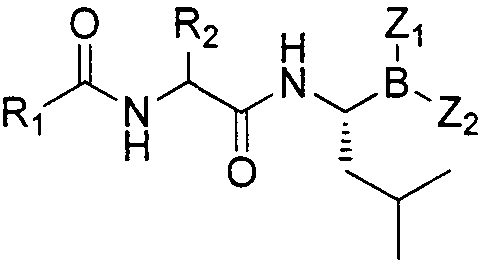
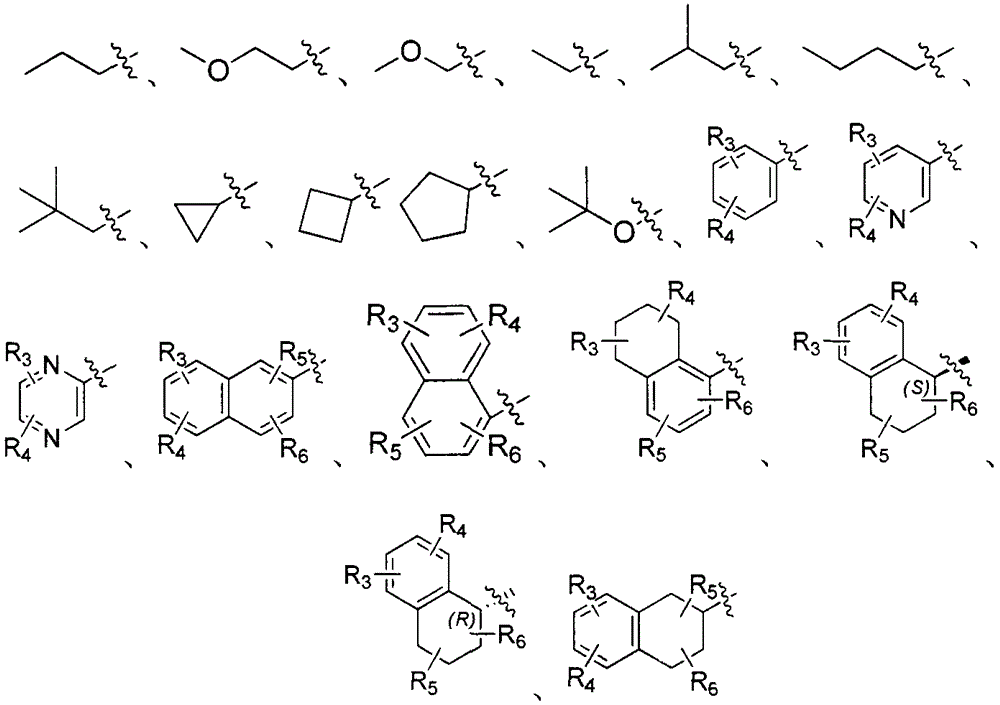
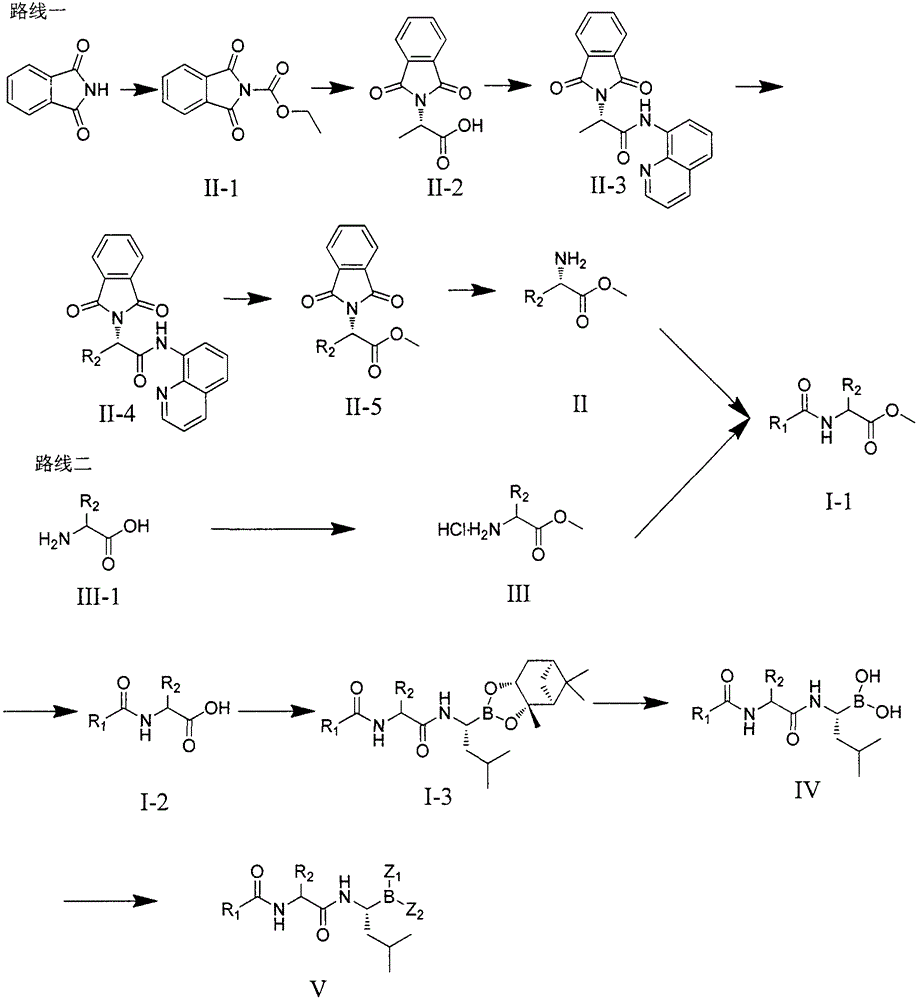
Dipeptide boric acid composed of carboxylic acid and alpha-amino acid as well as ester compound thereof, and preparation method and application of dipeptide boric acid and ester compound thereof
A technology of ester compounds and peptide boronic acid, which is applied in the field of preparation of new peptide boronic acid and its ester compounds, can solve the problems of patients with heavy economic burden, diarrhea and neuropathy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0058] Synthesis of the first part of the compound
[0059] The preparation of the compound of the present invention can be carried out according to the following process:
[0060] One, the preparation of compound (II)
[0061]
[0062] 1. Preparation of N-ethyl acetate phthalimide II-1:
[0063] Dissolve phthalimide in DMF, add triethylamine, add ethyl chloroacetate dropwise to the reaction system at 0°C, slowly rise to room temperature and react for 2 hours, pour the reaction solution into ice water, filter , the filter cake was washed with ice water, and vacuum-dried to obtain the pure compound (formula II-1).
[0064] 2. Preparation of N-phthaloyl-protected alanine II-2:
[0065] Compound II-1 and L-alanine were dissolved in H 2 O, add Na 2 CO 3 After reacting for 2 hours, add 1N HCl to adjust the pH value to 2, filter, and dry in vacuum to obtain the pure compound (formula II-2).
[0066] 3. Preparation of compound II-3:
[0067] Compound II-2 was dissolved in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com