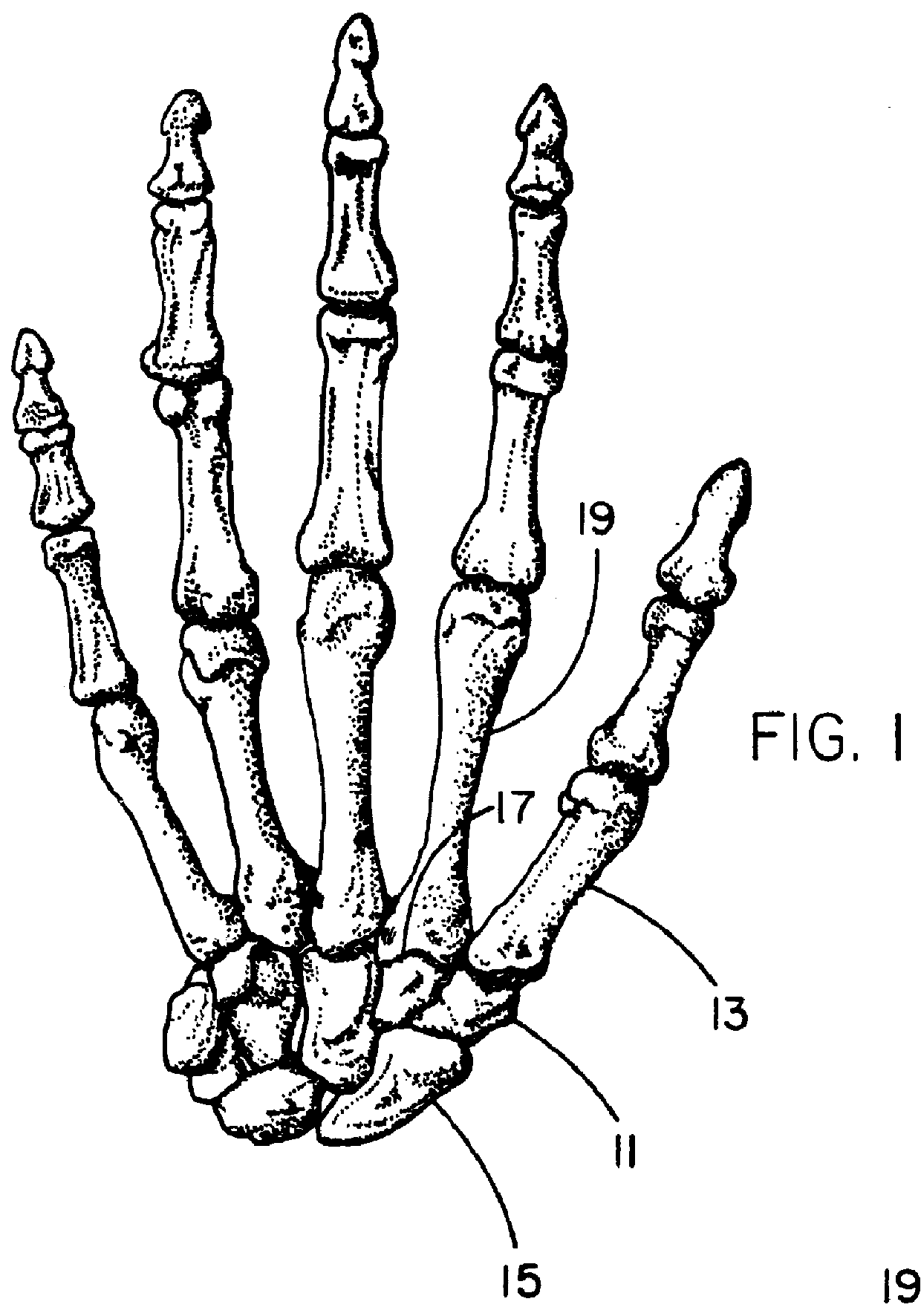
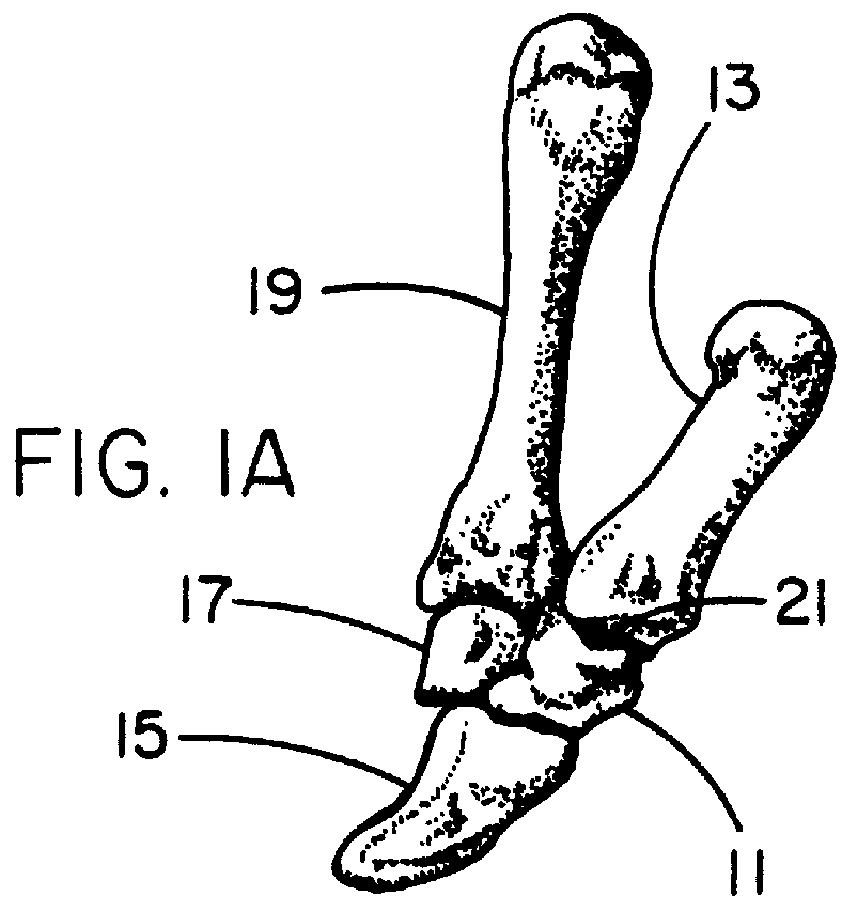
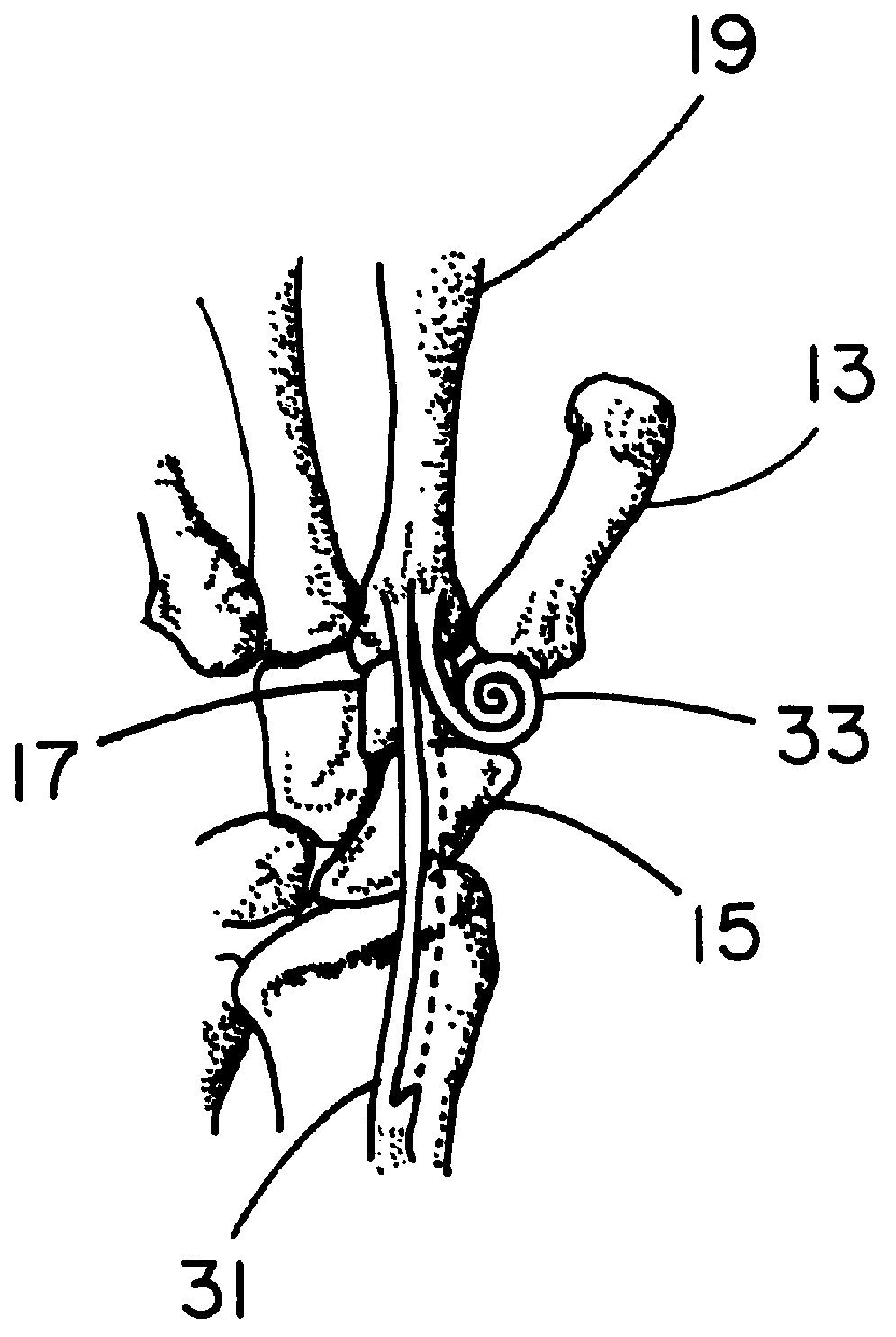
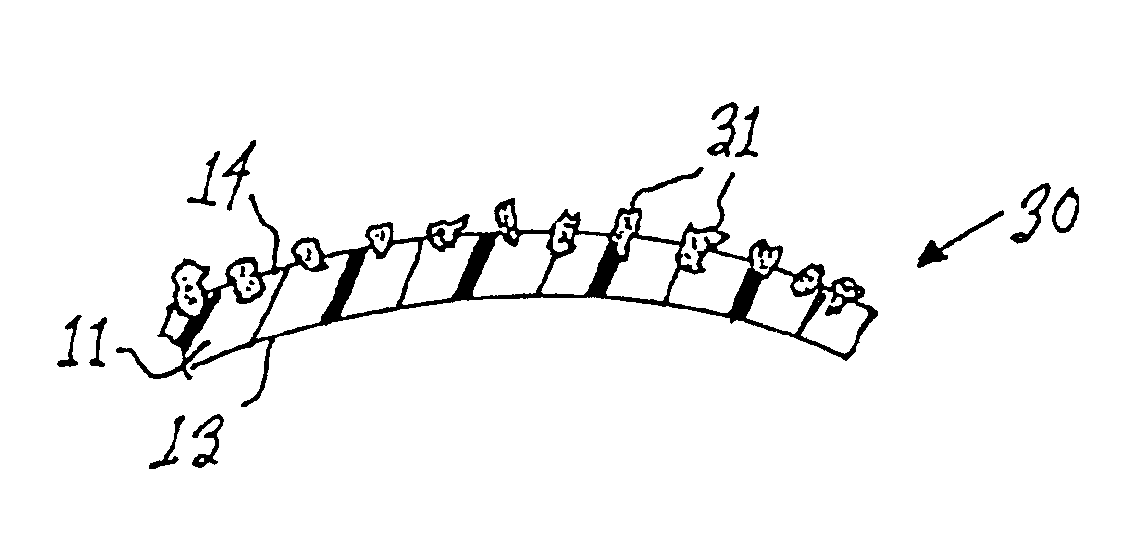
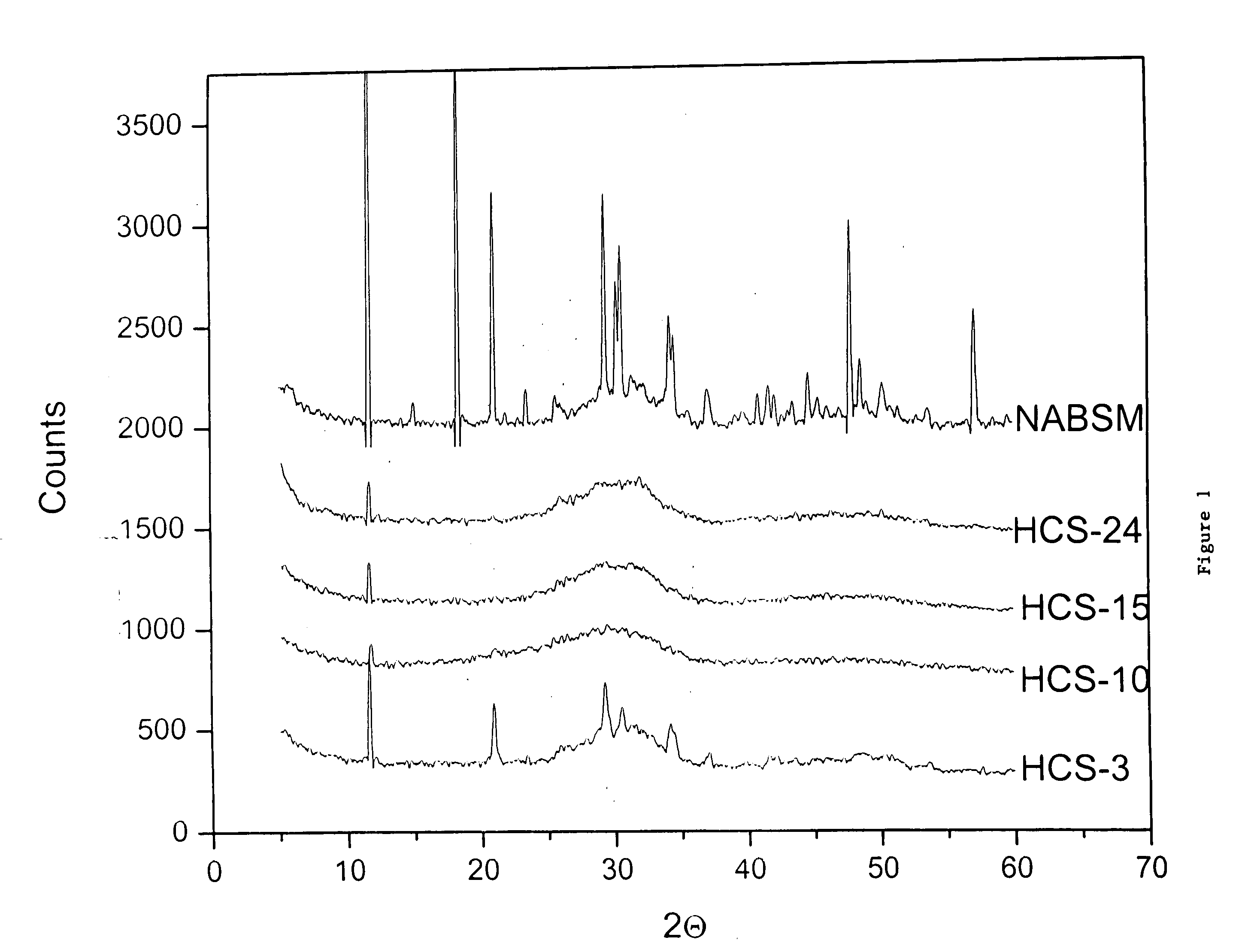
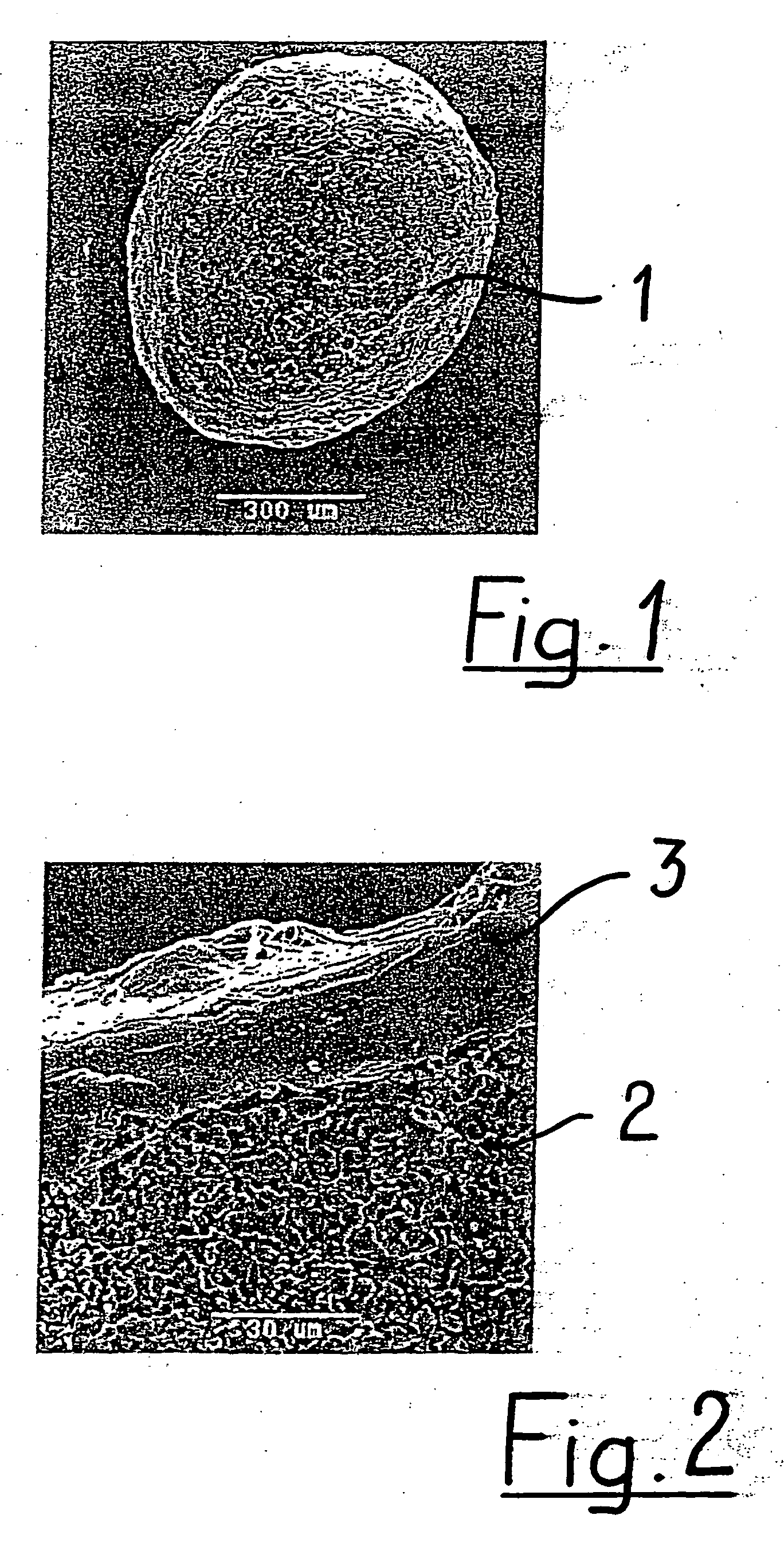
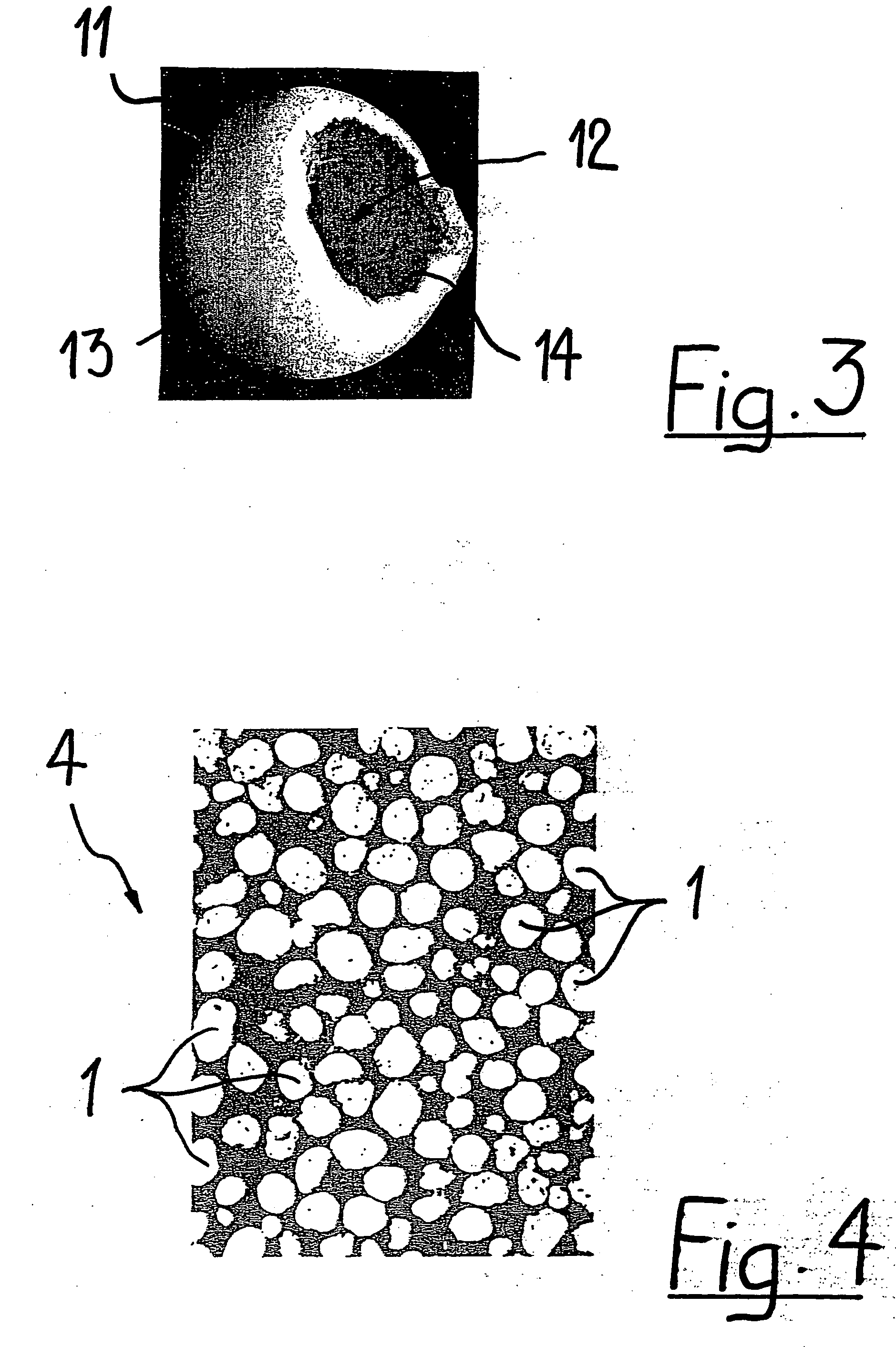
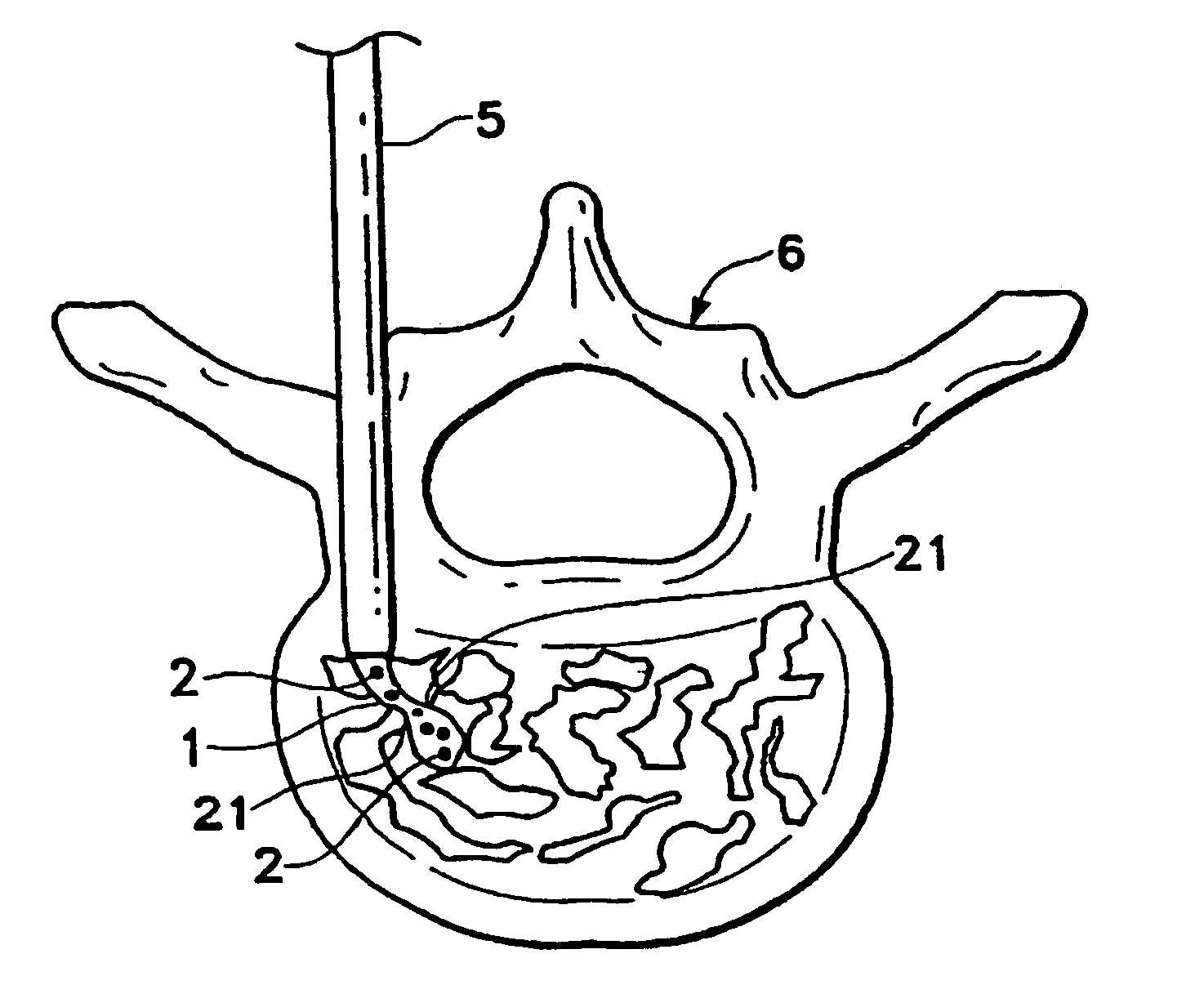
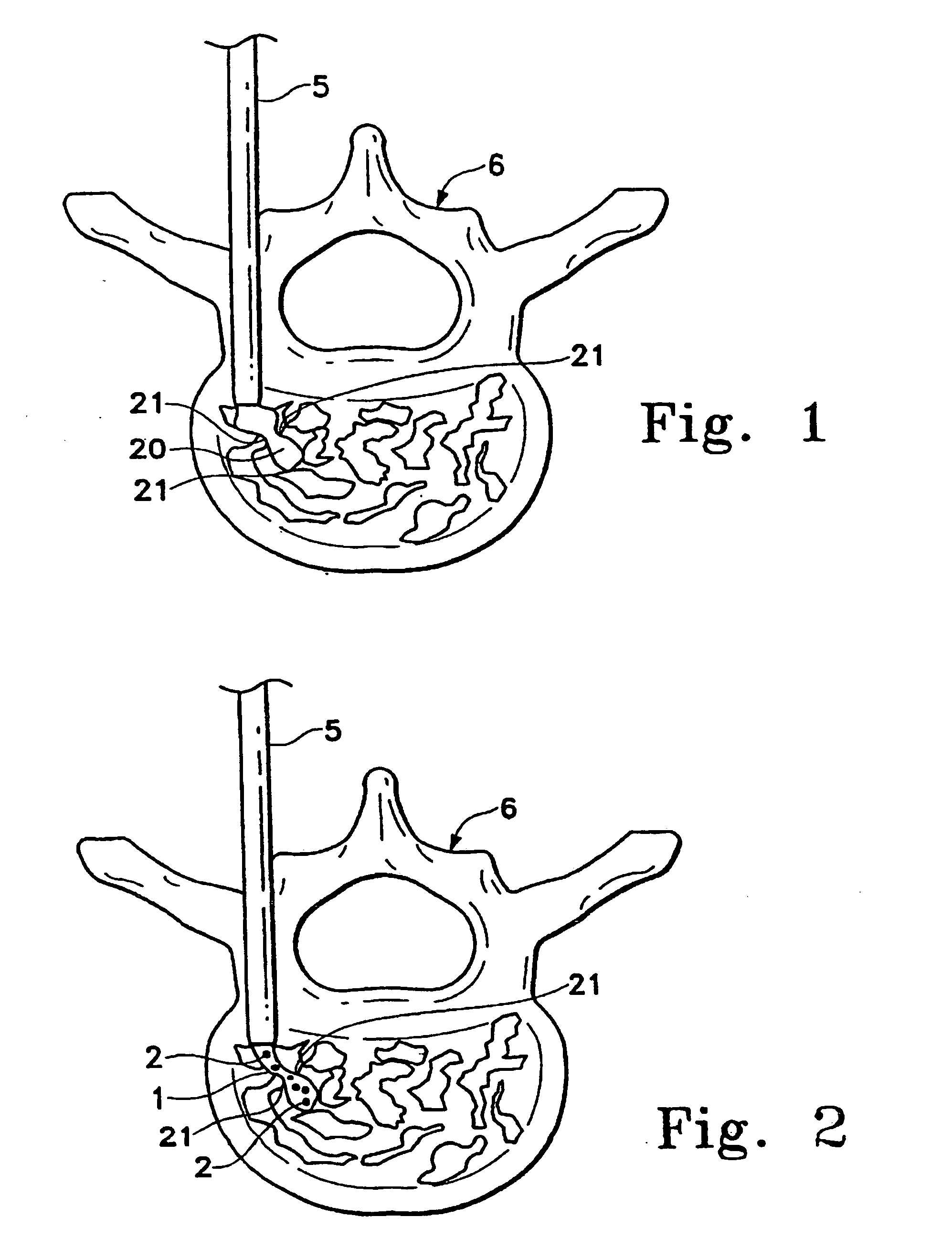
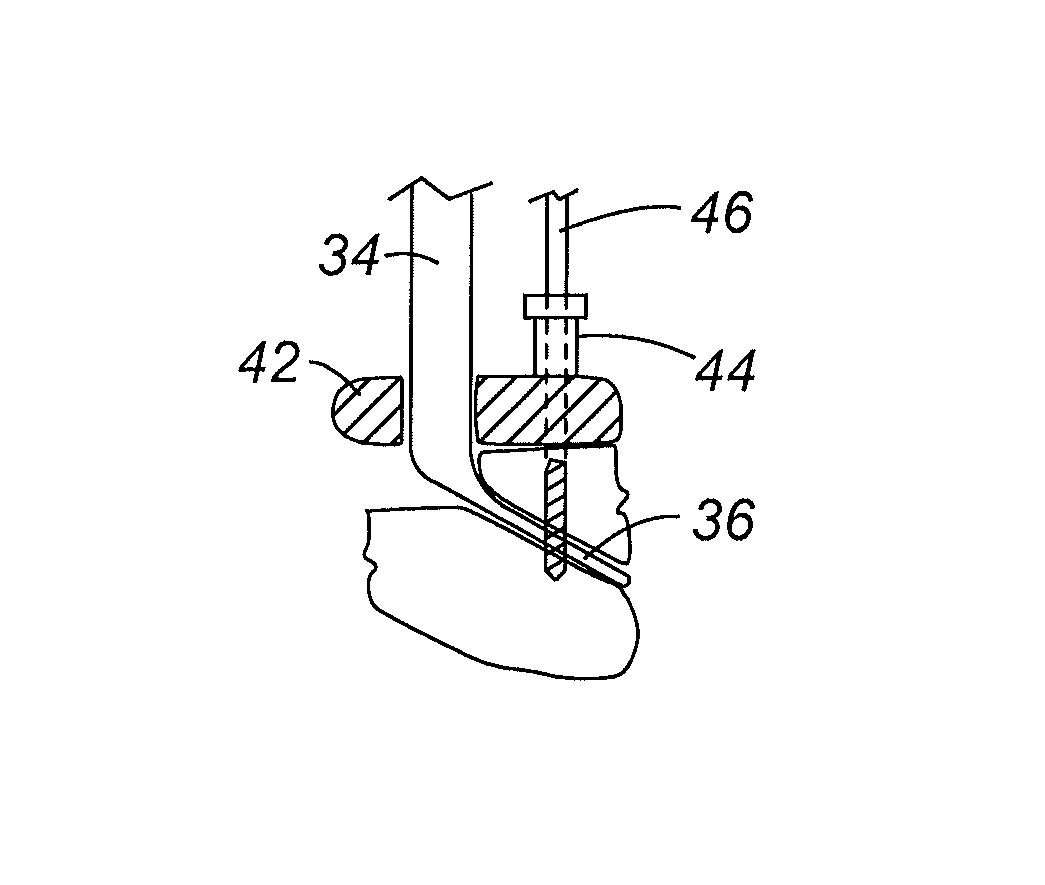
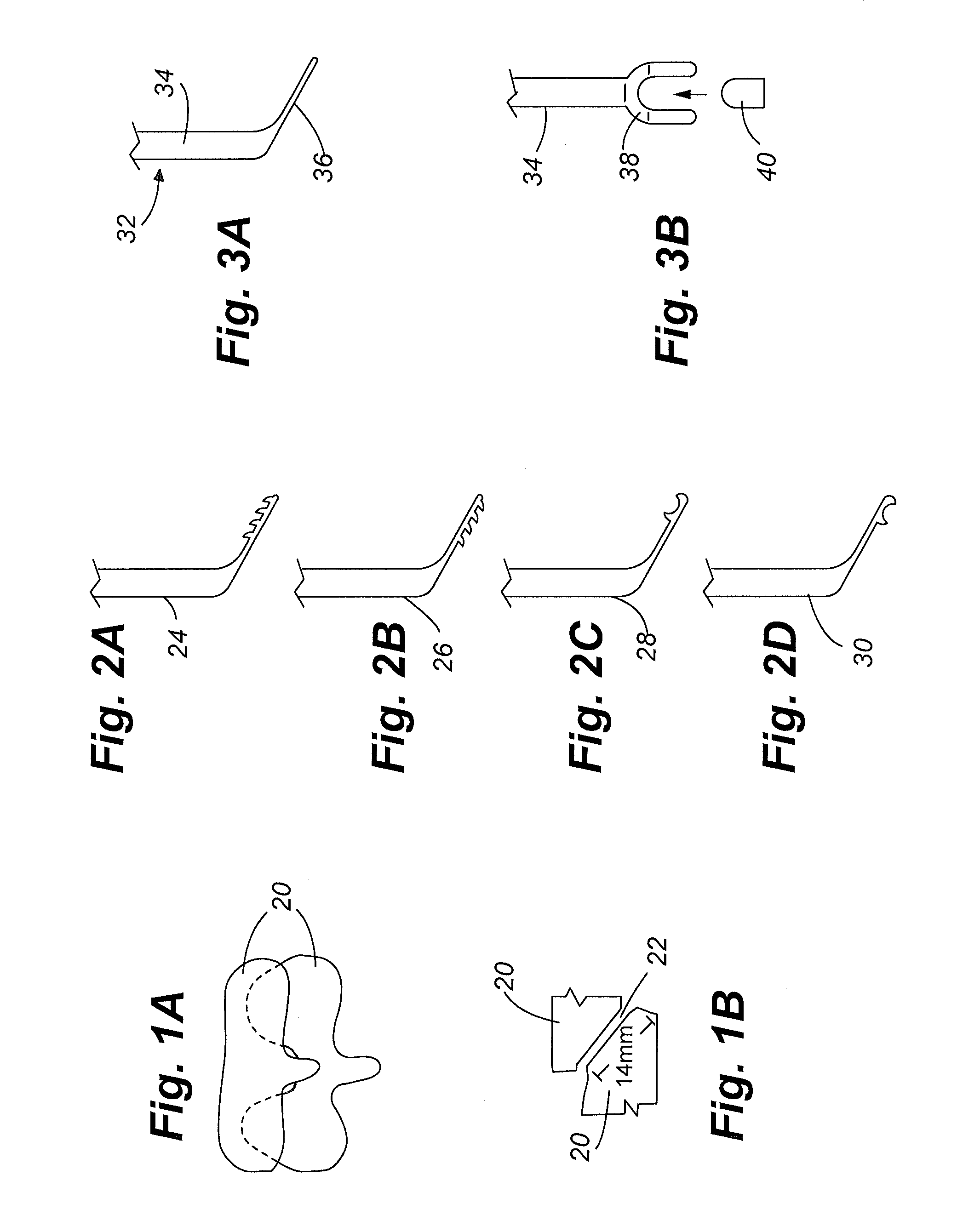
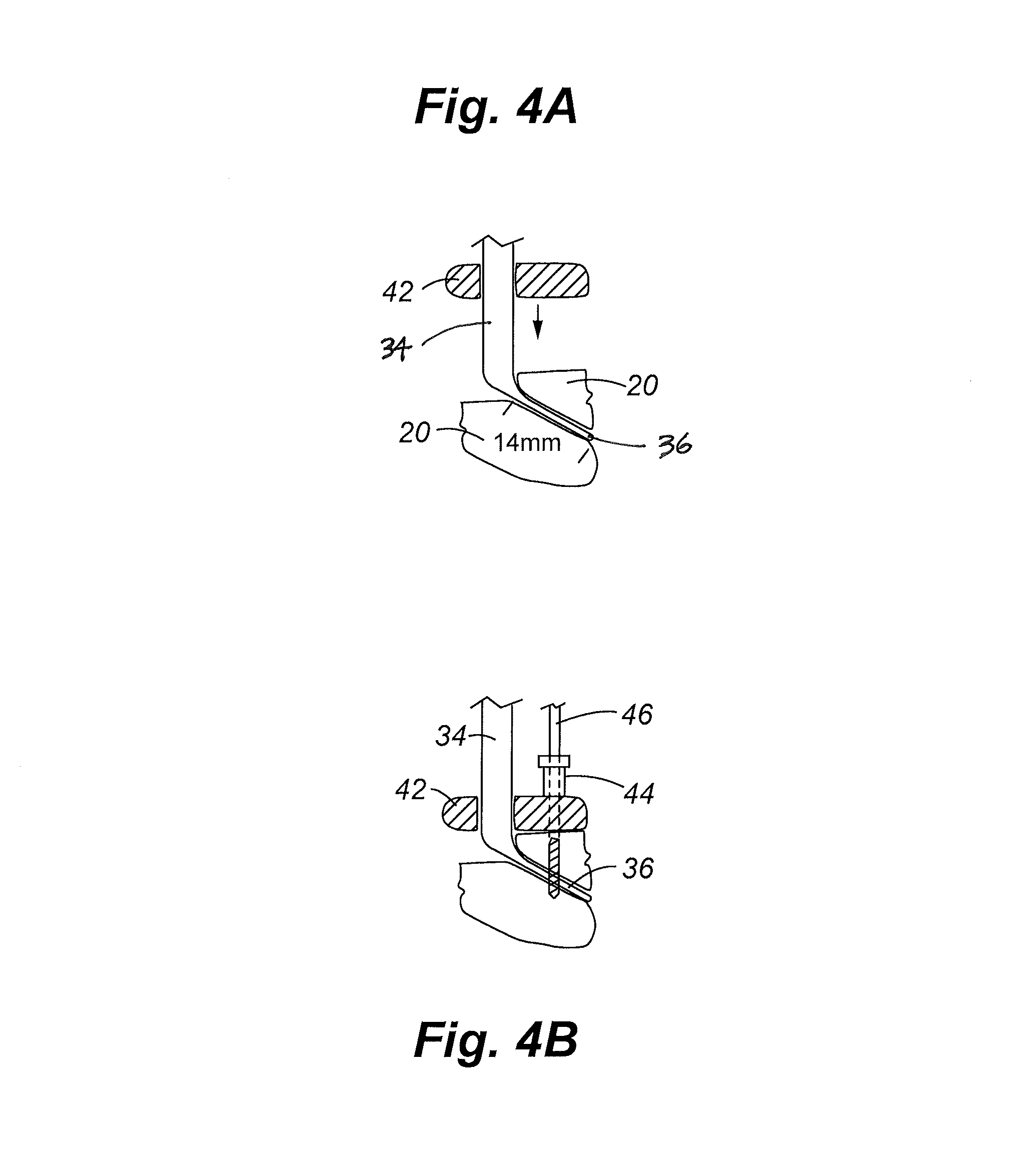
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1185 results about "Implant material" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
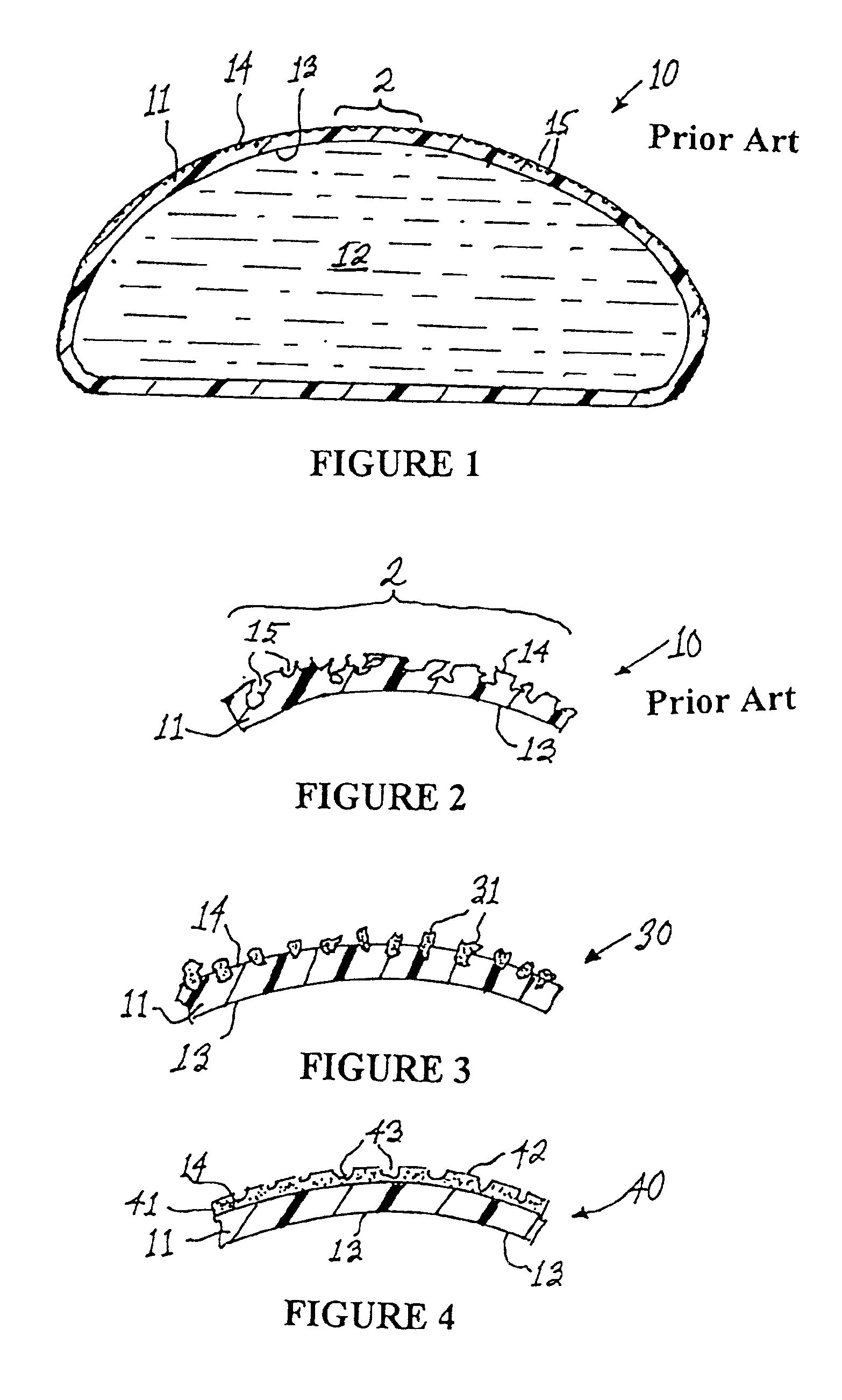
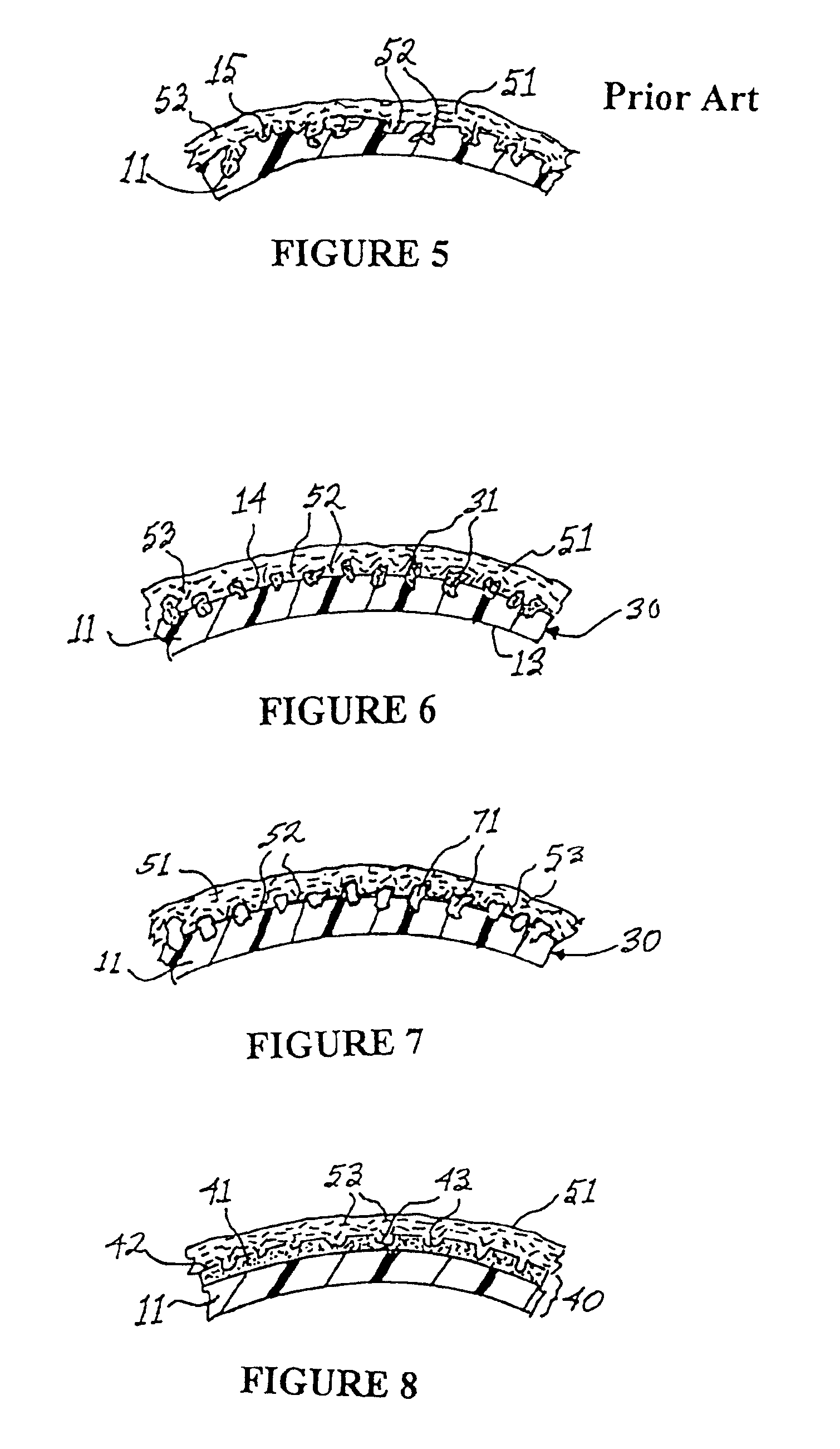
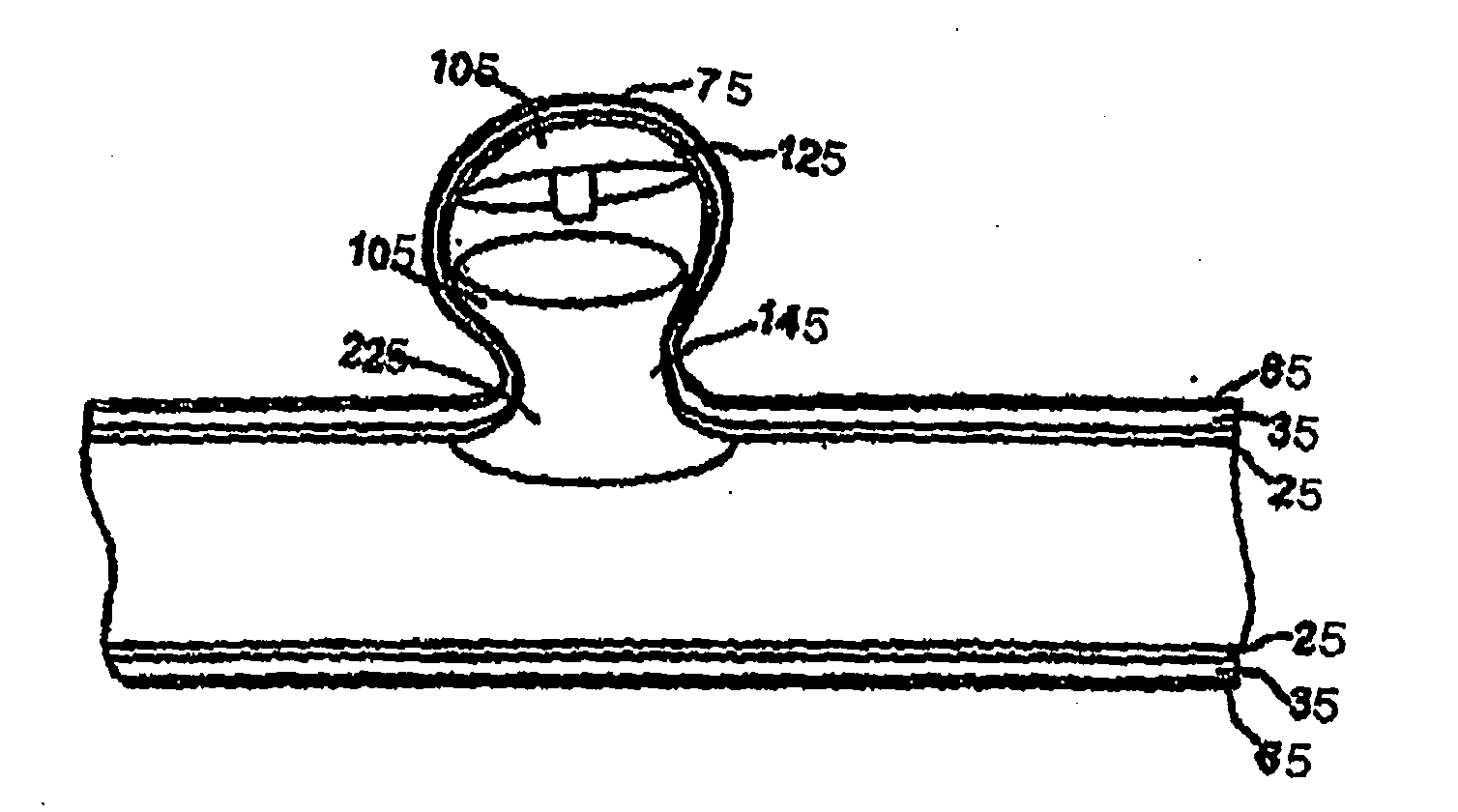
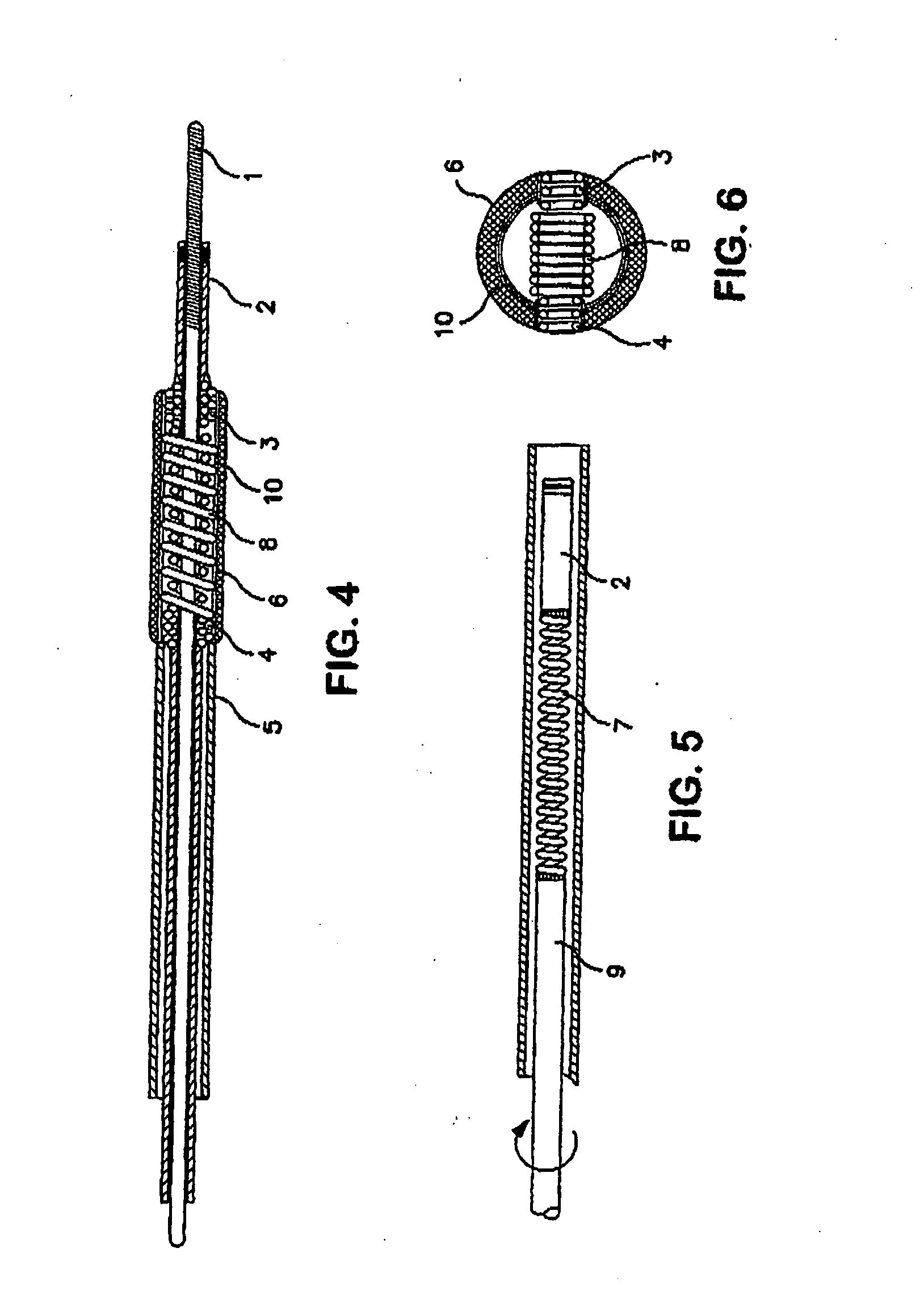
Apparatus and method for producing a reinforced surgical staple line
InactiveUS20080125812A1Easy to prepareEasy to useSuture equipmentsStapling toolsSurgical stapleBiomedical engineering
An apparatus for use with a surgical stapler to provide a reinforced surgical staple line. The apparatus includes an applicator that carries a first and second bioimplantable material connected by a hinge. An applicator clip may be provided to releasably secure the first and second bioimplantable material onto the applicator.
Owner:COOK BIOTECH
Composition comprising an agent providing a signal, an implant material and a drug
InactiveUS20060177379A1Facilitated releaseAvoid impairment of material compositionMaterial nanotechnologySurgeryTreatment effectBULK ACTIVE INGREDIENT
The present invention relates to compositions or combinations of materials for non-degradable and degradable implantable medical devices with regard to the setup of their signal generating properties and control of their therapeutic effectiveness, as well as to a method for the control of degradation of degradable or partially degradable medical devices composed like this, based on their signal generation, and to a method for supervision of their therapeutic effectiveness and / or the release of therapeutically active ingredients from such devices.
Owner:CINVENTION AG
Silver-containing, sol/gel derived bioglass compositions
Silver-containing, sol-gel derived bioactive glass compositions and methods of preparation and use thereof are disclosed. The compositions can be in the form of particles, fibers and / or coatings, among other possible forms, and can be used, for example, for treating wounds, improving the success of skin grafts, reducing the inflammatory response and providing anti-bacterial treatments to a patient in need thereof. Anti-bacterial properties can be imparted to implanted materials, such as prosthetic implants, sutures, stents, screws, plates, tubes, and the like, by incorporating the compositions into or onto the implanted materials. The compositions can also be used to prepare devices used for in vitro and ex vivo cell culture.
Owner:IMPERIAL INNOVATIONS LTD
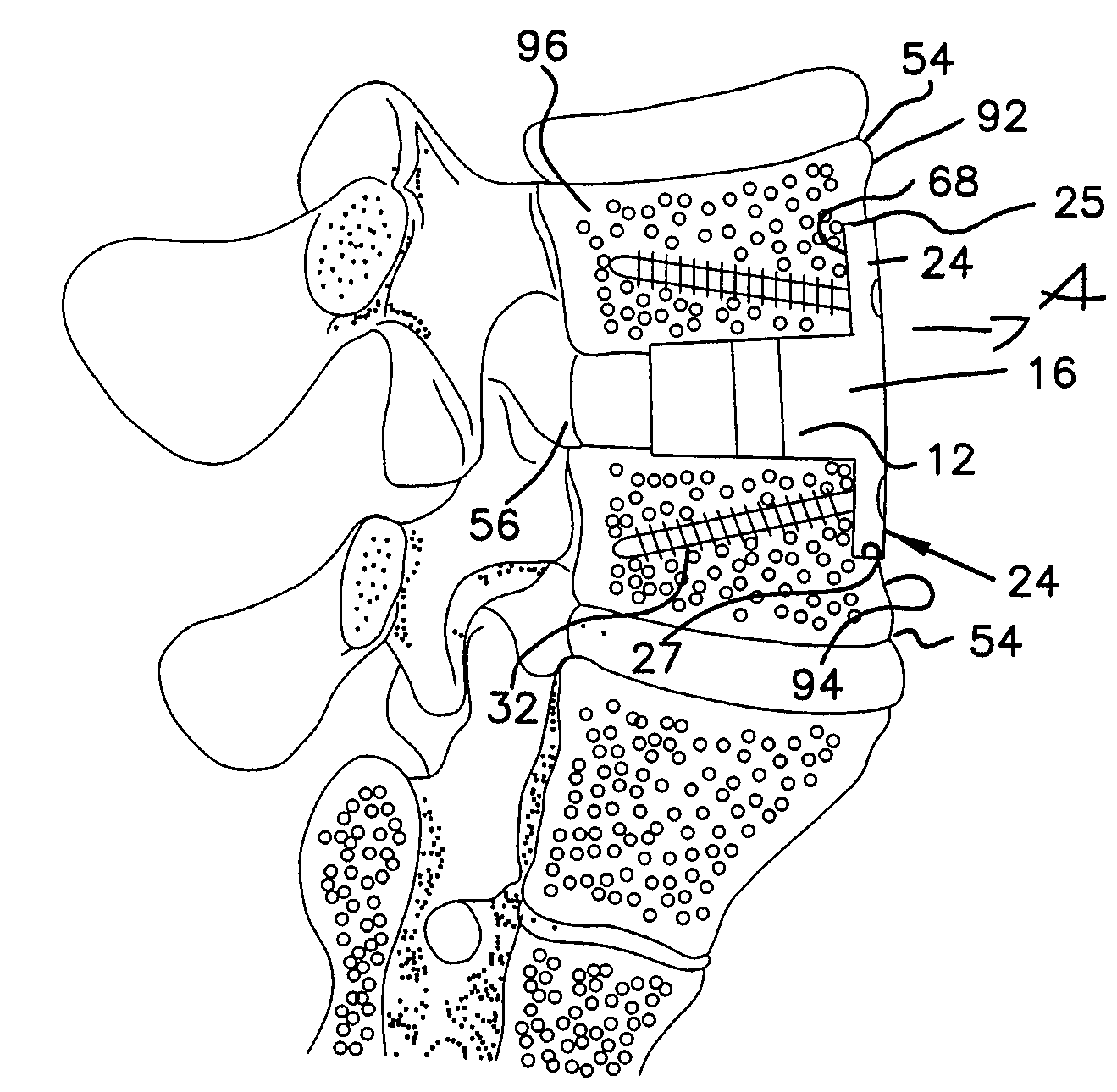
Vertebral implant for promoting arthrodesis of the spine
This invention provides a vertebral implant for impaction in a disc space to restore and / or maintain desired disc space height and spinal orientation. The implant has an elongated basis body having a generally lens-shape provided by convex upper and lower surfaces. Bearing surfaces are provided on the cross-edge surfaces of the endwalls. Grooves are provided in the upper and lower surfaces positioned between the bearing surfaces. The implant can be prepared from a wide variety of materials including metallic materials, synthetic materials, polymeric materials, ceramic materials, and composite materials including reinforced materials i.e. glass, fiber, and / or carbon fiber reinforced materials (CFRP). These preferred materials for fabricating implants in the present invention reduce costs, increase service life and provide excellent physiological compatibility. The non-metallic material can be selected to be either a substantially permanent material, a biodegradable material or a bioerodable material. Further, the implant material can be provided to be radio opaque to facilitate monitoring of bone ingrowth both into the implant and between the opposing endplates of the adjacent vertebrae.
Owner:WARSAW ORTHOPEDIC INC
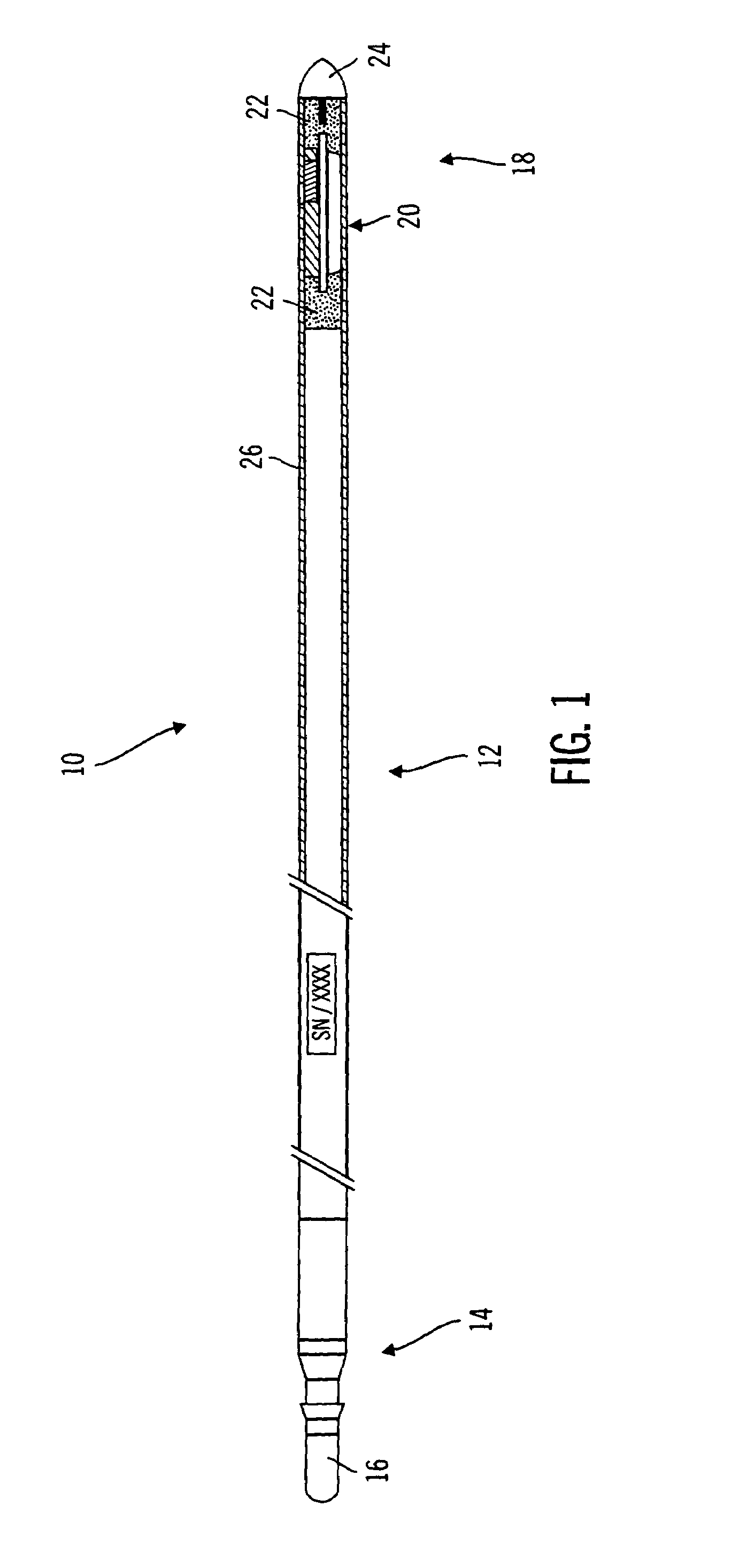
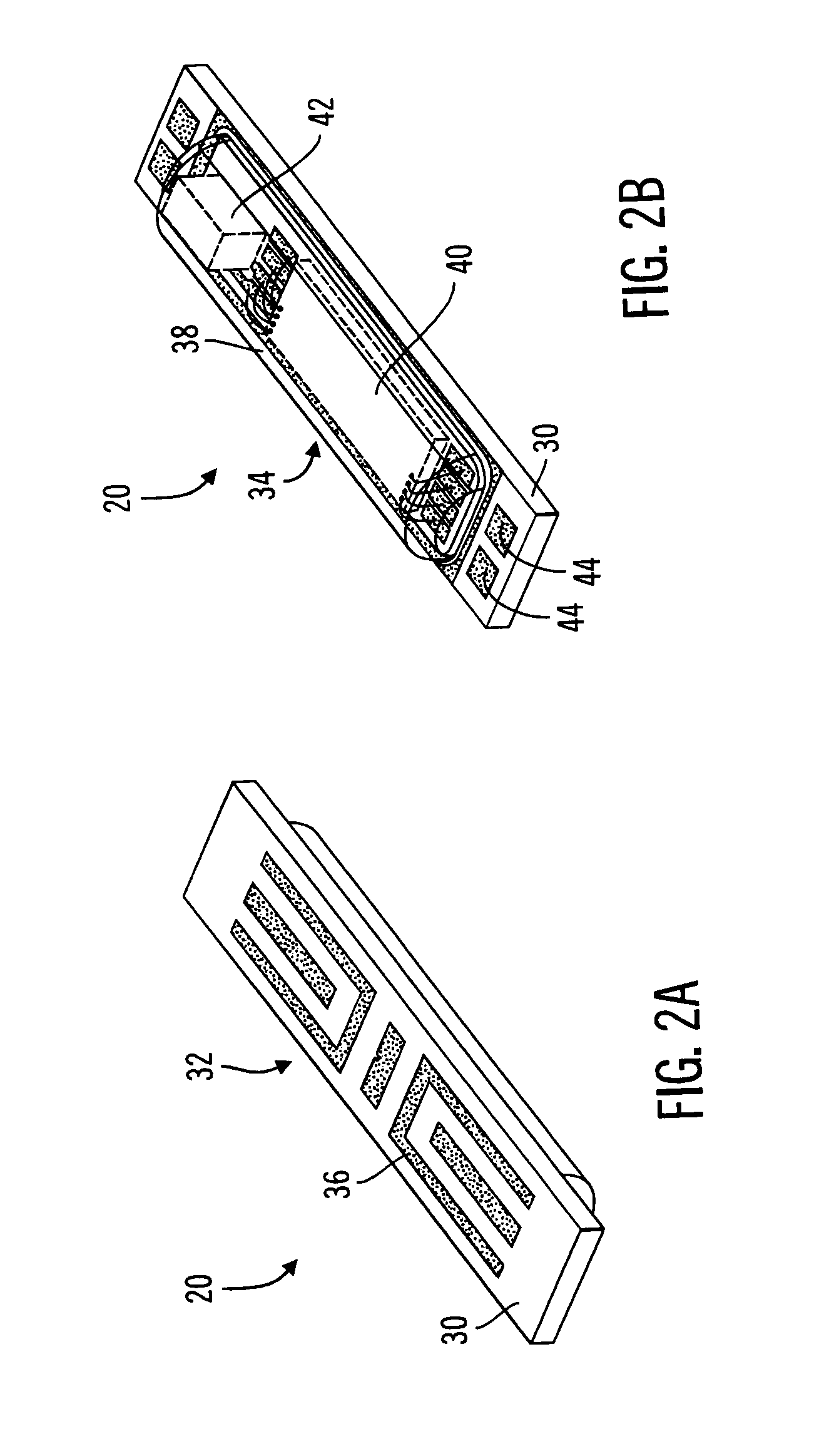
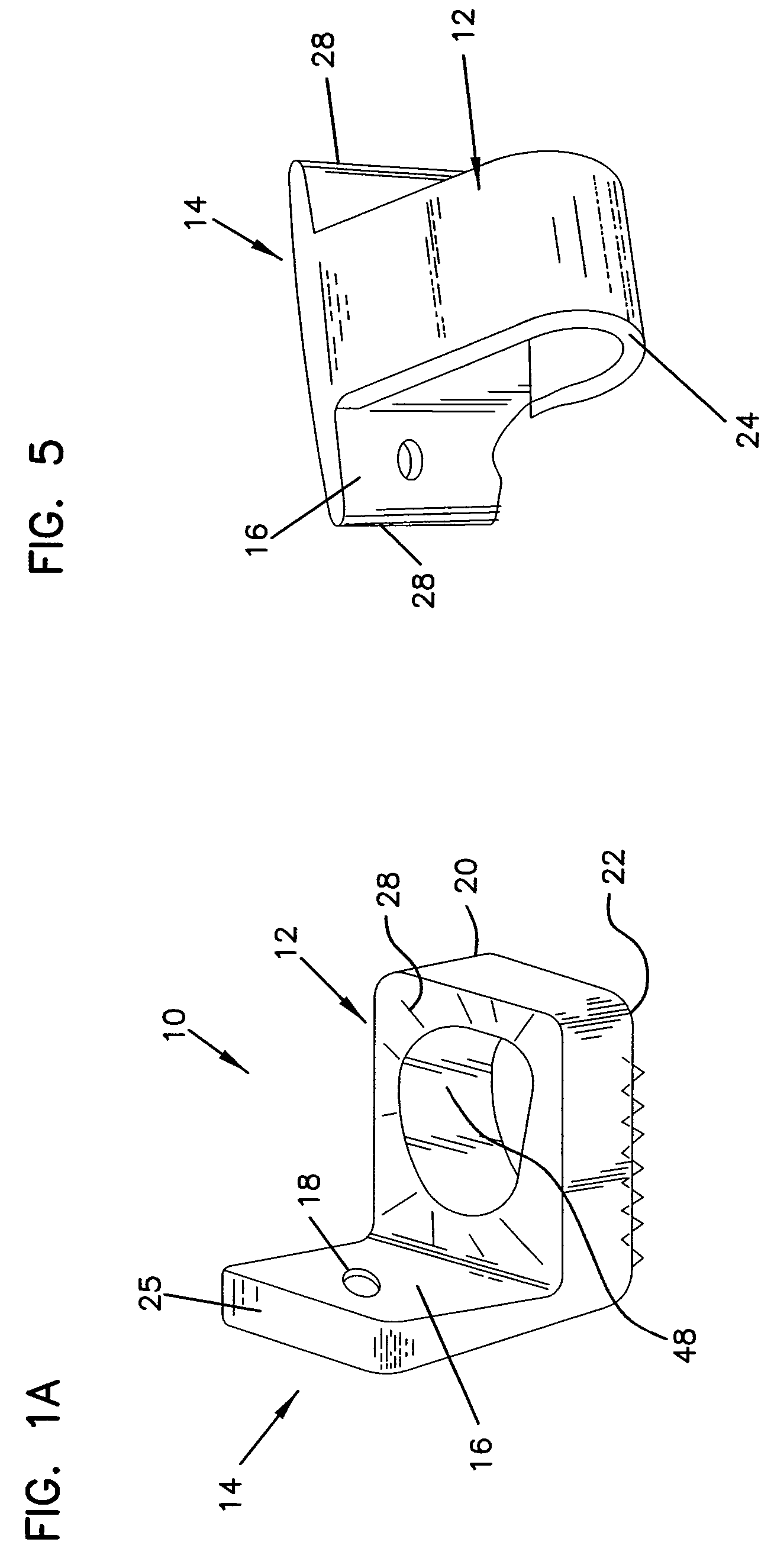
Method and apparatus for enhancing the integrity of an implantable sensor device
InactiveUS7162289B2Inhibited DiffusionExtended service lifeImmobilised enzymesBioreactor/fermenter combinationsProtein materialsLubrication
A method and apparatus for enhancing the integrity of an implantable sensor. Voids formed between an outer tubing and a sensor substrate or spacing element may be back-filled with a curable, implantable material, minimizing the extent to which unwanted fluids diffuse within the sensor. An enzyme or protein matrix pellet below the sensor window may be pre-treated with a reducing agent to enhance its bond stability, and to reduce undesired swelling that may cause the sensor window to detach or leak. The bonding between the enzyme pellet and a hydrogel layer may be reinforced by application of an intervening bonding layer of a protein material, such as human serum albumin (HSA). The size of the window may be minimized by minimizing the size of an underlying electrode, providing reduced flux and lengthening sensor. A coating may be deposited on the surface of the sensor leads, providing stiffening and lubrication.
Owner:MEDTRONIC MIMIMED INC
Methods for delivering tissue implant material with a high pressure applicator
InactiveUS7048743B2Increased margin of errorAvoid pollutionJoint implantsIntravenous devicesReady to useHigh pressure
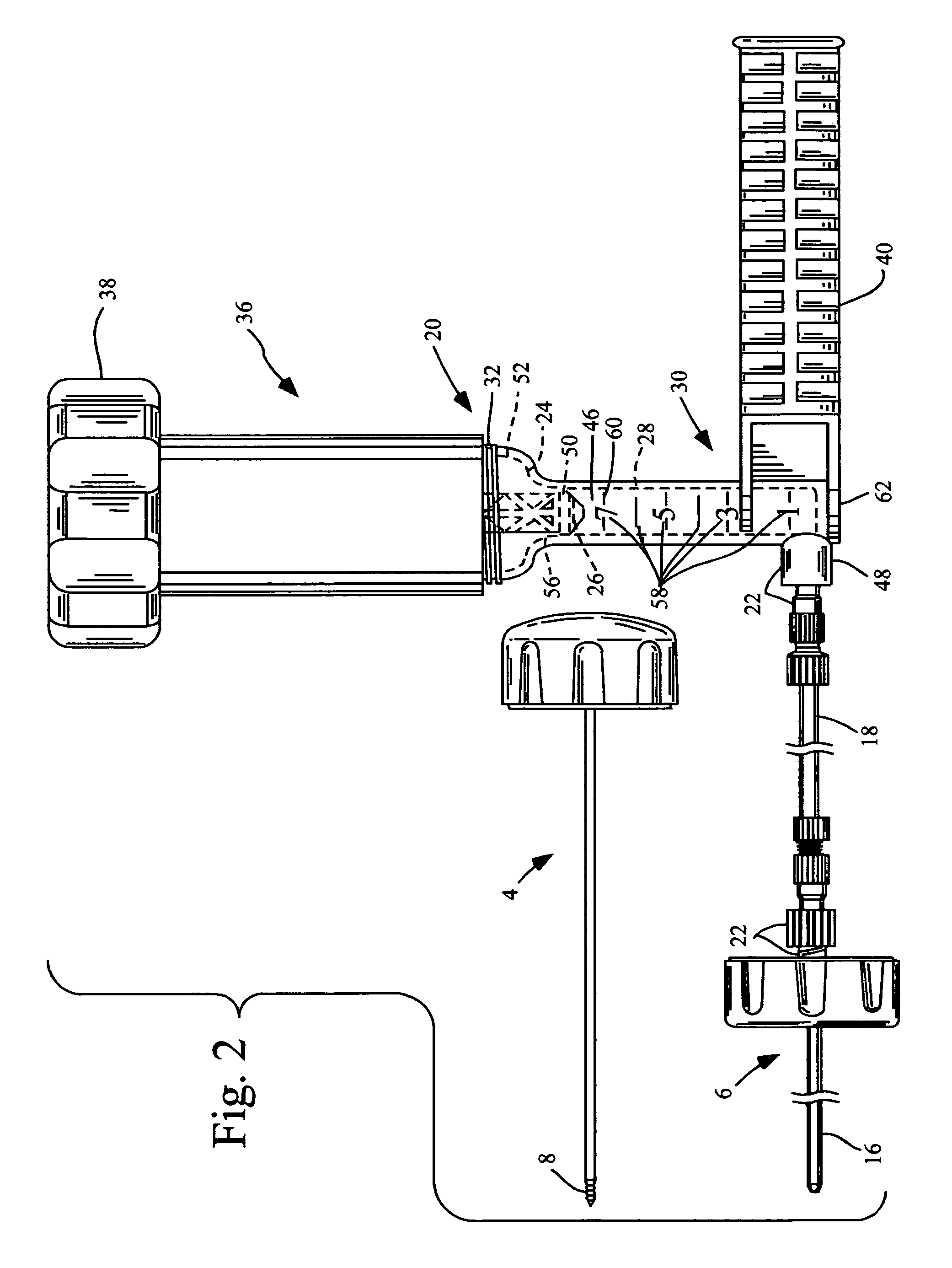
This relates to an improved delivery system for accurately loading and controlling the delivery of flowable material to a patient. Particularly, the system may be used in the injection of hard tissue implant materials such as PMMA under pressures up to about 4000 psi. The system includes an applicator with a first column having an implant material introduction section adapted to provide for effective loading of the implant material and a second column housing a piston. The introduction section has a larger size than that of a vessel section or bore in which the requisite pressure seal between the piston and bore wall is formed. The first column may include an introduction section flared open to an included larger funnel-like opening or a separate funnel may be used that interfaces with the introduction section to facilitate the introduction of implant material. Handles on the first and second columns to be turned relative to each other to advance the columns toward each other may be provided for manual actuation of the applicator to drive implant material through a cannula and deliver implant material to a desired site.
Owner:NEUROTHERM
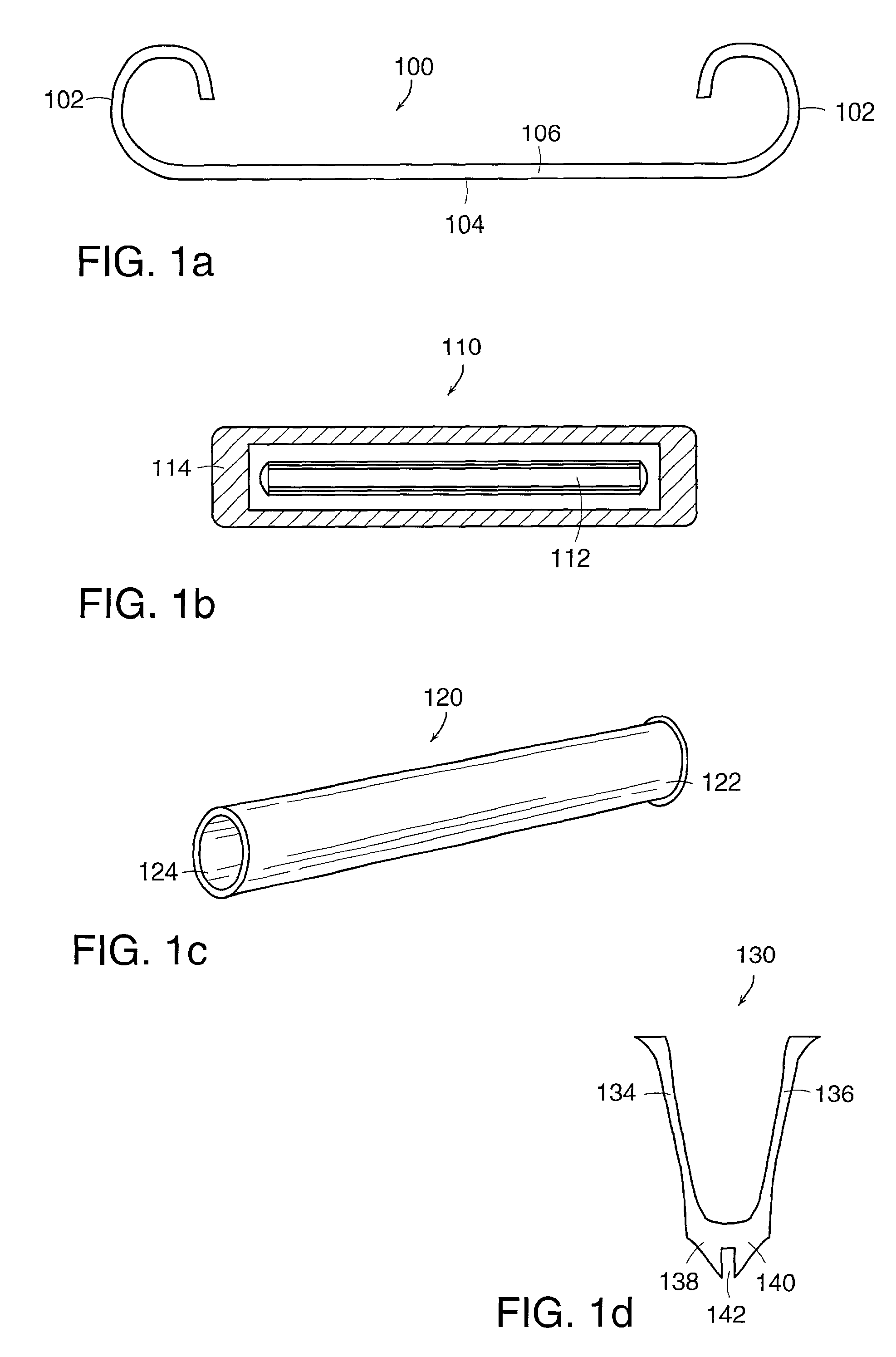
Linked slideable and interlockable rotatable components
InactiveUS8034109B2Precise directional and rotational controlInternal osteosythesisBone implantDistractionPermanent implant
Medical devices having one or more rotatable linkable components or segments, delivered in a first orientation relative to a component guide into a tissue cavity, such as the interbody vertebral space. After delivery, each segment may be rotated to a second, different orientation relative to the component guide, such as into a permanent vertical standing position. The segments achieve maximum distraction of the cavity space such as adjacent vertebra end plates, while using a minimal invasive surgical (MIS) approach. When the segments are tightened in place, the device provides long-term stability. The device can be used as a distraction instrument and / or permanent implant that can be used for interbody fusion, nuclear replacement, or anywhere in the body where a stable distraction of tissue and / or the implantation of material such as a device with an MIS approach is desired.
Owner:MORPHOGENY
Resorbable interposition arthroplasty implant
InactiveUS6017366AImprove permeabilityAvoid collisionFinger jointsWrist jointsTrapezium BoneSurgical department
A resorbable implant for interposition arthroplasty which is intended to fill a void between two adjacent bone ends, providing a cushion between the bone ends to prevent impingement of the bone ends while providing time for tissue to infiltrate into the space occupied by the implant. It has a protracted resorption time of preferably at least three months to allow time for the proliferation of load-bearing host fibrous tissue in place of the resorbed implant material. The implant preferably is porous with a pore size of greater than about 80 microns in order to enhance infiltration of fibrous tissue. The implant has a modulus in the range of 0.8 to 20 MPa which is soft enough to allow it to conform to surface irregularities of the adjacent bone surfaces while being hard enough to maintain a desired separation between those bones while tissue infiltration replaces the resorbable implant material. The implant may be preformed to the desired shape or alternatively may be formable by the surgeon by methods such as carving with a scalpel. A preferred application for the implant is as a trapezium bone replacement.
Owner:WL GORE & ASSOC INC
Medical implant having bioabsorbable textured surface
A hybrid medical implant having a biocompatible, nonabsorbable core portion and a bioabsorbable textured outer surface portion overlying the core portion. The hybrid implant is useful as a prosthesis for tissue augmentation and / or reconstruction. The core portion of the implant includes a body formed from a nonabsorbable, biocompatible implantable material such as silicone or urethane elastomer. The core portion may be either a solid body, a viscous gel body or a fluid-filled shell. The textured outer surface portion envelops the core portion and presents an irregular, bioabsorbable textured surface to the exterior environment. As a capsule forms around the implant following implantation, the irregular contour of the outer surface of the implant disorients structural proteins in the capsule to impede spherical contraction thereof. Either during the formation of the capsule and / or after the capsule is formed, the outer bioabsorbable surface portion of the implant is absorbed by the body of the host. After bioabsorbtion of the bioabsorbable outer surface portion, the remaining core portion of the implant remains enveloped by the capsule but unattached to capsular tissue. The outer bioabsorbable portion of the hybrid implant may include more than one biocompatible, bioabsorbable material.
Owner:WHD ELEKTRONISCHE PRUEFTECHN
Intervertebral implant with movement resistant structure
InactiveUS7833245B2Promote and accelerate new bone growthSupport of loadInternal osteosythesisBone implantMotion resistanceBiomedical engineering
An implant unit used in surgery has a body made from osteogenic implantable material and including an implant portion and a retaining portion, which is coupled to and extends transversely to the implant portion. The retaining portion is attached to the sidewall of the adjoining vertebral body or mammal bone to prevent displacement of the implant portion relative to the vertebral body or mammal bone and to accelerate fusion therebetween.
Owner:WARSAW ORTHOPEDIC INC
Immobilizing mediator molecules via anchor molecules on metallic implant materials containing oxide layer
A mediator molecule is immobilized on the surface of a metallic or ceramic implant material. An anchor molecule such as a dialdehyde having a functional group that covalently binds the mediator molecule is covalently bound to the surface, and the mediator molecule is coupled to the functional group of the anchor molecule. The implant material may be composed of titanium, titanium alloy, aluminum, stainless steel or hydroxylapatite. Oxide units on the surface of the implant material can be increased preferably by treating with hot chromic-sulphuric acid for 0.5 to 3 hours at a temperature between 100 to 250° C. prior to binding the anchor molecule. Also, prior to binding the anchor molecule, the surface of the implant material can be activated by reacting with a silane derivative. Mediator molecules include BMP protein, ubiquitin and antibiotics, and the implant material may be an artificial joint or coronary vessel support such as a stent.
Owner:MORPHOPLANT
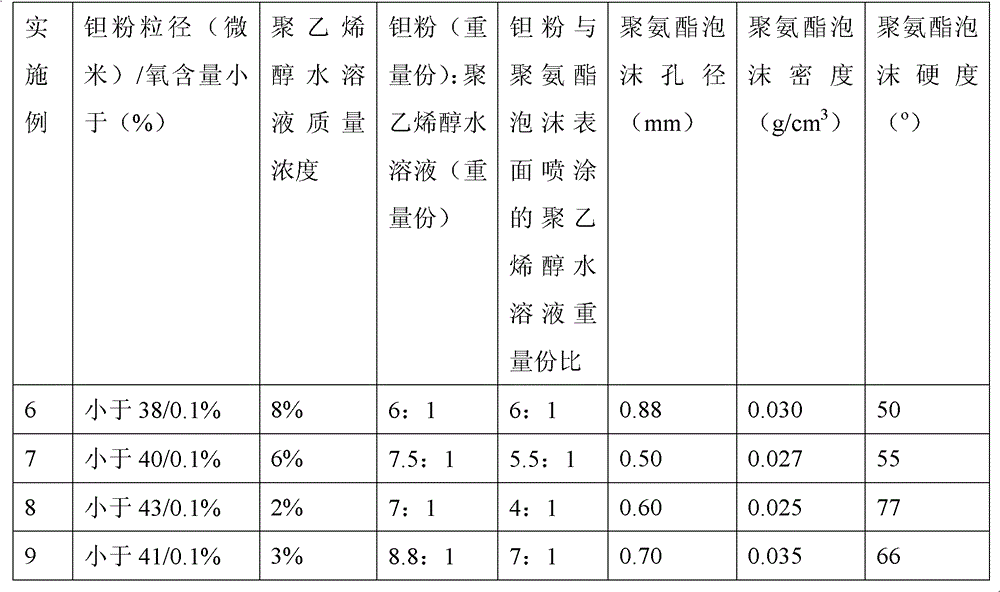
Method for preparing medical porous tantalum implant material
ActiveCN102796894AReduce contentImprove mechanical propertiesProsthesisPolyvinyl alcoholBiocompatibility Testing
The invention discloses a method for preparing a medical porous tantalum material. The method comprises the following steps of: mixing a poly ethanol aqueous solution and tantalum powder to obtain slurry, wherein the mass concentration of the poly ethanol aqueous solution is 2 to 8 percent; injecting the slurry into an organic foam by vibrating and pressurizing, wherein the vibrating frequency is 20 to 80 times / min; drying; degreasing; sintering, namely raising temperature to 1,500 to 1,800 DEG C at the speed of 10 to 20 DEG C / min under the vacuum degree of 10<-4> to 10<-3>Pa, preserving heat for 120 to 240 minutes, cooling to 200 to 300 DEG C along with a furnace, raising temperature to 1,500 to 1,800 DEG C at the speed of 10 to 20 DEG C / min again, preserving heat for 180 to 240 minutes, raising temperature to 2,000 to 2,200 DEG C at the speed of 5 to 10 DEG C / min, and preserving heat for 120 to 360 minutes; cooling; and performing thermal treatment, namely raising temperature to 800 to 900 DEG C at the speed of 10 to 20 DEG C / min under the vacuum degree of 10<-4> to 10<-3> Pa, preserving heat for 240 to 480 minutes, cooling to 400 DGE C at the speed of 2 to 5 DGE C / min, preserving heat for 120 to 300 minutes, and cooling to room temperature along with the furnace. The porous tantalum prepared by the method is very suitable to be used for the medical implant material for replacing bearing bone tissues, and biocompatibility and the mechanical property can be guaranteed simultaneously.
Owner:CHONGQING RUNZE PHARM CO LTD
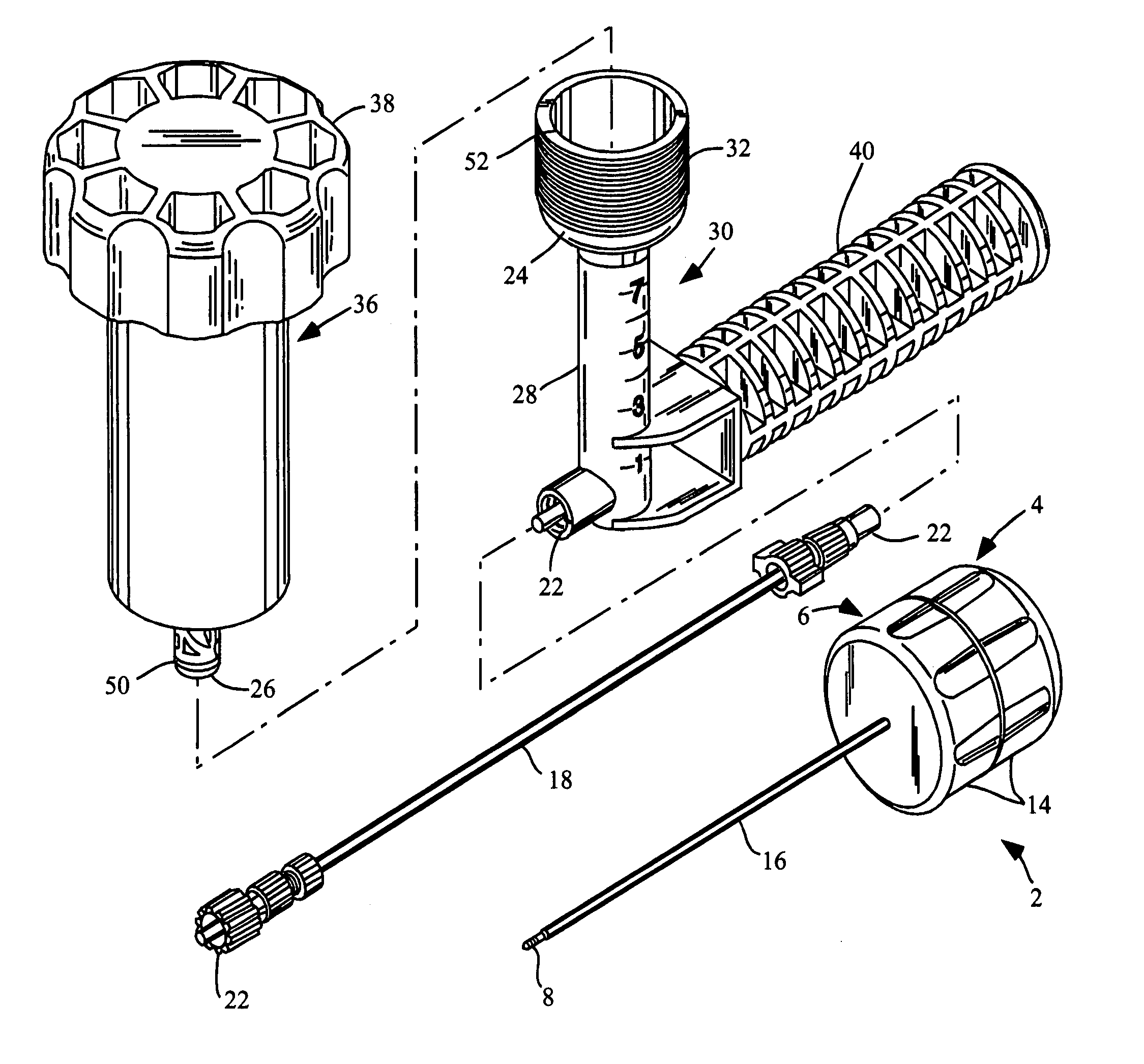
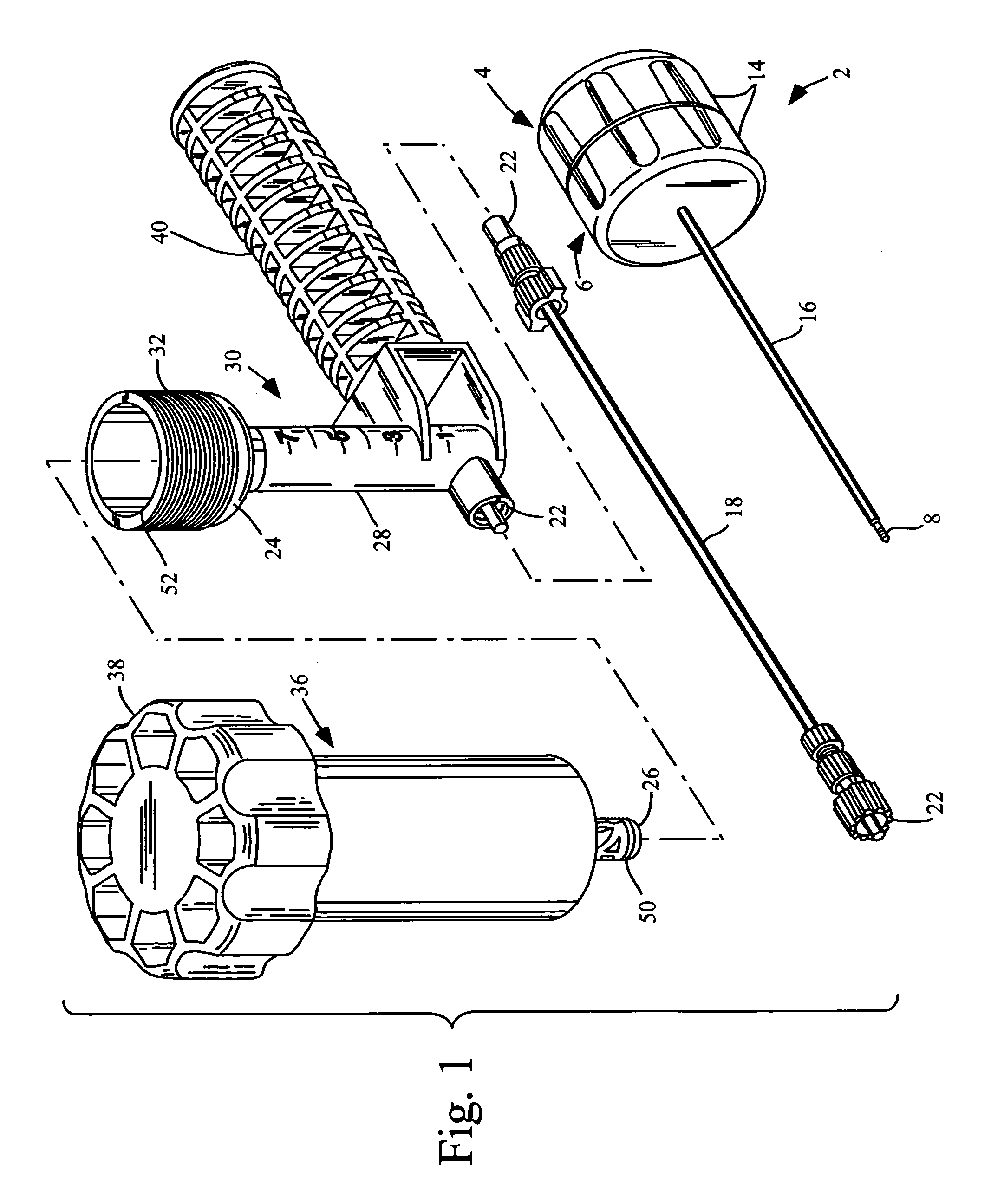
Remotely actuated system for bone cement delivery
An injection system for delivering viscous fluids is disclosed. Exemplary fluids included flowable hard tissue implant material such as Polymethylmethacrylate (PMMA). The system comprises a manual actuator, a control line and a dispenser. The control line may be flexible cable set within a housing running between the actuator and dispenser head. In which case, the actuator is designed to enable reciprocal movement of the cable relative to the cable housing. The actuator may be designed for one-handed use like a syringe. Components of the dispenser may be utilized as a mixing vessel prior to inserting a drive unit to enable fluid delivery. Prior to use, fluid is loaded into a portion section of the drive unit from a reservoir section of the dispenser head by retracing the cable via the retractor. Fluid is expelled from the dispenser portion of the system upon advancing the cable.
Owner:ARTHROCARE
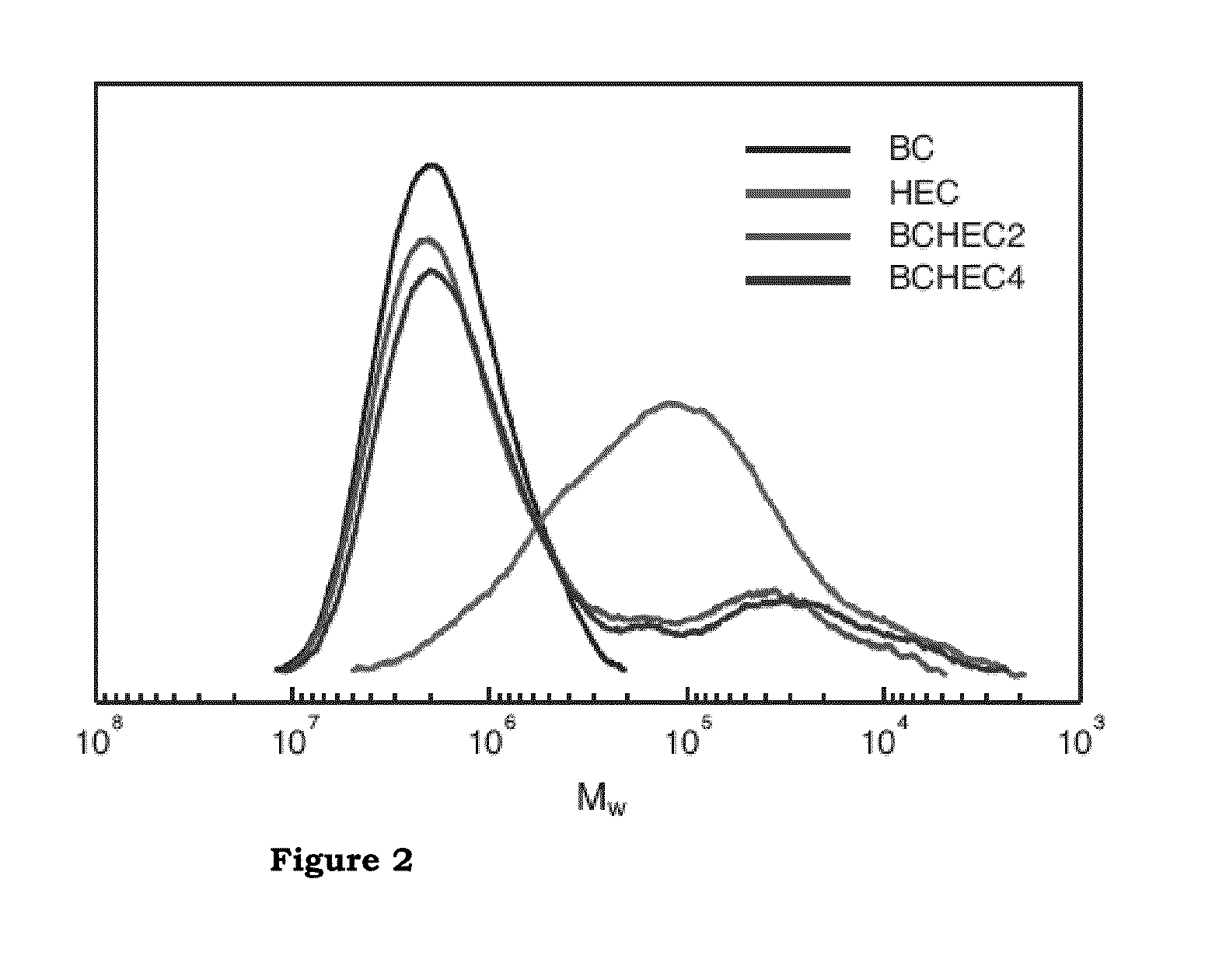
Method of producing and the use of microfibrillated paper
InactiveUS20100065236A1Non-fibrous pulp additionPulp properties modificationChemical structureEngineering
The present invention relates to a method of producing a cellulose based paper, the paper itself and the use thereof where the paper exhibits enhanced mechanical properties. The method involves providing a suspension of well dispersed modified cellulose at a low concentration. The properties and the chemical structure of the paper make it suitable for in vivo applications such as implant material.
Owner:SWETREE TECHOLOGIES AB
Controlling resorption of bioresorbable medical implant material
InactiveUS20020138154A1Different and fast resorption rateAnti-incontinence devicesCatheterPorosityBody fluid
The resorption of a medical implant can be controlled with the use of particles embedded in a resorbable bulk material forming the implant or portion thereof. The implant can be removed from a body of a mammal by natural biological mechanisms after use. The resorption of the implant can involve swelling and / or hydrolyzing of the particles within the implant upon contact with a body fluid such that porosity and flow of fluid within the bulk material of the implant is increased. Resorption of the implant may also involve the use of particles with magnetic properties embedded within the implant such that an applied magnetic field causes the particles to vibrate within the bulk material thereby increasing the porosity and thus the flow of fluid, hence facilitating resorption of the implant. The resorption rate of the implant can be controlled by modulating swelling, hydrolysis, or movement of the embedded particles.
Owner:BOSTON SCI SCIMED INC
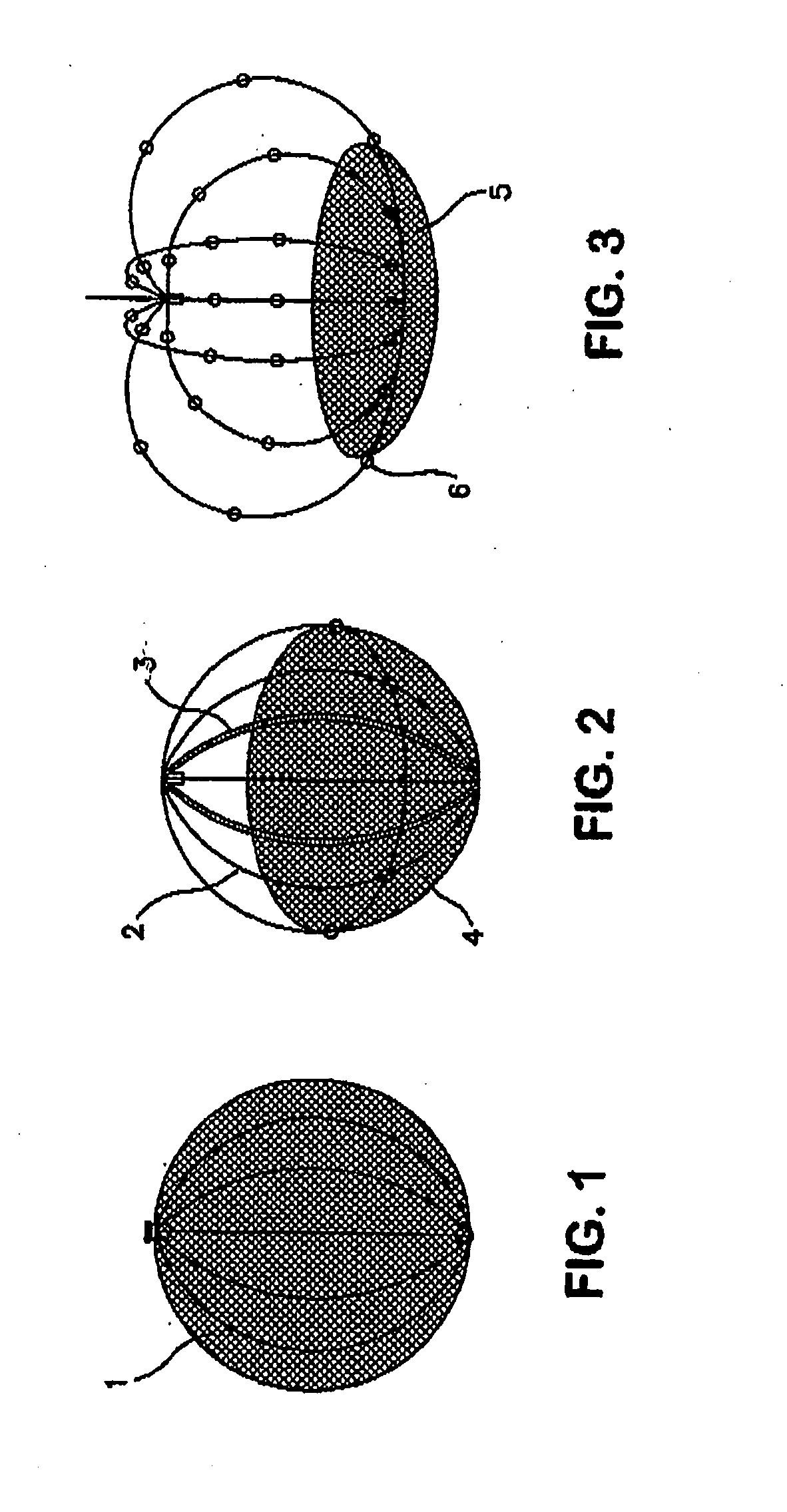
Self-Expandable Endovascular Device For Aneurysm Occlusion
The self-expandable endovascular apparatus for aneurysm occlusion of the invention comprises a deformable shape memory frame with at least a partial segment covering comprised of a matrix implant material. The device can be folded and / or stretched to adopt a narrow profile for loading into a coaxial delivery device and expands in place as it adopts its original shape on release from the device into an aneurysm. A method of treating an aneurysm, comprises the steps of: (a) providing the self-expandable endovascular apparatus inserted into a lumen of a delivery device comprising a proximal end and a distal end, the distal end having a distal tip; (b) advancing the distal tip of the delivery device into an opening in an aneurysm having an interior sac; (c) advancing the apparatus through the lumen into the opening; and (d) withdrawing the delivery device, whereby the apparatus expands into the sac and covers the opening.
Owner:BIOMERIX CORP
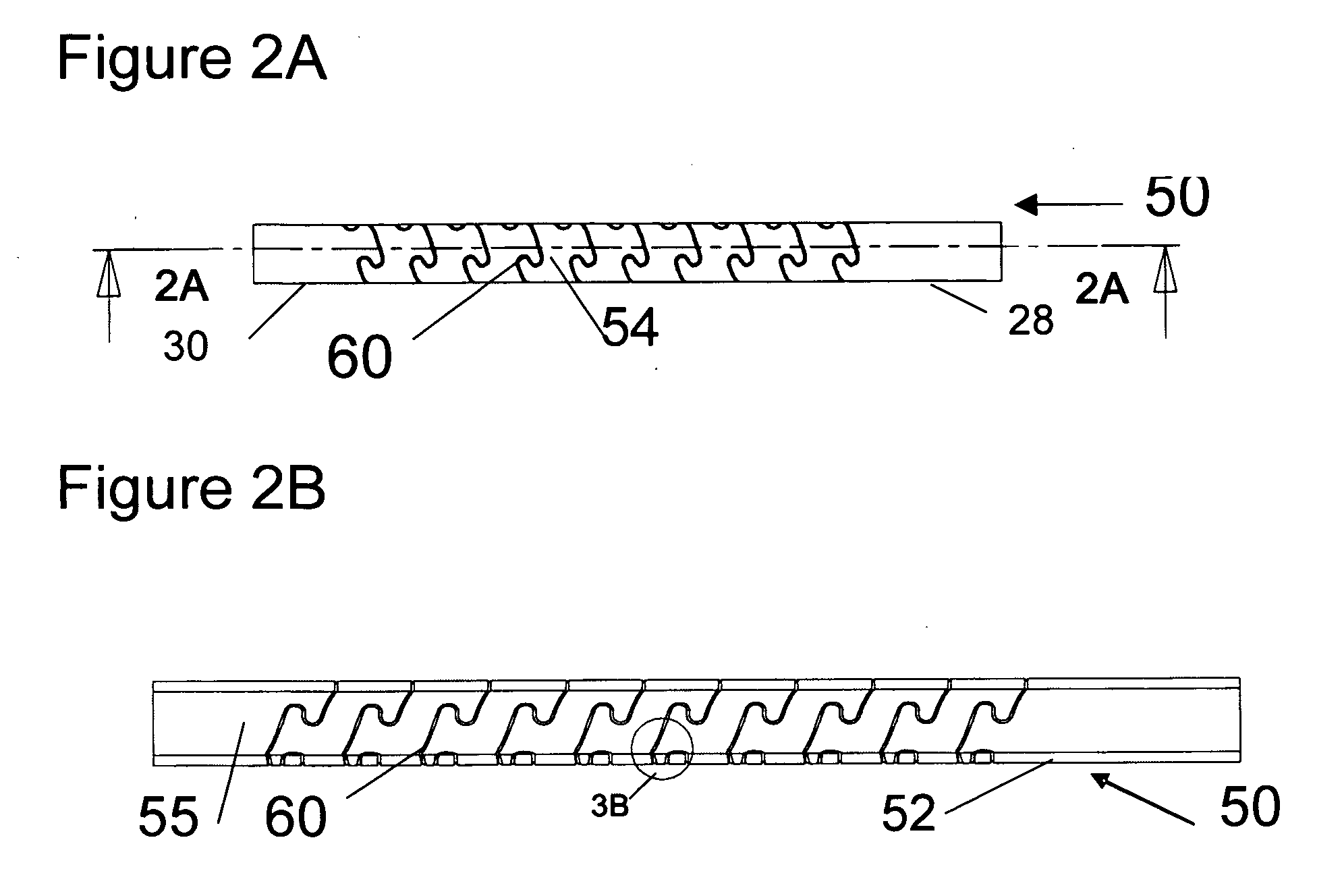
Flexible spine components
ActiveUS20080221620A1Increase flexibilityReduced flexibilitySuture equipmentsInternal osteosythesisElastomerDeformity
An improved flexible component used for dynamic stabilization of spinal segments for the treatment of vertebrae deformities and injuries and for the replacement of a complete or segment of the body of a vertebra in the spine is described. The flexible component is comprised of a solid, suitable implant material with a longitudinal bore the entire length and an appropriately formed slot which extends spirally around the shaft either continuously or segmentally. The flexible component may be encapsulated, fully or partially, in a suitable implant grade elastomeric resilient material. When used for a dynamic stabilization device, the component is attached to the vertebral bodies by pedicle screws know to those in the art. When used as a vertebral replacement device, attached to the component's opposite ends are members for attachment to the adjacent vertebra that allow for height and angular adjustment.
Owner:FLEX TECH
High pressure applicator
InactiveUS7572263B2Enhance generation of pressureImprove sealingJoint implantsIntravenous devicesCatheterBiomedical engineering
A pressure applicator for applying pressure to a flowable implant material, e.g., PMMA. A pressure applicator or driver includes a pair of columns which are engageable with one another, preferably by threads to generate a driving pressure. A handle is provided for the operator to grasp and steady the device as he turns the handle to apply pressure to the implantable material within the applicator. A luer-lock or other connecting device is provided for attaching the applicator to a cannula (or a connecting conduit that in turns connects with a cannula) that will deliver the implant material to the desired site. Pressures of about 1000-3000 psi may be generated by this device.
Owner:NEUROTHERM
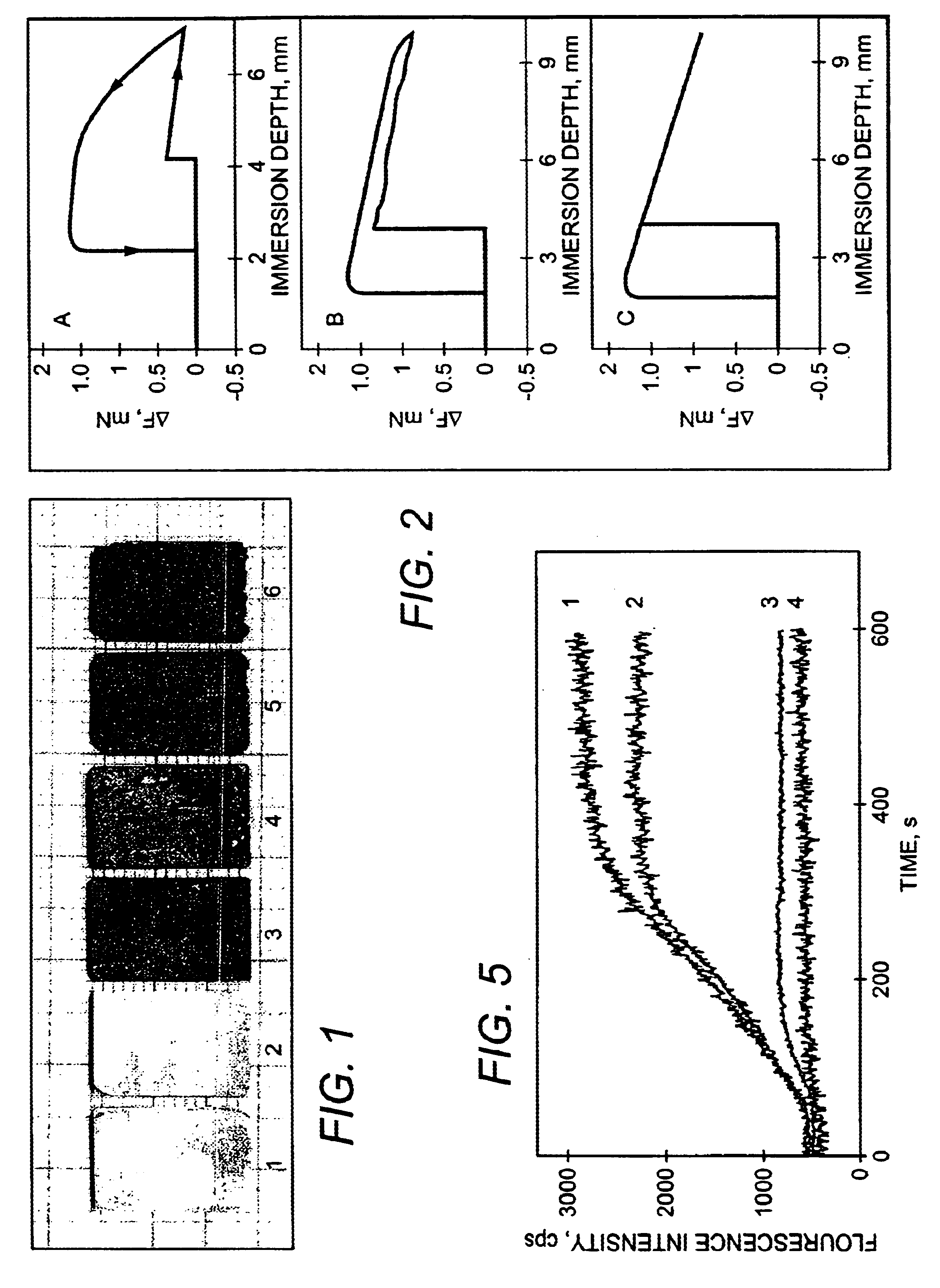
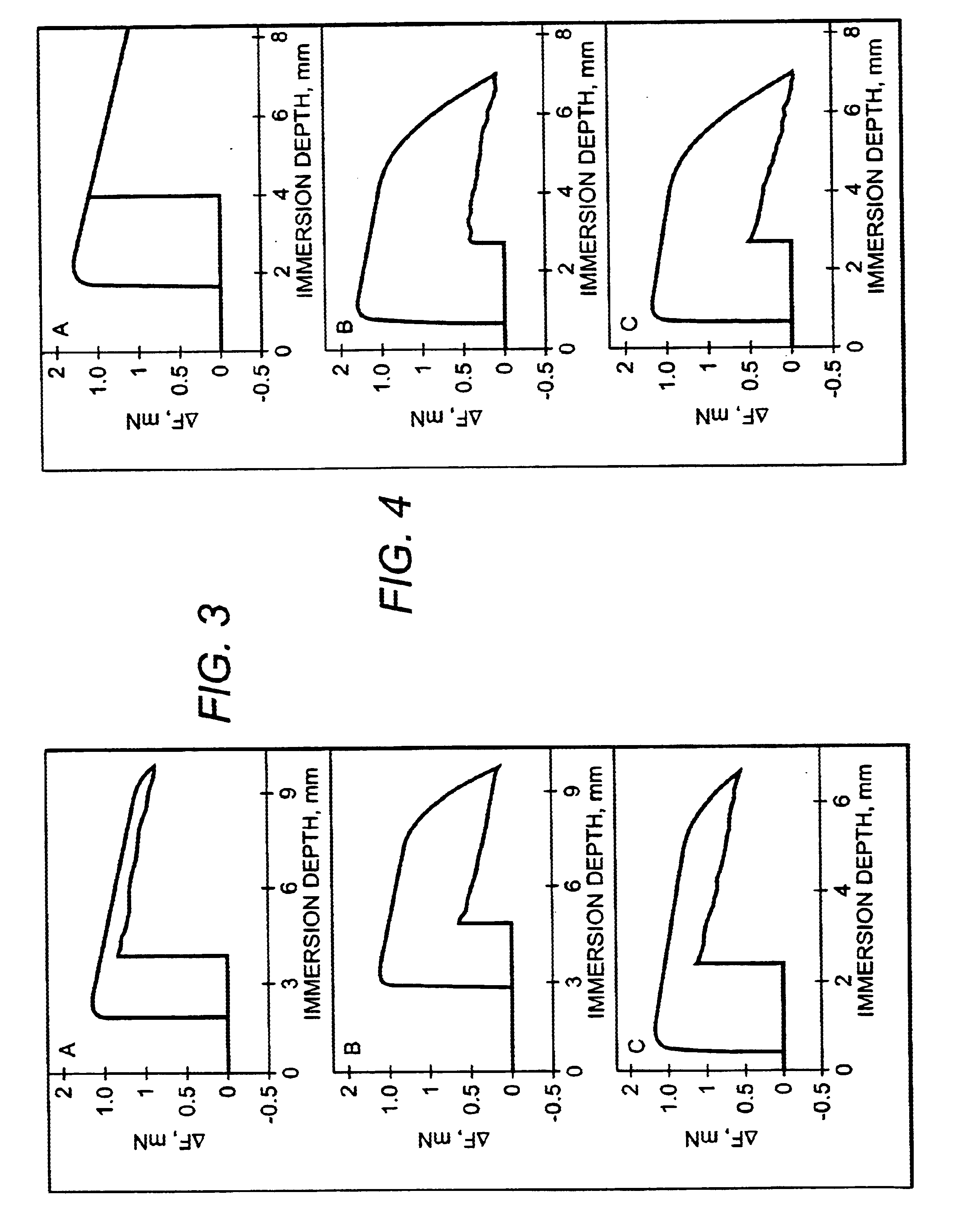
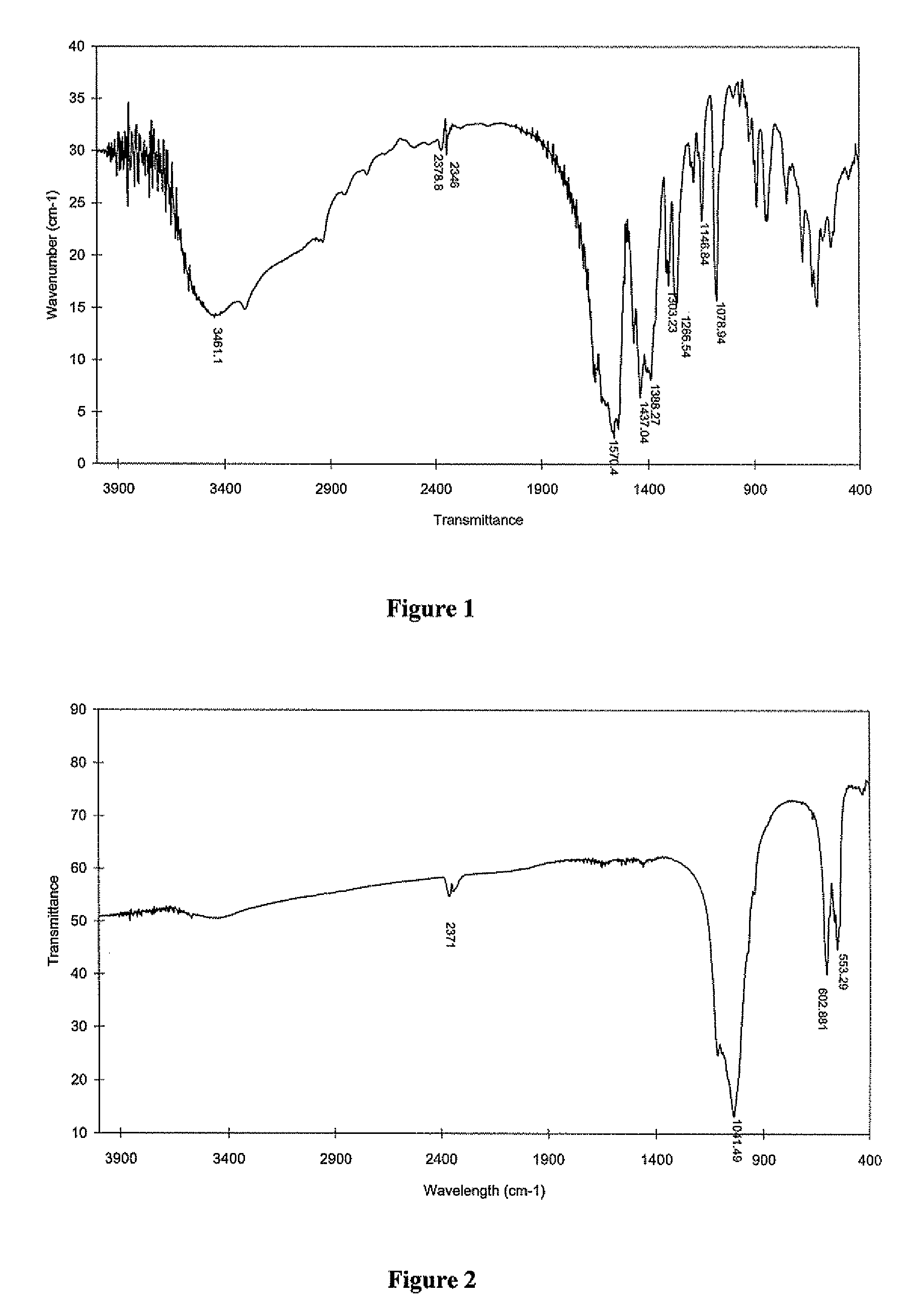
Bioactive spinal implant material and method of manufacture thereof
InactiveUS6987136B2High strengthGreat advantageImpression capsSurgical adhesivesMedicineSpinal implant
Bioactive spinal implant materials having optimized radiopacity, stiffness, and bioactivity properties for formulation of shaped bodies capable of withstanding large dynamic, compressive loads are provided. The invention also provides methods of making the optimized implant materials.
Owner:ORTHOVITA INC
Controlling resorption of bioresorbable medical implant material
InactiveUS6913765B2Avoids pain and sufferingReduces medical expense and lost productivityAnti-incontinence devicesPharmaceutical delivery mechanismPorosityMedicine
The resorption of a medical implant can be controlled with the use of particles embedded in a resorbable bulk material forming the implant or portion thereof. The implant can be removed from a body of a mammal by natural biological mechanisms after use. The resorption of the implant can involve swelling and / or hydrolyzing of the particles within the implant upon contact with a body fluid such that porosity and flow of fluid within the bulk material of the implant is increased. Resorption of the implant may also involve the use of particles with magnetic properties embedded within the implant such that an applied magnetic field causes the particles to vibrate within the bulk material thereby increasing the porosity and thus the flow of fluid, hence facilitating resorption of the implant. The resorption rate of the implant can be controlled by modulating swelling, hydrolysis, or movement of the embedded particles.
Owner:BOSTON SCI SCIMED INC
Glue for cartilage repair
ActiveUS7067123B2Promote migrationIncreased proliferationBiocidePeptide/protein ingredientsMedicineBone marrow cell
The invention is directed toward a sterile cartilage defect implant material comprising milled lyophilized allograft cartilage pieces ranging from 0.01 mm to 1.0 mm in size in a bioabsorbable carrier taken from a group consisting of sodium hyaluronate, hyaluronic acid and its derivatives, gelatin, collagen, chitosan, alginate, buffered PBS, Dextran or polymers with allogenic chondrocytes or bone marrow cells in an amount exceeding the natural occurrence of same in hyaline cartilage and adding a cell growth additive.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Osteoinductive bone material
ActiveUS20050084542A1Sufficient amountMaximize formabilityBiocideInorganic phosphorous active ingredientsNatural boneBone implant
Osteogenic bone implant compositions that approximate the chemical composition of natural bone are provided. The organic component of these implant compositions is osteoinductive despite the presence of the inorganic component and, further, is present in an amount sufficient to maximize the regenerative capabilities of the implant without compromising its formability and mechanical strength. The composition may be an osteoinductive powder, including demineralized bone matrix (DBM) particles, a calcium phosphate powder, and, optionally, a biocompatible cohesiveness agent. The powder may be combined with a physiologically-acceptable fluid to produce a formable, osteoinductive paste that self-hardens to form a poorly crystalline apatitic (PCA) calcium phosphate having significant compressive strength. The bone implant materials retain their cohesiveness when introduced at an implant site and are remodeled into bone in vivo. Methods for using these implant materials to repair damaged bone and a method of assaying the content of DBM particles, by weight, in a bone implant material are also provided.
Owner:ETEX
Porous biocompatible implant material and method for its fabrication
a biocompatible and biodegradable implant for a cavity in a bone of a living organism is made of a biocompatible and biodegradable granules which are selected from the group including biopolymers, bioglasses, bioceramics preferably calcium sulfate, calcium phosphate such as monocalcium phosphate monohydrate, monocalcium phosphate anhydrous, dicalcium phosphate dihydrate, dicalcium phosphate anhydrous, tetracalcium phosphate, calcium orthophosphate phosphate, α-tricalcium phosphate, β-tricalcium phosphate, apatite such as hydroxyapatite, or a mixture thereof. The biocompatible and biodegradable granules are provided with a coating, which comprises at least one layer of a biocompatible and biodegradable polymer which is selected from the group including poly((α-hydroxyesters), poly(orthoesters), polyanhydrides, poly(phosphazenes), poly(propylene fumarate), poly(ester amides), poly(ethylene fumarate), polylactide, polyglycolide, polycaprolactone, poly(glycolide-co-trimethylene carbonate), polydioxanone, co-polymers thereof and blends of those polymers. The biocompatible and biodegradable implants are obtained by fusing together the polymer-coated granules through polymer-linkage of the polymer coatings of neighboring granules.
Owner:COLLAGEN MATRIX
Enhanced visibility materials for implantation in hard tissue
InactiveUS20070191964A1Increase awarenessInternal osteosythesisSurgical adhesivesImplant materialVisibility
An enhanced visibility composition for implantation from a remote source, so that the composition can be readily observed under fluoroscopy or other imaging techniques is disclosed. The compositions include a biocompatible matrix, such as a hard tissue implant material for example, and radiopaque tracer particles mixed in the matrix. The radiopaque tracer particles have a particle size between about 120μ and 2200μ, more preferably about 350μ and 2200μ, even more preferably between about 450μ and 1600μ, and most preferably between about 570μ and 1150μ. Preferably the hard tissue implant and the radiopaque tracer particles are formed or prepared in a slurry. Optionally, the enhanced visibility composition may further include additional radiopaque contrast particles mixed in with the composition, which have a particle size between about 120μ and 350μ, preferably between about 120μ and 250μ.
Owner:ARTHROCARE
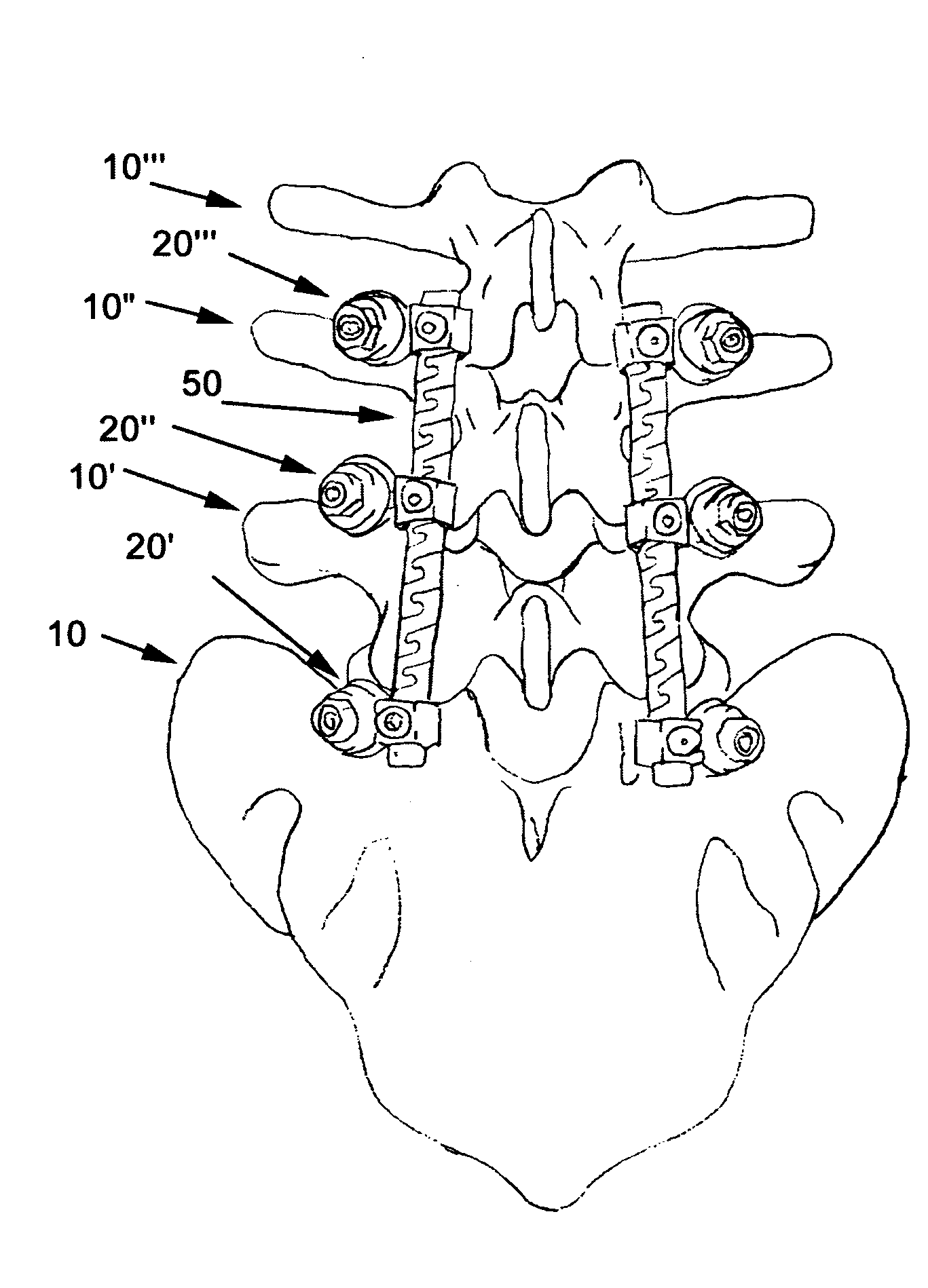
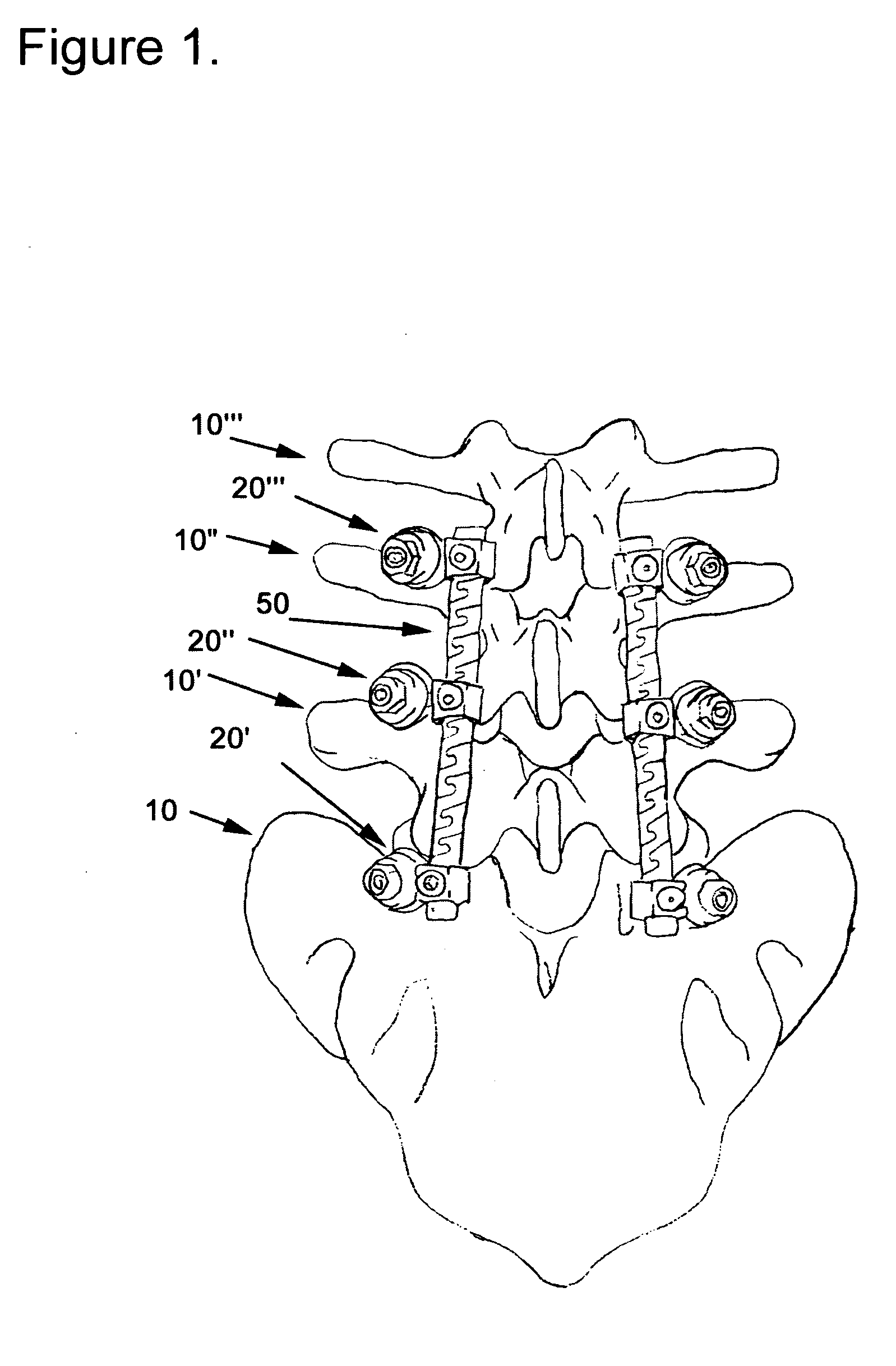
Apparatus and method of spinal implant and fusion
ActiveUS8366748B2Avoid undesired contact and interferenceReducing circumferenceInternal osteosythesisSurgical adhesivesSacroiliac jointSpinal implant
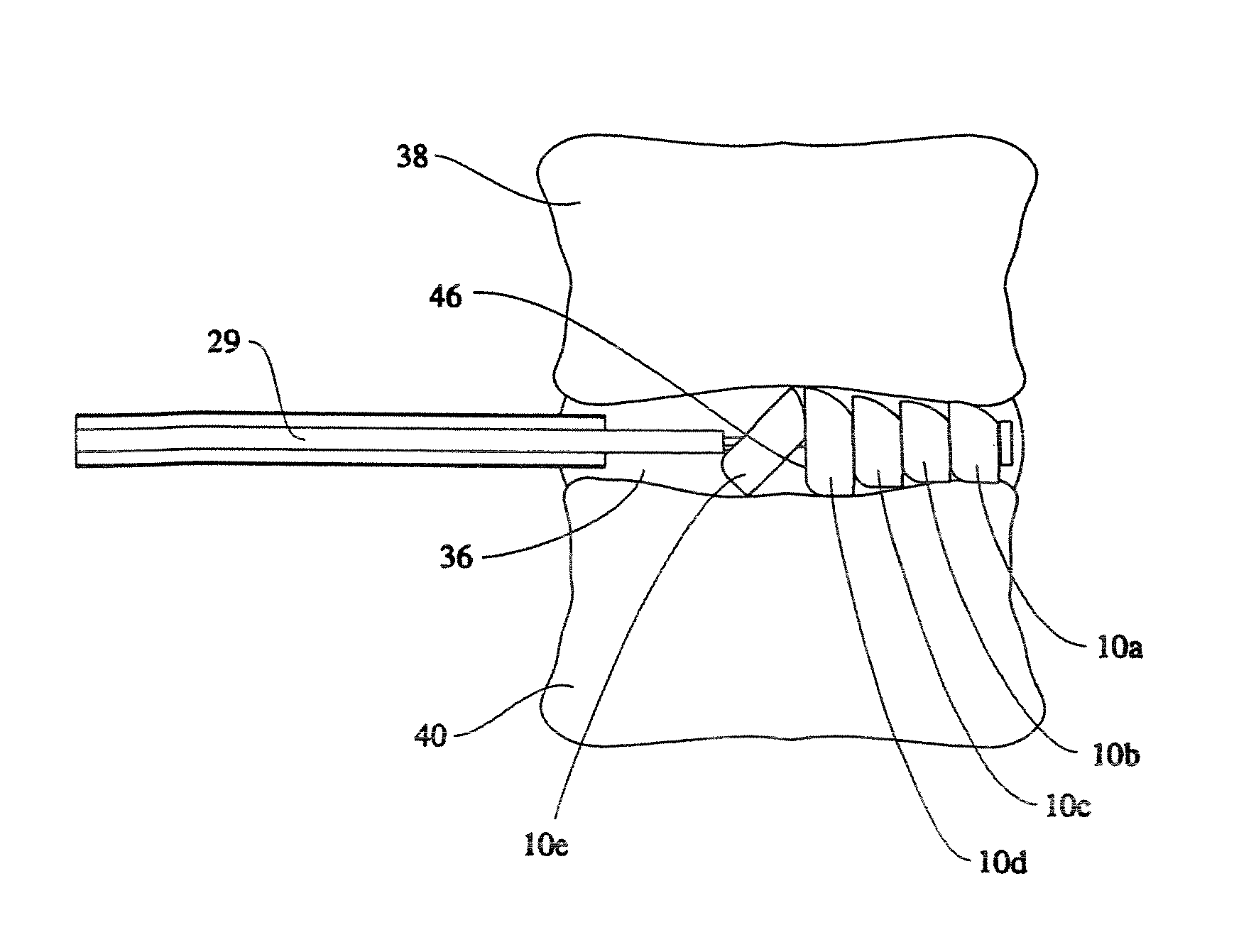
An apparatus and method of performing a minimally invasive posterior spine fusion. More specifically an apparatus with a handle and a forked head on the distal end of the handle is used to grasp implant material and introduce the material to an implant site. The shaft of the apparatus is shaped so as to allow the affixation of a drill guide and drill while simultaneously holding the implant material in the implant site. After removal of the boring tools and assembly of the fusing element, the apparatus can be selectively removed from the implant site. A method of achieving facet joint fusion with near simultaneous fixation is also disclosed.
Owner:KLEINER JEFFREY
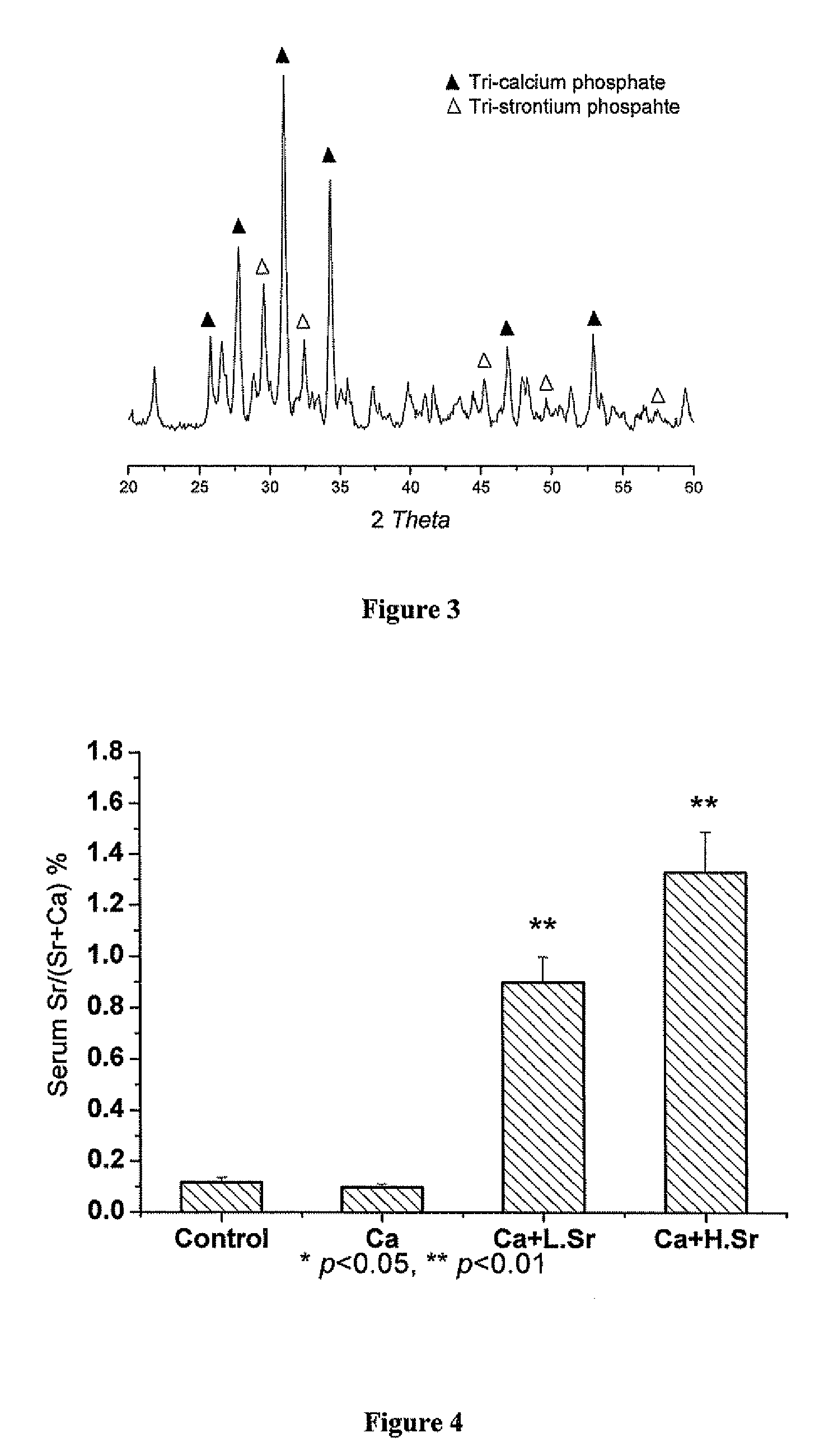
Strontium fortified calcium nano-and microparticle compositions and methods of making and using thereof
InactiveUS20080317807A1Good biocompatibilityPrevent looseningHeavy metal active ingredientsBiocideDiseasePhosphate
Compositions containing strontium fortified calcium nanoparticles and / or microparticles, and methods of making and using thereof are described herein. The strontium fortified calcium compounds contain calcium ions, calcium atoms, strontium ions, strontium atoms, and combinations thereof and one or more anions. Exemplary anions include, but are not limited to, citrate, phosphate, carbonate, and combinations thereof. The particles can be formulated for enteral or parenteral administration by incorporating the particles into a pharmaceutically carrier. The compositions can further contain one or more active agents useful for bone diseases or disorders, such as vitamin D, growth factors, and combinations thereof. The compositions can be used to treat or prevent one or more bone diseases or disorders of the bone, such as osteoporosis. Alternatively, the particles can be coated onto a substrate, such as the surface of an implant. The coatings can be used to improved biocompatibility of the implant, prevent loosening of the implant, reducing leaching of metal ions from metallic implants, and reduce corrosion. The coatings can be applied to the substrate using a variety of techniques well known in the art. In one embodiment, the coating is applied using electrophoretic deposition. The use of nano- and / or microparticles that provide high surface area helps to improve interfacial strength between the coating and the implant, which allows for the use of lower sintering temperatures. Lowering sintering temperatures minimizes or prevents thermal decomposition of the coating material and / or degradation of the implant material.
Owner:THE UNIVERSITY OF HONG KONG
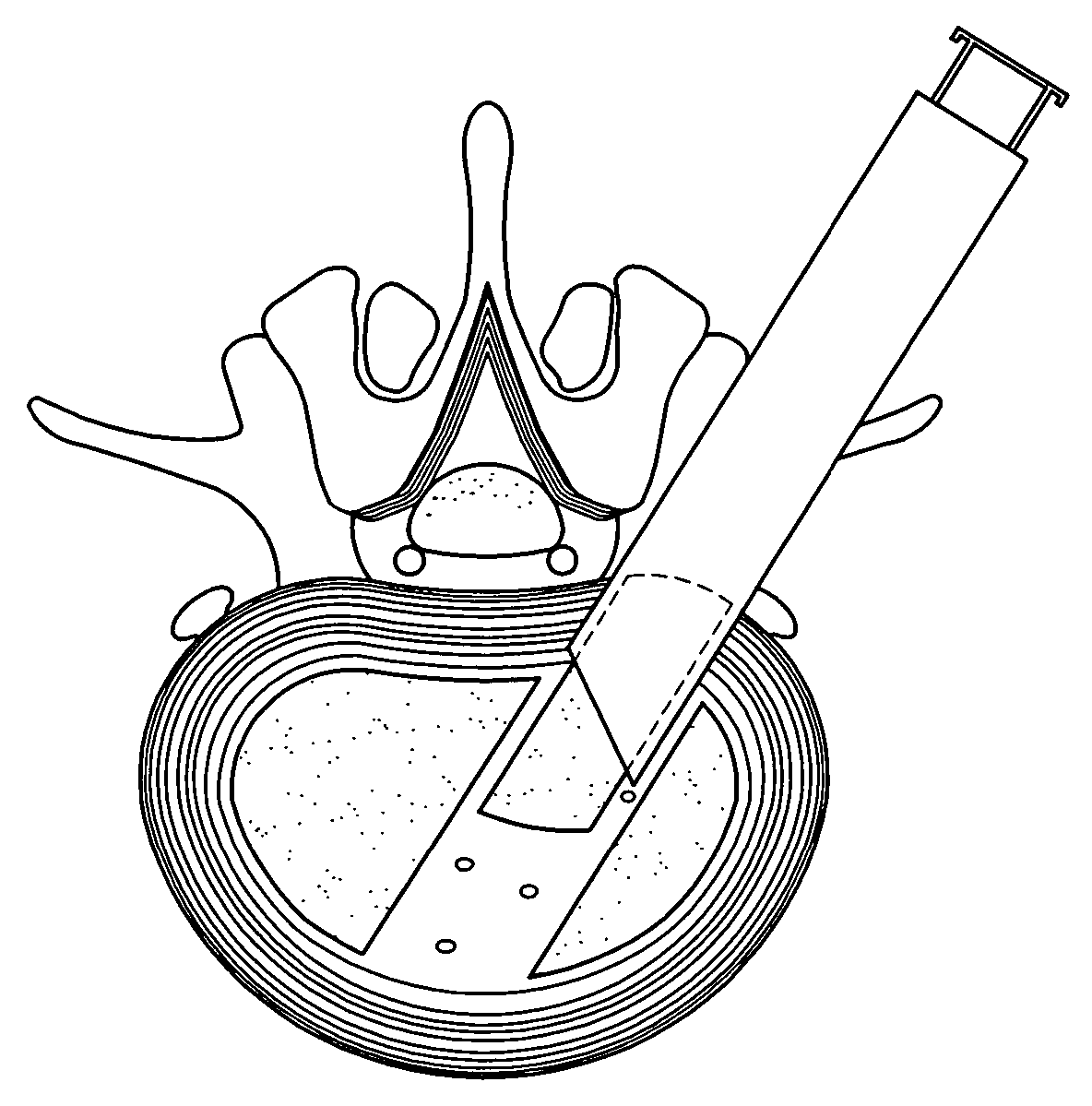
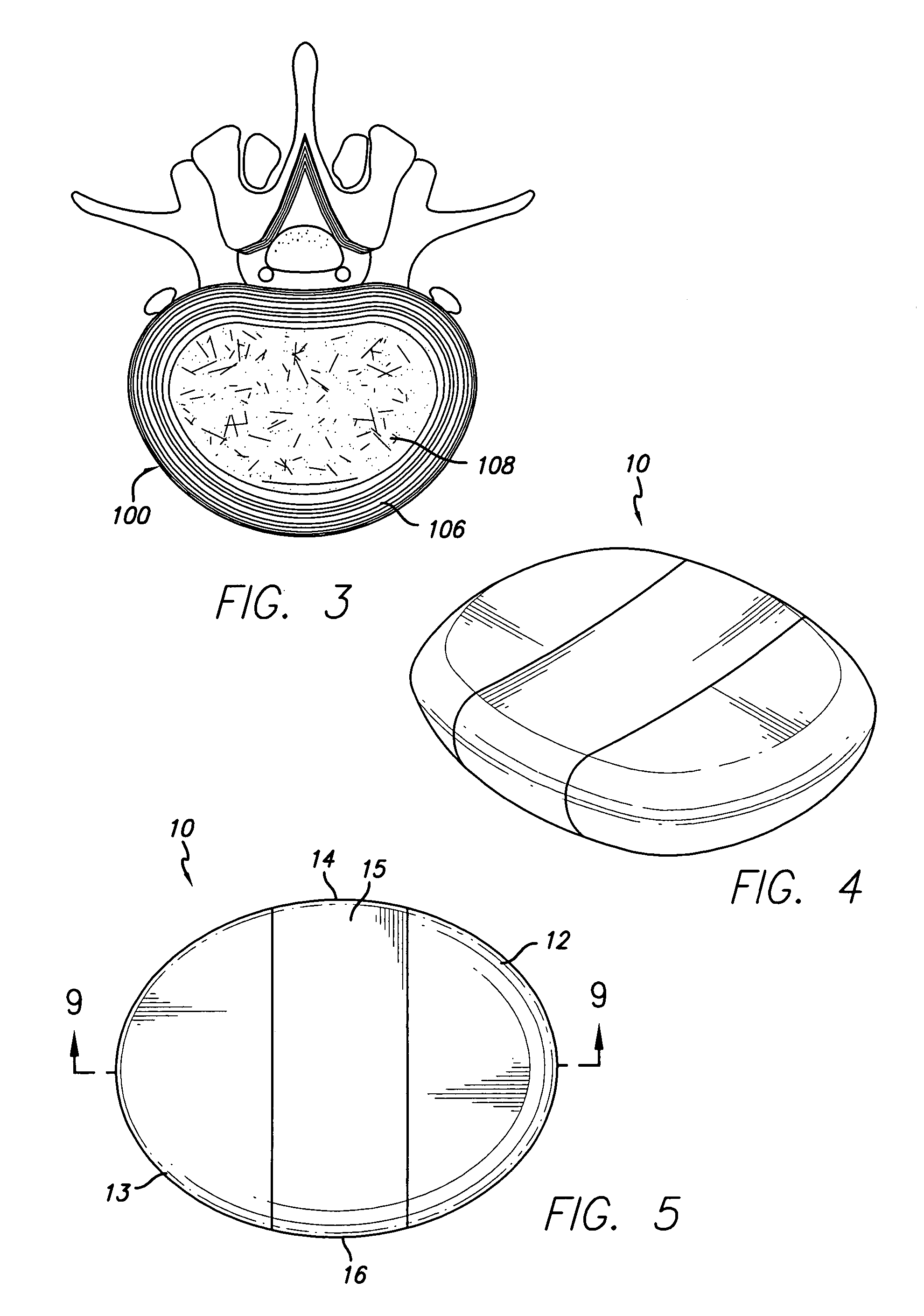
Implant material for minimally invasive spinal interbody fusion surgery
InactiveUS20060106462A1Easily be inserted into positionInternal osteosythesisBone implantSpinal columnHydroxyapatite ceramics
An implant device for spinal interbody fusion surgery has a shape substantially similar to a human excavated disc space, and includes a first modular end section, at least one modular middle section disposed adjacent to the first modular end section, a second modular end section disposed adjacent to the modular middle section and wherein when the first end section, the middle section and the second end section are placed adjacent to each other, the implant device has a shape with is substantially oval, viewed from top, and a cross section which is bi-convex. The preferred embodiment of the implant device is manufactured from human bone material that has been formed into the shape of the modular components. Alternative embodiments are manufactured from non-human material such as hydrooxyapetite, ceramics, coral and other biodegradable material. Non-resorptable plastic and metal can be used as internal structural members of the implant modules.
Owner:TSOU PAUL M
Pleated composite ePTFE/textile hybrid covering
InactiveUS7510571B2Enhanced tissue ingrowthHigh suture retention strengthStentsLayered productsFiberFibril
A composite multilayer implantable material having a first inner tubular layer formed of expanded polytetrafluoroethyene having a porous microstructure defined by nodes interconnected by fibrils, wherein said first layer has a plurality of pleated folds, a second tubular layer formed of textile material circumferentially disposed exteriorly to said first layer; and having an elastomeric bonding agent applied to one of said first layer or second layer and disposed within the pores of said microstructure for securing said first layer to said second layer.
Owner:LIFESHIELD SCI
Implantable materials having engineered surfaces and method of making same
Implantable materials having defined patterns of affinity regions for binding endothelial cells and providing for directed endothelial cell migration across the surface of the material. The affinity regions include photochemically altered regions of a material surface and physical members patterned on the material surface that exhibit a greater affinity for endothelial cell binding and migration than the remaining regions of the material surface.
Owner:VACTRONIX SCI LLC
Container for lyophilization and storage of tissue
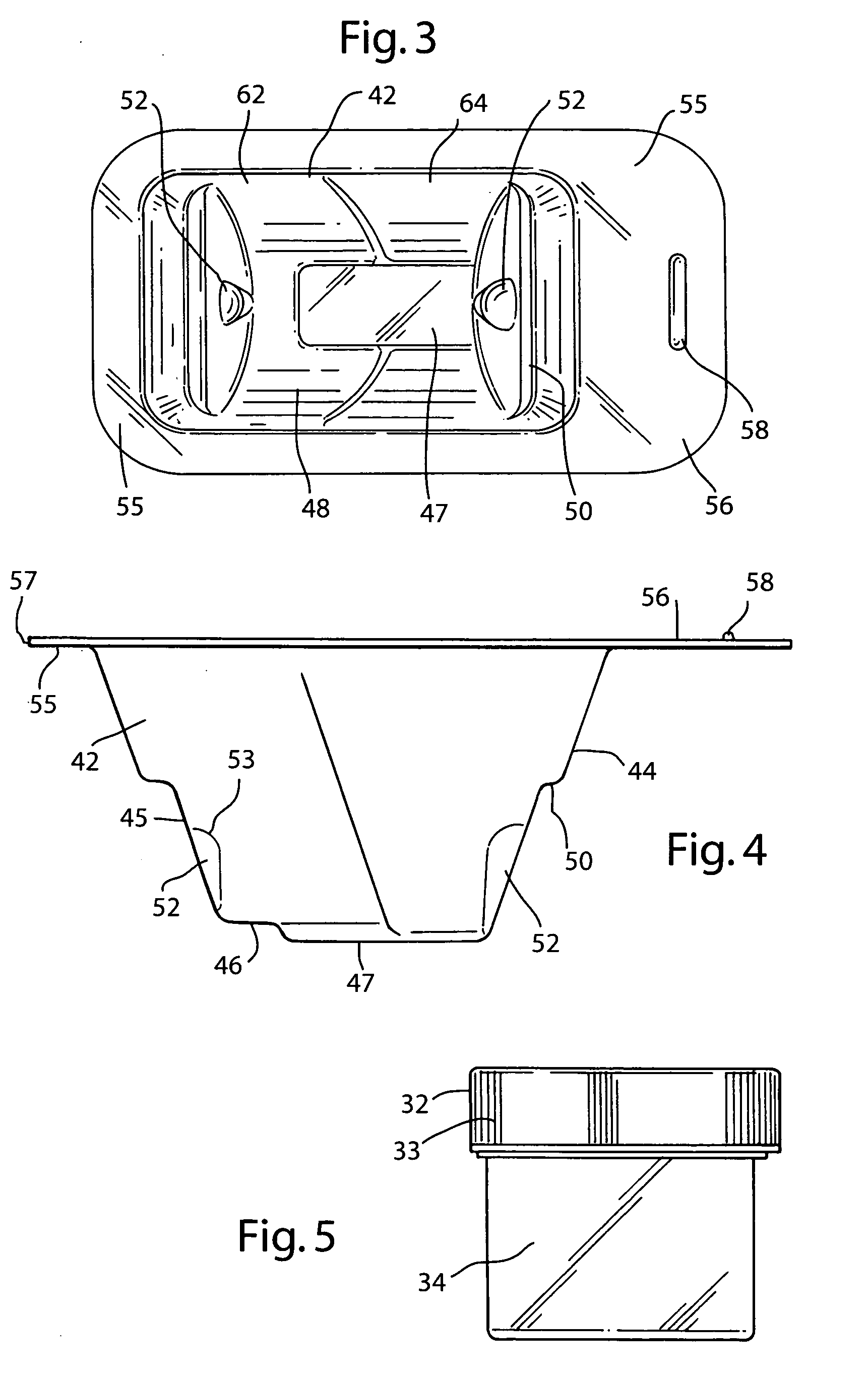
InactiveUS20070074989A1Resist absorption and discolorationEasy accessSurgical furnitureDiagnosticsBiomedical engineeringFlange
A sterile container assembly for storing sterile allograft tissue implant material is constructed with sidewalls, end walls and a base member defining an open faced cavity and a flange surrounding and extending outward from the cavity. A step and a spacer is formed in each end wall of the container and a cylindrical implant container sized to fit into the container cavity is positioned adjacent the end wall spacers. The implant container has a housing and a threadable cap with a vent hole formed therein which is covered by an insert member constructed of sintered PTFE mounted inside the cap. A foil cover is sealed to the flange of the container covering and sealing the container cavity and the cylindrical implant container mounted therein.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com