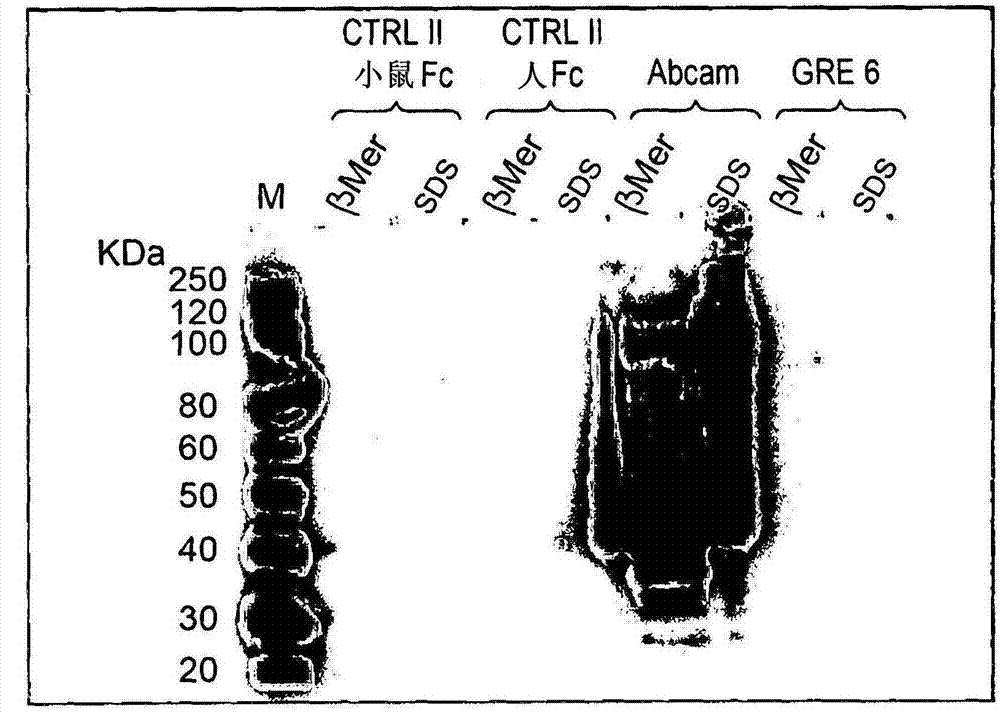
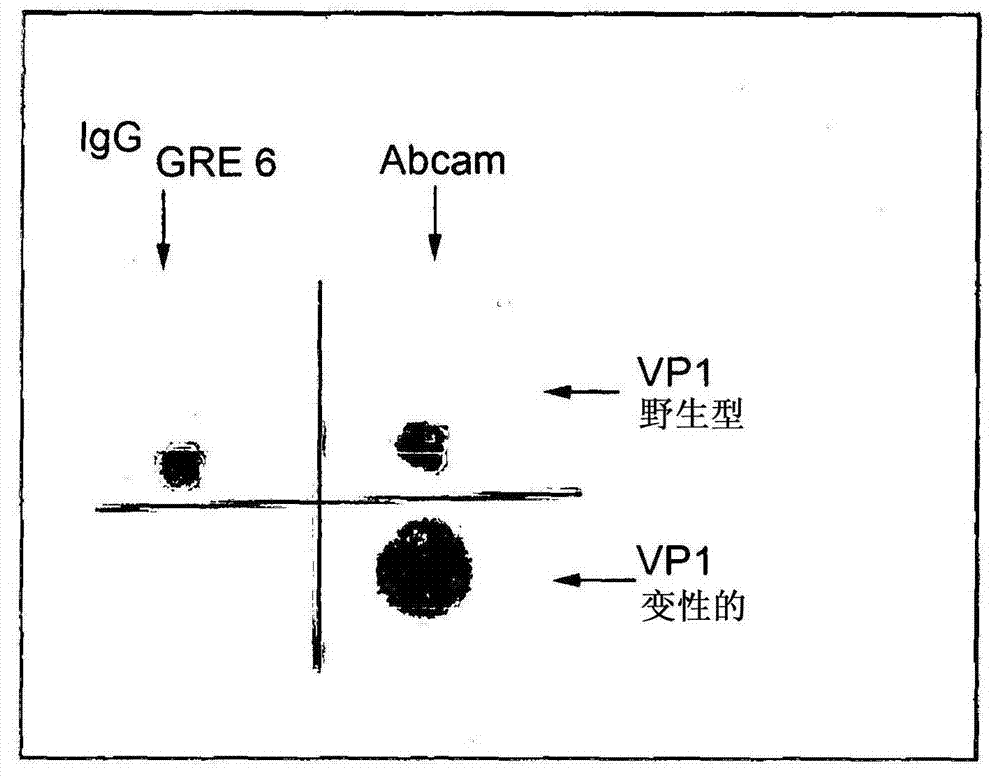
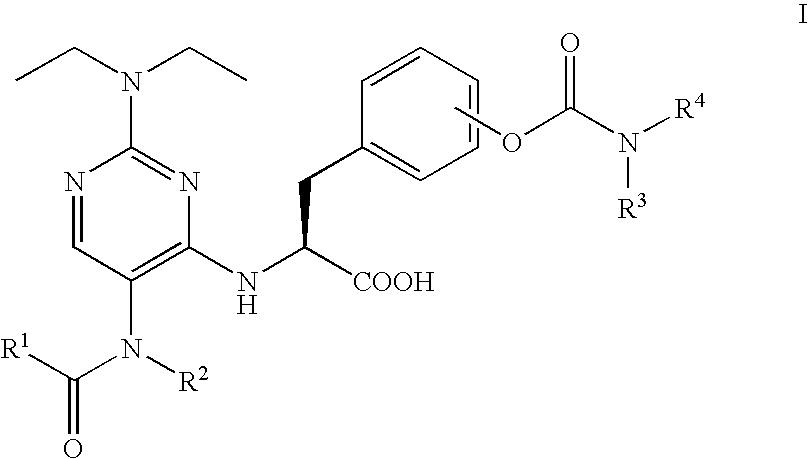
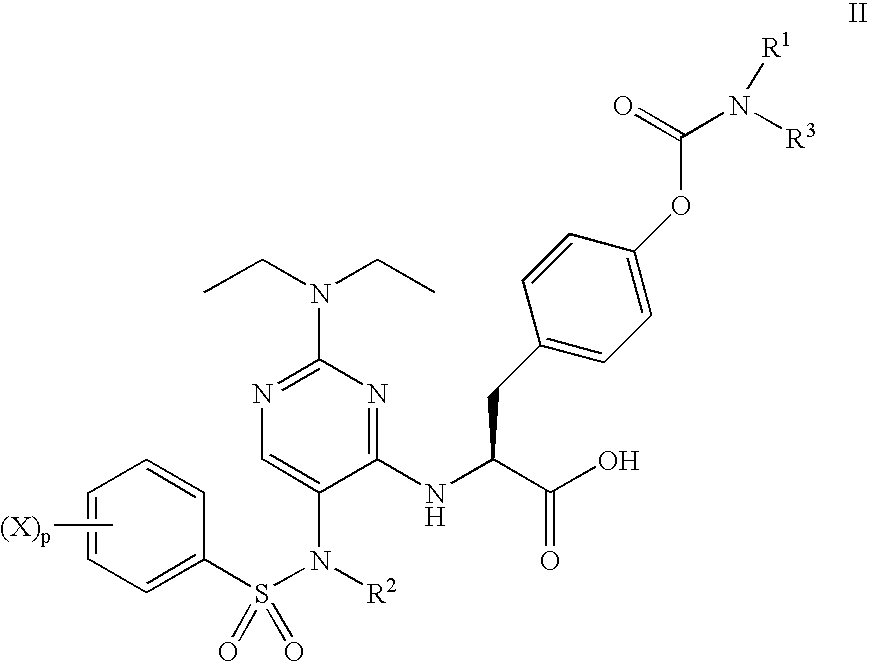
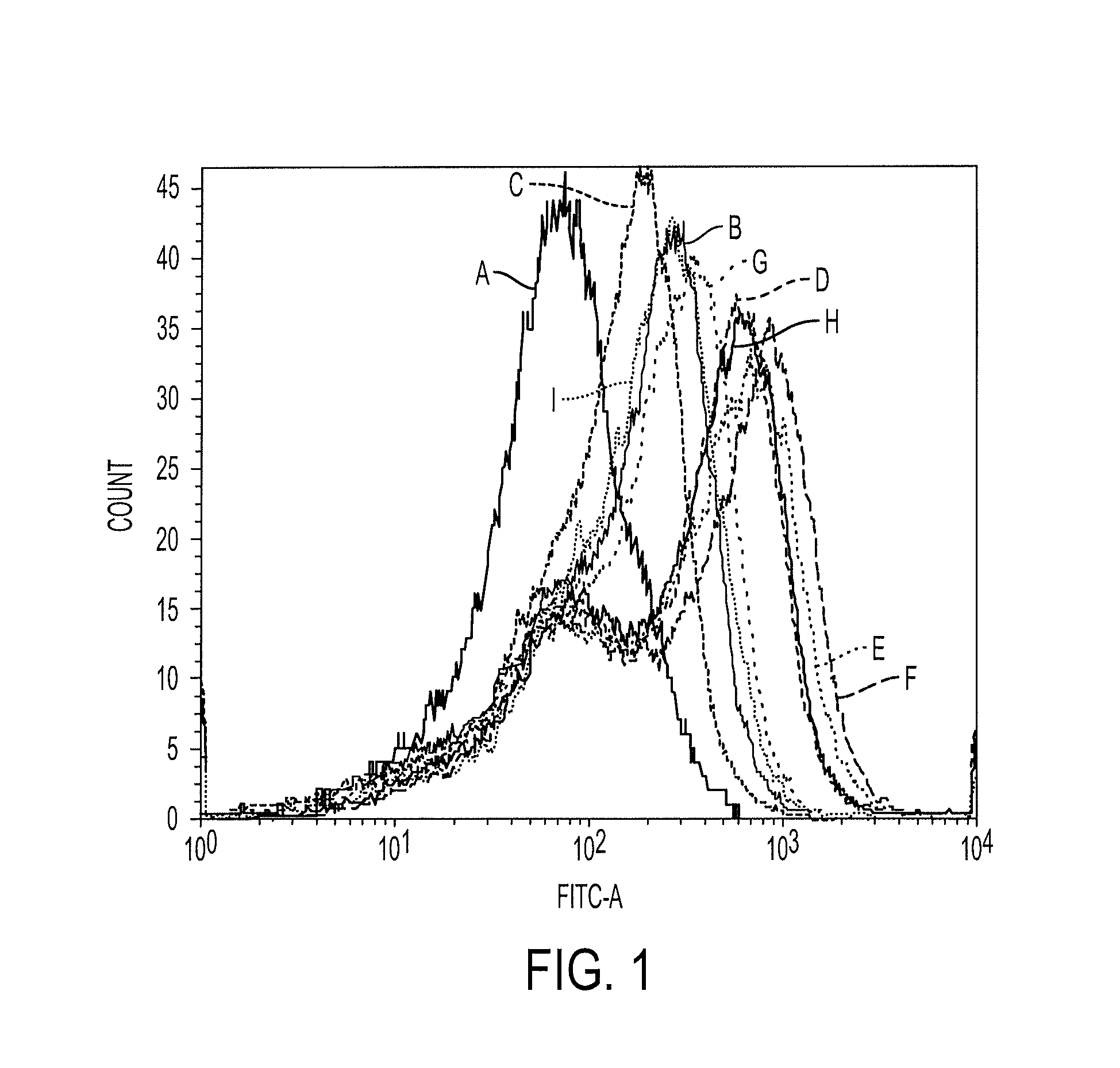
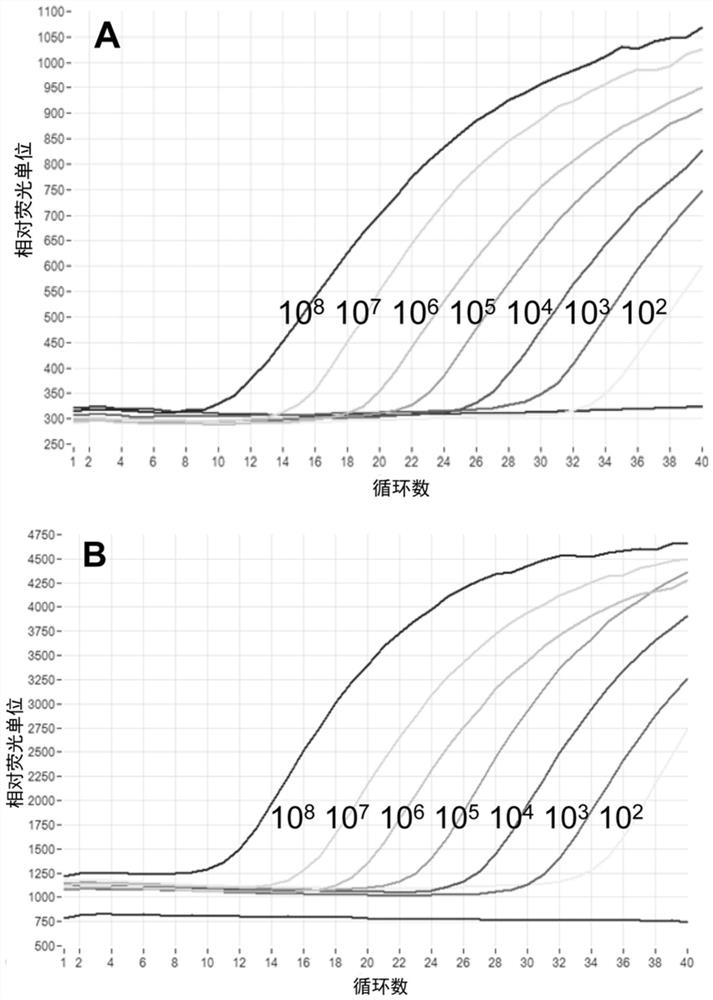
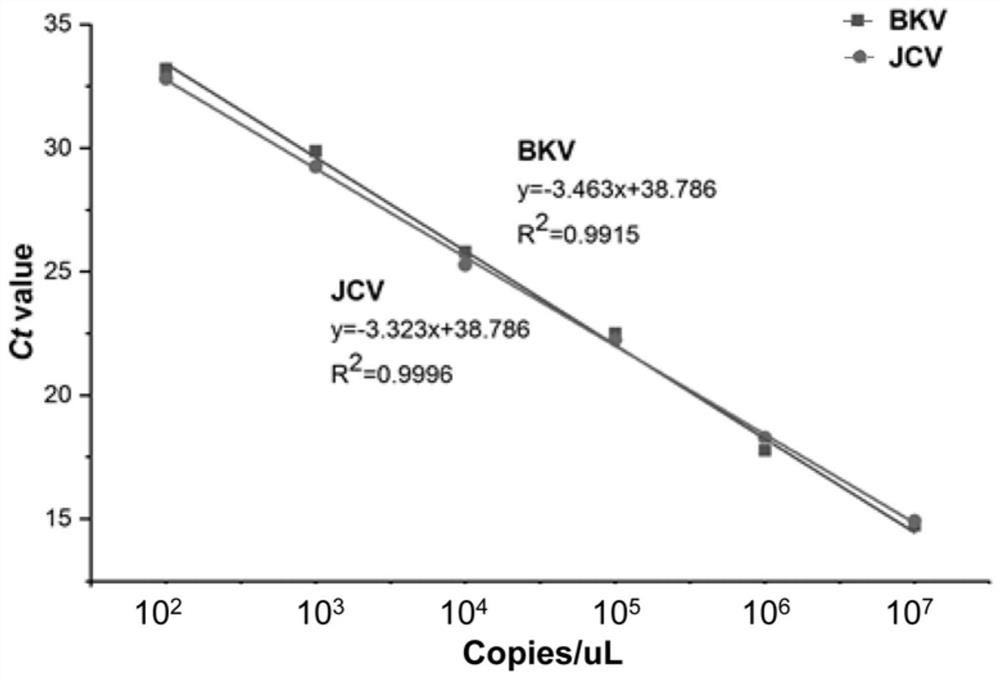
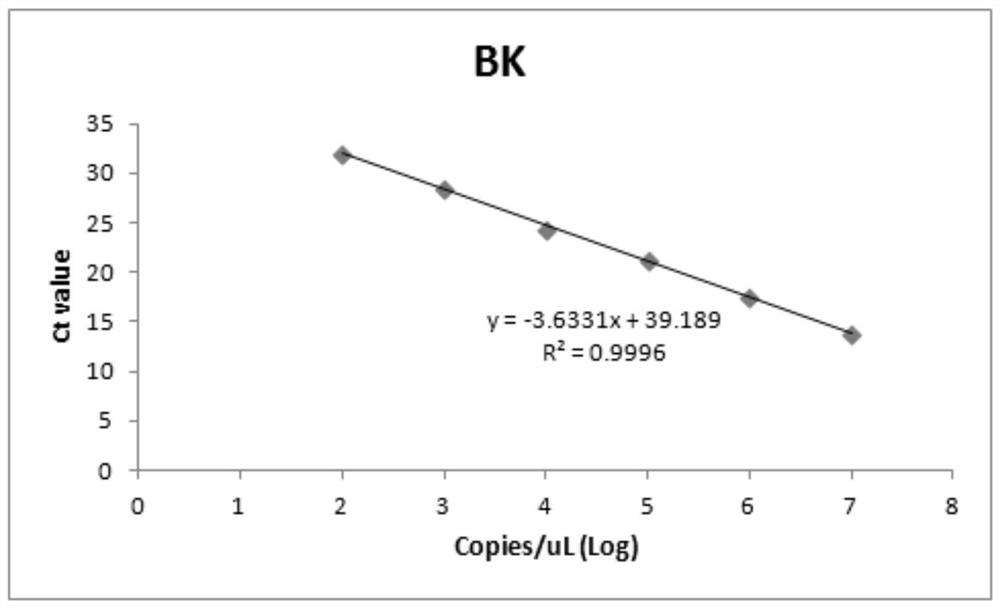
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Polyomavirus JC" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The JC virus or John Cunningham virus is a type of human polyomavirus (formerly known as papovavirus).
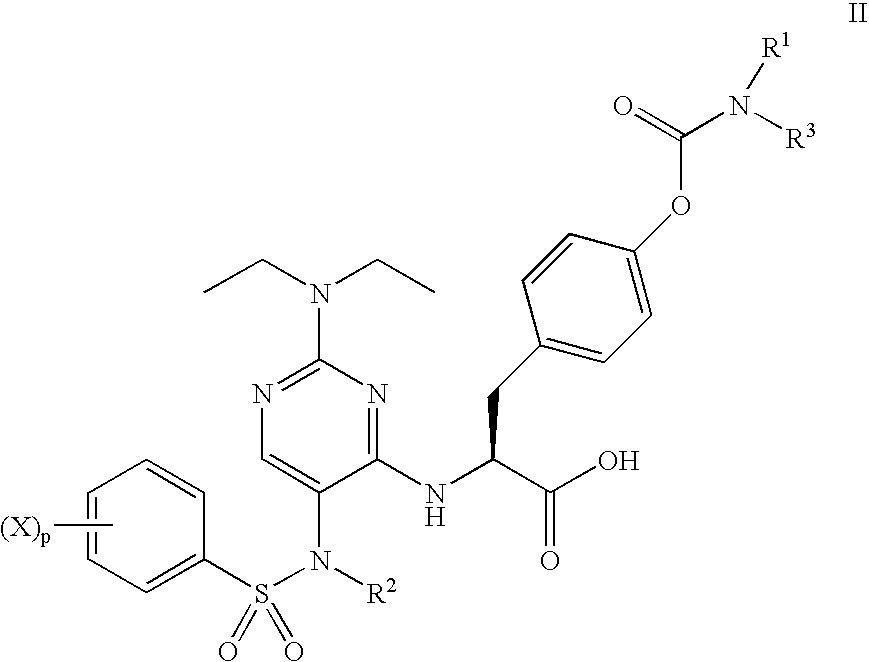
Methods of treating inflammatory and autoimmune diseases with alpha-4 inhibitory compounds
InactiveUS20080044382A1Improve securityMethod securityBiocideHeavy metal active ingredientsAutoimmune conditionPolyomavirus JC
Alpha 4 inhibitors are used in treatment of inflammatory and autoimmune diseases, such as multiple sclerosis, Crohn's Disease, rheumatoid arthritis and asthma. Rare occurrences of progressive multifocal leucoencephalopathy during treatment with an alpha-4 agent suggest the possibility that it may be related to such treatment. Monitoring for the JC virus and educating caregivers and patients about the manifestations of progressive multifocal leucoencephalopathy can improve the safety of alpha 4 inhibitor therapy.
Owner:ELAN PHARM INC +1
Polyomavirus cellular epitopes and uses therefor
ActiveUS20070026503A1Reduce frequencyPeptide/protein ingredientsViral antigen ingredientsRenal transplantPolyomavirus JC
The present invention relates to HLA-A*02-restricted cellular epitopes within the VP1 polypeptide of a human polyomavirus, BK virus, which is associated with polyomavirus-associated nephropathy in kidney transplant patients. Preferred peptides correspond to amino acids residues 107-116, 108-116 and 44-52 of BKV VP1, and are processed in vivo in natural infection with BKV. Effector T cell populations stimulated by the peptides represent functional CTLs as assessed by cytotoxicity and cytokine production, and are reactive against cells presenting both the BKV peptides above and the JC virus homolog sequences.
Owner:CITY OF HOPE
Compositions and methods for the treatment of progressive multifocal leukoencephalopathy (PML)
ActiveUS20130183289A1Reduce riskBiocideOther blood circulation devicesPolyomavirus JCViral infection
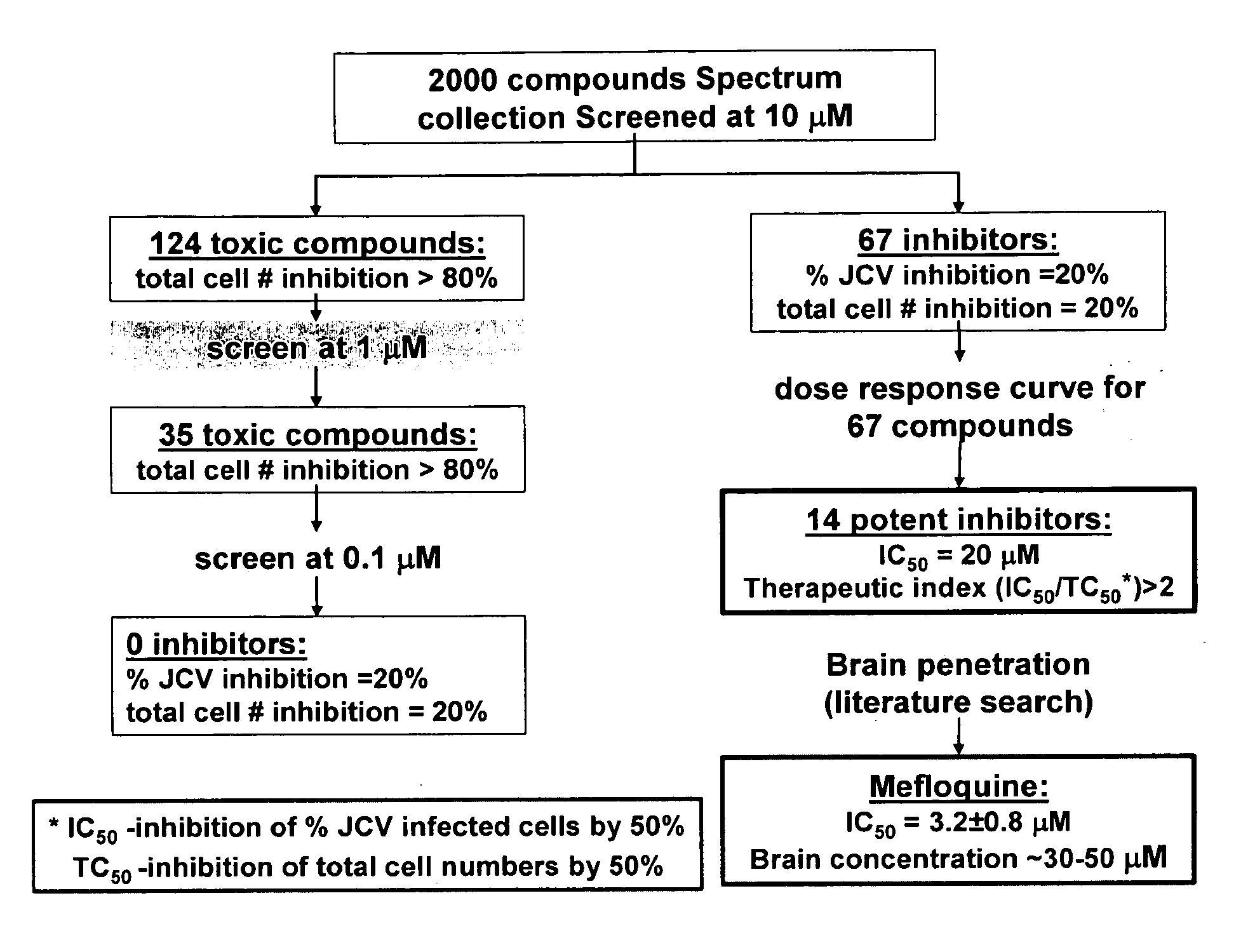
The invention relates to compositions, methods, and kits for treating subjects infected by or at risk of infection with a DNA virus (e.g., a JC Virus or a BK virus). Aspects of the invention are useful to prevent or treat DNA virus associated conditions (e.g., PML) in subjects that are immunocompromised. Compositions are provided that inhibit intracellular replication of DNA viruses.
Owner:BIOGEN MA INC
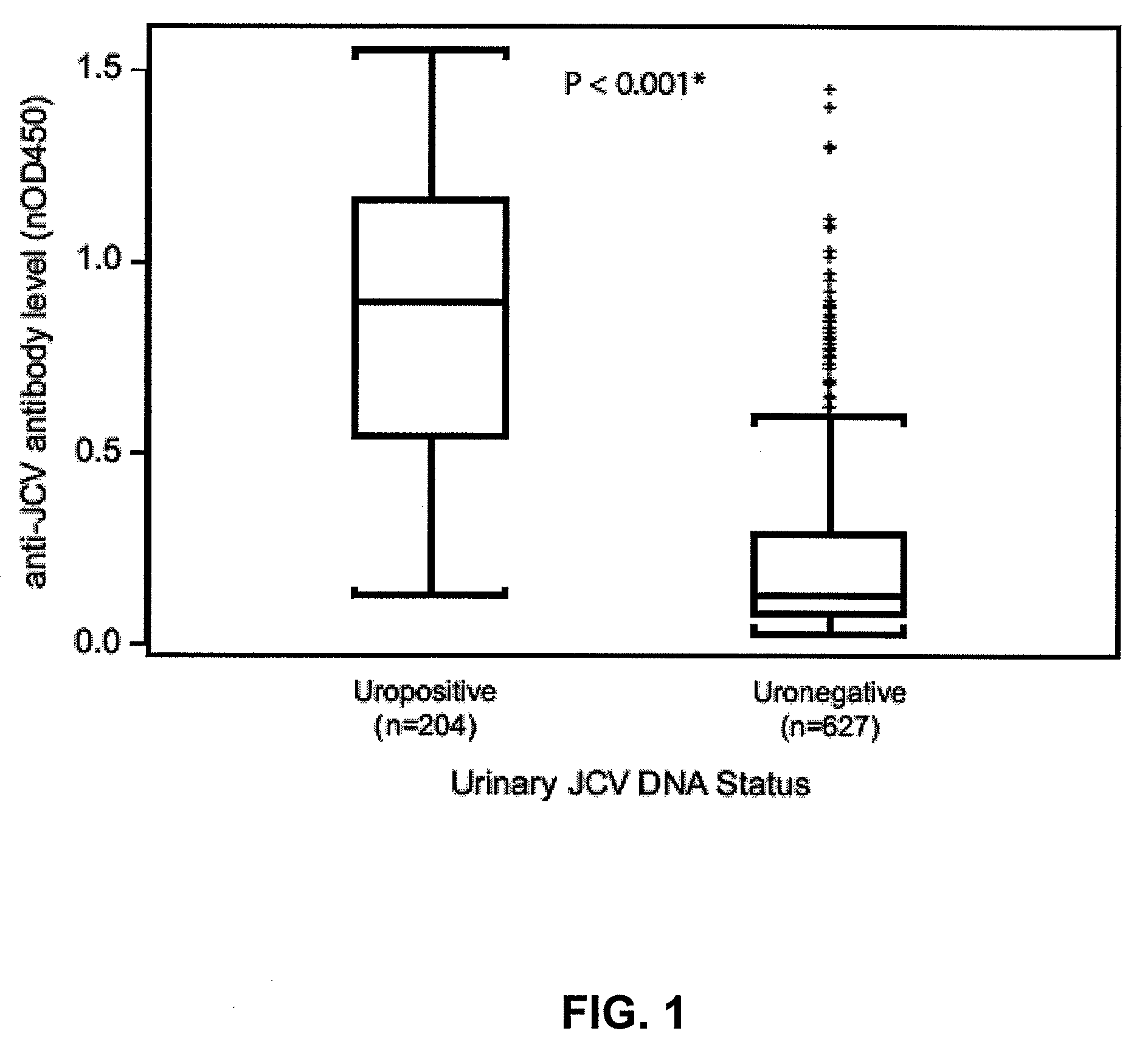
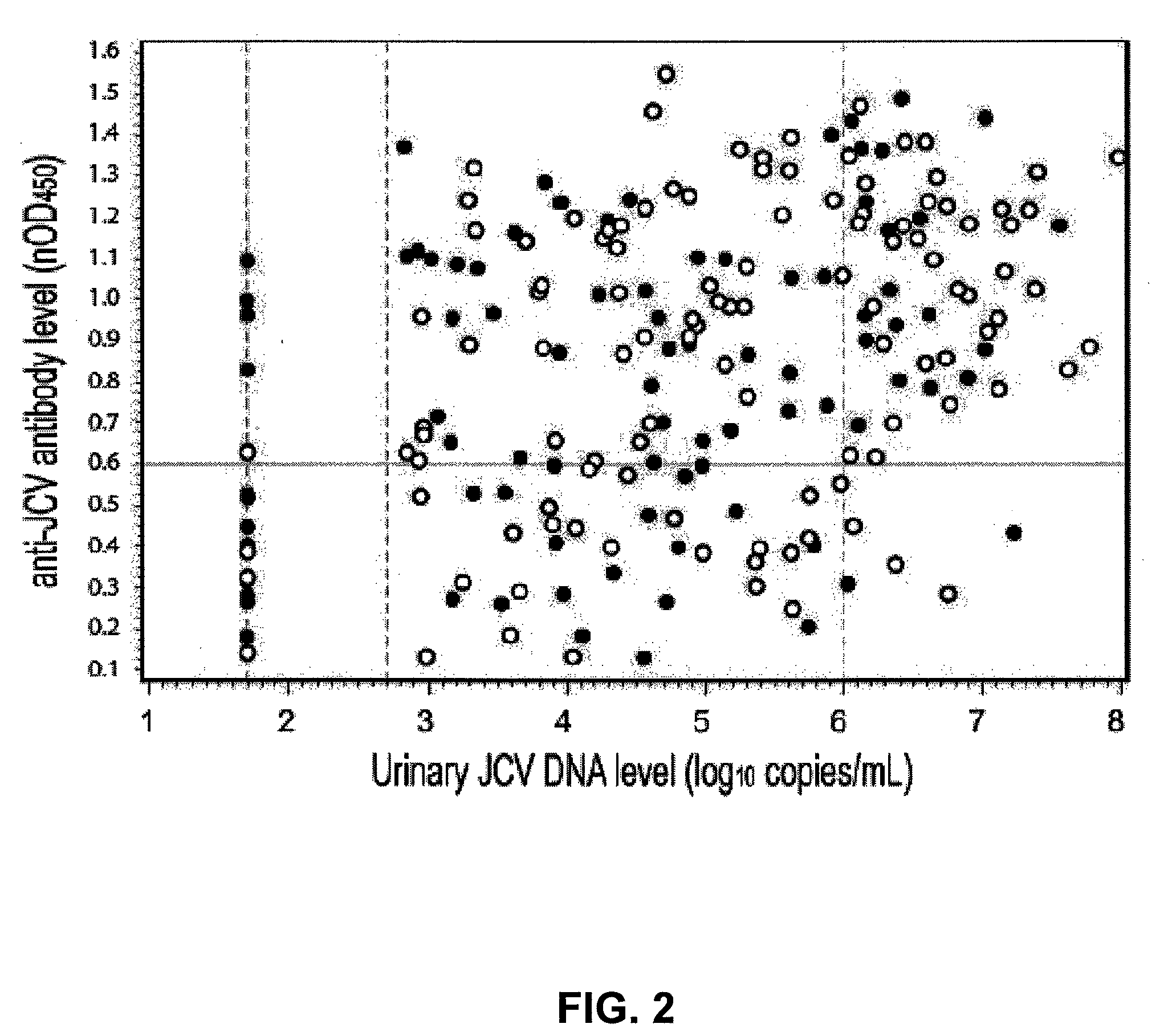
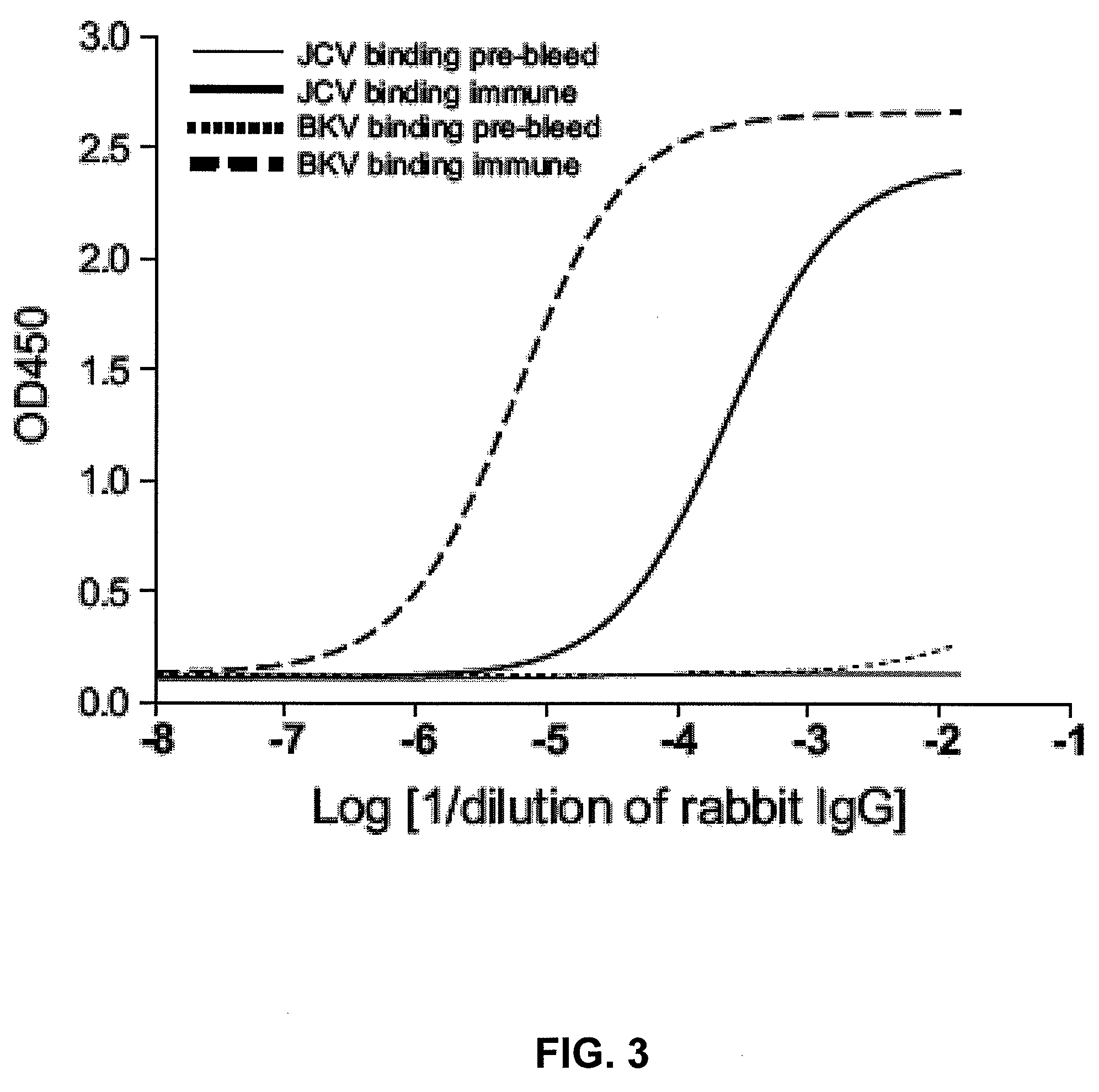
Assay for JC virus antibodies
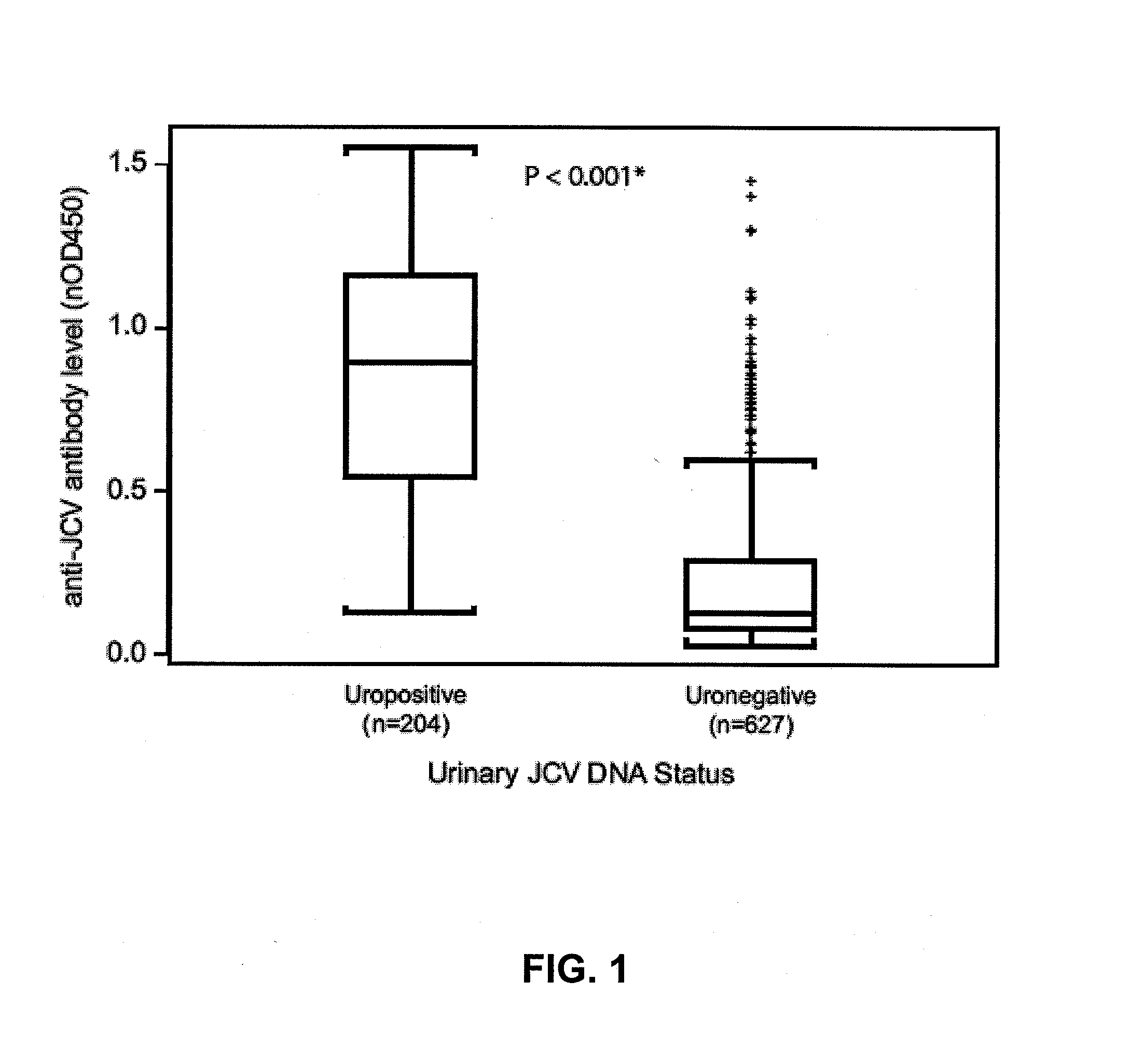
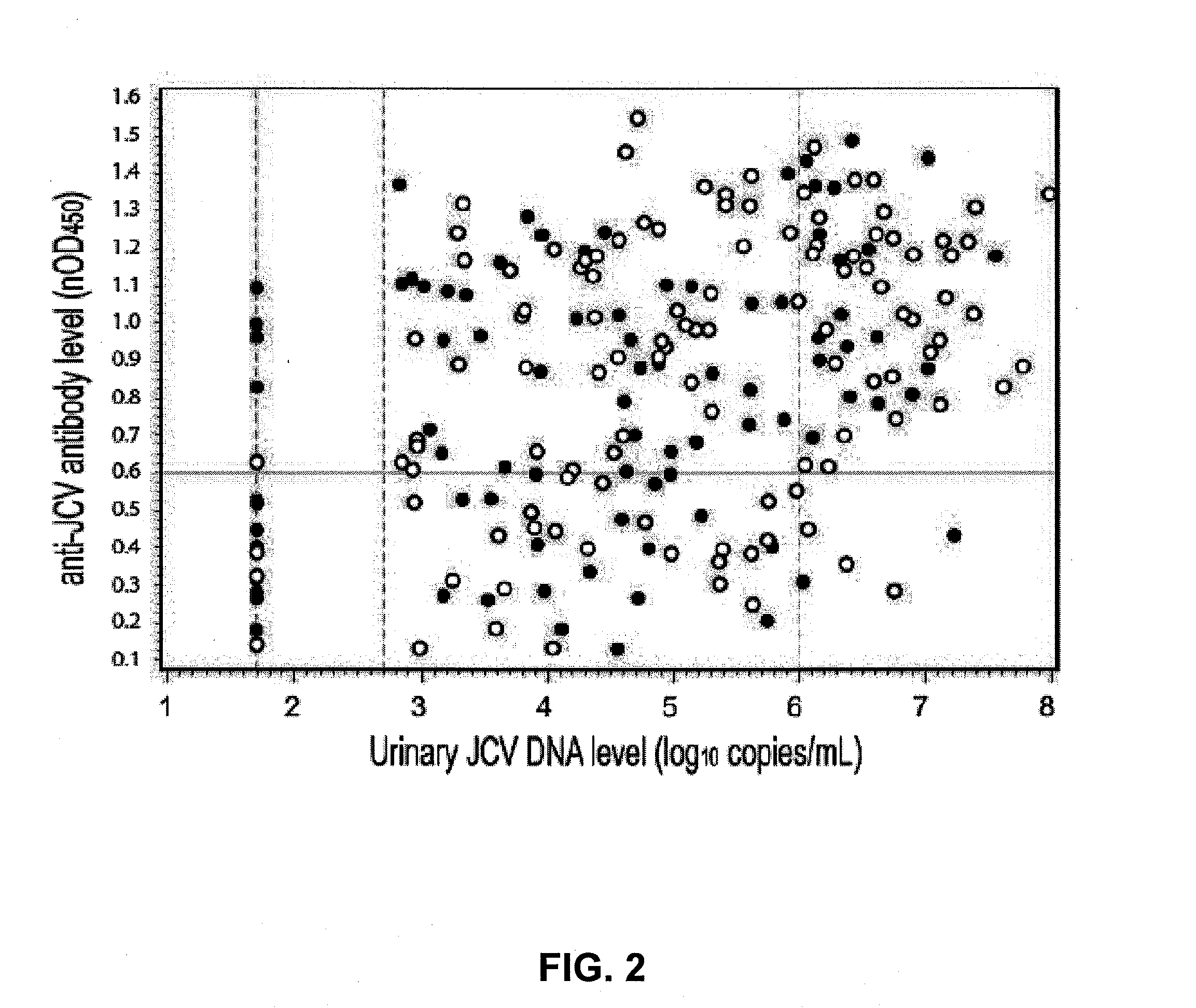
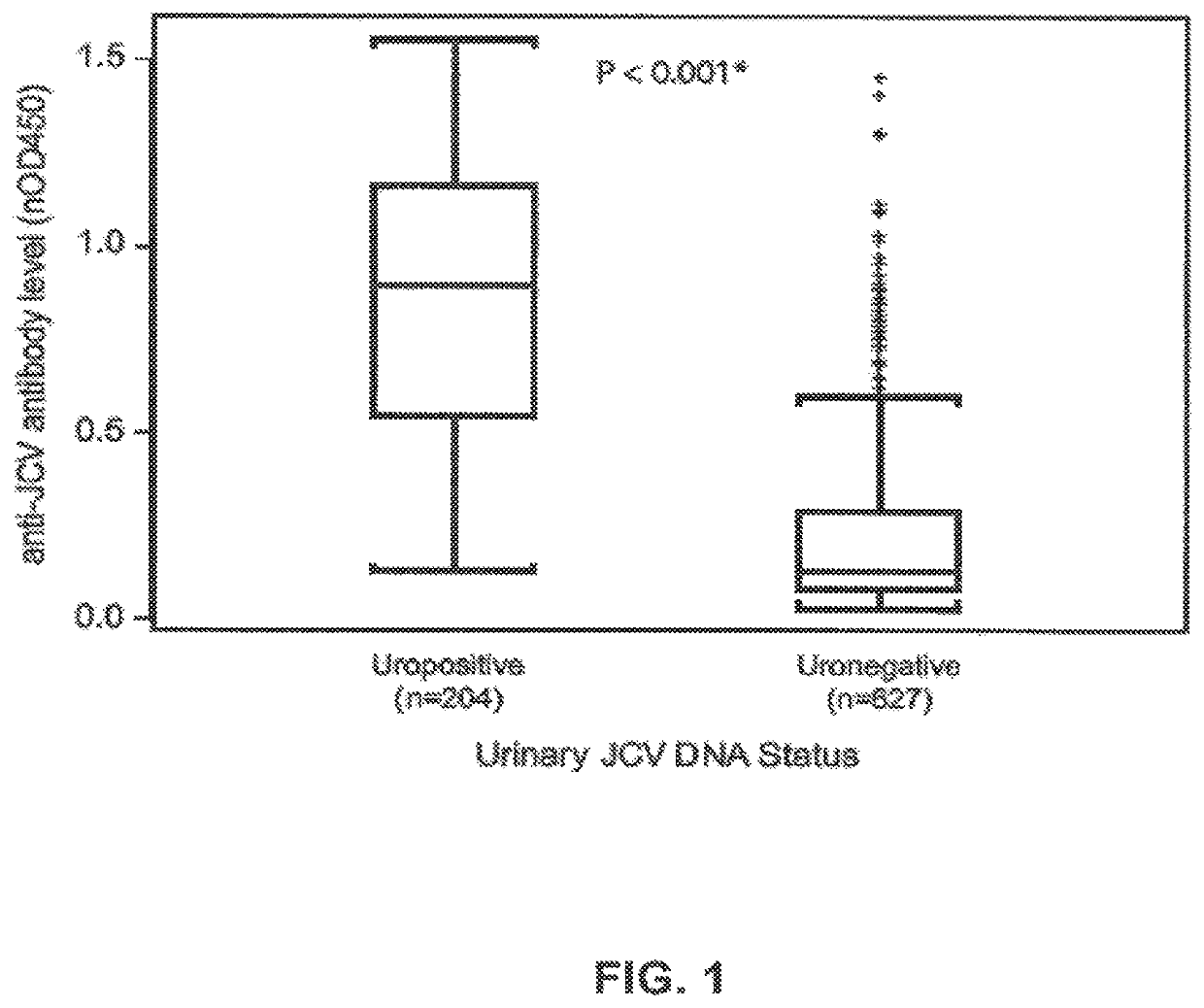
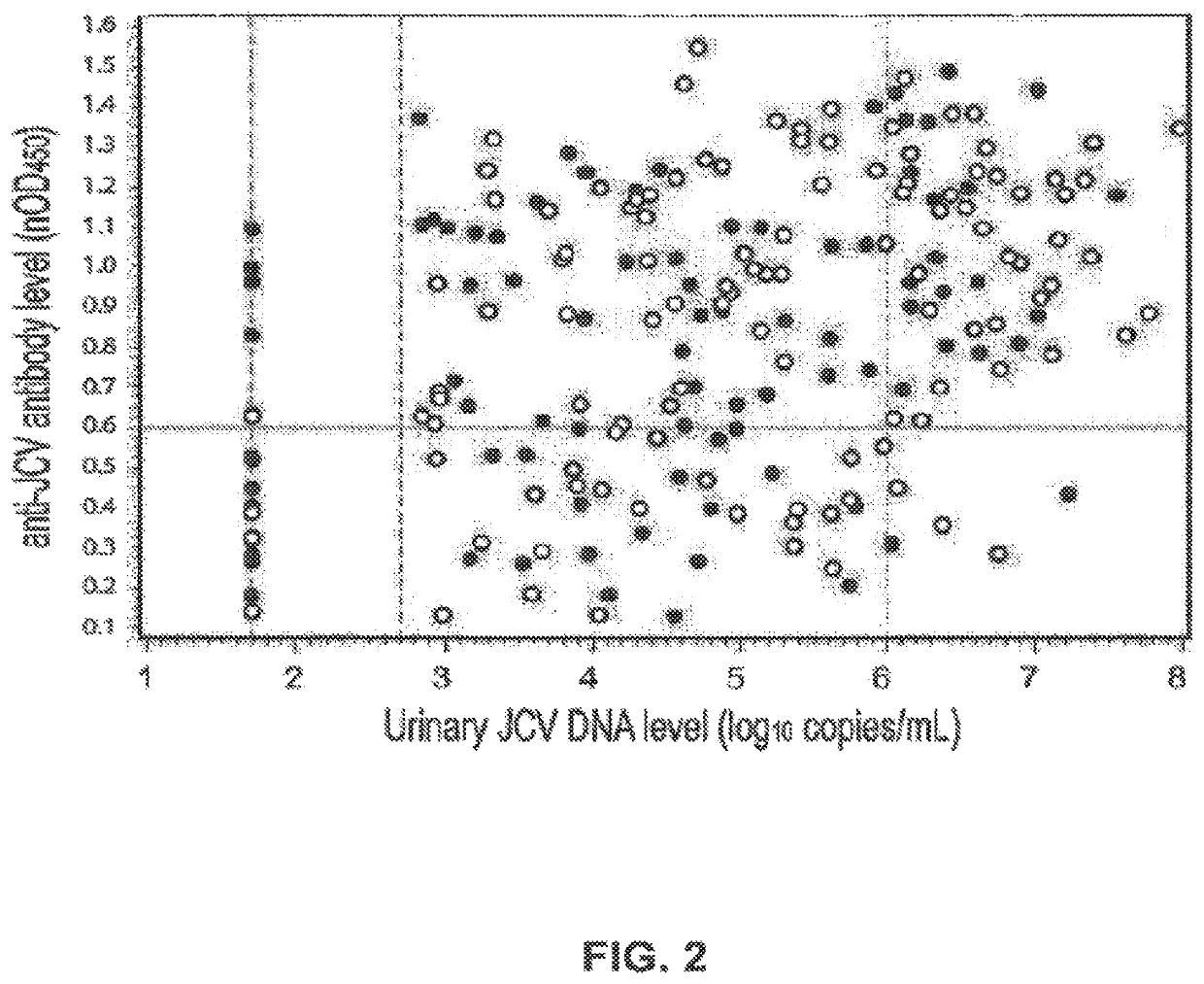
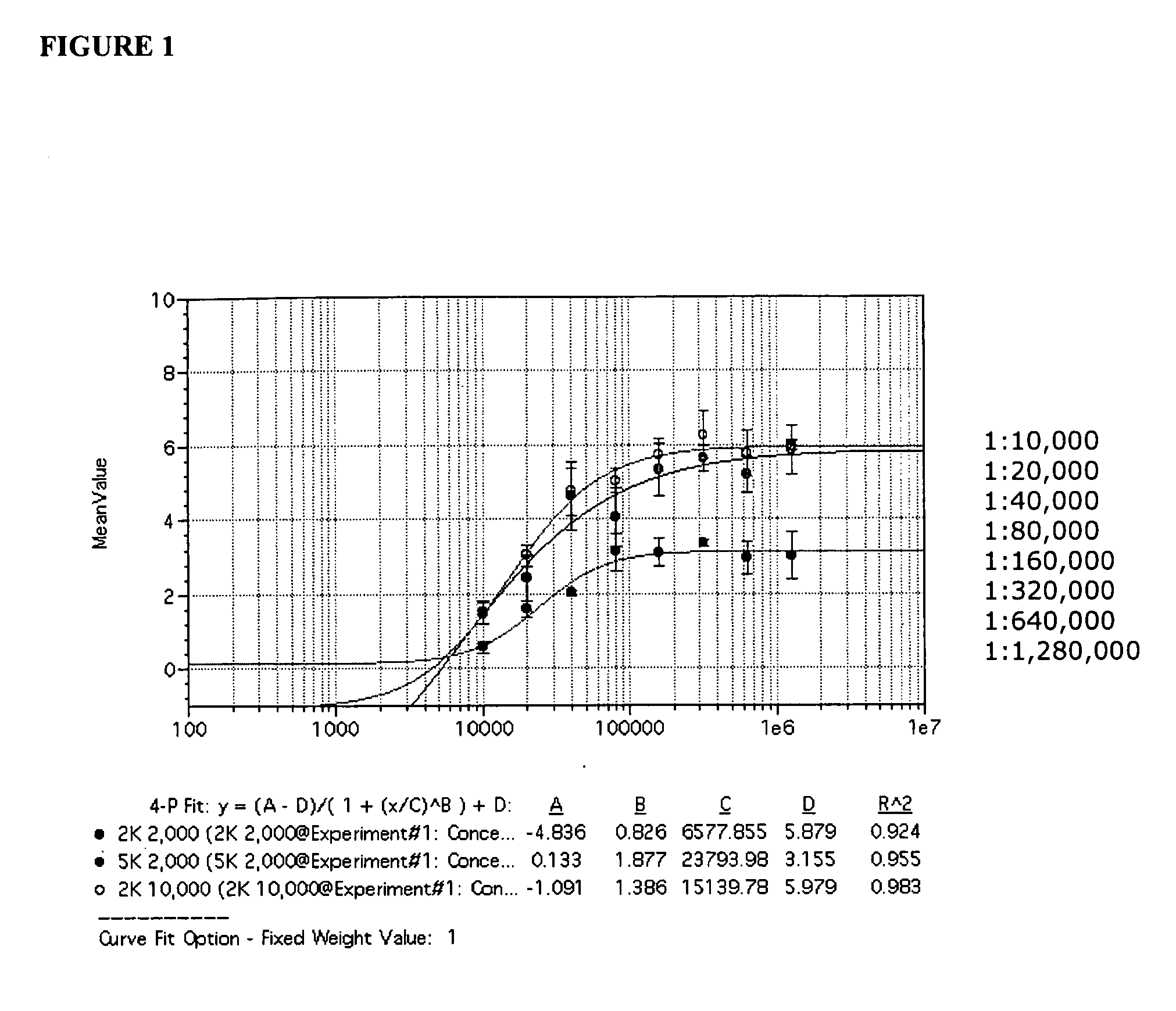
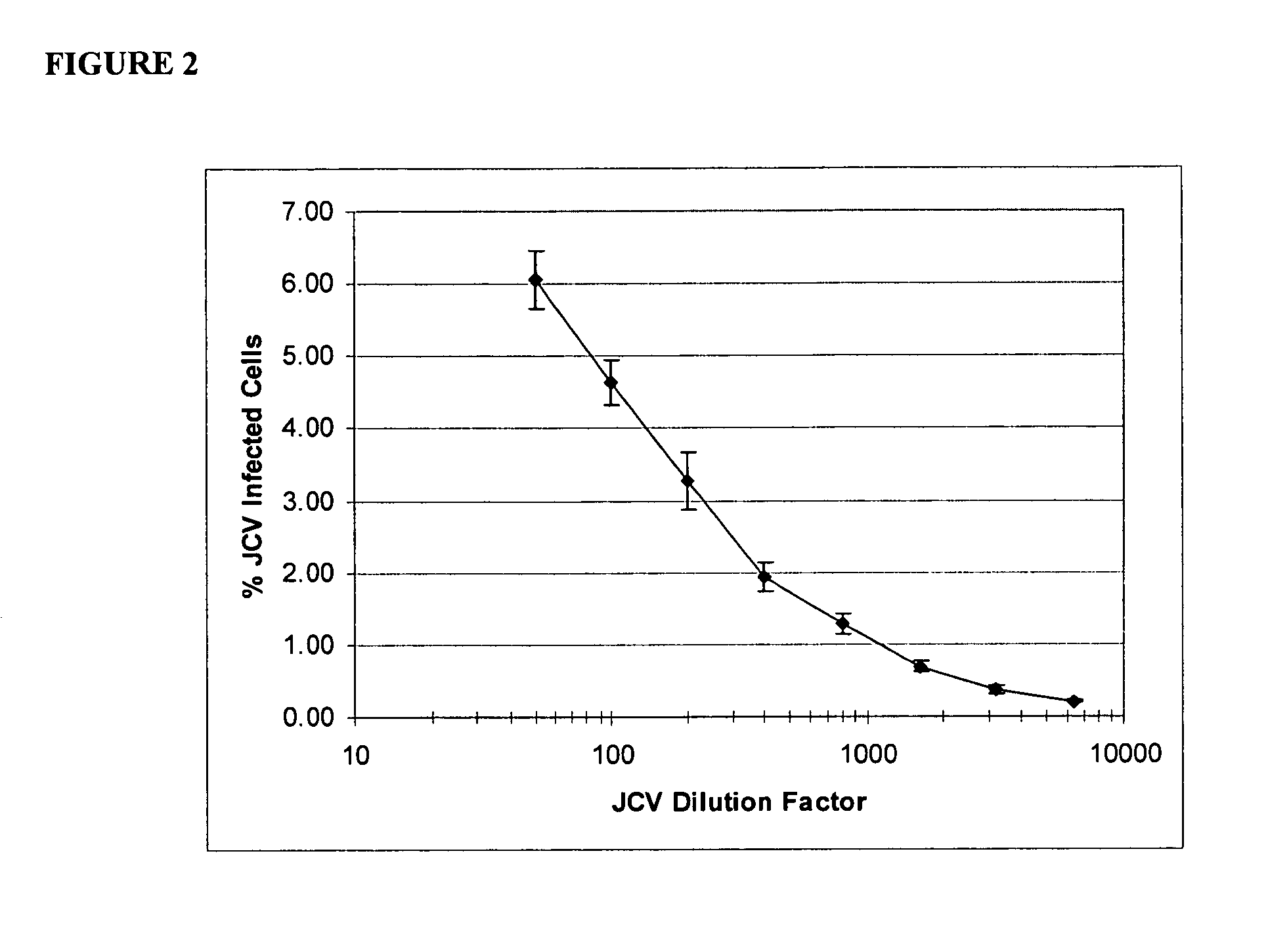
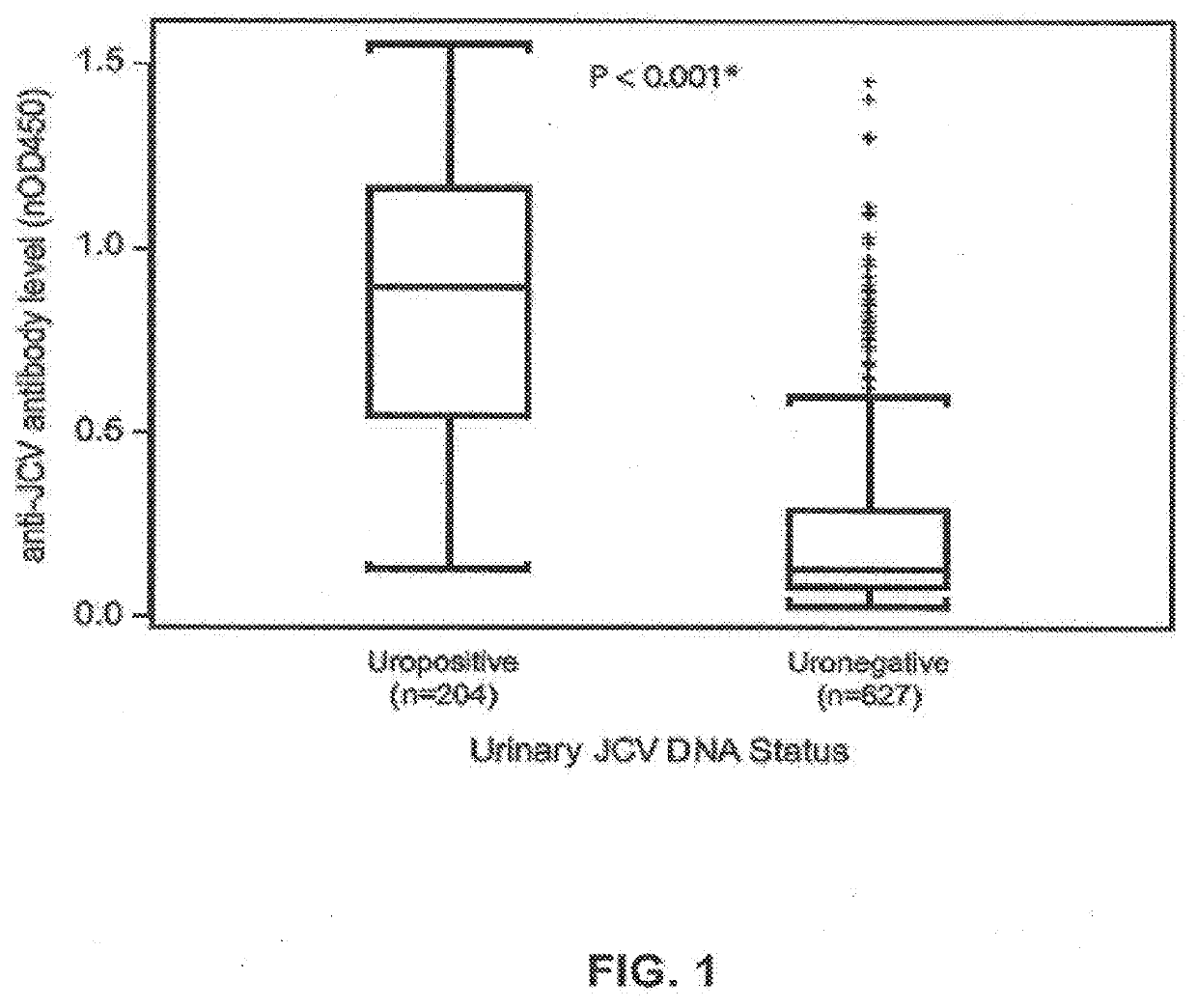
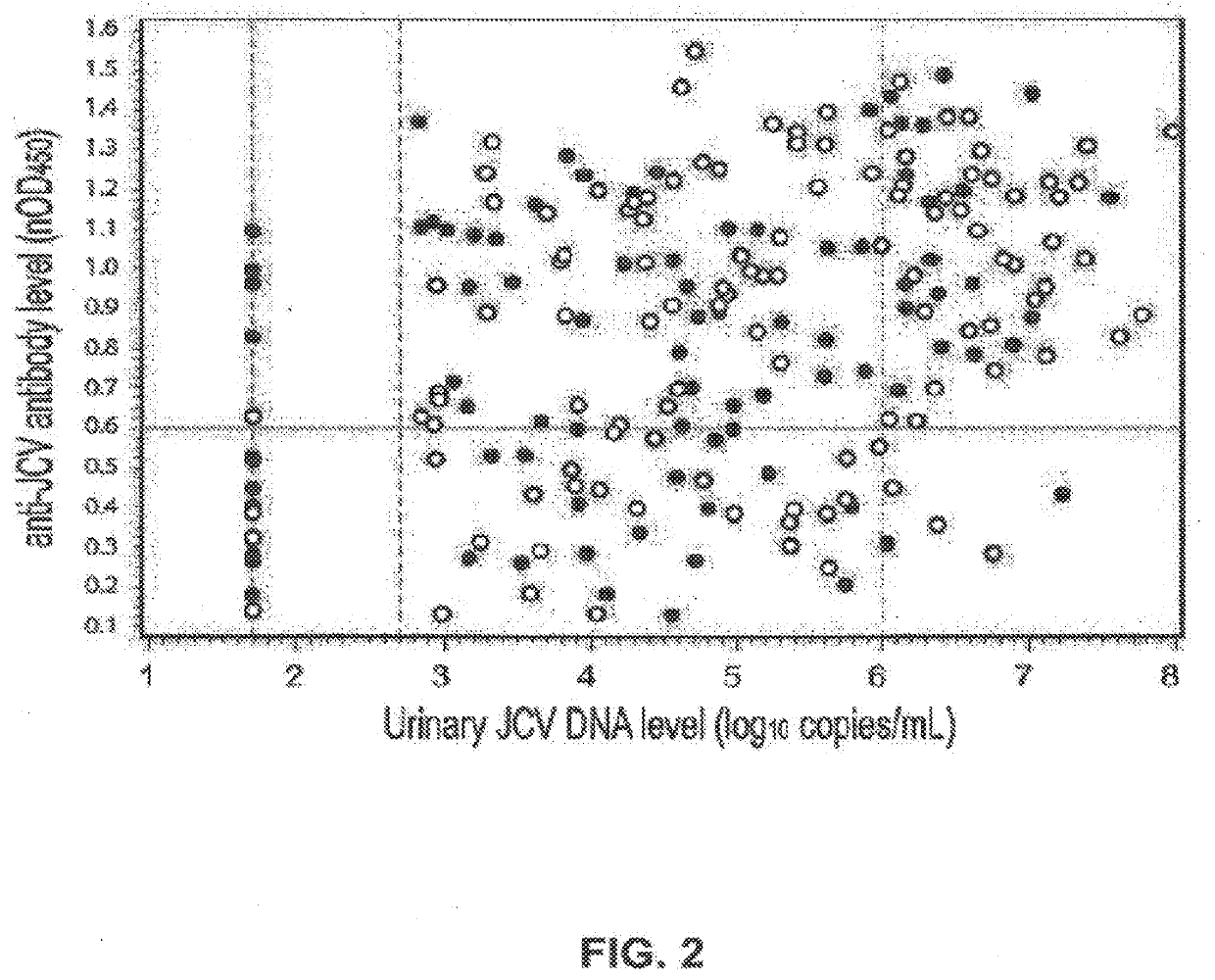
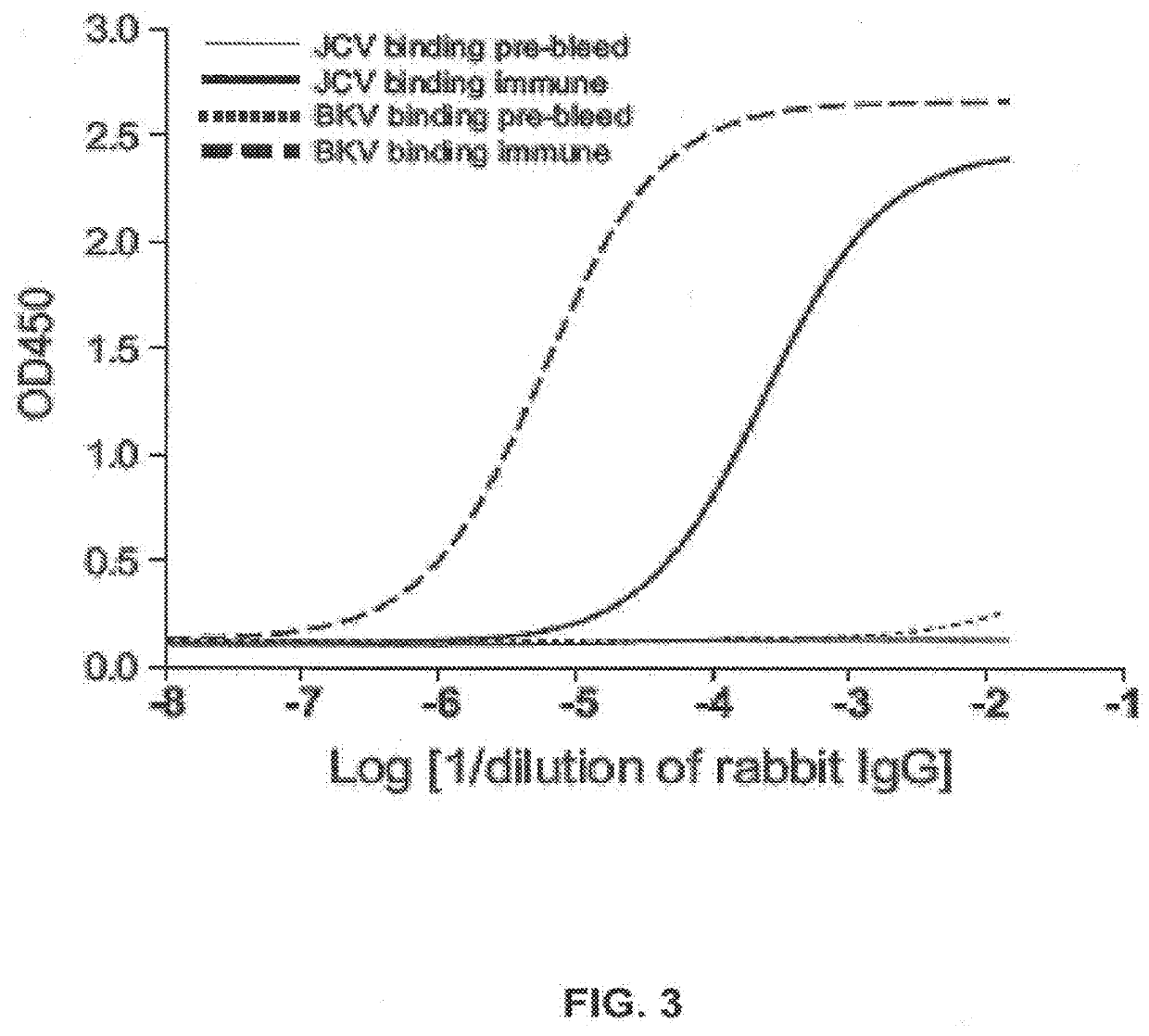
The disclosure relates to methods and reagents for analyzing samples for the presence of JC virus antibodies. Disclosed is a method that includes obtaining a biological sample from a subject (e.g., plasma, serum, blood, urine, or cerebrospinal fluid), contacting the sample with highly purified viral-like particles (HPVLPs) under conditions suitable for binding of a JCV antibody in the sample to an HPVLP, and detecting the level of JCV antibody binding in the sample to HPVLP. In one embodiment, determining the level of anti-JCV antibodies in the subject sample provides a method of identifying PML risk in a subject.
Owner:BIOGEN MA INC
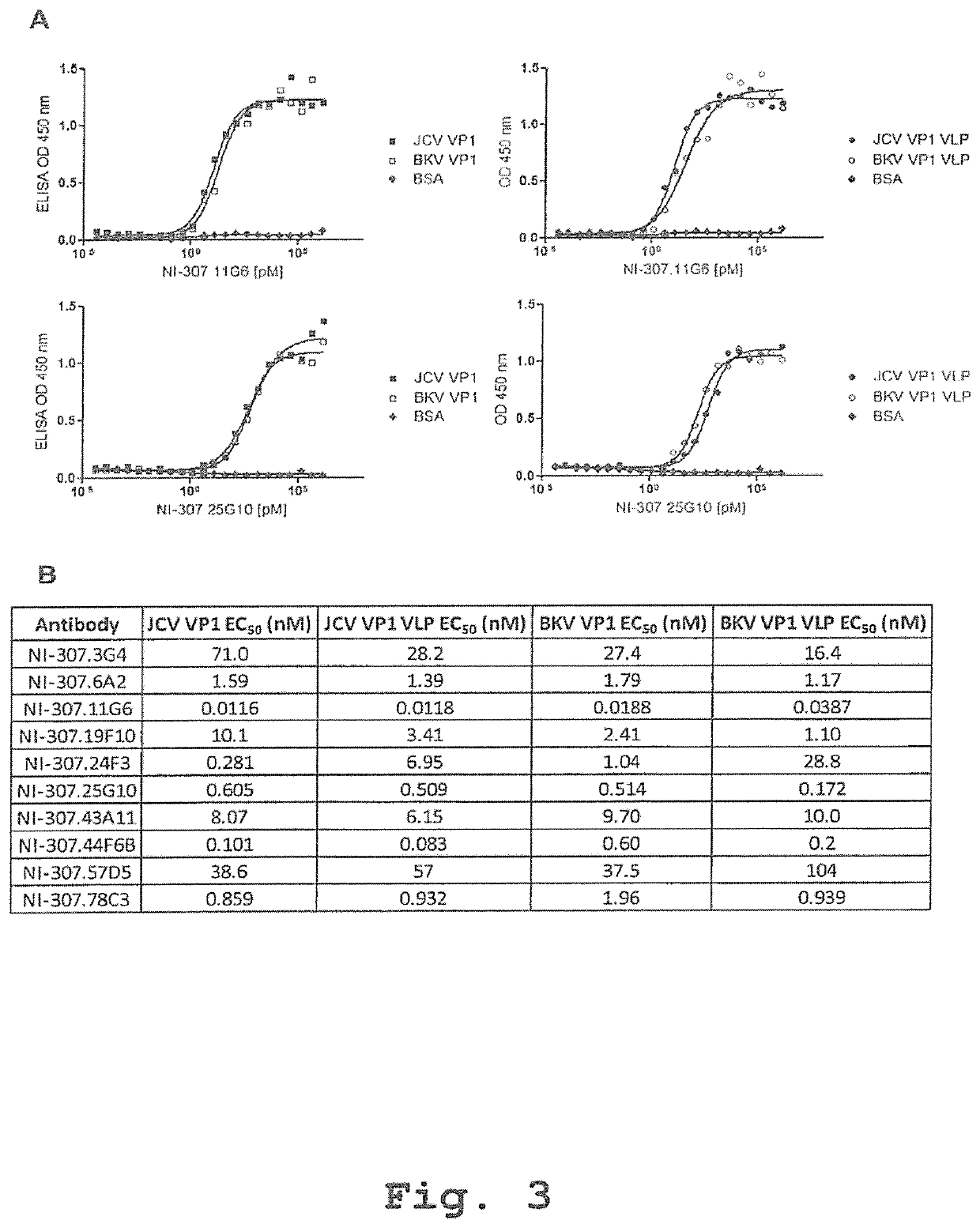
A human monoclonal antibody against the vp1 protein of jc virus
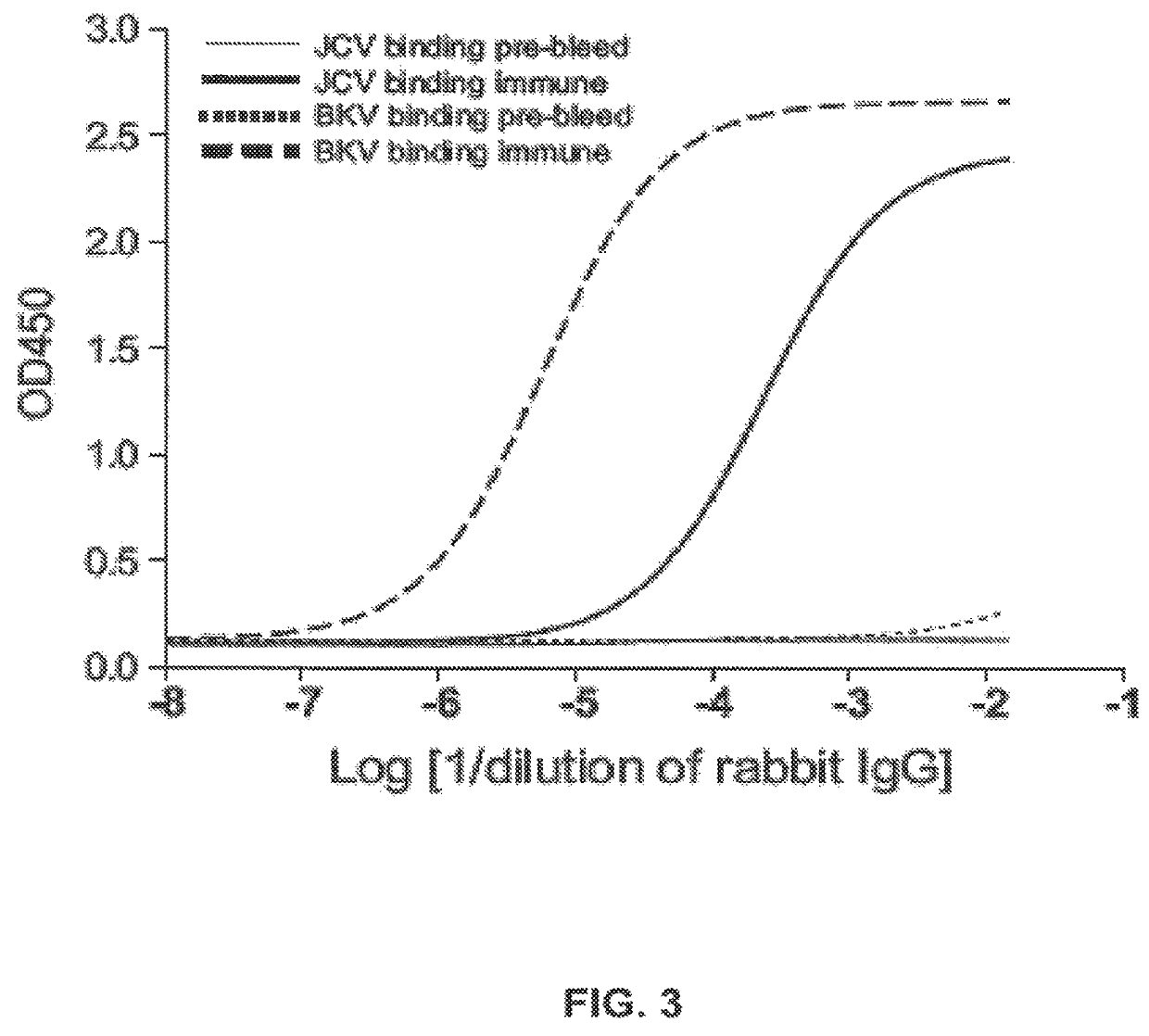
The invention relates to a human neutralizing monoclonal antibody directed against the VP1 protein of JC virus, that is the virus responsible for progressive multifocal leukoencephalopathy (PML), as well as the use thereof in a therapeutic or prophylactic treatment of a JCV infection or of a disease associated with a JCV infection, such as pro¬ gressive multifocal leukoencephalopathy (PML), and the use thereof in the diagnosis of JCV infections or of diseases associated with JCV infections.
Owner:POMONA RICERCA
Methods of treating inflammatory and autoimmune diseases with alpha-4 inhibitory compounds
InactiveUS8410115B2Improve securityMethod securityBiocideHeavy metal active ingredientsCrohn's diseaseAutoimmune responses
Owner:ELAN PHARM INC +1
Compositions and methods for inhibiting expression of a gene from the JC virus
The invention relates to a double-stranded ribonucleic acid (dsRNA) for inhibiting the expression of a gene from the JC Virus (JC virus genome), comprising an antisense strand having a nucleotide sequence which is less that 30 nucleotides in length, generally 19-25 nucleotides in length, and which is substantially complementary to at least a part of a gene from the JC Virus. The invention also relates to a pharmaceutical composition comprising the dsRNA together with a pharmaceutically acceptable carrier; methods for treating diseases caused by JC virus expression and the expression of a gene from the JC Virus using the pharmaceutical composition; and methods for inhibiting the expression of a gene from the JC Virus in a cell.
Owner:ALNYLAM PHARMA INC
Assay for jc virus antibodies
ActiveUS20130022961A1Treatment being stoppedMicrobiological testing/measurementInactivation/attenuationAssayPolyomavirus JC
The disclosure relates to methods and reagents for analyzing samples for the presence of JC virus antibodies. Disclosed is a method that includes obtaining a biological sample from a subject (e.g., plasma, serum, blood, urine, or cerebrospinal fluid), contacting the sample with highly purified viral-like particles (HPVLPs) under conditions suitable for binding of a JCV antibody in the sample to an HPVLP, and detecting the level of JCV antibody binding in the sample to HPVLP. In one embodiment, determining the level of anti-JCV antibodies in the subject sample provides a method of identifying PML risk in a subject.
Owner:BIOGEN MA INC
JC Virus Vaccine
InactiveUS20080057079A1Easy to useImprove stabilityViral antigen ingredientsAntiviralsPolyomavirus JCVirology
Owner:BAYLOR RES INST
Compositions and methods for inhibiting expression of a gene from the jc virus
InactiveUS20090062225A1Inhibit expressionOrganic active ingredientsNervous disorderDiseaseNucleotide
The invention relates to a double-stranded ribonucleic acid (dsRNA) for inhibiting the expression of a gene from the JC Virus (JC virus genome), comprising an antisense strand having a nucleotide sequence which is less that 30 nucleotides in length, generally 19-25 nucleotides in length, and which is substantially complementary to at least a part of a gene from the JC Virus. The invention also relates to a pharmaceutical composition comprising the dsRNA together with a pharmaceutically acceptable carrier; methods for treating diseases caused by JC virus expression and the expression of a gene from the JC Virus using the pharmaceutical composition; and methods for inhibiting the expression of a gene from the JC Virus in a cell.
Owner:ALNYLAM PHARMA INC
Primers, probe and kit used for detecting JC viruses (JCVs)
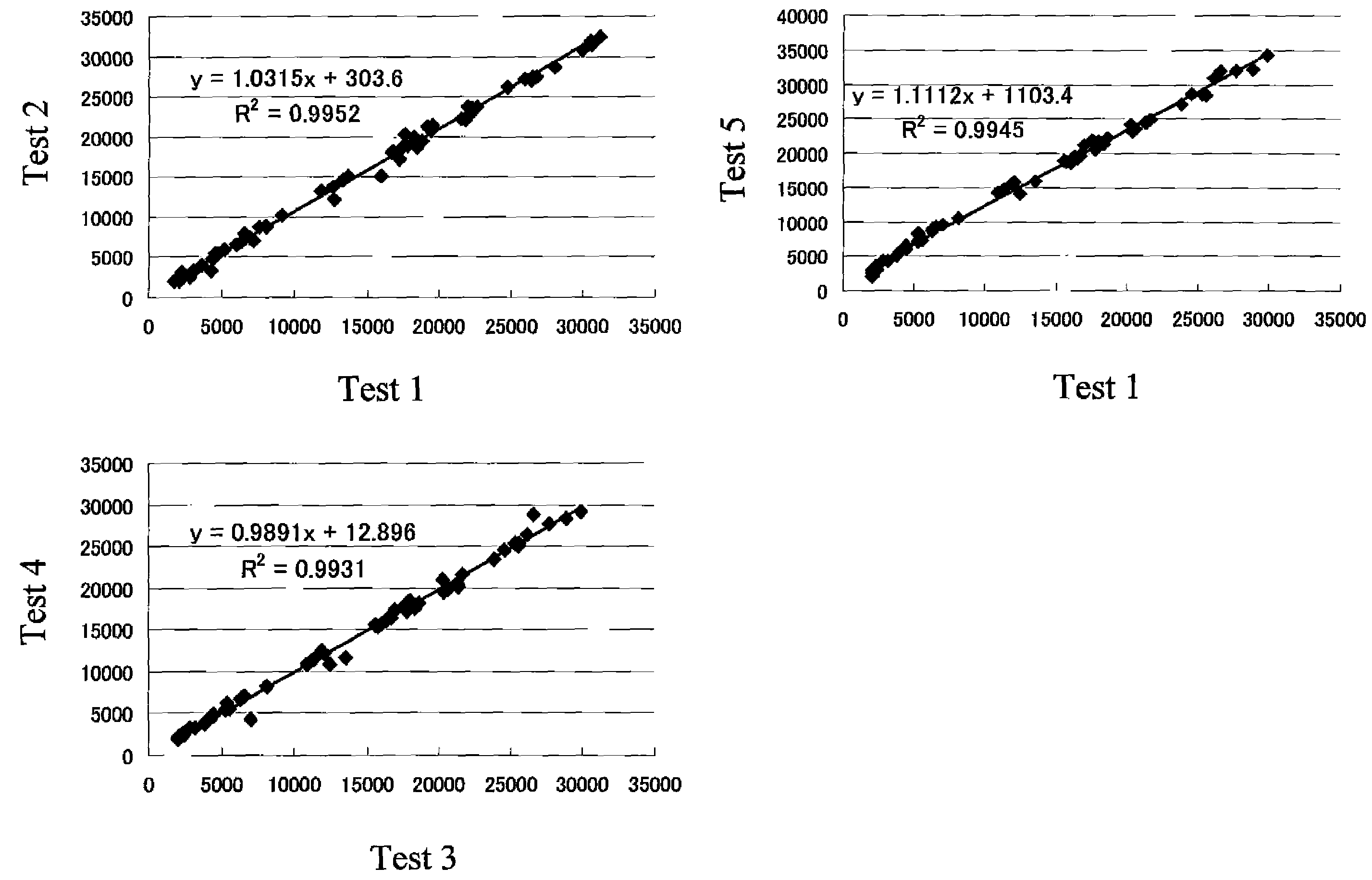
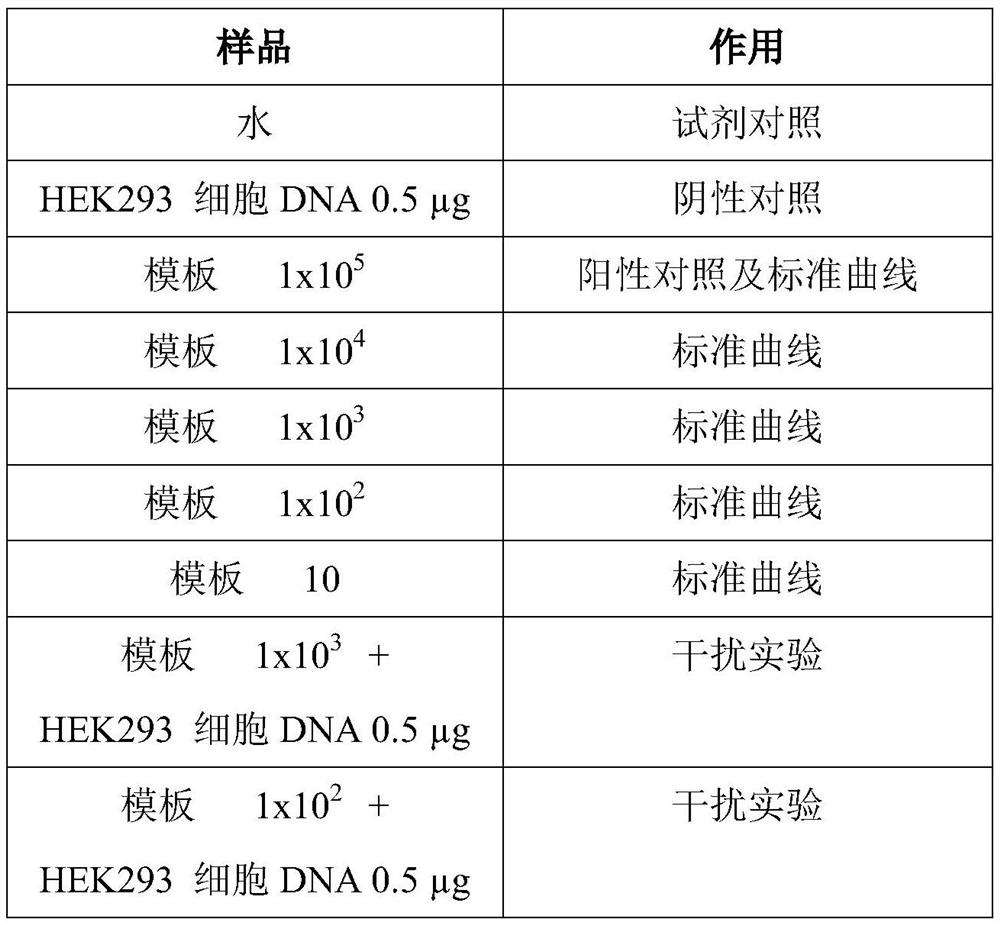
InactiveCN104745724AHigh sensitivityAccurate detectionMicrobiological testing/measurementMicroorganism based processesForward primerPolyomavirus JC
The invention discloses primers, probe and kit used for detecting JC viruses (JCVs), belonging to the technical field of biology. The primers and probe comprise a forward primer, a reverse primer and a probe, which are used for detecting JCVs. The kit comprises the primers and the probe. The primers, the probe and the kit have the characteristic of high sensitivity and can be used for accurately detecting whether samples are infected with the JCVs.
Owner:湖北永邦医疗科技有限公司
JC virus detection method, kit and application of kit
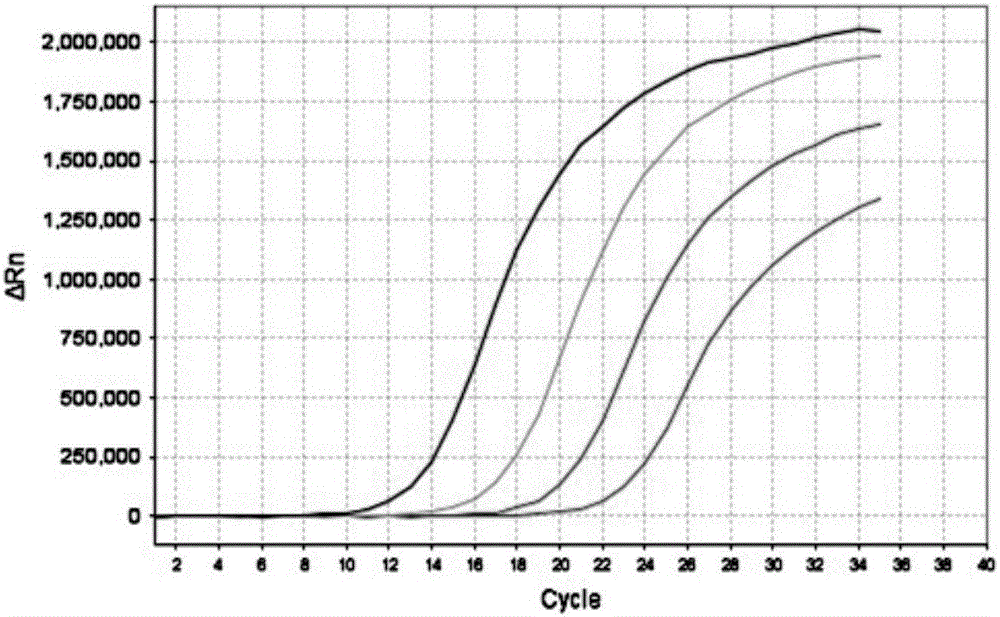
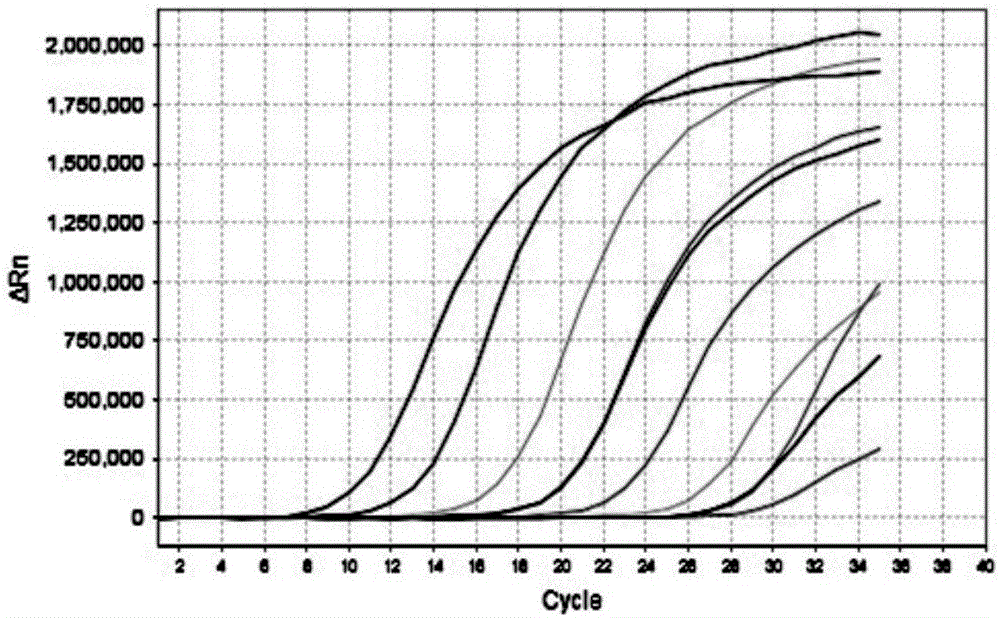
InactiveCN106048093AEfficient extractionAccurate CalibrationMicrobiological testing/measurementPolyomavirus JCMagnetic bead
The invention discloses a JC virus detection method. The JC virus detection method comprises the following steps: extracting JCV DNA from human urine sample by utilizing a paramagnetic particle method, thereby obtaining a sample to be detected; performing PCR amplification reaction; utilizing a fluorescent quantitative PCR meter to detect reaction results, and when collecting fluorescent signals, setting fluorescein corresponding to a fluorescent group at Taqman fluorescent probe JCV-FP 5' end to collect the fluorescent signals at 60 DEG. The invention further provides a kit for detecting the JC virus. The detection method is high in sensitivity, can detect to 500 copies / mL at the lowest extent; and meanwhile, the specificity of the detection method is good, and according to the detection method, and no cross reaction with other virus occurs, such as BK virus (BKV), hepatitis C virus (HCV), human cytomegalovirus (HCMV), and hepatitis B virus (HBV).
Owner:BEIJING SEARCH BIOTECH
Oligonucleotide probes and microarray for determining genome types
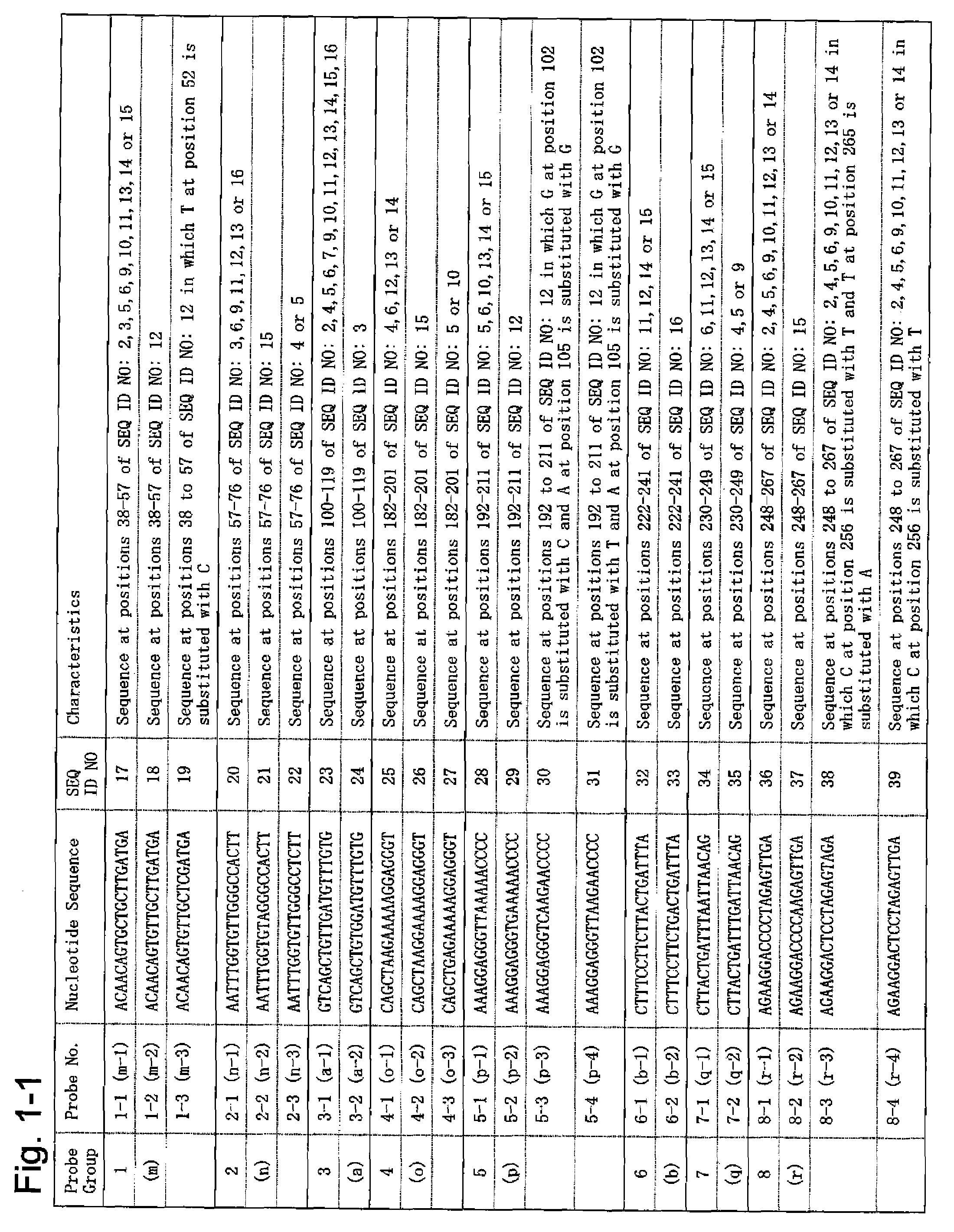
InactiveUS20080188376A1Determined quickly and convenientlyQuickly and conveniently determineSugar derivativesOrganic chemistry methodsPolyomavirus JCGenome
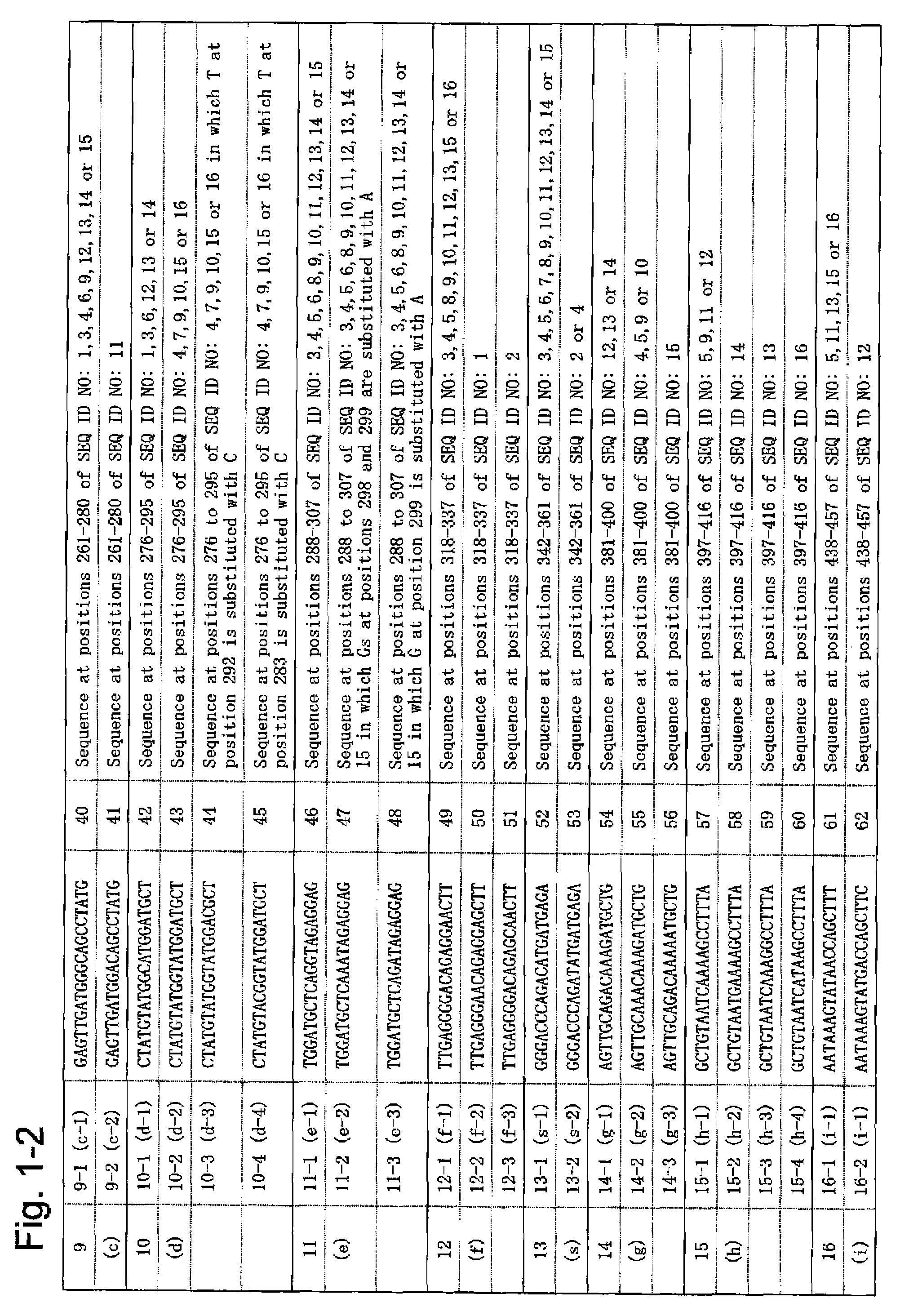
According to the present invention, a means of estimating the place of origin of a test subject by quickly and conveniently determining the genome type of JC virus infecting a test subject is provided. The present invention relates to a set of oligonucleotide probes for determining the genome type of JC virus infecting a test subject, such set containing: oligonucleotide probes (a-1) and (a-2); oligonucleotide probes (b-1) and (b-2); oligonucleotide probes (c-1) and (c-2); oligonucleotide probes (d-1), (d-2), (d-3), and (d-4); oligonucleotide probes (e-1), (e-2), and (e-3); oligonucleotide probes (f-1), (f-1), and (f-3); oligonucleotide probes (g-1), (g-2), and (g-3); oligonucleotide probes (h-1), (h-2), (h-3), and (h-4); oligonucleotide probes (i-1) and (i-2); oligonucleotide probes (j-1), (j-2), and (j-3); oligonucleotide probes (k-1), (k-2), and (k-3); and oligonucleotide probes (l-1) and (l-2).
Owner:TOYO KOHAN CO LTD +1
Compositions and methods for the treatment of progressive multifocal leukoencephalopathy (PML)
ActiveUS9233107B2Reduce riskOther blood circulation devicesBoron compound active ingredientsPolyomavirus JCDna viral
The invention relates to compositions, methods, and kits for treating subjects infected by or at risk of infection with a DNA virus (e.g., a JC Virus or a BK virus). Aspects of the invention are useful to prevent or treat DNA virus associated conditions (e.g., PML) in subjects that are immunocompromised. Compositions are provided that inhibit intracellular replication of DNA viruses.
Owner:BIOGEN MA INC
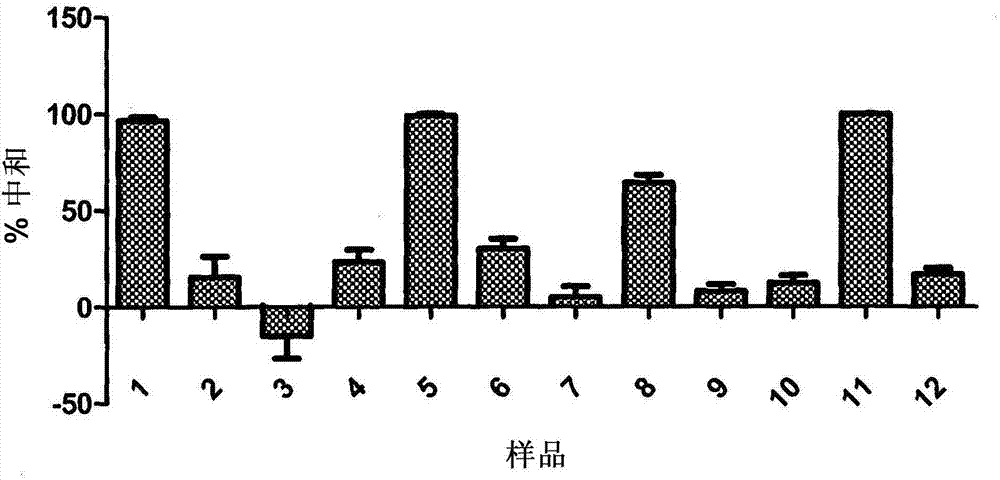
JCV neutralizing antibodies
ActiveUS9567391B2Immunoglobulins against virusesAntibody ingredientsPolyomavirus JCMonoclonal antibody
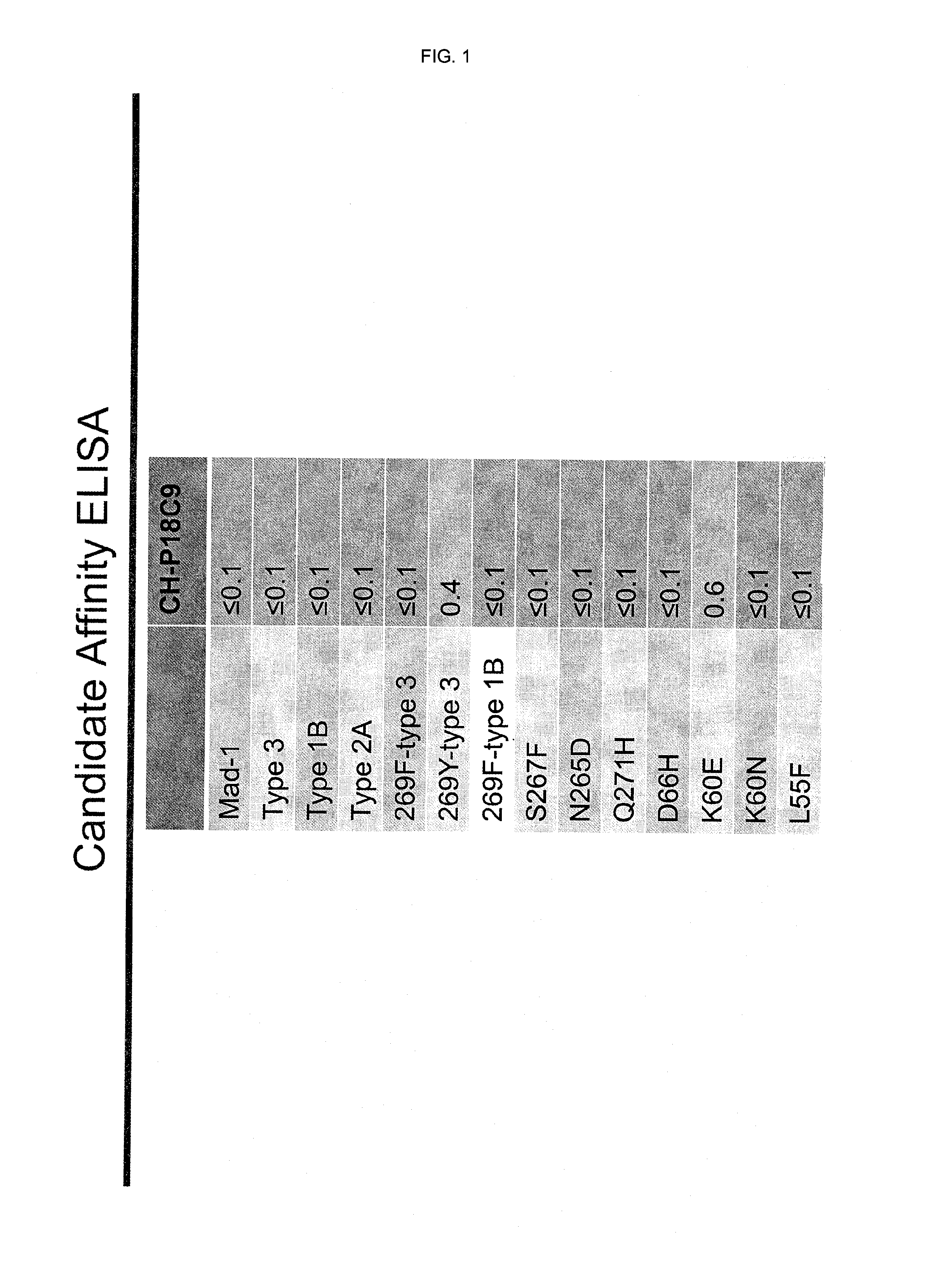
In one aspect, the disclosure provides neutralizing antibodies against JCV and methods for the treatment of PML. In some embodiments, aspects of the invention relate to an isolated JC-virus neutralizing monoclonal antibody against JCV capsid protein VPI (JCV-VP1). In some embodiments, the antibody suppresses infectivity of the JC-virus. In some embodiments, the antibody binds the sialic acid binding pocket of JCV-VPI. In some embodiments, the antibody binds JCV-VP 1 comprising one or more of the following mutations: S269F, S269Y, S267F, N265D, Q271 H, D66H, K60E, K60N and L55F.
Owner:BIOGEN MA INC
Assay for detection of jc virus dna
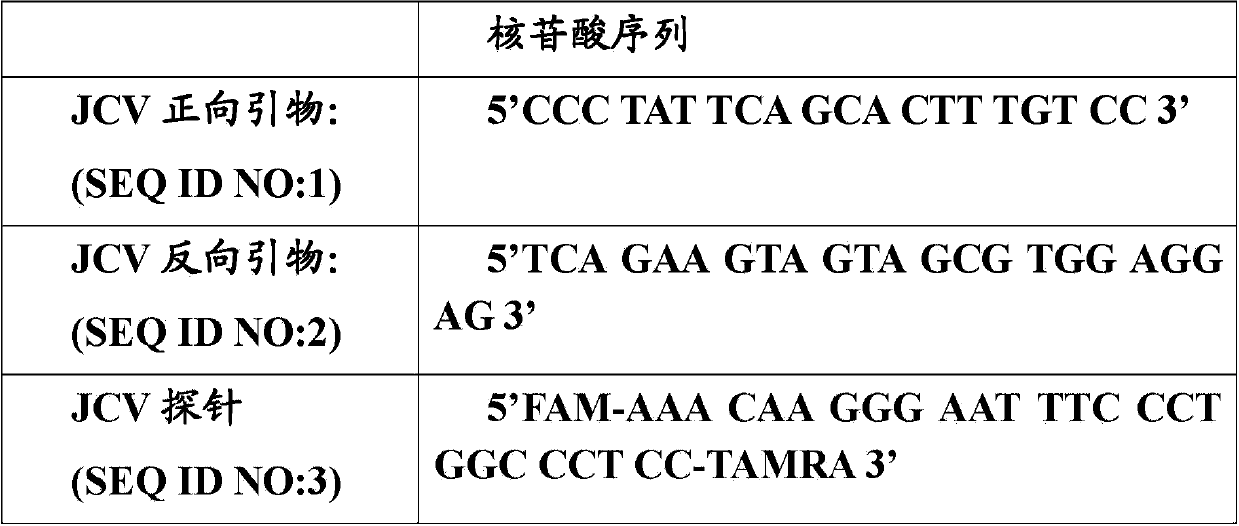
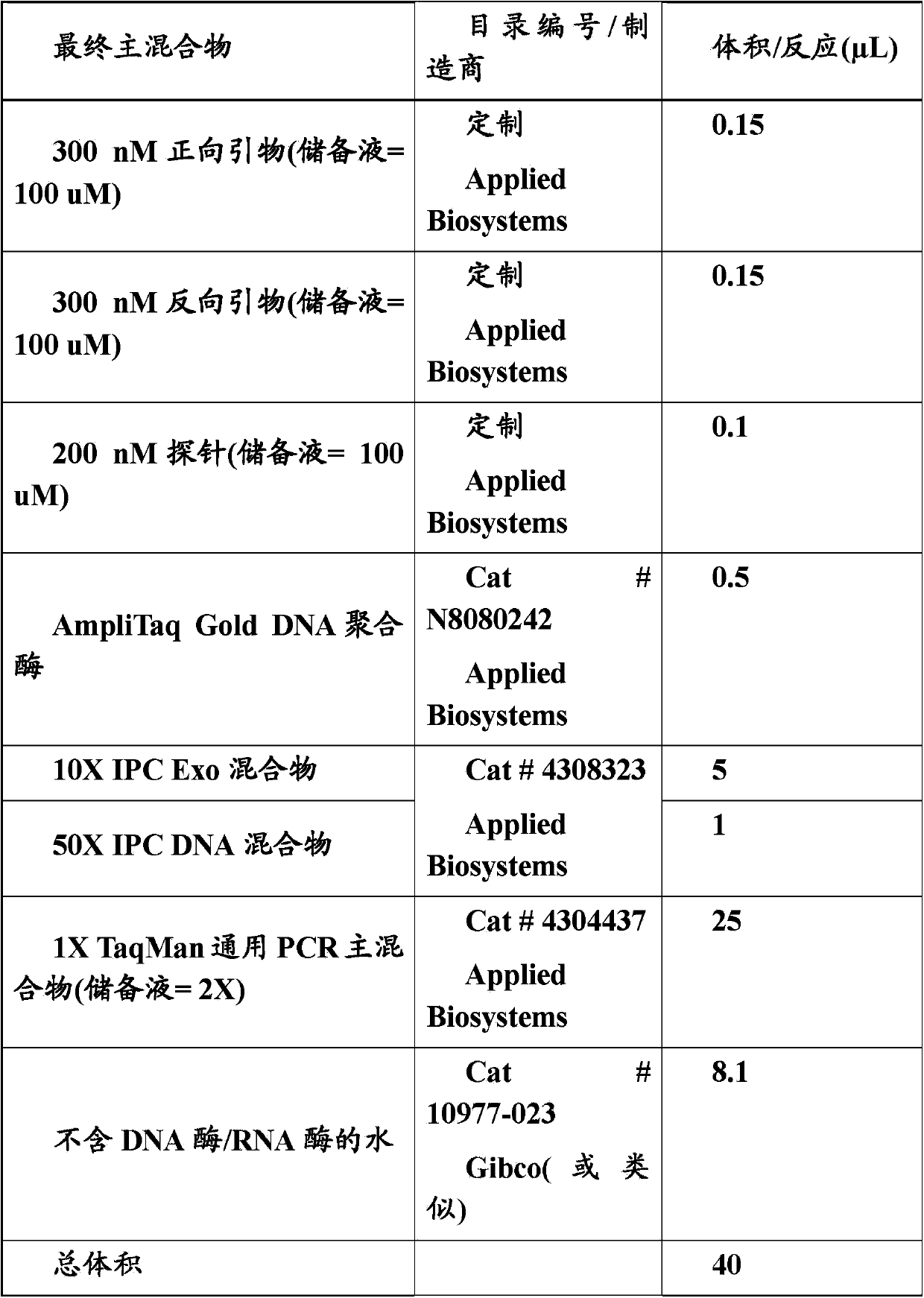
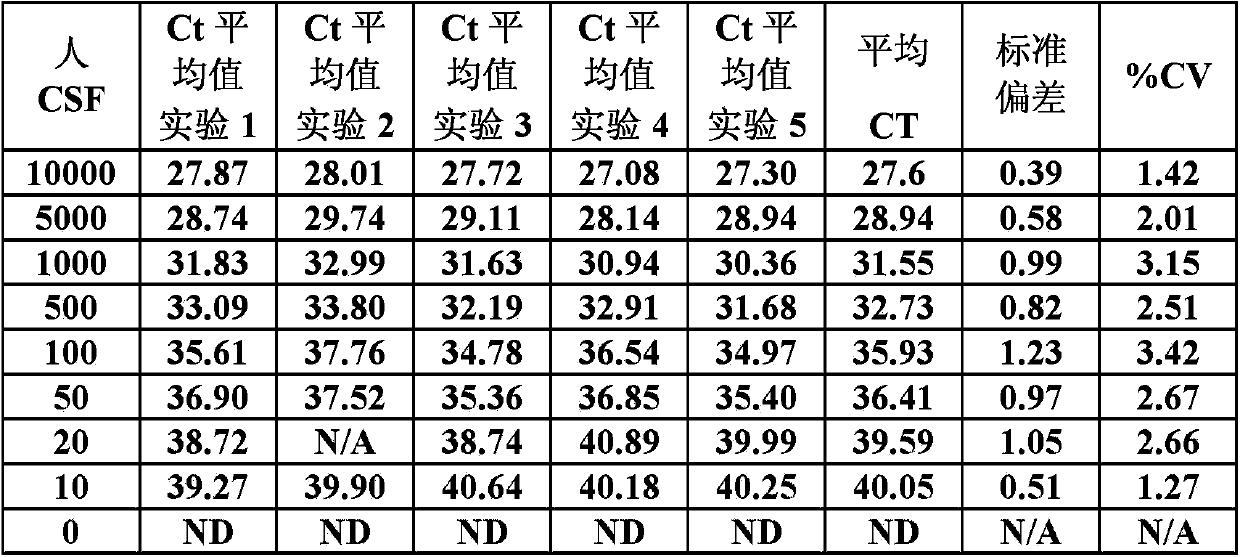
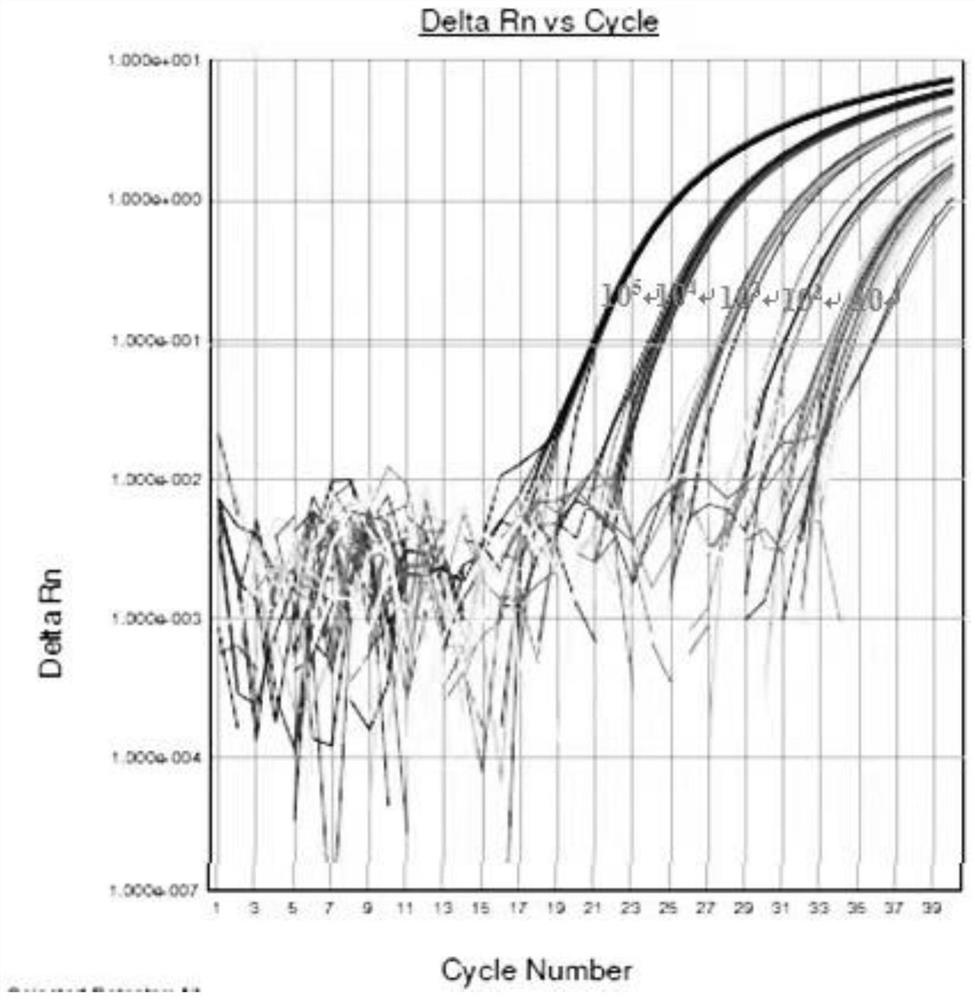
In one aspect, the disclosure provides methods for isolating nucleic acid from a Cerebrospinal Fluid (CSF) sample. In one aspect, the disclosure provides methods for determining the amount of JC virus DNA in a sample.
Owner:BIOGEN MA INC
Assay for detection of jc virus DNA
InactiveUS20140255915A1Raise the incident rateImproved prognosisSugar derivativesMicrobiological testing/measurementAssayDna test
In one aspect, the disclosure provides methods for isolating nucleic acid from a Cerebrospinal Fluid (CSF) sample. In one aspect, the disclosure provides methods for determining the amount of JC virus DNA in a sample.
Owner:BIOGEN IDEC MA INC
Polyomavirus cellular epitopes and uses therefor
ActiveUS7468186B2Reduce frequencyPeptide/protein ingredientsViral antigen ingredientsRenal transplantPolyomavirus JC
The present invention relates to HLA-A*02-restricted cellular epitopes within the VP1 polypeptide of a human polyomavirus, BK virus, which is associated with polyomavirus-associated nephropathy in kidney transplant patients. Preferred peptides correspond to amino acids residues 107-116, 108-116 and 44-52 of BKV VP1, and are processed in vivo in natural infection with BKV. Effector T cell populations stimulated by the peptides represent functional CTLs as assessed by cytotoxicity and cytokine production, and are reactive against cells presenting both the BKV peptides above and the JC virus homolog sequences.
Owner:CITY OF HOPE
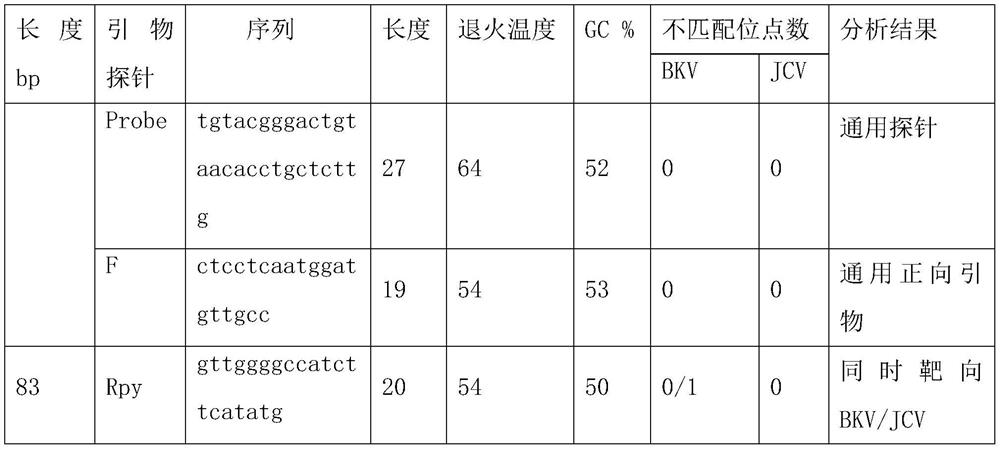
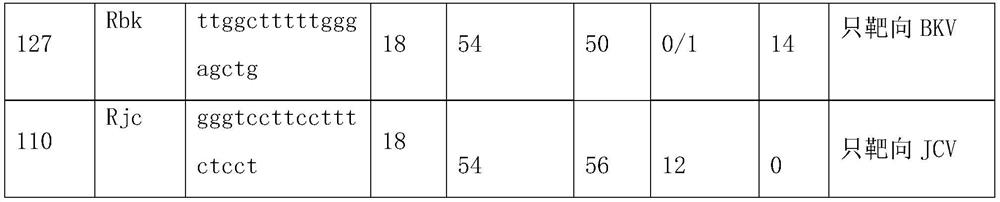
Primer group and probe group capable of simultaneously detecting BK virus and JC virus, and purpose
PendingCN112522440AEfficient identificationAccurate identificationMicrobiological testing/measurementMicroorganism based processesNucleotidePolyomavirus JC
The invention relates to the field of medical molecular biology detection, in particular to a primer and a probe capable of simultaneously detecting a BK virus and a JC virus, and a purpose. The invention provides a primer group capable of simultaneously detecting the BK virus and the JC virus. The primer group is designed on the basis of nucleotide sequences disclosed by SEQ ID NO.1 and SEQ ID NO.2, the above target sequence can meet a primer design requirement in a range of 100-200bp, meanwhile, the primer designed on the basis of the target sequence can effectively authenticate the BK virusand also can accurately authenticate the JC virus, and difficulty to design a fragment suitable for the primer is high.
Owner:苏州奥根诊断科技有限公司
Recombinant human antibodies for therapy and prevention of polyomavirus-related diseases
ActiveUS10450365B2High affinityReduce riskSenses disorderNervous disorderPolyomavirus JCVirus-like particle
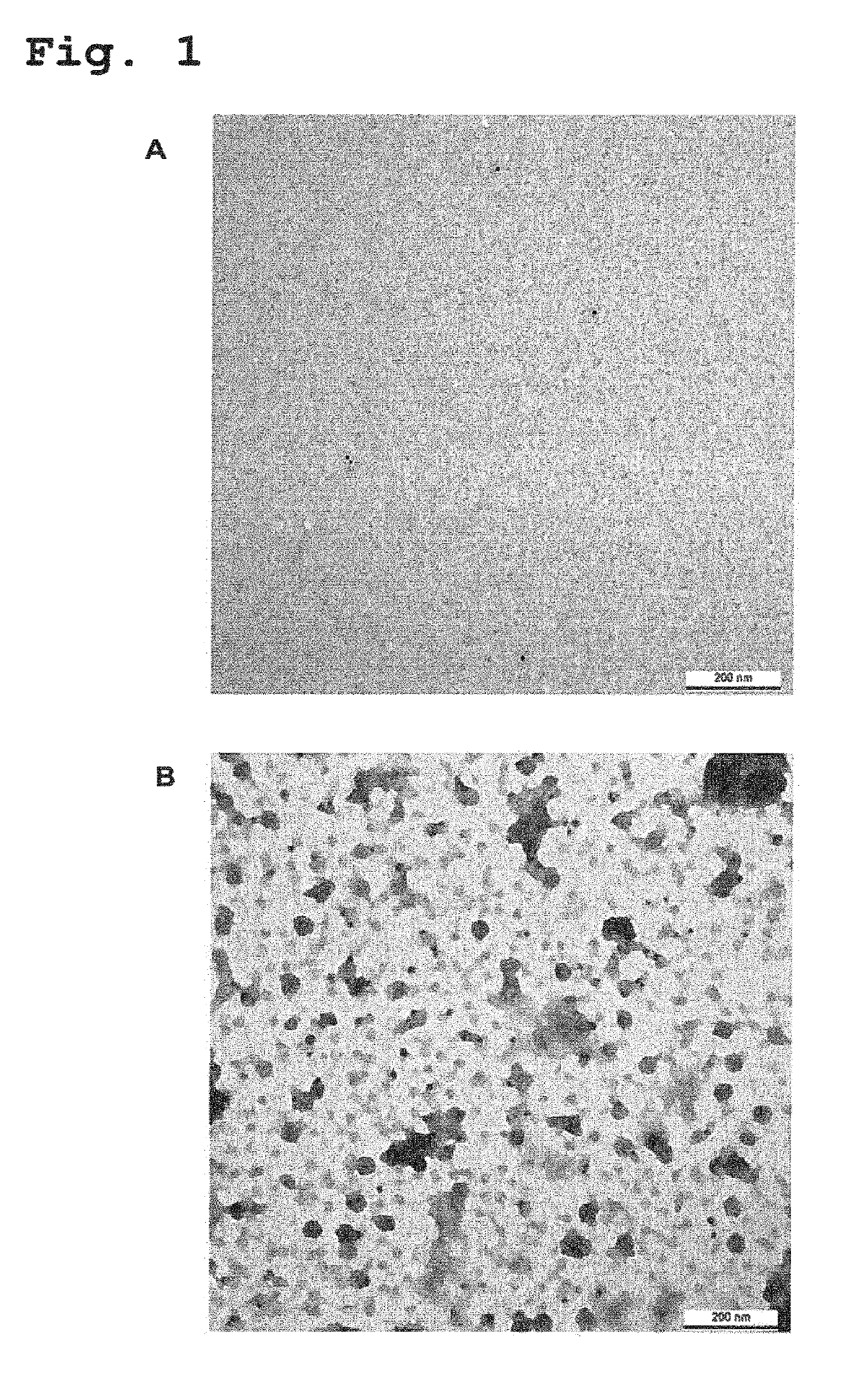
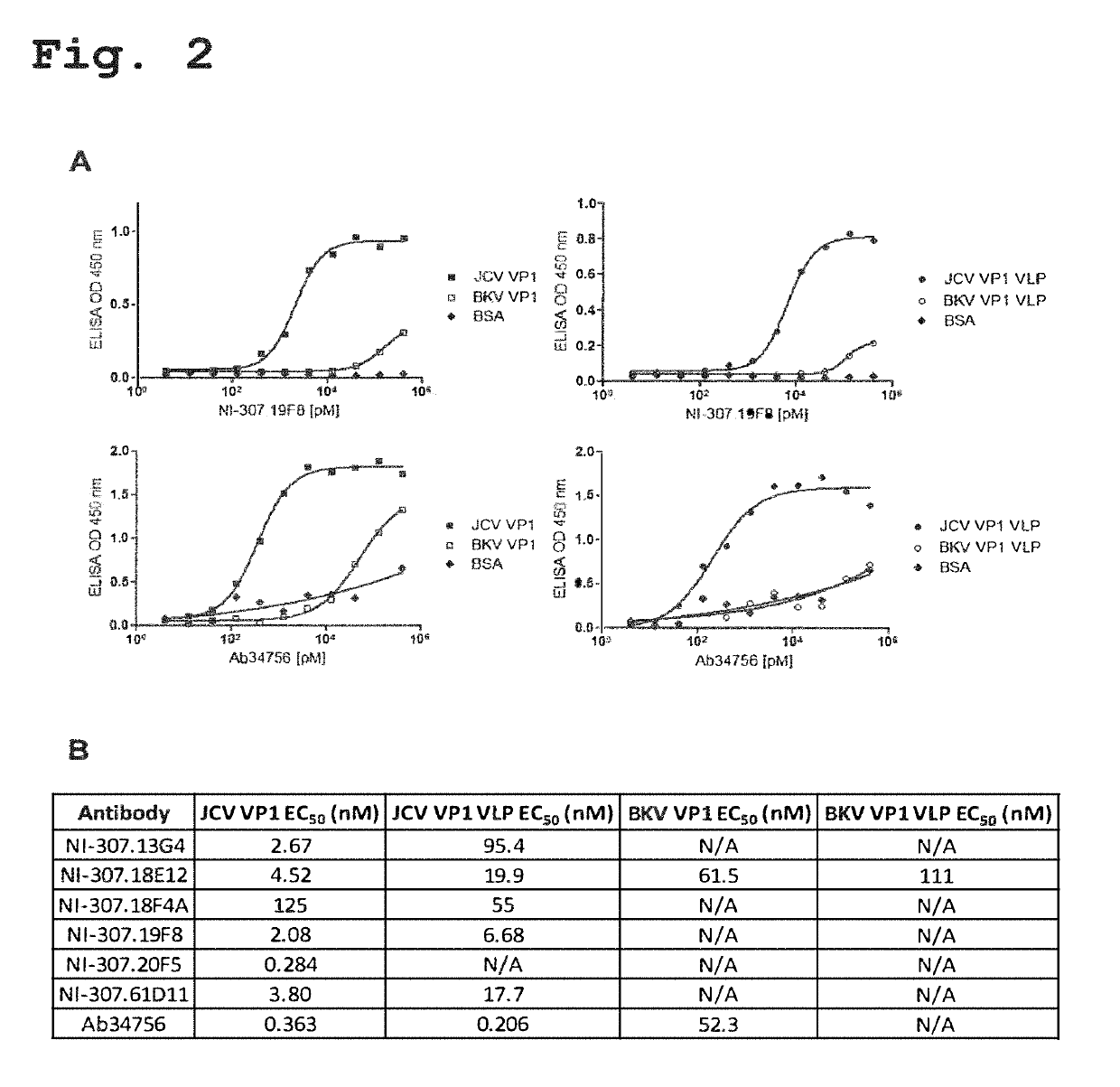
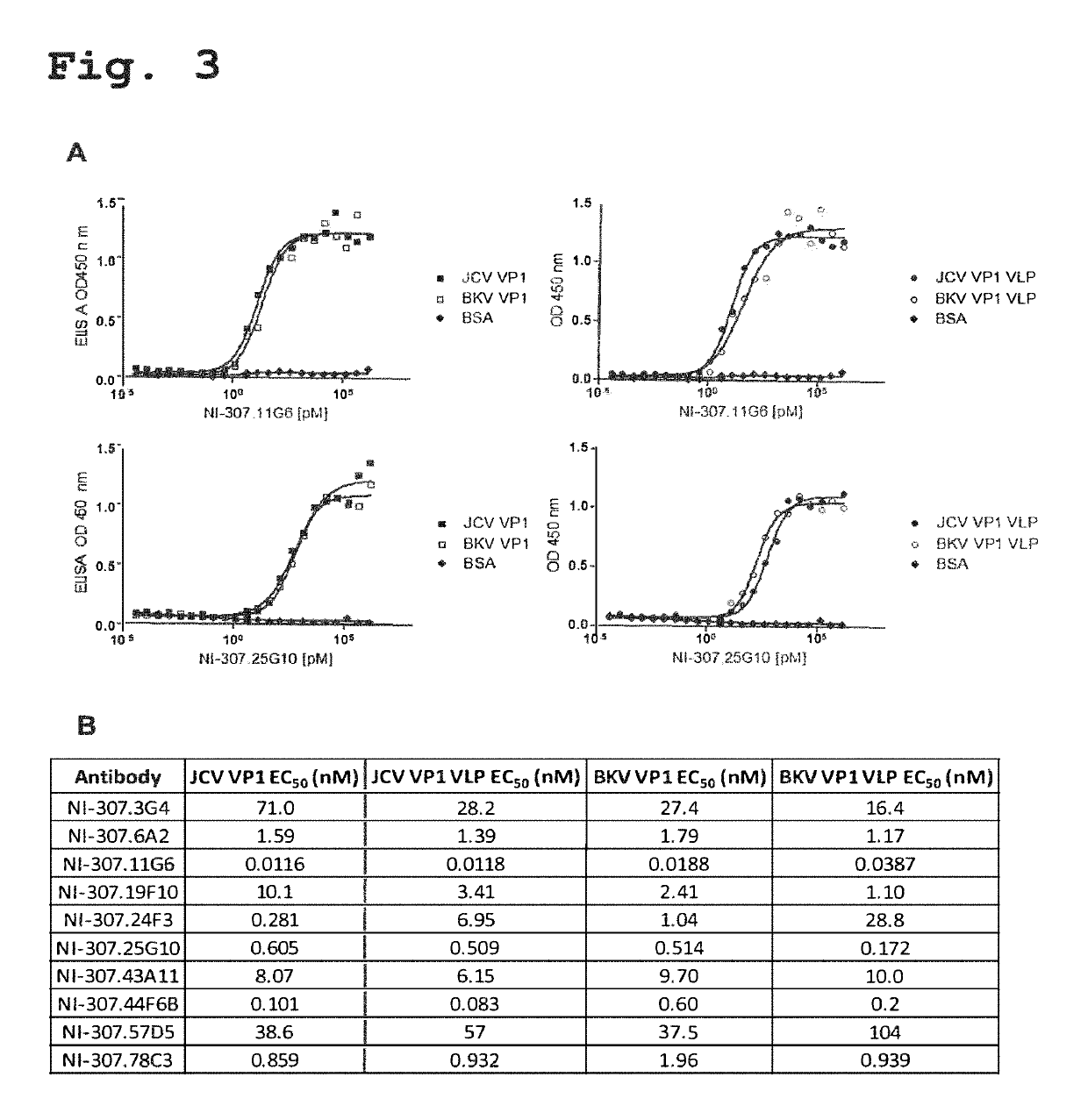
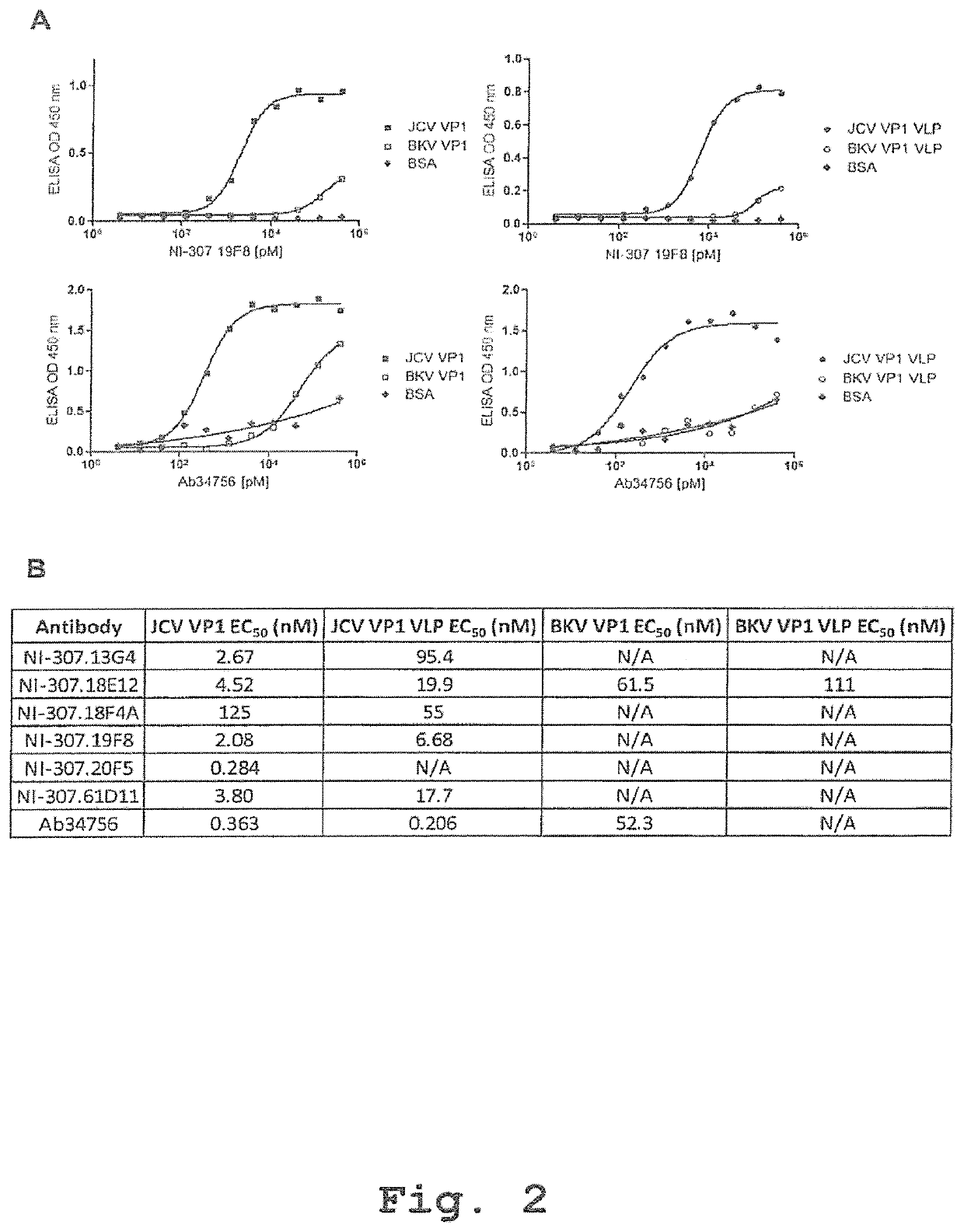
Provided are novel human-derived antibodies specifically recognizing polyomavirus polypeptides, preferably capable of binding to polyomaviruses of the type of JC virus (JCV) and / or BK virus (BKV) as well as methods related thereto. Furthermore, assays and kits related to antibodies specific for polyomaviruses, polyomavirus VP1 and or polyomavirus VP1 Virus-Like Particles (VLPs), preferably of the type of JCV and / or BKV, are disclosed. The human-derived antibodies as well as binding fragments, derivatives and variants thereof can be used in pharmaceutical and diagnostic compositions for polyomavirus targeted immunotherapy and diagnostics.
Owner:UNIV ZURICH +1
Method for detecting JC polyoma virus through real-time fluorescent quantitative PCR (Polymerase Chain Reaction)
PendingCN113913554ALow costShorten the timeMicrobiological testing/measurementForward primerPolyomavirus JC
The invention discloses a method for detecting JC polyoma virus through real-time fluorescent quantitative PCR (Polymerase Chain Reaction), provides a group of forward primers, reverse primers and probes for detecting JC virus in a biological product through real-time fluorescent quantitative PCR, and provides a PCR program for carrying out fluorescent quantitative PCR detection by utilizing the forward and reverse primers and the probes. The detection method provided by the invention can be used for detecting all JC viruses of different genotypes, is good in specificity, high in sensitivity and high in variant coverage, and compared with common virus cell culture detection, the detection time is greatly shortened, the labor and material cost is saved, and the detection method is suitable for complex detection of large-batch production of biological products.
Owner:SUZHOU YAOMING KANGDE INSPECTION TESTING
RNA-directed eradication of human jc virus and other polyomaviruses
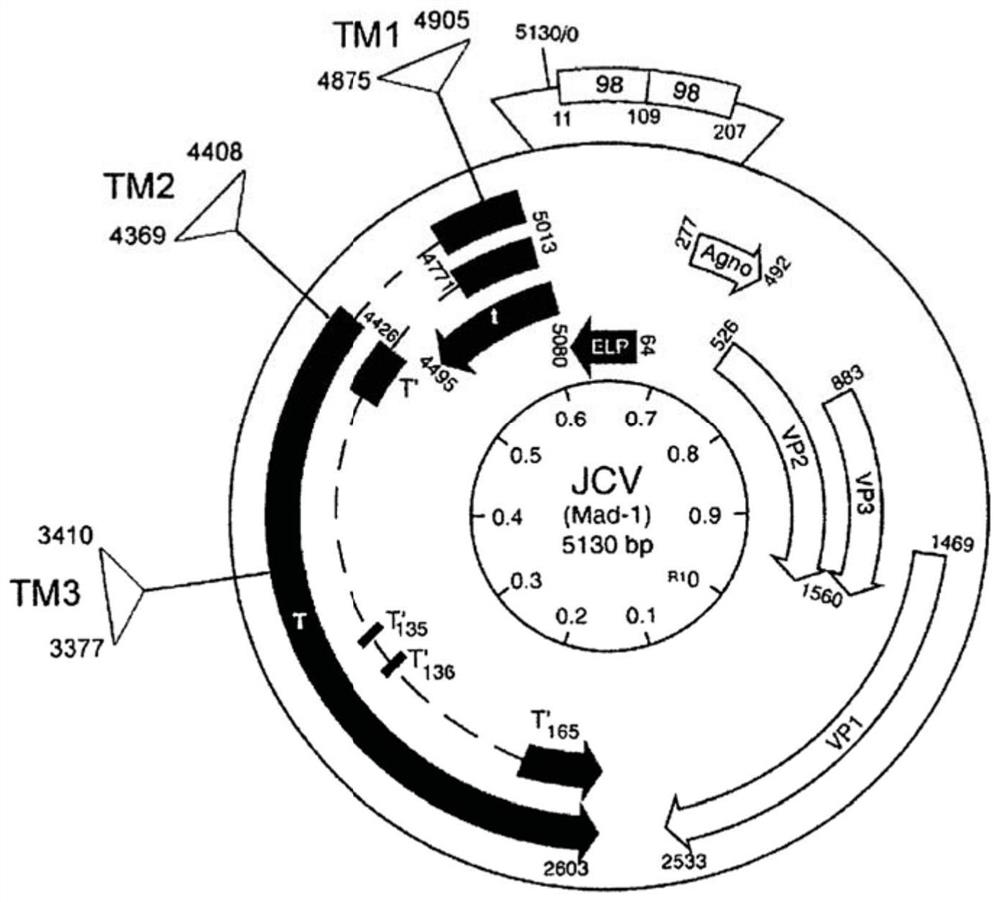
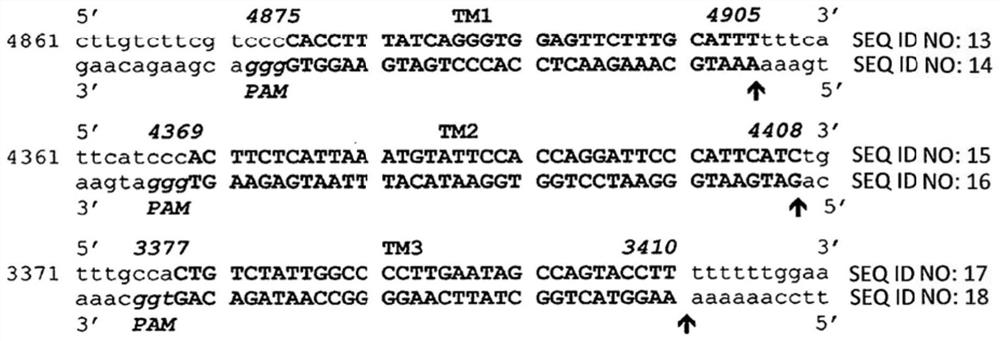
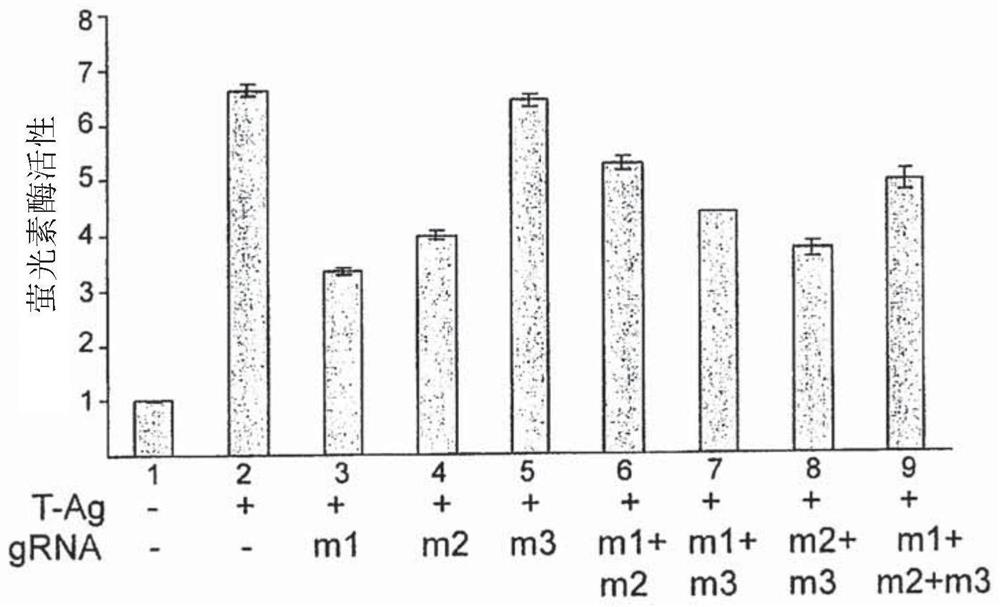
The present invention includes methods and compositions for the elimination of polyomaviruses, such as John Cunningham virus (JCV), from host cells, and the treatment of polyomavirus-associated diseases, such as progressive multifocal leukoencephalopathy (PML). The composition comprises an isolated nucleic acid sequence comprising a CRISPR-associated endonuclease and a guide RNA, wherein the guide RNA is complementary to a target sequence in a polyomavirus.
Owner:TEMPLE UNIVERSITY
Assay for JC virus antibodies
The disclosure relates to methods and reagents for analyzing samples for the presence of JC virus antibodies. Disclosed is a method that includes obtaining a biological sample from a subject (e.g., plasma, serum, blood, urine, or cerebrospinal fluid), contacting the sample with highly purified viral-like particles (HPVLPs) under conditions suitable for binding of a JCV antibody in the sample to an HPVLP, and detecting the level of JCV antibody binding in the sample to HPVLP. In one embodiment, determining the level of anti-JCV antibodies in the subject sample provides a method of identifying PML risk in a subject.
Owner:BIOGEN MA INC
Compositions and methods for the treatment of progressive multifocal leukoencephalopathy (PML)
ActiveUS20140199291A9Reduce riskBiocideOther blood circulation devicesPolyomavirus JCViral infection
The invention relates to compositions, methods, and kits for treating subjects infected by or at risk of infection with a DNA virus (e.g., a JC Virus or a BK virus). Aspects of the invention are useful to prevent or treat DNA virus associated conditions (e.g., PML) in subjects that are immunocompromised. Compositions are provided that inhibit intracellular replication of DNA viruses.
Owner:BIOGEN MA INC
Assay for jc virus antibodies
The disclosure relates to methods and reagents for analyzing samples for the presence of JC virus antibodies. Disclosed is a method that includes obtaining a biological sample from a subject (e.g., plasma, serum, blood, urine, or cerebrospinal fluid), contacting the sample with highly purified viral-like particles (HPVLPs) under conditions suitable for binding of a JCV antibody in the sample to an HPVLP, and detecting the level of JCV antibody binding in the sample to HPVLP. In one embodiment, determining the level of anti-JCV antibodies in the subject sample provides a method of identifying PML risk in a subject.
Owner:BIOGEN MA INC
Kit and method for simultaneously detecting herpes simplex virus, Carbosarcoma associated herpes virus, JC virus and EB virus
ActiveCN112195276AAvoid mutual interferenceShorten detection timeMicrobiological testing/measurementMicroorganism based processesPolyomavirus JCHerpes simplex virus DNA
The invention discloses a kit and method for simultaneously detecting herpes simplex virus, Carbosarcoma-associated herpes virus, JC virus and EB virus. According to the kit and the method, a real-time fluorescent quantitative PCR technology is applied, highly specific primers and probes for herpes simplex virus, Carbosarcoma-associated herpes virus, JC virus and EB virus are adopted, and whetherthe four viruses exist in a sample to be detected or not can be judged through one PCR reaction, so that the method is more convenient and quicker than a single fluorescent quantitative PCR method, and the cost is saved. The detection sensitivity of the kit to four viruses reaches 10 copies / mL, and the kit has good specificity and does not have cross reaction with other viruses; and meanwhile, thekit is good in amplification repeatability, and the precision is smaller than or equal to 2%.
Owner:浙江安维珞诊断技术有限公司
Gene editing methods and compositions for eliminating risk of jc virus activation and pml (progressive multifocal leukoencephalopathy) during immunosuppresive therapy
A method of eliminating the risk of JCV activation in a subject undergoing immunosuppressive therapy, by administering an effective amount of a gene editing composition directed toward at least one target sequence in the JCV genome, cleaving the target sequence in the JCV genome, disrupting the JCV genome, eliminating the JCV infection, eliminating the risk of JCV activation, and treating the subject with an immunosuppressive therapy. A pharmaceutical composition including at least one isolated nucleic acid sequence encoding a CRISPR-associated endonuclease and at least one gRNA having a spacer sequence complementary to a target sequence in a JCV DNA, the isolated nucleic acid sequences being included in at least one expression vector. Pharmaceutical compositions including at least one isolated nucleic acid sequence encoding at least one TALEN, at least one ZFN, or at least one argonaute protein, which target at least one nucleotide sequence of the JCV genome.
Owner:EXCISION BIOTHERAPEUTICS INC +1
Recombinant human antibodies for therapy and prevention of polyomavirus-related diseases
Provided are novel human-derived antibodies specifically recognizing polyomavirus polypeptides, preferably capable of binding to polyomaviruses of the type of JC virus (JCV) and / or BK virus (BKV) as well as methods related thereto. Furthermore, assays and kits related to antibodies specific for polyomaviruses, polyomavirus VP1 and or polyomavirus VP1 Virus-Like Particles (VLPs), preferably of the type of JCV and / or BKV, are disclosed. The human-derived antibodies as well as binding fragments, derivatives and variants thereof can be used in pharmaceutical and diagnostic compositions for polyomavirus targeted immunotherapy and diagnostics.
Owner:UNIV ZURICH +1
Reagent for detecting BK virus and JC virus and application
PendingCN113999937AIncreased risk of rejectionAvoid exclusionMicrobiological testing/measurementDiseasePolyomavirus JC
The invention relates to a reagent for detecting a BK virus and a JC virus and application, and belongs to the technical field of biological medicines. A section of JCV fragment notch region exists in highly conserved regions of BKV and JCV. A primer probe system for simultaneously targeting BKV and JCV is designed at a suitable position beyond the notch region, and a reverse primer is only designed in the notch region to specifically target BKV or JCV. The reagent is used for preliminary screening, BKV and JCV are targeted at the same time, and resource saving is facilitated; and BKV or JCV can be accurately judged, and the reagent is suitable for clinical popularization. According to the invention, BKV and JCV activation can be prevented, the treatment effect of related diseases can be evaluated in time, the defects and deficiencies of the existing detection system are made up, and the detection result is more reliable. BKV and JCV are taken into consideration, primary screening can be carried out by targeting BKV and JCV at the same time, BKV or JCV can be accurately judged, and timely immunosuppression adjustment is facilitated.
Owner:领致生物科技(昆山)有限公司
Primers, probe and kit for detecting JCV (JC virus)
InactiveCN107586883AHigh sensitivityAccurate detectionMicrobiological testing/measurementMicroorganism based processesForward primerPolyomavirus JC
The invention discloses primers, a probe and a kit for detecting JCV (JC virus) and belongs to the technical field of biology. The primers and the probe comprise a forward primer for detecting the JCV, a reverse primer for detecting the JCV and the probe for detecting the JCV. The kit comprises the primers and the probe. The primers, the probe and the kit have the characteristic of high sensitivity and can detect whether a sample is infected with the JCV accurately.
Owner:湖北朗德医疗科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com