Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Dose-Limiting" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
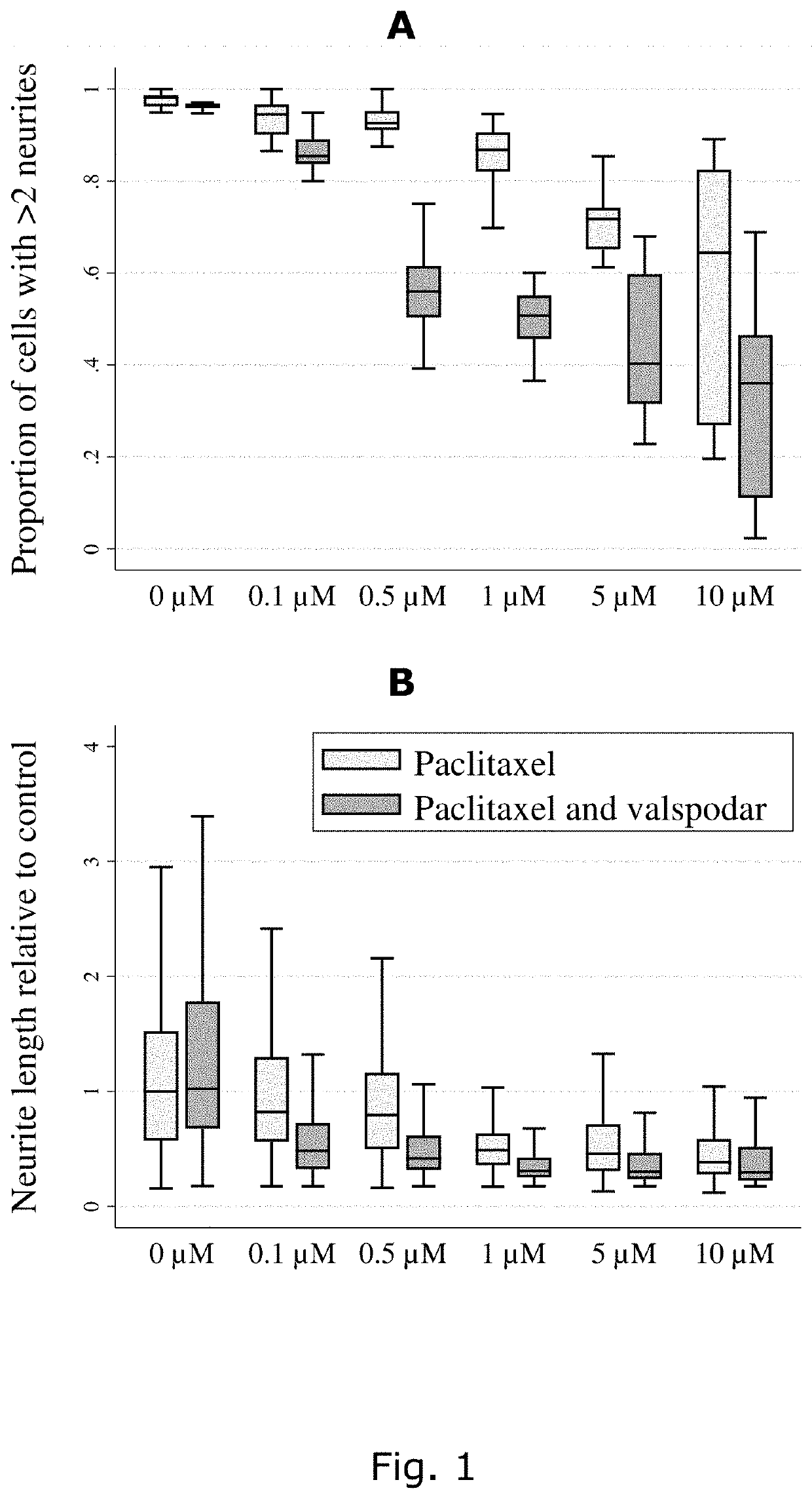
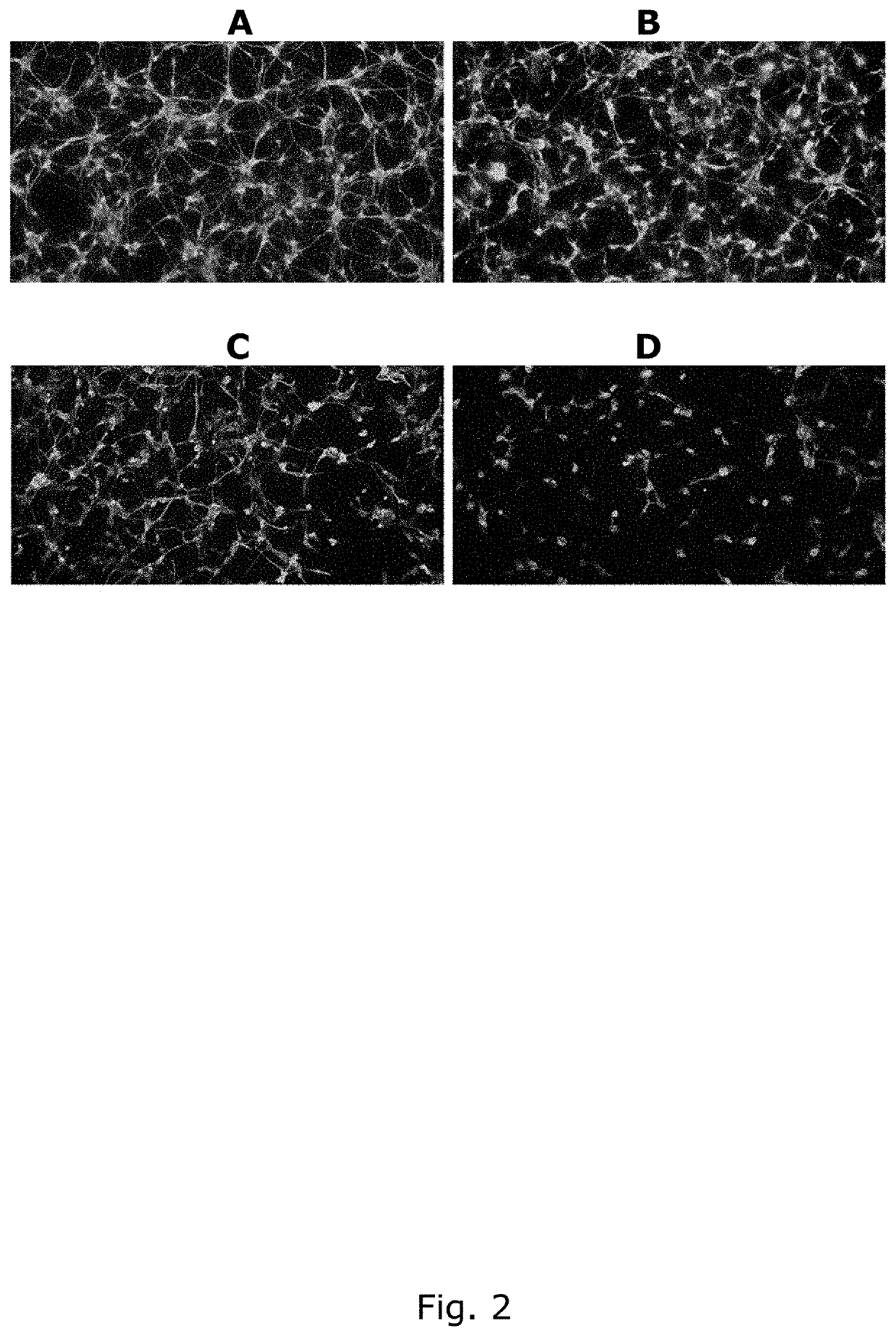
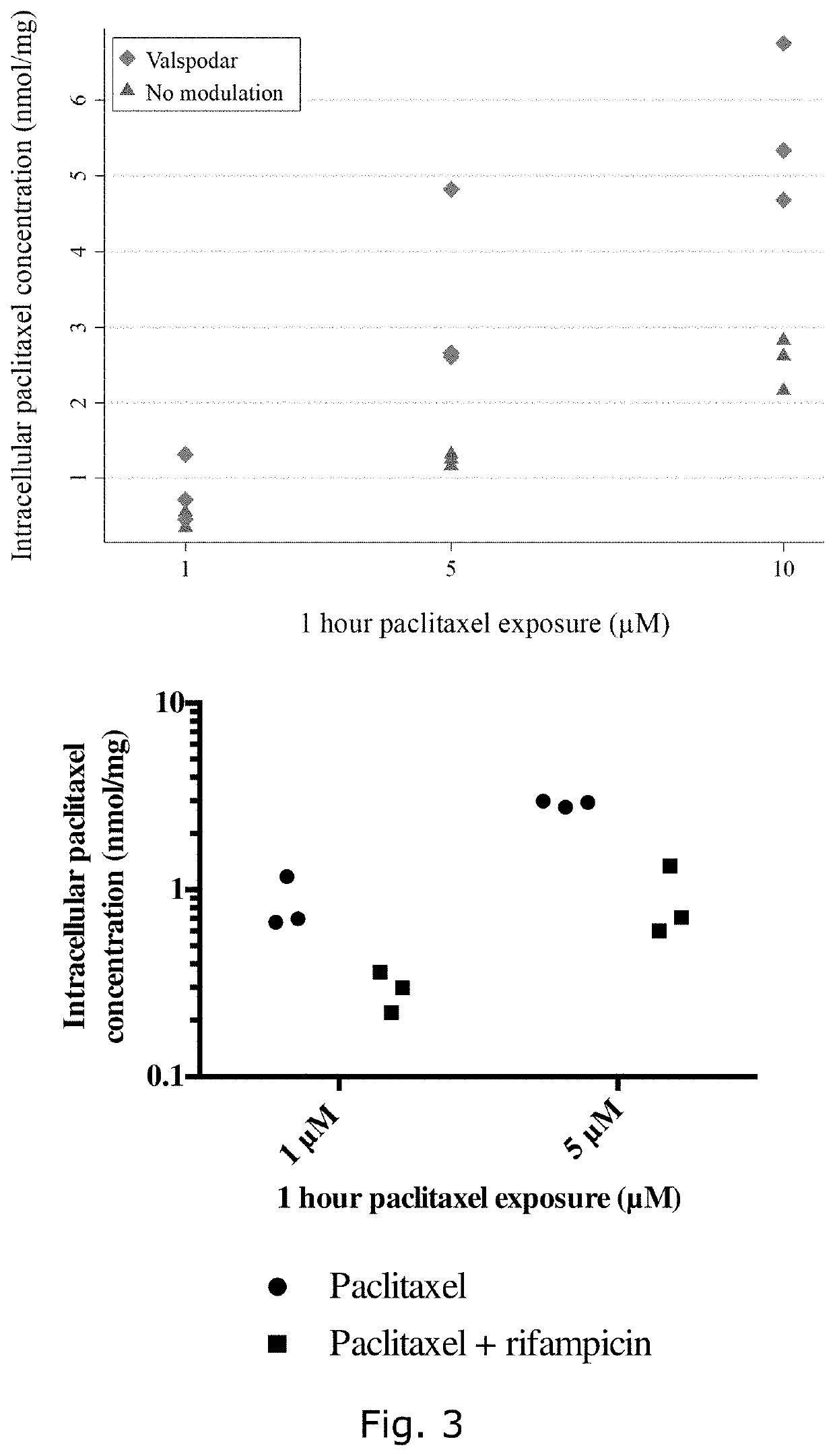
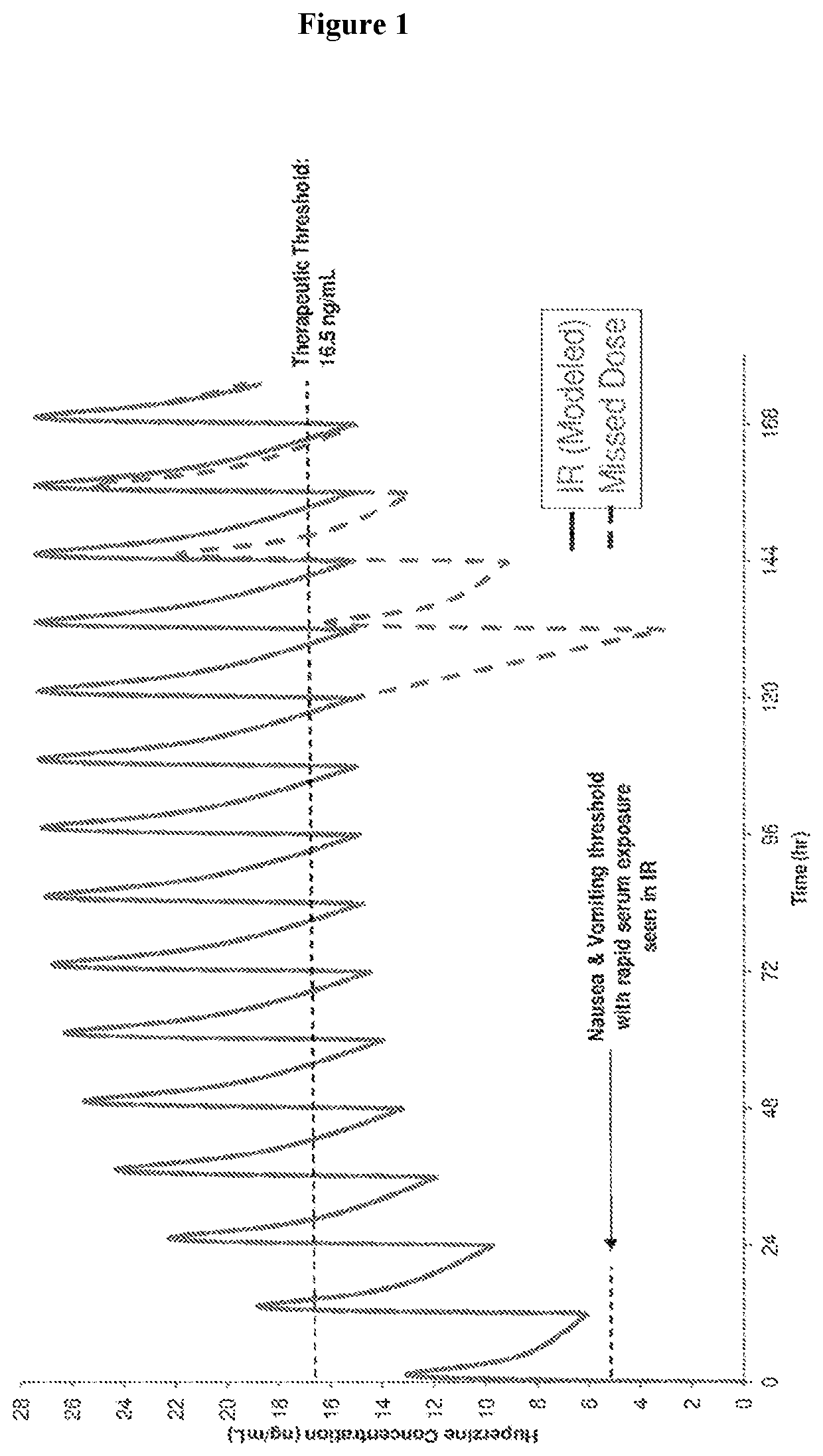
Describes side effects of a drug or other treatment that are serious enough to prevent an increase in dose or level of that treatment.
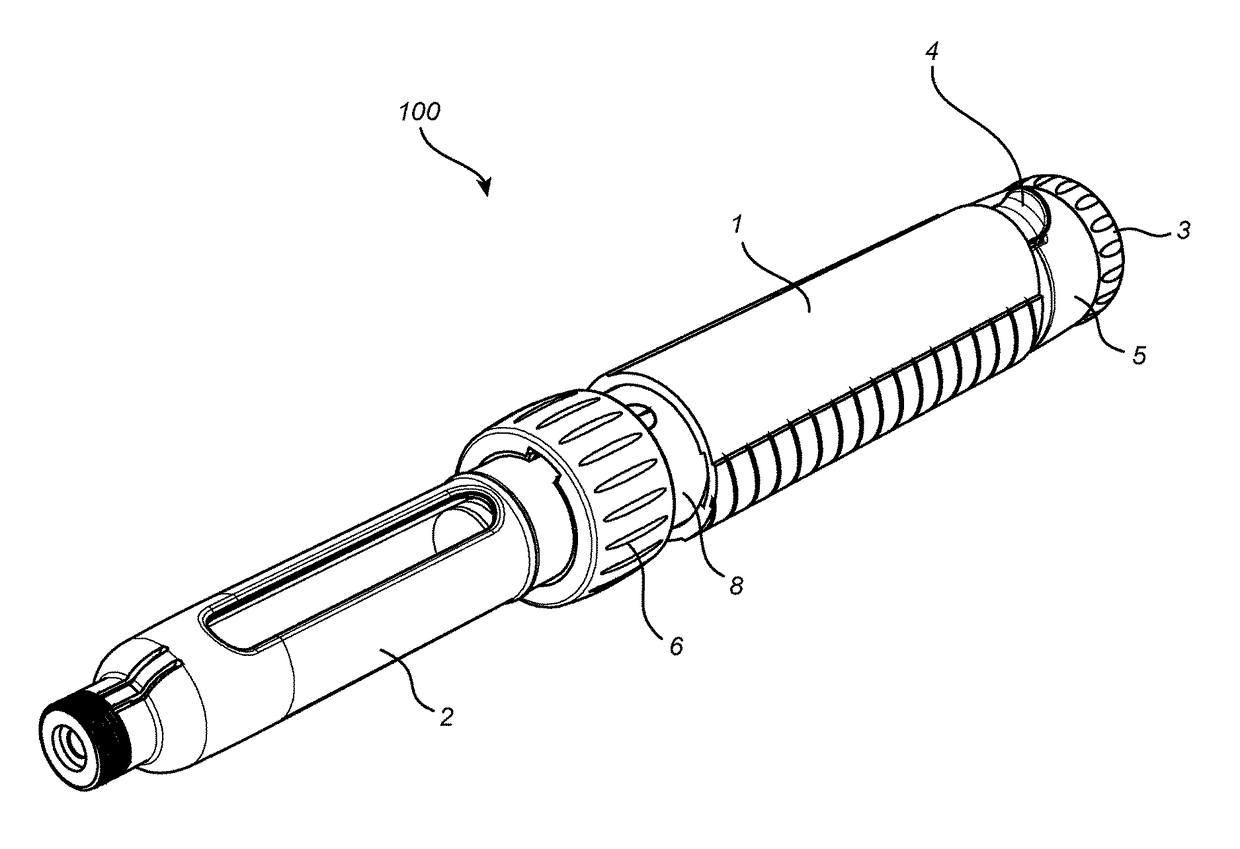
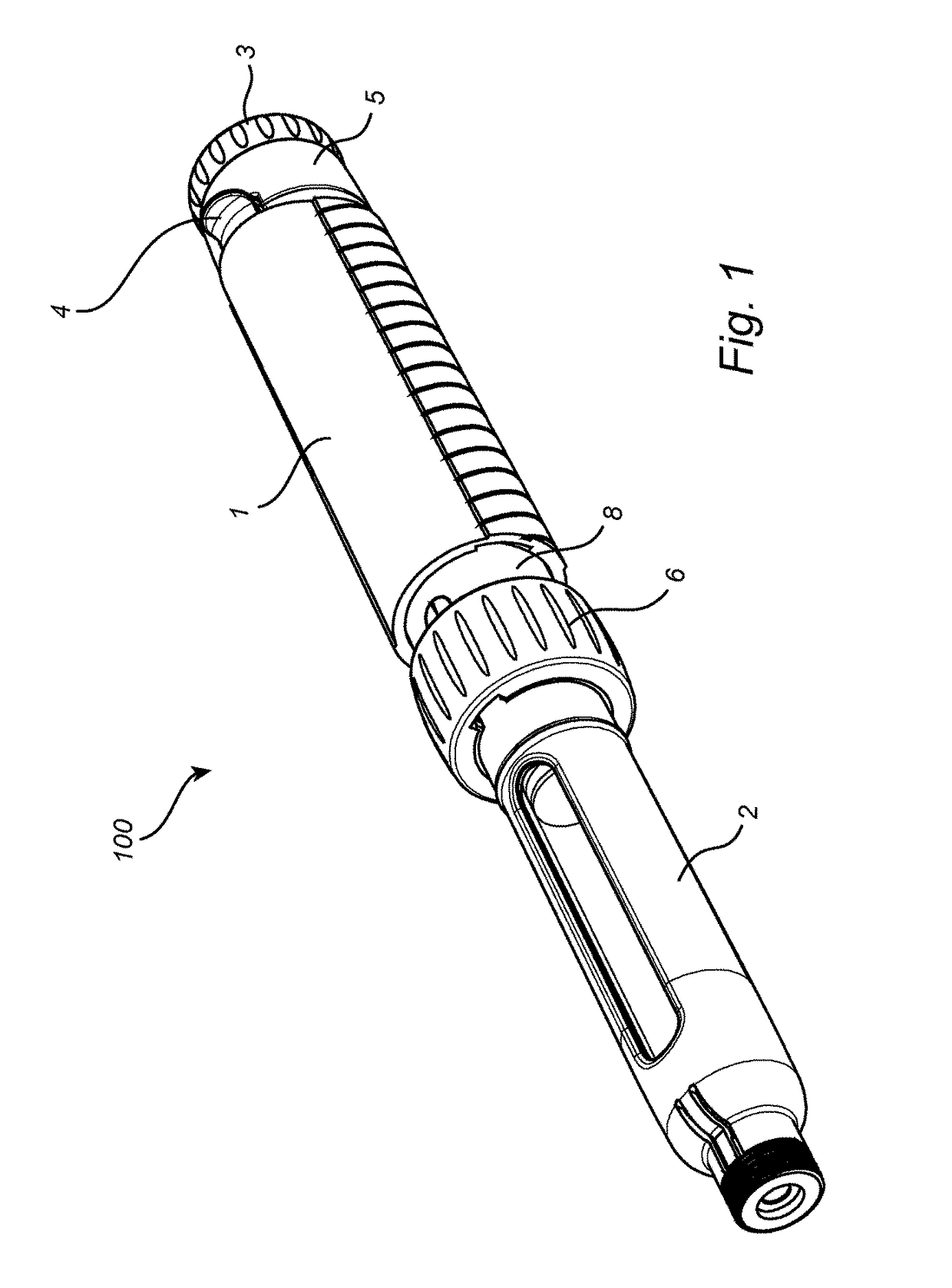
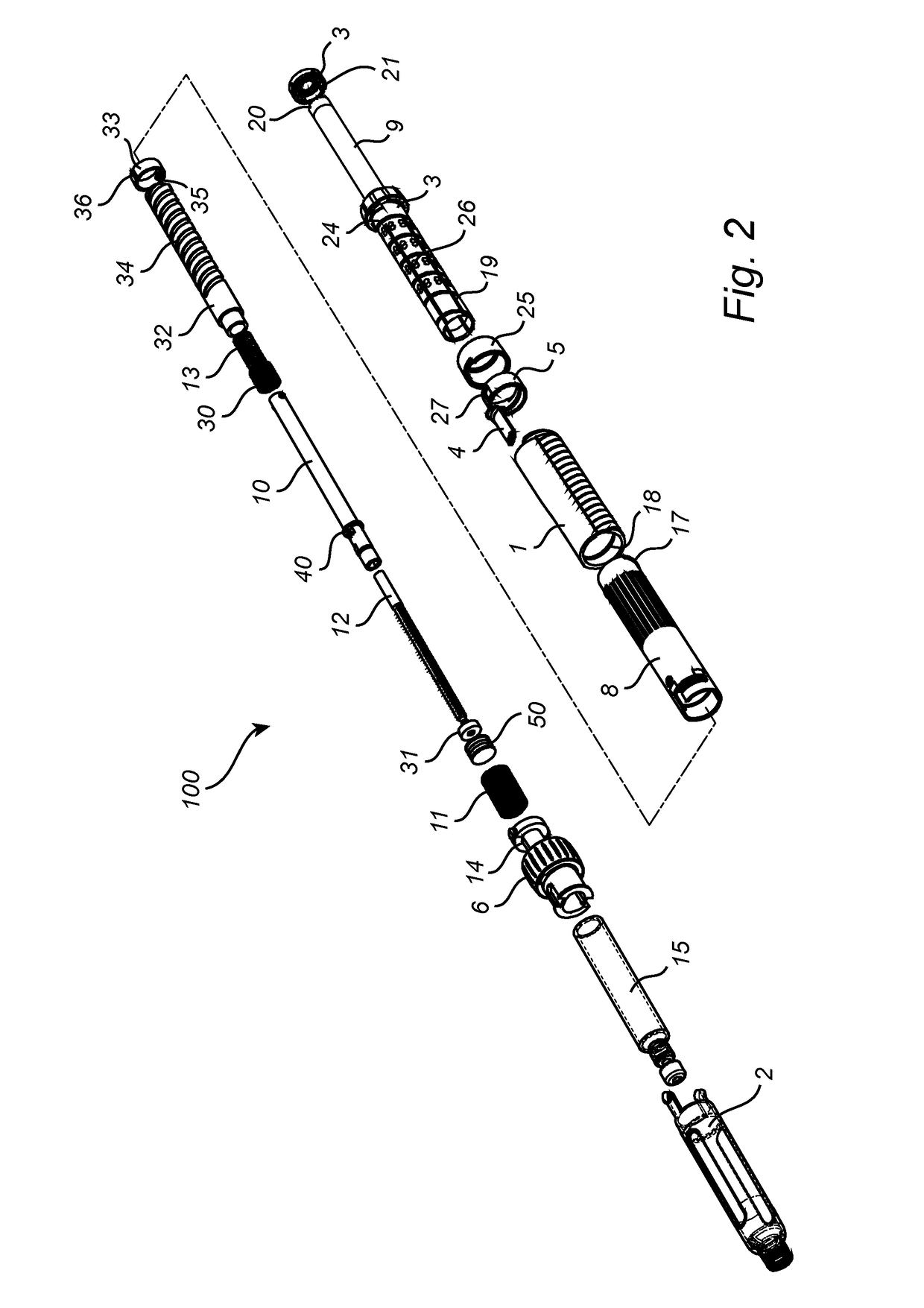
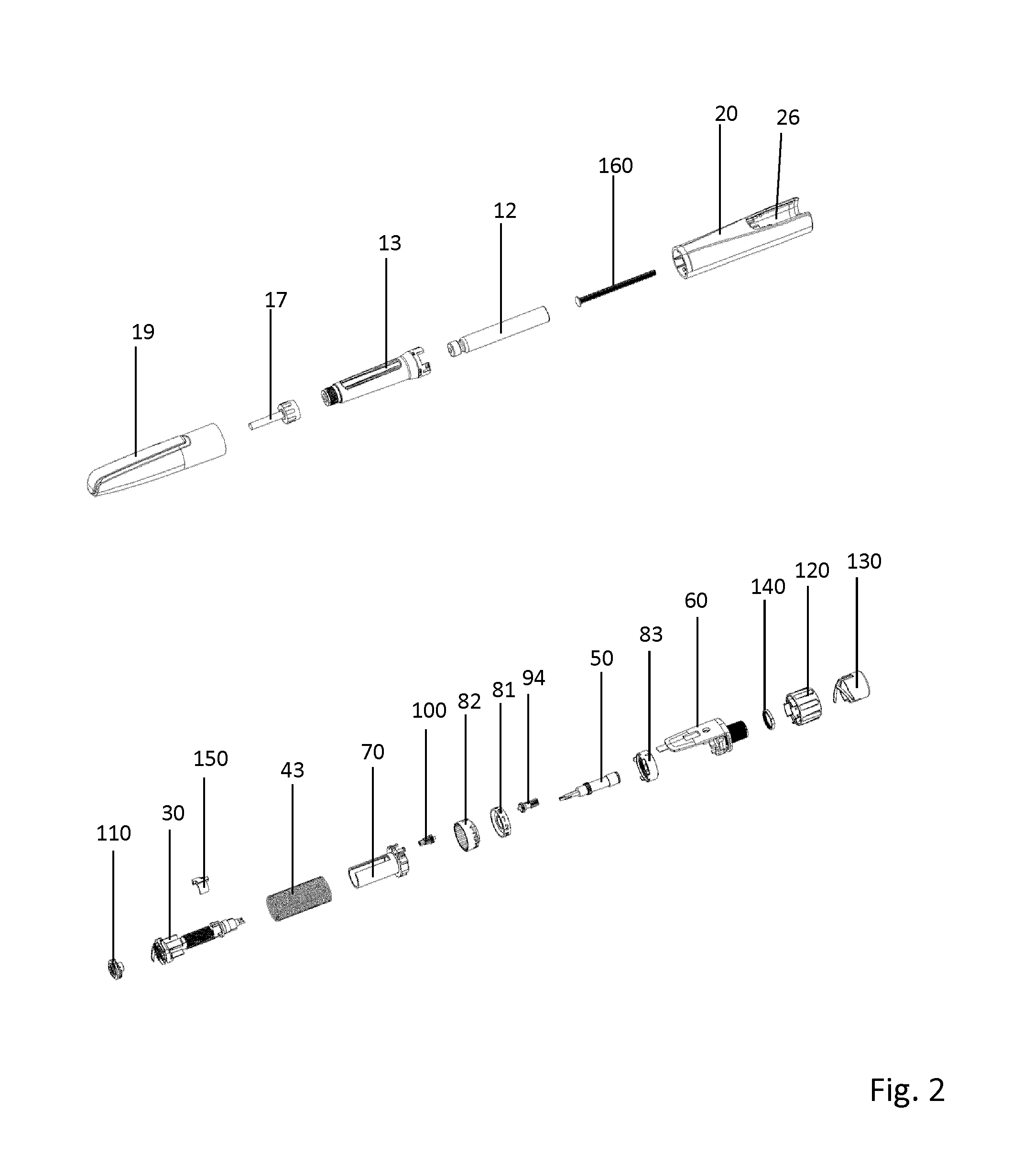
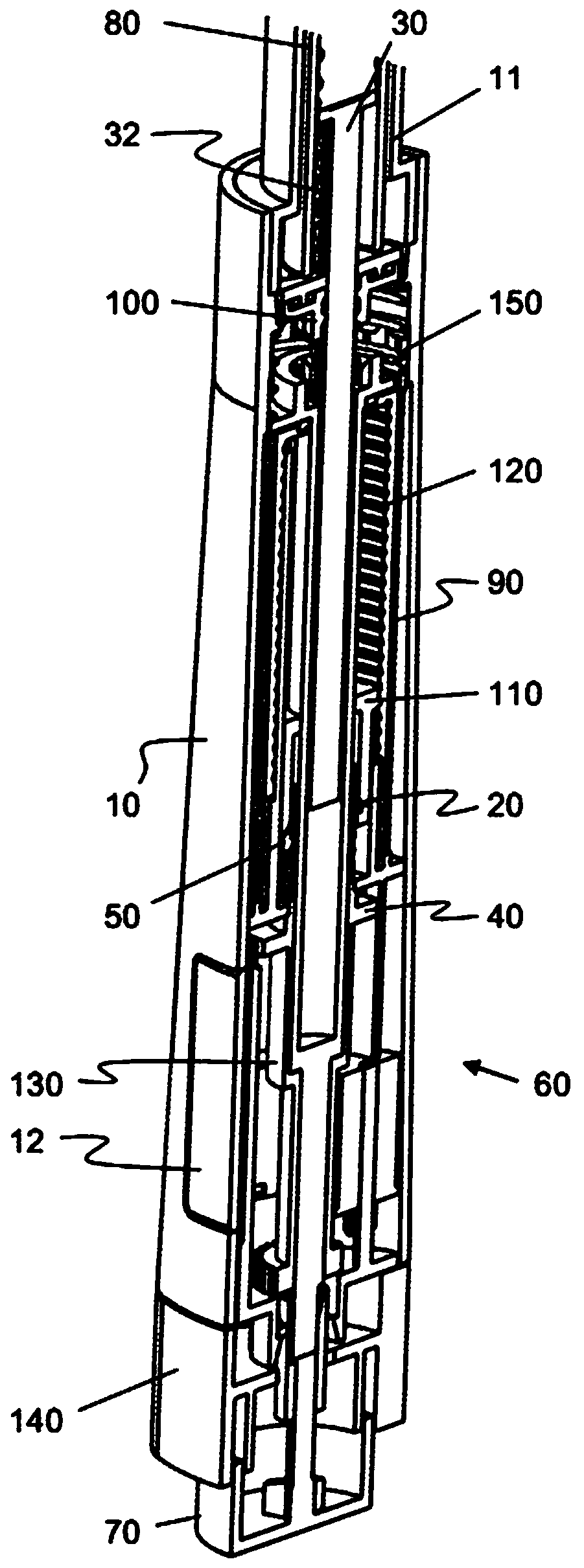
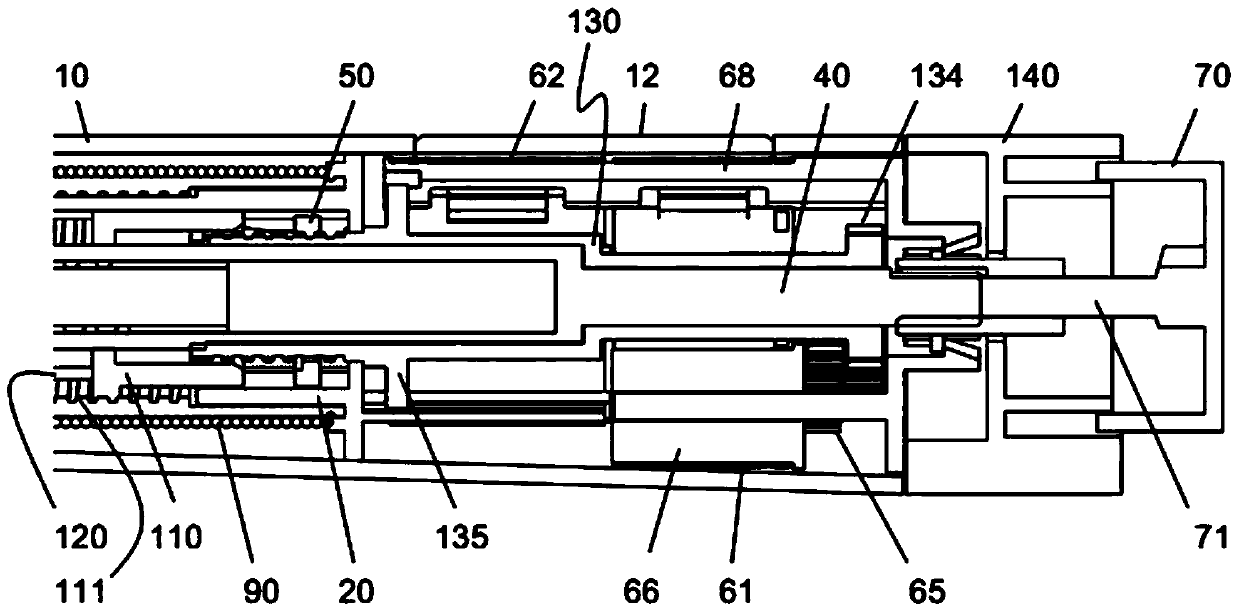
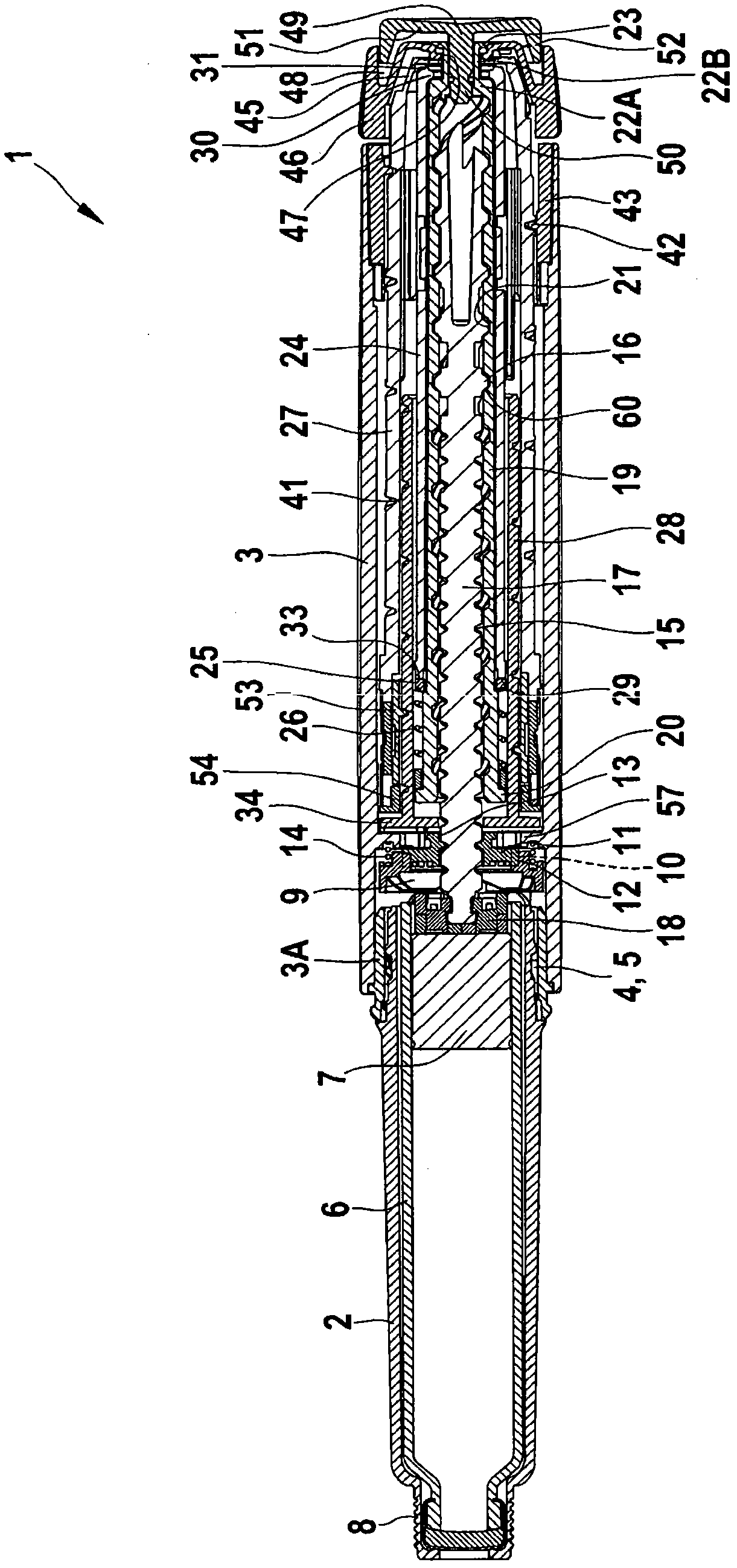
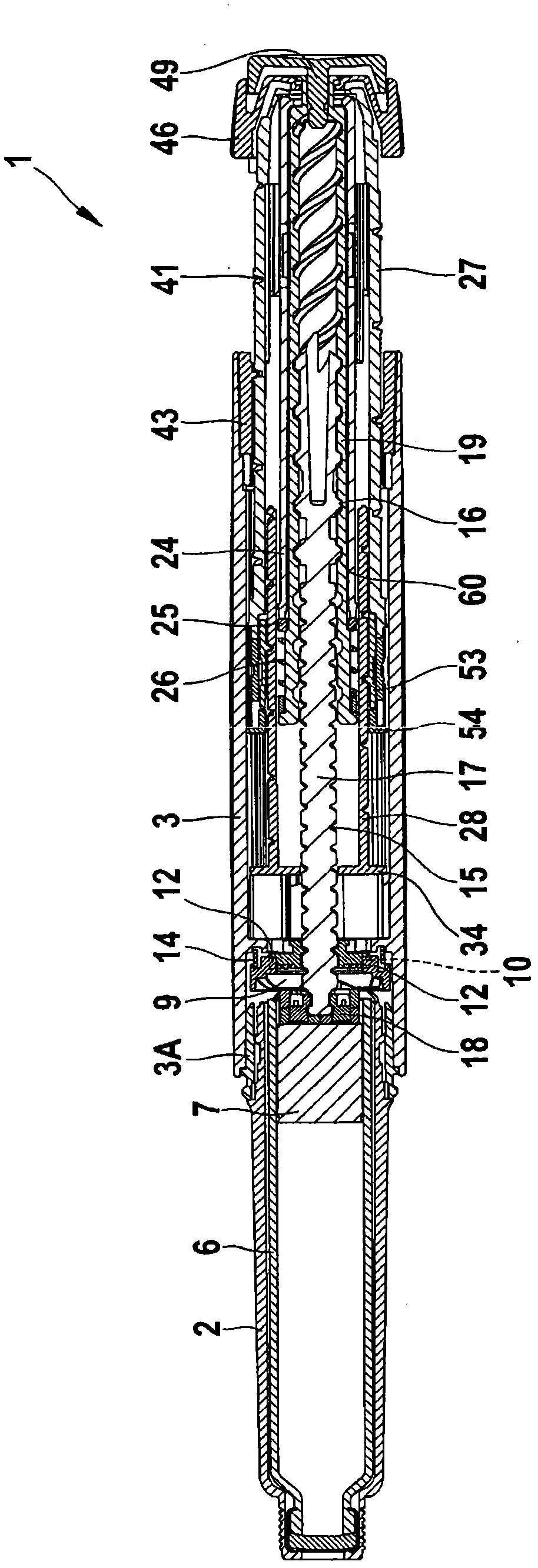
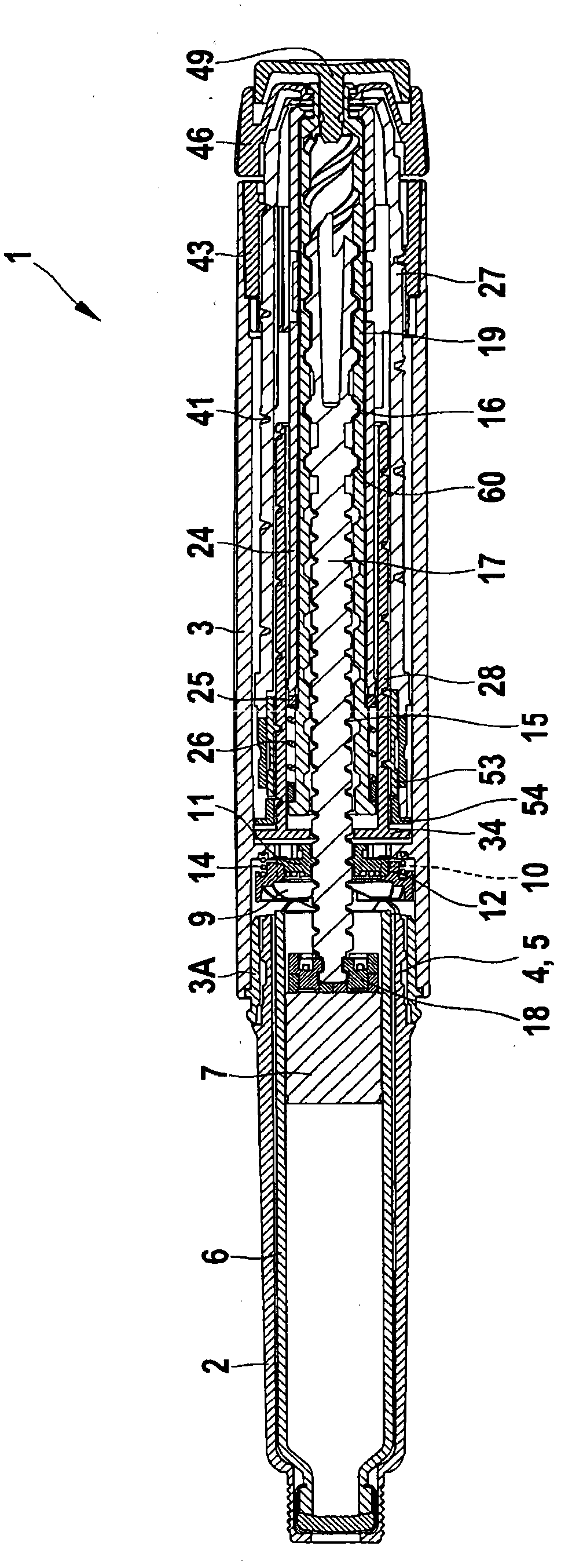
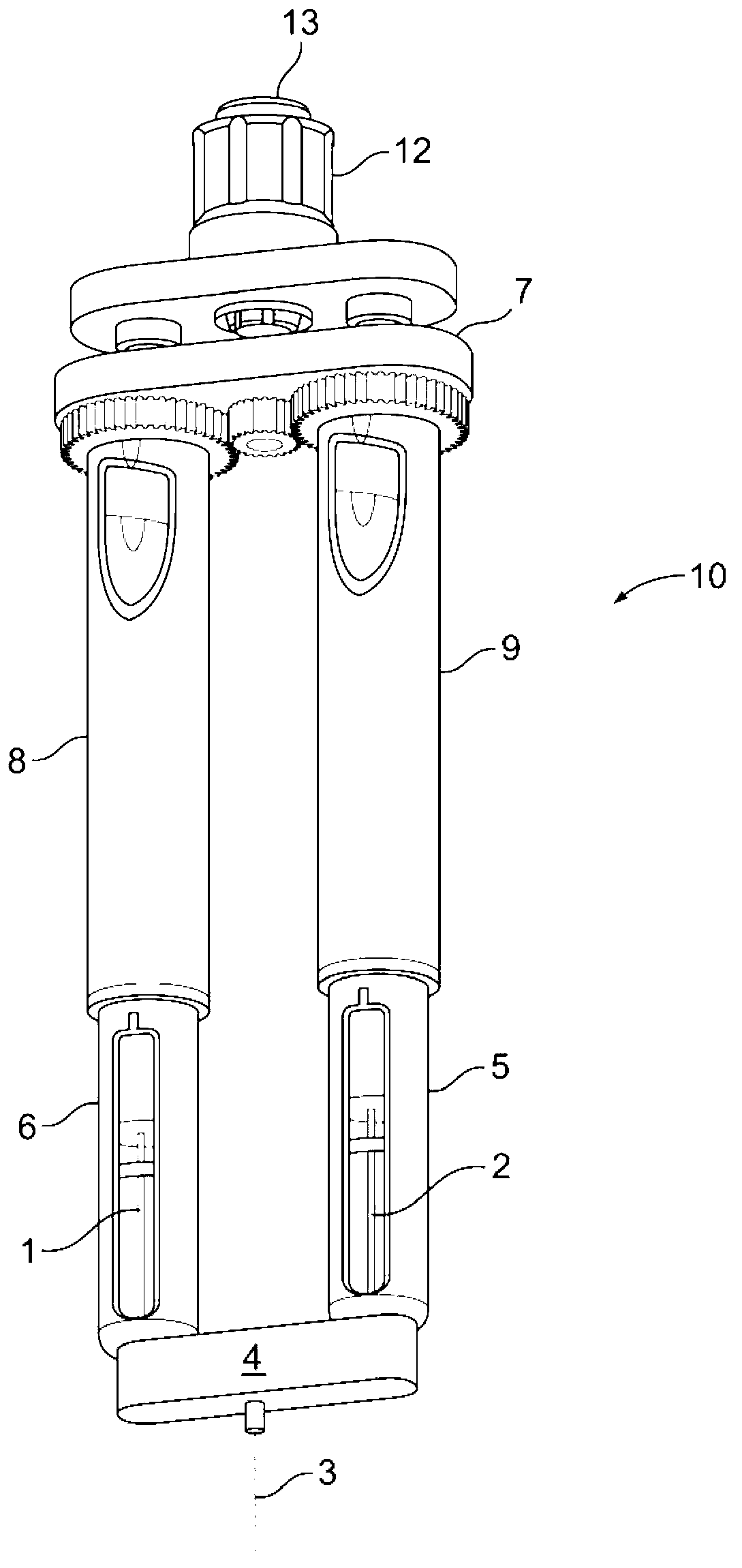
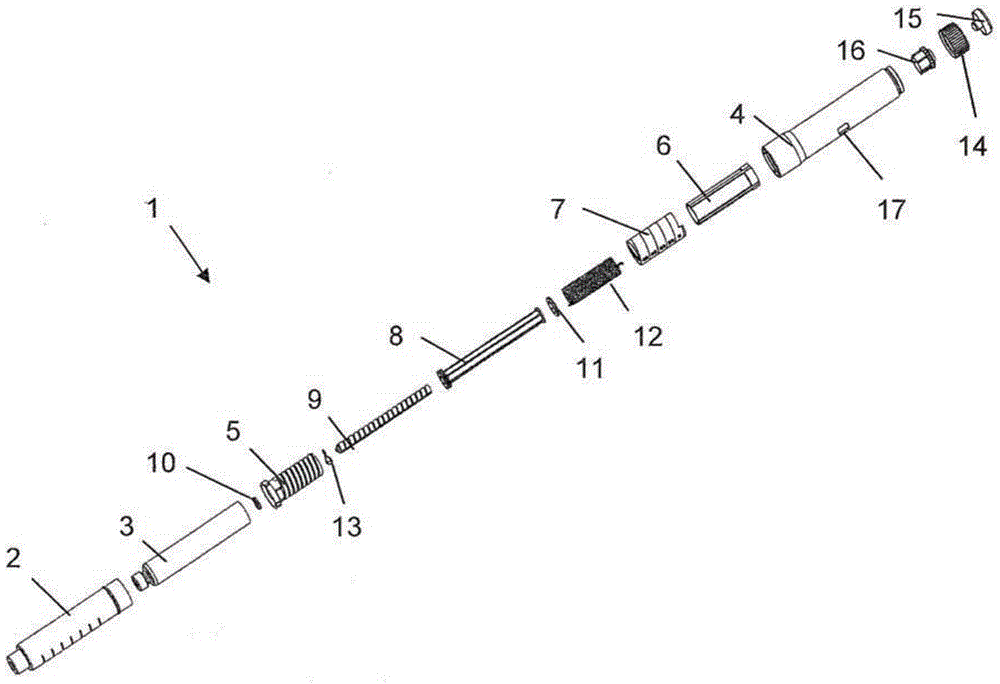
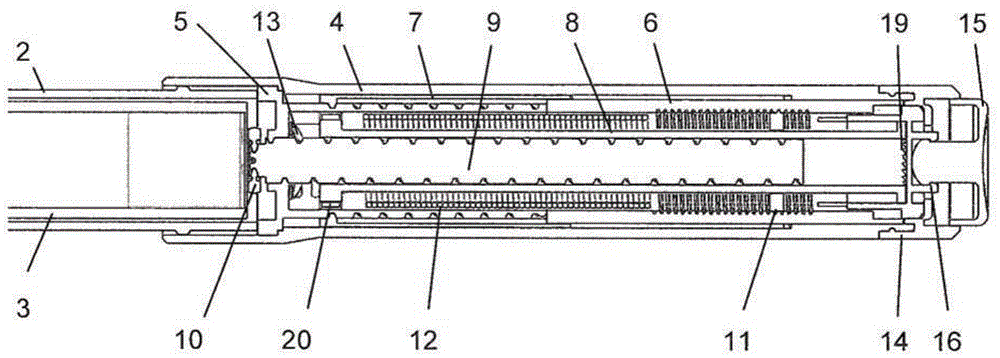
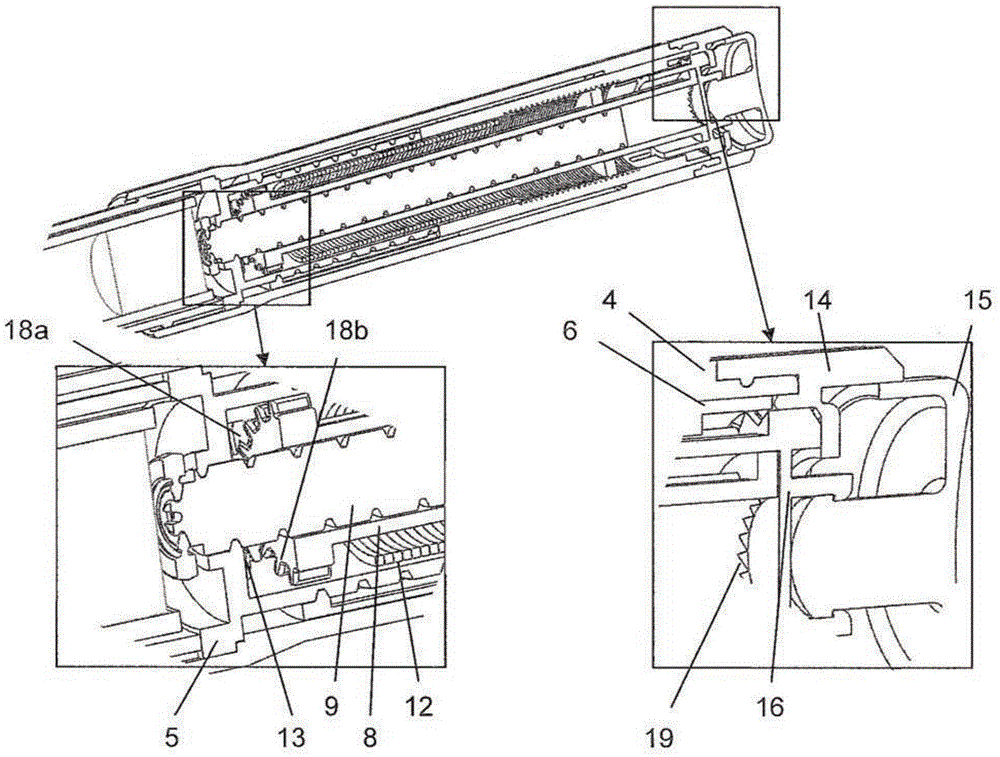
Medication delivery device
The present invention refers to a medication delivery device (1) comprising: a medication receptacle (6); a dosing mechanism comprising: a piston rod (17); a drive device (19); a dose setting member (27); and a dose limiting member (28) which prevents the setting of a dose of medication which exceeds an amount of medication contained in the medication receptacle (6); and a housing (3). The dose limiting member (28) is designed for axial movement in a proximal direction with respect to the piston rod (17) during dose setting and comprises a first stop element (35) and the piston rod (17) comprises a second stop element (36). The first and second stop elements (35, 35) stop an axial movement of the dose limiting member (28) in the proximal direction with respect to the piston rod (17) when the first and second stop elements (35, 36) catch, thereby limiting a movement of the dose setting member (27) for increasing a set dose of medication to be delivered. The dose limiting member (28) and the piston rod (17) only interact directly, when the first and second stop elements (35, 36) catch.
Owner:SANOFI AVENTIS DEUT GMBH
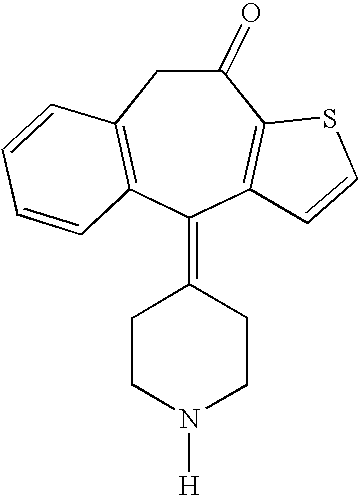
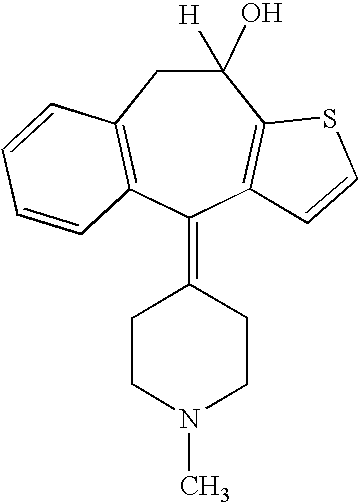
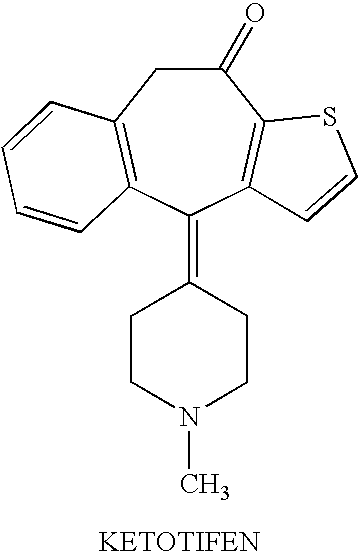
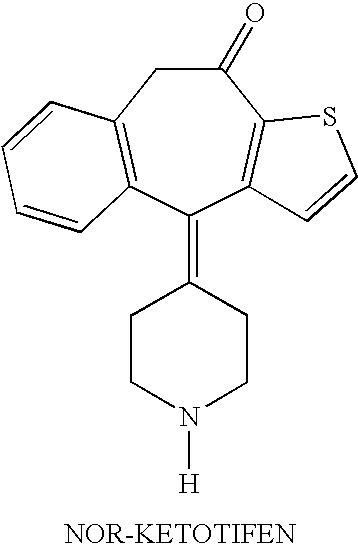
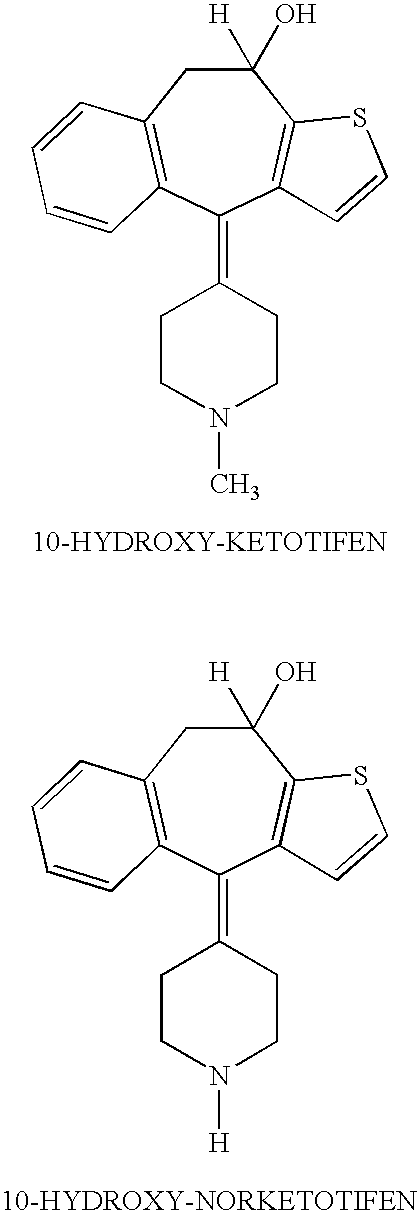
Optically active isomers of ketotifen and therapeutically active metabolites thereof
Racemic norketotifen, racemic 10-hydroxy-ketotifen, racemic 10-hydroxy-nor-ketotifen and optically active isomers of ketotifen, norketotifen, 10-hydroxy-ketotifen and 10-hydroxy-norketotifen were found to have antiallergic and anti-inflammatory effects while being devoid of the severe dose-limiting sedative side effects of ketotifen.
Owner:BRIDGE PHARMA INC
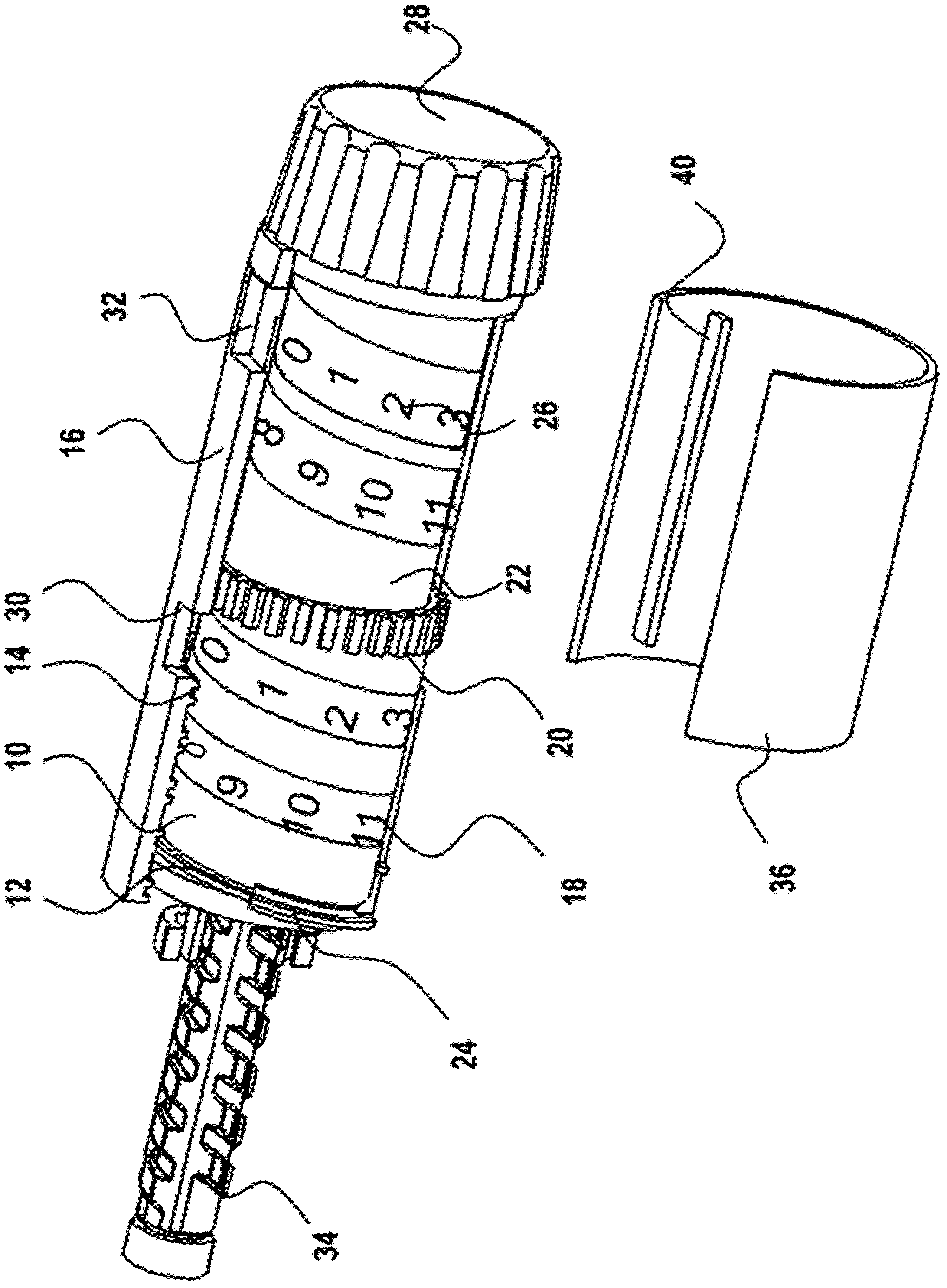
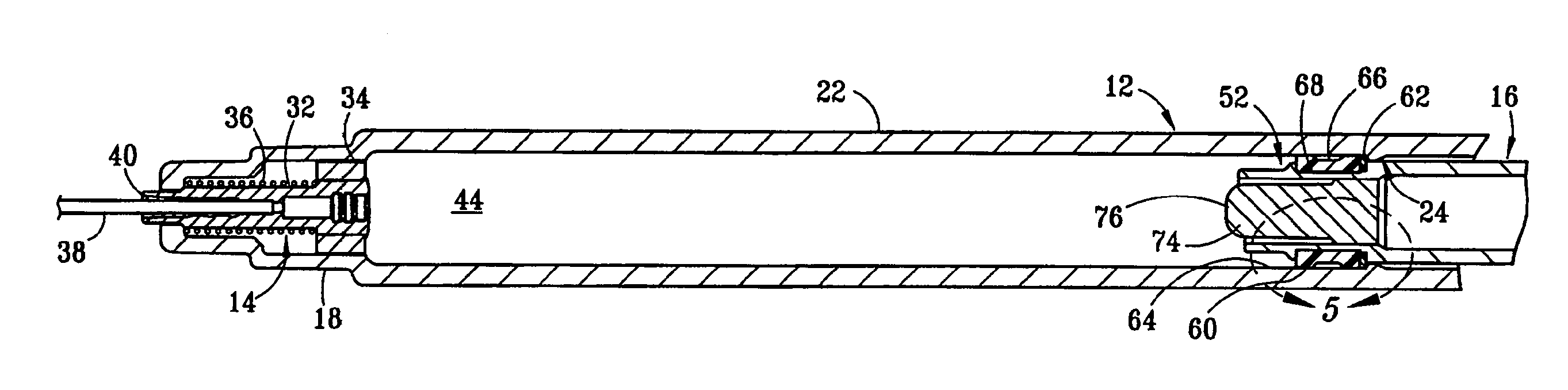
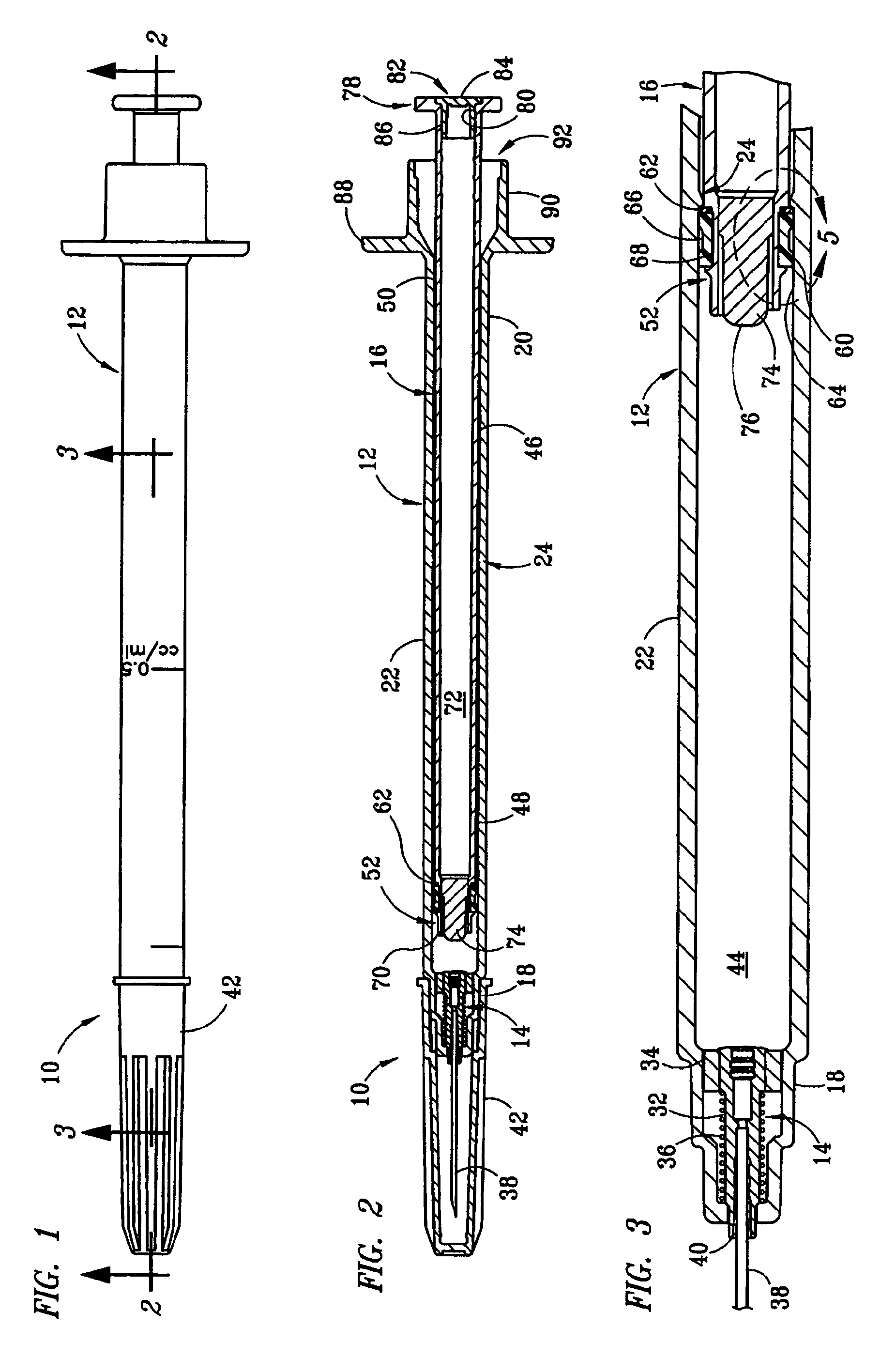
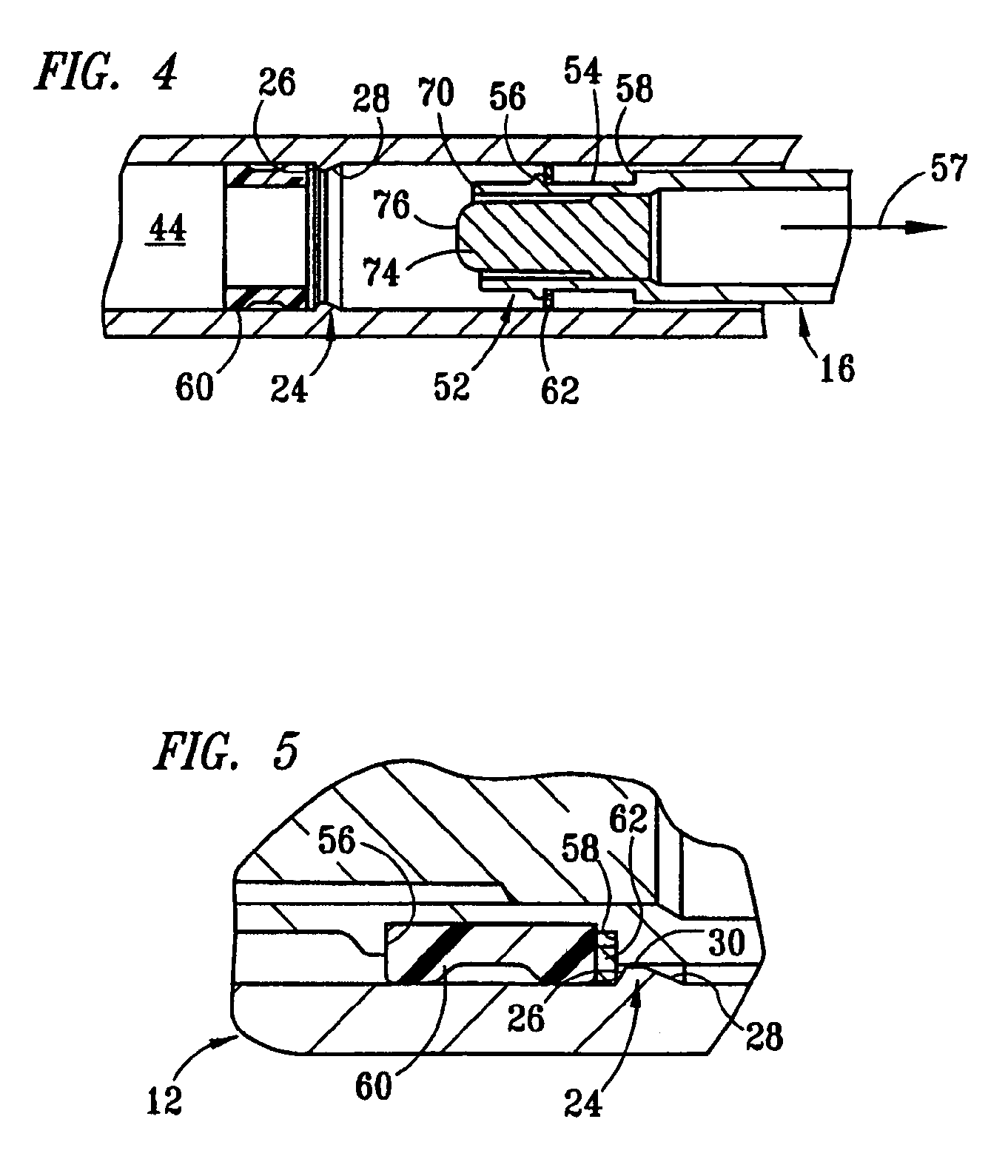
Dose setting mechanism
ActiveCN102413854AMovement effectively stopsMoving stopInfusion syringesIntravenous devicesDose limitBiomedical engineering
The present invention relates to a dose setting mechanism intended to be used in a medicament delivery device, comprising an elongated tubular distal housing (16) having opposite distal and proximal ends, wherein said distal housing comprises threads (14) on its inner surface, a first window (30), a second window (32), and at least one opening (38) on its elongated surface; a tubular dose limiting member (10) having opposite distal and proximal ends, wherein said dose limiting member comprises a first thread segment (12) on its outer circumference surface at its proximal end intended to cooperate with corresponding threads on the inner surface of the distal housing, first indicia (18) on its outer circumferential surface, a stop surface (42) at its proximal end, and a number of protrusions (20) arranged equidistant to each other along the circumference on the outer surface at its distal end; a tubular dose setting member (22) having opposite distal and proximal ends, wherein said distal housing is coaxially arranged inside the dose limiting member and comprises a second thread segment (24) having and end surface (44) on its outer circumferential surface at its proximal end intended to cooperate with the threads of the distal housing, second indicia (26) on its outer circumferential, and a dose setting knob (28) protruding through the distal end of the distal housing; and a removable lock member (36) attached to the distal housing, wherein said lock member comprises at least one elongated rib (40) on its inner circumferential surface which is intended to interact with the protrusions of the dose limiting member for locking the dose limiting member in a certain position when the lock member is attached to the distal housing.
Owner:SHL MEDICAL AG
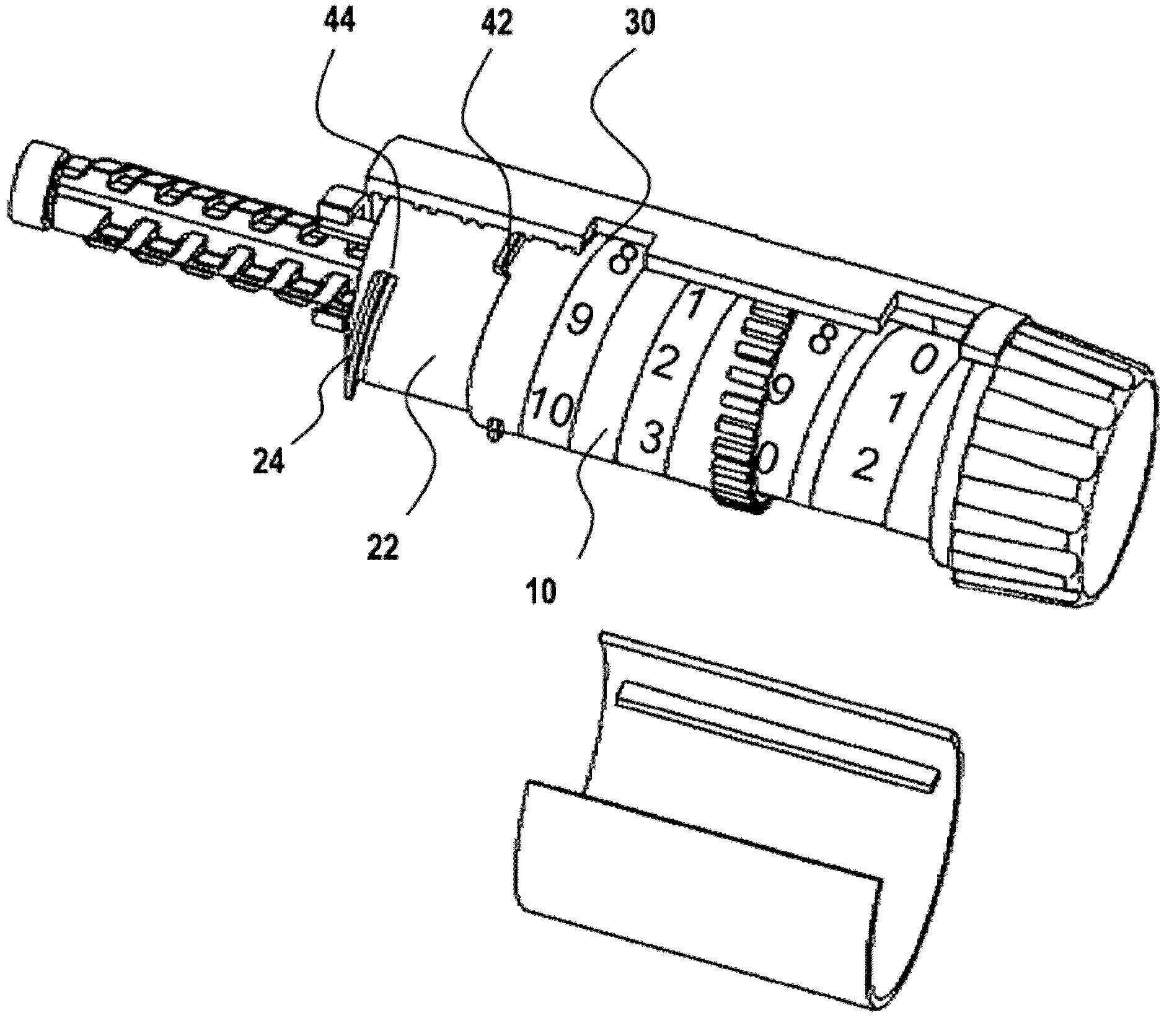
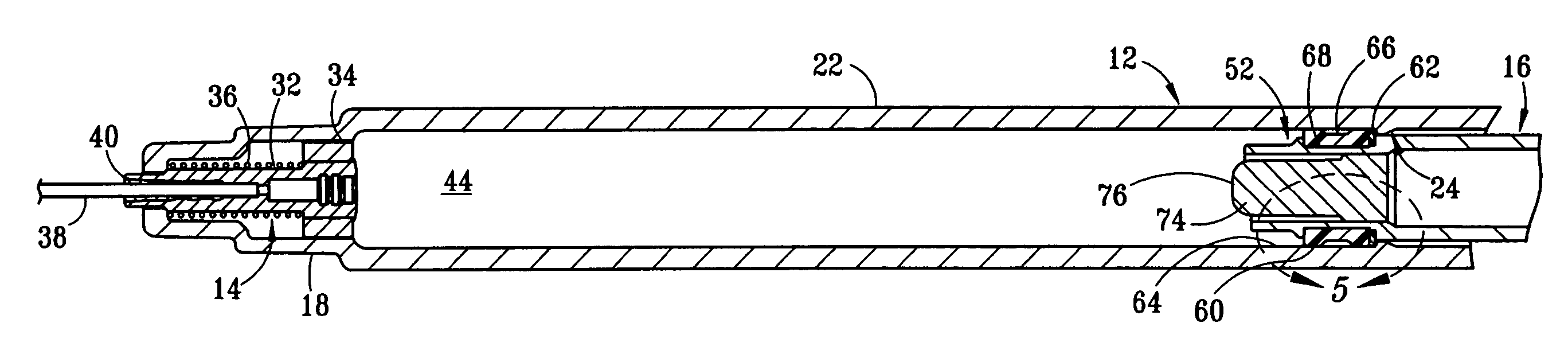
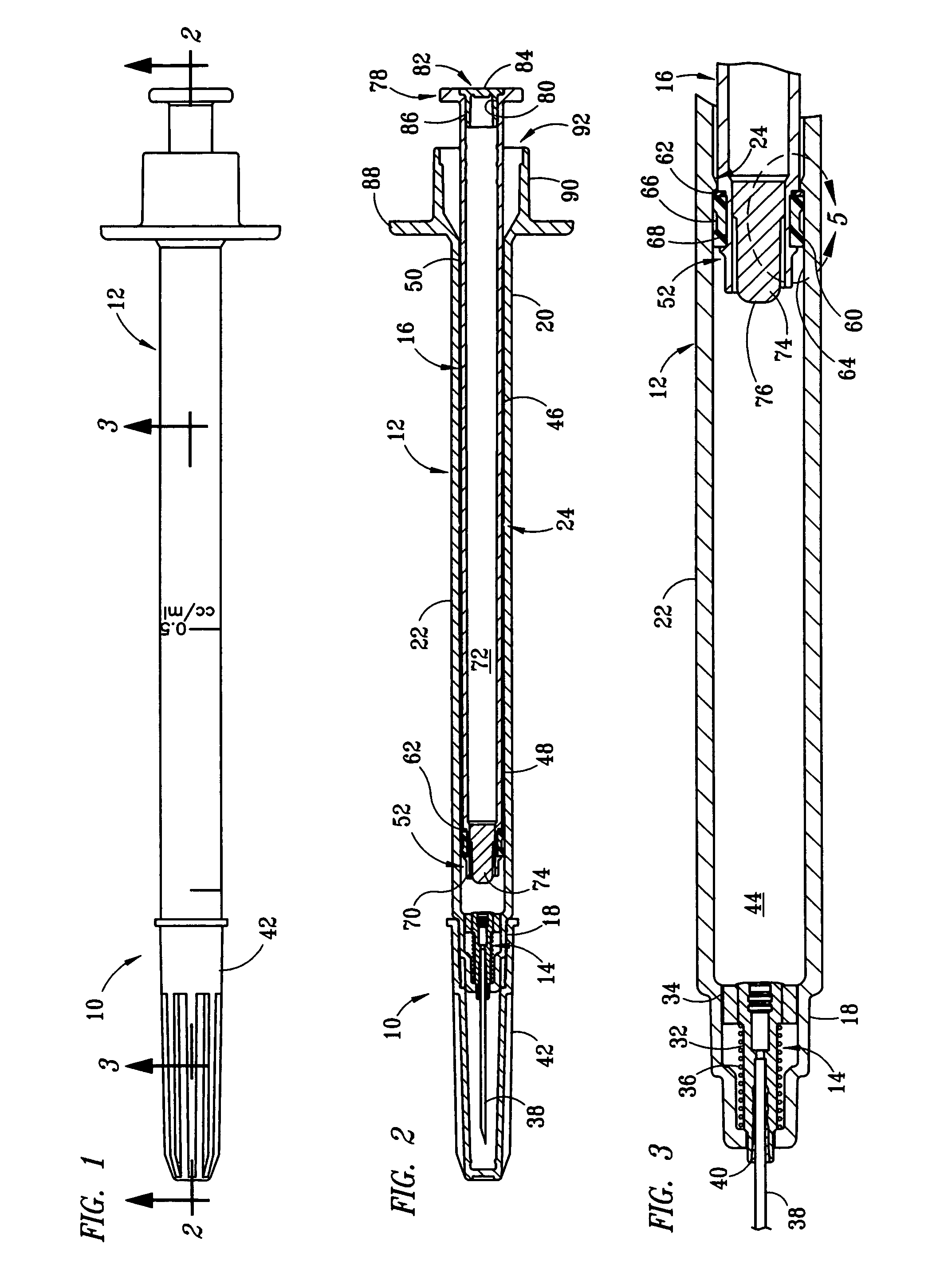
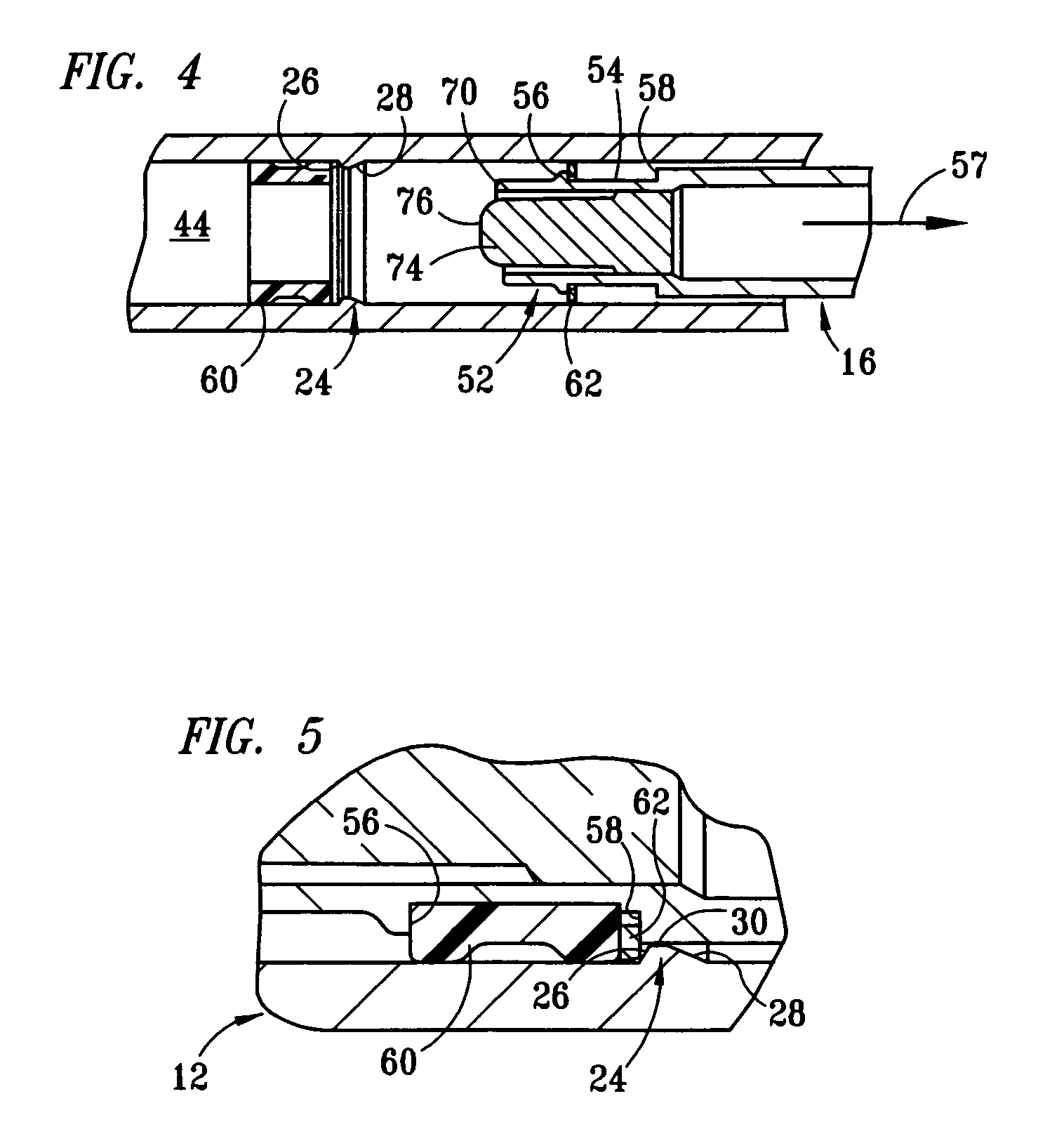
Fixed-dose syringe with limited aspiration
InactiveUS20060084919A1Prevent reuseReduce the possibilityMedical devicesIntravenous devicesEngineeringSyringe needle
A syringe configured with a limited maximum usable capacity. The syringe of the invention desirably has a retractable needle to prevent reuse. In the preferred embodiment, a dose-limiting structure includes a stop-ring member on the head of the plunger that abuts a constriction in the housing when the plunger is moved away from the needle to prevent the further rearward movement of the plunger. Preferably, the syringe of the invention is configured such that a user is tactilely signaled when the plunger has reached a position corresponding to a nominal fixed-dose. If the user attempts to force the stop-ring member beyond the constriction, the plunger seal is stripped off or removed from the plunger head and the syringe rendered inoperable. The features of the invention can also be applied to a nonretracting syringe.
Owner:RETRACTABLE TECH INC
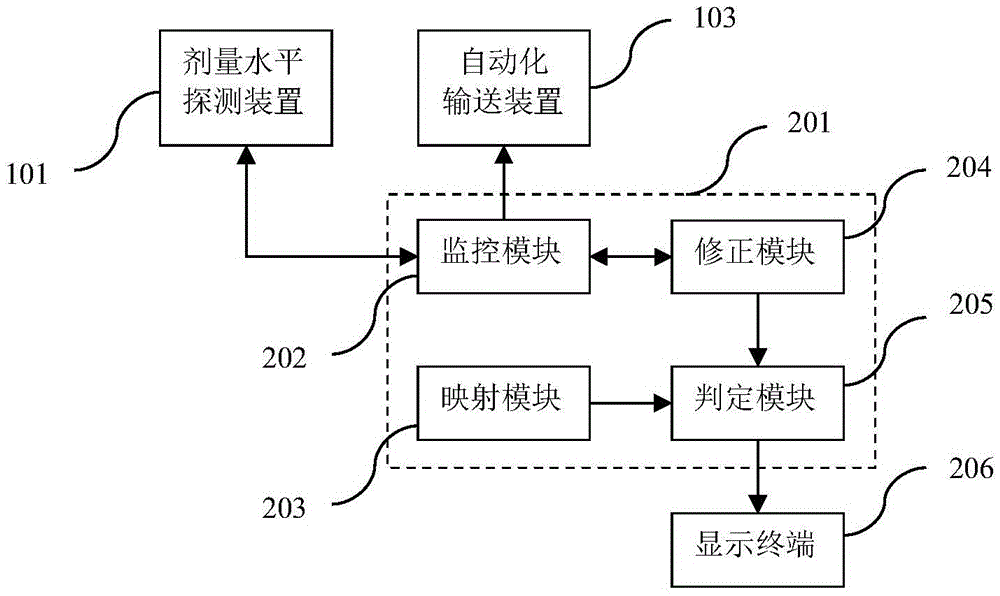
Radioactive solid waste detecting and classifying method and system
ActiveCN105665310AEasy to handleGood control effectX-ray spectral distribution measurementDosimetersEngineeringWaste treatment
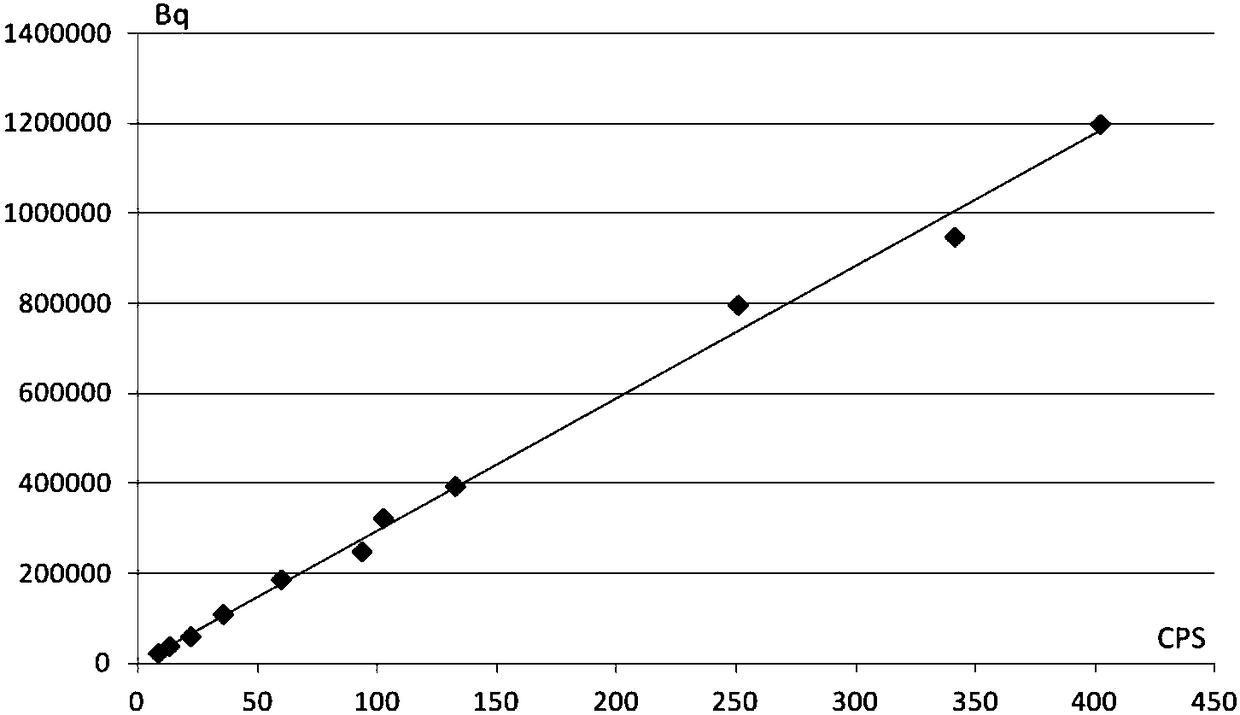
The invention belongs to the technical field of solid waste treatment, and discloses a radioactive solid waste detecting and classifying method. The method comprises the steps of: measuring a surface dose level, a pollution nuclide type and a radioactive specific activity of a radioactive solid waste sample; obtaining a fitting curve of the dose level and the radioactive specific activity, building a function relation of the two, and determining a dose limiting value range of the radioactive solid waste in a different-pollution-grade state; measuring a dose level of the radioactive solid waste; and comparing the dose level of the radioactive solid waste with the dose limiting value range thereof in the different-pollution-grade state, judging the pollution grade of the radioactive solid waste, and classifying and collecting the radioactive solid waste. The method compares the dose level of the radioactive solid waste with the dose level limiting value range through building the relation between the radioactive solid waste pollution grade and the dose level limiting value range, so that the pollution grade can be quickly judged, the classification and the collection are realized, and the treatment and management capacity of the radioactive solid waste is greatly promoted.
Owner:深圳市利美泰克自控设备有限公司
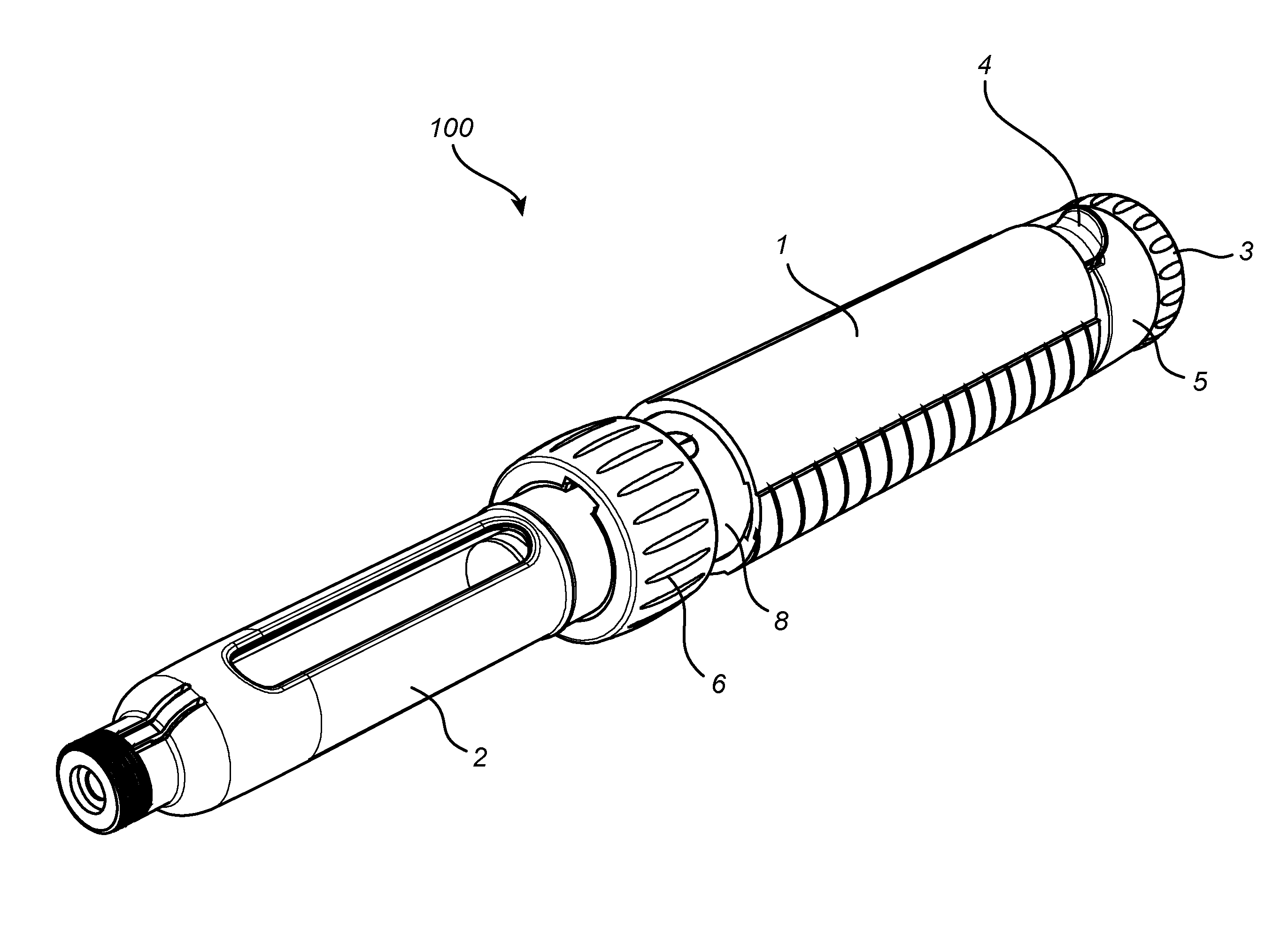
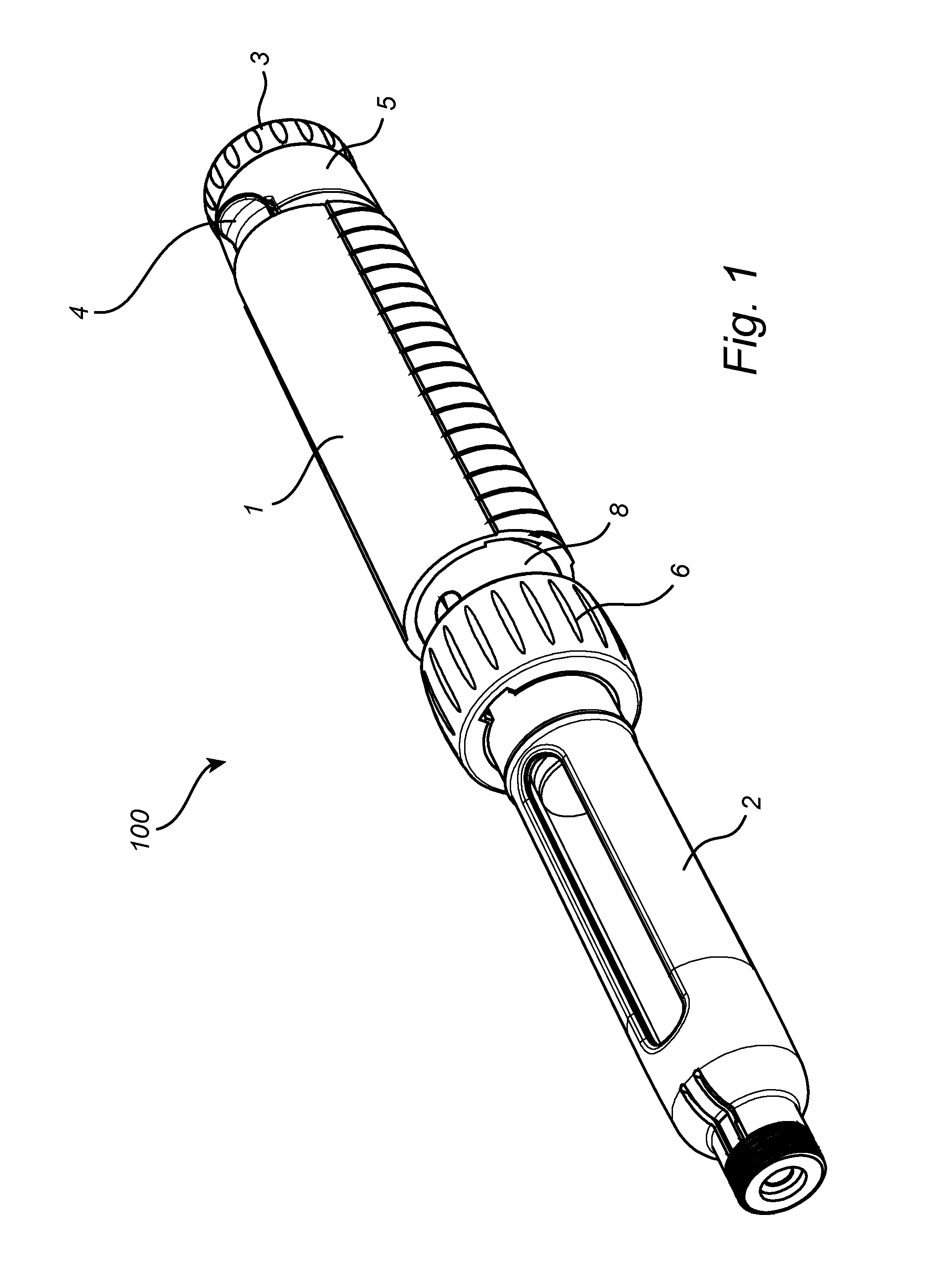
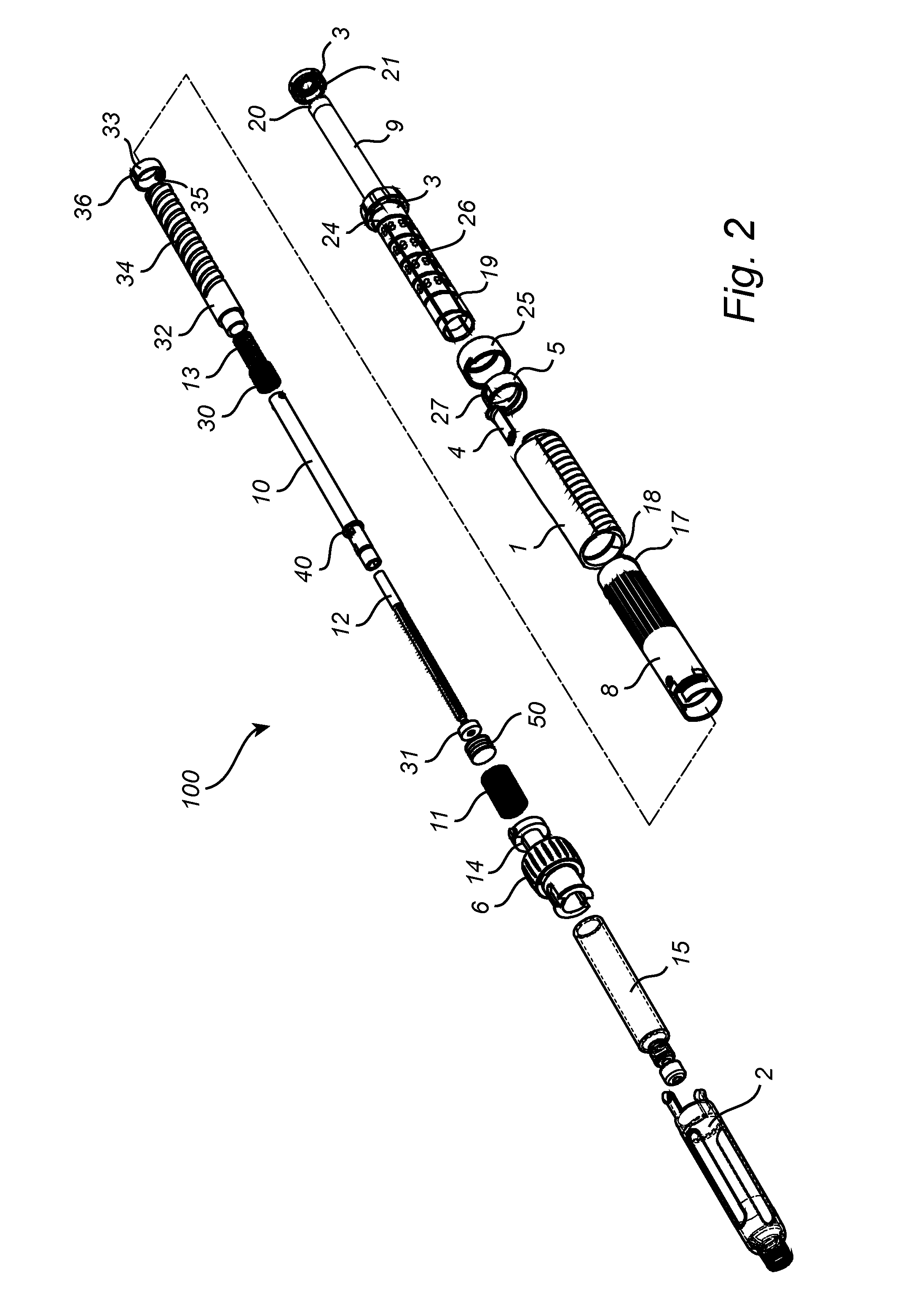
Medicament Delivery Device
ActiveUS20150151053A1Improve user friendlinessIncrease doseAmpoule syringesIntravenous devicesActuatorBiomedical engineering
The present invention relates to a medicament delivery device (100) having a a drive mechanism arranged to drive a plunger rod (12). A last dose limiting mechanism is arranged to interact with the drive mechanism and limit the distal end position of an actuator (3) when the plunger rod (12) has reached a pre-determined position between its distal end position and its proximal end position.
Owner:SHL MEDICAL AG
Drug delivery device
The invention relates to a drug delivery device having a dose limiting system. The drug delivery device includes a first dose setting mechanism (205) operably coupled to a primary reservoir (212) holding a first medicament (214). The first dose setting mechanism includes a first dose setter (208) and is a variable dose setting mechanism. The device further includes a second dose setting mechanism (204) operably coupled to a secondary reservoir (216) holding a second medicament, and the second dose setting mechanism includes a second dose setter (230). Still further, the device includes a dose limiting system (206). The dose limiting system operably couples the variable dose setting mechanism and the fixed dose setting mechanism. Further, the dose limiting system is configured to limit a settable amount of a dose of the second medicament a user can set using the second dose setter based on an amount of a variable dose that a user sets using the first dose setter.
Owner:SANOFI AVENTIS DEUT GMBH
Fixed-Dose Syringe with Limited Aspiration
InactiveUS20080221517A1Prevent reuseReduce the possibilityInfusion syringesMedical devicesEngineeringSyringe needle
A syringe configured with a limited maximum usable capacity. The syringe of the invention desirably has a retractable needle to prevent reuse. In the preferred embodiment, a dose-limiting structure includes a stop-ring member on the head of the plunger that abuts a constriction in the housing when the plunger is moved away from the needle to prevent the further rearward movement of the plunger. Preferably, the syringe of the invention is configured such that a user is tactilely signaled when the plunger has reached a position corresponding to a nominal fixed-dose. If the user attempts to force the stop-ring member beyond the constriction, the plunger seal is stripped off or removed from the plunger head and the syringe rendered inoperable. The features of the invention can also be applied to a nonretracting syringe.
Owner:RETRACTABLE TECH INC
Pen type drug injection device with dose limiting nut to prevent setting of a dose higher than the amount of drug remaining
The present invention relates to a pen type drug injection device (1; 101) with drive mechanism, comprises a housing (4, 5; 104, 105), a dose dial member (6; 106), a drive member (8; 108) engaging a lead screw (9; 109), a first clutch (18; 118) rotationally coupling the drive member (8; 108) and the housing in a coupled state and allowing relative rotation between the drive member and the housing in a de-coupled state, a second clutch (19; 119) rotationally coupling the drive member and the dial member in a coupled state and allowing relative rotation between the drive member and the dial member in a de-coupled state, and a nut (11; 111) coaxially arranged between the drive and the dial member and splined or keyed to the drive as well as threadedly engaged to the dial member, such that it moves axially on the drive member during dose setting but not during dose dispensing, and such that it eventually abuts an end stop on one of the drive or dial member, thereby limiting movement of the nut and preventing setting of a dose larger than the amount of drug remaining in the cartridge.
Owner:SANOFI SA
Optically active isomers of ketotifen and therapeutically active metabolites thereof
Racemic norketotifen, racemic 10-hydroxy-ketotifen, racemic 10-hydroxy-nor-ketotifen and optically active isomers of ketotifen, norketotifen, 10-hydroxy-ketotifen and 10-hydroxy-norketotifen were found to have antiallergic and anti-inflammatory effects while being devoid of the severe dose-limiting sedative side effects of Ketotifen.
Owner:BRIDGE PHARMA INC
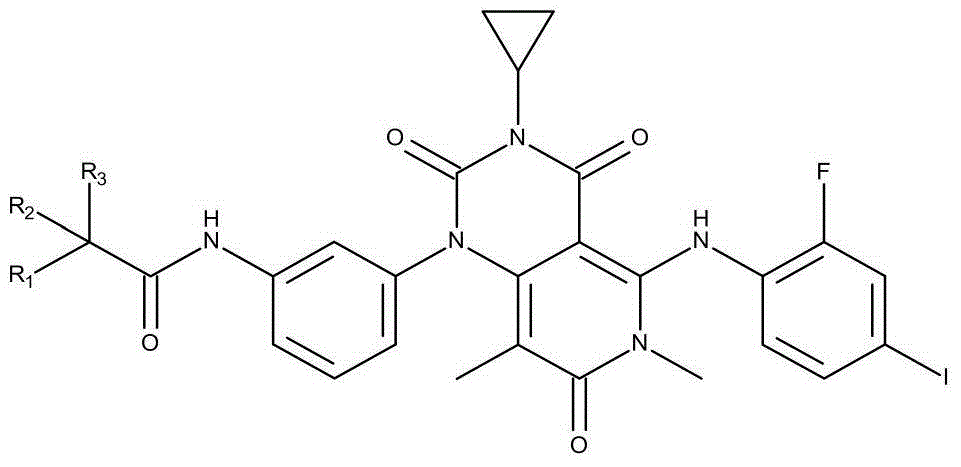
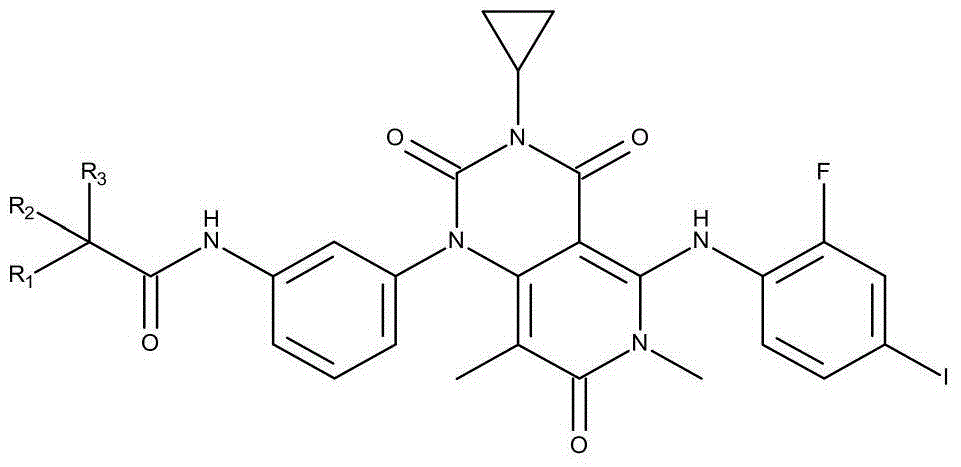
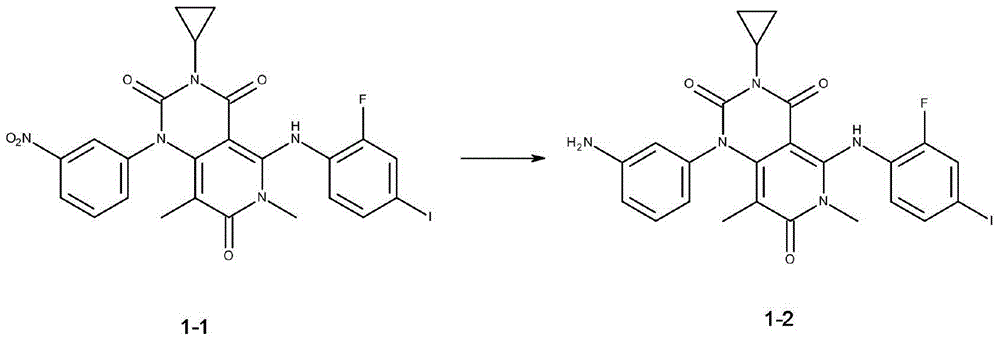
Novel derivative of N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N'-propyl and application of novel derivative
InactiveCN103724281AGood curative effectExtended half-lifeOrganic active ingredientsOrganic chemistryHalf-lifeToxicity
The invention discloses a novel derivative of N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N'-propyl and application of the novel derivative. The novel derivative has good medicine activity, can greatly prolong the half-life period of a medicine, prolongs the retention time of the medicine in a human body, and meanwhile, improves the concentration of the medicine in blood; therefore, a better curative effect is achieved. As the half-life period of the medicine is greatly prolonged, activity concentration of the medicine in the blood can be maintained for a longer period of time; under the cure condition of dose constrain, the curative effect is maintained and the dosage of the medicine is reduced, so that the problem of bad metabolism of the medicine is eliminated, the medicine toxicity is reduced, and the toxic and side effects in the medicine use process are reduced.
Owner:ZHENJIANG SAN AN PHARMA
Medicament delivery device
The present invention relates to a medicament delivery device (100) having a a drive mechanism arranged to drive a plunger rod (12). A last dose limiting mechanism is arranged to interact with the drive mechanism and limit the distal end position of an actuator (3) when the plunger rod (12) has reached a pre-determined position between its distal end position and its proximal end position.
Owner:SHL MEDICAL AG
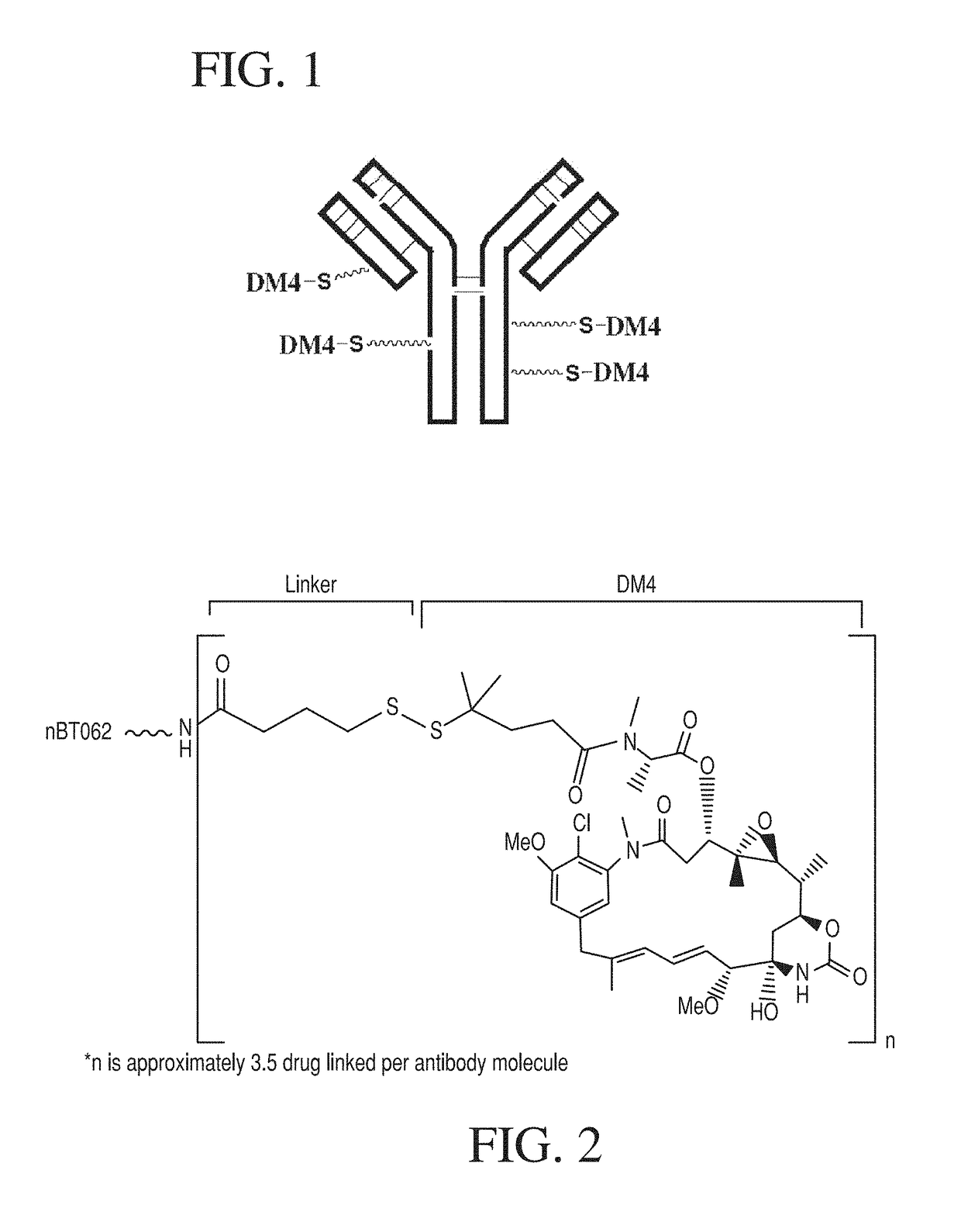
Uses of immunoconjugates targeting CD138
InactiveUS10117932B2Antibody ingredientsPharmaceutical non-active ingredientsDiseaseAntiendomysial antibodies
Owner:IMMUNOGEN INC
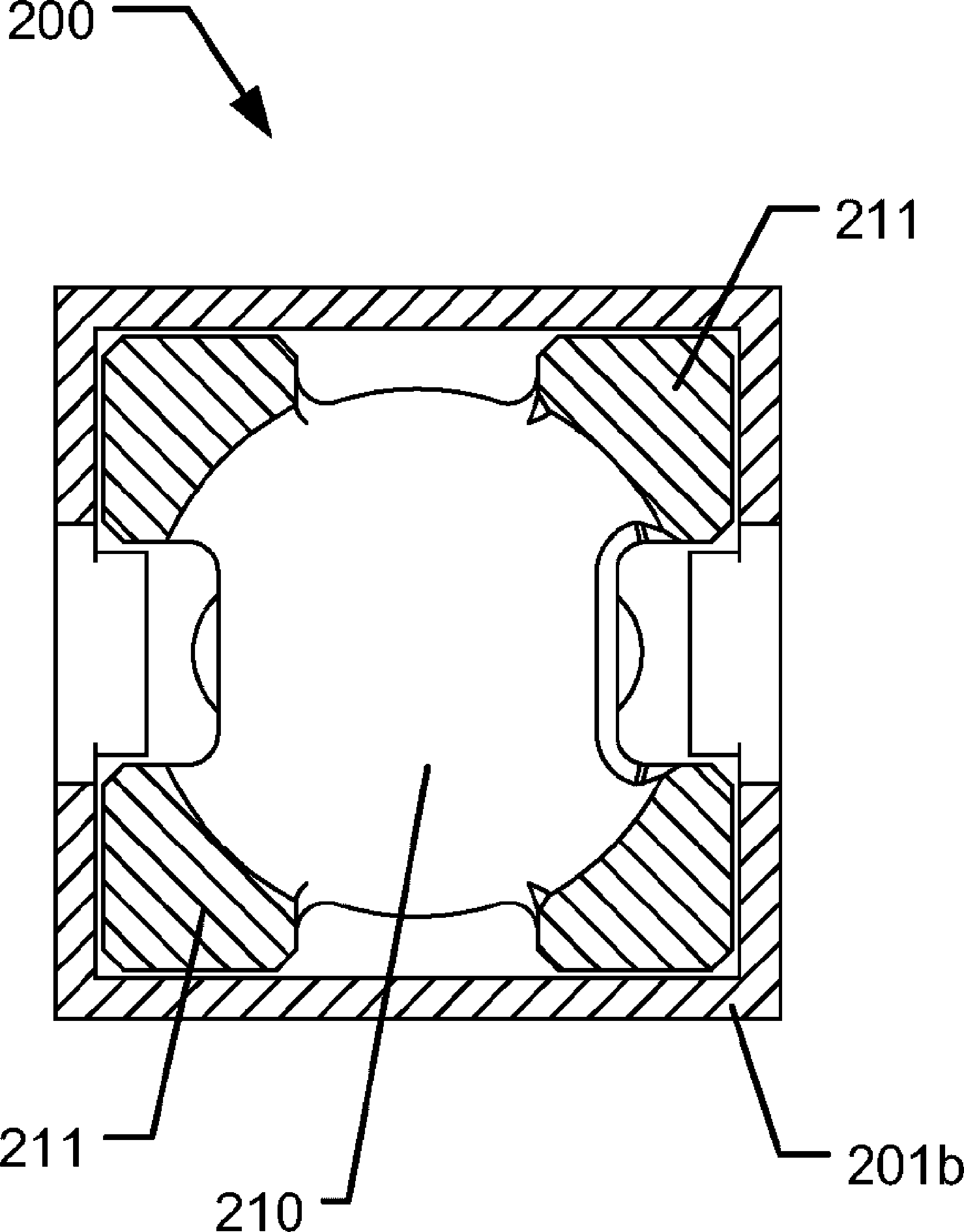
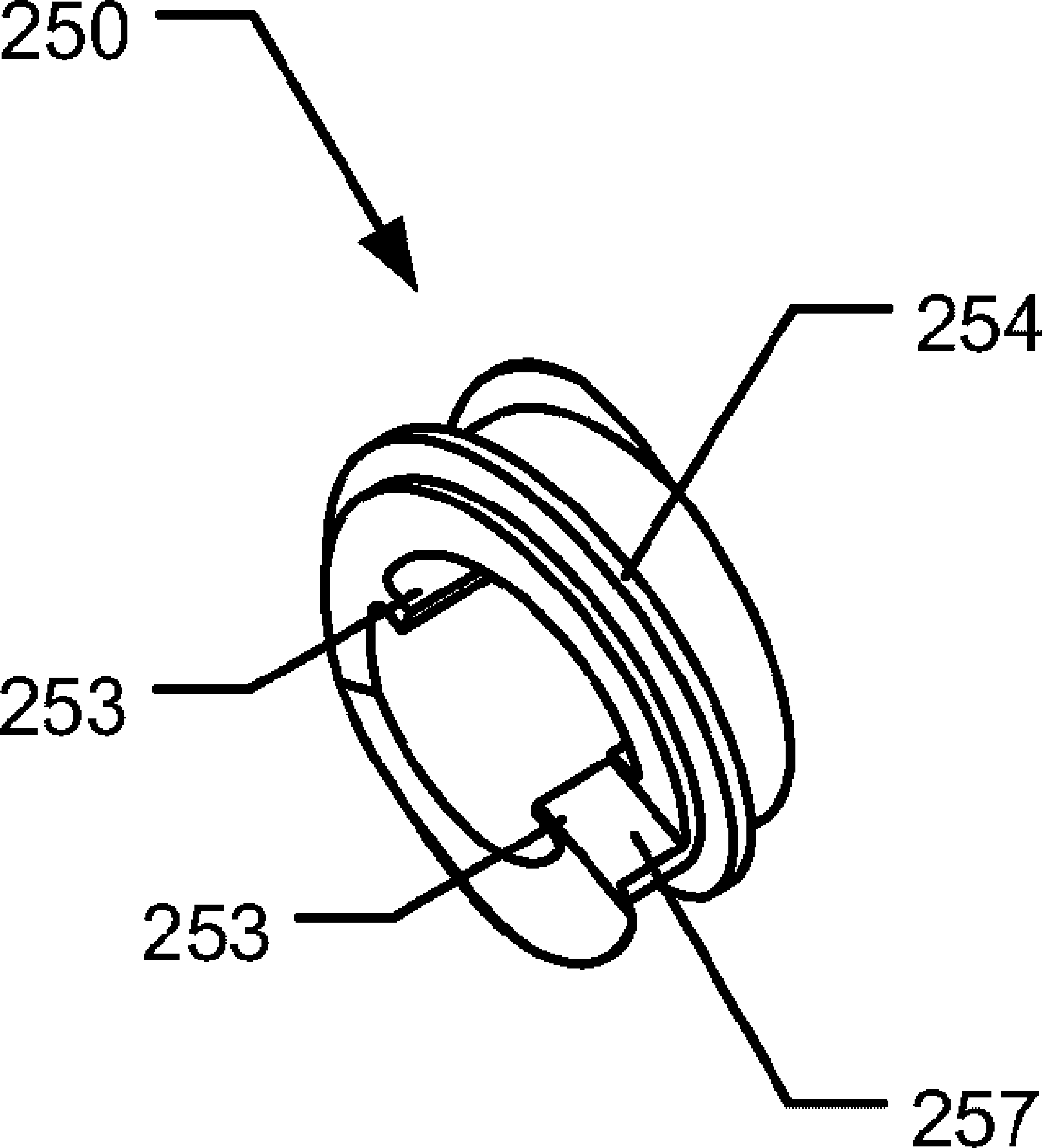
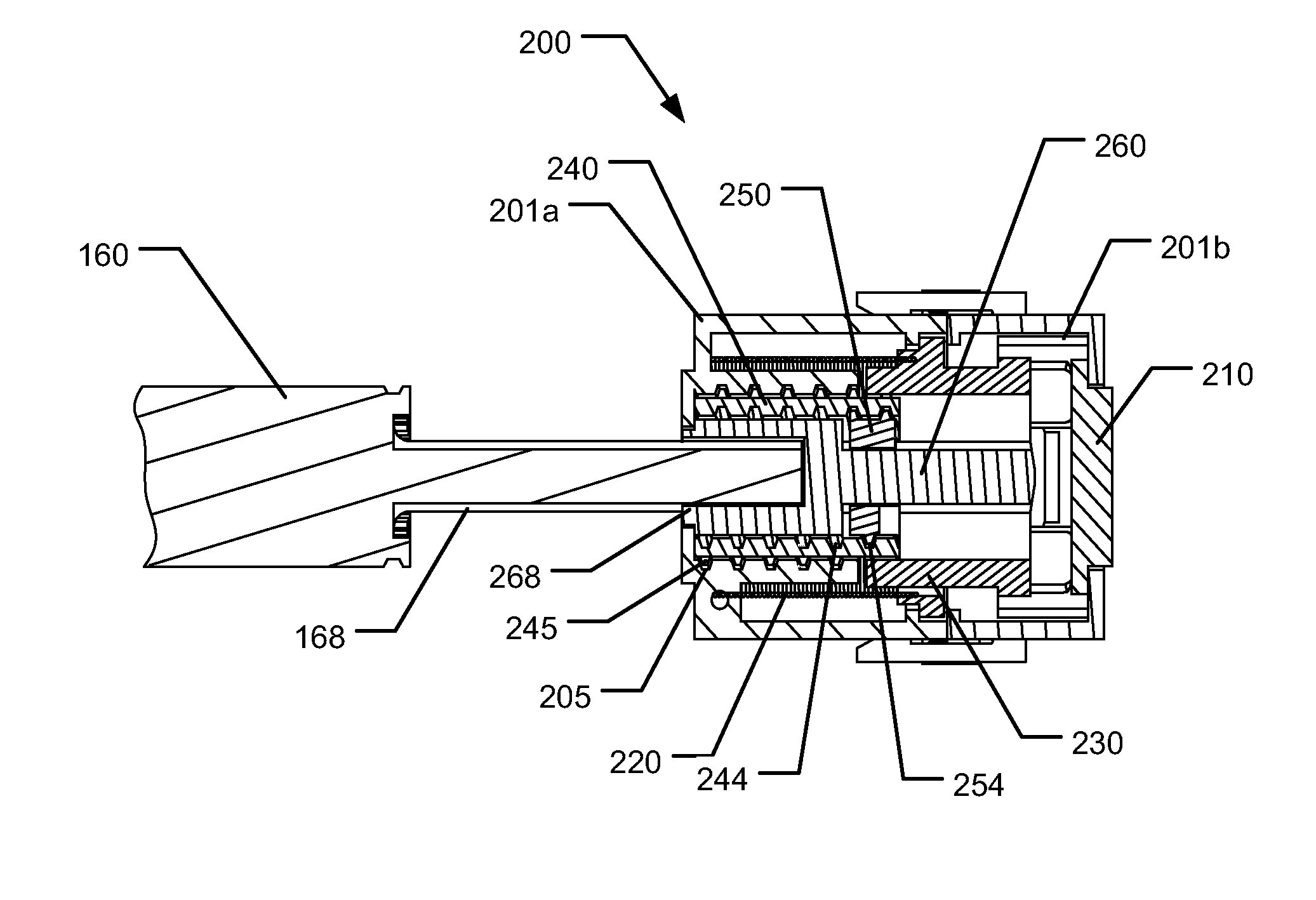
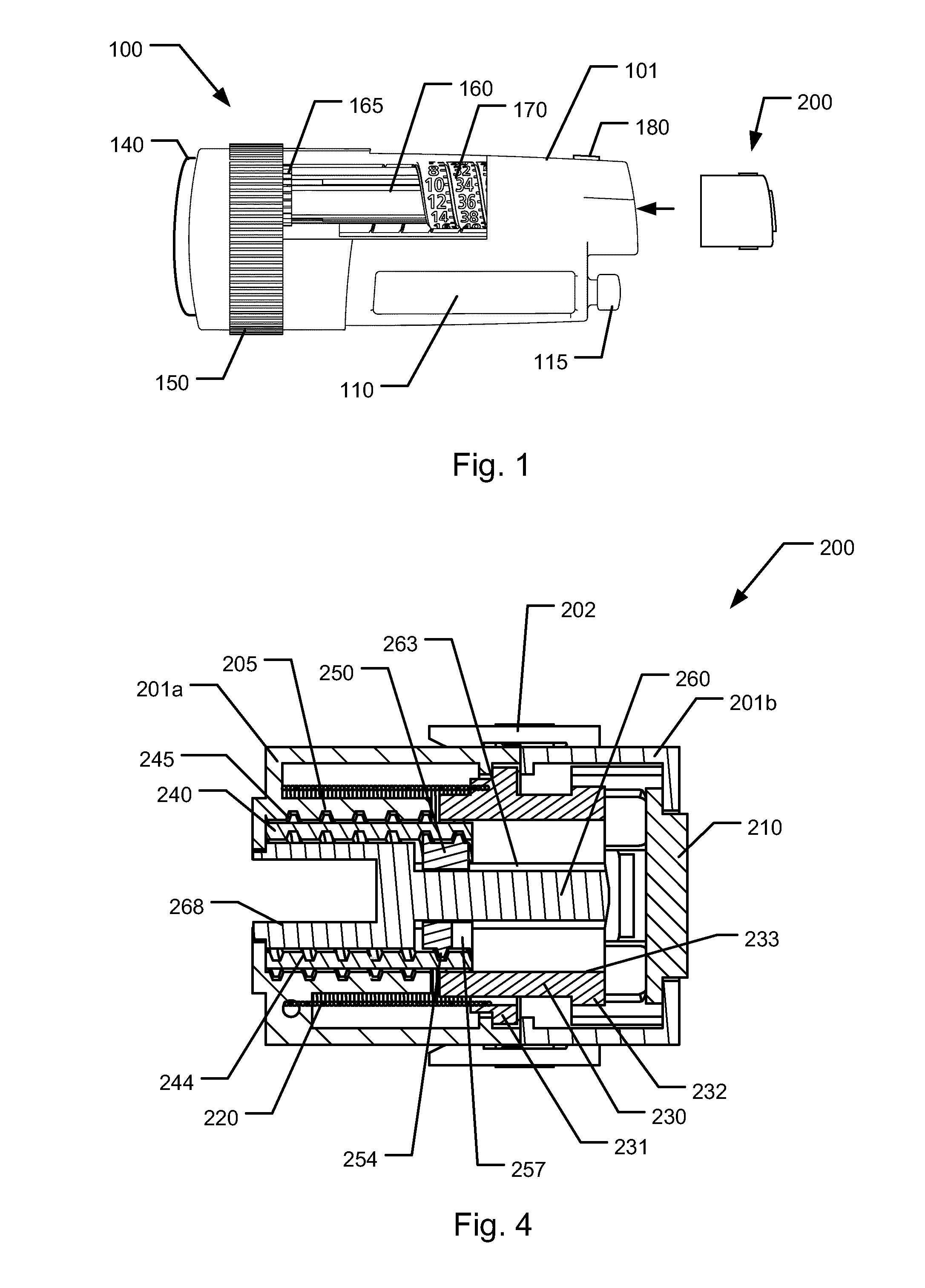
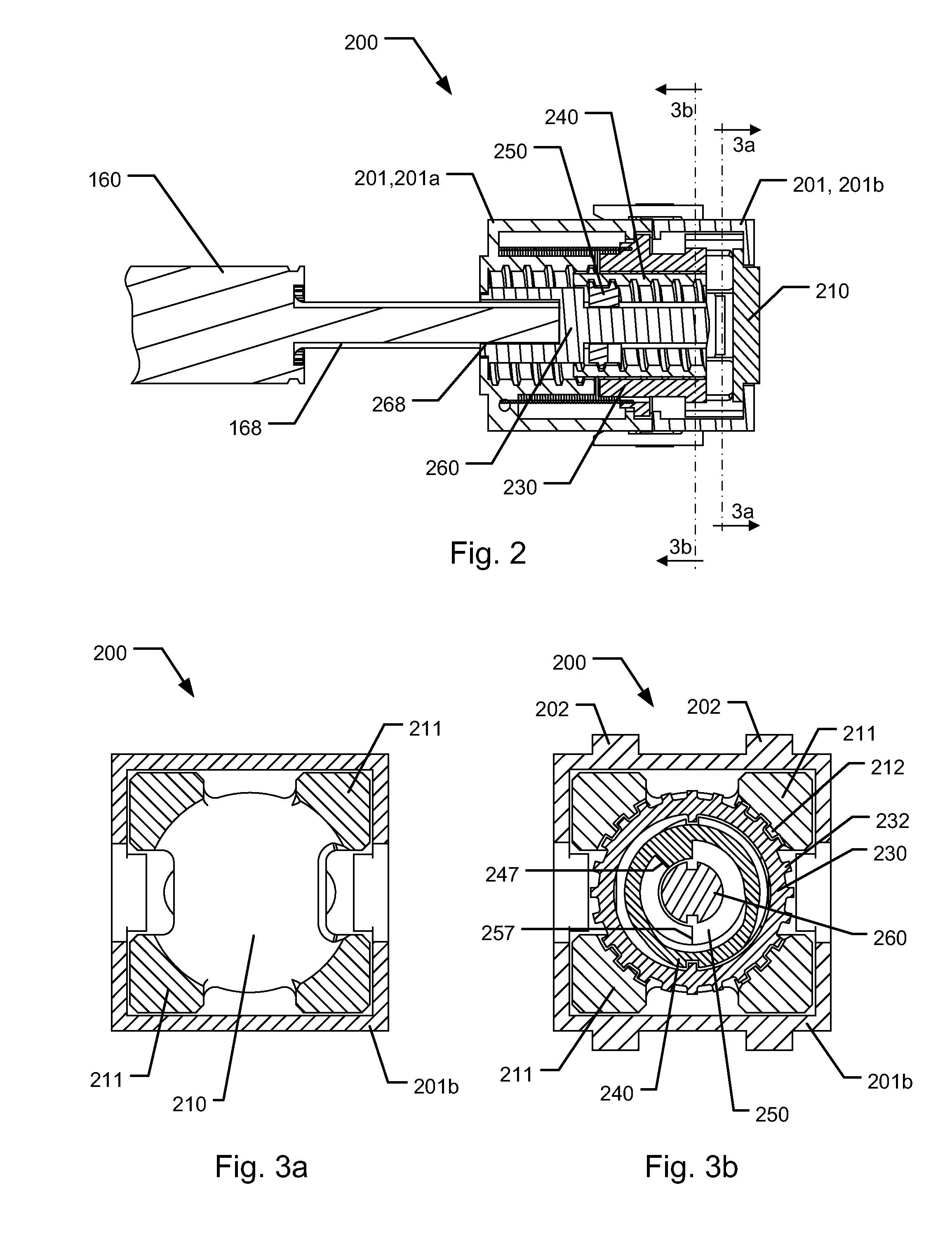
Medical injection system comprising a medical injection device and a dose limiter module
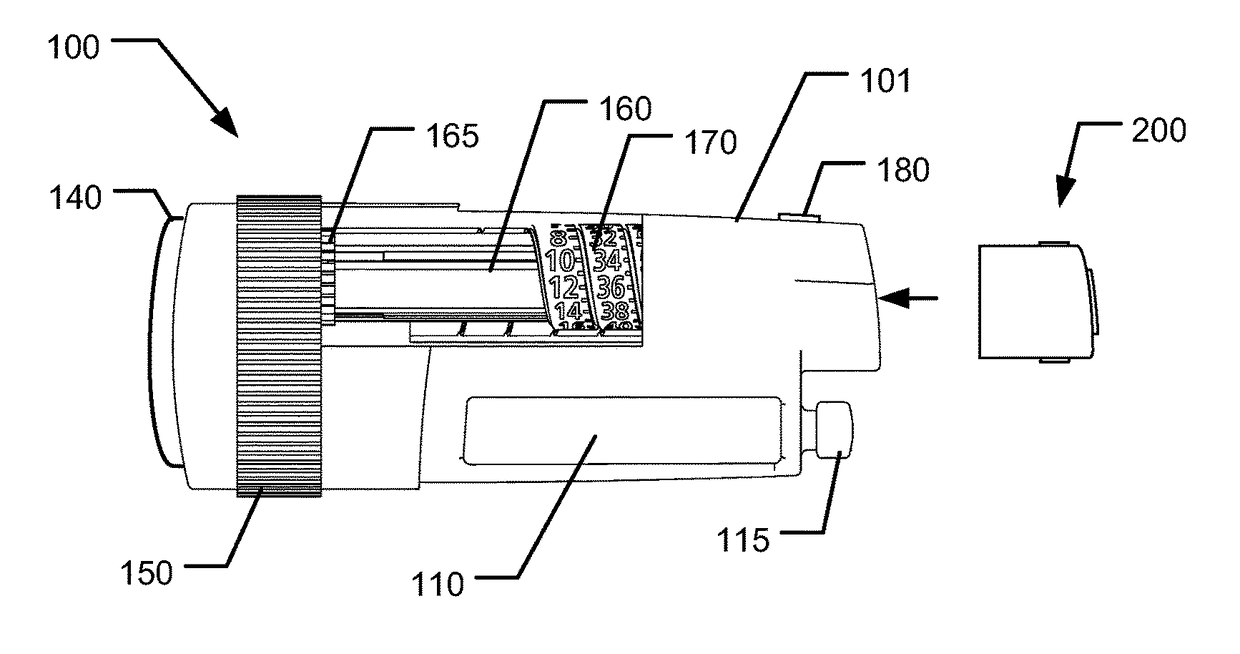
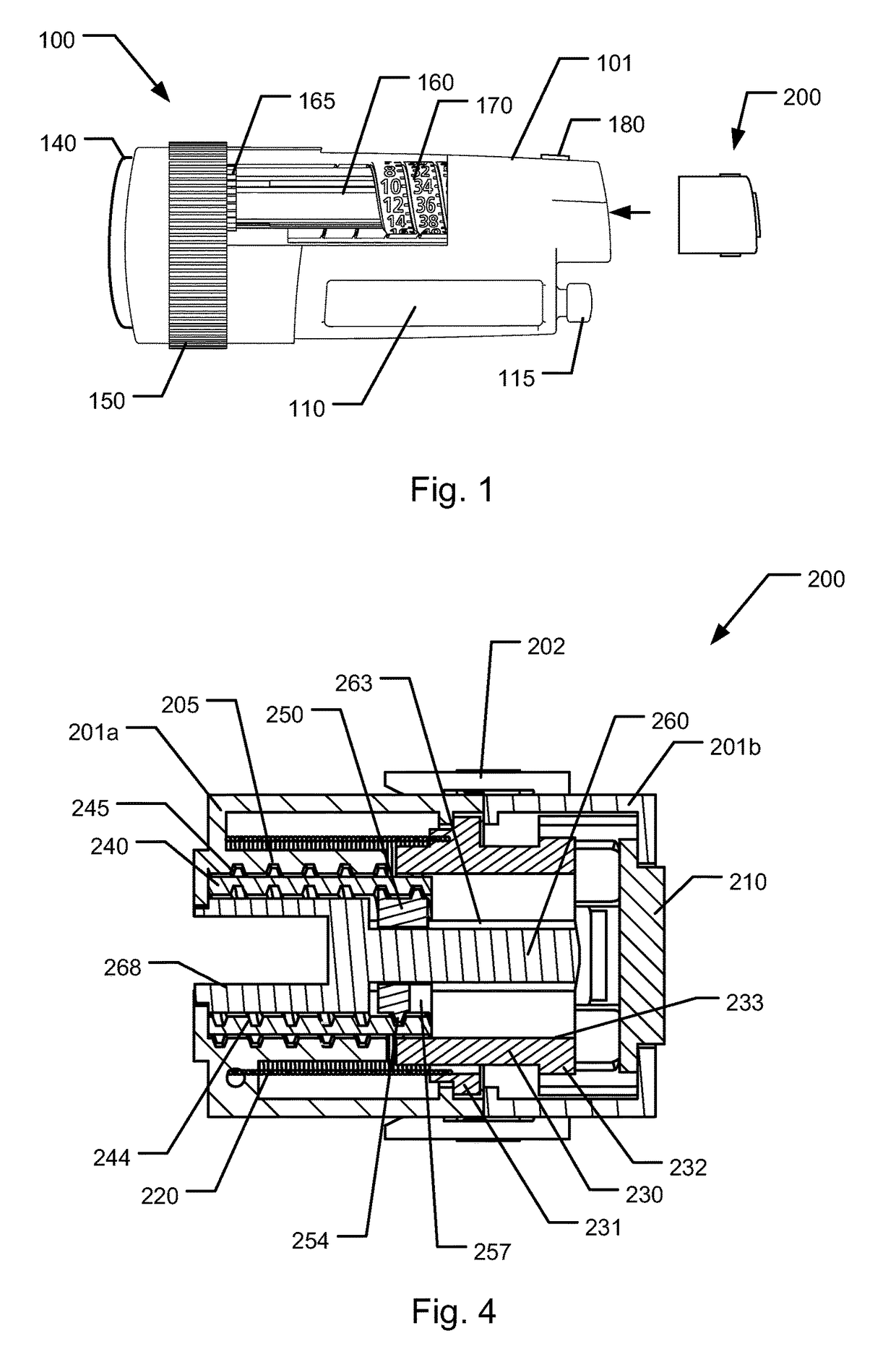
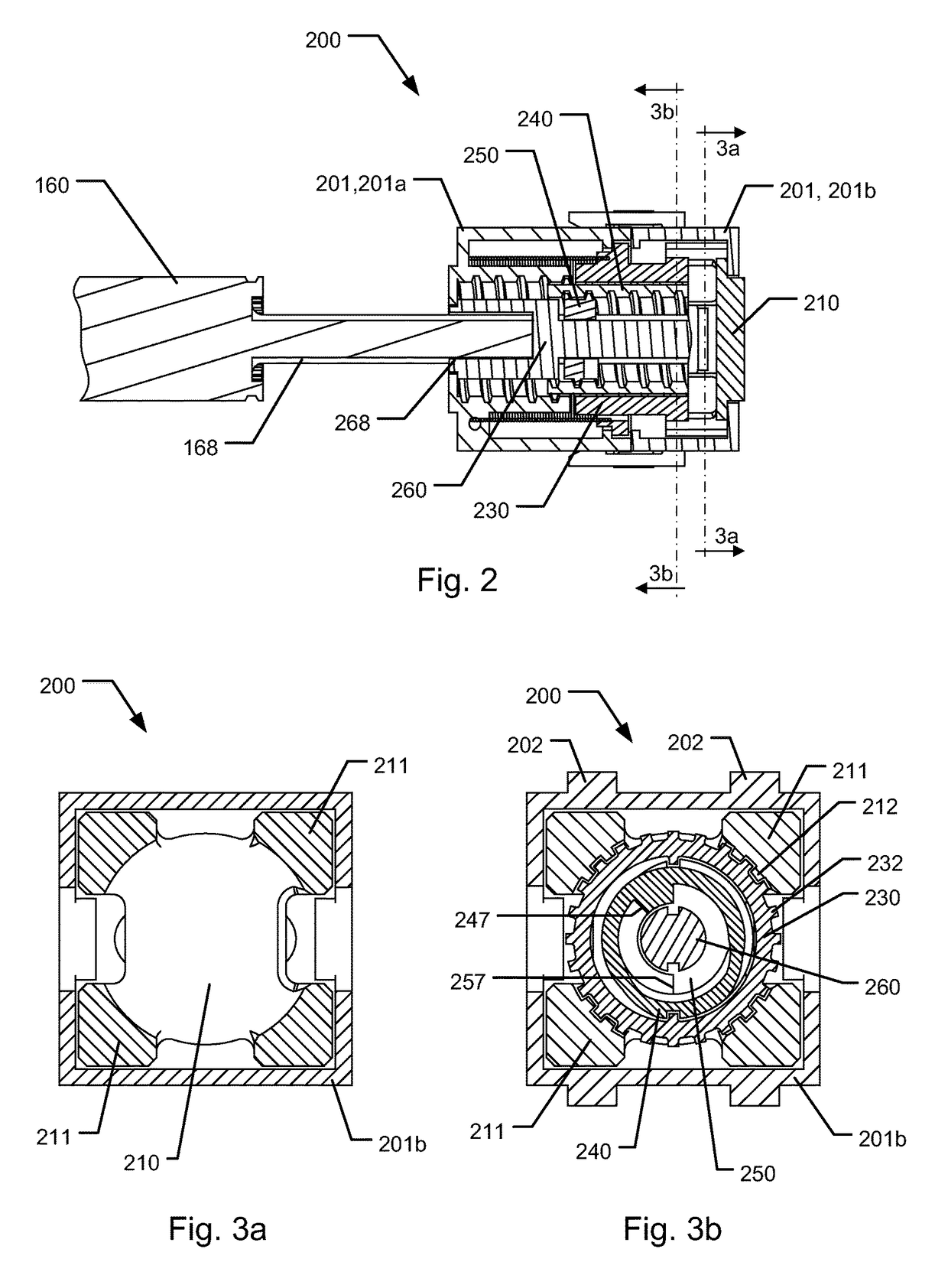
The present invention relates to a medical injection system comprising a medical injection device (100) of the type comprising a rotatable dose setting member (160). The system further comprises a dose limiter module (200) adapted for being releasably coupled to the injection device (100). The dose limiter module (200) comprises a dose limiter base (201) and a limiter (240) arranged relative to the dose limiter base (201) so that their relative position is adjustable to set an adjusted dose limiting position. When the dose limiter module (200) is coupled to the injection device (100), the limiter (240) cooperates with the dose setting member (160) to define a blocking means for preventing rotation of the dose setting member (160) beyond the adjusted dose limiting position. The dose limiter module (200) further comprises a user operable lock (210,212,232,223,243) configured for operation between a locked state and an unlocked state to respectively prevent and enable modification of the adjusted dose limiting position, the lock (210,212,232,223,243) being configured for maintaining the lock in the locked state at least when the dose limiter module (200) is not coupled with the injection device (100).
Owner:NOVO NORDISK AS
Medical Injection System Comprising a Medical Injection Device and a Dose Limiter Module
InactiveUS20140257195A1Easy procedureInfusion syringesIntravenous devicesInjection deviceBiomedical engineering
The present invention relates to a medical injection system comprising a medical injection device (100) of the type comprising a rotatable dose setting member (160). The system further comprises a dose limiter module (200) adapted for being releasably coupled to the injection device (100). The dose limiter module (200) comprises a dose limiter base (201) and a limiter (240) arranged relative to the dose limiter base (201) so that their relative position is adjustable to set an adjusted dose limiting position. When the dose limiter module (200) is coupled to the injection device (100), the limiter (240) cooperates with the dose setting member (160) to define a blocking means for preventing rotation of the dose setting member (160) beyond the adjusted dose limiting position. The dose limiter module (200) further comprises a user operable lock (210,212,232,223,243) configured for operation between a locked state and an unlocked state to respectively prevent and enable modification of the adjusted dose limiting position, the lock (210,212,232,223,243) being configured for maintaining the lock in the locked state at least when the dose limiter module (200) is not coupled with the injection device (100).
Owner:NOVO NORDISK AS
Alcoholism-dispelling and liver-protecting Chinese caterpillar fungus oligopeptide tableting candy
The present invention discloses alcoholism-dispelling and liver-protecting Chinese caterpillar fungus oligopeptide tableting candy. The tableting candy comprises the following components in parts by weight: 25-35 parts of Chinese caterpillar fungus polysaccharide compounds, 15-25 parts of kudzuvine root, 8-12 parts of Chinese yams, 14-18 parts of corn oligopeptide, 10-14 parts of Chinese wolfberryfruits, 16-20 parts of albumin peptide and the balance medical or food health-care accessory materials. The particularity of the Chinese caterpillar fungus polysaccharide compounds realizes the further enhancement of the functions of the albumin peptide, kudzuvine root powder, Chinese yams, corn oligopeptide and Chinese wolfberry fruits. The various ingredients are reasonably combined to realizethe purposes of accelerating alcohol metabolism, protecting liver and preventing liver damage. Moreover, the medicinally and edibly homologous raw materials are used; the food raw materials having thehighest safety level and not limited in use amount are reasonably combined; and the tableting candy has effects and safety, and is free of side effects and dose limiting value.
Owner:楼良水
Derivative of pyridine and (4,3-d) pyrimidine-1(2H)-base phenyl acetamide and application thereof
InactiveCN103819471AGood curative effectExtended half-lifeOrganic chemistryAntineoplastic agentsSide effectActivity concentration
The invention discloses a derivative of pyridine and (4,3-d) pyrimidine-1(2H)-base phenyl acetamide and an application thereof. The derivative has good pharmaceutical activity, can greatly prolong the half-life periods of drugs, prolongs the retention time of drugs in the human body, and meanwhile increases the drug concentration in the blood. Therefore, a better curative effect is achieved. As the half-life periods of drugs are greatly prolonged, the drugs can keep activity concentration for a longer time in the blood. Under the cure condition with dose limiting, the curative effect can be kept and the recommended dosage of drugs can be reduced at the same time, therefore the bad metabolism of drugs is eliminated, the drug toxicity is reduced, and the toxic and side effects in the drug using process are small.
Owner:ZHENJIANG SAN AN PHARMA
Pharmaceutical composition for treating hemangioma and vascular malformation sclerosis and preparation method and application thereof
PendingCN110946987AReduce dosageReduce the single doseAerosol deliverySaccharide peptide ingredientsPharmaceutical drugTherapeutic effect
The invention discloses a pharmaceutical composition for treating hemangioma or vascular malformation sclerosis. The pharmaceutical composition at least comprises bleomycin and a foam hardener. The invention further discloses a preparation method and application of the pharmaceutical composition. By using the pharmaceutical composition disclosed by the invention, the retention time of bleomycin ina local focus can be prolonged, and the contact area between bleomycin and a lesion vessel is increased. The embolization efficiency is improved, and a better treatment effect is achieved. Accordingto the composition disclosed by the invention, reduction of single dosage of bleomycin is realized, so that bleomycin plays a stronger treatment role within the limit of the lifetime dosage thereof.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Compositions and methods for treating synucleinopathies
The present invention describes the use of a 5HT3-antagonist, in combination with a 6-propylamino-4,5,6,7-tetrahydro-1,3-benzothiazole-2-amine, to reduce adverse effects and to facilitate the neuroprotective treatment of a patient suffering from a synucleinopathic disorder to enable a therapeutically effective 6-propylamino-4,5,6,7-tetrahydro-1,3-benzothiazole-2-amine daily dose without the dose-limiting adverse effects caused by pramipexole when administered alone.
Owner:CHASE THERAPEUTICS CORP
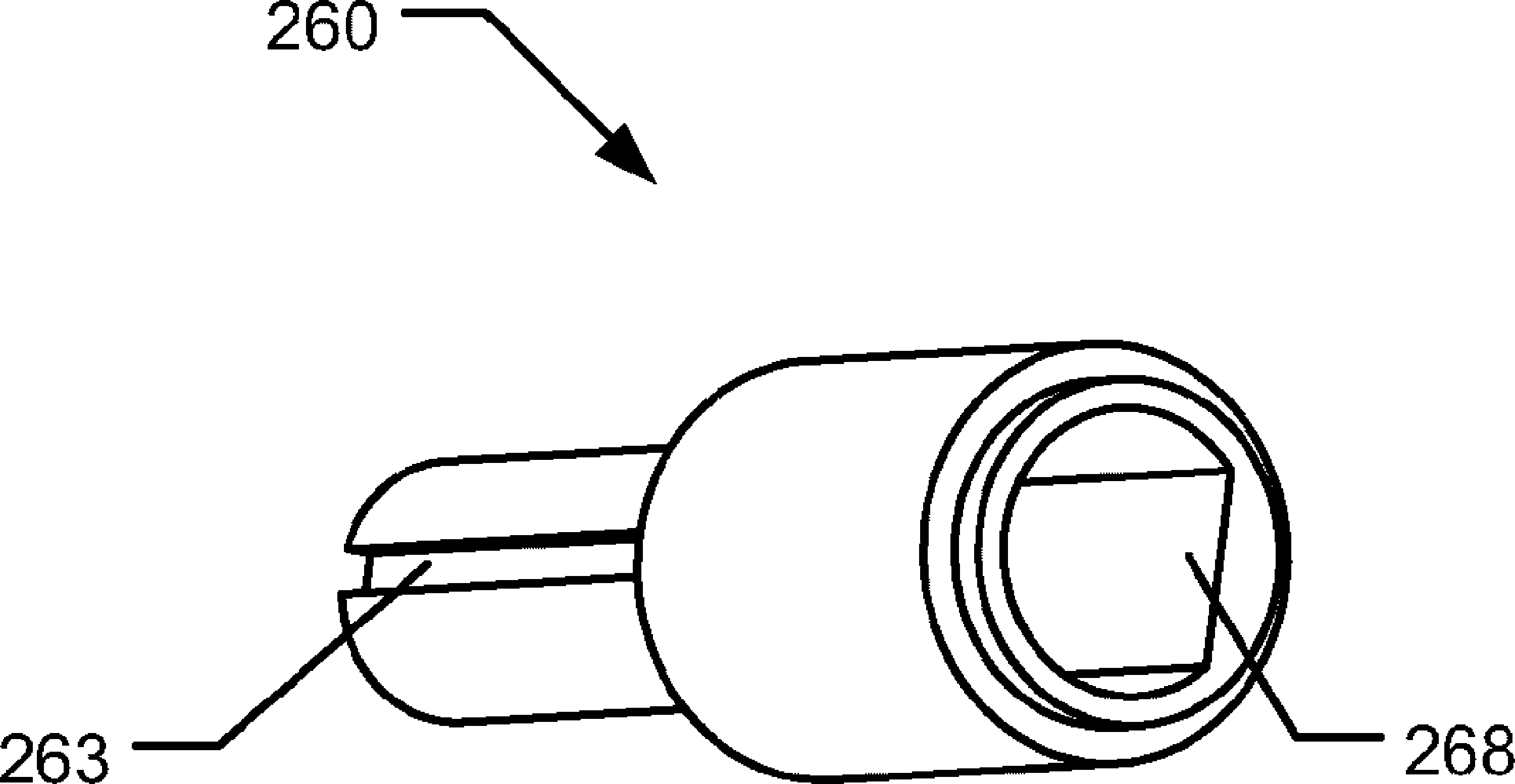
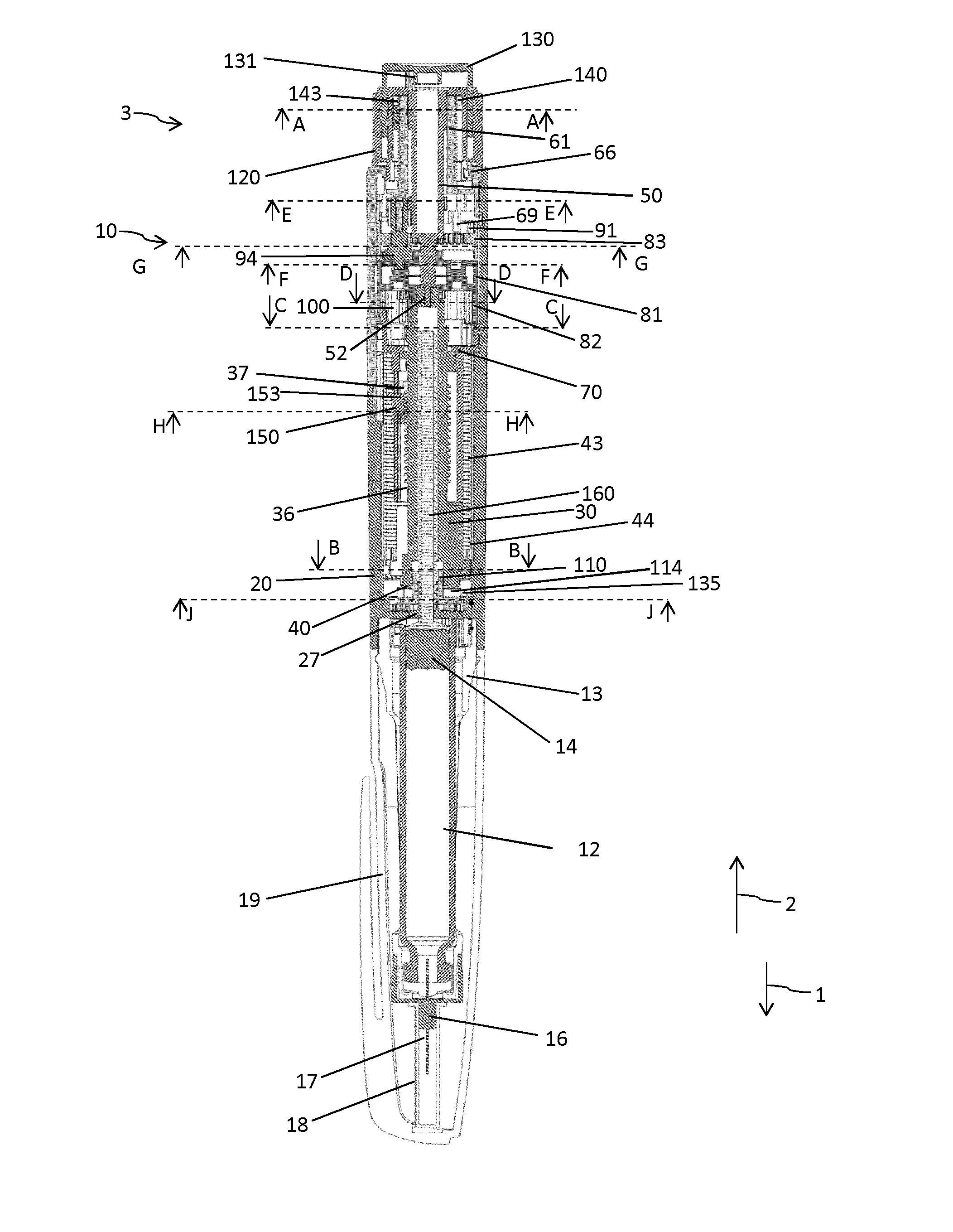
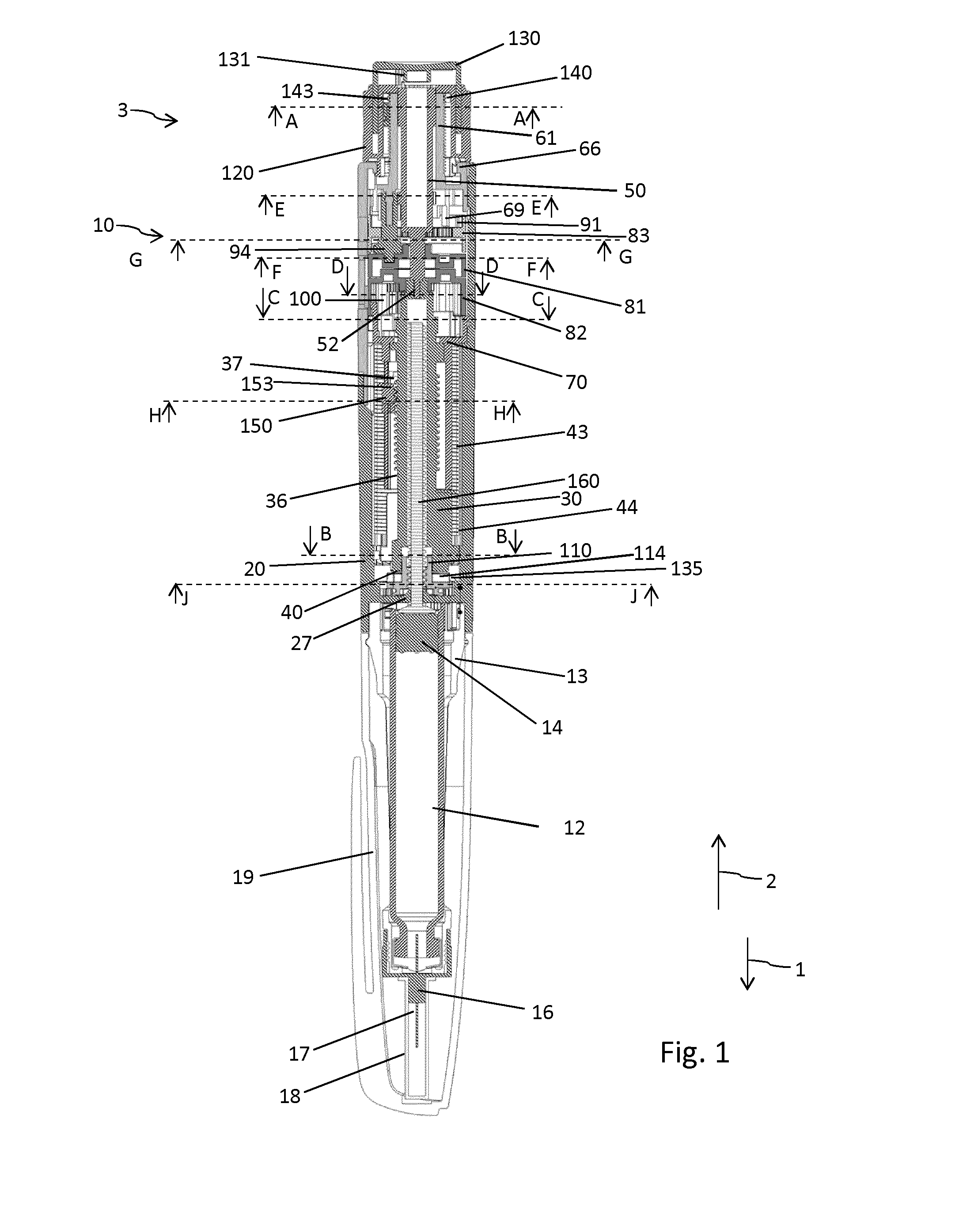
Drive mechanism for a drug delivery device
InactiveUS20160045667A1Improve securityEffective decouplingAutomatic syringesMedical devicesMedicinePharmaceutical drug
The present invention relates to a drive mechanism of a drug delivery device for dispensing of a dose of a medicament, the mechanism comprising: an elongated housing (20) extending in an axial direction (1, 2), a piston rod (160) to operably engage with a piston (14) of a cartridge (12) to displace the piston (14) in axial distal direction (1), a dose setting member (120; 220) rotatably arranged at a proximal end of the housing (20) for setting of a dose, a base member (60; 260) having a proximally extending shaft portion (61; 261) at least partially enclosed by the dose setting member (120;220), a last dose limiting member (140; 240) radially disposed between the dose setting member (120; 220) and the base member (60; 260) and having at least one radial stop (143; 243a, 243b) to limit a rotational displacement of the dose setting member (120; 220) relative to the base member (60; 260), wherein the last dose limiting member (140; 240) is rotatably locked but axially displaceable to one of the base member (60; 260) and the dose setting member (120; 220) and wherein the last dose limiting member (140; 240) is threadedly engaged with the other one of the base member (60; 260) and the dose setting member (120; 220).
Owner:SANOFI SA
Traditional Chinese medicine combined preparation for treating radiation esophagitis
InactiveCN104971237AGood treatment effectEasy to buyHydroxy compound active ingredientsDigestive systemClinical efficacySmoked Plum
The invention discloses a traditional Chinese medicine combined preparation for treating radiation esophagitis, which is composed of honeysuckles, mint, fructus forsythiae, radix ophiopogonis, bamboo leaves, liquorice, smoked plum, radix rehmanniae, pericarpium citri reticulatae, pseudo-ginseng powder, borneol and honey. The preparation has the effect that when a patient suffered from chest or head and neck malignant tumor receives radiotherapy, dose-limiting reactions, such as congestion, edema, erosion or inflammatory exudative change and even anabrosis, in mucous membrane of esophagus. The traditional Chinese medicine combined preparation can alleviate even cure these symptoms so that the radiotherapy on the patients can be completed with significant treatment effect. The preparation is prepared through a water-decoction extraction process so that the characteristic of original taste of a traditional Chinese medicine decoction and effective components are free from losing. In addition, the retention time of the decoction in mucous membrane of esophagus is prolonged, thereby improving clinical curative effects.
Owner:张梅 +1
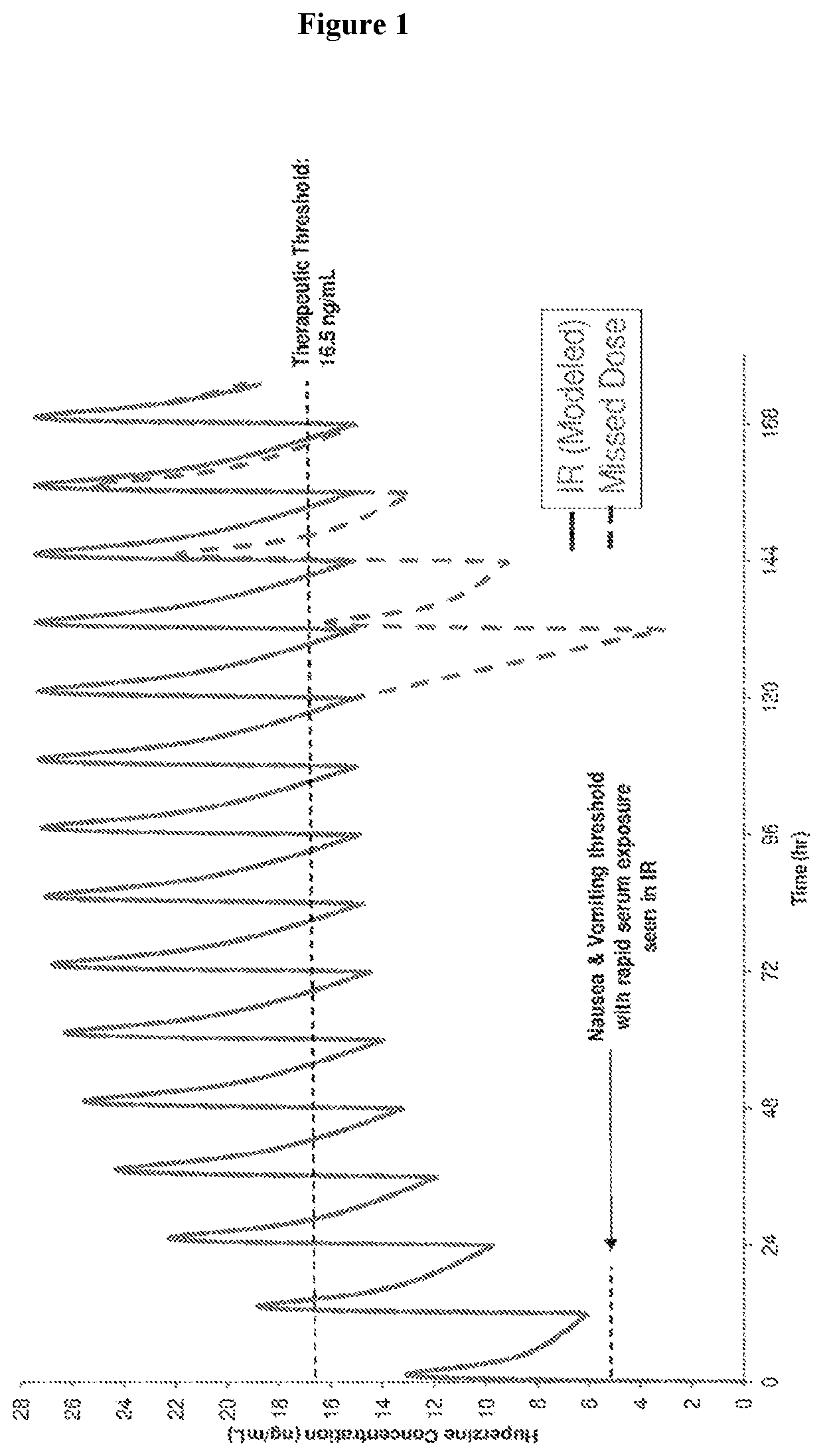
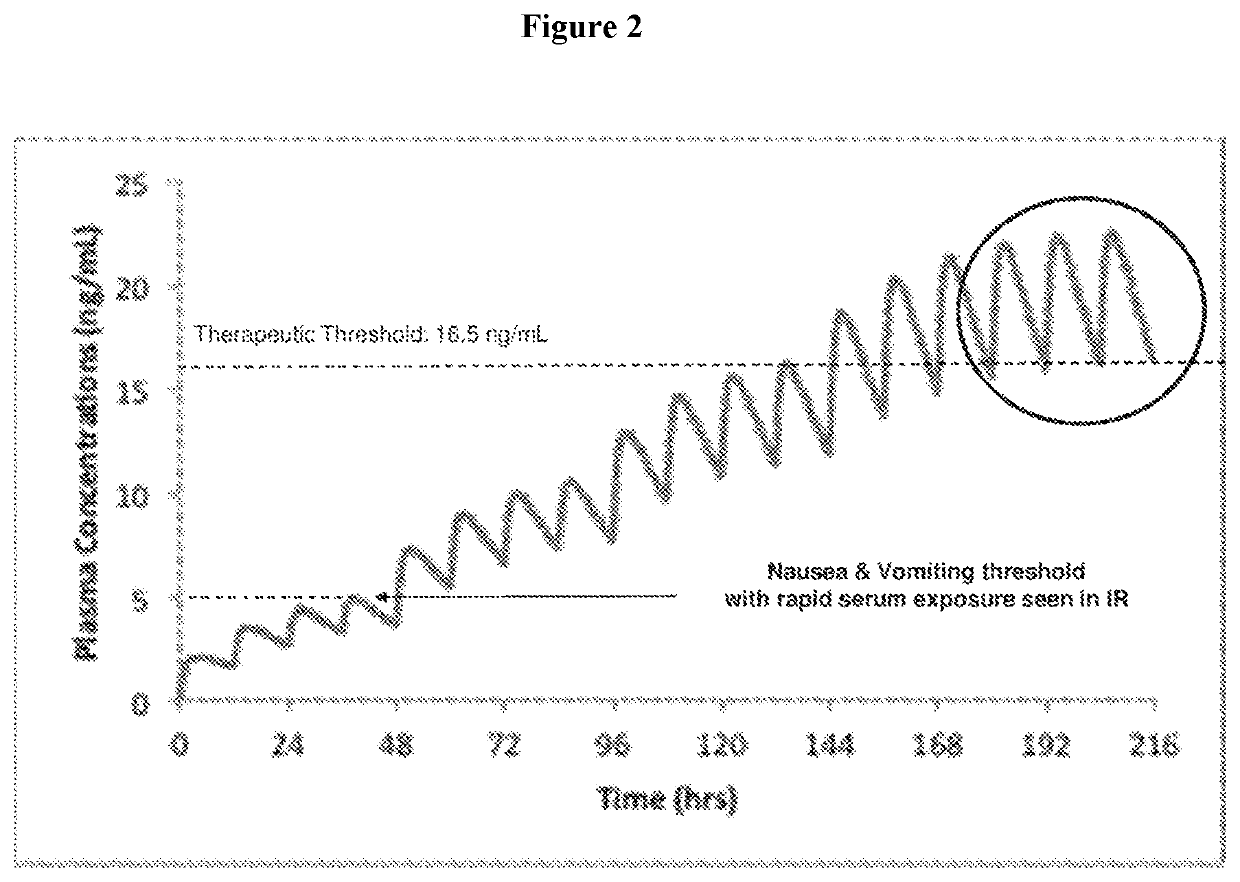
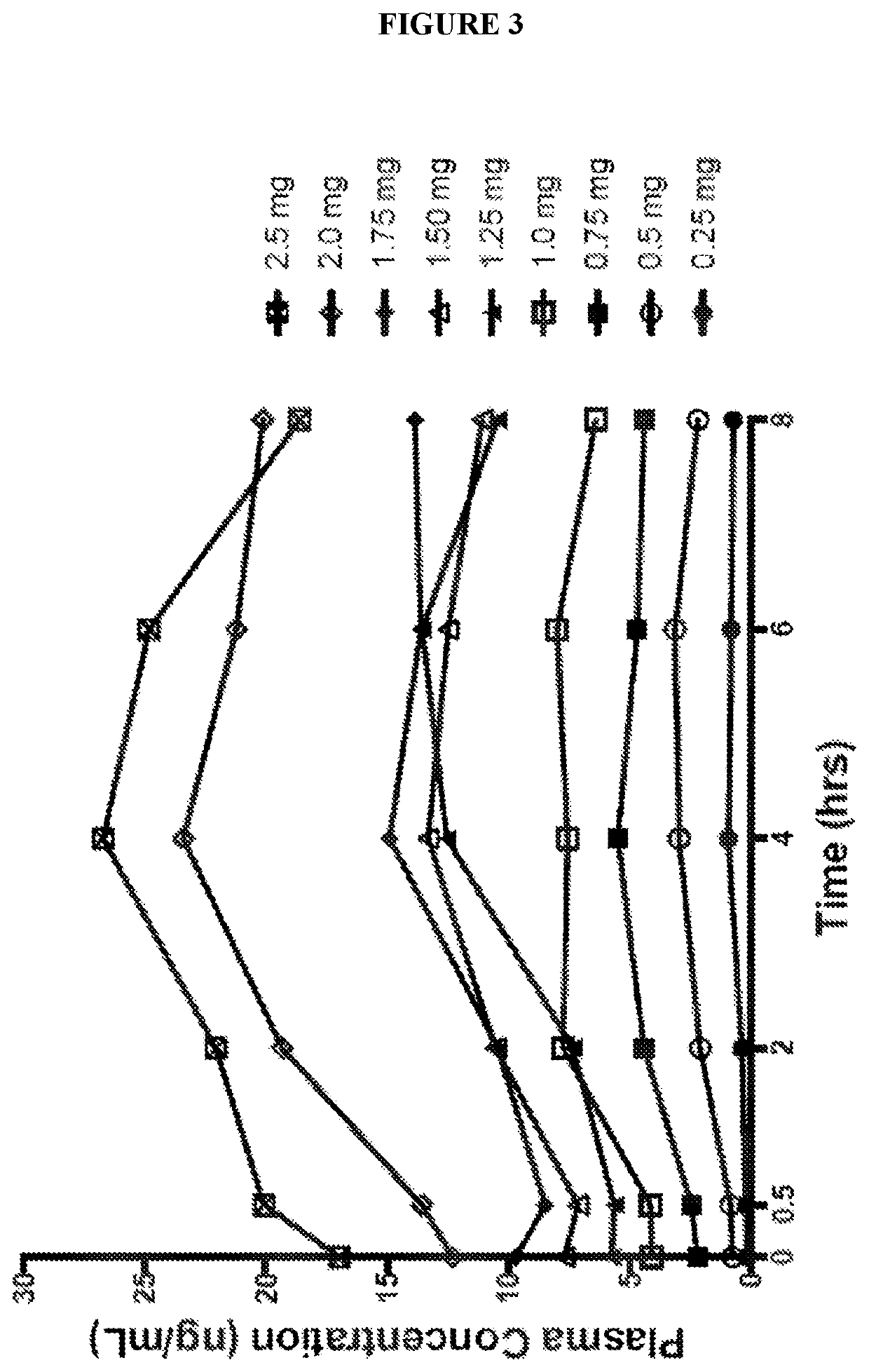
Use of higher doses of modified release huperzine formulations
ActiveUS11351120B2Reduce volatilityGood curative effectOrganic active ingredientsNervous disorderImmediate releasePharmaceutical drug
The present application discloses pharmaceutical compositions and methods of treating neurological disorders and seizure disorders with the high dose modified release compositions of huperzine. The pharmaceutical compositions and methods described herein, allow for higher dosing of huperzine, while avoiding rapid peak plasma levels, thereby avoiding the dose-limiting adverse events associated with the immediate release formulations.
Owner:SUPERNUS PHARM INC
Pharmaceutical compositions and methods utilizing neostigmine and an NK-1 antagonist for treating myasthenia gravis
The present invention describes the use of a NK1-antagonist, in constant combination with neostigmine, to facilitate the treatment of a patient suffering from myasthenia gravis by providing a therapeutically effective neostigmine bromide or methylsulfate daily dose without the dose-limiting gastrointestinal adverse effects.
Owner:DAS MG INC
P-gp inducers as protectors against chemotherapy-induced side effects, such as peripheral neuropathy (CIPN) and hair loss
PendingUS20220313649A1Avoid side effectsNervous disorderPharmaceutical delivery mechanismSide effectChemotherapy induced
The present invention relates to compositions comprising a P-gp inducer, for topical use in preventing one or more side effects of chemotherapeutics, which chemotherapeutics are transported out of cells by P-gp. Especially chemotherapy-induced peripheral neuropathy (CIPN) is a relevant side effect to treat / avoid, since CIPN may be a dose limiting side effect of chemotherapy. The invention also relates to kits and to cancer treatment of a subgroup of patients, which subgroup has been topically pretreated with a P-gp inducer.
Owner:SYDDANSK UNIV
Use of higher doses of modified release huperzine formulations
ActiveUS20200155456A1Reduce volatilityGood curative effectOrganic active ingredientsNervous disorderImmediate releasePharmaceutical drug
The present application discloses pharmaceutical compositions and methods of treating neurological disorders and seizure disorders with the high dose modified release compositions of huperzine. The pharmaceutical compositions and methods described herein, allow for higher dosing of huperzine, while avoiding rapid peak plasma levels, thereby avoiding the dose-limiting adverse events associated with the immediate release formulations.
Owner:SUPERNUS PHARM INC
Pharmaceutical compositions and method utilizing pyridostigmine and a nk-1 antagonist for treating myasthenia gravis
PendingUS20200155530A1Increased and great efficacyIncreased and great and safetyOrganic active ingredientsMuscular disorderSide effectPyridostigmine Bromide
The present invention describes the use of a NK1-antagonist, in constant combination with pyridostigmine, to facilitate the treatment of a patient suffering from myasthenia gravis by providing a therapeutically effective pyridostigmine bromide daily dose without the dose-limiting gastro-intestinal adverse effects.
Owner:DAS MG INC
Compositions and methods for treating synucleinopathies
The present invention describes the use of a 5HT3-antagonist, in combination with a 6-propylamino-4,5,6,7-tetrahydro-1,3-benzothiazole-2-amine, to reduce adverse effects and to facilitate the neuroprotective treatment of a patient suffering from a synucleinopathic disorder to enable a therapeutically effective 6-propylamino-4,5,6,7-tetrahydro-1,3-benzothiazole-2-amine daily dose without the dose-limiting adverse effects caused by pramipexole when administered alone.
Owner:CHASE THERAPEUTICS CORP
Method and system for detecting and classifying radioactive solid waste
ActiveCN105665310BEasy to handleGood control effectX-ray spectral distribution measurementDosimetersDose levelClassification methods
The invention belongs to the technical field of solid waste treatment, and discloses a radioactive solid waste detecting and classifying method. The method comprises the steps of: measuring a surface dose level, a pollution nuclide type and a radioactive specific activity of a radioactive solid waste sample; obtaining a fitting curve of the dose level and the radioactive specific activity, building a function relation of the two, and determining a dose limiting value range of the radioactive solid waste in a different-pollution-grade state; measuring a dose level of the radioactive solid waste; and comparing the dose level of the radioactive solid waste with the dose limiting value range thereof in the different-pollution-grade state, judging the pollution grade of the radioactive solid waste, and classifying and collecting the radioactive solid waste. The method compares the dose level of the radioactive solid waste with the dose level limiting value range through building the relation between the radioactive solid waste pollution grade and the dose level limiting value range, so that the pollution grade can be quickly judged, the classification and the collection are realized, and the treatment and management capacity of the radioactive solid waste is greatly promoted.
Owner:深圳市利美泰克自控设备有限公司
Medical injection system comprising a medical injection device and a dose limiter module
InactiveUS9604007B2Easy procedureInfusion syringesIntravenous devicesBiomedical engineeringInjection device
The present invention relates to a medical injection system comprising a medical injection device (100) of the type comprising a rotatable dose setting member (160). The system further comprises a dose limiter module (200) adapted for being releasably coupled to the injection device (100). The dose limiter module (200) comprises a dose limiter base (201) and a limiter (240) arranged relative to the dose limiter base (201) so that their relative position is adjustable to set an adjusted dose limiting position. When the dose limiter module (200) is coupled to the injection device (100), the limiter (240) cooperates with the dose setting member (160) to define a blocking means for preventing rotation of the dose setting member (160) beyond the adjusted dose limiting position. The dose limiter module (200) further comprises a user operable lock (210,212,232,223,243) configured for operation between a locked state and an unlocked state to respectively prevent and enable modification of the adjusted dose limiting position, the lock (210,212,232,223,243) being configured for maintaining the lock in the locked state at least when the dose limiter module (200) is not coupled with the injection device (100).
Owner:NOVO NORDISK AS
drug delivery device
Owner:SANOFI SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com






![Novel derivative of N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N'-propyl and application of novel derivative Novel derivative of N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N'-propyl and application of novel derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2d75608f-8c95-4dd3-92d1-9b30833ec10f/FDA0000429061800000011.png)
![Novel derivative of N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N'-propyl and application of novel derivative Novel derivative of N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N'-propyl and application of novel derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2d75608f-8c95-4dd3-92d1-9b30833ec10f/BDA0000429061810000021.png)
![Novel derivative of N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N'-propyl and application of novel derivative Novel derivative of N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N'-propyl and application of novel derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2d75608f-8c95-4dd3-92d1-9b30833ec10f/BDA0000429061810000041.png)