Protein antigen, coding gene thereof, and application of protein antigen and coding gene thereof to identification of mycoplasma hyopneumoniae inactivated vaccine antibody and natural infection antibody
A technology of Mycoplasma hyopneumoniae and protein antigen, applied in the application, genetic engineering, plant genetic improvement and other directions, can solve the problems of infection and indistinguishability, and achieve the effect of high sensitivity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
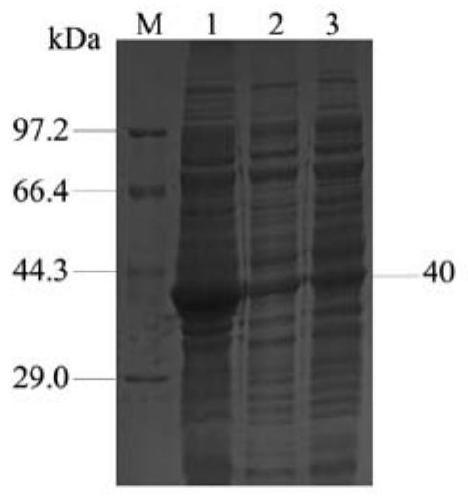
[0121] This example is used to illustrate the preparation method of the protein antigen provided by the present invention
[0122] 1. Construction of recombinant bacteria
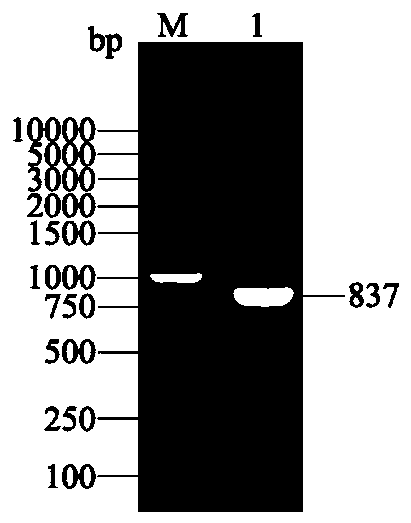
[0123] Entrust Chengdu Qingke Zixi Biotechnology Co., Ltd. to synthesize the nucleotide sequence shown in SEQ ID NO: 1 and the amplification primers of SEQ ID NO: 1. The upstream and downstream 5' ends of the amplification primers were respectively introduced with restriction endonucleases The sequences of enzymes BamHI and XhoI restriction sites (underlined) and corresponding protective bases (underlined wavy lines) are as follows:
[0124] Upstream primer (SEQ ID NO:8):
[0125] Downstream primer (SEQ ID NO:9):
[0126] The PCR reaction system is 50 μL: Primestar Max Premix 25 μL, DNA template 0.5 μL, upstream primer (10 pmol / μL) 0.5 μL, downstream primer (10 pmol / μL) 0.5 μL, ddH 2 O 23.5 μL.
[0127] The PCR reaction conditions were: pre-denaturation at 98°C for 5 min; pre-denaturation at 98°C ...
Embodiment 2
[0139] This embodiment is used to illustrate the collection and detection of pig serum
[0140] In the Mycoplasma hyopneumoniae negative pig farms, piglets were immunized with Mycoplasma hyopneumoniae vaccine Xiyifeng on the 28th day after weaning on the 21st day, boosted once on the 42nd day, and blood was collected from the anterior vena cava on the 63rd day. A total of 28 serum samples were collected.
[0141] 30 samples of commercial pig blood at the age of 90-200 days were collected from a pig farm positive for Mycoplasma hyopneumoniae.
[0142] The collected serum was detected by the Mycoplasma hyopneumoniae antibody kit of IDEXX Company, and 20 copies of Mycoplasma hyopneumoniae antibody-positive serum (hyperimmune serum) after vaccine immunization and 20 copies of Mycoplasma hyopneumoniae-positive pig farm antibody-positive serum (convalescent serum) were preserved.
Embodiment 3
[0144] This example is used to illustrate the determination and operation steps of the optimal reaction conditions of the ELISA kit
[0145] 1. Determination of optimal antigen coating concentration, blocking solution and blocking time
[0146] Dilute the purified protein with carbonate buffer. Concentrations were 0.25 μg / mL, 0.5 μg / mL, 1 μg / mL, 2 μg / mL, 4 μg / mL, 8 μg / mL, 100 μL was added to each well of the ELISA plate, incubated at 37 °C for 1 hour and then overnight at 4 °C. The washing solution was washed 5 times for 3 min each time, and 200 μL of PBS containing 2.5% by weight of skim milk powder was added to block for 0.5 h. The washing solution was washed 5 times for 3 min each time, and 3 parts of hyperimmune serum and 3 parts of convalescent serum were diluted 1:1000 with PBS containing 2.5% by weight skimmed milk powder, 100 μL was added to each well, and incubated at 37°C for 0.5 h. The washing solution was washed 5 times for 3 min each time, and the HRP-labeled ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com