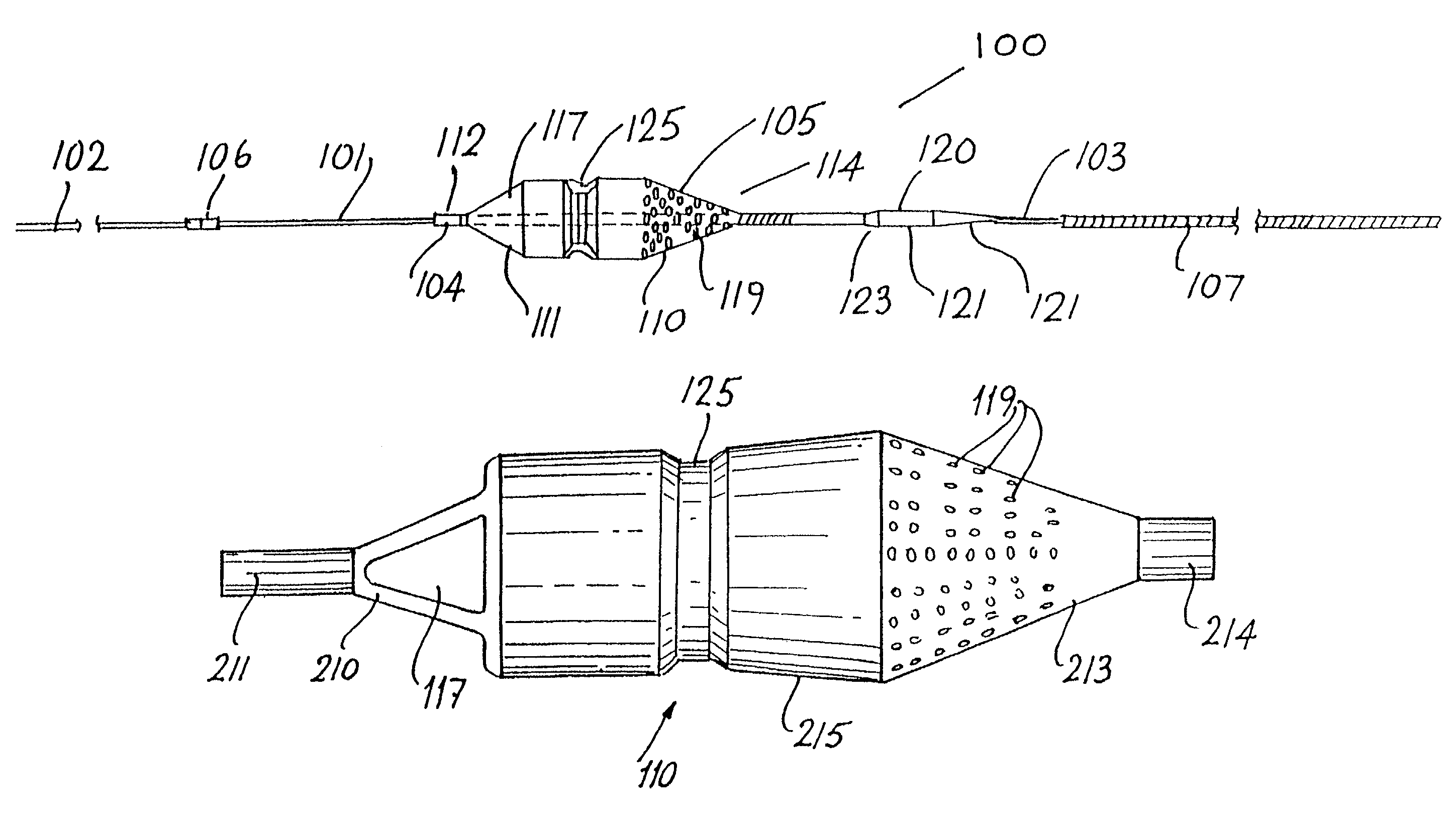
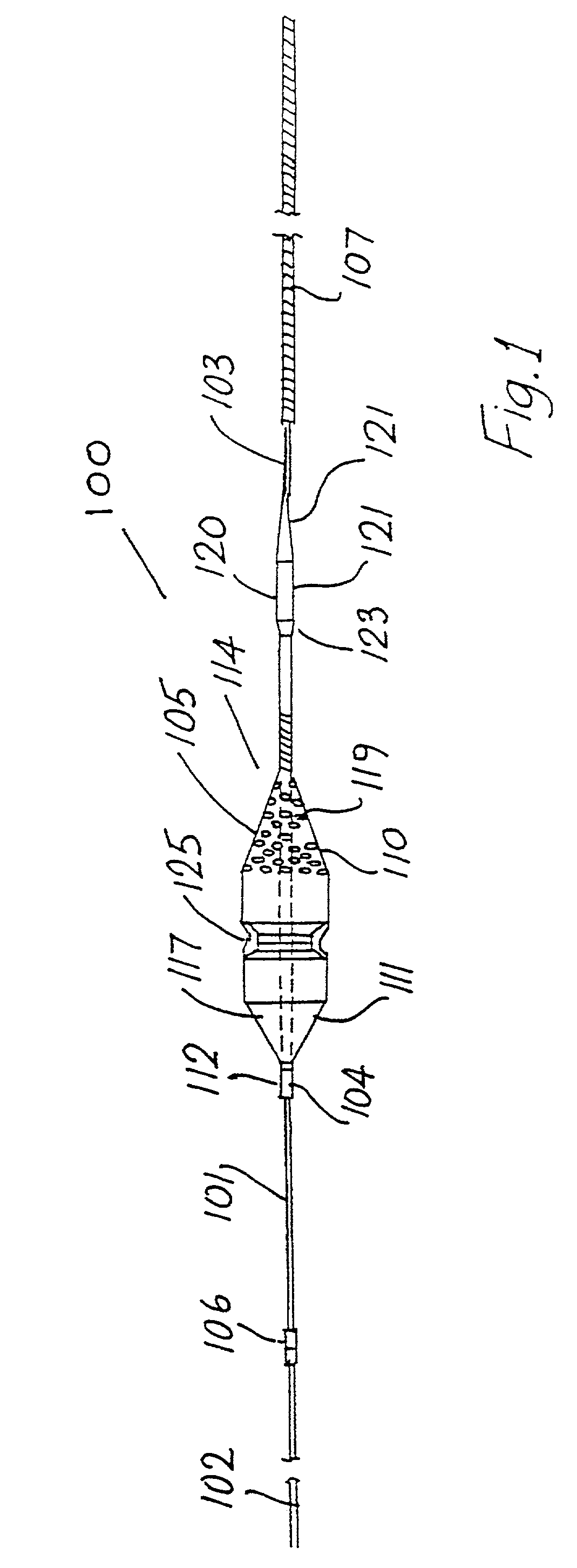
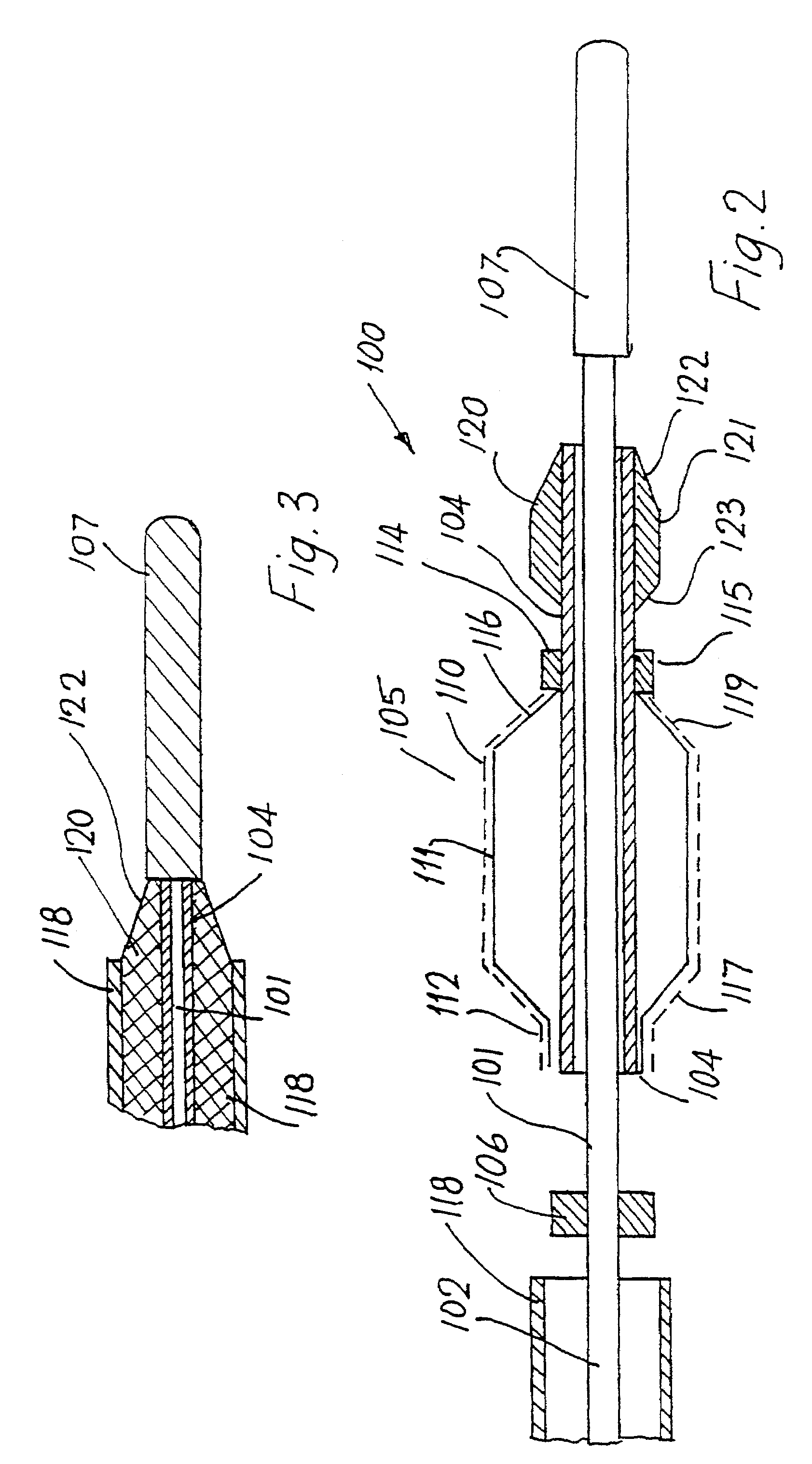
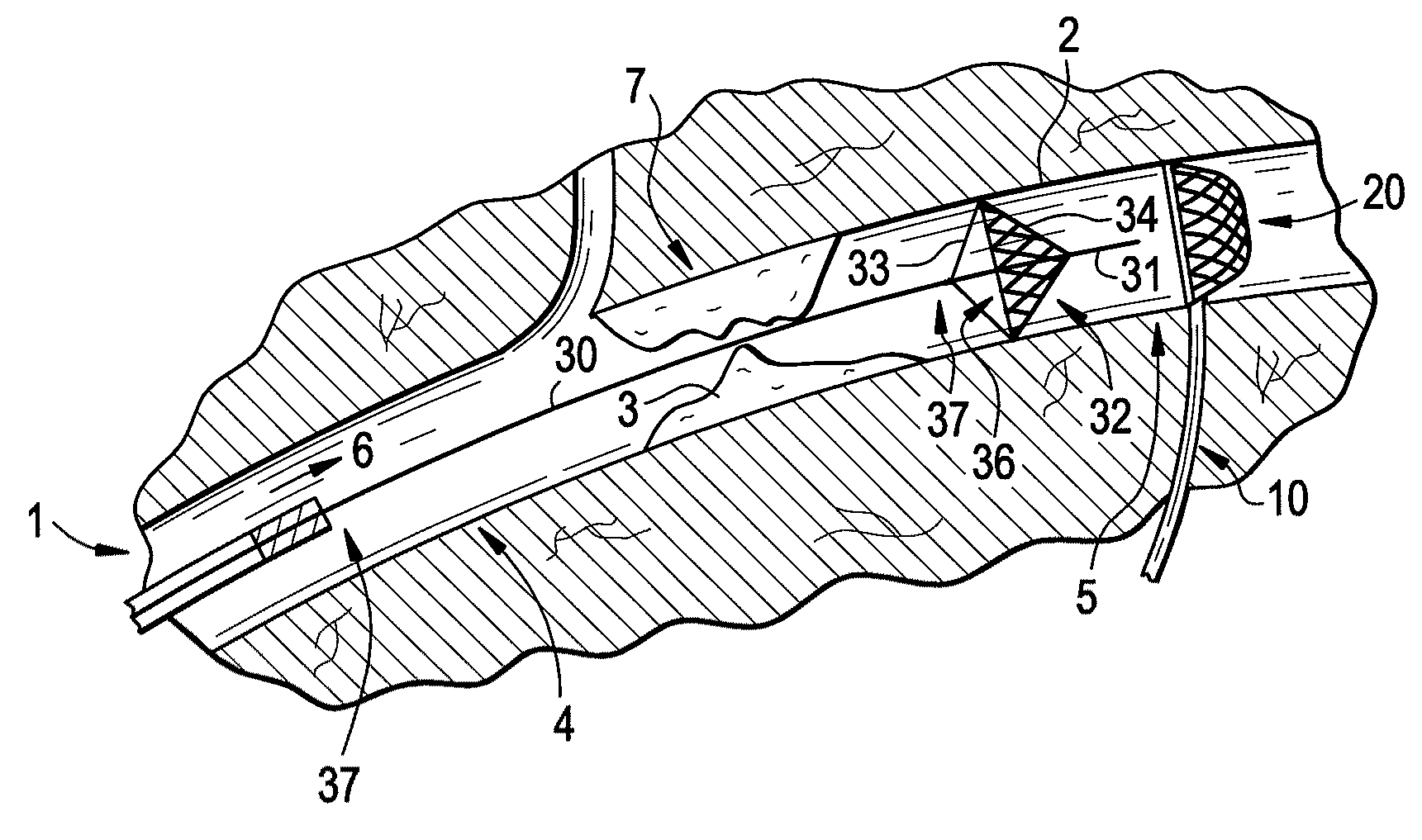
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
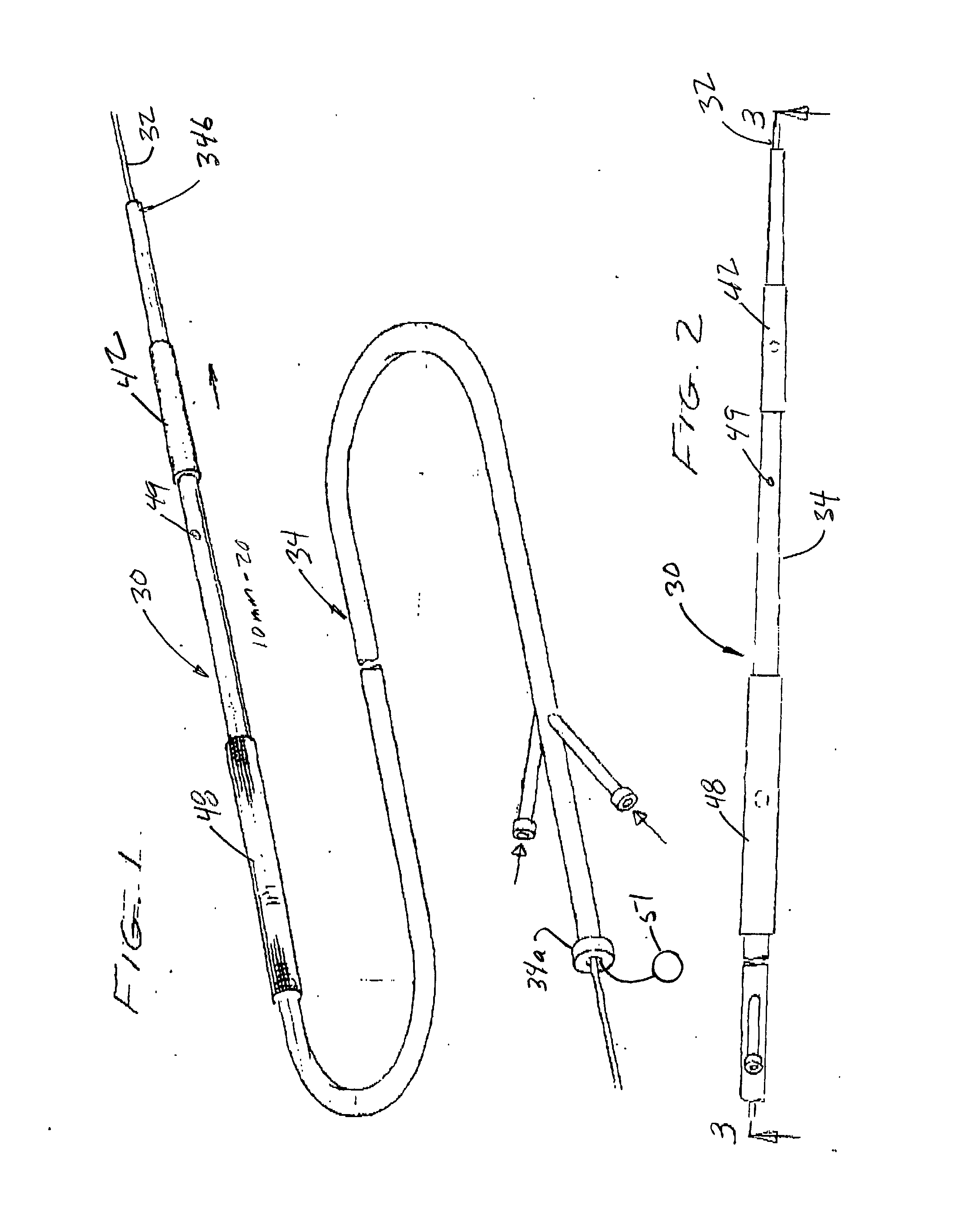
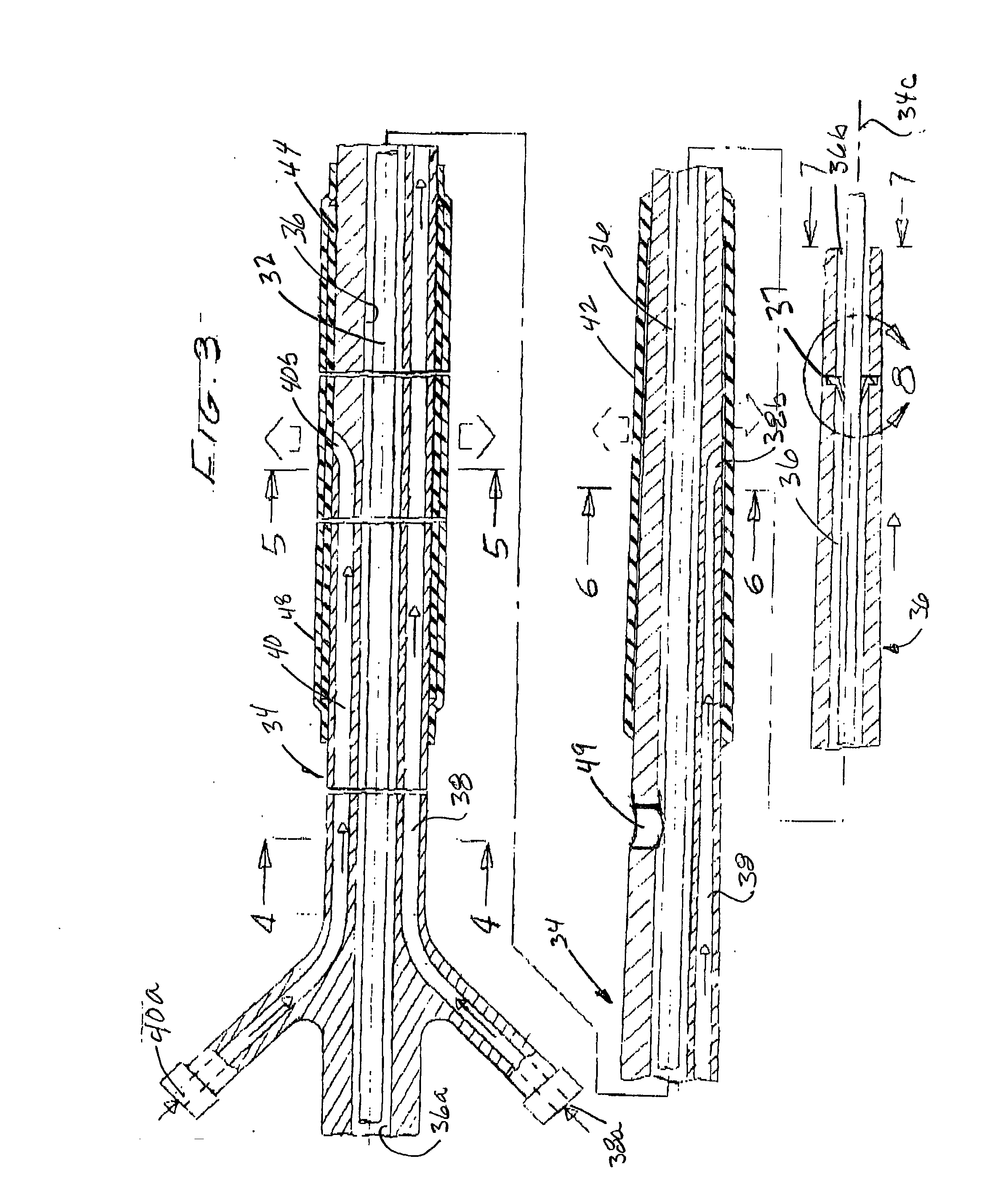
128 results about "Embolization material" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
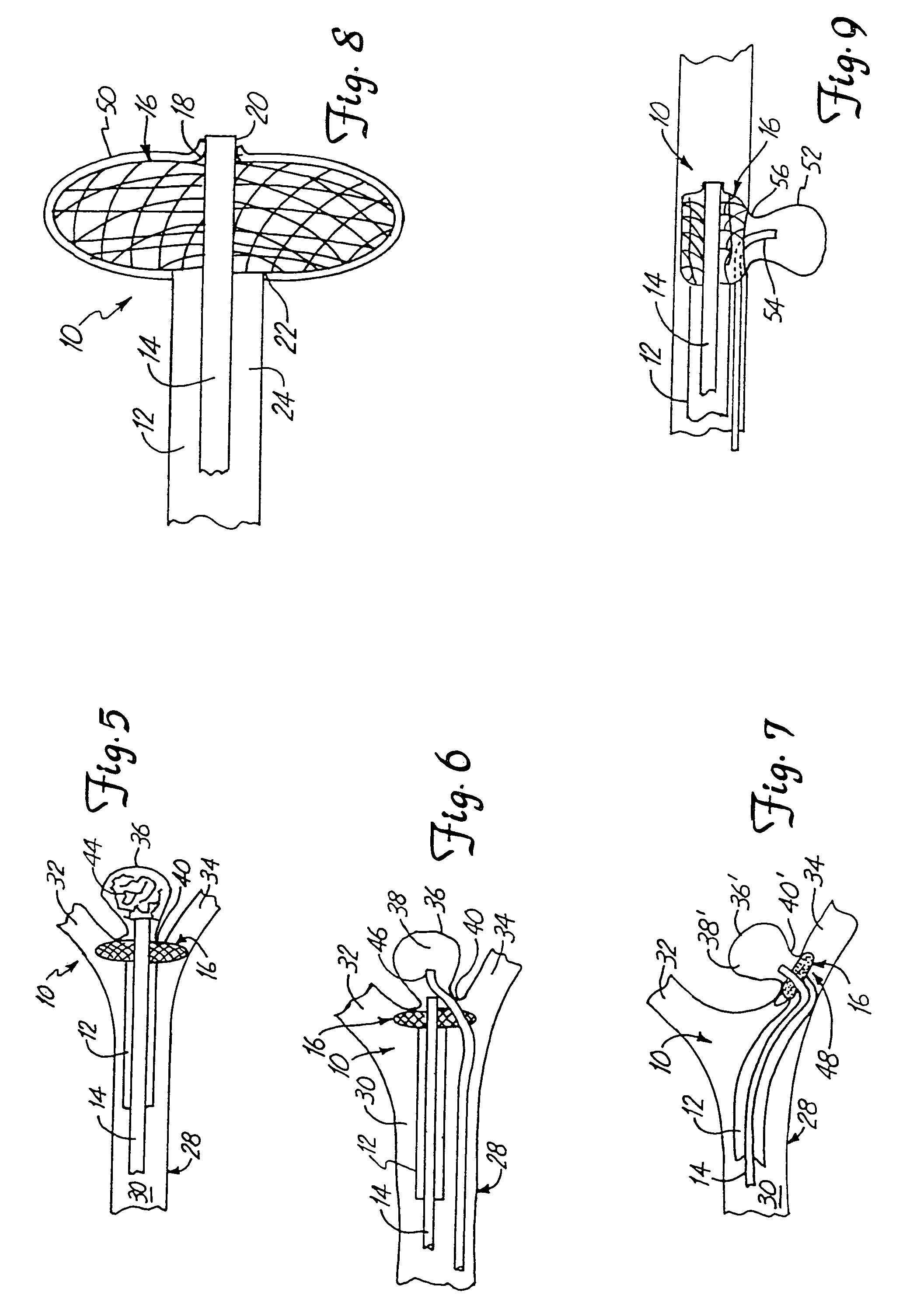
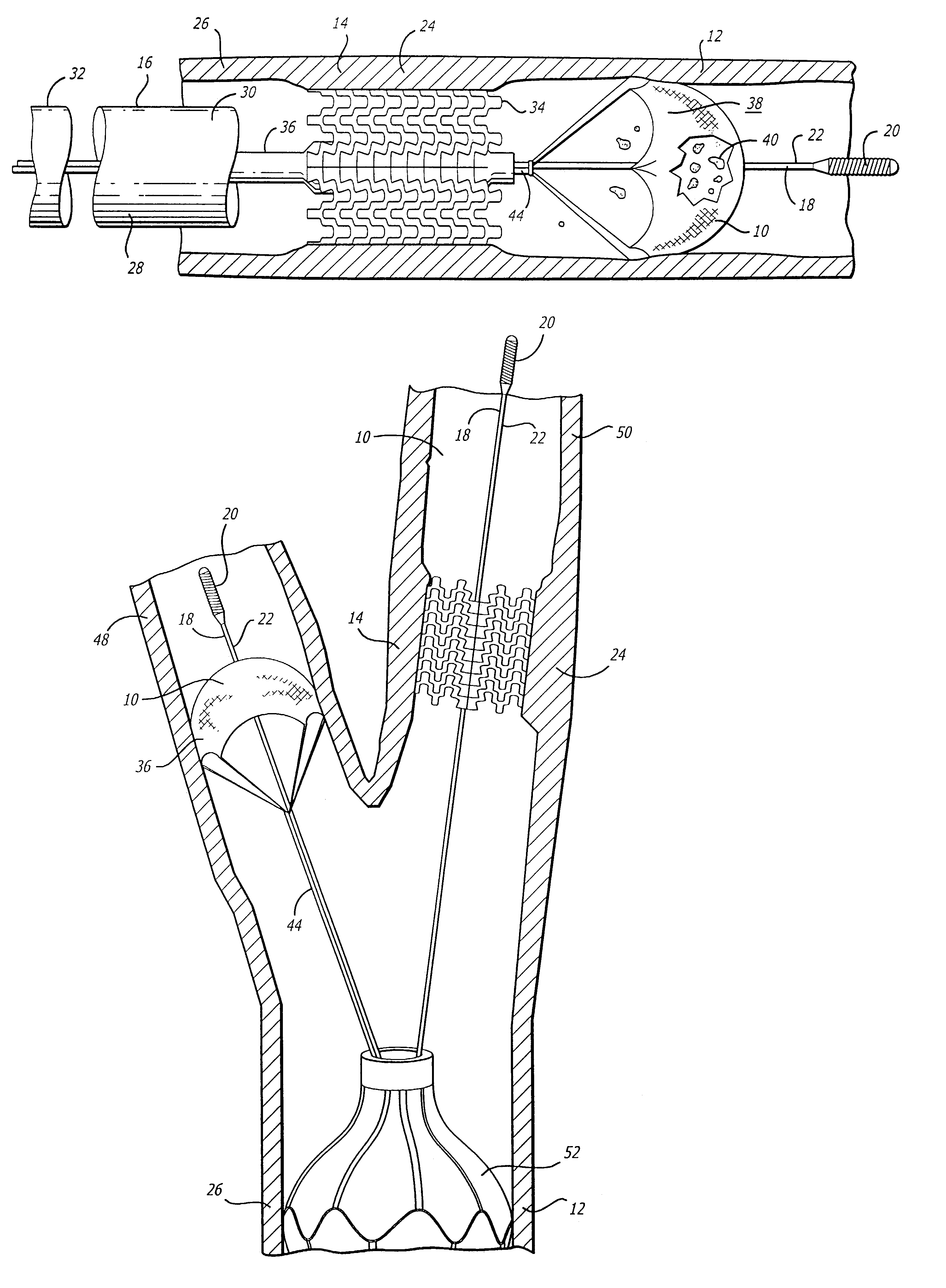
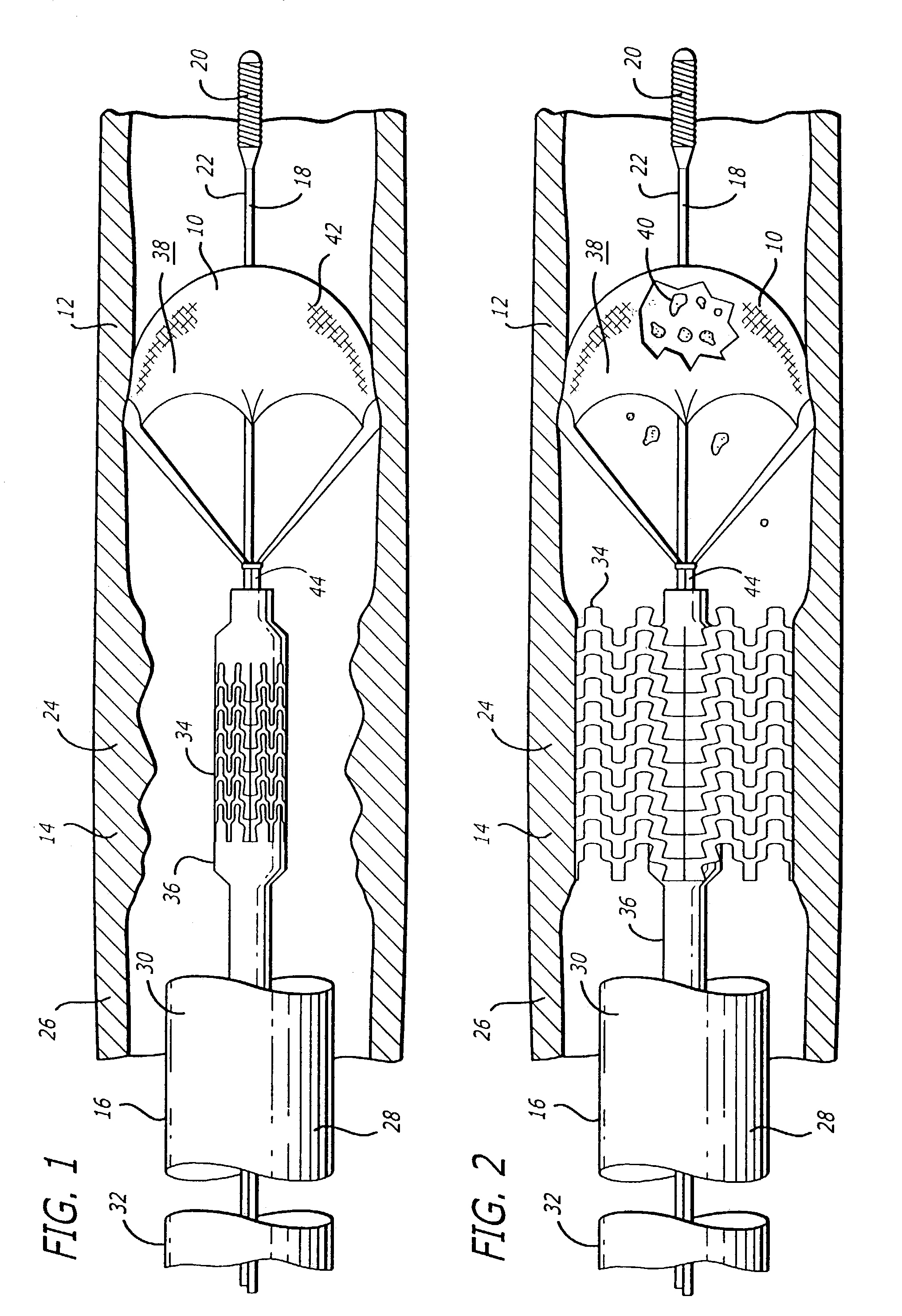
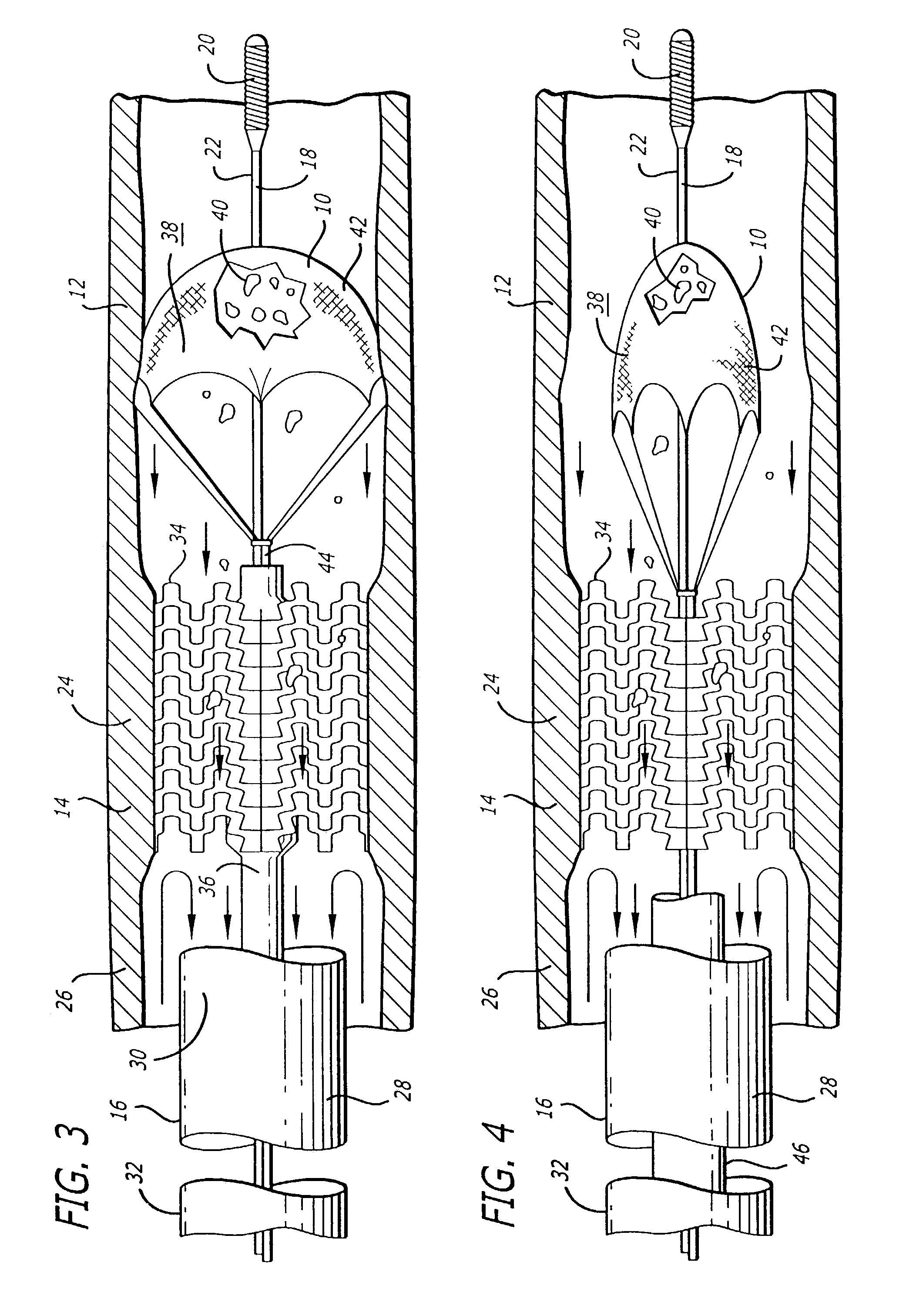
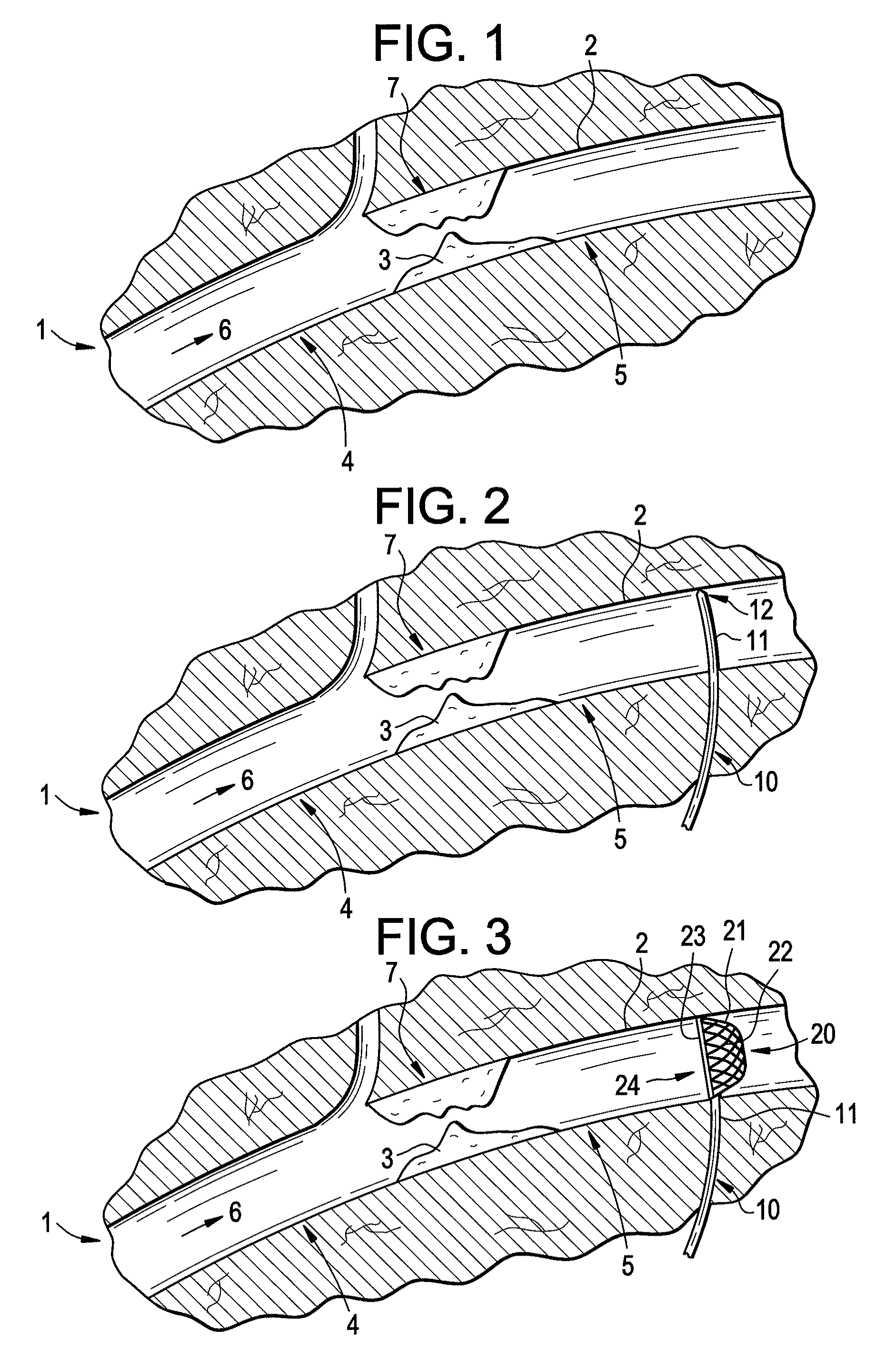
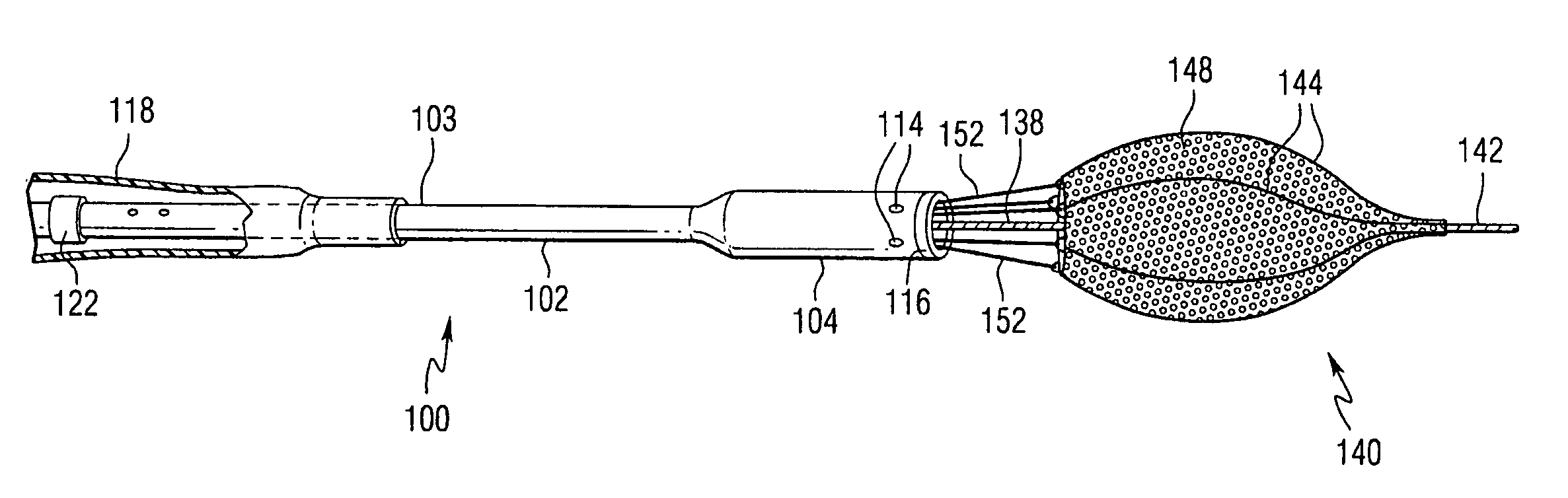
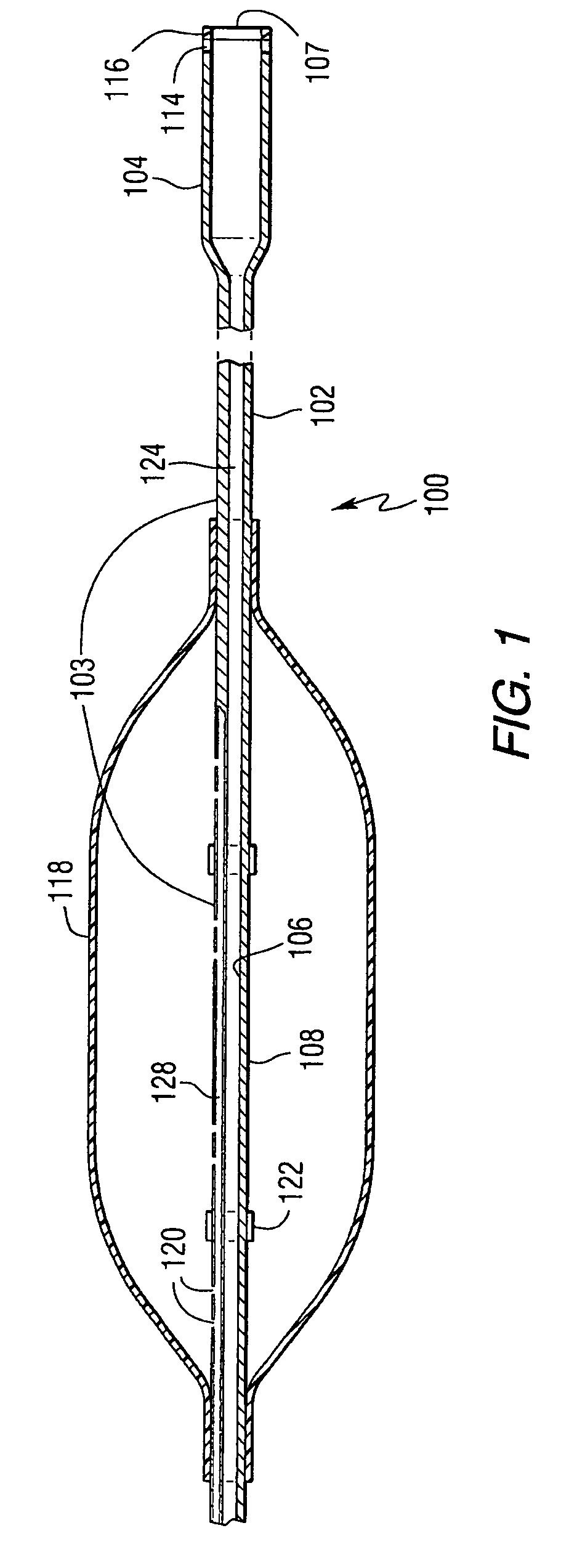
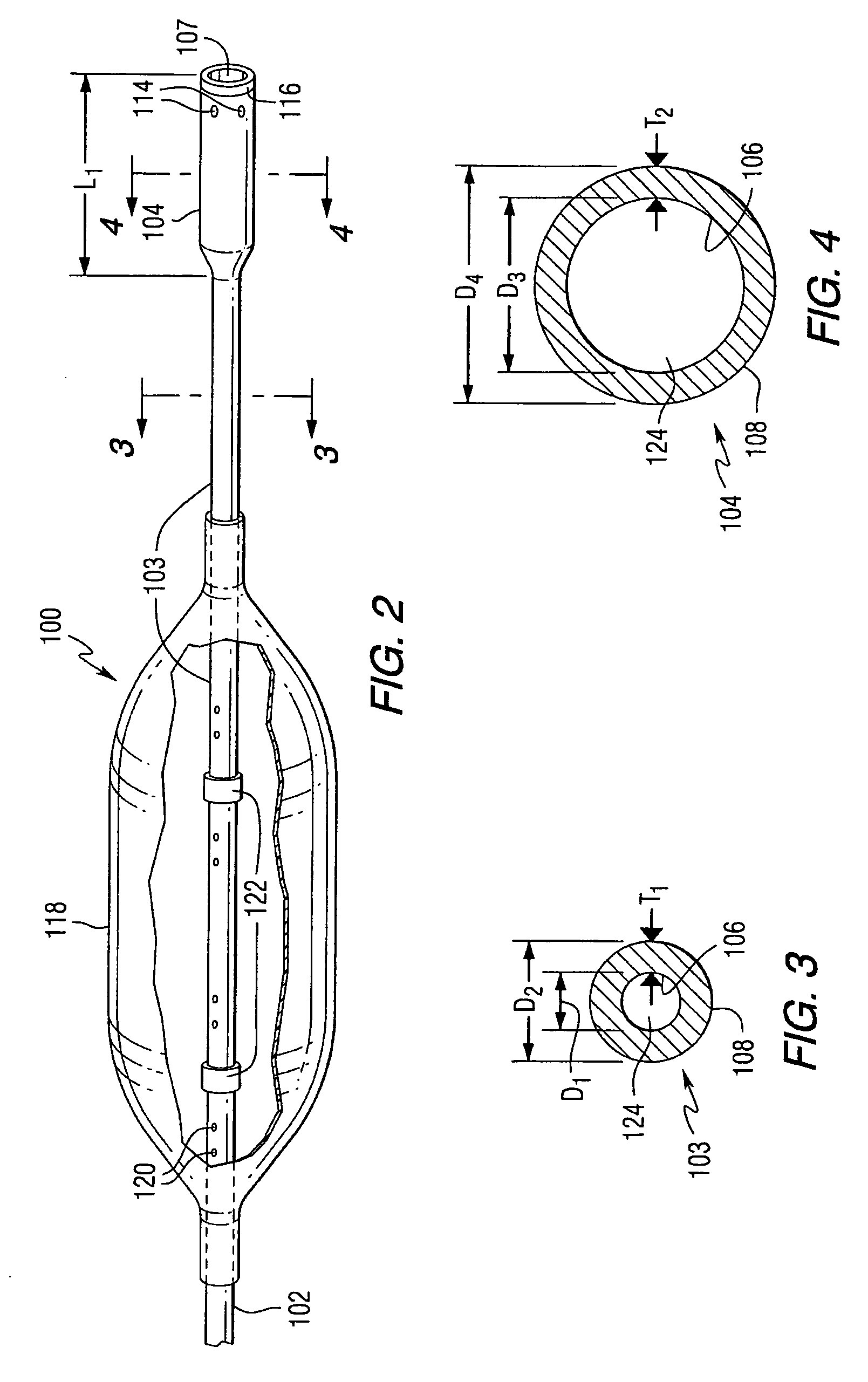
Embolization Materials. Therapeutic embolization is defined as the deliberate introduction of occluding material into a blood vessel in order to reduce or obstruct blood flow. Several factors determine the selection of an embolic material.
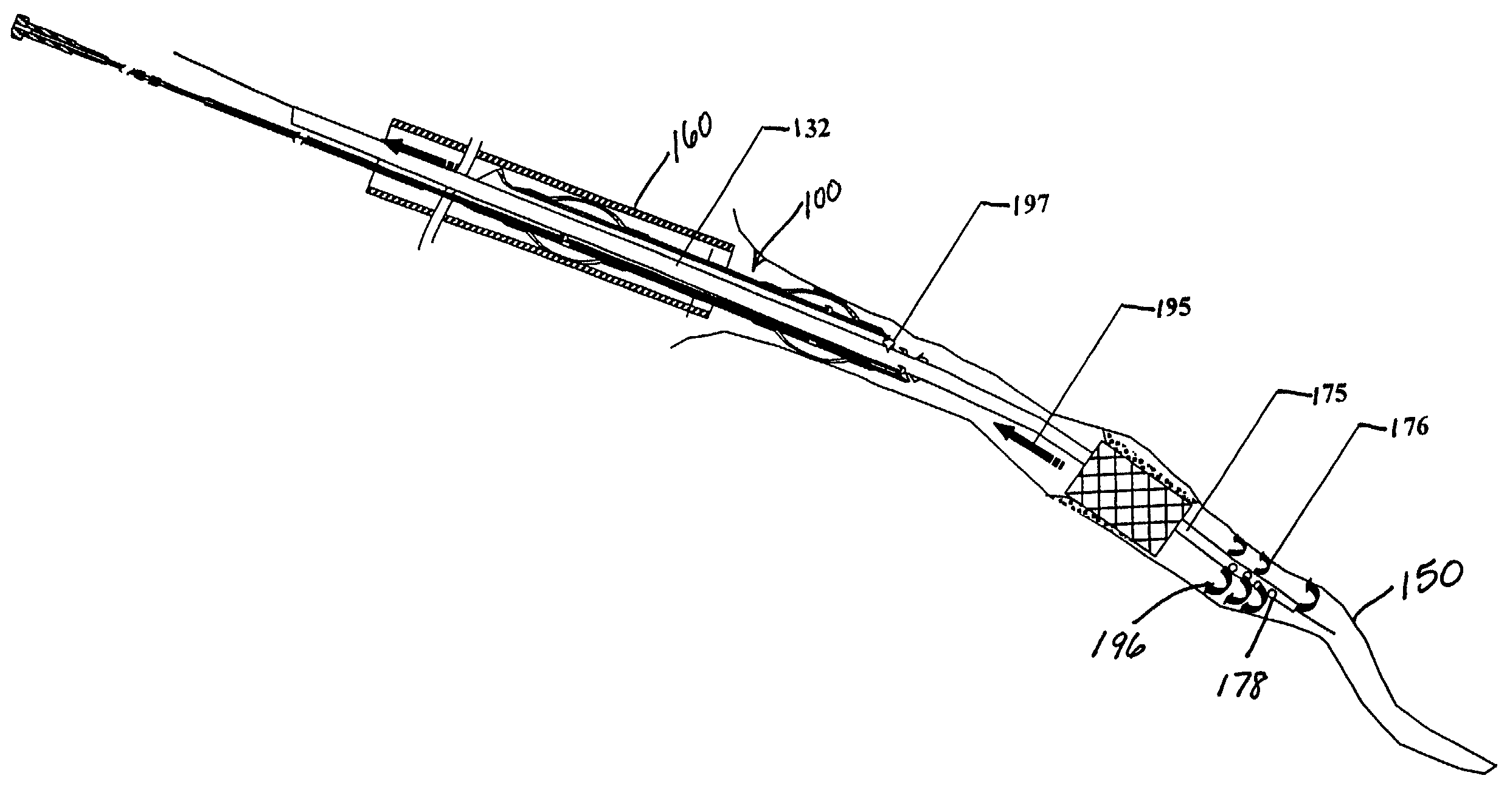
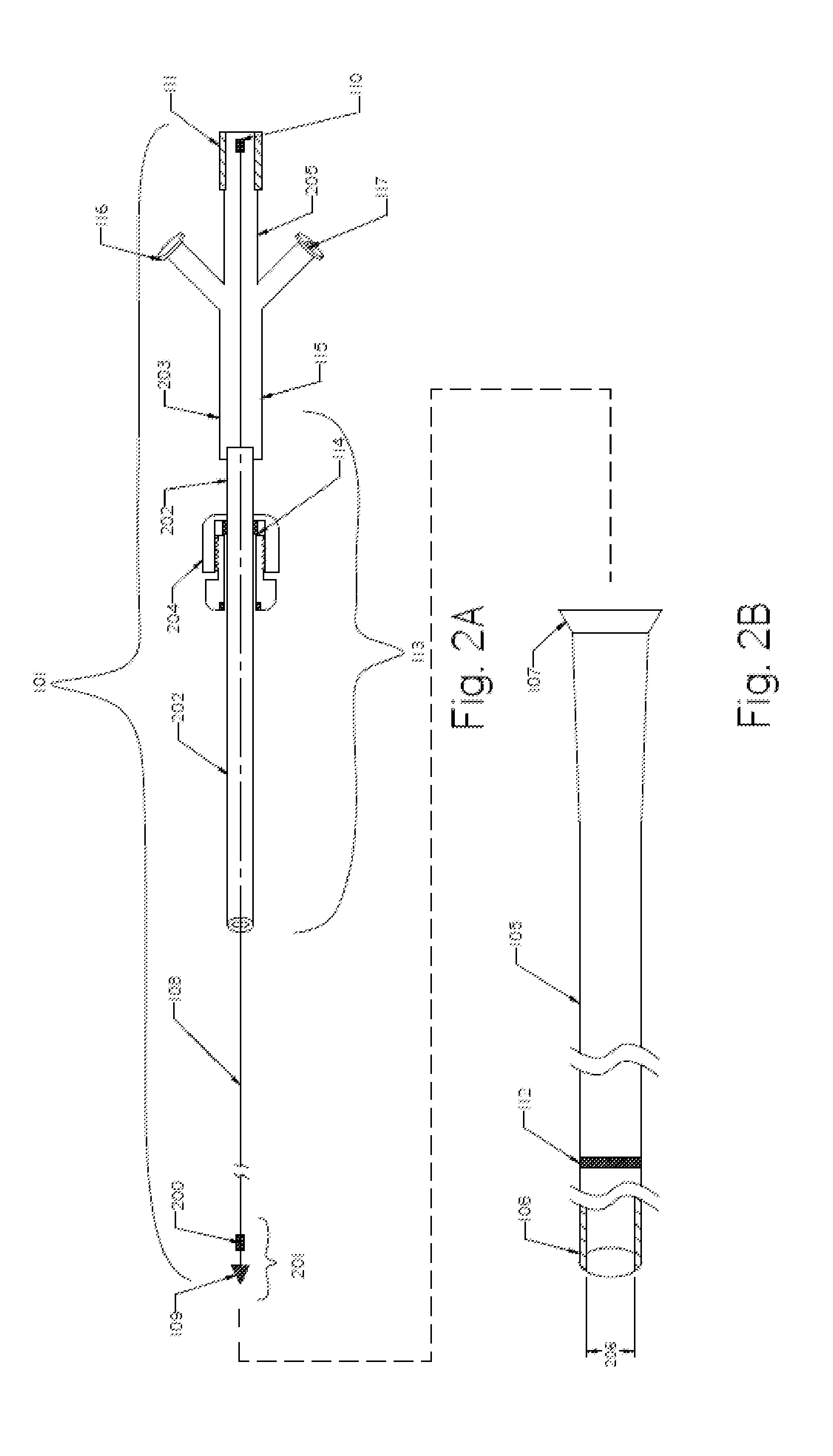
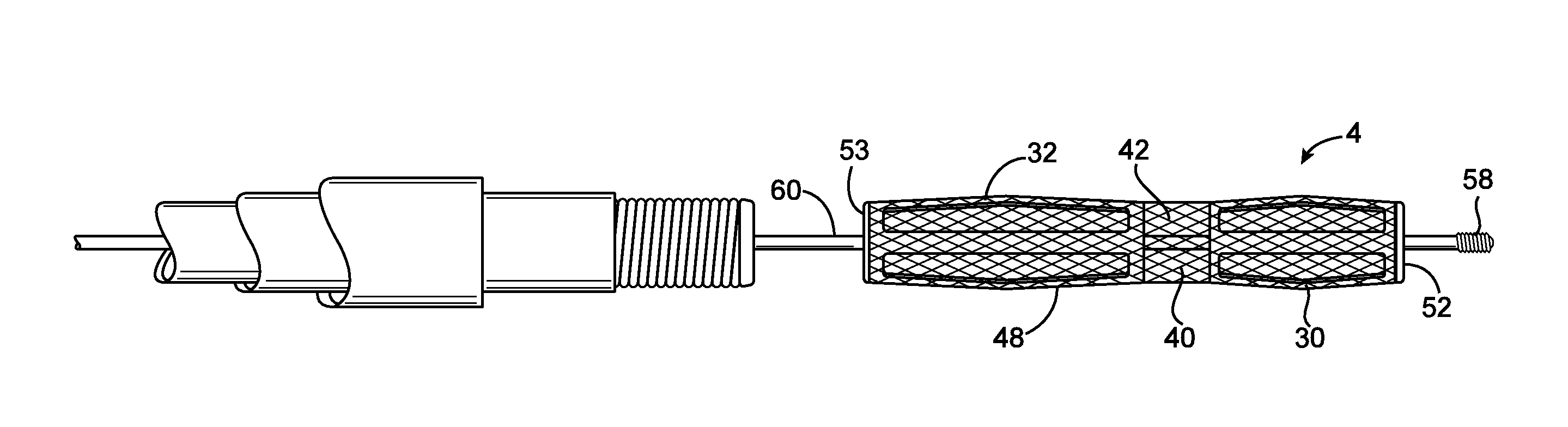
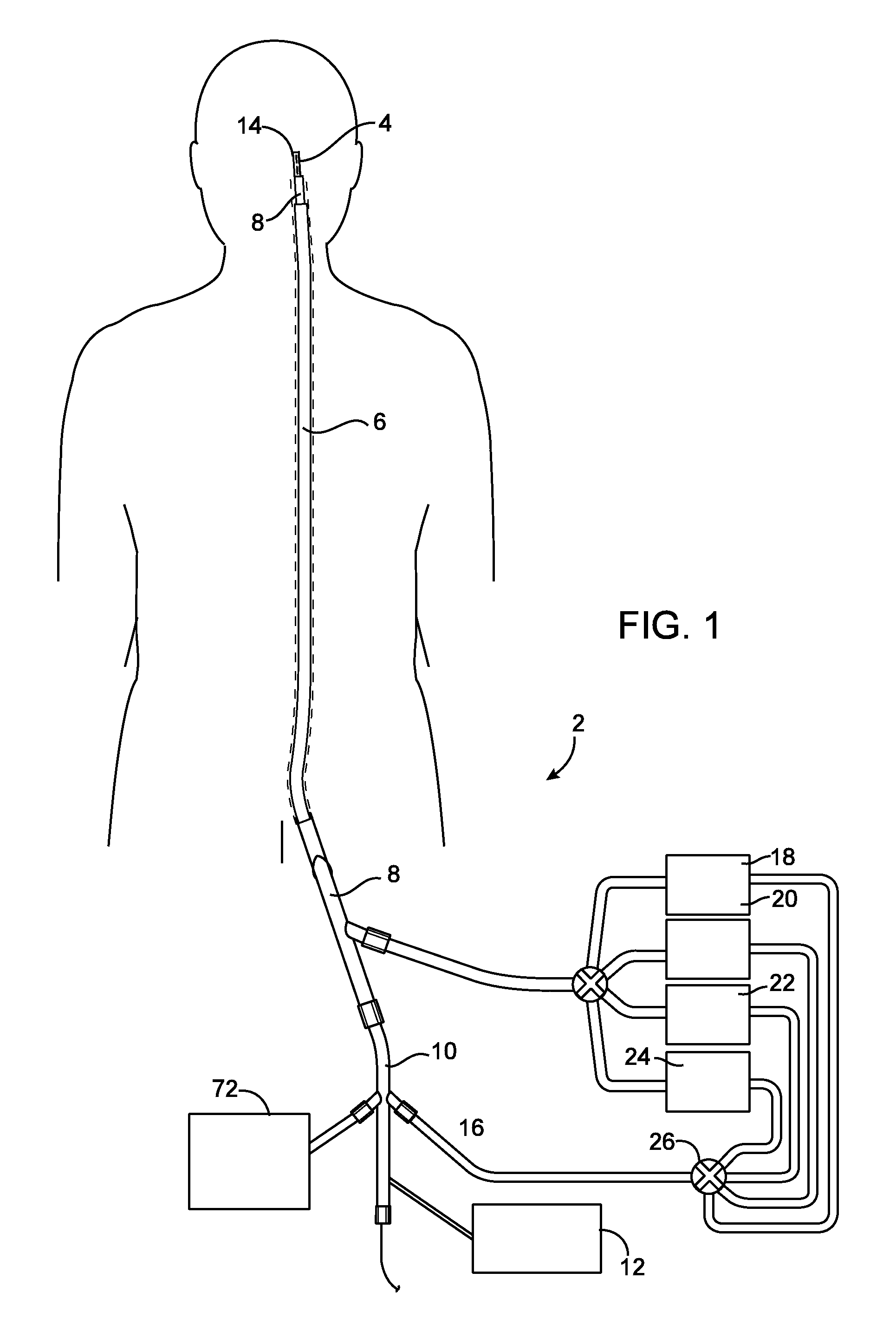
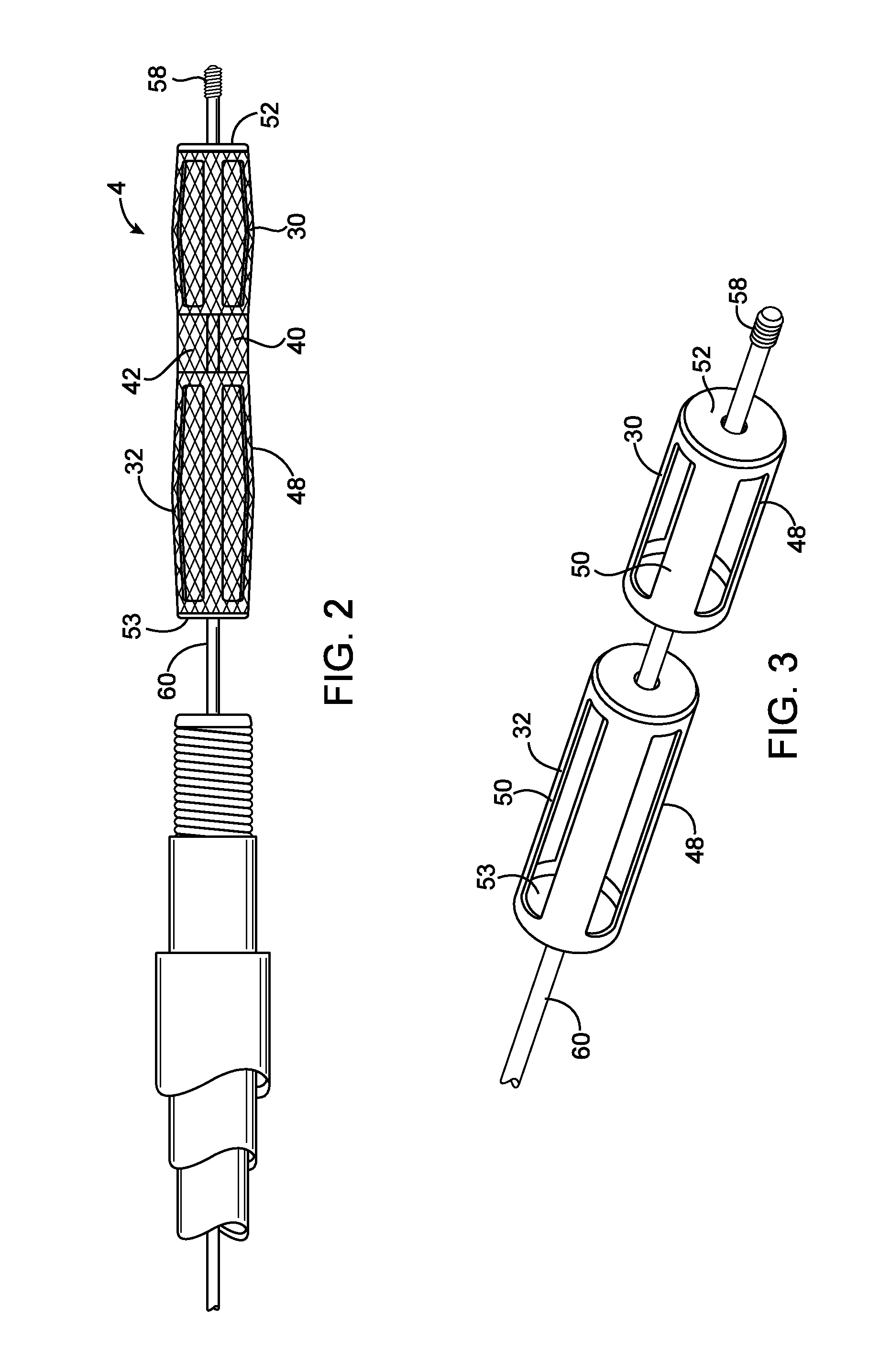
Emboli protection devices and related methods of use
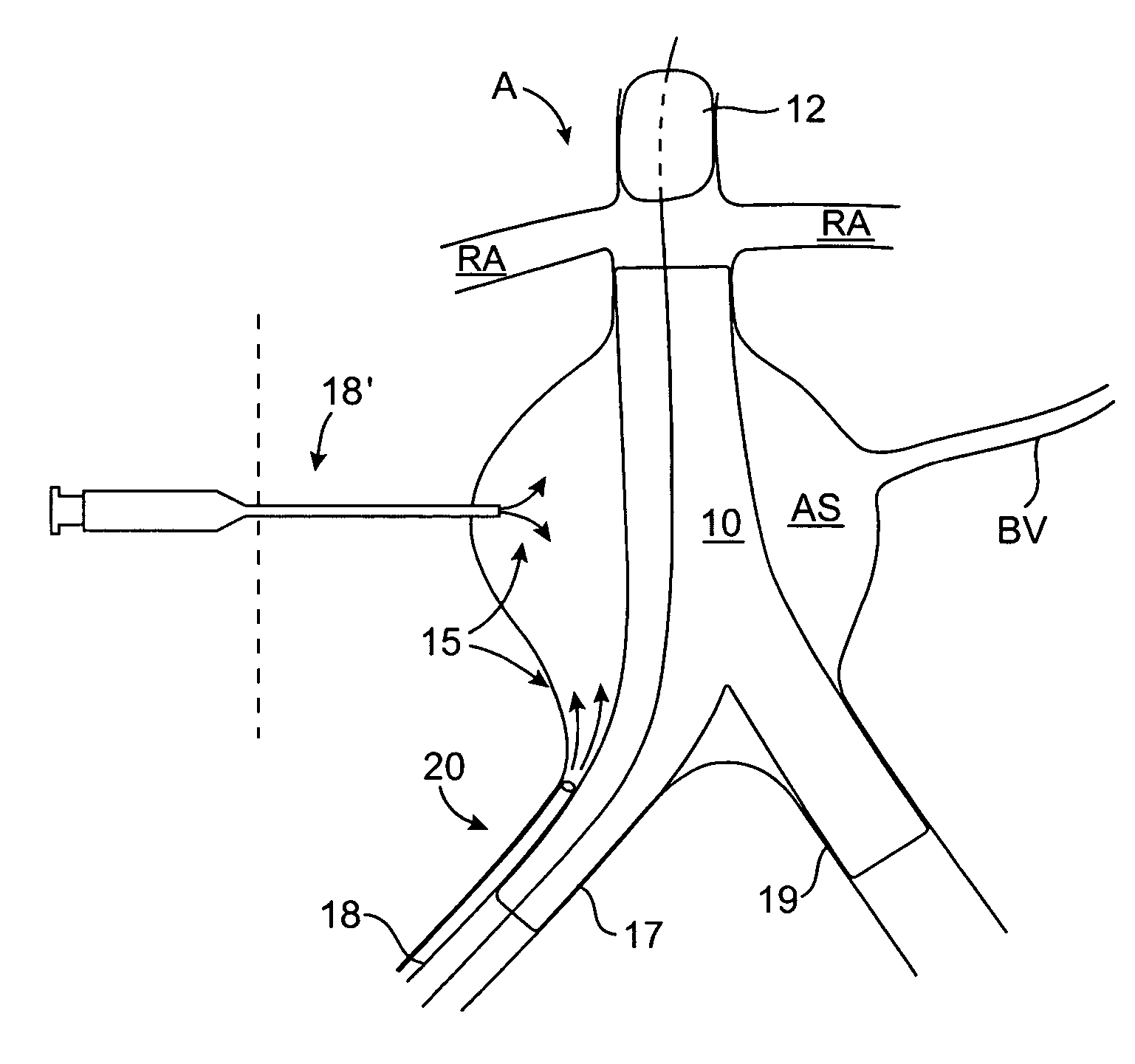
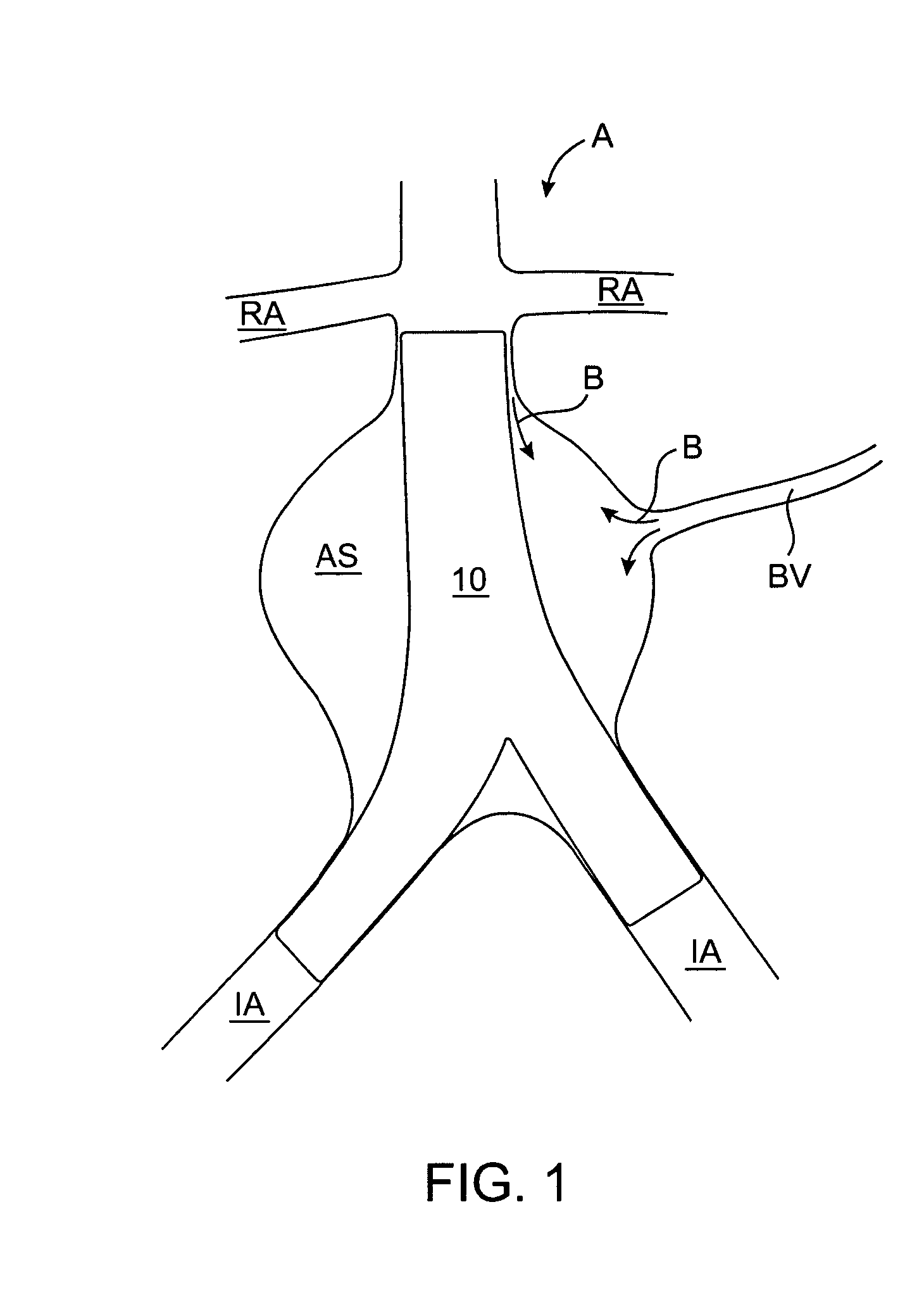
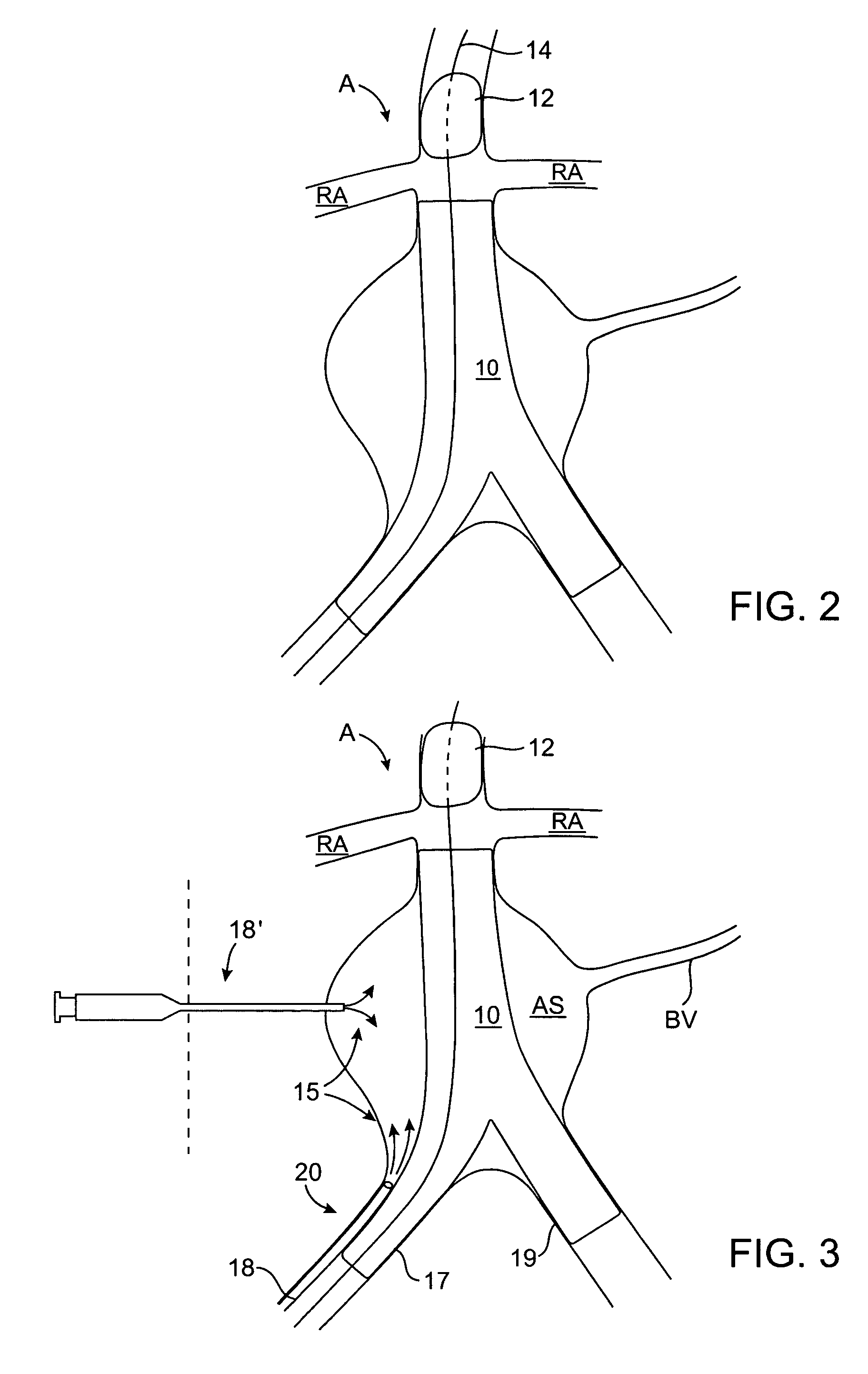
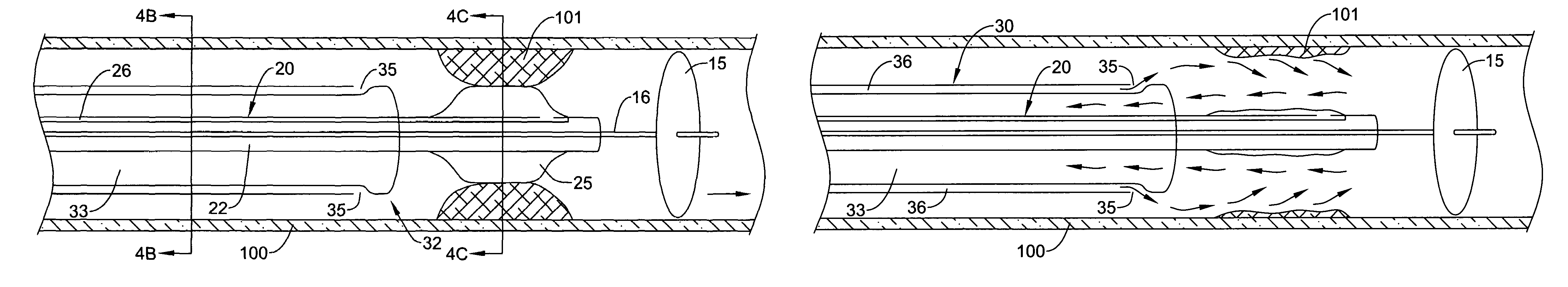
ActiveUS7374560B2Improve visualizationStopping normal blood flowStentsDilatorsRetrograde FlowEmbolization material
An evacuation sheath assembly and method of treating occluded vessels which reduces the risk of distal embolization during vascular interventions is provided. The evacuation sheath assembly includes an elongated tube defining an evacuation lumen having proximal and distal ends. A proximal sealing surface is provided on a proximal portion of the tube and is configured to form a seal with a lumen of a guided catheter. A distal sealing surface is provided on a distal portion of the tube and is configured to form a seal with a blood vessel. Obturator assemblies and infusion catheter assemblies are provided to be used with the evacuation sheath assembly. A method of treatment of a blood vessel using the evacuation sheath assembly includes advancing the evacuation sheath assembly into the blood vessel through a guide catheter. Normal antegrade blood flow in the blood vessel proximate to the stenosis is stopped and the stenosis is treated. Retrograde blood flow is induced within the blood vessel to carry embolic material dislodged during treating into the evacuation sheath assembly. If necessary to increase retrograde flow, the coronary sinus may be at least partially occluded. Alternatively, antegrade flow may be permitted while flow is occluded at the treatment site.
Owner:ST JUDE MEDICAL CARDILOGY DIV INC
Method and assembly for distal embolic protection
ActiveUS20050119688A1Reduce retrograde flowReduce flowHeart valvesSurgerySurgery procedureBiomedical engineering
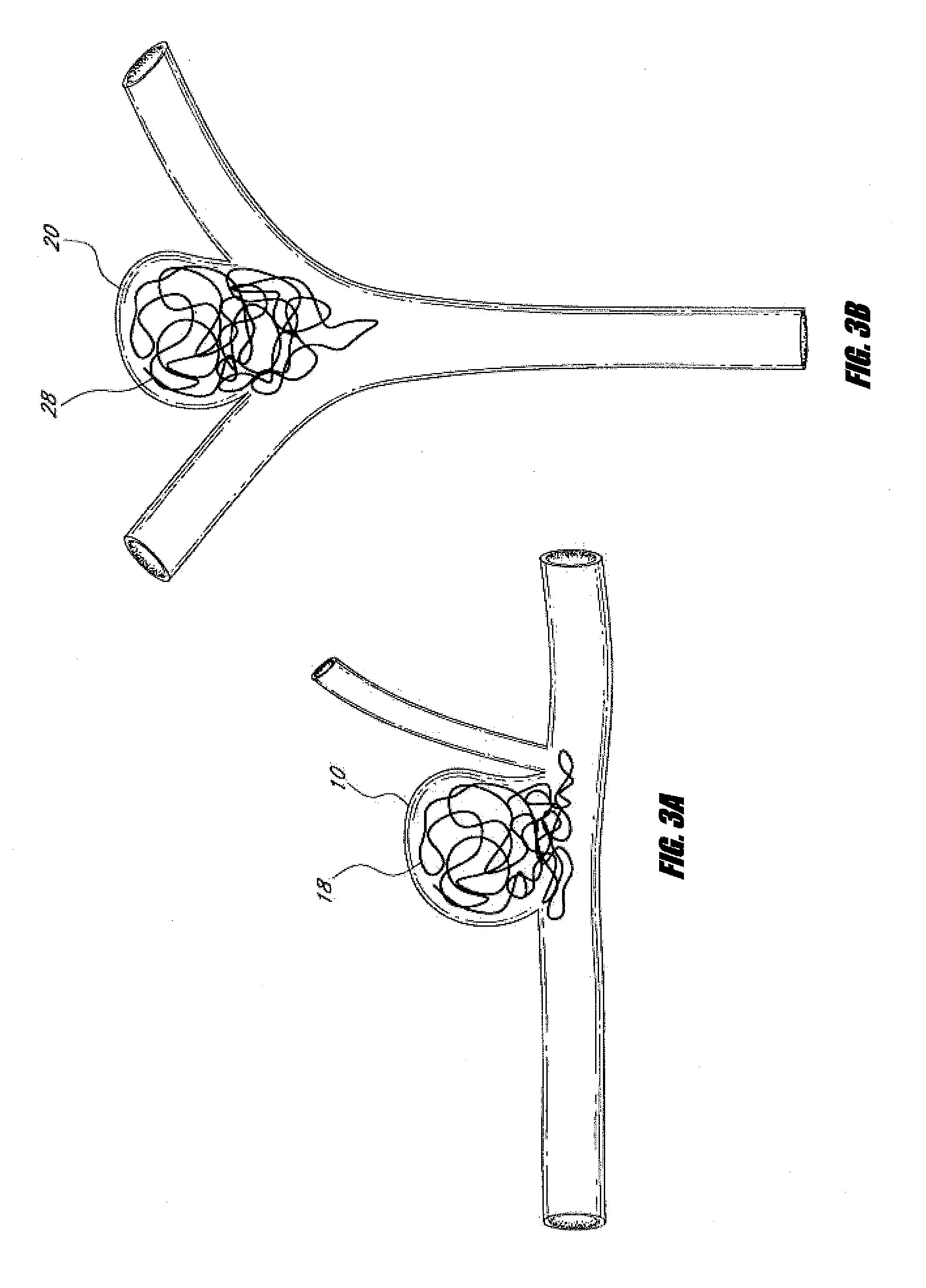
Methods and assemblies are described for capturing embolic material in a blood vessel or other body cavity during cardiovascular or valve replacement and repair surgery, wherein access is provided through the apical area of the patient's heart. The distal embolic protection assembly generally comprises a sleeve having a lumen, an actuating member having proximal and distal ends, wherein the actuating member is movably disposed within the lumen, and a filter assembly coupled to the distal end of the actuating member. The filter assembly generally comprises a porous bag having an open proximal end, a collapsible and expandable frame that is coupled to the open proximal end of the porous bag, and at least one support spine disposed at least a part of the longitudinal axis of the porous bag. The porous bag is configured such that it permits blood to perfuse freely through while capturing embolic material and other debris.
Owner:MEDTRONIC 3F THERAPEUTICS
Removable occlusion system for aneurysm neck
A system for treating an aneurysm in a vessel includes a delivery device having a delivery portion suitable for delivery of embolic material. The delivery device is placed in a neck of the aneurysm and an expandable member is placed proximate the neck. The expandable member is expanded to overlie substantially the entire neck. Embolic material is delivered to the aneurysm with a delivery device. The expandable member is held over the neck to inhibit movement of the embolic material out of the aneurysm. Blood is allowed to flow out of the aneurysm, past the neck of the aneurysm, and through the vessel while the expandable member is held over the neck of the aneurysm.
Owner:STRYKER CORP +1
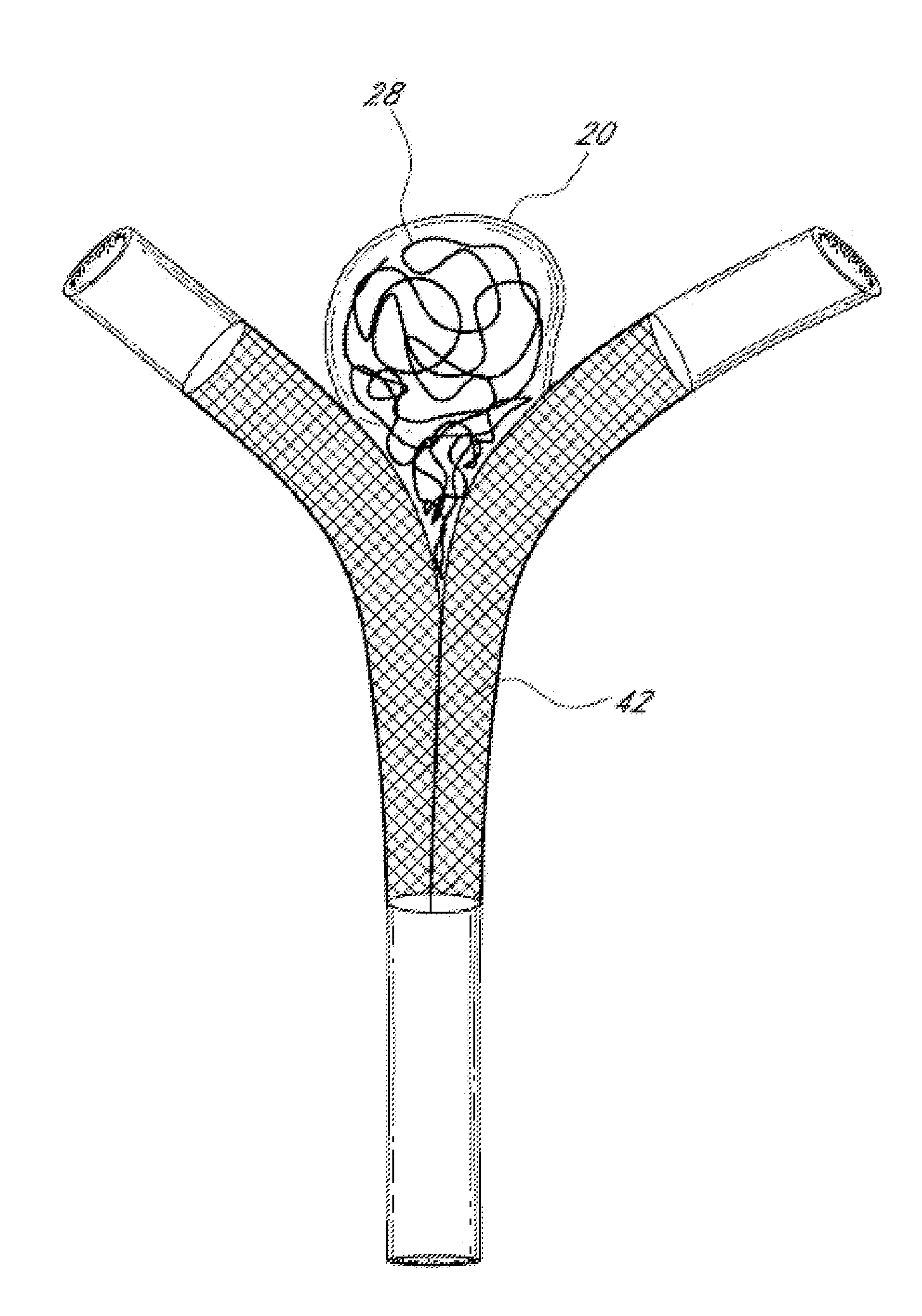
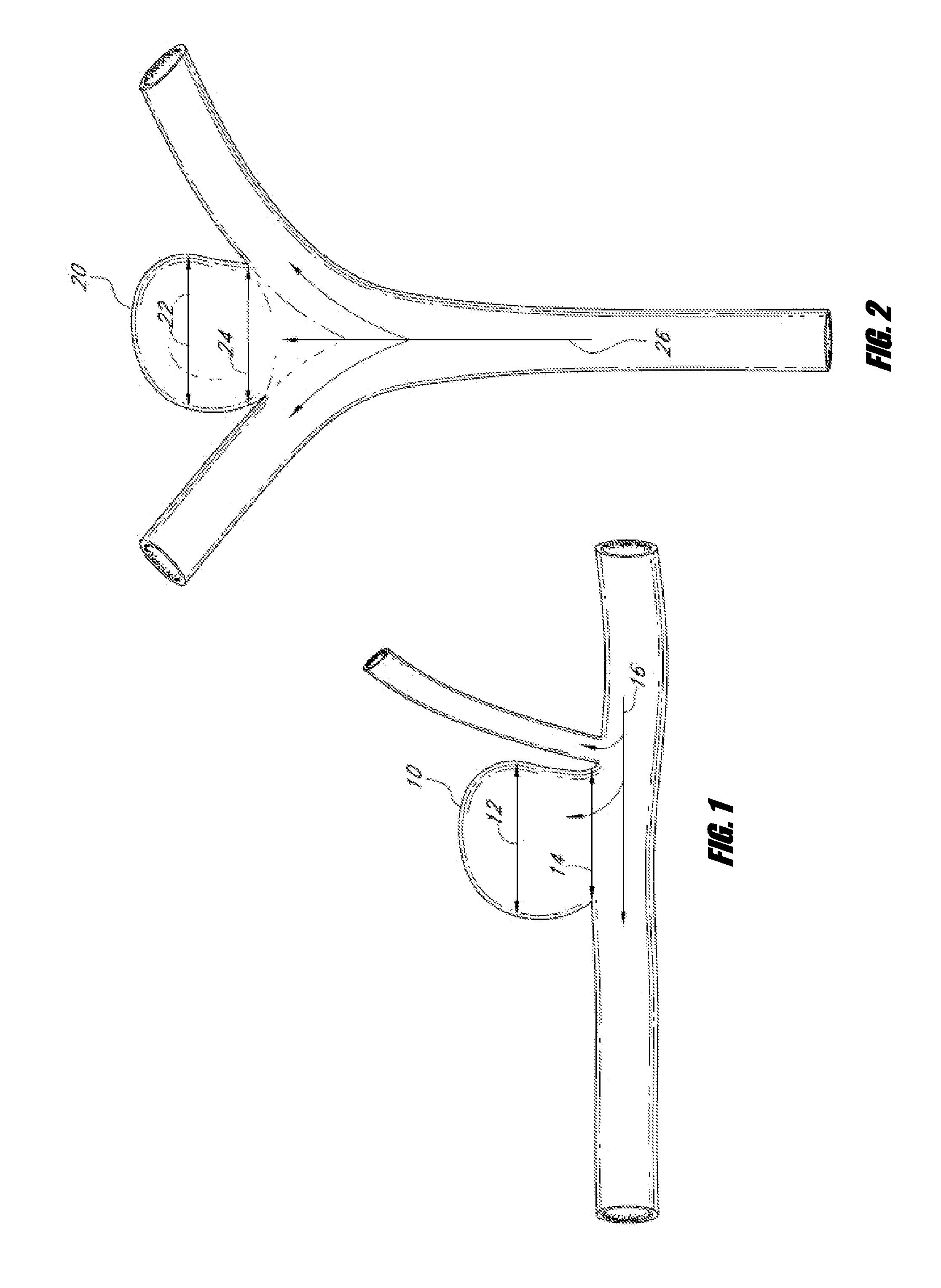
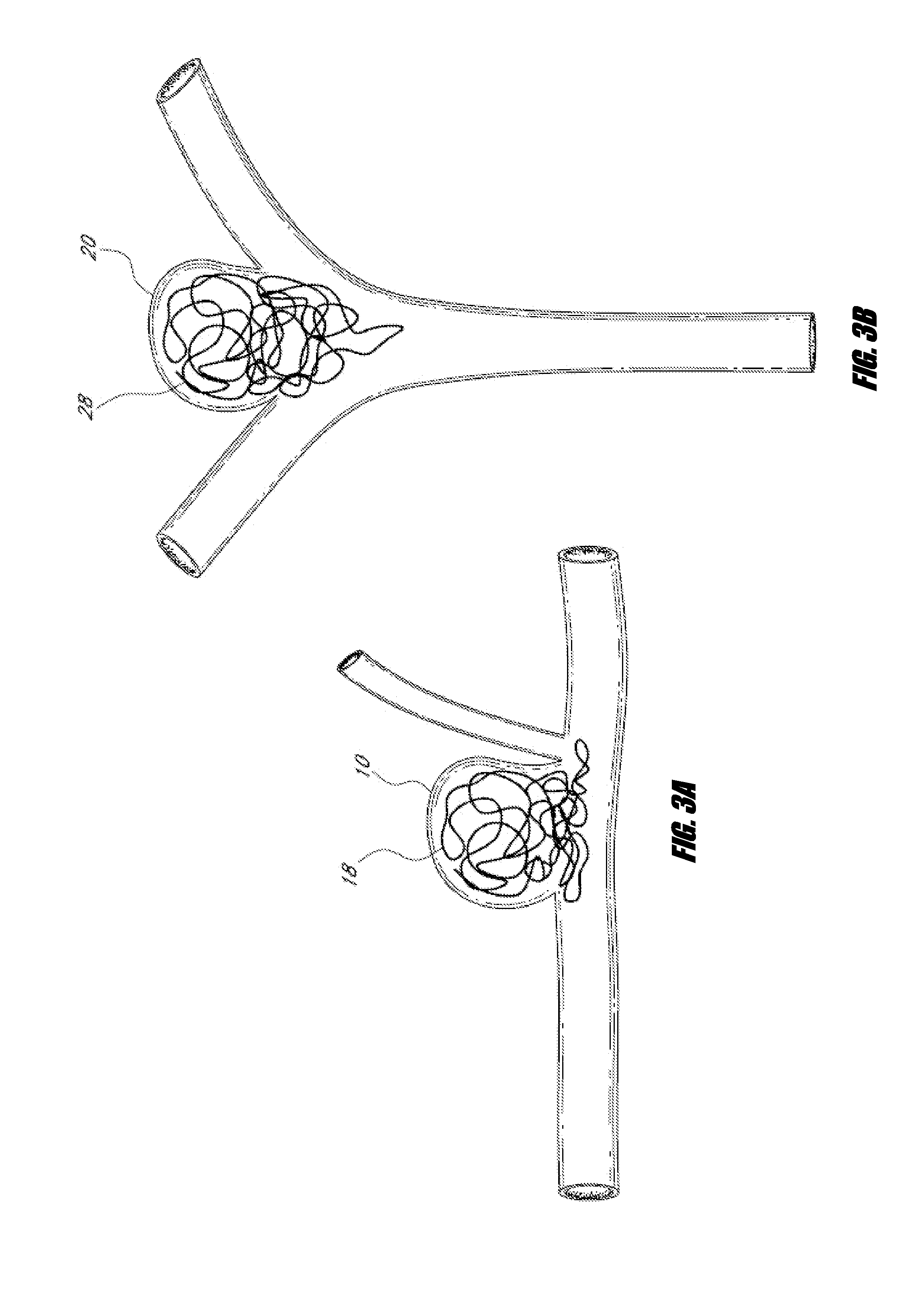
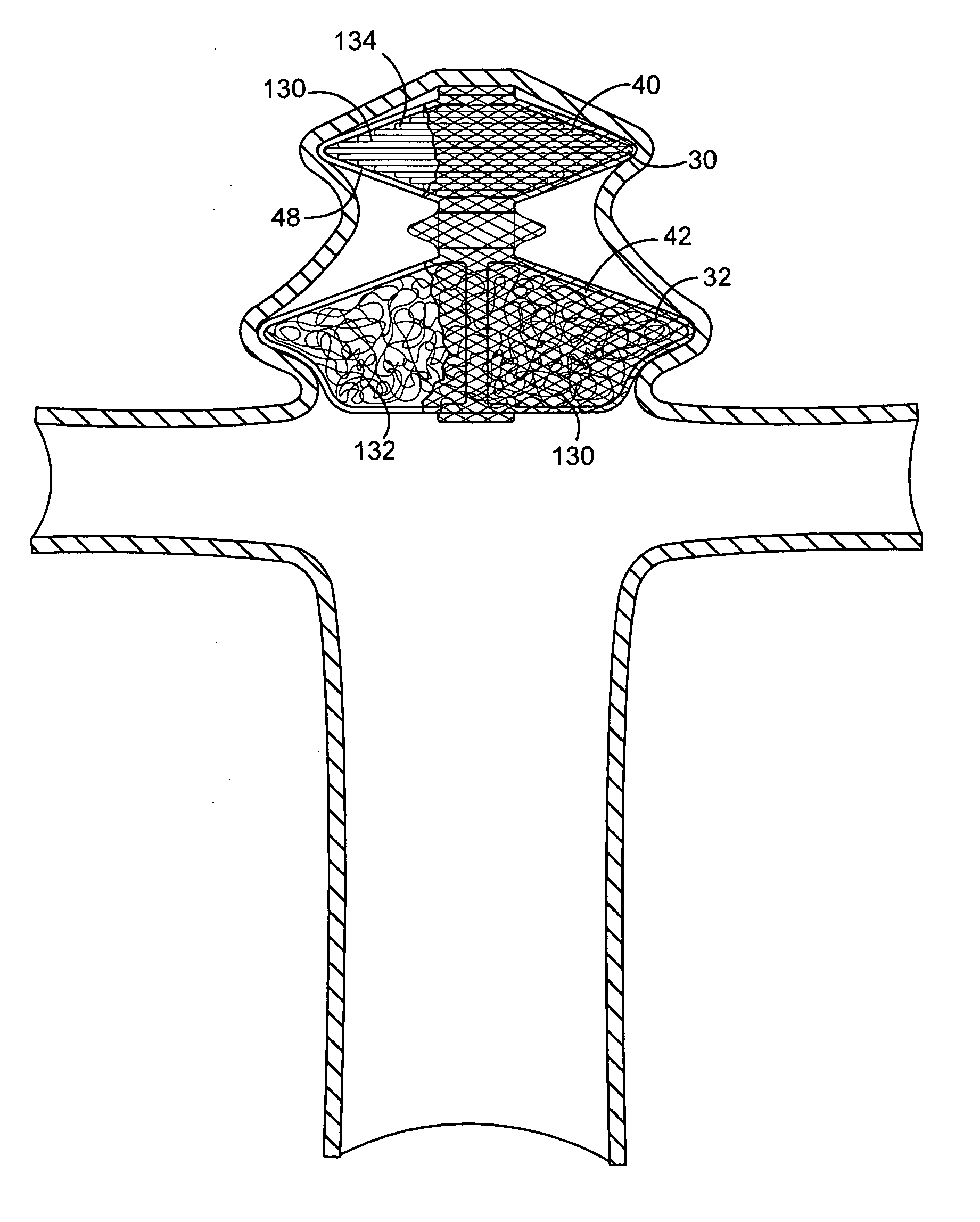
Vessel occlusion device for embolic protection system
A system used in a blood vessel when an interventional procedure is being performed in a stenosed or occluded region, which is capable of capturing embolic material which may be released into a blood vessel during a therapeutic interventional procedure at the site of a lesion in the blood vessel. The system is adapted to be utilized in a collateral blood supply system adapted to enable the flow of blood to bypass the blood vessel upon blocking thereof and to enable the reverse flow of blood through the blood vessel upon unblocking thereof. The system includes a guide wire, including a distal end, adapted to be positioned in a blood vessel relative to an interventional procedure site. A guide catheter, including a distal end, is adapted to enable the interventional procedure to be performed, and to be inserted over the guide wire and through a patient's vasculature to a position in the blood vessel relative to the interventional procedure site. An occluding device for occluding and blocking a blood vessel at a location relative to the interventional procedure site is adapted to be positionable at a location relative to he interventional procedure site, to be expandable so as to prevent and block the flow of blood past the occlusion, and to enable the capture of embolic material which may be released into the blood in the blood vessel during the therapeutic interventional procedure, and to be contracted to unblock the blood vessel and enable the recovery of captured embolic material.
Owner:ABBOTT CARDIOVASCULAR
Vascular remodeling device
ActiveUS20120143237A1Good flexibilityGood wall appositionStentsDilatorsIliac AneurysmEmbolization material
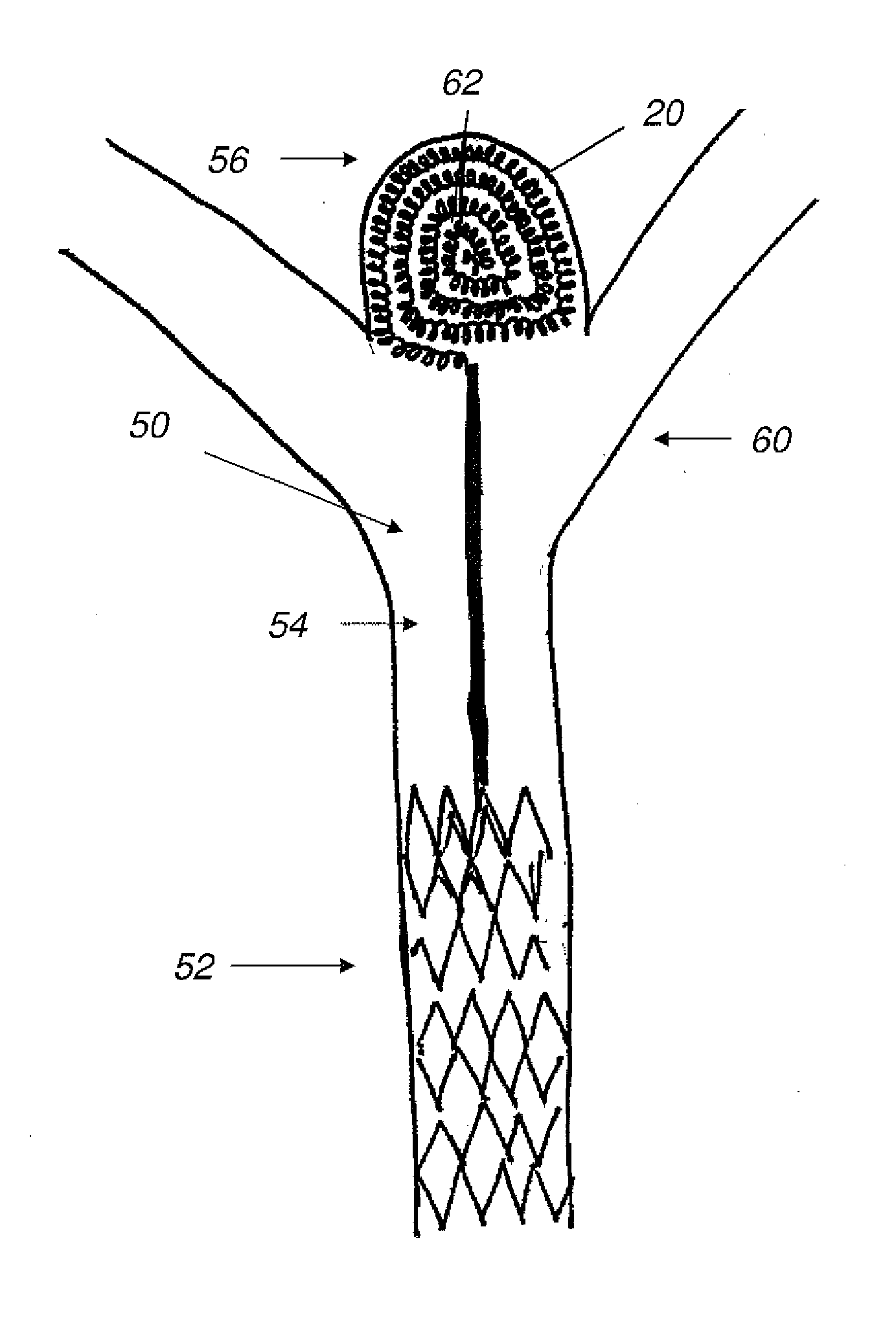
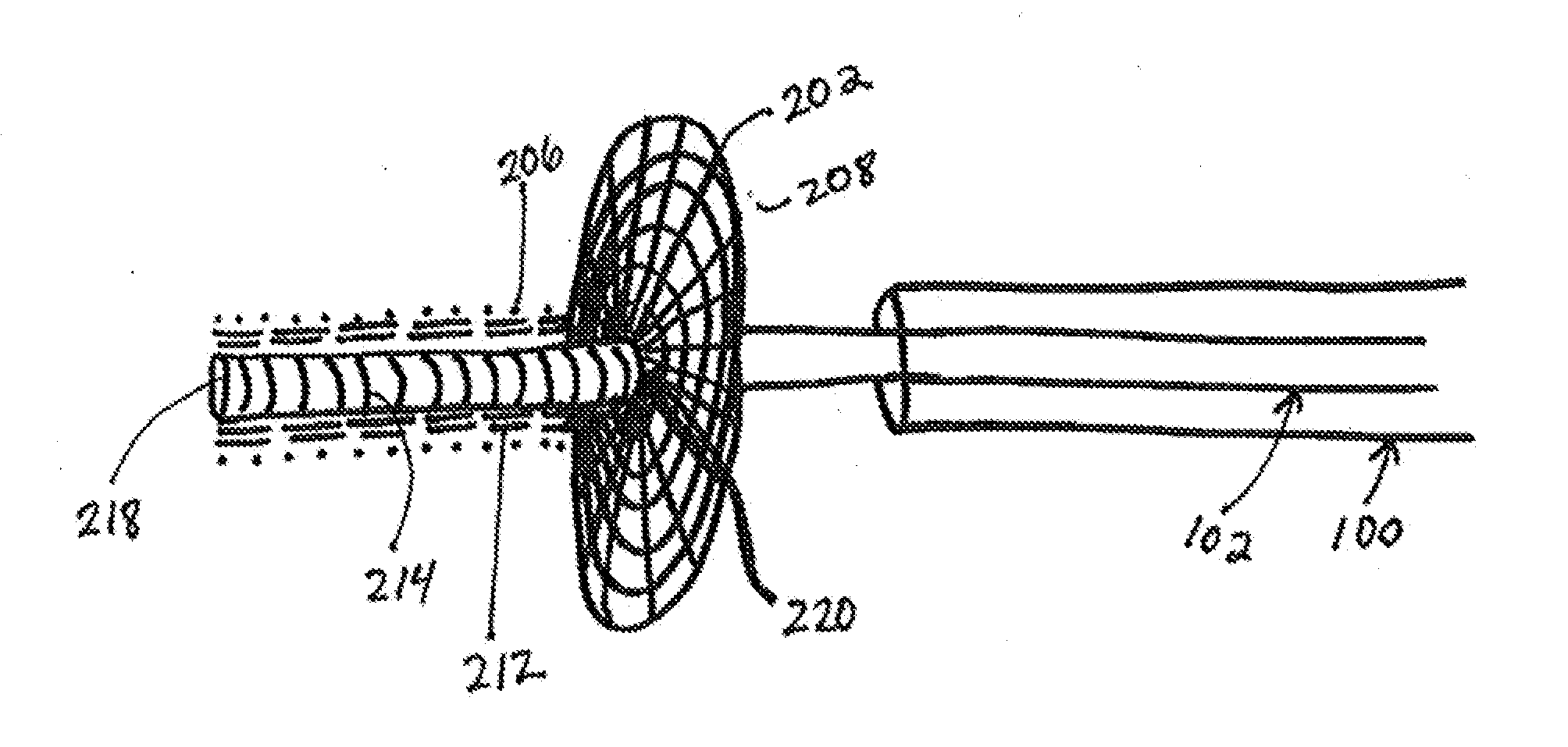
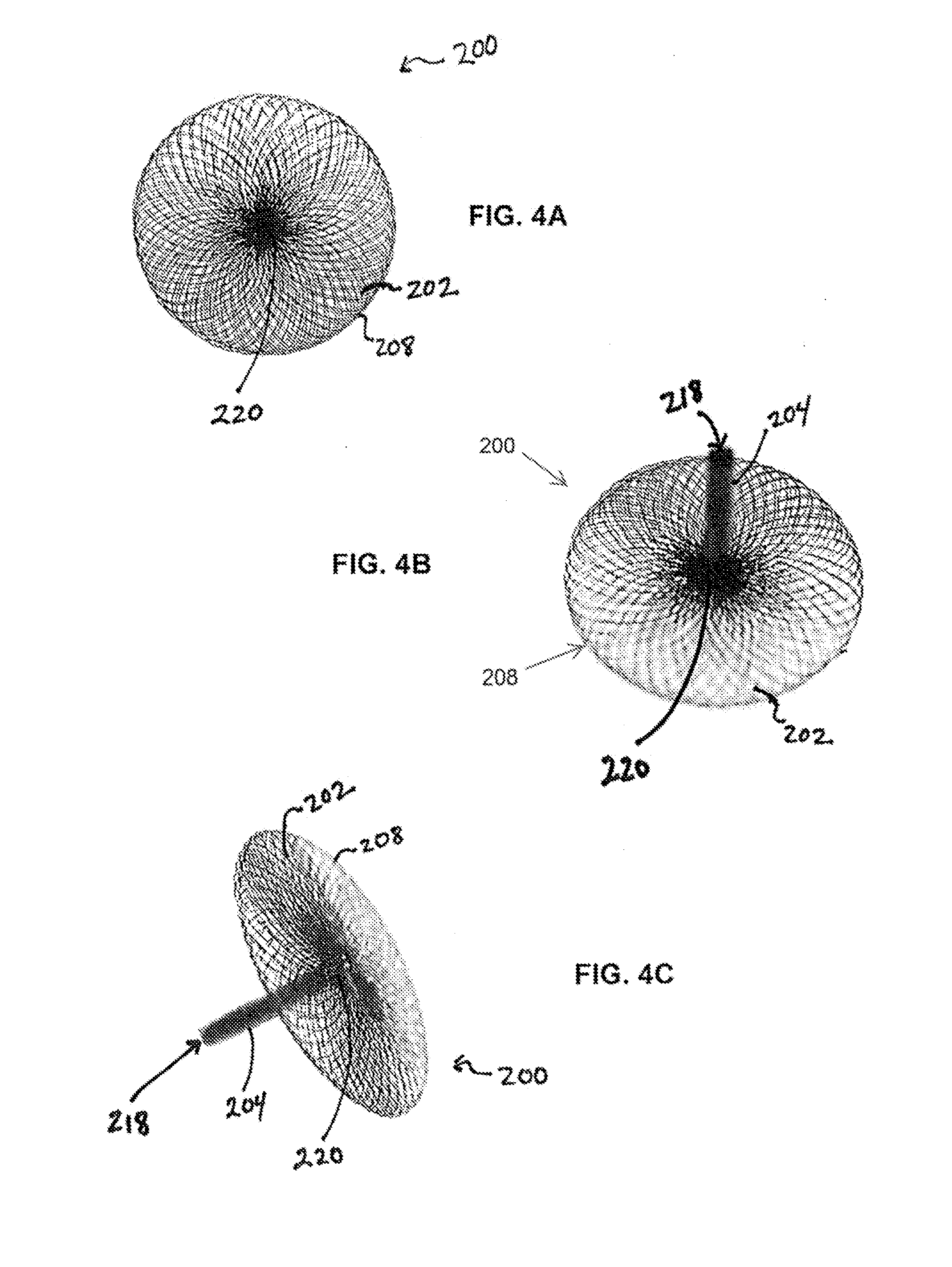
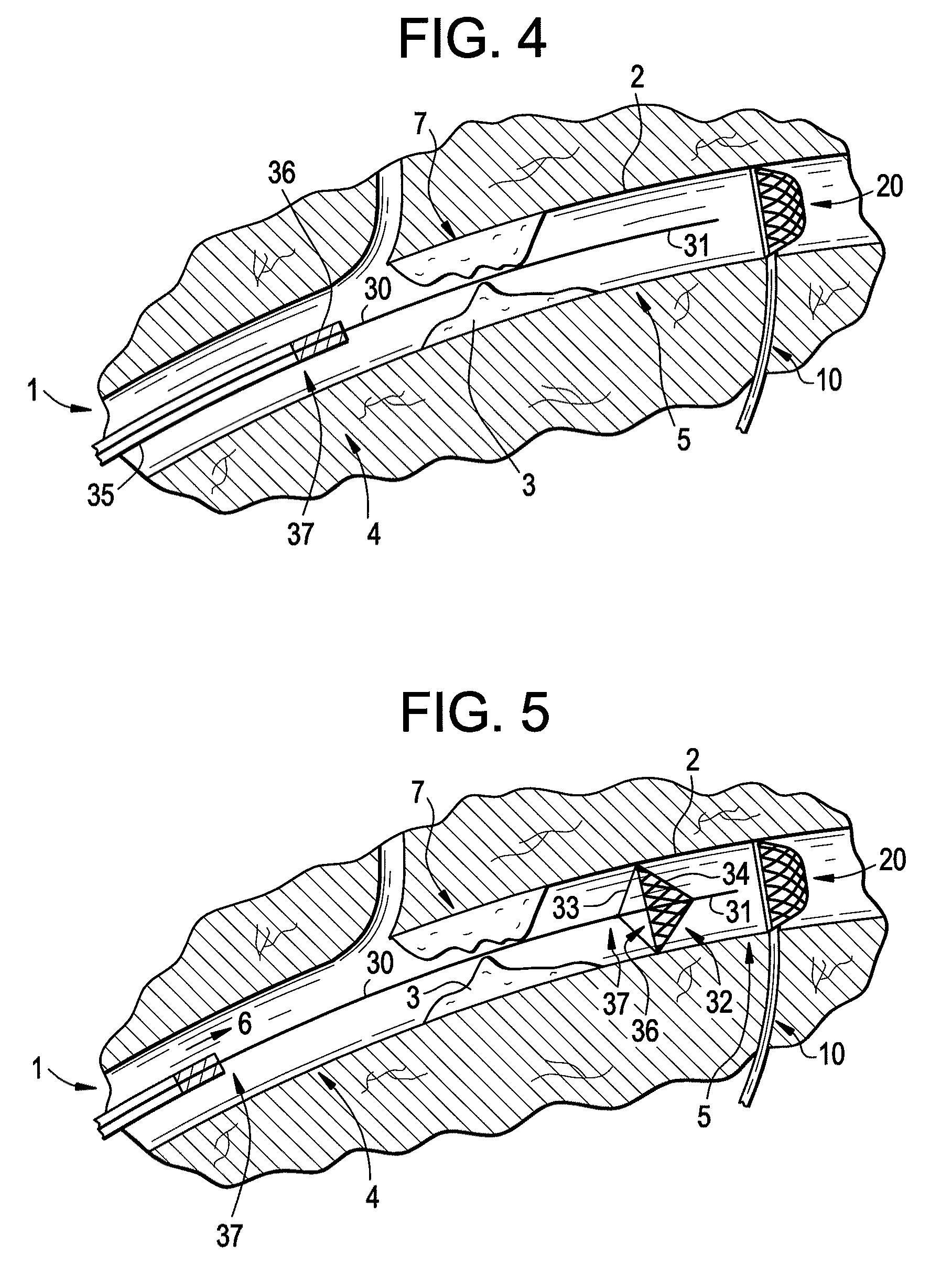
Described herein are vascular remodeling devices that include a proximal section, an intermediate section, and a distal section. During deployment, the proximal section can expand from a compressed delivery state to an expanded state and anchors the device in an afferent vessel of a bifurcation. The distal section expands from the compressed delivery state to an expanded state that may be substantially planar, approximately semi-spherical, umbrella shaped, or reverse umbrella shaped. The distal section is positioned in a bifurcation junction across the neck of an aneurysm or within an aneurysm. The intermediate section allows perfusion to efferent vessels. Before or after the device is in position, embolic material may be used to treat the aneurysm. The distal section can act as a scaffolding to prevent herniation of the embolic material. The device can be used for clot retrieval with integral distal embolic protection.
Owner:TYCO HEALTHCARE GRP LP
System and method for treating ischemic stroke
A thromboembolic removal system for treating ischemic stroke, including a guide and occlusion catheter, an aspiration catheter, an aspiration pump, and a thromboembolic separator. During aspiration of thromboembolic material through the aspiration catheter, the separator element is advanced from and retracted into the catheter lumen to break up or prevent clogs or flow restrictions formed by the thromboembolic material. Longitudinal channels in the separator allow aspiration through the aspiration catheter to continue even when the separator is disposed in the aspiration catheter lumen.
Owner:PENUMBRA
Emboli protection devices and related methods of use
An evacuation sheath assembly and method of treating occluded vessels which reduces the risk of distal embolization during vascular interventions is provided. The evacuation sheath assembly includes an elongated tube defining an evacuation lumen having proximal and distal ends. A proximal sealing surface is provided on a proximal portion of the tube and is configured to form a seal with a lumen of a guided catheter. A distal sealing surface is provided on a distal portion of the tube and is configured to form a seal with a blood vessel. Obturator assemblies and infusion catheter assemblies are provided to be used with the evacuation sheath assembly. A method of treatment of a blood vessel using the evacuation sheath assembly includes advancing the evacuation sheath assembly into the blood vessel through a guide catheter. Normal antegrade blood flow in the blood vessel proximate to the stenosis is stopped and the stenosis is treated. Retrograde blood flow is induced within the blood vessel to carry embolic material dislodged during treating into the evacuation sheath assembly. If necessary to increase retrograde flow, the coronary sinus may be at least partially occluded. Alternatively, antegrade flow may be permitted while flow is occluded at the treatment site.
Owner:ST JUDE MEDICAL CARDILOGY DIV INC
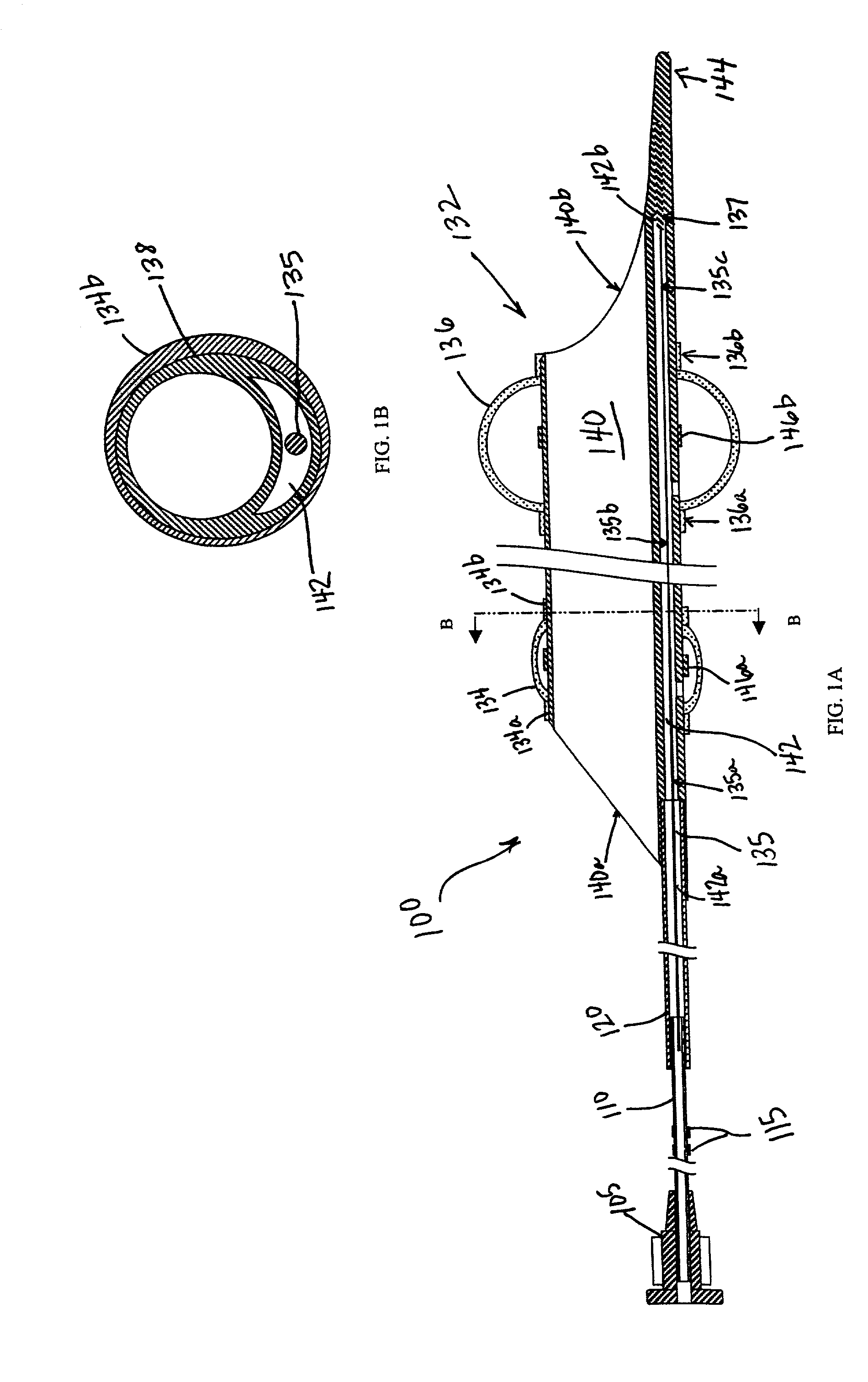
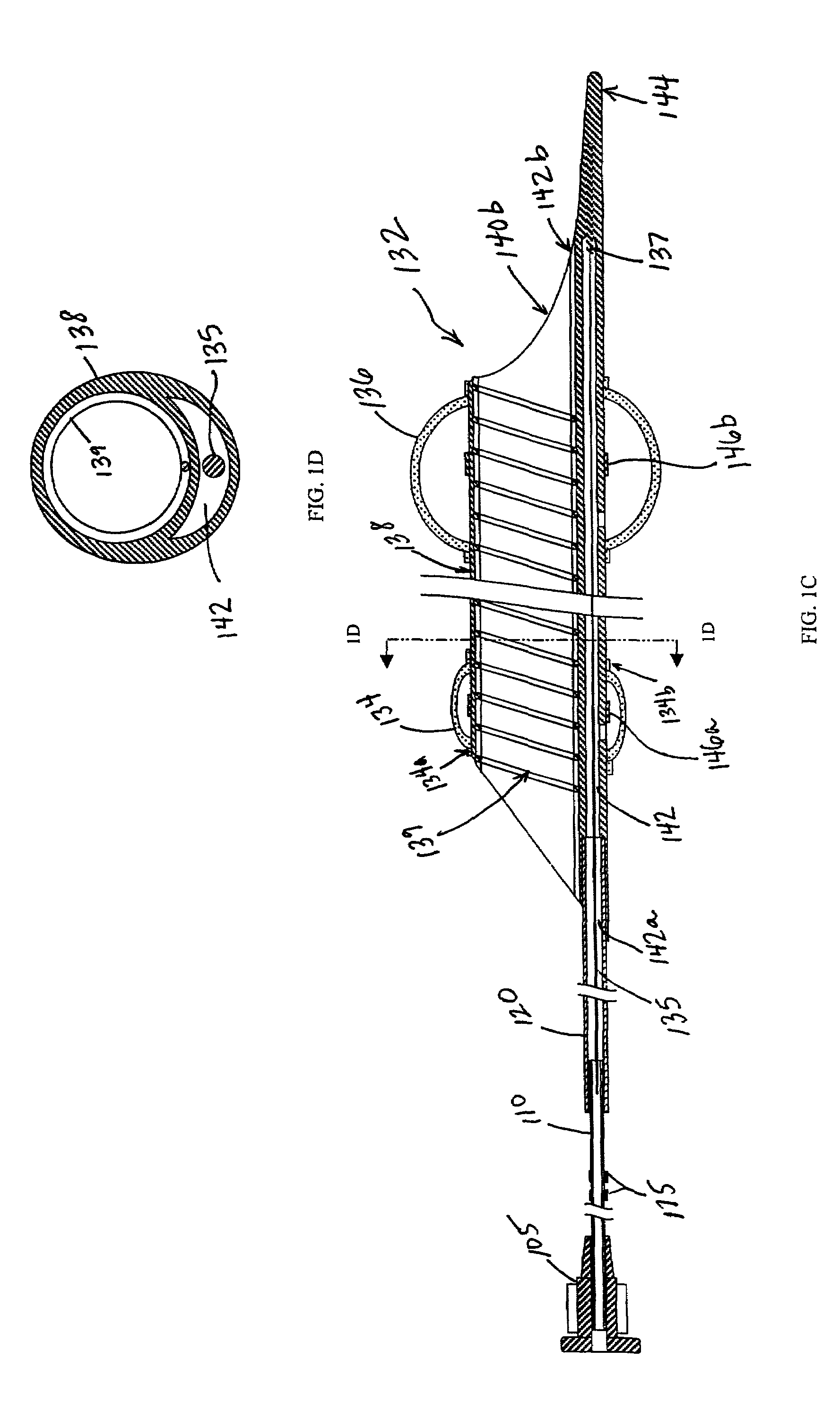
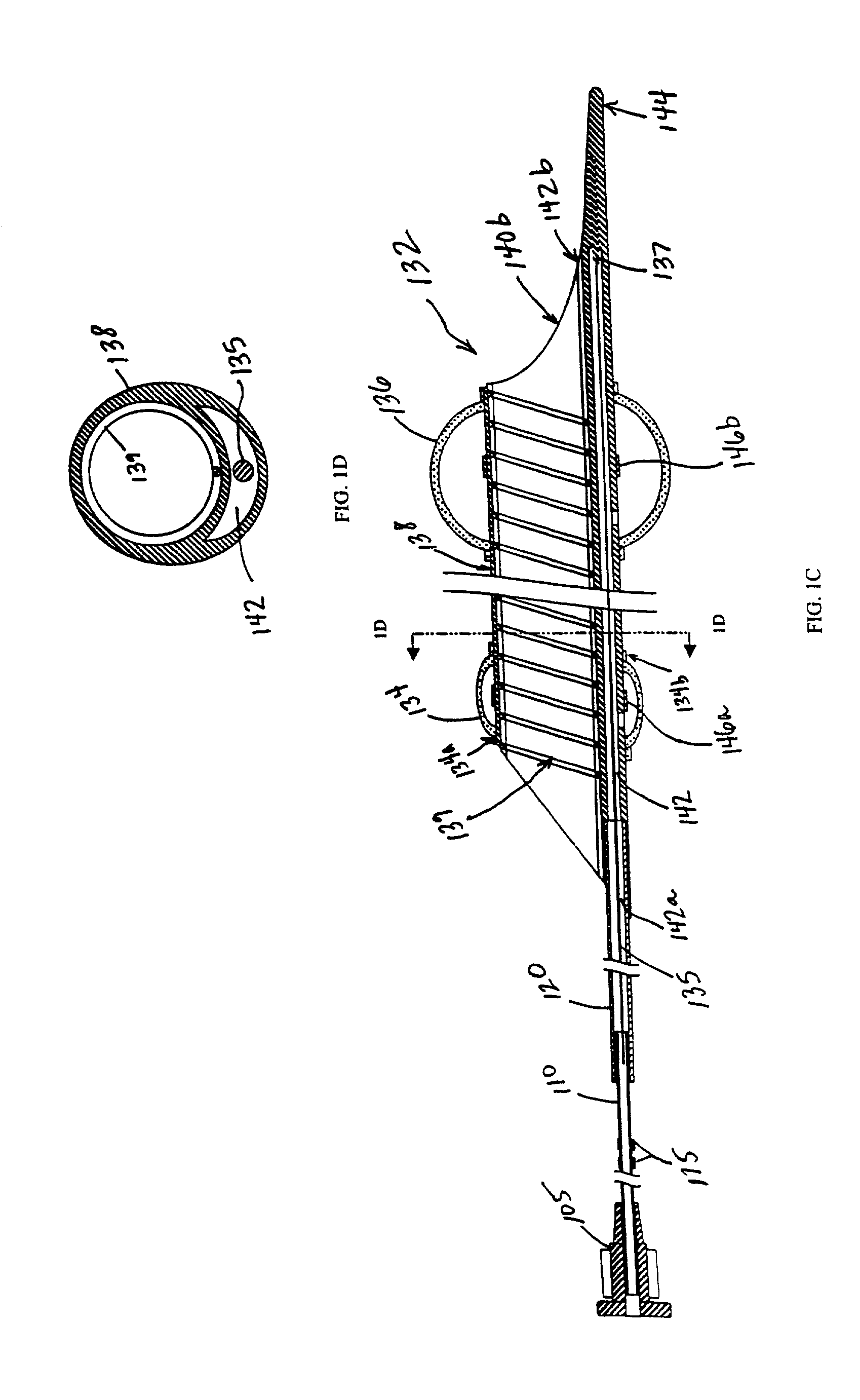
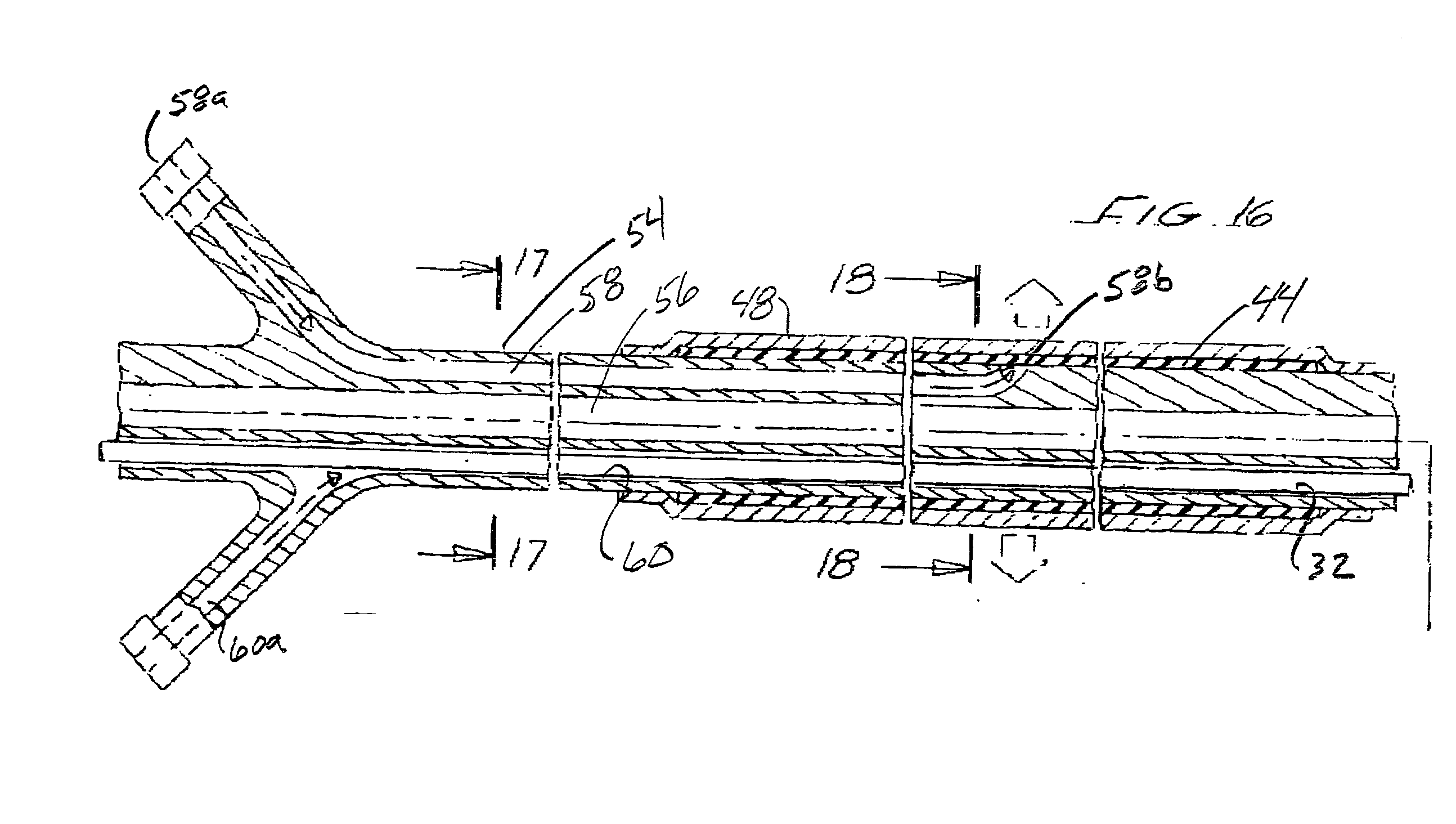
Distal protection apparatus with improved wall apposition
InactiveUS20060149313A1Easy loadingPrevent thrombosisSurgeryDilatorsEndovascular surgeryEmbolization material
In accordance with the present invention, a distal protection and embolic material retrieval device with improved apposition to both large and small vessel walls of varying geometries for enhancing the filtering of embolic material during intravascular procedures while allowing for the passage of blood is provided. The device includes a filter basket designed to maximize apposition of the filtering portion to that of the vessel wall and may include struts which provide circumferential support to the filtering membrane and thereby minimizing or eliminating in-folding of the filter basket. Thin film materials may also be utilized for the filtering membrane of the filter basket. In addition one can incorporate biological and / or pharmaceutical agents in combination with the present invention.
Owner:ARGUELLO EDWARD +3
Vascular remodeling device
A vascular remodeling device is provided that can comprise a proximal section, an intermediate section, and a distal section. During deployment, the proximal section can expand from a compressed delivery state to an expanded state and anchor the device in the afferent vessel of a bifurcation. The distal section can comprise at least one embolization coil that can be positioned within an aneurysm to treat the aneurysm and expand from the compressed delivery state to an expanded state upon deployment. The intermediate section can allow perfusion to efferent vessels. Before, during, and / or after the device is positioned, additional embolic material can be used to treat the aneurysm.
Owner:TYCO HEALTHCARE GRP LP
Implantable intraluminal protector device and method of using same for stabilizing atheromas
InactiveUS20030100940A1Process stabilityImprove propertiesStentsBlood vesselsThrombogenicityThrombus
An intraluminal device implantable in a body lumen having an atheroma therein in the vicinity of a side-branch vessel includes a mesh-like tube of bio-compatible material formed with liquid-permeable window openings. The mesh-like tube has an expanded condition in which the tube diameter is slightly larger than the diameter of the body lumen in which it is to be implanted, and the tube length is sufficient to cover the atheroma and the side-branch orifice, and to be anchored to the body lumen around the periphery of the atheroma, and a contracted condition wherein it is sufficiently flexible so as to be easily manipulatable through the body lumen to the site of the atheroma. The mesh-like tube, in its expanded condition, has window openings of a size and distribution such as to structurally stabilize the atheroma and to keep embolic material originating from the atheroma in place on the wall of the body lumen, while diverting embolic material of predetermined size present in the blood flowing through the mesh-like tube from the side-branch orifice, without substantially impeding the blood flow, or increasing the thrombogenitic properties, of the blood flowing into the side-branch orifice.
Owner:SURPASS MEDICAL
Occlusive Device
An aneurysm embolization device can include a body having a single, continuous piece of material that is shape set into a plurality of distinct structural components. For example, the device can have an expandable component and an atraumatic tip portion extending therefrom. Further, the tip portion can be configured to enable the device to be implanted within the aneurysm while tending to mitigate risk of puncturing the aneurysm dome or otherwise assist in framing the aneurysm in advance of placement of additional embolic material.
Owner:TYCO HEALTHCARE GRP LP
Aneurysm cover device for embolic delivery and retention
InactiveUS20110144669A1Improve durabilityImprove abilitiesOcculdersWound clampsAneurysm neckBrain aneurysm
An implant for treating brain aneurysms, especially terminal aneurysms, comprises a neck cover and elongate shaft removably secured to an embolic delivery catheter. As such, the shaft aids in directing and placing the cover at the aneurysm neck, protecting the delivery catheter from adhesion with the embolic material, and securing the cover in place with connection or adhesion of the shaft to the embolic material delivered through the catheter. The implant can be anchored at the aneurysm either by interface and / or adhesion of the shaft or shaft and cover with the resident embolic materials.
Owner:TYCO HEALTHCARE GRP LP
Vascular remodeling device
ActiveUS20120143317A1Good flexibilityGood wall appositionStentsOcculdersIliac AneurysmEmbolization material
Described herein are vascular remodeling devices that include a proximal section, an intermediate section, and a distal section. During deployment, the proximal section can expand from a compressed delivery state to an expanded state and anchors the device in an afferent vessel of a bifurcation. The distal section expands from the compressed delivery state to an expanded state that may be substantially planar, approximately semi-spherical, umbrella shaped, or reverse umbrella shaped. The distal section is positioned in a bifurcation junction across the neck of an aneurysm or within an aneurysm. The intermediate section allows perfusion to efferent vessels. Before or after the device is in position, embolic material may be used to treat the aneurysm. The distal section can act as a scaffolding to prevent herniation of the embolic material. The device can be used for clot retrieval with integral distal embolic protection.
Owner:TYCO HEALTHCARE GRP LP
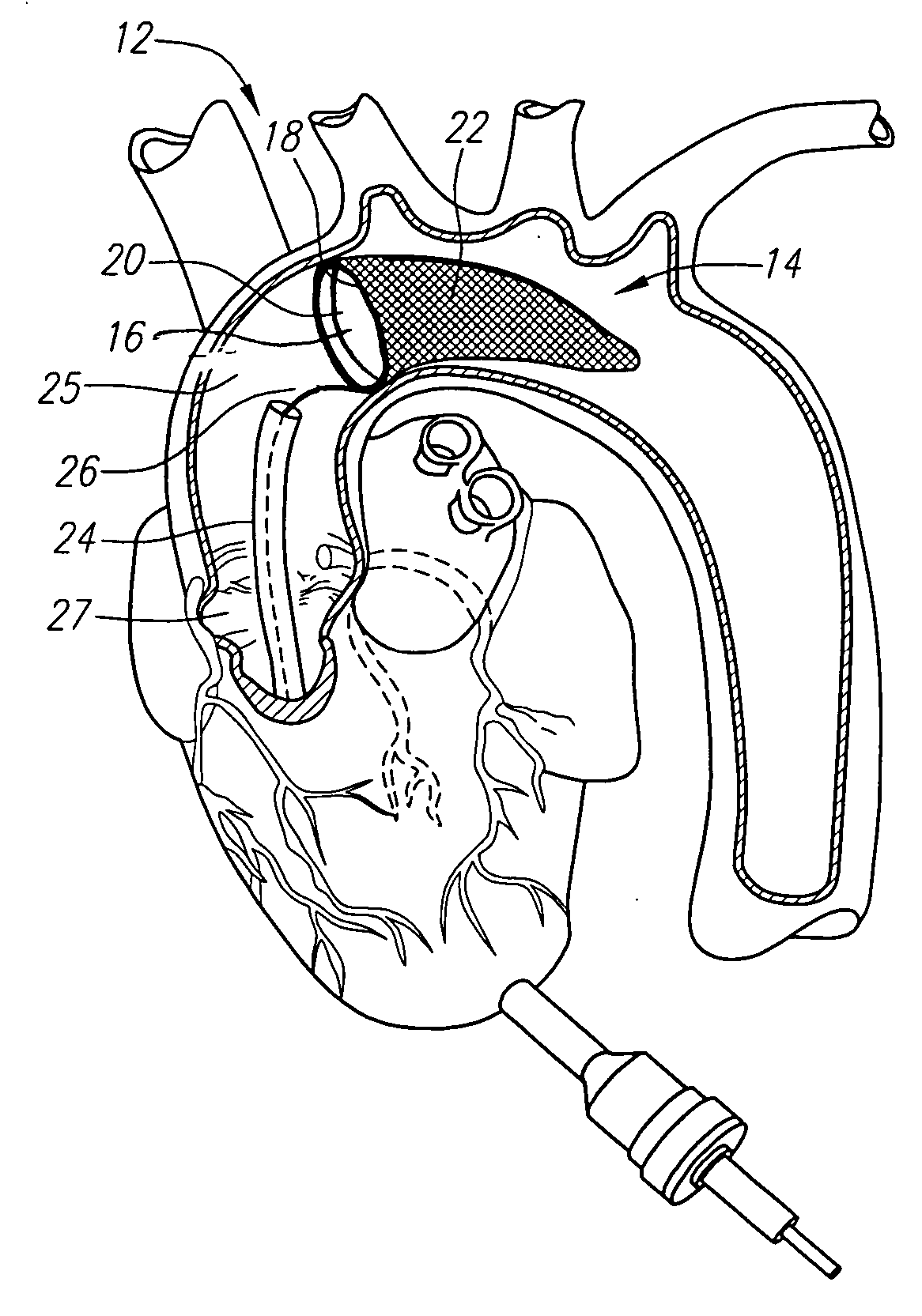
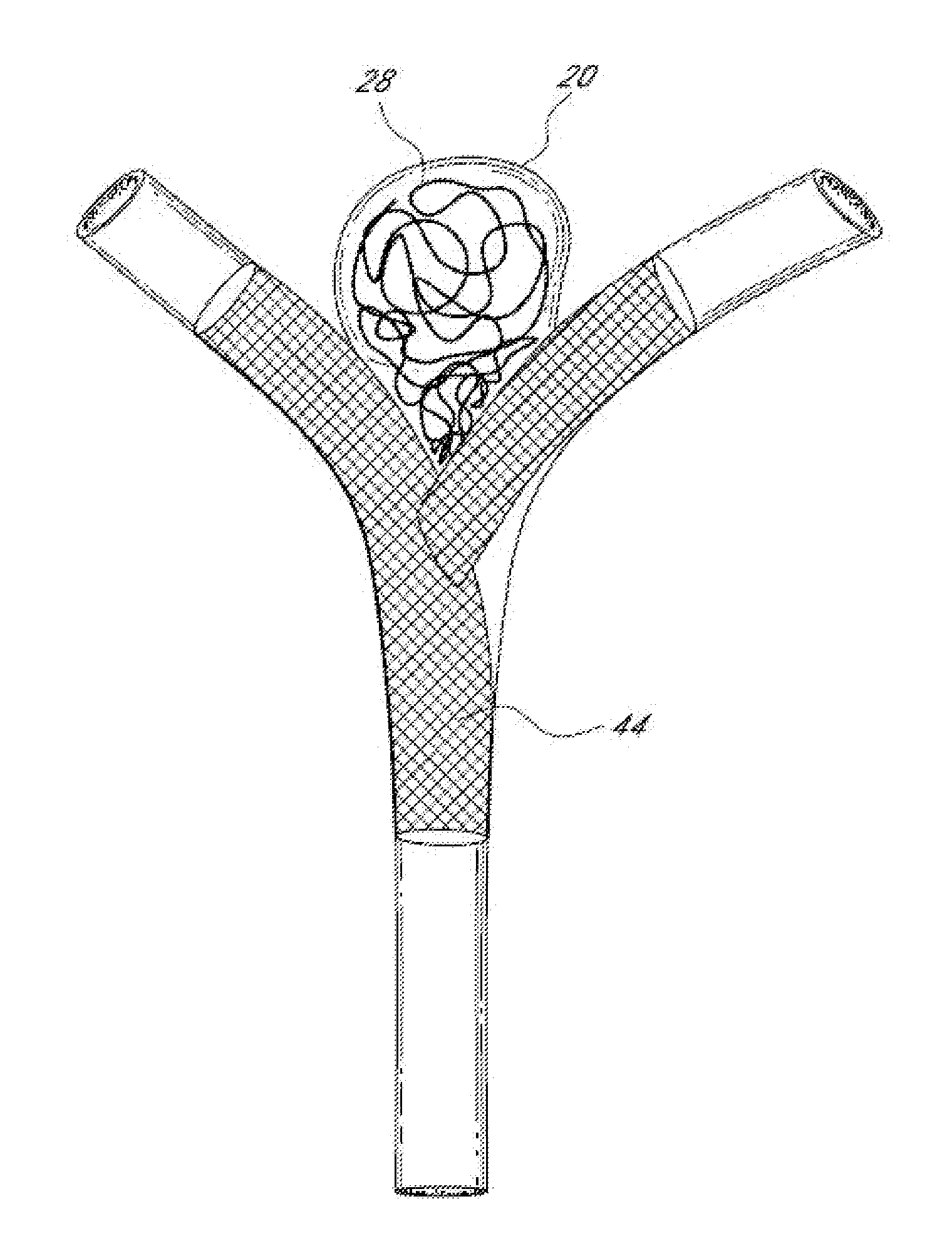
Intracardiac cage and method of delivering same
Owner:BOSTON SCI SCIMED INC
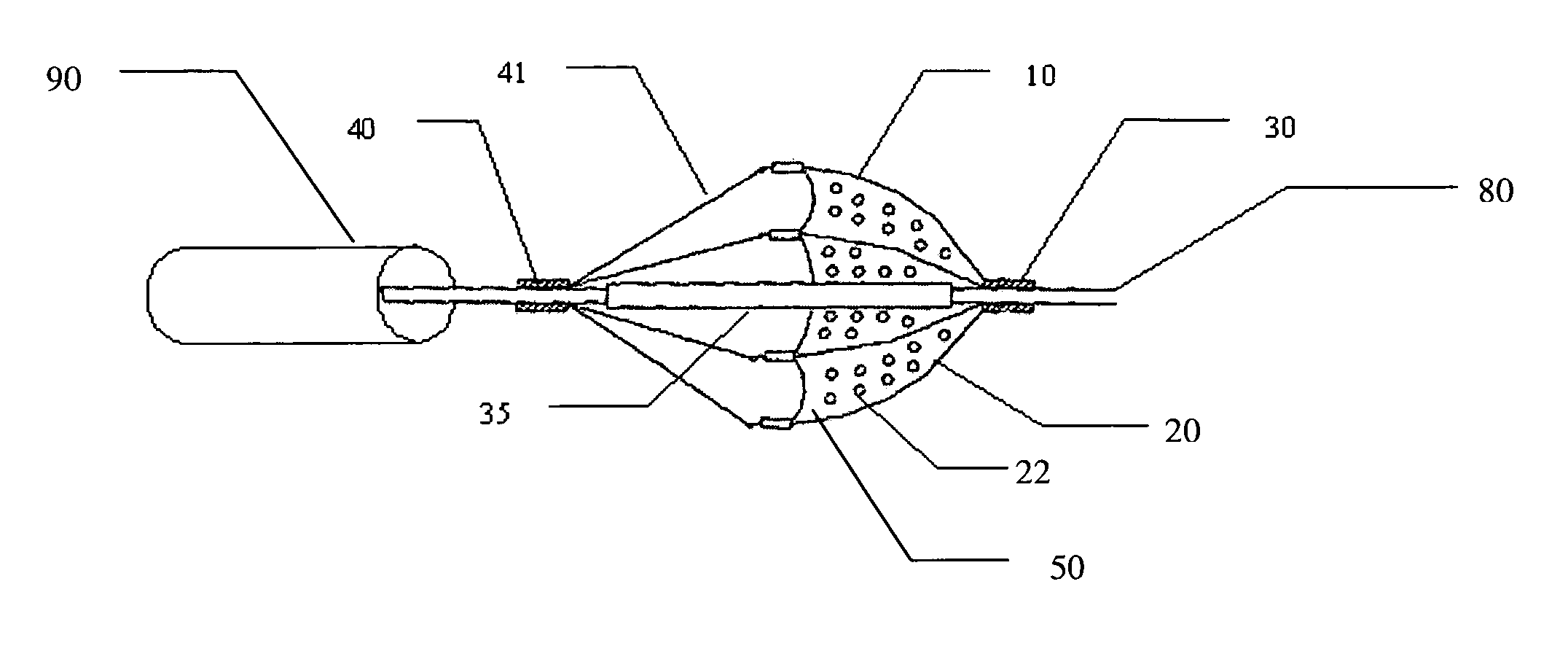
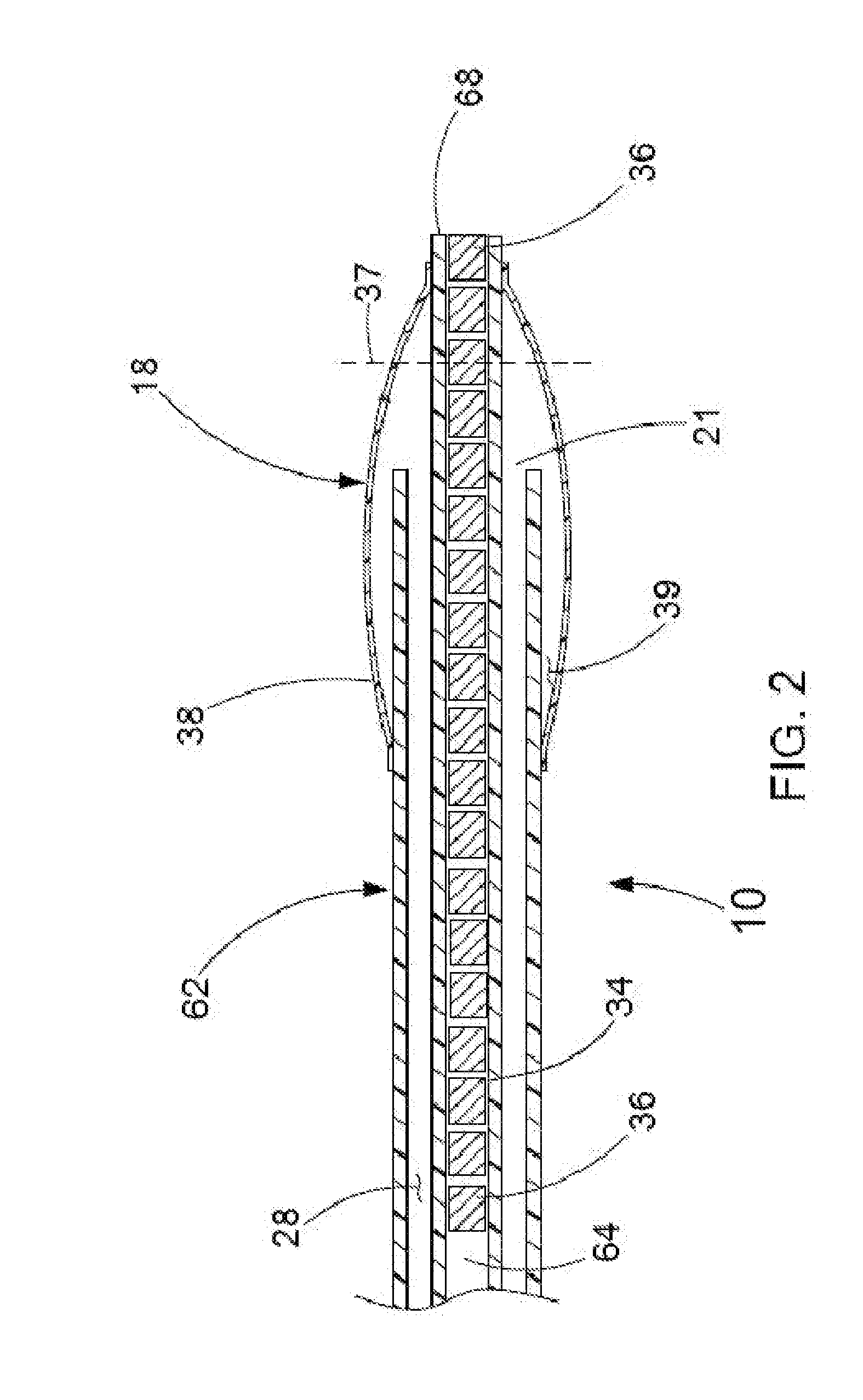
Filter element for embolic protection device
InactiveUS7491215B2Avoid distortionPrevent collapseHaemofiltrationUltrafiltrationEmbolic Protection DevicesDistal portion
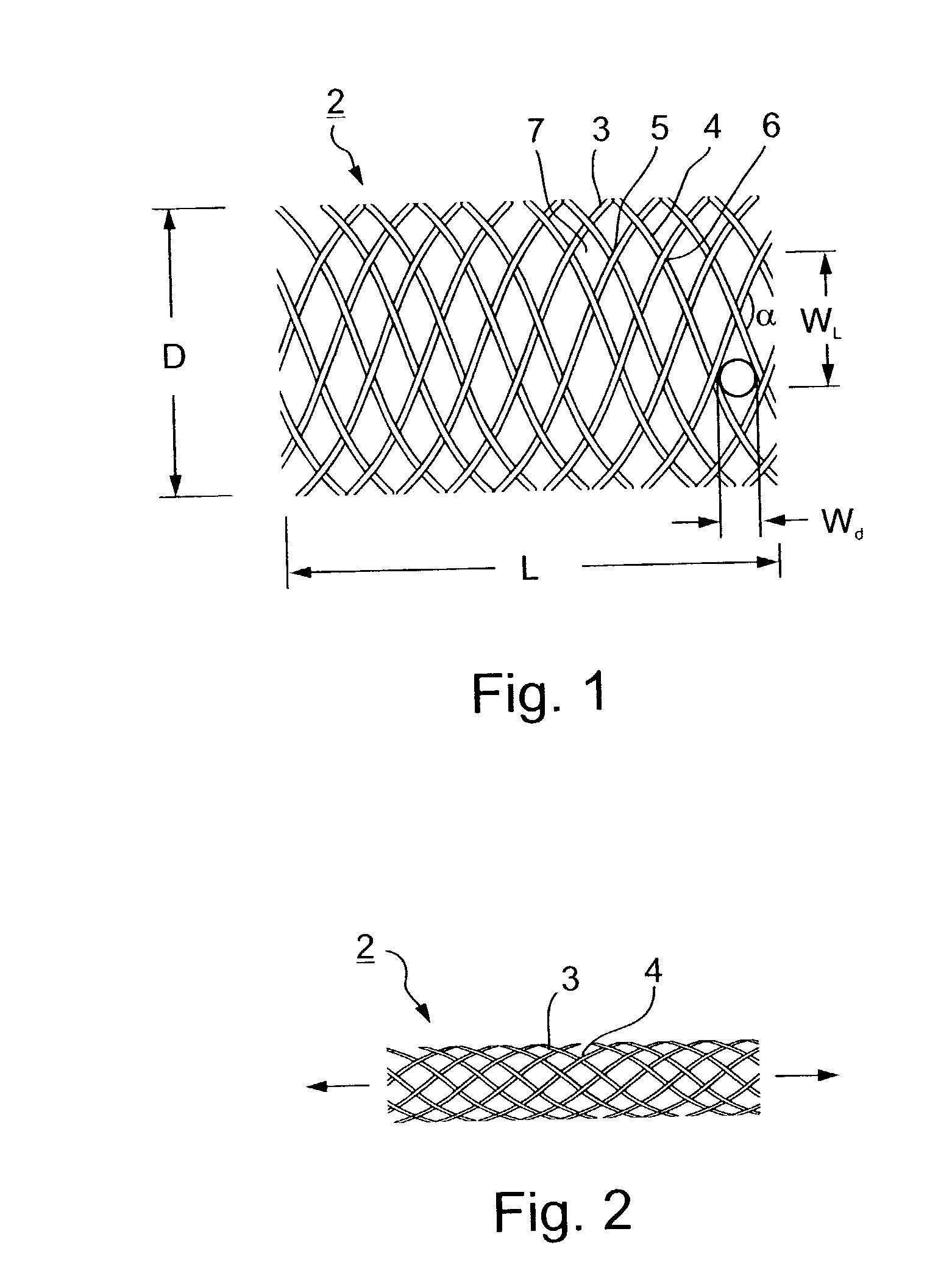
A collapsible filter element for a transcatheter embolic protection device comprises a collapsible filter body which is movable between a collapsed stored position for movement through a vascular system and an expanded position for extension across a blood vessel such that blood passing through the blood vessel is delivered through the filter element. A proximal inlet portion of the filter body has one or more inlet openings sized to allow blood and embolic material enter the filter body and a distal outlet portion of the filter body has a plurality of outlet openings sized to allow through-passage of blood, but to retain embolic material within the filter body. The filter body is at least partially of laminate construction comprising a membrane coated with a coating which is biocompatible, the thickness of the coating being from 4% to 40% of the thickness of the membrane. The coating may be of hydrophilic material. To facilitate retrieval of captured embolic material the distal portion and / or an intermediate portion of the filter membrane may be stretchable. The filter body may have regions of varying hardness or stiffness.
Owner:SALVIAC
Distal access embolic protection system and methods of using the same
A distal access embolic protection system and methods of using the same for the capture and removal of embolic material from a blood vessel during an interventional procedure are provided. A distal access embolic protection system is inserted through the wall of the blood vessel at a position distal to a stenosis and a distal access filter device is deployed at the point of insertion. After the filter is deployed and is filtering blood that is flowing across the treatment site, an interventional procedure can be performed at the treatment site. After the procedure, the filter device can be collapsed and removed from the blood vessel. A further method is provided wherein a second embolic protection system including a second filter is advanced to the treatment site using a transcatheter technique after the distal access filter has been deployed. A distal access embolic protection system is also provided.
Owner:SALVIAC
Methods and Devices for Removal of Thromboembolic Material
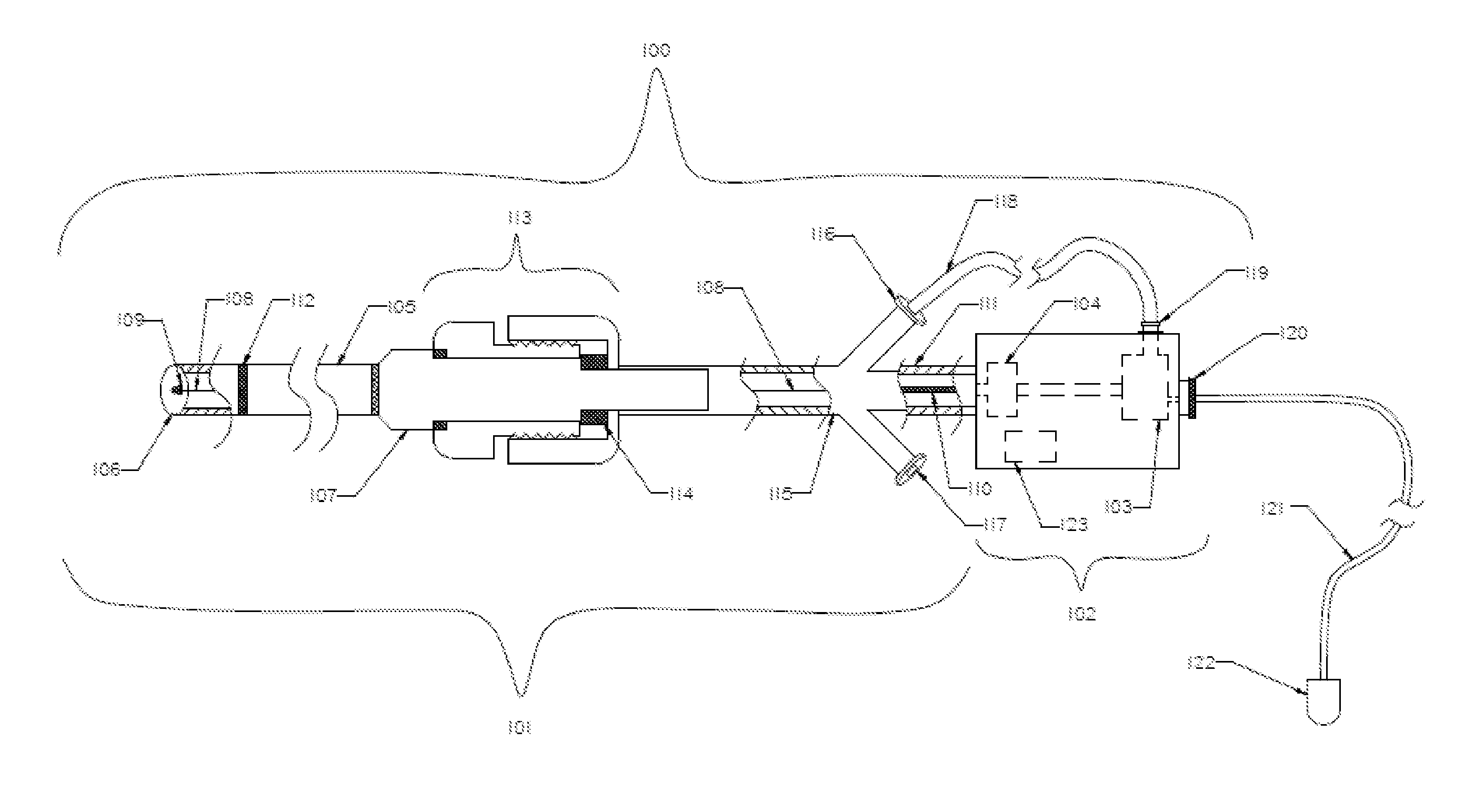
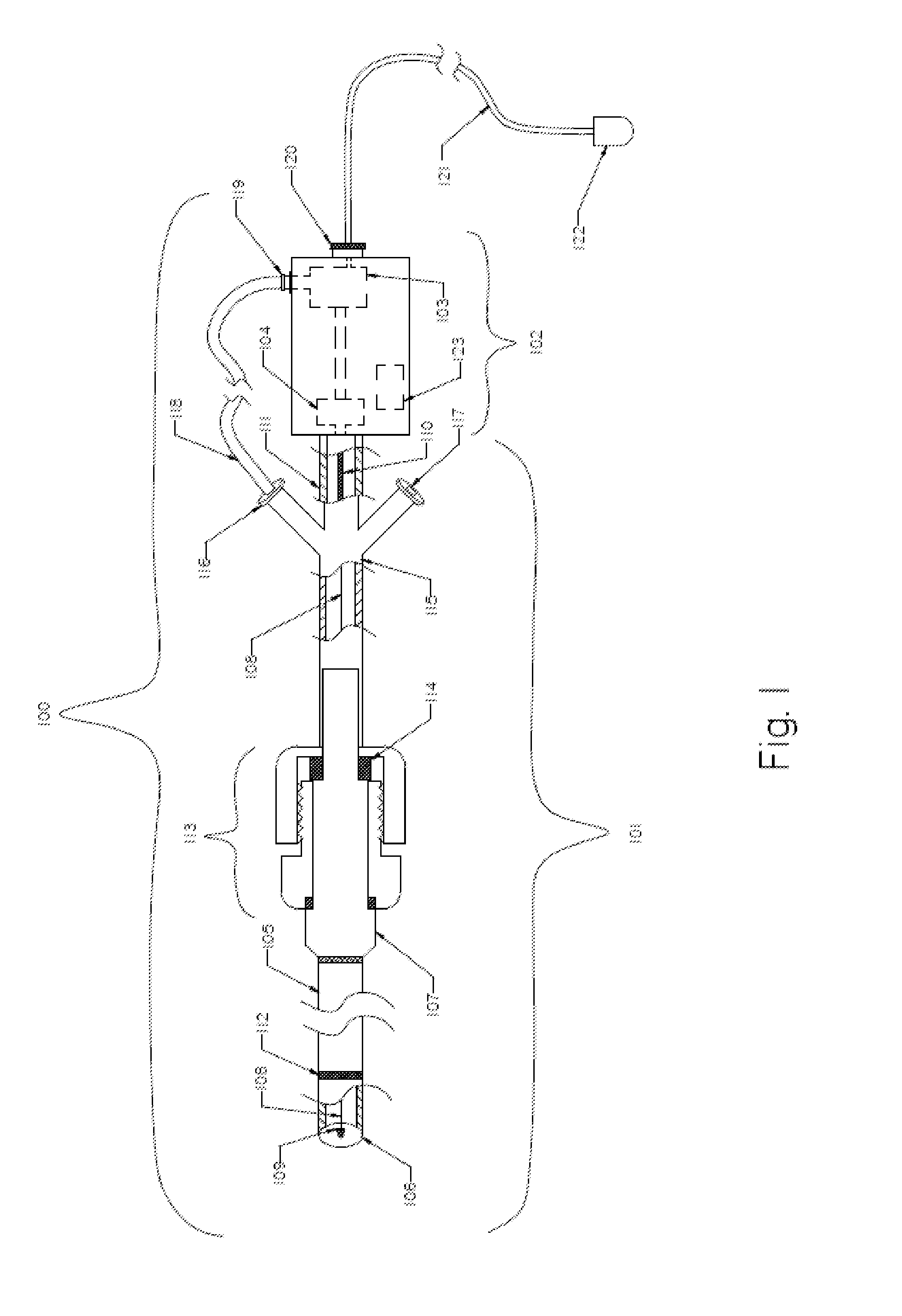
ActiveUS20160166266A1Easy to disassembleMinimize additional blood-lossBalloon catheterExcision instrumentsRotational energyEmbolization material
Methods and devices to remove thromboembolic material from the human body using rotational energy and aspiration are disclosed. A thromboembolic removal system includes an extraction device and drive unit. The extraction device is introduced to the treatment area and activated by the drive unit to separate, break apart, loosen or soften thromboembolic material and to facilitate its aspiration outside the patient.
Owner:PENUMBRA
Stent with combined distal protection device
InactiveUS20040054394A1Prevent escapePrevent travelStentsHaemostasis valvesCatheterEmbolization material
A method and apparatus for introducing a stent into a region of a major blood vessel within the human body in a manner which substantially reduces the risk of embolic material escaping to the vessel and causing a blockage at a downstream location. Additionally, the apparatus of the invention includes a uniquely designed stent delivery catheter having a central lumen through which a guide wire travels, the central lumen being provided with a valve means that is operable by the guide wire and when moved into a closed position by withdrawal of the guide wire, functions to prevent the flow of loose debris toward the proximal end of the catheter.
Owner:LEE DON W
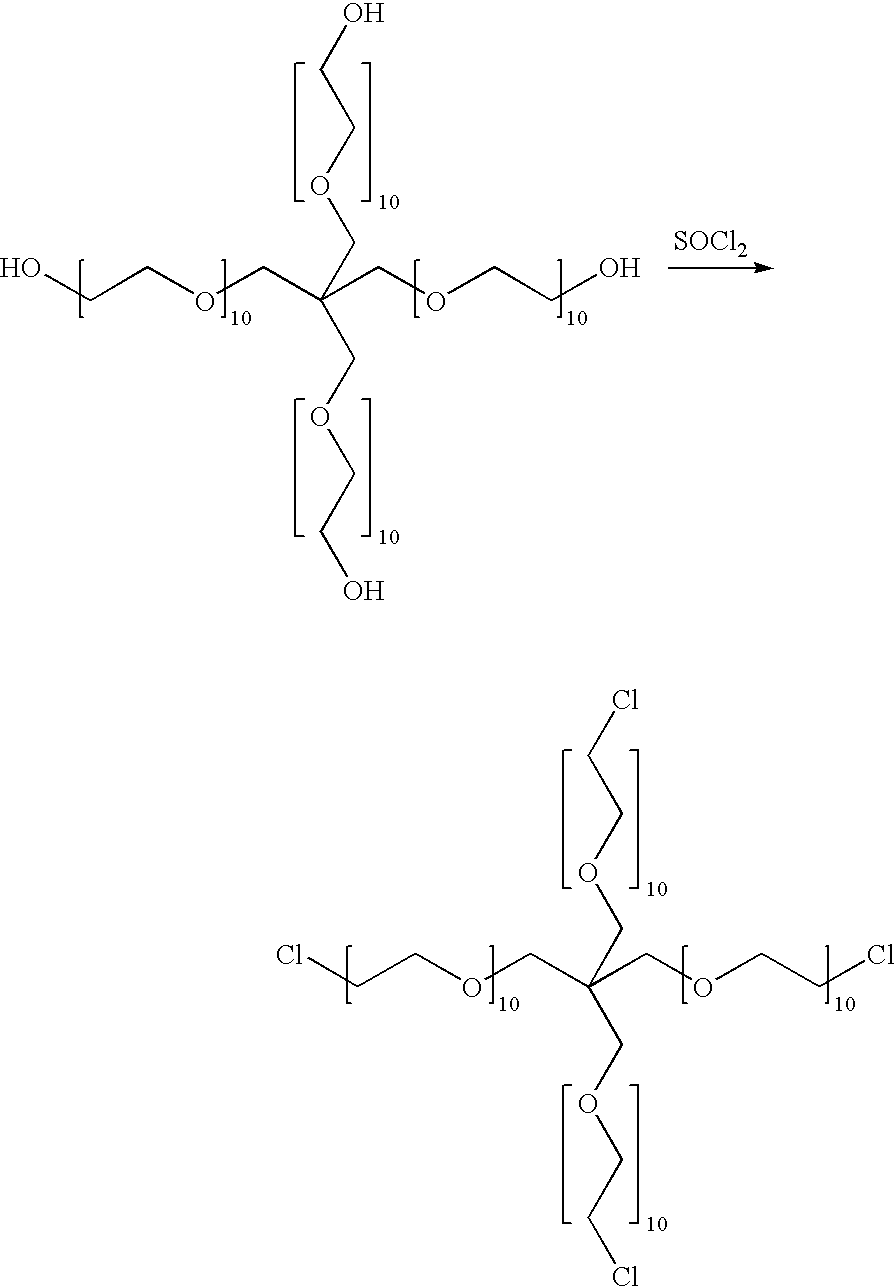
Vascular embolization material
InactiveUS20060069168A1Easy to observeImprove abilitiesSurgical adhesivesPharmaceutical non-active ingredientsSwelling ratioWater insoluble
This invention provides an embolization material used for blocking a blood vessel in vivo for stopping the blood flow. The most suitable embolization material has a water swelling ratio of 30% or more, is degradable in a phosphate buffered saline, is formed as virtually spherical particles, and is preferably composed of a water insoluble poly(ethylene glycol) copolymer, wherein when the film formed from said polymer is saturated with water, it has an elastic modulus in tension of 1500 MPa or less. The embolization material of this invention can reliably block a blood vessel at an intended site without causing cohesion or clogging in a catheter or in the blood vessel at other than the intended site. Thereafter, the blocked site concerned can be liberated from the embolized state by degradation, and the degraded components can be metabolized or excreted outside the body.
Owner:TORAY IND INC
Endoluminal prosthesis endoleak management
The present invention provides methods and compositions for managing endoleaks in a perigraft space around an endovascular graft. In one embodiment, a blood flow through the endovascular graft is temporarily reduced and an embolic material is delivered into the perigraft space while the blood flow through the endovascular graft is reduced. The embolic material may comprise polyethylene glycol diacrylate, pentaerthyritol tetra 3(mercaptopropionate), and a buffer.
Owner:BOSTON SCI CORP
Endoluminal occlusion-irrigation catheter with aspiration capabilities and methods of use
InactiveUS7731683B2Easy to disassembleThorough removalBalloon catheterSurgeryEmbolization materialIntravascular catheter
A catheter system comprising a guidewire, an endovascular catheter, and an aspiration catheter. The guidewire has an expandable occluder mounted on a distal end. The guidewire and the endovascular catheter are insertable into a lumen of the aspiration catheter. The aspiration catheter also includes infusion and aspiration lumen(s) and port(s). Methods of using the catheter system for treating a vascular lesion and removing embolic material during the procedure are also disclosed.
Owner:BOSTON SCI SCIMED INC
Method for embolization using liquid embolic materials
A method for embolization using liquid embolic materials is described. The method comprises the use of two liquid components. The first liquid component is an aqueous solution or dispersion comprising at least one oxidized polysaccharide. The second liquid component is either an aqueous solution or dispersion comprising at least one water-dispersible, multi-arm amine, or a water-dispersible multi-arm amine in the form of a neat liquid. The two components crosslink in situ to form a hydrogel that should act as an effective embolic agent.
Owner:EI DU PONT DE NEMOURS & CO
Devices and methods for treating vascular malformations
InactiveUS20110224776A1Ensure isolationProtect the neckStentsBalloon catheterEmbolization materialVascular malformation
A device for treating vascular malformations includes a primary coil and secondary windings. The primary coil provides resilience and structural integrity while the secondary windings fill interstitial spaces in the primary coil to isolate the vascular malformation. The device may have increased density along a central portion to isolate the malformation. In another aspect, the device may have a central opening through which embolic materials may be delivered.
Owner:STRYKER CORP
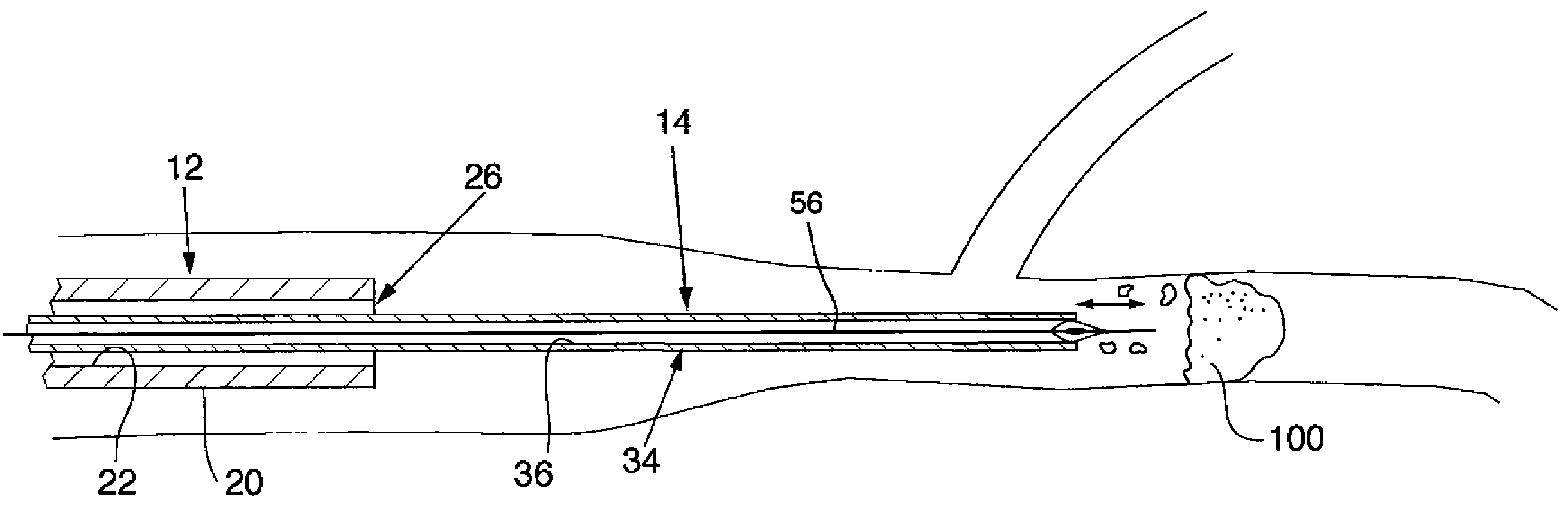
Vascular catheter with expanded distal tip for receiving a thromboembolic protection device and method of use
A vascular catheter including a radially expandable segment, such as an inflatable balloon, and an expanded distal tip having an increased storage volume is disclosed. The inflatable balloon segment of the catheter may be used to provide traditional balloon angioplasty to a portion of a blood vessel narrowed by stenosis, and the expanded distal tip of the catheter apparatus may be used to safely capture, store, and remove a thromboembolic protection device such as an embolic filter used to catch pieces of plaque and other embolic material dislodged during the balloon angioplasty procedure. The catheter of the present invention provides an effective means for dilating a narrowed portion of a blood vessel, as well as preventing the need for deploying a second catheter system to capture and retrieve the embolic filter. The present invention also greatly reduces the chance for plaque and other thromboembolic material to escape from the embolic filter and enter the patient's bloodstream.
Owner:WHOLEY MARK H +2
Devices and methods for treating vascular malformations
InactiveUS20110137332A1Ensure isolationProtect the neckStentsBalloon catheterVascular malformationEmbolization material
A device for treating vascular malformations includes a primary coil and secondary windings. The primary coil provides resilience and structural integrity while the secondary windings fill interstitial spaces in the primary coil to isolate the vascular malformation. The device may have increased density along a central portion to isolate the malformation. In another aspect, the device may have a central opening through which embolic materials may be delivered.
Owner:STRYKER CORP
Embolic occlusion of uterine arteries
InactiveUS20040202694A1Decreased blood flowSlow and stop flowSurgical adhesivesFemale contraceptivesWater basedUterine Disorder
A treatment procedure is disclosed which involves the short term, non-permanent occlusion of the patient's blood vessels by depositing a bioabsorbable embolic mass within the patient's blood vessel. The procedure is particularly suitable for treating uterine disorders by occluding a patient's uterine arteries. A therapeutically effective time period for occlusion of a uterine artery is from about 0.5 to about 48 hours, preferably about 1 to about 24 hours, with occlusion times of about 1 to about 8 hours being suitable in many instances. The embolic mass may bioabsorbable particulate with minimum transverse dimensions of about 100 to about 2000 micrometers, preferably about 300 to about 1000 micrometers. The particulate may be a polymeric material formed of polylactic acid, polyglycolic acid or copolymers thereof, or a swellable copolymer of lactic acid and polyethylene glycol. The embolic material may be delivered to an intracorporeal site as a biocompatible solution containing a solute which is relatively insoluble in a water based fluid and a solvent which is relatively soluble in the water based fluid, where the solute forms the embolic mass which occludes or partially occludes a body lumen or fills or partially fills a body cavity.
Owner:VASCULAR CONTROL SYST
Vessel embolic material comprising hydrogel and therapy with the use thereof
InactiveUS7476648B1Use of materialPeptide/protein ingredientsTissue regenerationVascular diseaseBiological body
According to the present invention, a vessel embolic material comprising a hydrogel containing a cell growth factor, which is applicable to embolic operations for various vascular disorders, excellent in high adaptability to a living body, can be easily handled, ensures the occlusion of the affected part and can maintain the occlusion for a long time, and a method of treatment using the same can be provided.
Owner:KAKEN PHARMA CO LTD +2
Occlusion device
A vascular occlusion device for occluding a body cavity includes an elongate member with a first lumen and a second lumen. An inflatable balloon is disposed about a distal end of the elongate member, and is inflated with inflating fluid introduced into the interior of the balloon by way of the first lumen. The device also includes a pressure regulation system that determines the pressure of embolization material being injected from the second lumen into the body cavity to occlude the body cavity.
Owner:COOK MEDICAL TECH LLC
Embolism material composition as well as preparation method and use thereof
ActiveCN103550834AGood biocompatibilityEasy to checkSurgeryX-ray constrast preparationsBiocompatibility TestingX ray image
The invention provides an embolism material composition as well as preparation method and use thereof, the embolism material composition is prepared from reactant raw materials, wherein the reactant raw materials comprise a biocompatibility material, a roentgenopaque substance and a magnetic resonance imaging substance, the roentgenopaque substance and the magnetic resonance imaging (MRI) substance are covered by the biocompatibility material. The embolism material composition not only can be directly detected by an X-ray image device, but also can be directly detected by the MRI, so that a doctor can select a detection method according to self condition of a patient and the medical device condition.
Owner:HYGEA MEDICAL TECH CO LTD
Aortic occluder with associated filter and methods of use during cardiac surgery
InactiveUS7306575B2Efficient fermentationEliminating and minimizing embolizationStentsBalloon catheterFiltrationArterial cannula
A balloon arterial cannula and methods for filtering blood. The devices generally include a mesh for filtering blood flowing within a blood vessel, particularly within an artery such as the aorta, a structure adapted to open and close the mesh within the blood vessel, a means to actuate the structure, and a balloon occluder which typically includes a flexible material enclosing a chamber. The methods generally include the steps of introducing a mesh into a blood vessel to capture embolic material, adjusting the mesh, if necessary, during the course of filtration, inflating the balloon occluder to occlude the vessel upstream of the mesh, and thereafter deflating the balloon occluder and removing the mesh and the captured foreign matter from the blood vessel. Additionally, visualization techniques are used to ensure effective filtration.
Owner:EDWARDS LIFESCIENCES CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com