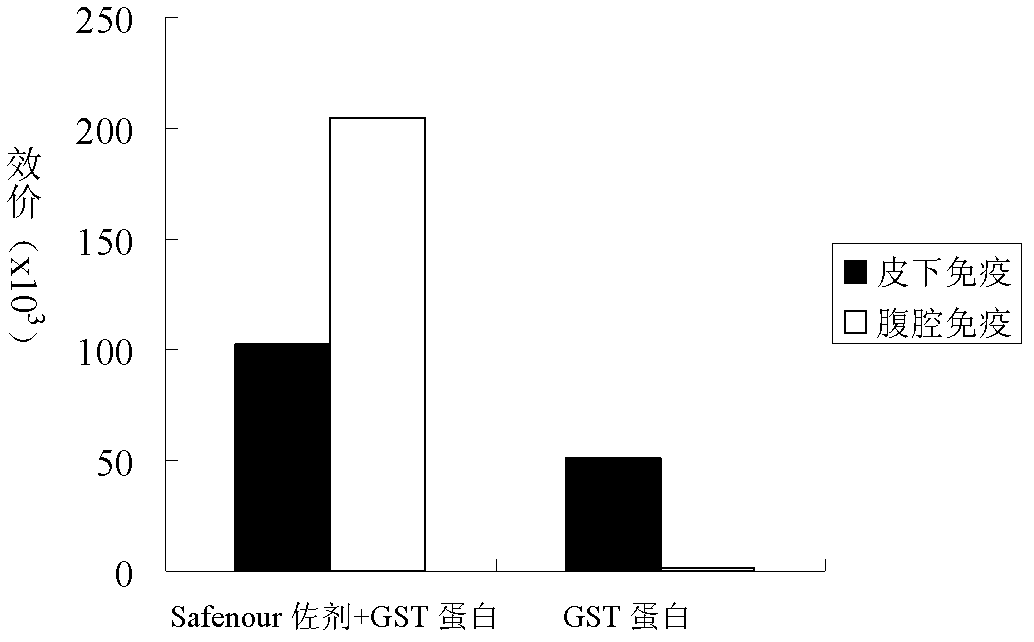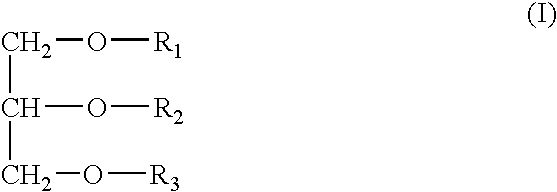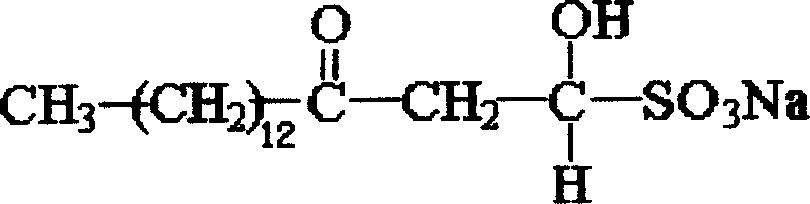Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
70 results about "Immunological Adjuvants" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vaccine composition containing synthetic adjuvant
ActiveUS20080131466A1Elicit immune responseAntibacterial agentsBacterial antigen ingredientsNatural productAdditive ingredient
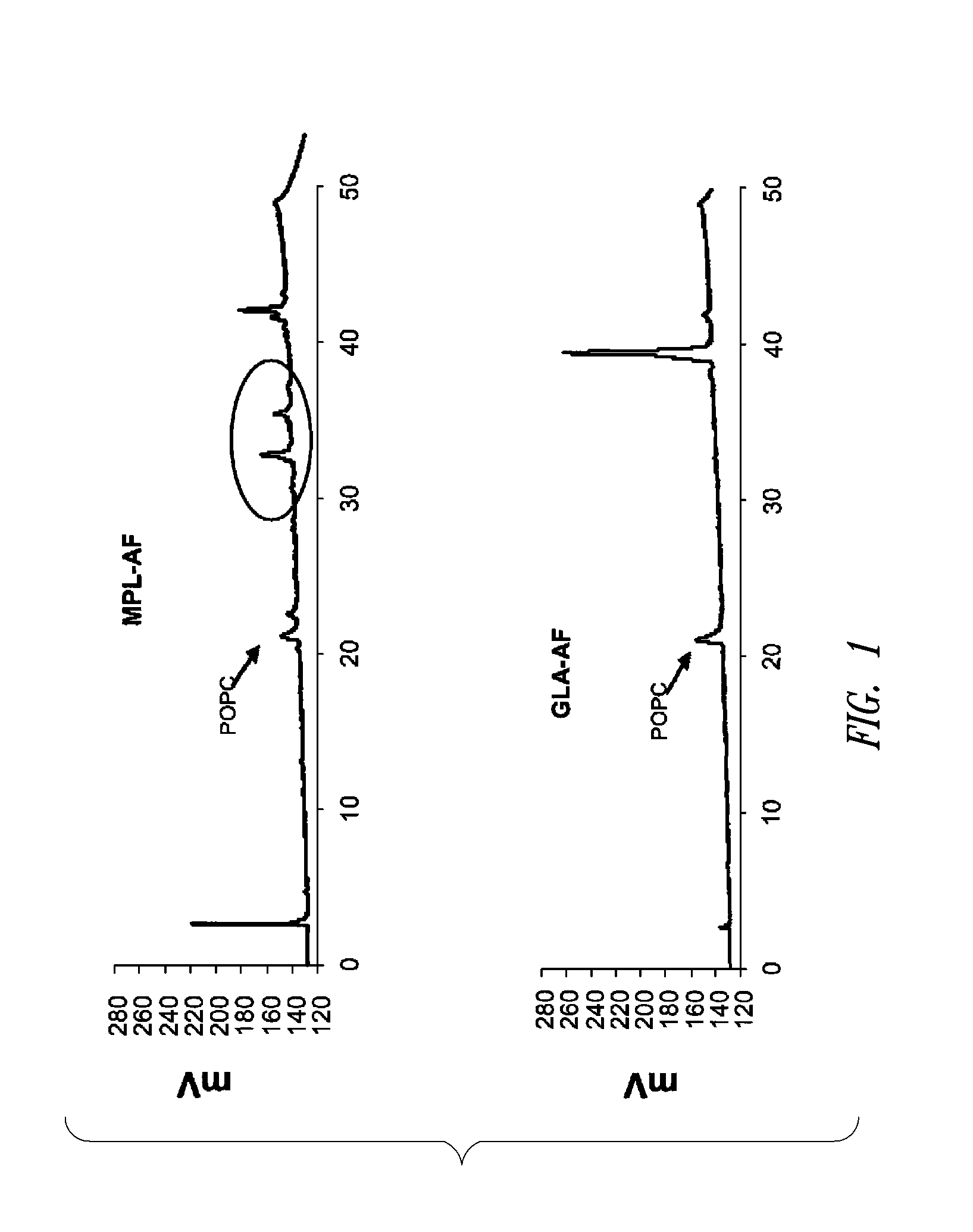
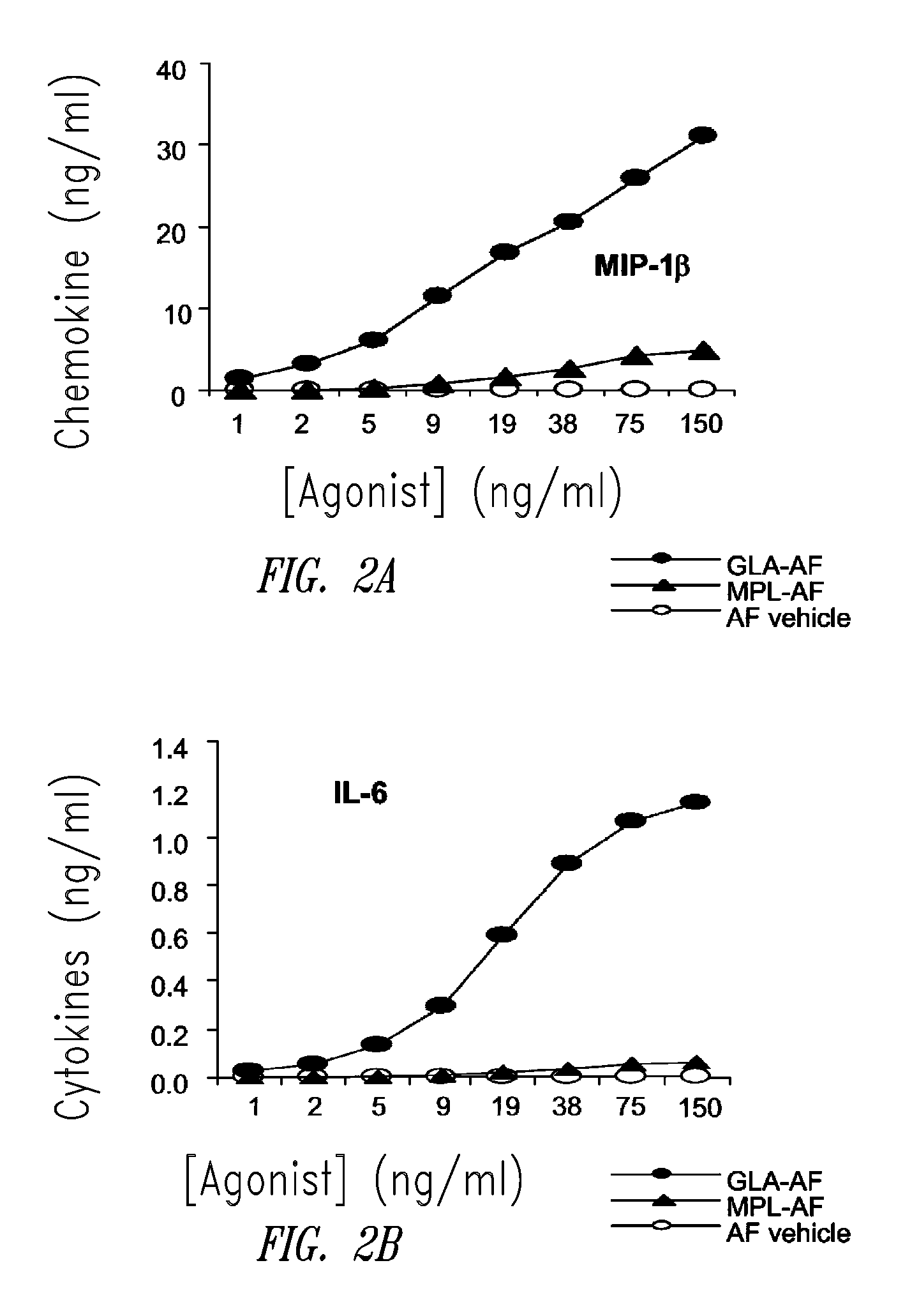
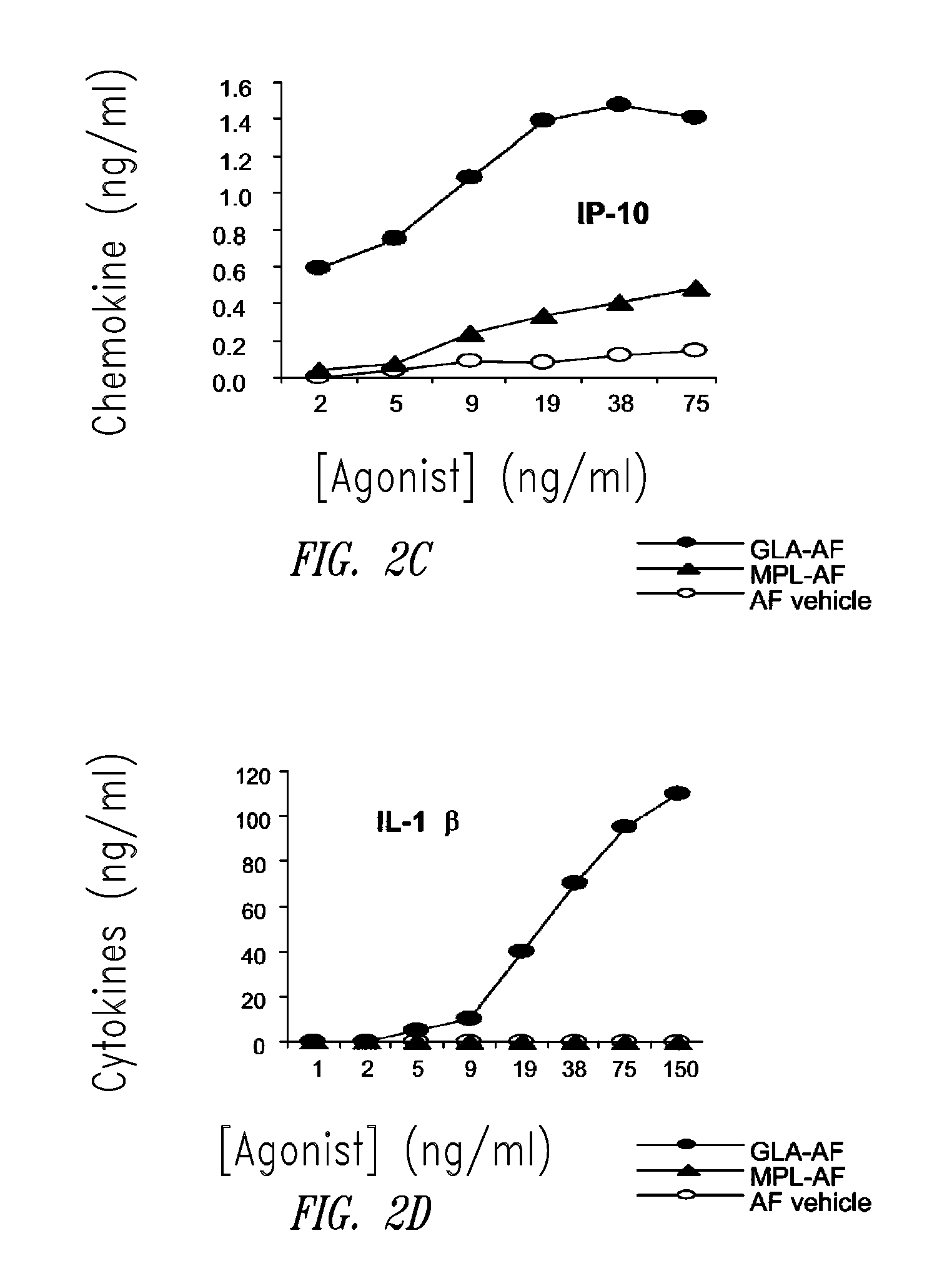
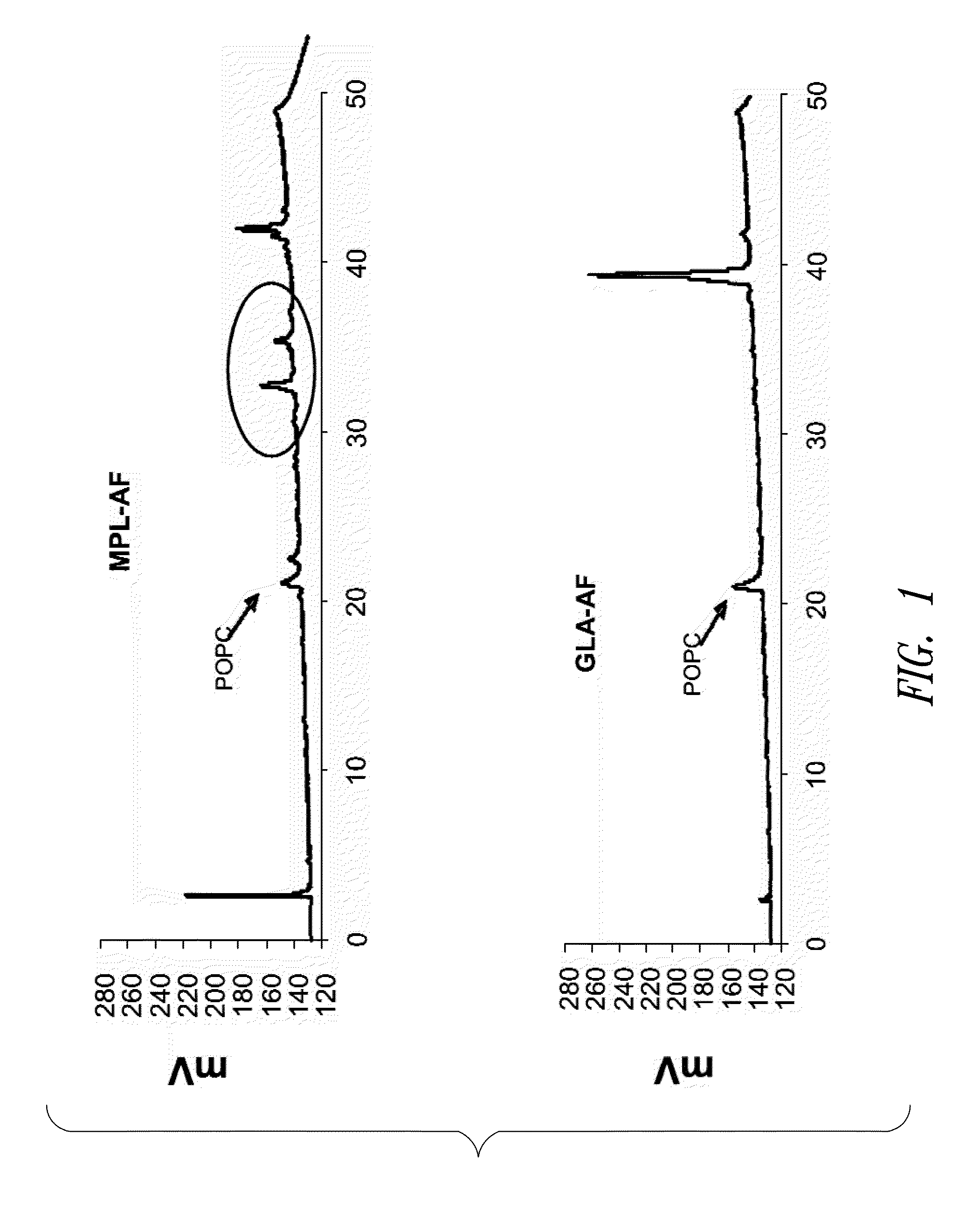
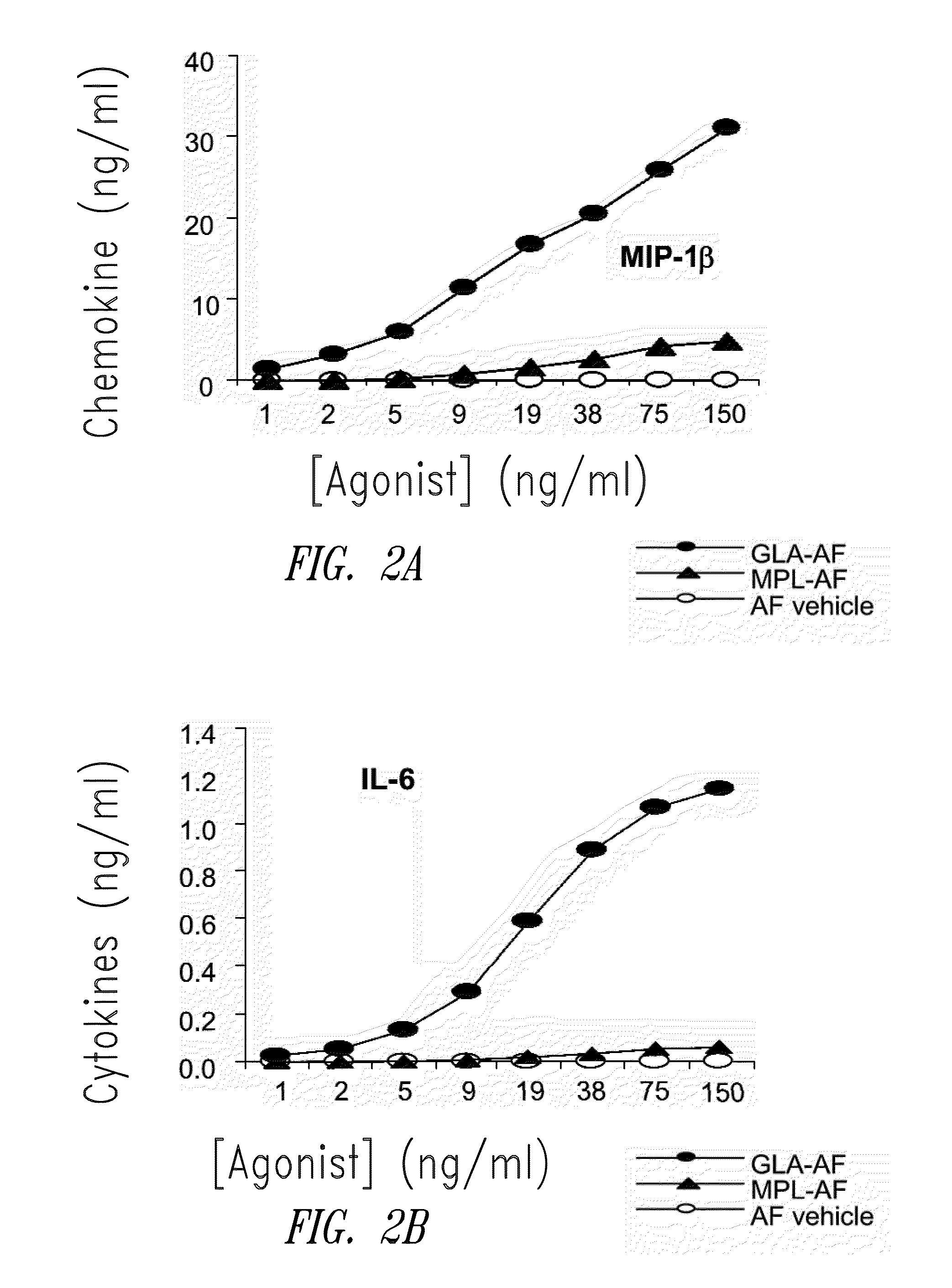
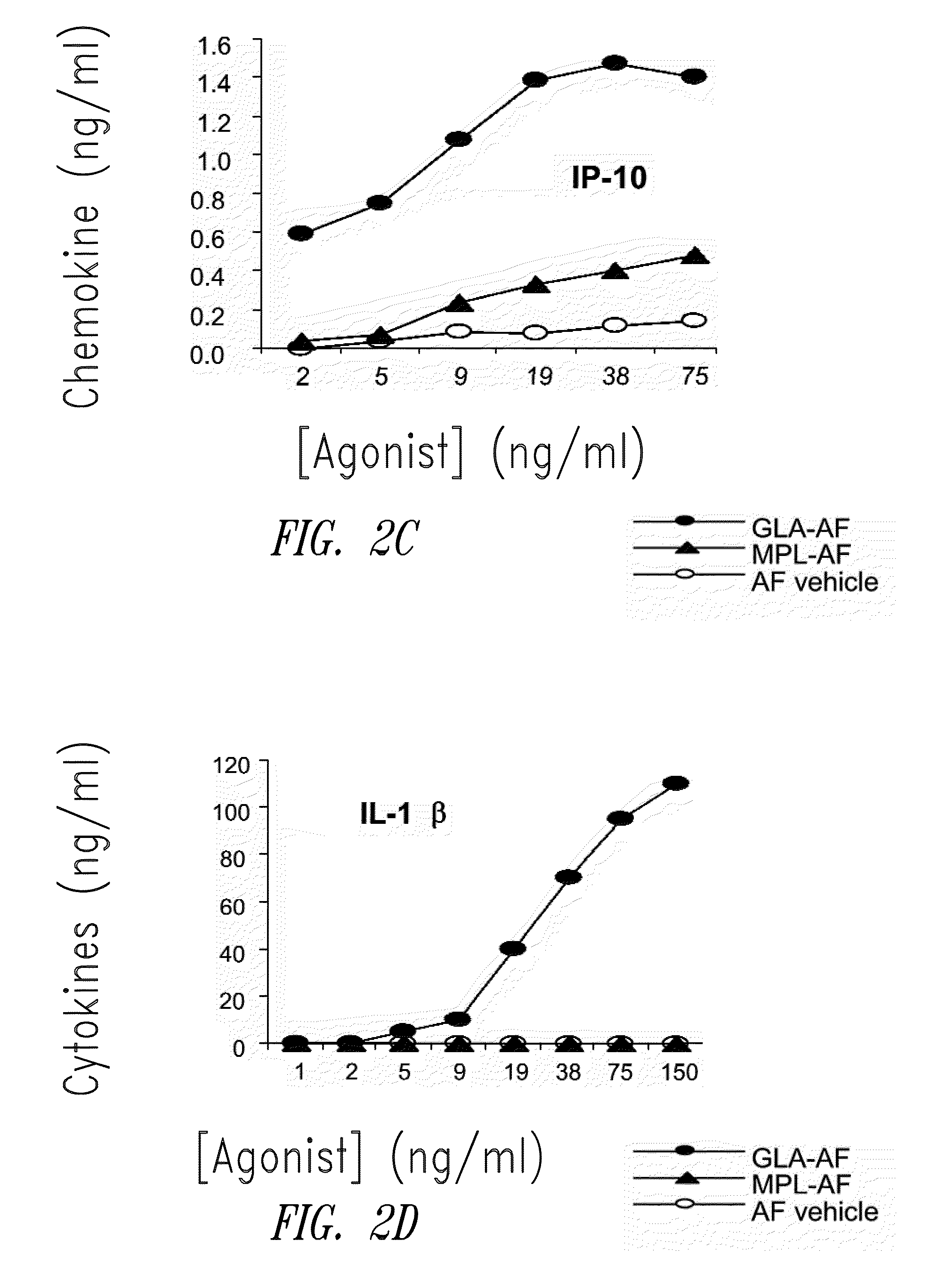
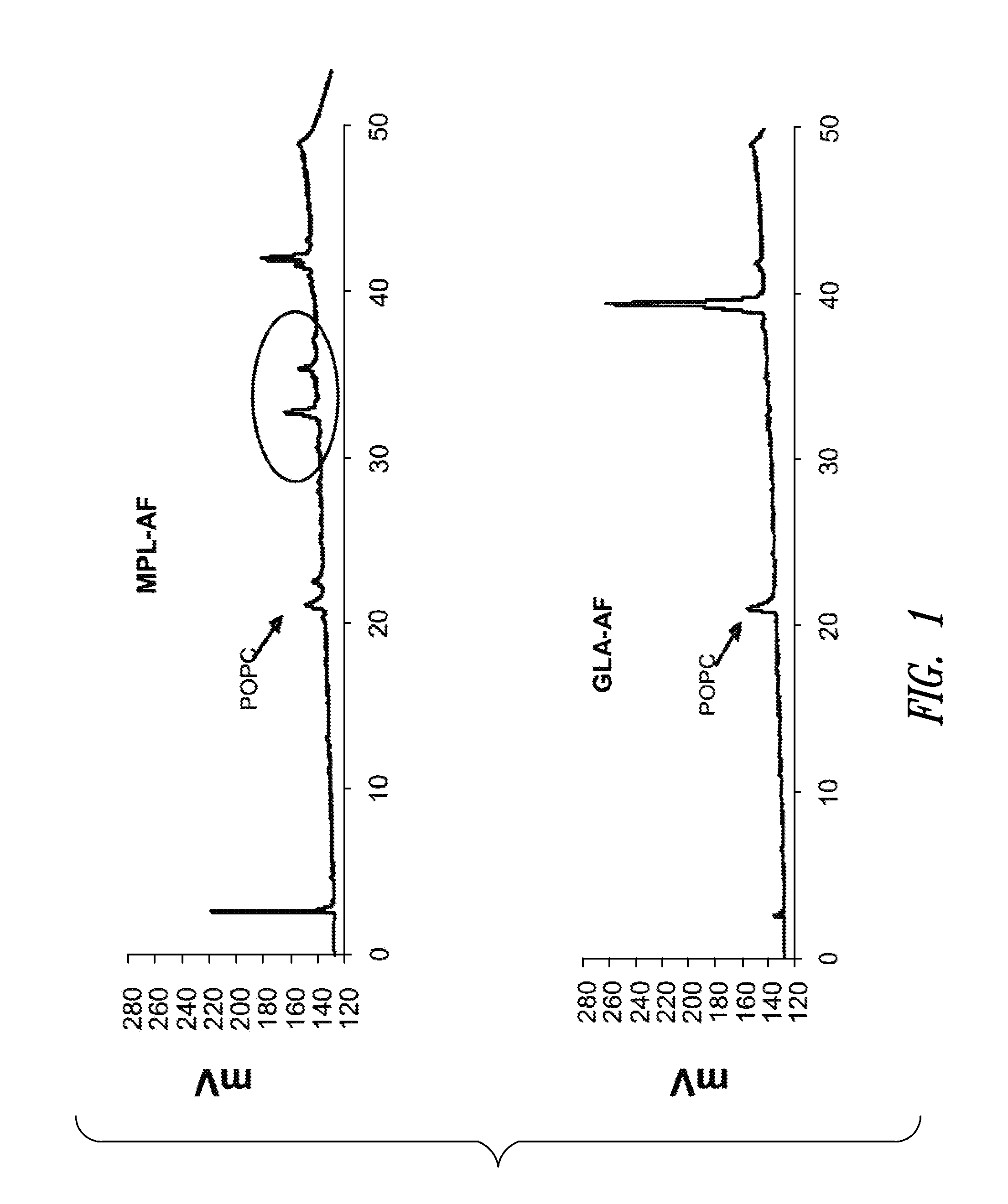
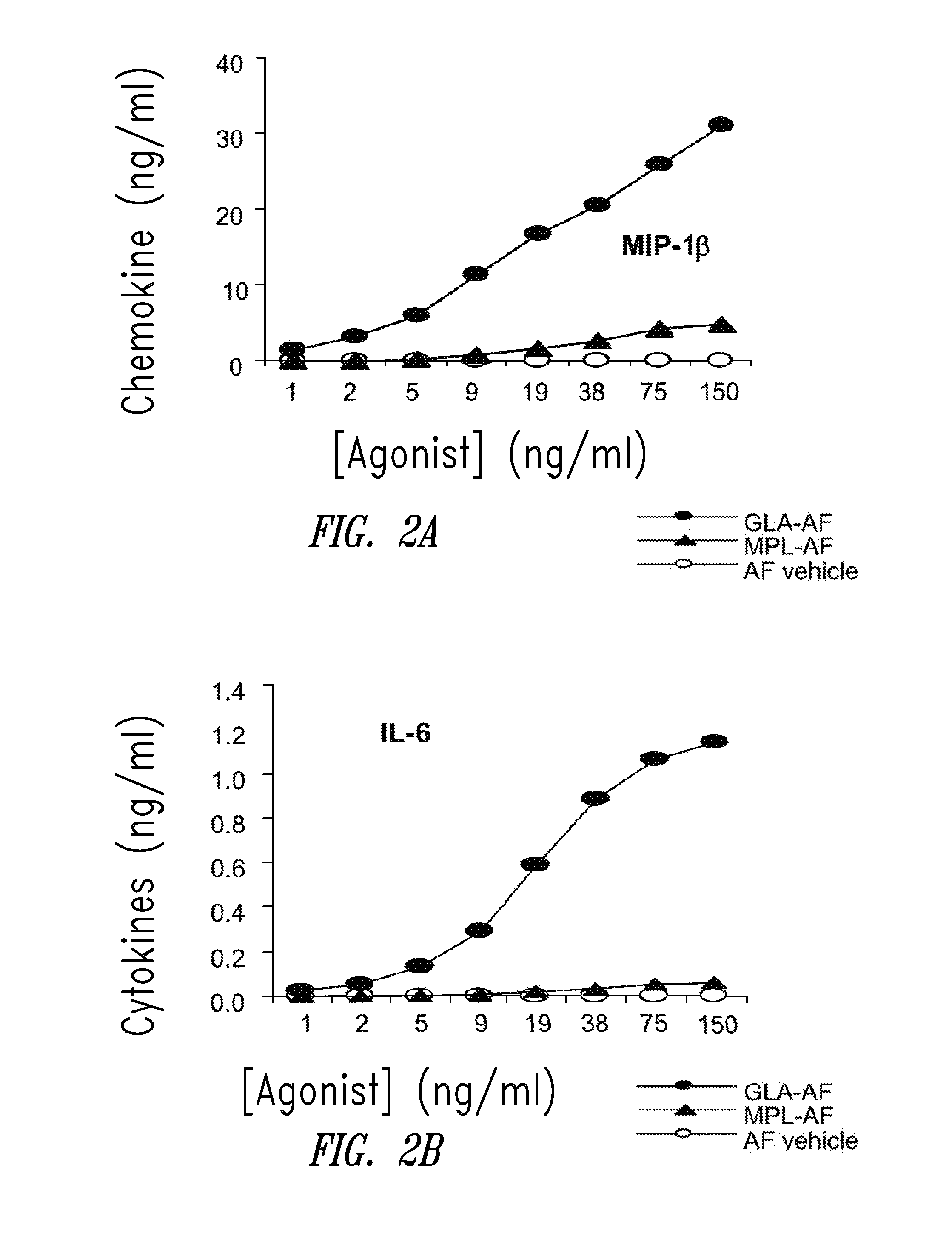
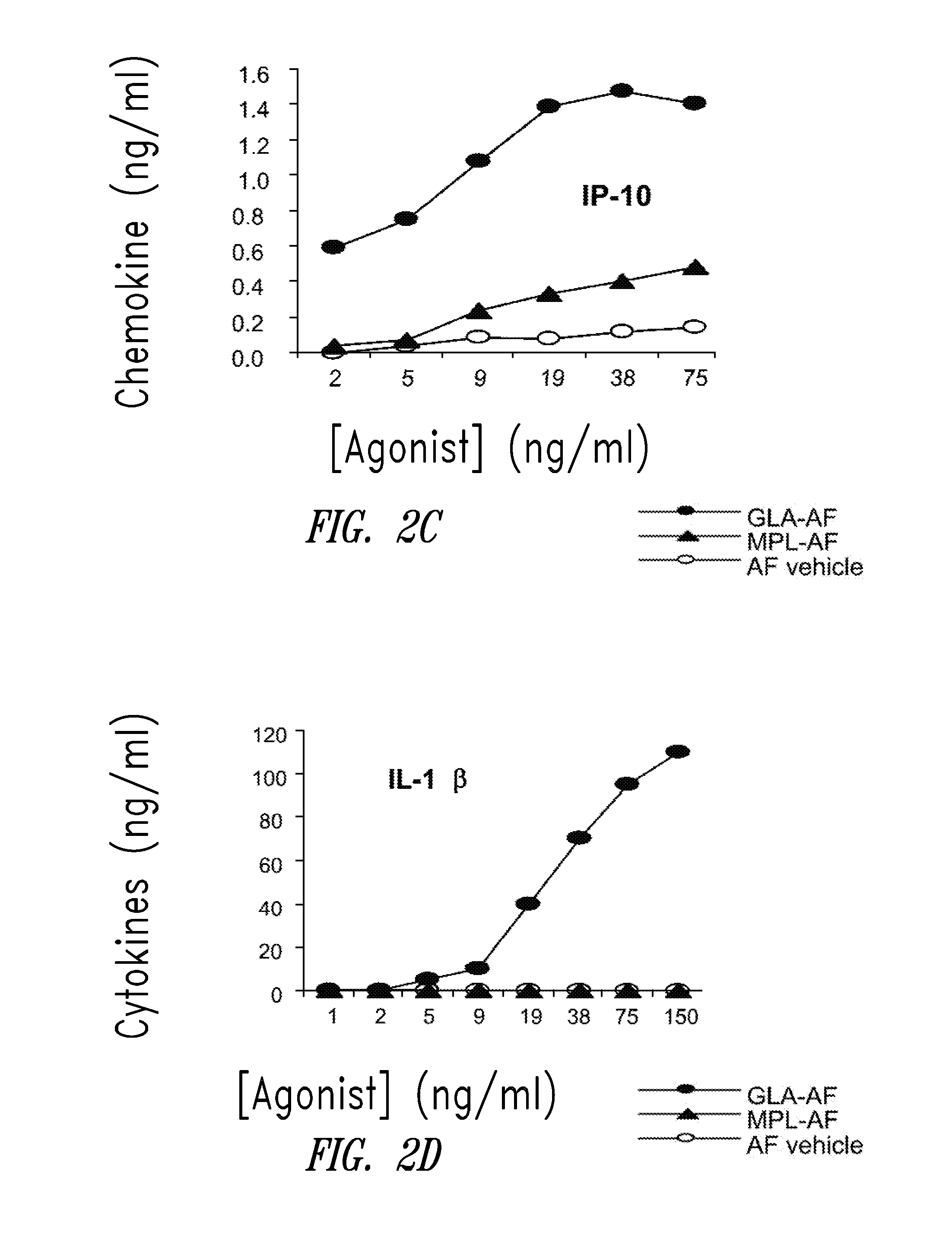
Compositions and methods, including vaccines and pharmaceutical compositions for inducing or enhancing an immune response are disclosed based on the discovery of useful immunological adjuvant properties in a synthetic, glucopyranosyl lipid adjuvant (GLA) that is provided in substantially homogeneous form. Chemically defined, synthetic GLA offers a consistent vaccine component from lot to lot without the fluctuations in contaminants or activity that compromise natural-product adjuvants. Also provided are vaccines and pharmaceutical compositions that include GLA and one or more of an antigen, a Toll-like receptor (TLR) agonist, a co-adjuvant and a carrier such as a pharmaceutical carrier.
Owner:ACCESS TO ADVANCED HEALTH INST
Vaccine composition containing synthetic adjuvant
InactiveUS20090181078A1Promote maturitySsRNA viruses negative-senseVertebrate antigen ingredientsNatural productPharmaceutical drug
Compositions and methods, including vaccines and pharmaceutical compositions for inducing or enhancing an immune response are disclosed based on the discovery of useful immunological adjuvant properties in a synthetic, glucopyranosyl lipid adjuvant (GLA) that is provided in substantially homogeneous form. Chemically defined, synthetic GLA offers a consistent vaccine component from lot to lot without the fluctuations in contaminants or activity that compromise natural-product adjuvants. Also provided are vaccines and pharmaceutical compositions that include GLA and one or more of an antigen, a Toll-like receptor (TLR) agonist, a co-adjuvant and a carrier such as a pharmaceutical carrier.
Owner:INFECTIOUS DISEASE RES INST
Adjuvant and Vaccine Compositions
InactiveUS20100226932A1Small sizeSnake antigen ingredientsInorganic non-active ingredientsSterolEmulsion
Abstract Compositions comprising an emulsion and aluminum salt nano- / micro-particles surface stabilized with at least one surfactant are useful as immunological adjuvants. The emulsion of these compositions comprises at least one oil; at least one surfactant; a plurality of surfactant vesicles; optionally at least one sterol; and an aqueous phase. The present invention also provides vaccines comprising one or more antigens combined with the emulsion and surface stabilized aluminum salt particles of the present invention, or one or more antigens combined with non-ionic surfactant vesicles.
Owner:NOVAVAX
Vaccine composition containing synthetic adjuvant
InactiveUS20110014274A1SsRNA viruses negative-senseBacterial antigen ingredientsNatural productDrug carrier
Compositions and methods, including vaccines and pharmaceutical compositions for inducing or enhancing an immune response are disclosed based on the discovery of useful immunological adjuvant properties in a synthetic, glucopyranosyl lipid adjuvant (GLA) that is provided in substantially homogeneous form. Chemically defined, synthetic GLA offers a consistent vaccine component from lot to lot without the fluctuations in contaminants or activity that compromise natural-product adjuvants. Also provided are vaccines and pharmaceutical compositions that include GLA and one or more of an antigen, a Toll-like receptor (TLR) agonist, a co-adjuvant and a carrier such as a pharmaceutical carrier.
Owner:INFECTIOUS DISEASE RES INST
Compounds for preparing immunological adjuvant
ActiveUS20070082875A1Useful in preparationBiocidePhosphorous compound active ingredientsDiseaseViral disease
The present invention provides methods for preparing TLR-4 receptor agonist E6020: and stereoisomers thereof, which compounds are useful as an immunological adjuvants when co-administered with antigens such as vaccines for bacterial and viral diseases. Also provided are synthetic intermediates.
Owner:EISIA R&D MANAGEMENT CO LTD
Immunological adjuvant, and its application in preparing vaccine and medicine for anti-virus
InactiveCN1718243AImprove immune activityReach clearAntiviralsAntibody medical ingredientsAnti virusDisease
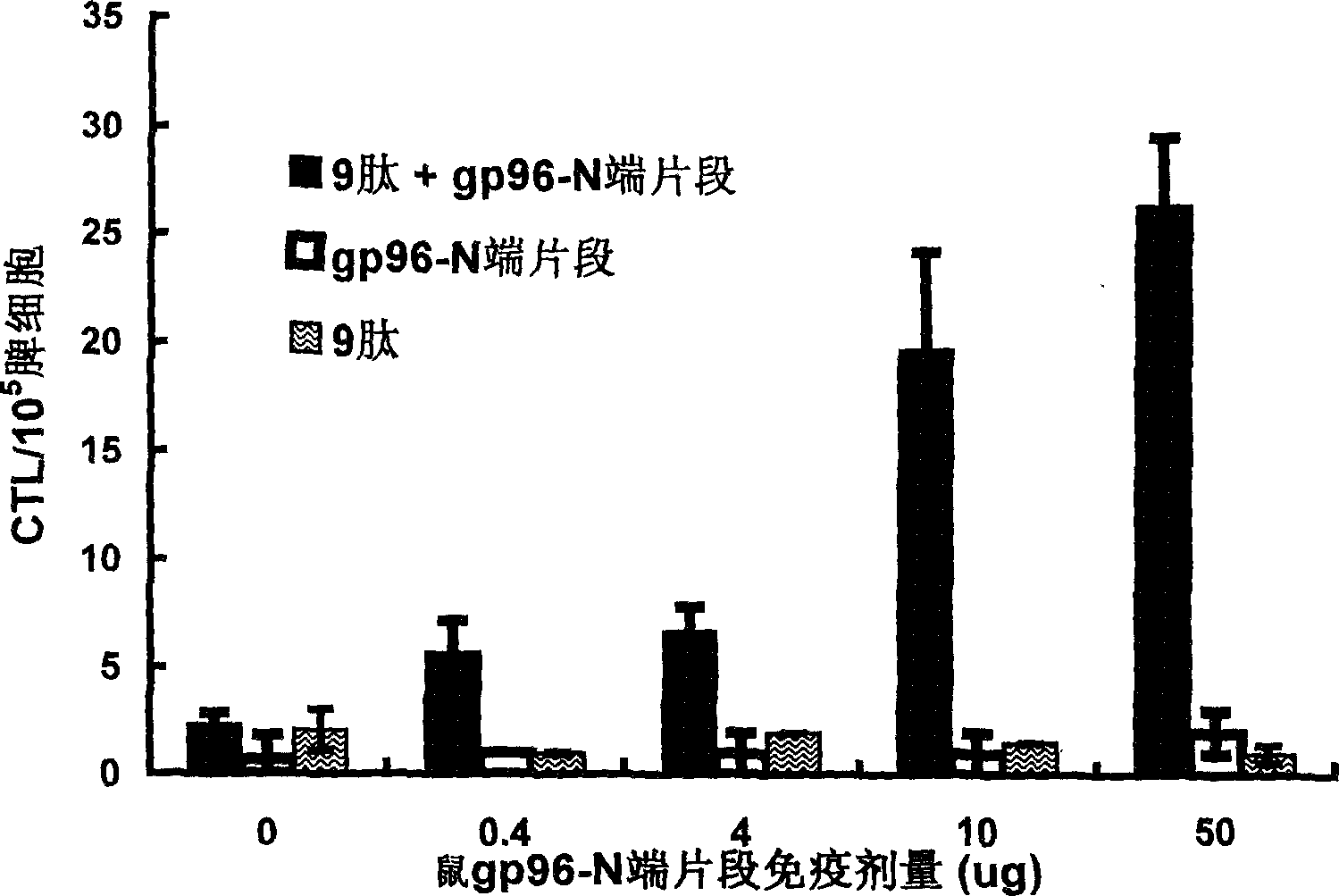
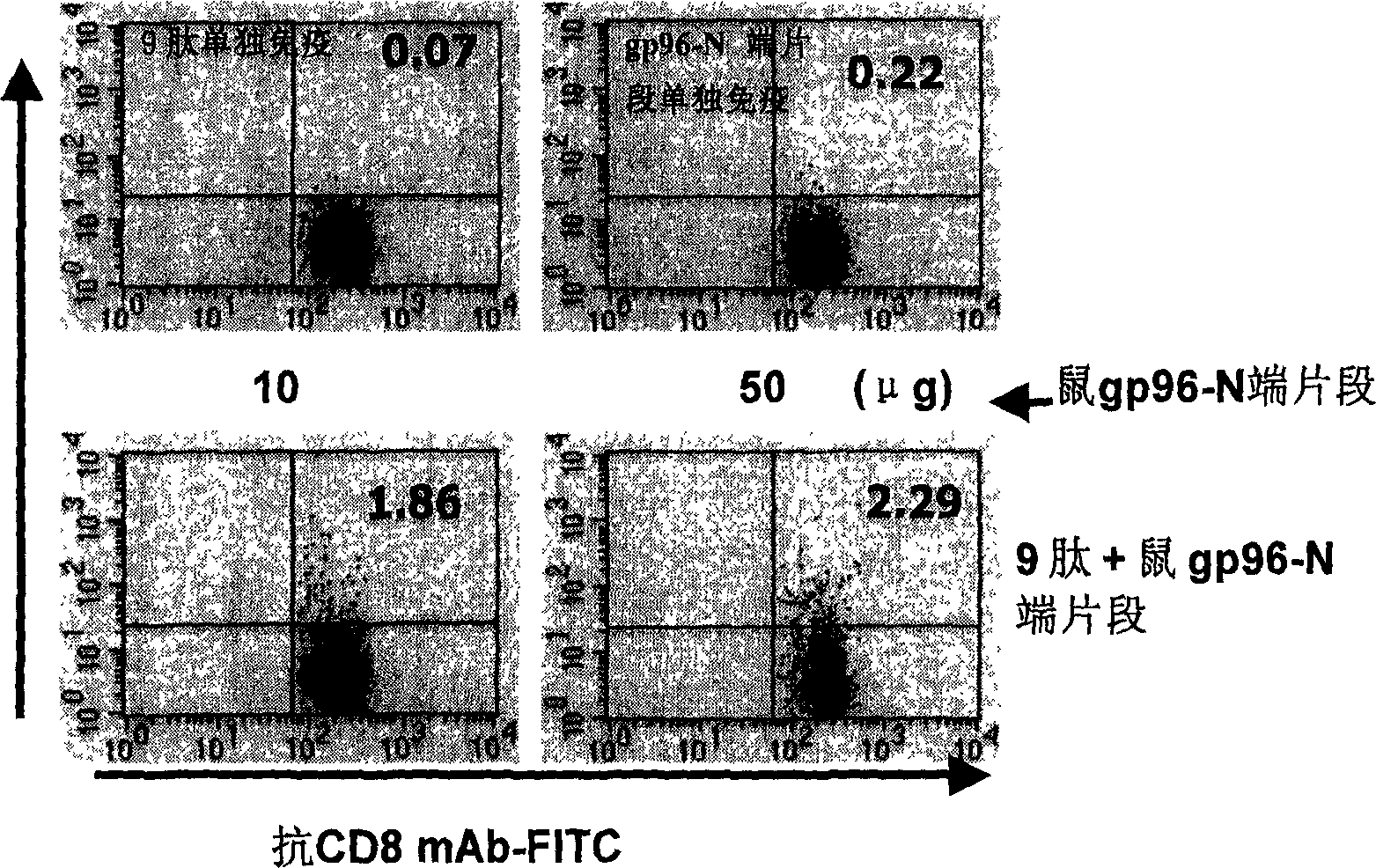
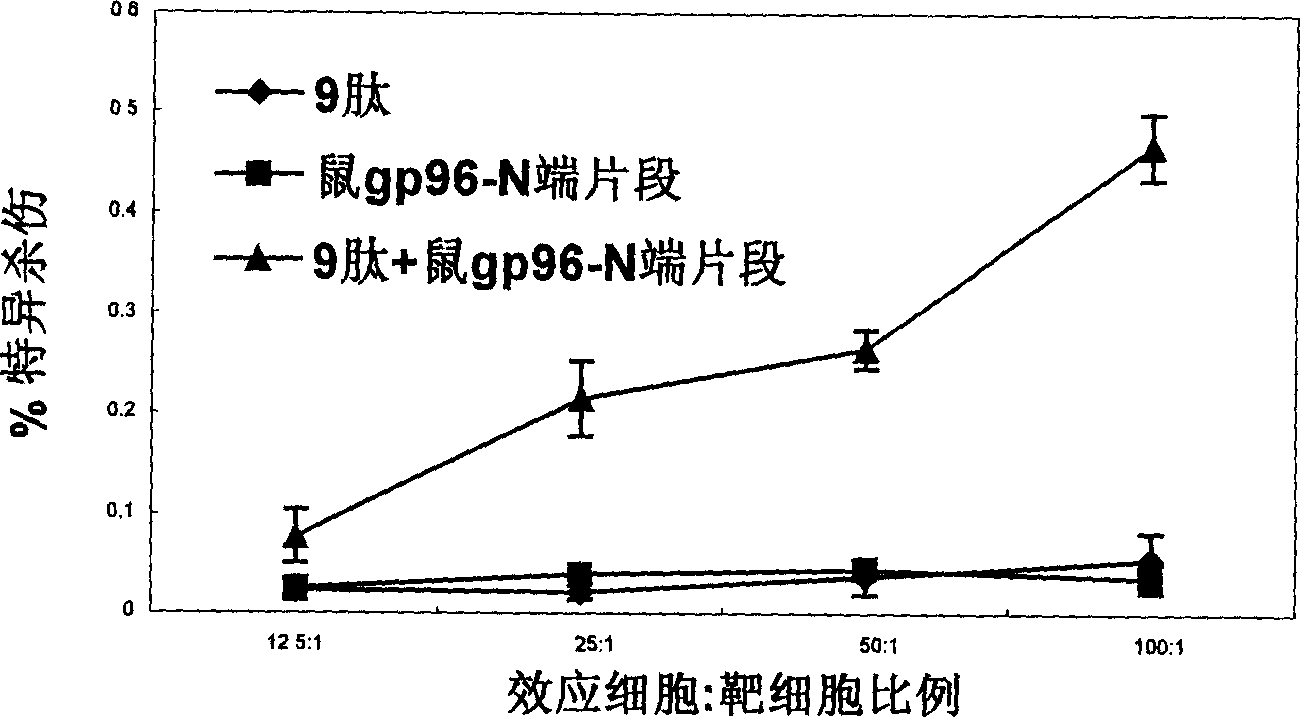
An immunoadjuvant used to prepare the antiviral vaccine or medicine for increasing the immune activity of the antigens for HBV, HCV, SARS coronavirus, fowl influenza virus, etc is a kind of human or animal's novel heat shock proteins gp96, hsp108 and hsp70.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Immunological adjuvant containing fucosan sulfate and application of immunological adjuvant
InactiveCN104383534AImproving immunogenicityGood effectAntiviralsAntibody medical ingredientsSide effectSulfate
The invention relates to an immunological adjuvant containing fucosan sulfate and application of the immunological adjuvant. The technical problems that at present, the kinds of immunological adjuvants are few, side effects cannot be avoided, and a great number of vaccines need to be provided when infectious diseases break out are solved. The immunological adjuvant consists of the following components in mass ratio: fucosan sulfate, ginsenoside and the rest of carriers, wherein the carriers consist of the following components in mass ratio: an emulsifying agent, an emulsifying auxiliary agent, oil phase and aqueous phase. The immunological adjuvant can be widely used in the preparation field of vaccines.
Owner:WEIHAI RENSHENG PHARMA GRP
Traditional Chinese medicine astragalus polysaccharide immunopotentiator
ActiveCN101884788AGood effectNo side effectsAntibody medical ingredientsCellular immunityNewcastle disease vaccine
The invention relates to a traditional Chinese medicine immunopotentiator (an astragalus polysaccharide immunopotentiator for short) prepared from astragalus extracts and sulfated epimedium polysaccharides, which belongs to the field of immunological adjuvants of livestock and poultry. 1,000ml of the liquid medicine is prepared from 40g of astragalus and 120g of epimedium herb. The preparation method of the immunopotentiator comprises the following steps of: decocting the astragalus with water for three times, then merging the obtained filter liquor and condensing the filter liquor into 500ml of astragalus solution; extracting the epimedium polysaccharides by using the epimedium herb water decoction and ethanol precipitate method, then decorating the polysaccharides by using the chlorosulfonic acid-pyridine method, and preparing the polysaccharides into 500ml of sulfated epimedium polysaccharide solution after carrying out distilled water dialysis on the polysaccharides; and mixing the astragalus solution and the sulfated epimedium polysaccharide solution, and then filtering, sub-packaging and sterilizing to obtain the traditional Chinese medicine astragalus polysaccharide immunopotentiator. The immune experiment shows that the astragalus polysaccharide immunopotentiator has the advantage of obviously improving the proliferation of peripheral blood lymphocyte of chicken and enhancing the cellular immunity, obviously improving the potency of a serum antibody, promoting the lymphocyte proliferation, enhancing the cellular immunity and humoral immunity, and improving the immune response of a vaccine by coordinately immunizing chickling by using the Newcastle disease vaccine.
Owner:NANJING AGRICULTURAL UNIVERSITY
Compound-type nano-vaccine and preparation method thereof
InactiveCN104645349AImprove Uptake PresentationImprove immune efficiencyGenetic material ingredientsMacromolecular non-active ingredientsDendritic cellBiocompatibility Testing
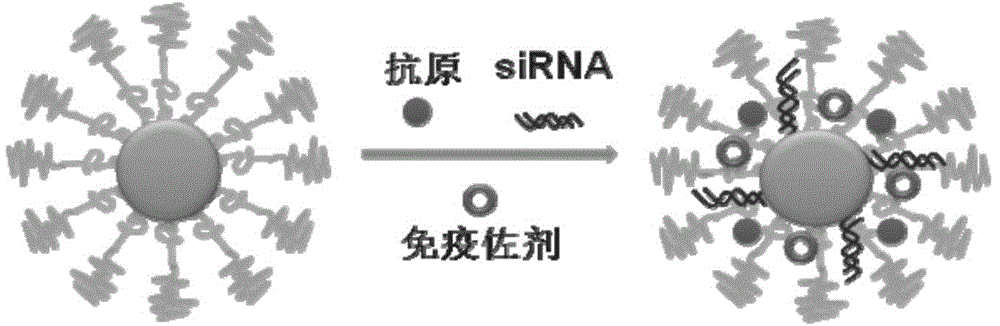
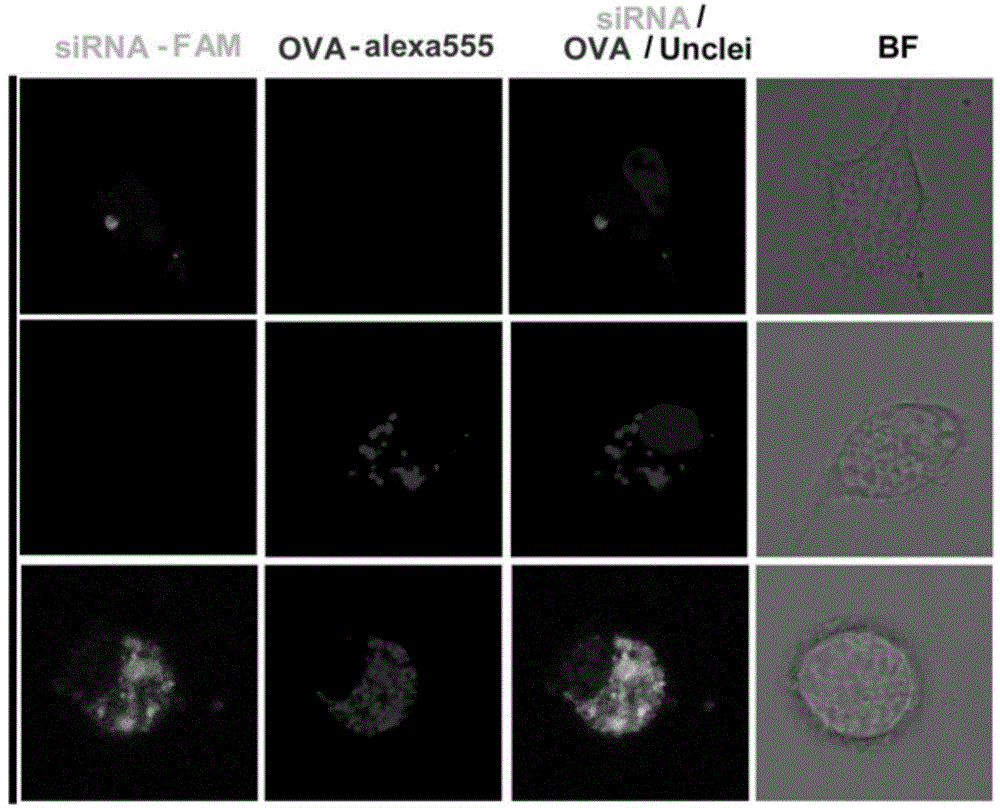
The invention discloses a compound-type nano-vaccine taking an amphiphilic three-block polymer of polyethylene glycol derivative-poly lysine-poly leucine as a nano-carrier bearing a tumor antigen, an immunological adjuvant and siRNA (si Ribonucleic Acid), and a preparation method of the compound-type nano-vaccine. According to the nano-vaccine, taking and presenting of the antigen by antigen-presenting cells can be improved by a nano-micelle bearing the antigen; siRNA is efficiently delivered to TADCs (Tumor-associated Dendritic Cells) to block immunosuppression signals of TADCs, and TADCs are induced and activated by collaboration with the immunological adjuvant, so that a tumor resisting effect of a tumor vaccine is improved; the nano-vaccine has the advantages that the adopted carrier of the nano-vaccine is good in biocompatibility and low in toxicity, and is degradable in a biological body; degradation products are nontoxic and harmless, and can be absorbed or metabolized; and the preparation method of the nano-vaccine is simple and convenient and feasible, good in stability and convenient for popularization.
Owner:SHENZHEN INST OF ADVANCED TECH
Immunoadjuvant
InactiveUS20060008478A1Prevent bad situationsEfficiently exhibit potent adjuvant activityPeptide/protein ingredientsCancer antigen ingredientsBiological bodyLiving body
An immunoadjuvant which can efficiently exhibit potent adjuvant activity and avoid conditions undesirable for living bodies, and comprises precipitates formed by coacervation of (a) a soluble protein (provided that a soluble protein contained in tuberculin is excluded), and (b) a mucopolysaccharide, and further comprises (c) a soluble protein contained in tuberculin wherein said (c) is coprecipitated with the precipitates.
Owner:RIKEN +1
Immunological adjuvant with immunity vegulating agent for treating and preventing diabetic from insulin-dependent
InactiveCN1831012AReduce immune damageDoes not induce atherosclerosisPeptide/protein ingredientsMetabolism disorderInsulin dependent diabetesInsulin dependent
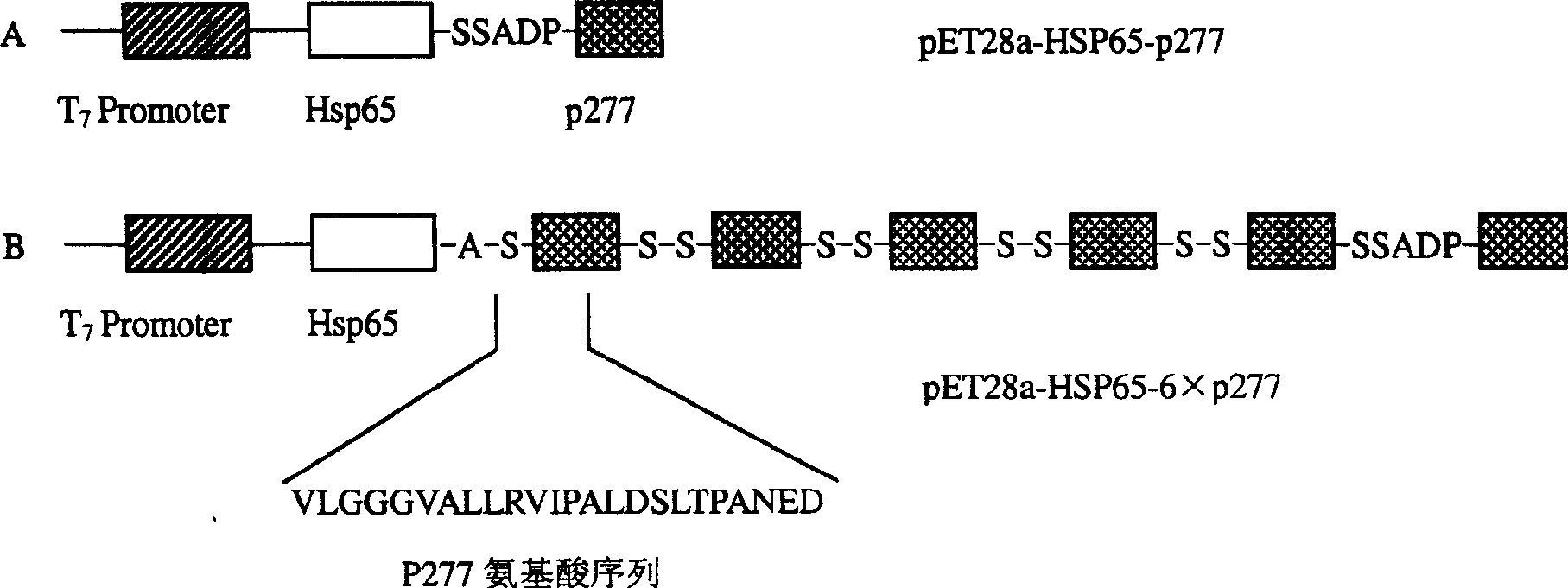
The invention provides the immunomodulator with the free adjuvant having the function of preventing and curing the insulin-dependent diabetes mellitus. Aiming at the weak immunogenicity, the antigen epitope polypeptide gene (a length of antigen epitope p277 rooted from human HSP60) is associated repeatedly six times and inserted repeatedly into the backward position of the heat shock protein HSP65 gene rooted from the mycobacterium bovis, the fusing expression between the repeat series connecting antigen polyeptide and the HSP65 is realized. The produced fusing albumen did not need the adjuvant; the antigen polyeptide don't need be coupled with the carrier chemistry to the immunity. The fusing albumen can inspire the organism to produce the high titer aiming at p277 by means of the mucous membrane immunity, so the nosogeny of NOD chmice diabetic are depressed highly. The invention can prevent the 1 type and 1.5 type diabete characterized in depending on the insulin.
Owner:CHINA PHARM UNIV
Immunogenic Compositions Containing Anthrax Antigen, Biodegradable Polymer Microparticles, And Polynucleotide-Containing Immunological Adjuvant
InactiveUS20080317784A1Quick buildReadily apparentAntibacterial agentsPowder deliveryPaenibacillus lactisNucleotide
Immunogenic compositions and kits, as well as methods of stimulating immune responses and methods of immunization using the same. The compositions and kits comprise: (a) an antigen derived from Bacillus anthracis; (b) polymer microparticles comprising a biodegradable polymer; and (c) a polynucleotide-containing immunological adjuvant.
Owner:CHIRON CORP
A kind of bacillus amyloliquefaciens wh3 and its preparation method and application
InactiveCN102286408AOral lowLow injection toxicityBacteriaMicroorganism based processesFreund adjuvantSclerotinia
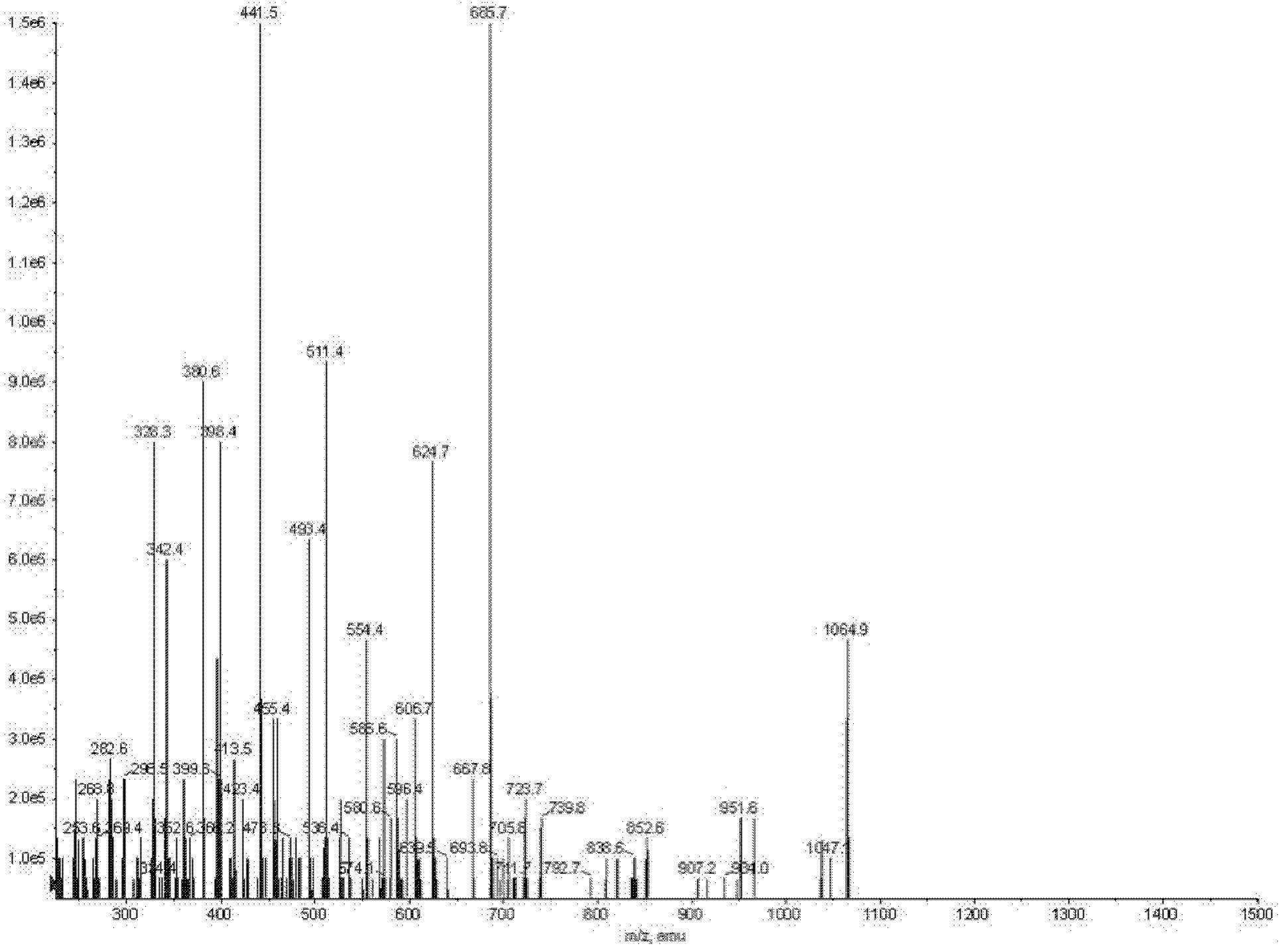
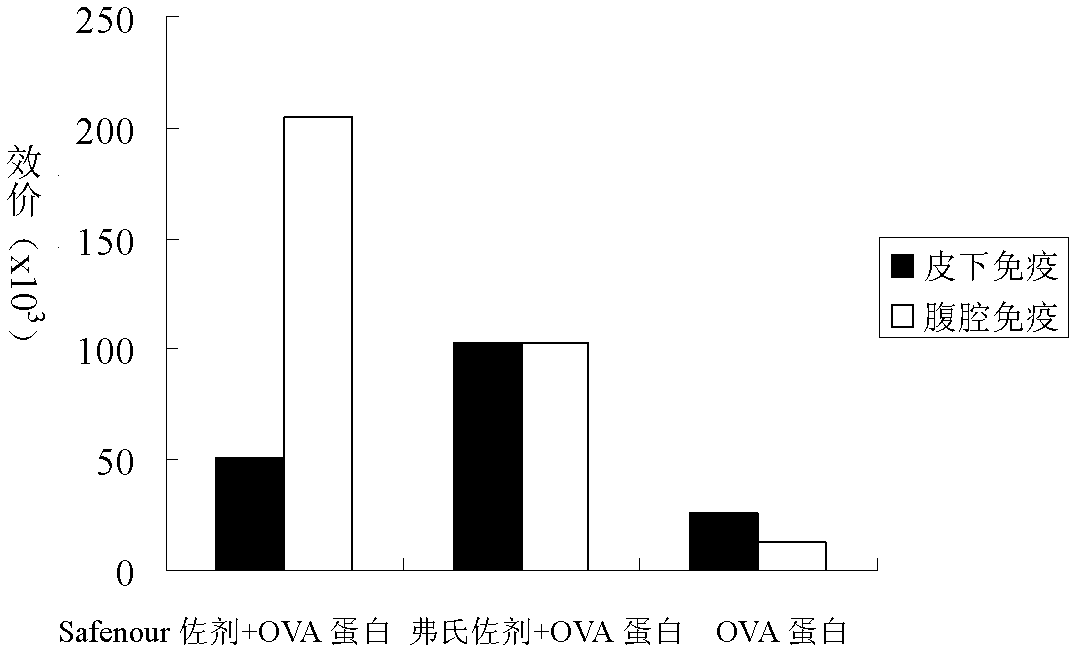
The invention discloses a Bacillus amyloliquefaciens WH3 strain, and a preparation method and application thereof. The preparation method comprises the following steps: 1, separation and identification of bacteria: separating bacteria resistant to rape sclerotinia rot from rape seedlings, and carrying out 16SrDNA and morphological identification to determine that the WH3 strain obtained by separation is Bacillus amyloliquefaciens; 2, separation, purification and identification of an antifungal active substance Safenour: fermenting the WH3 strain, extracting the antifungal active substance, separating and purifying through a sephadex column, and carrying out MALDI-TOF (matrix-assisted laser desorption / ionization-time of flight ) mass spectrometry on the active antifungal substance to inferthat the substance is a ring type polypeptide; and 3, application of the strain in the preparation of vaccines and immunological adjuvants. After the Safenour used as the adjuvant is mixed with a protein antigen and mice are respectively immunized through oral administration and injection of the mixture, effective body fluid and cellular immune response can be activated, and a high-titer specificantibody can be detected in the blood serum. The Safenour has low production cost and high stability, does not need to be emulsified when mixed with the antigen, and has a better immunoenhancement effect in comparison with Freund adjuvants and cholera toxin B subunits.
Owner:武汉光谷世傲生物科技有限公司
Production of squalene from hyper-producing yeasts
ActiveUS20110243969A1Lower unit costIncrease productionFungiSnake antigen ingredientsBiotechnologyYeast
A method for preparing purified yeast is disclosed, where the squalene source is a yeast that hyper-produces squalene. The squalene is useful for pharmaceutical purposes. For instance, it can be used to prepare an oil-in-water emulsion, and the emulsion is particularly suitable for use as an immunological adjuvant.
Owner:NOVARTIS AG
Compounds for preparing immunological adjuvant
Owner:EISIA R&D MANAGEMENT CO LTD
Compound immunological adjuvant and vaccine
ActiveCN101850117AHigh activityImprove immune responseViral antigen ingredientsAntiviralsMicroorganismRecombinant peptide
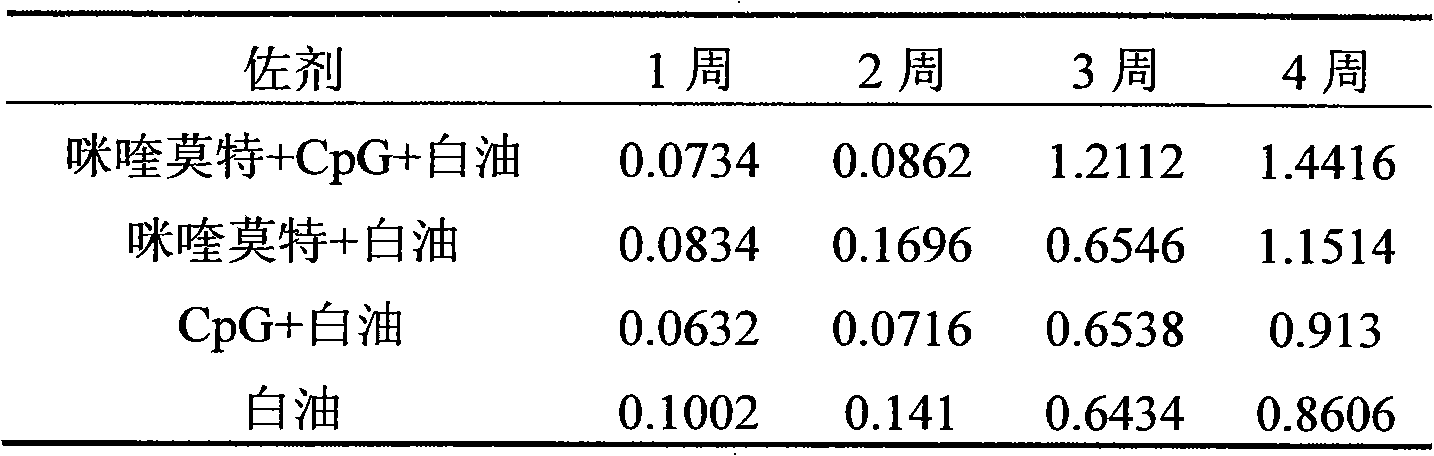
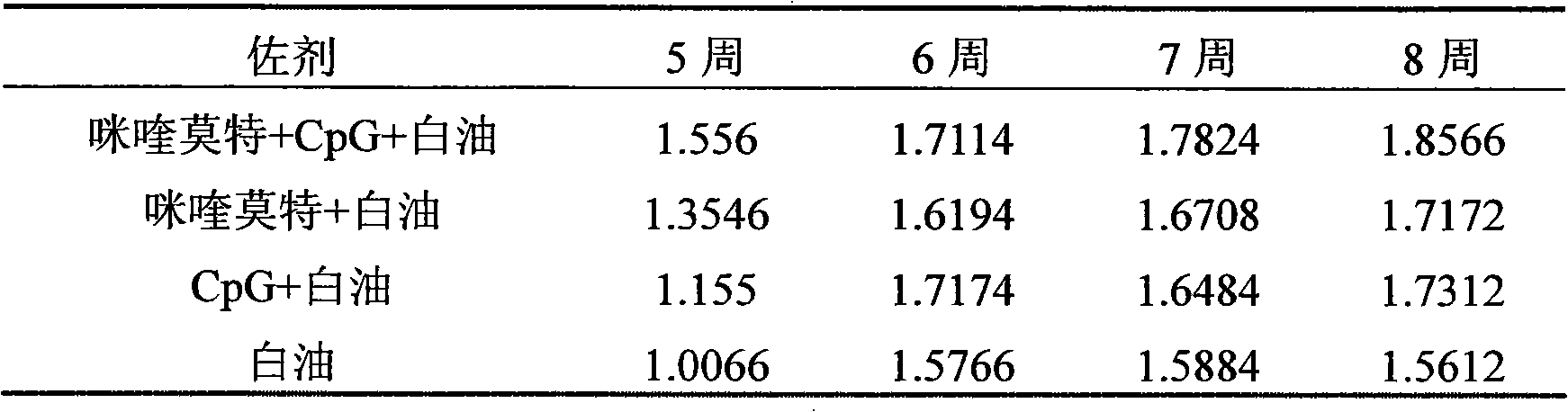
The invention relates to a compound immunological adjuvant comprising a water phase solution and an oil phase solution which are respectively prepared, wherein the water phase solution is a CpG water solution the concentration of which is 0.5 to 2.0mg / mL; the oil phase solution is an imiquimod white oil solution the concentration of which is 0.25 to 1.0mg / mL; and the volume ratio of the water phase solution to the oil phase solution is 1:6. The invention also relates to a vaccine prepared from one or more antigens selected from attenuated live full microorganisms, inactivated microorganisms, recombinant peptide and proteins, synthetic peptide and cracked microorganisms and the water phase solution and the oil phase solution in the compound immunological adjuvant according to the volume ratio of 1:1:6. By the compounding of imiquimod, CpG and a white oil component, the compound immunological adjuvant has an obvious synergistic effect and enhances the immunological activity reactions of Th1 and Th2, so that the activity of stimulated immunological cells is obviously enhanced.
Owner:国家兽用生物制品工程技术研究中心 +2
Immunomodulatory compounds and methods of use thereof
Owner:EISIA R&D MANAGEMENT CO LTD
Building method for autovaccine by aiming at human TNF(Tumor Necrosis Factor)-alpha molecule
InactiveCN102370979AMaintain immunogenicityRemove natural biological activityBacteriaAntipyreticL929 cellEscherichia coli
The invention discloses a building method for autovaccine in-vivo induced by aiming at human TNF(Tumor Necrosis Factor)-alpha molecule. With a step-by-step cloning method, a fusion gene of hTNF-TT830-844, hTNF-HEL46-61 and hTNF-PADRE is built; point mutation (T439-A,C440-G) is introduced into a natural human TNF gene to optimize a mRNA (Ribonucleic Acid) secondary structure; the fusion gene is cloned into a pET22b prokaryotic expression vector, and efficient expression is achieved in the bacterial strain of escherichia coli; three T accessory cell epitope peptides are introduced between the epitope peptide structure domains of hTNF by the computer-aided analysis and is fused with the hTNF-alpha to overcome the immunological tolerance of an organism for the autologous protein, and therefore the organism generates high-level humoral immune response; the generated high-level hTNF-alpha neutralizing polyclone antibody can neutralize killing activity of the hTNF-alpha on L929 cells in vitro; the hTNF-PADRE has the strongest immunogenicity; the high-level antibody can be induced under the condition of using no immunological adjuvant; and the vaccine has favorable protection and curing action on mouse models suffering from rheumatoid arthritis induced by the II-type collagen, cachexia and the like induced by LPS (lipopolysaccharide).
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Compound ginseng-astragalus immunopotentiator
InactiveCN101019885AGood effectNo side effectsOrganic active ingredientsImmunological disordersIrritationT lymphocyte
The compound ginseng-astragalus immunopotentiator for enhancing immunological function of animal is injection, each 1000 ml of which contains ginsenoside 0.75 g and astragalus polysaccharide 3.0 g except water for injection. The compound ginseng-astragalus immunopotentiator may be used alone to enhance the cellular immunity and humoral immunity or as immunological adjuvant to result in excellent immunopotentiating effect. Pharmacological test shows that the immunopotentiator can raise the expression of chicken's peripheral blood T lymphocyte IL-2mRNA and IFN-gamma-mRNA. Safety test shows that the immunopotentiator has no irritation and no toxicity.
Owner:NANJING AGRICULTURAL UNIVERSITY
Preparation method of immune-enhanced recombinant PRRSV virus-like particle subunit vaccine
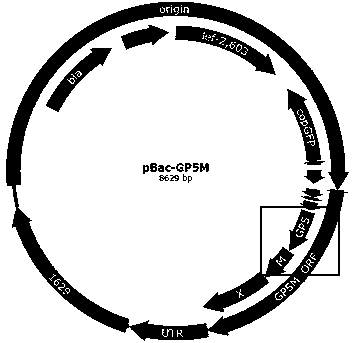
ActiveCN109402145ABroad-spectrum cross-immunogenicityImproving immunogenicityViral antigen ingredientsVirus peptidesBaculovirus expressionVirus-like particle
The invention discloses a preparation method of an immune-enhanced recombinant PRRSV virus-like particle subunit vaccine. The subunit vaccine is prepared from PRRSV virus-like particles and compound immunological adjuvants. By a genetic engineering means, a PRRSV GP5-M gene is modified, and PRRSV virus-like particles with high immunogenicity are prepared by constructing a rhabdovirus expression vector. In addition, by improving the immunological adjuvants, the immuno-enhanced recombinant PRRSV virus-like particle subunit vaccine is obtained, and the subunit vaccine has better immunization effects.
Owner:陕西诺威利华生物科技有限公司
Immunological adjuvant
InactiveUS20050163787A1Small volumeSnake antigen ingredientsPharmaceutical non-active ingredientsVaccinationAutoimmune condition
A pharmaceutical composition to be administered to mammals via the mucosal route consisting of a vehicle system comprising mono- / dietherglycerides conjugated with a water soluble polymer groups selected from PEG's containing 2-30 polyoxyethylene units, an antigen and optionally a bacterial toxin for the augmentation of immune responses for vaccination, immunisation, treatment of allergy, treatment of cancer, treatment of infectious disease, treatment of an autoimmune disease, Alzheimer's, substance addiction or the treatment of a disease which is fully or partially controlled by the body immune system.
Owner:LYFJATHROUN HF BIOPHARML RES
Compound, medicament, vaccine composition and nanocapsules
InactiveUS20110244044A1Effective targetingStrong and durable immune responseBiocideOrganic active ingredientsPolyelectrolyteAnionic polymers
The present invention relates to a compound comprising a polyelectrolyte and, covalently linked thereto, an immunological adjuvant and / or cell targeting ligand, wherein the covalently linked entity can have both adjuvant and cell targeting characteristics. The compound is used in the preparation of hydrophilic vaccine nanoparticles, which preferably have an antigenic compound or therapeutic agent, or genetic information encoding such compounds or agents entrapped in their matrix, or covalently linked to their surfaces. Vaccine compositions comprising the particles of the invention are advantageous, because a strong and long-lasting immune response is obtained following administration of a single dose. In a preferred embodiment, the polyelectrolyte of the compound is an anionic polymer, and the particle comprises a matrix comprising chitosan.
Owner:MEDIPOL
Research and application of Mycobacterium extract
InactiveCN101518549AStimulate immune responseHas immunogenic activityAntibacterial agentsPowder deliverySide effectMedicine
The invention relates to a method for extracting effective components of Mycobacterium, aims at removing lipid components, reducing side effects, remaining effective components thereof and leading to a function of immunological adjuvant, and comprises the strain of Mycobacterium and application of the extract thereof on the prevention and treatment of tuberculosis.
Owner:中国人民解放军总医院第二附属医院
Nasal cavity immunity composite adjuvant for avian influenza inactivation antigen
InactiveCN101085348ALow costImprove immunityAntiviralsAntibody medical ingredientsNasal cavityLocal immunity
The invention relates to compound intranasai immunization adjuvant used for deactivated avian influenza antigen, belongs to biotechnical field, and is special for the application of deactivated avian influenza antigen. The compound adjuvant is mixed or combined by CpG DNA and sodium cholate according to weight ratio of 5:2 according to dosage of immunological adjuvant of 0.1mg / kg. through combining with deactivated avian influenza antigen and intranasai immunization, the inventive compound intranasai immunization adjuvant used for deactivated avian influenza antigen can effectively local immunity of mucous membrane and humoral immunity of whole body, avoid immunization schedule through intramuscular injection, reduce stress reaction of chickens, provide guarantee for improving chicken quality, and provide an ideal Immunization approach for prevention of avian influenza.
Owner:NANJING AGRICULTURAL UNIVERSITY
Radix achyranthis bidentatae crude polysaccharide, radix achyranthis bidentatae polysaccharide component, radix achyranthis bidentatae homogeneous polysaccharide, and preparation methods and uses of radix achyranthis bidentatae crude polysaccharide, radix achyranthis bidentatae polysaccharide component and radix achyranthis bidentatae homogeneous polysaccharide
ActiveCN107098984AHigh activityEasy to adjustOrganic active ingredientsAntibody ingredientsTraditional medicineAchyranthes
The present invention belongs to the technical field of medicine, and particularly to the field of vaccines and immunizations, more particularly to a radix achyranthis bidentatae crude polysaccharide, a radix achyranthis bidentatae polysaccharide component and a radix achyranthis bidentatae homogeneous polysaccharide extracted from radix achyranthis bidentatae, wherein the radix achyranthis bidentatae crude polysaccharide is selected from a radix achyranthis bidentatae crude polysaccharide 1 and a radix achyranthis bidentatae crude polysaccharide 2, wherein the radix achyranthis bidentatae crude polysaccharide 1 mainly comprises fructose, has a molecular weight of 1000-3000 Da, and preferably contains a small amount of glucose, and the radix achyranthis bidentatae crude polysaccharide 2 mainly comprises arabinose, glucose, Rhamnose, galactose and galacturonic acid, and has a molecular weight of 1.0*10<4>-2.0*10<5>Da. The present invention further relates to a pharmaceutical composition containing the polysaccharide, preparation methods of the polysaccharides, and vaccine uses of the polysaccharides. According to the present invention, the radix achyranthis bidentatae crude polysaccharide, the radix achyranthis bidentatae polysaccharide component and the radix achyranthis bidentatae homogeneous polysaccharide respectively have good immunoadjuvant activity and good immunomodulatory effects, and have the potential in preparation of vaccine adjuvants and immunomodulatory drugs.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Ultrasonic microvesicle as immuno adjuvant and vaccine carrier
ActiveCN1935259AImprove expression levelImprove immune activityPeptide/protein ingredientsCarrier-bound antigen/hapten ingredientsVaccine antigenTarget tissue
The present invention belongs to the field of biomedical engineering, in the more concrete, said invention relates to a new type ultrasonic microvesicle which can be used as immunological adjuvant and vaccine carrier. It is characterized by that the vaccine antigen substance is adhered on the microvesicle surface and / or covered in microvesicle interior so as to synthesize the vaccine antige carried ultrasonic microvesicle. When it is used, the vaccine antigen carried ultrasonic microvesicle can systematically or locally act on target tissue, the ultrasonic wave can be used to break the microvesicle of target tissue area, and orietationally release vaccine antigen substance so as to raise the immunological activity of antigen.
Owner:许川山 +2
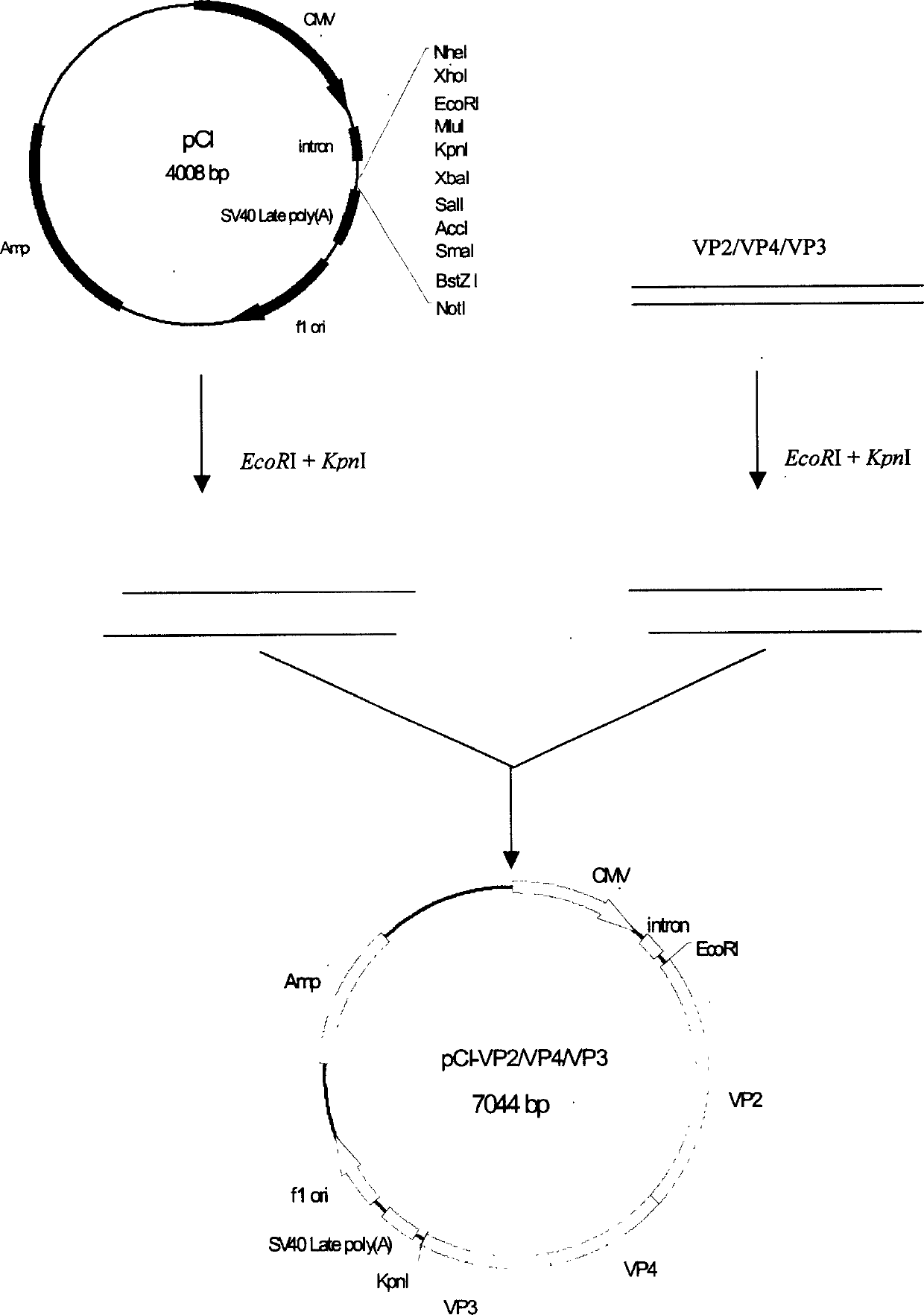
Infectious bursal disease virus (IBDV) polyprotein gene (VP2/VP4/VP3), eukaryon expressing plasmid, DNA vaccine
InactiveCN1467293AImproving immunogenicityNo missingViral antigen ingredientsAntiviralsVp2 geneA-DNA
A polymer protein (VP2 / VP4 / VP3) gene of infectious Fabricius bursa virus (IBDV) for intensifying the immunogenicity of vaccine and a recombinant carrier using pCI as eukaryon expression vector and containing said coding sequence are disclosed. The expression ability of said recombinant carrier increase by 10-40 times than normal eukaryon carrier. A DNA vaccine for infectious Fabricius bursa virus is also disclosed, which contains said eukaryon expression plasmid and immunological adjuvant and features high safety, stability and effect.
Owner:ZHEJIANG UNIV
Poplar bark lipoid adjuvant and its preparing method and using method
InactiveCN1531970AEasy to store and transportDoes not affect exportAntibody medical ingredientsAllergyImmunological Adjuvants
The present invention relates to immunological adjuvant, and is especially a kind of poplar bark lipoid used as immunological adjuvant and its preparation process and usage. The adjuvant is prepared with coarse extracted lipoid liquid and through stirring, filtering and cooling under certain condition in bacteria-free environment. The adjuvant is easy to maintain and transport without needing special apparatus. The mixture of the adjuvant and antigen in certain ratio is used to immunize animal to produce immunity fast and with less allergy. Immunizing hen with the mixture can also raise laying rate.
Owner:SHANDONG BINZHOU BOLAIWEI BIOTECH
Solidified tissue immunological adjuvant
InactiveCN1622829AStrong cell stimulating effectEffective treatmentBacterial antigen ingredientsAntineoplastic agentsBiological bodyMicroorganism
An immunological adjuvant having a potent cell-stimulation effect and a high safety to living bodies, being prepared from a solidified material selected from the group consisting of tissues, cells and components thereof of animals involving humans, containing fragments from which soluble components have been removed by washing with an organic solvent and / or hot water, and having microorganism-origin soluble components immobilized in the fragments.
Owner:CELL MEDICINE
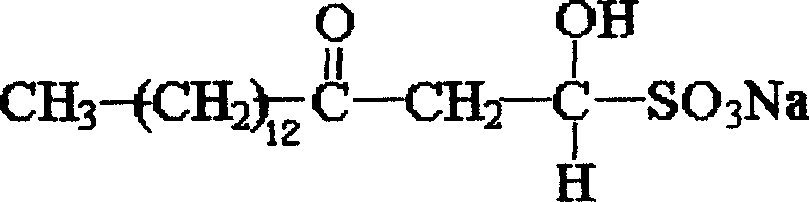
Sodium myristyl aldehyde sulfate and its application as immunoadjuvant
InactiveCN101019845AImprove immune activityImprove immunityImmunological disordersAntibody medical ingredientsSulfateImmunological Adjuvants
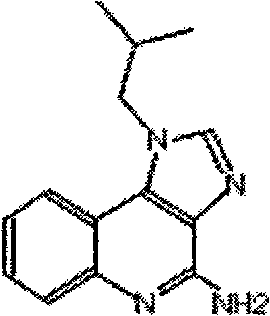
The present invention provides one kind of immunological active matter and immunological adjuvant compound, sodium myristyl aldehyde sulfate. Sodium myristyl aldehyde sulfate has molecular formula of C16H31O5SNa and molecular structure as shown. The present invention proposes the application of sodium myristyl aldehyde sulfate in providing animal with immunological activity and as immunological adjuvant.
Owner:SOUTHWEST UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com