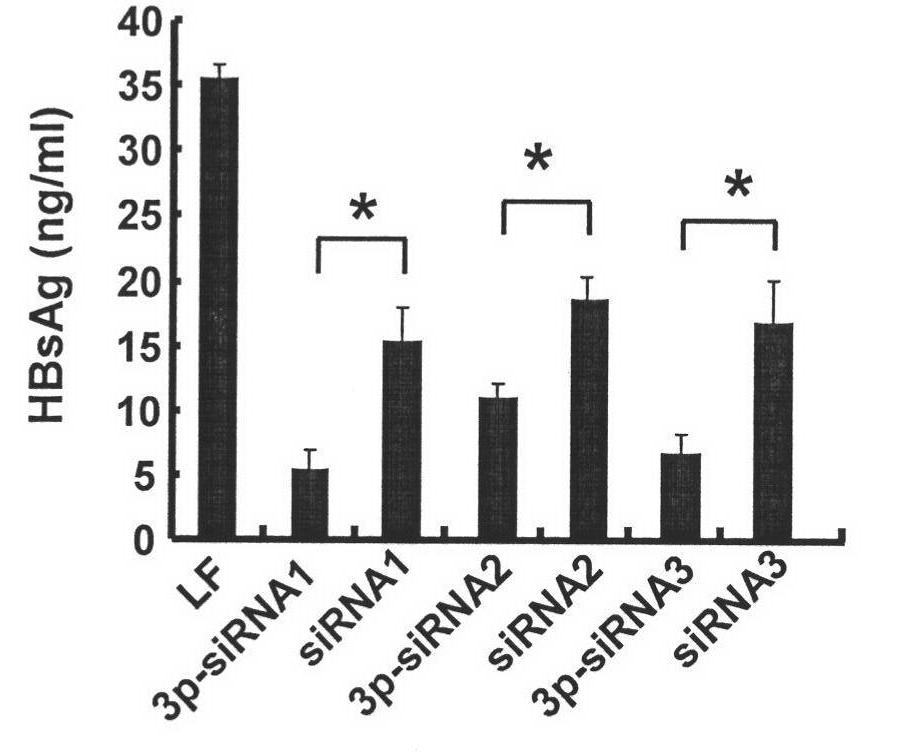
siRNA for treating hbv
A technology for HBV and treatment, applied in the field of genetic engineering, can solve the problems of chronic HBV patients obtaining expected results and being in a state of immune tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0062] 1. Synthesis of chemically synthesized siRNA:
[0063] Design siRNA for HBV X gene: According to the siRNA design principle, three pairs of siRNA were handed over to Guangzhou Ruibo Company to directly synthesize the siRNA sequence, see Table 1, and the sequence table SEQ ID NO.1-SEQ ID NO.3.
[0064] 2. In vitro transcription of 3p-siRNA:
[0065] (1) The target and sequence are completely consistent with chemically synthesized siRNA.
[0066] (2) Synthesize single-stranded DNA corresponding to the HBx-siRNA sequence with T7promoter, see Table 2.
[0067] (3) Preparation of double-stranded oligo DNA: the synthesized single-stranded oligo DNA was dissolved in DEPC-treated water to prepare a 100 pmol / μl DNA solution.
[0068] ① Prepare Oligo DNA annealing reaction solution according to the components shown in Table 3.
[0069] ②Place the Oligo DNA annealing reaction solution above on a PCR amplification instrument, treat it at 95°C for 2 minutes, cool it to 25°C for 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com