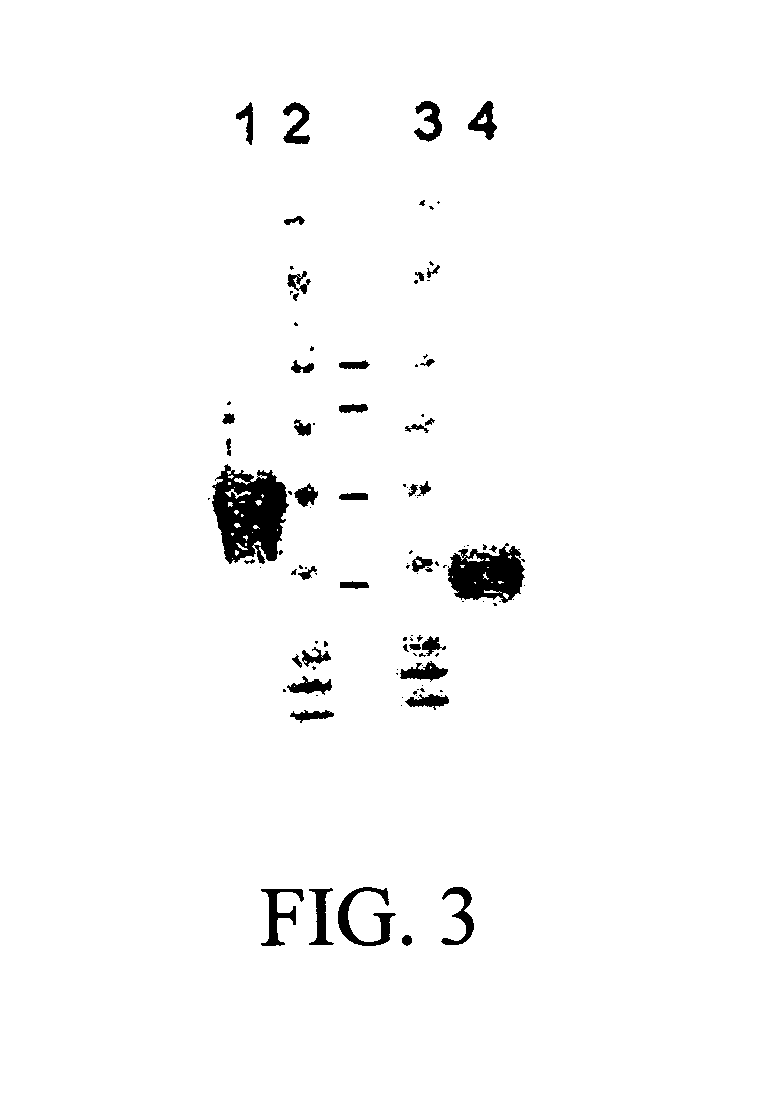
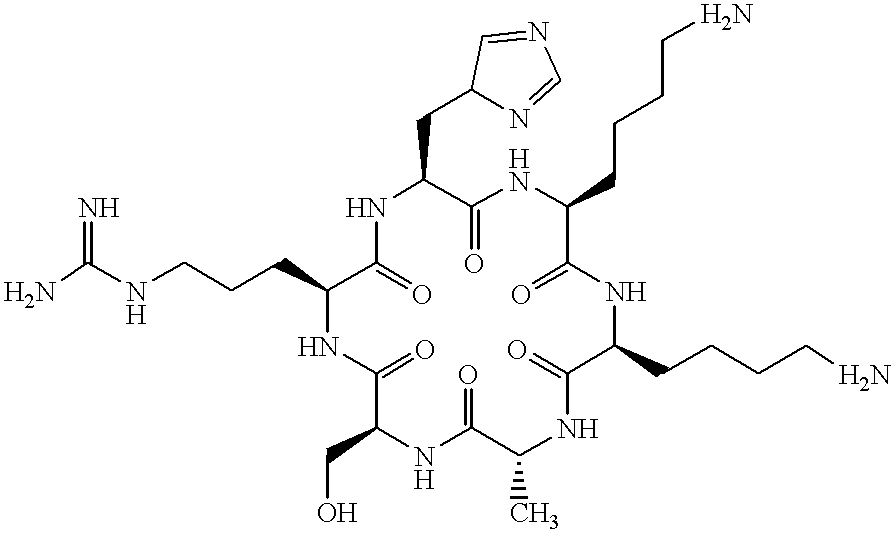
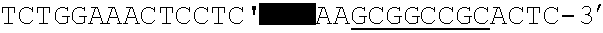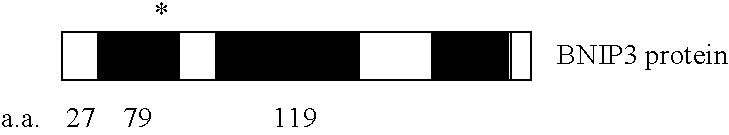Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
907results about "Oncogene translation products" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
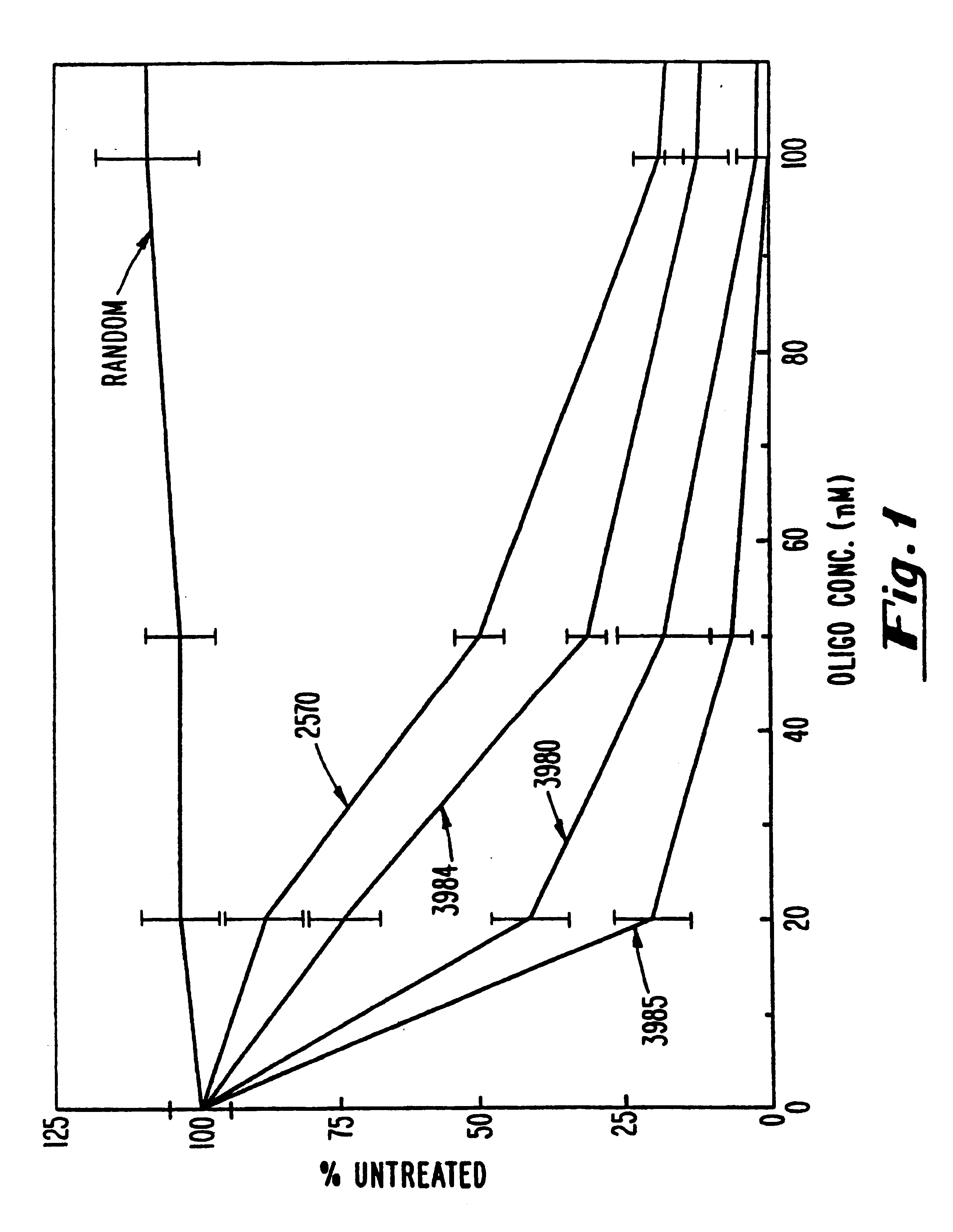
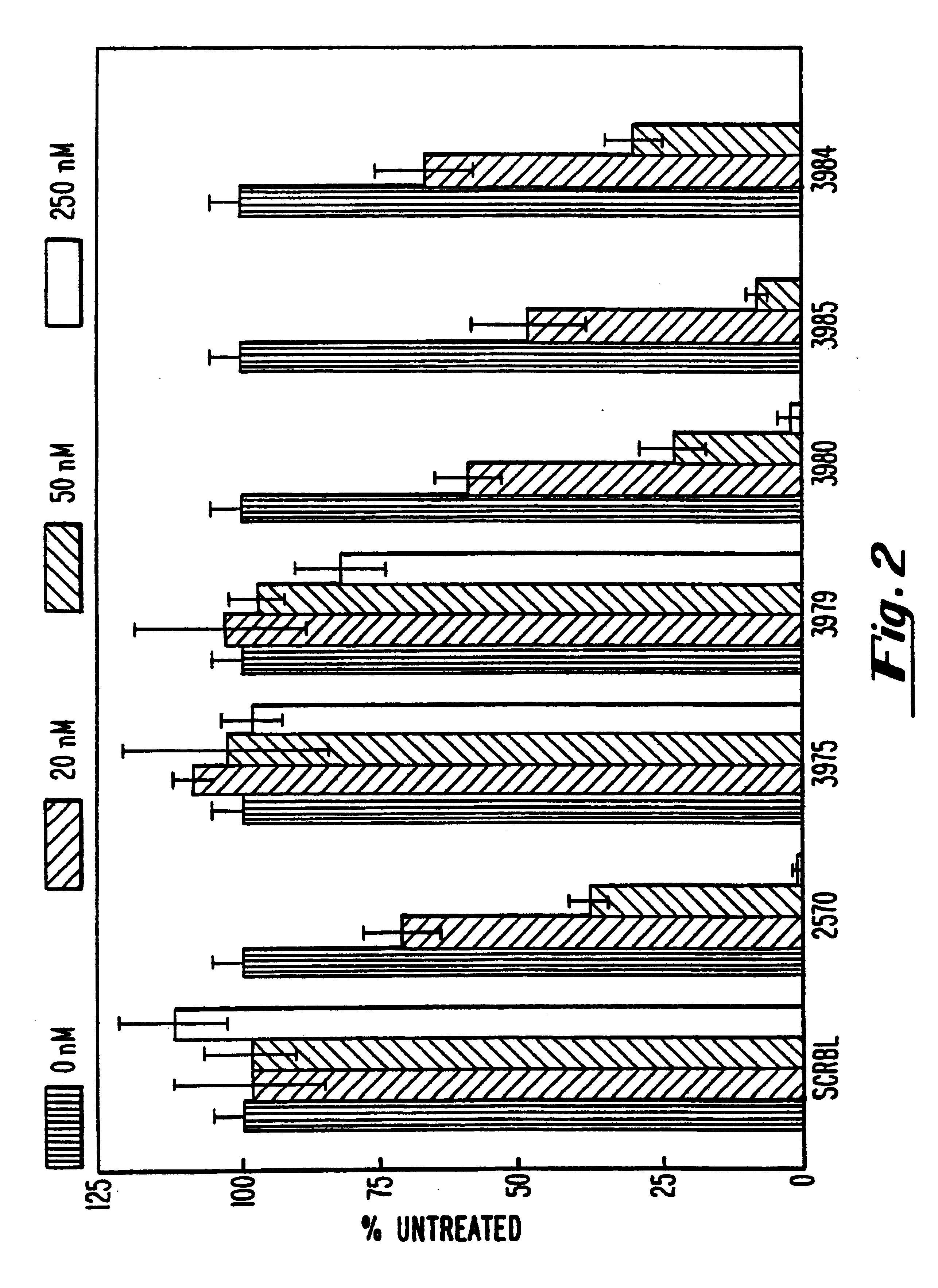
Gapped 2' modified oligonucleotides
InactiveUS6326199B1High affinityAvoid degradationHydrolasesPeptide/protein ingredientsNucleotideNuclease
Oligonucleotides and other macromolecules are provided that have increased nuclease resistance, substituent groups for increasing binding affinity to complementary strand, and sub-sequences of 2'-deoxy-erythro-pentofuranosyl nucleotides that activate RNase H enzyme. Such oligonucleotides and macromolecules are useful for diagnostics and other research purposes, for modulating protein in organisms, and for the diagnosis, detection and treatment of other conditions susceptible to antisense therapeutics.
Owner:IONIS PHARMA INC
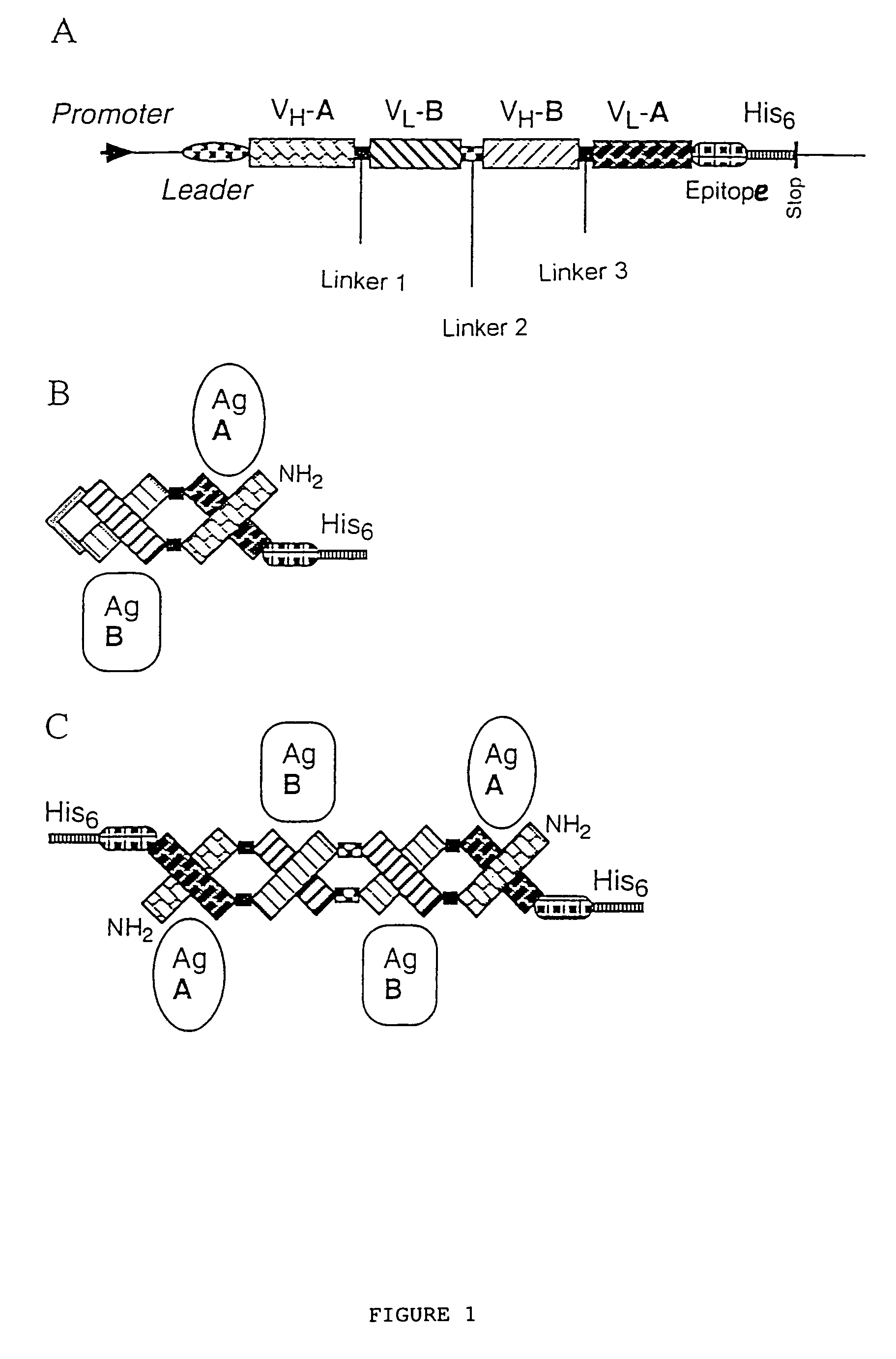
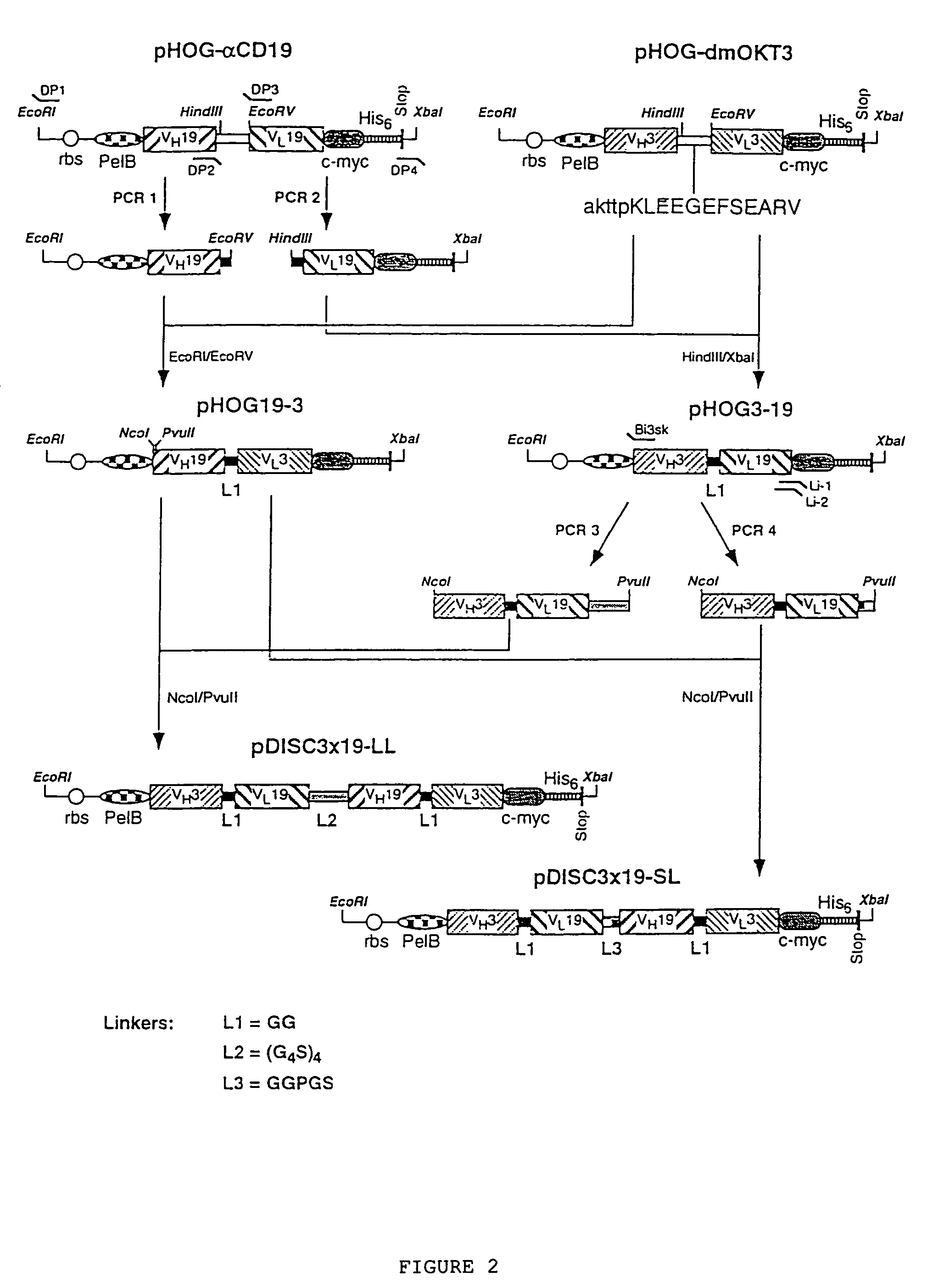
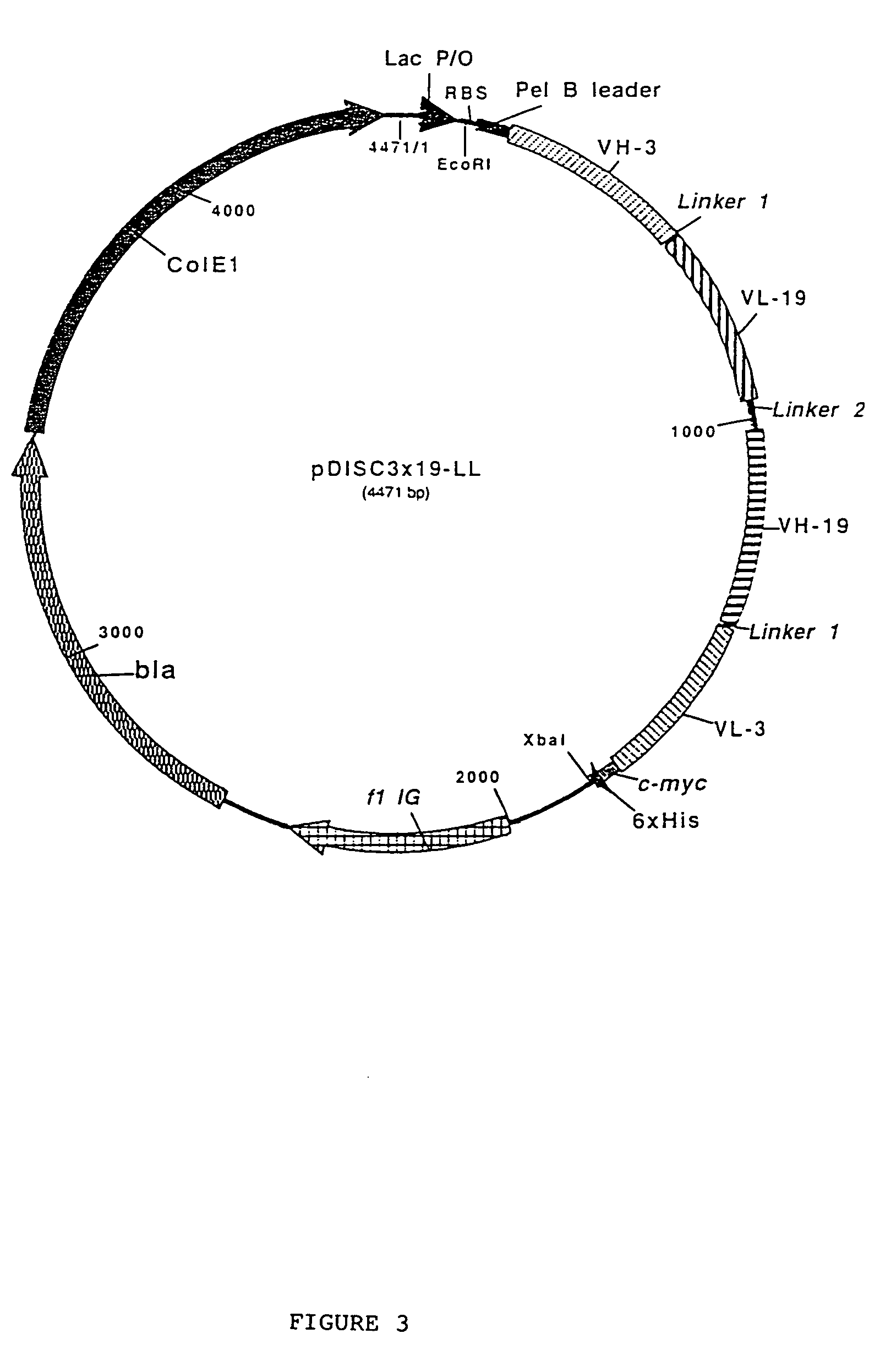
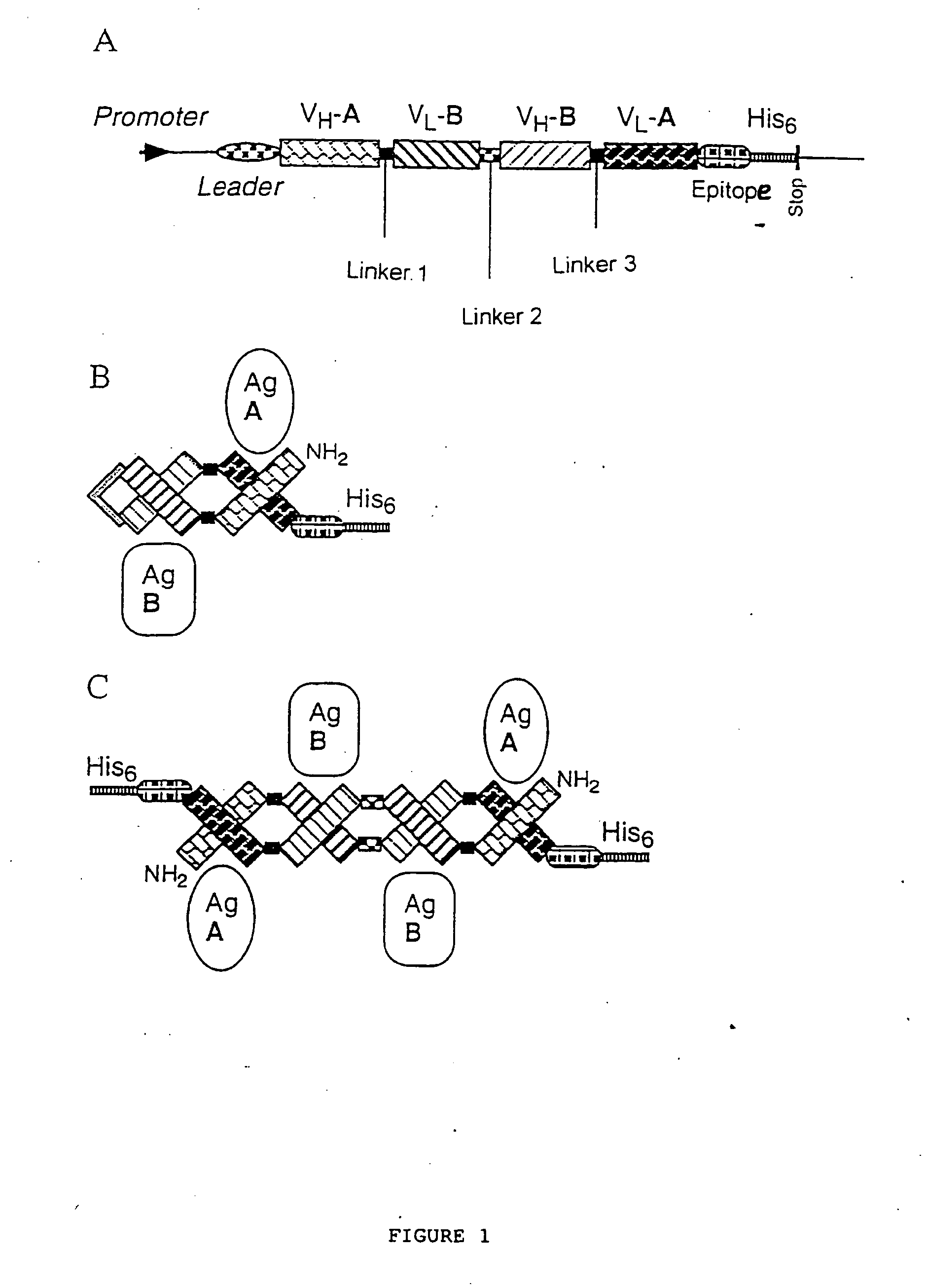
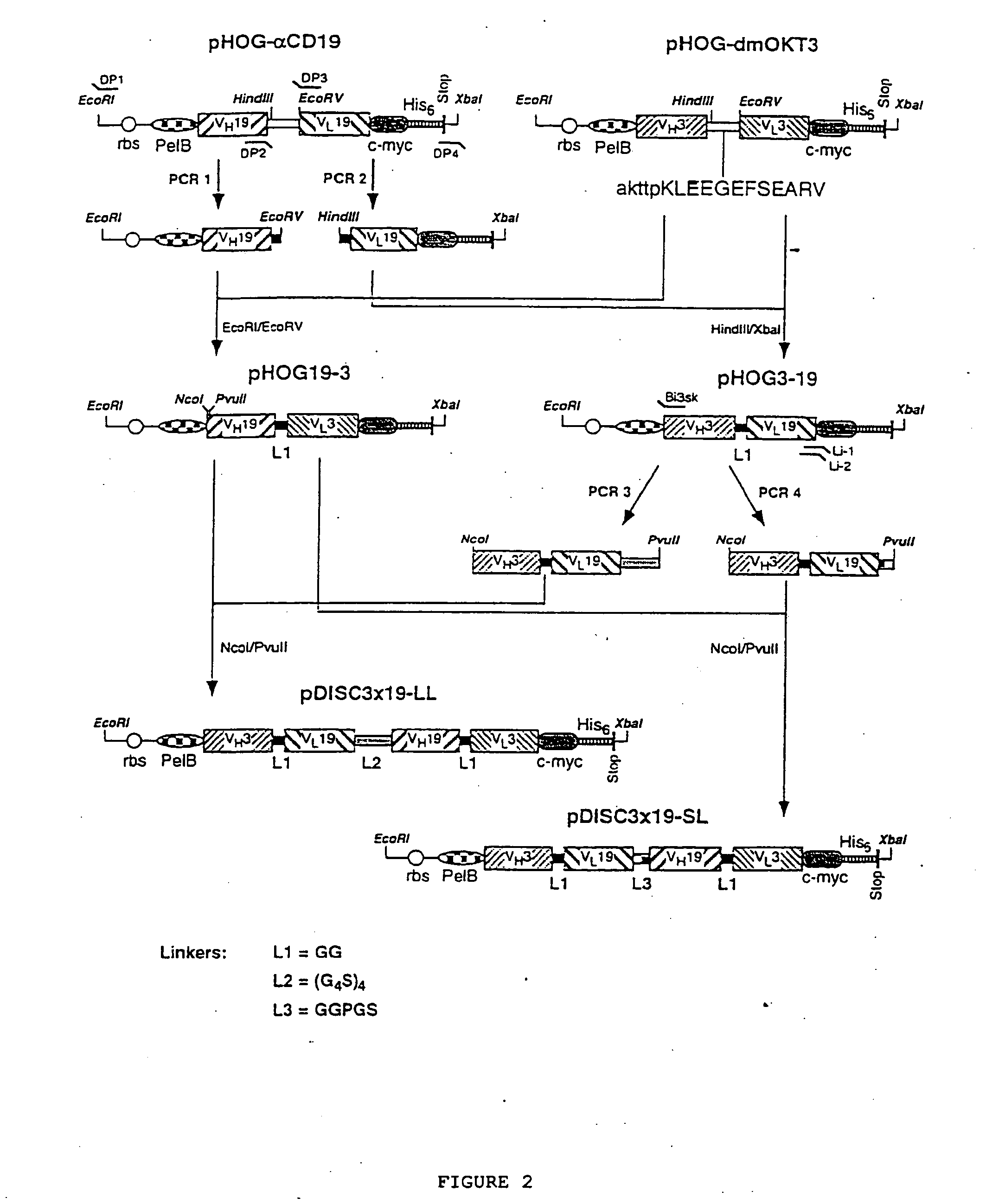
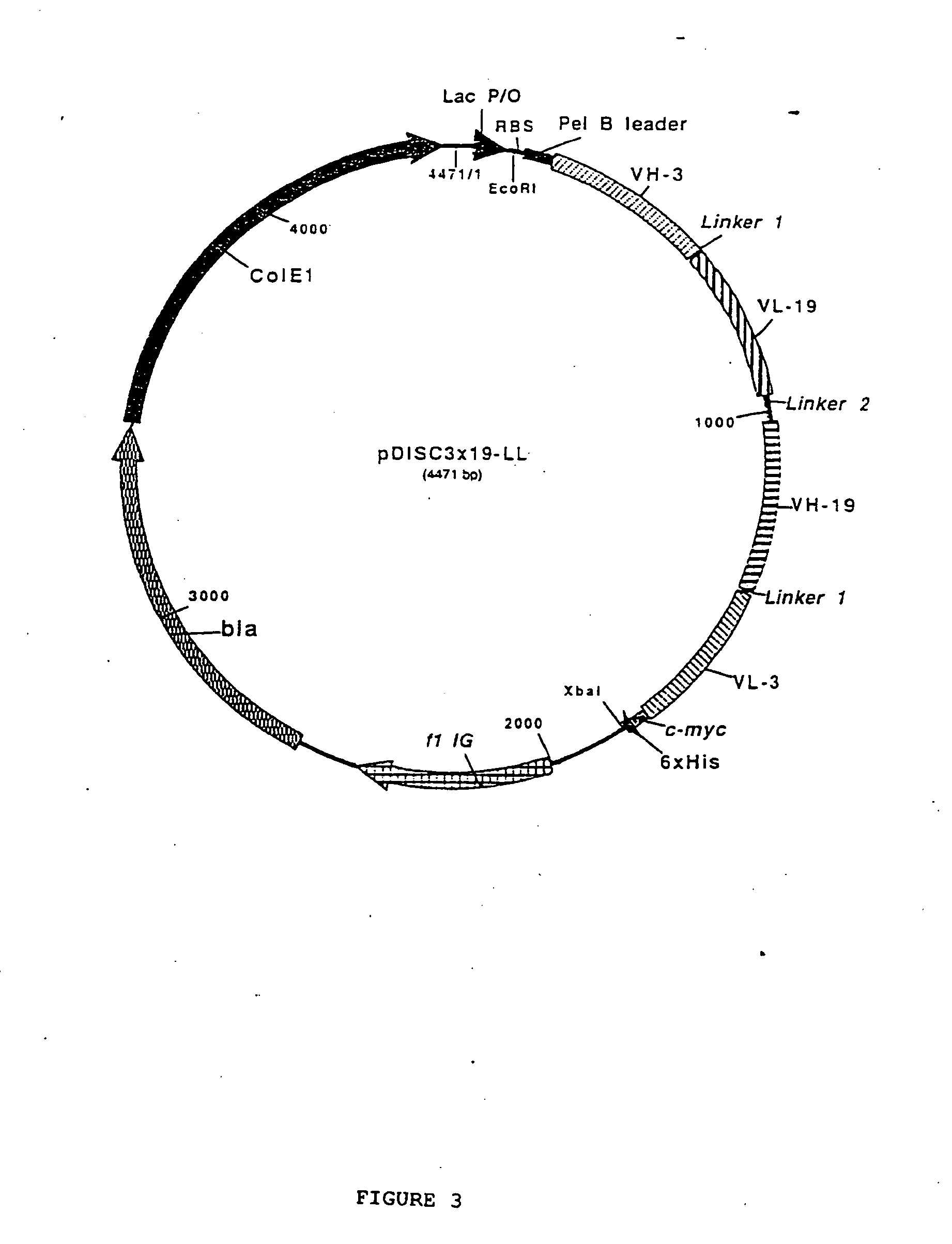
Multivalent antibody constructs
InactiveUS7129330B1Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsVariable domainPlasmid
The present invention relates to multivalent Fvantibody construct having at least four variable domains which are linked with each over via the peptide linkers 1, 2 and 3. The invention also concerns expression plasmids which code for such an Fvantibody construct and a method of producing the Fvantibody constructs as well as their use.
Owner:DEUTES KREBSFORSCHUNGSZENT STIFTUNG DES OFFENTLICHEN RECHTS
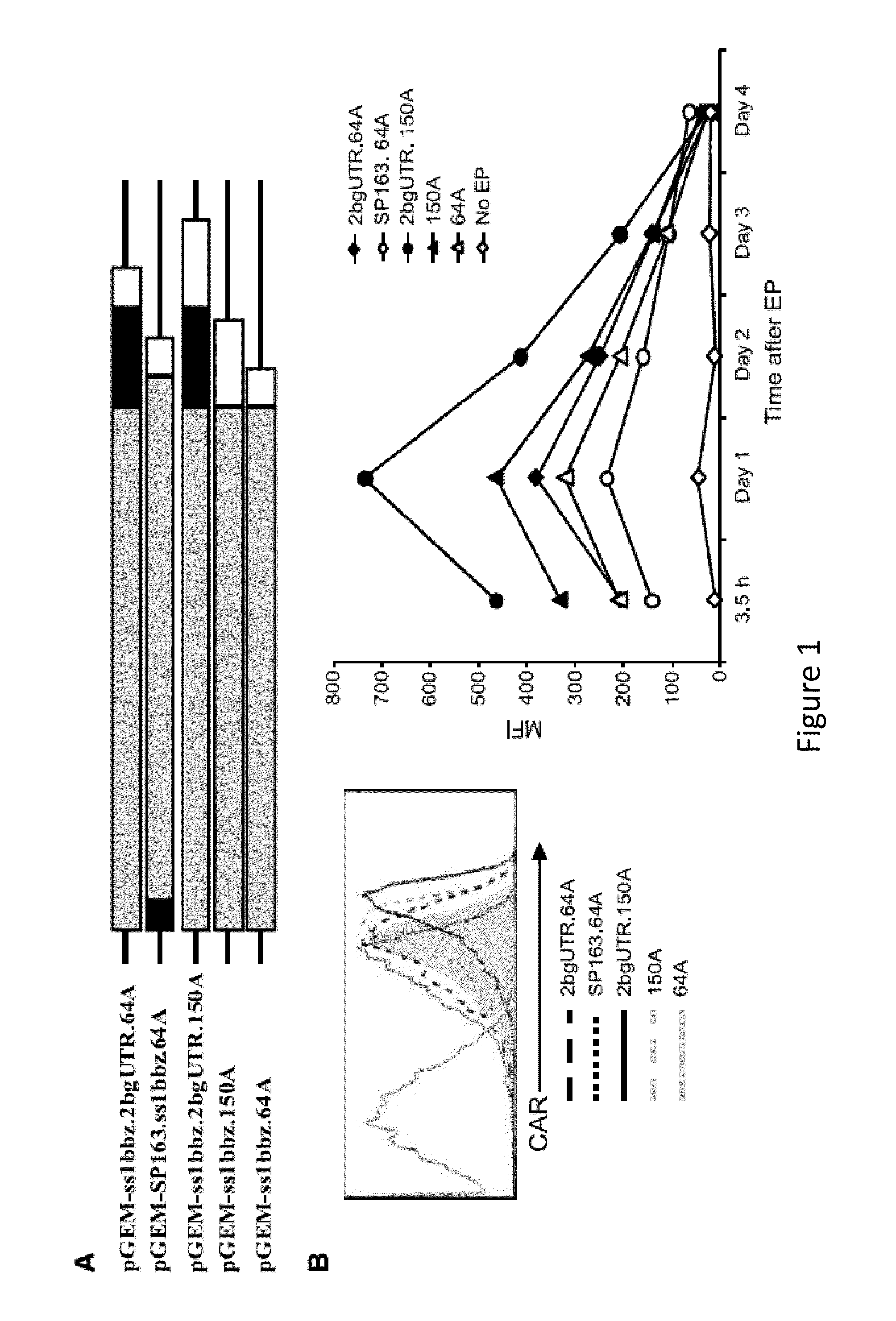
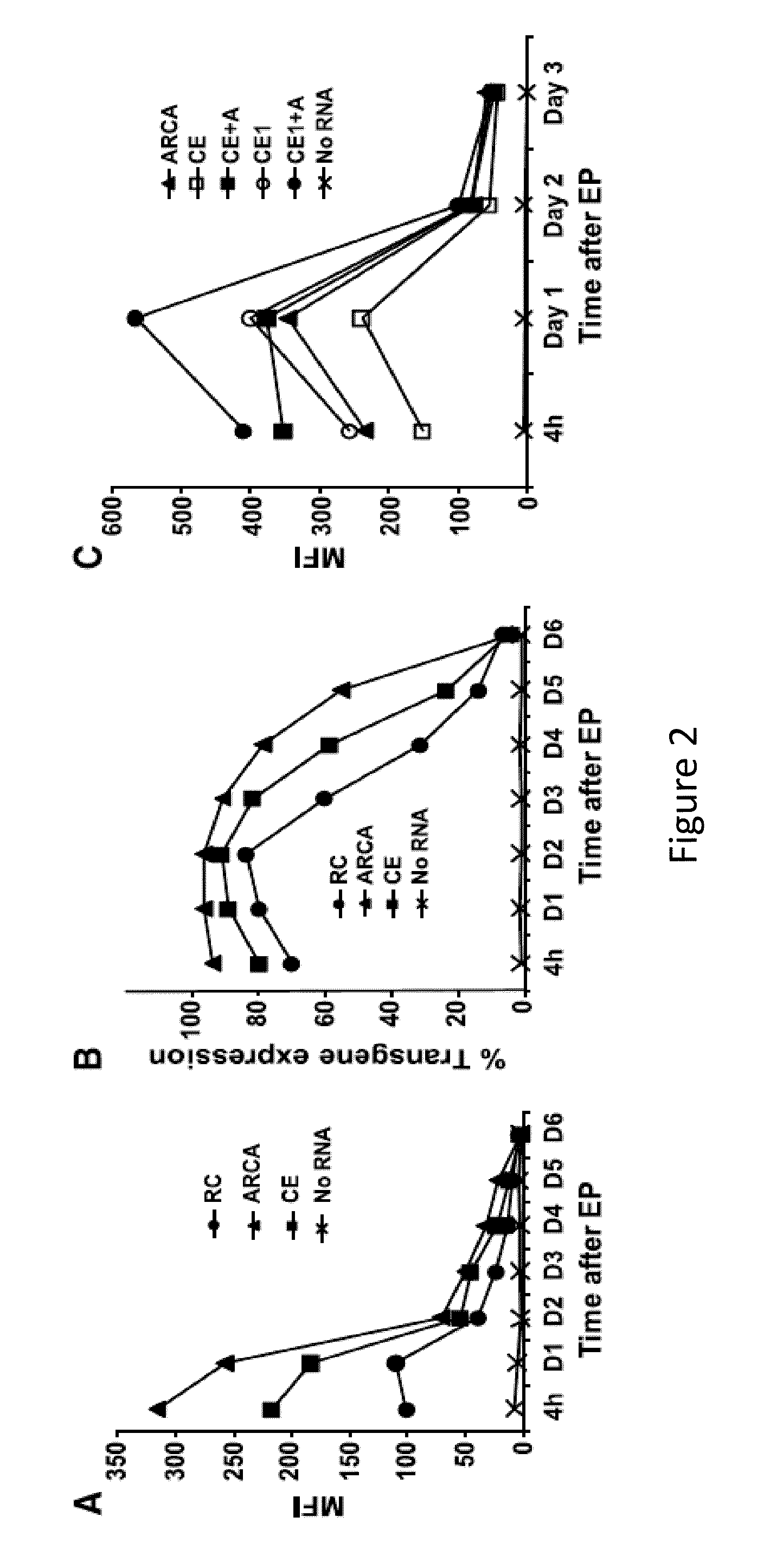
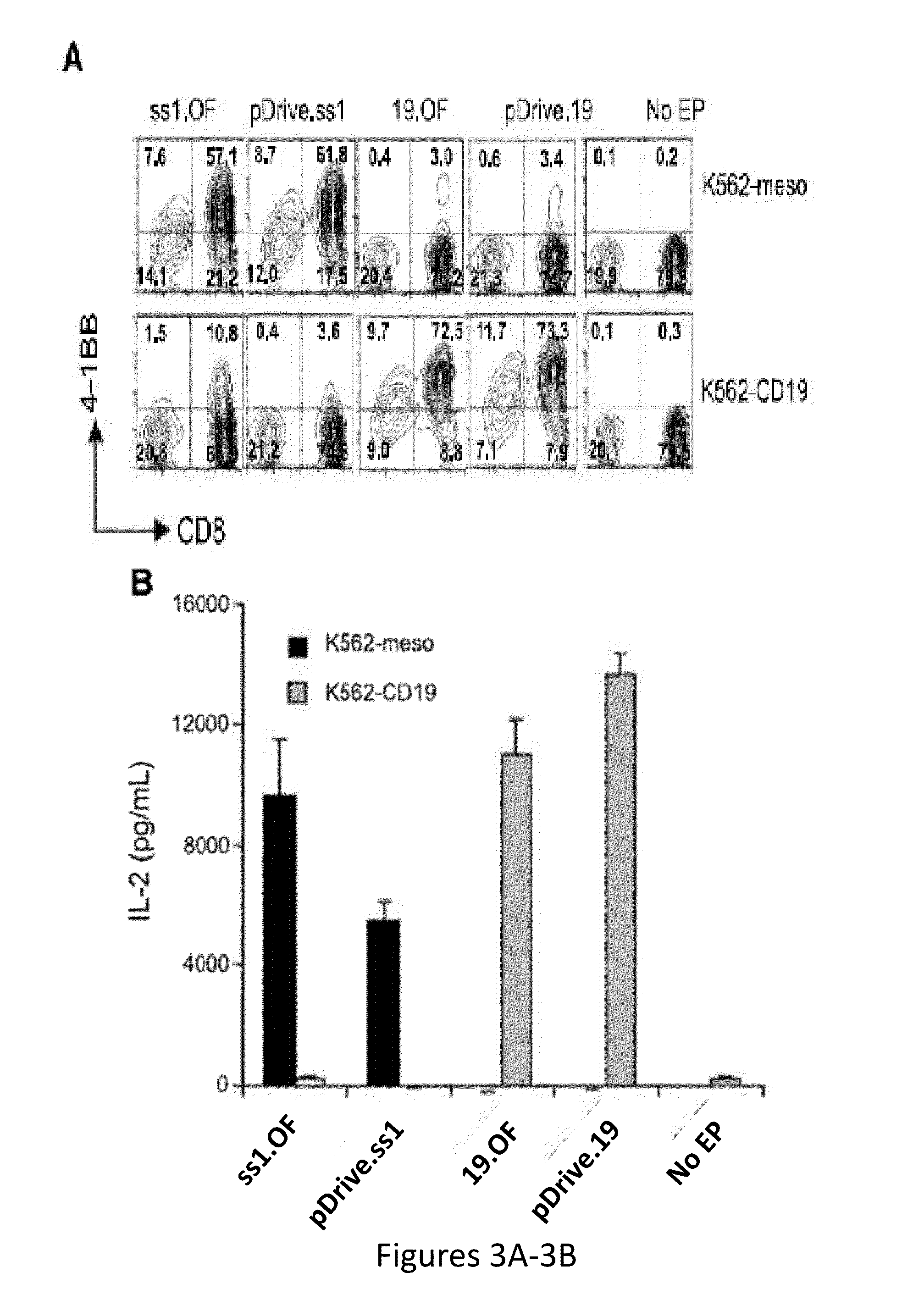
RNA engineered t cells for the treatment of cancer
ActiveUS20140227237A1Reduce riskLimited half-lifeBiocideVirusesAntigen receptorChimeric antigen receptor
The present invention relates to compositions and methods for generating RNA Chimeric Antigen Receptor (CAR) transfected T cells. The RNA-engineered T cells can be used in adoptive therapy to treat cancer.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS20070036799A1Good curative effectEnhanced ADCC activityAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsTherapeutic antibodyWild type
Owner:MACROGENICS INC
Pancreatic cancer associated antigen, antibody thereto, and diagnostic and treatment methods
The present invention is directed to an antigen found on the surface of rat and human pancreatic cancer cells and provides antibodies of high specificity and selectivity to this antigen as well as hybridomas secreting the subject antibodies. Methods for both the diagnosis and treatment of pancreatic cancer are also provided. This tissue marker of pancreatic adenocarcinoma, an approximately 43.5 kD surface membrane protein designated PaCa-Ag1, is completely unexpressed in normal pancreas but abundantly expressed in pancreatic carcinoma cells. Moreover, a soluble form of PaCa-Ag1 exists, having a molecular weight about 36 to about 38 kD, that is readily identified in sera and other body fluids of pancreatic cancer patients, using a subject antibody.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Peptides and peptidomimetics with structural similarity to human P53 that activate P53 function
InactiveUS6245886B1Improve the immunityLow toxicityPeptide/protein ingredientsP53 proteinWild typeP53 Mutation
The present invention provides peptides and peptidomimetics corresponding to part or to the entirety of the region encompassed by residues 360-386 of human p53, said peptides and peptidomimetics characterized by the ability to activate DNA binding of wild-type p53 and to select tumor-derived p53 mutants. Pharmaceutical compositions of the compounds of the invention and methods of using these compositions therapeutically are also provided.
Owner:BAYER HEALTHCARE LLC +1
Methods for the treatment, diagnosis, and prognosis of cancer
InactiveUS20060160762A1Reduce the overall heightImprove the level ofPeptide/protein ingredientsGenetic material ingredientsCancer cellCancer treatment
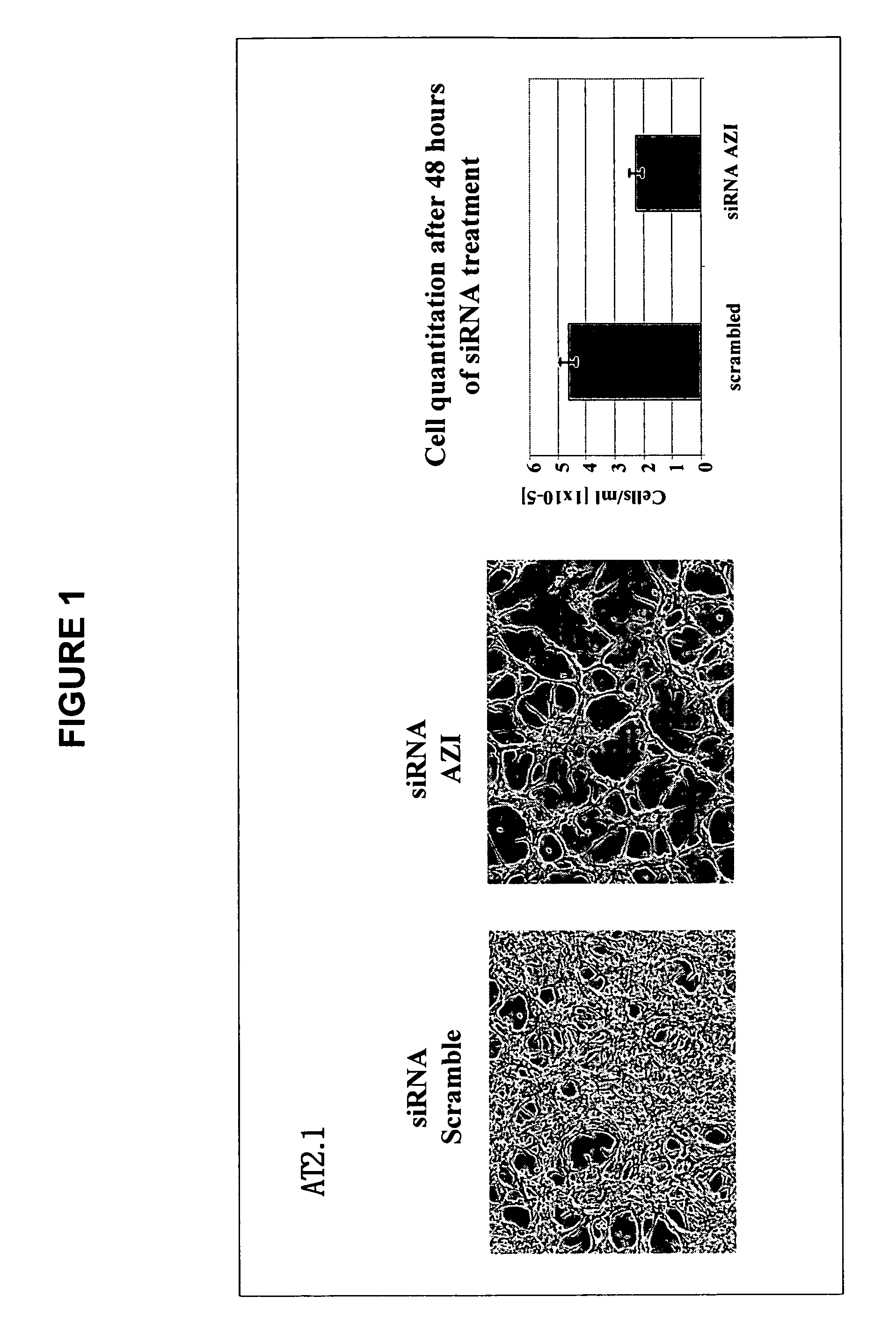
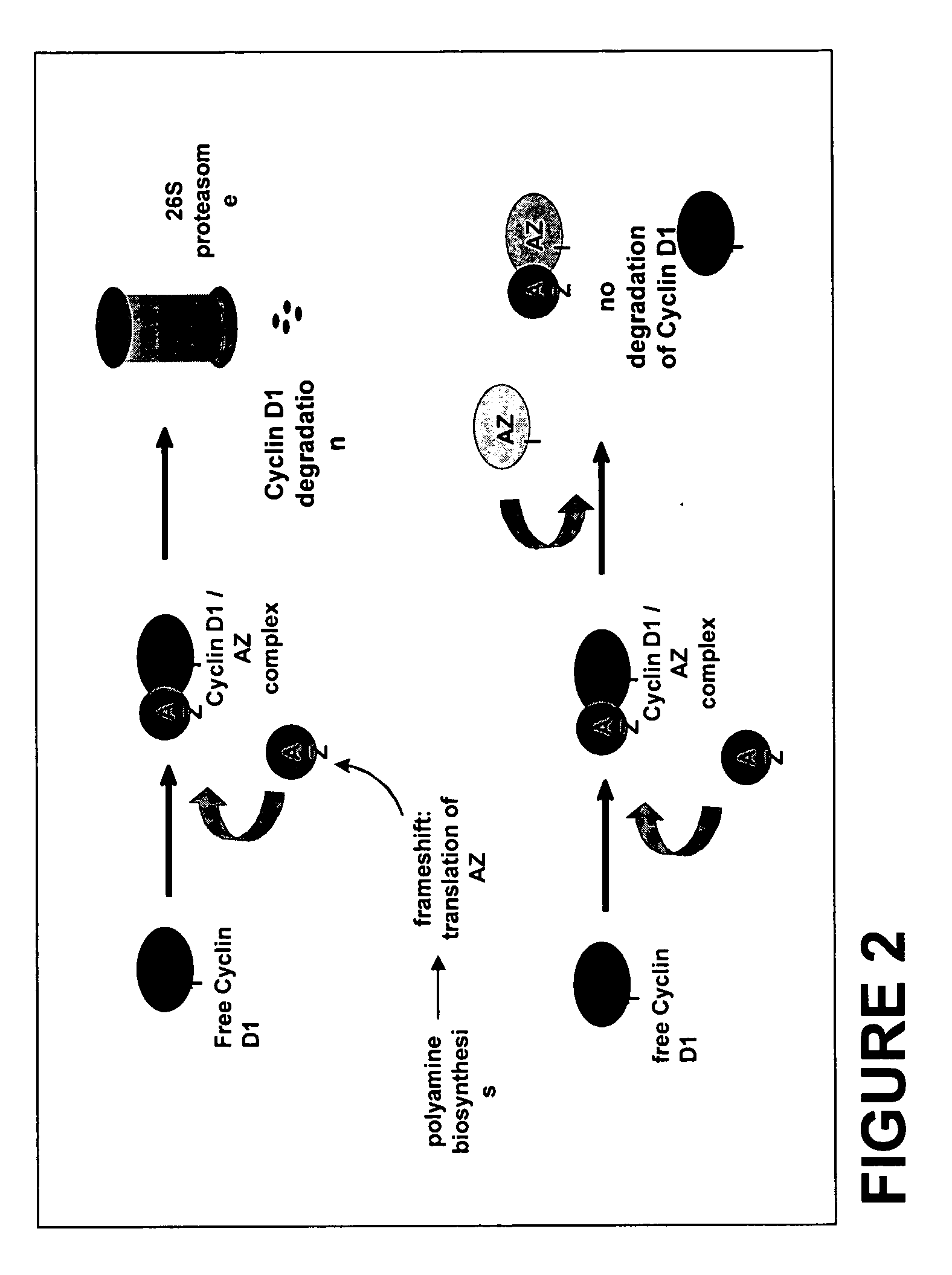
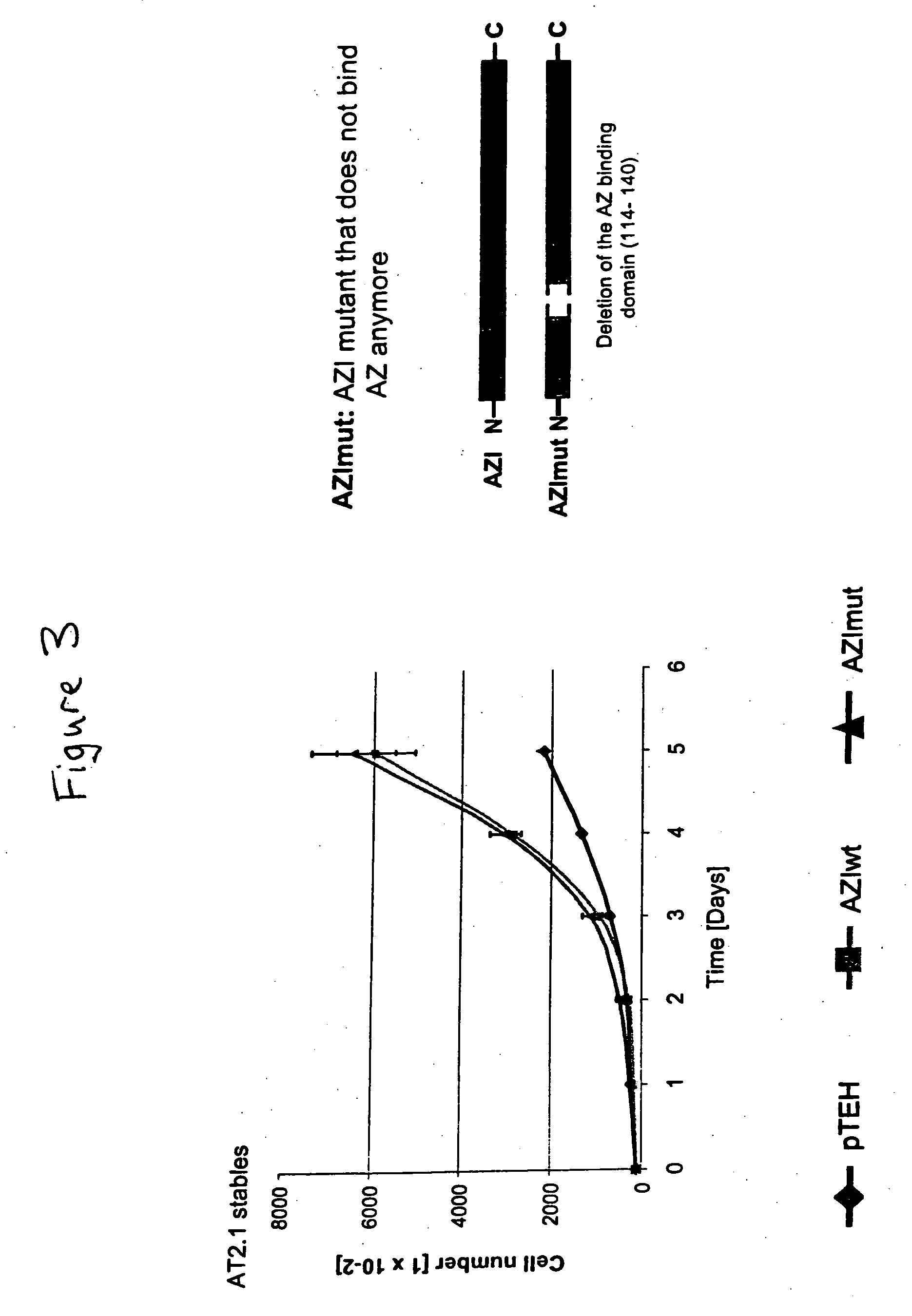
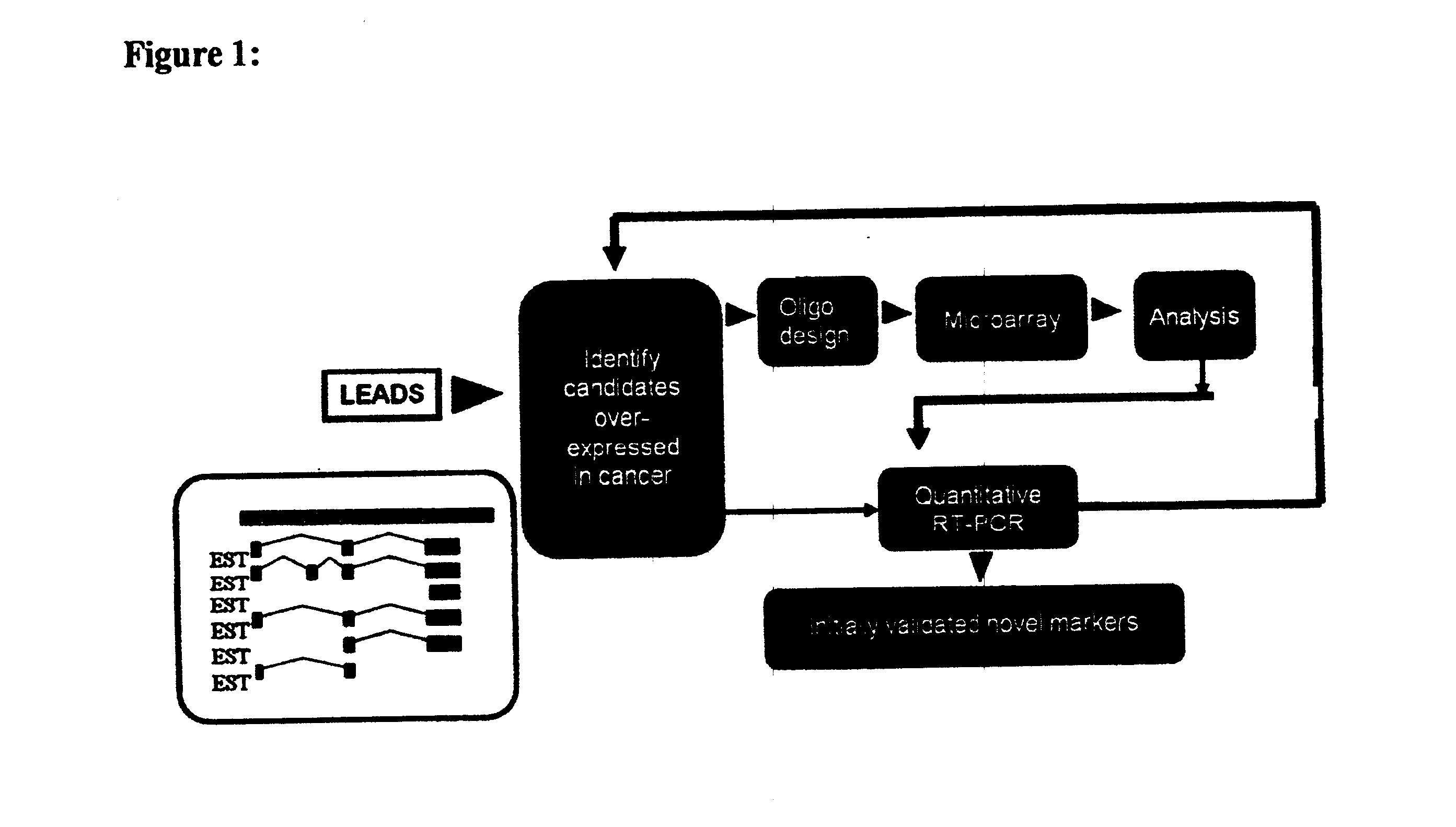
We have discovered that antizyme inhibitor (AZI) is expressed at increased levels in highly proliferating cells. We have also discovered that inhibiting antizyme inhibitor, including inhibiting its expression, reduces the growth of cancer cells. The present invention is directed to the use of inhibitors of antizyme inhibitor for the treatment of cancer, the use of antizyme inhibitor for the diagnosis and prognosis of cancer, and methods for identifying novel cancer treatments.
Owner:CHILDRENS MEDICAL CENT CORP
Novel nucleotide and amino acid sequences, and assays and methods of use thereof for diagnosis of prostate cancer
InactiveUS20080014590A1High degreeOvercomes these deficiencies of the background artPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsOncologyProstate hyperplasia
Novel markers for prostate cancer that are both sensitive and accurate. Furthermore, these markers are able to distinguish between prostate cancer and benign prostate hyperplasia (“BPH”). These markers are overexpressed in prostate cancer specifically, as opposed to normal prostate tissue and / or BPH. The measurement of these markers, alone or in combination, in patient samples provides information that the diagnostician can correlate with a probable diagnosis of prostate cancer. The markers of the present invention, alone or in combination, show a high degree of differential detection between prostate cancer and non-cancerous states.
Owner:COMPUGEN
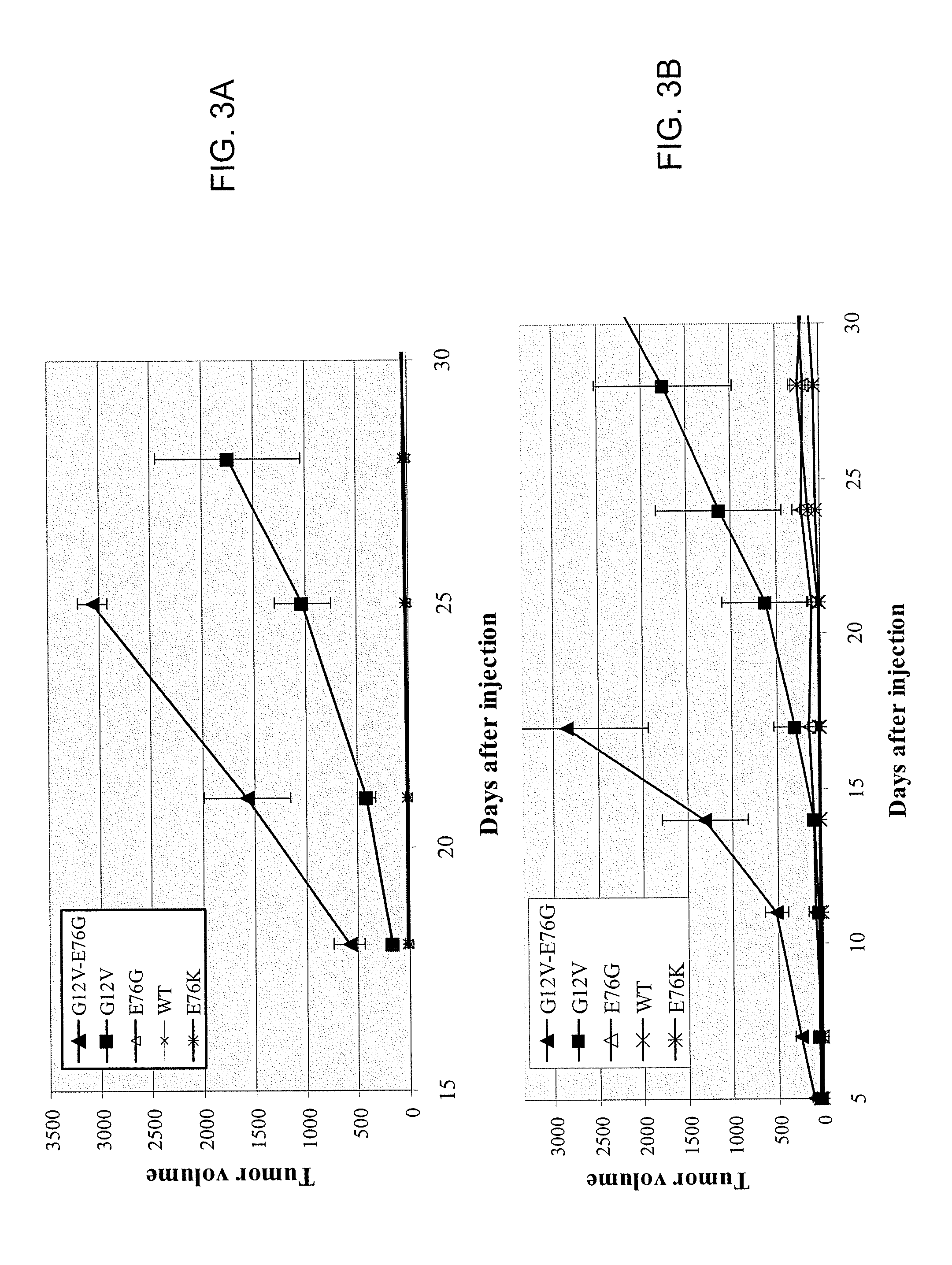
Ras mutation and compositions and methods related thereto
Disclosed are newly discovered Ras mutations and combinations of mutations, proteins and peptides and fusion proteins containing these mutations, nucleic acid molecules encoding such proteins, peptides, and fusion proteins, and a variety of tools and diagnostic, therapeutic, and screening methods associated with the use of such mutations.
Owner:GLOBE IMMUNE INC
Method of diagnosing, monitoring, staging, imaging and treating prostate cancer
InactiveUS7432064B2Microbiological testing/measurementOncogene translation productsProstate cancerImmunology
Owner:DIAZYME LAB INC
Diagnosis of diseases associated with the immune system by determining cytosine methylation
InactiveUS20030143606A1Bioreactor/fermenter combinationsBiological substance pretreatmentsCytosineOligomer
The present invention relates to chemically modified genomic sequences of genes associated with the immune system, to oligonucleotides and / or PNA-oligomers directed against the sequence, for the detection of the methylation status of genes, associated with the immune system as well as to a method for ascertaining genetic and / or epigentic parametres of genes, associated with the immune system.
Owner:EPIGENOMICS AG
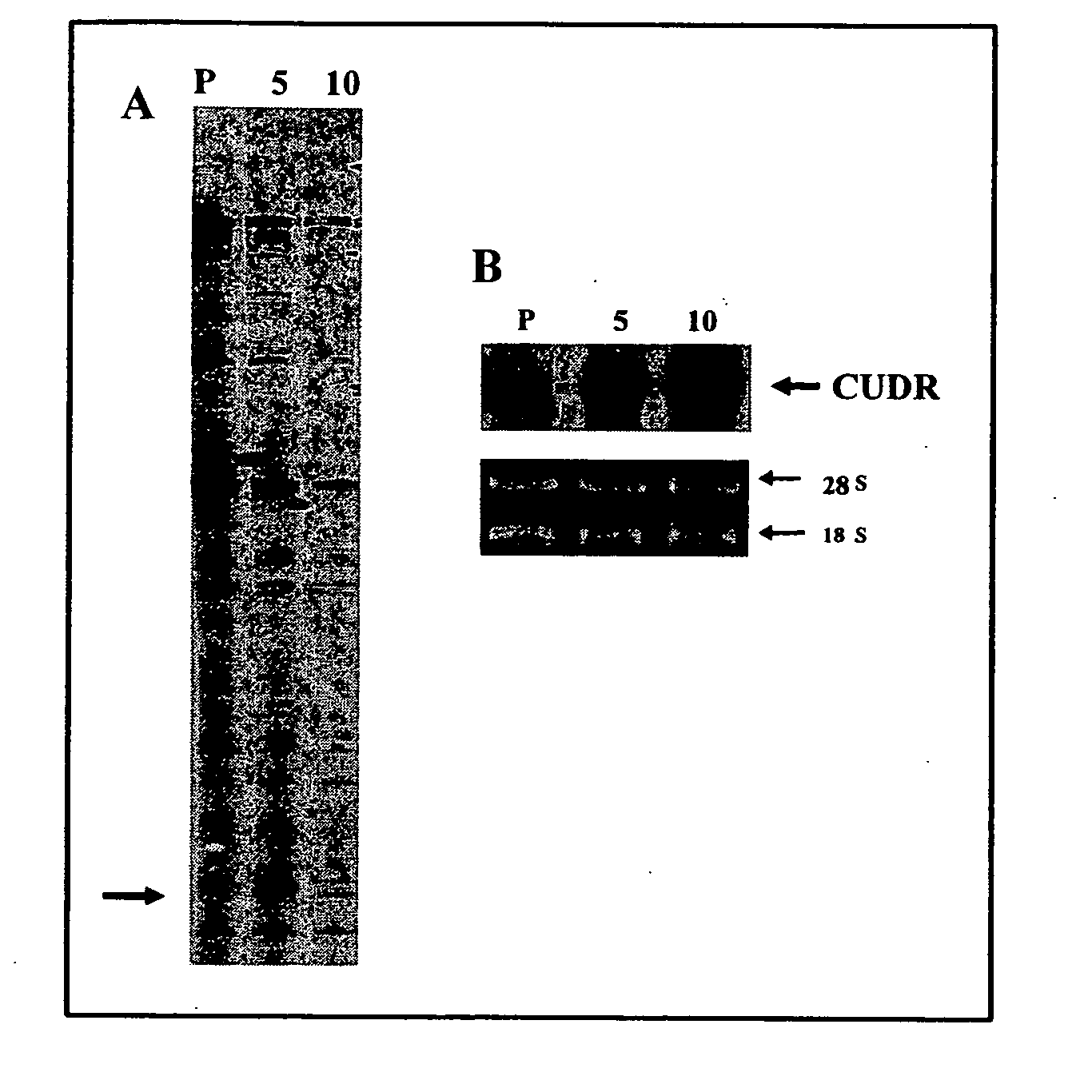
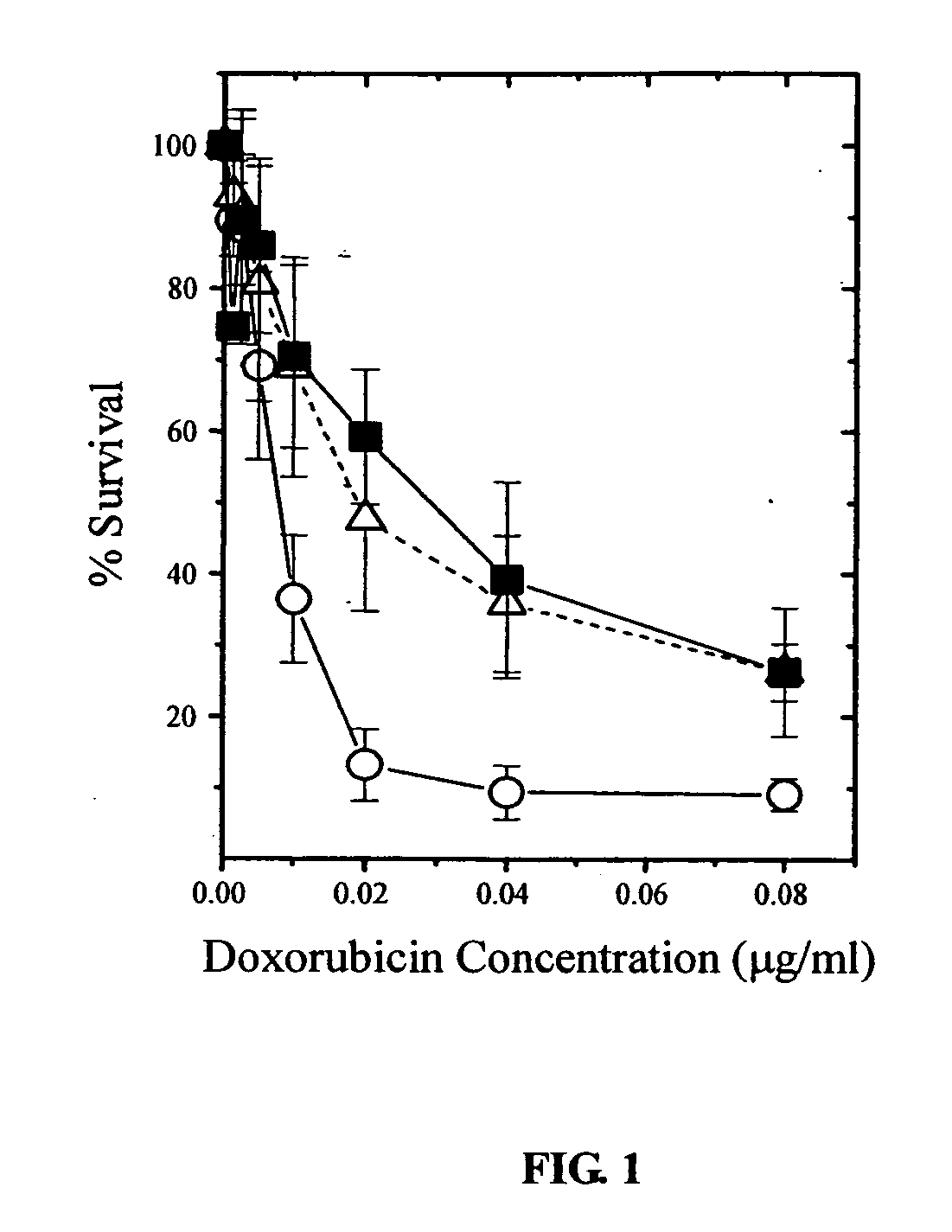
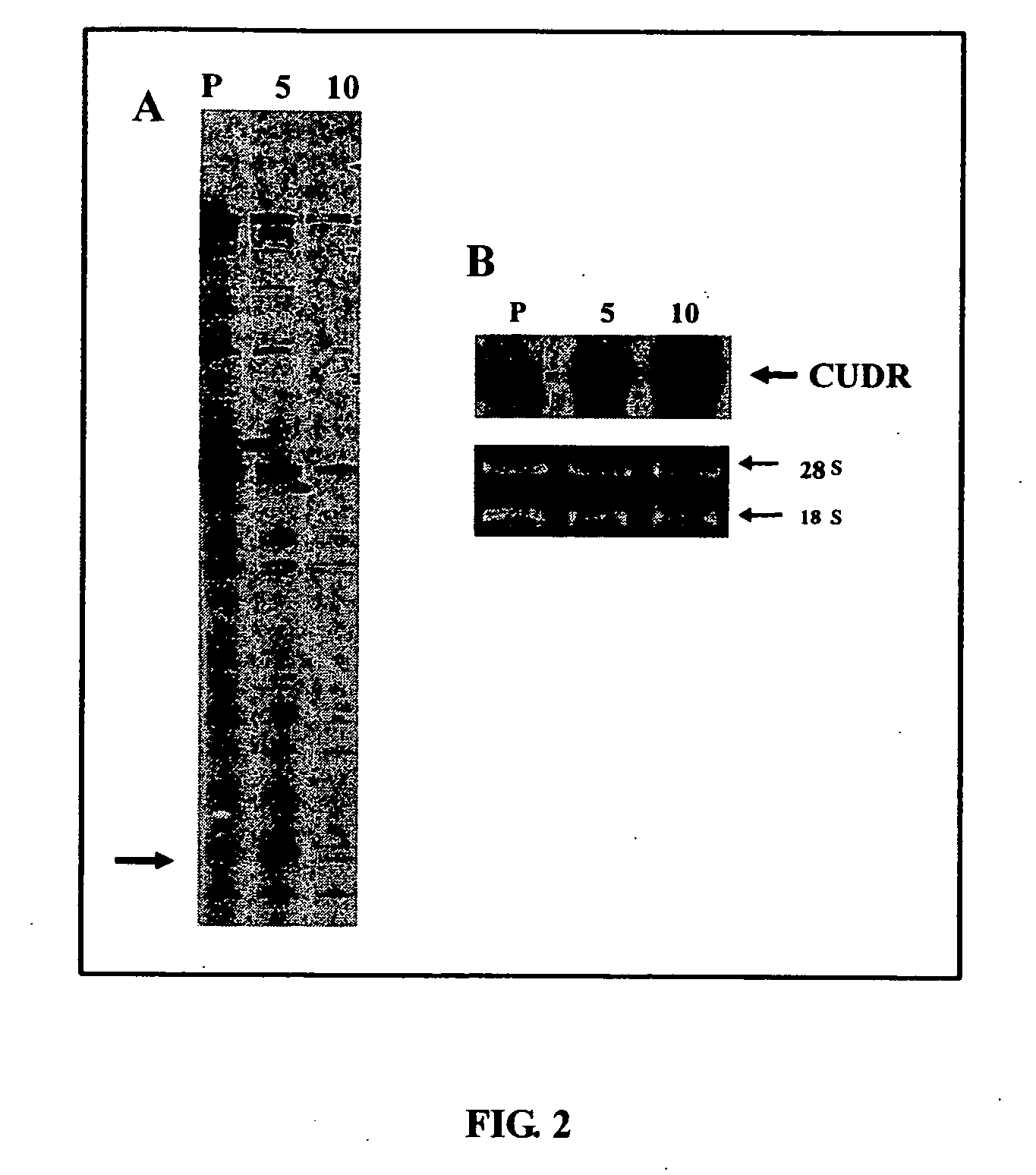
CUDR as biomarker for cancer progression and therapeutic response
InactiveUS20080044828A1Sugar derivativesMicrobiological testing/measurementHuman cancerCancer therapy
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Antibody to cytokine response gene 2(CR2) polypeptide
InactiveUS6057427AHigh incidenceEliminate the effects ofCytokine-induced proteinsPeptide/protein ingredientsAntibody fragmentsAmino acid
PCT No. PCT / US96 / 08992 Sec. 371 Date Jun. 5, 1996 Sec. 102(e) Date Jun. 5, 1996 PCT Filed Jun. 5, 1996Disclosed are an isolated antibody or antibody fragment that selectively binds a polypeptide encoded by Cytokine Response gene 2 (CR2), and in particular, selectively binds to a first polypeptide having the sequence of residues 1-60 of SEQ. ID No: 4. Also disclosed is a composition containing the antibody or antibody fragment and a diluent or carrier. Also disclosed are methods, using the present antibody or antibody fragment, of isolating or purifying a peptide comprising an amino acid sequence of residues 1-60 of SEQ. ID No: 4 or an antibody binding fragment thereof that is at least 10 to 30 amino acids long, or a fusion protein comprising any of these.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
High fidelity detection of nucleic acid differences by ligase detection reaction
InactiveUS7244831B2Improve fidelityAlteration in rateSugar derivativesMicrobiological testing/measurementBiologyOligonucleotide
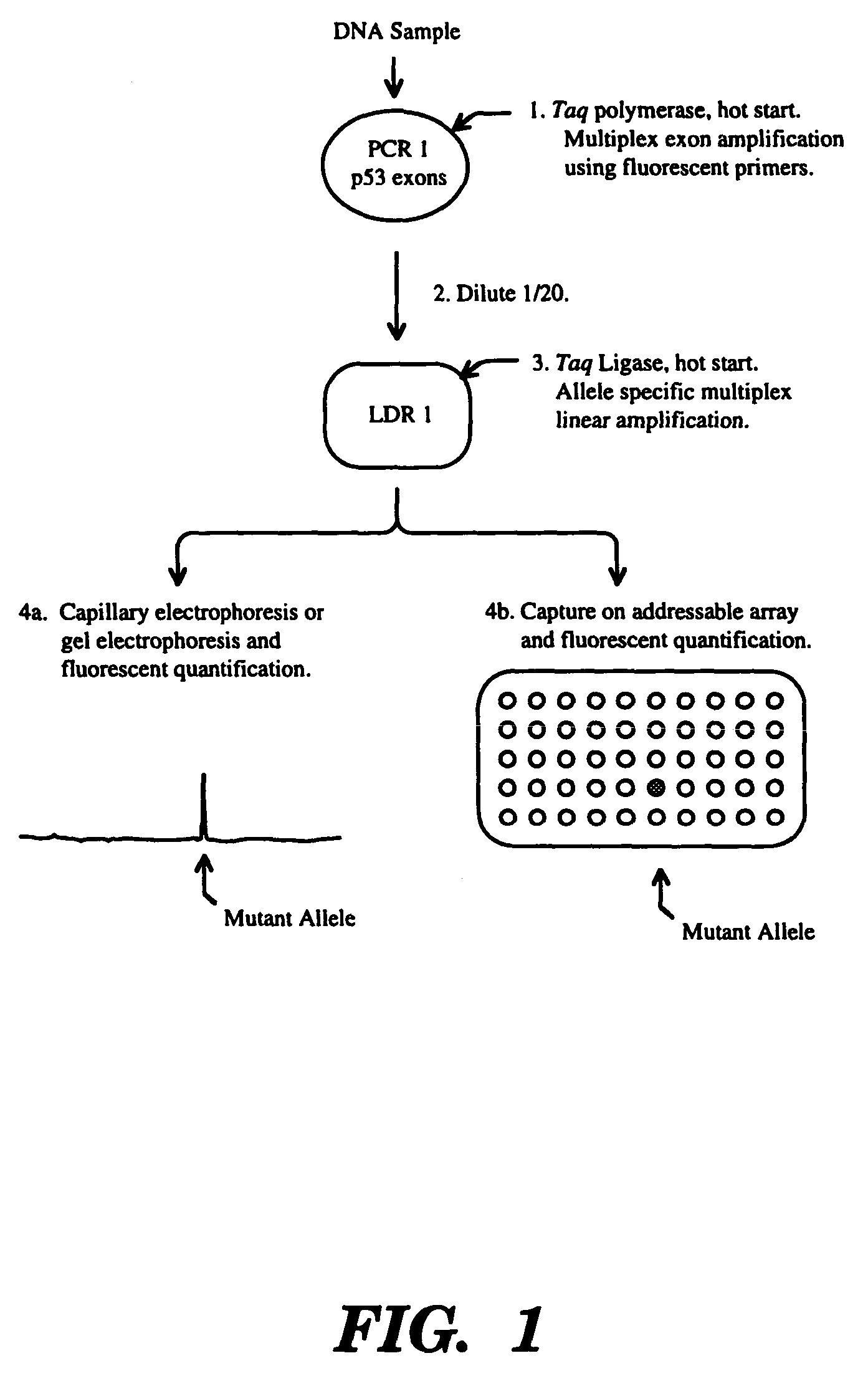
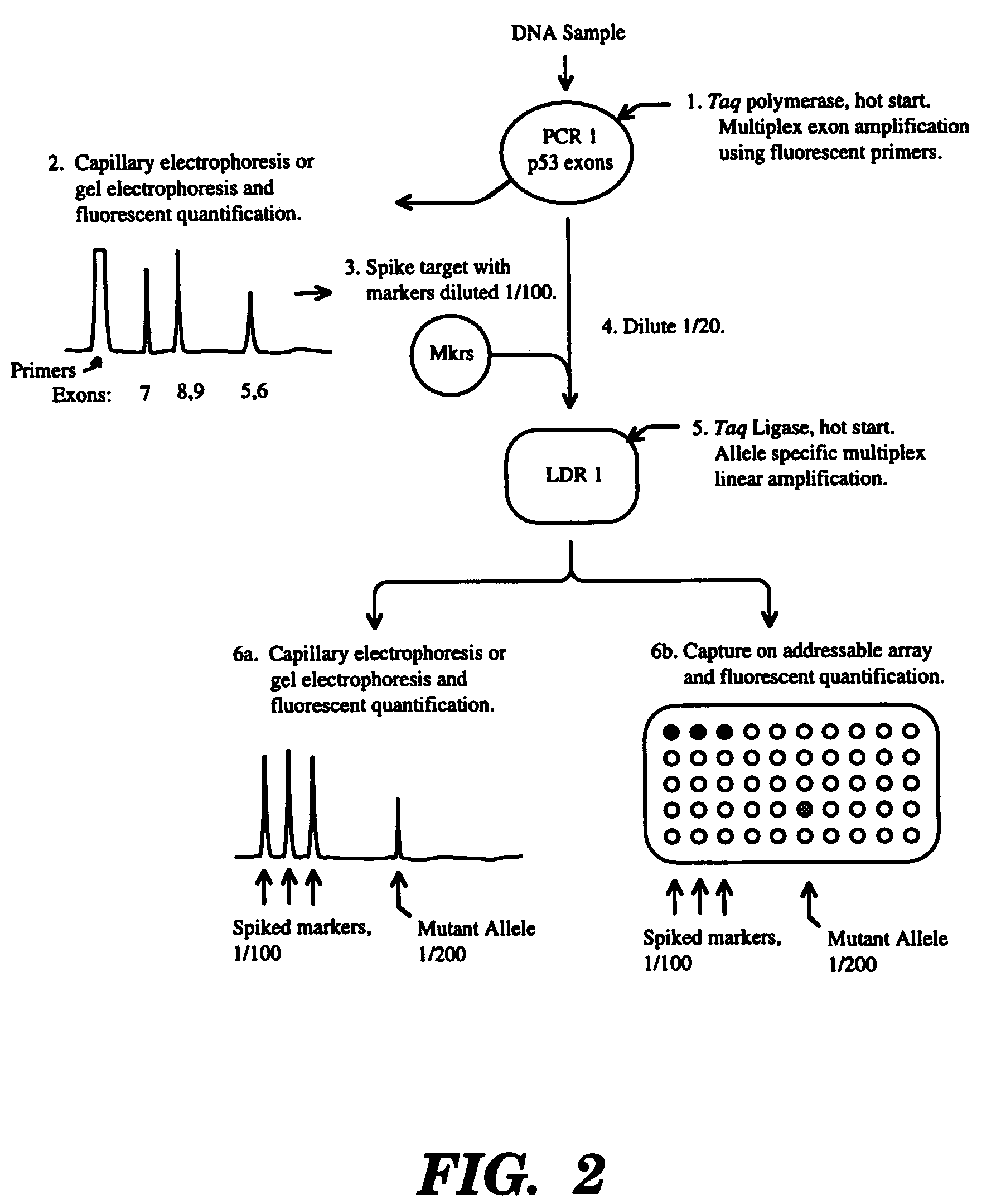
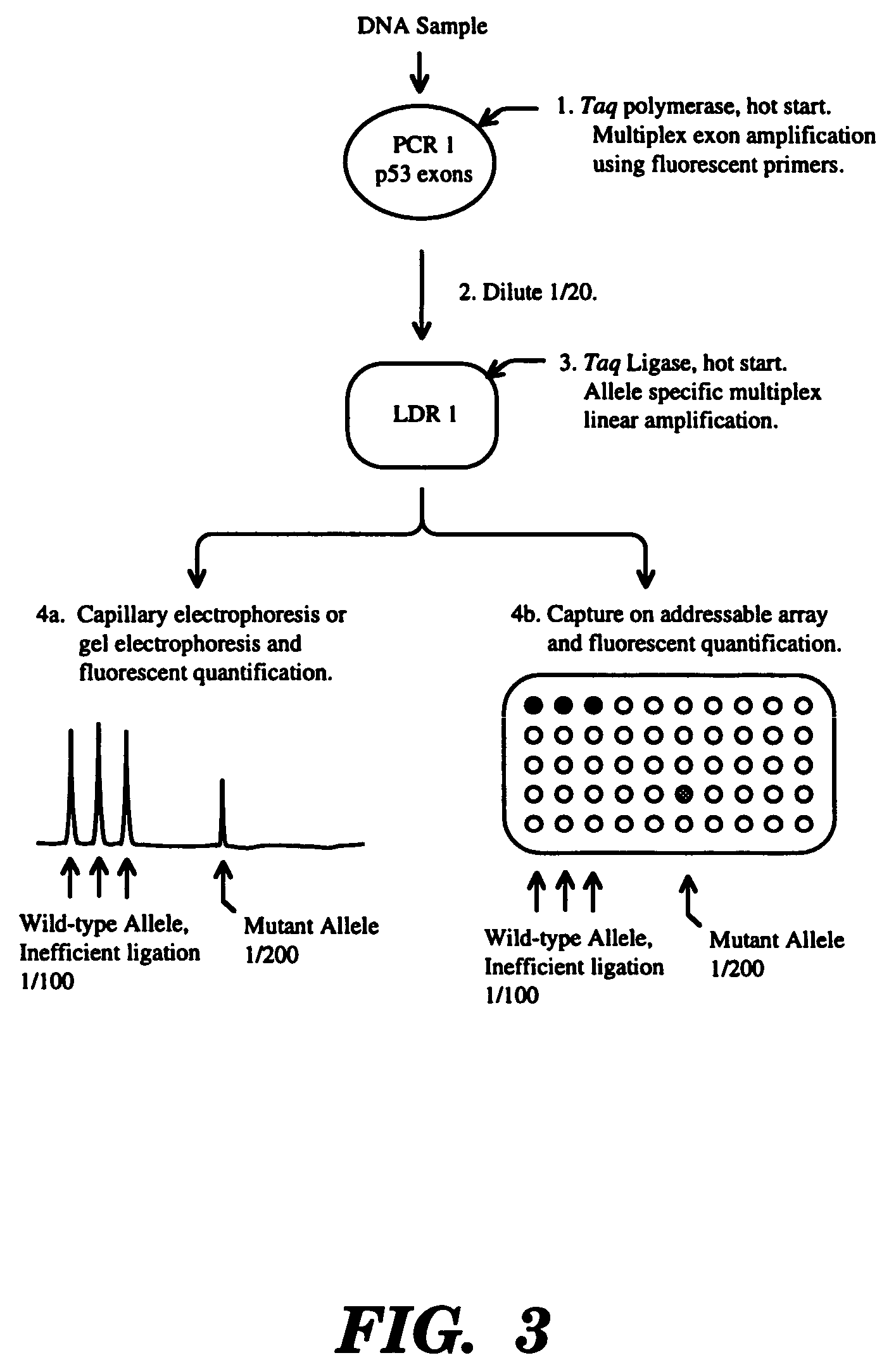
Ligase detection reaction is utilized to distinguish minority template in the presence of an excess of normal template with a thermostable ligase. This process can be carried out with a mutant ligase, thermostable ligase, or a modified oligonucleotide probe. This procedure is particularly useful for the detection of cancer-associated mutations. It has the advantage of providing a quantitative measure of the amount or ratio of minority template.
Owner:CORNELL RES FOUNDATION INC +1
Soluble Form of Carbonic Anhydrase IX (s-CA IX), Assays to Detect s-CA IX, CA IX's Coexpression with HER-2/neu/c-erbB-2, and CA IX-Specific Monoclonal Antibodies to Non-Immunodominant Epitopes
InactiveUS20080176258A1Improve efficiencyIncrease resourcesBiological material analysisDepsipeptidesImmunodominant EpitopesMonoclonal antibody
Disclosed herein among other MN / CA IX-related inventions are new MN / CA IX-specific antibodies generated from MN / CA IX-deficient mice, preferably monoclonal antibodies and immunoreactive fragments and engineered variants thereof. Subsets of the new antibodies are to either the proteoglycan-like (PG) domain or to the carbonic anhydrase (CA) domain of MN / CA IX, and methods are provided by which antibodies can be prepared to the other MN / CA IX domains. Such new MN / CA IX-specific antibodies, fragments and variants are useful diagnostically / prognostically and therapeutically for cancer and precancer. Particularly preferred are the new monoclonal antibodies, fragments and variants that are specific for the non-immunodominant epitopes of MN / CA IX, which antibodies are, among other uses, useful to detect soluble MN / CA IX (s-CA IX) in body fluids, alone but preferably in combination with antibodies specific to the immunodominant epitopes of MN / CA IX, for example, in a sandwich assay.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI
Inducing cellular immune responses to hepatitis B virus using peptide and nucleic acid compositions
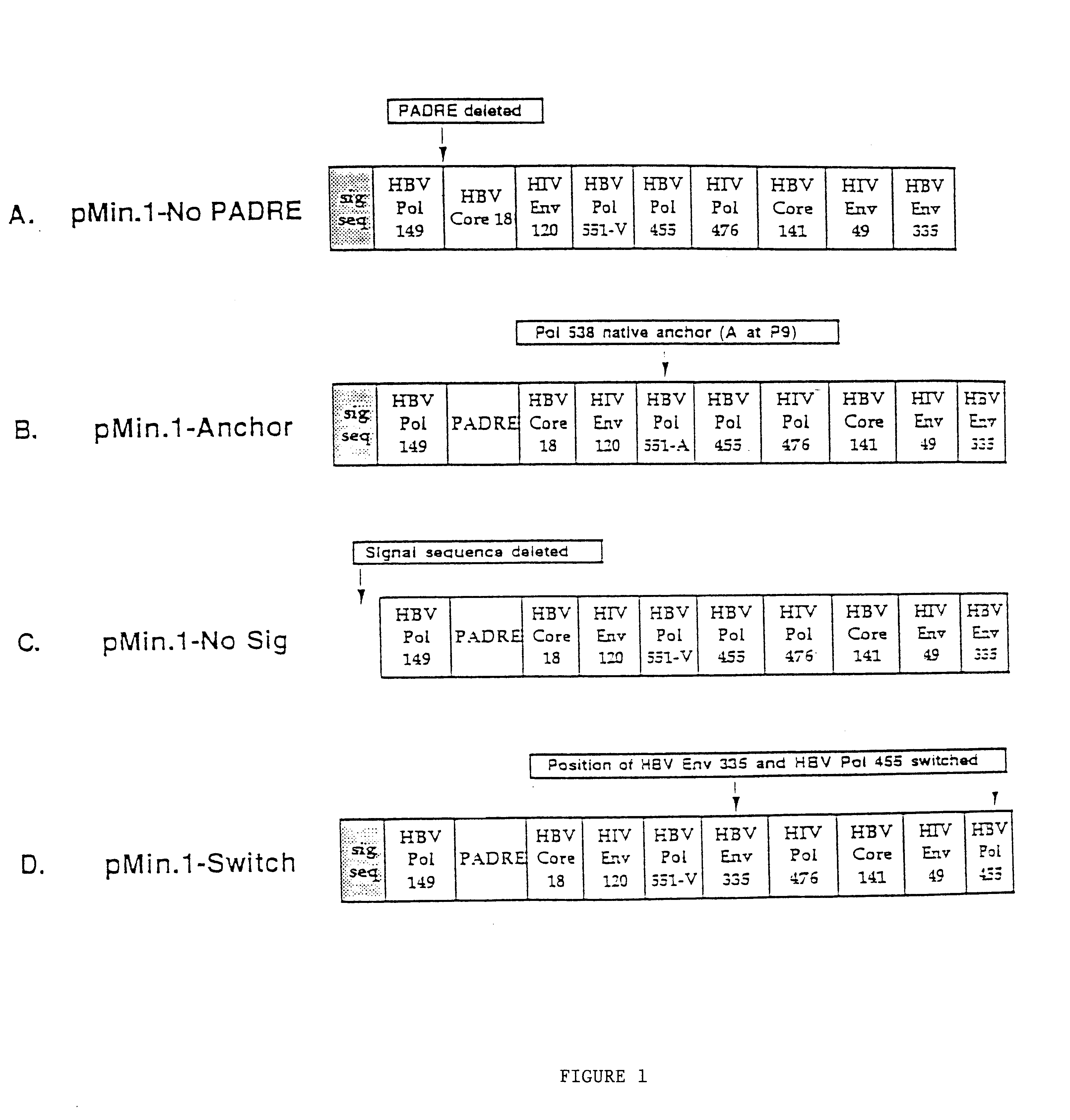
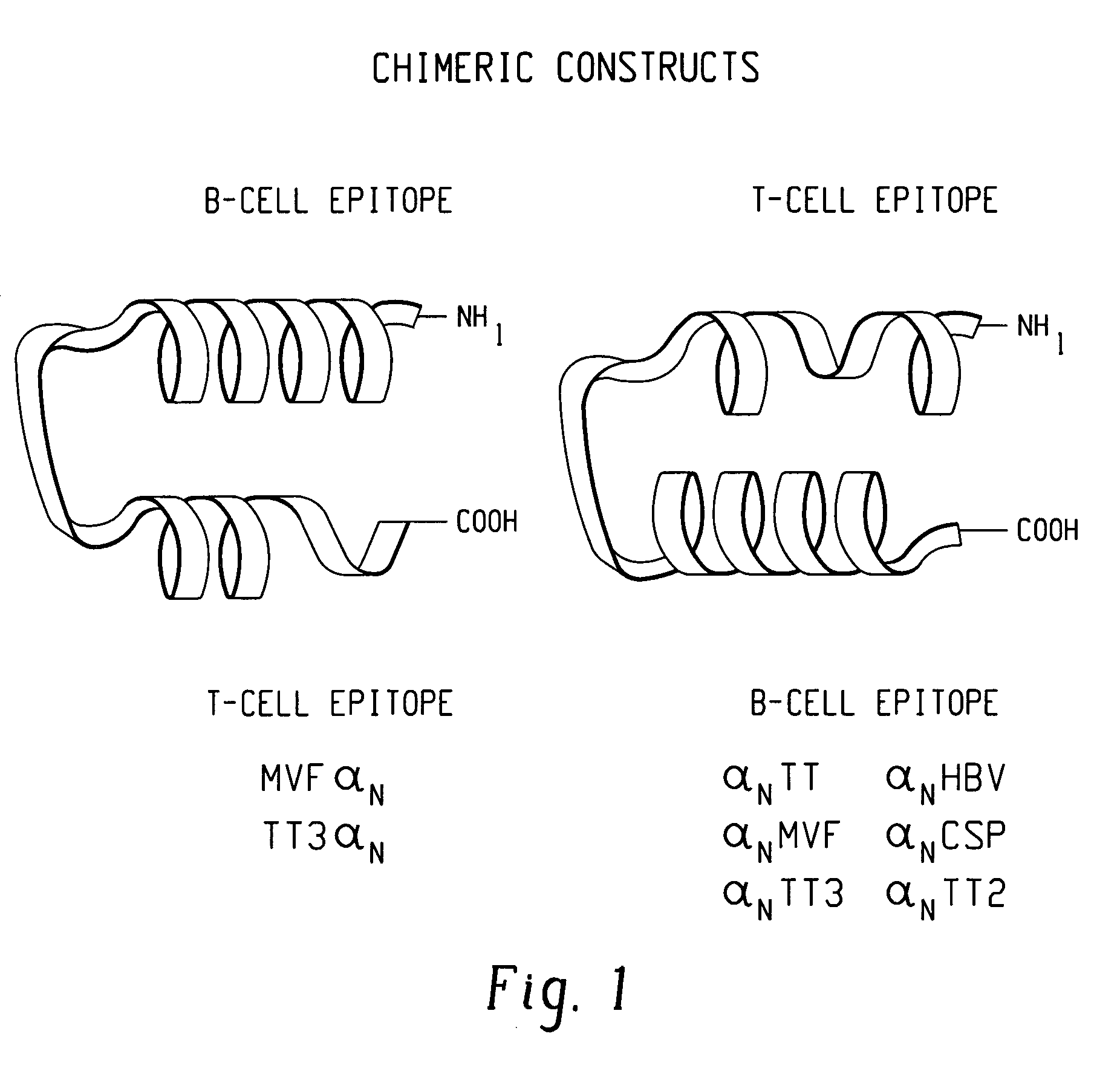
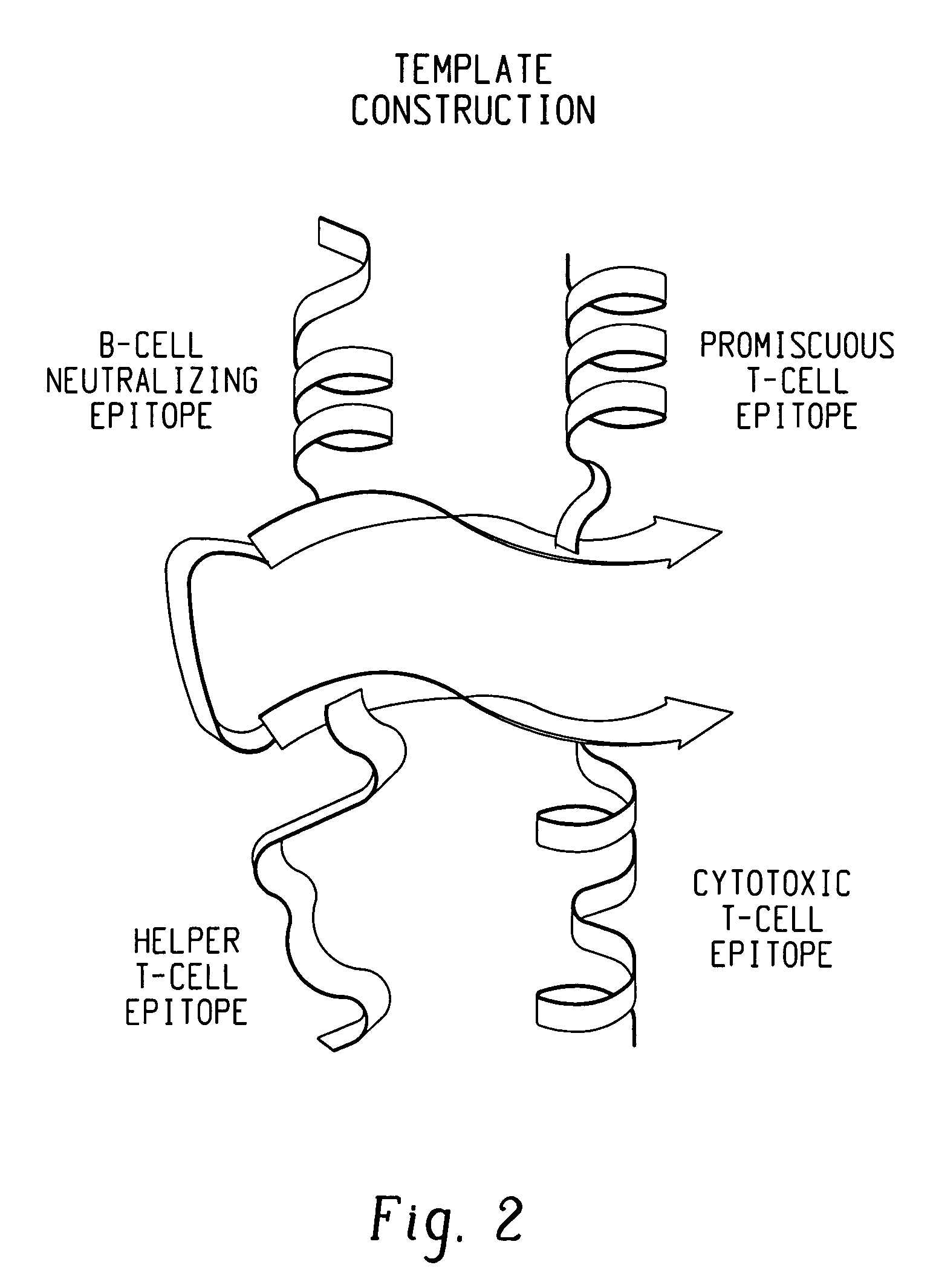
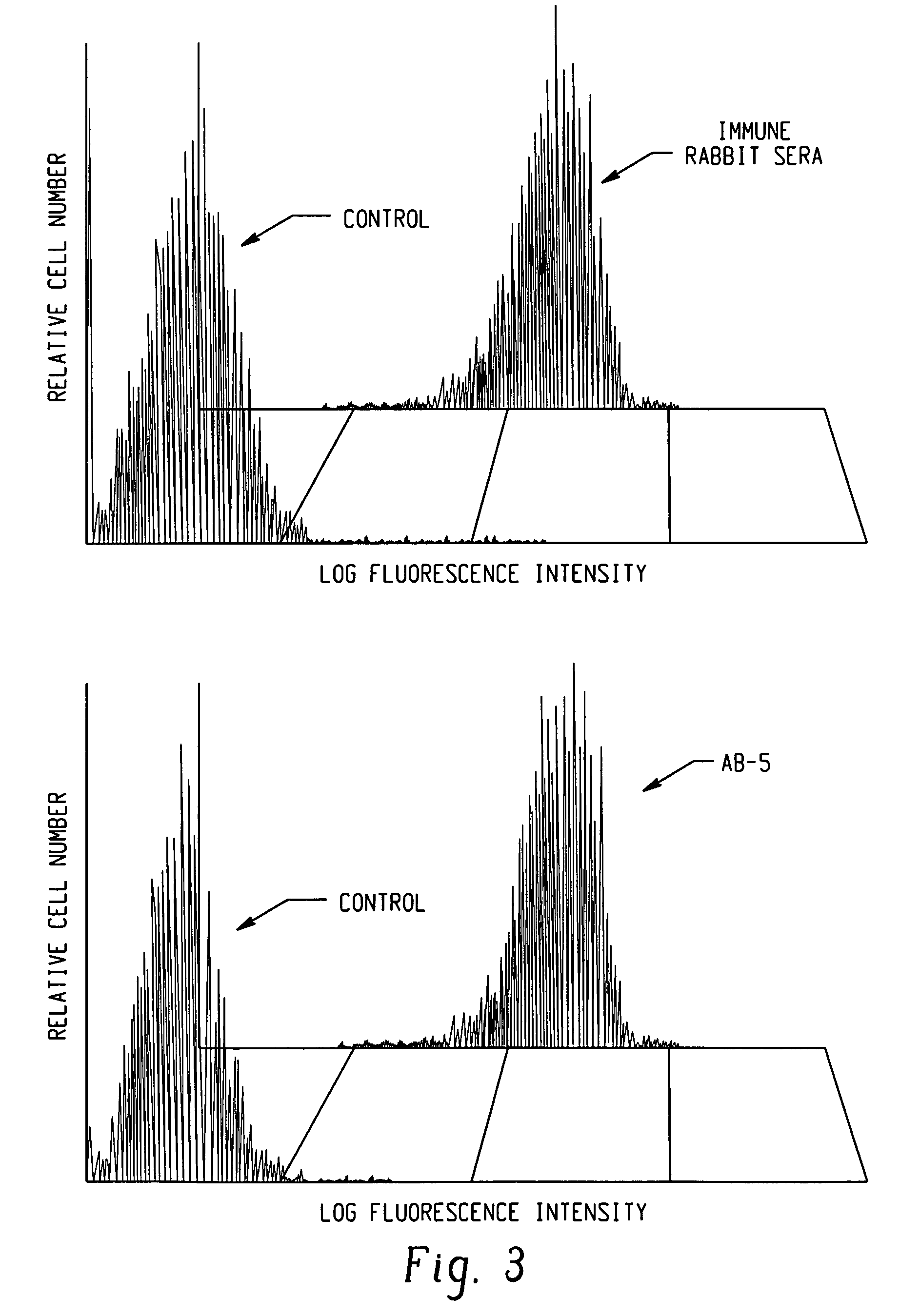
This invention uses our knowledge of the mechanisms by which antigen is recognized by T cells to develop epitope-based vaccines directed towards HBV. More specifically, this application communicates our discovery of pharmaceutical compositions and methods of use in the prevention and treatment of HBV infection.
Owner:PHARMEXA
Multivalent antibody contructs
InactiveUS20070031436A1Polypeptide with localisation/targeting motifAnimal cellsEukaryotic plasmidsVariable domain
The present invention relates to a multivalent Fv antibody construct having at least four variable domains which are linked with each over via the peptide linkers 1, 2 and 3. The invention also concerns expression plasmids which code for such an Fv antibody construct and a method of producing the Fv antibody constructs as well as their use.
Owner:DEUTES KREBSFORSCHUNGSZENT STIFTUNG DES OFFENTLICHEN RECHTS
Methods for detecting cancer and monitoring cancer progression
InactiveUS20060281089A1Sugar derivativesMicrobiological testing/measurementSolid tumorCancer research
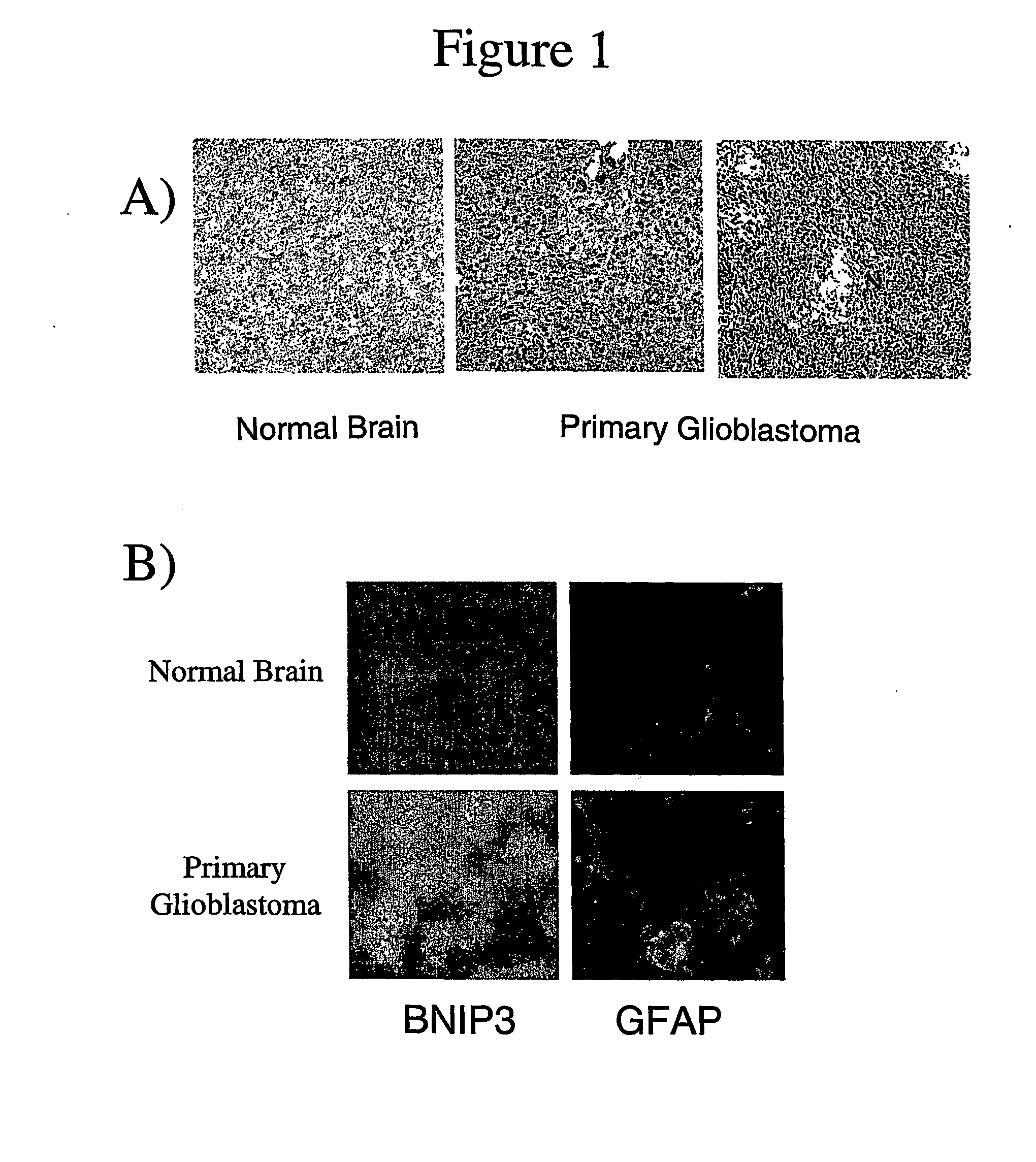
Mutant BNIP3 plays a functionally important role in the development of solid tumors and in resistance to chemotherapy and radiation treatments. The invention relates to methods of detecting cancer, methods of monitoring the progression of cancer, methods of identifying patients with cancer that is resistant to chemotherapy or radiation treatments, and diagnostic kits for performing the methods of the invention.
Owner:UNIVERSITY OF MANITOBA
Cupredoxin derived transport agents and methods of use thereof
ActiveUS20060149037A1Facilitate entryPeptide/protein ingredientsMammal material medical ingredientsCancer cellProtein transduction domain
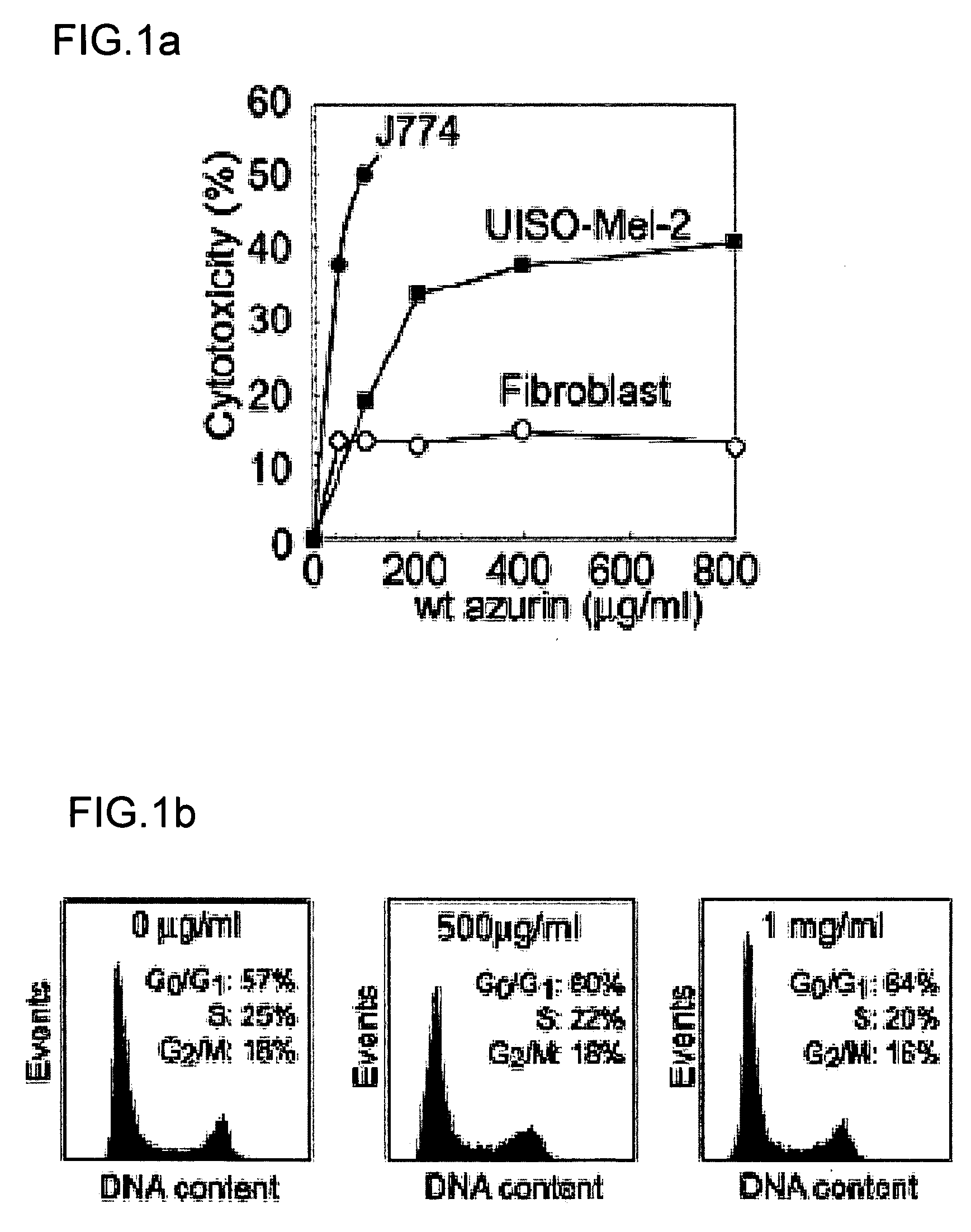
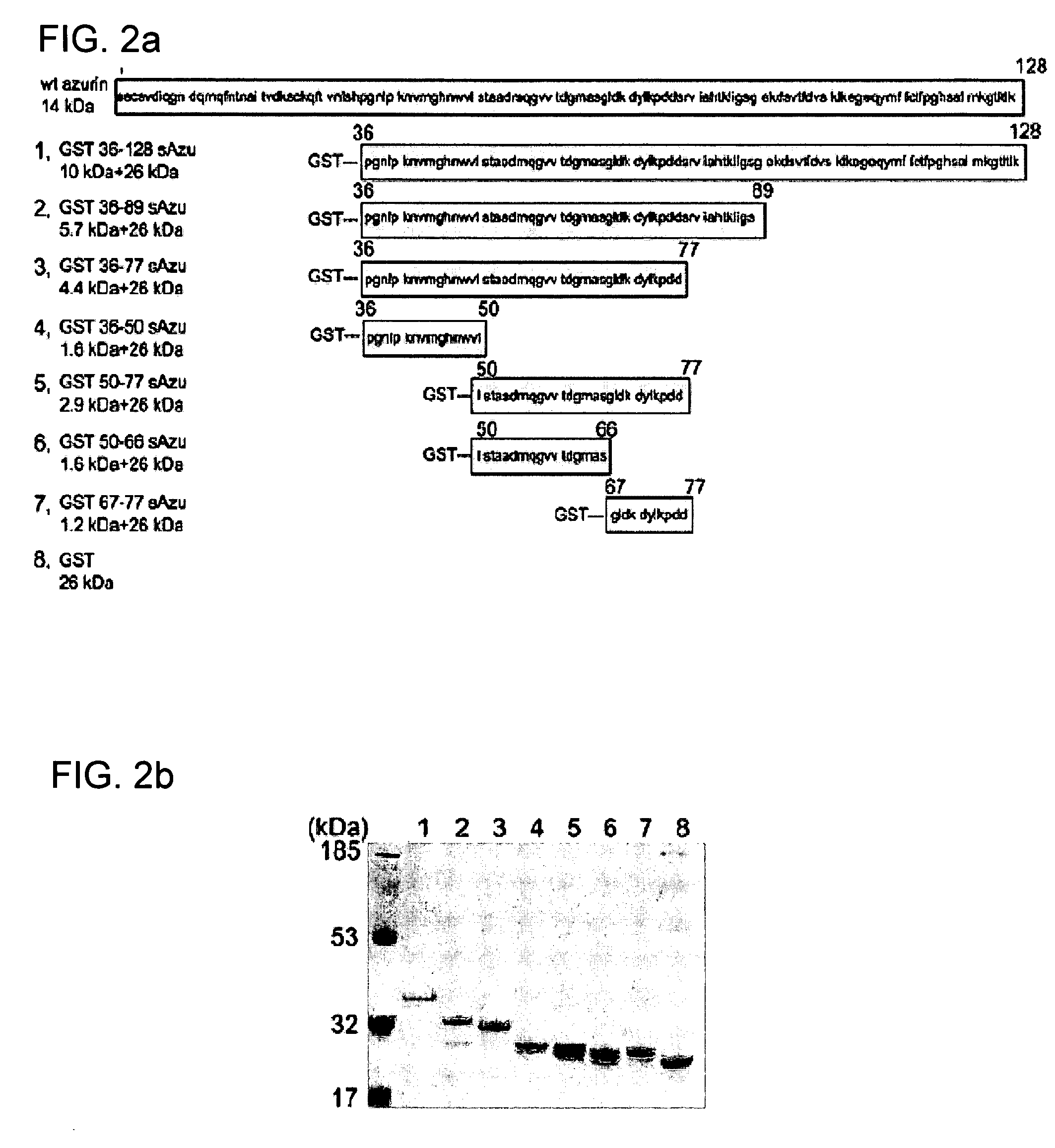
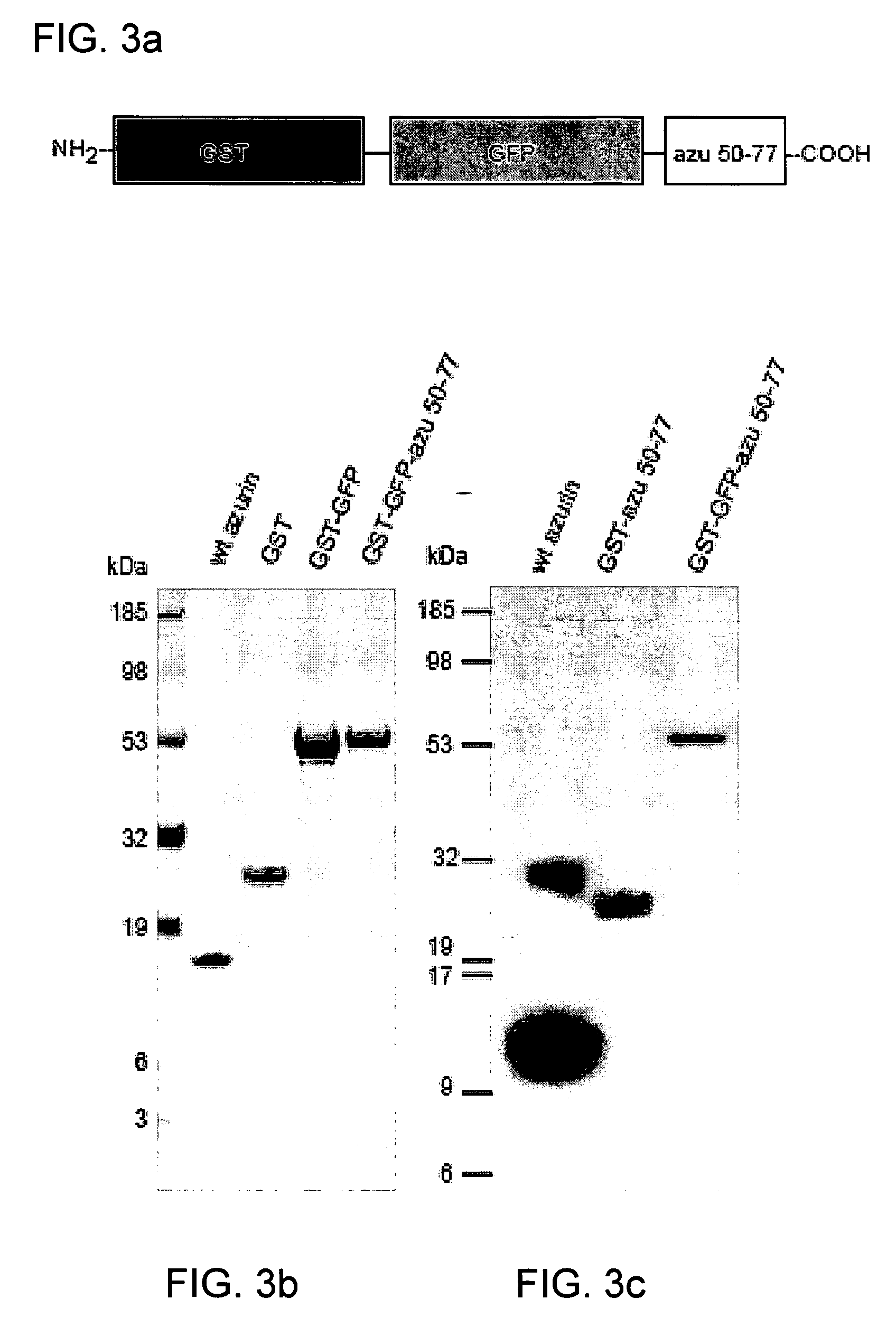
The present invention discloses methods and materials for delivering a cargo compound into a cancer cell. Delivery of the cargo compound is accomplished by the use of protein transduction domains derived from cupredoxins. The invention further discloses methods for treating cancer and diagnosing cancer.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Soluble Form of Carbonic Anhydrase IX (s-CA IX), Assays to Detect s-CA IX, CA IX's Coexpression with HER-2/neu/c-erbB-2, and CA IX-Specific Monoclonal Antibodies to Non-Immunodominant Epitopes
InactiveUS20080176310A1Improve efficiencyIncrease resourcesOxidoreductasesFermentationKilodaltonWestern blot
Disclosed herein is the discovery of a soluble MN / CA IX (s-CA IX) in body fluids, such as, urine and serum. Said s-CA IX comprises the extracellular domain of CA IX or portions thereof. The predominant s-CA IX species is the extracellular domain comprising a proteoglycan-like (PG) domain and carbonic anhydrase (CA) domain, and having a molecular weight of about 50 / 54 kilodaltons (kd) upon Western blot. A smaller s-CA IX form of about 20 to about 30 kd comprising the CA domain or parts thereof, not linked to the PG domain, has also been found in body fluids. Diagnostic / prognostic methods for precancer and cancer that detect or detect and quantitate said s-CA IX in body fluids, are described. Also disclosed herein is the coexpression of CA IX and HER-2 / neu / c-erbB-2 that provides parallel, alternative and potentially synergistic diagnostic / prognostic and therapeutic strategies for precancer and cancer. Further disclosed are new MN / CA IX-specific antibodies generated from MN / CA IX-deficient mice, preferably monoclonal antibodies and immunoreactive fragments and engineered variants thereof. Such new MN / CA IX-specific antibodies, fragments and variants are useful diagnostically / prognostically and therapeutically for cancer and precancer. Particularly preferred are the new monoclonal antibodies, fragments and variants that are specific for the non-immunodominant epitopes of MN / CA IX, which antibodies are, among other uses, useful to detect soluble MN / CA IX (s-CA IX) in body fluids, alone but preferably in combination with antibodies specific to the immunodominant epitopes of MN / CA IX, for example, in a sandwich assay.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI
Soluble Form of Carbonic Anhydrase IX (s-CA IX), Assays to Detect s-CA IX, CA IX's Coexpression with Her-2/neu/c-erbB-2, and CA IX-Specific Monoclonal Antibodies to Non-Immunodominant Epitopes
InactiveUS20080177046A1Good curative effectImprove efficiencyImmunoglobulins against animals/humansBiological material analysisKilodaltonWestern blot
Disclosed herein is the discovery of a soluble MN / CA IX (s-CA IX) in body fluids, such as, urine and serum. Said s-CA IX comprises the extracellular domain of CA IX or portions thereof. The Predominant s-CA IX species is the extracellular domain comprising a proteoglycan-like (PG) domain and carbonic anhydrase (CA) domain, and having a molecular weight of about 50 / 54 kilodaltons (kd) upon Western blot. A smaller s-CA IX form of about 20 to about 30 kd comprising the CA domain or parts thereof, not linked to the PG domain, has also been found in body fluids. Diagnostic / prognostic methods for precancer and cancer that detect or detect and quantitate said s-CA IX in body fluids, are described. Also disclosed herein is the coexpression of CA IX and HER-2 / neu / c-erbB-2 that provides parallel, alternative and potentially synergistic diagnostic / prognostic and therapeutic strategies for precancer and cancer. Further disclosed are new MN / CA IX-specific antibodies generated from MN / CA IX-deficient mice, preferably monoclonal antibodies and immunoreactive fragments and engineered variants thereof. Such new MN / CA IX-specific antibodies, fragments and variants are useful diagnostically / prognostically and therapeutically for cancer and precancer. Particularly preferred are the new monoclonal antibodies, fragments and variants that are specific for the non-immunodominant epitopes of MN / CA IX, which antibodies are, among other uses, useful to detect soluble MN / CA IX (s-CA IX) in body fluids, alone but preferably in combination with antibodies specific to the immunodominant epitopes of MN / CA IX, for example, in a sandwich assay.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI
Gapped 2' modified oligonucleotides
InactiveUS6146829AHigh affinityAvoid degradationSugar derivativesPeptide/protein ingredientsNucleaseOrganism
Oligonucleotides and other macromolecules are provided that have increased nuclease resistance, substituent groups for increasing binding affinity to complementary strand, and sub-sequences of 2'-deoxy-erythro-pentofuranosyl nucleotides that activate RNase H enzyme. Such oligonucleotides and macromolecules are useful for diagnostics and other research purposes, for modulating protein in organisms, and for the diagnosis, detection and treatment of other conditions susceptible to antisense therapeutics.
Owner:IONIS PHARMA INC
Polypeptides and polynucleotides for enhancing immune reactivity to HER-2 protein
Compositions for stimulating the immune system and for treating malignancies associated with overexpression of the HER-2 protein are provided. Such compositions include immunogenic epitopes of the HER-2 proteins and chimeric and multivalent peptides which comprise such epitopes. The present invention also relates to polynucleotides which encode the chimeric peptides. Also provided are pharmaceutical compositions comprising such immunogenic compositions. Methods for stimulating an immune response to HER-2 protein are provided. Methods for treating breast cancer, ovarian cancer, prostate cancer, colon cancer and lung cancer are provided.
Owner:OHIO STATE INNOVATION FOUND
Isolated peptides consisting of amino acid sequences found in SSX or NY-ESO-1 molecules, which bind to HLA molecules
The invention relates to members of the SSX family of genes, as well as their uses. Also a part of the invention are peptides derived from SSX molecules and the NY-ESO-1 molecule, which form complexes with HLA molecules, leading to lysis of cells presenting these complexes, by cytolytic T cells.
Owner:LUDWIG INST FOR CANCER RES
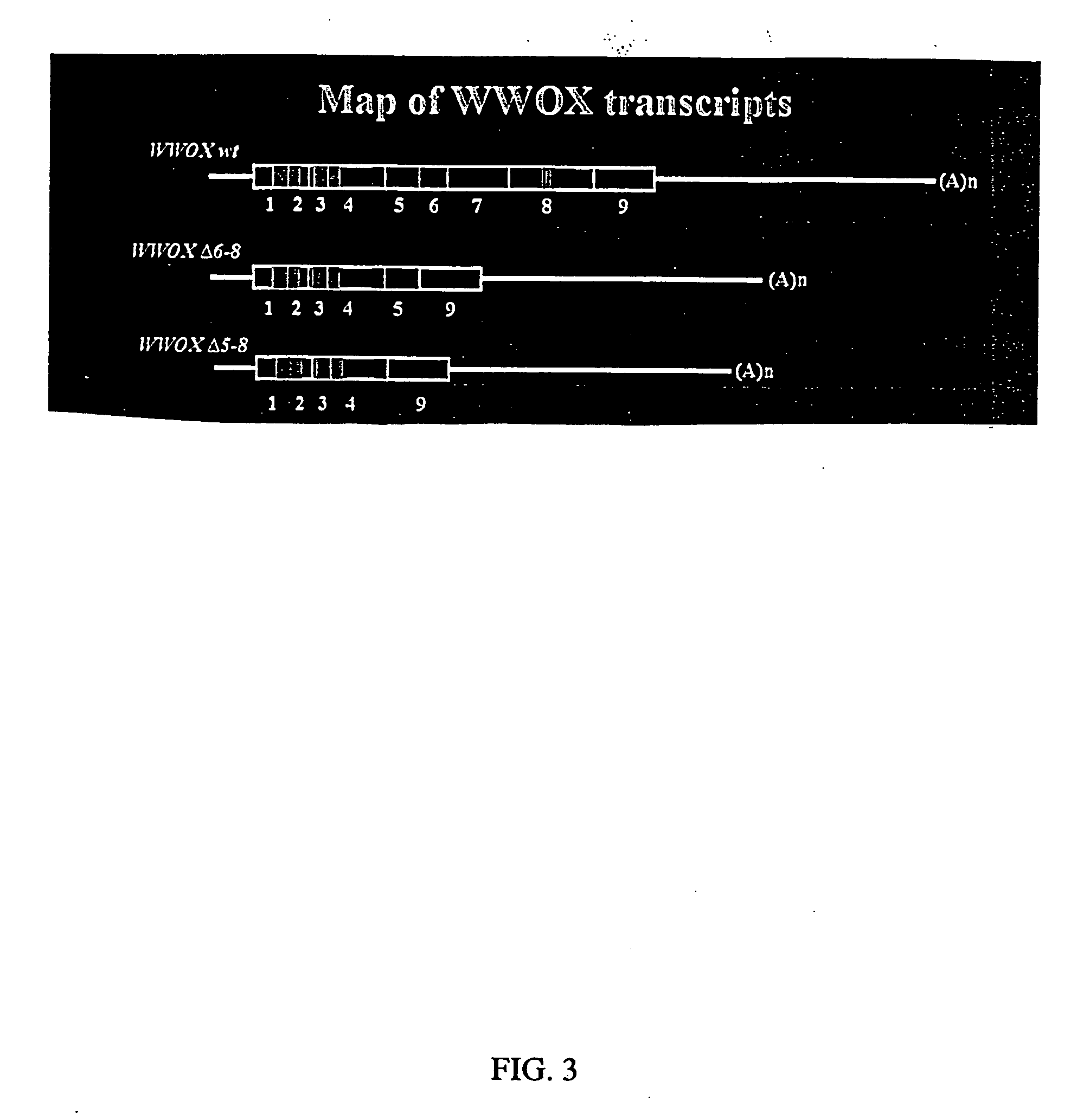
Wwox: a tumor suppressor gene mutated in multiple cancers
InactiveUS20060024780A1Sugar derivativesPeptide preparation methodsAbnormal tissue growthTumour suppressor gene
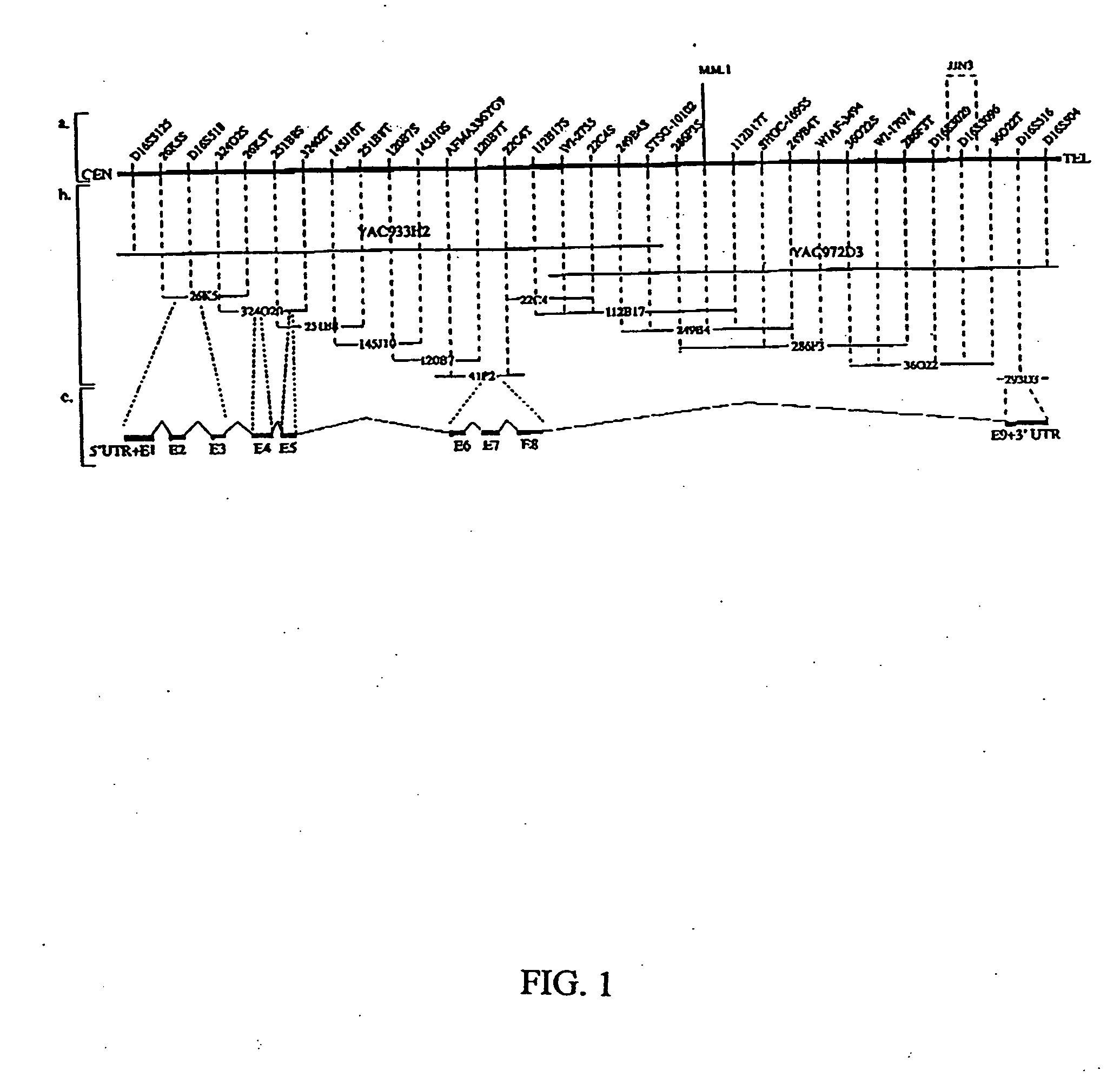
The present invention provides the isolation and cloning of WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3-24.1, a region frequently affected in several cancers. This gene encodes a tumor suppressor with apoptotic functions. The invention provides WWOX nucleic acid- and polypeptide-based cancer therapies. The invention also provides methods for cancer detection, diagnosis and prognosis involving WWOX nucleic acids and polypeptides.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Tumor antigen based on products of the tumor suppressor gene WT1
Owner:INT INST OF CANCER IMMUNOLOGY INC
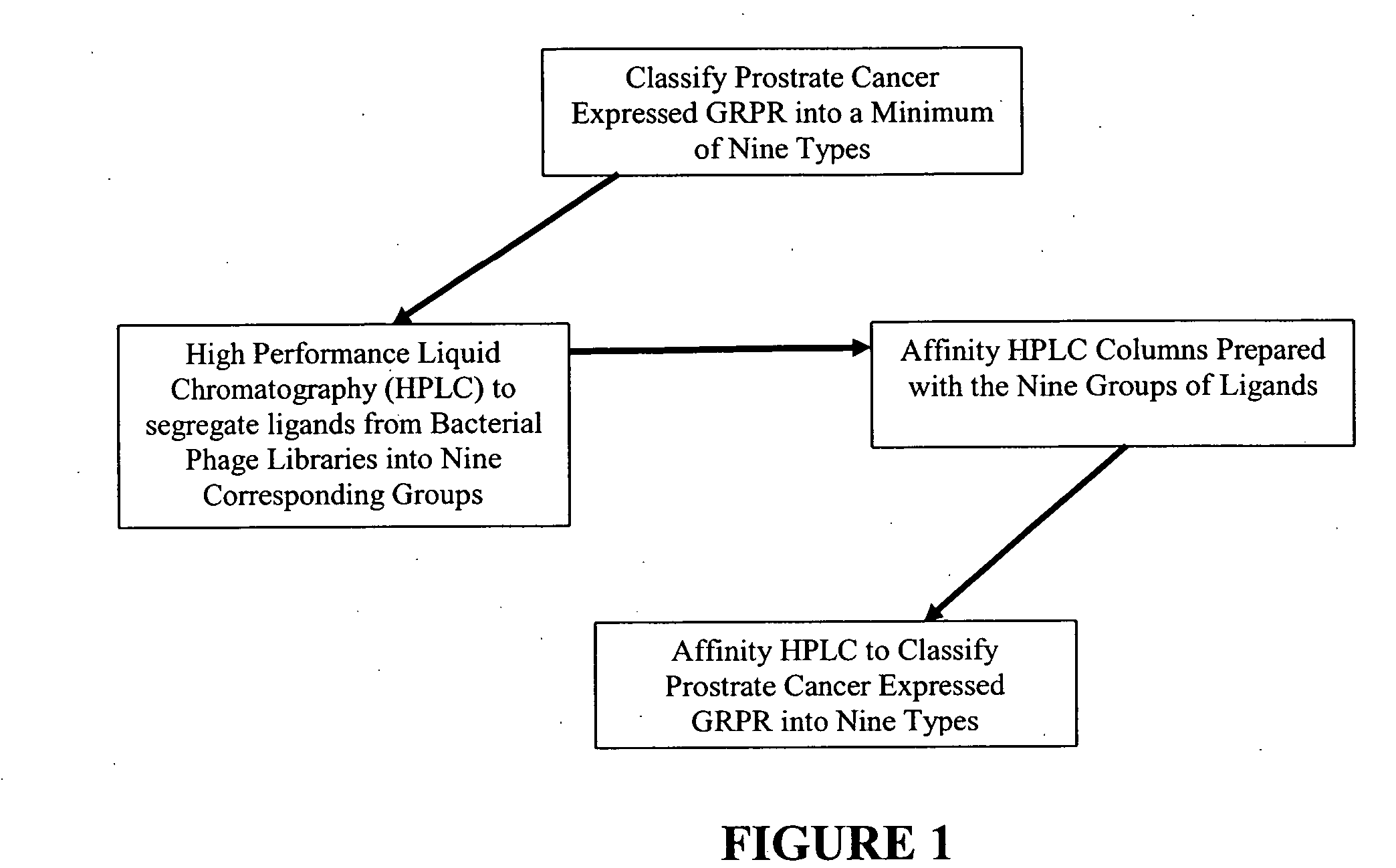
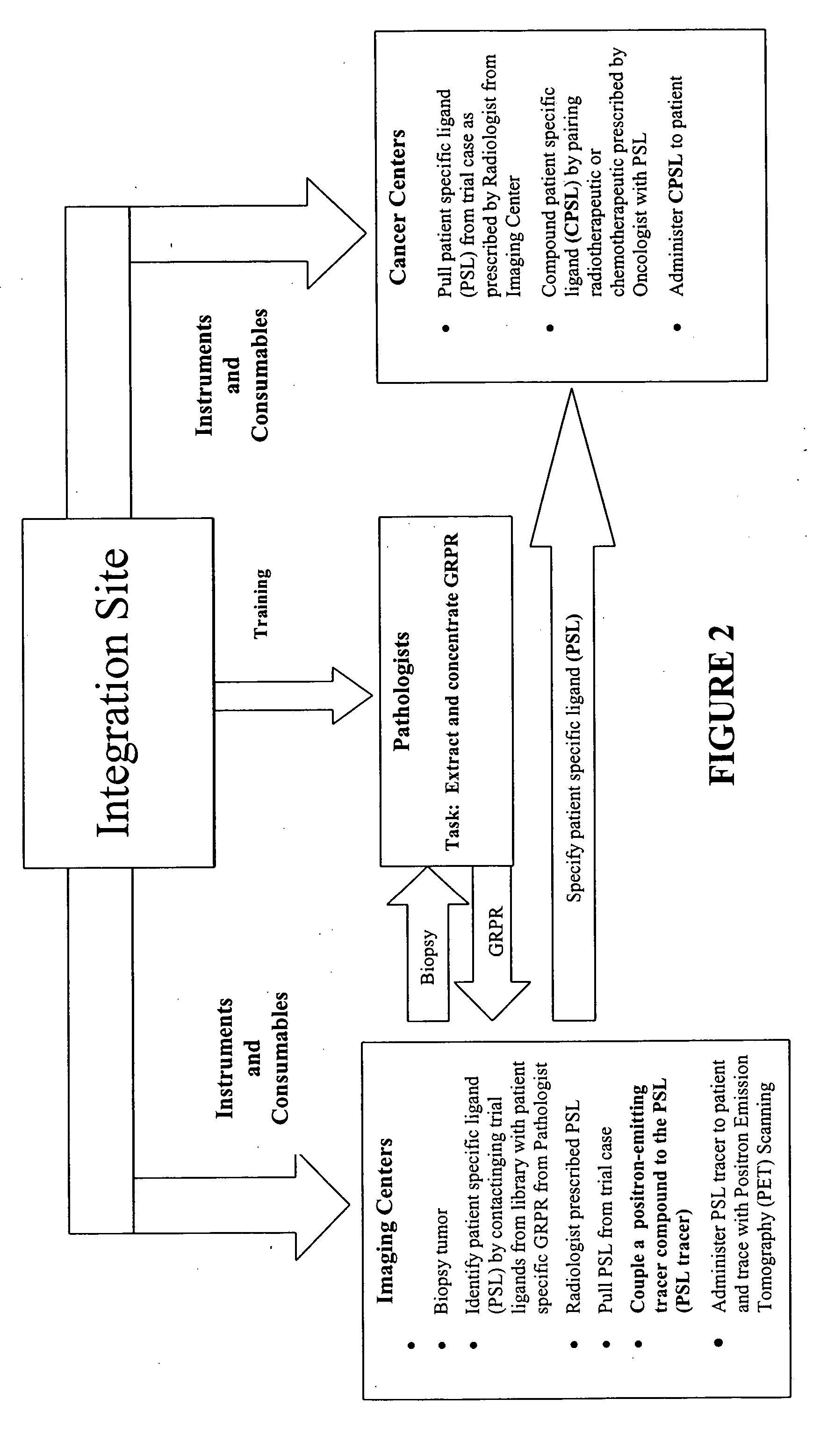
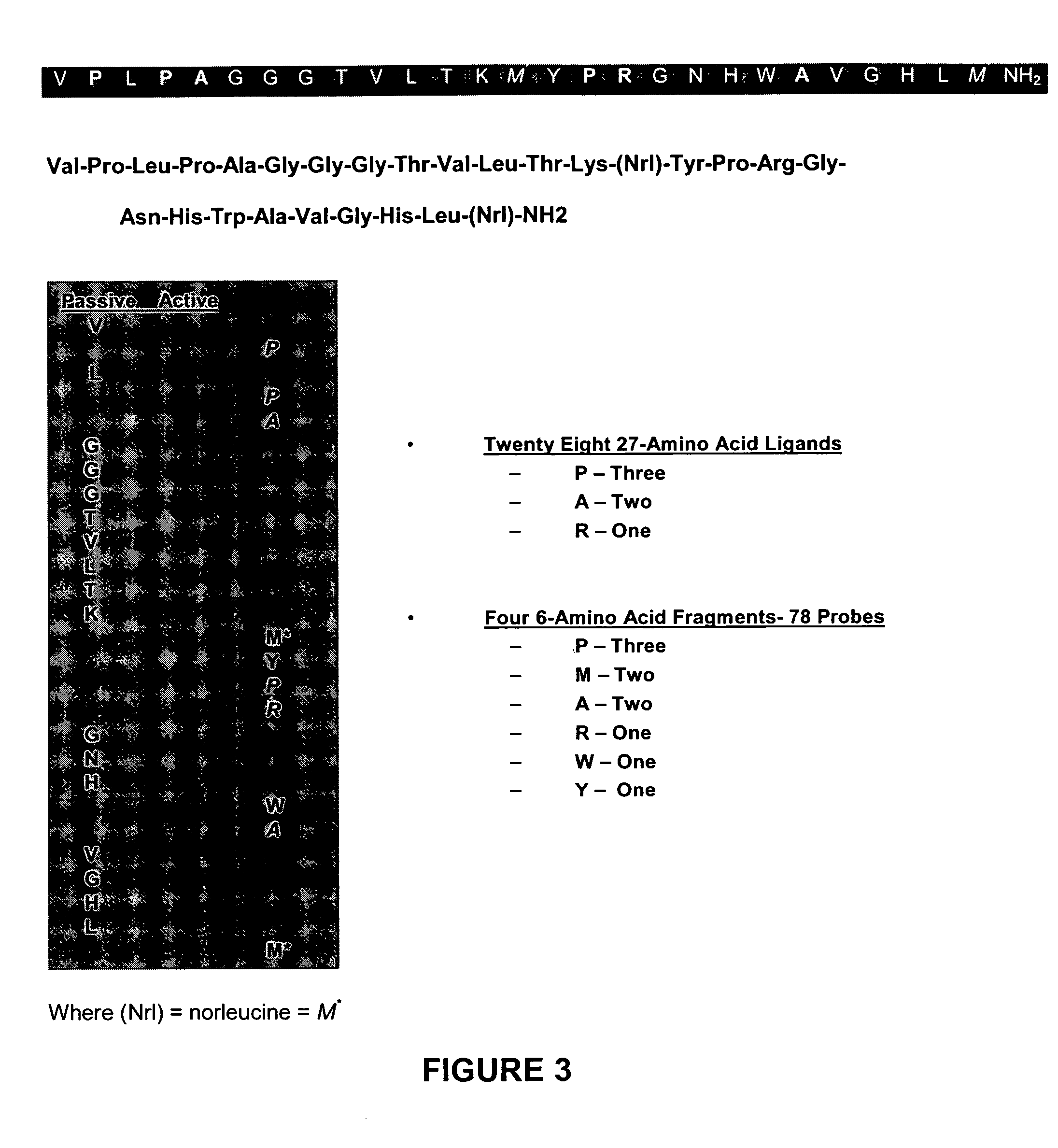
Method, compositions and classification for tumor diagnostics and treatment
InactiveUS20060160157A1Quick and easy determinationImprove survivalCompound screeningApoptosis detectionAbnormal tissue growthSide effect
The present invention is directed towards classifying tumor biomarkers, particularly membrane receptors, and more particularly the gastrin-releasing peptide (GPR) receptors, identified in patient samples, then linking therapeutic agents (chemical, radiological, or biological) to patient-specific ligands that bind to such receptors, clinicians can produce diagnostic and treatment compositions and implement treatment regimens which, by using the classified and identified biomarkers, and due to their improved accuracy, increase success and decrease undesired side effects from such treatments.
Owner:ZUCKERMAN MATHEW MARK
Anti-5T4 antibodies and uses thereof
InactiveUS8044178B2In-vivo radioactive preparationsImmunoglobulins against cell receptors/antigens/surface-determinantsAntibodyAntiendomysial antibodies
Owner:KODANGATTIL SREEKUMAR RAMAN +1
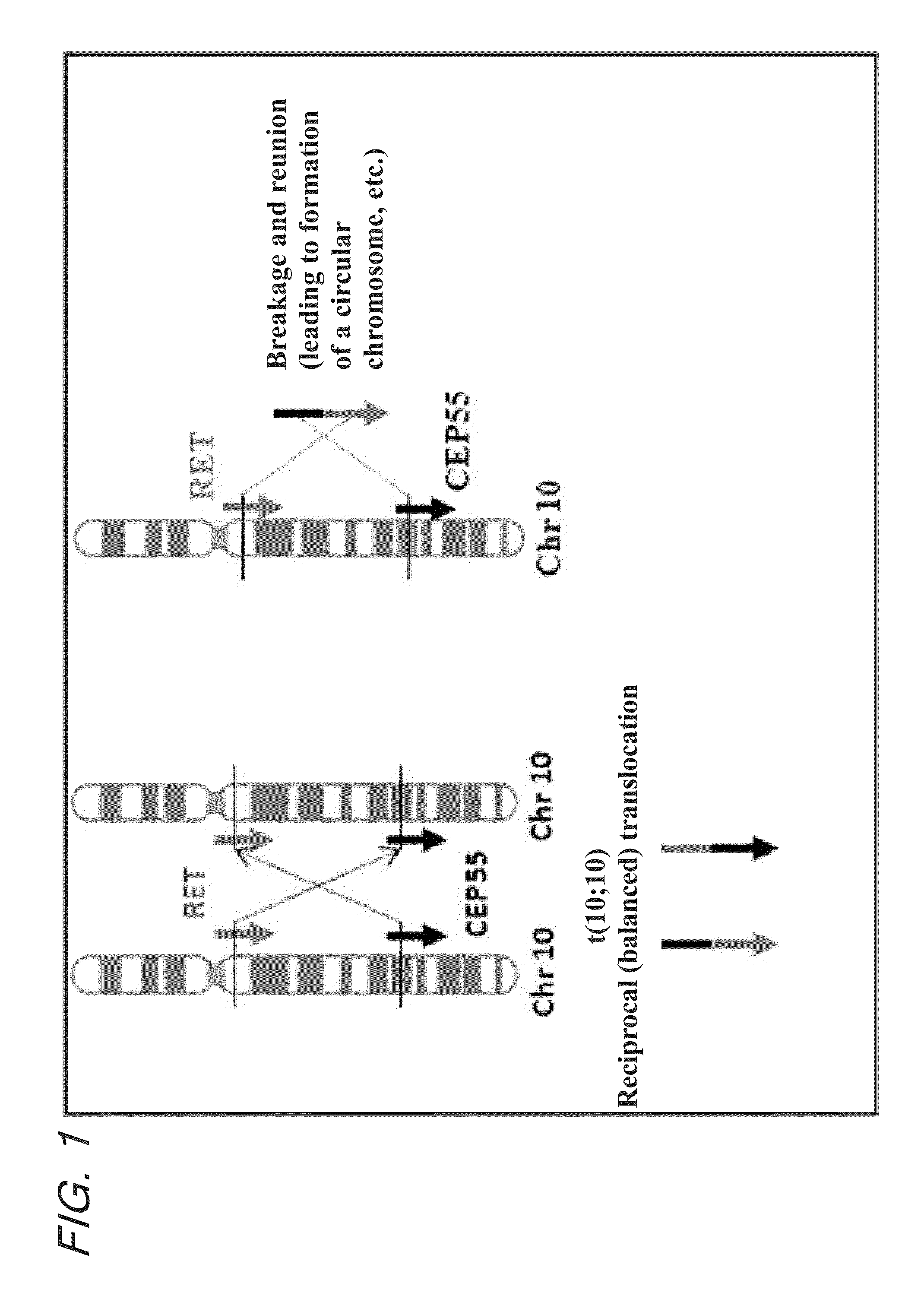
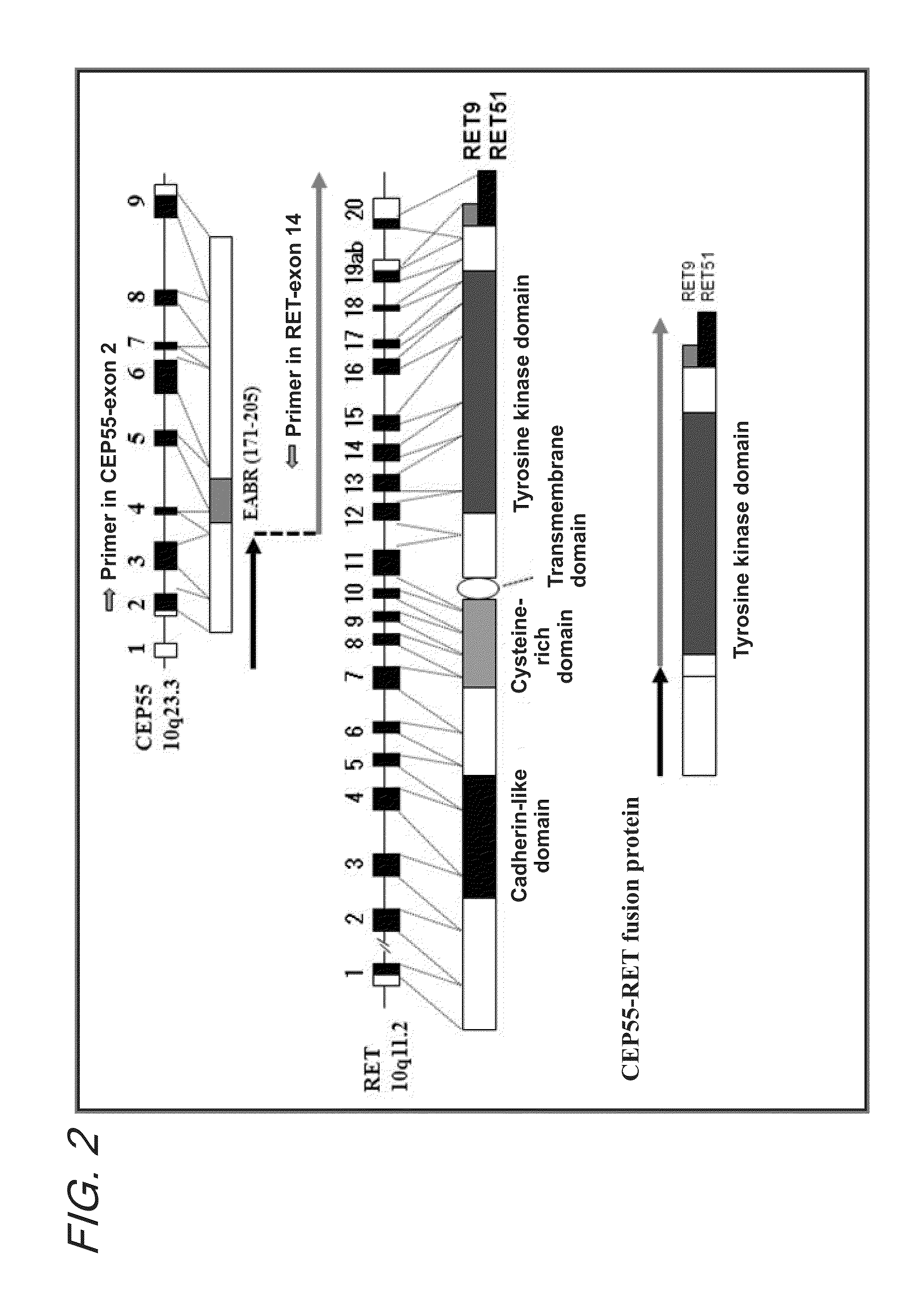
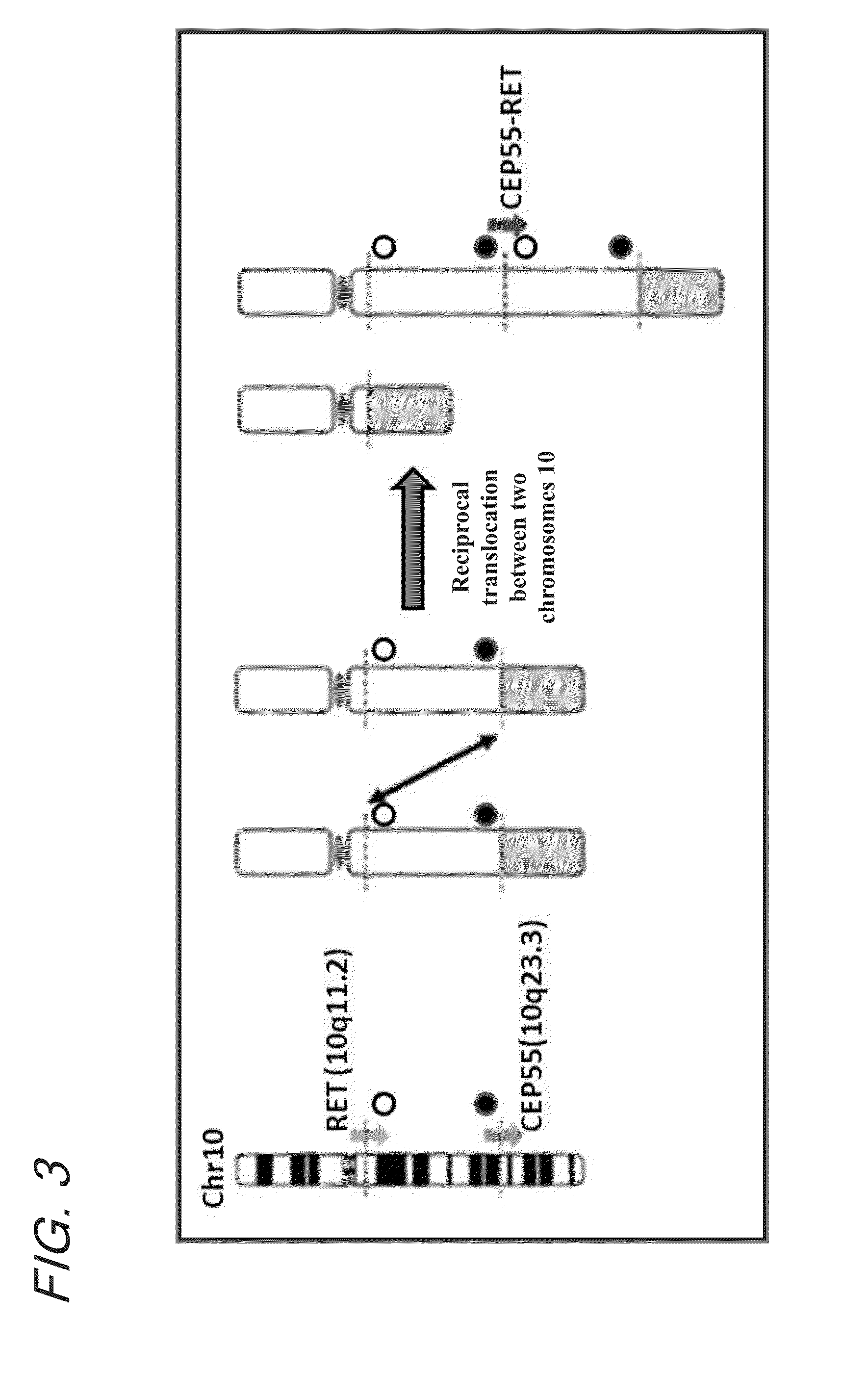
Fusion gene of cep55 gene and ret gene
InactiveUS20150177246A1Efficient detectionEffective treatment of cancerBiocideAntibody mimetics/scaffoldsRet geneTyrosine-kinase inhibitor
In order to identify genes that can serve as indicators for predicting the effectiveness of drug treatments in cancers and provide novel methods for predicting the effectiveness of treatments with drugs targeting said genes, transcriptome sequencing was performed of diffuse-type gastric cancer. As a result, in-frame fusion transcripts between the CEP55 gene and the RET gene were identified. It was also found that said gene fusions induce activation of RET protein, thereby causing canceration of cells. Further, it was demonstrated that the RET protein activation and canceration caused by said gene fusion can be suppressed by using a RET tyrosine kinase inhibitor, and that treatments with a RET tyrosine kinase inhibitor are effective in patients with detection of said gene fusion.
Owner:NAT CANCER CENT +1
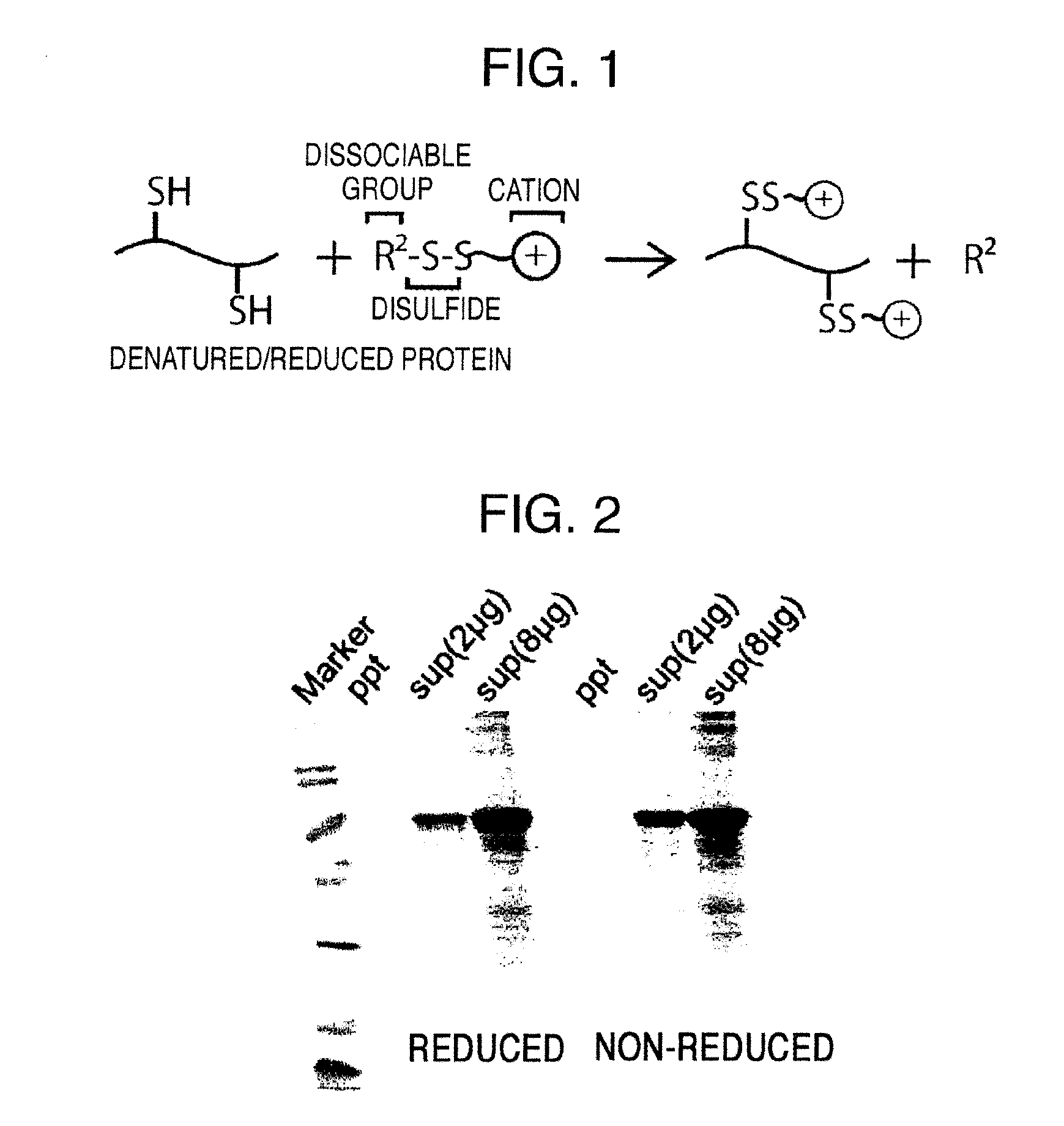
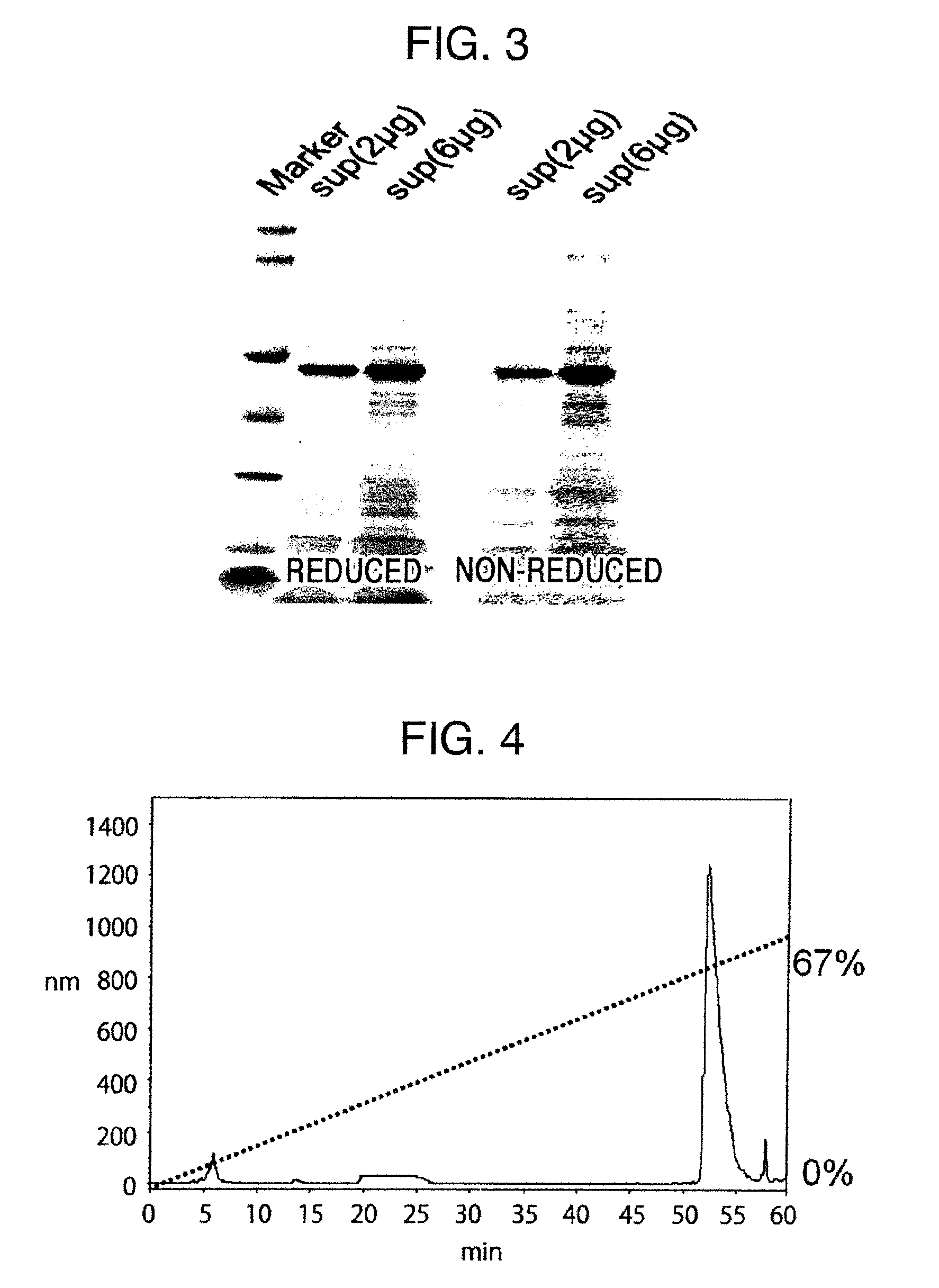
Method for producing reagent for antibody detection and use thereof
ActiveUS20150064801A1Efficiently solubilizeEffective recoveryTumor rejection antigen precursorsCell receptors/surface-antigens/surface-determinantsProtein moleculesAntigenic protein
The present invention provides the following: a method for efficiently producing a reagent for detecting an antibody that specifically binds with an insoluble antigen protein present in a liquid sample; a reagent for antibody detection produced by the production method; and a use of the antibody. In a step for solubilizing an antigen protein, it is possible to efficiently solubilize and recover the antigen protein by using a cationizing agent; therefore, when compared to conventional methods, it is possible to efficiently produce a reagent for detecting an antibody that has bound to multiple antigen protein molecules in a carrier.
Owner:FUTAMI JUNICHIRO +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com