Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
97 results about "Vascular malformation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
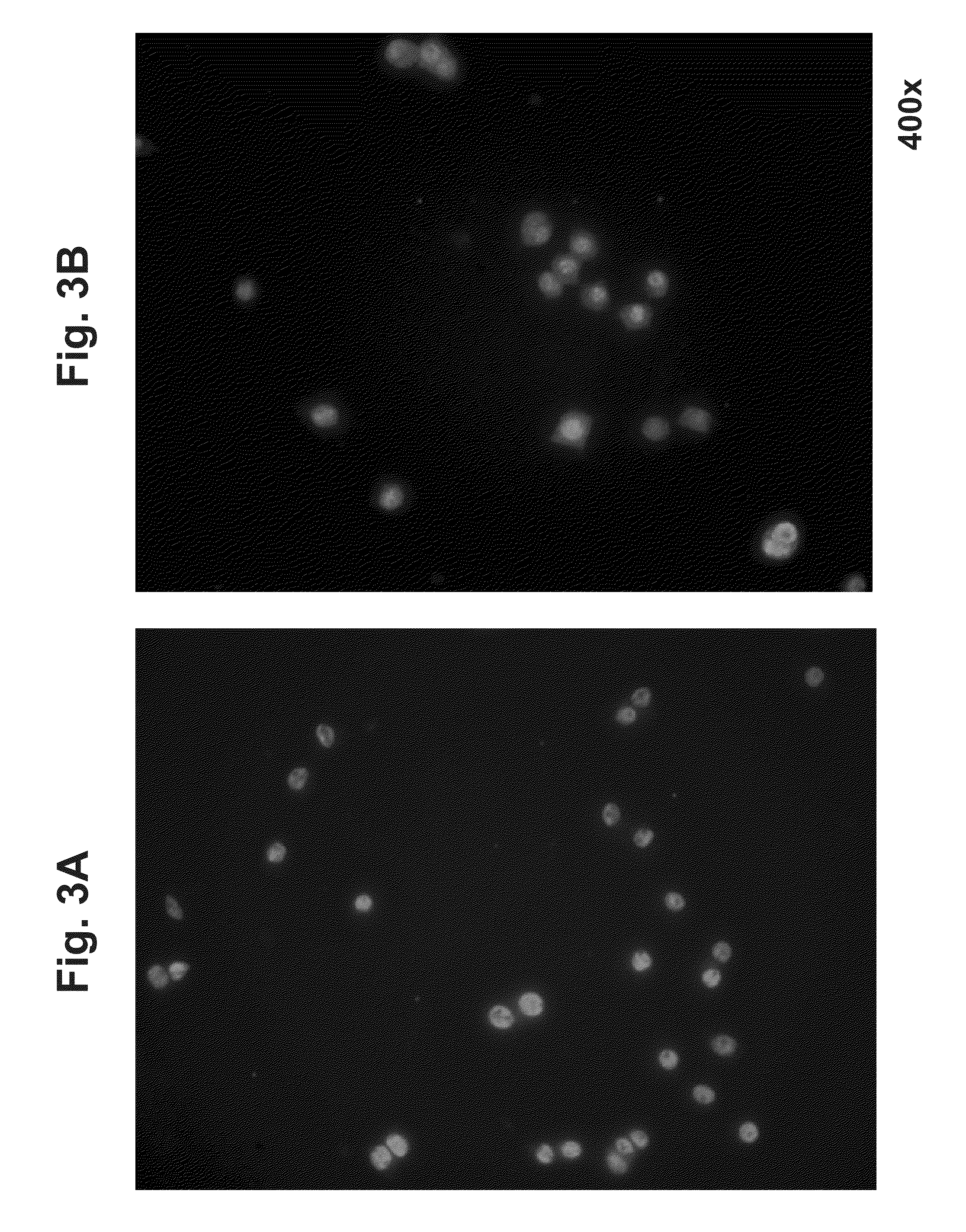
Vascular malformation, is a blood vessel abnormality. There are many types, but the most common is arteriovenous malformation. It may cause aesthetic problems as it has a growth cycle and can continue to grow throughout life.
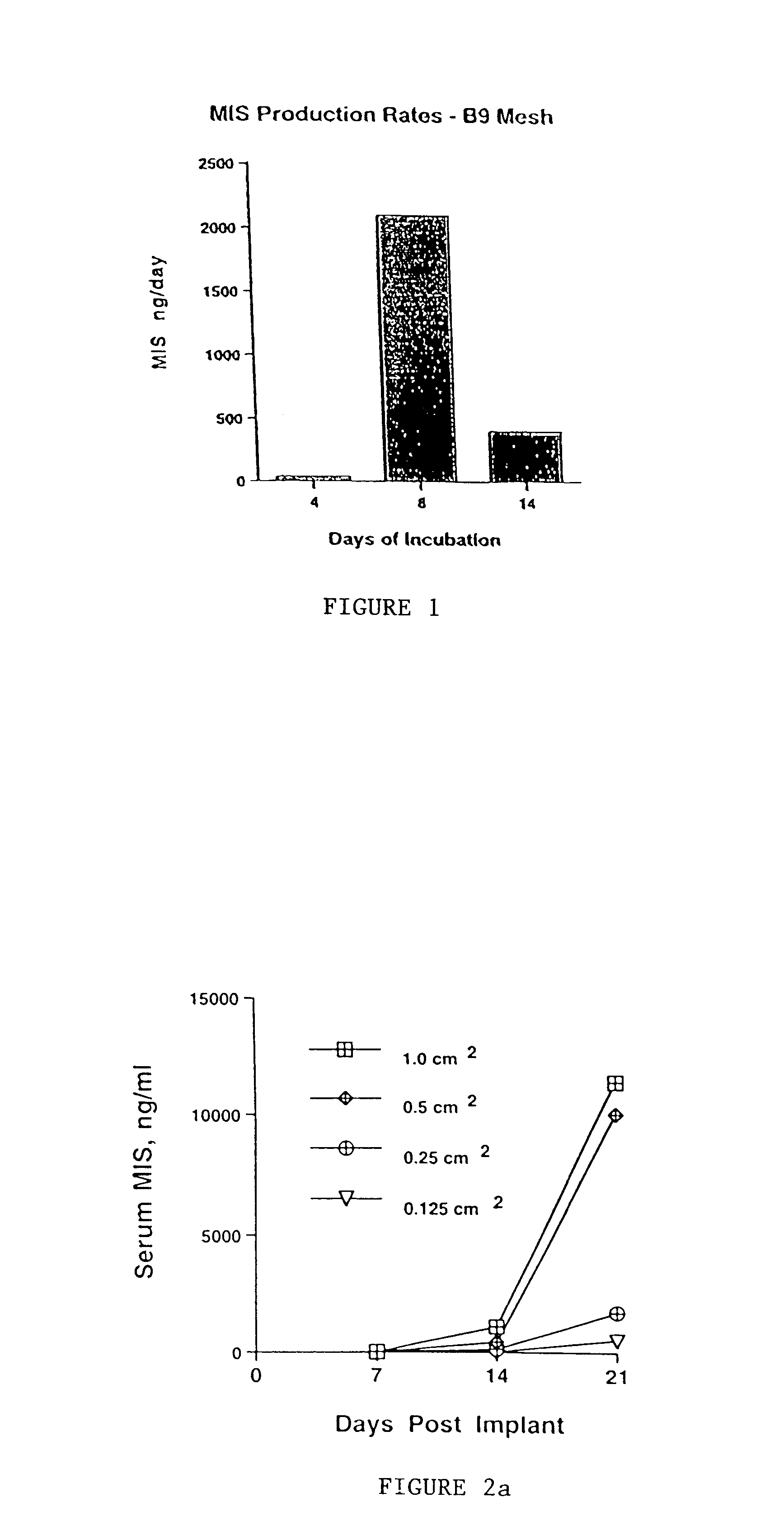
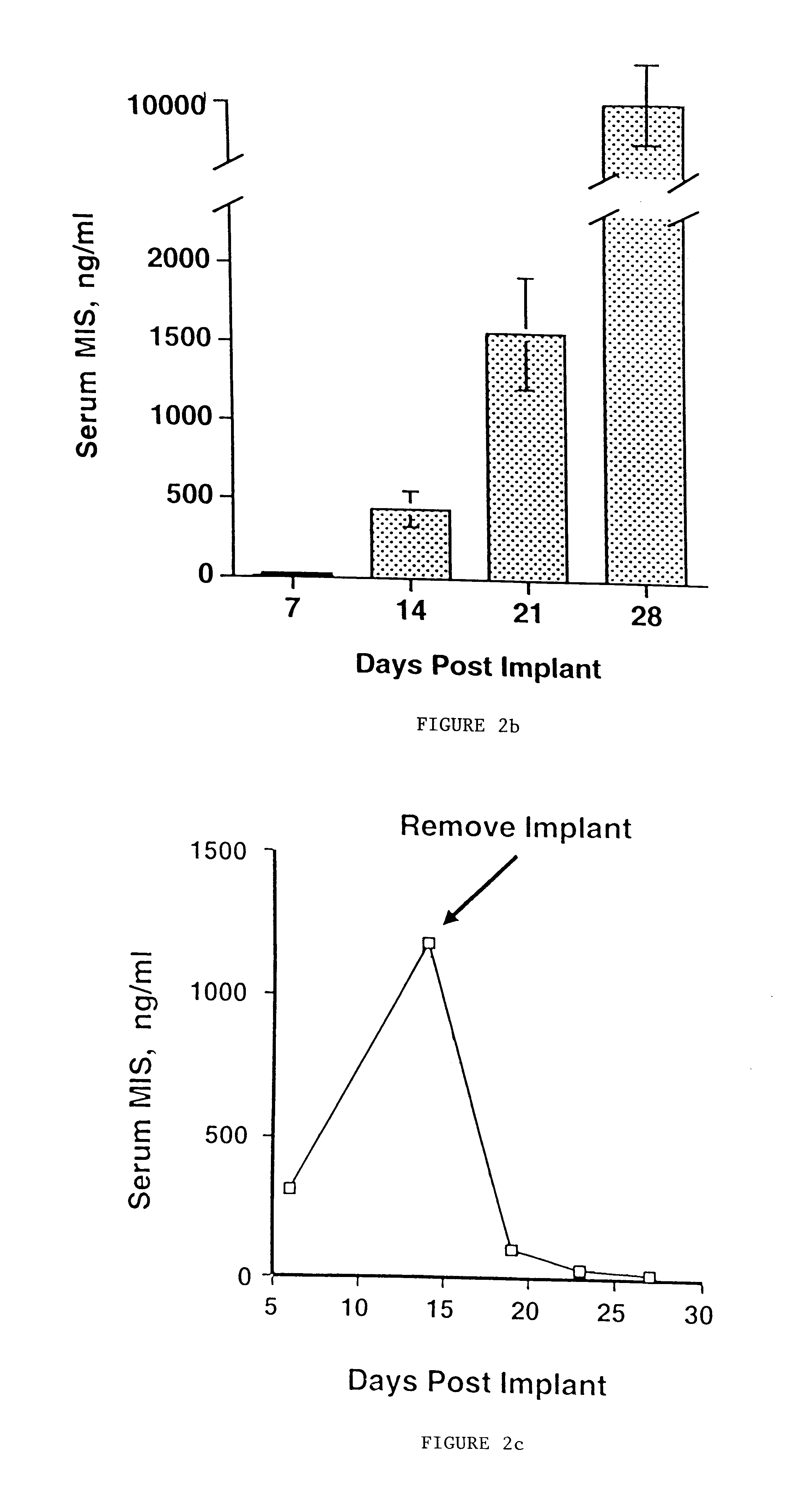
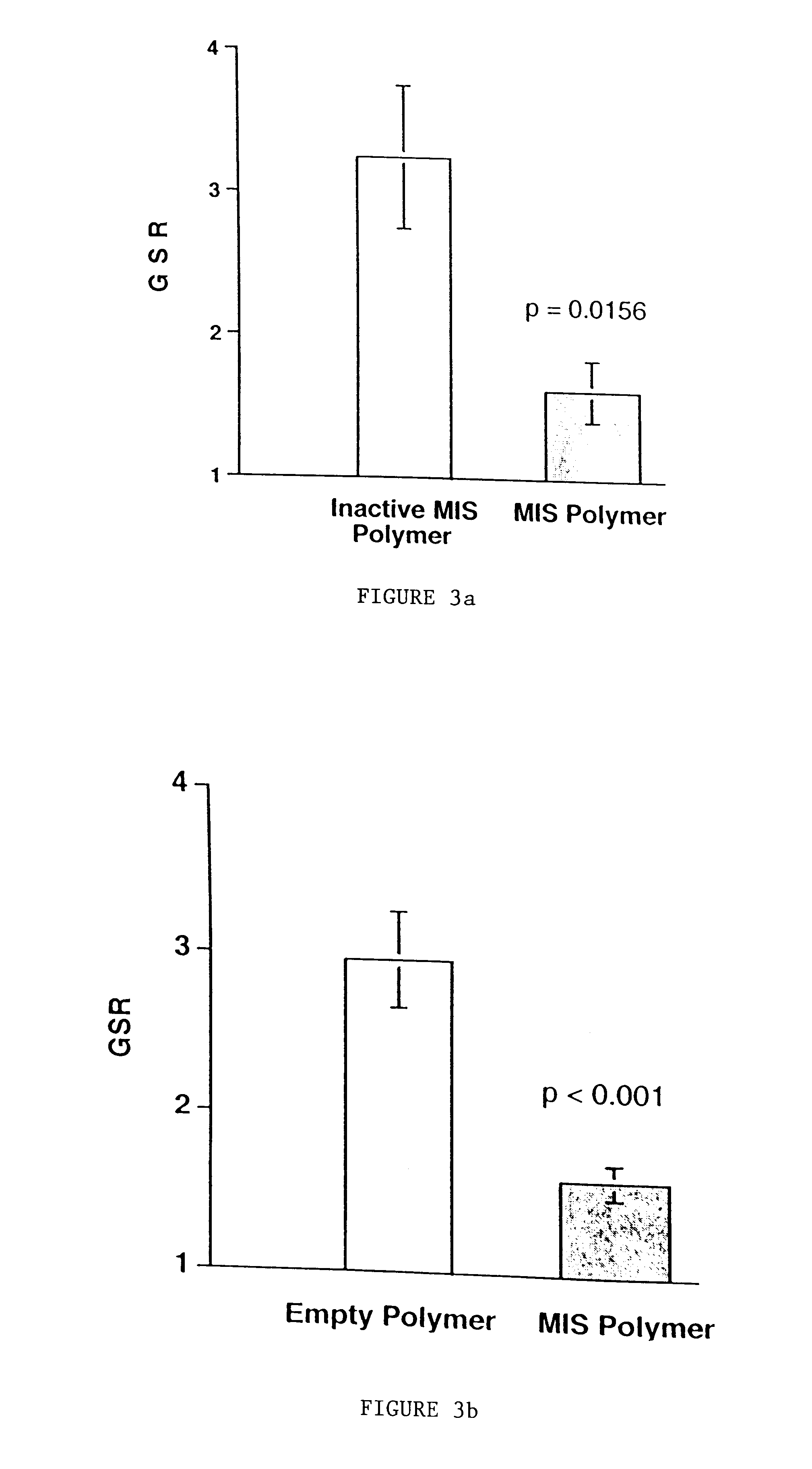
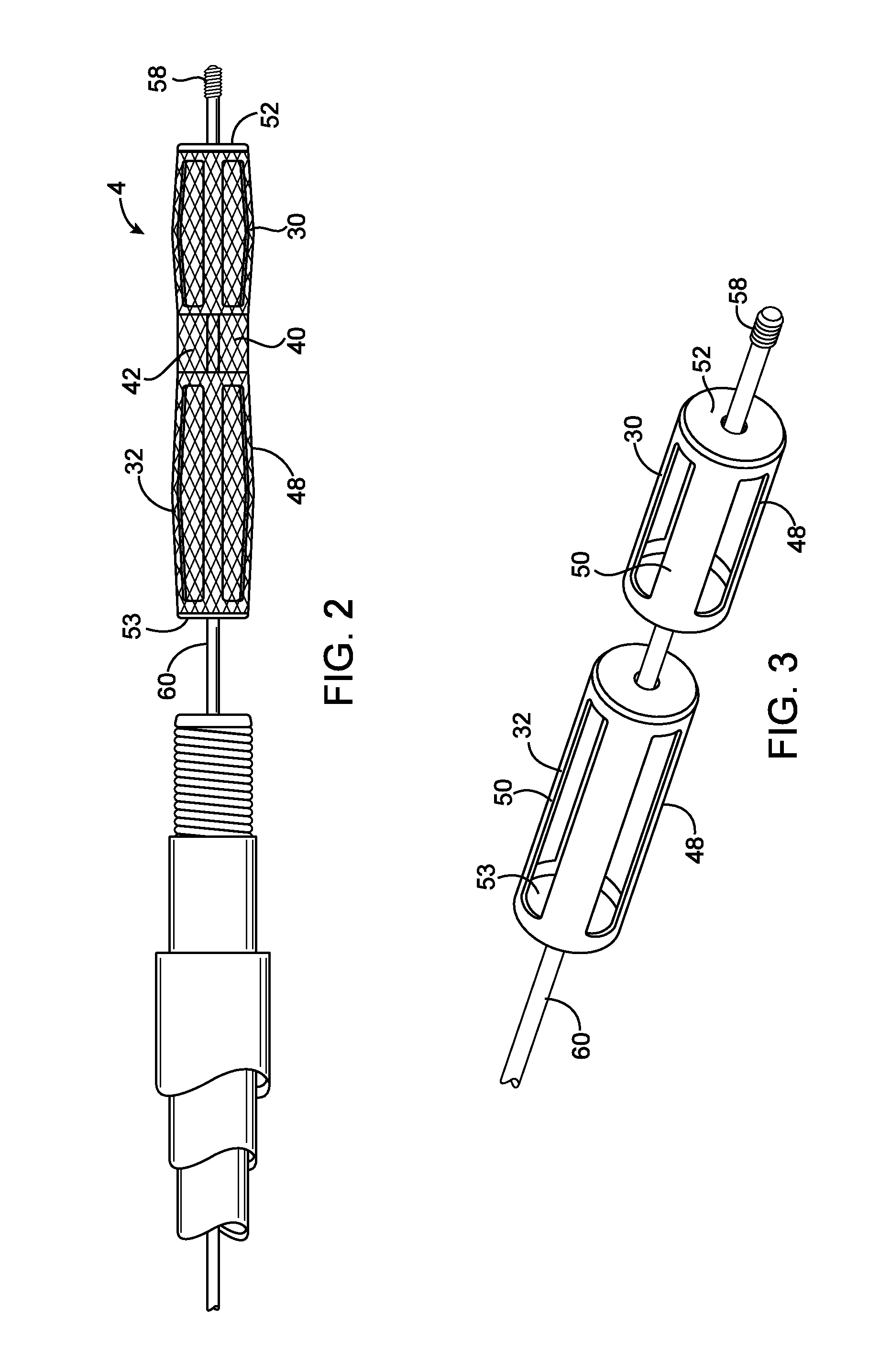
Delivery of therapeutic biologicals from implantable tissue matrices
Normal cells, such as fibroblasts or other tissue or organ cell types, are genetically engineered to express biologically active, therapeutic agents, such as proteins that are normally produced in small amounts, for example, MIS, or other members of the TGF-beta family Herceptin(TM), interferons, andanti-angiogenic factors. These cells are seeded into a matrix for implantation into the patient to be treated. Cells may also be engineered to include a lethal gene, so that implanted cells can be destroyed once treatment is completed. Cells can be implanted in a variety of different matrices. In a preferred embodiment, these matrices are implantable and biodegradable over a period of time equal to or less than the expected period of treatment, when cells engraft to form a functional tissue producing the desired biologically active agent. Implantation may be ectopic or in some cases orthotopic. Representative cell types include tissue specific cells, progenitor cells, and stem cells. Matrices can be formed of synthetic or natural materials, by chemical coupling at the time of implantation, using standard techniques for formation of fibrous matrices from polymeric fibers, and using micromachining or microfabrication techniques. These devices and strategies are used as delivery systems via standard or minimally invasive implantation techniques for any number of parenterally deliverable recombinant proteins, particularly those that are difficult to produce in large amounts and / or active forms using conventional methods of purification, for the treatment of a variety of conditions that produce abnormal growth, including treatment of malignant and benign neoplasias, vascular malformations (hemangiomas), inflammatory conditions, keloid formation, abdominal or plural adhesions, endometriosis, congenital or endocrine abnormalities, and other conditions that can produce abnormal growth such as infection. Efficacy of treatment with the therapeutic biologicals is detected by determining specific criteria, for example, cessation of cell proliferation, regression of abnormal tissue, or cell death, or expression of genes or proteins reflecting the above.
Owner:THE GENERAL HOSPITAL CORP
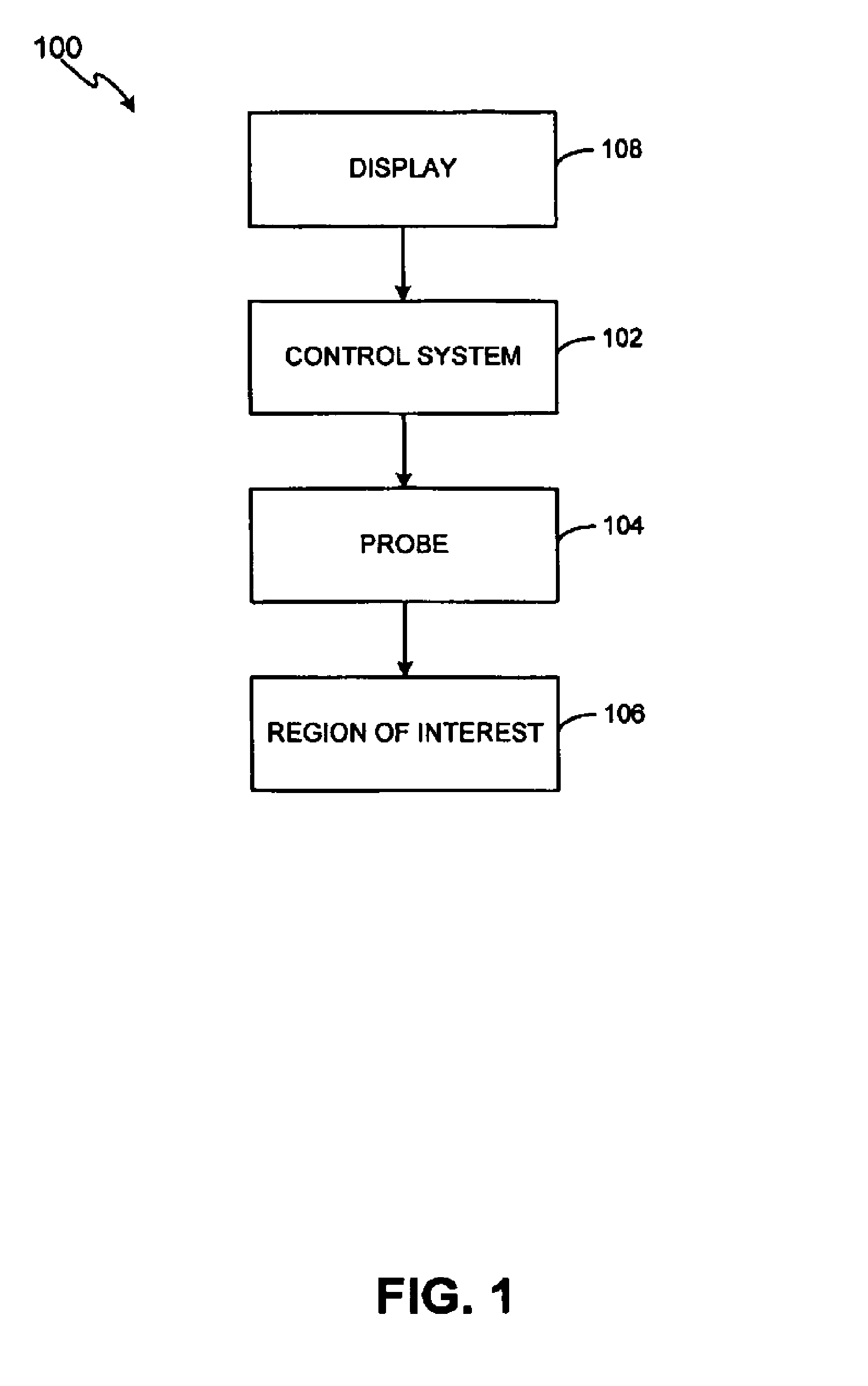
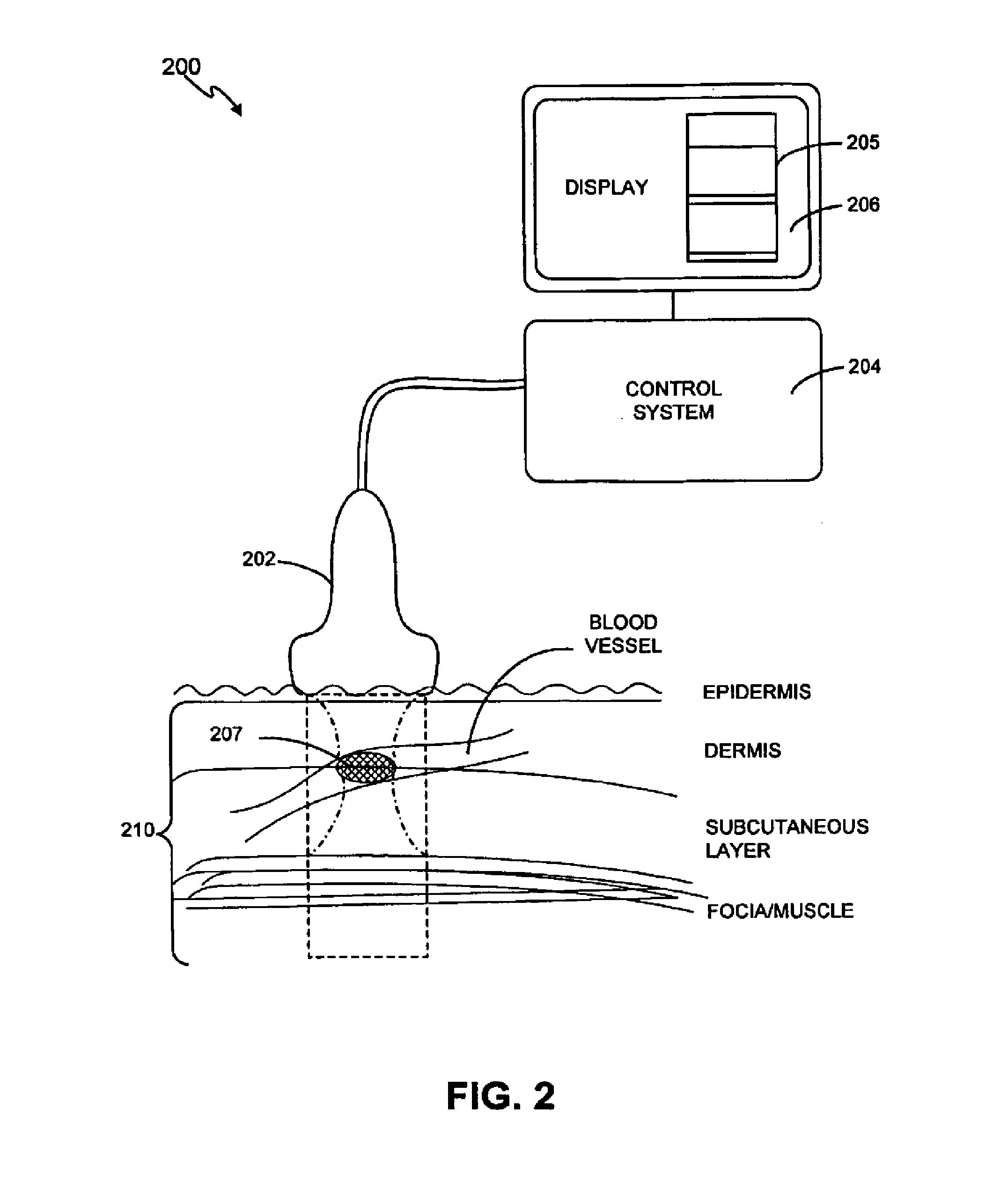
Noninvasive treatment of blood vessels
InactiveUS20120330197A1Good effectHighly focusedUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyVascular diseaseBlood vessel spasm
A non-invasive method and system for using ultrasound energy for the treatment of conditions resulting from vascular disorders is provided. In one embodiment, an image-treatment approach can be used to locate the blood vessel to be treated and then to ablate it non-invasively, while also monitoring the progress of the treatment. In another embodiment, a transducer is configured to deliver ultrasound energy to the regions of the superficial tissue (e.g., skin) such that the energy is deposited at the particular depth at which the vascular malformations are located below the skin surface. The ultrasound transducer can be driven at a number of different frequency regimes such that the depth and shape of energy concentration can match the region of treatment.
Owner:GUIDED THERAPY SYSTEMS LLC
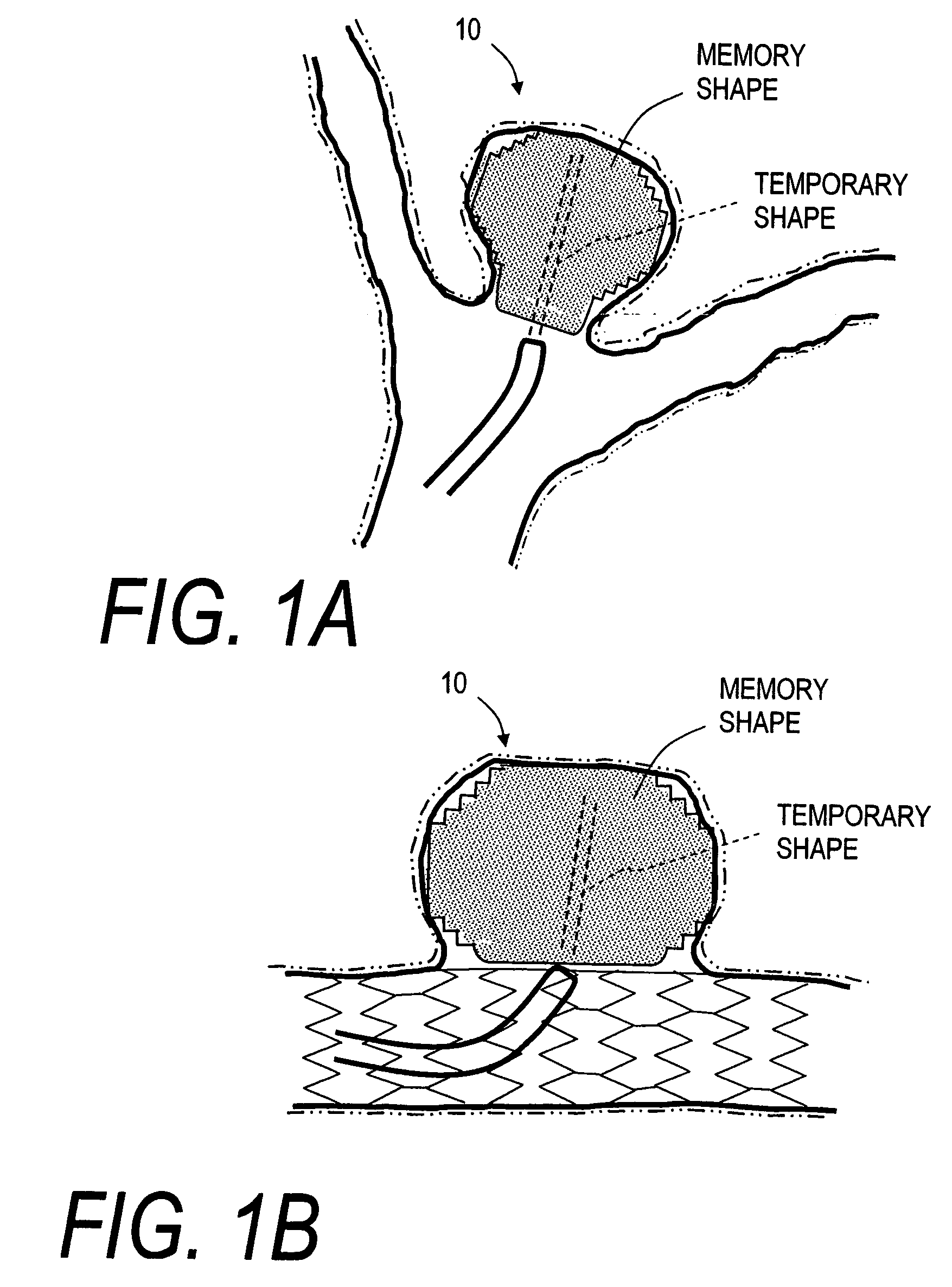
Endovascular occlusion devices and methods of use
InactiveUS20050267570A1Rapid formation of thrombusWithout riskStentsOcculdersVascular malformationAnterior Cerebral Artery Aneurysm
An implantable stent-like device for treating and occluding arteriovascular malformations (AVMs), fistulas, varicose veins or the like. More particularly, this invention relates to an implant body that includes an open-cell shape-transformable polymer structure that provides stress-free means for occluding an AVM without applying additional pressures an any distended walls of the AVM. In one embodiment, the shape-transformable polymer is a shape memory polymer implant body that self-deploys from a temporary shape to a memory shape. In another embodiment, the shape memory polymer structure is capable of a temporary compacted shape for carrying about the struts of an expandable stent for self-deployment to occlude an aneurysm.
Owner:SHADDUCK JOHN H
In-Situ Forming Foams for Embolizing or Occluding a Cavity
The present invention provides systems and methods for occluding and / or embolizing a cavity within a patient by delivering a prepolymer material into or onto a cavity and forming an expanding foam within the cavity. The inventions methods are applicable to occluding a variety of cavities, including blood vessels, aneurysms, left arterial appendages, vascular malformations and the like.
Owner:ARSENAL MEDICAL
Apparatus and method for treatment of cerebral aneurysms, arterial-vascular malformations and arterial fistulas
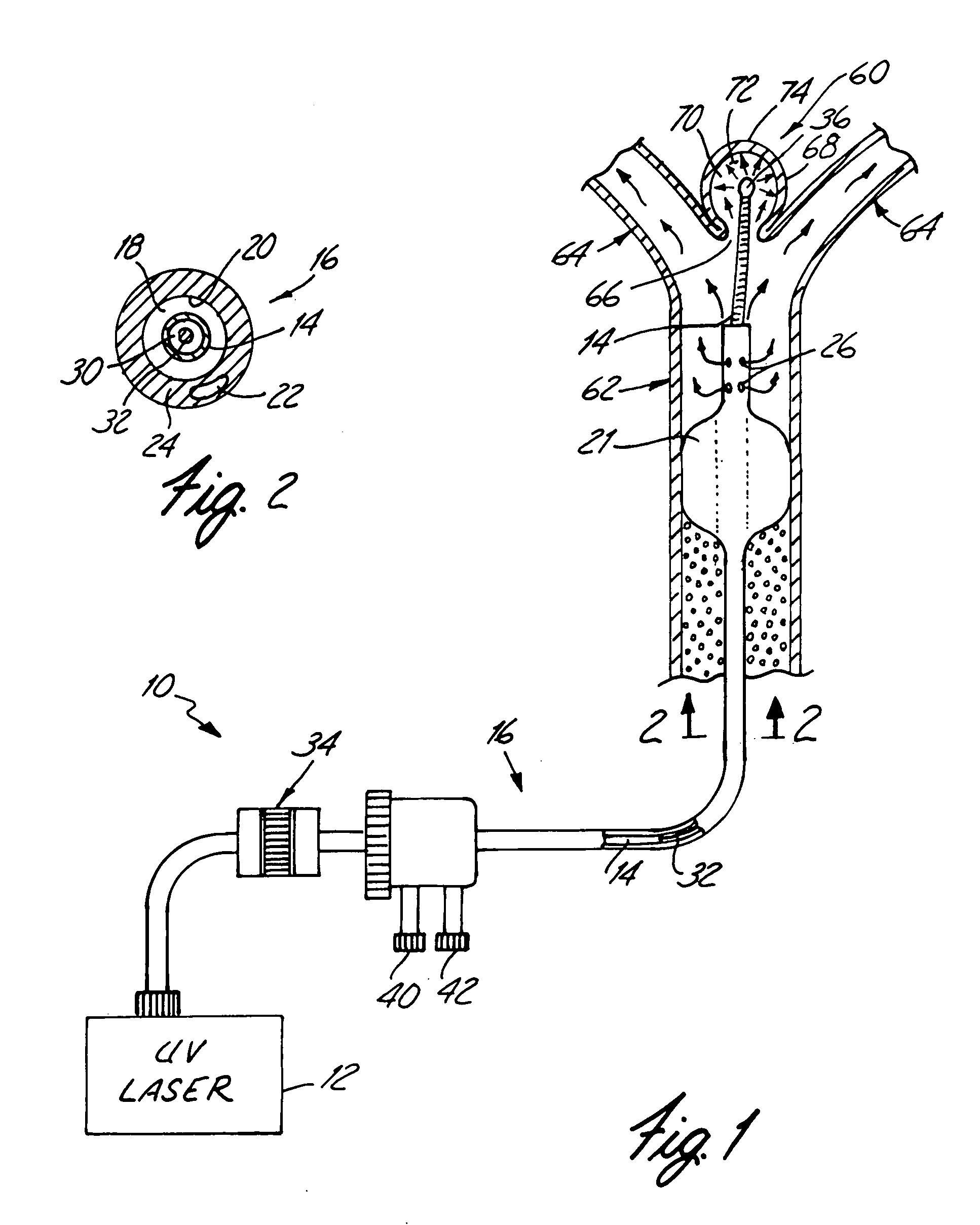
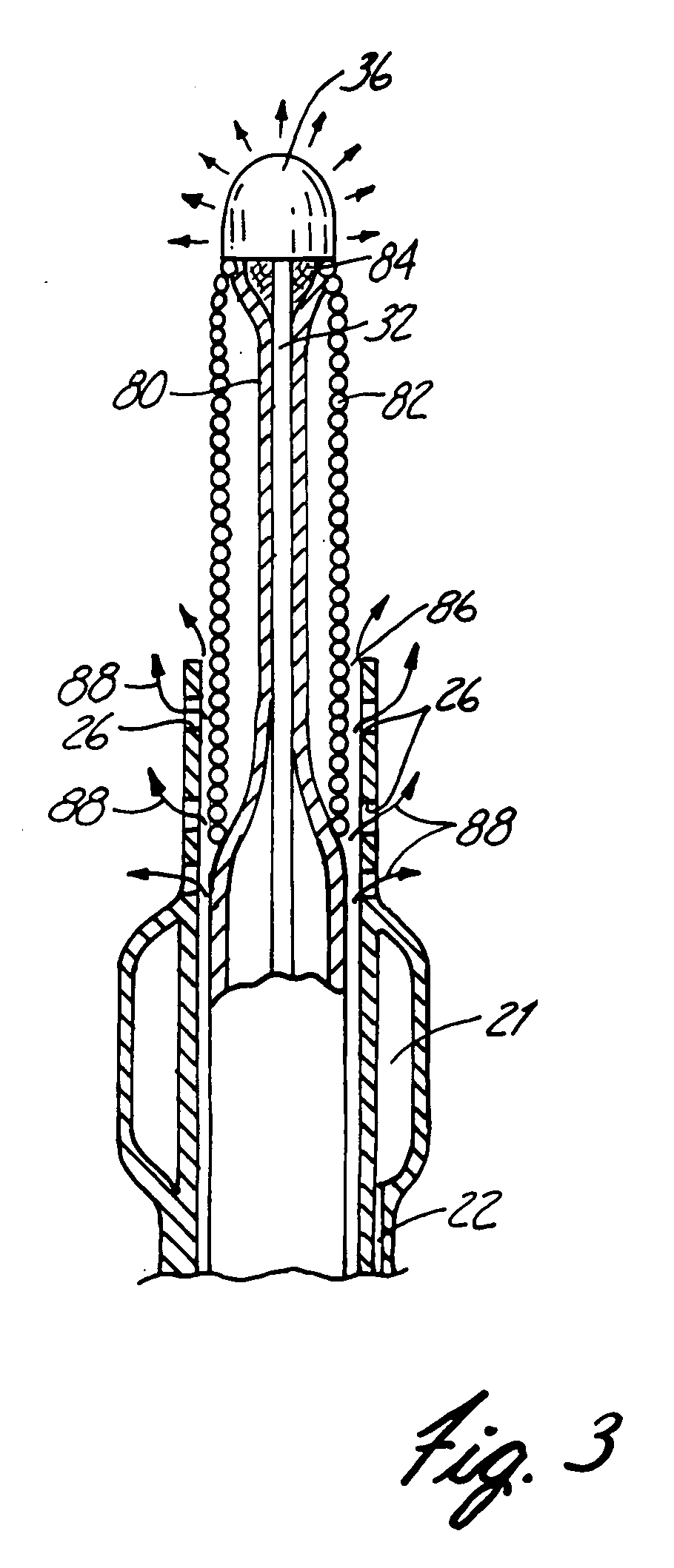
An apparatus and method for stabilization of aneurysm is disclosed. The apparatus comprises an ultraviolet radiation generator for generating UV radiation having a wavelength, strongly absorbed by the DNA and a catheter including means for delivering the ultraviolet radiation to the aneurysm. The distal end of the catheter is placed inside the aneurysm. Stabilization of the aneurysm is achieved by forming a mural arterial thrombus inside the aneurysm. To make irradiation possible, the blood is displaced from the aneurysm by a steady stream of UV radiation transparent fluid. The injury to the endothelium that triggers the thrombus formation is caused by UV radiation delivered to the aneurysm. In several days after intervention the thrombus becomes fully organized, leaving on the inside surface of the aneurysm a thick layer of fibrotic tissue that stabilizes the aneurysm.
Owner:MINNESOTA MEDICAL PHYSICS LLC
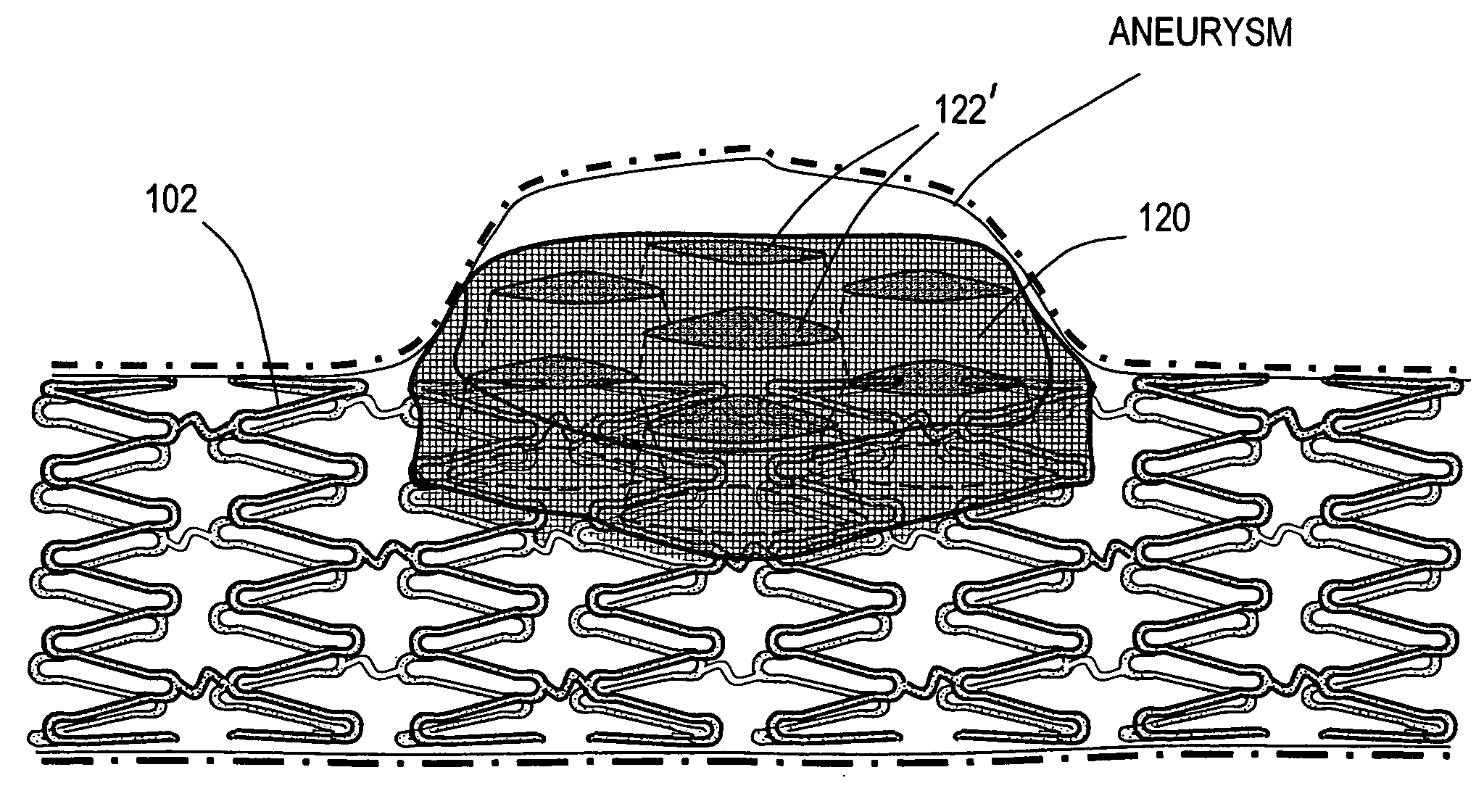
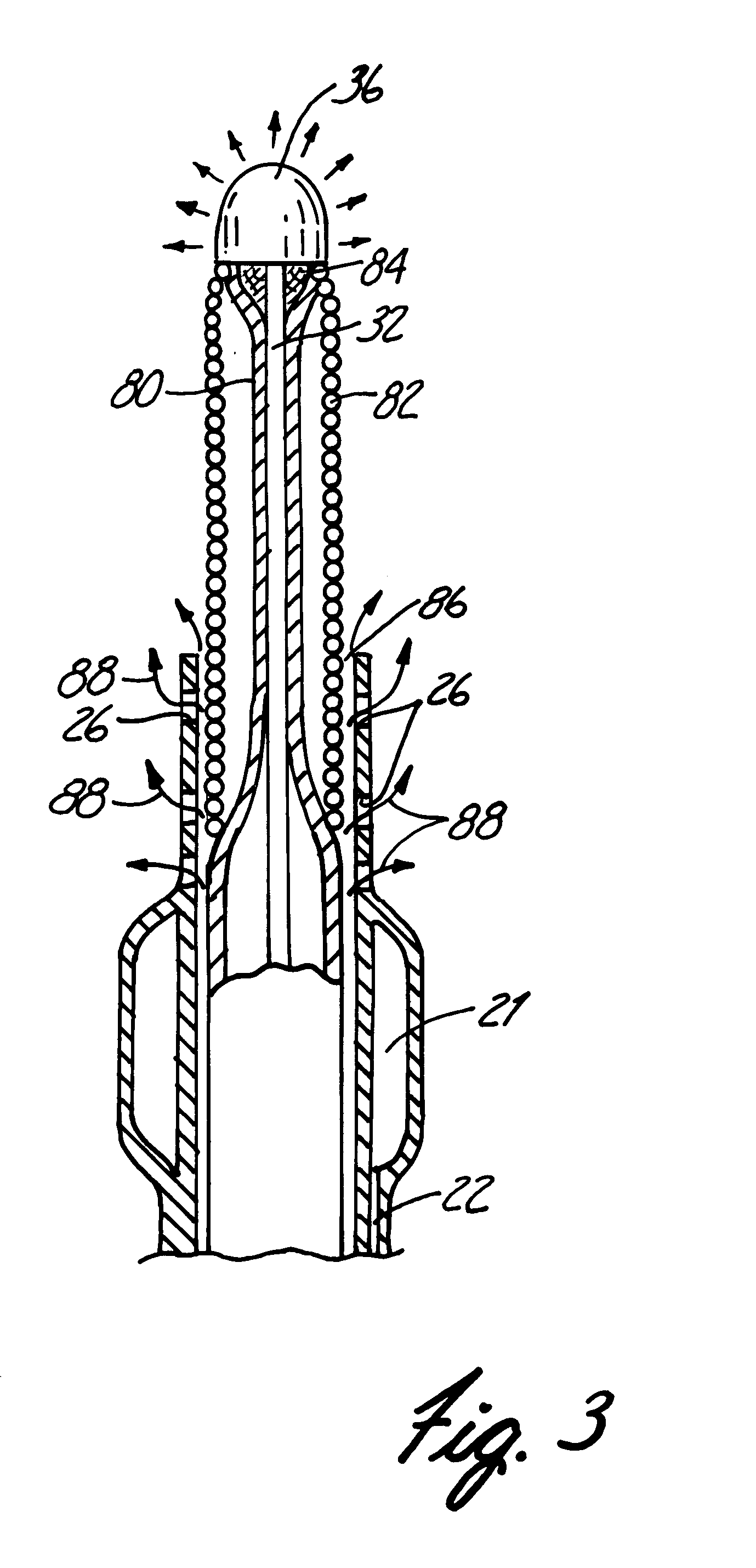
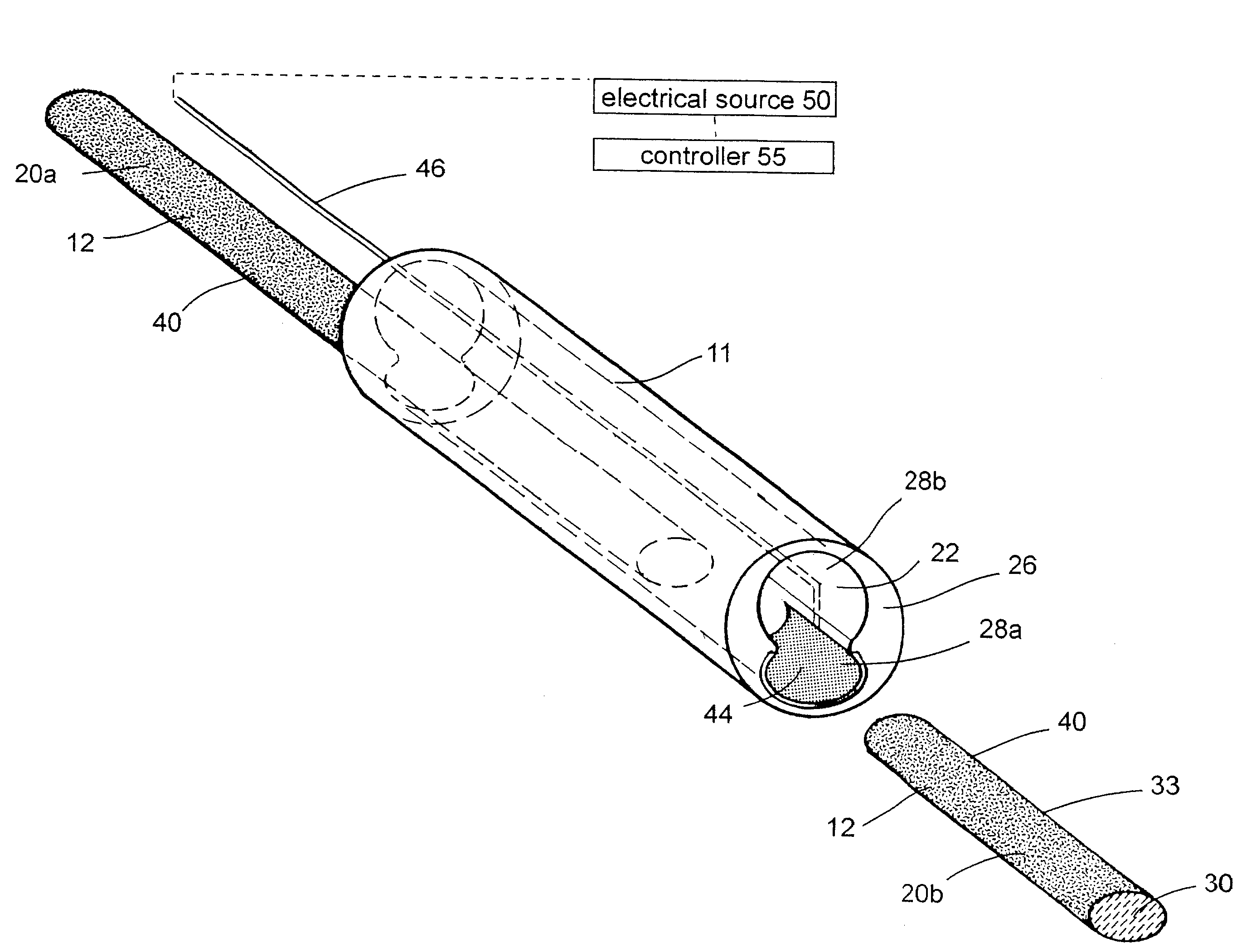
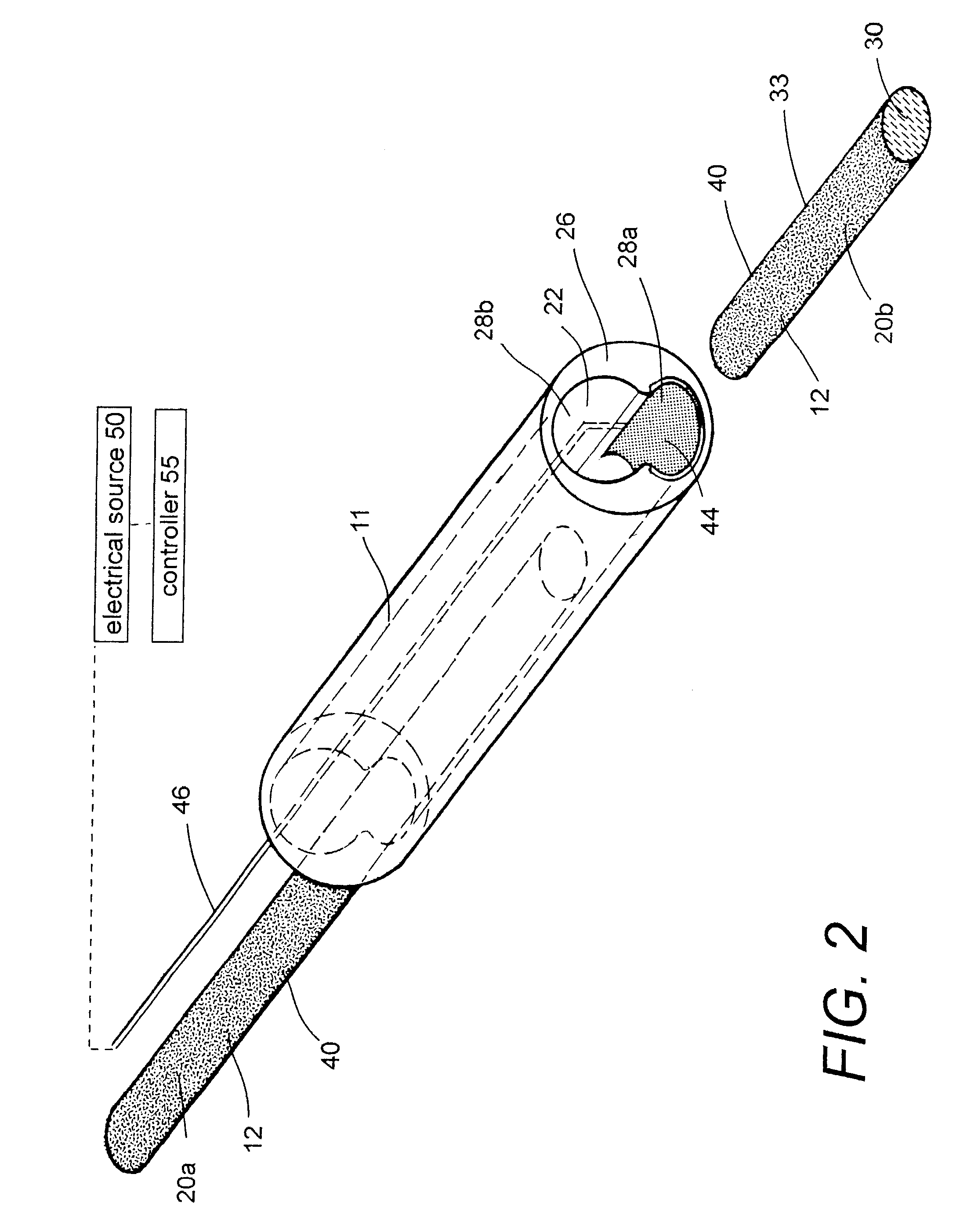
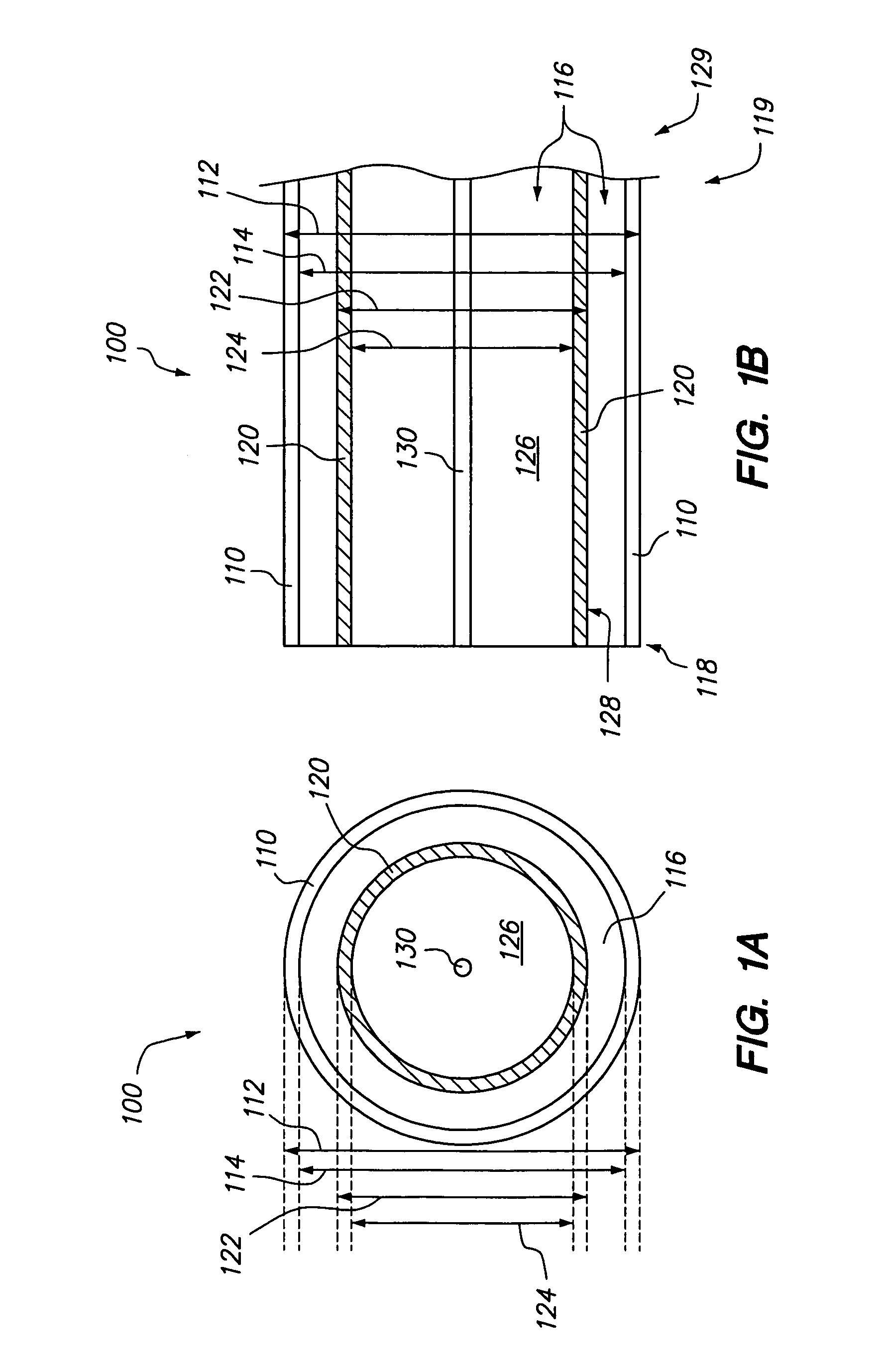
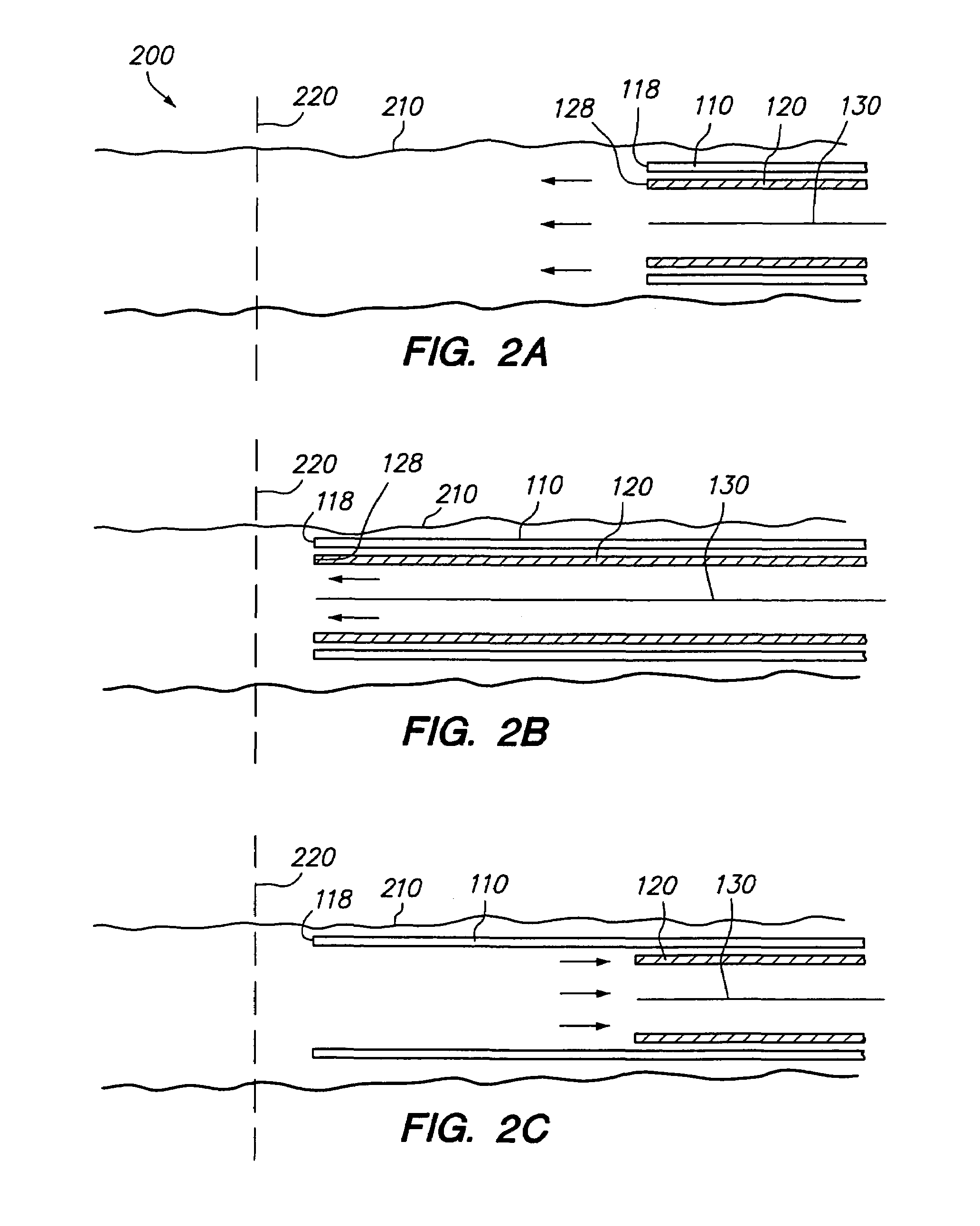
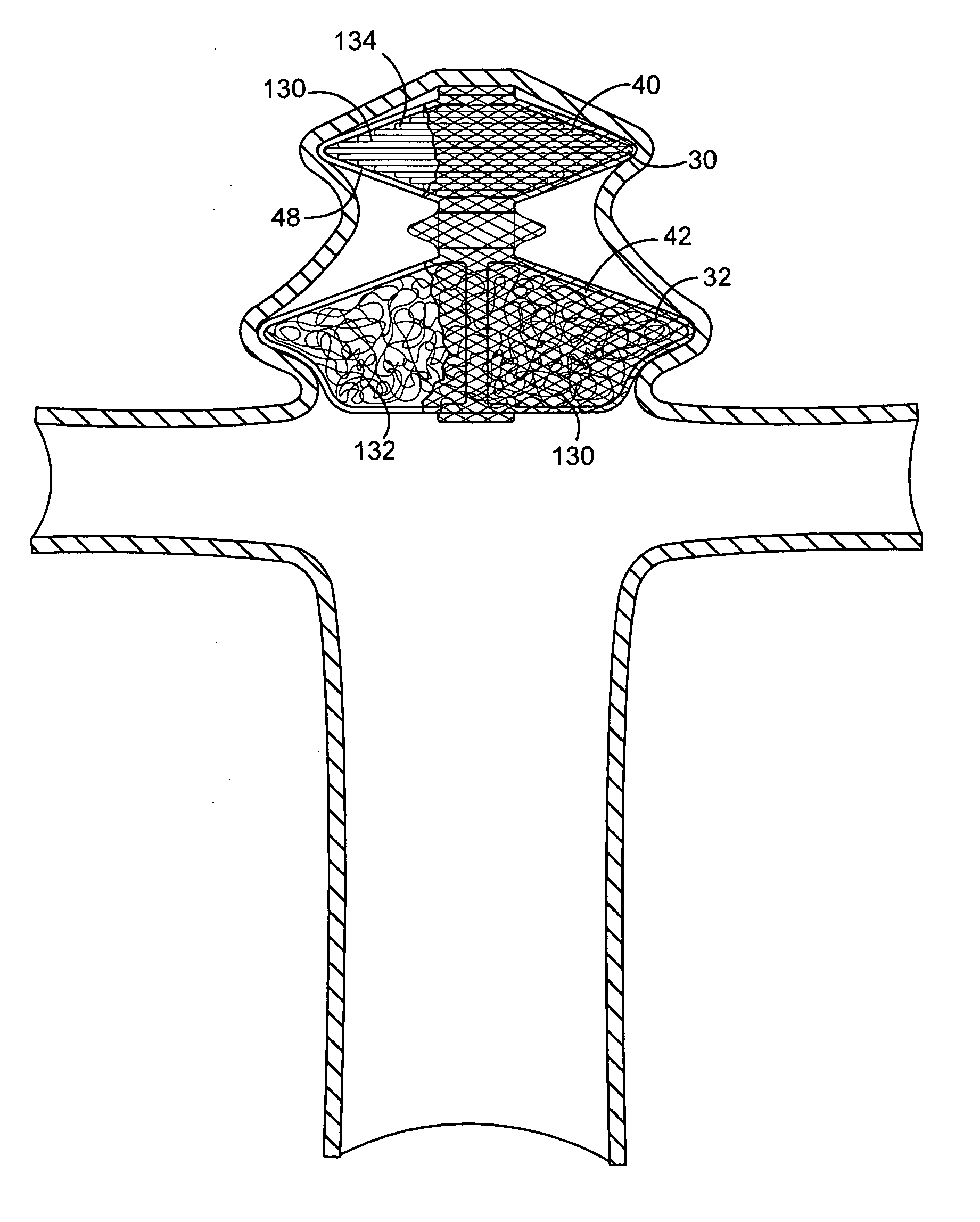
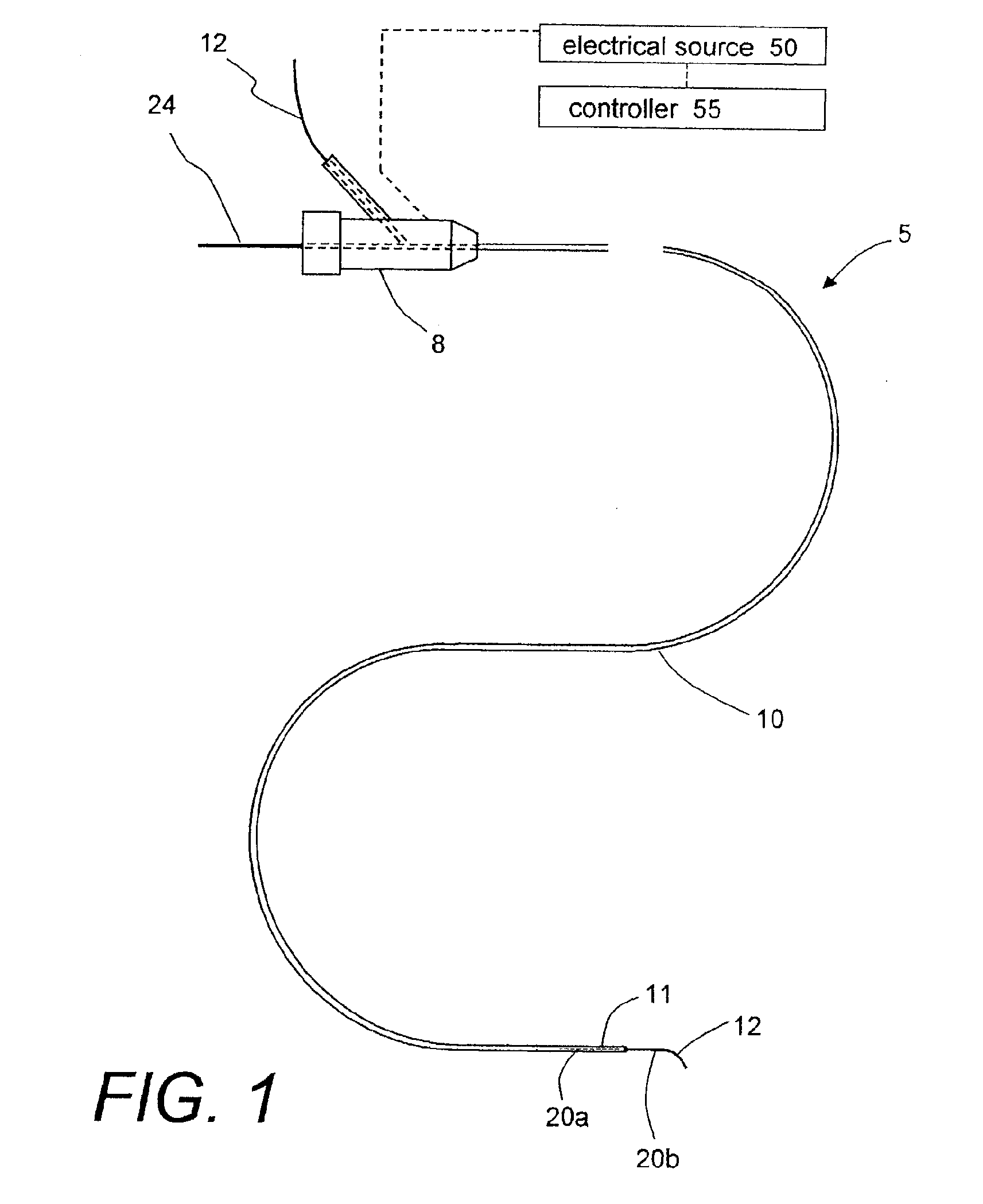
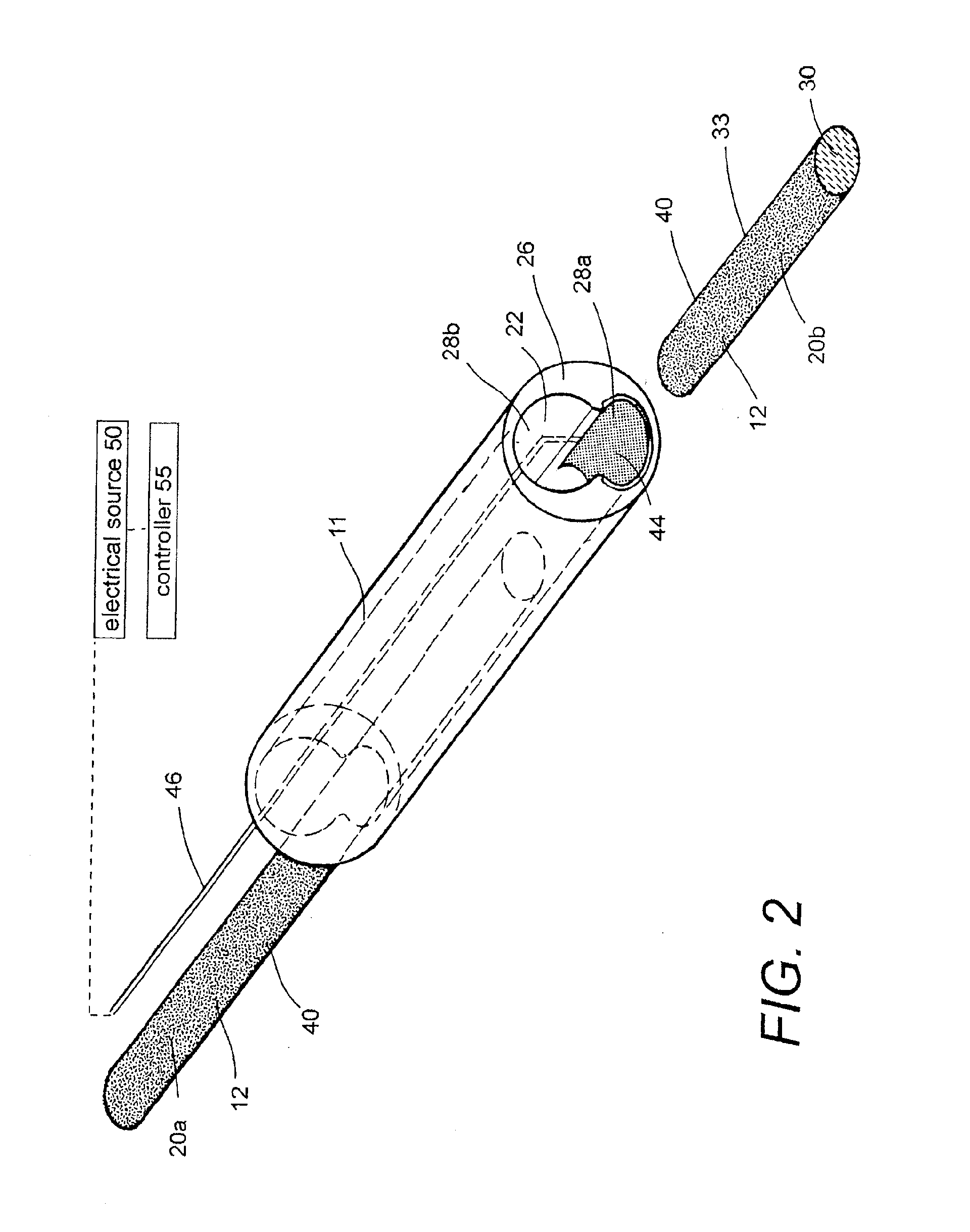
Polymer matrix devices for treatment of vascular malformations
ActiveUS7306598B2Control ratePromote formationStentsDiagnostic recording/measuringWide necked aneurysmVascular malformation
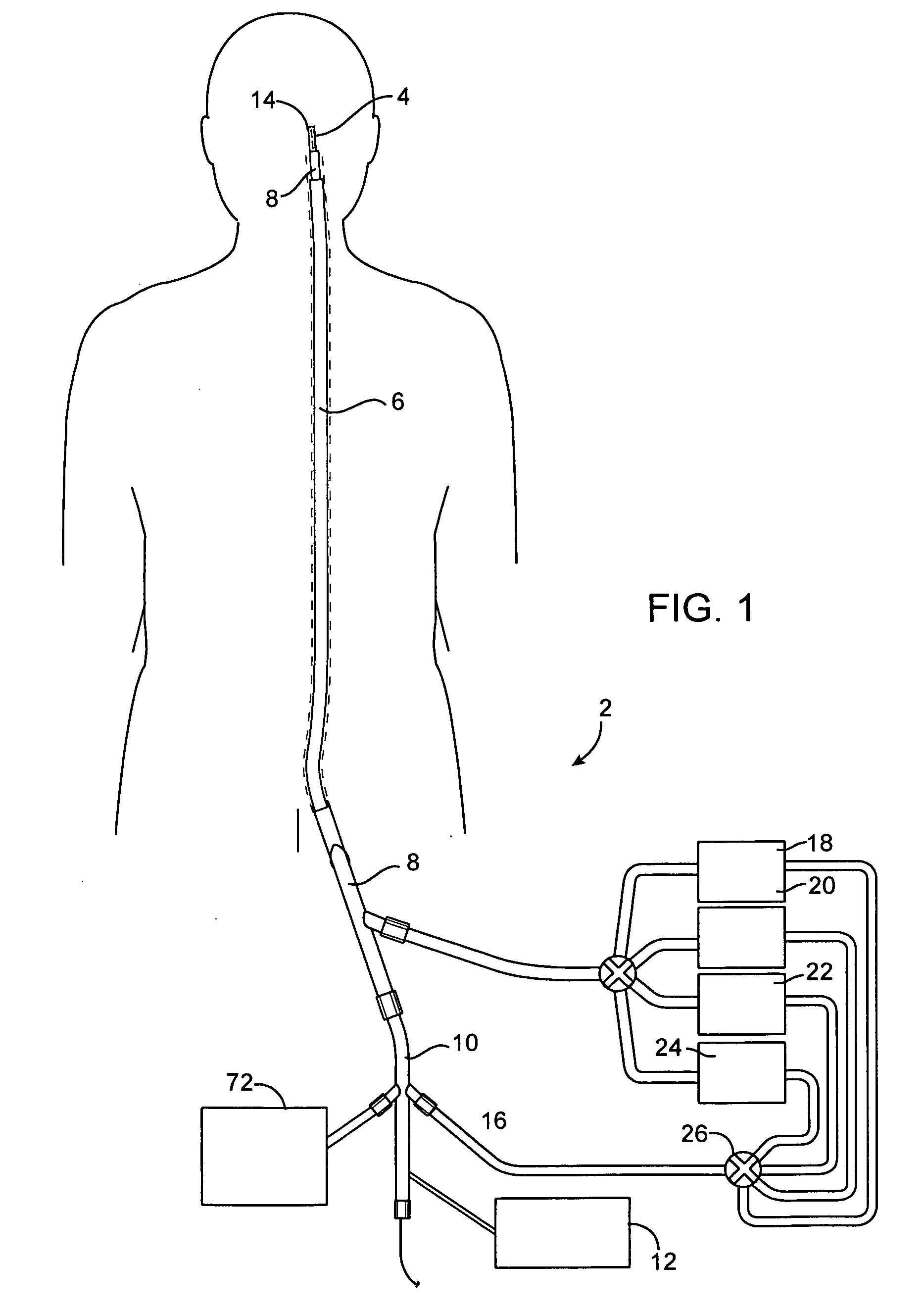
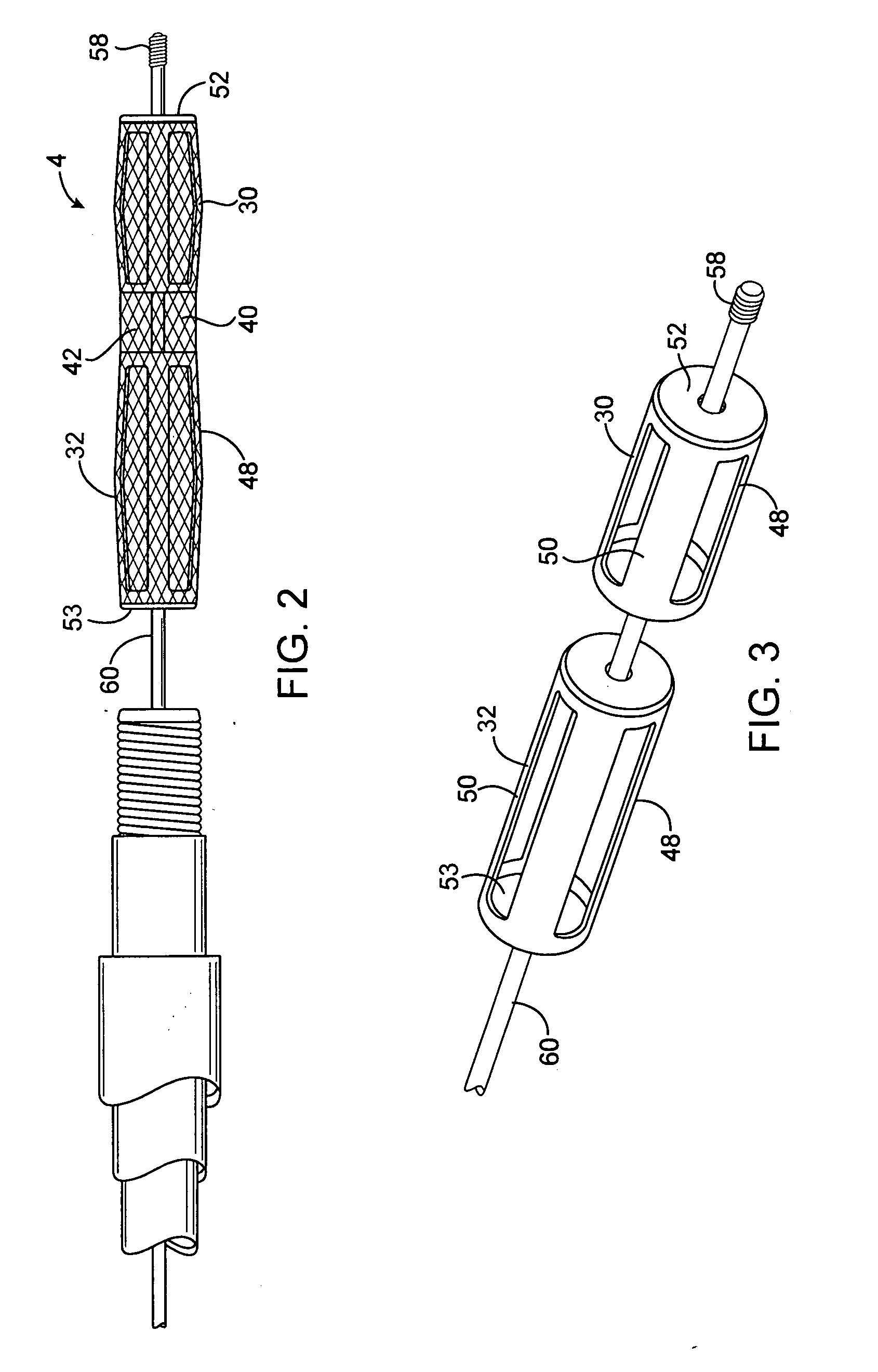
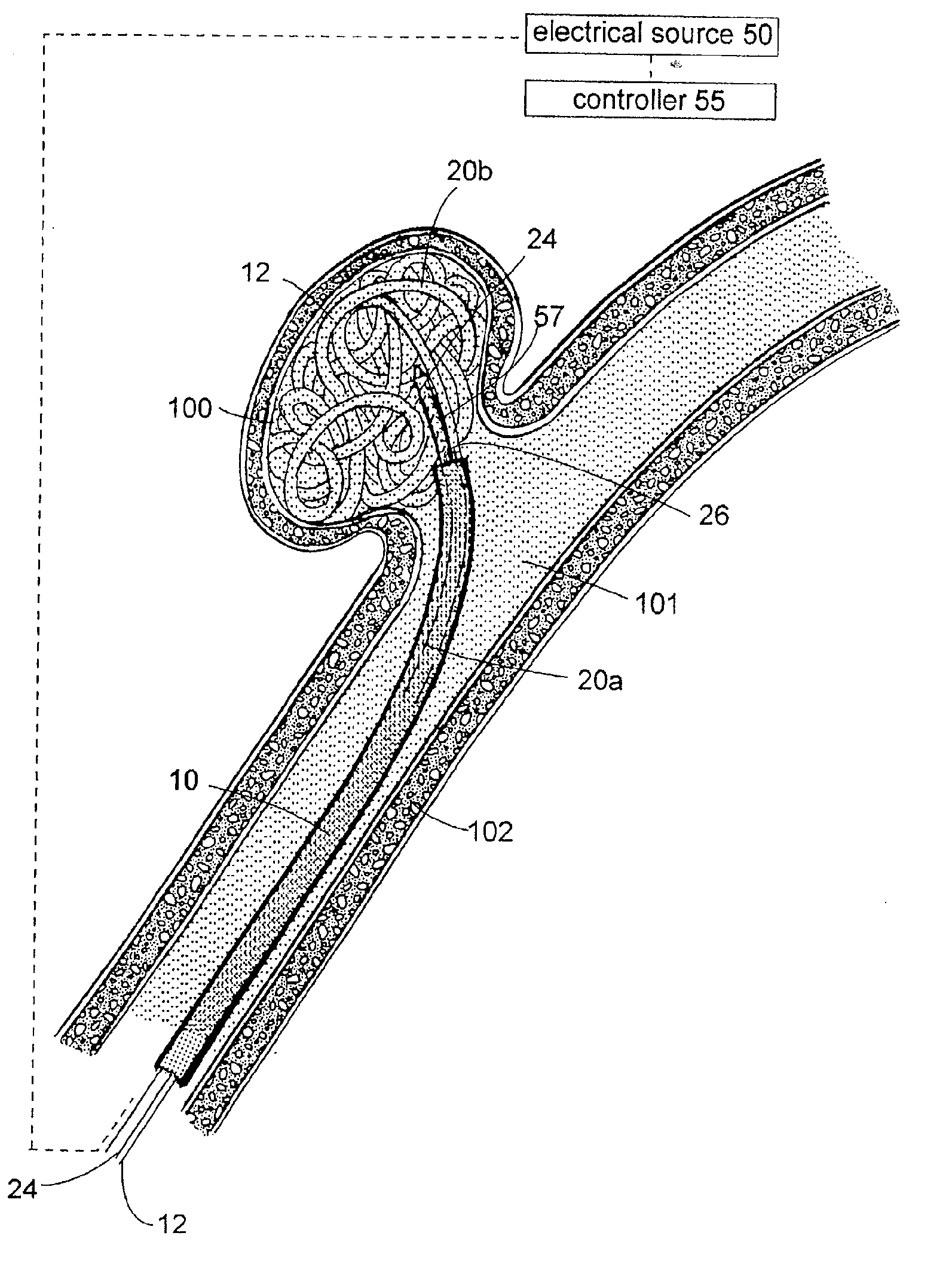
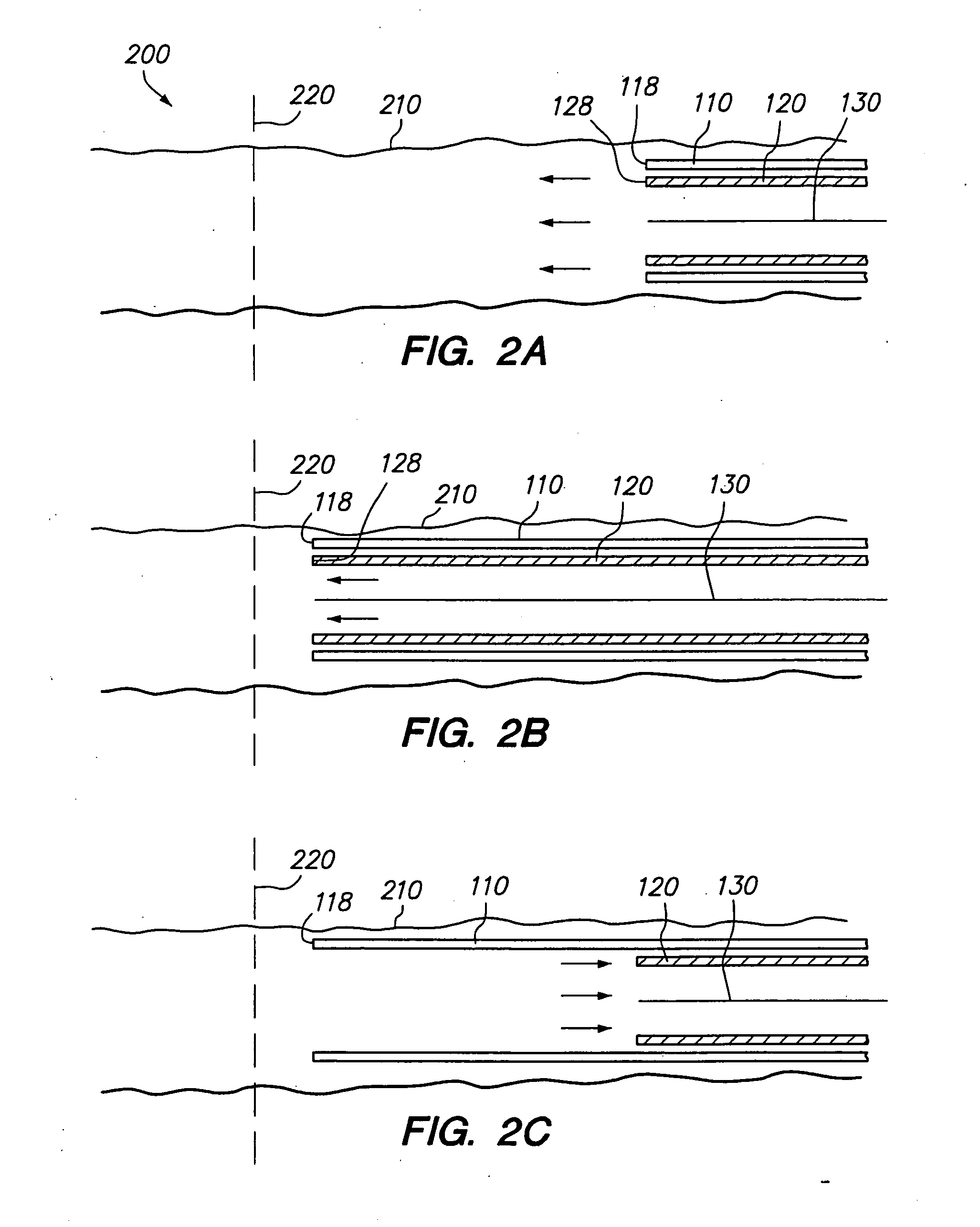
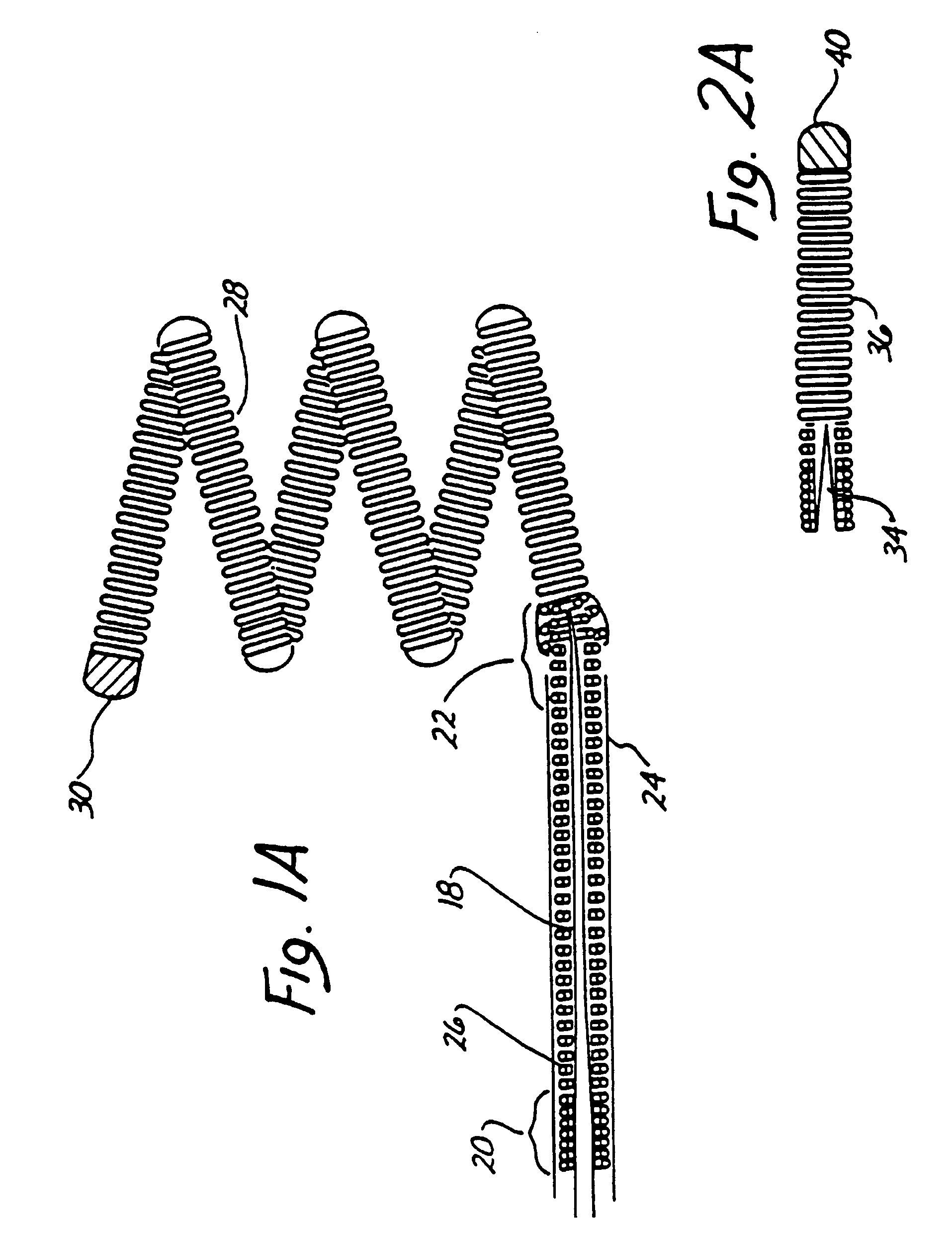
A system for treating a wide-neck aneurysm comprising a mesh-like sleeve fabricated from a class of polymer filaments that carry conductive particles therein to provide the filaments with a specified resistivity. The releasable mesh-like sleeve is introduced to the site of a targeted vascular malformation by the working end of a catheter that carries an electrode arrangement at its distal terminus. The system further provides an electrical source and controller (i) that modulates power delivery to the polymer matrix which can then fuse the sleeve to the wall of a blood vessel to span across the vascular malformation.
Owner:DFINE INC
Method and system for delivering an implant utilizing a lumen reducing member
A method and system for inserting an implant, such as vaso-occlusive device, an embolic containment device, or a stent into a vascular space to a vascular site in a body utilizing a lumen-reducing catheter. The method and system can be used to treat aneurysm, tumors and other vascular malformations. A guide is inserted into the vascular space. First and second catheters are inserted into the vascular space along the guide. The first or delivery catheter defines a first cavity, and the second or lumen-reducing catheter defines a second, smaller cavity. The second catheter is inserted within the first catheter. In one embodiment, an implant is advanced together with the first and second catheters to a vascular site. In an alternative embodiment, after the first and second catheters are positioned, the guide and the second catheter are removed from the first cavity, and an implant is inserted through the first cavity. With these configurations, radial movement of the guide is restricted to the smaller, second cavity rather than the larger, first cavity.
Owner:STRYKER EURO OPERATIONS HLDG LLC +1
Devices and methods for treating vascular malformations
InactiveUS20110224776A1Ensure isolationProtect the neckStentsBalloon catheterEmbolization materialVascular malformation
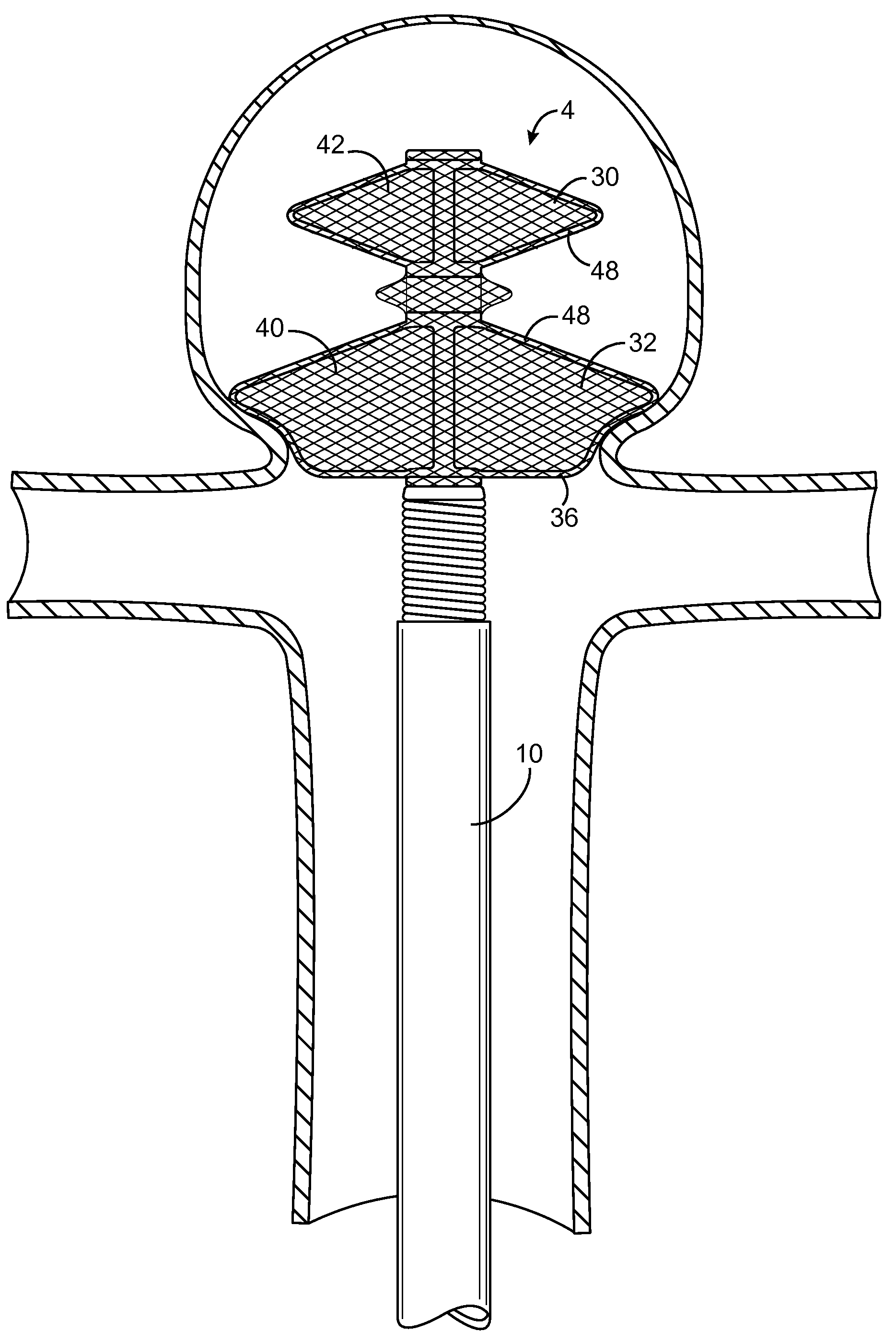
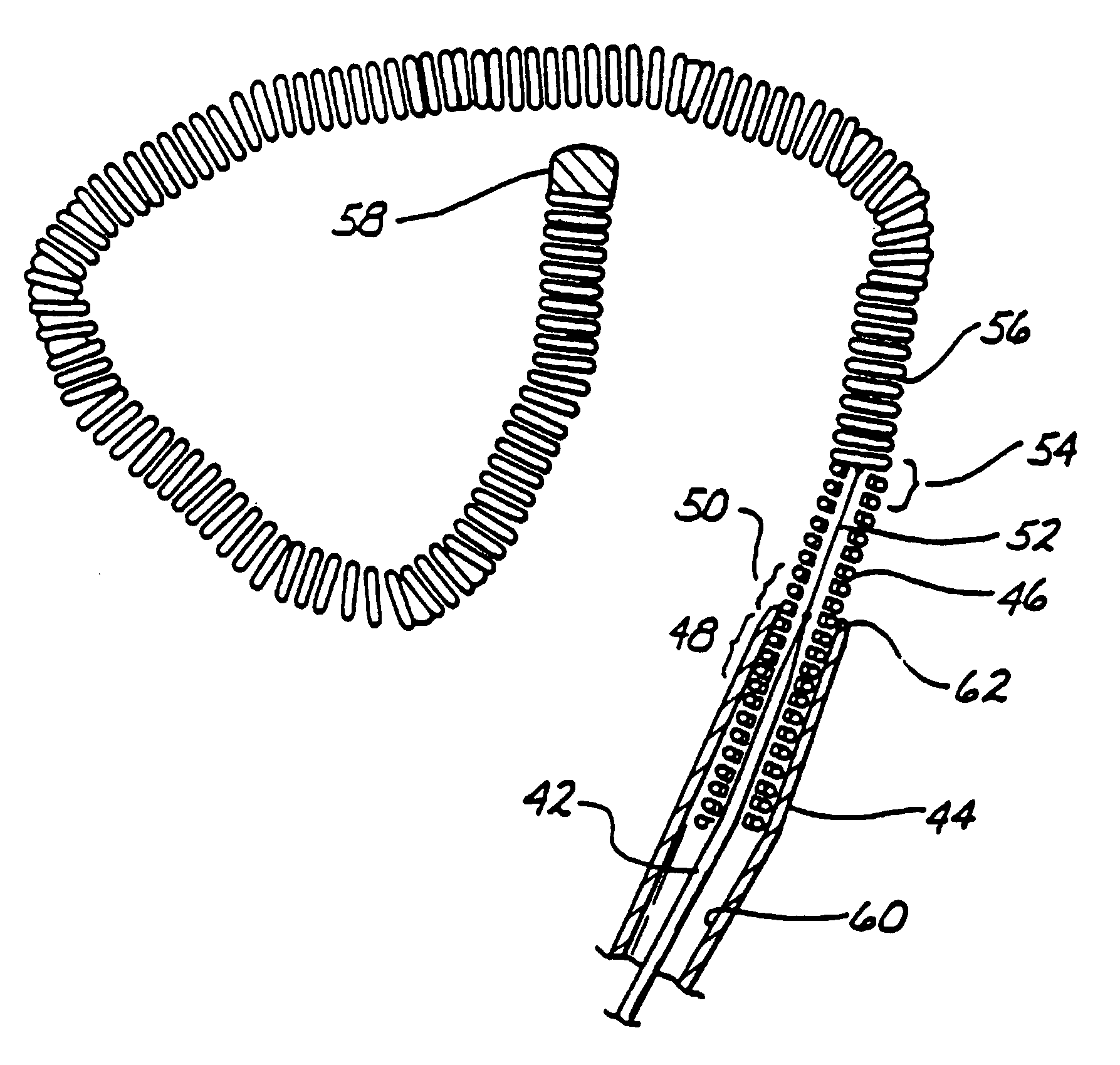
A device for treating vascular malformations includes a primary coil and secondary windings. The primary coil provides resilience and structural integrity while the secondary windings fill interstitial spaces in the primary coil to isolate the vascular malformation. The device may have increased density along a central portion to isolate the malformation. In another aspect, the device may have a central opening through which embolic materials may be delivered.
Owner:STRYKER CORP
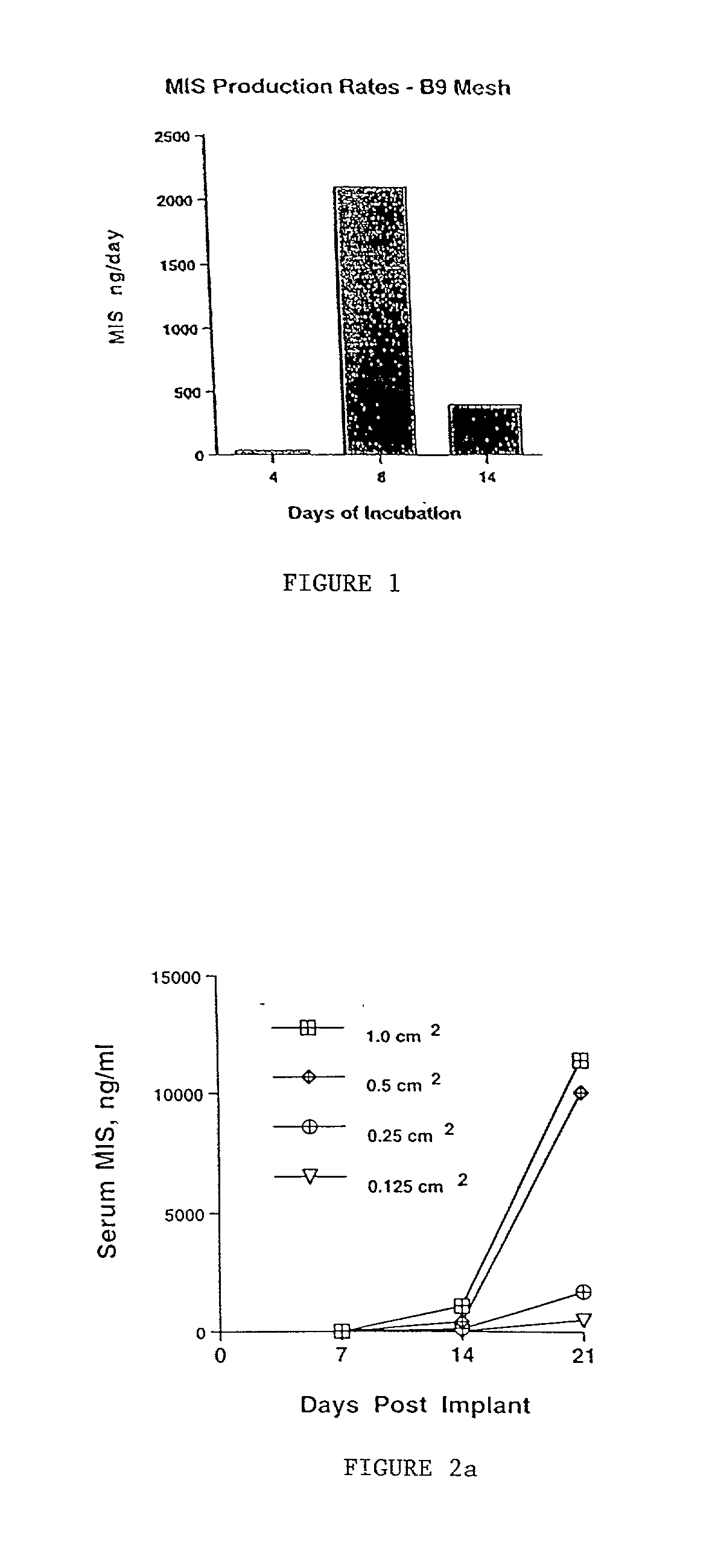
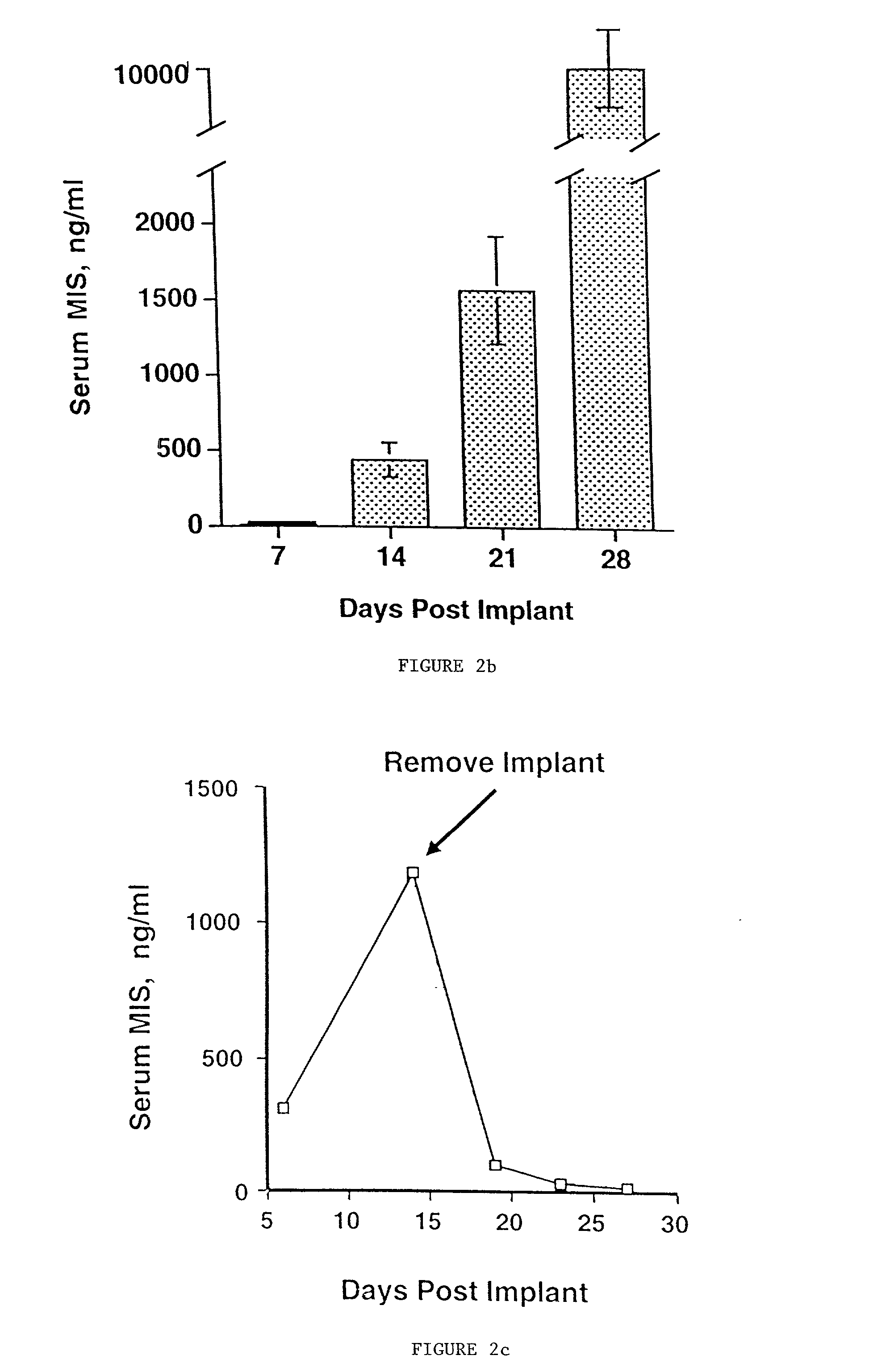
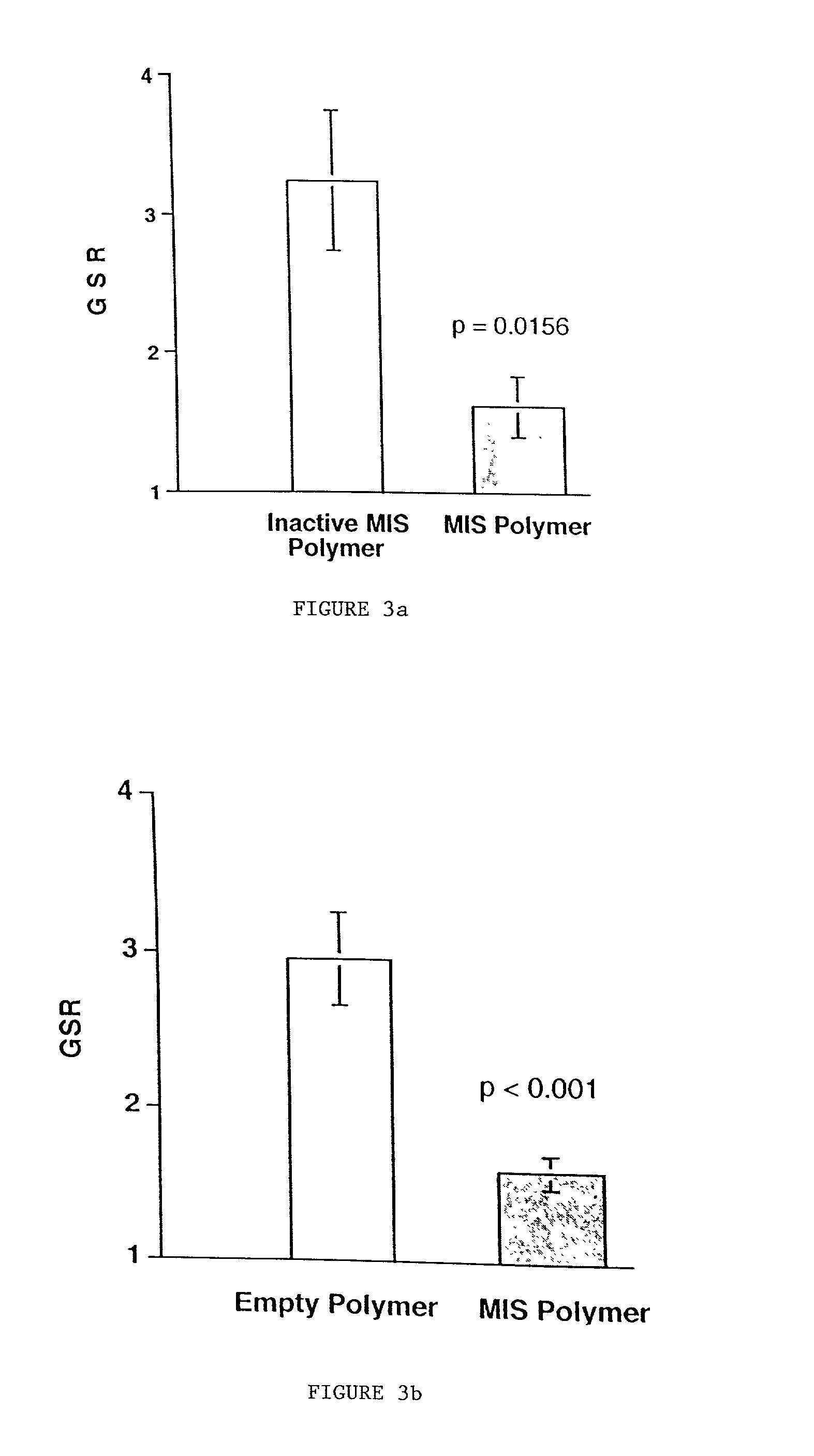
Delivery of therapeutic biologicals from implantable tissue matrices
InactiveUS20020031500A1Many of effectMany of inconveniencePowder deliveryBiocideProgenitorActive agent
Normal cells, such as fibroblasts or other tissue or organ cell types, are genetically engineered to express biologically active, therapeutic agents, such as proteins that are normally produced in small amounts, for example, MIS, or other members of the TGF-beta family Herceptin(TM), interferons, andanti-angiogenic factors. These cells are seeded into a matrix for implantation into the patient to be treated. Cells may also be engineered to include a lethal gene, so that implanted cells can be destroyed once treatment is completed. Cells can be implanted in a variety of different matrices. In a preferred embodiment, these matrices are implantable and biodegradable over a period of time equal to or less than the expected period of treatment, when cells engraft to form a functional tissue producing the desired biologically active agent. Implantation may be ectopic or in some cases orthotopic. Representative cell types include tissue specific cells, progenitor cells, and stem cells. Matrices can be formed of synthetic or natural materials, by chemical coupling at the time of implantation, using standard techniques for formation of fibrous matrices from polymeric fibers, and using micromachining or microfabrication techniques. These devices and strategies are used as delivery systems via standard or minimally invasive implantation techniques for any number of parenterally deliverable recombinant proteins, particularly those that are difficult to produce in large amounts and / or active forms using conventional methods of purification, for the treatment of a variety of conditions that produce abnormal growth, including treatment of malignant and benign neoplasias, vascular malformations (hemangiomas), inflammatory conditions, keloid formation, abdominal or plural adhesions, endometriosis, congenital or endocrine abnormalities, and other conditions that can produce abnormal growth such as infection. Efficacy of treatment with the therapeutic biologicals is detected by determining specific criteria, for example, cessation of cell proliferation, regression of abnormal tissue, or cell death, or expression of genes or proteins reflecting the above.
Owner:THE GENERAL HOSPITAL CORP
Devices and methods for treating vascular malformations
InactiveUS20110137332A1Ensure isolationProtect the neckStentsBalloon catheterVascular malformationEmbolization material
A device for treating vascular malformations includes a primary coil and secondary windings. The primary coil provides resilience and structural integrity while the secondary windings fill interstitial spaces in the primary coil to isolate the vascular malformation. The device may have increased density along a central portion to isolate the malformation. In another aspect, the device may have a central opening through which embolic materials may be delivered.
Owner:STRYKER CORP
Polymer matrix devices for treatment of vascular malformations
InactiveUS20080086196A1Control ratePromote formationStentsOcculdersVascular malformationWide necked aneurysm
A system for treating a wide-neck aneurysm comprising a mesh-like sleeve fabricated from a class of polymer filaments that carry conductive particles therein to provide the filaments with a specified resistivity. The releasable mesh-like sleeve is introduced to the site of a targeted vascular malformation by the working end of a catheter that carries an electrode arrangement at its distal terminus. The system further provides an electrical source and controller (i) that modulates power delivery to the polymer matrix which can then fuse the sleeve to the wall of a blood vessel to span across the vascular malformation.
Owner:DFINE INC
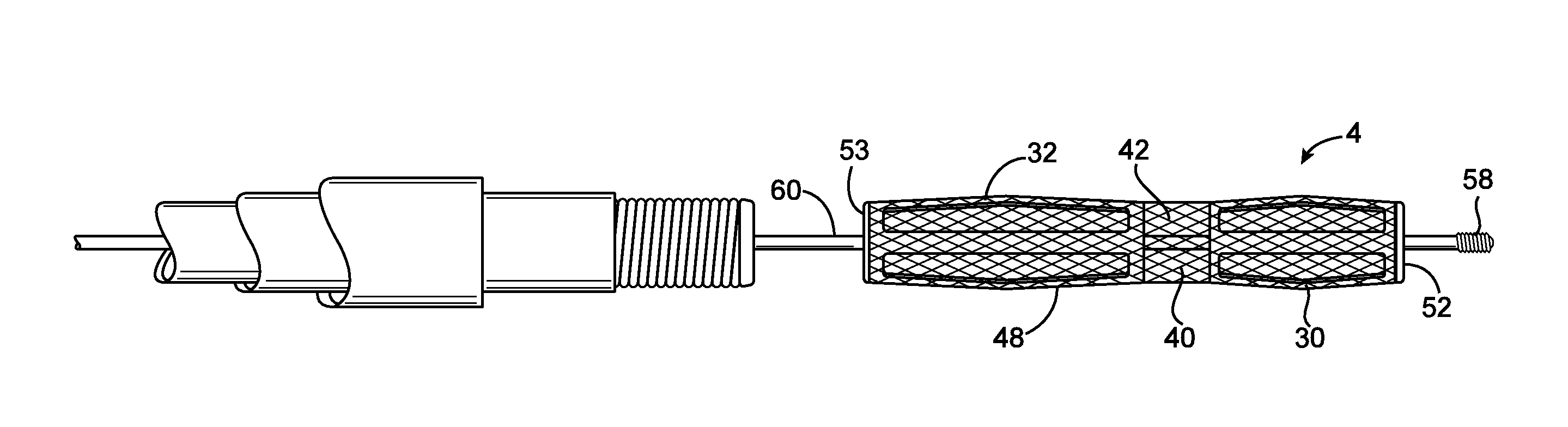
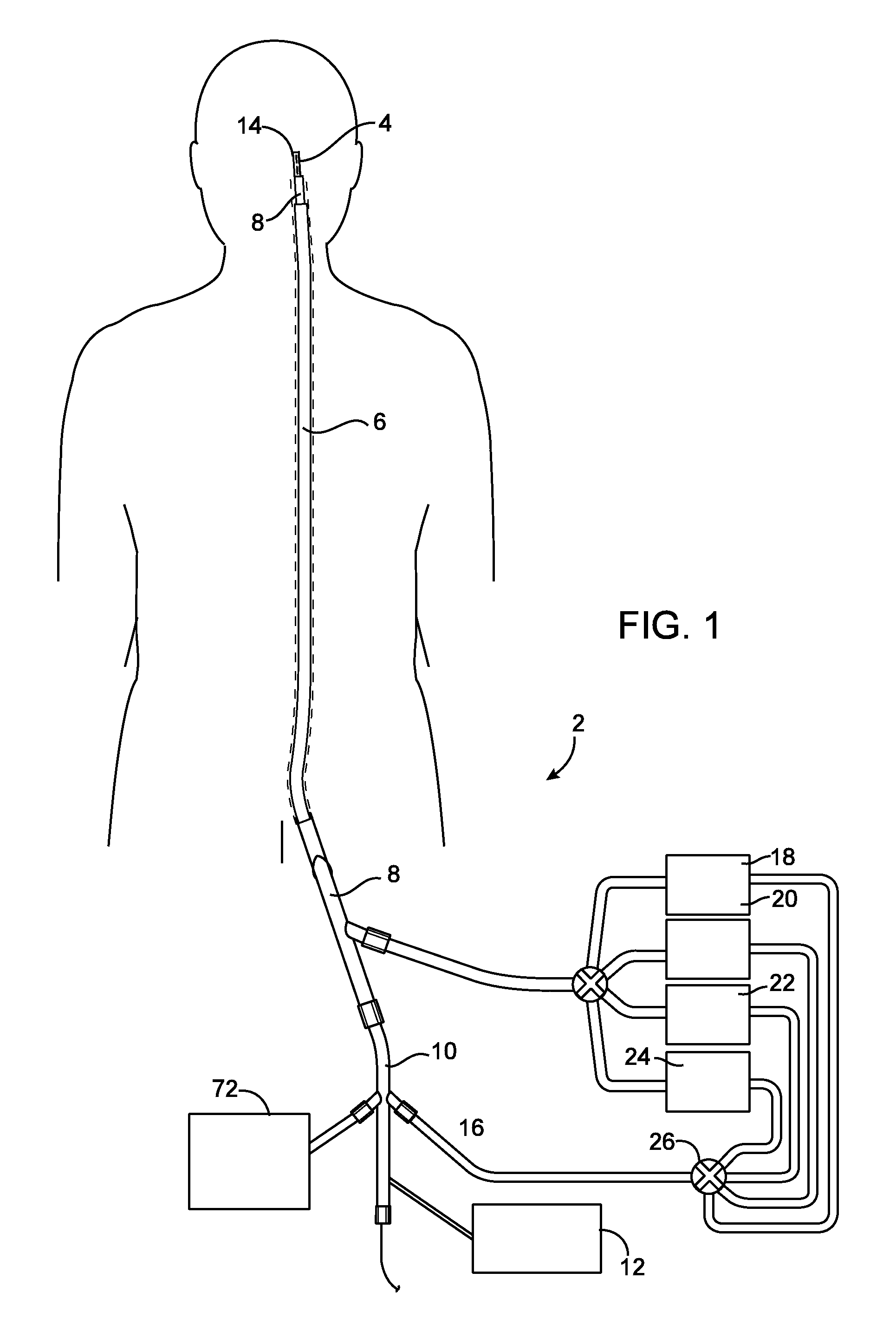
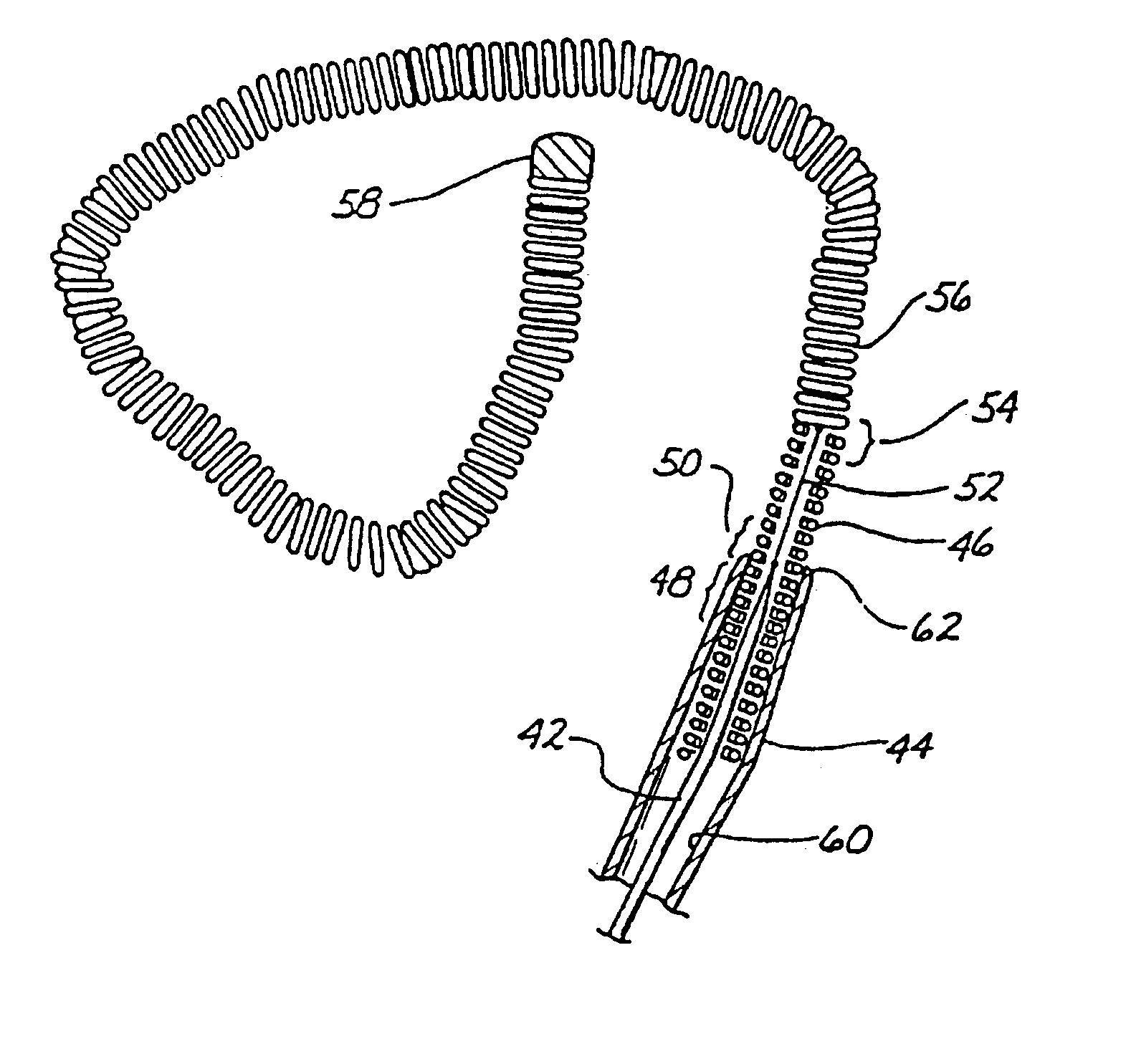
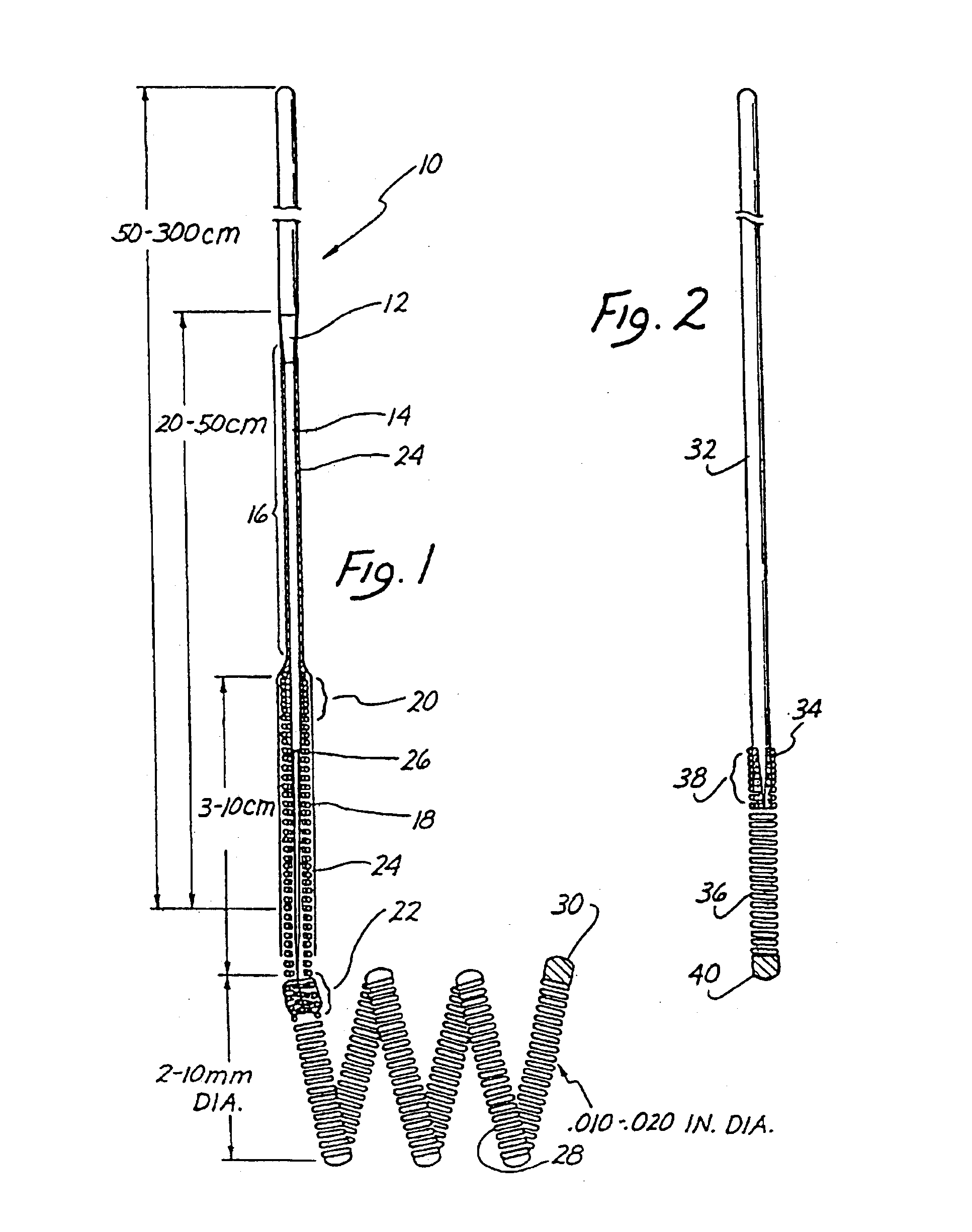
Endovascular electrolytically detachable wire and tip for the formation of thrombus in arteries, veins, aneurysms, vascular malformations and arteriovenous fistulas
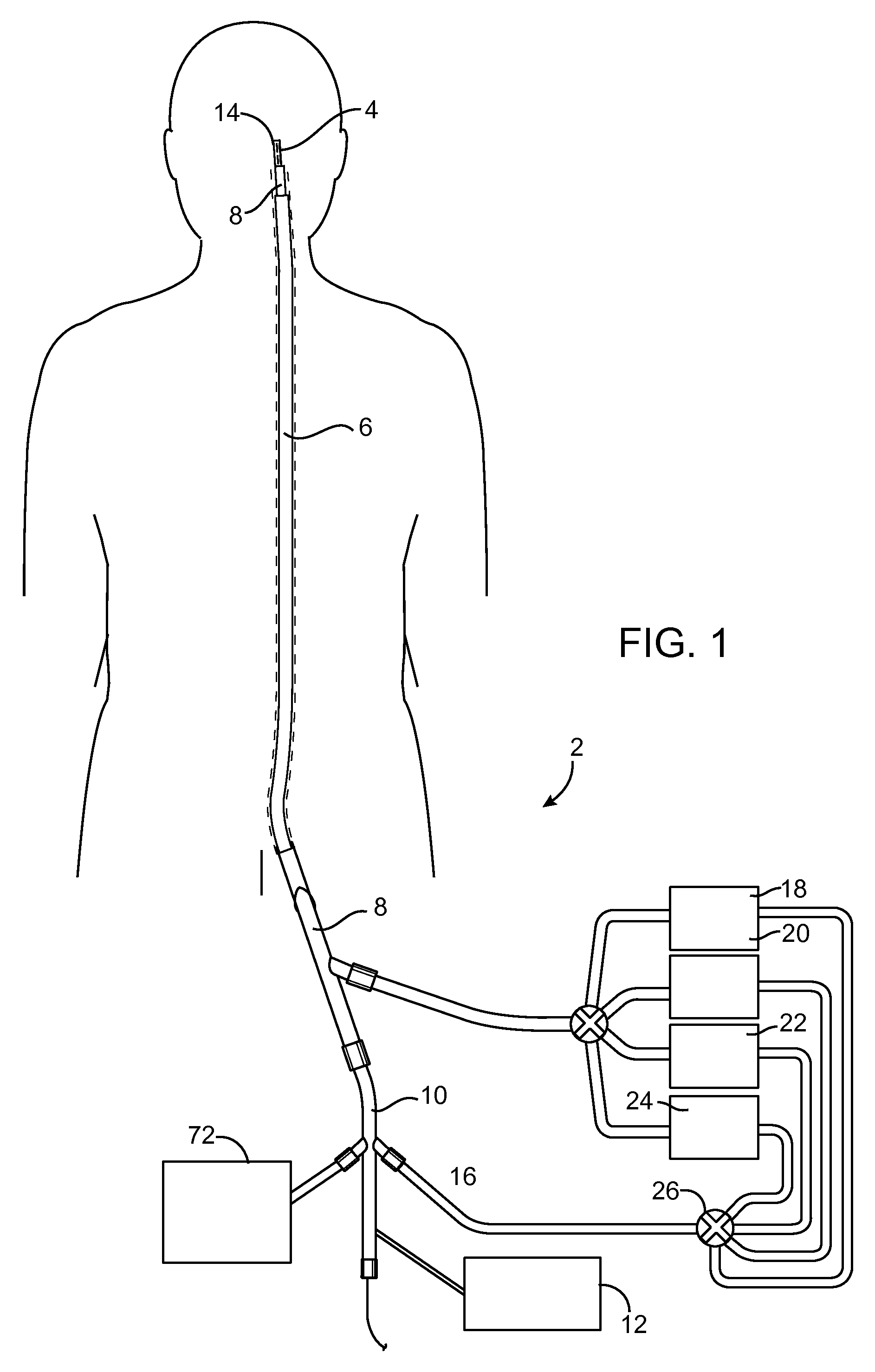
An artery, vein, aneurysms vascular malformation or arterial fistula is occluded through endovascular occlusion by the endovascular insertion of a platinum wire and / or tip into the vascular cavity. The vascular cavity is packed with the tip to obstruct blood flow or access of blood in the cavity such that the blood clots in the cavity and an occlusion if formed. The tip may be elongate and flexible so that it packs the cavity by being folded upon itself a multiple number of times, or may pack the cavity by virtue of a filamentary or fuzzy structure of the tip. The tip is then separated from the wire mechanically or by electrolytic separation of the tip from the wire. The wire and the microcatheter are thereafter removed leaving the tip embedded in the thrombus formed within the vascular cavity. Movement of wire in the microcatheter is more easily tracked by providing a radioopaque proximal marker on the microcatheter and a corresponding indicator marker on the wire. Electrothrombosis is facilitate by placing the ground electrode on the distal end of the microcatheter and flowing current between the microcatheter electrode and the tip.REEAXMINATION RESULTS The questions raised in reexamination request 90 / 007,231, filed Oct. 4, 2004 have been considered and the results thereof are reflected in this reissue patent which constitutes the reexamination certificate required by 35 U.S.C. 307 as provided in 37 CFR 1.570(e), for ex parte reexaminations, or the reexamination certificate required by 35 U.S.C. 316 as provided in 37 CFR 1.99(e) for inter partes reexaminations.
Owner:RGT UNIV OF CALIFORNIA
Thermo-reversible polymer for intralumenal implant
InactiveUS7485317B1Low toxicityEasy injectionPowder deliverySurgical adhesivesSolubilityVascular tissue
An intralumenal implant material, which comprises, a polymer having a sol-gel transition temperature in an aqueous solution thereof, shows a substantial water-insolubility at a temperature higher than the sol-gel transition temperature, and shows a thermo-reversible water-solubility at a temperature lower than the sol-gel transition temperature. Such an intralumenal implant is capable to be endovascularly or percutaneuosly delivered into a vascular lumen in a liquid state at the temperature lower than the sol-gel transition temperature, is capable to be instantly converted into a gel state in the vascular lumen at the blood temperature higher than the sol-gel transition temperature and is capable of occluding aneurysms, vascular tumors or vascular malformation. Such intralumenal implant material shows excellent biocompatibility and mechanical matching for the vascular tissue and the surrounding tissue because it is a highly water-containing hydrogel. In addition, biologically active substances for promoting a prompt neo-endothelium formation and / or endothelialization can be easily incorporated into such an intralumenal implant material.
Owner:RGT UNIV OF CALIFORNIA
Method and system for delivering an implant utilizing a lumen reducing member
A method and system for inserting an implant, such as vaso-occlusive device, an embolic containment device, or a stent into a vascular space to a vascular site in a body utilizing a lumen-reducing catheter. The method and system can be used to treat aneurysm, tumors and other vascular malformations. A guide is inserted into the vascular space. First and second catheters are inserted into the vascular space along the guide. The first or delivery catheter defines a first cavity, and the second or lumen-reducing catheter defines a second, smaller cavity. The second catheter is inserted within the first catheter. In one embodiment, an implant is advanced together with the first and second catheters to a vascular site. In an alternative embodiment, after the first and second catheters are positioned, the guide and the second catheter are removed from the first cavity, and an implant is inserted through the first cavity. With these configurations, radial movement of the guide is restricted to the smaller, second cavity rather than the larger, first cavity.
Owner:STRYKER EURO OPERATIONS HLDG LLC +1
Devices and methods for treating vascular malformations
InactiveUS20110082491A1Protect the neckSmall sizeStentsBalloon catheterIliac AneurysmVascular malformation
The invention is also directed to a device for treating an aneurysm which has a cover covering the neck of the aneurysm and a lateral portion extending into the aneurysm. The invention is also directed to a cover which is used to cover the neck of the aneurysm thereby isolating the aneurysm from the parental vessel.
Owner:STRYKER CORP
Apparatus and method for treatment of cerebral aneurysms, arterial-vascular malformations and arterial fistulas
An apparatus and method for stabilization of aneurysm is disclosed. The apparatus comprises an ultraviolet radiation generator for generating UV radiation having a wavelength, strongly absorbed by the DNA and a catheter including means for delivering the ultraviolet radiation to the aneurysm. The distal end of the catheter is placed inside the aneurysm. Stabilization of the aneurysm is achieved by forming a mural arterial thrombus inside the aneurysm. To make irradiation possible, the blood is displaced from the aneurysm by a steady stream of UV radiation transparent fluid. The injury to the endothelium that triggers the thrombus formation is caused by UV radiation delivered to the aneurysm. In several days after intervention the thrombus becomes fully organized, leaving on the inside surface of the aneurysm a thick layer of fibrotic tissue that stabilizes the aneurysm.
Owner:CHORNENKY VICTOR I +1
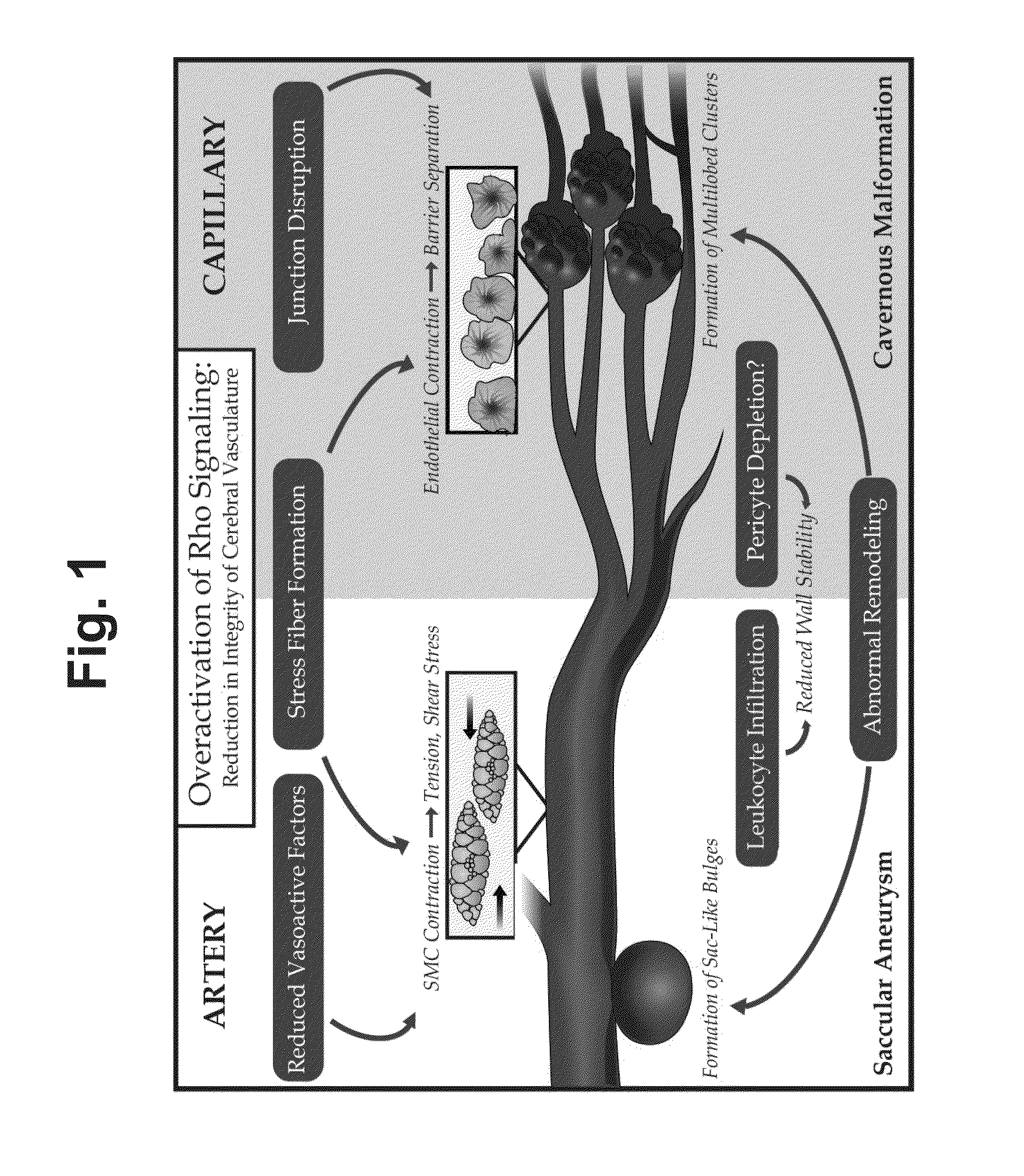
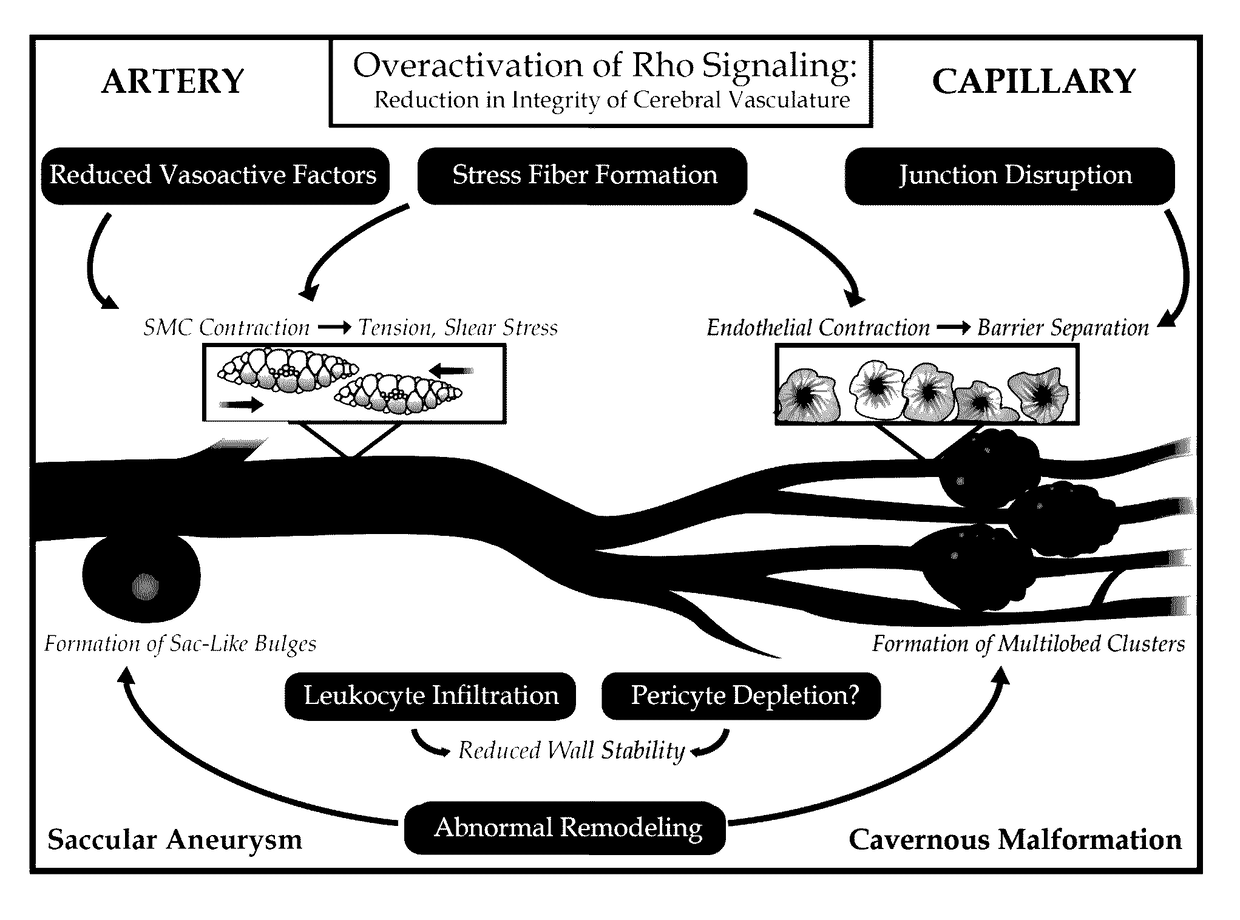
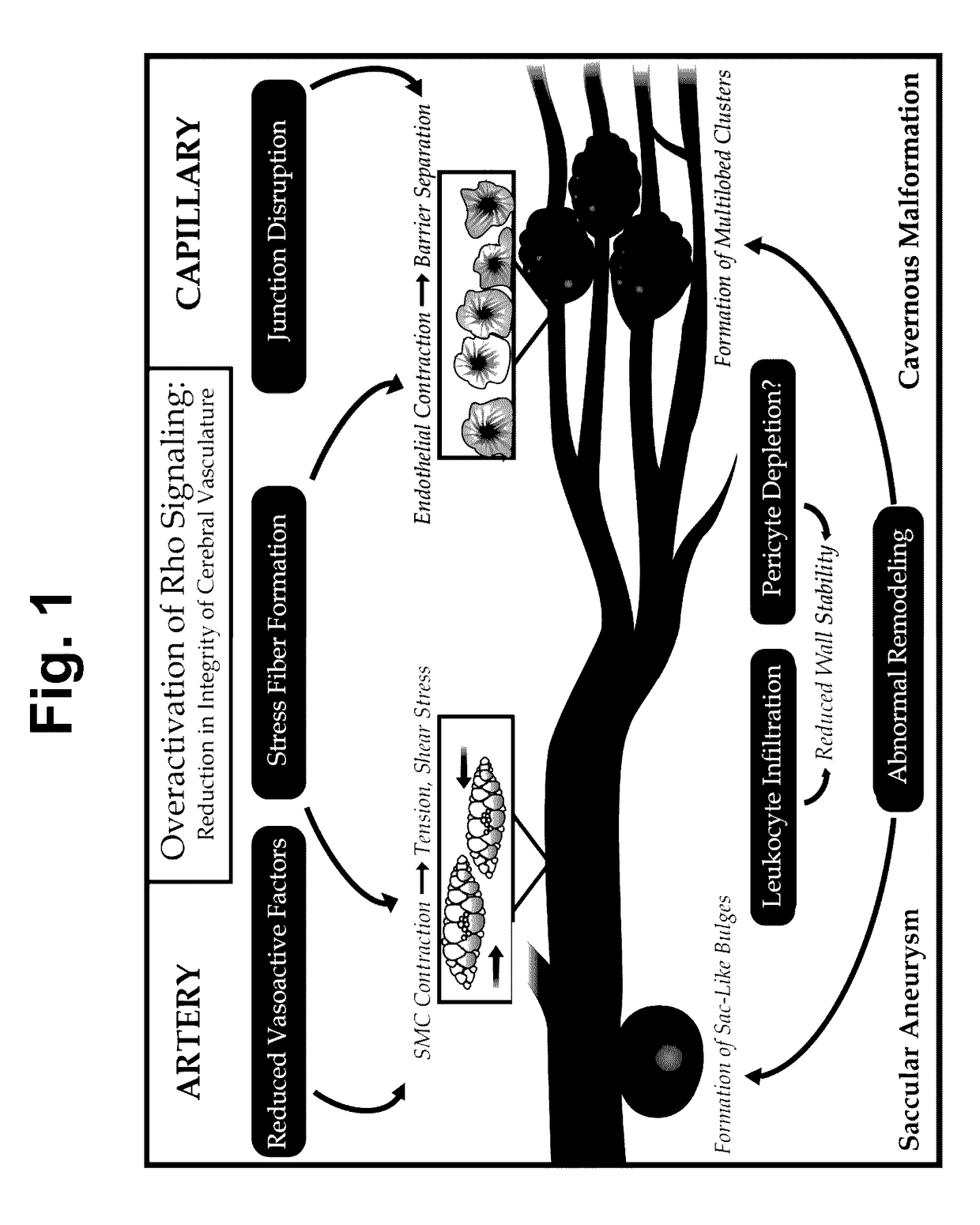
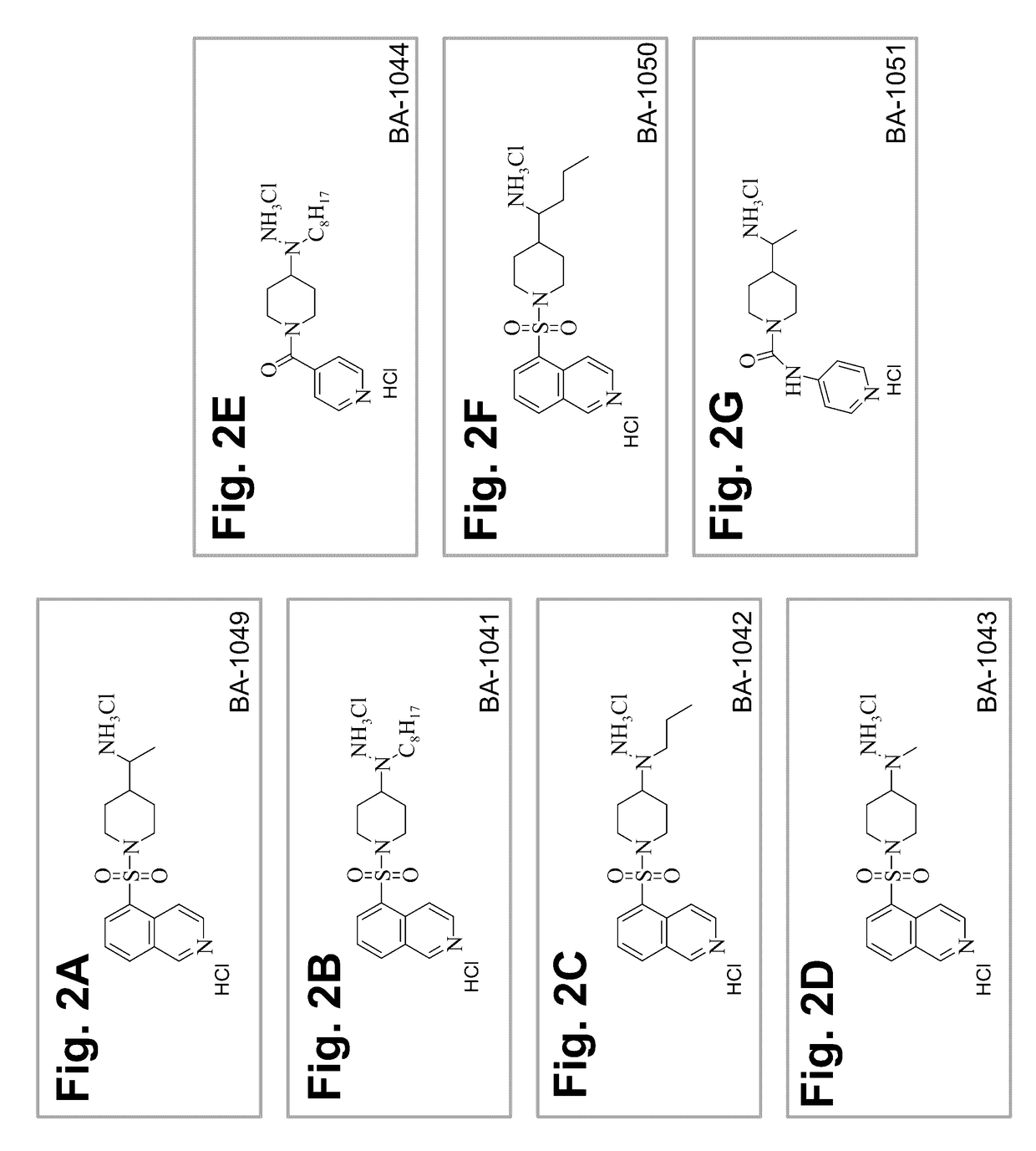
Treatment of cerebral cavernous malformations and cerebral aneurysms with rho kinase inhibitors
ActiveUS20160213664A1Stop CCM formationReduce in quantityOrganic active ingredientsTyrosine-kinase inhibitorRho kinase inhibitor
Owner:BIOAXONE BIOSCI
Endovascular electrolytically detachable wire and tip for the formation of thrombus in arteries, veins, aneurysms, vascular malformations and arteriovenous fistulas
Owner:RGT UNIV OF CALIFORNIA
Implant comprising a non-woven fabric
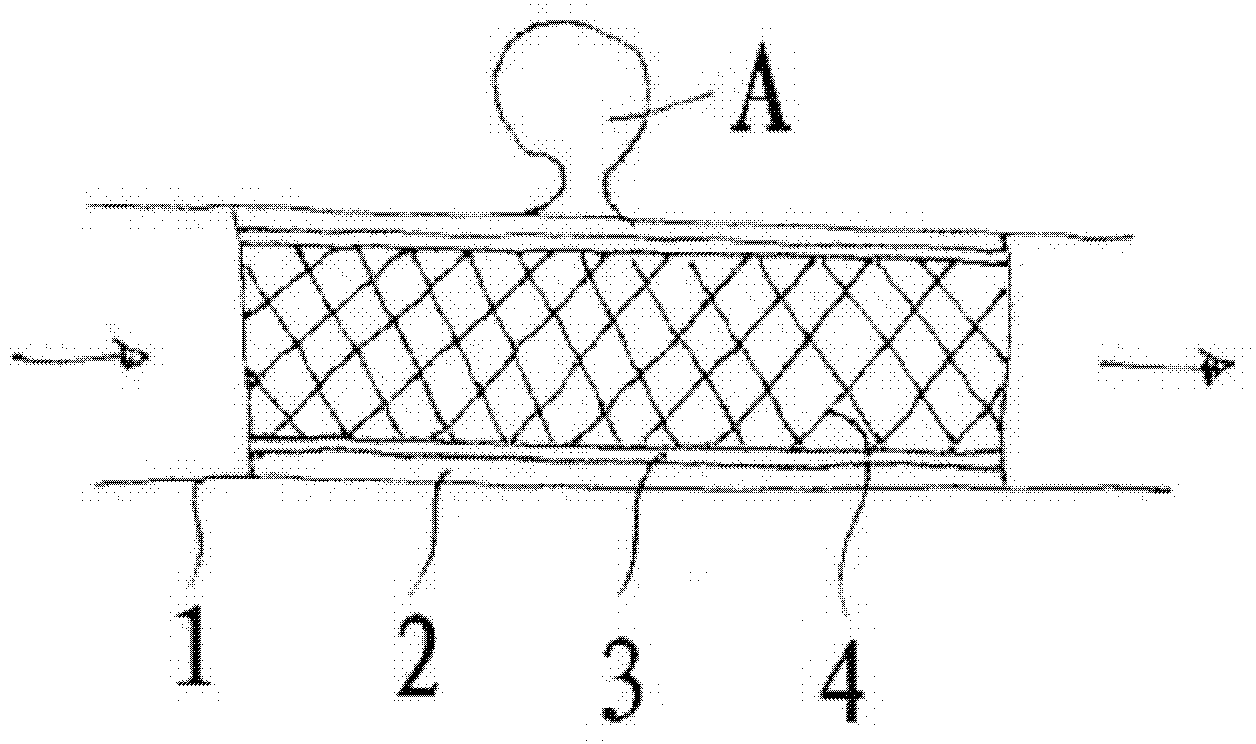
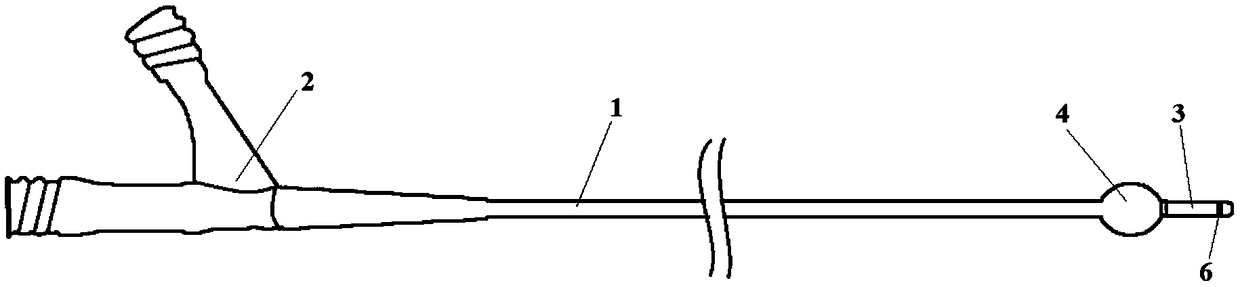
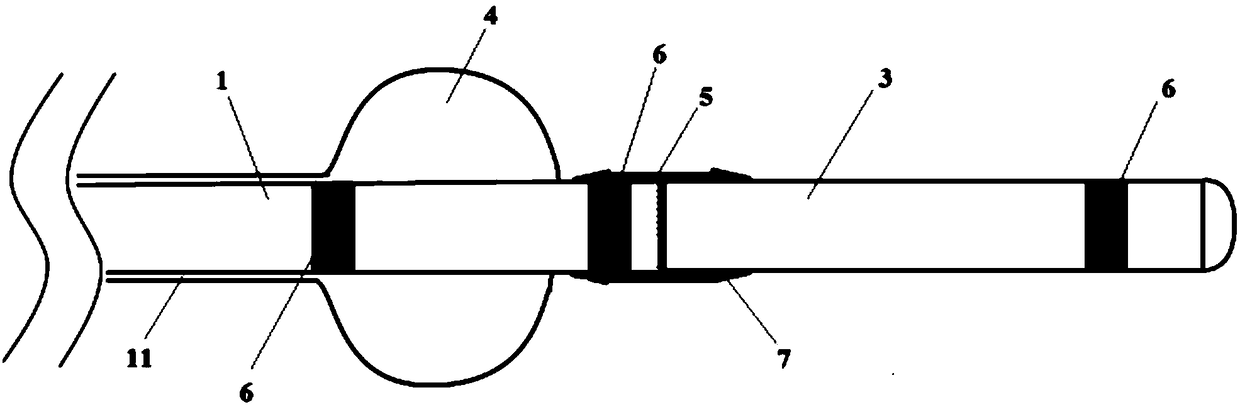
The invention relates to a membrane implant for treating vascular malformations, for the endovascular implantation into the vessel to be treated, the membrane implant consisting of an expandable stent (4) and a membrane (2, 3, 5, 11) connected to the stent (4). Said membrane (2, 3, 5, 11) covers mesh of the stent (4) at least in a center region and is designed as a non-woven fabric that contains synthetic filaments. The membrane (2, 3, 5, 11) is integrally connected to the stent (4) and is porous in at least some sections.
Owner:PHENOX
Magnetic resonance imaging detectable in-situ liquid crystal precursor embolism composition, preparation and application thereof
The invention discloses a magnetic resonance imaging detectable in-situ liquid crystal precursor embolism composition which comprises the following components: an MRI water-soluble or water-insoluble contrast medium, a liquid crystal material, a physiological acceptable water-soluble organic solvent and water. The following components can be added according to the treatment goal and the clinical demand: an X ray-impermeable water-soluble or water-insoluble contrast medium and / or drugs. The MRI detectable liquid embolism composition disclosed by the invention has the characteristics of low viscosity, no toxicity, low cost and fast curing, can be injected, and is mainly used in local embolism treatment and relevant imageological examination of tumors, vascular malformation and hemostasis.
Owner:HYGEA MEDICAL TECH CO LTD
DDX24 gene mutation and application thereof
ActiveCN108018296APromote formationPromote migrationHydrolasesMicrobiological testing/measurementDiseaseCell migration
The invention discloses DDX24 gene mutation and an application thereof. The DDX24 gene SNP (single-nucleotide polymorphism) mutation comprises Glu271Lys, Lys11Glu and Arg436His. The DDX24 gene mutation Glu271Lys, Lys11Glu or Arg436His is remarkably correlated with development of vessels. By means of interference to DDX24, cell migration and vascular formation can be promoted, and occurrence of vascular malformation is reminded. The occurrence condition of the vascular malformation of a human body can be effectively predicted by detecting SNP loci of DDX24, the DDX24 gene mutation can be used for screening genetic defects and is beneficial to true judgment of types of abnormal diseases of the vessels, misdiagnosis is reduced, and specific treatment is convenient.
Owner:THE FIFTH AFFILIATED HOSPITAL SUN YAT SEN UNIV
Balloon microcatheter with detachable tip
PendingCN108939257ASimple designInhibit refluxBalloon catheterMedical devicesCatheter hubIntervention treatment
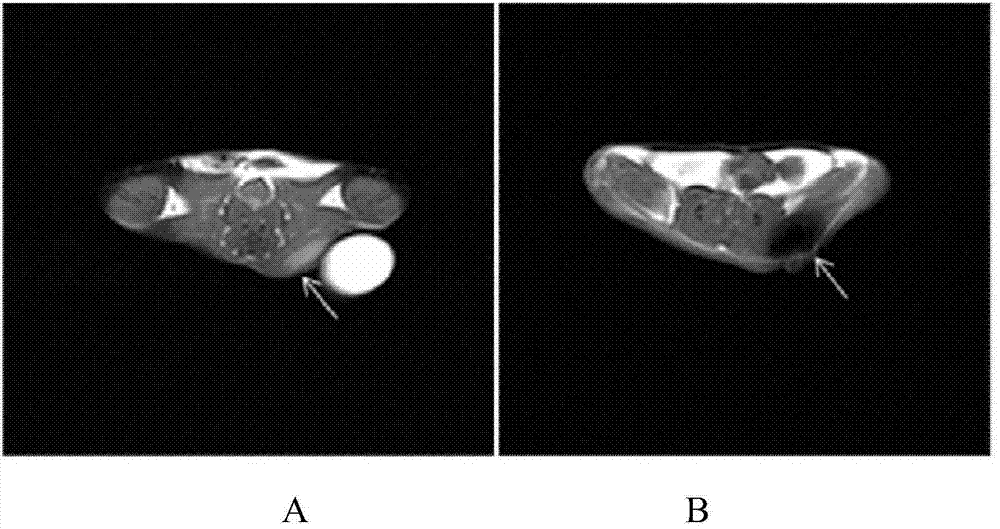
The invention relates to a balloon microcatheter with a detachable tip. The balloon microcatheter comprises a microcatheter body, one end of the microcatheter body is communicated with a catheter base, and the other end of the microcatheter body is connected with the detachable microcatheter tip; a cannula is coaxially arranged outside the microcatheter body, and the two ends of the cannula are communicated with the catheter base and an inflatable balloon on the microcatheter body respectively. The balloon microcatheter has the advantages that the curative embolization rate of minimally invasive intervention treatment of cerebrovascular malformation can be increased, complications caused when a liquid emboliaztion agent flows back and hemorrhage related to incomplete embolization can be reduced, the treatment process is simplified, and the economic burden is relieved.
Owner:SHANGHAI CHANGHAI HOSPITAL
Magnetic resonance imaging detectable liquid embolism composition and preparation and application thereof
ActiveCN103536972AGood curative effectImprove securitySurgeryNMR/MRI constrast preparationsProton magnetic resonanceWater insoluble
The invention discloses a magnetic resonance imaging (MRI) detectable liquid embolism composition which comprises the following components: an MRI water-soluble or water-insoluble contrast medium, an embolism material, a physiological acceptable water-soluble organic solvent and water. The following components can be added according to the treatment goal and the clinical demand: an X ray-impermeable water-soluble or water-insoluble contrast medium and / or drugs. The MRI detectable liquid embolism composition disclosed by the invention has the characteristics of low viscosity, no toxicity, low cost and fast curing, can be injected, and is mainly used in local embolism treatment and relevant imageological examination of tumors, vascular malformation and hemostasis.
Owner:HYGEA MEDICAL TECH CO LTD
Detachable micro-catheter with double-coating at the head end
PendingCN108434580AAvoid premature inflationImprove passabilityMedical devicesCatheterDouble coatingCatheter
The invention relates to a detachable micro-catheter with double-coating at the head end. The detachable micro-catheter with double-coating at the head end comprises a microcatheter body, a catheter base connected at one end of the microcatheter body, and a detachable microcatheter head connected at the other end of the microcatheter body, the outer surface of the microcatheter head is coated withan expandable inner layer coating, and the inner layer coating is also tightly sealed and tightly coated with a variable loose outer layer coating. The detachable micro-catheter with double-coating at the head end has the beneficial effects that the curative embolization rate of the minimally invasive interventional treatment of the cerebral vascular malformation can be improved, the complications caused by the return flow of the liquid embolism agent and the bleeding due to embolism are reduced, the treatment process is simplified, and the economic burden is reduced.
Owner:SHANGHAI CHANGHAI HOSPITAL
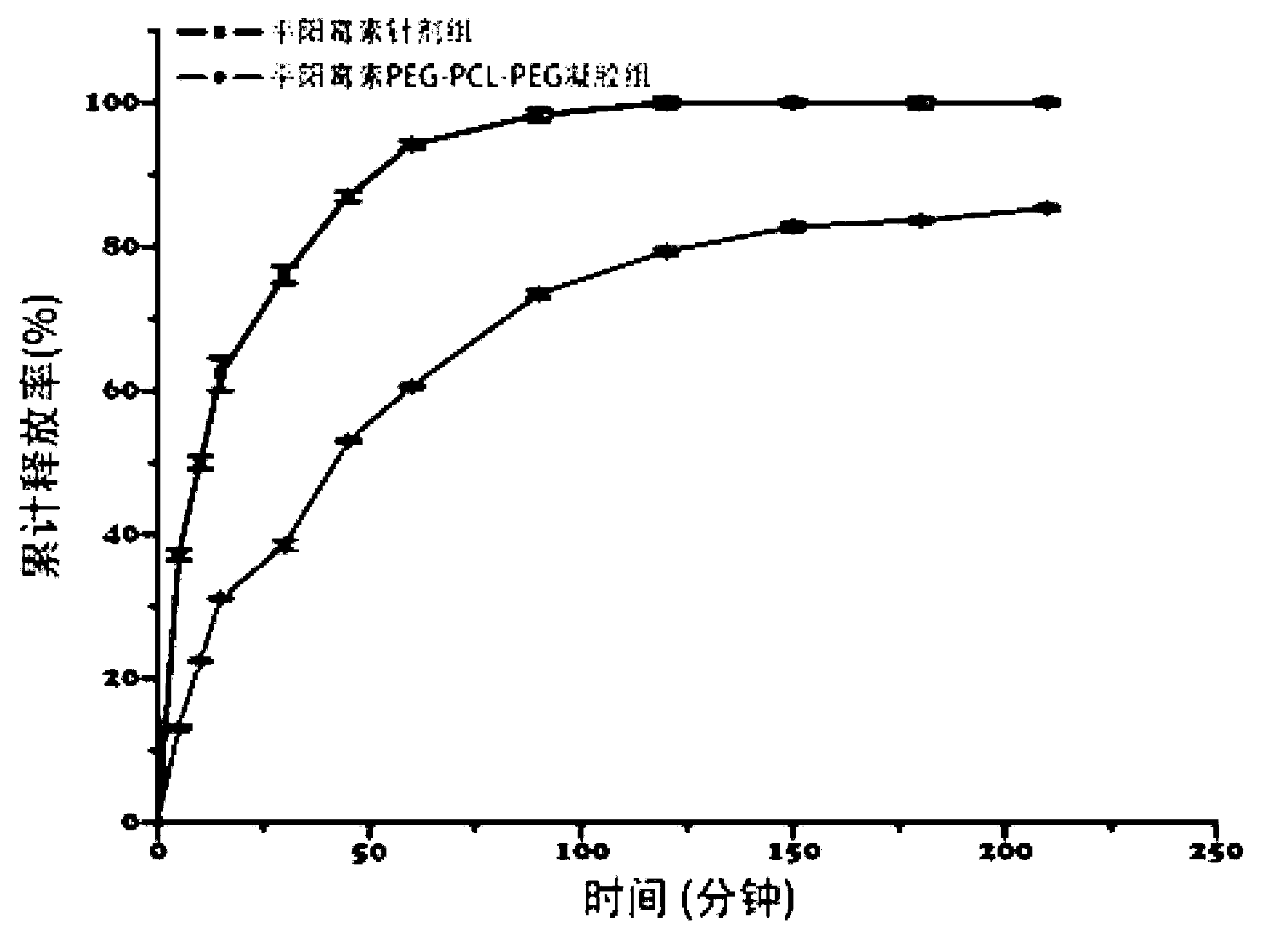
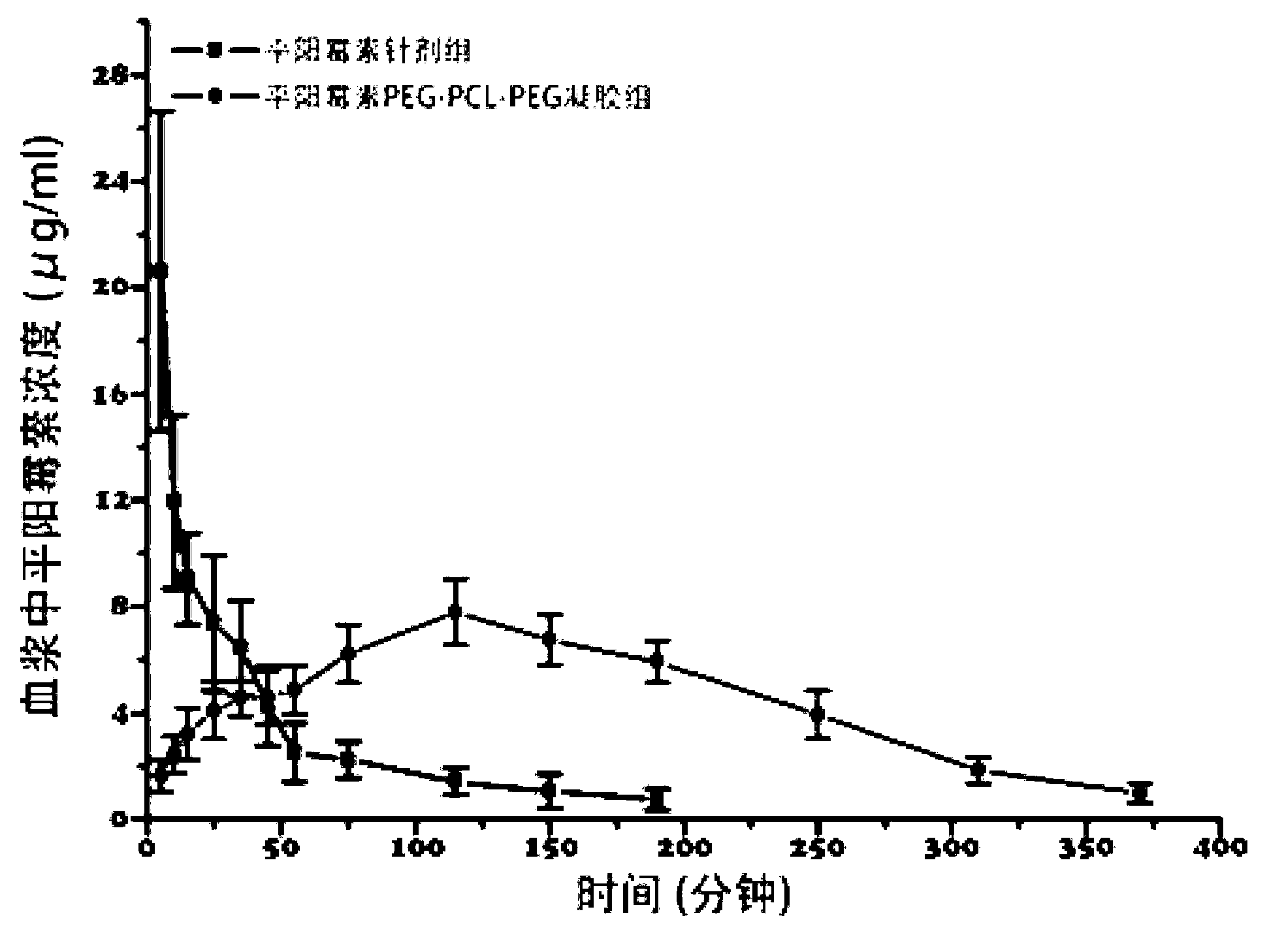
Pingyangmycin polyethylene glycol (PEG)-polycaprolactone (PCL)-polyethylene glycol (PEG) temperature-sensitive slow-release gel, as well as preparation method and application of same
ActiveCN102836418AExtended half-lifeExtension of timeAerosol deliveryOintment deliverySide effectHalf-life
The invention discloses a pingyangmycin polyethylene glycol (PEG)-polycaprolactone (PCL)-polyethylene glycol (PEG) temperature-sensitive slow-release gel, as well as a preparation method and the application of the gel. The Pingyangmycin PEG-PCL-PEG temperature-sensitive slow-release gel mainly consists of two parts comprising PEG (polyethylene glycol)-PCL (polycaprolactone)-PEG (polyethylene glycol) co-polymer and Pingyangmycin, is liquid at room temperature, and is solid gel under the in vivo 37 DEG C condition; the gel system has significant slow-release effect, thereby having functions in prolonging the half-life period and the acting time of the Pingyangmycin, reducing drug concentration in plasma, and reducing systemic toxic and side effects. The gel can be formed in situ after the medicine is injected in a high-flow-speed vessel, and then location embolism is realized, and so as to realize the, and muscularization and closing of muscle is caused, so that the sclerotherapy and the interventional therapy are combined effectively, the gel has excellent biocompatibility and degradability, is beneficial for treating hemangiomas, vascular malformations and cancers, particularly, a new selection is provided to the treatment of the vein malformation and partial malformation of cancers.
Owner:WUHAN UNIV
Biological marker relevant to vascular malformation and relevant detection reagent kit
ActiveCN109852689AMicrobiological testing/measurementDNA/RNA fragmentationVascular diseaseWhole genome sequencing
The invention relates to the technical field of biology, in particular to a set of biological markers and a relevant detection reagent kit. The invention provides the use of a combination of a substance for detecting Q346P mutation in a TEK gene or an active fragment thereof, a substance for detecting Q279R / R574P mutation in a MMP9 gene or an active fragment thereof, a substance for detecting Y1213Y mutation in a FLT1 gene or an active fragment thereof, and a substance for detecting X278X mutation in a TNFAIP6 gene or an active fragment thereof in a prepared reagent kit. The reagent kit is used for diagnosing vascular diseases and / or assessing the risk of the vascular diseases. The biological marker provided by the invention can avoid complete genome sequencing, so that needed sequencing data number can be greatly reduced, and the biological marker has excellent detection sensibility and specificity, and is low in cost, high in depth and accurate in detection on relevant mutation.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Treatment of cerebral cavernous malformations and cerebral aneurysms with Rho kinase inhibitors
ActiveUS9687483B2Reduce in quantityReduce formationHeterocyclic compound active ingredientsTyrosine-kinase inhibitorKinase
Owner:BIOAXONE BIOSCI
Use of phosphoinositide 3-kinase inhibitors for treatment of vascular malformations
ActiveUS20180117055A1Reduces vascular abnormalityReduce exceptionOrganic active ingredientsCardiovascular disorderGain of function mutationKinase
The present disclosure relates to methods of treating a vascular malformation in a subject expressing a gain-of-function mutation in a PIK3CA gene comprising administering, to the subject, an effective amount of an agent that inhibits phosphoinositide 3-kinase (“PI3K”).
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Compound for treating vascular malformation
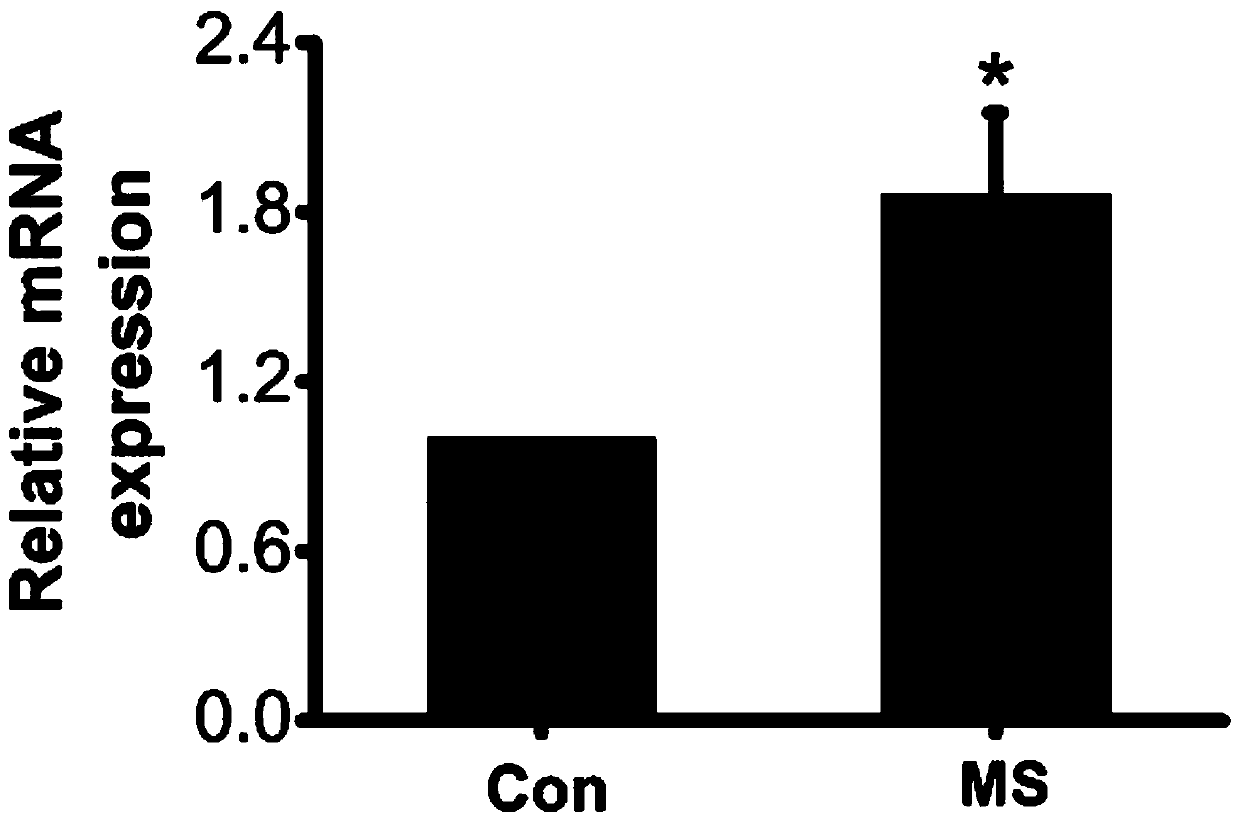
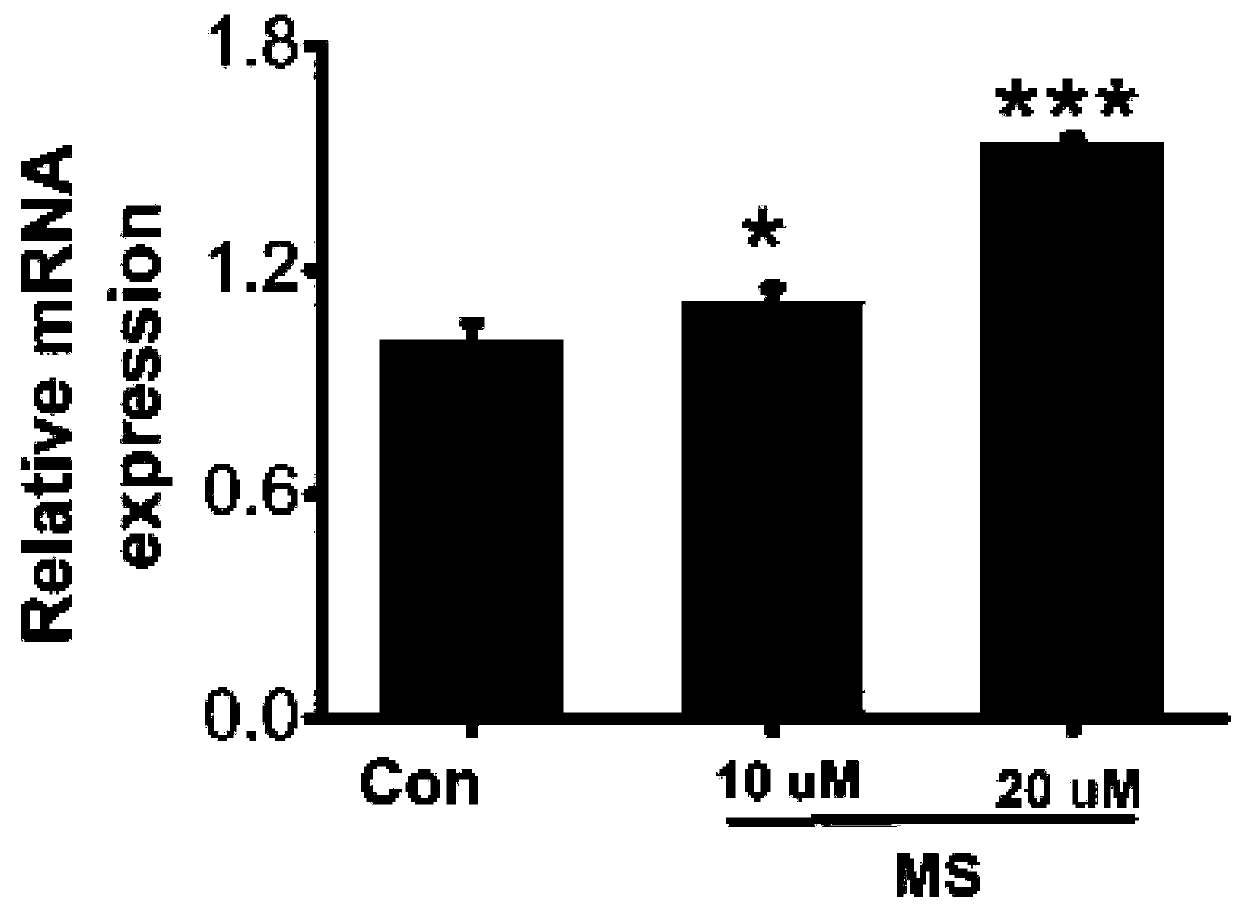
InactiveCN110496124ATherapeuticHas improved effectDigestive systemRespiratory disorderVascular malformationDeformity
The invention discloses a compound for treating vascular malformation. The inventor researches and discovers that a CysLTR1 antagonist can up-regulate expression in a vascular smooth muscle cell DDX24, and the CysLTR1 antagonist has a certain treating or improvement effect on the vascular malformation.
Owner:THE FIFTH AFFILIATED HOSPITAL SUN YAT SEN UNIV
Treatment of cerebral cavernous malformations and cerebral aneurysms with rho kinase inhibitors
ActiveUS20170246181A1Stop CCM formationReduce in quantityOrganic active ingredientsPharmaceutical delivery mechanismTyrosine-kinase inhibitorRho kinase inhibitor
Owner:BIOAXONE BIOSCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com