Binding protein-toxin conjugates comprising anthracyclines, and use thereof in immune-oncological applications
A technology for binding proteins and conjugates, which is applied in the field of binding protein-toxin conjugates, and can solve problems such as no efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0081] According to one embodiment of the present invention, the binding protein binds to at least one target selected from the group consisting of:
[0082] ·ROR1
[0083] ·CS1
[0084] ·HER2
[0085] Mesothelin (MN), and / or
[0086] ROR2.
[0087] Among them, ROR1 is a preferred target.
[0088] These targets and antibodies against them are described in the following publications:
[0089] · US2019112385A1 (mesothelin),
[0090] ·US2019153092A1, WO2019016381(ROR1),
[0091] ·WO2019030240A1(CS-1),
[0092] · US2018028682 (HER2) and
[0093] · WO2019016392A1 (ROR2),
[0094] Its content is incorporated herein by reference.
[0095] Preferably, said target is of human origin. In particular, when referred to herein, ROR1 preferably refers to human ROR1.
[0096] The term "immune checkpoint inhibitor" as used herein refers to any binding agent or compound suitable to act against an immune checkpoint protein. In particular, "immune checkpoint inhibitor" refers to any ...
Embodiment 1
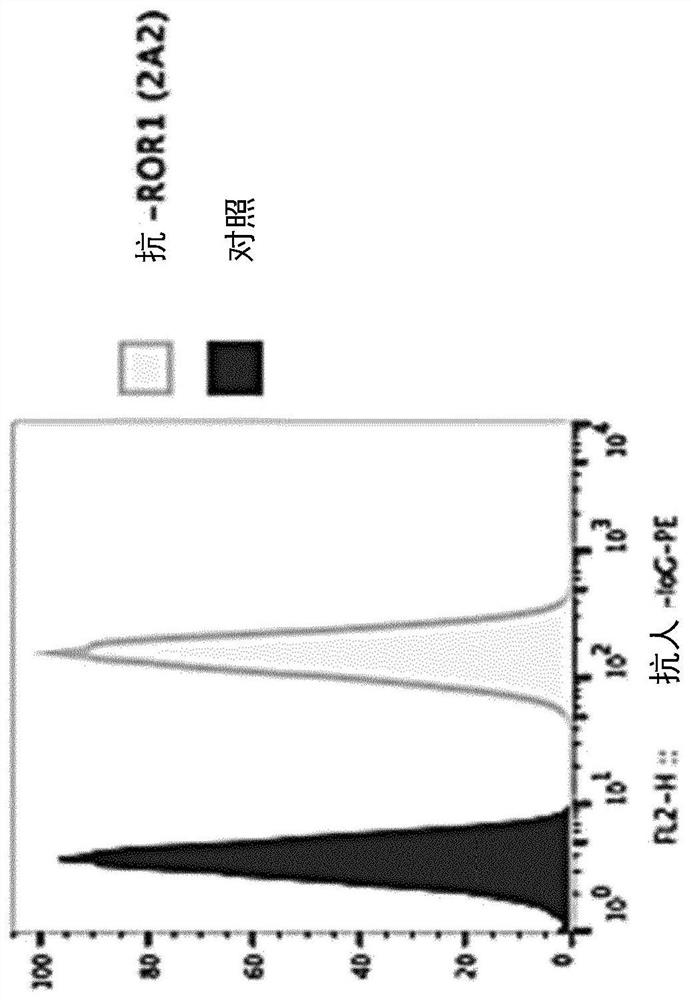
[0258] Example 1. Production of purified, recombinant anti-human ROR1 and isotype control antibodies
[0259] Expression vectors: antibody variable region coding regions were generated by total gene synthesis (GenScript), using MNFGLRLIFLVLTLKGVQC as a leader sequence, and human IgH-γ1 and IgL-κ or IgL-λ constant regions as appropriate in expression vector pCB14 Assemble. This vector is a derivative of the episomal mammalian expression vector pCEP4 (Invitrogen), carries the EBV origin of replication, encodes the EBV nuclear antigen (EBNA-1) to allow extrachromosomal replication, and contains a puromycin selectable marker in place of the initial tide Mycin B resistance gene.
[0260] Expression and purification: The pCB14-based expression vector was transfected into HEK293T cells, using LTX reagent with PLUS TM (Thermo Fisher Scientific, Reinach, Switzerland, 15388100); followed by incubation for 1 day (37°C, 5% CO 2 , growth medium: Duchenne's modified Eagle medium (DMEM)...
Embodiment 2
[0265] Example 2. SMAC-Technology for mAbs TM Conjugation of glycine modified toxins to form ADCC
[0266] Sortase A. According to the technique described in WO2014140317A1, the recombinant and affinity-purified sortase A based on Staphylococcus aureus was produced in Escherichia coli.
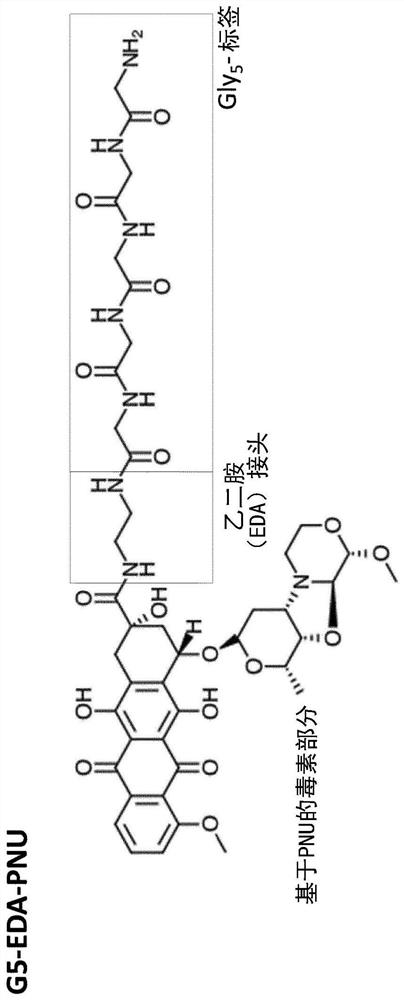
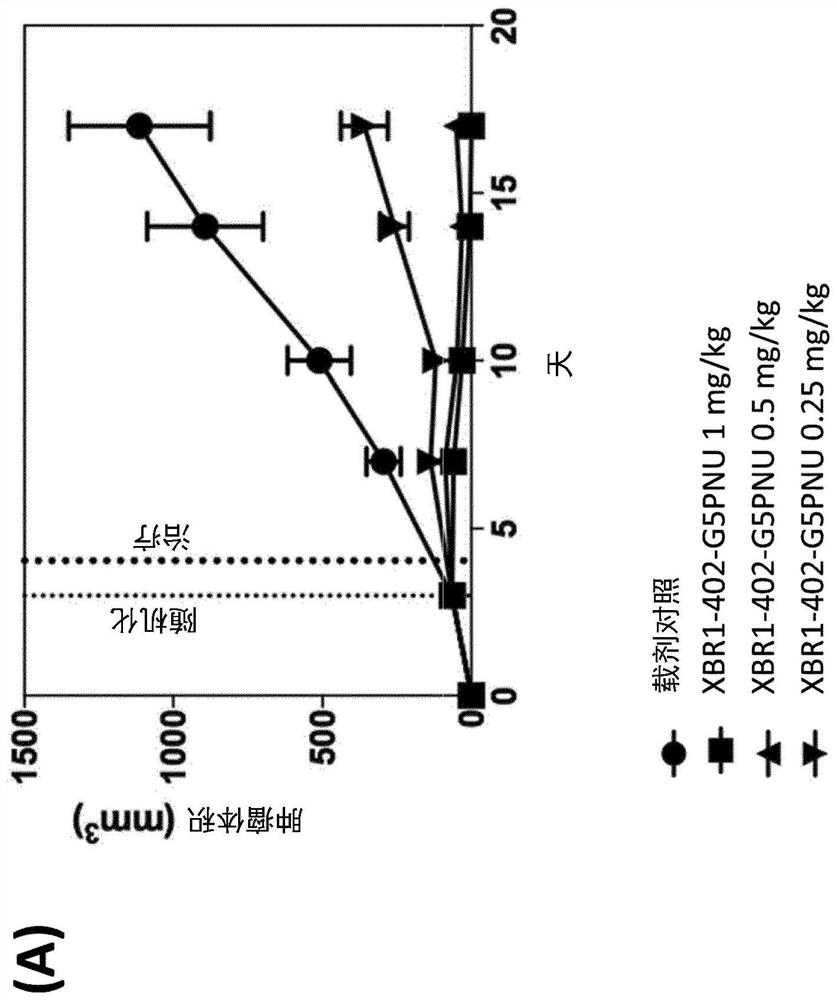
[0267] Production of glycine-modified toxins. For the production of SMAC-technologies TM The conjugated ADC, Pentaglycine-EDA-PNU derivative (G5-EDA-PNU) was produced by Concortis (eg figure 1 shown). The identity and purity of the pentaglycine-modified toxin were confirmed by mass spectrometry and HPLC, respectively. G5-EDA-PNU showed >95% purity as determined by HPLC chromatography.
[0268] Sortase-mediated antibody conjugation. Conjugate antibodies to the above toxins according to Table 2: LPETG-tagged mAb [10 μM] with glycine-modified toxin [200 μM] and 3 μM sortase A in listed conjugation buffer at 25 °C Incubate for 3.5h. The reaction was terminated by passing it over a protein A ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com