Vaccine composition containing synthetic adjuvant
a technology of adjuvants and compositions, applied in the field of pharmaceutical and vaccine compositions, can solve the problems of high production cost, inconsistency from lot to lot, and curtail the use of adjuvants derived from natural products, and achieve the effect of increasing maturation with gla stimulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
GLA Aqueous Formulation
[0200]This example describes the preparation of a GLA-containing adjuvant aqueous formulation. The aqueous formulation of GLA (GLA-AF) contains Water For Injection (WFI), GLA (Avanti Polar Lipids, Inc., Alabaster, Ala.; product number 699800), and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC). The formulation was prepared by adding a solution of ethanol and POPC to a pre-weighed amount of GLA. This wetted GLA was sonicated for 10 minutes to disperse the GLA as much as possible. The GLA was then dried under nitrogen gas. The dried GLA and POPC were reconstituted with WFI to the correct volume. This solution was sonicated at 60° C. for 15-30 minutes until all the GLA and POPC were in solution. For long term storage, GLA-AF formulations must be lyophilized. The lyophilization process consisted of adding glycerol to the solution until it was 2% of the total volume. Then the solution was placed in vials in 1-10 mL amounts. The vials were then run through ...
example 2
GLA HPLC Analysis
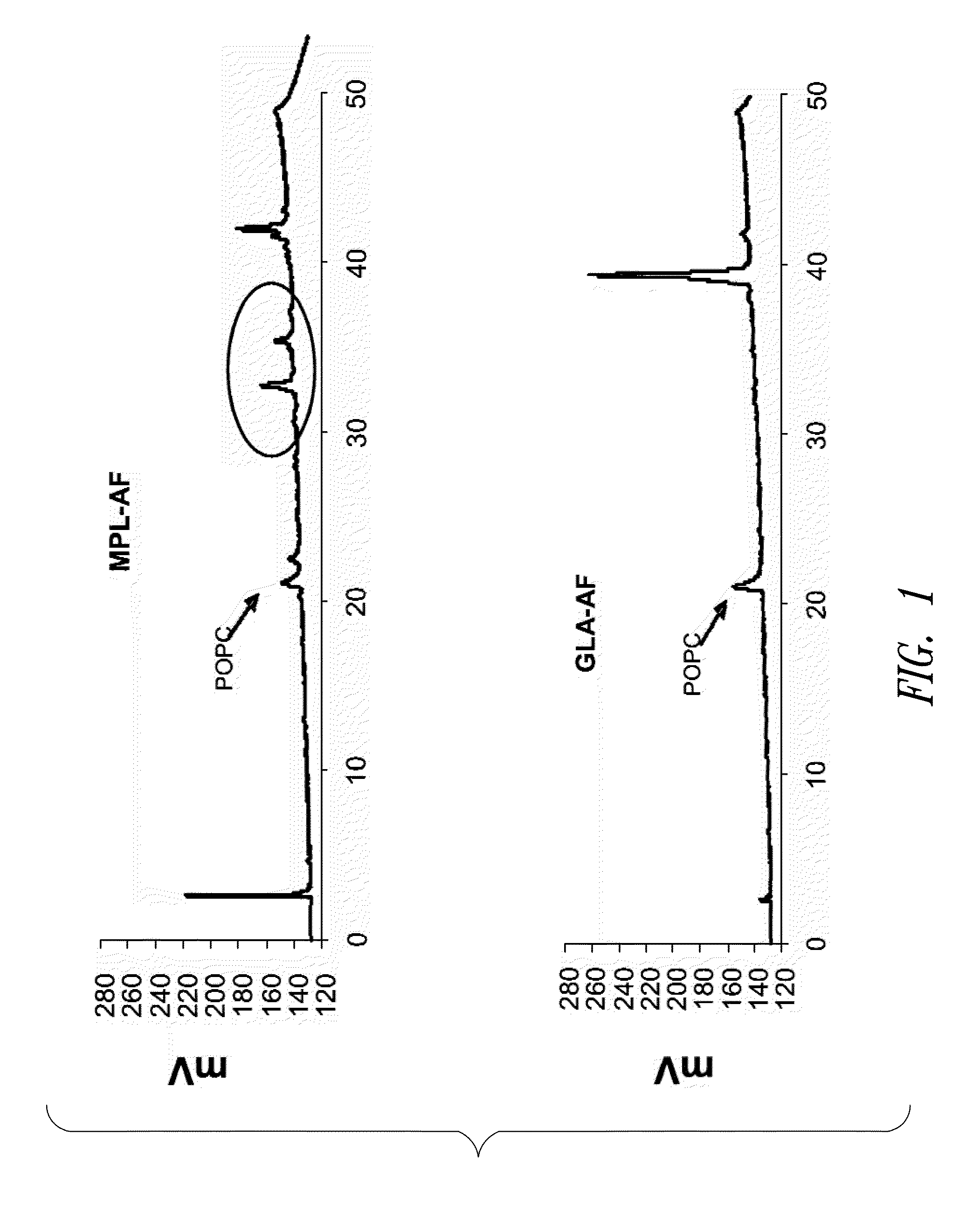
[0201]This example describes HPLC analysis of a GLA-containing adjuvant aqueous formulation. After the formulation was manufactured (see Example 1 above), certain release and stability tests were conducted to ensure product quality and reproducibility. All formulations were tested for release and long-term stability using High Performance Liquid Chromatography (HPLC), Dynamic Light Scattering (DLS) and a visual examination. HPLC chromatograms were collected using an Agilent 1100 system and an ESA Corona CAD detector. The method was run using a methanol to chloroform gradient on a Waters Atlantis C18 column. The injections included 2.5 μg of GLA (Avanti Polar Lipids, Inc., Alabaster, Ala.; product number 699800, GLA-AF) or MPL® (GSK Biologicals, Rixensart, Belgium, MPL-AF) respectively, and 0.27 μg of synthetic phosphocholine (POPC) which was used as a solubilizing agent.
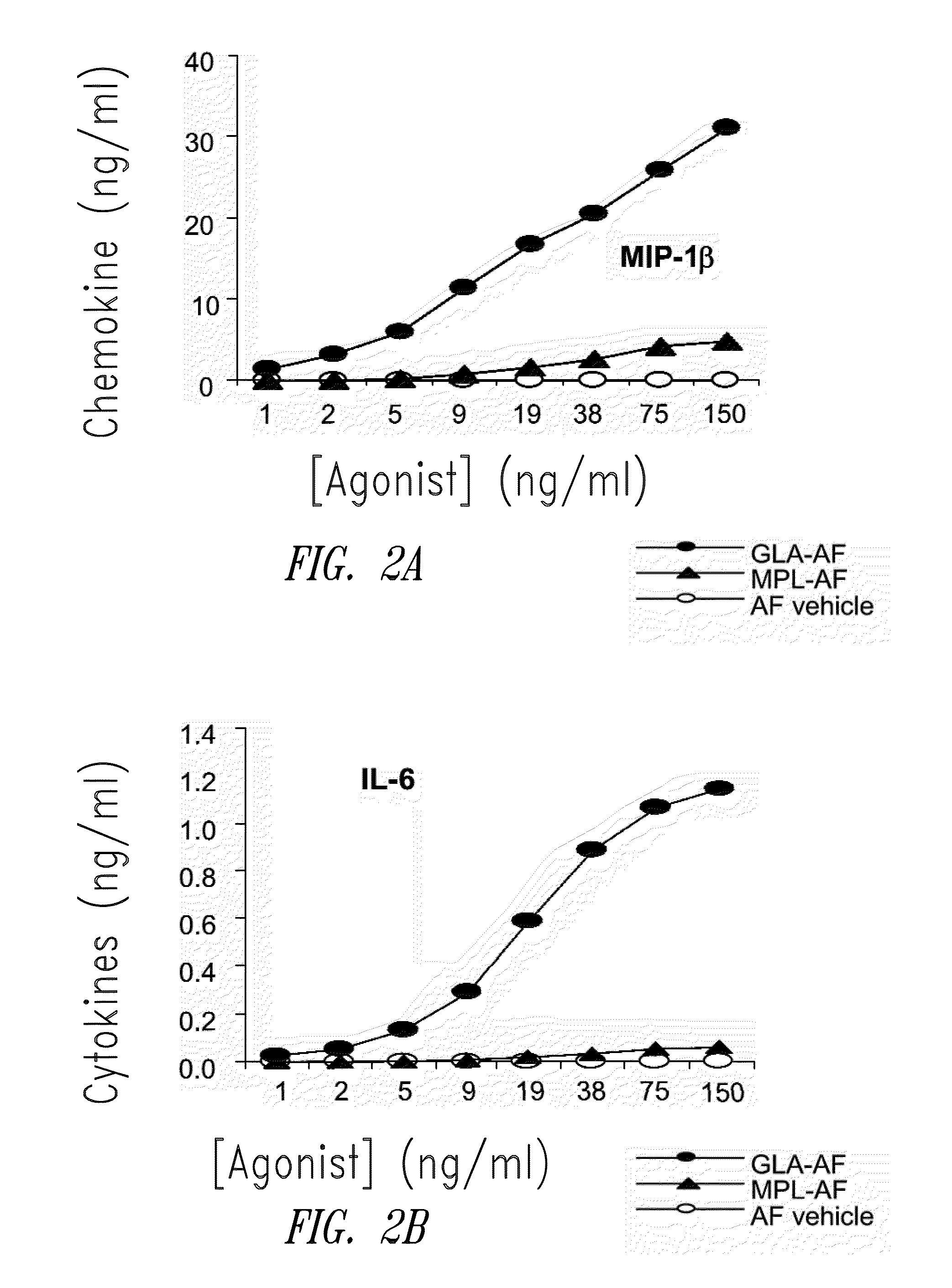
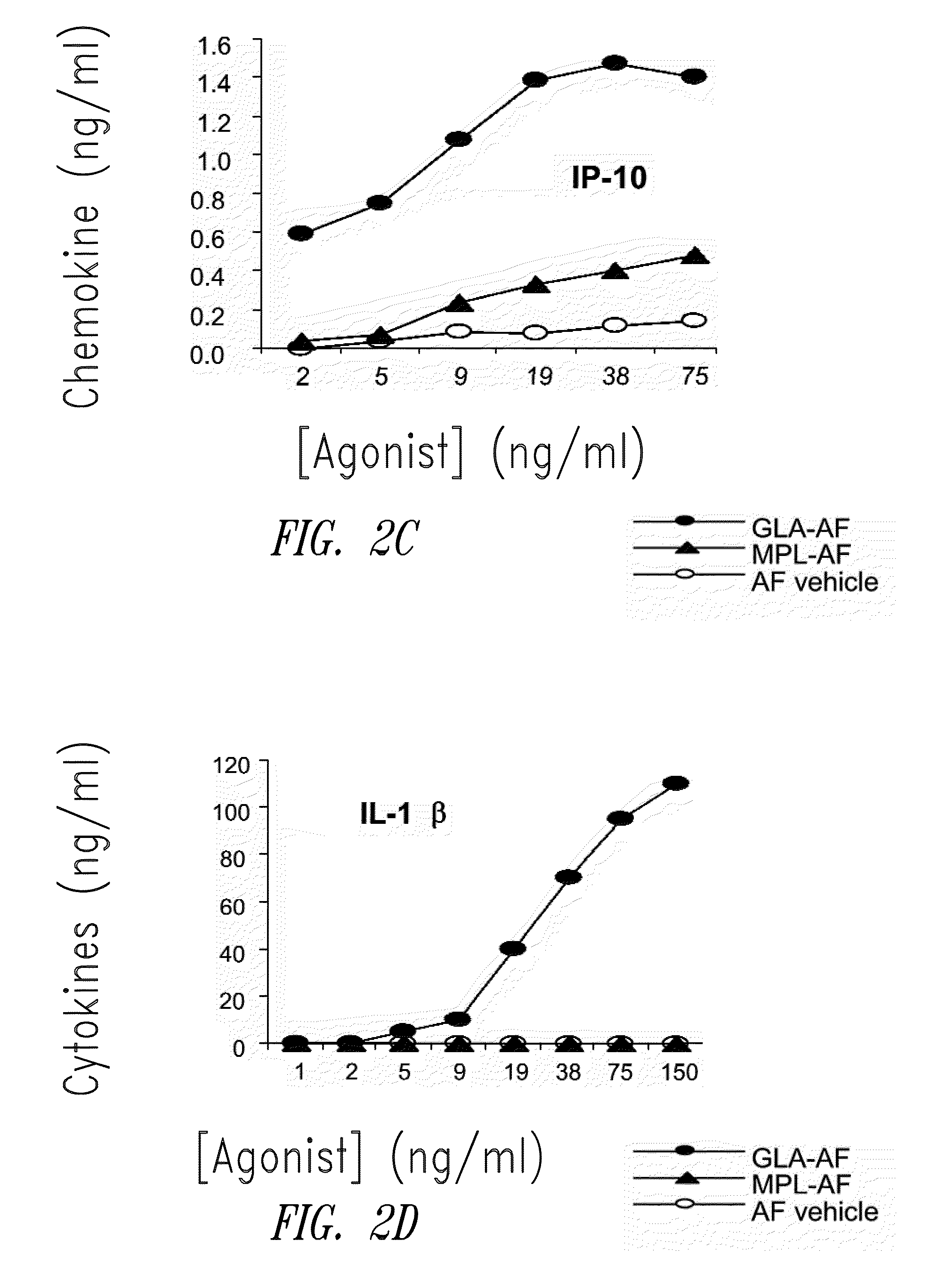
[0202]FIG. 1 shows HPLC data demonstrating the number and amounts of contaminating materials in M...
example 3
GLA Oil Formulation
[0204]This example describes preparation of one milliliter of a GLA-containing adjuvant oil formulation. GLA (100 micrograms; Avanti Polar Lipids, Inc., Alabaster, Ala.; product number 699800) was emulsified in squalene (34.3 mg) with glycerol (22.7 mg), phosphotidylcholine or lecithin (7.64 mg), Pluronic® F-68 (BASF Corp., Mount Olive, N. J.) or similar block co-polymer (0.364 mg) in 25 millimolar ammonium phosphate buffer (pH=5.1) using 0.5 mg D,L-alpha-tocopherol as an antioxidant. The mixture was processed under high pressure until an emulsion formed that did not separate and that had an average particle size of less than 180 nm. The emulsion was then sterile-filtered into glass unidose vials and capped for longer term storage. This preparation may be used for at least three years when stored at 2-8° C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com