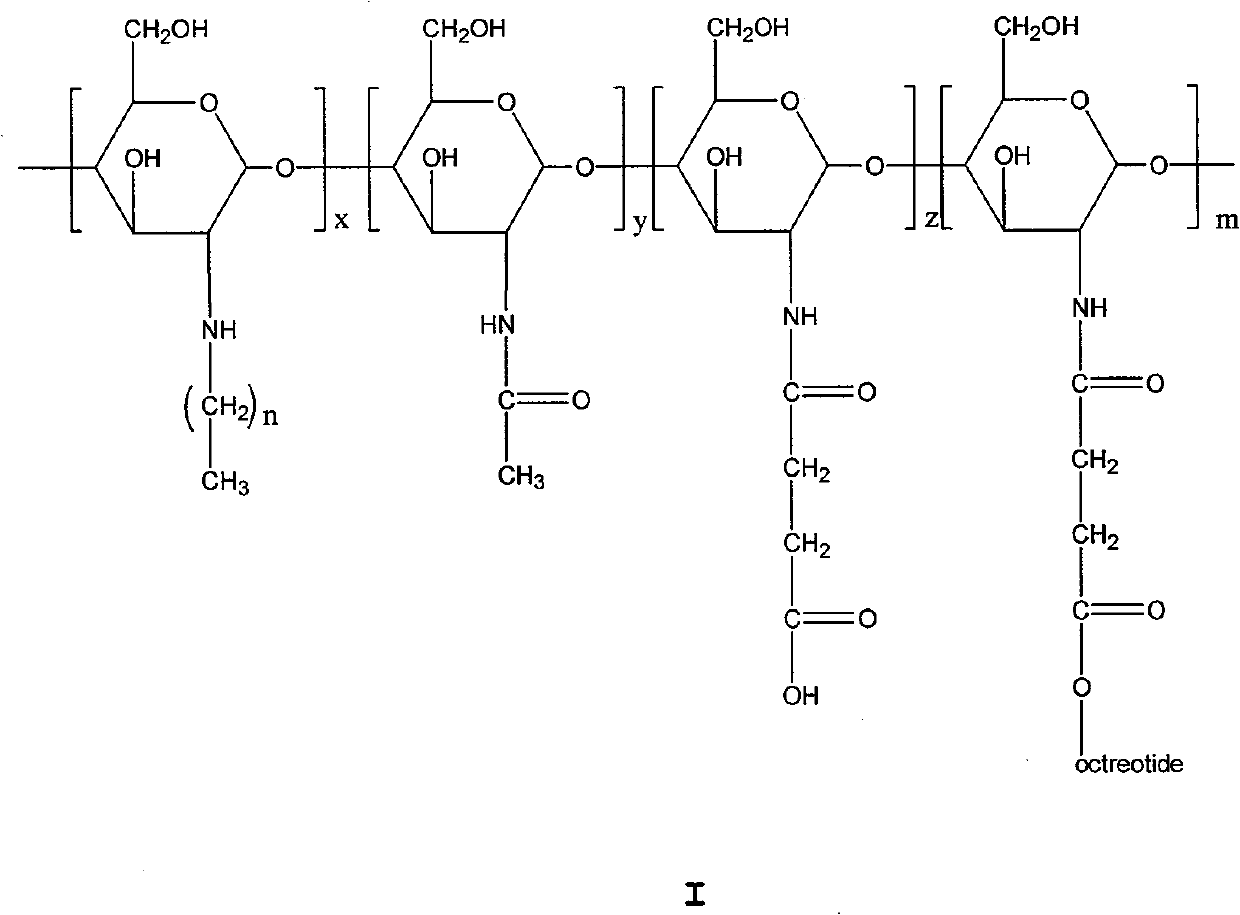
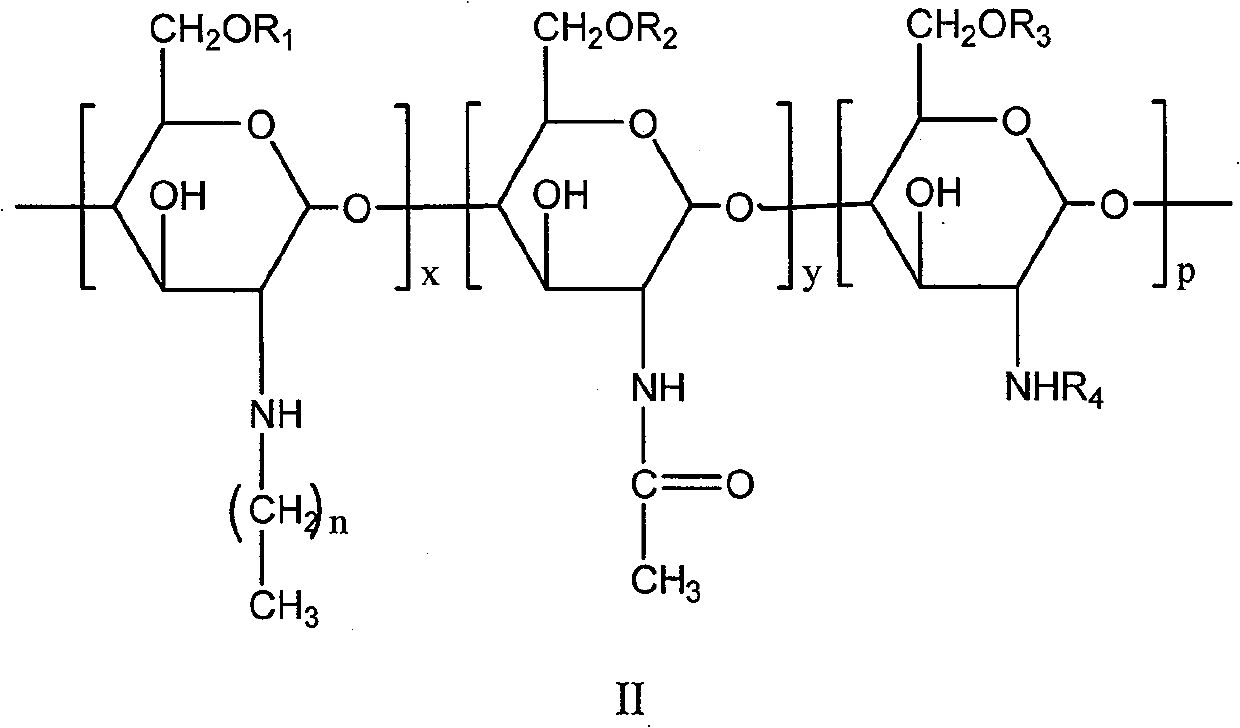
Chitose derivates using octreotide as target ligand and use thereof in medicament
A technology of chitosan derivatives and drugs, applied in the field of polymer chemistry, to achieve the effect of reducing toxic side effects and improving delivery efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1. Preparation of N-octyl chitosan (NOC)
[0038] Add 3.5 g of chitosan, 105 mL of water, and 2.2 mL of acetic acid to a 500 mL three-necked flask, and stir for 2 hours. 105 mL of anhydrous methanol and 1 mL of acetic anhydride were added to the three-necked flask, and the reaction was incubated for 6 h, and the pH of the reaction solution was adjusted to 7 with 1 molL of NaOH. Add 10.6mL octanal, react at room temperature for 36h, slowly add 5g KBH 4 , the reaction solution was reduced at room temperature for 24h, the pH of the reaction solution was adjusted to 7, filtered, washed twice with water, 4 times with methanol, 2 times with ether, and dried to obtain 4.0g of yellow powder (octyl substitution degree 65%).
[0039] 2. Preparation of N-octyl-N-succinyl chitosan (NONSC)
[0040] Take 1 g of N-octyl chitosan, suspend it in 50 mL of dimethyl sulfoxide, stir for 1 h, add 0.56 g of succinic anhydride, stir vigorously at 80 °C for 24 h, adjust the pH of the reaction...
Embodiment 2
[0048] 1. Preparation of N-nonylchitosan (NNC)
[0049] Use chitosan to react with acetic anhydride and nonanal, and use KBH 4 Reduction, that is, the preparation method is the same as the preparation of NOC in Example 1.
[0050] 2. Preparation of N-nonyl-N-succinyl chitosan (NNNSC)
[0051] It is prepared by reacting N-nonyl chitosan with succinic anhydride, and the preparation method is the same as the preparation of NONSC in Example 1.
[0052] 3. Preparation of Octreotide Grafted N-Nonyl-N-Succinyl Chitosan (NNNSOC)
[0053] It is prepared by reacting N-nonyl-N-succinyl chitosan with octreotide, and the preparation method is the same as the preparation of NONSOC in Example 1.
[0054] NNNSOC:
[0055] FT-IR: 3428, 2951, 2871, 1728, 1669, 1656, 1551, 1426, 1381, 1315, 1258, 1231, 1151, 1116, 1071, 1036, 868cm -1 .
[0056] 1 H NMR (500MHz, D 2 O): 7.5-6.9 (arom Phe), 4.6-4.5 (Hα Thr, Hα Lys, H 1 ), 4.2(HβThr), 4.1-4.0(HβThr-ol), 4.0-3.4(Hβ 3 , H 4 , H 5 , H 6 ...
Embodiment 3
[0059] 1. Preparation of N-dodecyl chitosan (NLC)
[0060] Use chitosan to react with acetic anhydride and lauraldehyde, and use KBH 4 Reduction, that is, the preparation method is the same as the preparation of NOC in Example 1.
[0061] 2. Preparation of N-dodecyl-N-succinyl chitosan (NLNSC)
[0062] It is prepared by reacting N-dodecyl chitosan with succinic anhydride, and the preparation method is the same as the preparation of NONSC in Example 1.
[0063] 3. Preparation of Octreotide Grafted N-Dodecyl-N-Succinyl Chitosan (NLNSOC)
[0064] It is prepared by reacting N-dodecyl-N-succinyl chitosan with octreotide, and the preparation method is the same as the preparation of NONSOC in Example 1.
[0065] NLNSOC:
[0066] FT-IR: 3423, 2956, 2863, 1729, 1668, 1656, 1549, 1420, 1381, 1316, 1259, 1236, 1158, 1112, 1066, 1033, 869cm -1 .
[0067] 1 H NMR (500MHz, D 2 O): 7.5-6.9 (arom Phe), 4.6-4.5 (Hα Thr, Hα Lys, H 1 ), 4.2 (Hβ Thr), 4.1-4.0 (Hβ Thr-ol), 4.0-3.3 (H 3 ,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com