Compositions and methods for the treatment of progressive multifocal leukoencephalopathy (PML)
a technology of encephalopathy and compositions, applied in the field of compositions and methods for treating subjects infected with dna virus, can solve the problems of no specific antiviral therapy that has been proven effective for treating pml, and achieve the effects of inhibiting jcv activity, and reducing the risk of infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Detection of JCV and JCV Variants by PCR
[0174]Nucleic acids are isolated from a biological sample using established protocols (e.g., cell lysis). Because the viral DNA may have integrated in the genomic DNA or may still be present as a smaller entity, both genomic DNA and shorter DNA sequences may be isolated and subjected to PCR analysis. Upon isolation the nucleic acids are resuspended in a buffer that will facilitate PCR analysis. Buffers that facilitate PCR analysis are known to the skilled artisan and are also commercially available from manufacturers of PCR enzymes (e.g., New England Biolabs, Beverly, Mass.). Nucleotide primers are designed to result in the amplification of a JCV gene. PCR amplification is an established laboratory technique and comprises the addition of nucleotide primers, a polymerase and single nucleotides, and polymerase buffer and subjection this mixture to cycles of annealing, amplification and dissociation resulting in the amplification of a desired DNA...
example 2
Detection of JCV and JCV Variants Using ELISA
[0175]Proteins and peptides are isolated from a biological sample using standard laboratory techniques. Both the cellular proteins and proteins of non-cellular components can be subjected to the analysis. In one assay the sample is interrogated for the presence of JCV polypeptides. The polypeptides are detected using sandwich ELISA comprising antibodies specific for JCV polypeptides. The antibodies are generated by inoculating animals (e.g., rabbits) with the JCV polypeptides of the invention resulting in polyclonal antibodies. If so desired, cells can be harvested from the inoculated animal to generate monoclonal antibodies. Methods for the generation of both polyclonal and monoclonal antibodies are routine in the art. The antibodies against JCV polypeptide and JCV polypeptide variants are immobilized on a solid surface (e.g., a 96-well plate), with one antibody type per well or surface area. The biological samples comprising the polypep...
example 3
Treatment of JCV Infection
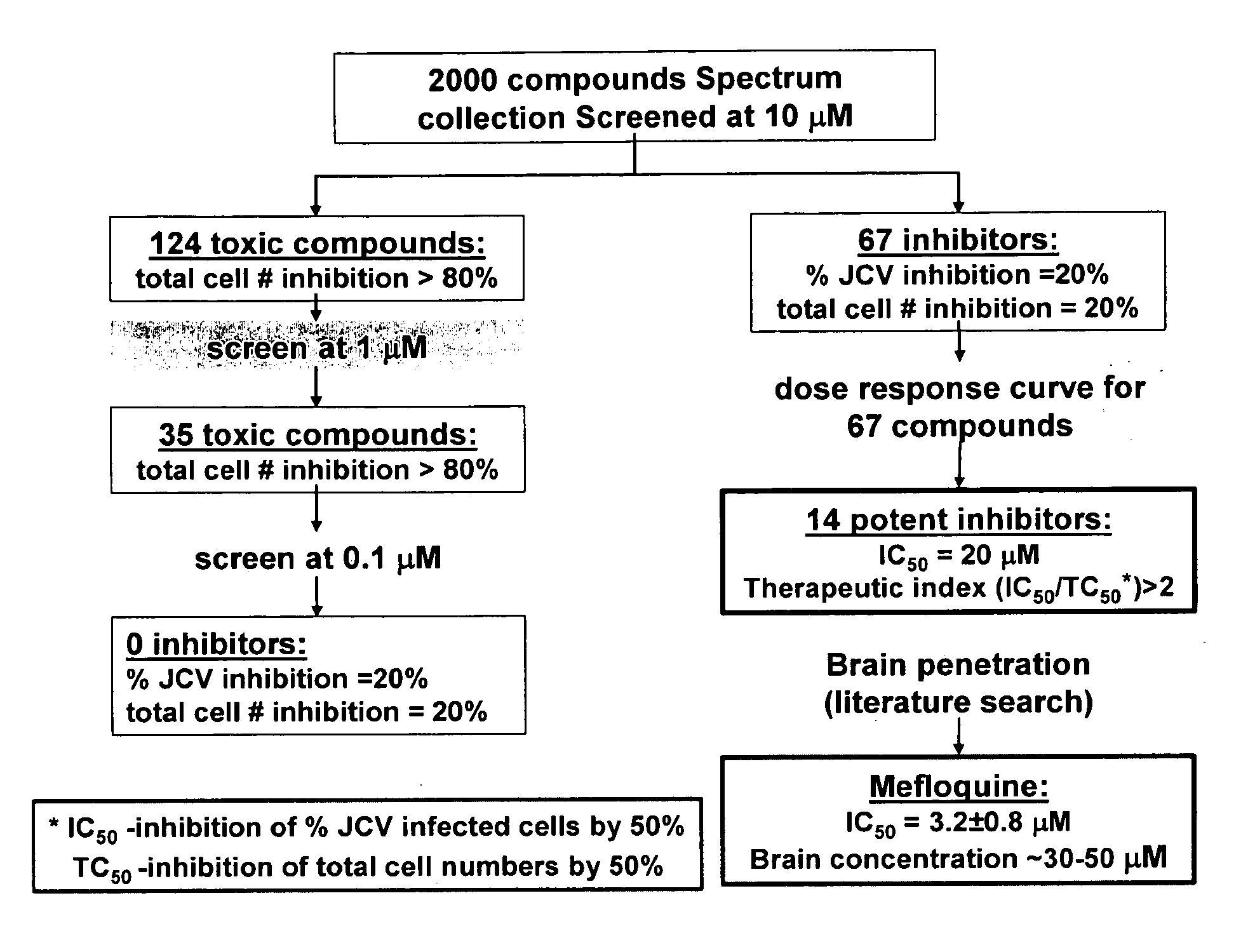
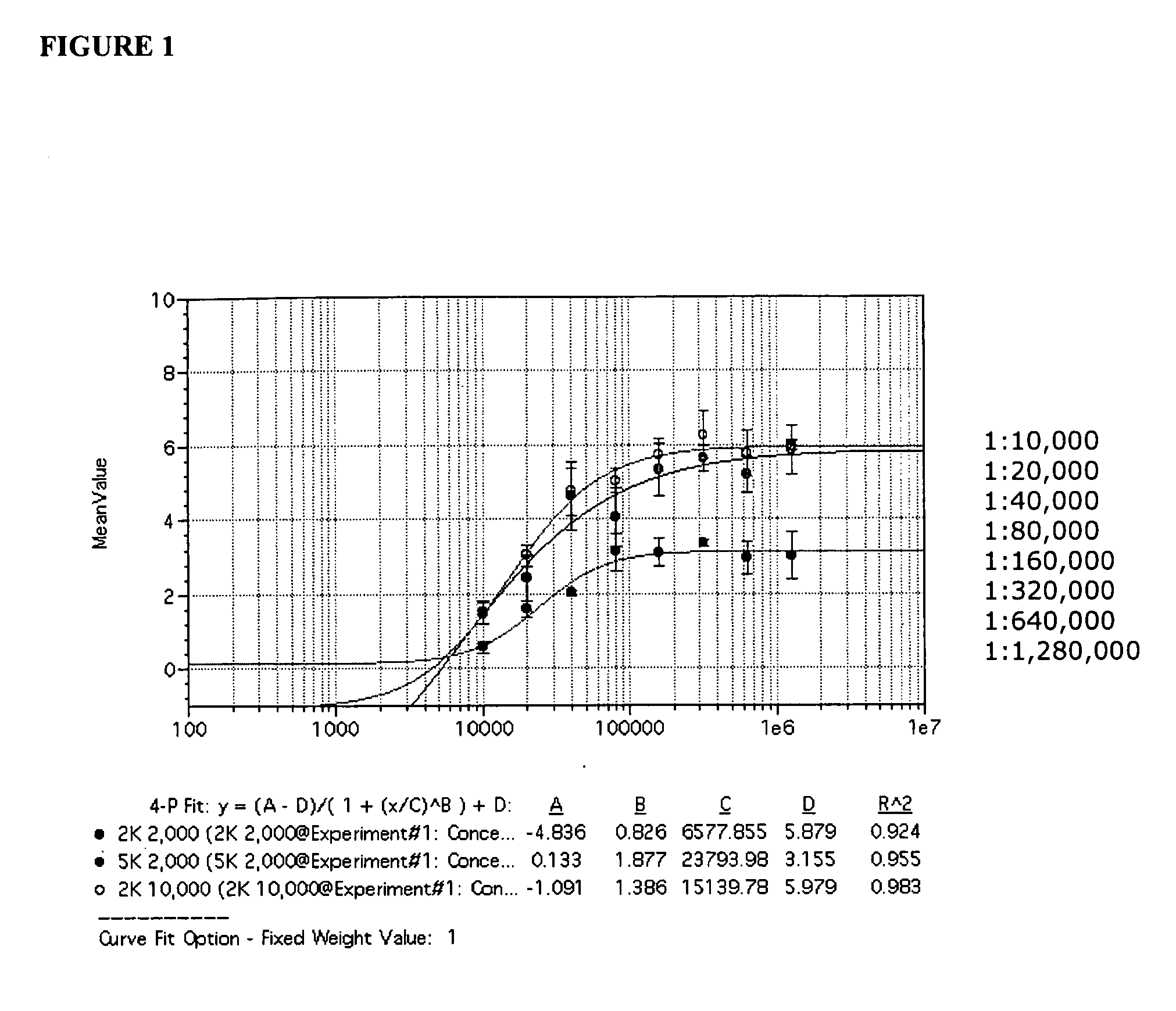
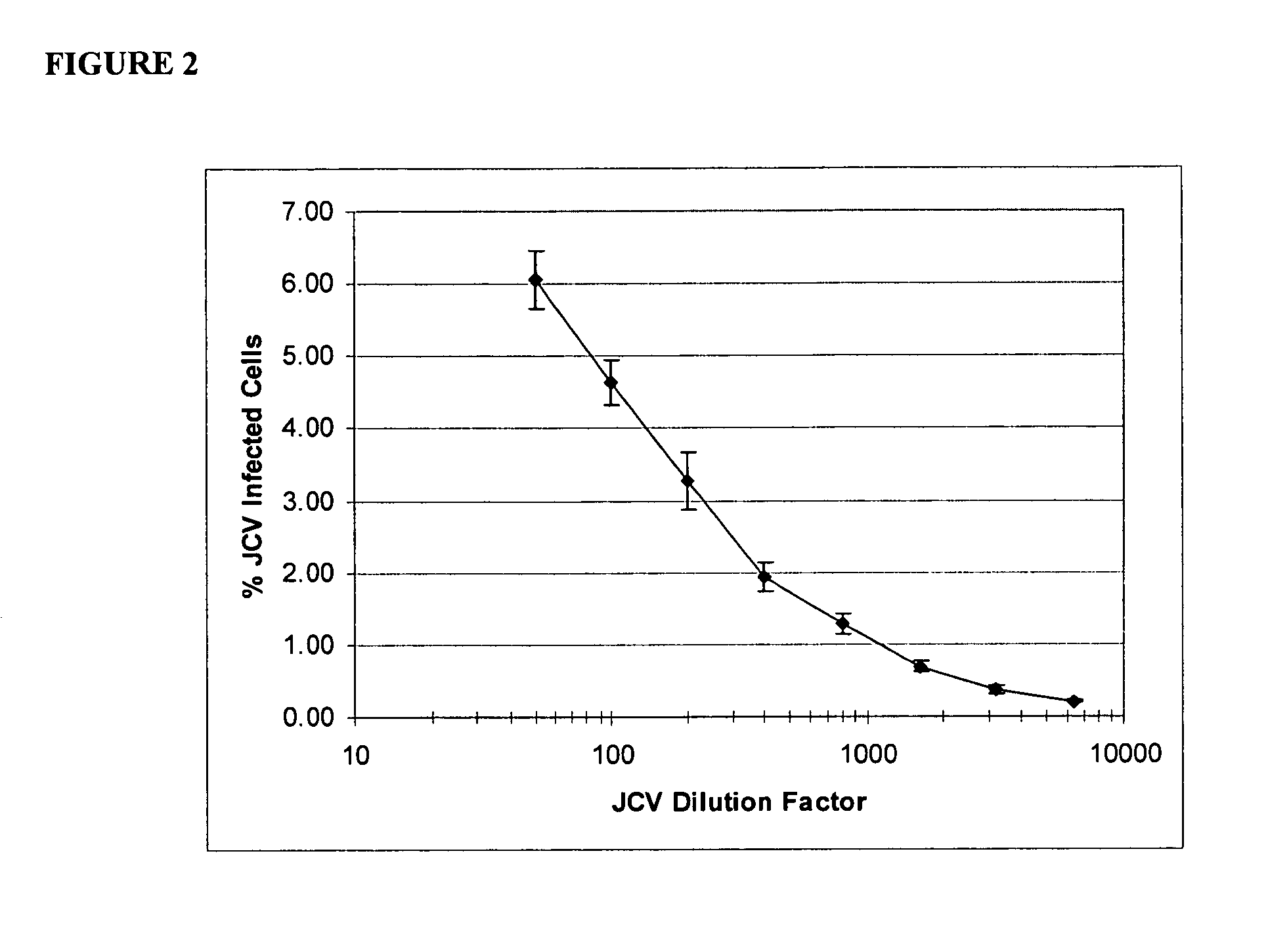
[0177]SV40 transformed glial cells were seeded in 10% FBS media. The cells were seeded at 2000 cells per well in 75 μl of media per well. On day 2, the media was removed and replaced by 35 μl of a 1× concentrated drug aliquot combined with a 100× diluted JCV Turbo aliquot in 35 μl of 2% FBS media (JCV Turbo is a hybrid Mad-1 / SVEΔ virus constructed by insertion of the regulatory region of SV40 into the regulatory region of the Mad-1 strain of JCV (Mad-1 / SVE), Vacante et al., Virology 1989, 170: 353-361). The cells were incubated for an hour, after which another 65 uL aliquot of 1× concentrated drug in 2% FBS was added. On day 5 the cells were stained with DAPI (for total cell number) and with a mouse monoclonal antibody against SV40 VP1, which cross reacts with the JCV VP1 protein. The JCV VP1 protein is displayed on the cell surface when a cell is infected by JVC1. The ability of the drug to suppress or inhibit JCV infection is shown in Table 2. The informa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com