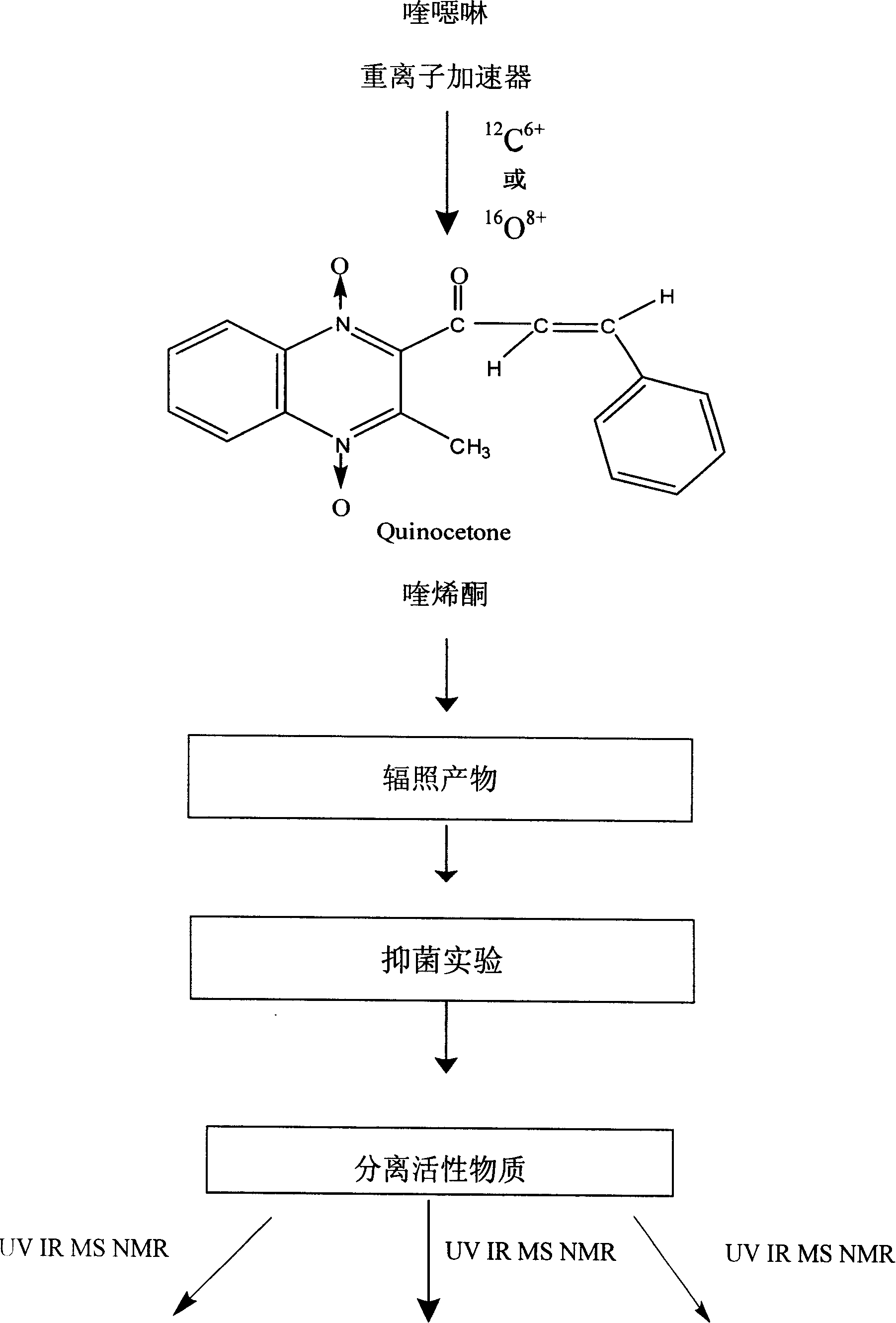
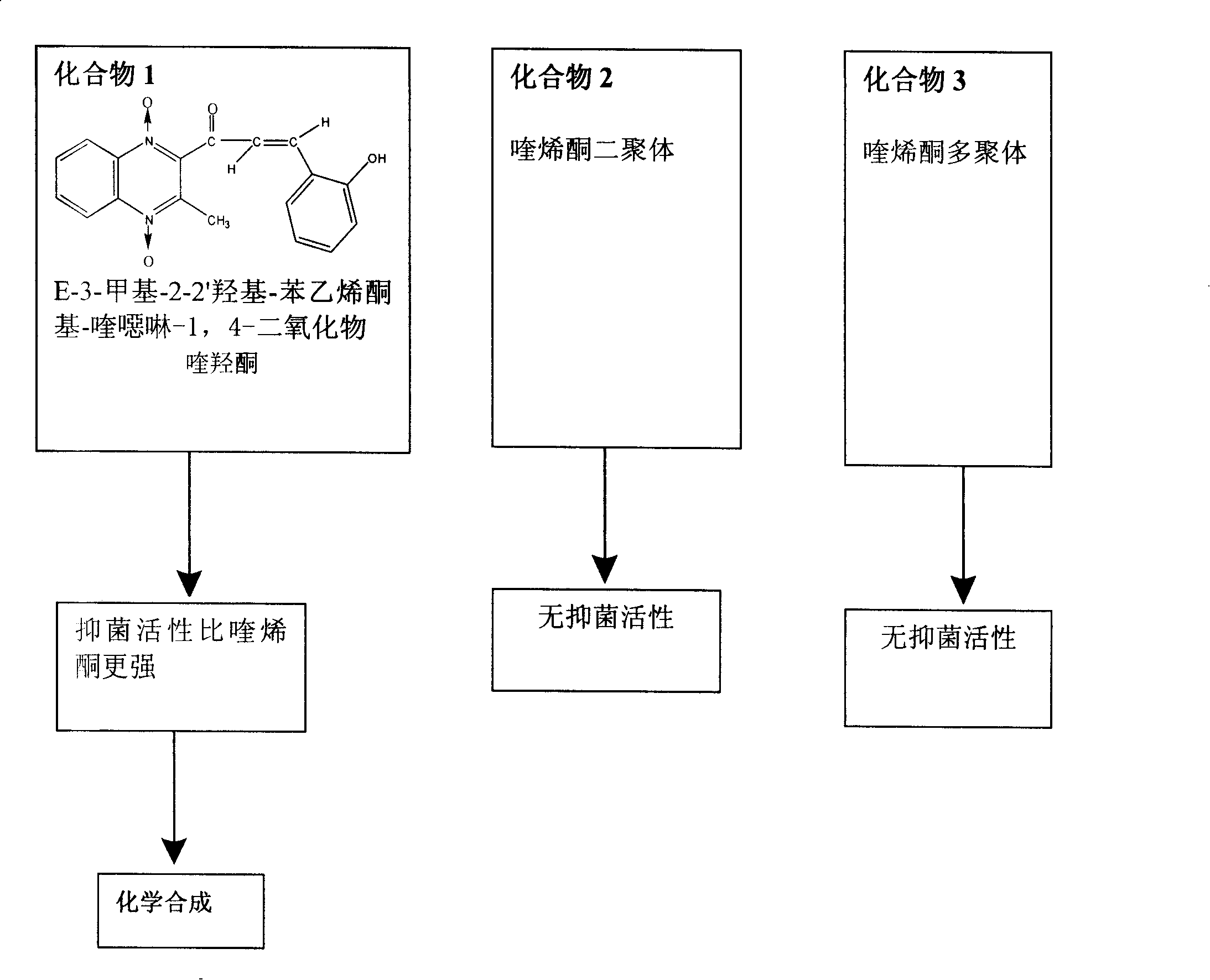
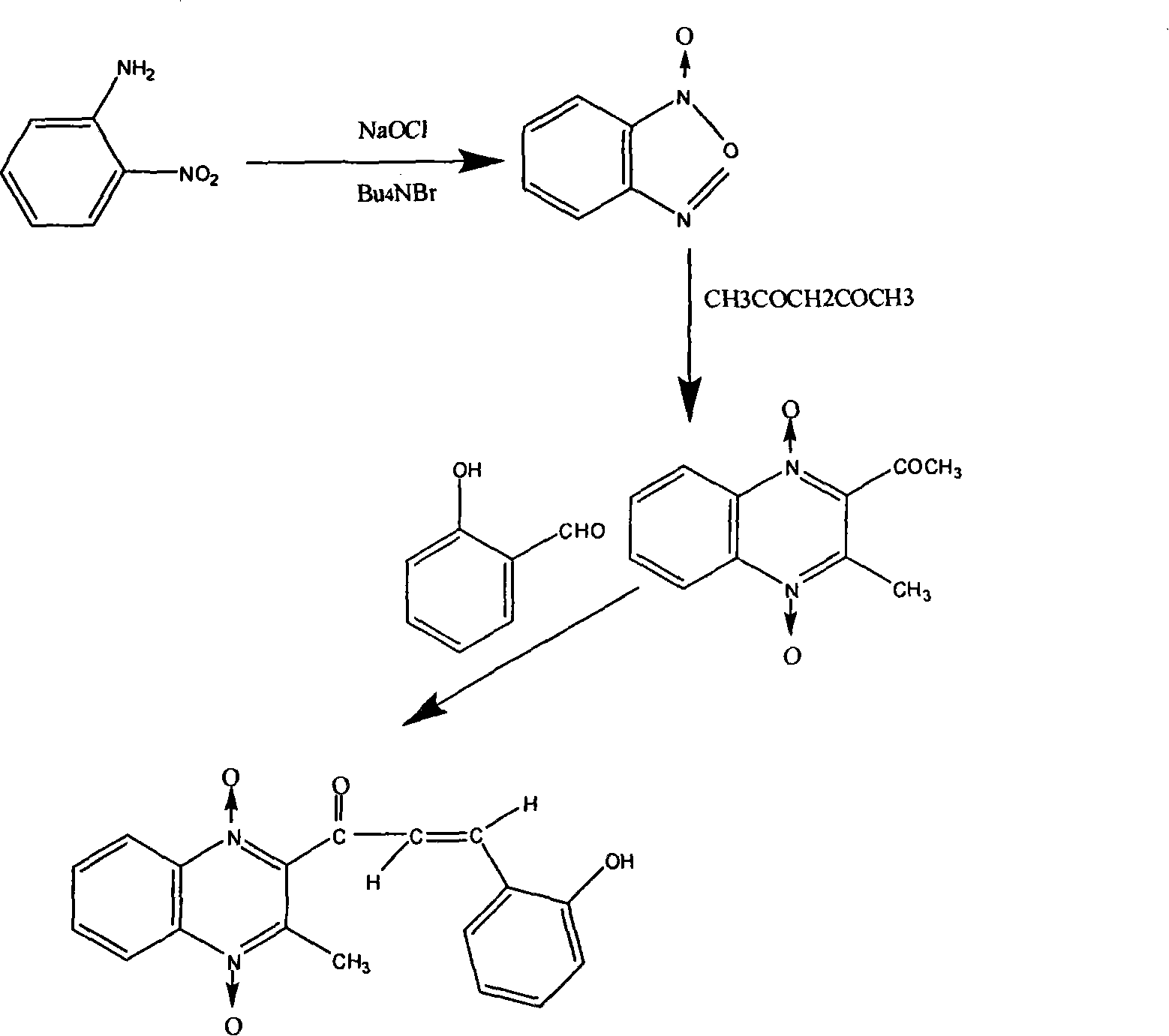
Chemical synthesis technique of quinoxaline
A chemical synthesis, quinolone technology, applied in the direction of organic chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0083] The implementation process of three kinds of chemical synthesis techniques of quinolone is described as follows now:
[0084] The synthetic technique of the first kind of operational route:
[0085] (1) Dissolve 18.3g (0.133mol) of o-nitroaniline in 70g of methanol, add 25.3g (0.266mol) of 50% aqueous sodium hydroxide solution, continue stirring at 0-10°C, and slowly add 127g ( 0.185mol) sodium hypochlorite aqueous solution (the available chlorine content is 5.2%), the dropwise addition is completed in about one hour, then continue stirring at 010° C. for 1 hour, and the water phase is separated. The methanol solution of benzofurazan-1-oxide was obtained, washed with water, and filtered to obtain 17.4 g of benzofurazan-1-oxide.
[0086] (2) Dissolve 16.2g of benzofurazan-1-oxide and 13.5g of acetylacetone in 30ml of triethylamine, let the solution stand at room temperature for 18 hours, collect the precipitate, and dry it to obtain 16g The 2-acetyl-2-methylquinoxaline...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com