Research and application of Mycobacterium extract
A technology of mycobacteria and bacteria, applied in the direction of antibacterial drugs, bacterial antigen components, medical raw materials derived from bacteria, etc., can solve safety problems and other problems, achieve the effect of shortening the course of treatment, reducing side effects, and improving curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the preparation of mycobacterial preparation
[0038] According to the mycobacterium cell composition, cell wall structure and active ingredients, the extraction process was formulated. Taking Mycobacterium light flavinus as an example, BCG, Mycobacterium light yellow, Mycobacterium vaccae, etc. cultured on the improved Sutong medium were collected by filtration, washed and centrifuged twice with normal saline, and the wet weight of the bacteria was accurately weighed , add 3 times (V / W) acetone by volume and weight, extract for 2-10 hours, and extract 2 times in total. Wash twice with normal saline and once with pH8.0 20mmol / L Tris.Cl. pH8.0 20mmol / LTris.Cl suspended bacteria, 1mg / ml lysozyme digested at 37°C for 4-10 hours. Centrifuge, wash the cells with PBS, and dilute the suspended cells with PBS to 20mg / ml.
[0039] Preparation of mycobacterium preparations by high-pressure crushing: the above-mentioned bacterial suspension is homogenized and crus...
Embodiment 2
[0060] Example 2: Effects of Mycobacterium Preparations Immunizing Mice on the Production of Cytokines by Mouse Spleen Lymphocytes
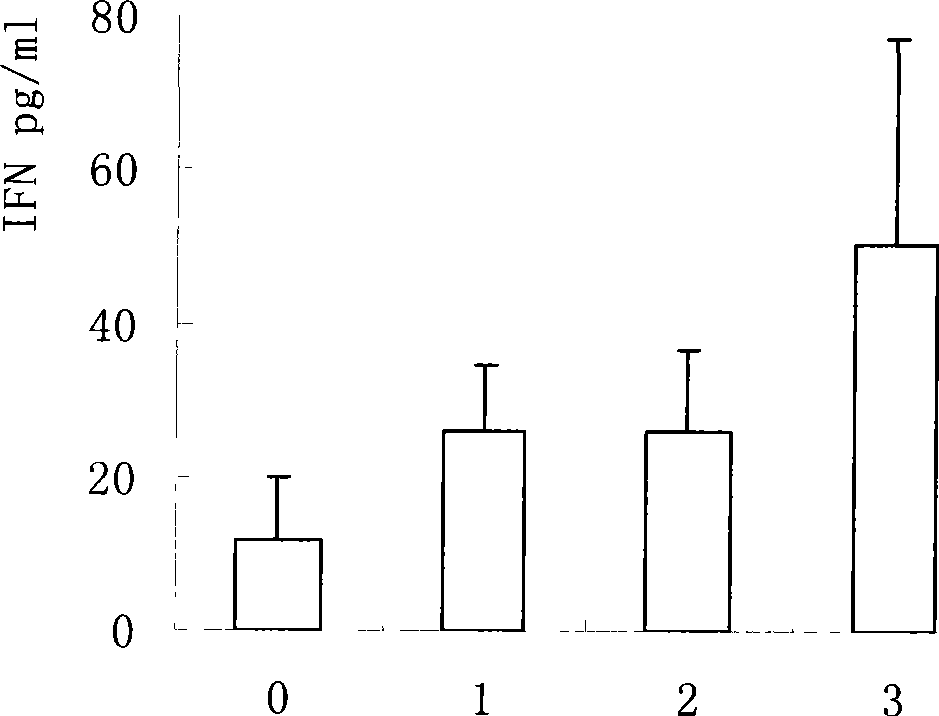
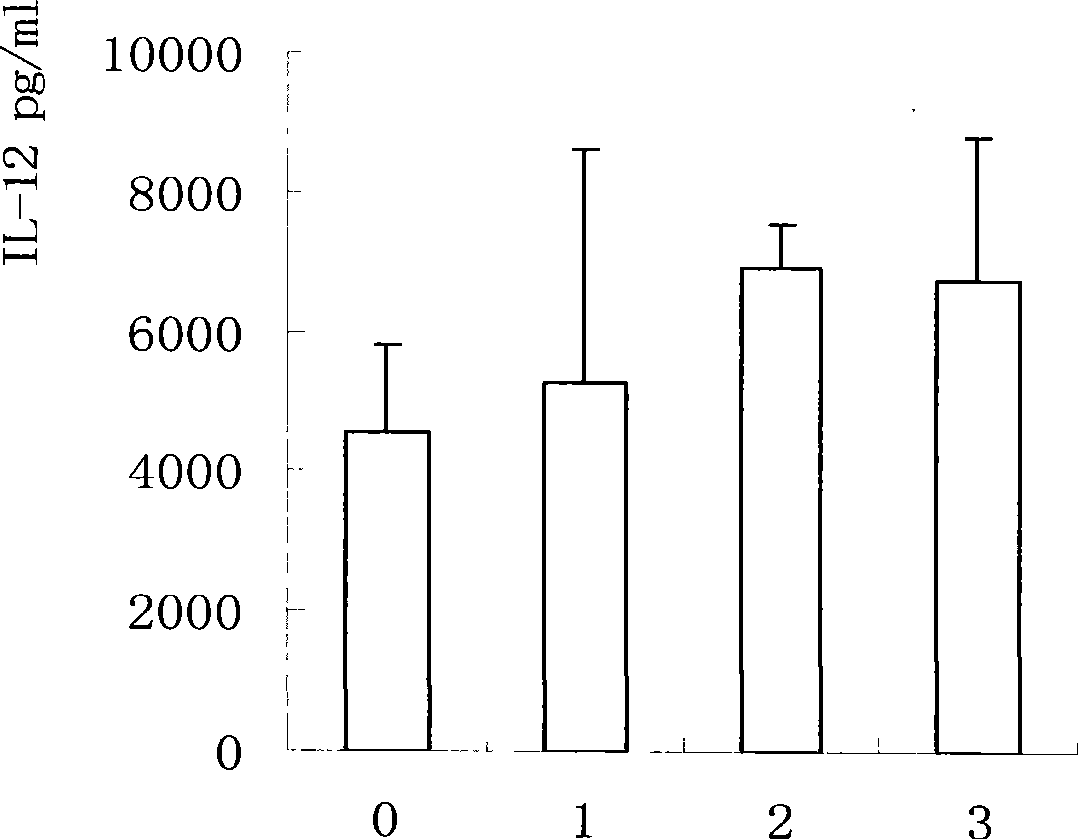
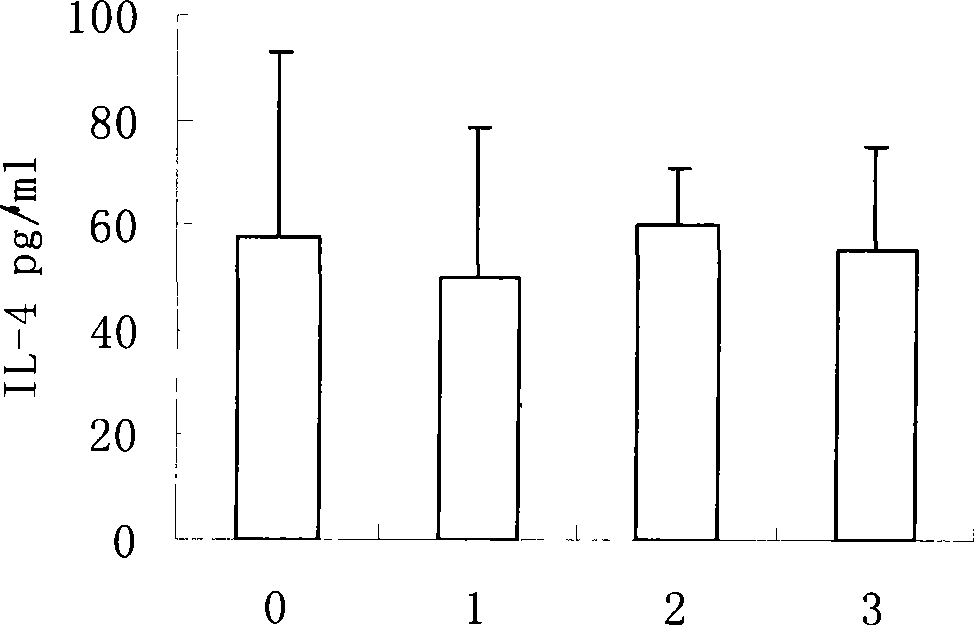
[0061] Mice were immunized with different doses, and an equal volume of normal saline was injected as a control. Two weeks after the last immunization, the spleen was aseptically taken and grinded to adjust the splenocyte concentration to 5×10 6 / ml, dispense into 96-well plates, 100 μl per well, 37°C, 5% CO 2 After culturing for 72 hours, the supernatant was collected by centrifugation and stored at -20°C. The mouse IL-12, IL-4, IFN-γELISA kit produced by BD company detects the cytokines in the culture supernatant of splenic lymphocytes, and the results are shown in Figure 1-3 .
[0062] Cytokines results showed that mycobacterial preparations could stimulate the secretion of Th1 cytokines IL-12 and IFN-γ, and reduce the expression of Th2 cytokines IL-4. This has important implications for TB prevention.
Embodiment 3
[0063] Embodiment 3: the effect of the mycobacterium preparation of the present invention immunizing mice on the production of NO by mouse macrophages
[0064] Nitric oxide (NO), synthesized by nitric oxide synthase (NO synthase), is a very important physiological intracellular and intercellular signaling molecule. The increase of its level is conducive to the body's response to parasitic cells. Injury and removal of bacteria and viruses. Nitric oxide itself is extremely unstable, and there is no good way to directly measure it.
[0065] NaNO 2 It is a stable product formed by the metabolism of nitric oxide, so the determination of NaNO 2 Equivalent to the determination of the cumulative production of nitric oxide.
[0066] In vitro culture of macrophages can induce the production of NO, and the produced NO is rapidly oxidized to NO 2 - , by detecting NO 2 - Can reflect the level of NO production.
[0067] Immunize mice with different doses, and inject an equal volume ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com