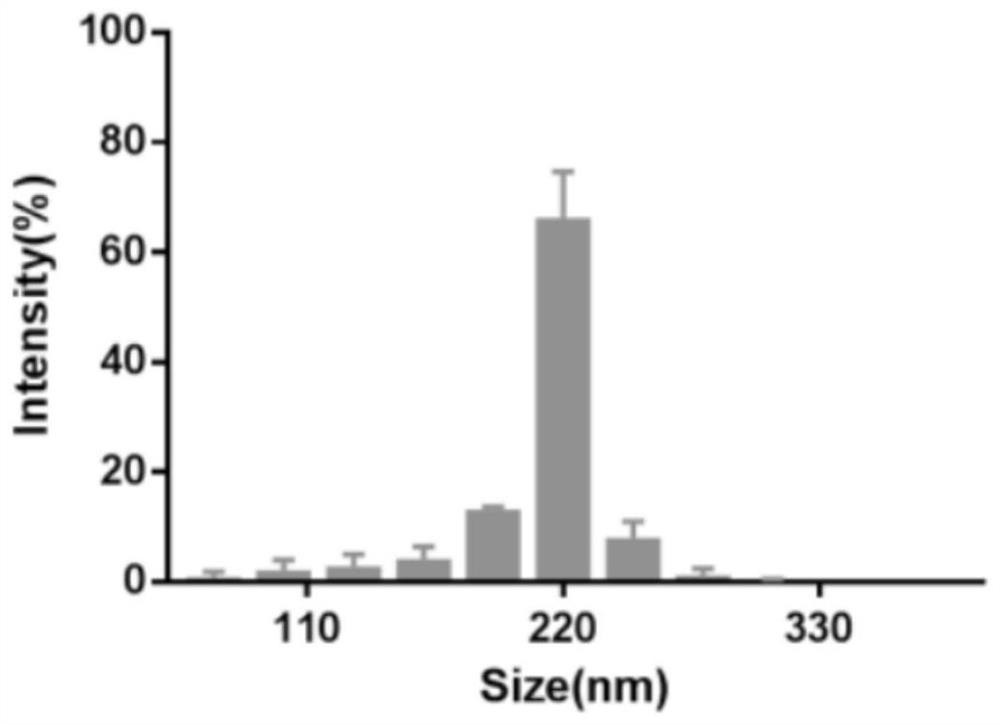
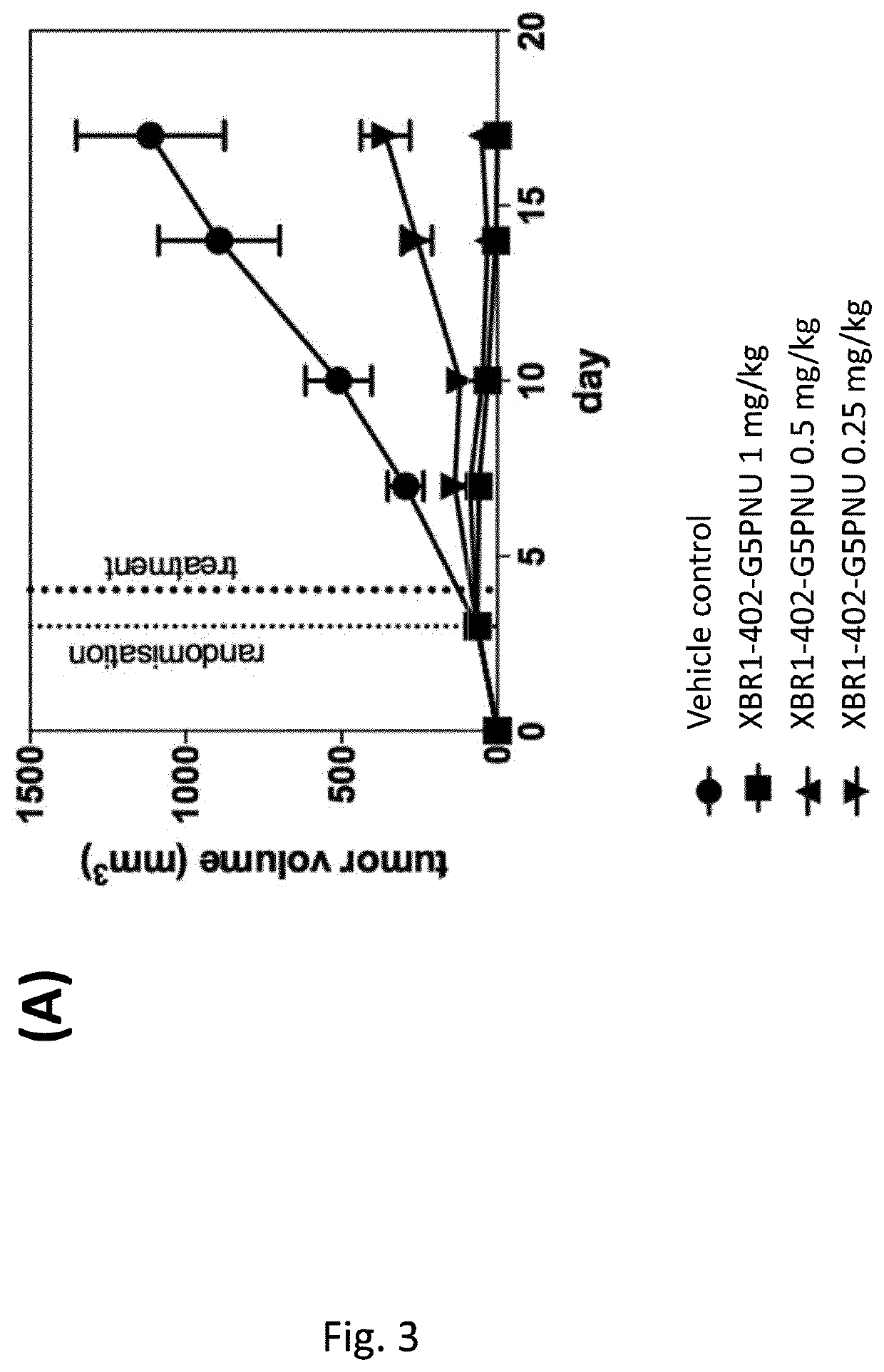
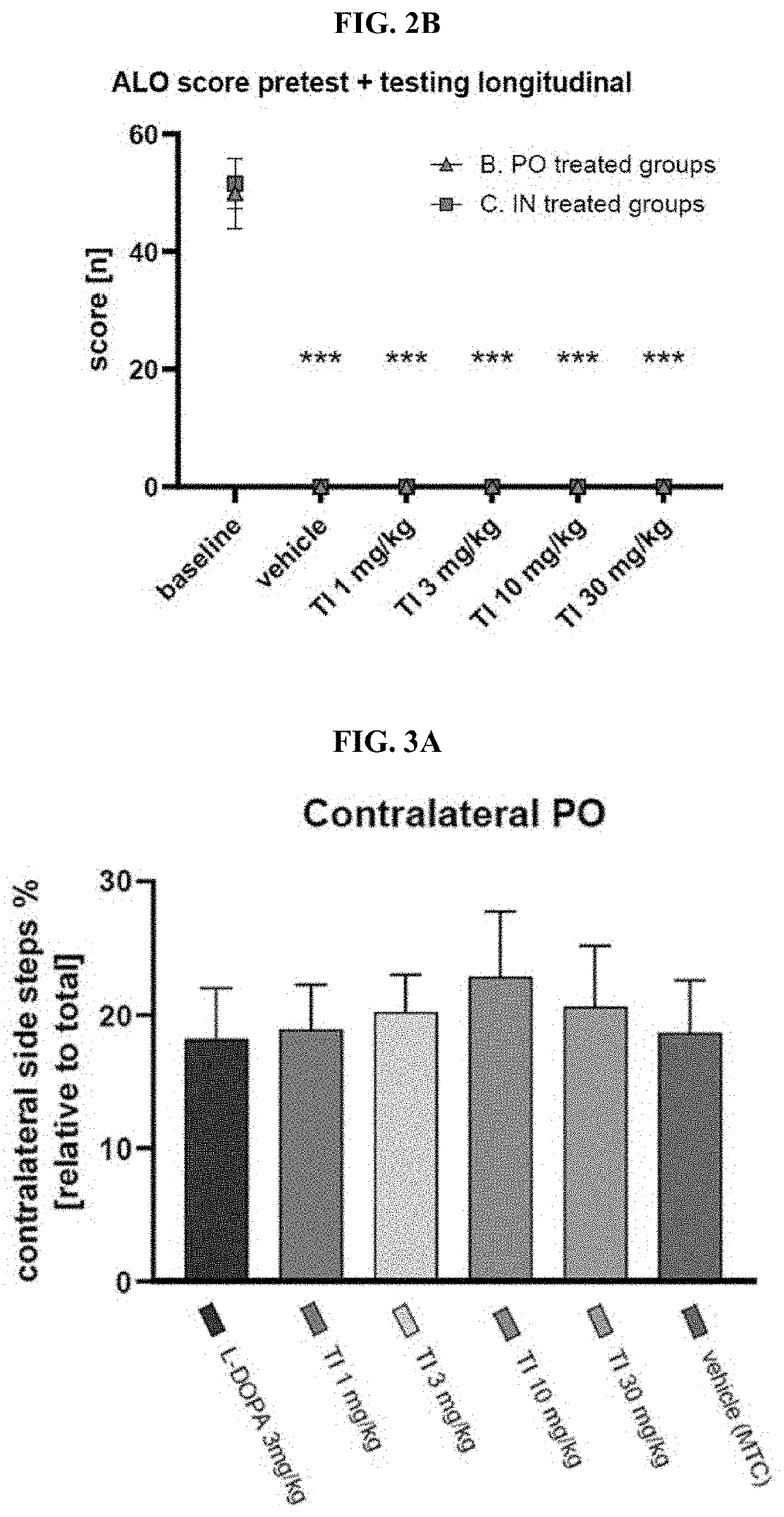
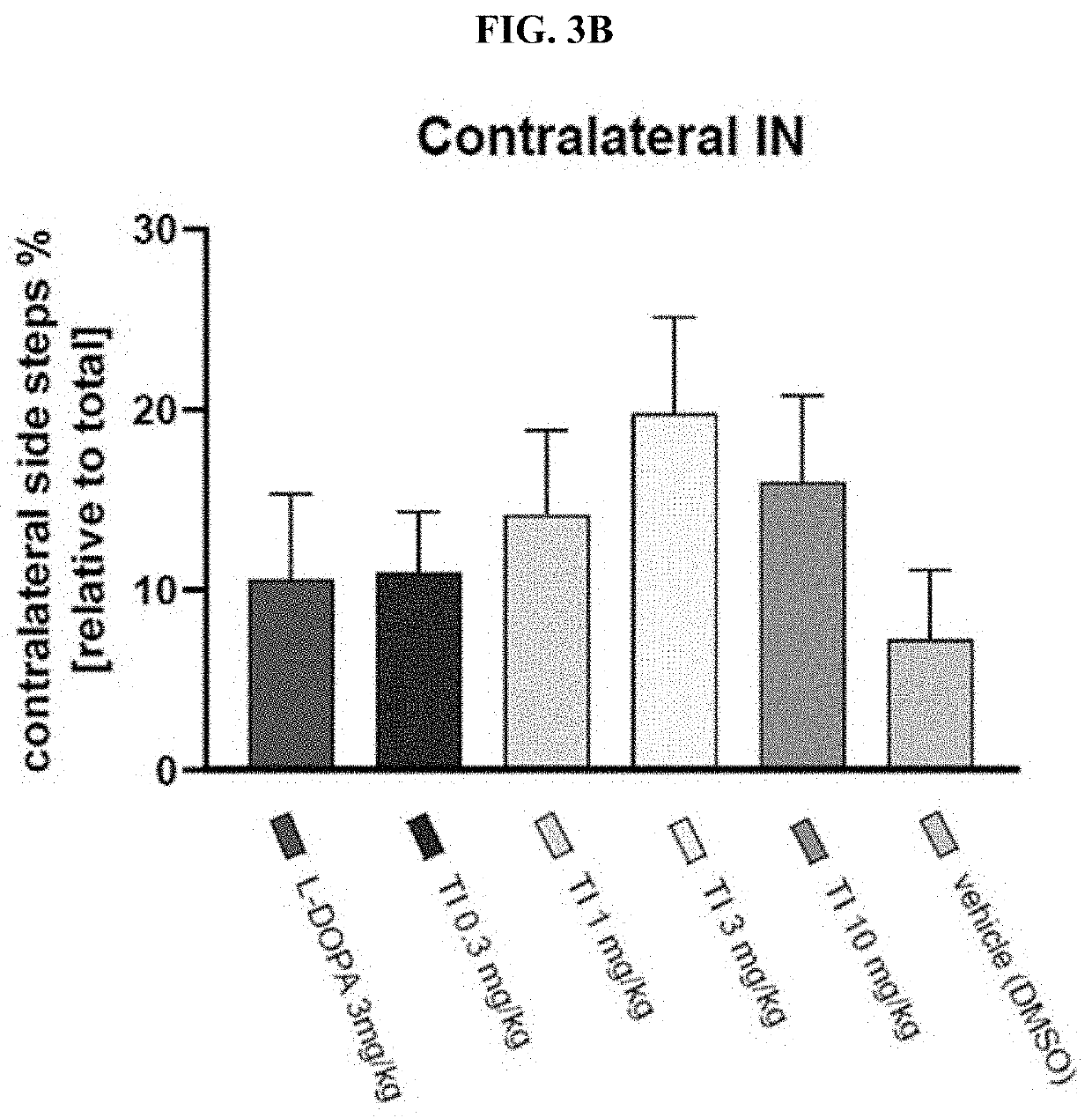
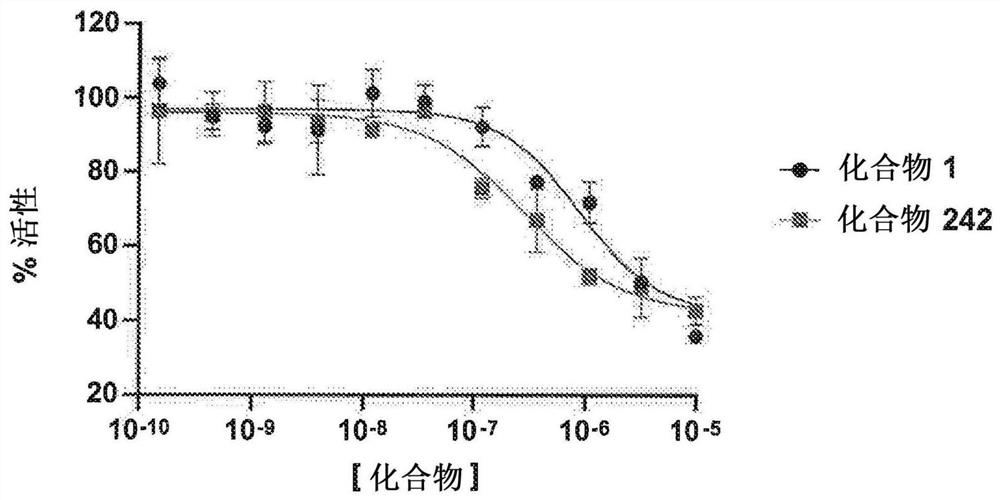
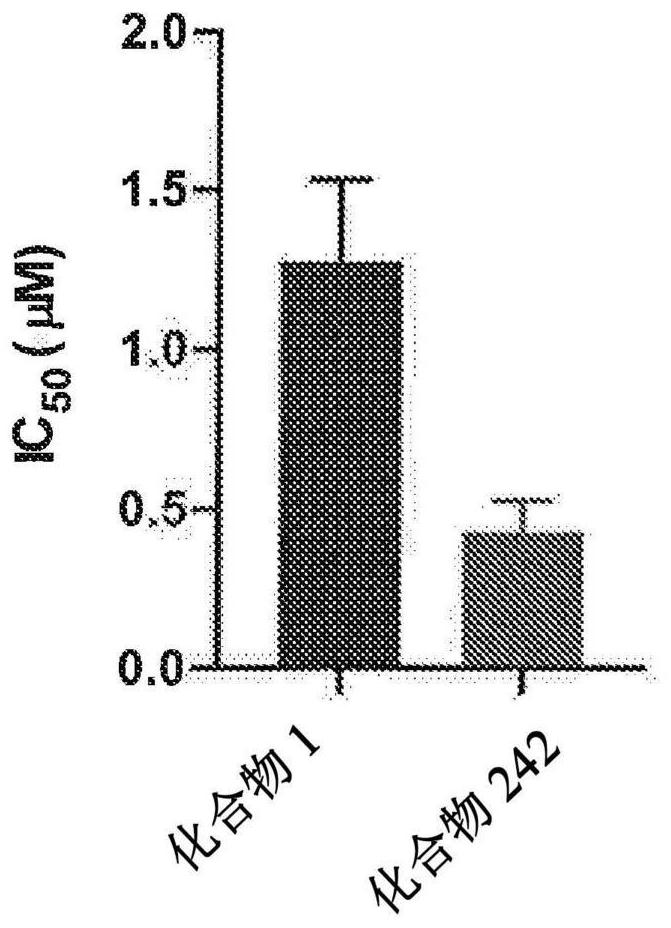
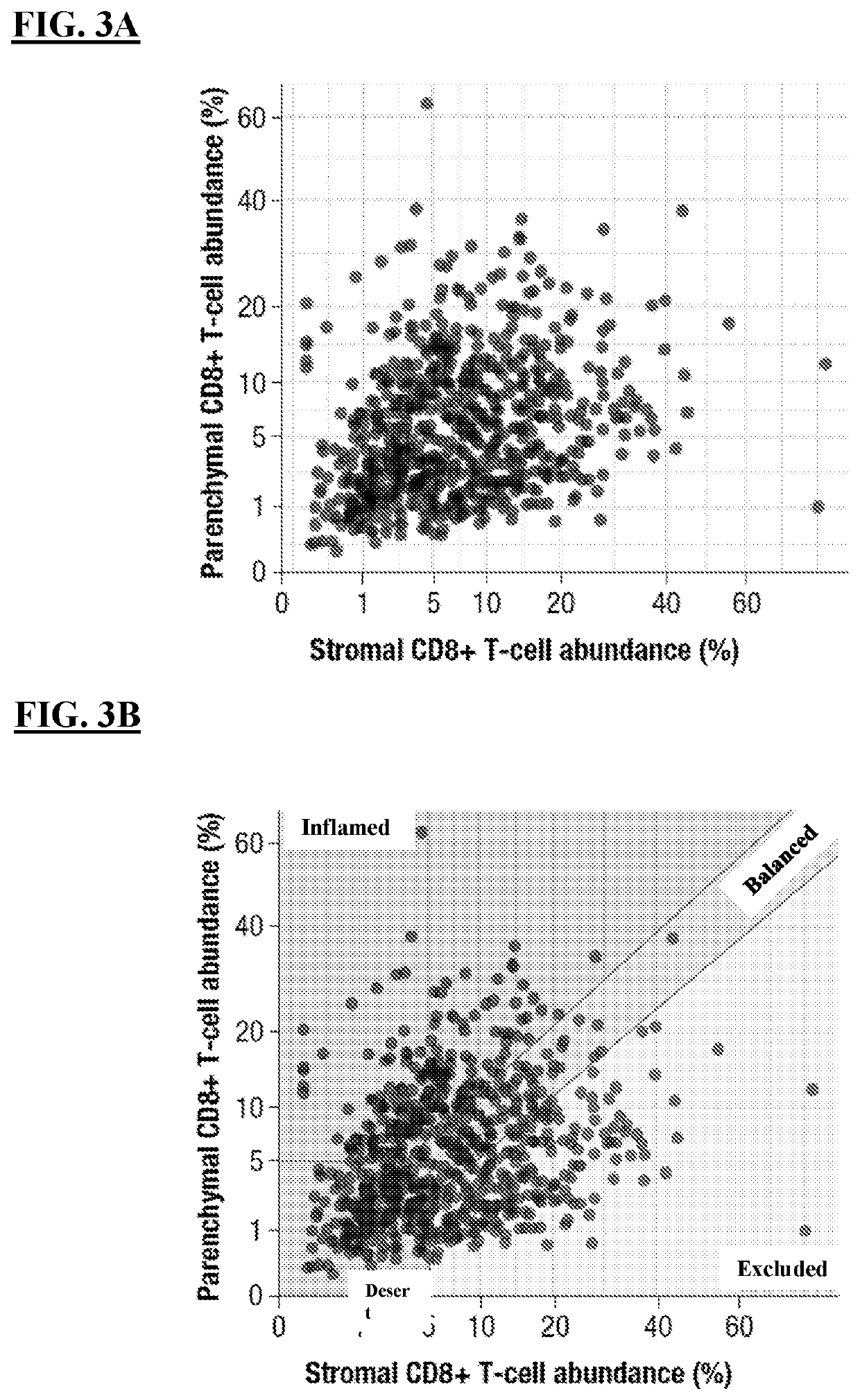
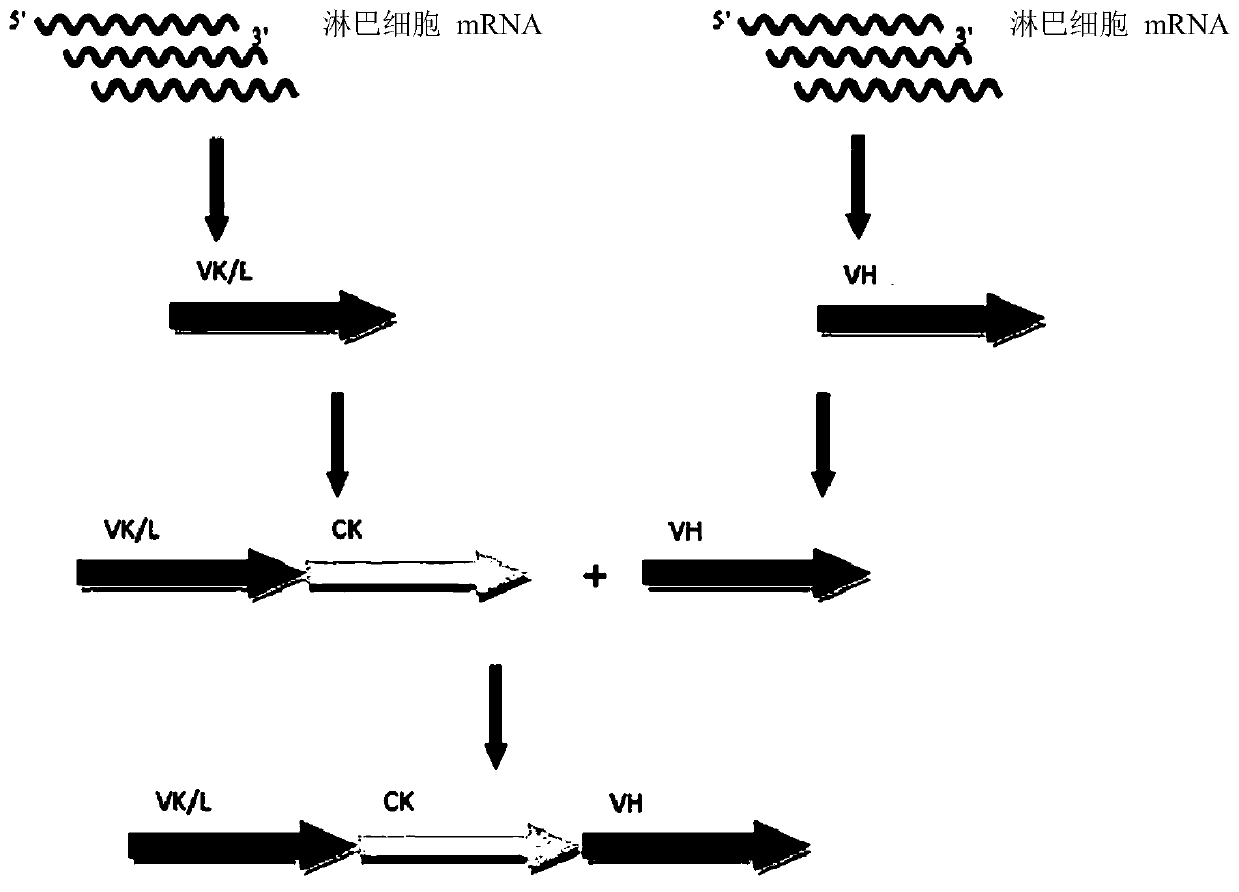
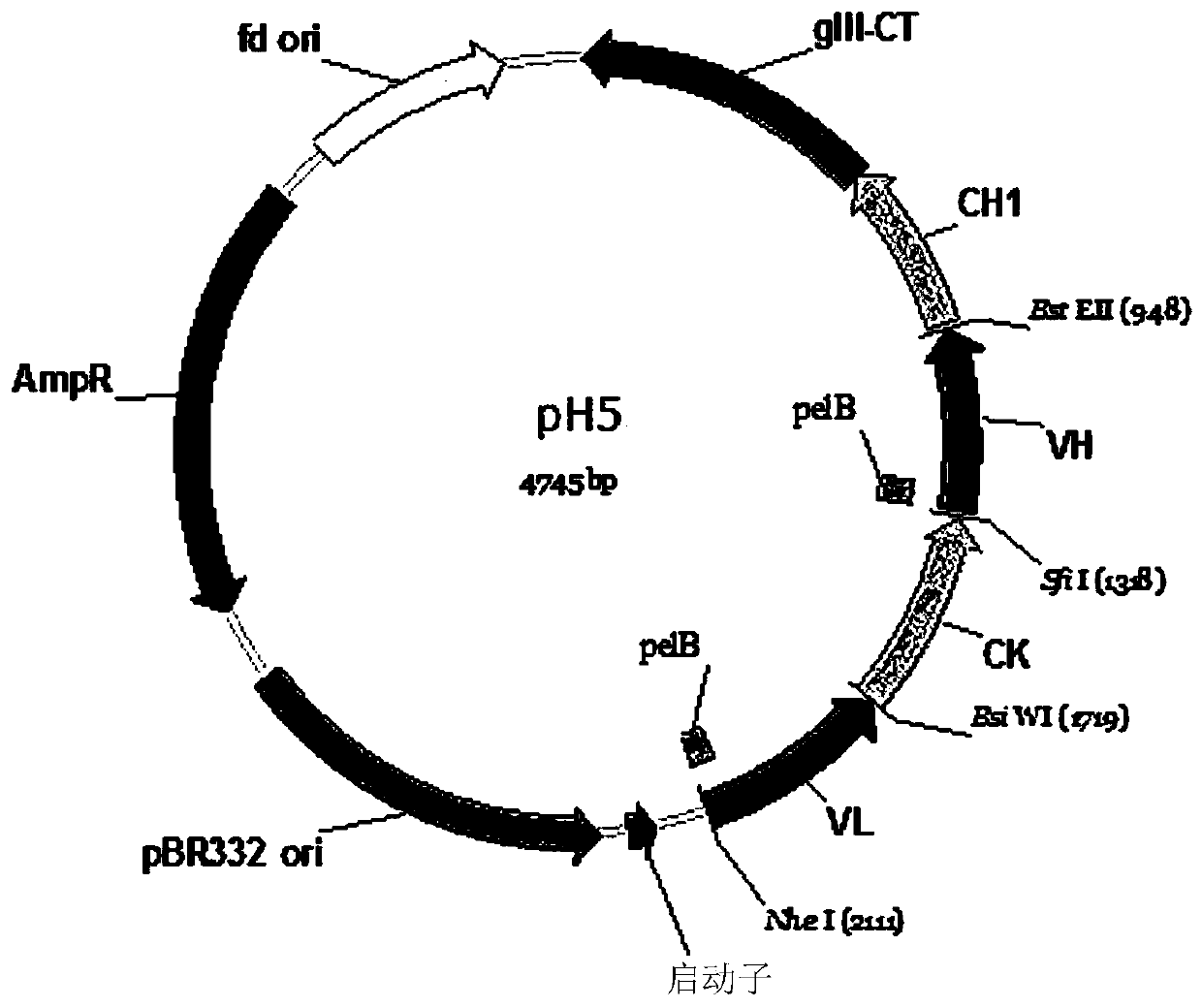
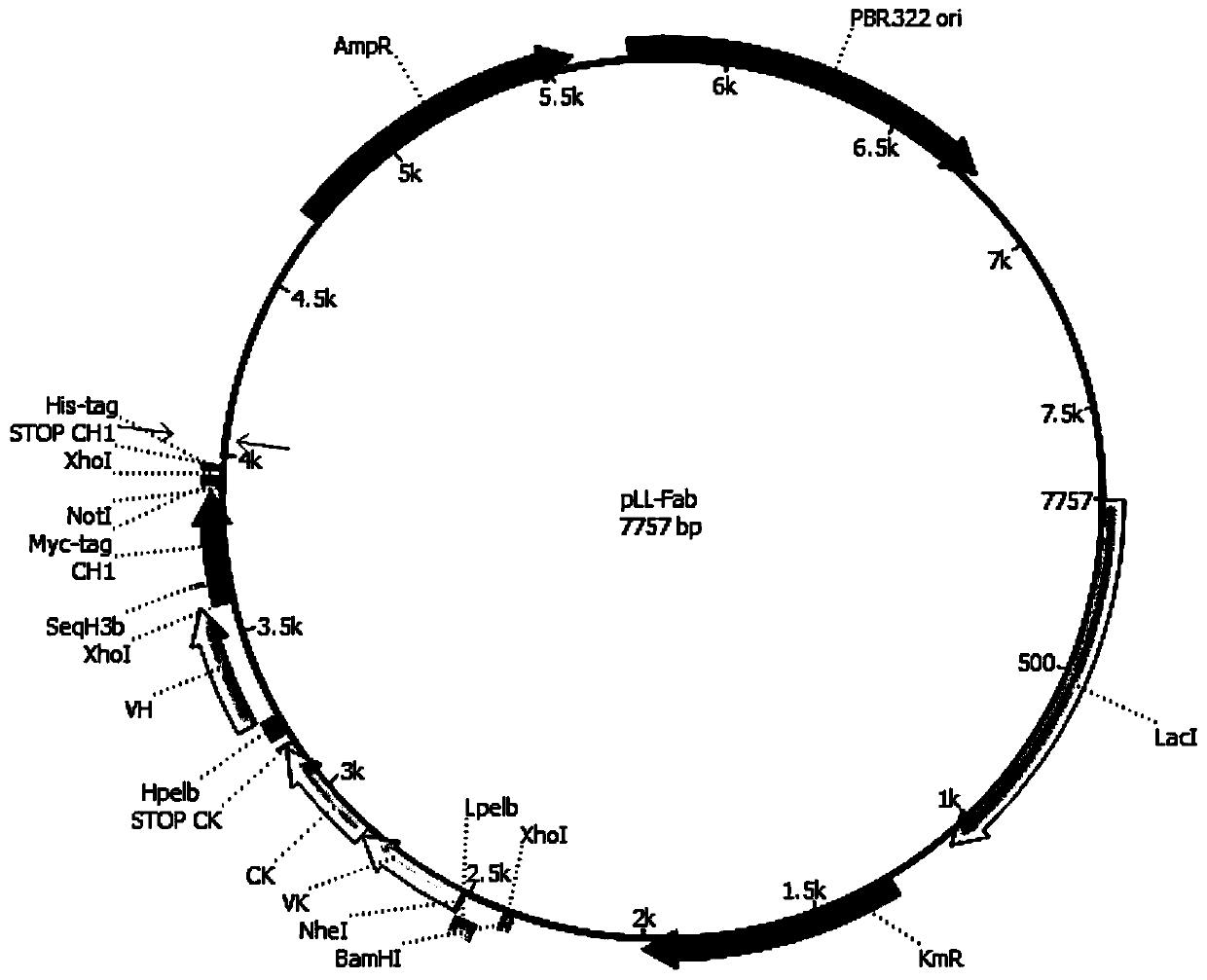
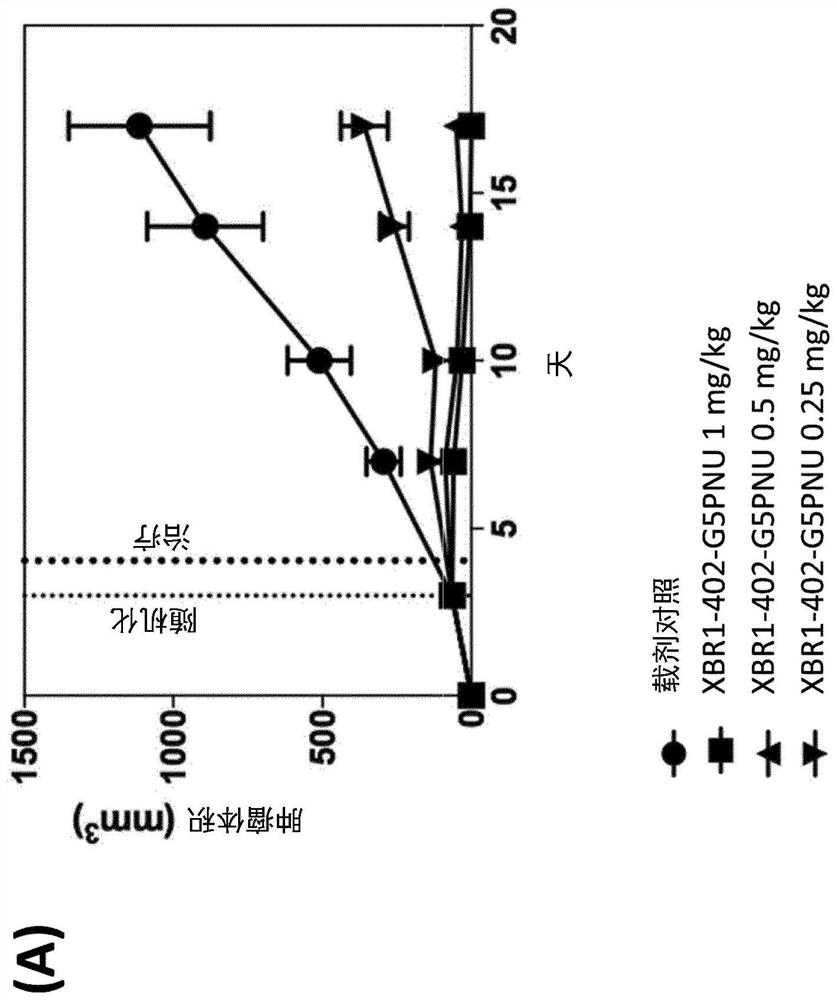
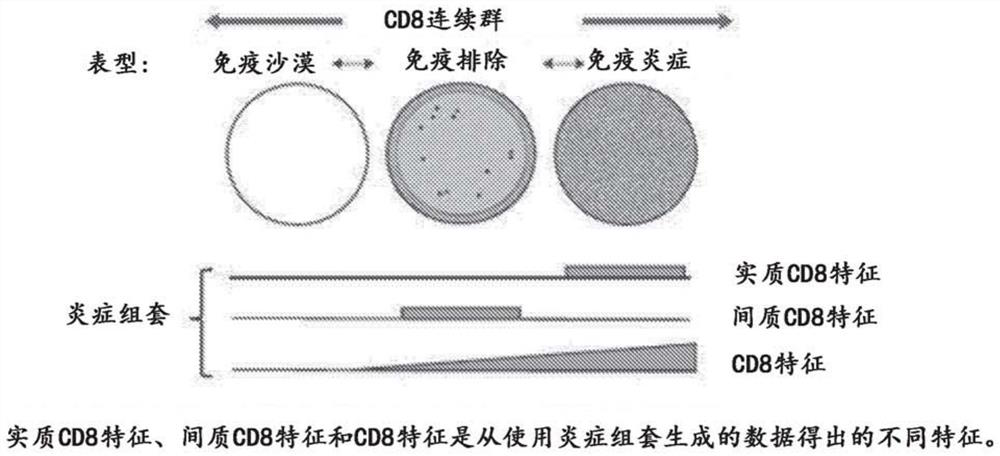
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Immunooncology" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immuno-Oncology is the practice and study of employing immune-based therapies to treat cancer. Several drugs are currently being developed by companies and undergoing clinical trials within this new category. Understanding how cancer evades the immune system is the foundation of immuno-oncology. The body’s immune system to give an immune response against cancer is immuno-oncology's primary focus.
Method of Treatment of Neuroendocrine Tumors That Over-Express Somatostatatin Receptors
InactiveUS20180185524A1Reducing tumorigenicityPrevent proliferationRadioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsImmunooncologySomatostatin receptor
The present invention relates to methods of treating cancers that over-express somatostatin receptors. More specifically, the invention provides a combined therapy in which a combination of Peptide Receptor Radionuclide Therapy (PRRT) and Immuno Oncology therapy (I-O therapy) are administered for the treatment of neuroendocrine tumors.
Owner:ADVANCED ACCELERATOR APPLICATIONS SA
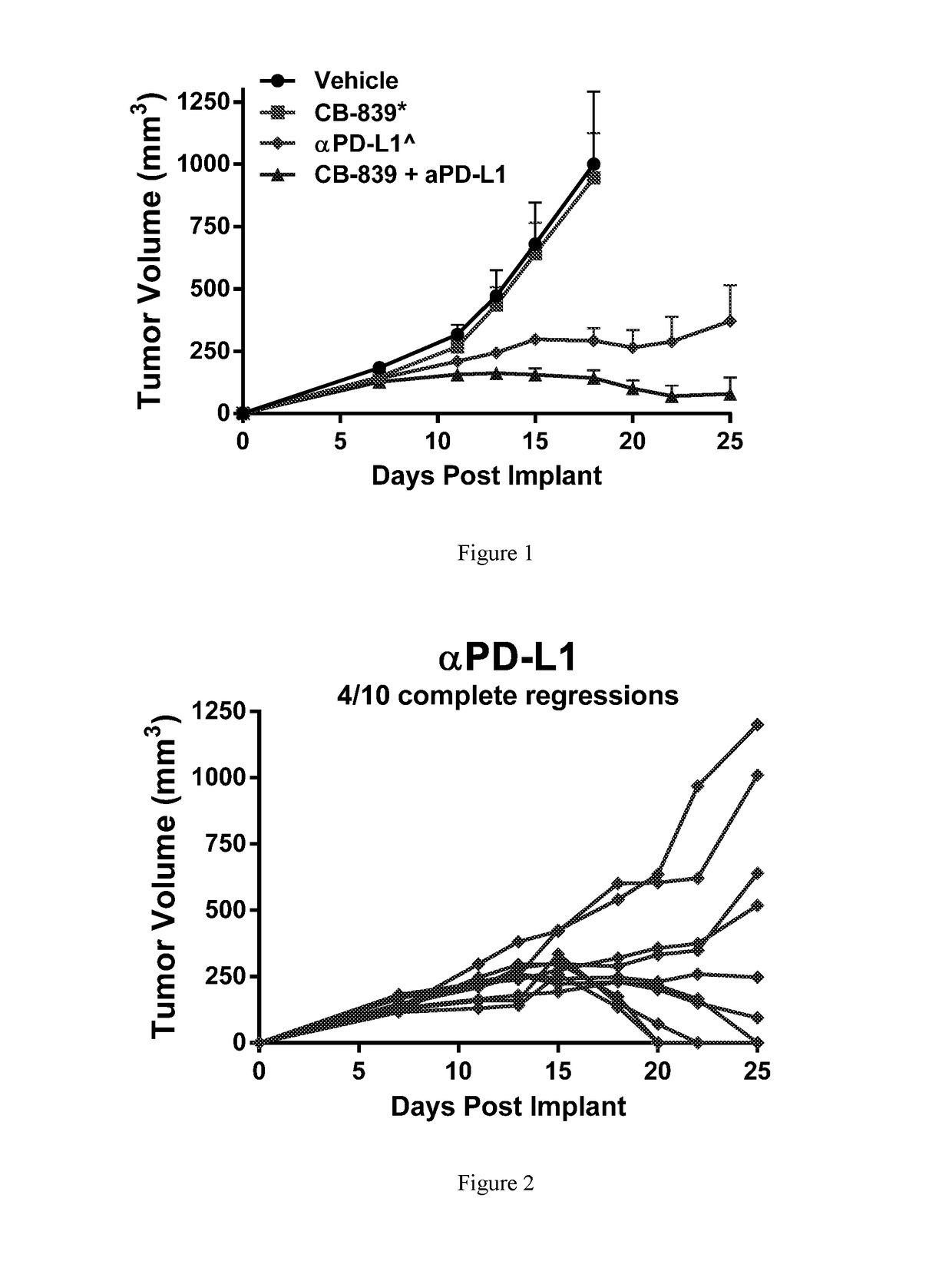
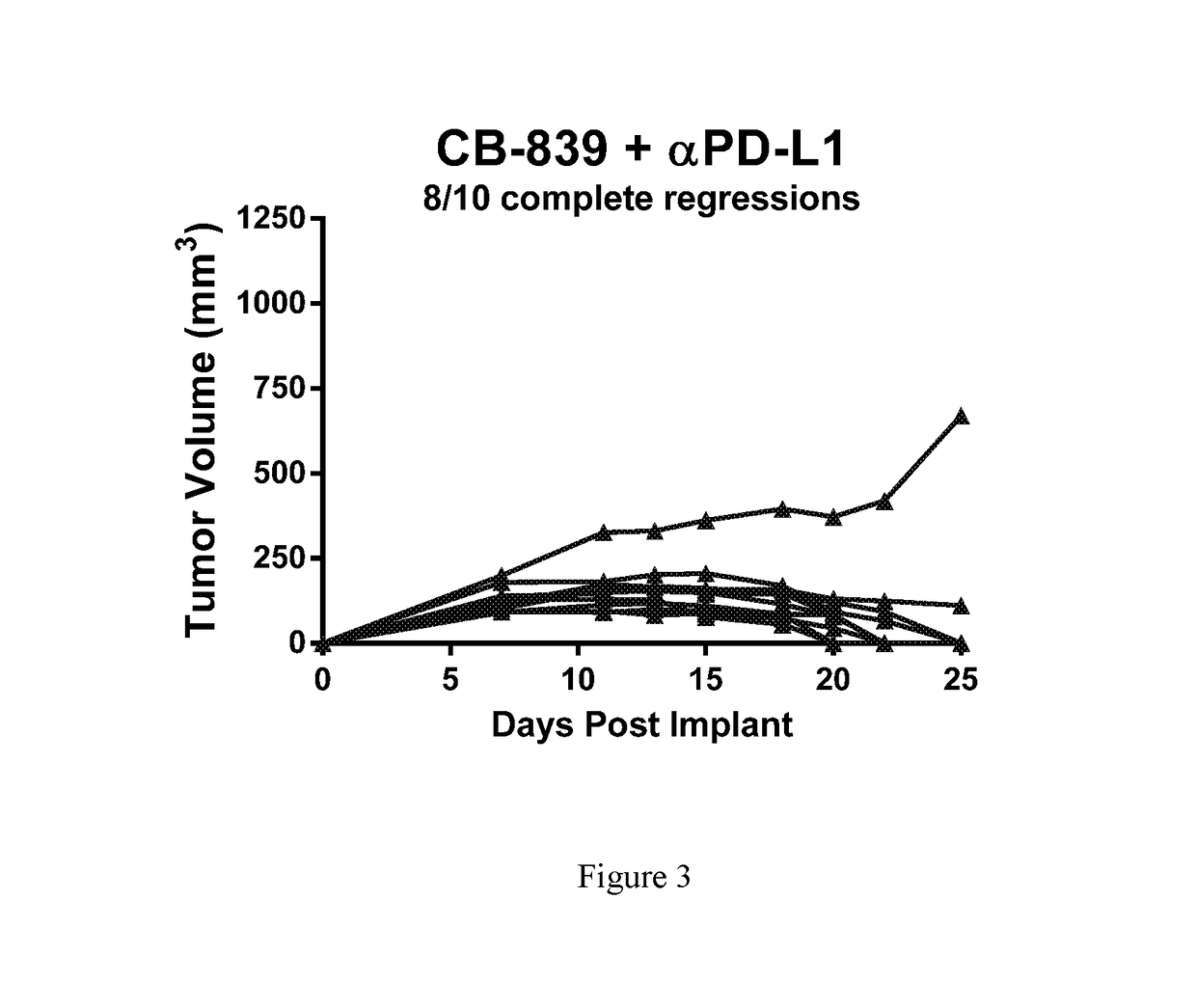
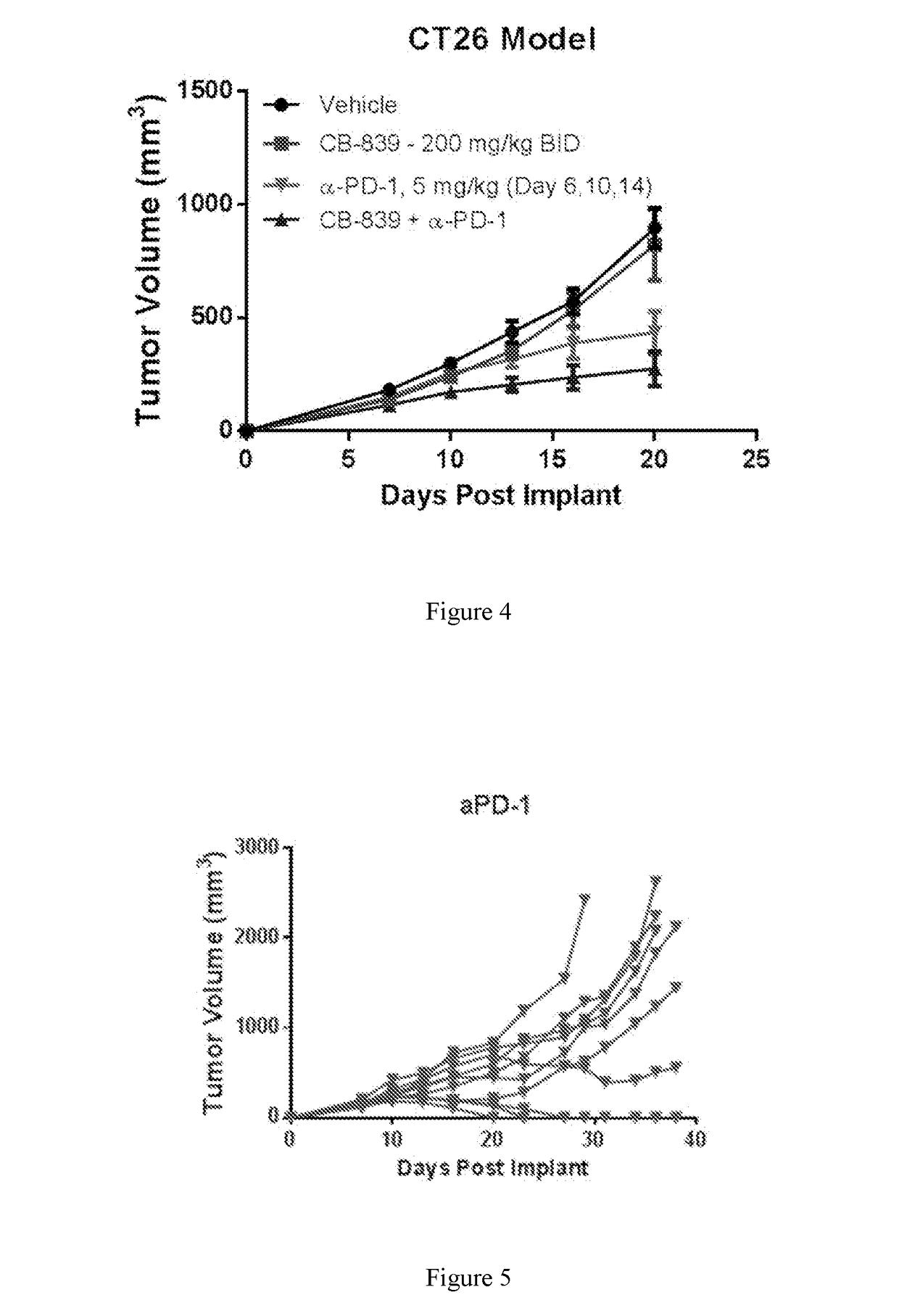
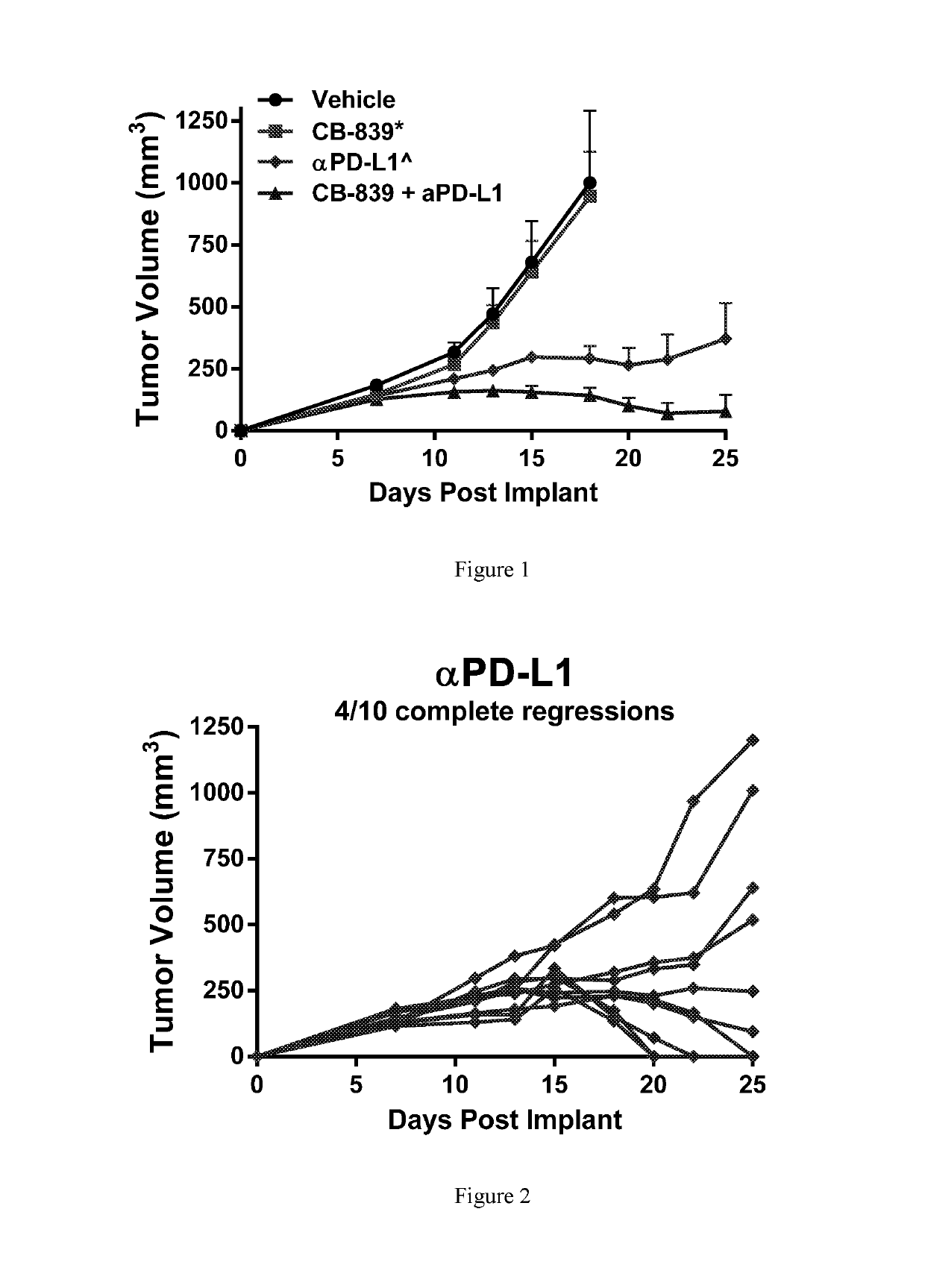
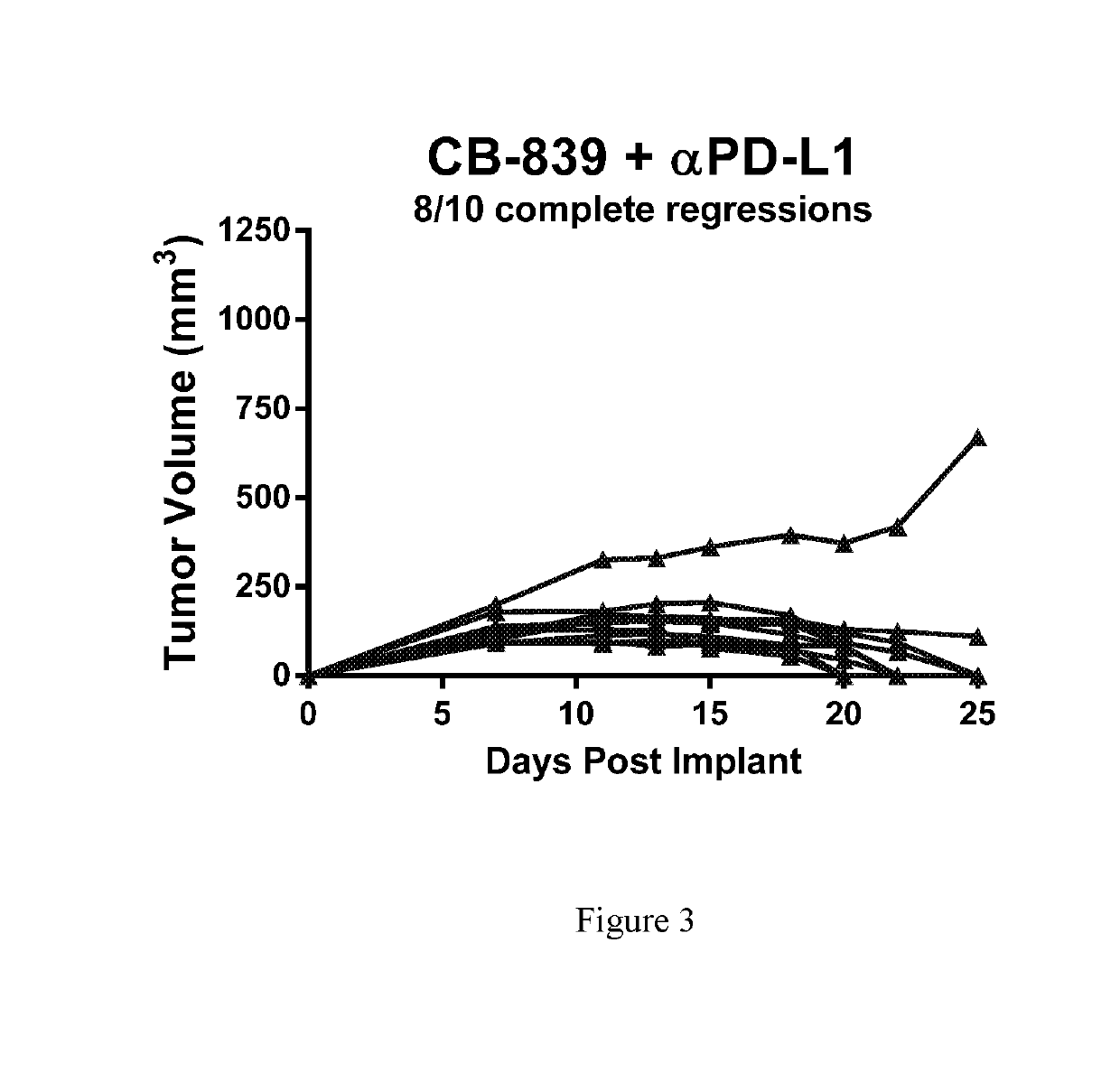
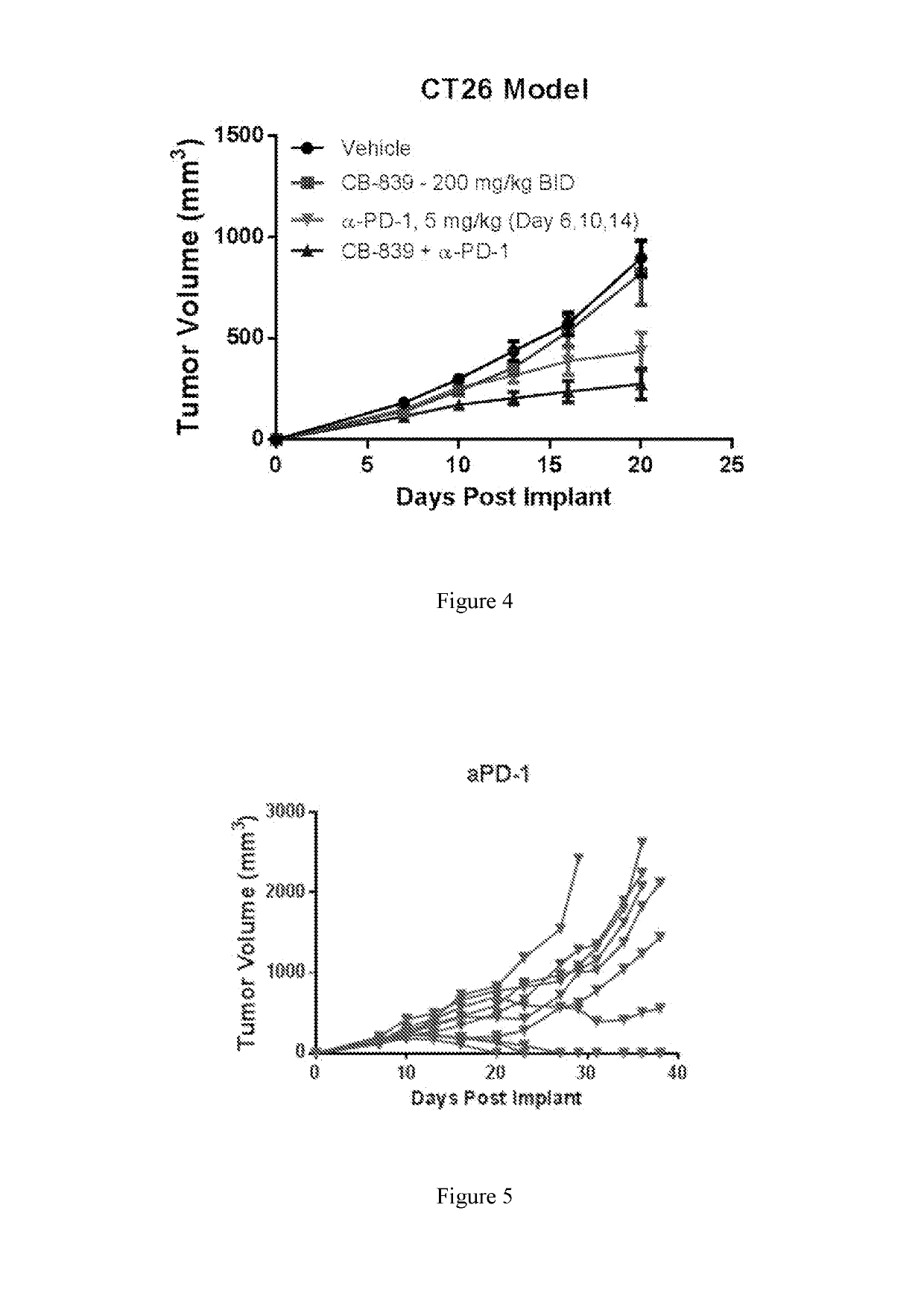
Combination therapy with glutaminase inhibitors and immuno-oncology agents
InactiveUS20170095473A1Chronic infectionAntibacterial agentsOrganic active ingredientsDiseaseImmunooncology
The invention relates to methods of treating cancer, myeloproliferative diseases, or immunological or neurological diseases with a combination of a glutaminase inhibitor and an immuno-oncology therapeutic agent, such as an inhibitor of arginase, CTLA-4, indoleamine 2,3-dioxygenase, and / or PD-1 / PD-L1.
Owner:CALITHERA BIOSCIENCES INC
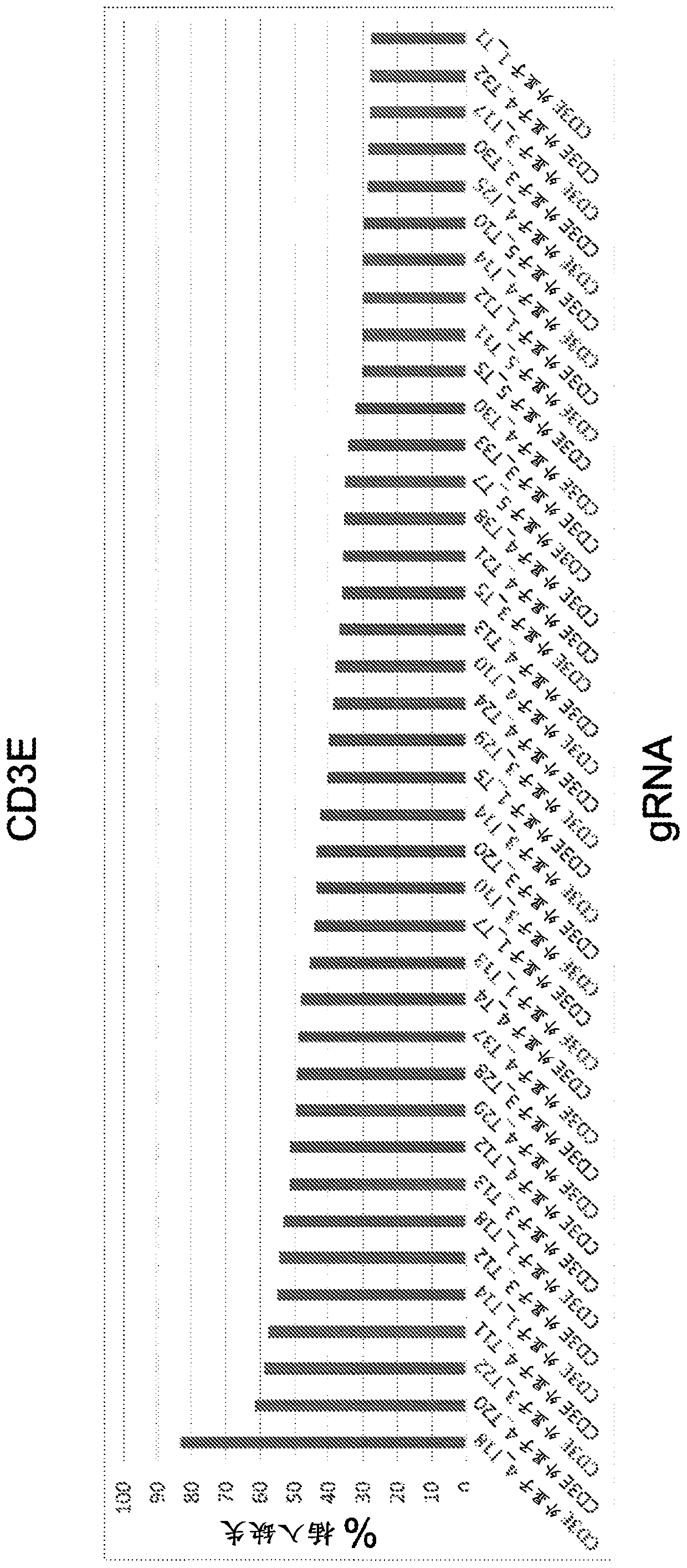
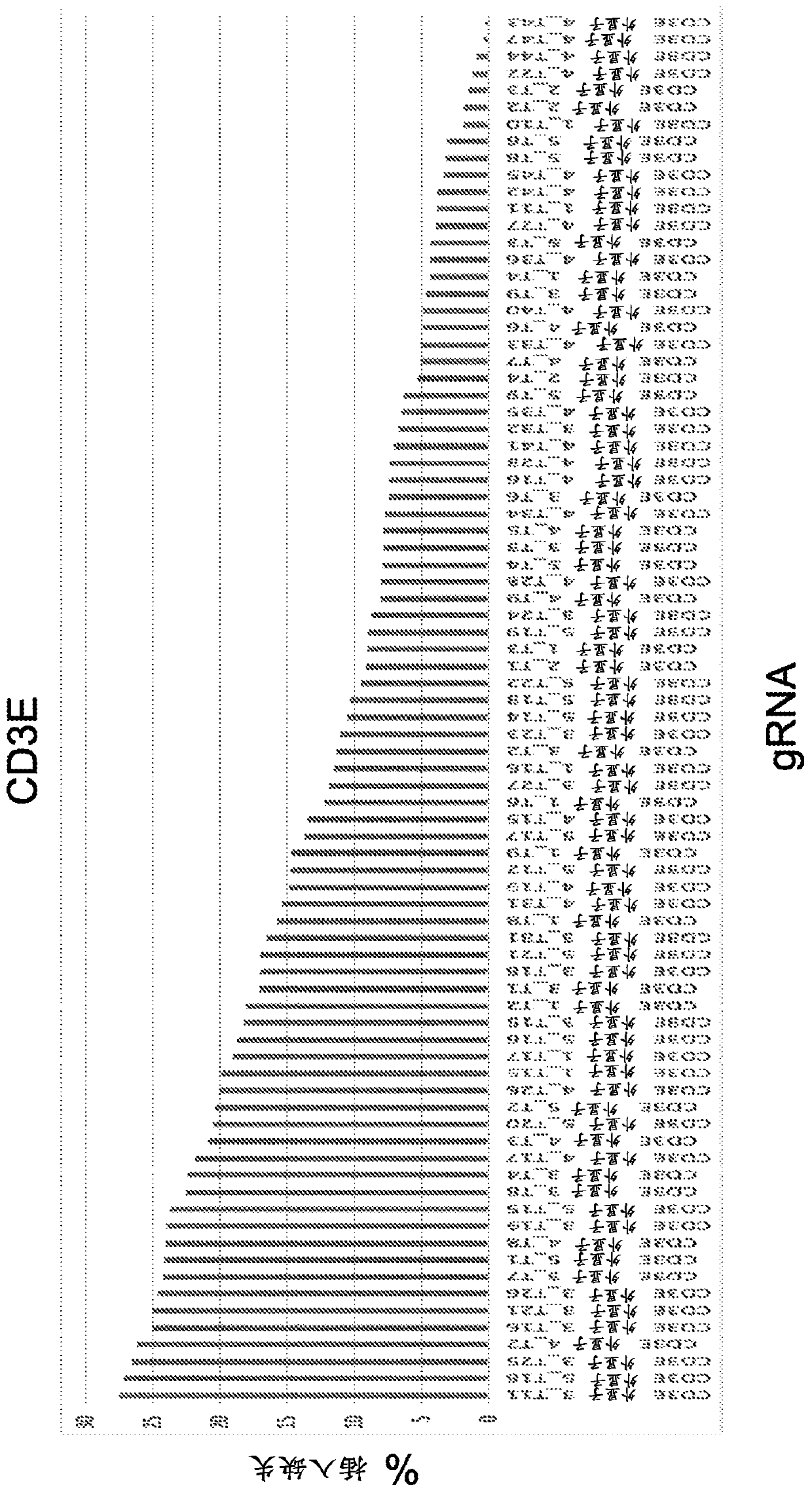
Materials and methods for engineering cells and uses thereof in immuno-oncology
The present invention disclsoes materials and methods for producing genome-edited cells engineered to express a chimeric antigen receptor (CAR) construct on the cell surface, and materials and methodsfor genome editing to modulate the expression, function, or activity of one or more immuno-oncology related genes in a cell, and materials and methods for treating a patient using the genome-edited engineered cells.
Owner:CRISPR THERAPEUTICS AG
Combination therapy with glutaminase inhibitors and immuno-oncology agents
The invention relates to methods of treating cancer, myeloproliferative diseases, or immunological or neurological diseases with a combination of a glutaminase inhibitor and an immuno-oncology therapeutic agent, such as an inhibitor of arginase, CTLA-4, indoleamine 2,3-dioxygenase, and / or PD-1 / PD-L1.
Owner:CALITHERA BIOSCIENCES INC
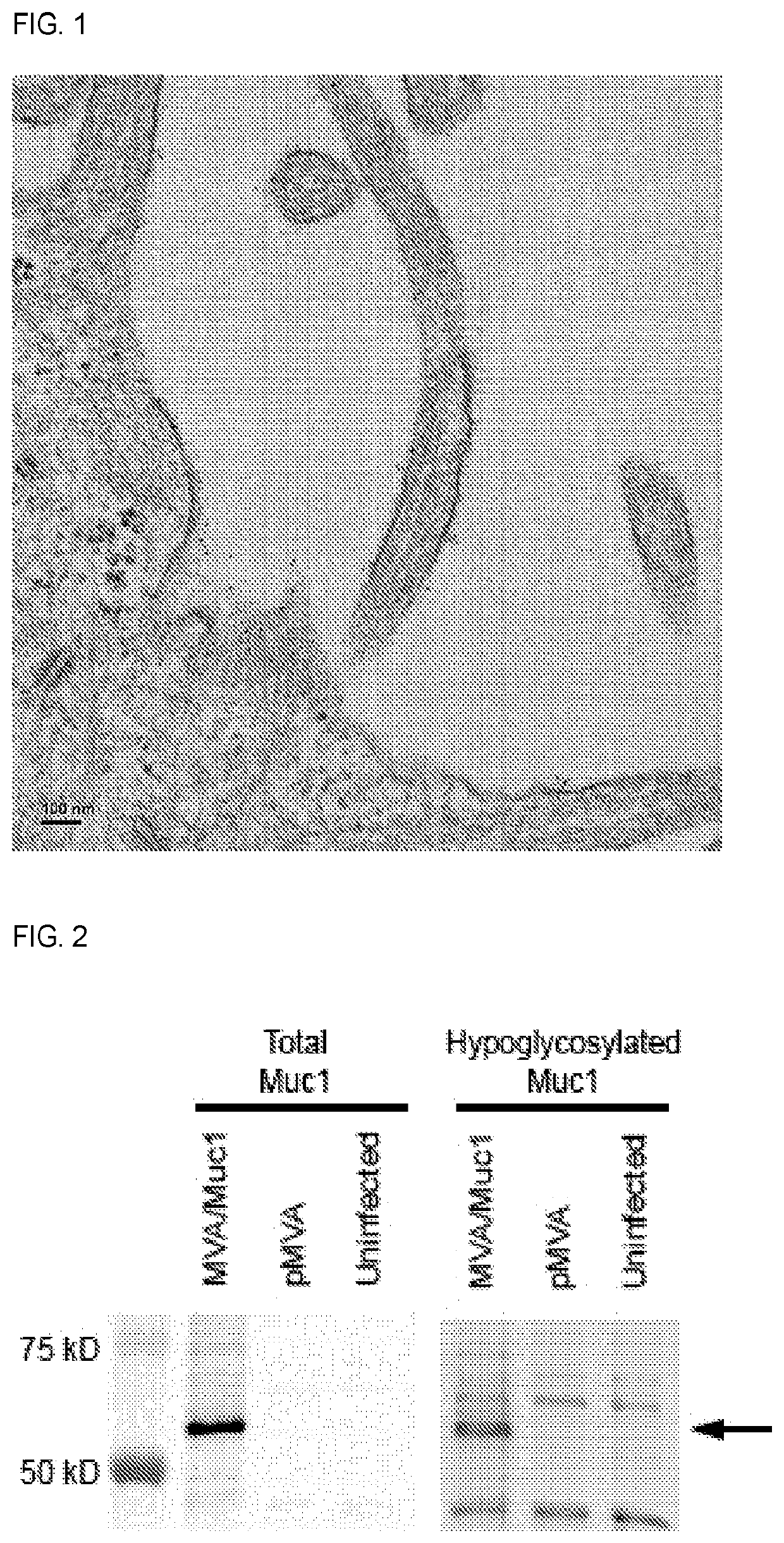
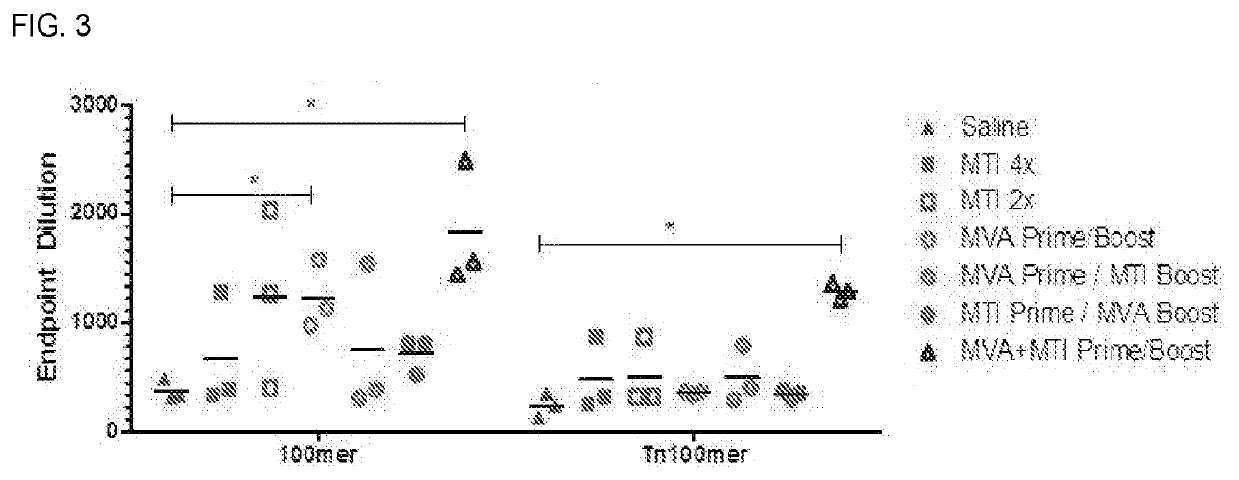
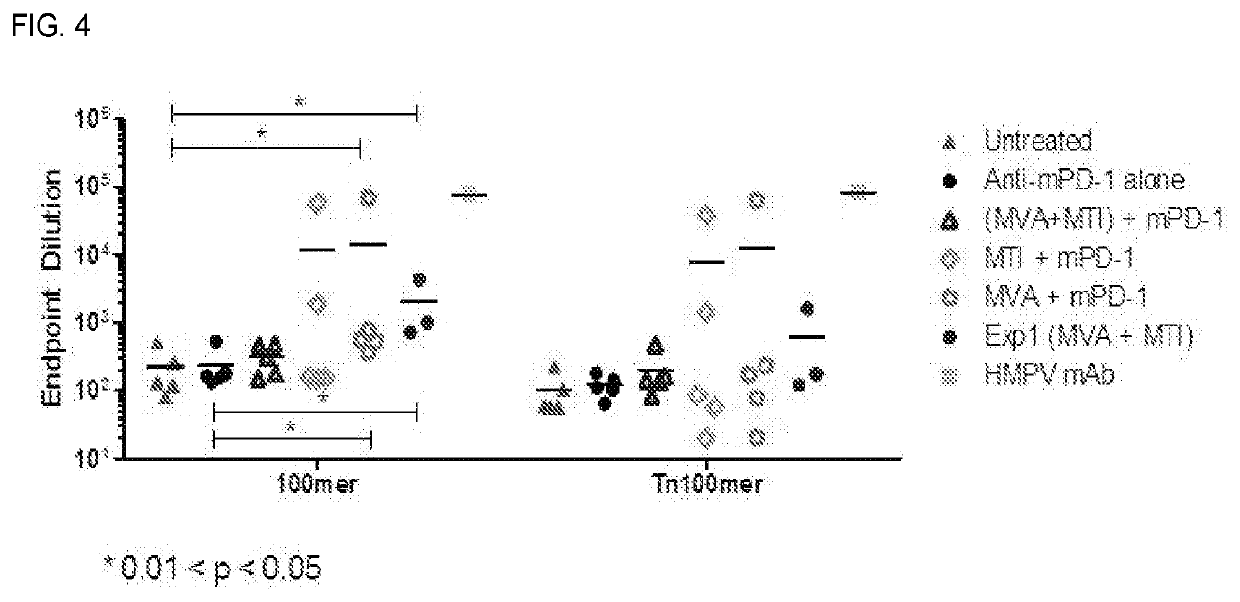
Immuno-Oncology Compositions and Methods for Use Thereof
InactiveUS20210220469A1Stimulate immune responseReduce and prevent growthSsRNA viruses negative-senseViral antigen ingredientsModified vaccinia AnkaraImmunooncology
The compositions and methods are described for generating an immune response to a tumor associated antigen such as MUC-1. The compositions and methods described herein relate to a modified vaccinia Ankara (MVA) vector encoding one or more viral antigens for generating a protective immune response to MUC-1 in the subject to which the vector is administered and boosting the immune response by administering a MUC-1 peptide. The compositions and methods of the present invention are useful both prophylactically and therapeutically and may be used to prevent and / or treat neoplasms and associated diseases.
Owner:GEOVAX INC
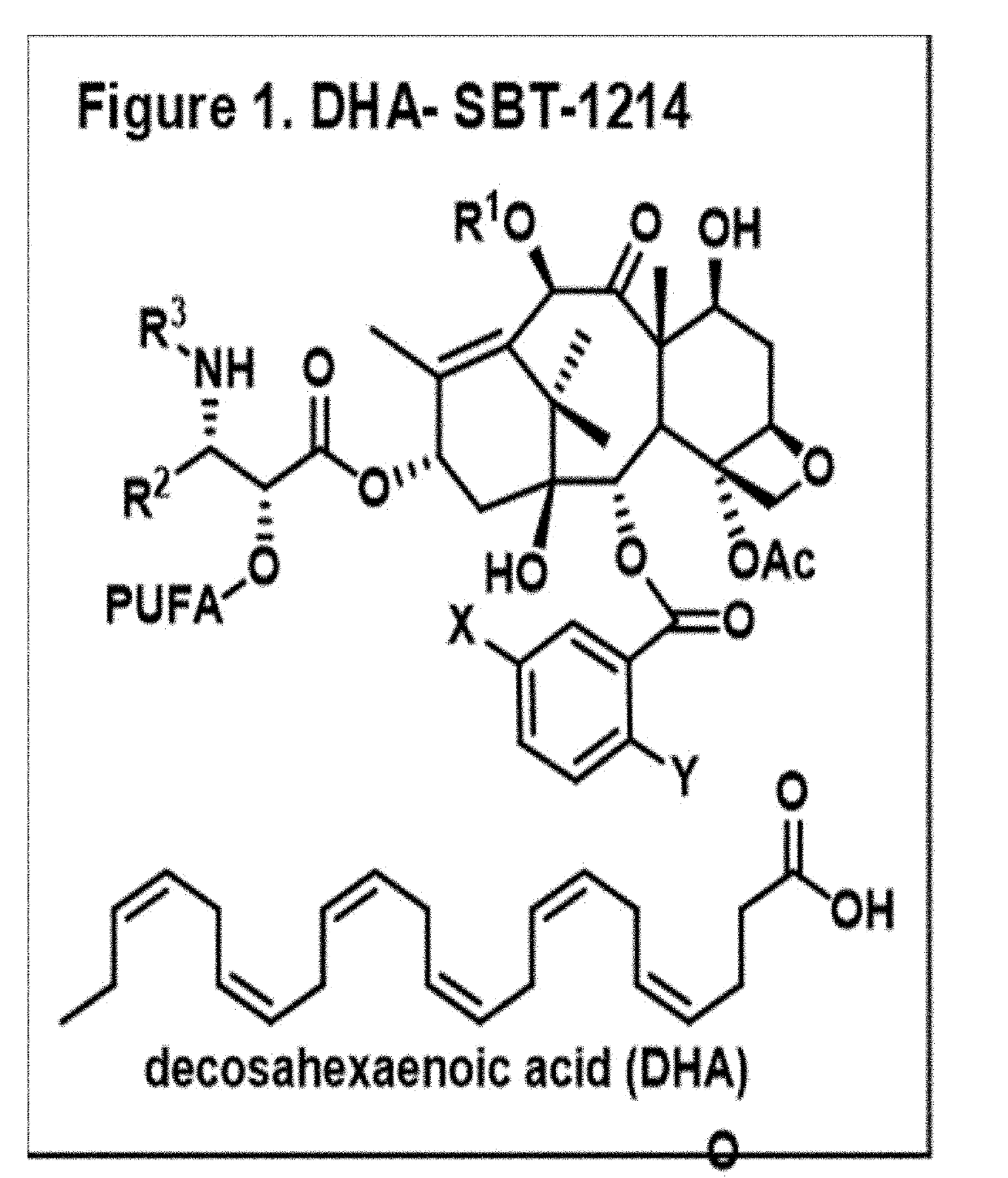
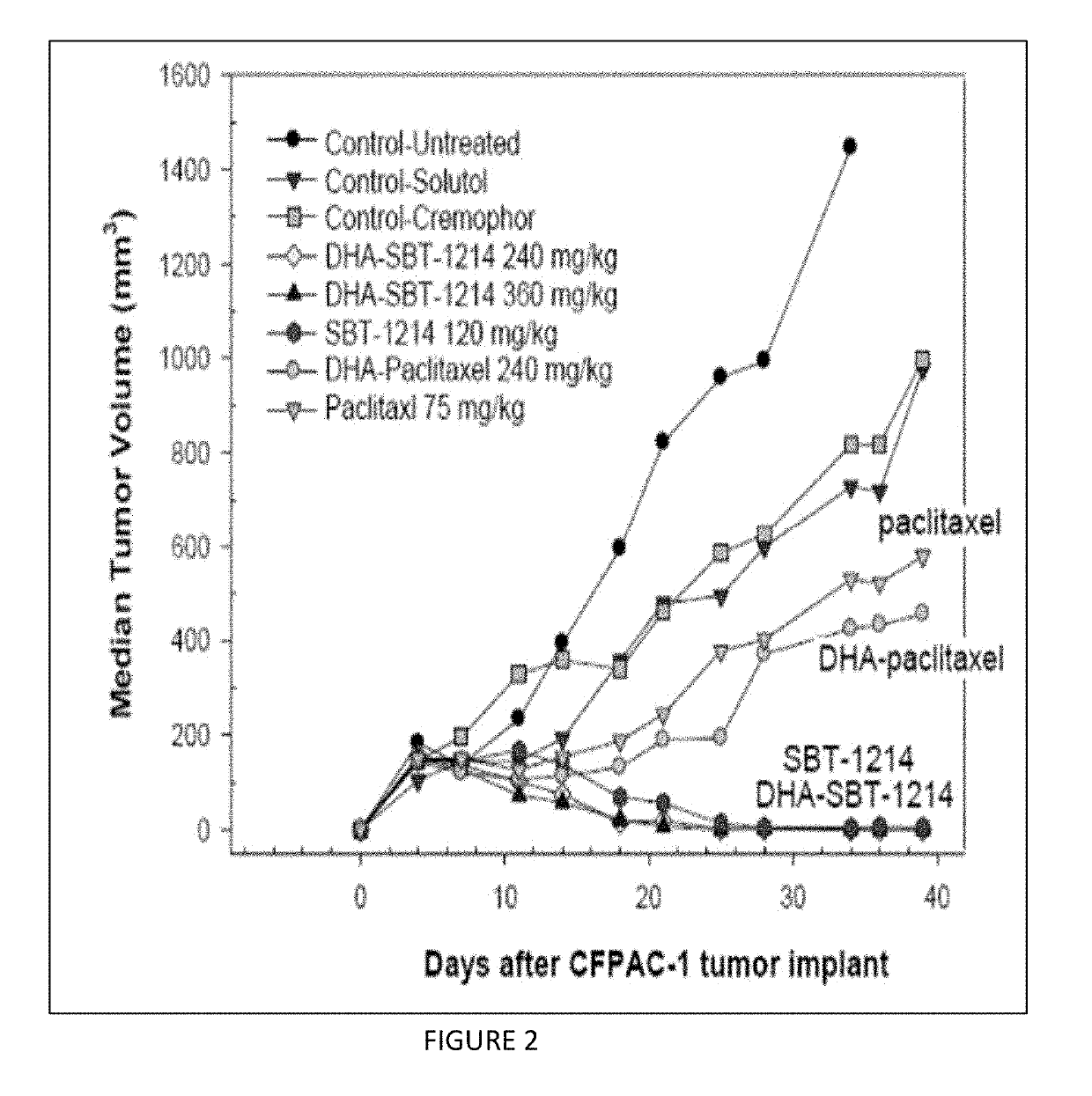
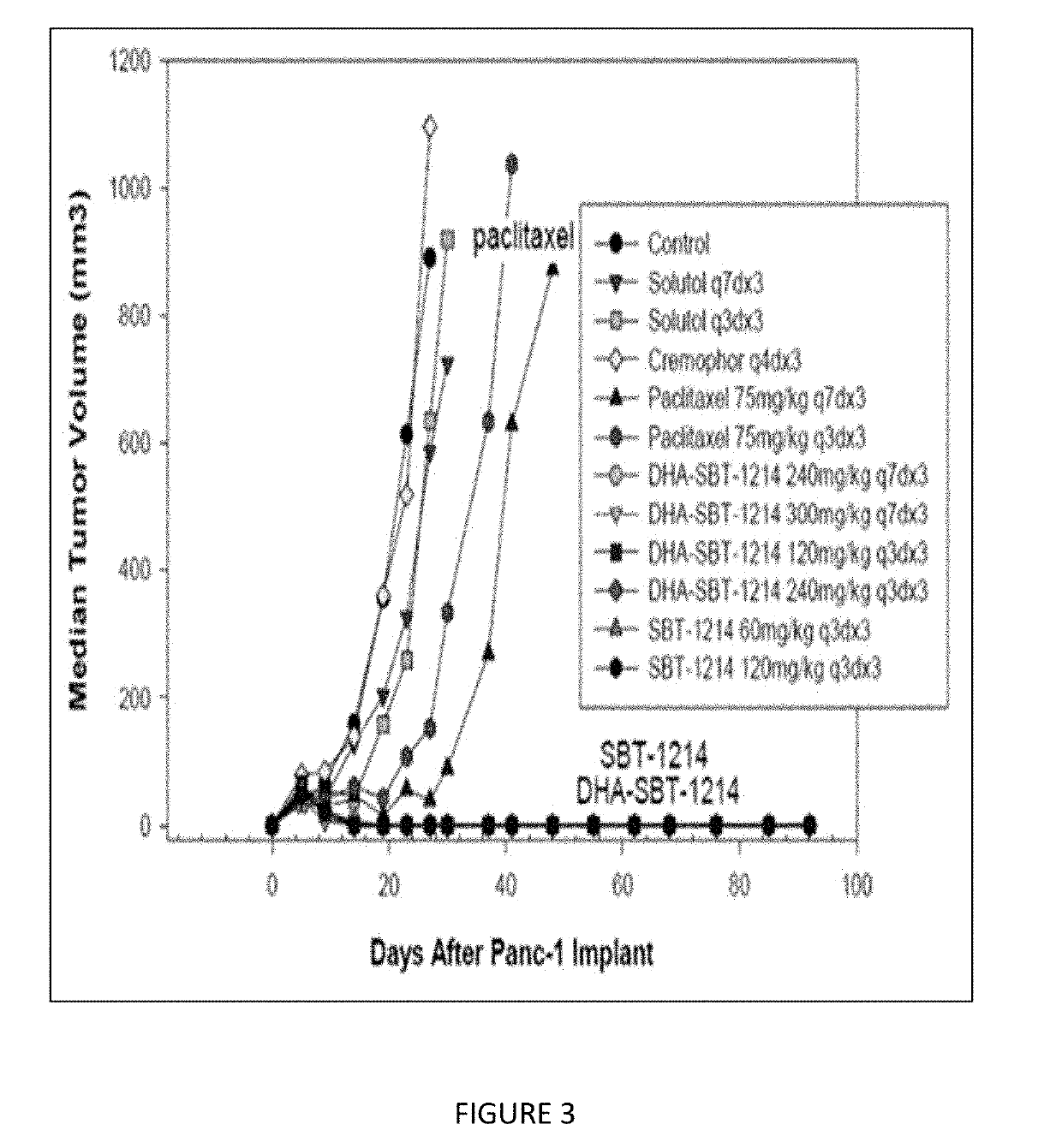
Combination taxoid nanoemulsion with immunotherapy in cancer
ActiveUS11497713B2Good curative effectOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsImmunooncologyEfficacy
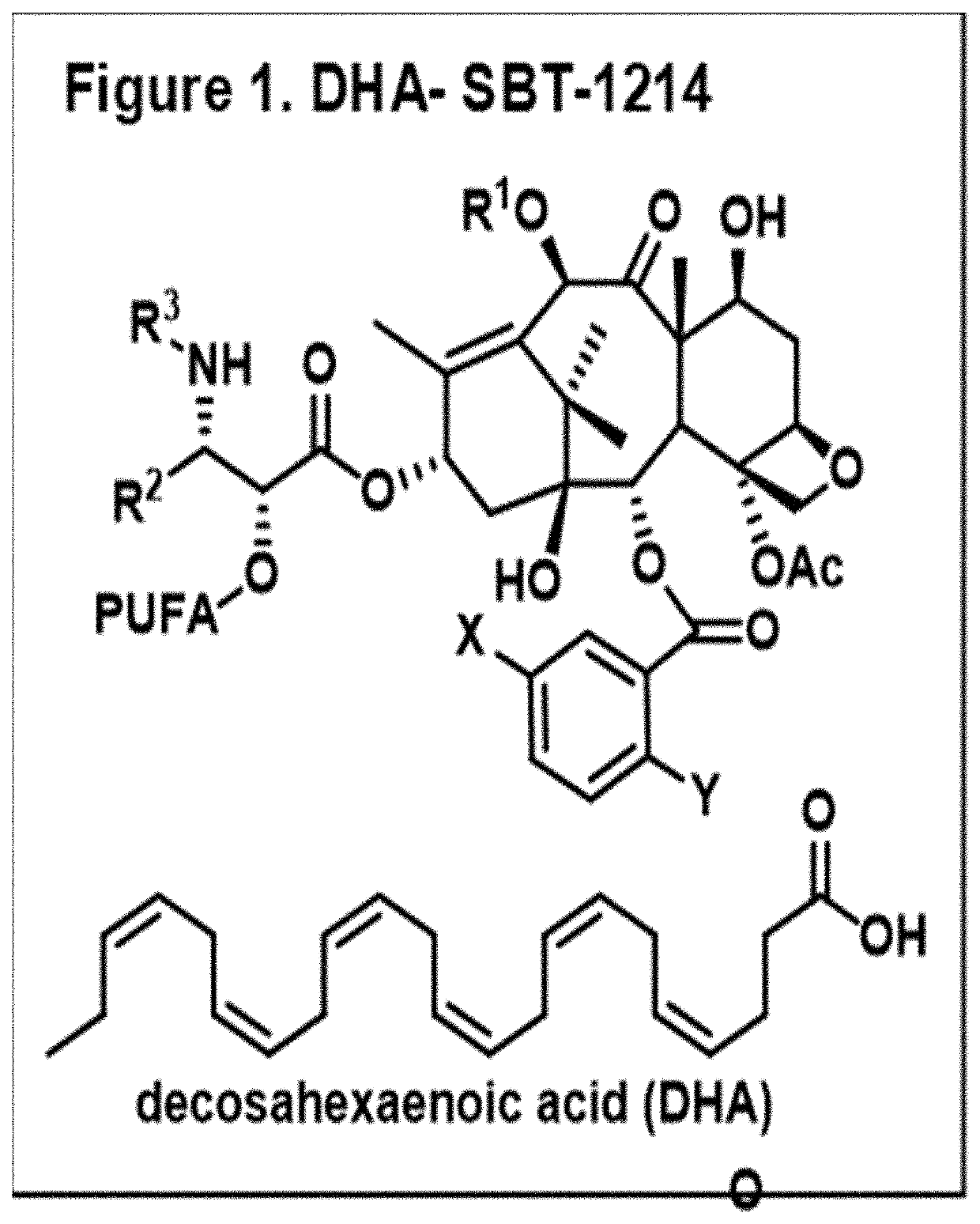
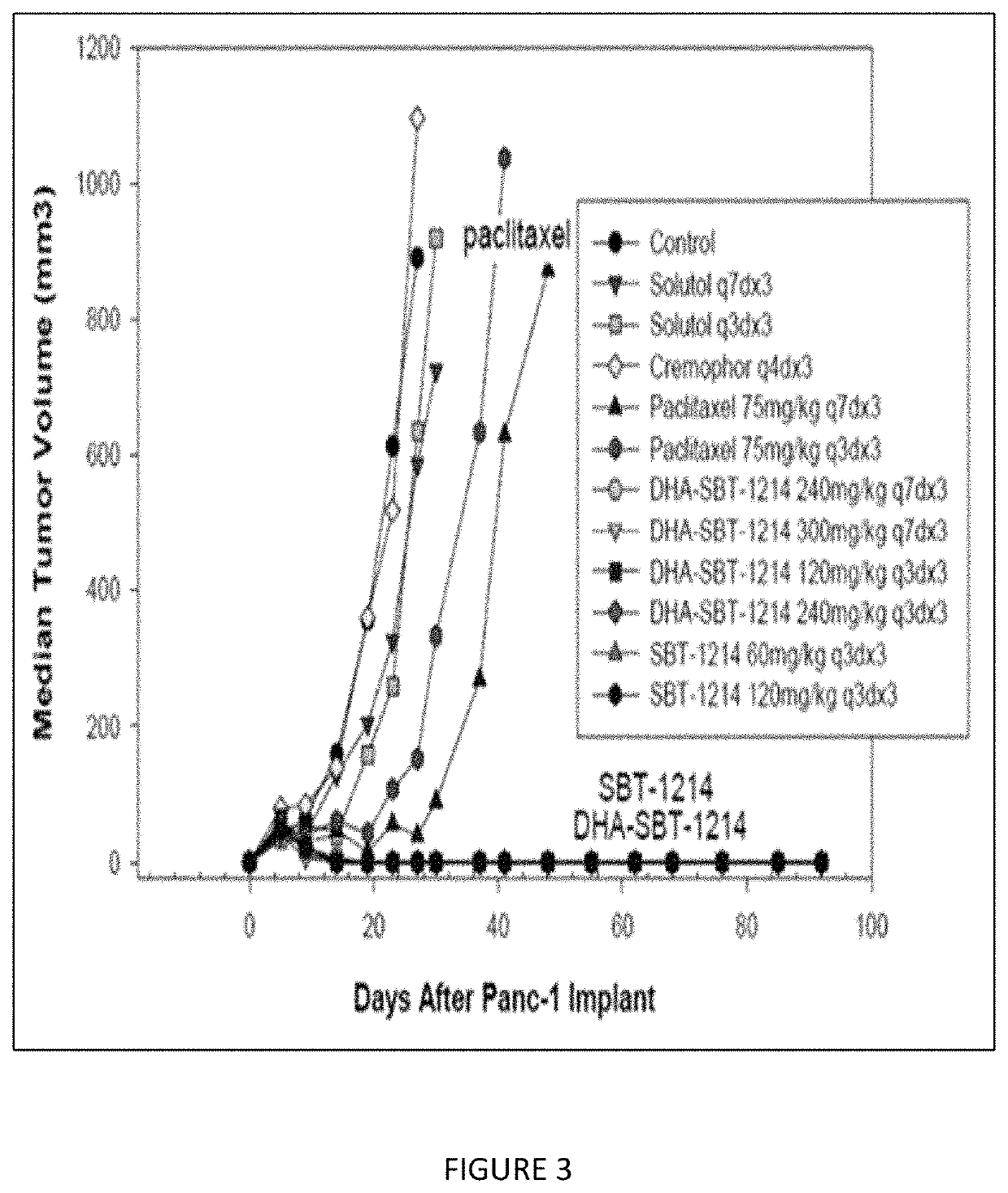
A composition of an omega-3 polyunsaturated fatty acid (PUFA)-taxoid conjugate formulated in an oil-in-water nanoemulsion (NE) drug delivery system in combination with an immune-oncology (IO) agent to enhance therapeutic efficacy in refractory cancers, such as PDAC. A method of treating cancer, by administering an effective amount of a pharmaceutical composition including an omega03 PUFA-taxoid conjugate in combination with an IO agent encapsulated in an NE drug delivery system to a subject in need of treatment, and treating cancer.
Owner:TARGAGENIX INC +2
Tumor-targeted delivery system for in-vivo in-situ induction of CAR-T cells and application of tumor-targeted delivery system
ActiveCN113384690AOvercoming Systemic Security IssuesAvoid process problemsAntibody medical ingredientsAntineoplastic agentsImmunooncologyNanocarriers
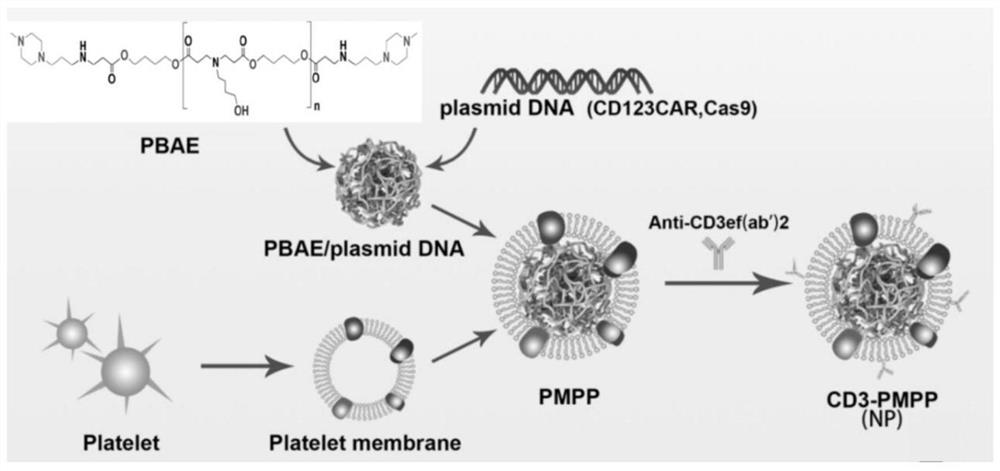
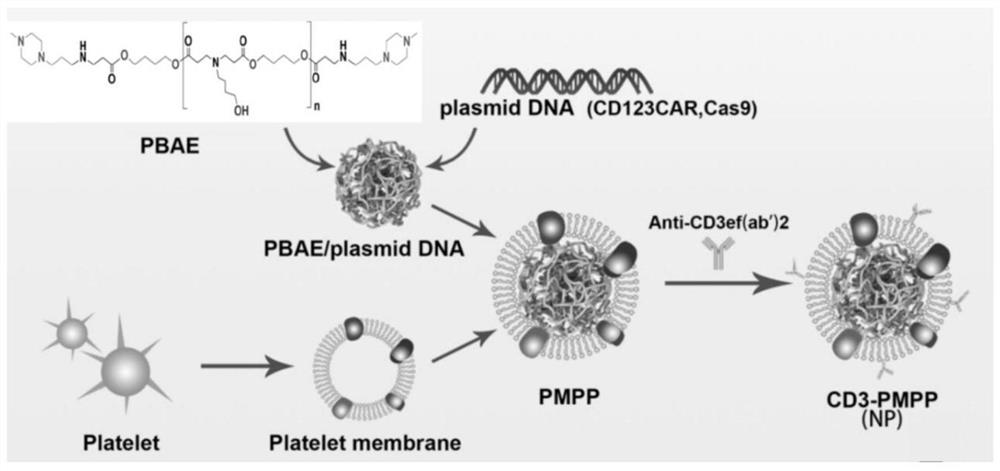
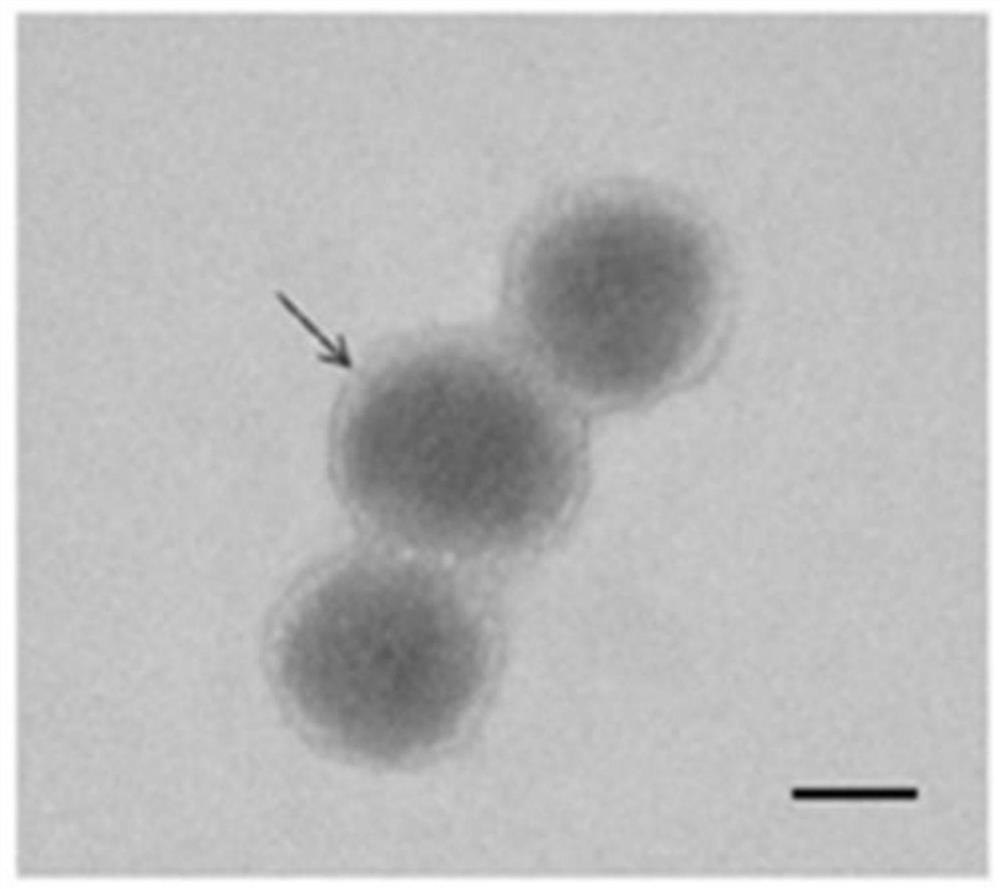
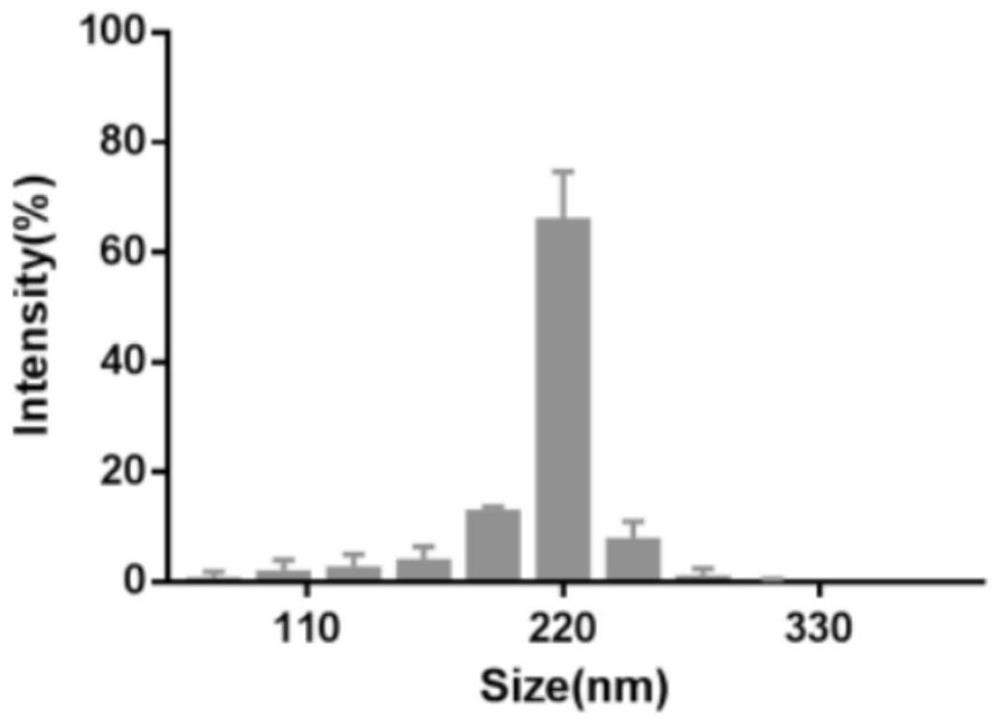
The invention discloses a tumor-targeted delivery system for in-vivo in-situ induction of CAR-T cells and application of the tumor-targeted delivery system, and belongs to the technical field of immunooncology. The tumor-targeted delivery system comprises nanoparticles and a nano-carrier formed by wrapping the nanoparticles with a platelet membrane, wherein the nanoparticles are formed by assembling a cationic polymer, CAR gene plasmids and a CRISPR system, and the CRISPR system is delivered to a tumor through the nano-carrier to achieve in-situ editing of the T cells. The tumor-targeted delivery system achieves in-situ editing of the T cells in the solid tumor, improves the tumor microenvironment, enhances the proliferation and durability of the CAR-T cells, and has higher safety, simple process and low cost compared with traditional CAR-T.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
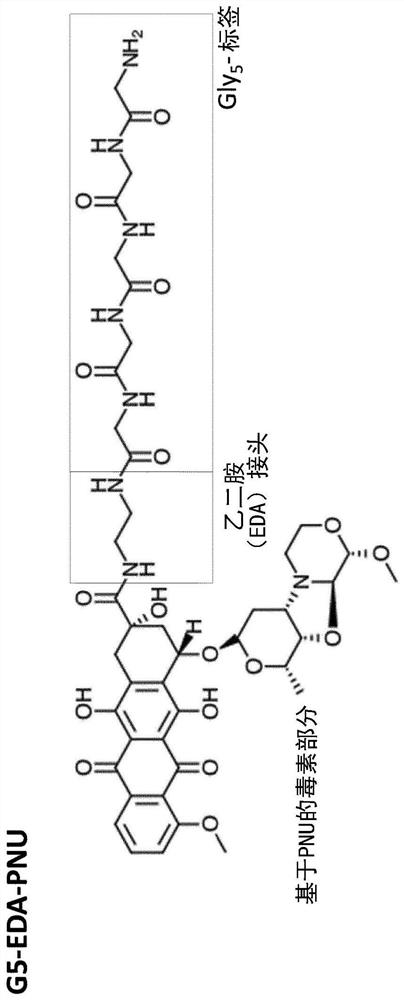
Binding protein-toxin conjugates comprising anthracyclines, and use thereof in immune-oncological applications
PendingUS20210379194A1Tetracycline active ingredientsPharmaceutical non-active ingredientsImmunooncologyToxin Conjugates
The present invention relates to binding protein-toxin conjugates comprising one or more anthracycline toxin moieties, and the use thereof in immunooncological applications.
Owner:NBE THERAPEUTICS
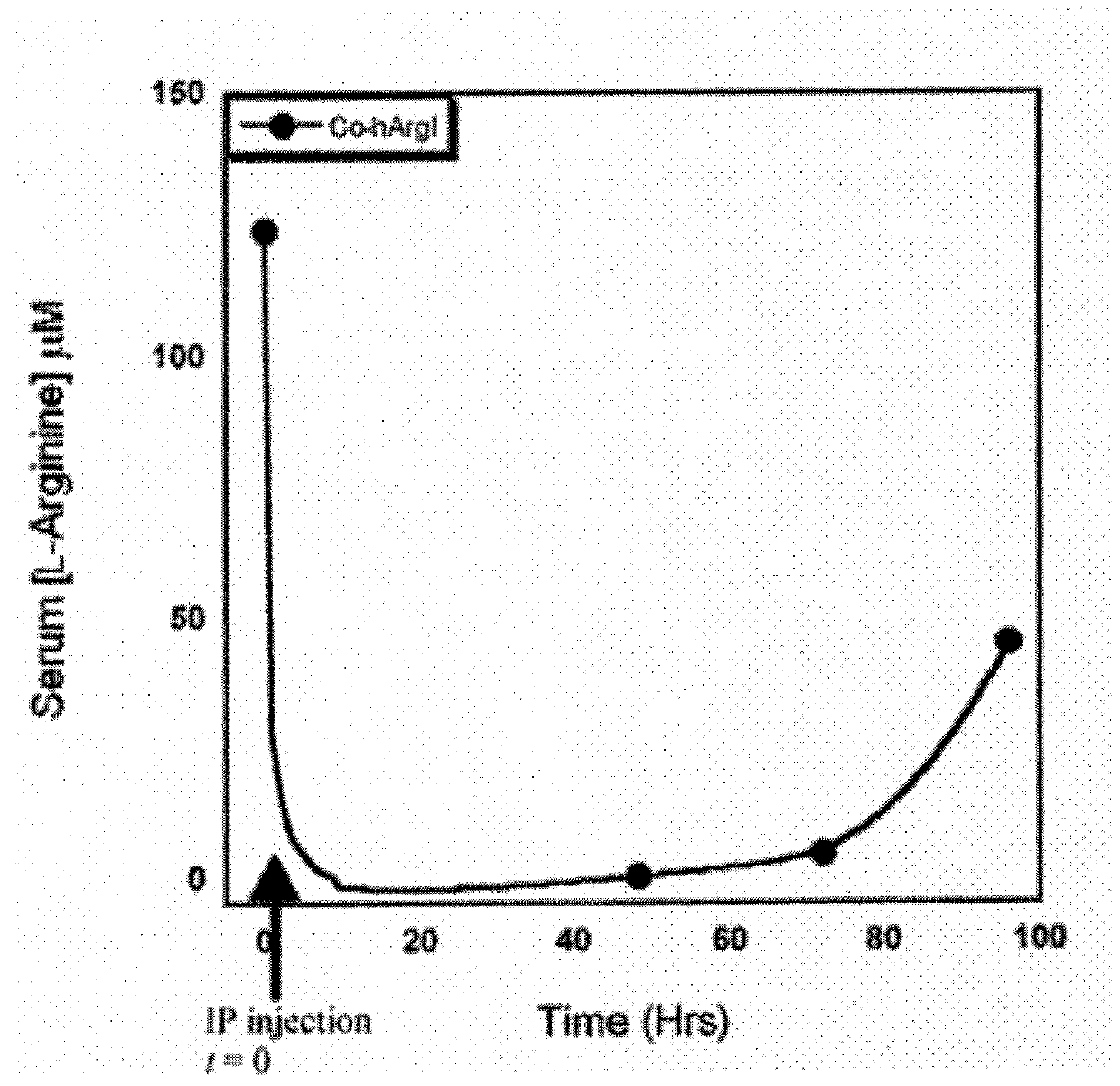
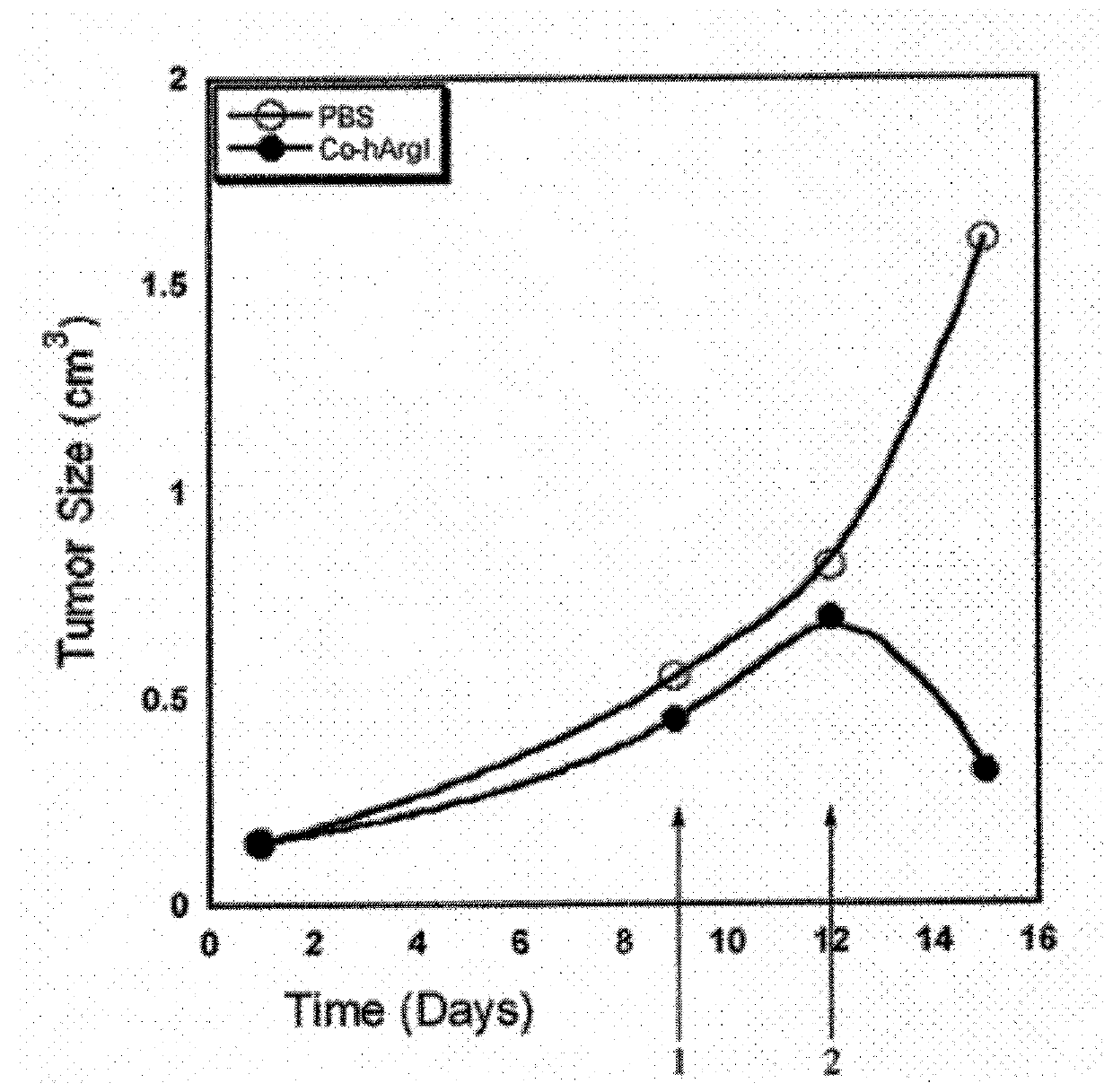
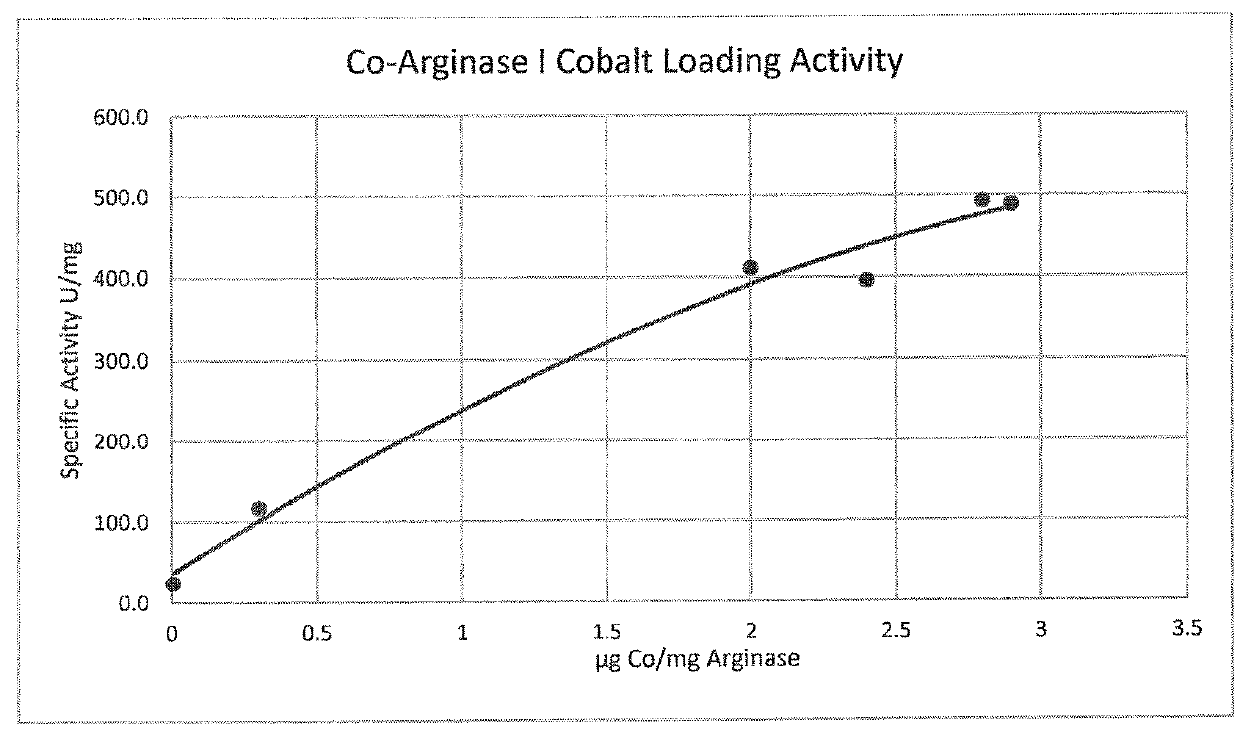
Compositions and Methods for Treating Cancer with Arginine Depletion and Immuno Oncology Agents
ActiveUS20180177853A1Promote growthReduce proliferationPeptide/protein ingredientsHydrolasesImmunooncologyArginine
Owner:AERASE
Tumor targeted radionuclide therapy and molecular imaging of her2+ cancers and other neoplasms
ActiveUS20200085981A1Reduce spreadModulate pathologic angiogenesisImmunoglobulins against growth factorsRadioactive preparation carriersImmunooncologyTumor targeting
Methods and compositions for treating, diagnosing and staging cancers, in particular overexpressing the Human Epidermal growth factor Receptor 2 protein (HER2+) given rise to in breast, gastric, gastroesophageal, ovarian, pancreatic cancer and brain tumors, which may be metastatic to the brain or other site. More specifically, the invention provides for Targeted Radionuclide Therapy (TRNT) with a compound of the invention having a peptide that targets the HER2+ cells, a second component for combining metals into complexes through a ring structure (DOTA), and a third radioisotope component, Lu-177 and Ga-68, in which embodiments further include a companion diagnostic, and in which embodiments further include anti-integrin precision medicines for cancers expressing αvβ3 and αvβ5 integrins, HER2+, vascular endothelial growth factor, vitronectin, fibronectin, tenascin, reelin, kindlin and talin. TRNT may be administered alone or in combination with standard-of-care; an immunooncologic and / or chemotherapeutic, adjuvantly or neoadjuvantly.
Owner:SATZ STANLEY
Prevention and/or treatment of CNS disorders
PendingUS20220152034A1Improve immunityLow cytotoxicityOrganic active ingredientsNervous disorderImmunooncologyNervous system
Adenosine receptor (e.g., A2A and / or A1 receptor) antagonist compounds and compositions including said compounds are disclosed. The present disclosure also provides methods of using said compounds and compositions for modulating (e.g., inhibiting or antagonizing) A2A and / or A1 receptor in a biological system. The compounds and compositions find use in various therapeutic applications including the treatment of central nervous system or neurodegenerative diseases, such as Parkinson's disease. The compounds and compositions may also find use in various therapeutic applications including the treatment of cancer and in immuno-oncology.
Owner:IL DONG PHARMA CO LTD
Compounds
The present invention relates to a compound of formula (Ia), or a pharmaceutically acceptable salt or hydrate thereof, wherein: the group X-Y is -NHSO2- or -SO2NH-; R1 is H or alkyl; R2 is selected from COOH and a tetrazolyl group; R3 is selected from H, Cl and alkyl; R4 is selected from H, Cl and F; R5 is selected from H, alkyl, alkynyl, alkenyl, haloalkyl, SO2-alkyl, Cl, alkoxy, OH, CN, hydroxyalkyl, alkylthio, heteroaryl, cycloalkyl, heterocycloalkyl and haloalkoxy; R6 is H; R7 is selected from H, CN, haloalkyl, Cl, F, SO2-alkyl, SO2NR13R14, optionally substituted heteroaryl and alkyl; R8 is selected from H, alkyl, haloalkyl and halo; R9 is H, C1-C3-alkyl, or halo; R10 and R11, together with the nitrogen to which they are attached, form an azepanyl group, wherein (a) said azepanyl group is substituted by one or more substituents, or (b) one or two carbons in said azepanyl group are replaced by a group selected from O, NH, S and CO, and said azepanyl group is optionally further substituted; or R10 and R11, together with the nitrogen to which they are attached, form an azetidinyl, pyrrolidinyl or piperidinyl group wherein (a) said azetidinyl, pyrrolidinyl or piperidinyl group is substituted by one or more substituents, or (b) one or two carbons in said azetidinyl, pyrrolidinyl or piperidinyl group are replaced by a group selected from NH, S and CO; or R10 and R11, together with the nitrogen to which they are attached, form an 8, 9 or 10-membered bicyclic heterocycloalkyl group, wherein one or two carbons in the bicyclic heterocycloalkyl ring are optionally replaced by a group selected from O, NH, S and CO, and said bicyclic heterocycloalkyl group is optionally substituted; or R10 and R11, together with the nitrogen to which they are attached, form a 6 to 12-membered bicyclic group containing a spirocyclic carbon atom, wherein one or two carbons in the bicyclic group are optionally replaced by a group selected from O, NH, S and CO, and said bicyclic group is optionally substituted, or said bicyclic group is optionally fused to a 5 or 6-membered aryl or heteroaryl group; R13 and R14 are each independently H or alkyl. Further aspects of the invention relate to such compounds for use in the field of immune-oncology and related applications.
Owner:GREJ VULF TERAPYUTIKS LTD
Methods of identifying a subject suitable for an immuno-oncology (i-o) therapy
PendingUS20220259669A1Shrink tumorSmall sizeCompound screeningApoptosis detectionImmunooncologyAntiendomysial antibodies
The present disclosure provides methods of identifying a subject suitable for an immunooncology (I-O) therapy comprising measuring the expression of one or more of STAT1, IFNγ, NECTIN2, and CSFIR. In some aspects, the I-O therapy comprises administering an anti-PD-1 antibody or antigen-binding portion thereof or an anti-PD-L1 antibody or antigen-binding portion thereof to the subject.
Owner:BRISTOL MYERS SQUIBB CO
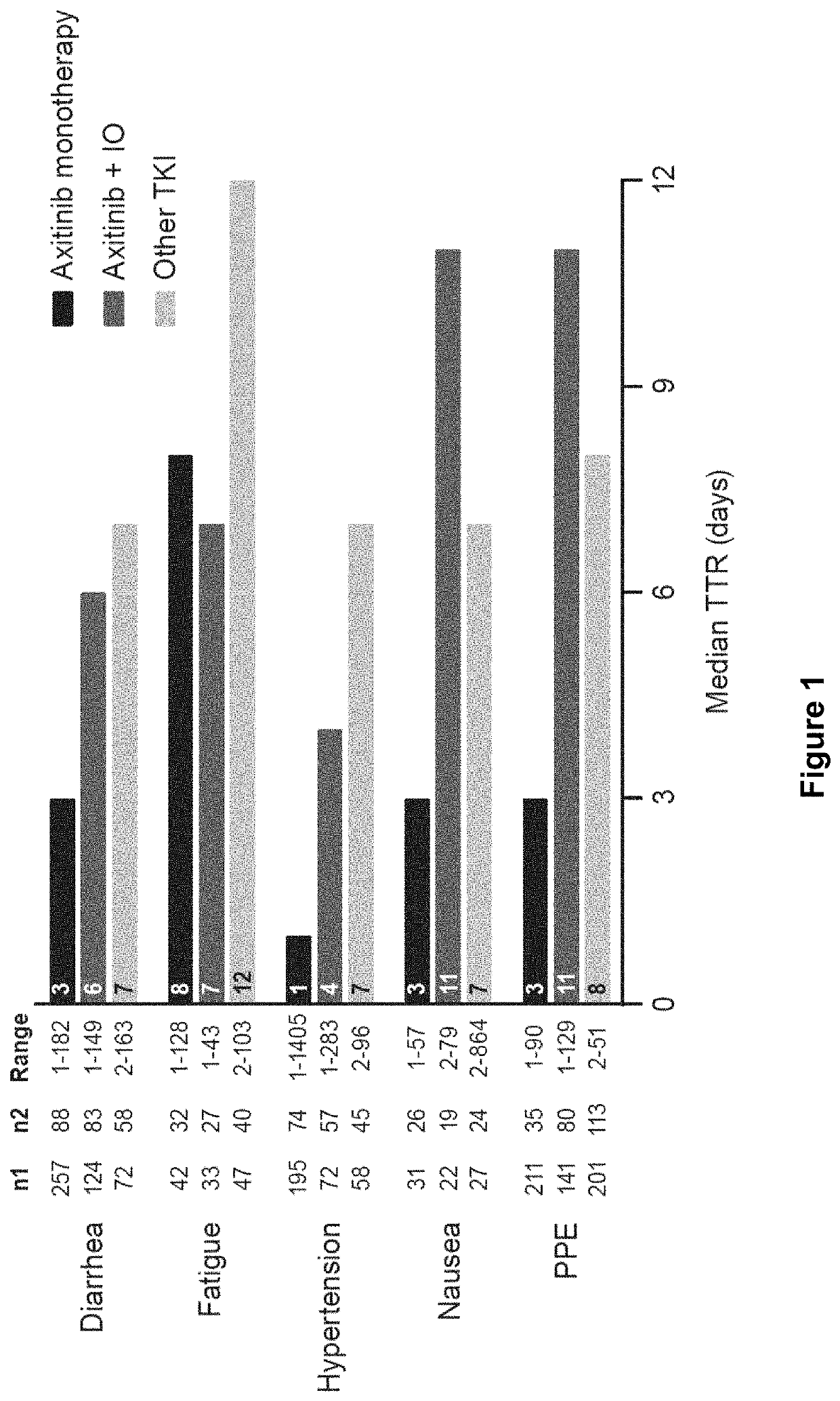
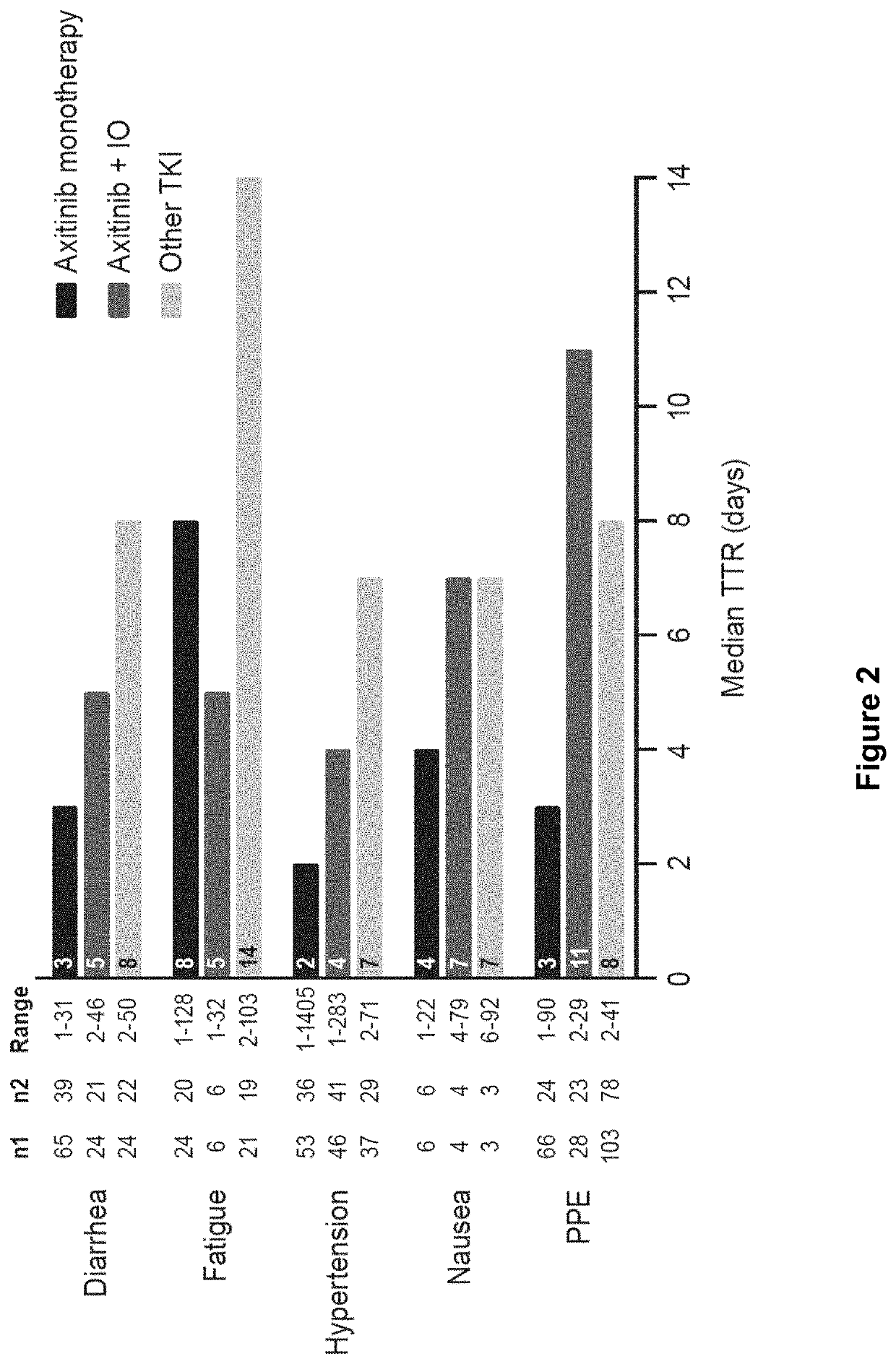
Time to resolution of axitinib-related adverse events
PendingUS20220168293A1Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsImmunooncologyPharmaceutical medicine
This invention relates to a method of managing an adverse event in a renal cell carcinoma (RCC) patient undergoing treatment with axitinib, or a pharmaceutically acceptable salt thereof, wherein said method comprises interrupting axitinib, or a pharmaceutically acceptable salt thereof, treatment for at least 1-7 days to allow the adverse event to resolve before restarting treatment. Additionally, the invention relates to a method of managing an adverse event in an RCC patient undergoing treatment with a combination of axitinib, or a pharmaceutically acceptable salt thereof, and an immune-oncology (IO) agent, wherein said method comprises interrupting axitinib, or a pharmaceutically acceptable salt thereof, treatment for at least 4-11 days to allow the adverse event to resolve before restarting axitinib, or a pharmaceutically acceptable salt thereof, treatment.
Owner:PFIZER INC
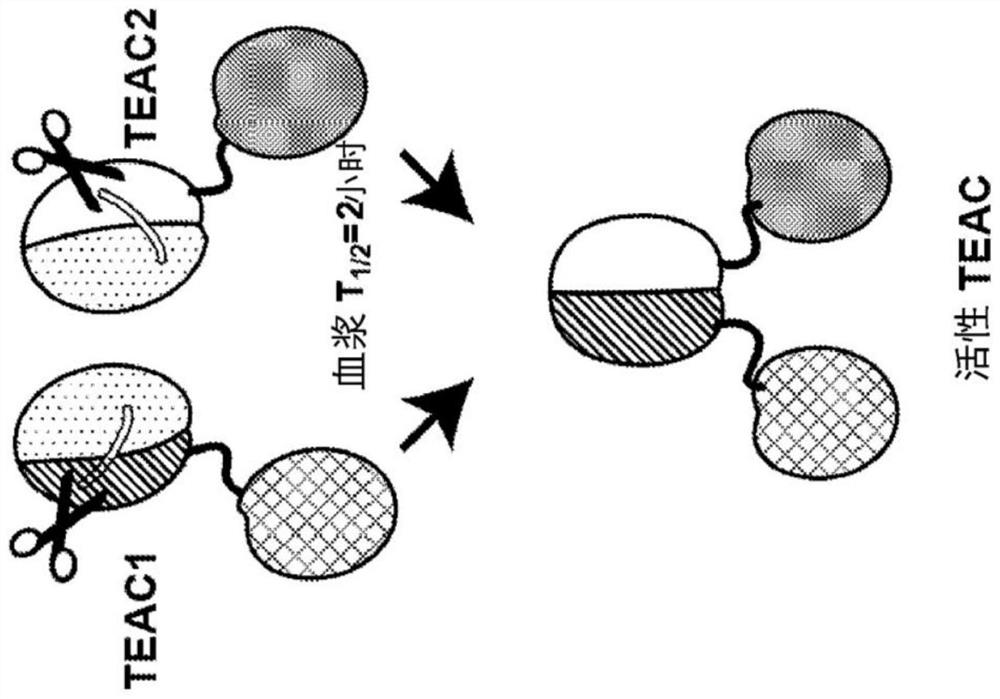
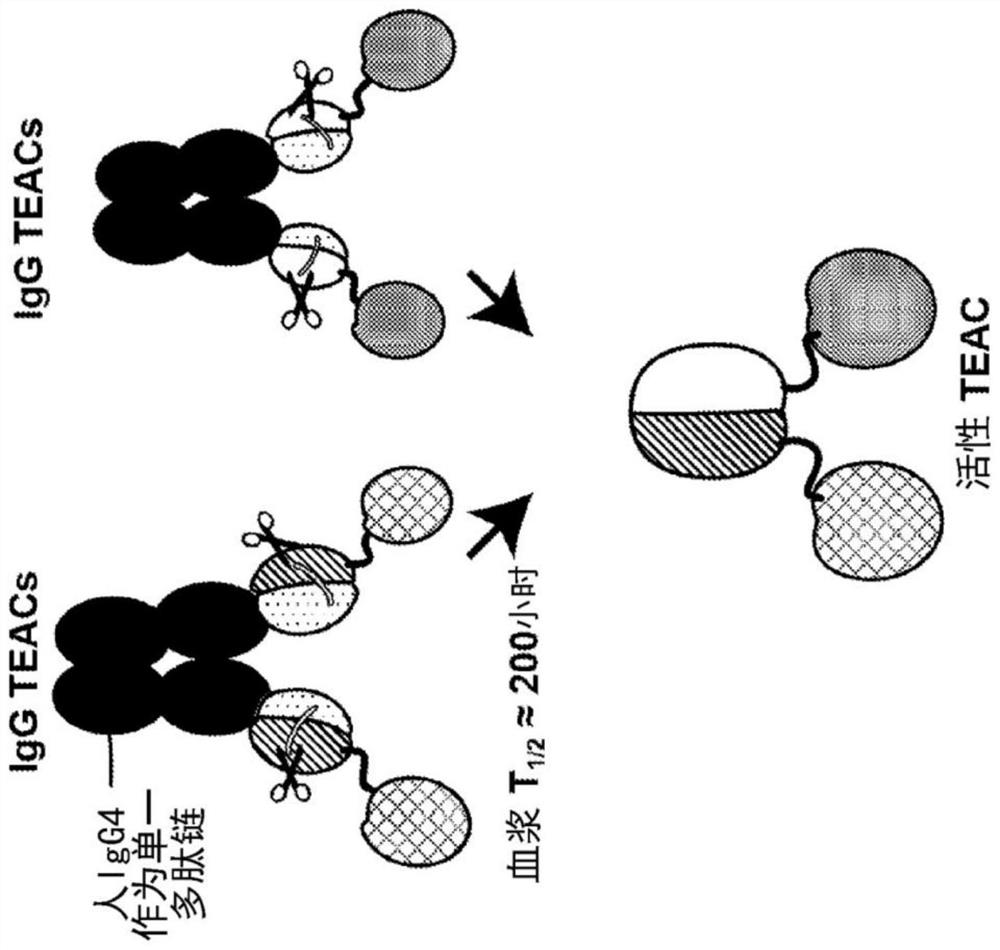
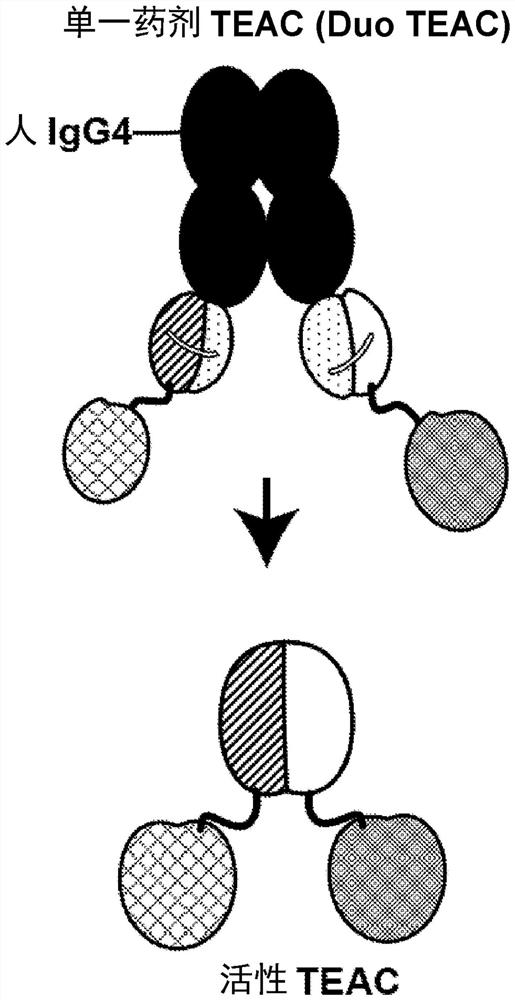
TEAC and ATTAC immunooncology compositions and methods
PendingCN114364696AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsImmunooncologyTumor target
The present disclosure provides a targeted T cell cement (TEAC) and an antibody tumor targeting assembly complex (ATTAC) for targeting cancer. The TEACs or ATTACs described herein may have, for example, a longer half-life or comprise multiple components in a single agent.
Owner:THE GENERAL HOSPITAL CORP +1
Combination taxoid nanoemulsion with immunotherapy in cancer
ActiveUS20190183796A1Good curative effectOrganic active ingredientsAntibody ingredientsImmunooncologyOMEGA-3 POLYUNSATURATED FATTY ACIDS
A composition of an omega-3 polyunsaturated fatty acid (PUFA)-taxoid conjugate formulated in an oil-in-water nanoemulsion (NE) drug delivery system in combination with an immune-oncology (IO) agent to enhance therapeutic efficacy in refractory cancers, such as PDAC. A method of treating cancer, by administering an effective amount of a pharmaceutical composition including an omega03 PUFA-taxoid conjugate in combination with an IO agent encapsulated in an NE drug delivery system to a subject in need of treatment, and treating cancer.
Owner:TARGAGENIX INC +2
Methods of treating gastrointestinal immune-related adverse events in immune oncology treatments
PendingUS20190040140A1Reduce gi-irAEsLow efficacyImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsImmunooncologyDiarrhea
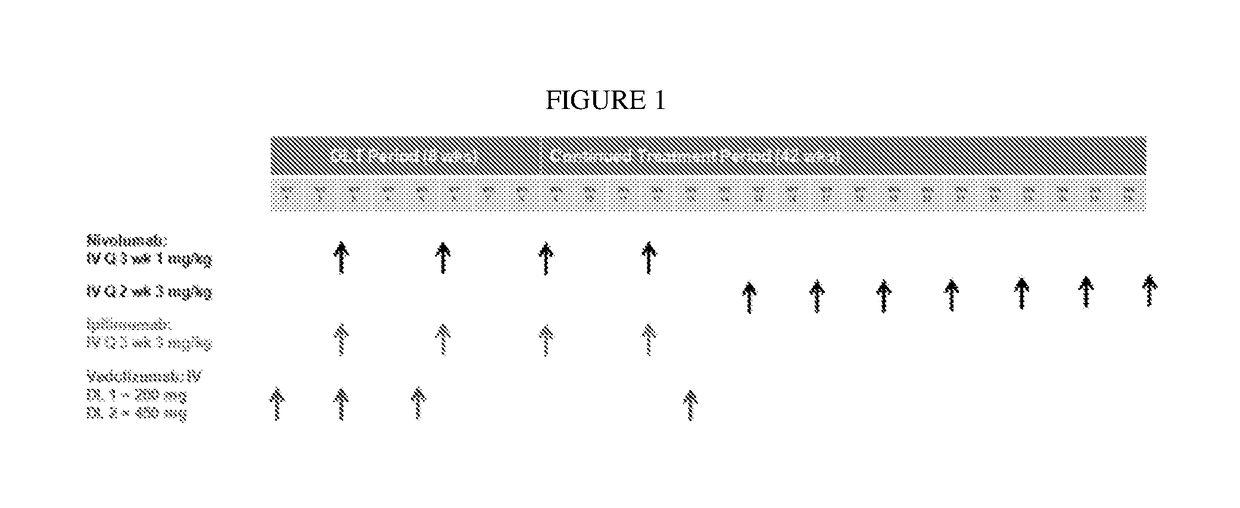
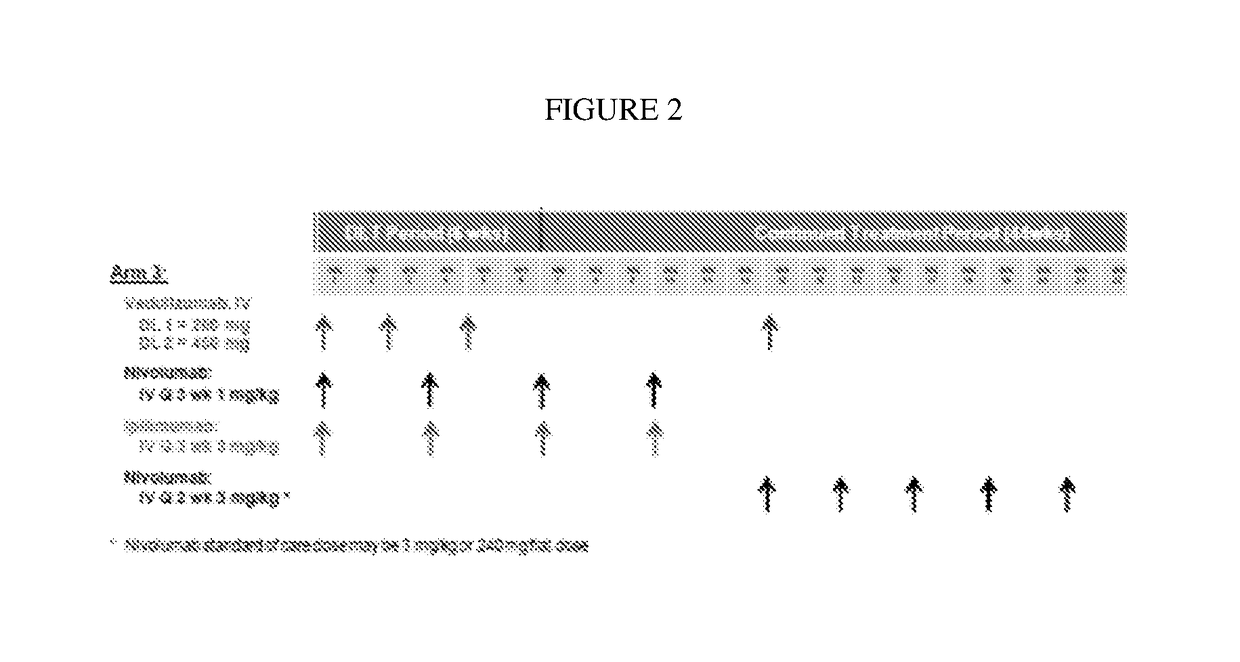
The invention provides, inter alia, methods of reducing gastrointestinal immunerelated adverse events, such as colitis and diarrhea, in subjects undergoing an immune treatment, such as an immune oncology treatment, such as anti-CTLA4 antibody and anti-PD-1 antibody combination treatment for melanoma. In certain aspects, the methods encompass administering a therapeutically effective amount of a polypeptide that inhibits MAdCAM-integrin binding, such as an anti-α4β7 integrin antibody, such vedolizumab or a related antibody.
Owner:TAKEDA PHARMA CO LTD
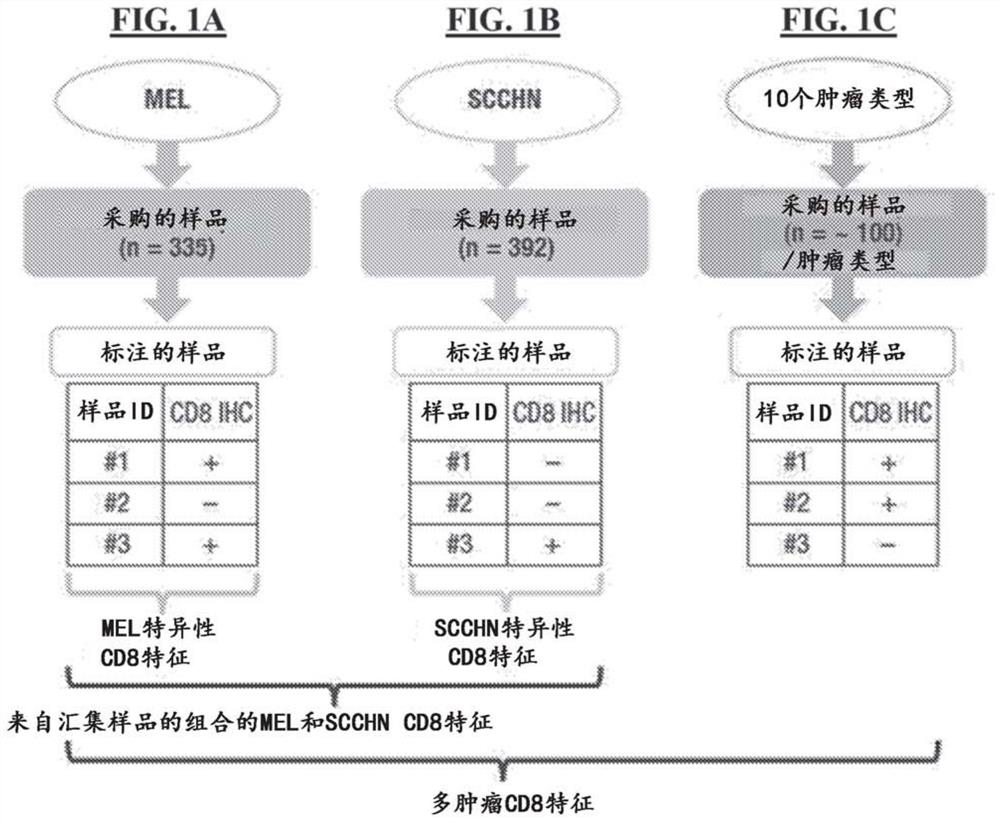
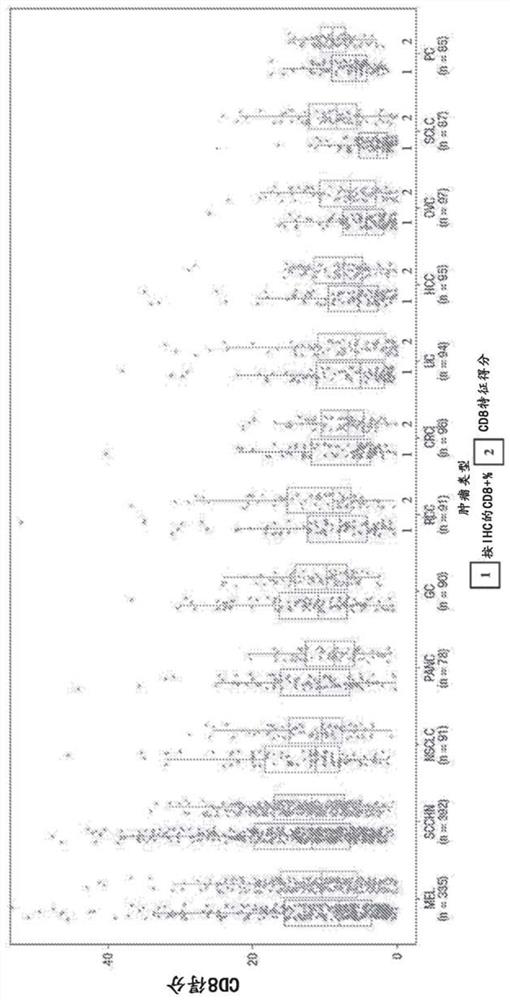
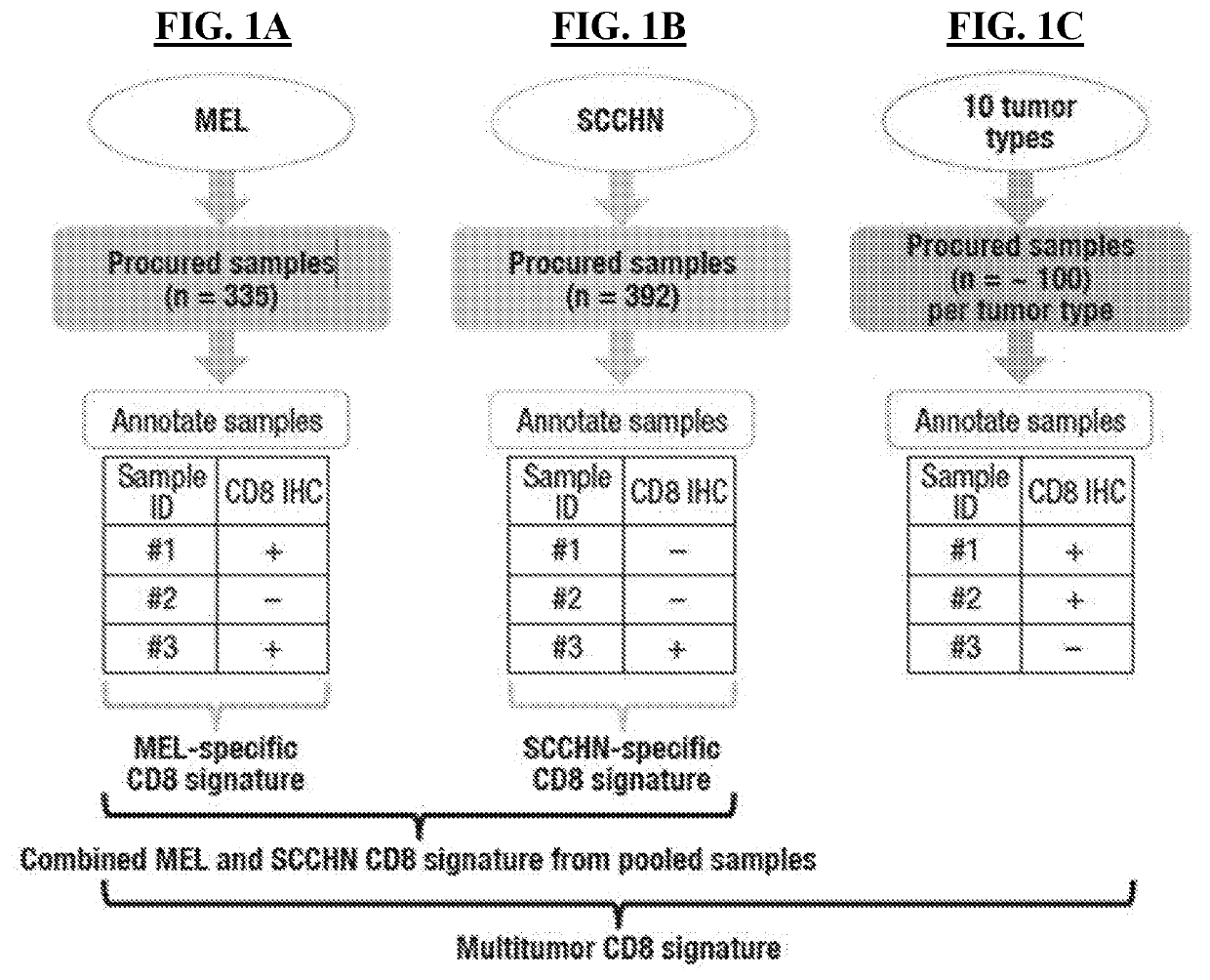
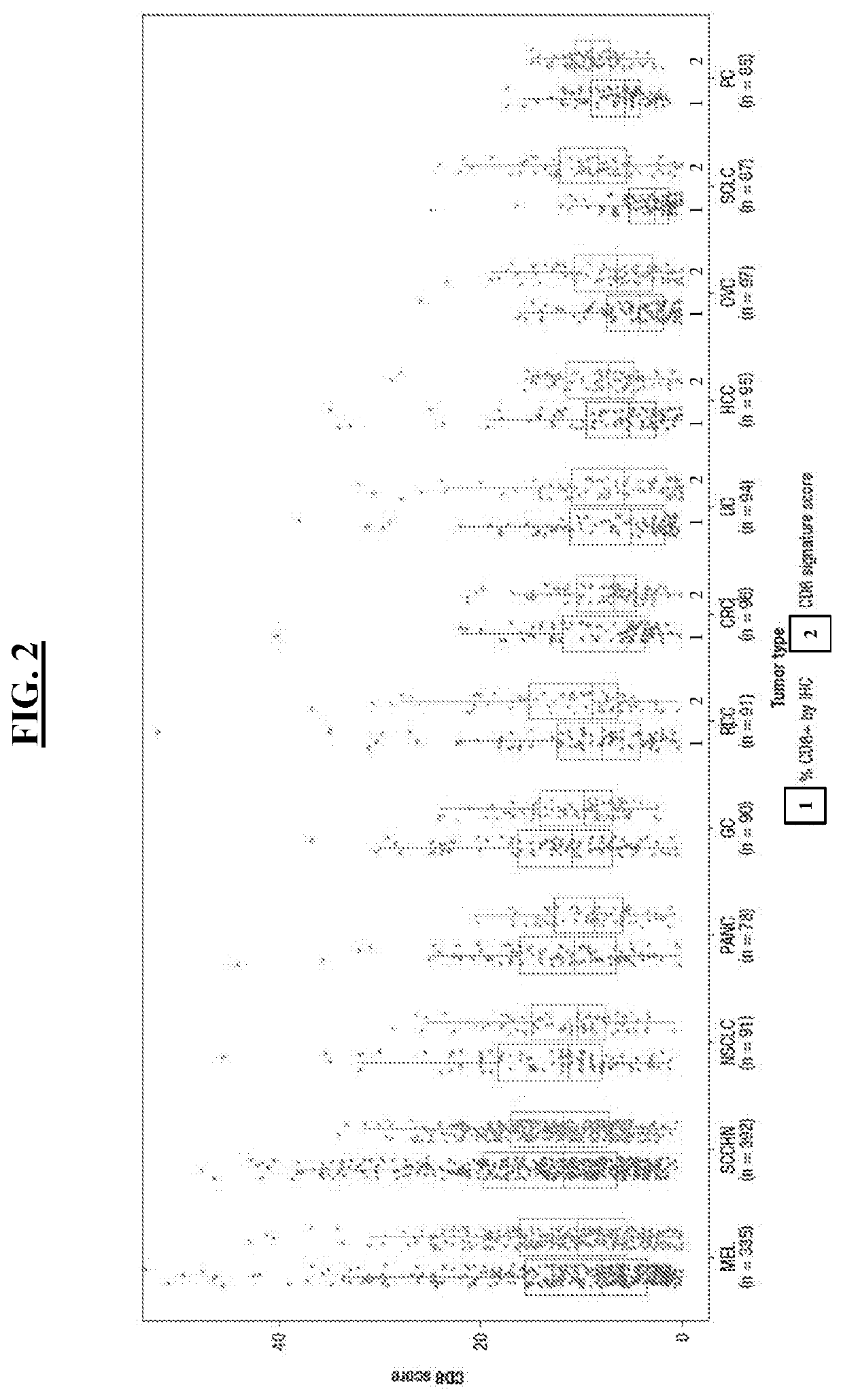
Multi-tumor gene signature suitable for immunooncology therapy
PendingCN114174538AMicrobiological testing/measurementAntibody ingredientsImmunooncologyAntigen binding
The present disclosure provides a method of identifying a subject suitable for immunooncology (I-O) therapy comprising measuring expression of one or more genes of a generic tumor inflammation genome set. In some aspects, the method further comprises administering an I-O therapy to the subject. In some aspects, the I-O therapy comprises administering to the subject an anti-PD-1 antibody, or an antigen-binding portion thereof, or an anti-PD-L1 antibody, or an antigen-binding portion thereof.
Owner:BRISTOL MYERS SQUIBB CO
Use of tivozanib to treat subjects with refractory cancer
Disclosed is a method of treating cancer, e.g., refractory cancer, with tivozanib. The methods disclosed include, for example, administering tivozanib as a second or third-line therapy to subjects suffering from refractory advanced renal cell carcinoma where traditional therapies as well as more recent targeted and immune-oncology therapies have not adequately treated the subject.
Owner:AVEO PHARM INC
In vivo in situ induced CAR-T cell delivery system targeting tumor and its application
ActiveCN113384690BOvercoming Systemic Security IssuesAvoid process problemsAntibody medical ingredientsAntineoplastic agentsImmunooncologyNanocarriers
The invention discloses a delivery system for targeting tumors in vivo in situ inducing CAR-T cells and applications thereof, belonging to the technical field of immuno-oncology. The delivery system includes nanoparticles and a nanocarrier formed by wrapping the nanoparticles with a platelet membrane; wherein, the nanoparticles are assembled from a cationic polymer, a CAR gene plasmid and a CRISPR system, and the CRISPR is delivered through the nanocarrier The system enables in situ editing of T cells into the tumor. The present invention realizes in situ editing of T cells in solid tumors, improves the tumor microenvironment, and enhances the proliferation and persistence of CAR-T cells. Compared with traditional CAR-T, the invention has higher safety, simple process and low cost.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Multi-tumor gene signature for suitability to immuno-oncology therapy
PendingUS20220363760A1Shrink tumorSmall sizeMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsAntigen bindingImmunooncology
The present disclosure provides methods of identifying a subject suitable for an immunooncology (I-O) therapy comprising measuring the expression of one or more genes of a pantumor inflammation gene panel. In some aspects, the method further comprises administering an I-O therapy to the subject. In some aspects, the I-O therapy comprises administering an anti-PD-1 antibody or antigen-binding portion thereof or an anti-PD-L1 antibody or antigen-binding portion thereof to the subject.
Owner:BRISTOL MYERS SQUIBB CO
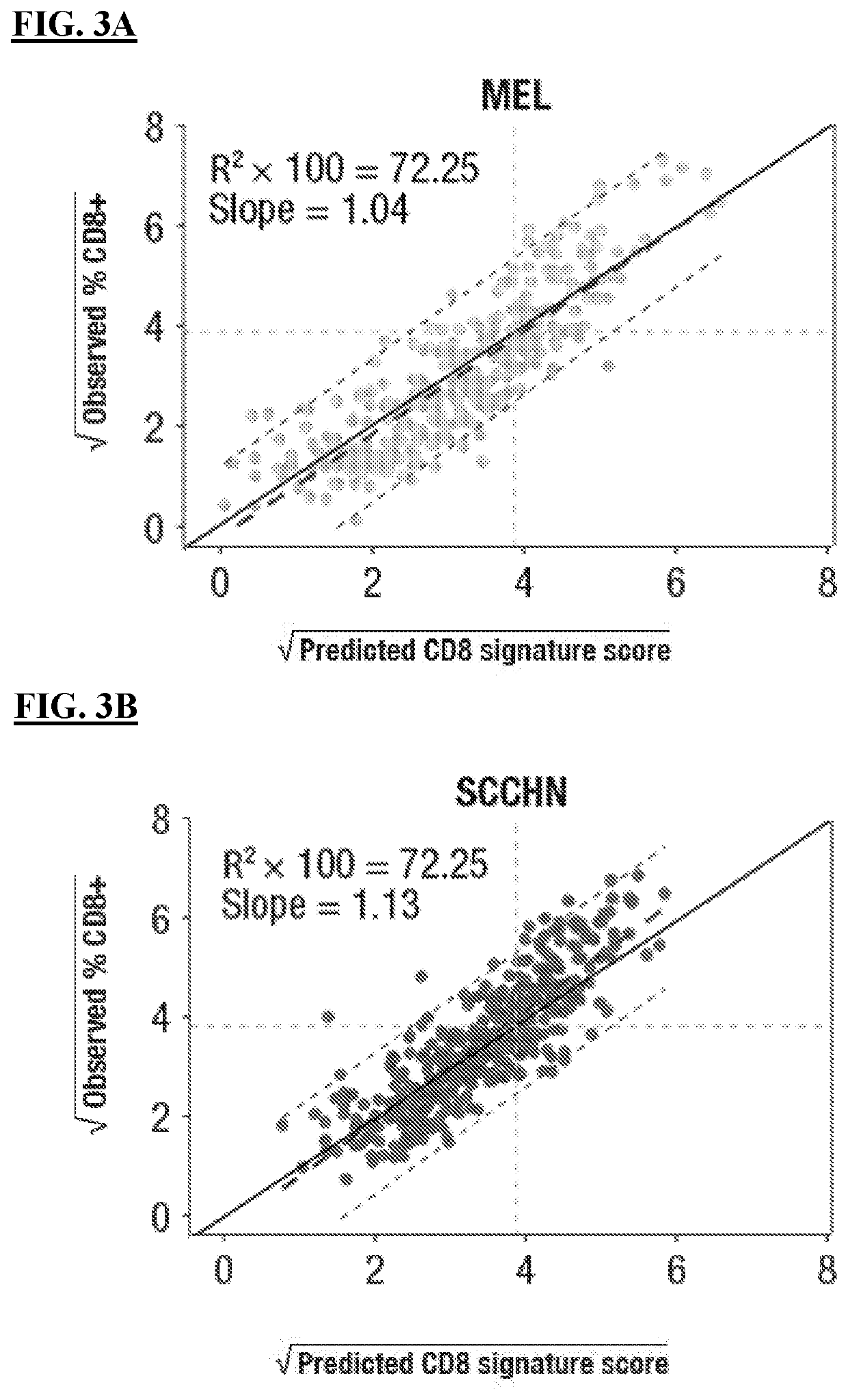
Cell localization signature and combination therapy
PendingUS20220233691A1Shrink tumorSmall sizeMicrobiological testing/measurementAntibody ingredientsImmunooncologyAntiendomysial antibodies
The present disclosure provides methods of identifying a subject suitable for an immuno-oncology (I-O) therapy comprising measuring the expression of one or more of STAT1, IFNγ, NECTIN2, and CSF1R. In some aspects, the I-O therapy comprises administering an anti-PD-1 antibody or antigen-binding portion thereof or an anti-PD-L1 antibody or antigen-binding portion thereof to the subject.
Owner:BRISTOL MYERS SQUIBB CO
Immuno-oncology mesodermal progenitor (ioMP) cell
ActiveUS10316291B2Good immune regulationIncreasing and decreasing T cell responseCulture processMammal material medical ingredientsImmunooncologyMedicine.oncology
The invention relates to immuno-oncology mesodermal progenitor (ioMP) cells and their use in therapy.
Owner:CELL THERAPY LTD
Anti-pd-1 antibodies, method for producing same and method for using same
PendingCN110023335AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsImmunooncologyAntigen
The present invention relates to biotechnology and comprises isolated monoclonal antibodies, in particular human monoclonal antibodies, which specifically bind to PD-1 with high affinity. The antibodies according to the invention may be chimeric, humanized or human antibodies, or antigen-binding fragments thereof, and may be used as a medicinal agent in oncology and immuno-oncology, for treating diseases related to various disorders of cell proliferation and development. The invention also relates to methods for producing the indicated antibodies, and to a method for treating human diseases using said antibodies.
Cannabinoids and derivatives for promoting immunogenicity of tumor and infected cells
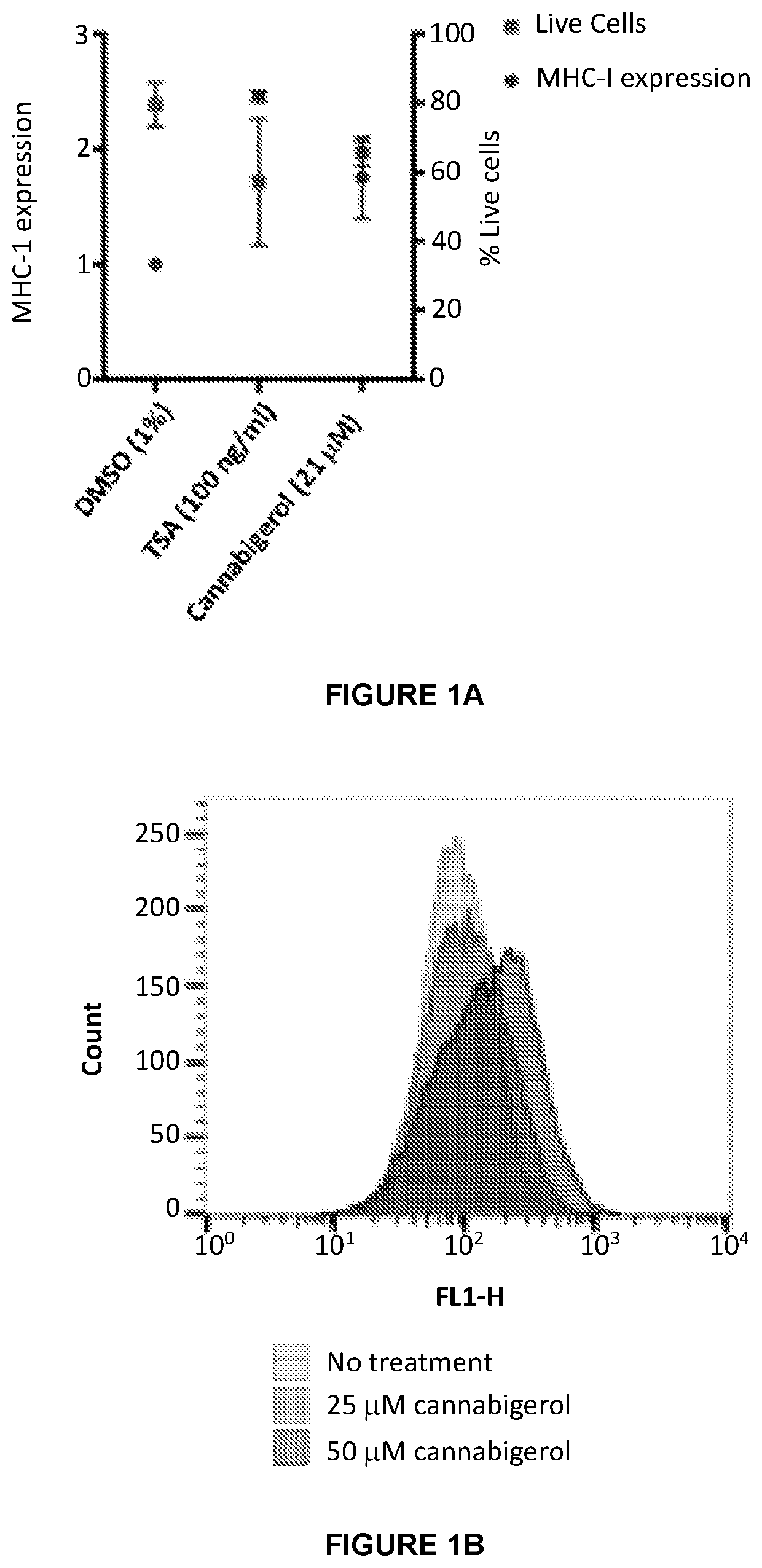
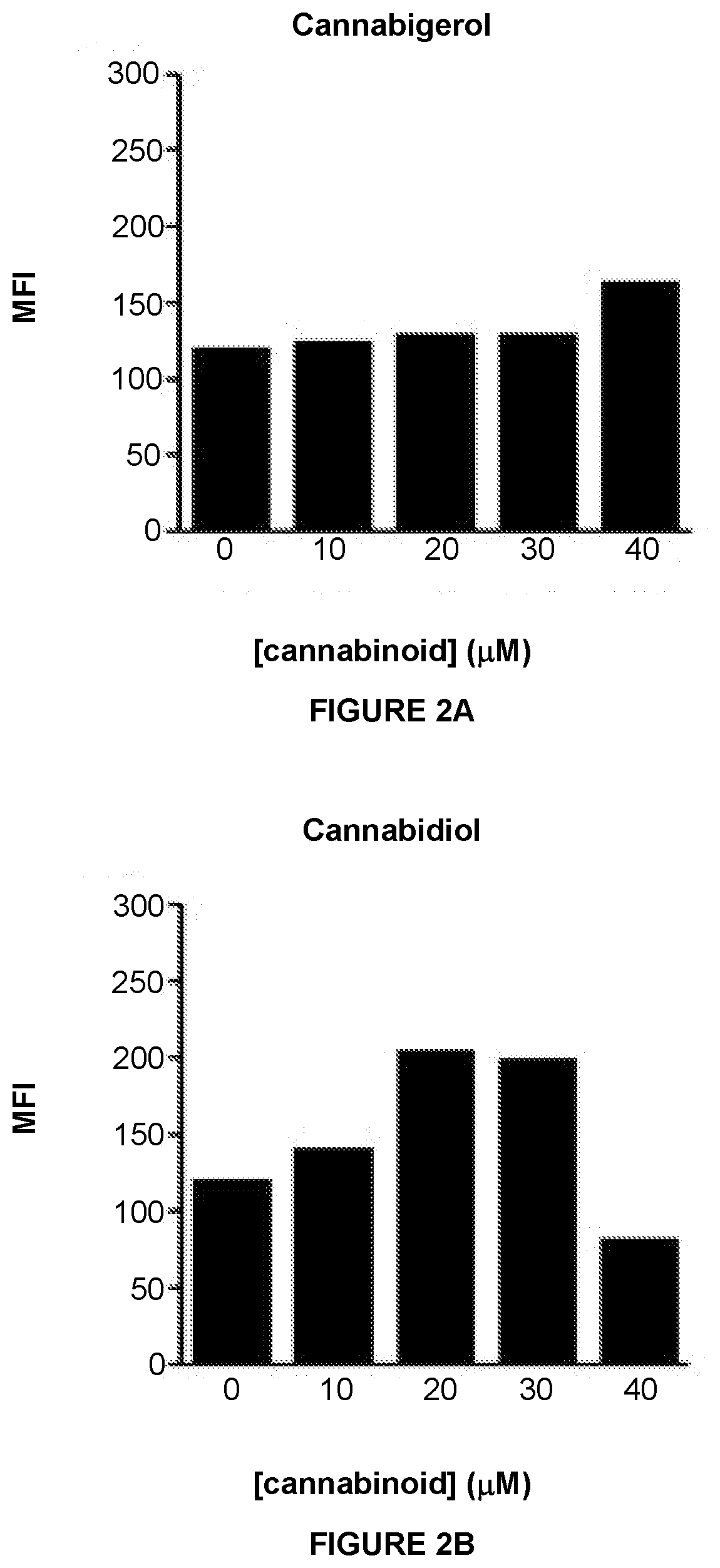
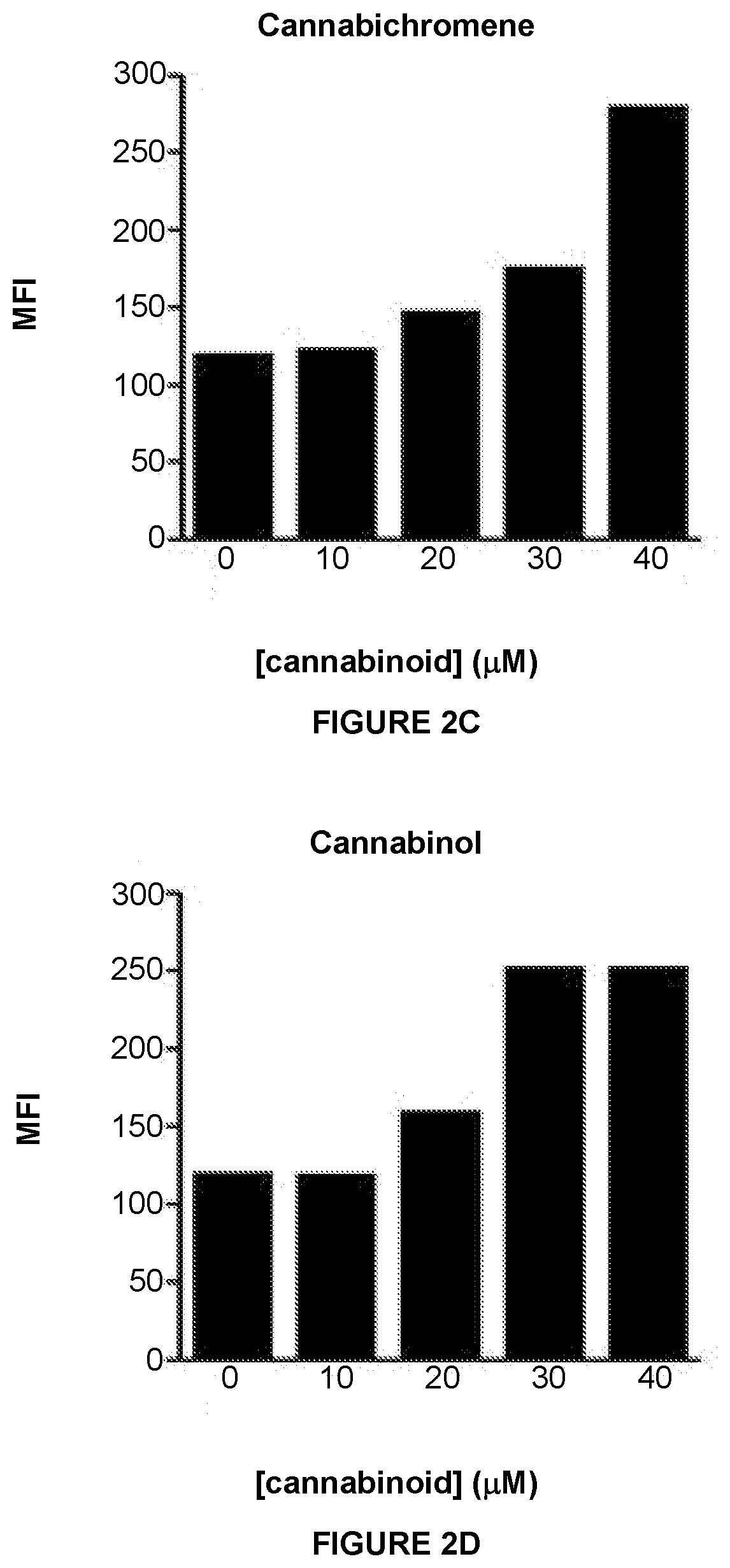
InactiveUS20210038559A1Improving immunogenicityEasy to demonstratePeptide/protein ingredientsHydroxy compound active ingredientsMHC class IImmunooncology
This invention provides processes and compositions for enhancing the immunogenicity of tumor or infected cells by increasing expression of major histocompatibility complex (MHC) class I surface molecules. Tumor cells often circumvent immune recognition by reducing or eliminating MHC expression. The lack of MHC on their surface allows tumor cells to evade immune detection and enables uncontrolled growth. Similarly, certain viral or microbial infections result in MHC class I downregulation and the resulting immune evasion. With a unique reporter assay, we have determined that some cannabinoid compounds and structural analogs can increase MHC class I expression on tumor cells grown in cell culture. The increase of tumor and infected cell MHC class I expression will enable detection and destruction by cytotoxic T-lymphocyte cells. The process and compositions increase the immunogenicity of the target cells, e.g. tumor or infected cells, to enhance their destruction by cytotoxic lymphocytes. When administered in combination, such compositions may enhance the activity of immune-oncology and anti-infectious disease agents.
Owner:PASCAL BIOSCI INC
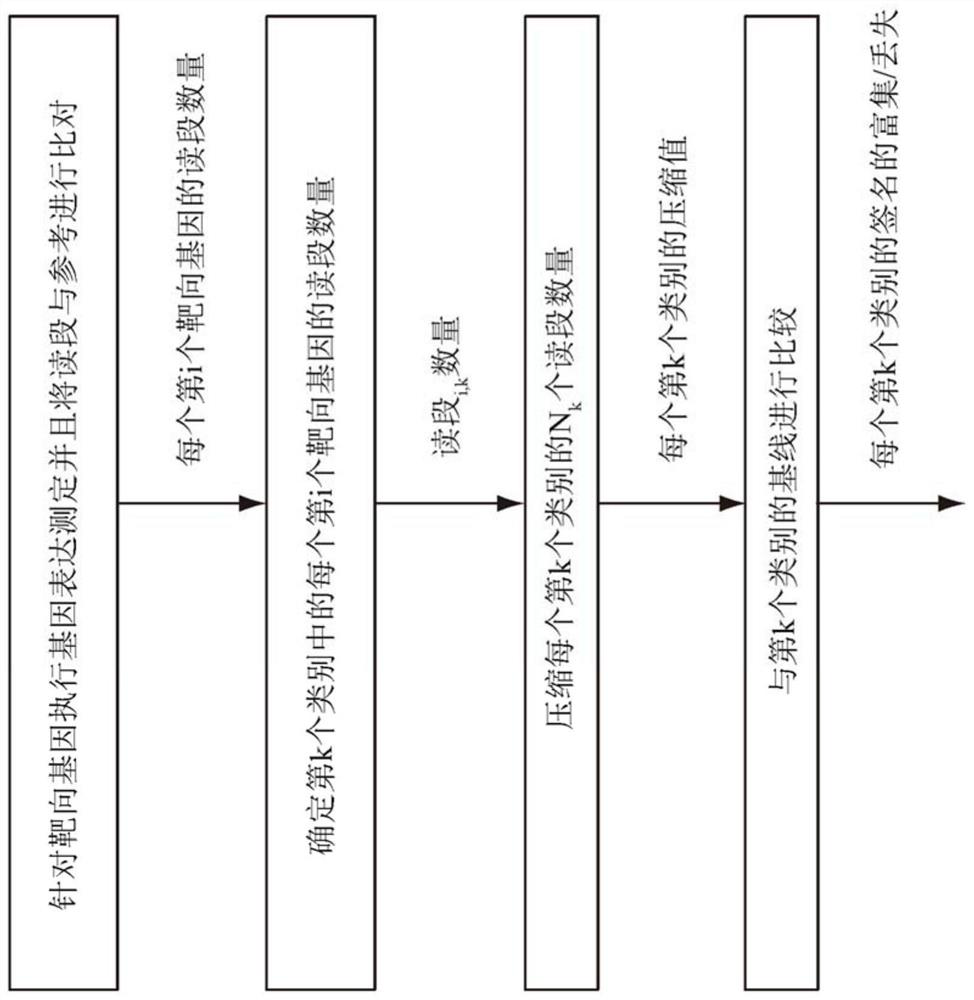
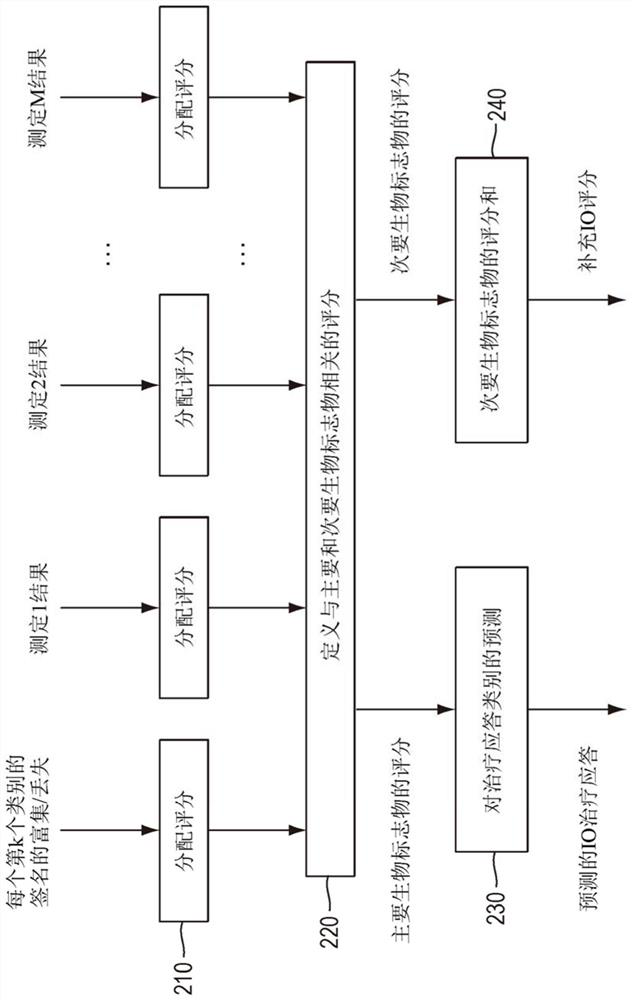
Methods for context based compression of genomic data for immuno-oncology biomarkers
The method includes compressing numbers of reads data for targeted genes of a gene expression assay performed on a test sample. The targeted genes are organized into categories. Each category represents a functional context associated with the targeted genes in that category. The numbers of reads corresponding to targeted genes each category is compressed to form a compressed value for the category. The compressed value is compared to a baseline value for the category to determine an enrichment or a loss of a signature corresponding to the functional context of the category. The method may include analyzing information from multiple assays performed on the test sample, assigning a score value to each assay result and predicting a response to immune-oncology treatment based on the assigned scores.
Owner:LIFE TECH CORP
Binding protein-toxin conjugates comprising anthracyclines, and use thereof in immune-oncological applications
PendingCN112805037ATetracycline active ingredientsPharmaceutical non-active ingredientsImmunooncologyToxin Conjugates
The present invention relates to binding protein-toxin conjugates comprising one or more anthracycline toxin moieties, and the use thereof in immunooncological applications.
Owner:NBE THERAPEUTICS
Method of Treatment of Neuroendocrine Tumors That Over-Express Somatostatatin Receptors
PendingUS20220143227A1Reducing tumorigenicityPrevent proliferationImmunoglobulins against cell receptors/antigens/surface-determinantsRadioactive preparation carriersImmunooncologyChalone receptors
The present invention relates to methods of treating cancers that over-express somatostatin receptors. More specifically, the invention provides a combined therapy in which a combination of Peptide Receptor Radionuclide Therapy (PRRT) and Immuno Oncology therapy (I-O therapy) are administered for the treatment of neuroendocrine tumors.
Owner:ADVANCED ACCELERATOR APPLICATIONS SA
Cell localization features and combination therapy
PendingCN114174537AMicrobiological testing/measurementAntibody ingredientsImmunooncologyAntiendomysial antibodies
The present disclosure provides a method of identifying a subject suitable for immunooncology (I-O) therapy, the method comprising measuring the expression of one or more of STAT1, IFN [gamma], NECTIN2, and CSF1R. In some aspects, the I-O therapy comprises administering to the subject an anti-PD-1 antibody, or an antigen-binding portion thereof, or an anti-PD-L1 antibody, or an antigen-binding portion thereof.
Owner:BRISTOL MYERS SQUIBB CO
Tumor targeted radionuclide therapy and molecular imaging of HER2+ cancers and other neoplasms
ActiveUS11357874B2Reducing tumorigenicityPrevent proliferationImmunoglobulins against growth factorsRadioactive preparation carriersImmunooncologyTumor targeting
Methods and compositions for treating, diagnosing and staging cancers, in particular overexpressing the Human Epidermal growth factor Receptor 2 protein (HER2+) given rise to in breast, gastric, gastroesophageal, ovarian, pancreatic cancer and brain tumors, which may be metastatic to the brain or other site. More specifically, the invention provides for Targeted Radionuclide Therapy (TRNT) with a compound of the invention having a peptide that targets the HER2+ cells, a second component for combining metals into complexes through a ring structure (DOTA), and a third radioisotope component, Lu-177 and Ga-68, in which embodiments further include a companion diagnostic, and in which embodiments further include anti-integrin precision medicines for cancers expressing αvβ3 and αvβ5 integrins, HER2+, vascular endothelial growth factor, vitronectin, fibronectin, tenascin, reelin, kindlin and talin. TRNT may be administered alone or in combination with standard-of-care; an immunooncologic and / or chemotherapeutic, adjuvantly or neoadjuvantly.
Owner:SATZ STANLEY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com