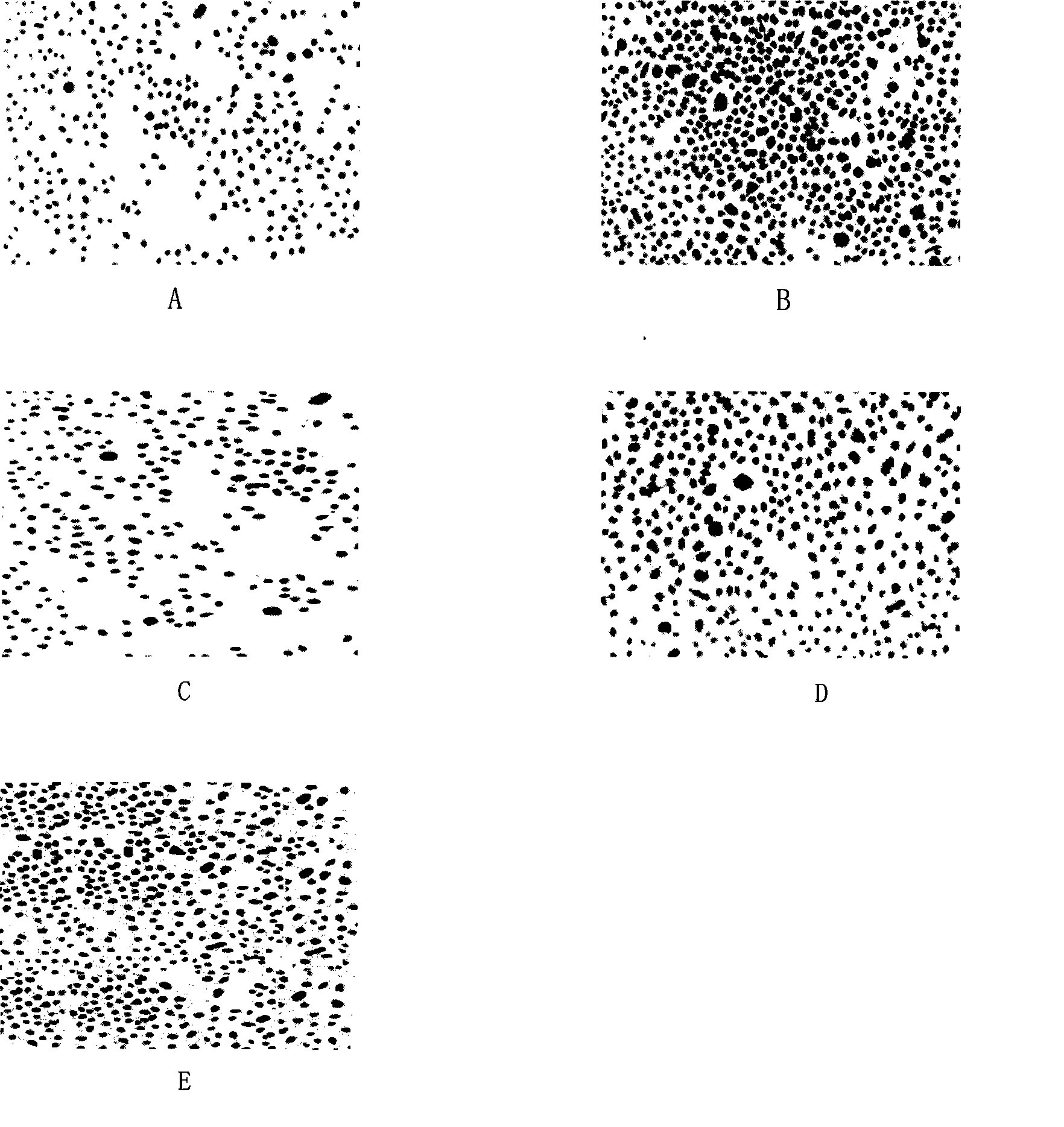
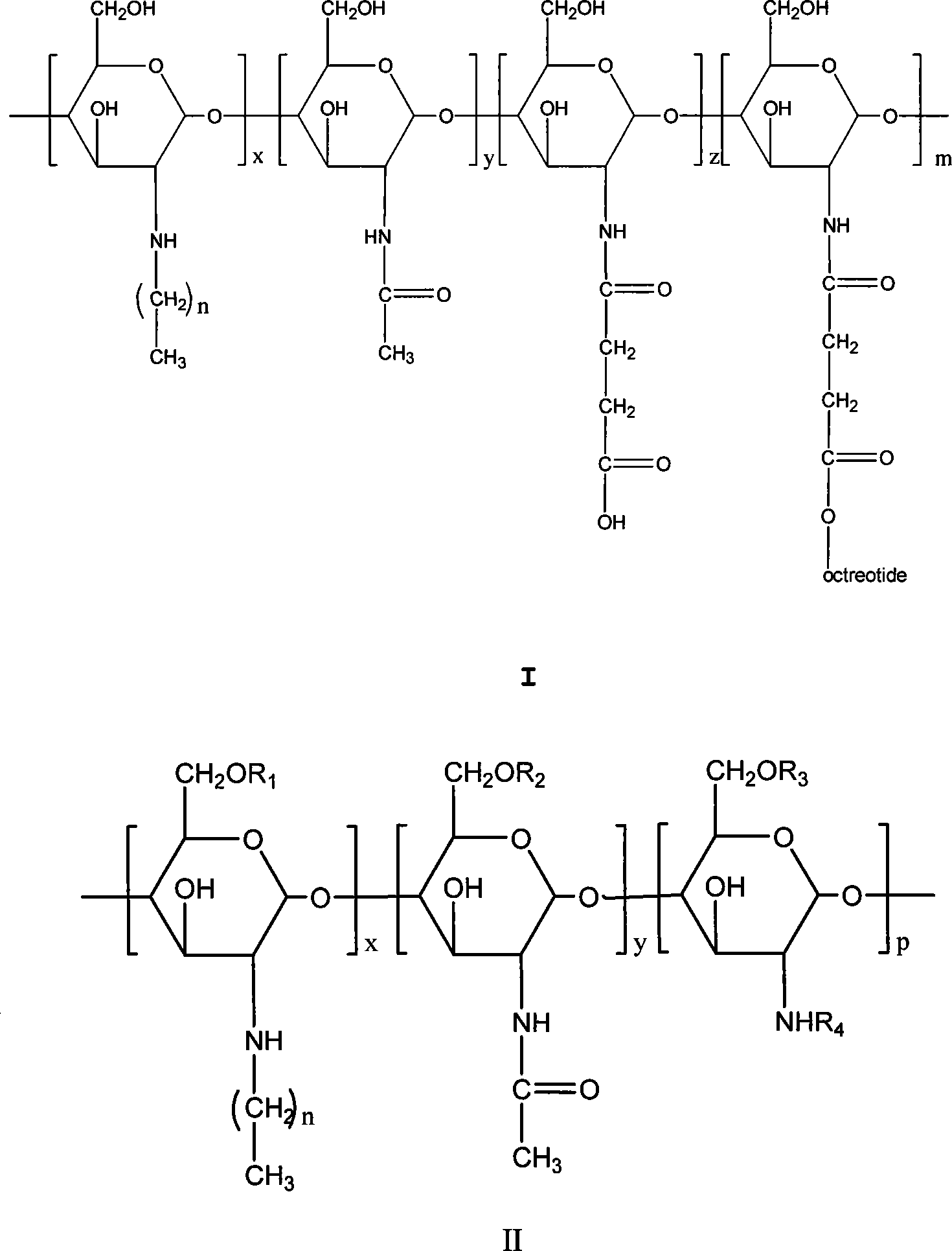
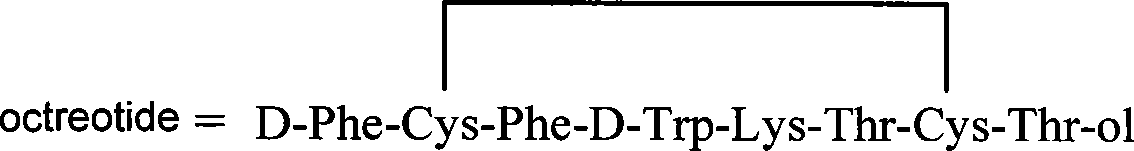Chitose derivates using octreotide as target ligand and use thereof in medicament
A technology for chitosan derivatives and drugs, which is applied in the field of polymer chemistry to reduce toxic side effects and improve delivery efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1. Preparation of N-octyl chitosan (NOC)
[0039] Add 3.5g of chitosan, 105mL of water, and 2.2mL of acetic acid into a 500mL three-necked bottle and stir for 2 hours, raise the temperature to 30°C, and keep stirring for 2h. Add 105 mL of anhydrous methanol and 1 mL of acetic anhydride to the three-necked flask, keep the reaction for 6 hours, and adjust the pH of the reaction solution to 7 with 1 molL NaOH. Add 10.6mL octanal, react at room temperature for 36h, slowly add 5g KBH 4 , reduction reaction at room temperature for 24 hours, adjusted the pH of the reaction solution to 7, filtered, washed twice with water, four times with methanol, twice with ether, and dried to obtain 4.0 g of yellow powder (octyl substitution degree 65%).
[0040] 2. Preparation of N-octyl-N-succinyl chitosan (NONSC)
[0041] Take 1g of N-octyl chitosan, suspend it in 50mL dimethyl sulfoxide, stir for 1h, add 0.56g of succinic anhydride, stir vigorously at 80°C for 24h, adjust the pH of the...
Embodiment 2
[0049] 1. Preparation of N-nonyl chitosan (NNC)
[0050] Use chitosan to react with acetic anhydride and nonanal, use KBH 4 Reduction, that is, the preparation method is the same as that of NOC in Example 1.
[0051] 2. Preparation of N-nonyl-N-succinyl chitosan (NNNSC)
[0052] It is prepared by reacting N-nonyl chitosan with succinic anhydride, and the preparation method is the same as that of NONSC in Example 1.
[0053] 3. Preparation of N-nonyl-N-succinyl chitosan grafted with octreotide (NNNSOC)
[0054] It is prepared by reacting N-nonyl-N-succinyl chitosan with octreotide, and the preparation method is the same as that of NONSOC in Example 1.
[0055] NNNSOC:
[0056] FT-IR: 3428, 2951, 2871, 1728, 1669, 1656, 1551, 1426, 1381, 1315, 1258, 1231, 1151, 1116, 1071,
[0057] 1036,868cm -1 .
[0058] 1 H NMR (500MHz, D 2 O): 7.5-6.9 (arom Phe), 4.6-4.5 (Hα Thr, Hα Lys, H 1 ), 4.2 (Hβ Thr), 4.1-4.0 (Hβ Thr-ol), 4.0-3.4 (H 3 , H 4 , H 5 , H 6 ), 3.2 (-NH-C H ...
Embodiment 3
[0061] 1. Preparation of N-Lauryl Chitosan (NLC)
[0062] Use chitosan to react with acetic anhydride and lauryl aldehyde, use KBH 4 Reduction, that is, the preparation method is the same as that of NOC in Example 1.
[0063] 2. Preparation of N-dodecyl-N-succinyl chitosan (NLNSC)
[0064] It is prepared by reacting N-dodecyl chitosan with succinic anhydride, and the preparation method is the same as that of NONSC in Example 1.
[0065] 3. Preparation of N-dodecyl-N-succinyl chitosan grafted with octreotide (NLNSOC)
[0066] It is prepared by reacting N-dodecyl-N-succinyl chitosan with octreotide, and the preparation method is the same as that of NONSOC in Example 1.
[0067] NLNSOC:
[0068] FT-IR: 3423, 2956, 2863, 1729, 1668, 1656, 1549, 1420, 1381, 1316, 1259, 1236, 1158, 1112, 1066, 1033, 869cm -1 .
[0069] 1 H NMR (500MHz, D 2 O): 7.5-6.9 (arom Phe), 4.6-4.5 (Hα Thr, Hα Lys, H 1 ), 4.2 (Hβ Thr), 4.1-4.0 (Hβ Thr-ol), 4.0-3.3 (H 3 , H 4 , H 5 , H 6), 3.2 (-NH...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com