Tumor-targeted delivery system for in-vivo in-situ induction of CAR-T cells and application of tumor-targeted delivery system
A delivery system and cell technology, applied in the field of immuno-oncology, can solve problems that do not involve solid tumor treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
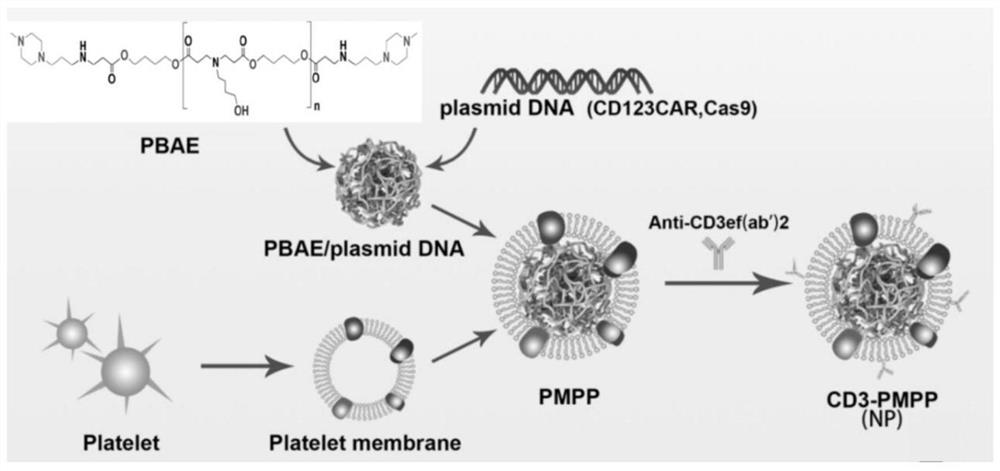
[0039] Example 1 Delivery system for in situ induction of CAR-T cells targeting tumors
[0040] 1. Preparation of PBAE / pDNA
[0041] Dilute PBAE, CD123 gene and CRISPR plasmids (purchased by the company) in 25mM sodium acetate solution respectively, add the PBAE solution dropwise to the same volume of CRISPR plasmid solution and mix, then add the CD123 gene plasmid and mix well, let it stand for 15 minutes at room temperature, and automatically Assemble and construct PBAE / pDNA nanoparticles (such as figure 1 shown), the ratio of CD123 gene and CRISPR plasmid is 1:1, and the ratio of PBAE and CD123 gene plasmid is 30:1.
[0042] 2. Preparation of platelet membrane-wrapped PBAE / pDNA nanocarriers
[0043] After C57BL / 6J mice were anesthetized, the living heart was taken into an anticoagulant tube, added platelet separation solution, centrifuged at 300g for 15 minutes, the first layer of plasma layer was taken into a sterile centrifuge tube, and the same volume of sample diluent...
Embodiment 2
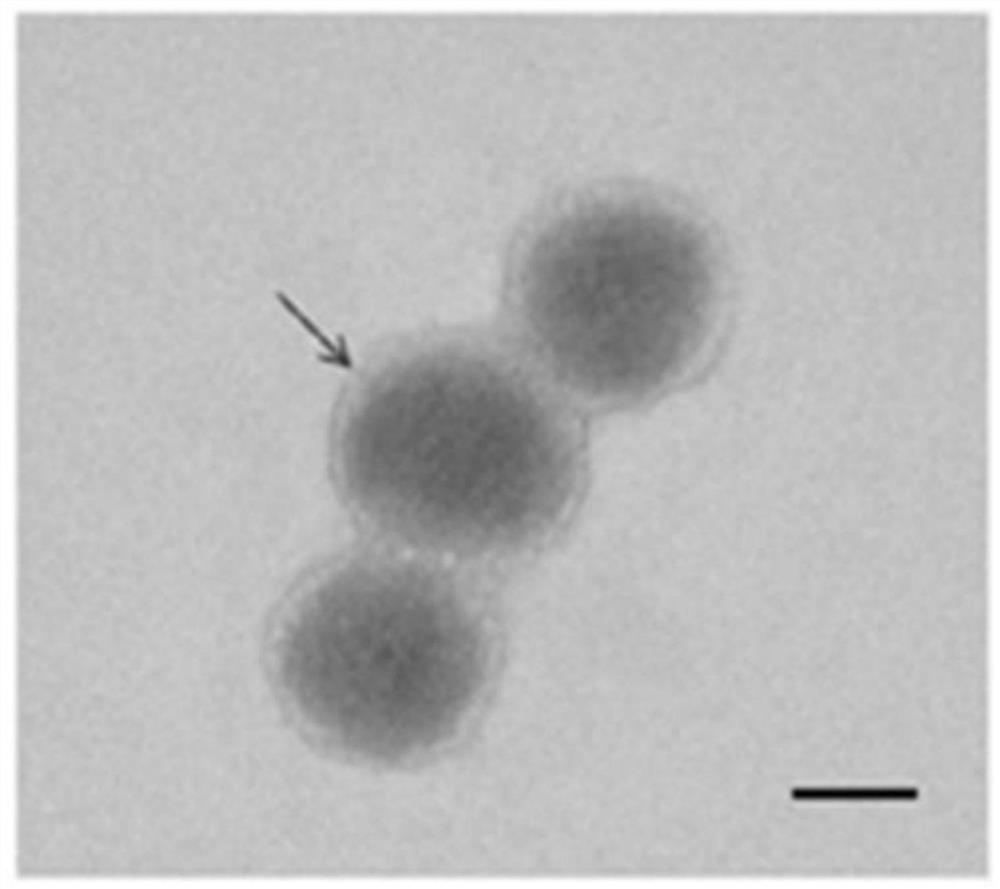
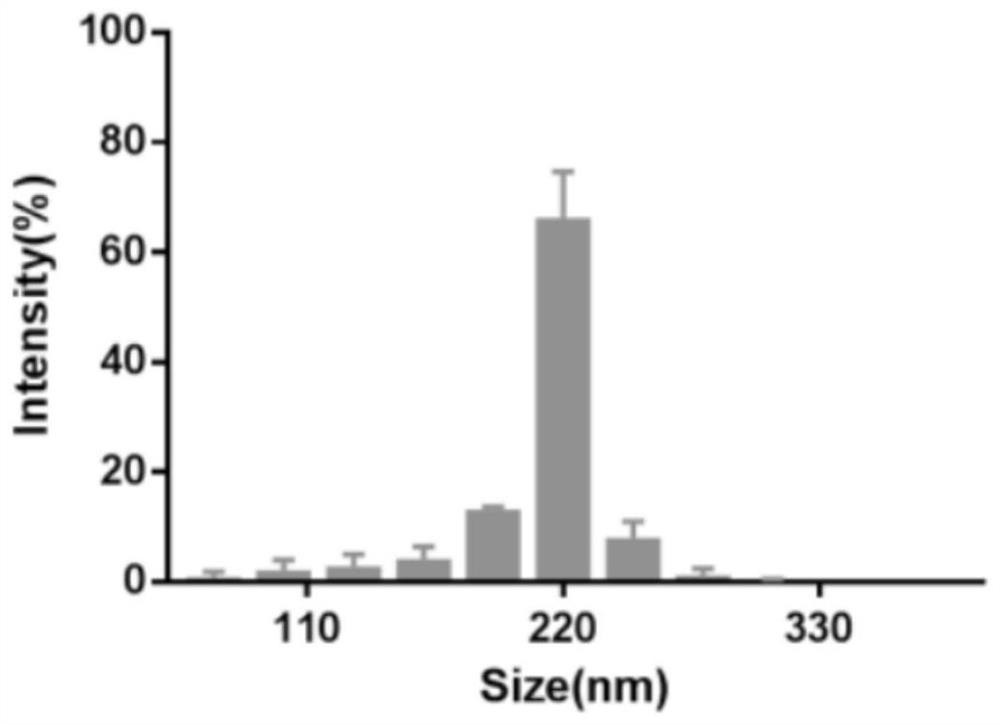
[0045] Characterization and Identification of Embodiment 2 Nanoparticles
[0046] Utilize transmission electron microscope to observe the form of the nano-carrier prepared in embodiment 1, draw 5ul nano-carrier and dilute 3 times with deionized water, then draw a small amount of diluted sample and drip on the copper grid, the result is as follows figure 2 As shown, the particle size of nanometer is 150-200nm.
[0047] Utilize the dynamic light scattering laser nanometer particle size analyzer to measure the size and zeta potential of nanoparticles, take 1mL samples of nanocarriers respectively and put them into the particle size analyzer sample cell and the potential measurement sample cell, the results are as follows image 3 with Figure 4 As shown, the main particle size of nanoparticles is distributed at 220nm, and the Zeta potential of the last wrapped nanometer is -18mV.
Embodiment 3
[0048] Example 3 Efficiency Detection of In vitro Transfection of Plasmids into T Cells by Nanoparticles
[0049] After the pure pDNA, PBAE / pDNA and platelet membrane-wrapped PBAE / pDNA groups were cultured with mouse spleen-derived T cells (PBMC), the transfection efficiency was observed with a confocal microscope at 20 min and 120 min after transfection. The result is as Figure 5 As shown, the platelet membrane-wrapped PBAE / pDNA nanocarrier has a good transfection efficiency compared with the plasmid pDNA group alone.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com