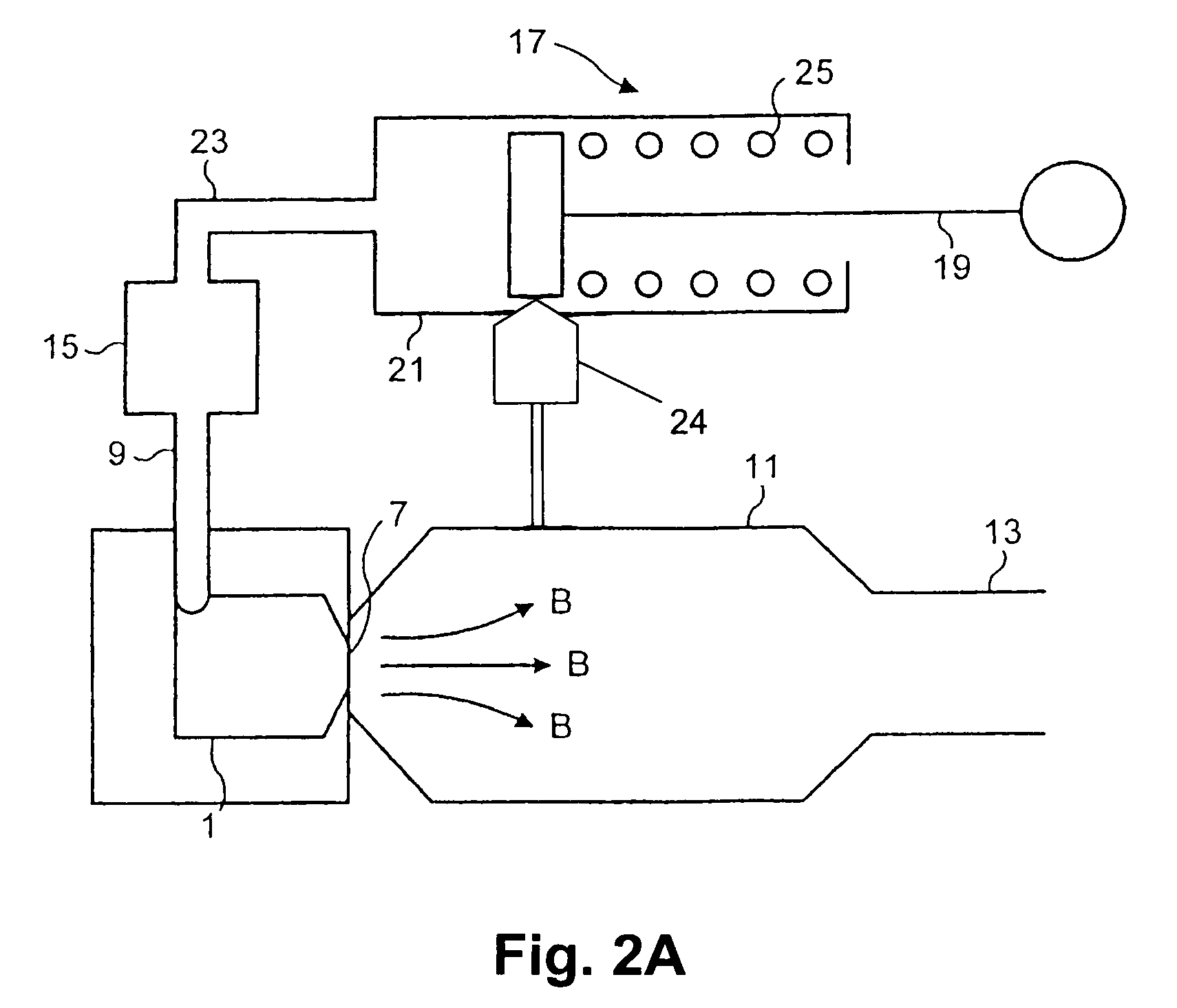
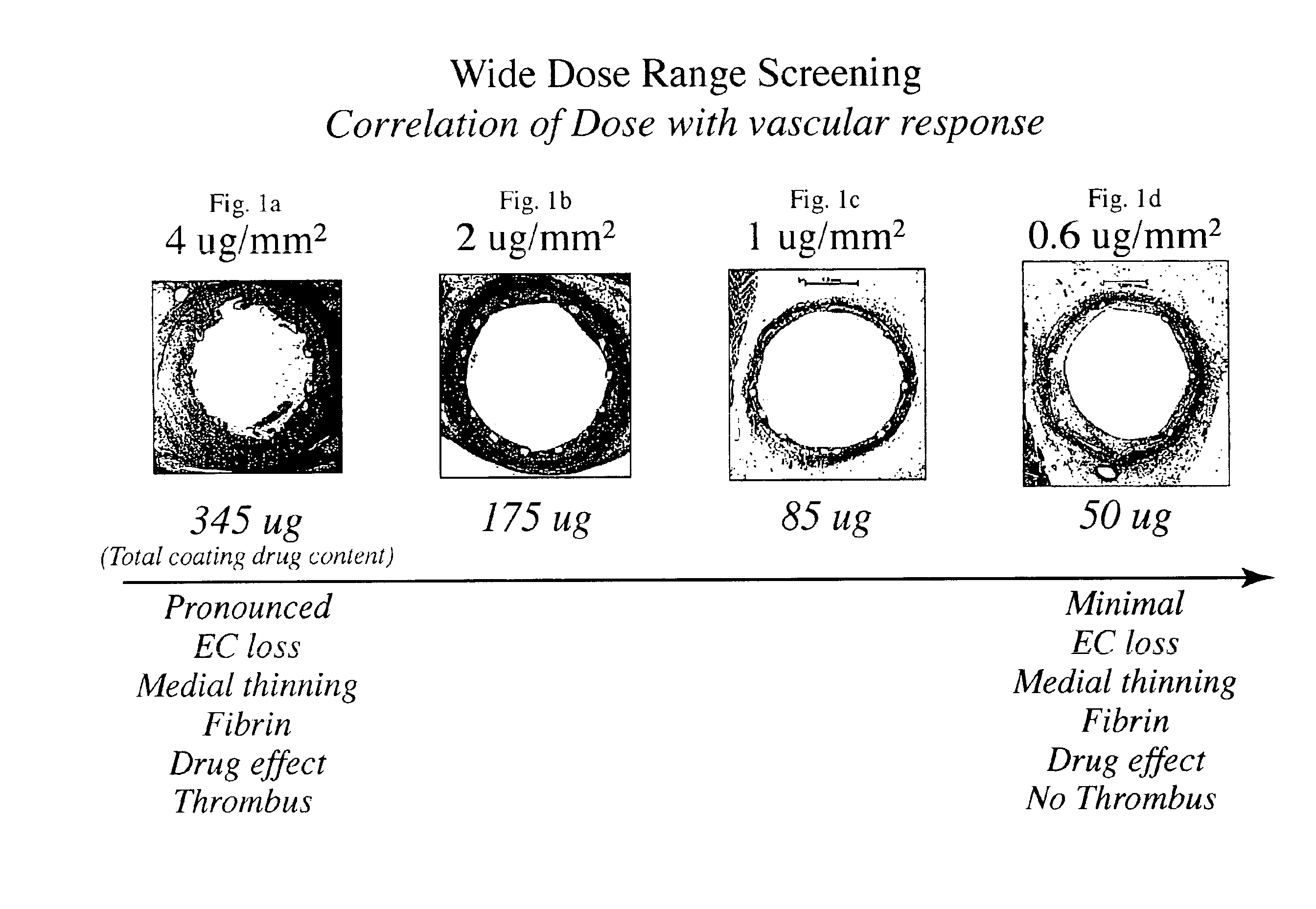
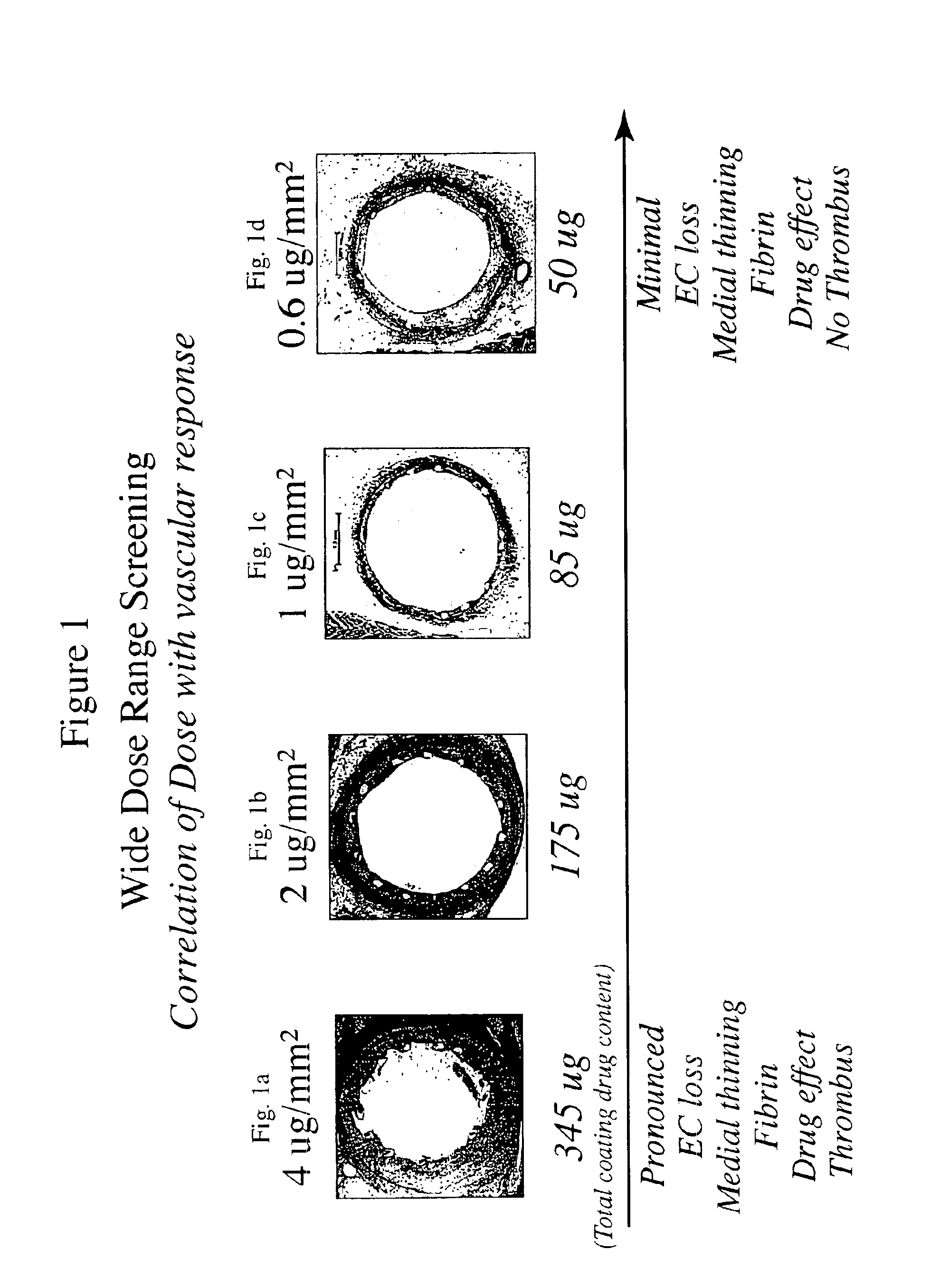
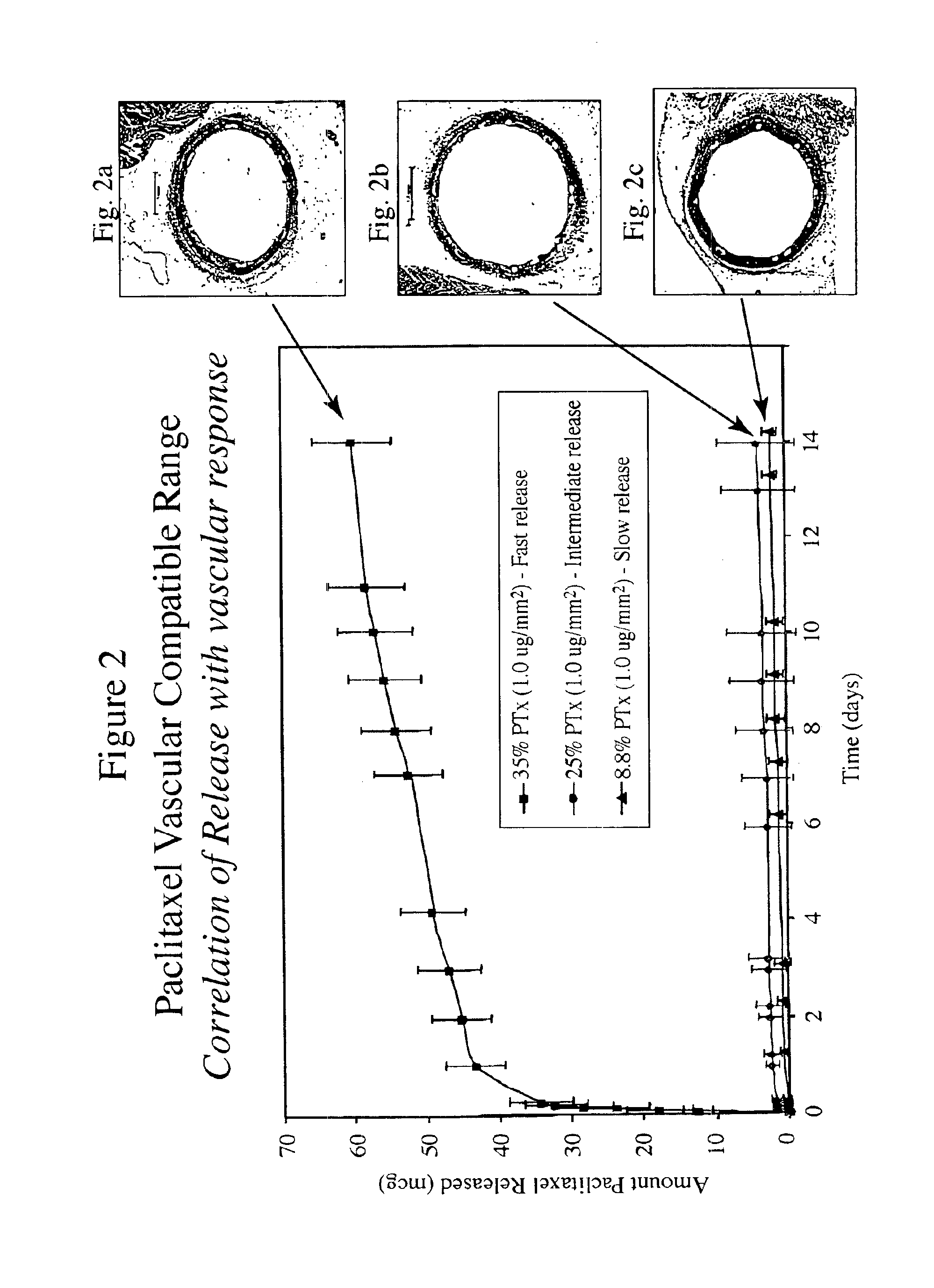
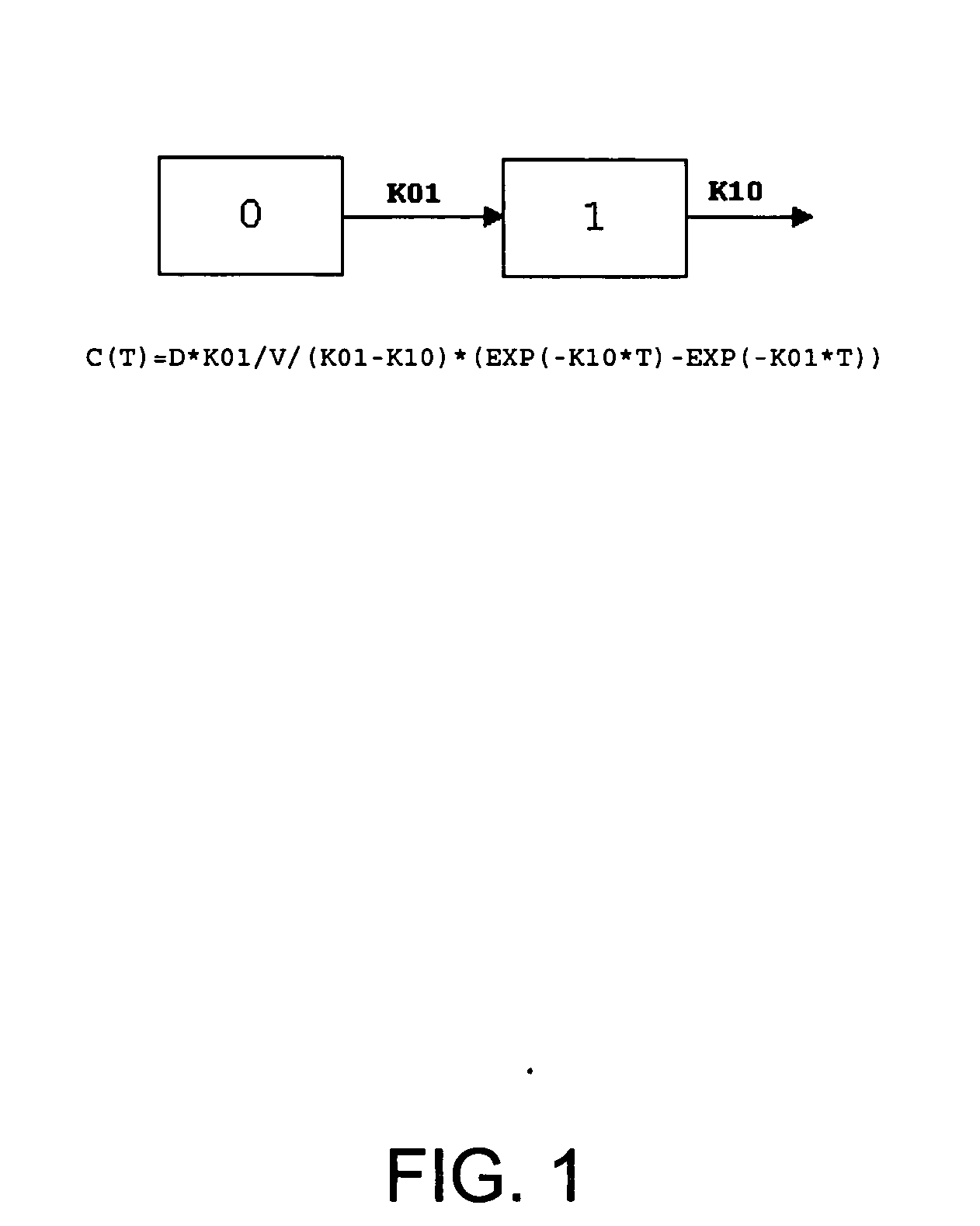
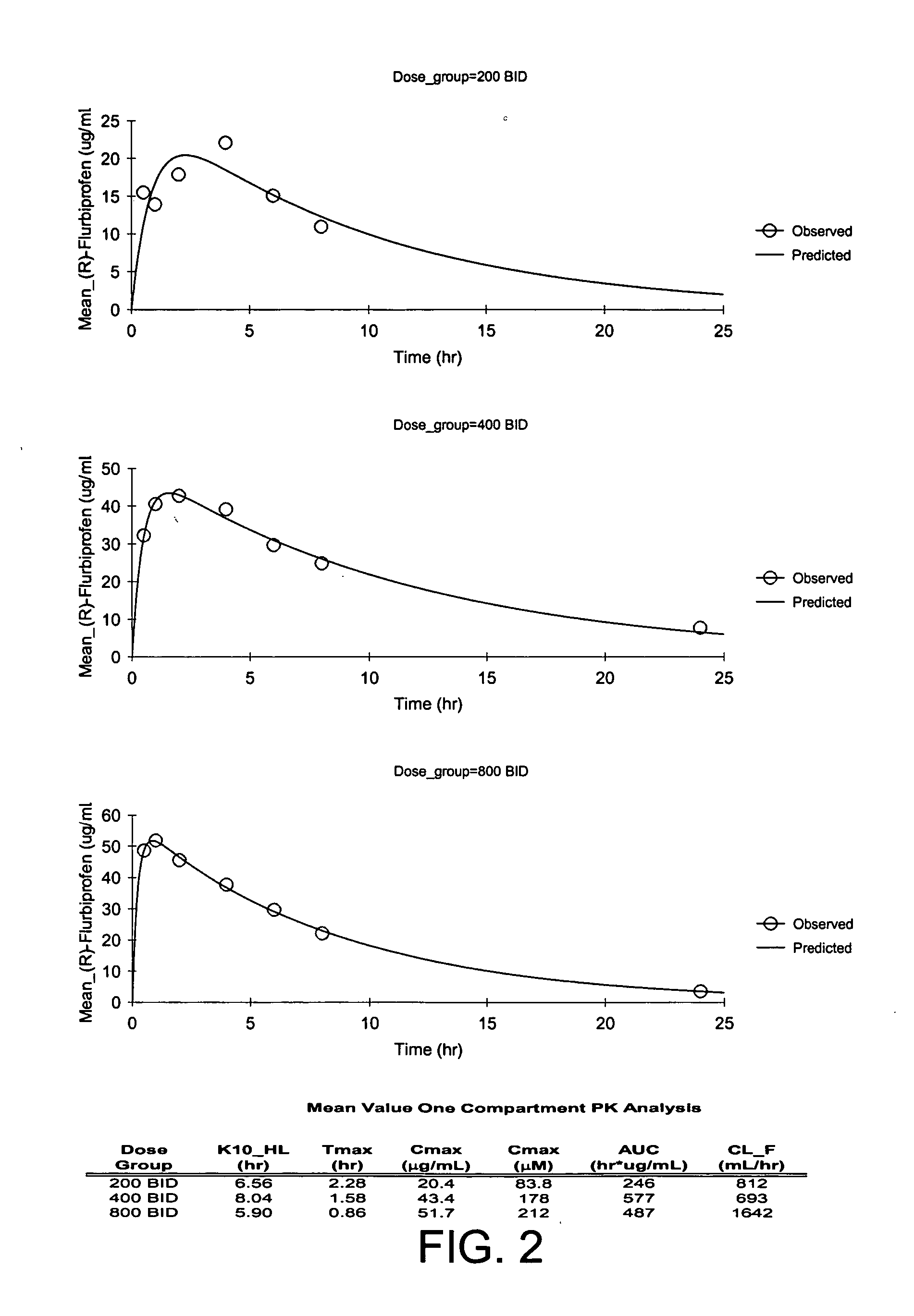
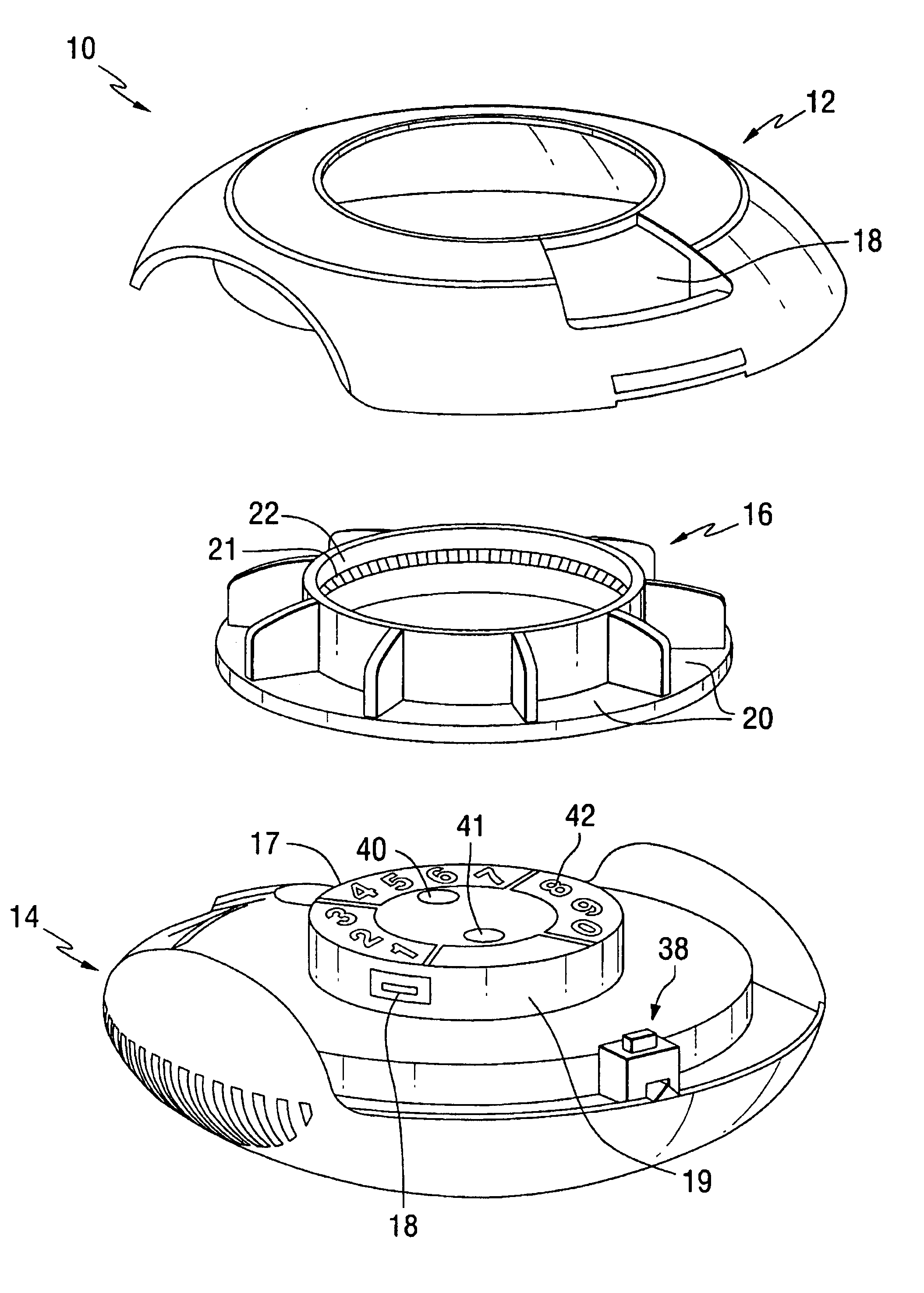
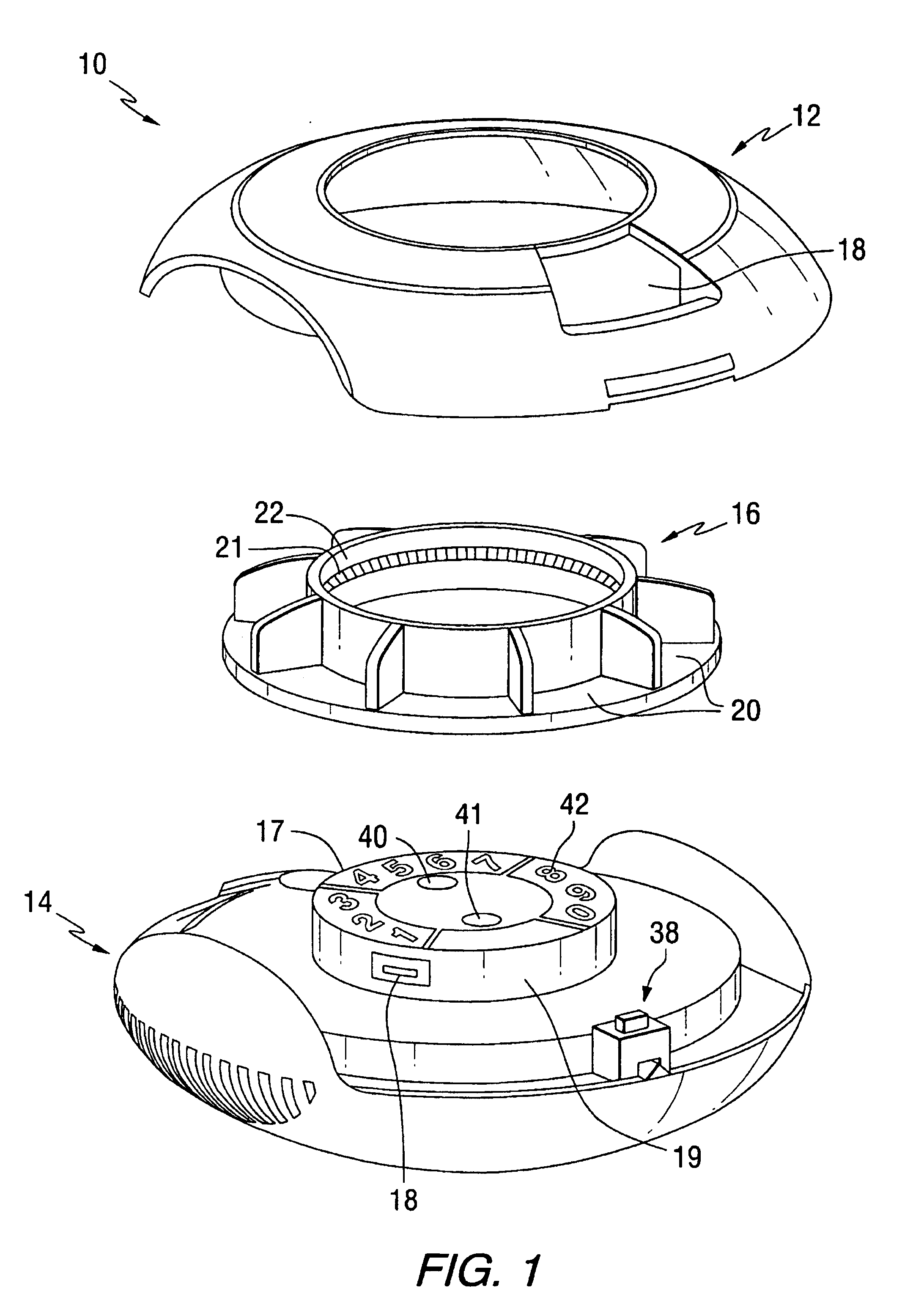
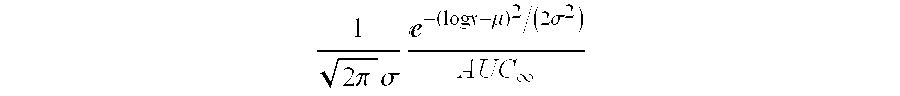Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
390 results about "Drug doses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
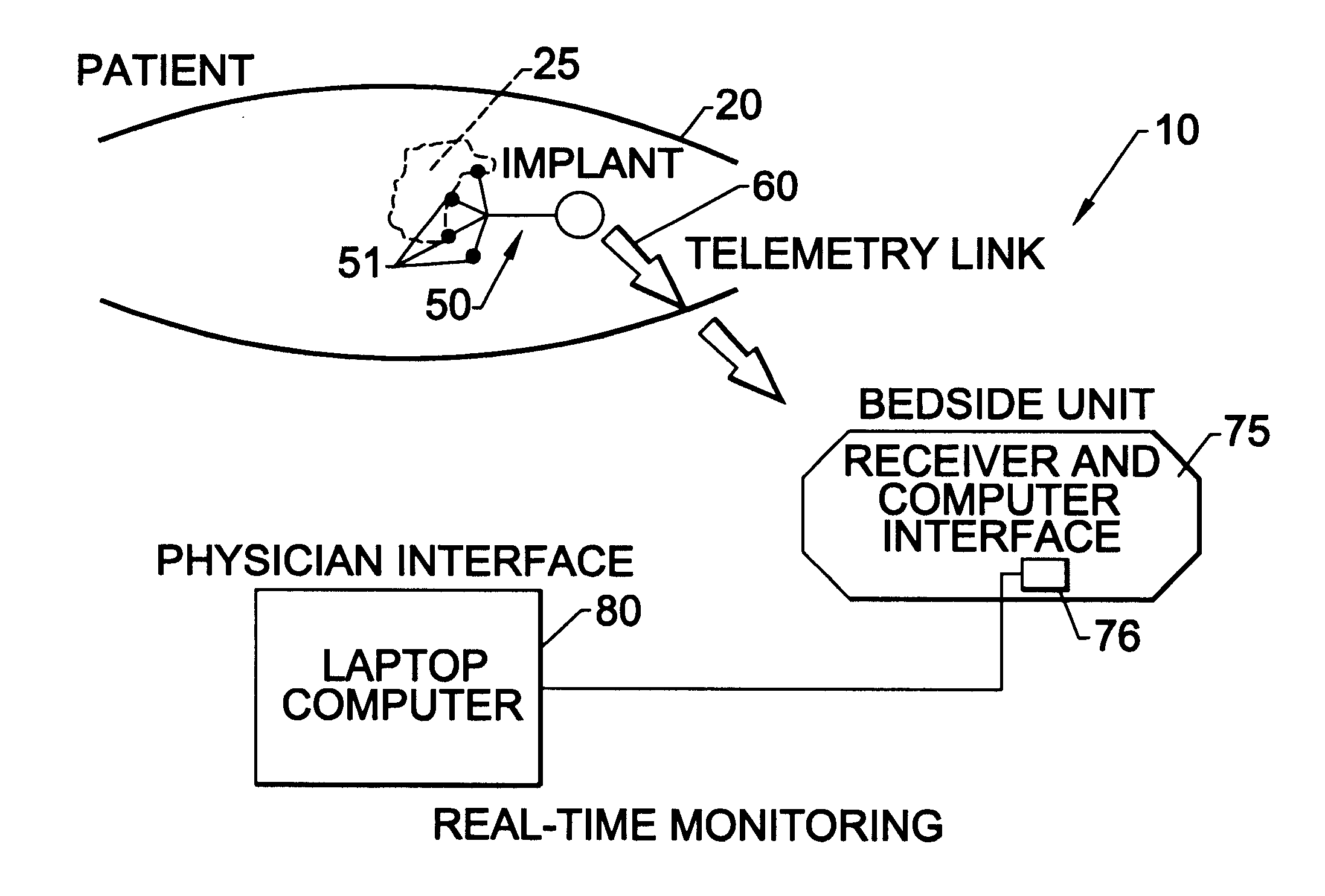
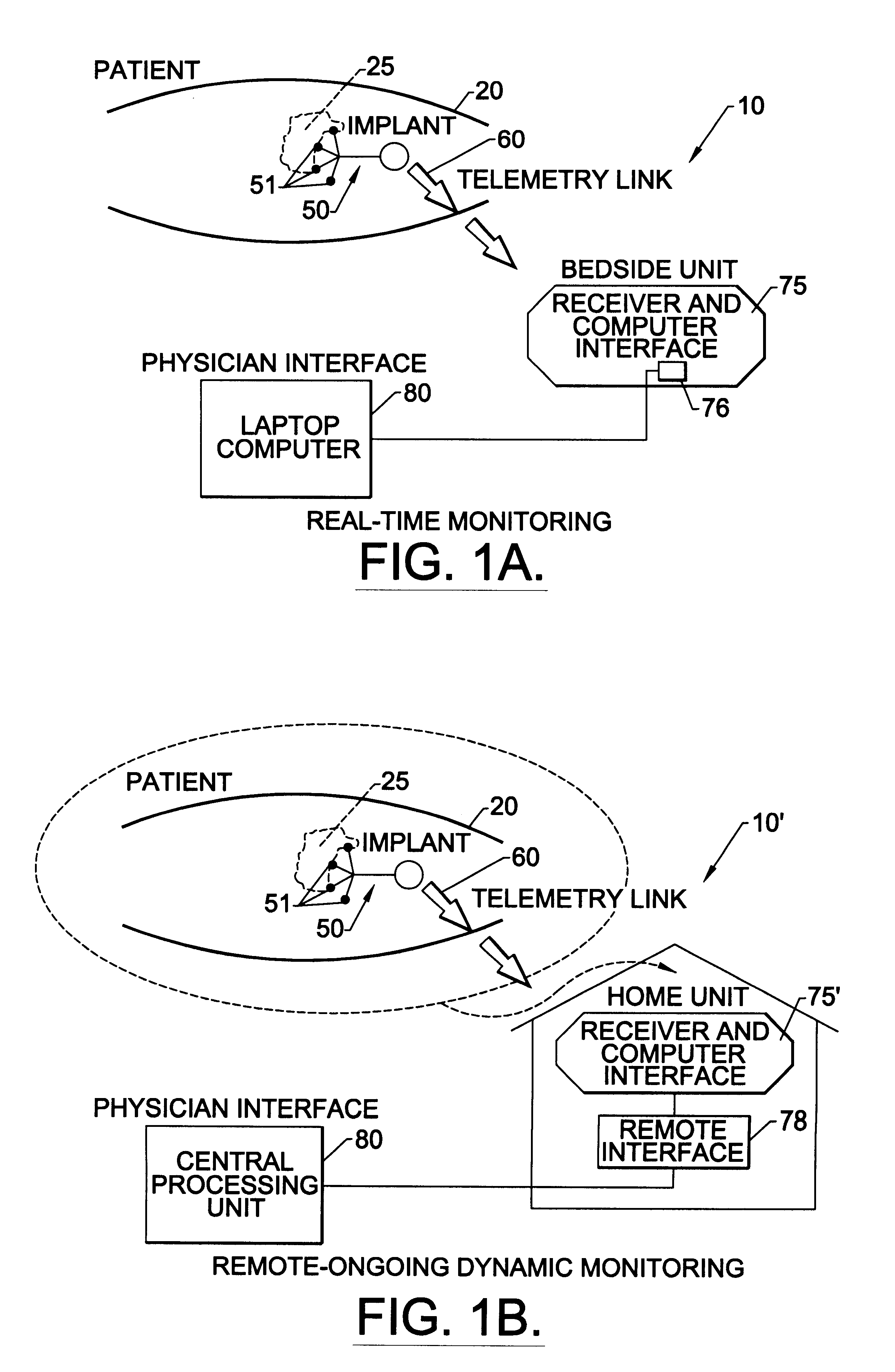
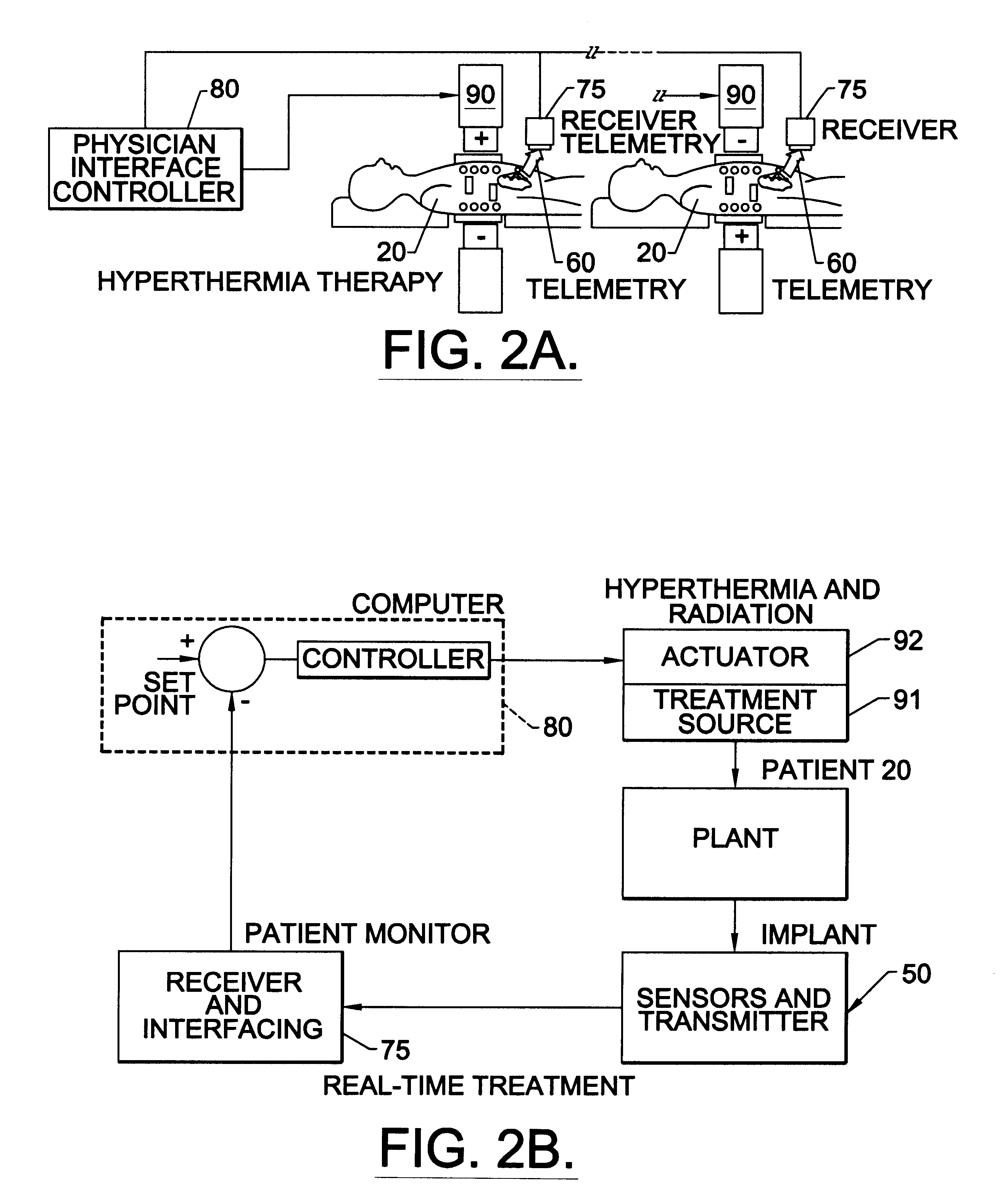
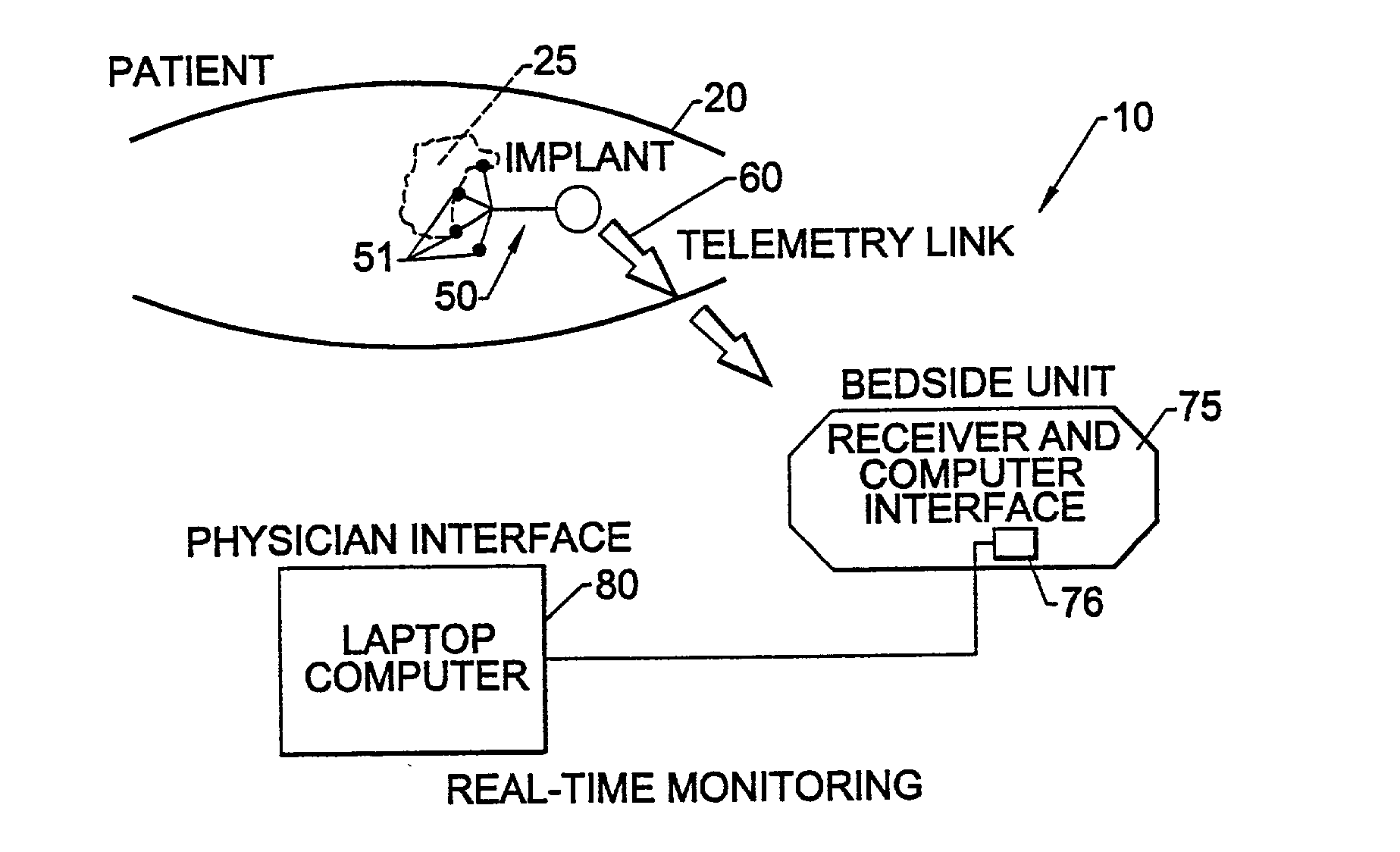
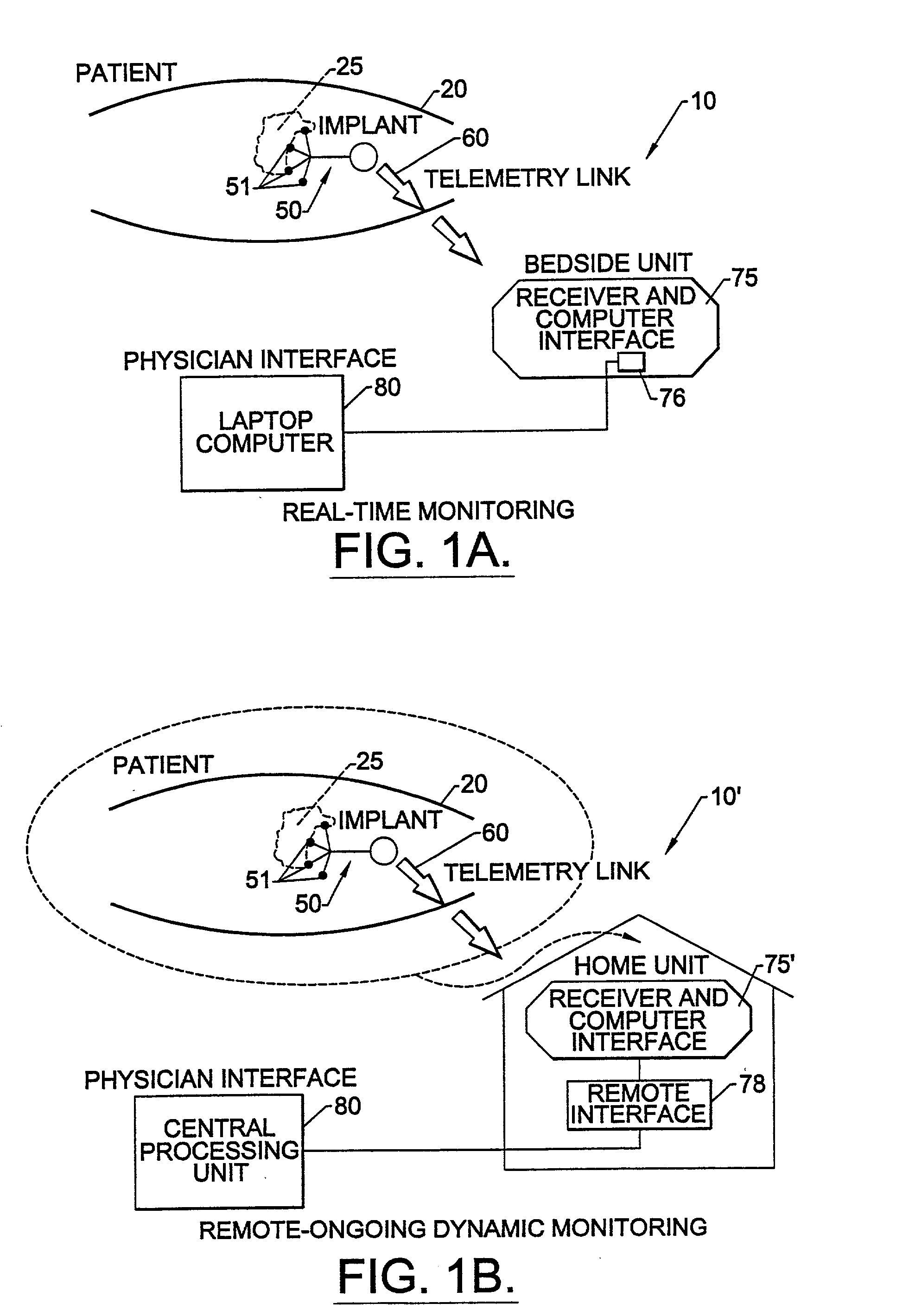
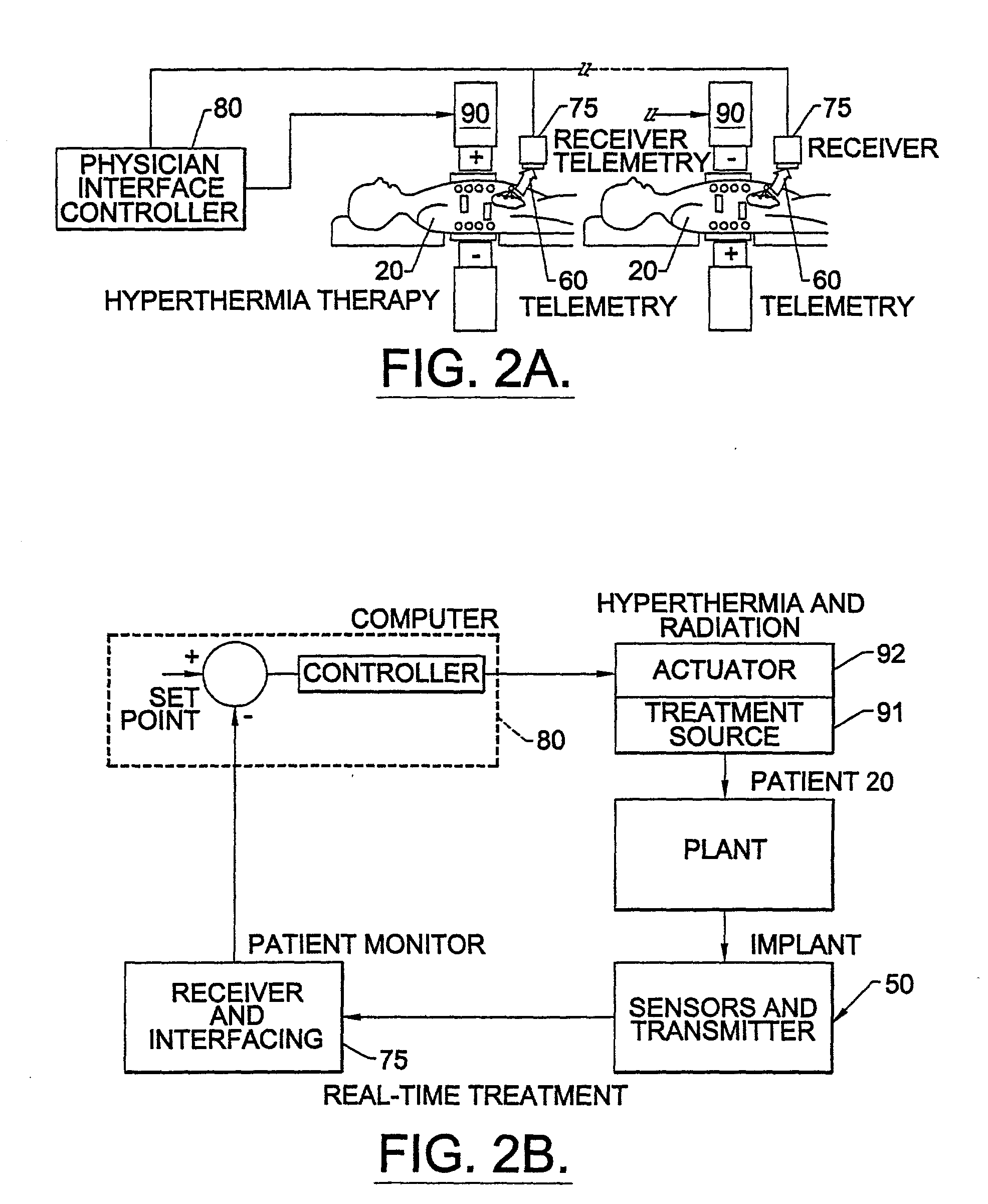
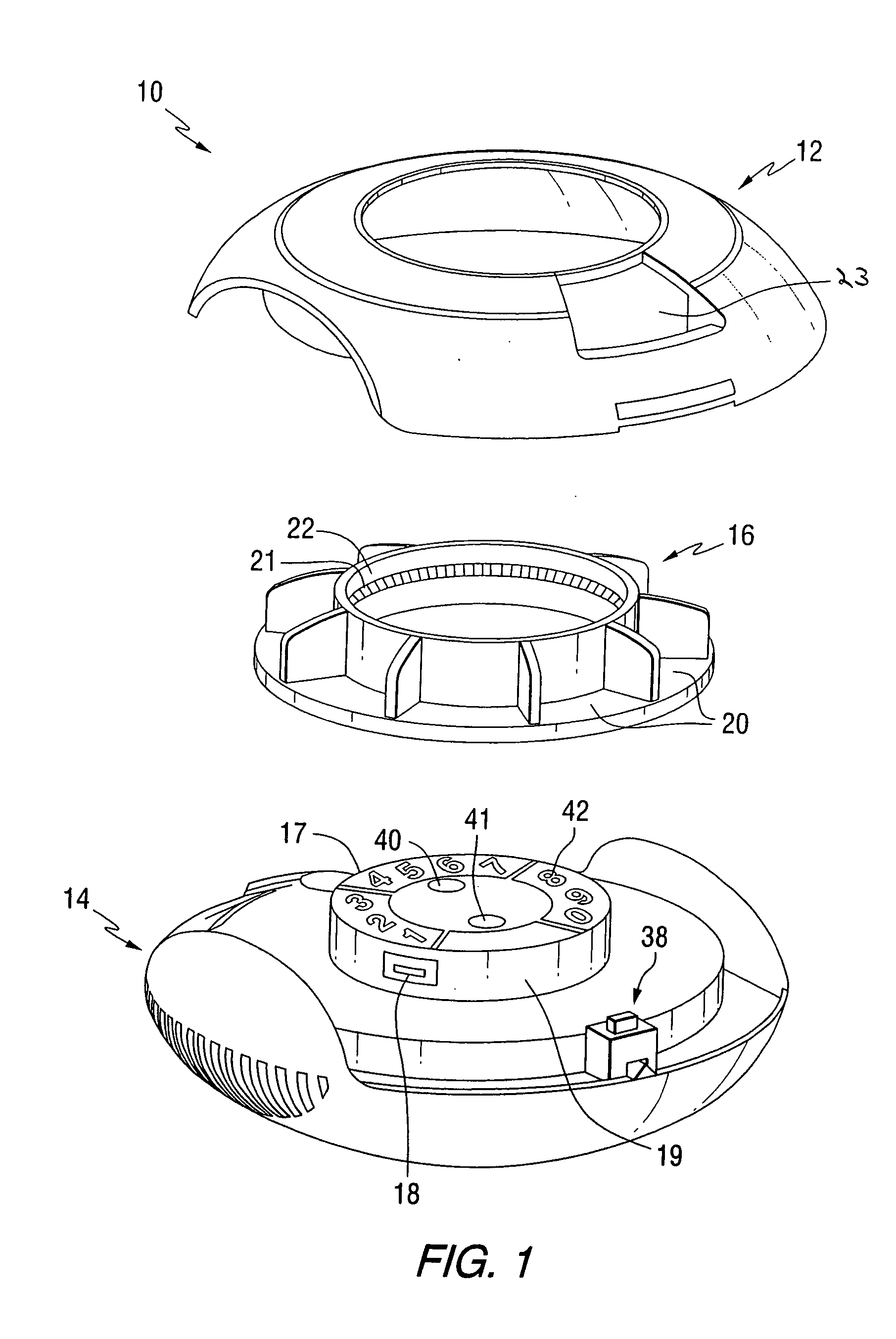
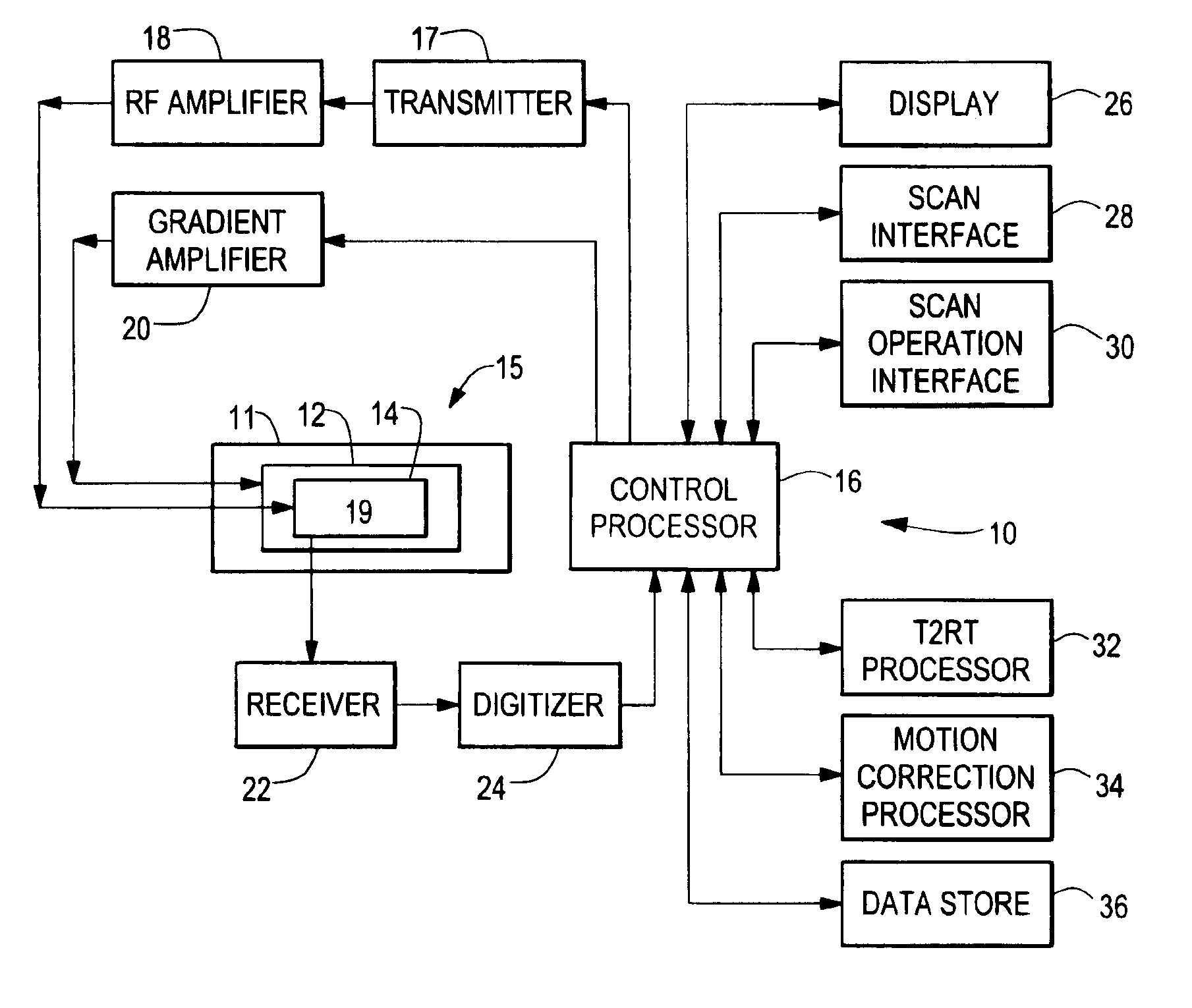
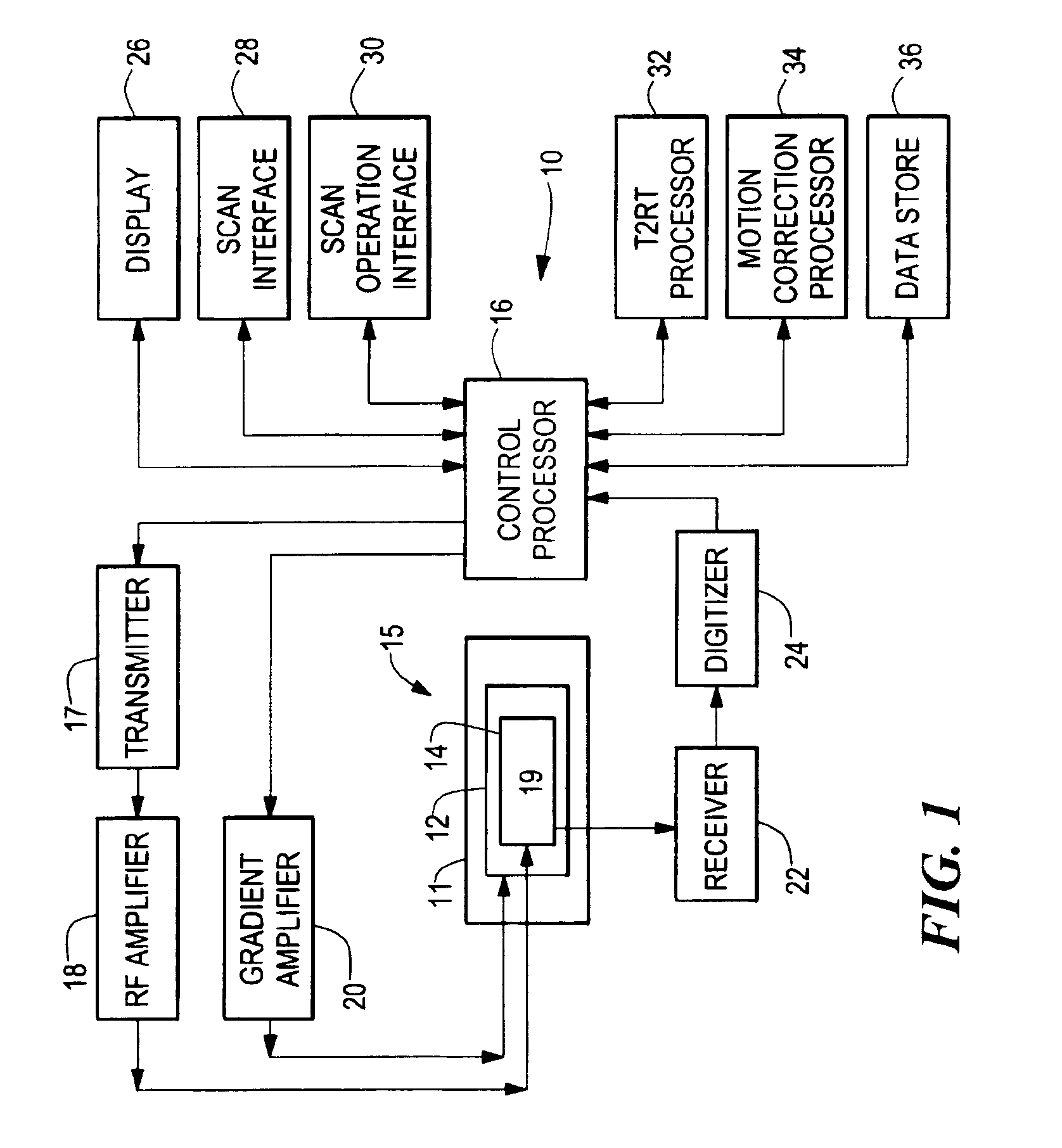
Methods, systems, and associated implantable devices for dynamic monitoring of physiological and biological properties of tumors
InactiveUS6402689B1Enhanced and favorable treatmentMinimize couplingMechanical/radiation/invasive therapiesSurgeryDynamic monitoringEngineering
Methods of monitoring and evaluating the status of a tumor undergoing treatment includes monitoring in vivo at least one physiological parameter associated with a tumor in a subject undergoing treatment, transmitting data from an in situ located sensor to a receiver external of the subject, analyzing the transmitted data, repeating the monitoring and transmitting steps at sequential points in time and evaluating a treatment strategy. The method provides dynamic tracking of the monitored parameters over time. The method can also include identifying in a substantially real time manner when conditions are favorable for treatment and when conditions are unfavorable for treatment and can verify or quantify how much of a known drug dose or radiation dose was actually received at the tumor. The method can include remote transmission from a non-clinical site to allow oversight of the tumor's condition even during non-active treatment periods (in between active treatments). The disclosure also includes monitoring systems with in situ in vivo biocompatible sensors and telemetry based operations and related computer program products.
Owner:NORTH CAROLINA STATE UNIV +1
Methods, systems, and associated implantable devices for dynamic monitoring of physiological and biological properties of tumors
InactiveUS20020137991A1Enhanced and favorable treatmentMechanical/radiation/invasive therapiesSurgeryAbnormal tissue growthDynamic monitoring
Owner:VTQ IP HLDG +1
Decision information system for drug delivery devices
ActiveUS6928338B1Sampled-variable control systemsControlling ratio of multiple fluid flowsDoses rateGeneral purpose computer
Decision information systems, methods, and computer programs for better informing decisions to use multiple drugs in drug delivery devices, including implantable devices, for drug administration. Executable computer programs and logic embodying methods of the invention can calculate consistent multiple drug mixture amounts and drug delivery flow rates. One program accepts user input indicating a desired first drug dose rate, an initial first drug concentration, a desired second drug dose rate, an initial second drug concentration, and the reservoir size of the drug delivery device. The program method calculates a first drug amount and a second drug amount to combine in a mixture as well as a first drug true concentration in the mixture. The drugs can be mixed consistent with the physician's instructions using the program output. The first drug true concentration can be entered into a programmer device as the only drug concentration entered. Another program calculates a consistent first drug, second drug, and diluent amount to be added to a mixture for injection into a fixed flow rate, implantable drug delivery device. Methods preferably output true concentrations and dose rates for all drugs to be added and most preferably show all calculations used to arrive at the flow rate and mixture amount calculations. Yet another program receives a new desired drug dose rate for a previously filled device. The program accepts the existing mixture volume and true drug concentrations for a partially depleted device and calculates a new mixture flow rate to achieve the desired dose rate using the existing mixture. The methods can be implemented as executable computer programs in programmer devices, general purpose computers, servers, handheld computers, and personal digital assistants.
Owner:MEDTRONIC INC
Communications system for an implantable medical device and a delivery device
InactiveUS8002700B2Reduce deliveryFacilitate communicationElectrotherapyDrug and medicationsCommunications systemData center
A closed loop system for monitoring drug dose, intake and effectiveness includes a drug delivery device in data communications with at least one implantable medical device. The system is preferably implemented in a web-enabled environment in which a remote data center communicates with the implantable devices (IMDs) in a patient via a programmer or the pill dispenser. A physician, clinician, or other user may access the remote data center to review and monitor the IMDs or the drug delivery regime remotely. The system further provides a dynamic drug management system, compatible with a web-enabled interactive data communication environment that accurately monitors dose and specific drug effectiveness in a patient to enhance patient care.
Owner:MEDTRONIC INC
Patient controlled timed medication dispenser
InactiveUS7896192B2Small article dispensingContainer/bottle contructionMedication DispenserMedication dose
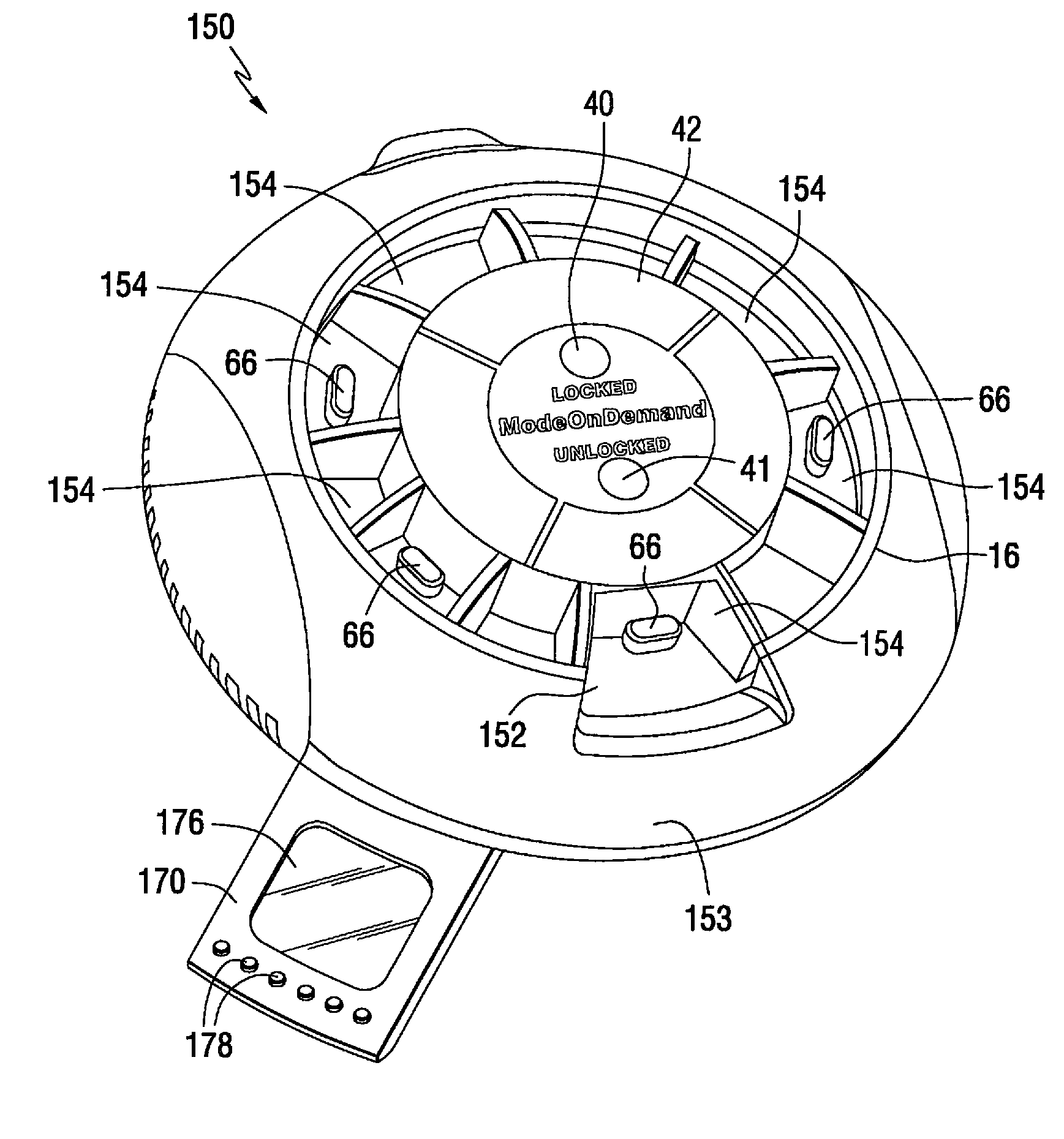
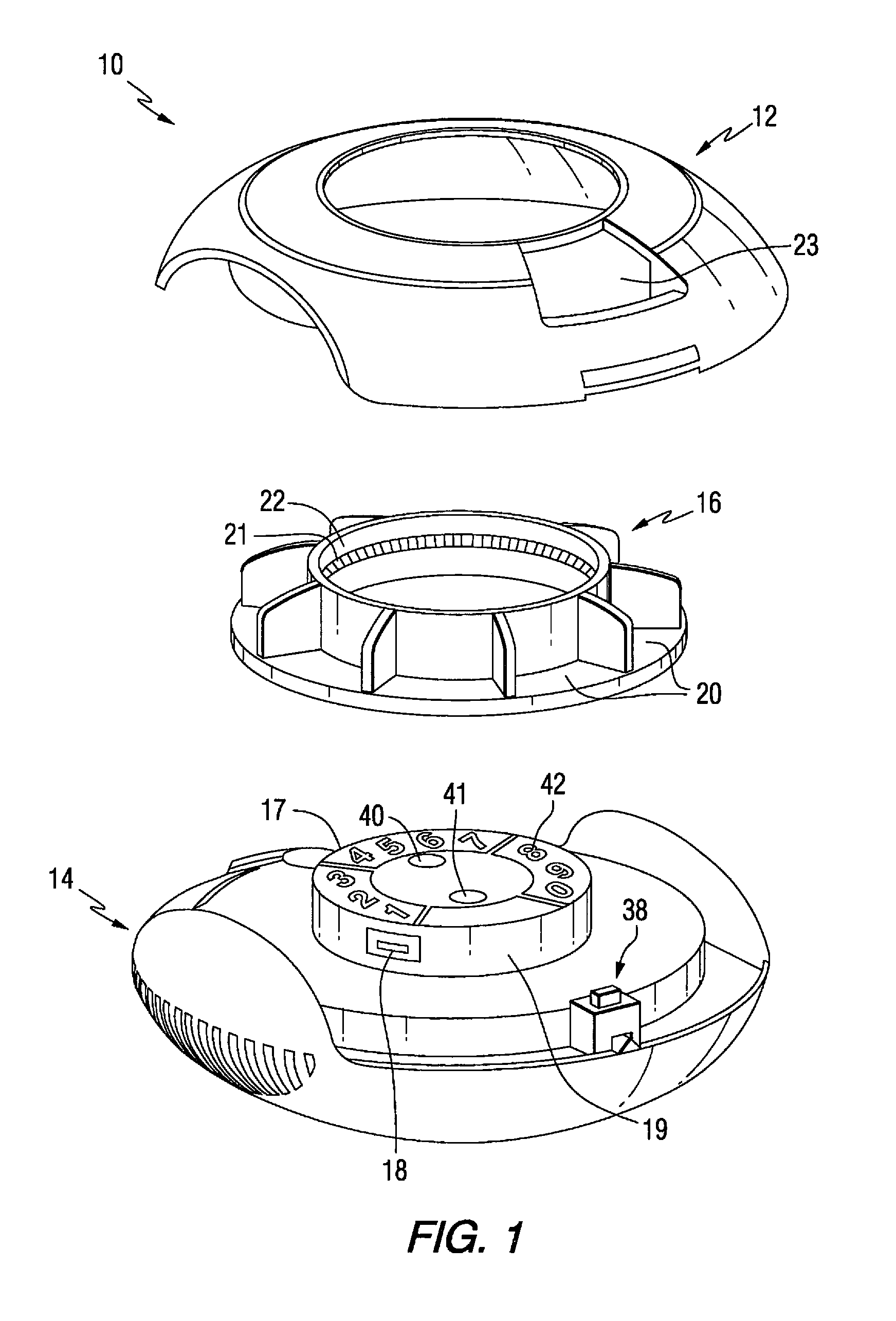
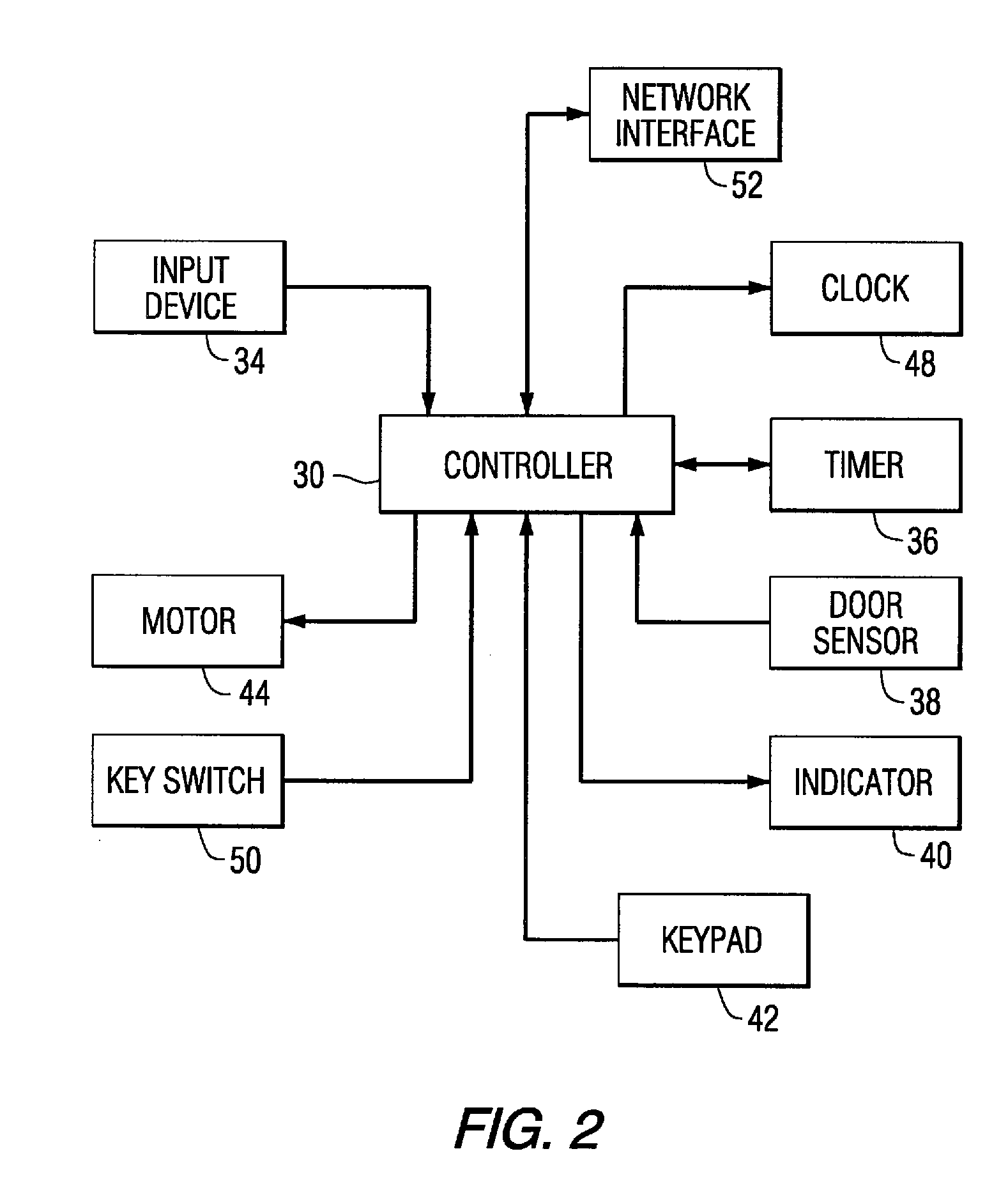
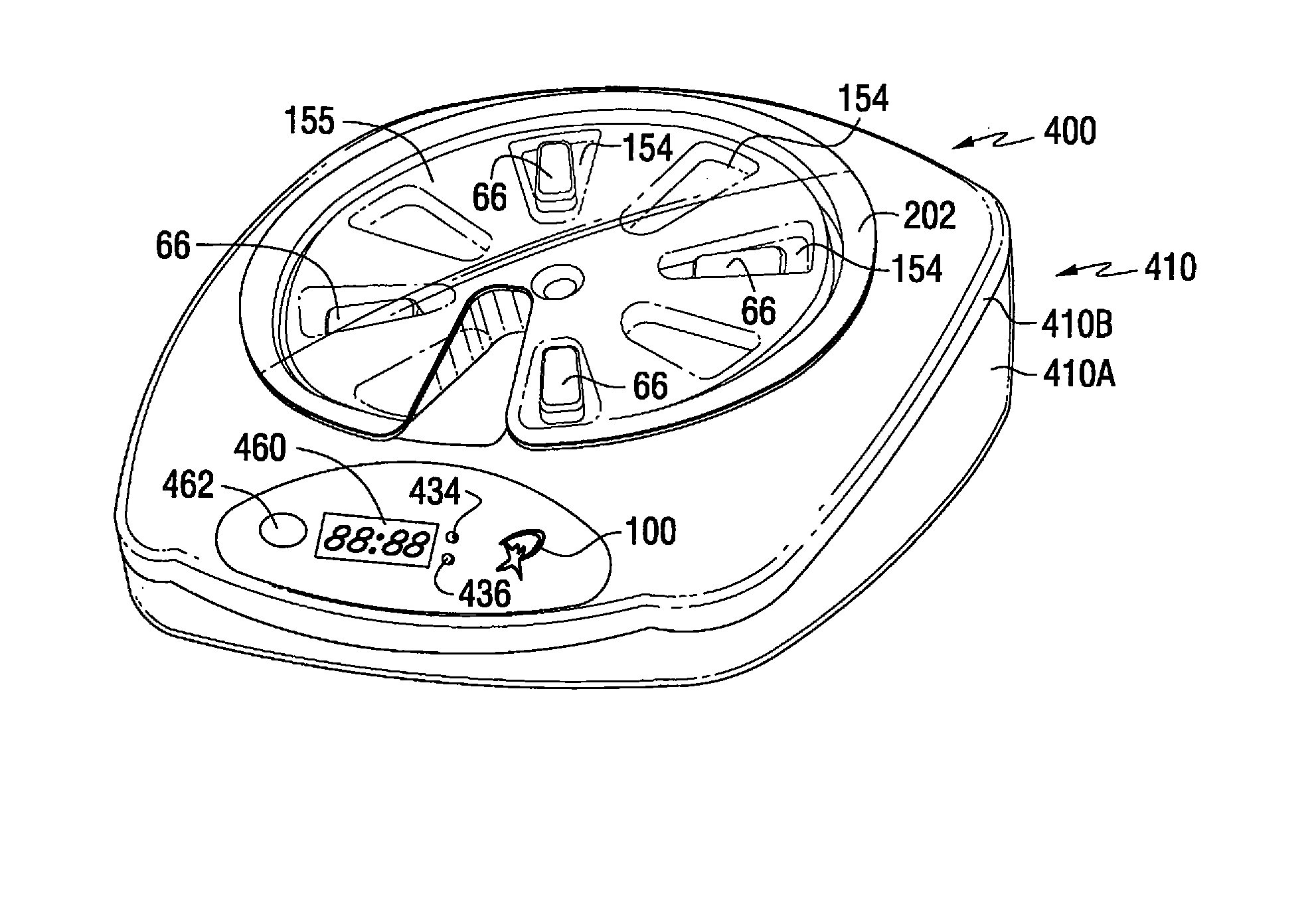
A medication on demand dispenser. The dispenser provides patient access to medications prescribed to be available on an as-needed basis but with a prescribed minimum time interval between doses. The dispenser permits access to a single medication dose after each minimum time interval has elapsed. After a drug dose is presented to the patient, the dispenser prevents access to the next dose until the minimum time interval has elapsed.
Owner:AVANCEN MOD
Patient controlled timed medication dispenser
InactiveUS20080203107A1Small article dispensingContainer/bottle contructionMedication DispenserMedication dose
A medication on demand dispenser. The dispenser provides patient access to medications prescribed to be available on an as-needed basis but with a prescribed minimum time interval between doses. The dispenser permits access to a single medication dose after each minimum time interval has elapsed. After a drug dose is presented to the patient, the dispenser prevents access to the next dose until the minimum time interval has elapsed.
Owner:AVANCEN MOD
Inhalers
InactiveUS7246617B1Reduce depositionIncrease effective viscosityRespiratorsLiquid surface applicatorsDrug dosesPharmacology
Owner:ETV CAPITAL
Optimized dosing for drug coated stents
The inventors have found that both the drug dose and drug release profiles are significant factors for the safety and efficacy of drug coated stents. The inventors have identified optimum dosing and release kinetics for drug coated stents. In particular, the inventors have determined dosing and release kinetics that permit the delivery of the lowest effective drug dosage, thus enhancing patient safety and minimizing any side effects from the drug.
Owner:BOSTON SCI SCIMED INC
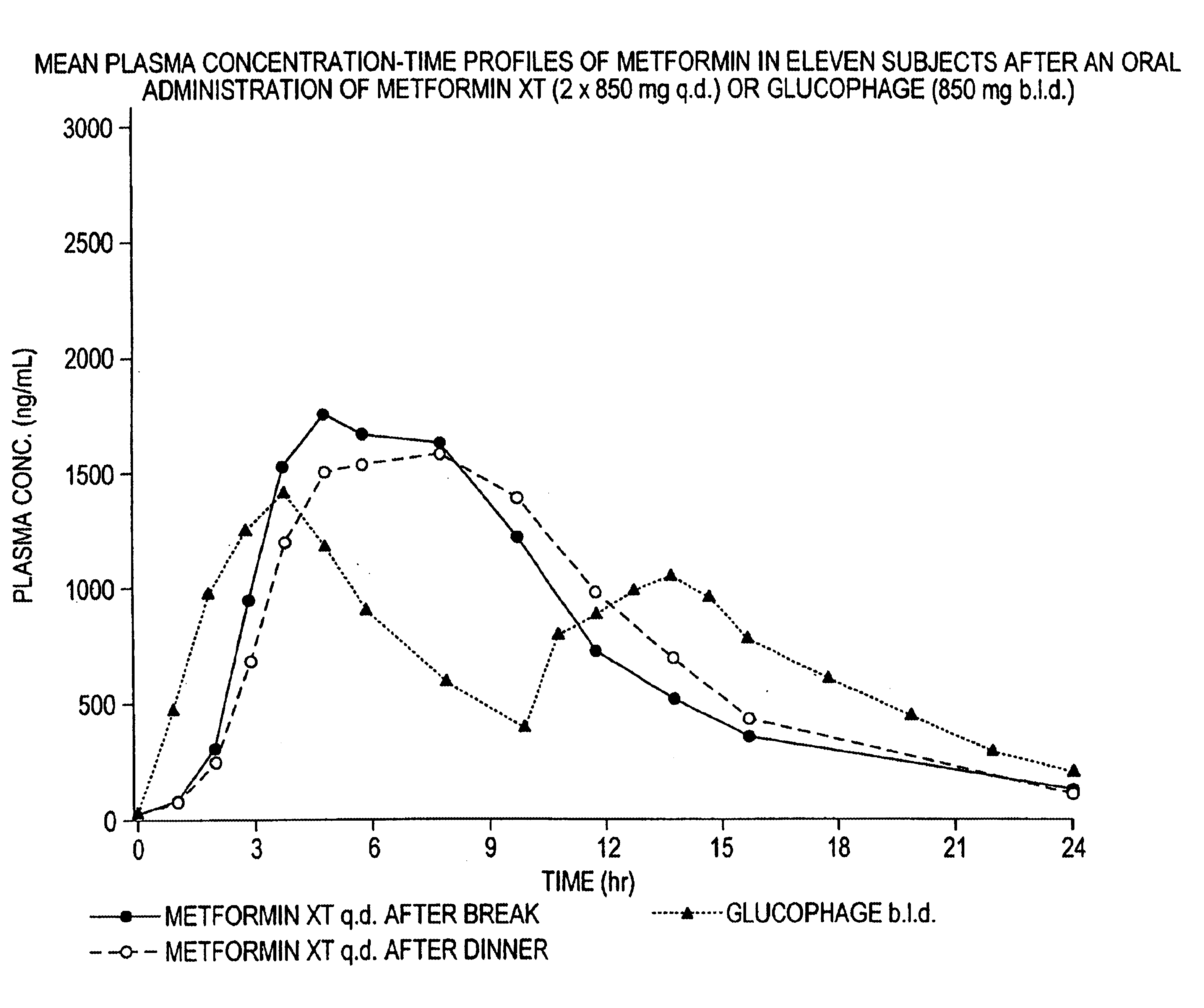
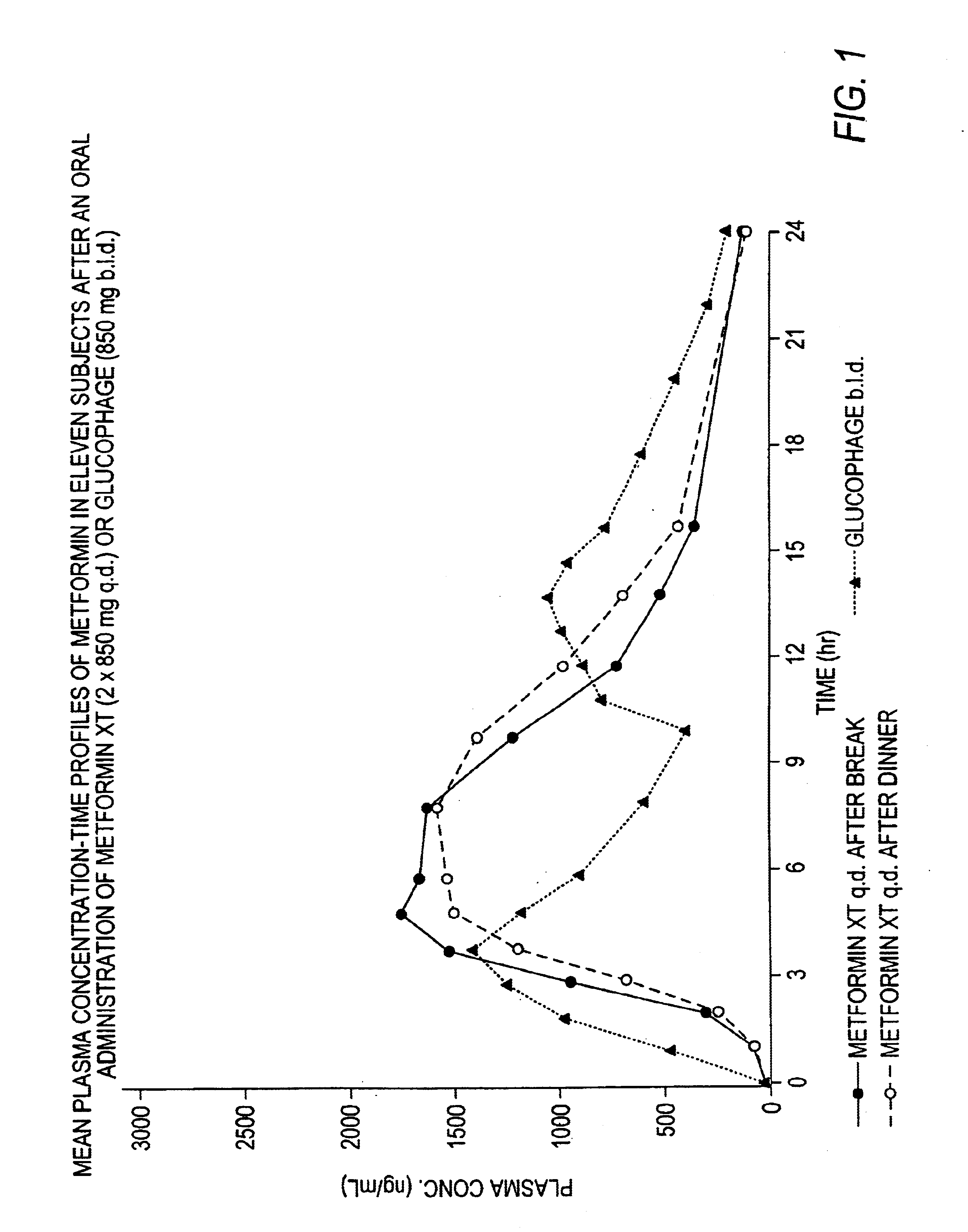
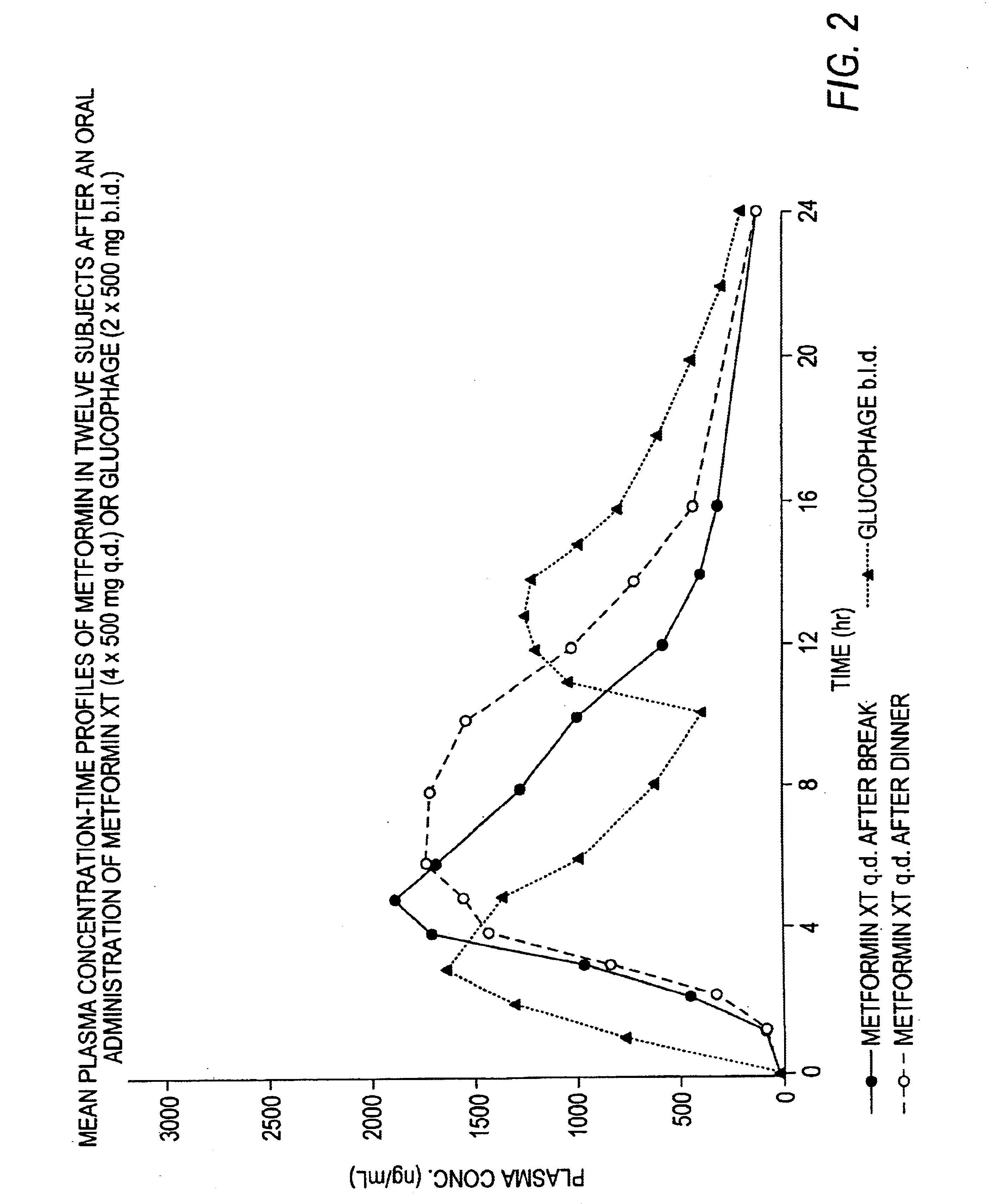
Controlled release metformin compositions
InactiveUS6866866B1Effective controlImprove bioavailabilityOrganic active ingredientsCoatingsCo administrationBlood plasma
A composition for treating patients having non-insulin-dependent diabetes mellitus (NIDDM) by administering a controlled release oral solid dosage form containing preferably a biguanide drug such as metformin, on a once-a-day basis. The dosage form provides a mean time to maximum plasma concentration (Tmax) of the drug which occurs at 5.5 to 7.5 hours after oral administration on a once-a-day basis to human patients. Preferably, the dose of drug is administered at dinnertime to a patient in the fed state.
Owner:ANDRX LABS
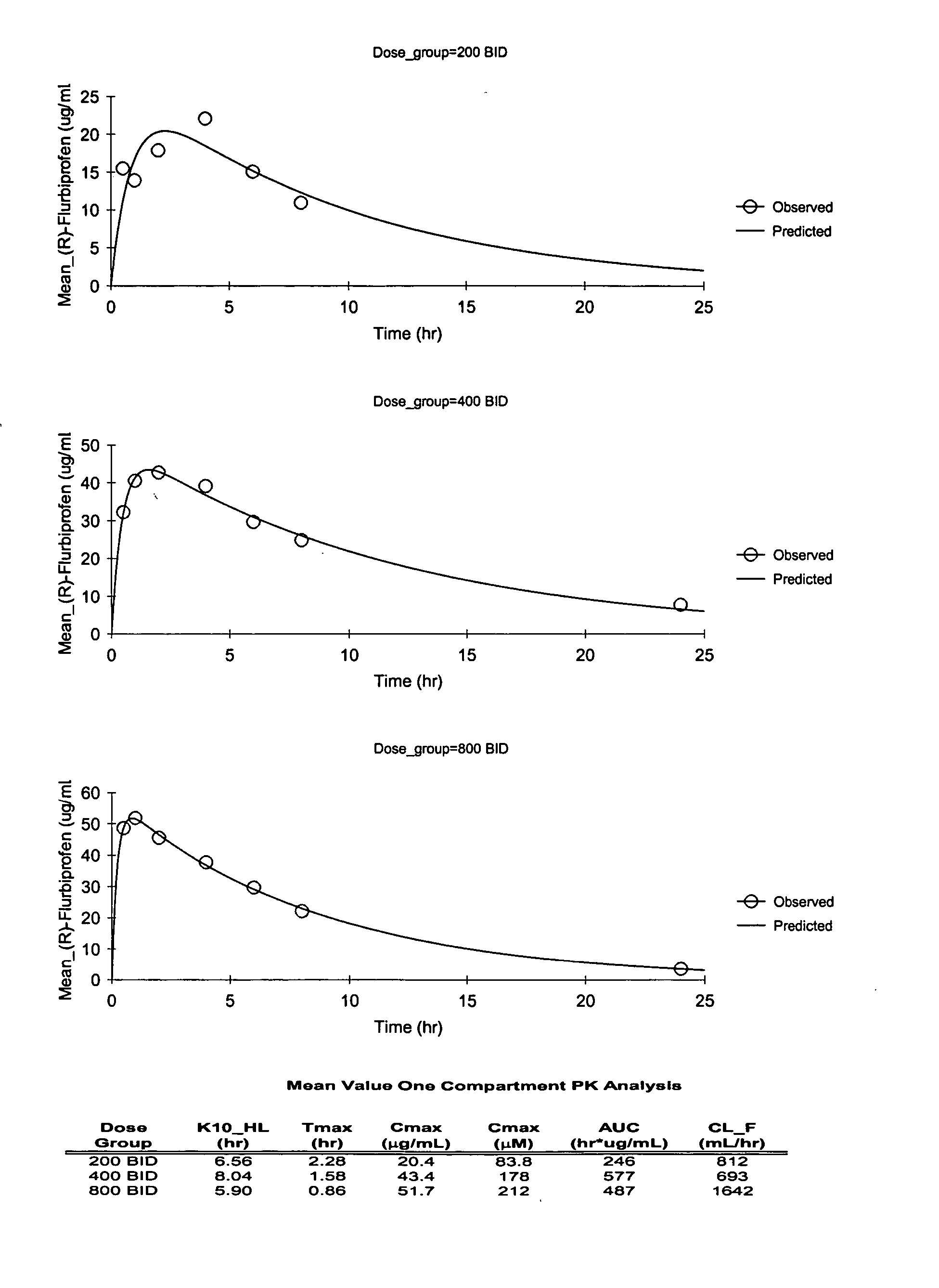
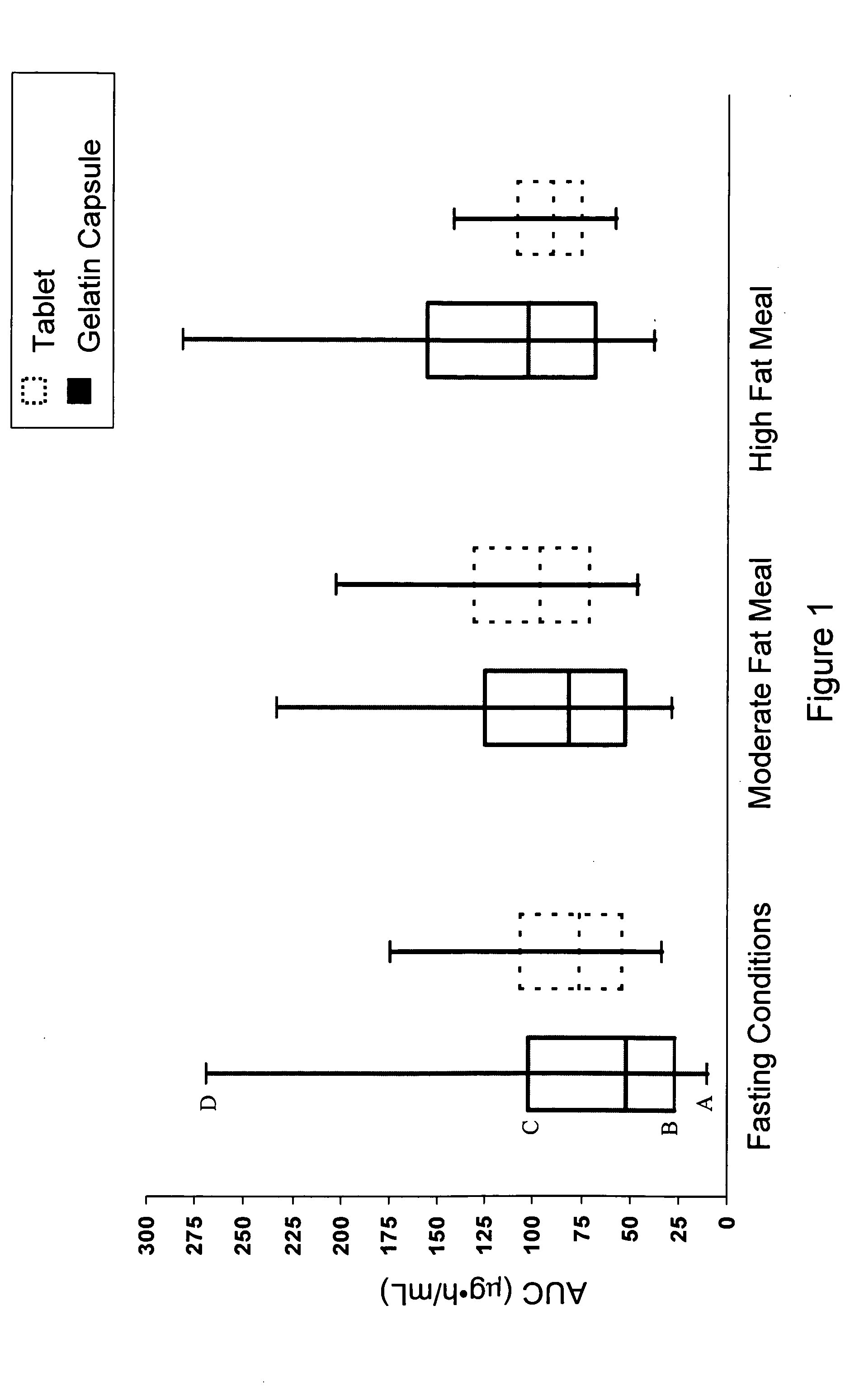
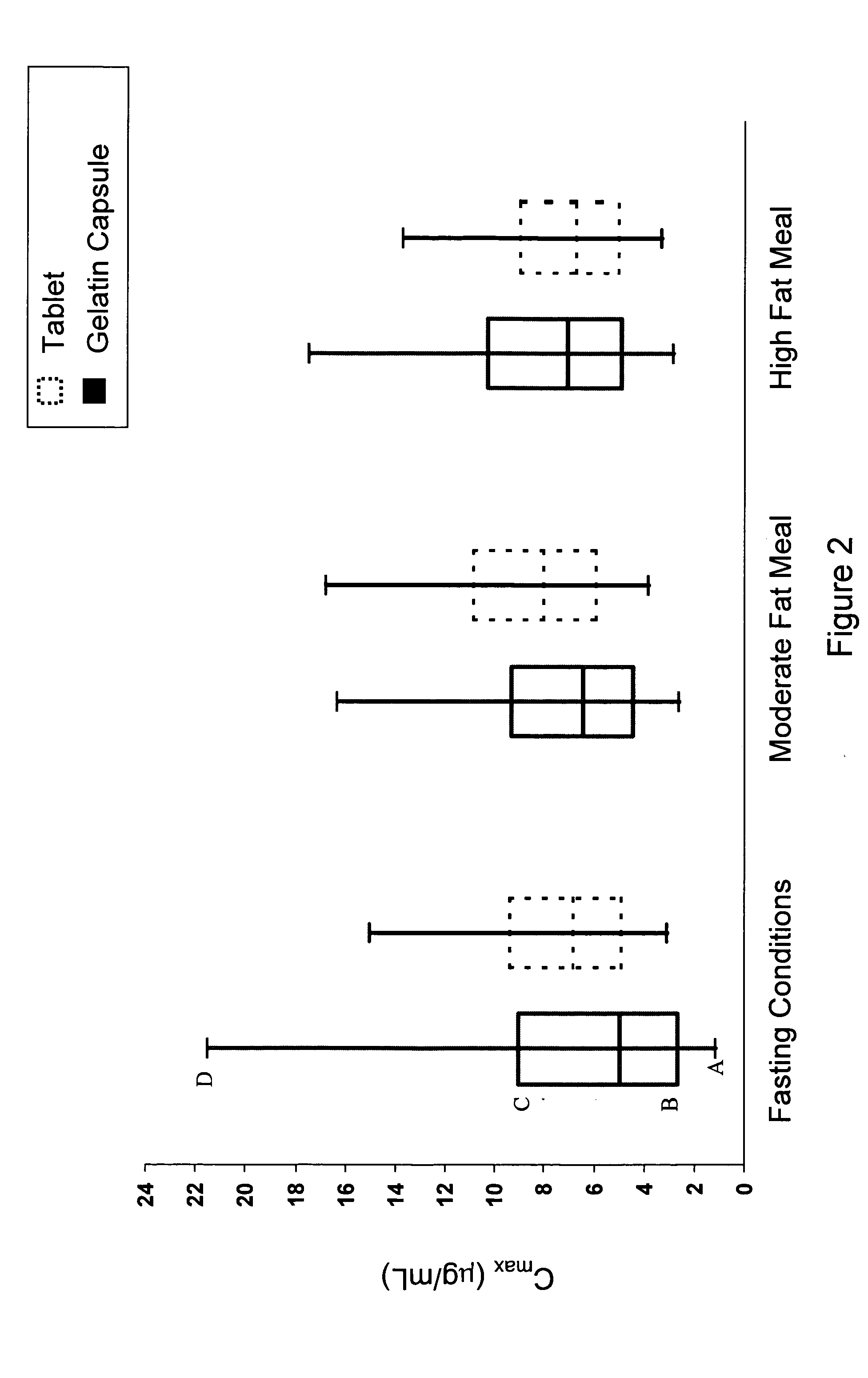
Pharmaceutical methods, dosing regimes and dosage forms for the treatment of Alzheimer's disease
InactiveUS20050042284A1Improving and lessening rate of declineReduction of the decline in saidBiocideNervous disorderDosing regimenGuideline
In general, the invention relates to a pharmaceutical dose having R-flurbiprofen as the active ingredient that upon oral administration of a single dose to a fasting subject provides a Cmax of about 30-95 μg per mL. When the dose is administered to an individual having mild-to-moderate Alzheimer's disease (or desiring protection against Alzheimer's disease) twice daily for at least 4 months according to the described guidelines, an improvement or lessening in decline of cognitive function as characterized by cognition tests is observed in the patient. The composition of the invention is formulated with one or more pharmaceutically acceptable excipients, salts or carriers.
Owner:MYRIAD GENETICS
Patient controlled timed oral medication dispenser
ActiveUS7044302B2Promotes autonomyPromotes patient autonomySmall article dispensingContainer/bottle contructionMedication DispenserOral medication
The oral medication delivery device provides patient access to medications prescribed to be available on an as-needed basis, but with a minimum time intervals between doses. The required time interval between drug accessibility is programmed into the device when the medication tray carrying the multiple doses is loaded into the device. The device allows access to a single dose of the medication after each minimum time interval has elapsed. When the drug dose is removed from the device, the medication tray locks until the next minimum time interval has elapsed.
Owner:AVANCEN MOD
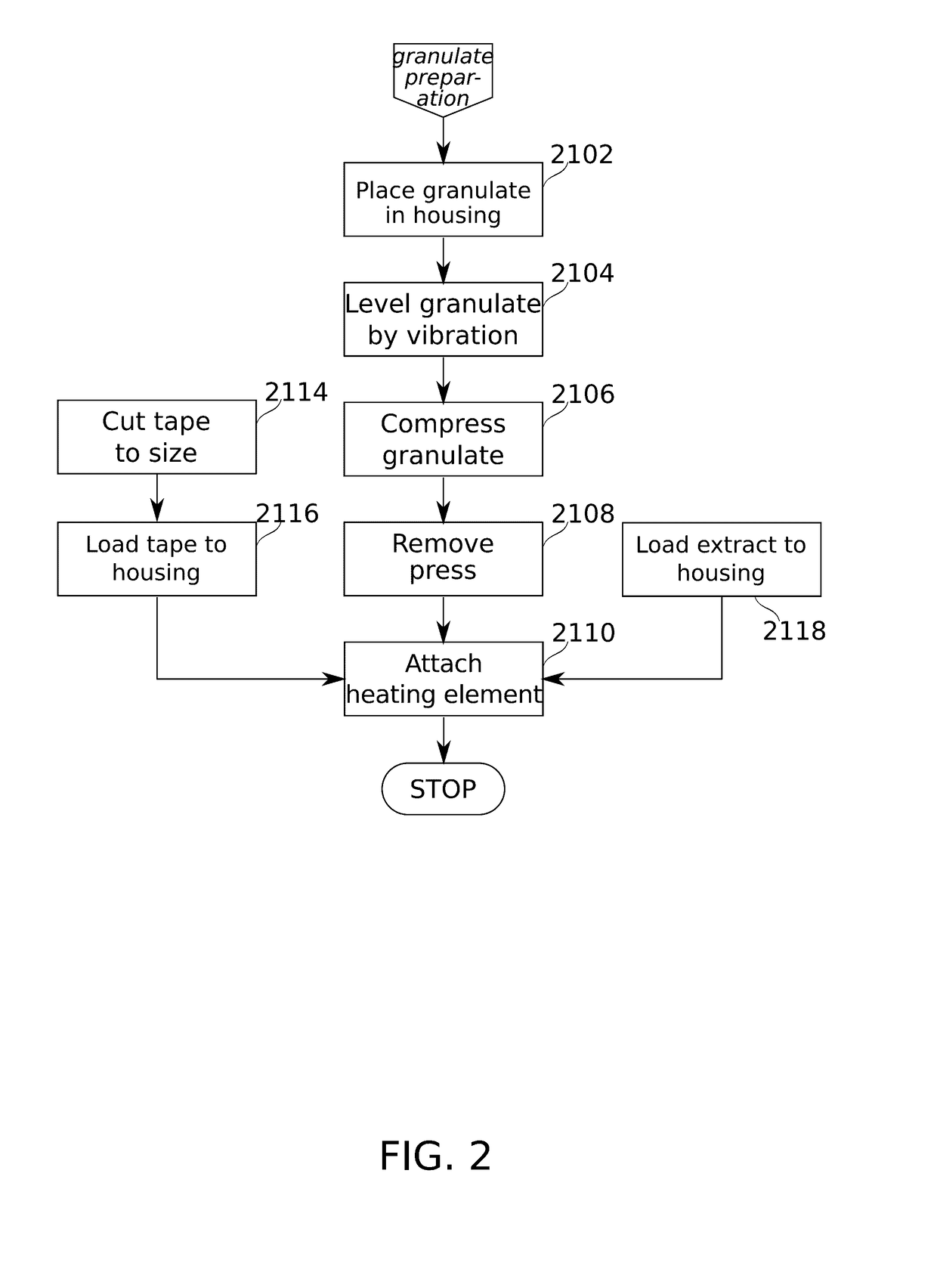
Drug dose cartridge for an inhaler device
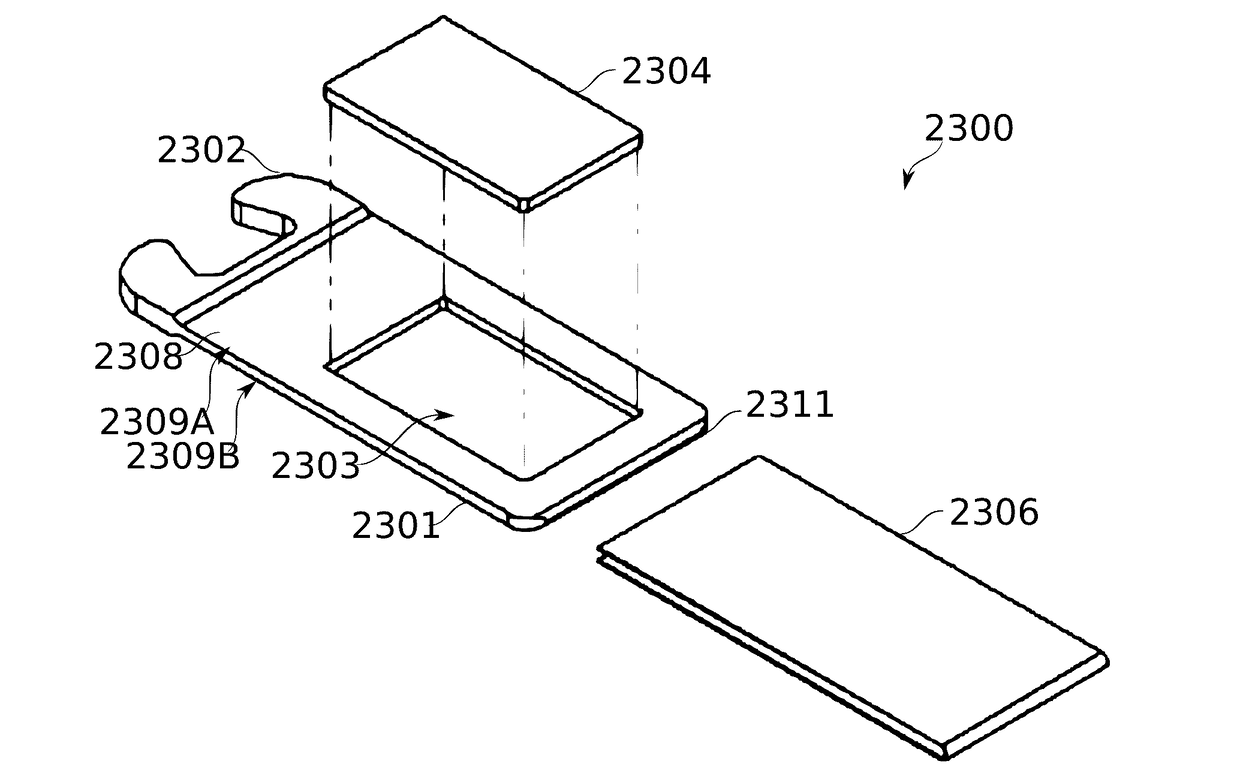
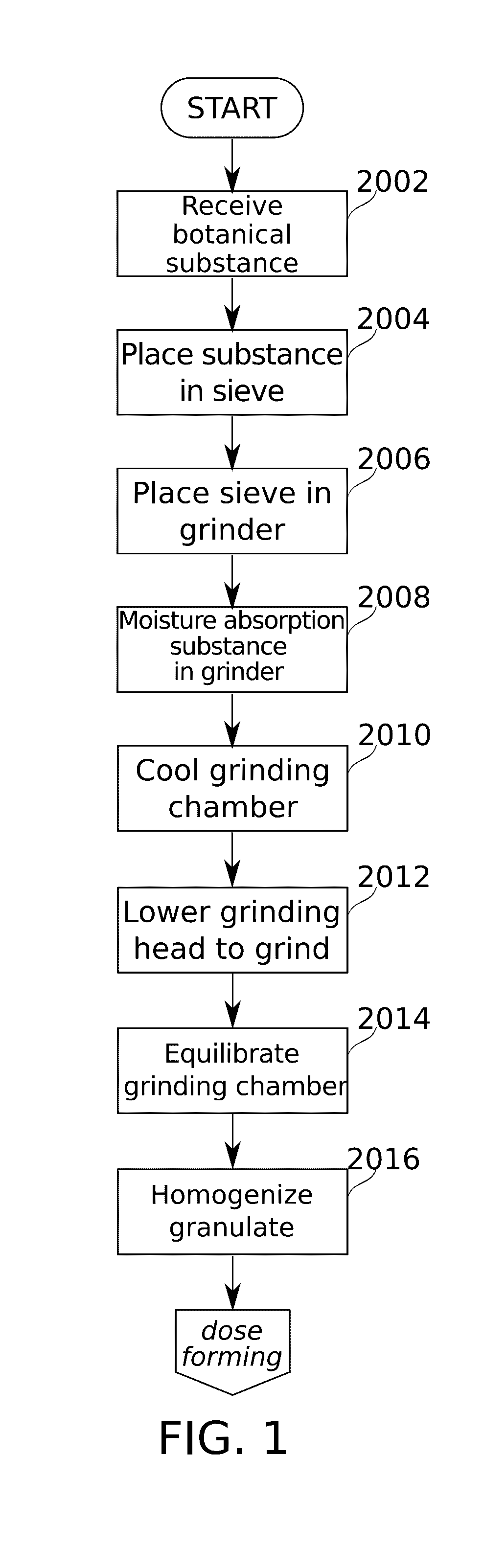
ActiveUS20170095624A1Hamper dispensingAntibacterial agentsOrganic active ingredientsEnvironmental healthDrug doses
Devices and methods are described for preparing, managing, and / or administering metered doses of substances for vaporized administration. In some embodiments, dose cartridges comprising at least one botanical substance include a heating element integrated into the cartridge in close contact with the botanical substance. In some embodiments, cartridge-mounted doses are stored in a magazine, optionally in carousel form, before use. Transport of a cartridge from a magazine to an electrically operated vaporizing chamber which activates the heating element is provided by a mechanical pickup means.
Owner:SYQE MEDICAL
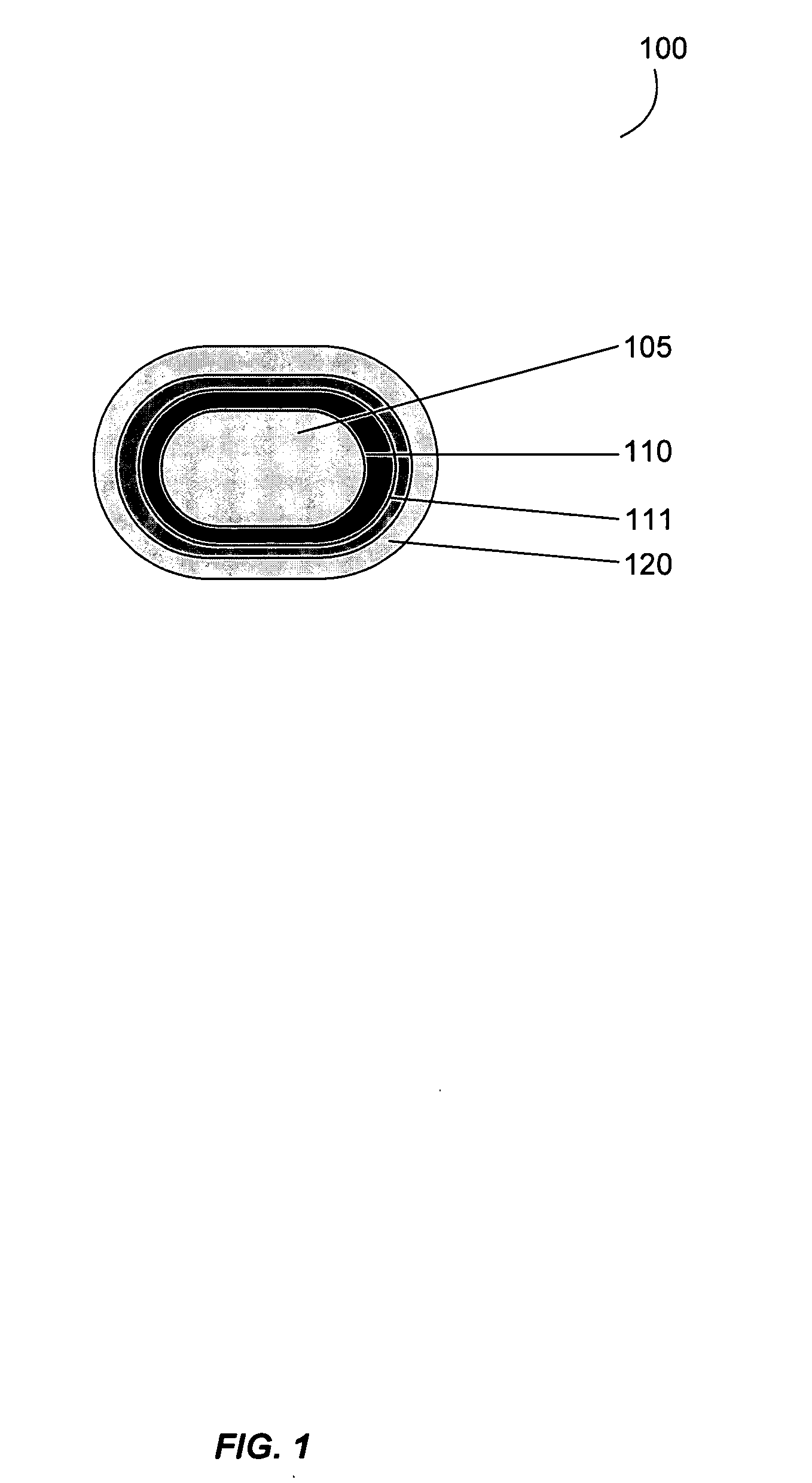
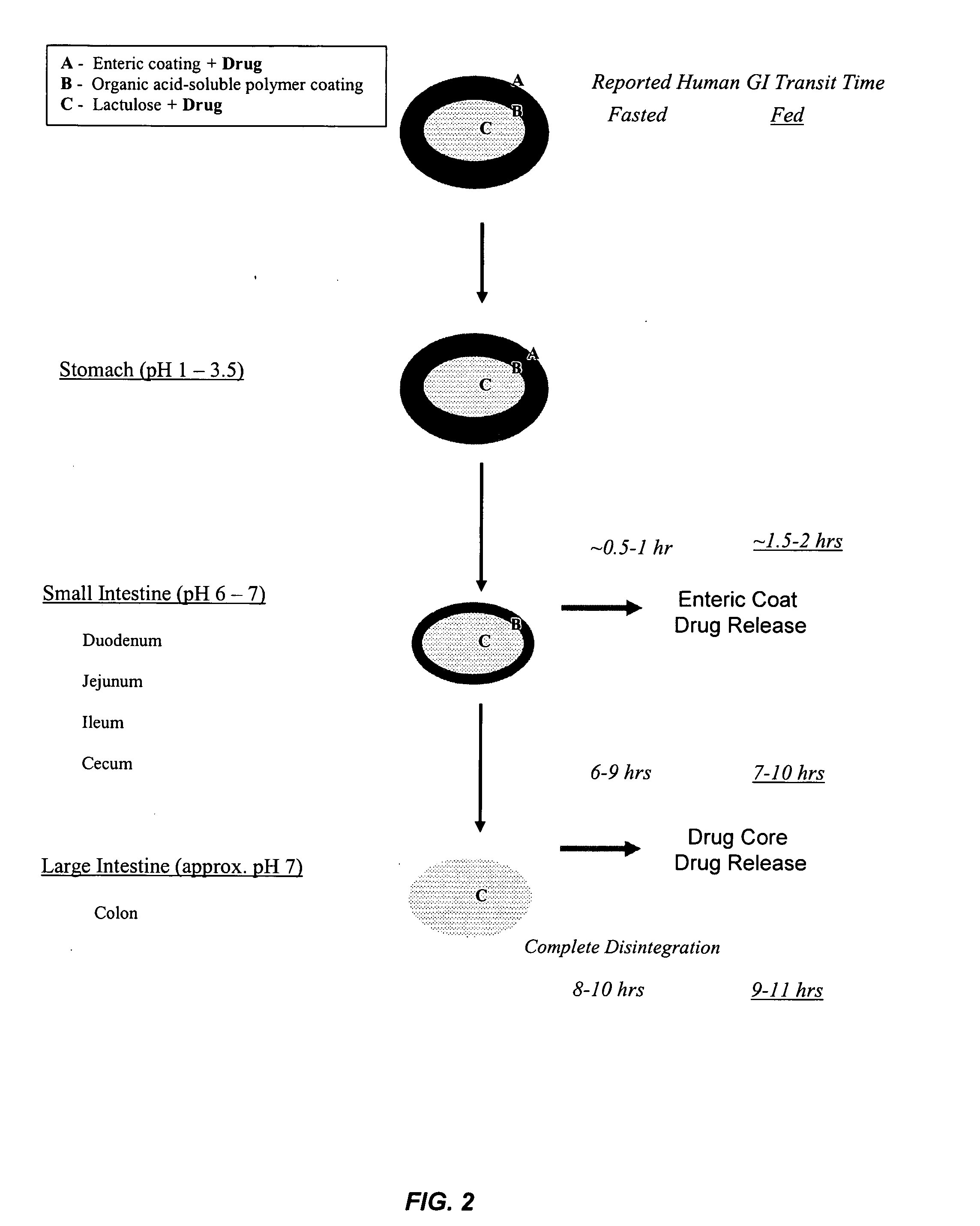
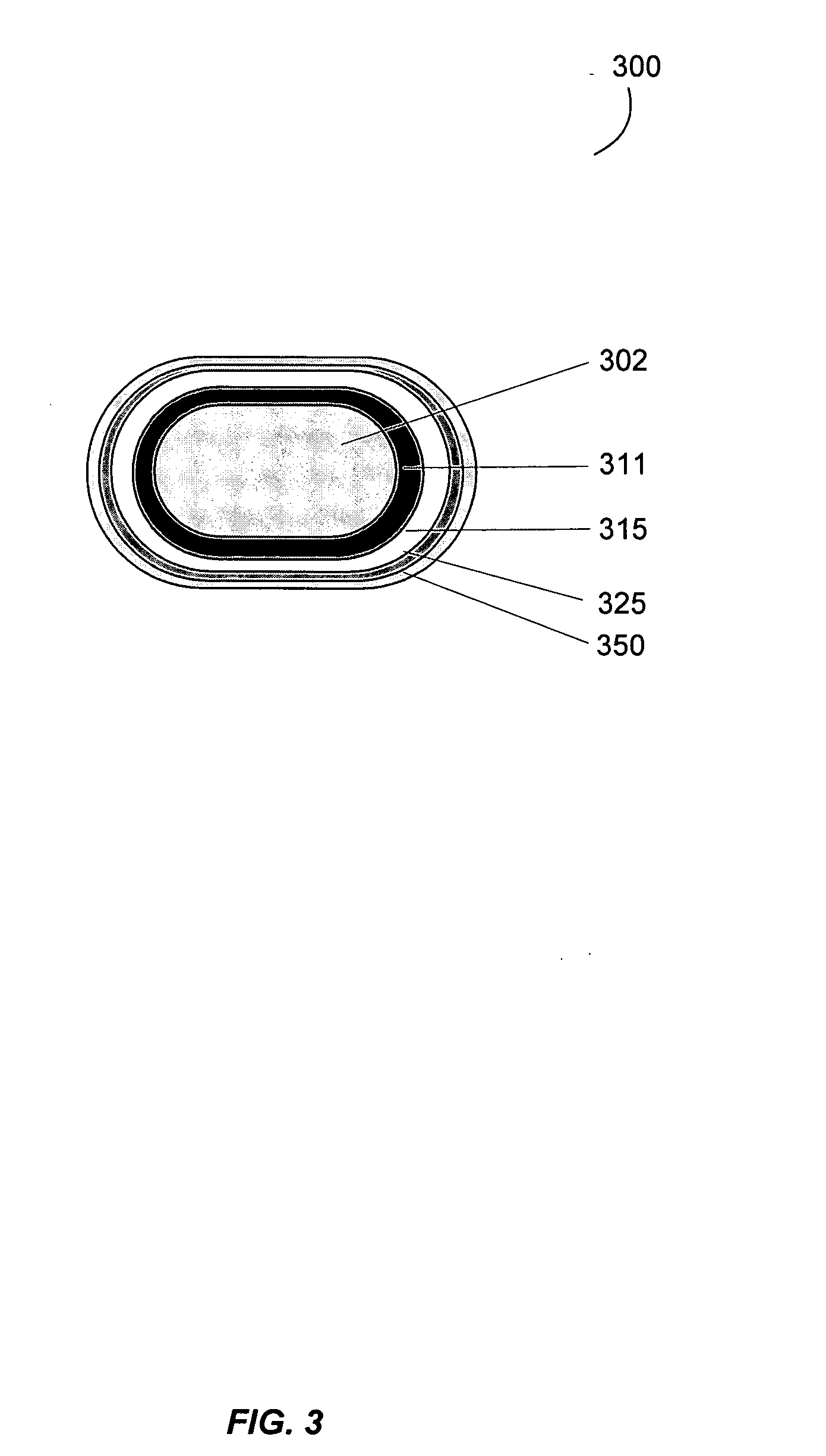
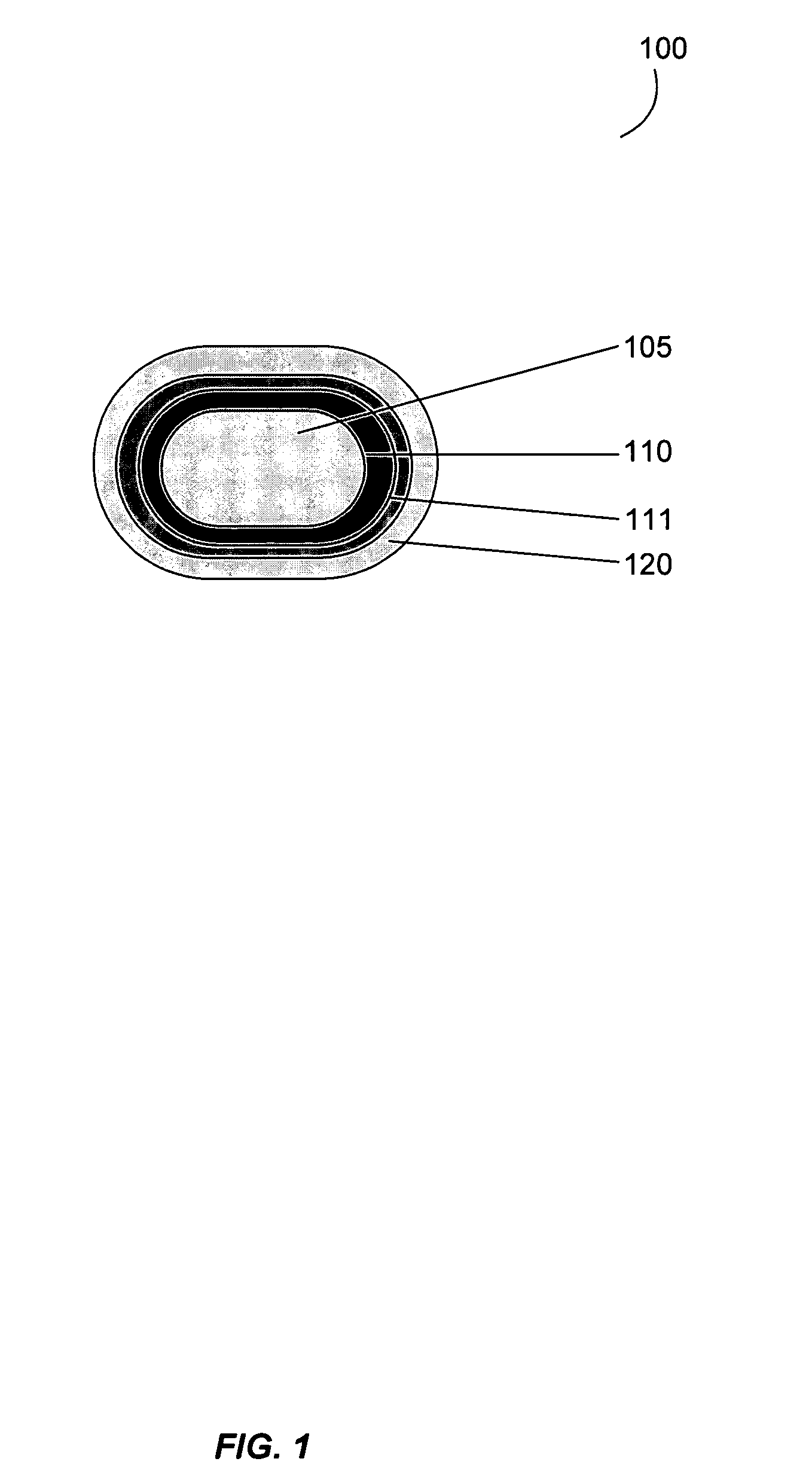
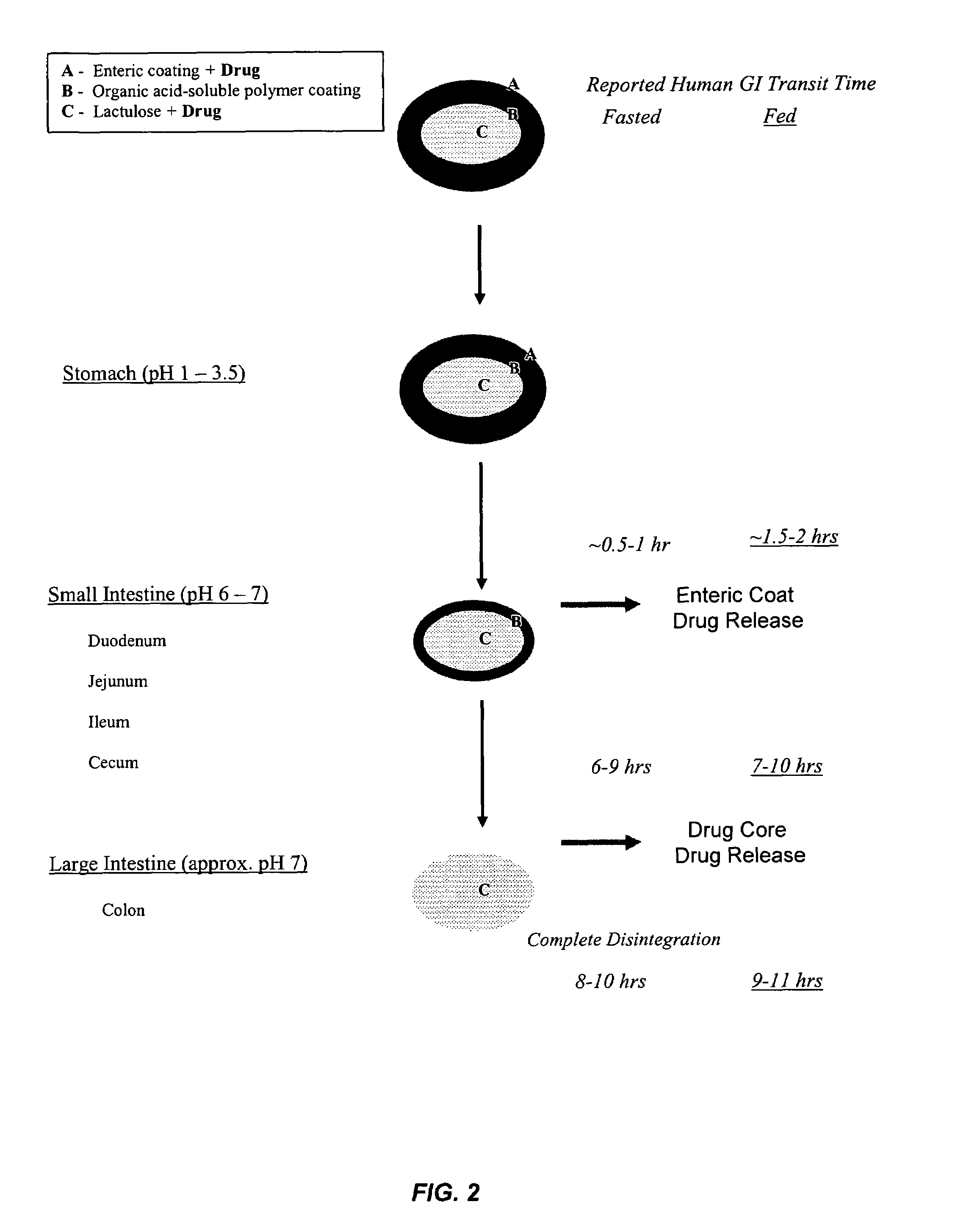
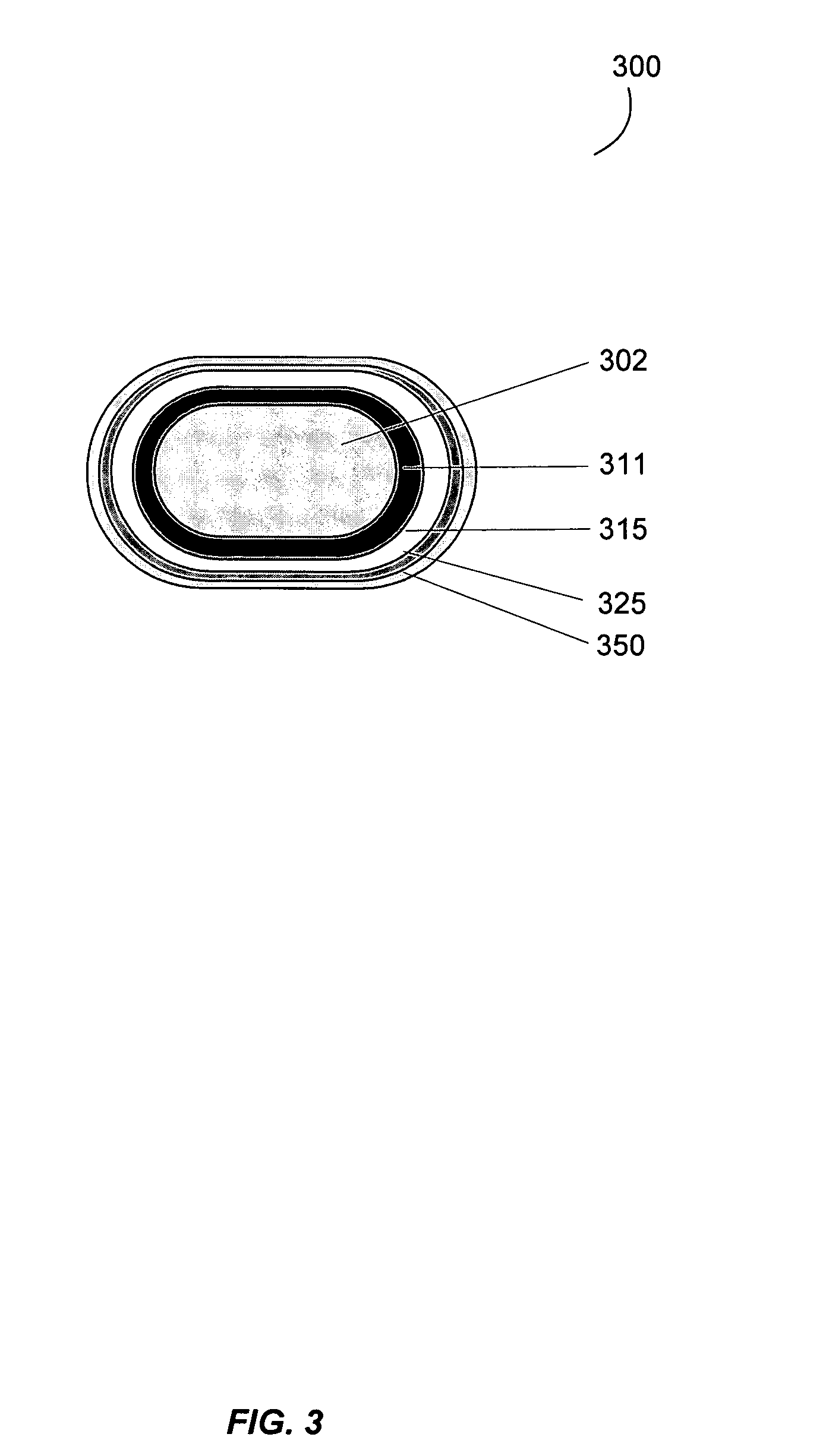
Gastrointestinal-specific multiple drug release system
InactiveUS20050208133A1Reduce dosing frequencyAntibacterial agentsPowder deliveryMedicineSmall intestine
The present invention provides compositions and methods for the multiple release of a drug in the gastrointestinal tract of a subject through the use of an oral multiple drug release system. The system provides site-specific release of the drug to both the small intestine and the colon in the form of multiple controlled doses for long-lasting efficacy, thereby reducing the drug dosing frequency.
Owner:ASTELLAS PHARMA INC
Patient controlled timed medication dispenser
ActiveUS20050258066A1Small article dispensingCoin-freed apparatus detailsMedication DispenserMinimum time
The medication delivery device provides patient access to medications prescribed to be available on an as-needed basis, but with a minimum time intervals between doses. The device permits access to a single dose of the medication after each minimum time interval has elapsed. When the drug dose is removed from the device, the medication tray locks until the next minimum dosage time interval has elapsed.
Owner:AVANCEN MOD
Solid pharmaceutical dosage formulation
InactiveUS20050143404A1Reduce the burden onEase complicated treatment regimenBiocideOrganic active ingredientsHIV Protease InhibitorDrug doses
The present invention provides a pharmaceutical dosage formulation, and more particularly, to a pharmaceutical dosage formulation comprising an HIV protease inhibitor.
Owner:ABBVIE INC
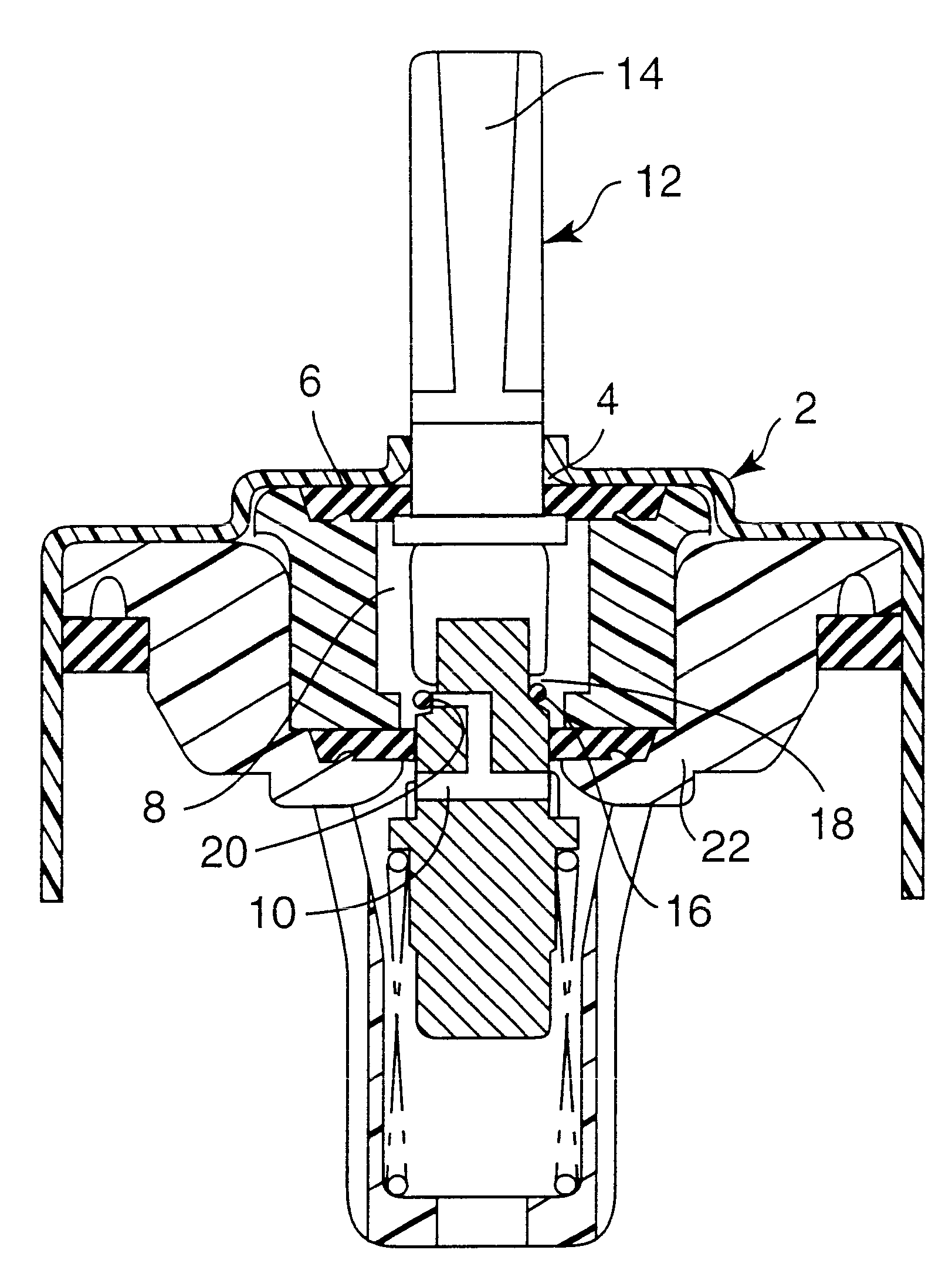
Medication delivery device
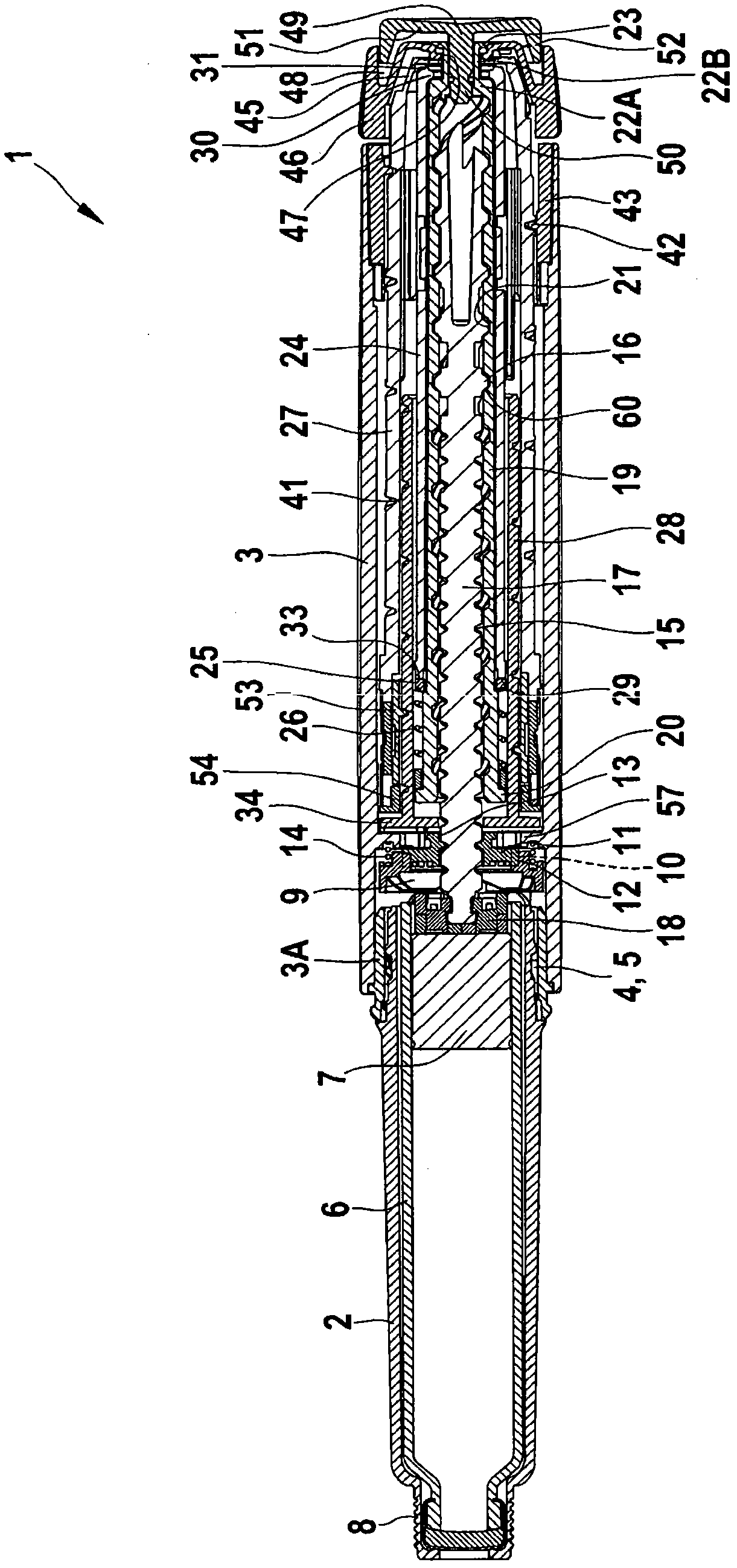
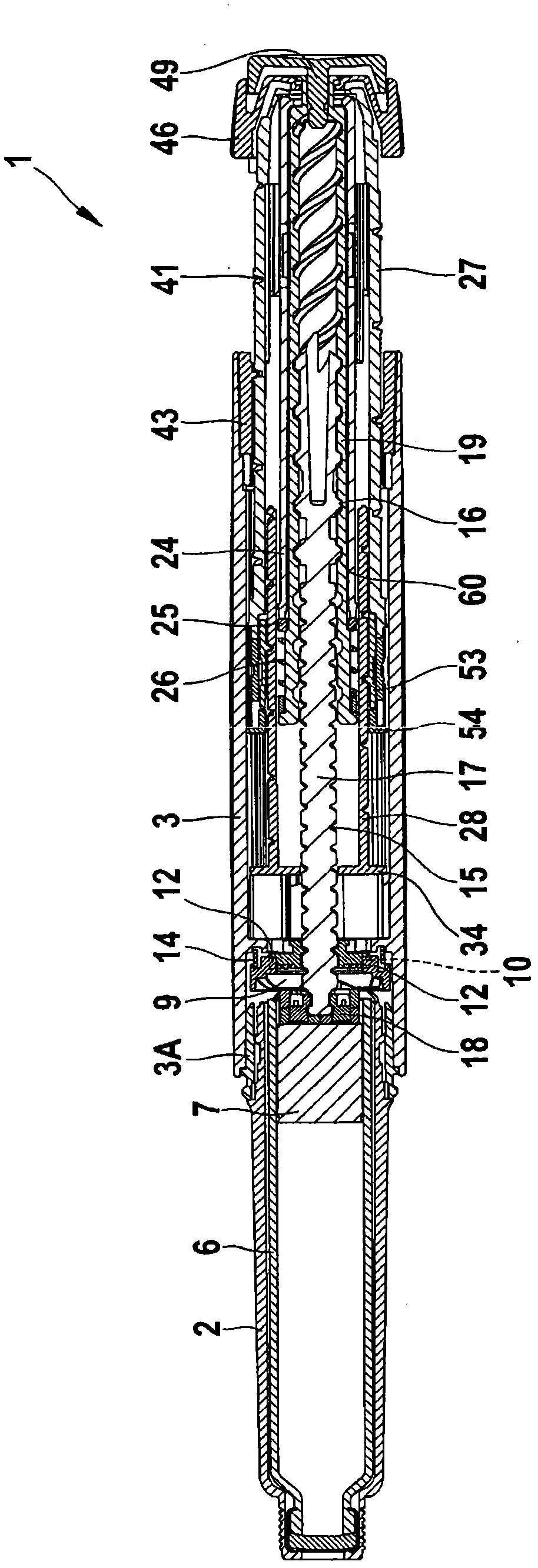
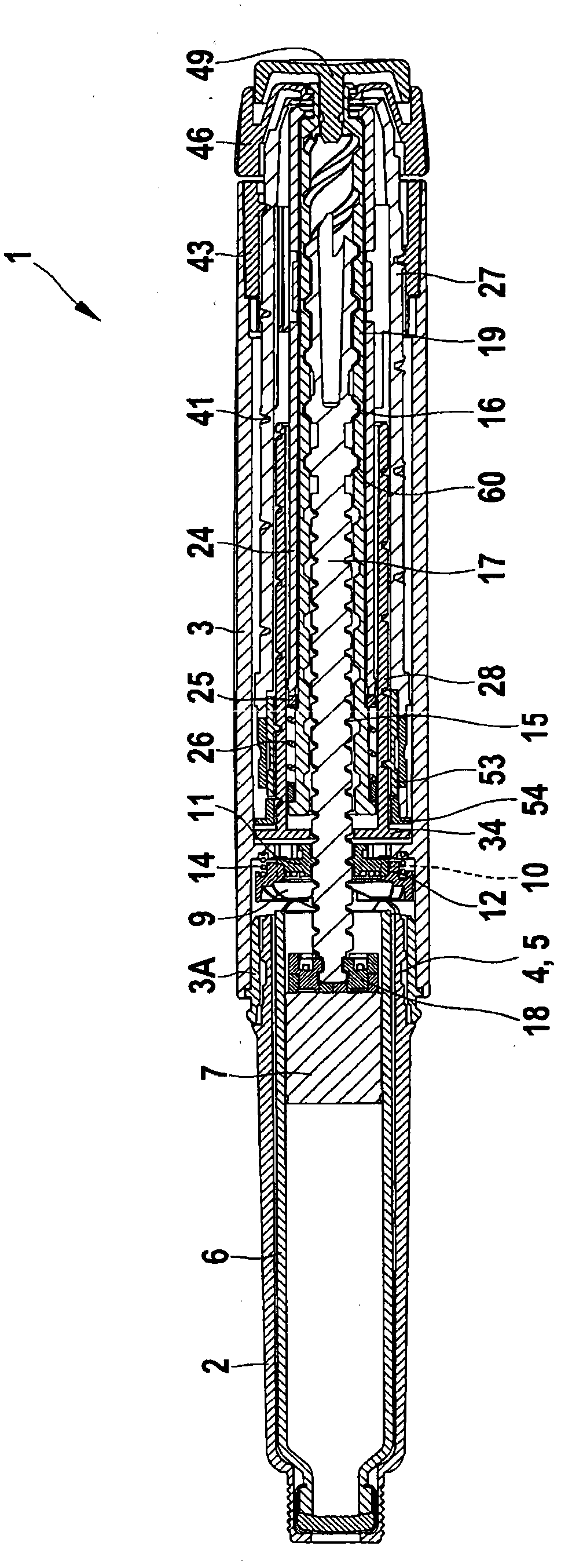
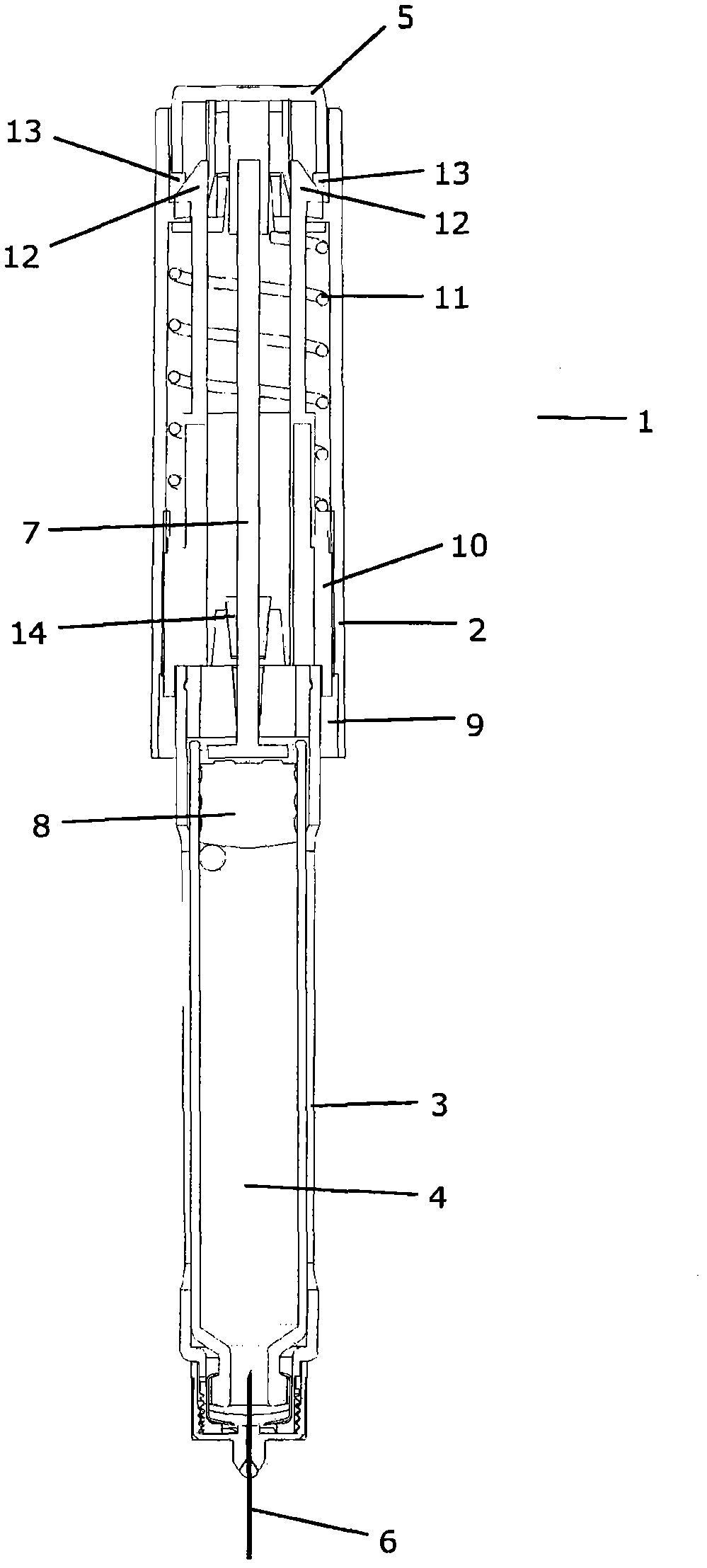
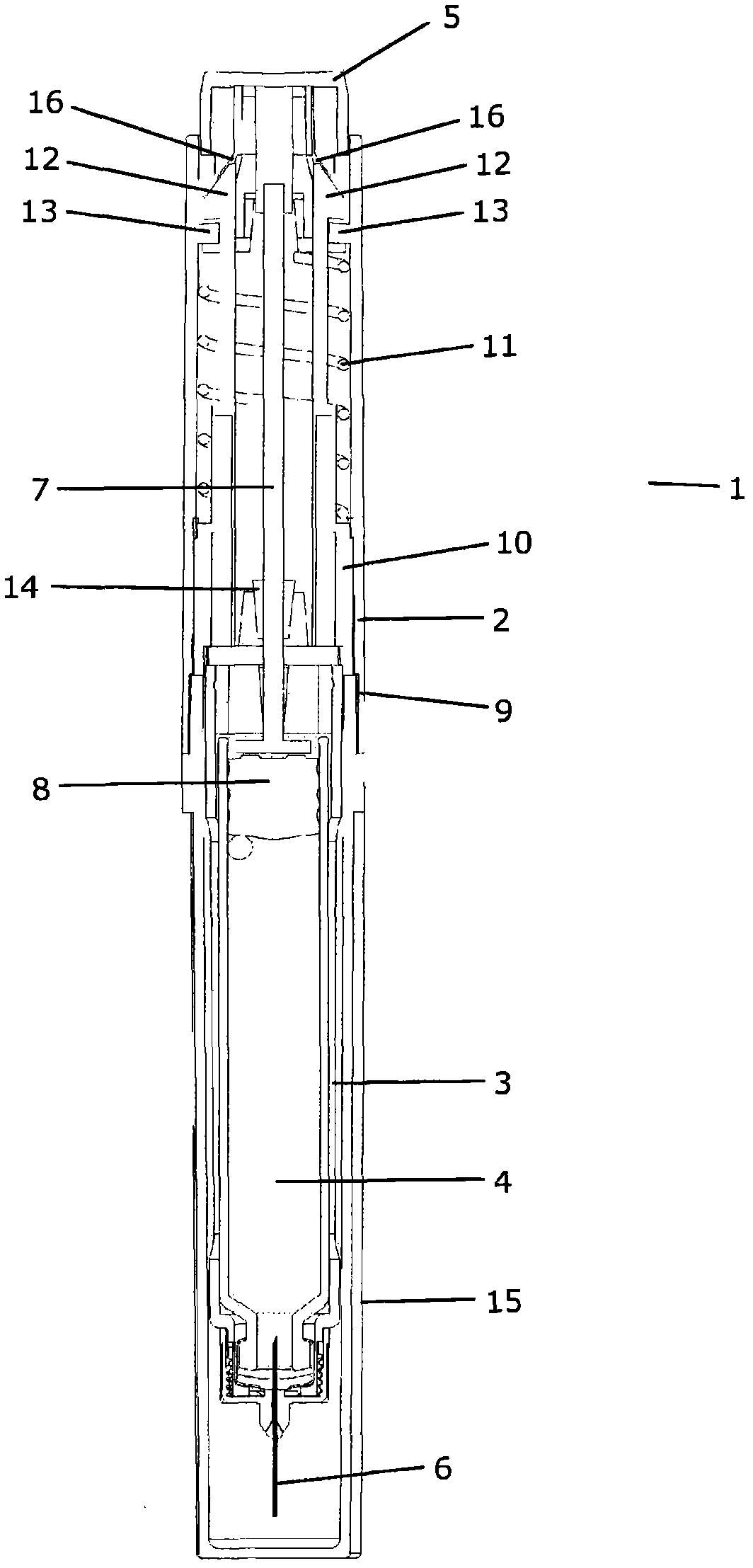
The present invention refers to a medication delivery device (1) comprising: a medication receptacle (6); a dosing mechanism comprising: a piston rod (17); a drive device (19); a dose setting member (27); and a dose limiting member (28) which prevents the setting of a dose of medication which exceeds an amount of medication contained in the medication receptacle (6); and a housing (3). The dose limiting member (28) is designed for axial movement in a proximal direction with respect to the piston rod (17) during dose setting and comprises a first stop element (35) and the piston rod (17) comprises a second stop element (36). The first and second stop elements (35, 35) stop an axial movement of the dose limiting member (28) in the proximal direction with respect to the piston rod (17) when the first and second stop elements (35, 36) catch, thereby limiting a movement of the dose setting member (27) for increasing a set dose of medication to be delivered. The dose limiting member (28) and the piston rod (17) only interact directly, when the first and second stop elements (35, 36) catch.
Owner:SANOFI AVENTIS DEUT GMBH
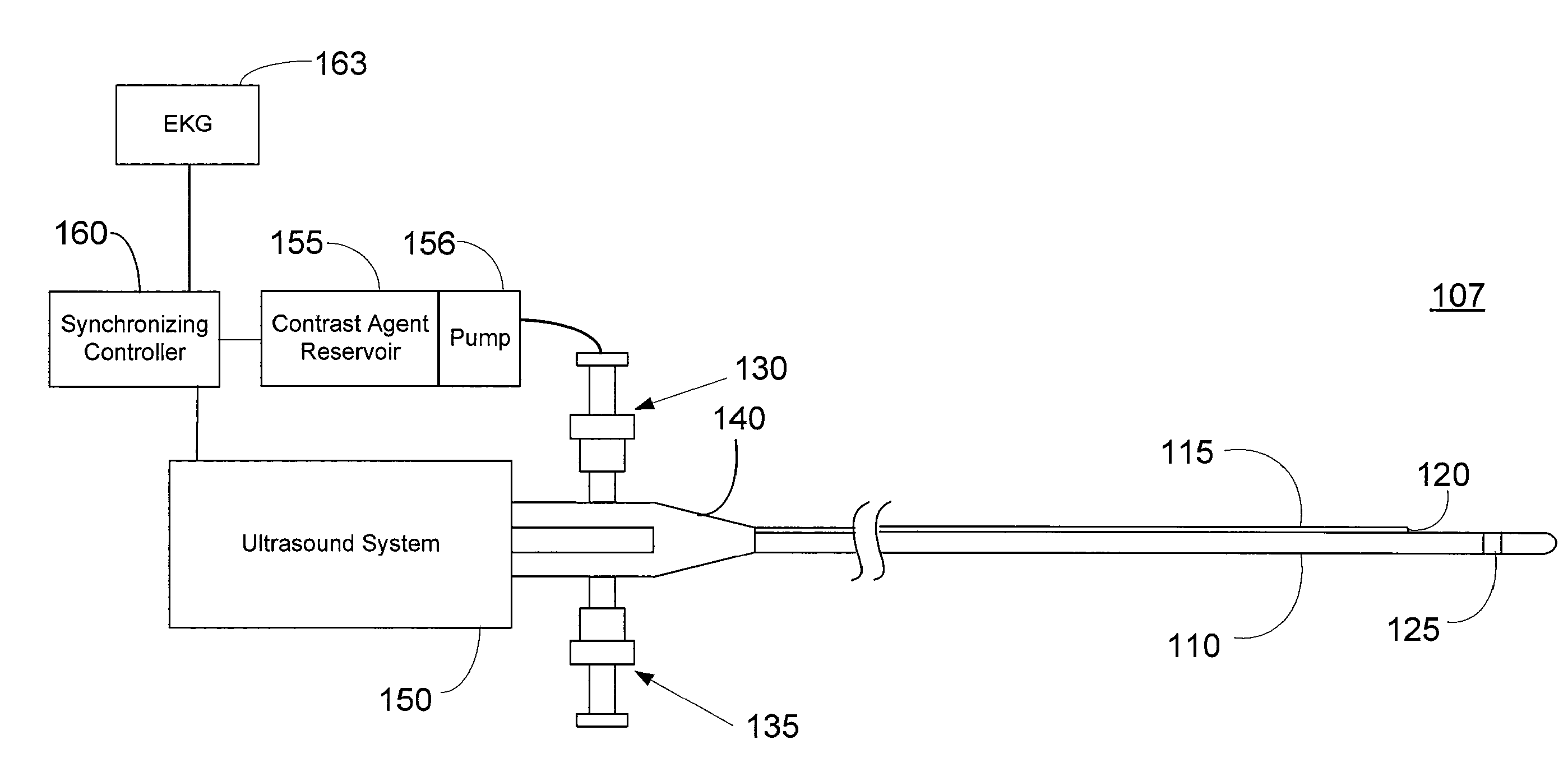
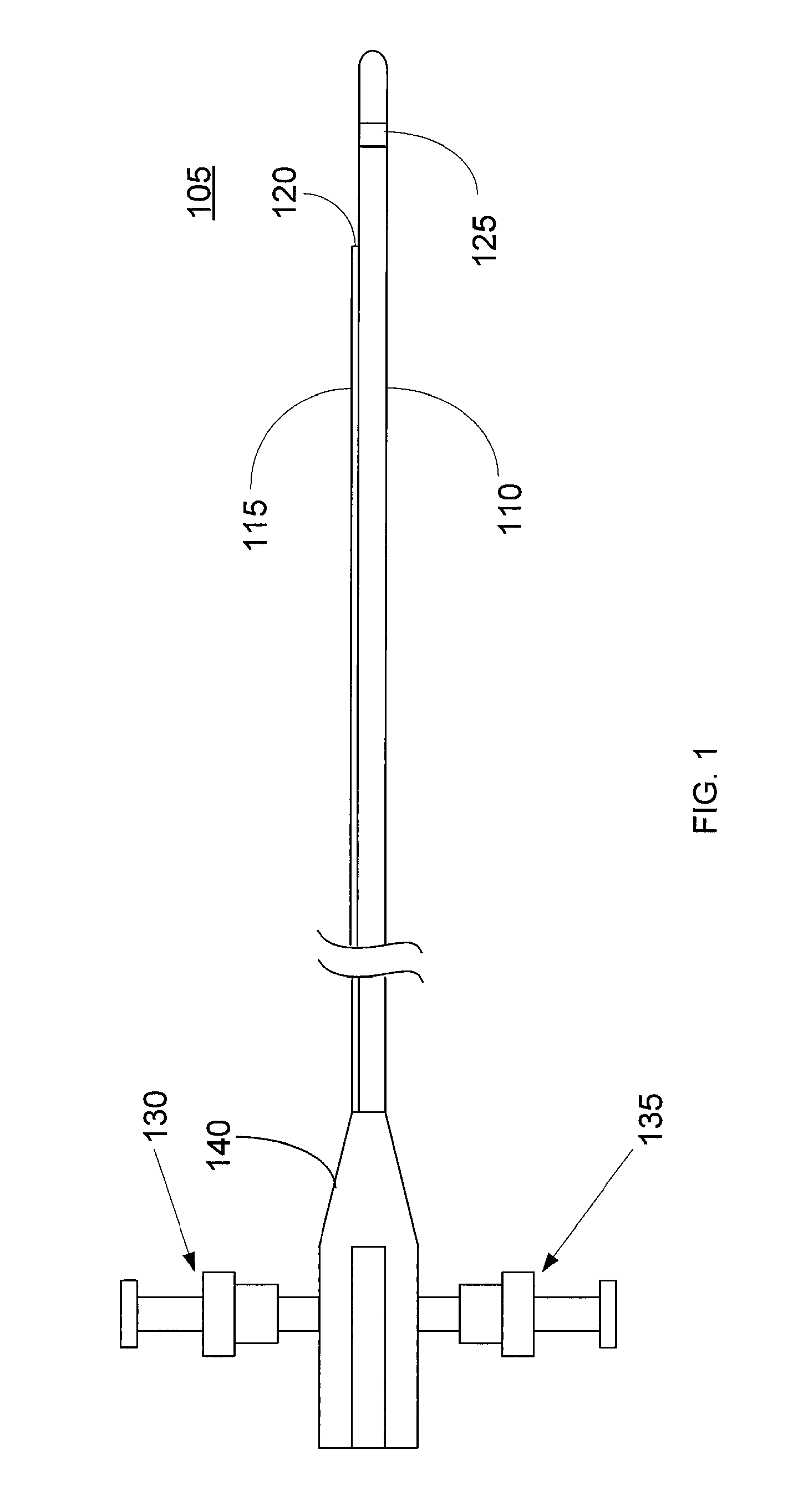
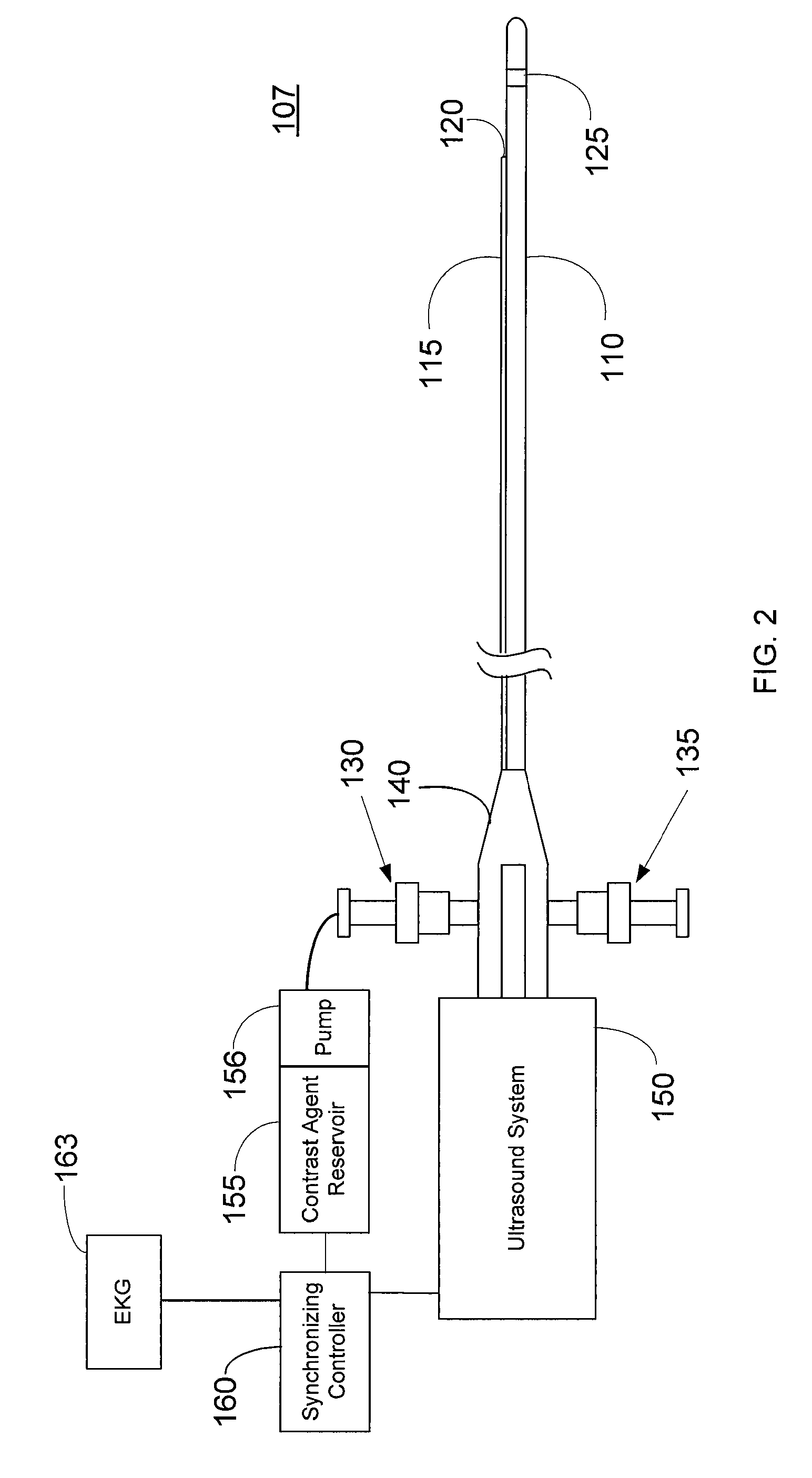
Imaging Catheter With Integrated Contrast Agent Injector
InactiveUS20090234231A1Lower the volumeSynchronization is simpleUltrasound therapyMulti-lumen catheterUltrasound imagingControlled drugs
Described herein are systems and methods that integrate the injection of contrast agents with imaging catheters. In an embodiment, an imaging catheter comprises a catheter sheath and an imager, e.g., ultrasound transducer. The imaging catheter further comprises a contrast lumen having one or more exit ports for injecting contrast agent into the patient. The contrast lumen extends along the catheter sheath and may be external to or integrated into the catheter sheath. Preferably, the exit port of the contrast lumen is positioned along the catheter sheath at a relatively short known distance from the imager. The catheter may include multiple contrast lumens for injecting different types of contrast agents. In an embodiment, a synchronizing controller is provided to automatically synchronize the injection of contrast agent with imaging. In another embodiment, drug-filled microbubbles in combination with ultrasound imaging are used to deliver a controlled drug dose to a specific treatment site.
Owner:BOSTON SCI SCIMED INC
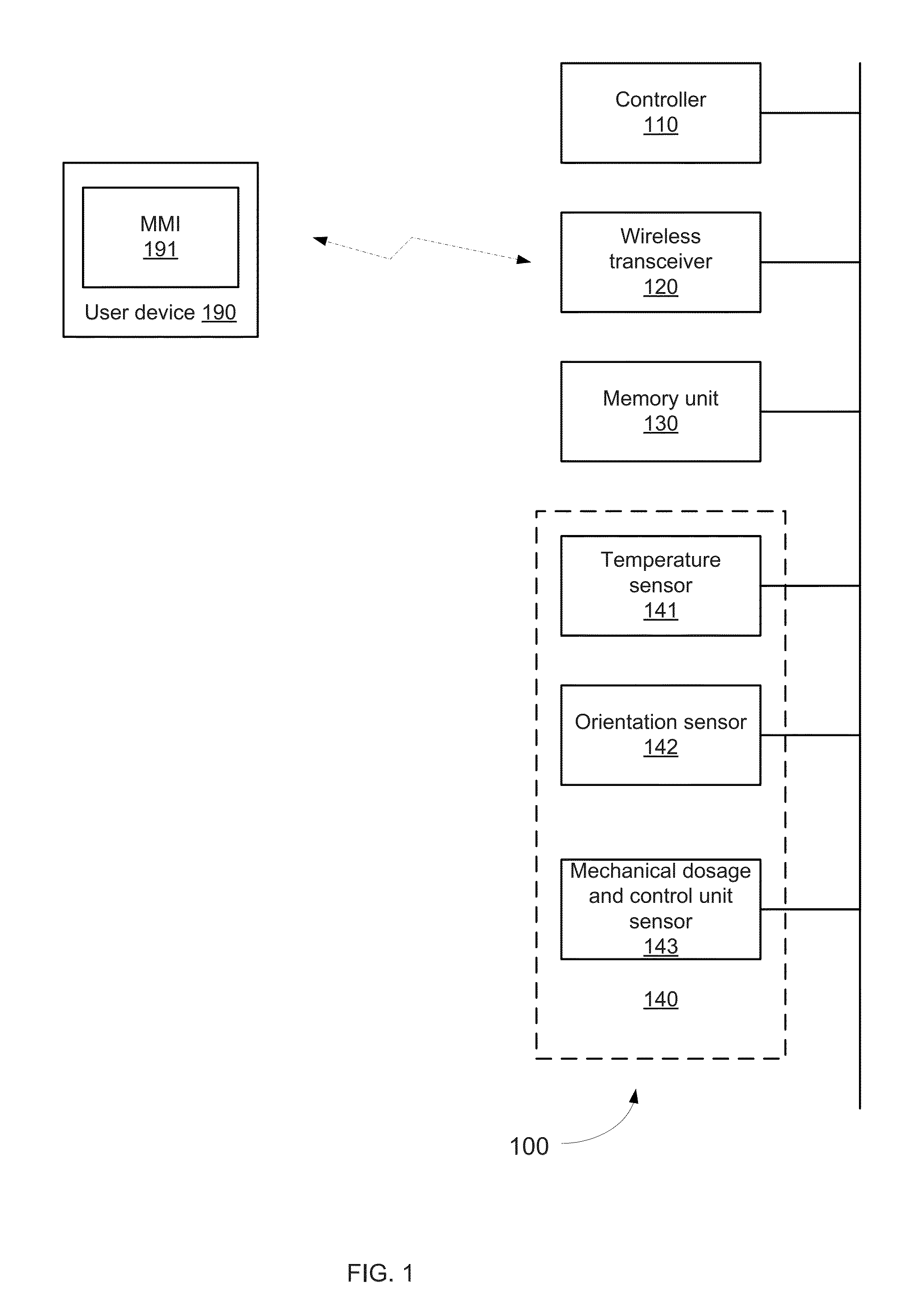
Device and method for drug dosing with administration monitoring, in particular for insulin pen integrated with smart phone apps.
InactiveUS20150246179A1Memory architecture accessing/allocationDrug and medicationsWireless transceiverHand held
A hand held drug administration unit that may include a controller, sensors, a wireless transceiver, a memory unit, and a mechanical dosage and injection control unit that is controlled by a user; wherein the sensors are configured to generate detection signals indicative of a progress of a drug provision process; wherein the controller is configured to process the detection signals and to determine the progress of the injection process and to provide a notification regarding the progress of the injection process
Owner:ZUR NISSIM +1
Patient Controlled Timed Medication Dispenser
InactiveUS20100305750A1Small article dispensingElectric signal transmission systemsMedication DispenserMedication dose
A medication dispenser for permitting administration of a medication dose to an authorized user only after a predetermined minimum-dosing interval has elapsed. The medication dispenser comprises: a medication tray having a substantially circular shape and comprising a plurality of compartments containing a medication dose, the compartments disposed about a circumference thereof; regions between two consecutive dose-carrying compartments comprising an empty compartment or a blank region; an enclosure for supporting the dispenser, the enclosure including an opening; and a controller for controlling the medication tray to align one of the plurality of compartments containing a medication dose with the opening after the minimum dosing interval has elapsed and after a person has been authenticated as an authorized user, thereby permitting the authorized user to access the medication dose through the opening, wherein the medication dispenser remains in a dose-accessible configuration for a predetermined hold period, after which the medication tray is controlled to align a next successive empty compartment or blank region with the opening.
Owner:CONLEY N SHARON
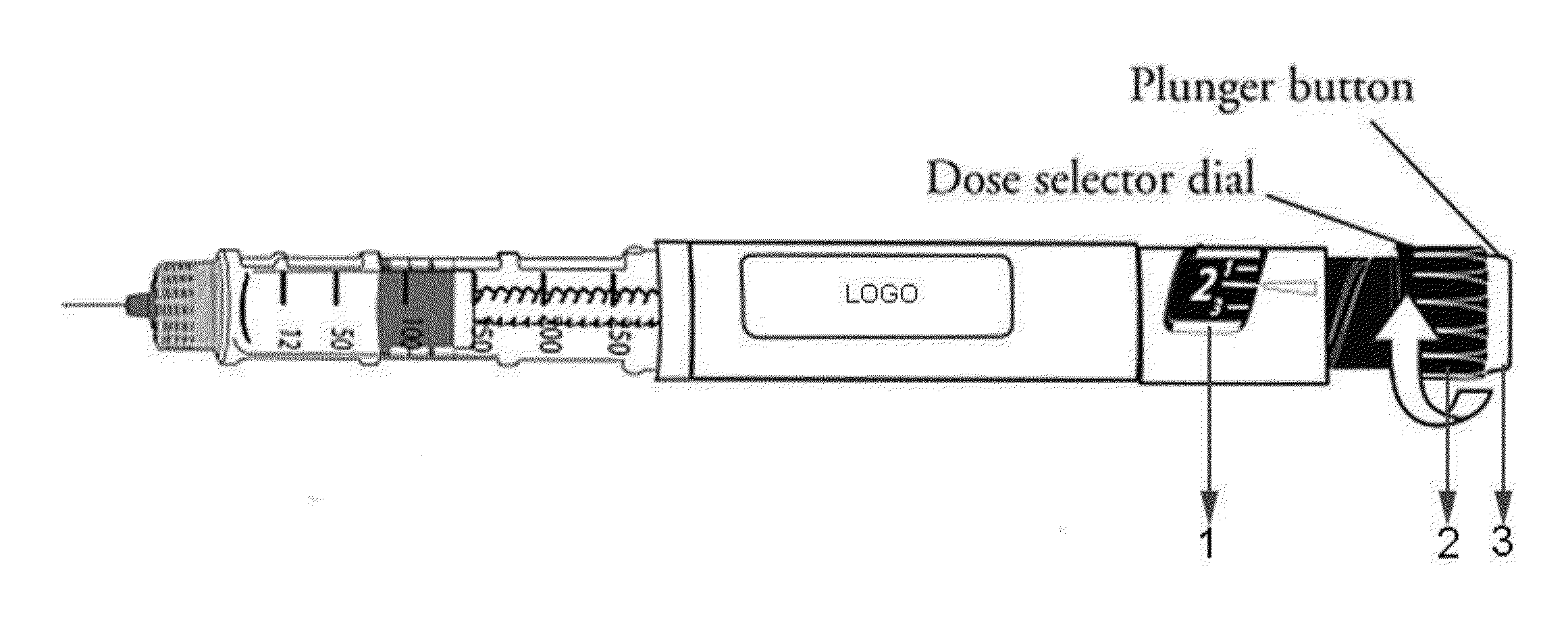
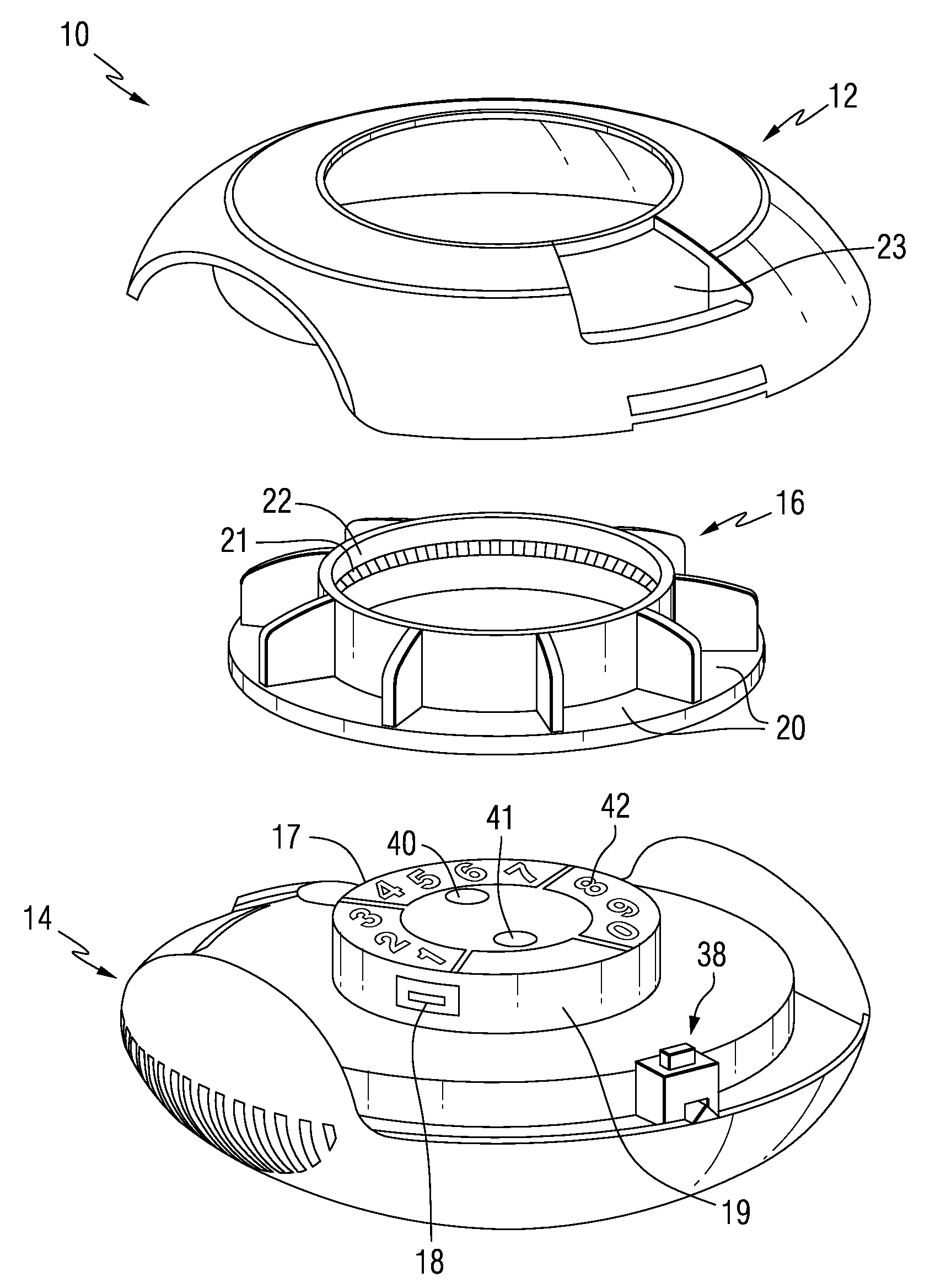
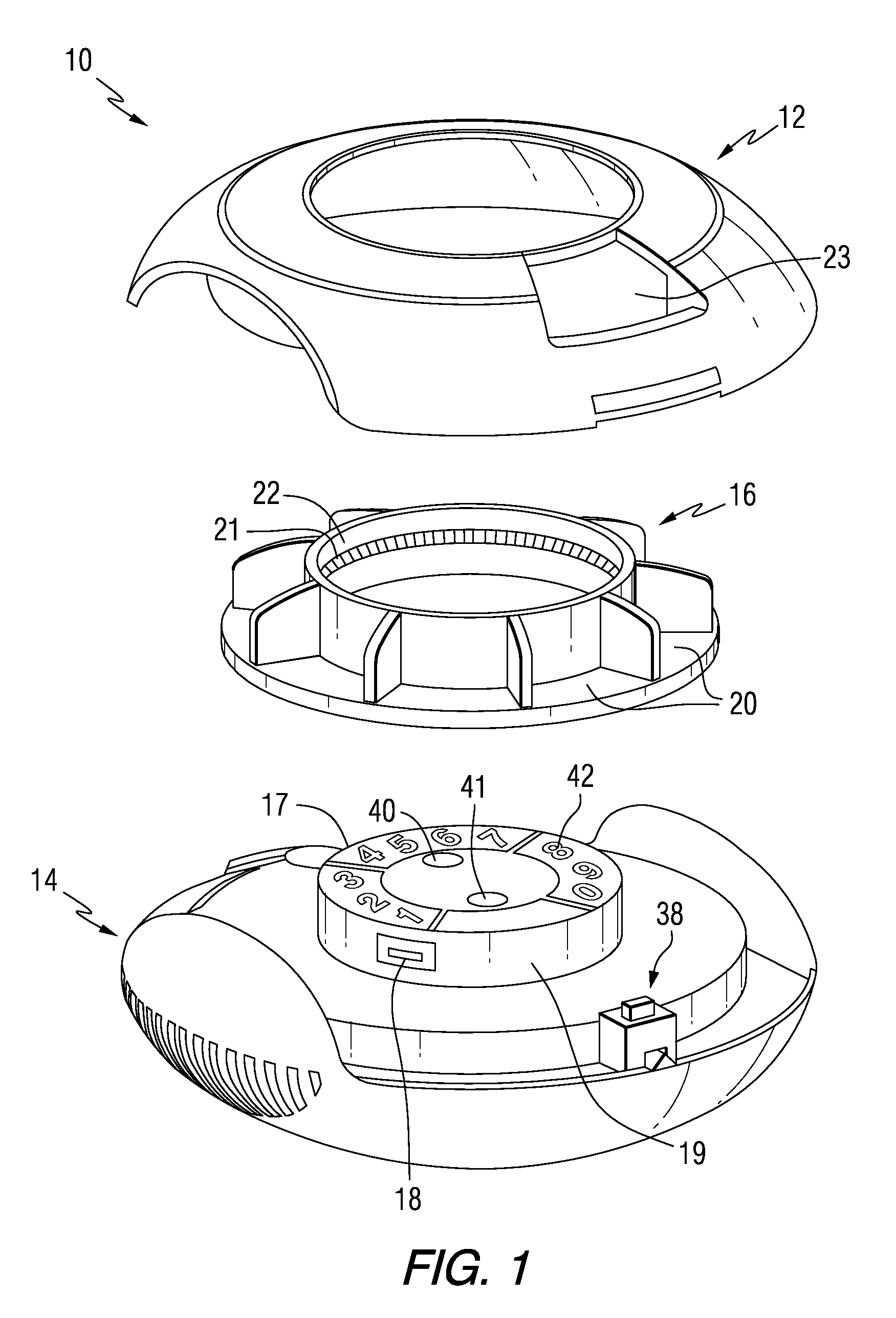
Drug delivery injection pen with add-on dose capturing and display module
A drug injector (1) comprising expelling means for expelling a dose of drug from a reservoir, a release member (13) for releasing the drug expelling means, and an actuation member (33) adapted to be moved by a user between an initial, intermediate and actuated position in which the release member is moved to release the drug expelling means. The injector further comprises an electronic capturing system for capturing data representing a dose of drug to be expelled, and a switch (38) for starting initialization of the data capture system, the switch being actuated when the actuation member is positioned in its intermediate position. A spring (34) provides a biasing force against movement of the actuation member between its initial and intermediate position. Thereby the electronic data capturing system is allowed to initialize during the actuation member's movement between the intermediate and the actuated position.
Owner:NOVO NORDISK AS
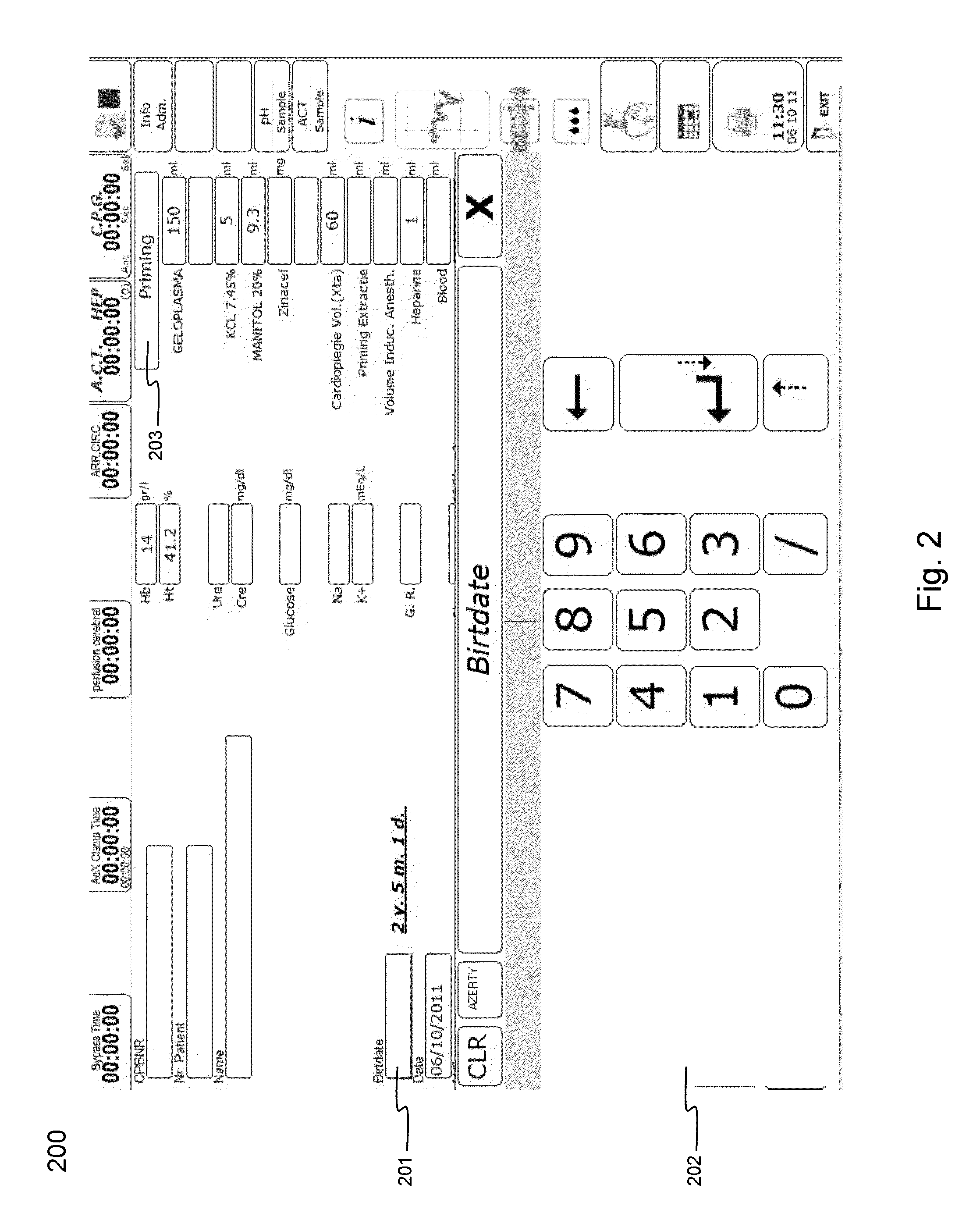
Cardiopulmonary bypass or cpb monitoring tool
InactiveUS20130094996A1Well decideEasy to calculateOther blood circulation devicesLocal control/monitoringGraphical user interfaceBody surface area
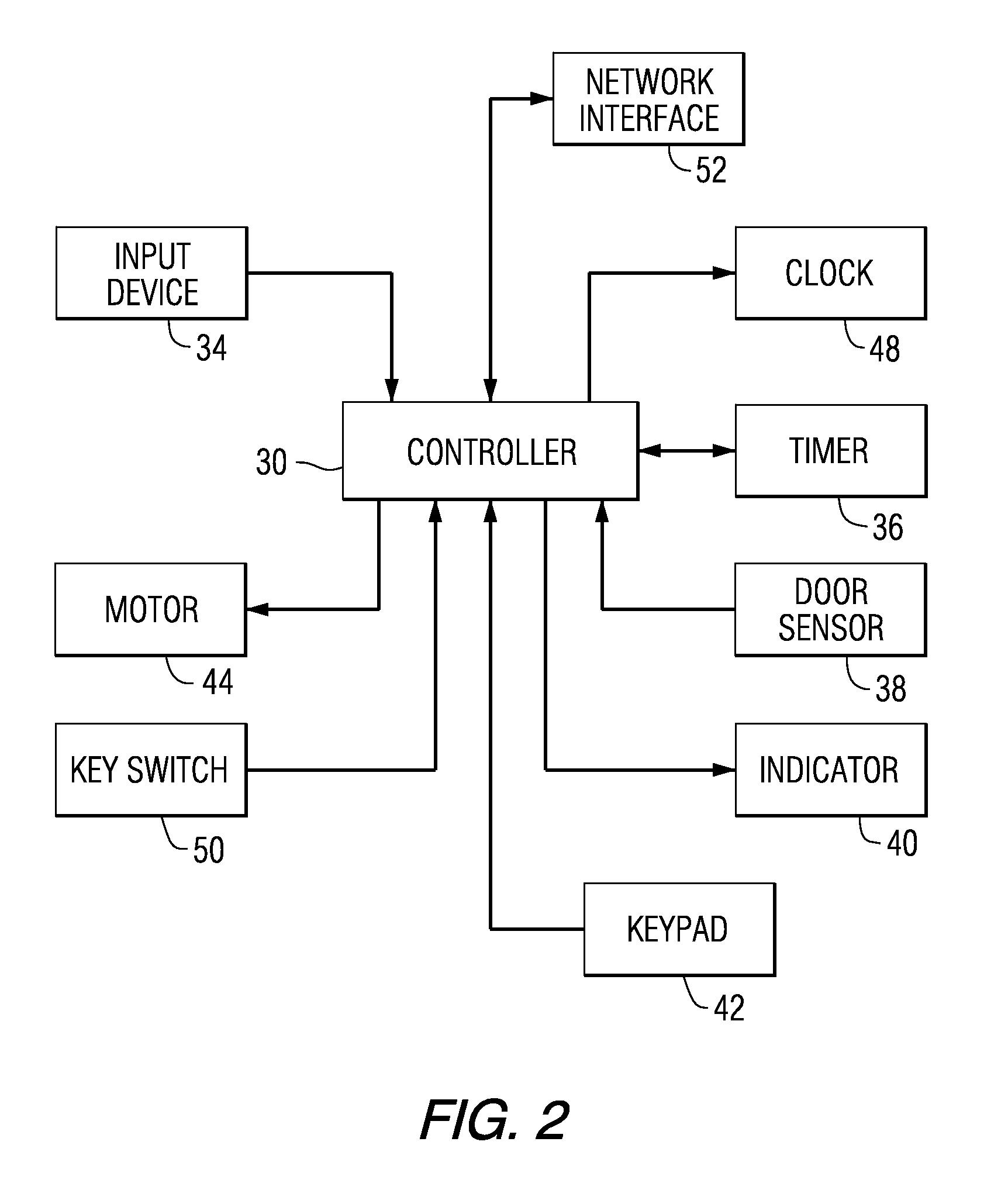
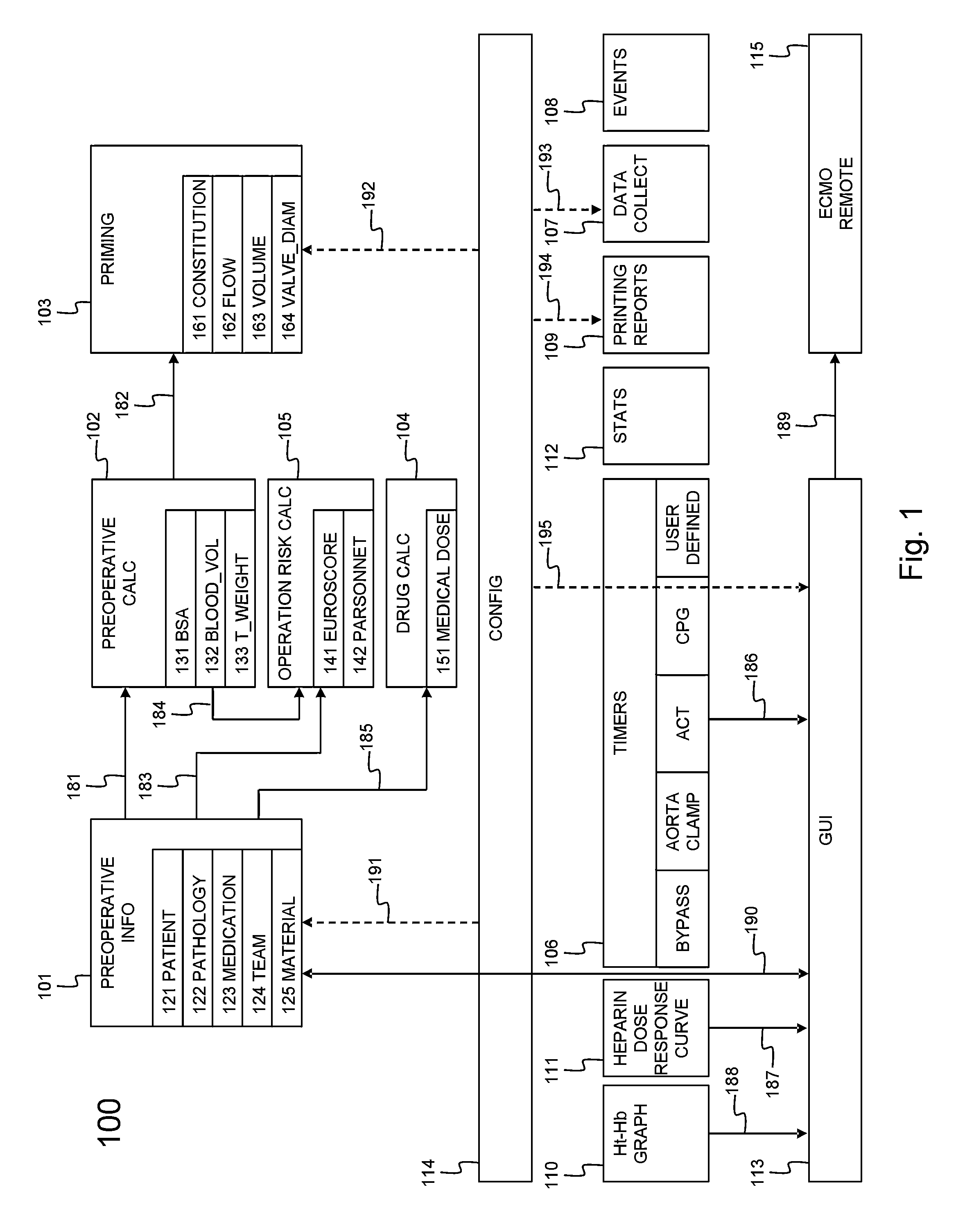
A cardiopulmonary bypass or CPB monitoring tool includes: a preoperative information module; a preoperative calculation module able to estimate a body surface area, blood volume, and theoretical weight; a priming module able to determine priming constitution, volume and flow to achieve a hemodilution target; an operation risk module for calculating operation risk; a drug calculation module able to determine medication doses; a timer module with timers that can be activated during operation; a data collection module with an interface and drivers enabling data collection from a wide variety of extracorporeal pumps and oxygenators during operation; an events module with retroactive manipulation of the time of an event; a printing report generation module; a graphic user interface; and a configuration module.
Owner:HEARTWARE INC
Drug Inventory System
InactiveUS20120259655A1Facilitates ordering dosesDrug and medicationsMedical practises/guidelinesElectronic communicationThe Internet
A system and method for managing drug dose inventory for a medical practice includes a server in electronic communication with a database and an interface with practice management software. The system allows users in a medical practice to track drug dose orders, receipt, and the administration of drug doses to patients, and billing for the doses, with a user computer that accesses the server over the Internet.
Owner:GENERAL MEDICAL SERVICES
Device for injecting apportioned doses of liquid drug
InactiveCN101912650APrevent axial movementAmpoule syringesAutomatic syringesInjection equipmentDrug doses
A mechanical injection device (1) for injecting apportioned doses of liquid drug. The injection device (1) comprises a dose setting means, an injection means, a removable cap (15) and a cap receiving part (9) adapted to abut or engage with the cap (15) when the cap (15) is mounted on the injection device (1). The dose setting means is operatively coupled to the cap receiving part (9) in such a manner that mounting and / or dismounting of the cap (15) on / from the injection device (1) causes the dose setting means to set a dose. Thereby a correct dose of drug is automatically set during a cap on / cap off cycle. Since such a cycle is normally performed between two subsequent injections, the number of steps required to be performed by the user is reduced.
Owner:NOVO NORDISK AS
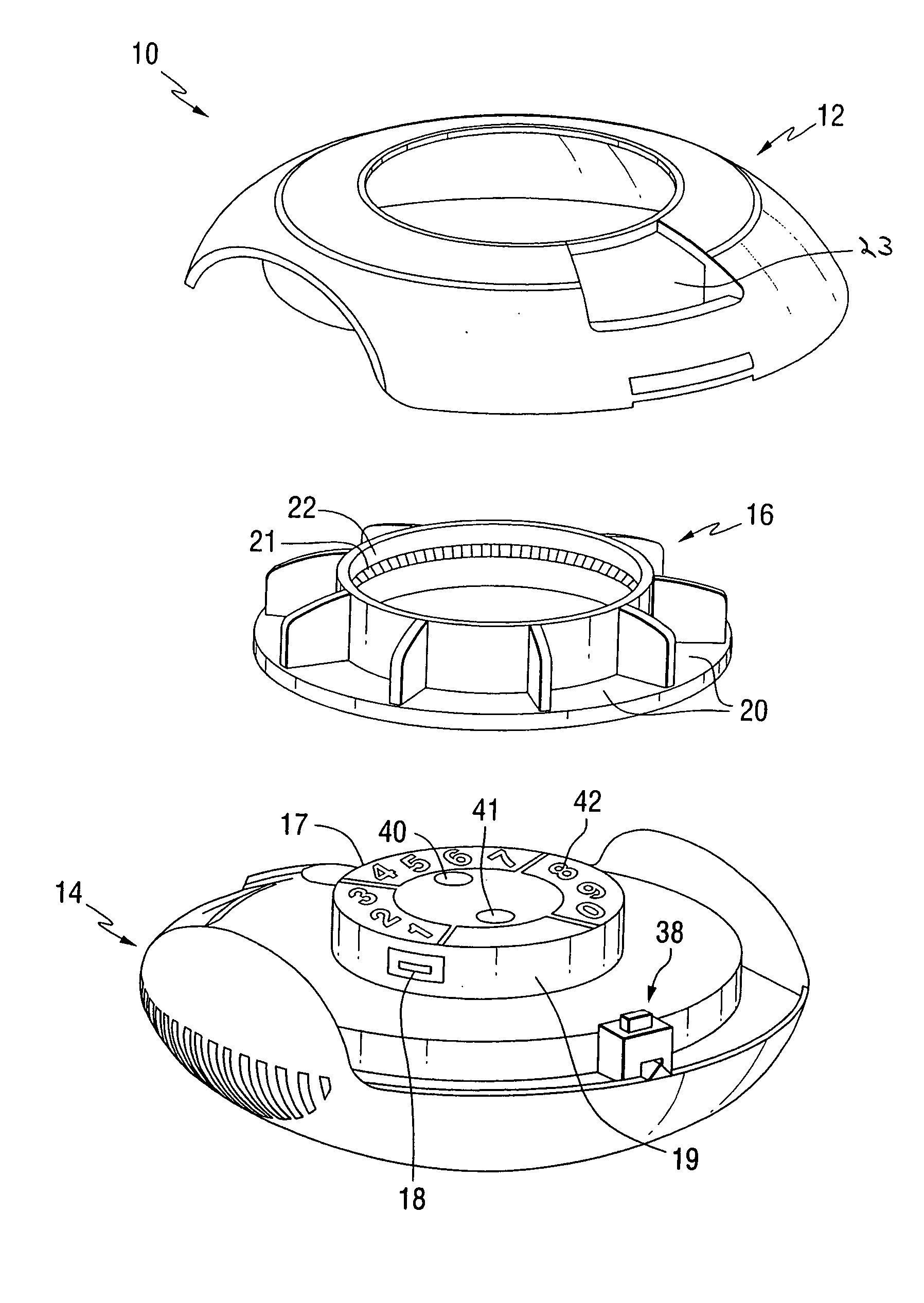
Dry powder inhaler devices, multi-dose dry powder drug packages, control systems, and associated methods
InactiveUS20060191534A1Evenly dispersedFacilitate dispersion and releaseRespiratorsLiquid surface applicatorsPharmaceutical formulationDecentralised system
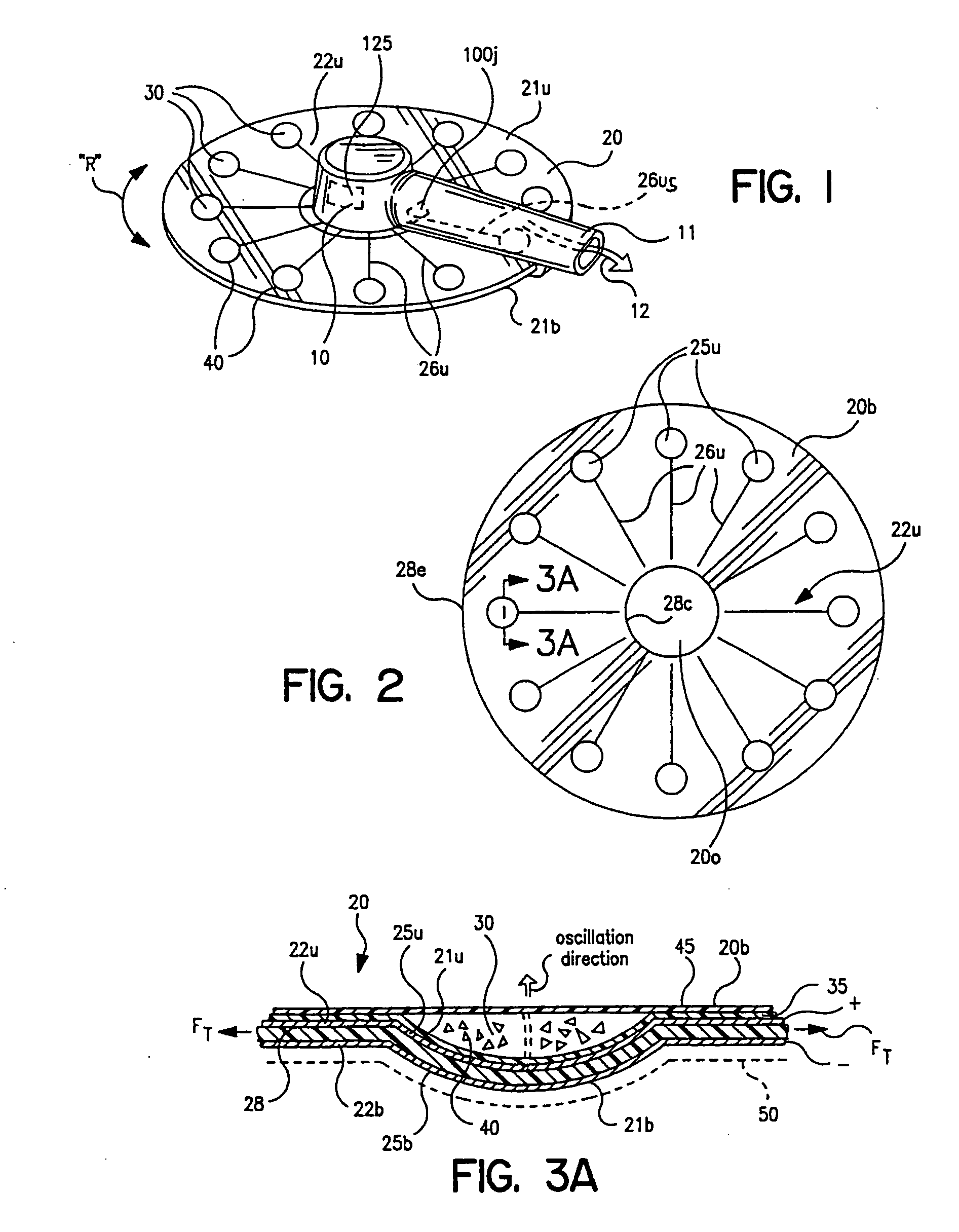
Dry powder inhalers with integrated active energy patient assist dispersal systems are configured with control systems which provide adjustable energy output responsive to the user's inspiratory capabilities and / or the flowability of the dry powder drug being administered. The multi-dose dry drug package includes a piezoelectric polymer substrate (such as PVDF) which flexes to deform and provide mechanical oscillation in a selected region of the package corresponding to the dry powder drug dose in the exit flow path and is thus actively dispersed into the exit flow path of the inhaler during the user's inspiratory activity. Control systems employ fuzzy logic models of the flowability of particular drug formulations (also being able to compensate or allow for the particular type of excipient used) and / or adjust for the real-time measured inspiratory efforts of the user. Manufacturing process control systems can adjust certain parameters in response to a fuzzy logic model of the flowability of the dry powder and other conditions associated with the dry powder drug being produced and / or dispensed.
Owner:HICKEY ANTHONY J +1
Gastrointestinal-specific multiple drug release system
InactiveUS7670624B2Reduce dosing frequencyAntibacterial agentsPowder deliverySmall intestineDrug doses
Owner:ASTELLAS PHARMA INC
Method for providing optimal drug dosage
InactiveUS6898455B2Accurately determineGood estimateSensorsPsychotechnic devicesSick personAttention deficits
A method for treating attention deficit / hyperactivity disorder (ADHD) includes performing an objective ADHD behavioral assay for a patient, administering a drug to the patient at varying doses, and performing an fMRI on the patient for the varying drug doses to determine an optimal dosage for the patient. The method can further include performing a further objective ADHD behavioral assay after the drug administration.
Owner:THE MCLEAN HOSPITAL CORP
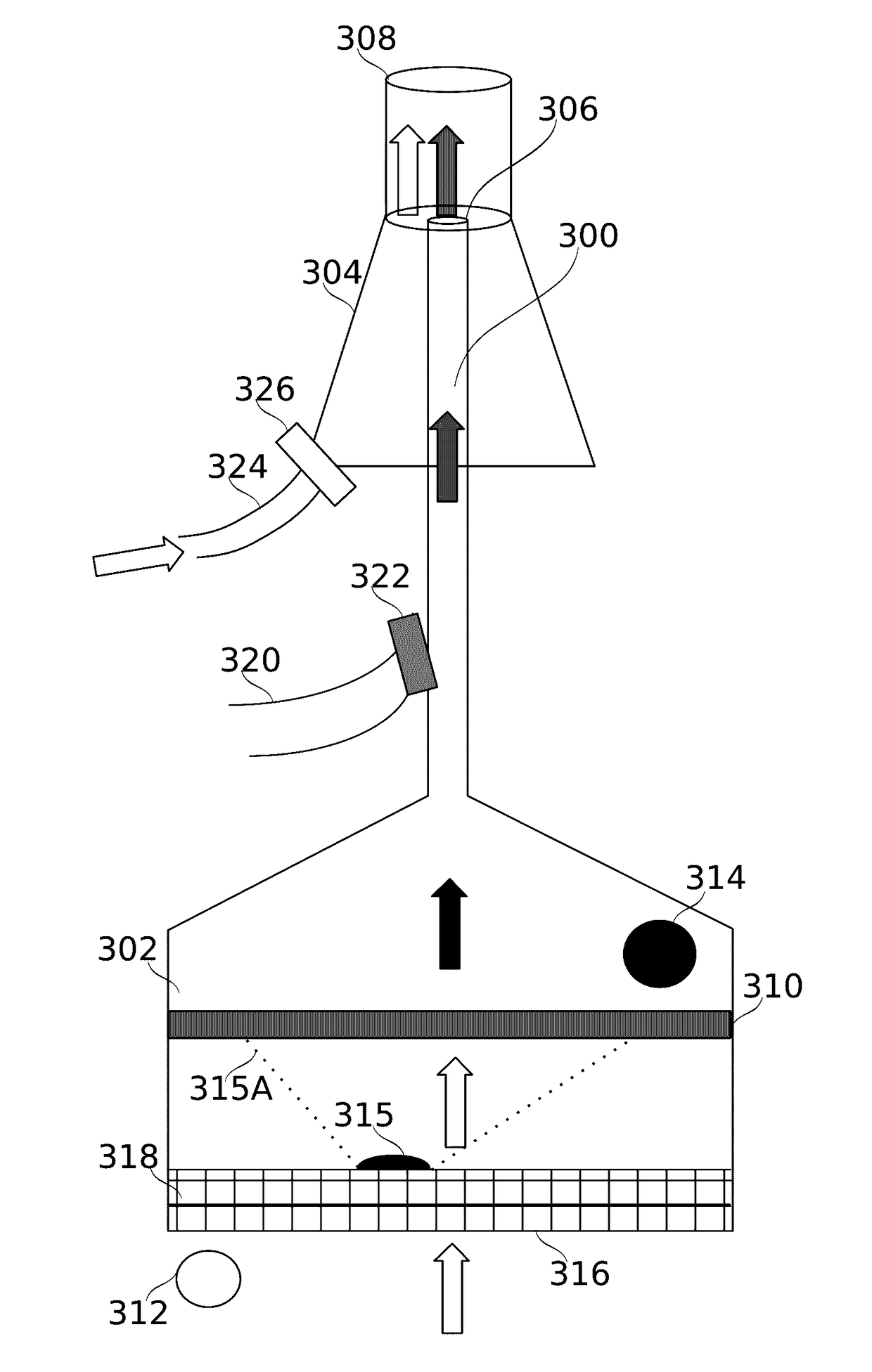
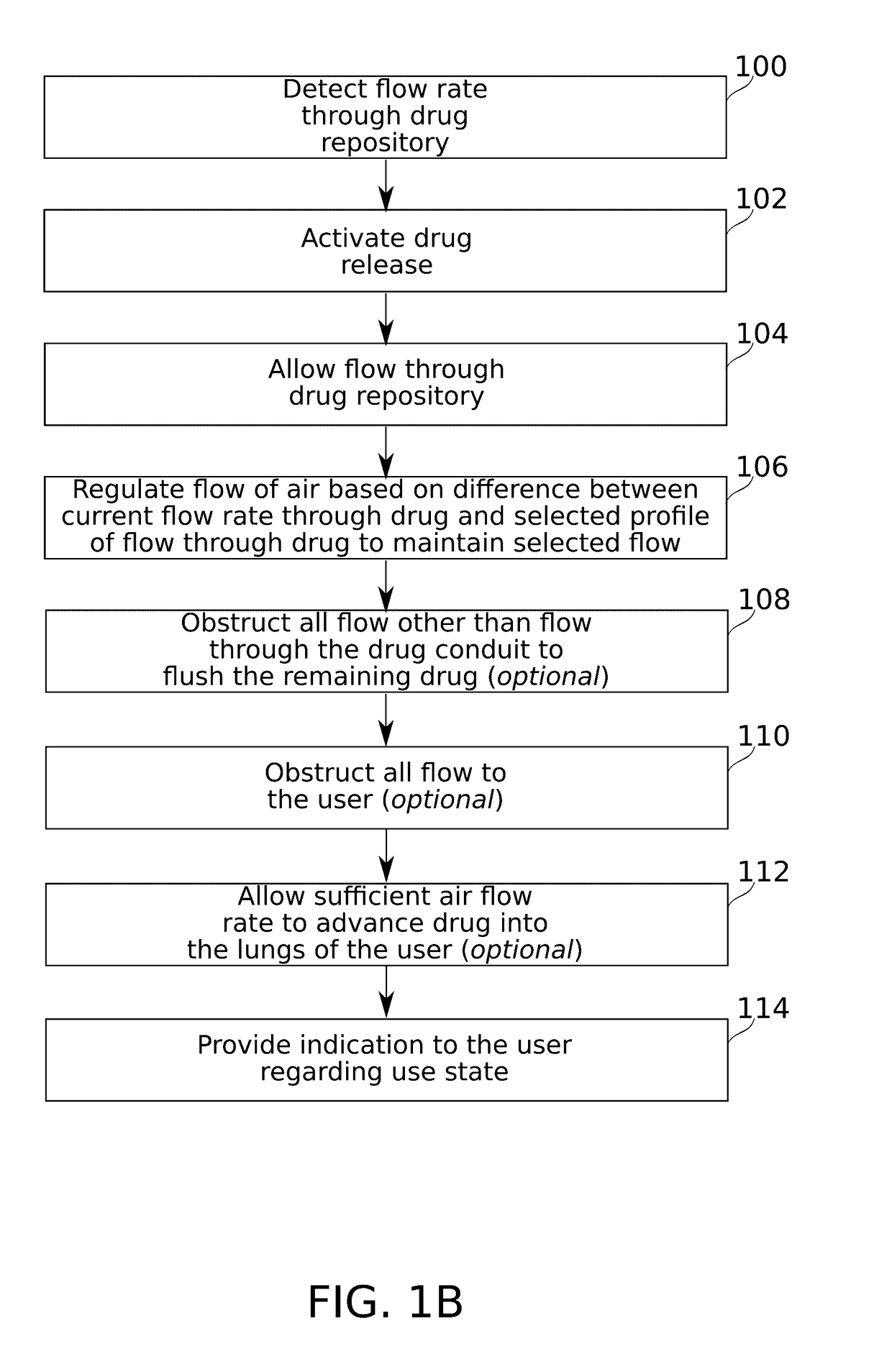
Flow regulating inhaler device
ActiveUS20170106153A1Reduce probabilityAntibacterial agentsOrganic active ingredientsInsertion stentDrug doses
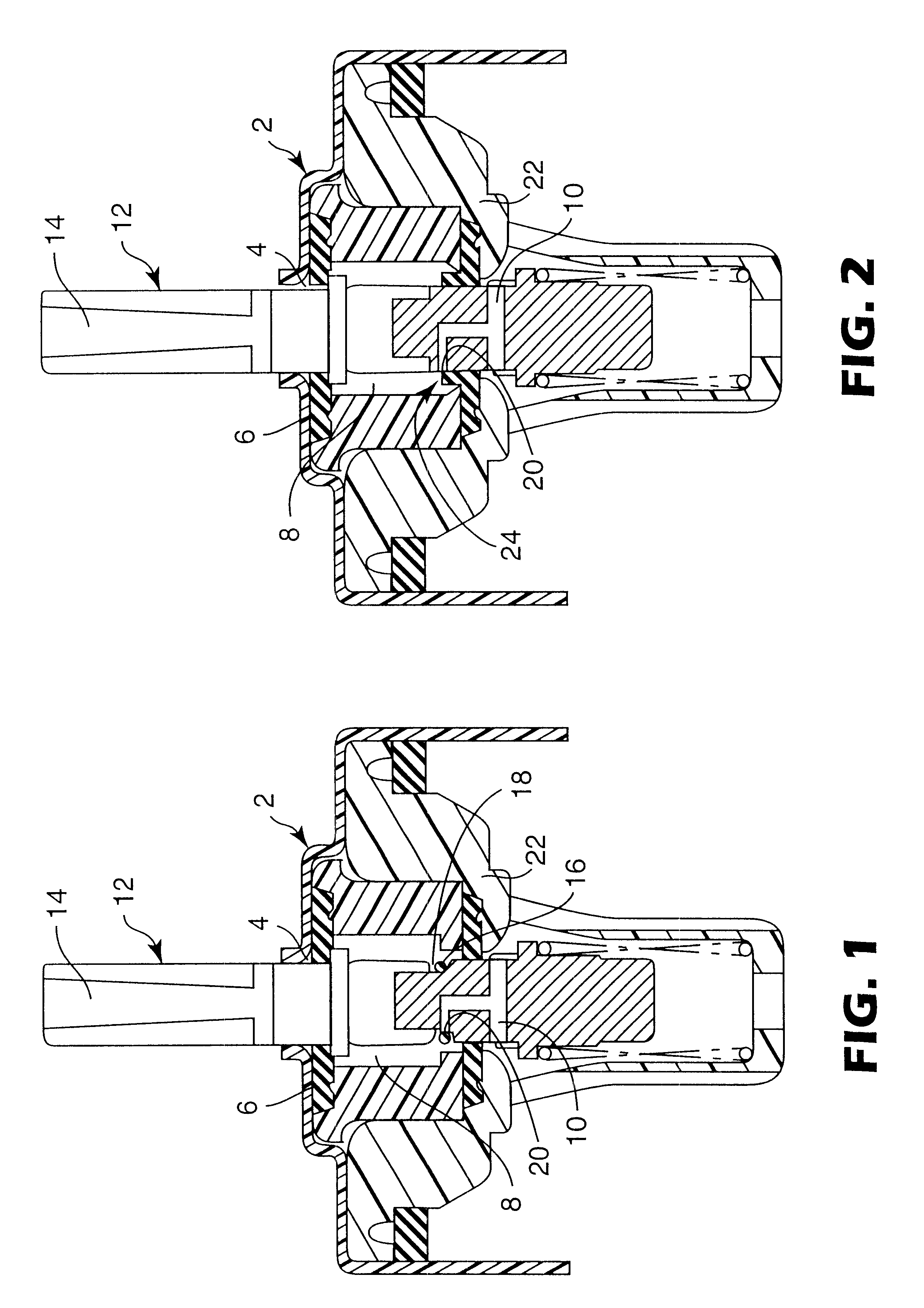
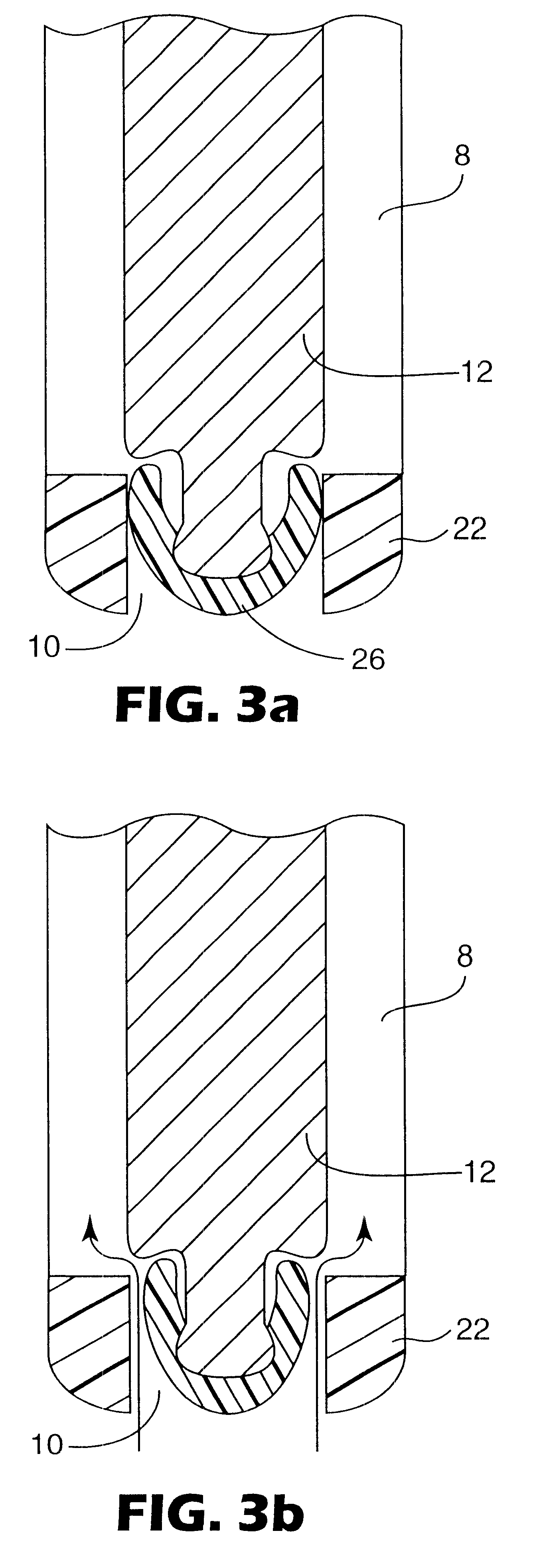
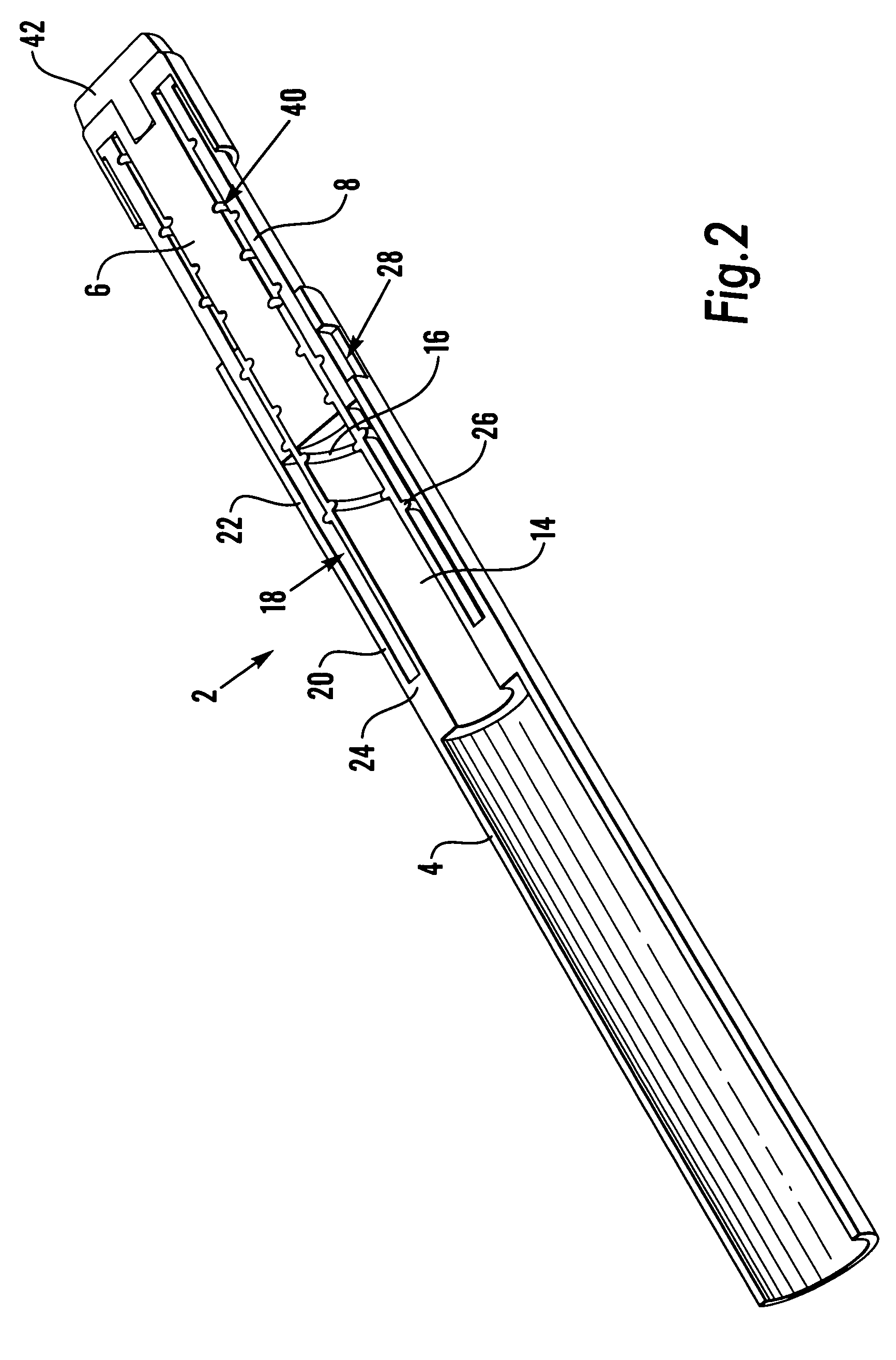
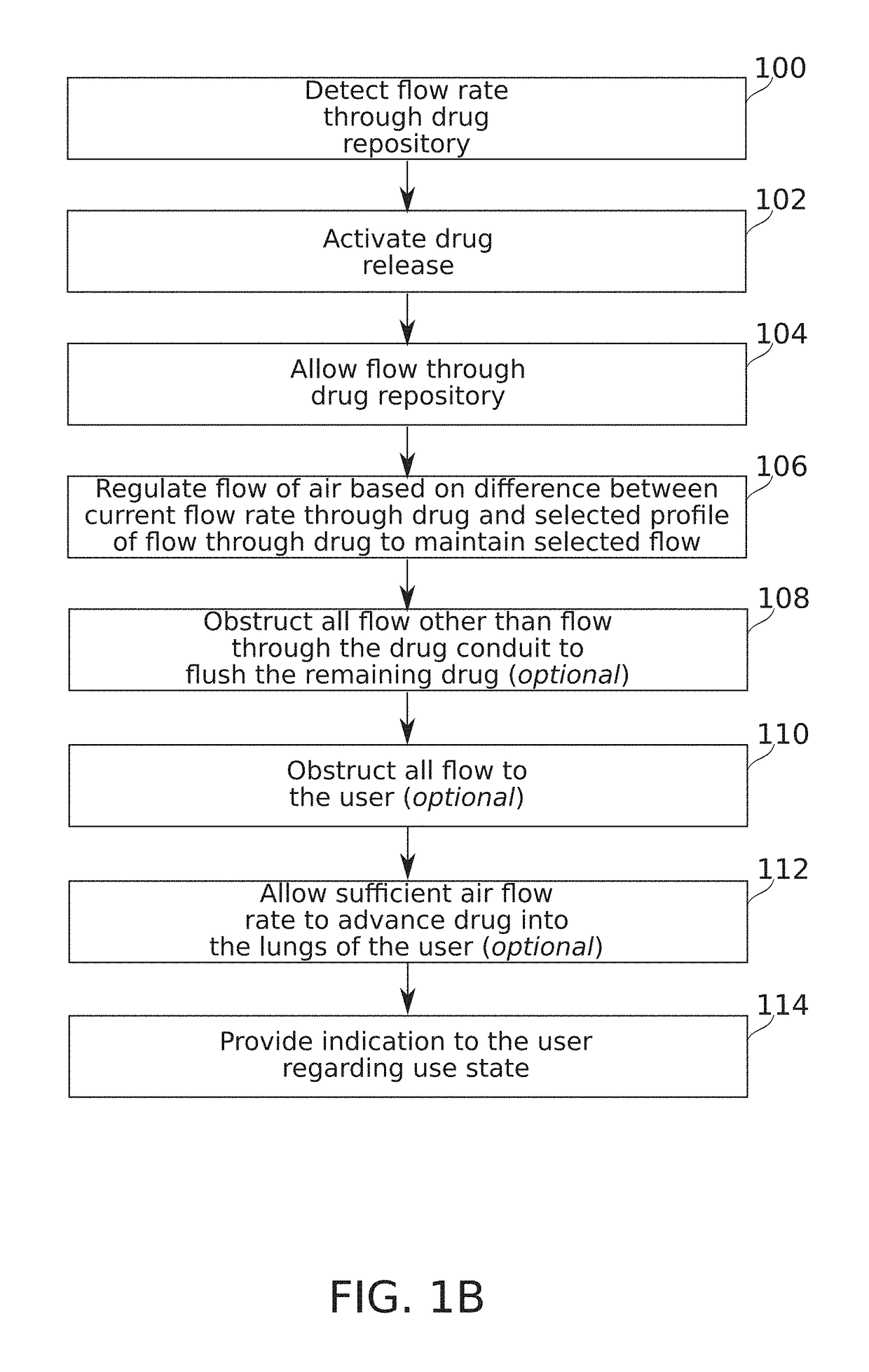
Some embodiments of the invention relate to an inhaler device for pulmonary delivery of at least one substance from a drug dose cartridge to an inhaling user, comprising: a first conduit for conducting a carrier airflow to a proximal opening of a mouthpiece for use by the user; a holder configured to position the dose cartridge within the carrier airflow; and a second conduit for conducting a shunting airflow to the mouthpiece without passing through the dose cartridge position. In some embodiments, a controller connected to a valve controls a rate of carrier airflow, for example by controlling the shunting airflow, based on a sensor indication of airflow rate and a target airflow profile.
Owner:SYQE MEDICAL
Metered dose dispensing aerosol valve
InactiveUS6454140B1Loss of primeDose is reduced and eliminatedLiquid dispensingDrug dosesMetered dose aerosol
Owner:KINDEVA DRUG DELIVERY LP
Medicament delivery device
The present invention relates to a medicament delivery device such as an injector for self-administration of a medicament. Those using such devices are often infirm or have impaired vision. There is accordingly a dead for a medicament delivery device in which an amount of a dosage of medicament to be delivered can be selected relatively quickly by the firm and infirm alike and in which the amount of the dosage may readily be controlled and determined. Also there is a need to display the amount of the dialed dosage of medicament on the rotatable knob in figures sufficiently large and clear to be legible by those having a degree of impaired vision. There is disclosed a dose dial mechanism for a medicament delivery device having a housing, the dose dial mechanism comprising a dose dial element having a first thread for engagement with the housing and a dial sleeve member having a second thread for engagement with the housing; in which the dial sleeve member is coupled to the dose dial element so as to allow relative axial movement and inhibit relative rotational movement therebetween, and in which the pitch of the second thread is different from the pitch of the first thread so as to cause a different axial displacement of the dial sleeve member during axial displacement of the dose dial element.
Owner:SANOFI AVENTIS DEUT GMBH
Flow regulating inhaler device
Some embodiments of the invention relate to an inhaler device for pulmonary delivery of at least one substance from a drug dose cartridge to an inhaling user, comprising: a first conduit for conducting a carrier airflow to a proximal opening of a mouthpiece for use by the user; a holder configured to position the dose cartridge within the carrier airflow; and a second conduit for conducting a shunting airflow to the mouthpiece without passing through the dose cartridge position. In some embodiments, a controller connected to a valve controls a rate of carrier airflow, for example by controlling the shunting airflow, based on a sensor indication of airflow rate and a target airflow profile.
Owner:SYQE MEDICAL LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com