Method for preparing rotigotine and derivative thereof
A compound and a selected technology, applied in the directions of organic chemistry methods, chemical instruments and methods, separation of optically active compounds, etc., can solve the problems of low yield, difficulty in industrial production, expensive preparation of rotigotine, etc., and achieve easy operation. , good social and economic benefits, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
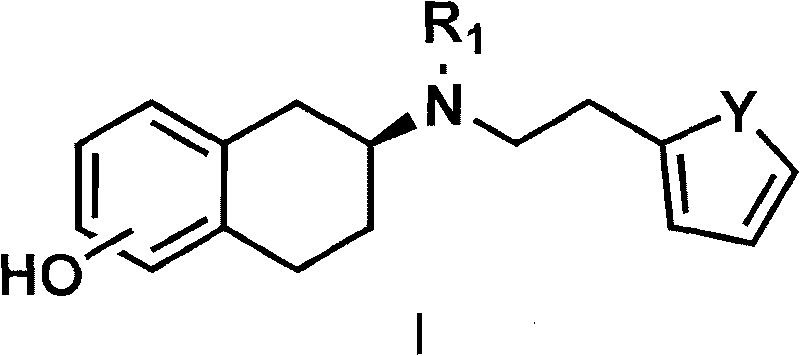
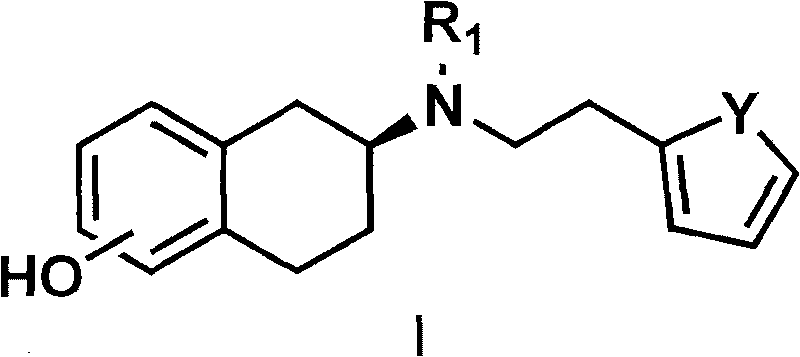
[0054] The preparation of embodiment 1 rotigotine
[0055] Preparation of 2-(N-n-propylamino)-5-methoxytetralin
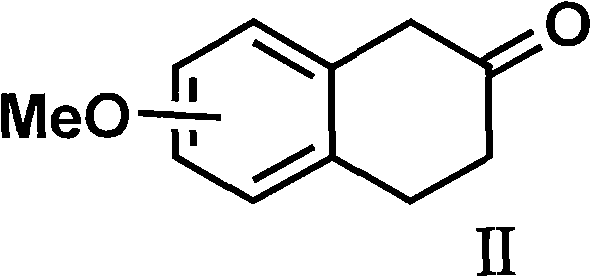
[0056] 5-Methoxy-2-tetralone (3.52 g, 20 mmol) and propylamine (3.3 mL, 40 mmol) were dissolved in 50 mL of absolute ethanol, and a catalytic amount of p-toluenesulfonic acid was added , heated to reflux and stirred for 2 to 5 hours; the reaction mixture was cooled to 0°C, under stirring, slowly added sodium borohydride (1.14 g, 30 mmol), then slowly warmed up to 30°C, and continued to stir at this temperature 24 hours. Remove the organic solvent, add 100 ml of 10% sodium hydroxide solution, stir for 1 hour, and then extract with 100 ml of dichloromethane for three consecutive extractions. The organic solvent was dried over anhydrous sodium sulfate, filtered, and the solvent was extracted to obtain 3.2 g (62%) of an oil.
[0057] Preparation of Chiral Organic Phosphonic Acids
[0058] Slowly add 1 equivalent of potassium hydroxide ethanol solution dropwise to 1...
Embodiment 2
[0070] The preparation of embodiment 2 rotigotine hydrochloride
[0071] Preparation of 2-(N-n-propylamino)-5-methoxytetralin
[0072] 5-methoxy-2-tetralone (3.52 g, 20 mmol) and propylamine (3.3 ml, 40 mmol) were dissolved in 50 ml of absolute ethanol, then a catalytic amount of camphorsulfonic acid was added, heated to reflux Stir for 2-5 hours; cool the reaction mixture to room temperature, add a catalytic amount of palladium carbon catalyst, add potassium borohydride and continue stirring at room temperature for 24 hours. Suction filtration was performed to remove palladium carbon, washed with 50 ml of dichloromethane, the organic liquid was combined and concentrated to obtain 3.3 g of oil.
[0073] Preparation of Chiral Organic Phosphonic Acids
[0074] Mix 1 equivalent of o-chlorobenzaldehyde and 2.5 equivalents of isobutyraldehyde evenly, control the temperature, slowly add 1 equivalent of potassium hydroxide ethanol solution dropwise, then react overnight at 60-65°C,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com