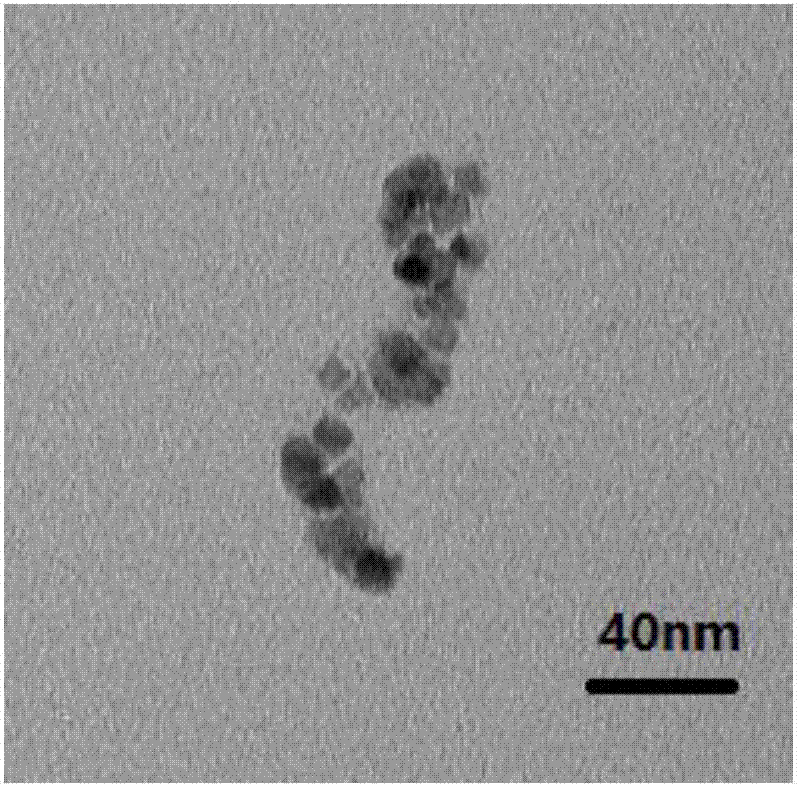
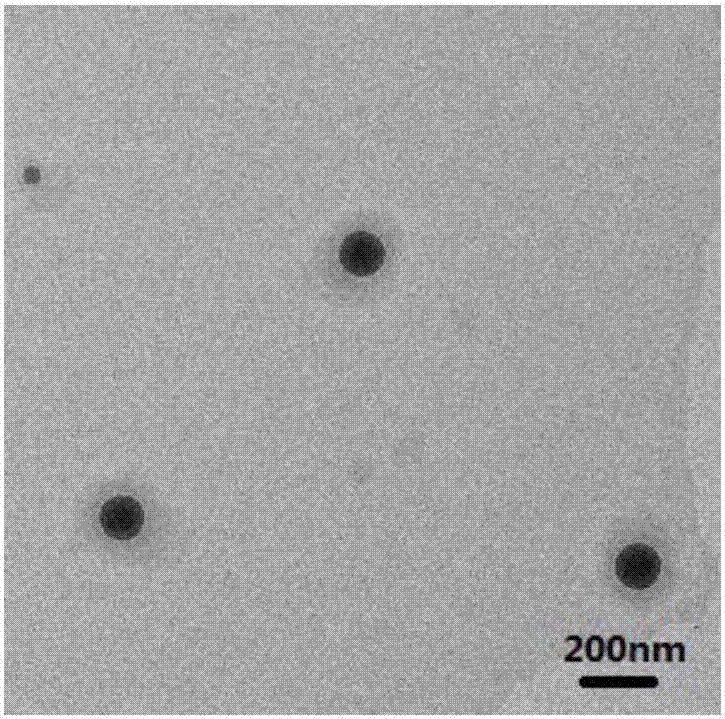
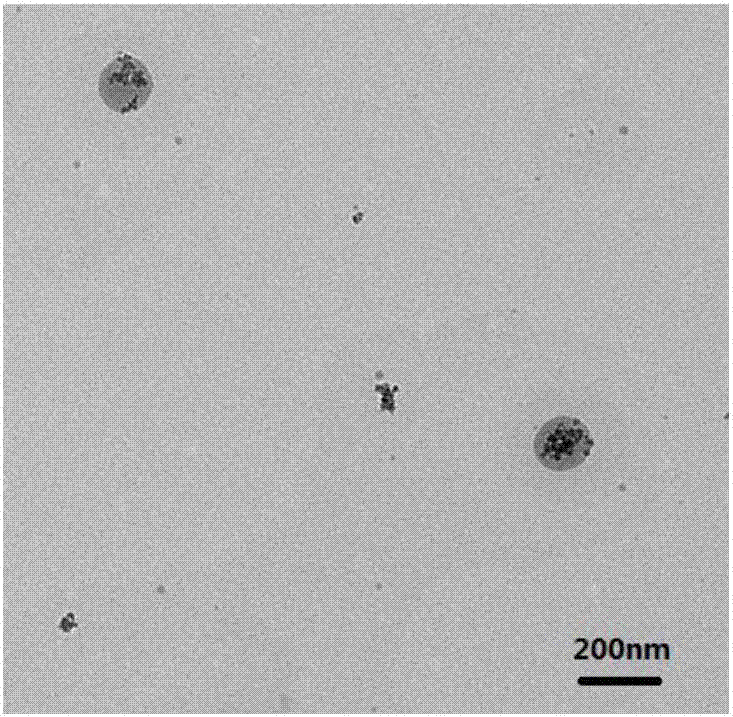
Fe3O4/octreotide modified nano-liposome and preparing method thereof
A nano-liposome and octreotide technology, which is applied in the field of medicine, can solve the problems of inability to realize targeted drug delivery and precise release, and achieve the effects of simple preparation process, improved therapeutic effect, and good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]Using the natural fat-soluble antineoplastic drug-oleanolic acid as a template drug, 1.0 mg oleanolic acid (purchased from Huakang Pharmaceutical Raw Material Factory, Shifang City, Sichuan Province), 40 mg soybean lecithin (purchased from Shenyang Tianfeng Biopharmaceutical Co., Ltd. ) and 4.0mg of cholesterol (purchased from Tianjin Damao Chemical Instrument Supply Station) were dissolved in 2mL of absolute ethanol, and a lipid phase solution was formed by magnetic stirring; 1mg of PEG and 1mg of glutamic acid were dissolved in 8mL of phosphate buffer as water phase solution, adjust the pH of the aqueous phase solution to 6.0 with 1 mol / L NaOH solution, and add 8 μL Tween-80. Under magnetic stirring, slowly and uniformly drop 2 mL of lipid phase into 8 mL of water phase solution at 30°C at a speed of 80 rpm to obtain a liposome suspension, and finally continue rotary evaporation for 1.5 h to remove any impurities in the liposome suspension. water and ethanol to obtain ...
Embodiment 2
[0039] Taking natural fat-soluble antineoplastic drug-betulinic acid as a template drug, 1.5mg betulinic acid (purchased from Nanjing Zelang Pharmaceutical Technology Co., Ltd.), 45mg soybean lecithin (purchased from Shenyang Tianfeng Biopharmaceutical Co., Ltd.) and 4.5 mg mg cholesterol (purchased from Tianjin Damao Chemical Instrument Supply Station) was dissolved in 2 mL of absolute ethanol, and a lipid phase solution was formed by magnetic stirring. Dissolve 1.0 mg of PEG and 1.5 mg of glutamic acid in 9 mL of phosphate buffer as an aqueous phase solution, adjust the pH of the aqueous phase solution to 6.2 with a concentration of 1 mol / L NaOH solution, and add 11 μL of Tween-80. Under magnetic stirring, 2 mL of lipid phase was slowly and uniformly dropped into 9 mL of aqueous phase solution at 34° C. at a speed of 90 rpm to obtain a liposome suspension. Finally, the rotary evaporation was continued for 1.7 h to remove the absolute ethanol in the liposome suspension to obt...
Embodiment 3
[0044] Taking the natural fat-soluble antineoplastic drug-ursolic acid as a template drug, 3.0mg ursolic acid (purchased from Huakang Pharmaceutical Raw Material Factory, Shifang, Sichuan), 50mg soybean lecithin (purchased from Shenyang Tianfeng Biopharmaceutical Co., Ltd.) and 5.0 mg of cholesterol (purchased from Tianjin Damao Chemical Instrument Supply Station) was dissolved in 2 mL of absolute ethanol, and a lipid phase solution was formed by magnetic stirring. Dissolve 2.5mg of PEG and 4.5mg of glutamic acid in 10mL of phosphate buffer as the aqueous phase solution, adjust the pH of the aqueous phase solution to 7.0 with a concentration of 1mol / L NaOH solution, and add 12 μL of Tween-80. Under magnetic stirring, 2 mL of lipid phase was slowly and uniformly dropped into 10 mL of aqueous phase solution at 40° C. at a speed of 110 rpm to obtain a liposome suspension. Finally, the rotary evaporation was continued for 2 h to remove the absolute ethanol in the liposome suspensi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com