Derivative of pyridine and (4,3-d) pyrimidine-1(2H)-base phenyl acetamide and application thereof
A technology of phenylacetamide and derivatives, applied in the field of derivatives of compounds, can solve problems such as poor metabolism, small toxic and side effects, and achieve the effects of less side effects, small dosage, and elimination of bad metabolism problems.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
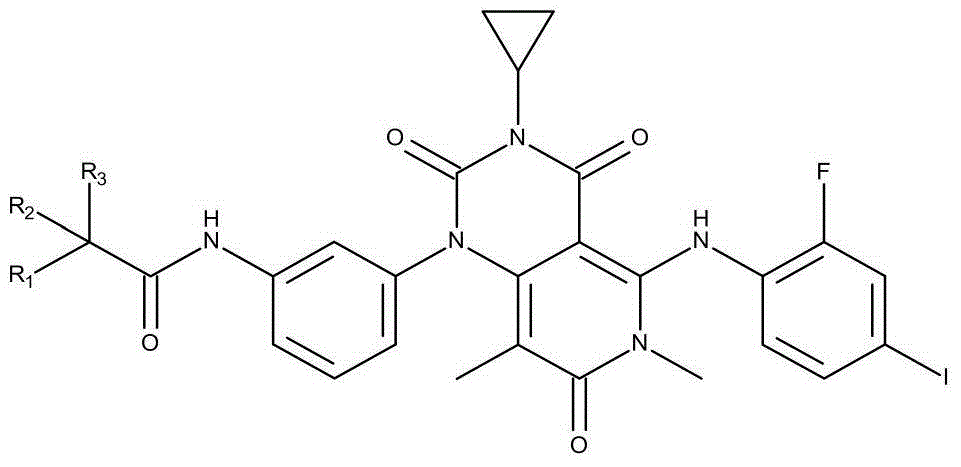
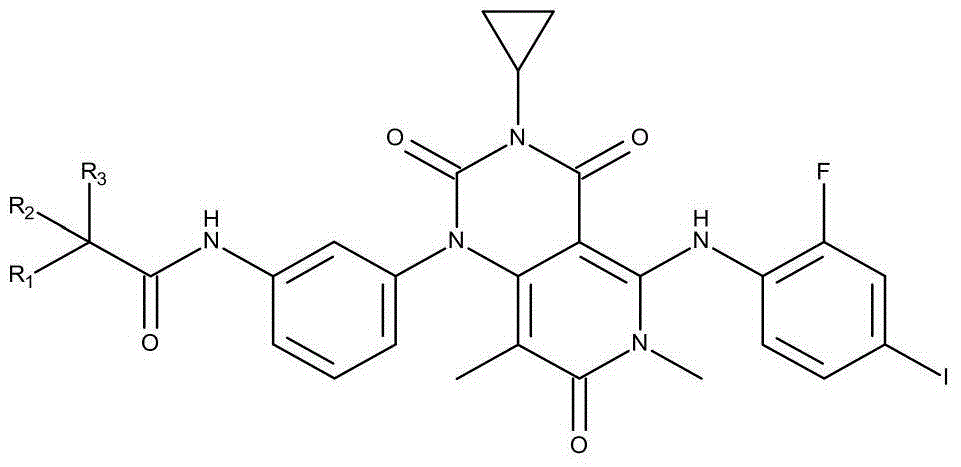
[0021] Preparation of N-[3-[3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-3,4,6,7-tetrahydro-6,8-dimethyl-2 ,4,7-Trioxo-pyrido[4,3-d]pyrimidin-1(2H)-yl}phenyl)acetamide-1,1,1-d3.
[0022] The synthesis of derivatives described in the present invention comprises reduction and acetylation 2 steps:
[0023] The preparation steps are as follows:
[0024] (1) Reduction steps
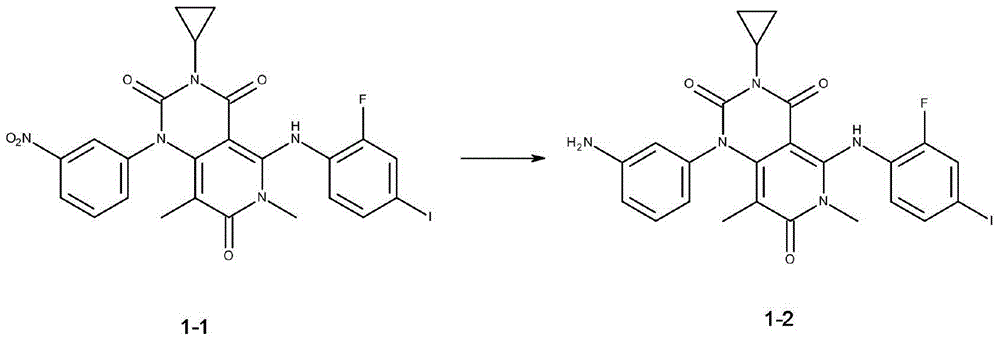
[0025] Preparation of [3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-6,8-dimethyl-1-(3-aminophenyl)-pyrido[4,3- d] pyrimidine-2,4,7(1H,3H,6H)-trione 1-2, the reaction equation is as follows:
[0026]
[0027] Under anhydrous, oxygen-free and argon protection, 3.78 grams of [3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-6,8- Dimethyl-1-(3-nitrophenyl)-pyrido[4,3-d]pyrimidine-2,4,7(1H,3H,6H)-trione 1-1 and 40 ml of dichloromethane . 3.27 grams of sodium bisulfite and 2 milliliters of water were added under stirring at room temperature, and the reaction was stirred for 3-5 hours at a reaction tempera...
Embodiment 2
[0033] Preparation of N-[3-[3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-3,4,6,7-tetrahydro-6,8-dimethyl-2 , 4,7-trioxo-pyrido[4,3-d]pyrimidin-1(2H)-yl}phenyl)acetamide-1-d1.
[0034]
[0035] The preparation method is the same as in Example 1, and the acetic acid-d3 anhydride in the embodiment 1 is replaced by acetic acid-d2 anhydride.
Embodiment 3
[0037] Preparation of N-[3-[3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-3,4,6,7-tetrahydro-6,8-dimethyl-2 ,4,7-Trioxo-pyrido[4,3-d]pyrimidin-1(2H)-yl}phenyl)acetamide-1,1-d2.
[0038]
[0039] The preparation method is the same as in Example 1, and the acetic acid-d3 anhydride in the embodiment 1 is replaced by acetic acid-d1 anhydride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com