in general, to a methodology or the generation of nonsegmented negative-strand RNA viruses (Pringle, 1991) from cloned deoxyribonucleic acid (cDNA). Such rescued viruses are suitable for use as vaccines, or alternatively, as plasmids in somatic gene therapy applications. The invention also relates to cDNA molecules suitable as tools in this methodology and to helper cell lines allowing the direct rescue of such viruses. Measles virus (MV) is used as a model for other representatives of the Mononegavirales, in particular the family Paramyxoviridae.
Owner:CRUCELL SWITZERLAND AG
cDNA Corresponding to the antigenome of nonsegmented negative stranded RNA viruses and process for the production of such viruses
The present invention relates, in general, to a methodology for the generation of nonsegmented negative-strand RNA viruses (Pringle, 1991) from cloned deoxyribonucleic acid (cDNA). Such rescued viruses are suitable for use as vaccines, or alternatively, as plasmids in somatic gene therapy applications. The invention also relates to cDNA molecules suitable as tools in this methodology and to helper cell lines allowing the direct rescue of such viruses. Measles virus (MV) is used as a mode for other representatives of the Mononegavirales, in particular the family Paramyxoviridae.
Owner:CRUCELL SWITZERLAND AG
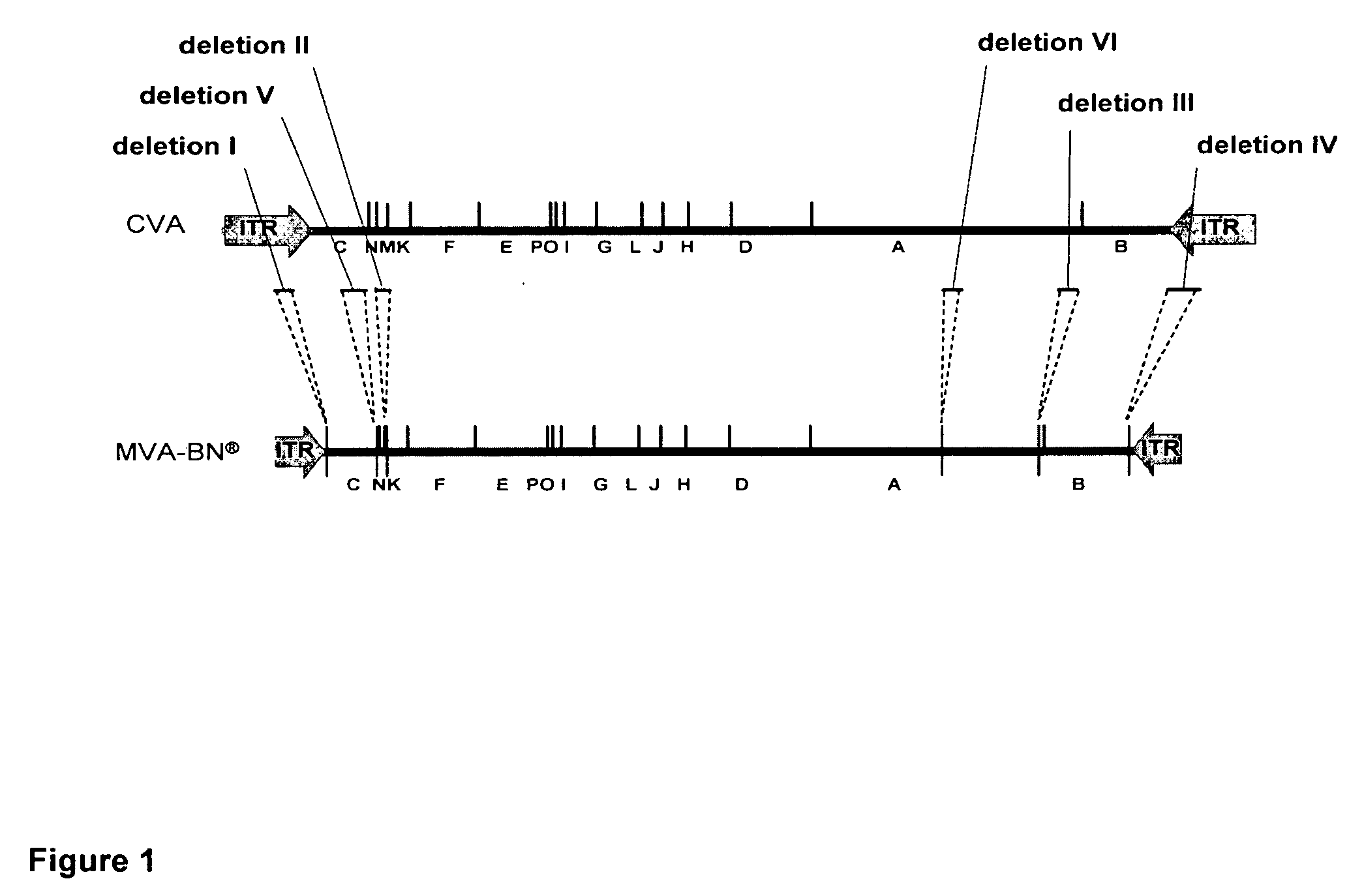
Recombinant modified vaccinia virus measles vaccine
InactiveUS20110052627A1Improve toleranceMinor side effectsSsRNA viruses negative-senseViral antigen ingredientsHemagglutinin proteinVaccinia
The invention concerns methods, compositions and kits for use in preparing a medicament and vaccine for measles virus comprising an Attenuated Modified Vaccinia Virus Ankara (MVA) strain encoding hemagglutinin protein, fusion protein, and nucleoprotein of measles virus (MVA-Measles). The recombinant virus induced superior cellular and humoral responses to the measles virus when compared to Measles vaccine Rouvax®. Both T cell and B cell immune responses to the recombinant MVA were observed not only in adult animals, but also in newborn and juvenile animals. Results in adult humans showed that MVA-Measles induces a strong immune response, is safe and well tolerated.
Owner:BAVARIAN NORDIC AS
Preparation method and application of gene chip for detecting important respiratory pathogenic viruses
InactiveCN102586475AStrong specificityImprove the consistency rateMicrobiological testing/measurementOligonucleotide chipMeasles virus IgG
The invention relates to a gene chip for detecting important respiratory pathogenic viruses. A preparation method of the gene chip comprises the following steps of: preparing a specific primer; preparing a virus specific probe; preparing an oligonucleotide chip; establishing an RT-PCR (Reverse Transcription-Polymerase Chain Reaction) system; establishing a hybrid system; preparing a visual detection reagent; and establishing a developing method. The gene chip prepared by the invention can be used for simultaneously discriminating nine general respiratory pathogenic viruses comprising A and B type influenza viruses, parainfluenza viruses type 1 and 2, human metapneumovirus, respiratory syncytial virus, measles virus, rubella virus and mumps virus, provides a new solution for quickly detecting the general respiratory pathogenic viruses at high throughout and can provide guidance for monitoring, clinically diagnosing and treating the respiratory pathogenic viruses.
Owner:深圳市普瑞康生物技术有限公司 +1
Preparation method of human measles immune sensor based on graphdiyne and silver nanoparticle cage
ActiveCN105842314ASpecific surface area enlargementFacilitates electron transferBiological material analysisMaterial electrochemical variablesSignalling moleculesEngineering
The invention relates to a preparation method of a human measles immune sensor based on graphdiyne and a silver nanoparticle cage, and belongs to the technical field of novel biological sensing and detection. Graphdiyne is of a carbon-carbon triple-bond linear structure with an sp hybridized state and has the advantages of zero cis-trans-isomer, high conjugation and the like; due to excellent electrical, optical and photoelectric properties, graphdiyne is a key material of a novel electronic and photoelectric device of the next generation. Meanwhile, by the use of good pi-pi conjugation effect, a high-flux load of signal molecules is realized, and a signal error caused by instable combination is reduced. Therefore, the human measles immune sensor based on graphdiyne and the silver nanoparticle cage can sensitively detect measles viruses.
Owner:UNIV OF JINAN
One-step real-time fluorescence quantitative RT-PCR (reverse transcription-polymerase chain reaction) kit for measles virus
InactiveCN102140547AShort detection time periodImprove detection efficiencyMicrobiological testing/measurementFluorescence/phosphorescenceMeasles virus IgGQualitative analysis
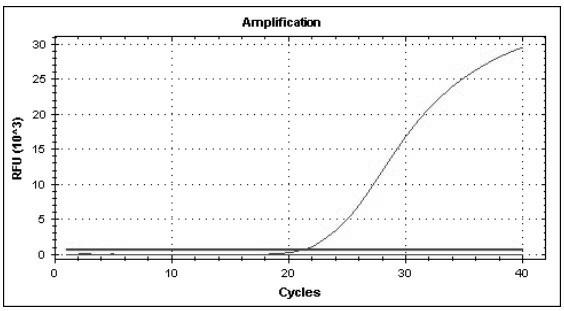
The invention discloses a one-step real-time fluorescence quantitative RT-PCR (reverse transcription-polymerase chain reaction) kit for measles virus, relating to the PCR kits in the biotechnology field. The kit comprises a pair of specific primers, a specific fluorescent probe, positive control, negative control, one-step 5*RT-PCR buffer and Enzyme Mix of the measles virus, wherein the specific primers are F:5'-ACASRGTGATCAAARTGRRARYGAGCTC-3' and R:5'-GYCCTGCCATGGYYTGCAC-3'; and the specific probe is FP: FAM-ATCYGATRCAGTRTCAATT-MGB-NQF. The kit has the following advantages and positive effects: the detection time period is short and the detection efficiency is high; the specificity and accuracy rate in detecting the virus are high; quantitative analysis of the virus can be carried out while carrying out qualitative analysis of the virus; the detection sensitivity is higher than the sensitivity in the common PCR and immunological detection methods; the kit is easy to operate and popularize; and the experimental results have good repeatability.
Owner:WUHAN UNIV
Medicaments and methods for treating mesothelioma
The present invention relates to the use of at least one attenuated measles virus for the manufacture of a medicament intended for treating malignant mesothelioma in an individual.
Owner:CENT NAT DE LA RECHERCHE SCI
Chromatography based purification strategies for viruses
ActiveUS10894079B2SsRNA viruses negative-senseSsRNA viruses positive-senseMeasles virus IgGInfectious virus
The present invention provides purification strategies for sterically demanding, i.e. large and pleomorphic, infectious virus particles or VLPs derived therefrom, preferably having a measles virus scaffold to yield fractions or compositions with a significantly reduced content of contaminating host cell DNA and a reduced content of further process-related impurities. Further provided are methods of propagating and purifying infectious virus particles having a measles virus scaffold suitable to provide a preparation having a strongly reduced content of contaminating host cell DNA and a reduced content of further process-related impurities for immunogenic or anti-tumor purposes. In addition, immunogenic and vaccine compositions based on the above methods are provided. Finally, there are provided immunogenic or vaccine compositions produced by the disclosed methods, which are suitable for use in immunogenic or prophylactic vaccination treatment of a subject in need thereof.
Owner:MERCK SHARP & DOHME LLC
Non-coding region and P.M gene sequence and vector construction of long-47 measles virus
The present invention provides coding sequences and structures of all the non-coding regions and P and M coding regions of Longchun-47 measles virus, protein amino acid sequence and structure of P and M coding regions, the use of the non-coding region sequences in constituting the vaccine virus carrier and the constituting process of the carrier. These data and method may be used in recombining multiple effect vaccine or gene treatment via utilizing the vaccine strain virus to constitute expression vector and may be also used in molecular biological diagnosis and reagent development for measles virus infection.
Owner:中国预防医学科学院病毒学研究所
Recombinant measles virus expressing chikungunya virus polypeptides and their applications
ActiveCN104918952AAvoid infectionSsRNA viruses negative-senseSsRNA viruses positive-senseVirus-like particleChikungunya
The invention relates to recombinant Measles virus expressing Chikungunya virus polypeptides, and concerns in particular virus like particles (VLP) that contain envelope and capsid proteins of a Chikungunya virus at their surface. These particles are recombinant infectious particles able to replicate in a host after an administration. The invention provides means, in particular nucleic acids, vectors, cells and rescue systems to produce these recombinant infectious particles. The invention also relates to the use of these recombinant infectious particles, in particular under the form of a composition, more particularly in a vaccine formulation, for the treatment or prevention of an infection by Chikungunya virus.
Owner:INST PASTEUR +2
System and method for rescuing measles viruses and recombinant measles viruses
PendingCN106957859AImprove rescue efficiencyImprove stabilitySsRNA viruses negative-senseInactivation/attenuationGreen fluorescent proteinPolymerase L
The invention discloses a system and a method for rescuing measles viruses and recombinant measles viruses for expressing GFP (Green Fluorescent Protein) and application. The system provided by the invention comprises the following components: 1) a full-length transcription plasmid pT7MVS191 or pT7MVS191-ATU-GFP containing a measles virus anti-genome; 2) a helper plasmid 1 containing an encoding gene of nucleocapsid protein of the measles viruses; 3) a helper plasmid 2 containing an encoding gene of phosphoprotein of the measles viruses; and 4) a helper plasmid 3 containing an encoding gene of RNA (Ribonucleic Acid) polymerase of the measles viruses. The invention further provides a method for rescuing the measles viruses and the recombinant measles viruses. By adopting the system and the method, the measles viruses and the recombinant measles viruses can be effectively rescued; and the system and the method can be used for developing and applying therapeutic or preventive recombinant measles virus products.
Owner:CHENGDU INST OF BIOLOGICAL PROD
Recombinant measles viruses expressing novel coronavirus proteins and application thereof
PendingCN113480618ASsRNA viruses negative-senseSsRNA viruses positive-senseVirus ProteinMeasles virus IgG
The present invention relates to recombinant measles viruses expressing novel coronavirus proteins, such as S proteins or trimers or functional fragments thereof, vaccines comprising the recombinant measles viruses, and the use of such vaccines for the prevention or treatment of novel coronavirus infections.
Owner:ZHEJIANG UNIV
Measles subunit vaccine
InactiveUS20090175903A1SsRNA viruses negative-senseViral antigen ingredientsMeasles Virus AntigenAdjuvant
Compositions and methods for making and using therapeutic formulations of measles virus antigens with a Proteosome-based adjuvant are provided. The measles virus antigens may be derived from a variety of sources, such as from recombinant production or from a split antigen preparation. The measles vaccine formulations may be used, for example, in methods for treating or preventing a measles virus infection and eliciting a protective immune response.
Owner:MCGILL UNIV
Construction of F gene replaced chimeric measles attenuated strain
ActiveCN113293149ASsRNA viruses negative-senseViral antigen ingredientsMeasles virus IgGImmunogenicity
The invention provides a construction method and application of an F gene replaced chimeric measles attenuated strain. Specifically, the invention provides the chimeric measles virus attenuated strain, and the attenuated strain is a measles virus rMV / F (H1a) with the preservation number of CCTCCNO: V202101. The invention also provides a vaccine composition containing the F gene substituted chimeric measles attenuated strain or the derivative virus strain thereof as an active ingredient and a preparation method of the vaccine composition. Compared with the existing measles vaccine strain in the market, the chimeric virus provided by the invention has stronger replication ability and better immunogenicity.
Owner:SHANGHAI KING CELL BIOTECHNOLOGY CO LTD +1
Cysteic acid derivatives of anti-viral peptides
InactiveCN101687911AImprove solubilitySimple purification processPeptide/protein ingredientsVirus peptidesHuman Parainfluenza VirusCysteic acid
This invention relates to C34 peptide derivatives having improved aqueous solubility that are inhibitors of viral infection and / or exhibit antifusogenic properties. In particular, this invention relates to C34 derivatives having inhibiting activity against human immunodeficiency virus (HIV), respiratory synctial vims (RSV), human parainfluenza virus (HPV), measles virus (MeV). and simian immunodeficiency virus (SIV) with long duration of action for the treatment of the respective viral infections.
Owner:CONJUCHEM BIOTECH INC
Mutations responsible for attenuation in measles virus or human respiratory syncytial virus subgroup B
InactiveCN1294628ASsRNA viruses negative-senseVirus peptidesHuman respiratory virusAnti-Measles Virus
Isolated, recombinantly-generated, attenuated measles viruses and respiratory syncytial subgroup B viruses having defined attenuating mutations are described. Vaccines are formulated comprising such viruses and a physiologically acceptable carrier. The vaccines are used for immunizing an individual to induce protection against measles virus or respiratory syncytial subgroup B virus.
Owner:WYETH HOLDINGS CORP
Poxvirus-canine destemper virus (CDV) recombinants and compositions and methods employing the recombinant
InactiveUS20030082204A1Improve securityImprove security levelSsRNA viruses negative-senseSsRNA viruses positive-senseHemagglutininCanine distemper virus Antigen
Attenuated recombinant viruses containing DNA coding for a canine distemper virus antigen or measles M or N antigen, as well as methods and compositions employing the viruses, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least one of: canine distemper virus fusion protein, canine distemper virus hemagglutinin glycoprotein, canine distemper nucleocaspid portein, canine distemper matrix protein, measles virus nucleocaspid protein, and measles virus matrix protein. The recombinant viruses and gene products therefrom are useful for eliciting protection against canine distemper virus and / or measles virus, and, the gene products and antibodies elicited thereby are useful in assays. Additionally, DNA from the recombinants is used for probes or for generating PCR primers.
Owner:CONNAUGHT TECH
Dengue virus chimeric polyepitope composed of fragments of non-structural proteins and its use in an immunogenic composition against dengue virus infection
ActiveUS10316066B2SsRNA viruses negative-senseSsRNA viruses positive-senseStructural proteinPolynucleotide
The present invention is directed to a dengue virus chimeric polyepitope composed of fragments of non-structural proteins and its use in an immunogenic composition against dengue virus infection. The present invention provides means, in particular polynucleotides, vectors, cells and methods to produce vectors expressing said chimeric polyepitopes, in particular vectors consisting of recombinant measles virus (MV) particles. The present invention also relates to the use of the recombinant MV particles, in particular under the form of a composition or of a vaccine, for the prevention and / or treatment of a dengue virus infection.
Owner:INST PASTEUR +2
Recombinant canine measles virus expressing luciferase
PendingCN111733170AGrowth kinetics were not significantly affectedSsRNA viruses negative-senseMicrobiological testing/measurementFluorescenceMeasles virus IgG
The invention aims to provide a recombinant canine measles virus expressing luciferase NLuc. The structure of a recombinant CMV nucleic acid fragment for rescuing and expressing NLuc is 5'-N-P-NLuc-M-F-H-L-3', wherein N, P, M, F, H and L are structural genes of the virus; NLuc is ORF of luciferase, the upstream of the ORF contains an M gene initiation sequence and a Not I restriction enzyme cutting site, and the downstream contains a Pme I restriction enzyme cutting site and a P gene termination sequence. The rCMV-NLuc rescued by a reverse genetic technology has a high expression level on NLucin cells, and can emit macroscopic light blue fluorescence under the action of a luminescent substrate furimazine. The insertion of an exogenous gene does not significantly affect the growth kineticsof the virus, and the exogenous gene is relatively stable in the virus passage process.
Owner:QINGDAO AGRI UNIV
Anti-measles cancer immunotherapy
InactiveUS20110014231A1Readily availableSsRNA viruses negative-senseMicrobiological testing/measurementSpecific populationImmunogenicity
Owner:MOR RES APPL
DNA molecules producing custom designed replicating and non-replicating negative stranded RNA viruses and uses there of
ActiveUS20190211355A1Improving oncolytic potencyEasy to detectSsRNA viruses negative-senseSsRNA viruses positive-senseNegative strandCancer cell
This invention comprises: compositions comprising a derivative, plasmids, a reagent kit and methods of making these compositions a derivative, vaccine- and non-vaccine-compositions of above for causing death of cancer cells that form part of a tumour and virus infected Dengue, Measles and other diseased cells; the derivative comprising replicating as well as non-replicating derivatives of an attenuated negative stranded RNA virus belonging to family paramyxoviridae, including Measles Virus, comprising a single additional transcriptional unit carrying either only one or two or more non-viral genes, and the non-replicating derivatives being free from contaminating replicating Measles Virus (b) a Measles Virus packaging cell line for making above compositions, expressing the M, F and H proteins of MV stably. And (c) a reagent kit for producing the Measles Virus derivatives described above.
Owner:JOSHI VISHWAS DATTATRAYA
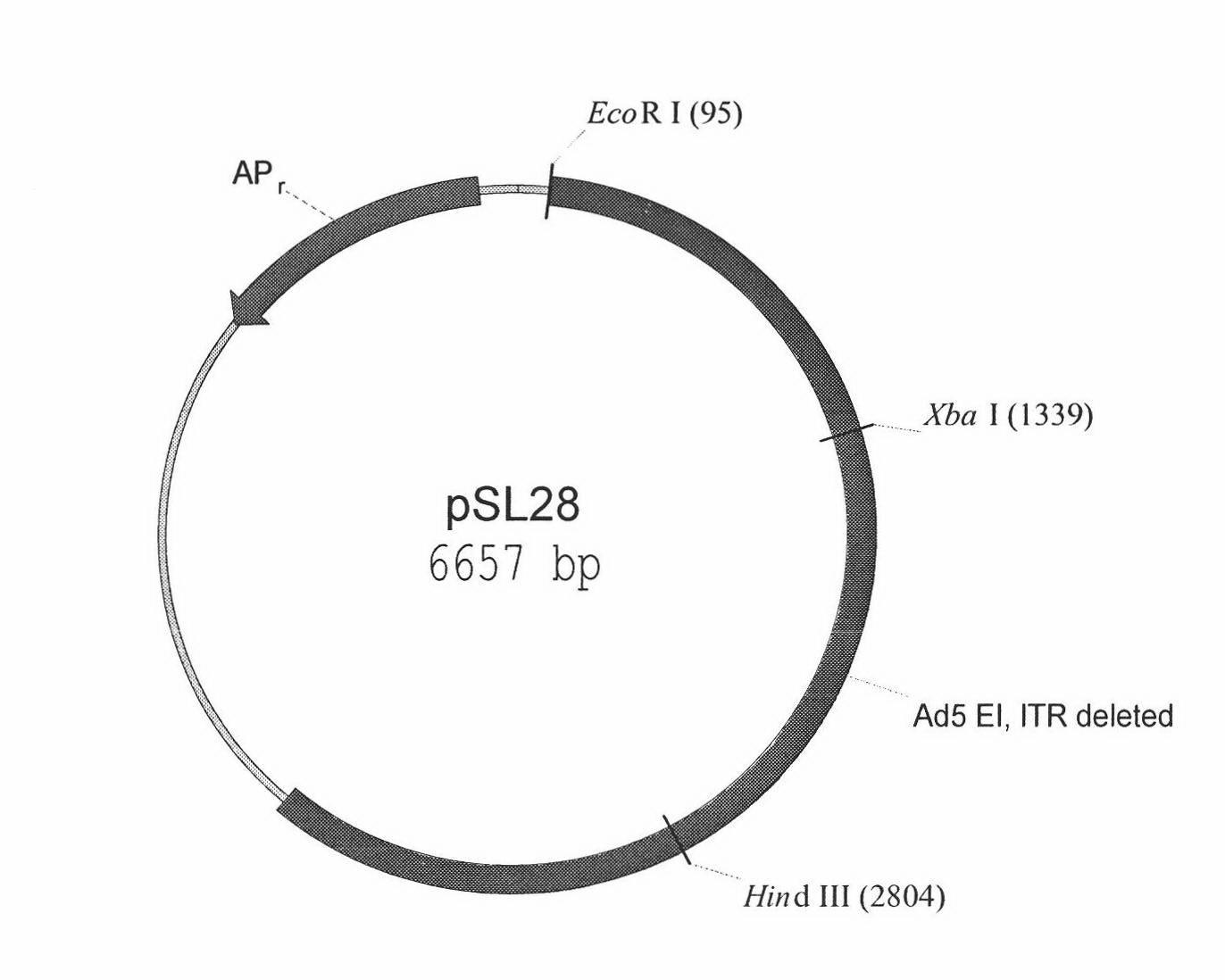
Human embryo retina transformed cell line for producing biological products
The invention constructs a human embryo retina transformed cell line SL-2 by transforming adenovirus gene fragments into human embryo retina cells. The cell line can be used for producing biological products such as adenovirus and the like, and has obvious advantages. The cell line can also be used for producing influenza virus, herpes simplex virus, measles virus, rotavirus and other viruses, polypeptides and other biological products.
Owner:梁旻 +1
Nucleic acid sequence for isothermal amplification detection of measles virus, kit, detection method and application
PendingCN111411169AStrong specificityGuaranteed accuracyMicrobiological testing/measurementDNA/RNA fragmentationMeasles virus IgGNucleic acid sequencing
The invention relates to the field of virus detection, in particular to a nucleic acid sequence for isothermal amplification detection of measles virus, a kit, a detection method and application. Thenucleic acid sequence comprises strand displacement primer sequences shown as SEQ ID No. 1 and SEQ ID No. 2, probes shown as SEQ ID No. 3 and SEQ ID No. 4, and a cross amplification primer sequence shown as SEQ ID No. 5. The kit and the detection method have the technical advantages of good specificity, high sensitivity, simple steps and high repeatability.
Owner:苏秀兰 +1
H1a genotype chimeric measles virus attenuated strain as well as preparation method and application thereof
ActiveCN113999824AImprove protectionSolve the problem of not being able to adapt to chicken embryo cellsSsRNA viruses negative-senseSsRNA viruses positive-senseNucleotideHemagglutinin protein
The invention relates to the technical field of virus gene engineering, in particular to an H1a genotype chimeric measles virus attenuated strain and a preparation method and application of the H1a genotype chimeric measles virus attenuated strain. The method comprises the following steps: by taking a measles virus attenuated strain as a skeleton, and replacing the fusion protein coding gene F and the hemagglutinin protein coding gene H in the measles virus attenuated strain with nucleotide sequences of the fusion protein coding gene F and the hemagglutinin protein coding gene H containing the H1a genotype measles virus; and according to the present invention, the existing measles vaccine attenuated strain (such as Schwarz) is adopted as the skeleton, the immunogenic proteins F and H are respectively replaced with the H1a genotype related proteins F and H, and the constructed H1a genotype chimeric measles virus attenuated strain has the excellent protection effect on the H1a genotype measles virus.
Owner:BEIJING CELL FUSION BIOTECHNOLOGY CO LTD +1
immune combination for inducing broad-spectrum neutralizing antibodies against HIV-1
PendingCN111494623AStimulate maturation and differentiationExtension of timeViral antigen ingredientsAntiviralsEnterovirusAntiendomysial antibodies
The invention relates to the field of vaccines, in particular to a vaccine composition for inducing an HIV-1 broad-spectrum neutralizing antibody. A combined vaccine form is adopted, HIV-1 membrane protein is adopted as immunogen for the first time, and vaccine presentation forms include but are not limited to recombinant plasmid vaccines, recombinant protein subunit vaccines, recombinant virus vector vaccines, virus-like particle vaccines, nanoparticle vaccines, attenuated live vaccines and inactivated virus vaccines; the virus vector vaccine for expressing the HIV-1 membrane protein is adopted in the second injection, so that the titer and broad spectrum of an induced anti-HIV-1 neutralizing antibody can be remarkably improved; wherein the virus vector comprises but is not limited to poxvirus, adenovirus, herpes simplex virus, measles virus, enterovirus and rhabdovirus. And the third needle and the fourth needle are protein nanoparticle vaccine and virus vector vaccine combinations or vice versa. Inoculation modes of the vaccine include but are not limited to muscle inoculation, intracutaneous inoculation, subcutaneous inoculation, nasal dripping, aerosol inhalation, genital tract, rectum, oral administration and the like. Animal experiment results prove that the vaccine combination is safe, can continuously generate a high-titer broad-spectrum neutralizing antibody, and canbe used for preventing and treating AIDS in the future.
Owner:SHANGHAI PUBLIC HEALTH CLINICAL CENT
Tumor quantum biology early stage diagnosis-tumor ultraviolet absorption spectrum energy measurement technology
A result of the detection of normal and abnormal absorption spectrum of MCF-7, HeLa cells, measles viruses and the like through an ultraviolet absorption spectrum energy measurement technique shows that characteristic absorption peak curves mean the interaction of DNA and proteins in cells. The shape of curves of a same substance with different concentrations is completely same. A cancer cell gene isomer is a long-lived excited nuclide, and has a blue shift of 5-8nm from the maximum absorption wavelength of proto-oncogene, so a nuclear binding energy-gene threshold value is the measurement of the nuclear stability degree. The maximum gene barrier height lambdamax is 255.84nm, and DNA fission (canceration) is carried out when the maximum gene barrier exceeds gene saddle point height. A true tumor early stage diagnosis technology is used for detecting the gene threshold value barrier height. The multiplication of cancer cells and the secretion of oncofetal antigens appear after the cell canceration, and the cancer cells and the oncofetal antigens are cancer later stage detection products. The ultraviolet absorption spectrum energy measurement technology can rapidly detect the gene threshold value DNA quantum and momentum (Schrodinger quantum mechanics), and is excellent as a tumor early diagnosis technology.
Owner:王汉成
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com