Synthesis of anti-hepatitis B medicine LQC-X and application thereof
A technology of LQC-X1 and reaction, which is applied in the fields of chemistry and biological sciences, and can solve problems such as high price, virus rebound, and large side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1L
[0039] Synthesis of Preparation Example 1LQC-X1
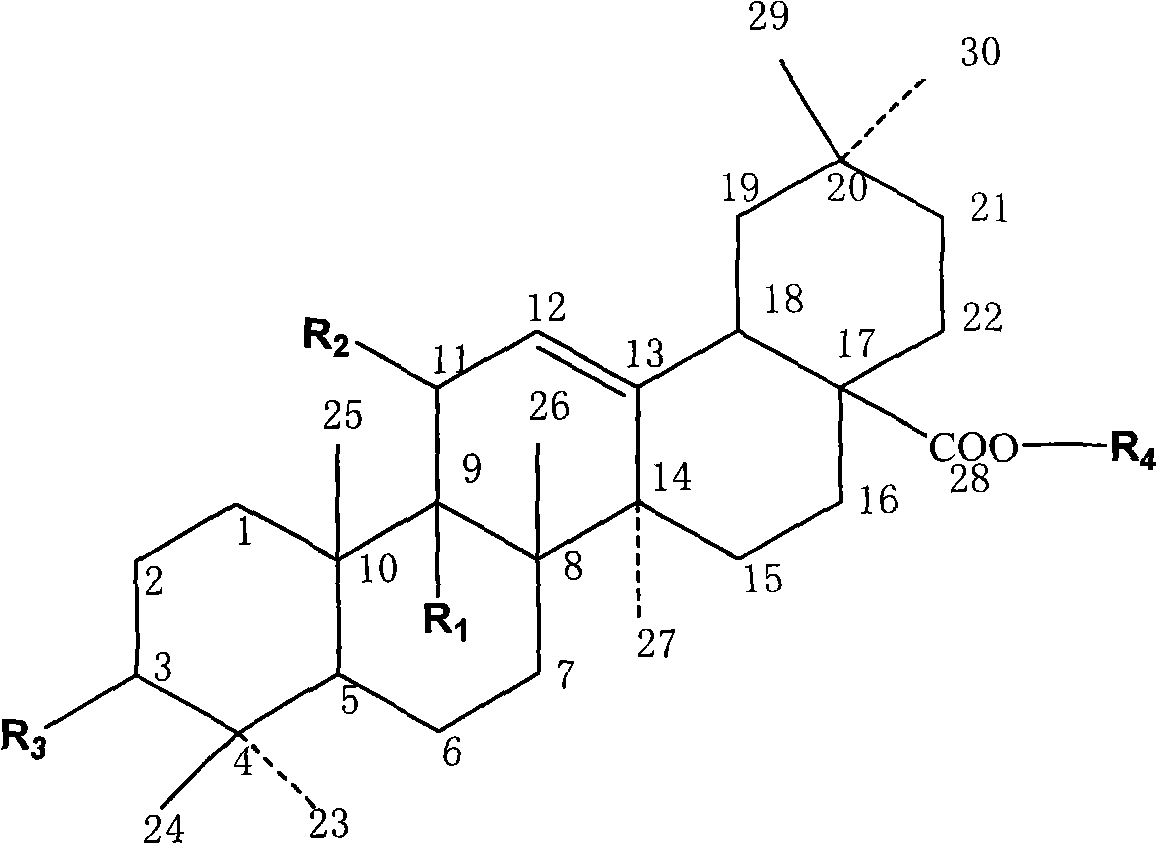
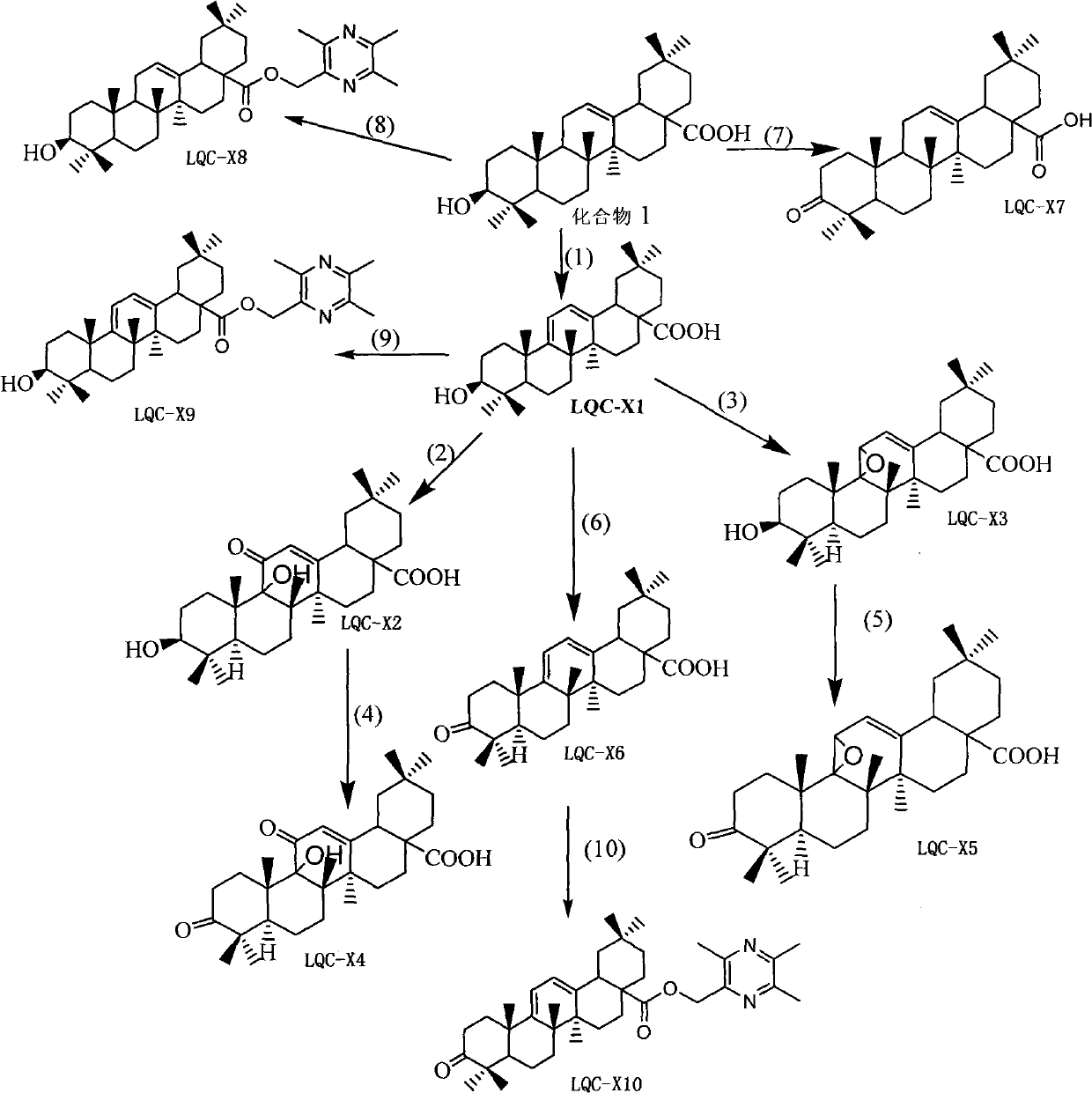
[0040] Put 9.12g (0.02mol) of oleanolic acid, 400ml carbon tetrachloride, and 4.27g N-bromosuccinimide (about 0.024mol) in a 500ml reaction bottle, light, magnetically stir and heat to reflux at 74°C for 4 Hours, reacted to no raw material, and the reaction ended. Recover the reaction solution to dryness, dissolve the recovered solid with 200ml of ethyl acetate, filter it while it is hot, and place it at room temperature for 24 hours to crystallize to obtain a large amount of coarse crystals, recrystallize once with ethyl acetate to obtain LQC-X1, white flakes 7.13 g of crystals were obtained, and the reaction yield was 78.5%. m.p.276.7-278.4°C.
[0041] LQC-X1 related spectral data:
[0042] ESI-MS: [M-1] - 453
[0043] 1 H-NMR (500MHZ, CDCl 3 ): 083, 0.93, 0.97, 1.00, 1.05, 1.06, 1.20 (3H, each, s, 7×CH 3 ) 2.99 (m, 1H, 3-OH), 3.28 (m, 1H, H-3), 5.58 (d, 1H, J=5.5HZ, H-12), 5.62 (d, 1H, J=5.5HZ, H-11).1.000~2.500 (24...
preparation Embodiment 2L
[0045]Synthesis of Preparation Example 2LQC-X2
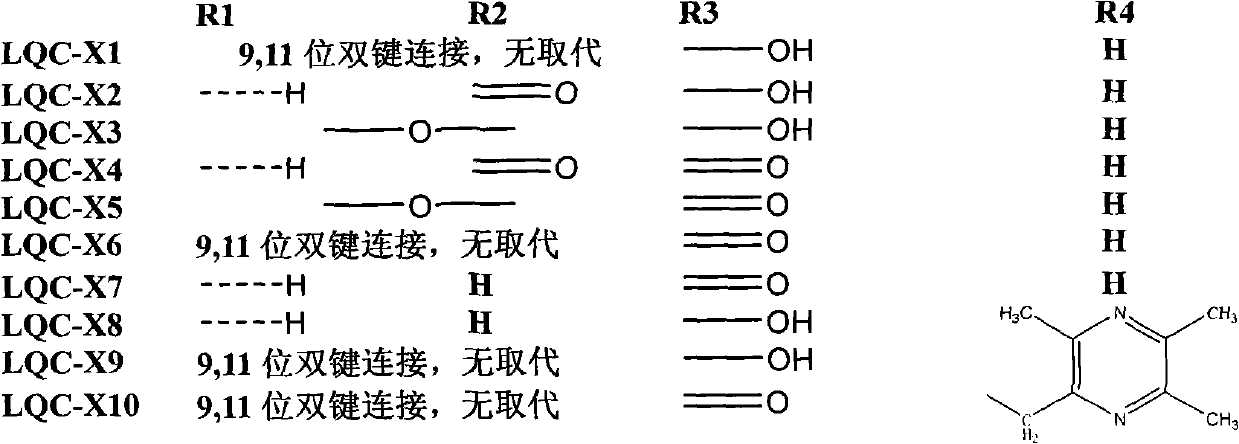
[0046] Weigh about 4.54g (0.01mol) of LQC-X1, dissolve it in 500ml of dichloromethane, add 200mg of Eosiy (alcohol solution), ventilate, and react with light for 10h. After the reaction, wash twice with 3 times the amount of saturated sodium bicarbonate solution, wash with water until neutral, remove water with anhydrous sodium sulfate, recover dichloromethane, pass through a silica gel column, and elute with petroleum ether: ethyl acetate = 5:1 . LQC-X2 was obtained, 2.05 g in total, and the reaction yield was 42.0%.
[0047] LQC-X2 related spectral data:
[0048] ESI-MS[M-1] - 487
[0049] 1 H-NMR (500MHZ, CDCl 3 ): 0.86, 0.98, 0.99, 1.02, 1.08, 1.28, 1.27 (3H, each, s, 7×CH 3 ), 3.01 (1H, m, H-3), 3.25 (1H, m, 3-OH), 6.02 (1H, s, H-12), 1.000~2.500 (24H, methylene in the three-layer core structure base and methine hydrogen signals);
[0050] 13 C-NMR (500MHZ, CDCl 3 ): 36.7(C-1), 27.2(C-2), 77.9(C-3), 40.5(C-4), 50...
preparation Embodiment 3L
[0051] Synthesis of Preparation Example 3LQC-X3
[0052] Weigh about 4.54g (0.01mol) of LQC-X1, dissolve it in 500ml of dichloromethane, add 200mg of Eosiy (alcohol solution), ventilate, and react with light for 10h. After the reaction, wash twice with 3 times the amount of saturated sodium bicarbonate solution, wash with water until neutral, remove water with anhydrous sodium sulfate, recover dichloromethane, pass through a silica gel column, and elute with petroleum ether: ethyl acetate = 5:1 . The LQC-X3 reaction yield was about 10%.
[0053] LQC-X3 related spectral data:
[0054] 1 H-NMR (500MHZ, CDCl 3 ): 0.83, 0.96, 0.99, 1.03, 1.05, 1.20, 1.21 (3H, each, s, 7×CH 3 ), 3.22(1H, m, H-3), 4.33(1H, d, J=2.5HZ, H-11), 5.38(1H, d, J=2.5HZ, H-12), 1.000~2.500(24H , the methylene and methine hydrogen signals in the triple core structure);
[0055] 13 C-NMR (500MHZ, CDCl 3 ): 37.0(C-1), 27.2(C-2), 78.4(C-3), 39.3(C-4), 50.8(C-5), 17.6(C-6), 34.2(C-7) , 37.7(C-8), 78.9(C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com