Anti-pd-1 antibodies, method for producing same and method for using same
A PD-1, antibody technology, applied in biochemical equipment and methods, antibodies, antibody medical components, etc., can solve problems such as no significant cross-reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0207] Designing Natural Human Antibody Fab-Library MeganLibTM
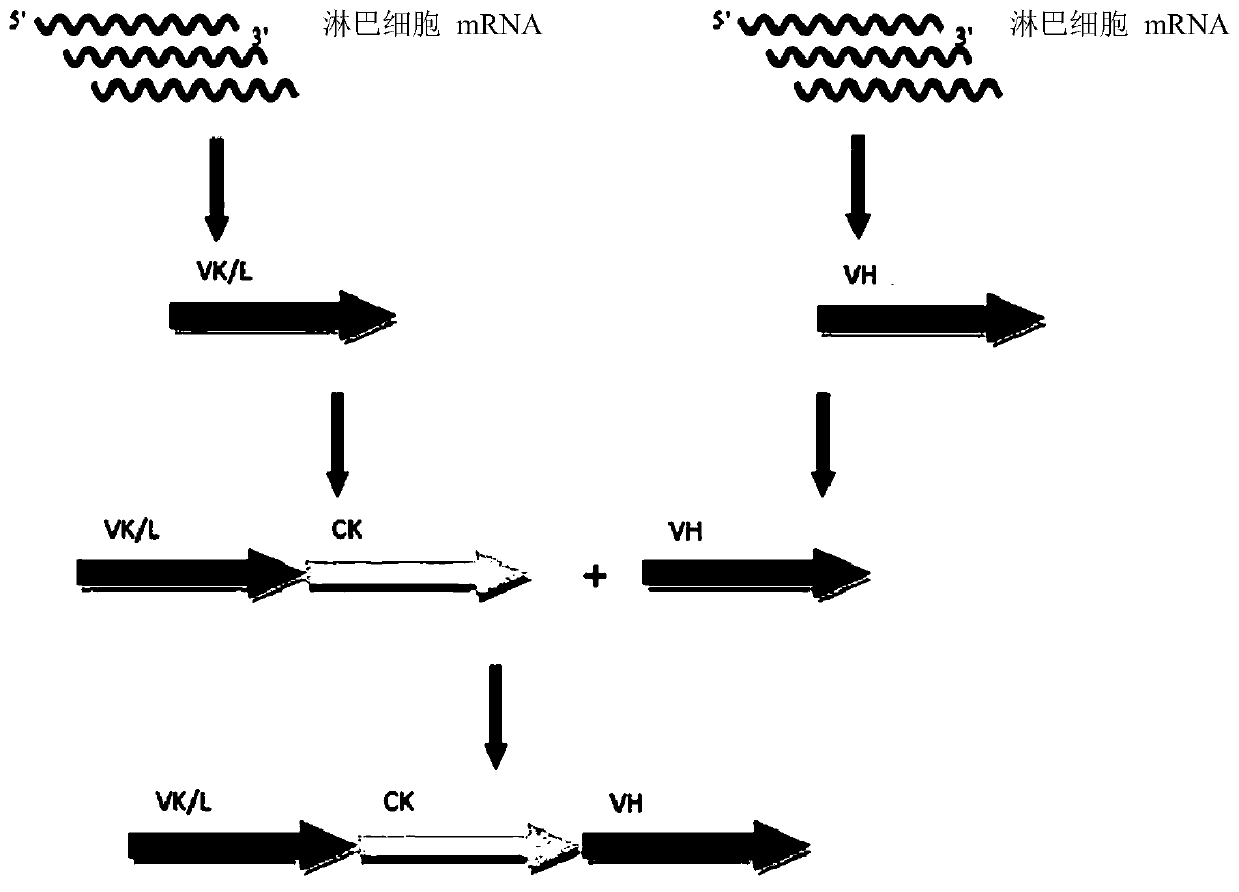
[0208] Total RNA in B lymphocytes from blood samples of more than one thousand individual human donors was isolated using a kit (RNeasy Mini Kit) according to the suggested protocol (Qiagen, Germany). RNA concentration determination was performed using Nanovue kit (GE Healthcare); the quality of isolated RNA was detected by 1.5% agarose gel electrophoresis.
[0209] Reverse transcription reactions were performed using MMLV RT kit (Anolen) according to the recommended protocol with MMuLV reverse transcriptase and random hexamer oligonucleotides as primers.
[0210] Reverse transcription products were used as substrates in a two-stage polymerase chain reaction to obtain genes with variable domains flanked by restriction sites; using oligonucleotide kits and [Journal of Biological Chemistry, June 1999 , 25; 274(26):18218-30] for the reaction.
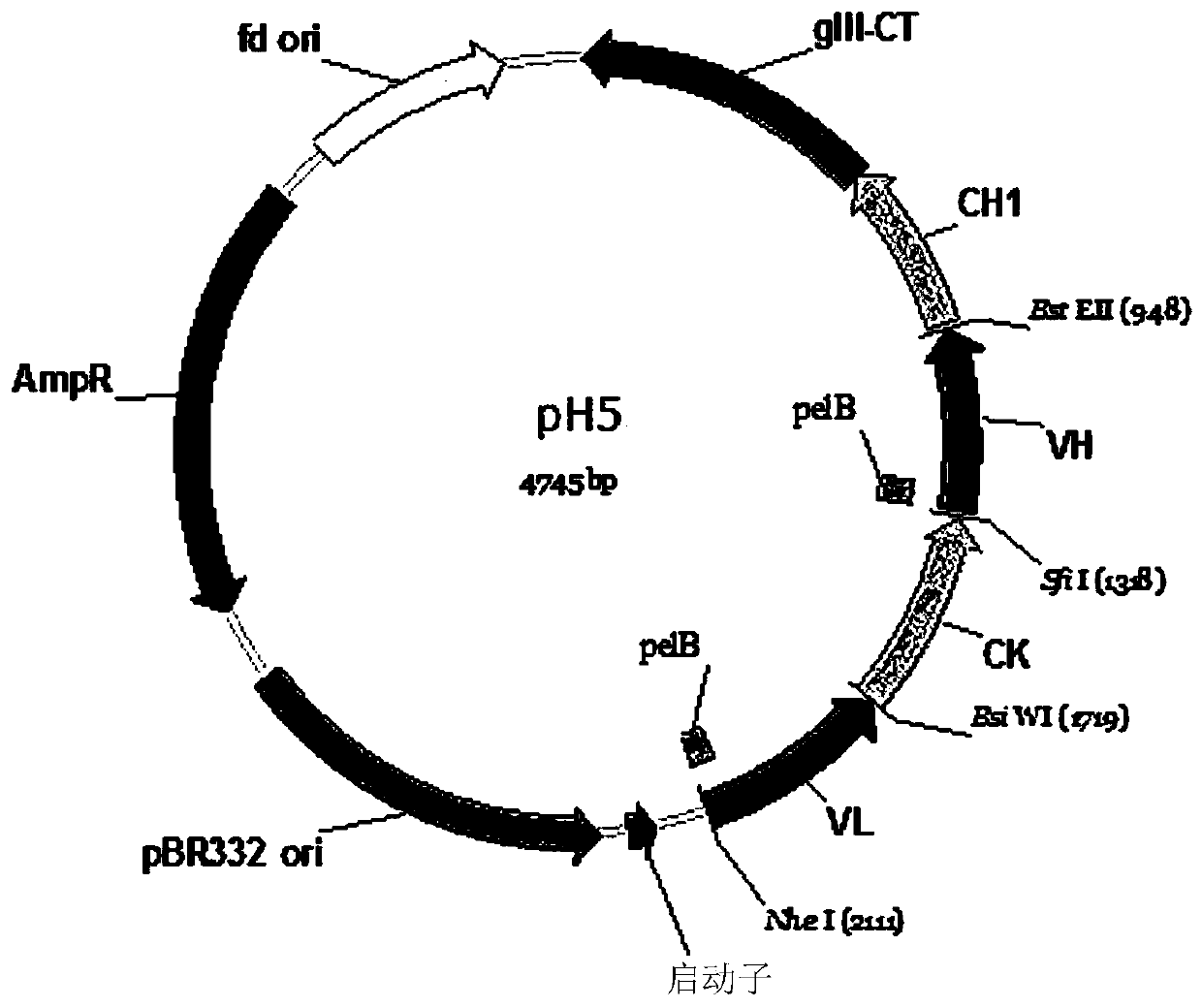
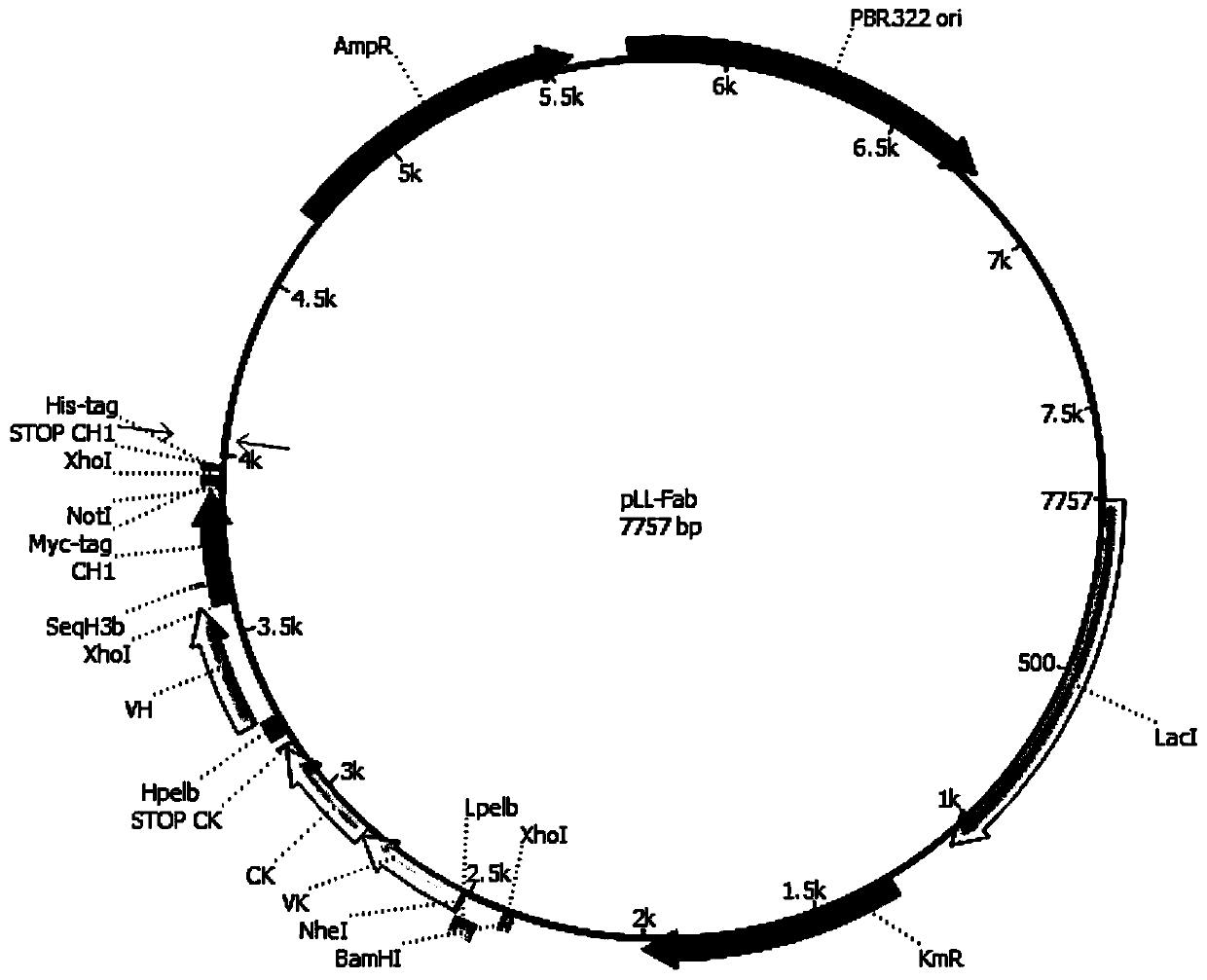
[0211] The obtained DNA preparation VL-CK-VH ( figure 1 ) was treated...
Embodiment 2
[0213] Selection of fab-libraries of phage antibodies
[0214] Specific anti-PD-1 phage Fab-antibodies were isolated from MeganLibTM's combinatorial library phage Fab-display library. Selection was performed using recombinant human PD-1, under conditions as previously described (Journal of Biochemistry, June 25, 1999, 274(26):18218-30; Nature Biotechnology, March 1996, 14(3) :309-14; Journal of Molecular Biology, 5 Dec. 1991, 222(3):581-97). For the selection process by the panning method, human PD-1 in 50 mM carbonate buffer (pH 9.5) was adsorbed on the surface of a high pipette (Nanken, Denmark) overnight at 4°C. In addition, the tubes were washed with PBS (pH 7.4) and then blocked with a solution containing PBS (pH 7.4)-non-fat milk (0.5% w / v) for 1 hour. Then, 2-4 ml of phage solution (10 13 phage particles / ml) - non-fat milk (0.5% w / vol) was transferred to a tube with antigen and the system was incubated for 1 hour with agitation. Unbound phage were removed by a serie...
Embodiment 3
[0216] Analysis of Fab specific binding to human PD-1
[0217] ELISA was used to measure the binding of the tested Fab fragments to human PD-1. A Fab with published Nivolumab sequence (Bristol-Myers Squibb) was used as a positive control. For the analysis of specific binding, ELISA plate wells (medium binding from Grena First Biochemical) were filled with 50 μl of PD1-H6F (0.5 μg / ml in 1× carbonate coating buffer, pH 9.5) Coat, seal and incubate overnight at 4°C. All further stages were carried out according to standard ELISA protocols utilizing high performance automation platforms based on robotic systems such as Genetix Qpix2xt (Megumolecular) and Tecan Friedham EVO 200 (Tecan). Non-specific binding was blocked by adding blocking buffer BB (200 [mu]l 0.5% non-fat milk in PBS). The plate was incubated on a shaker at room temperature for 1 hour. After washing with PBS-Tween, each cell was coated with 50 μl of the cell supernatant containing the test Fab mixed with an equa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com