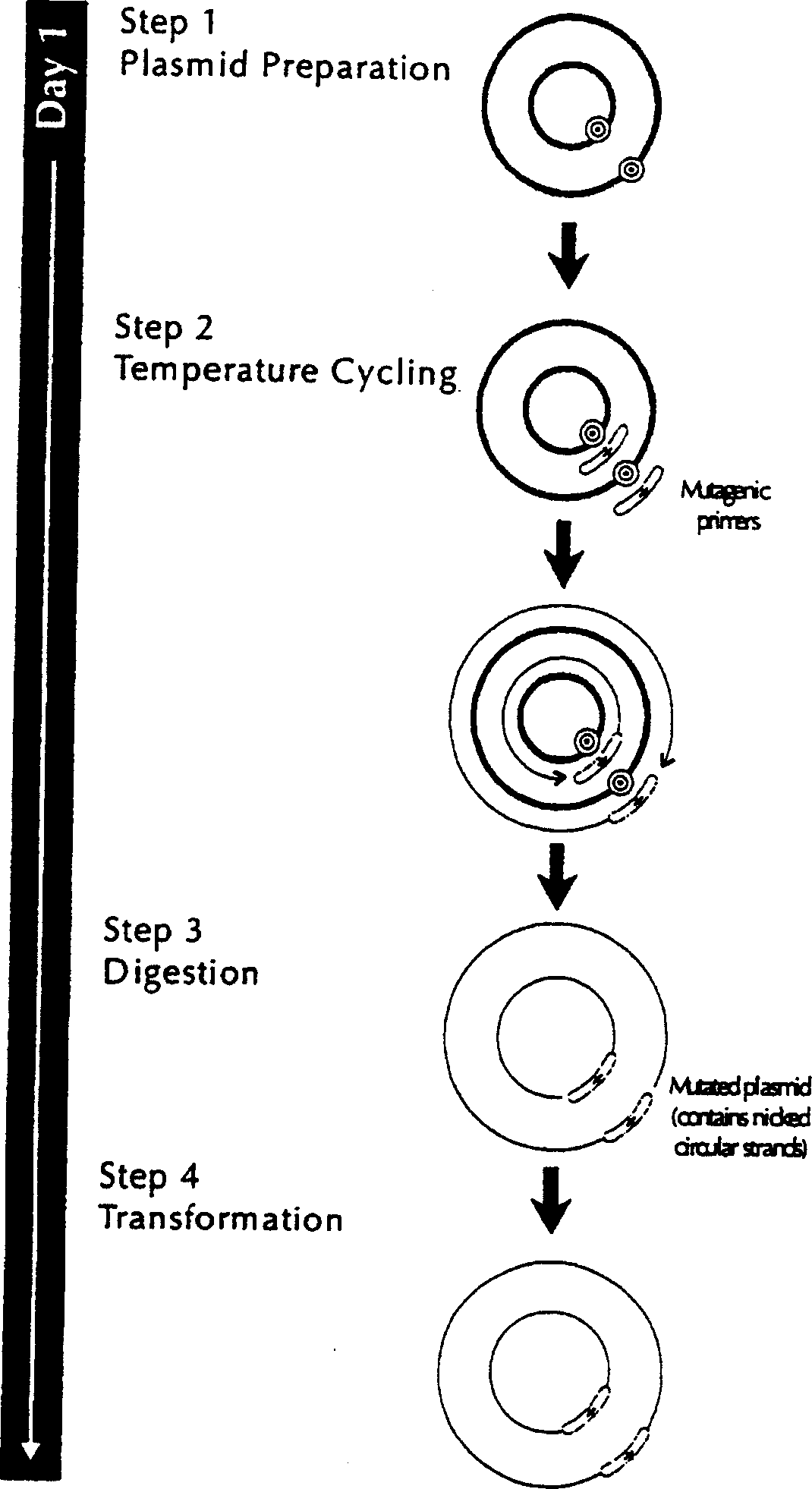
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "Viral Fusion Proteins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Rotavirus subunit vaccine
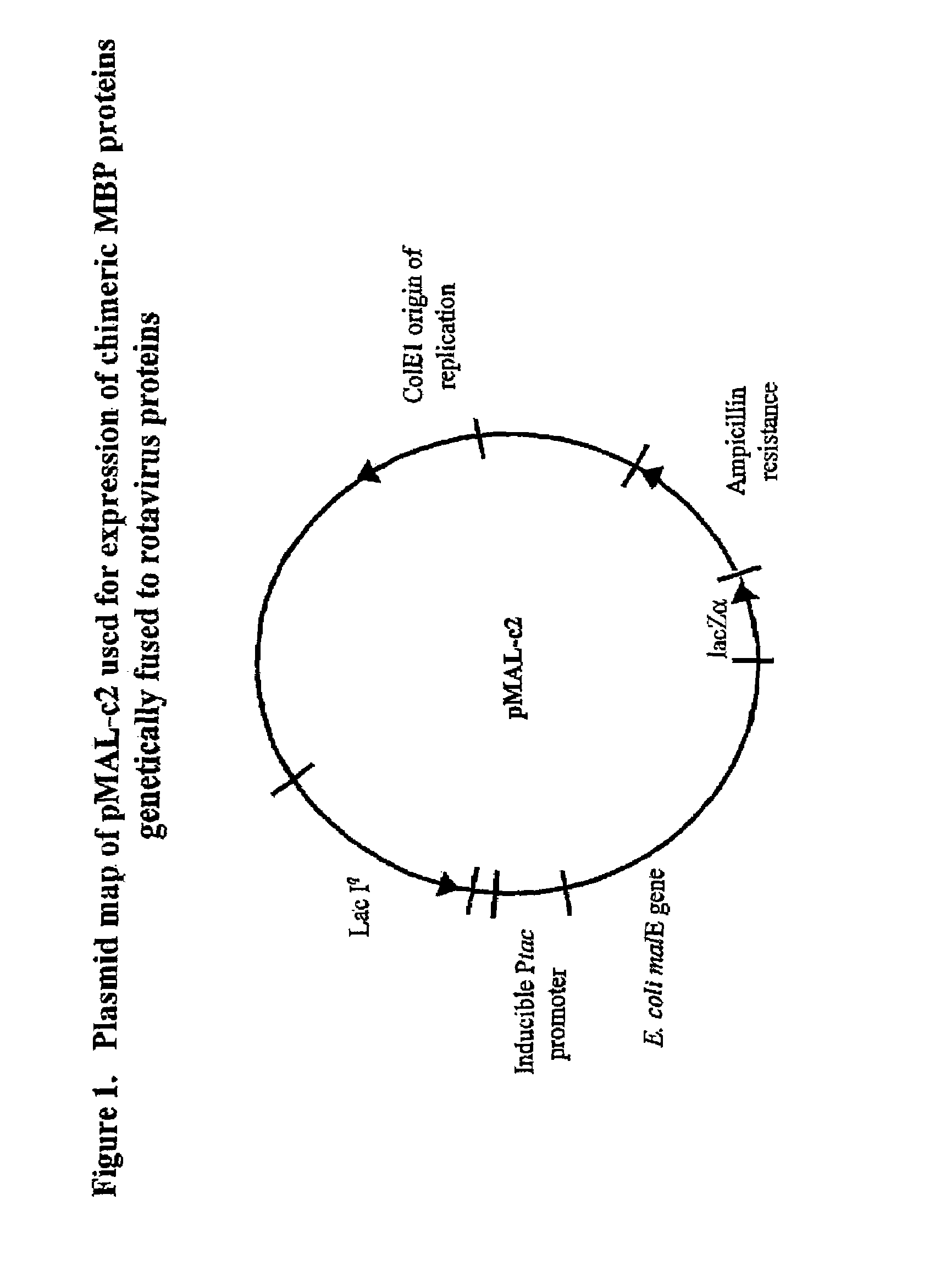
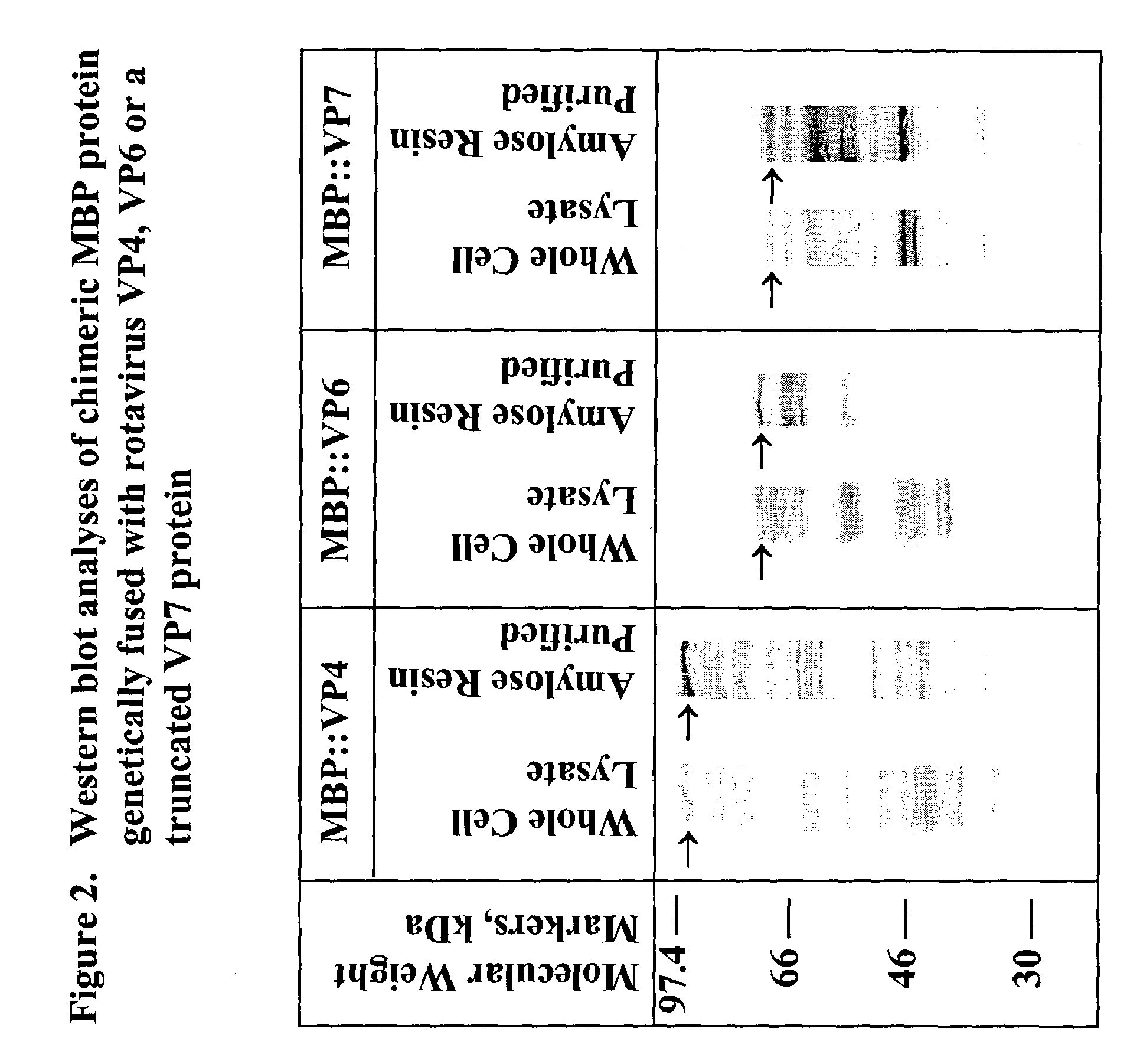
The present invention is directed to the generation and use of recombinant rotavirus fusion proteins as immunogens to produce a protective immune response from immunized individuals. In one embodiment, the present invention contemplates a recombinant rotavirus fusion protein vaccine composition comprising a rotavirus subunit protein or immunogenic fragment thereof, and an adjuvant in combination with the recombinant rotavirus subunit fusion protein. In one aspect of this embodiment, the recombinant rotavirus fusion protein comprises a rotavirus subunit protein and a fusion partner protein in genetic association with the rotavirus subunit protein, wherein the fusion partner protein does not interfere with expression and immunogenicity of the rotavirus subunit protein, the fusion partner protein prevents complex formation by the rotavirus subunit protein, and the fusion partner protein facilitates purification of the recombinant rotavirus fusion protein. In another aspect of this embodiment, the rotavirus subunit protein is selected from the group consisting of VP1, VP2, VP3, VP4, VP6, VP7, NSP1, NSP2, NSP3, NSP4 or NSP5. In yet another aspect of this embodiment, the rotavirus subunit protein is VP6.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
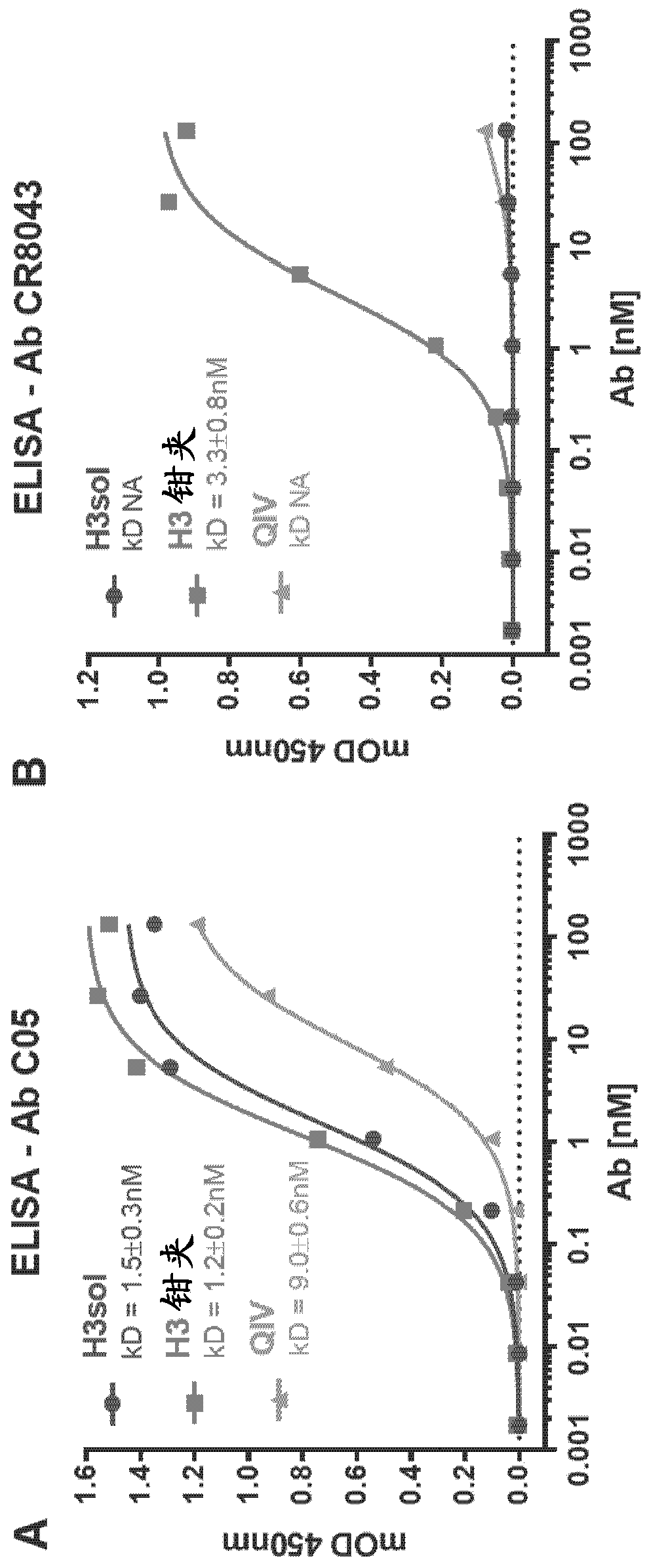
ELISA (enzyme linked immunosorbent assay) kit for detecting PPRV (peste des petits ruminants virus) antibody
ActiveCN107238702AEasy to monitorStrong specificitySsRNA viruses negative-senseAntibody mimetics/scaffoldsAntigenElisa kit
The invention discloses an ELISA (enzyme linked immunosorbent assay) kit for detecting a PPRV (peste des petits ruminants virus) antibody. The ELISA kit for detecting the PPRV antibody comprises an envelope antigen, wherein the envelope antigen is a recombinant PPRV H-F fusion protein which is a protein of a) or b) as follows: a) protein formed by an amino acid sequence shown in SEQ ID No.2; b) soluble protein obtained from the amino acid sequence shown in SEQ ID No.2 through substitution and / or deletion and / or addition of one or several amino acid residues. The ELISA kit for detecting the PPRV antibody not only can be used for diagnosing PPR (peste des petits ruminants), but also can be used for evaluating a vaccine immunity effect, further can detect PPR rapidly and accurately and is beneficial to clinical monitoring of PPR, thereby playing a positive role in better control of spread of PPR in China.
Owner:CHINA ANIMAL DISEASE CONTROL CENT
Fusogenic, self-propagating blebs as immunogenic compositions
InactiveUS20070128222A1Avoid infectionReduce severitySsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousCytopathic effect
Self-propagating, fusogenic blebs are produced from cells infected with a population of Venezuelan Equine Encephalitis virus replicon particles (VRP). The self-propagating, fusogenic nature of the blebs is derived from expression of heterologous genes encoding viral fusion proteins that are incorporated into the replication defective replicon particles. The resulting blebs can be harvested from supernatants of cells displaying severe cytopathic effects. The blebs are used to make immunogenic compositions and devise methods of immunizing mammals against paramyxoviruses such as parainfluenza virus type 3.
Owner:WYETH HOLDINGS CORP
Coronavirus fusion protein, and preparation method and application thereof
ActiveCN111499765AHigh expressionHigh sensitivitySsRNA viruses positive-senseViral antigen ingredientsEscherichia coliNucleotide
The invention belongs to the technical field of antigen preparation processes, and particularly relates to a coronavirus fusion protein, and a preparation method and application thereof. According tothe coronavirus fusion protein provided by the invention, the nucleotide sequence of the RBD structural domain of a 2019-nCoV S protein and the nucleotide sequence of a 2019-nCoV N protein are subjected to complete gene synthesis and then are constructed into an insect cell expression vector to prepare a recombinant plasmid containing the RBD structural domain sequence of the 2019-nCoV N protein and the RBD structural domain sequence of the 2019-nCoV S protein, escherichia coli is transformed by the recombinant plasmid to obtain a recombinant plasmid containing the gene sequence of the fusionprotein, and host cells are transfected by the recombinant plasmid to complete then fusion expression of the RBD structural domains of the 2019-nCoV N protein and the 2019-nCoV S protein. The expression process of the fusion protein is simple, meanwhile, and when the fusion protein is applied to a 2019-nCoV antibody detection kit, the sensitivity of the detection kit is remarkably improved, the specificity is also improved, the detection rate is efficiently increased, and the overall use performance is more optimized.
Owner:四川携光生物技术有限公司
Fusogenic, self-propagating blebs as immunogenic compositions
InactiveUS7541038B2SsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousCytopathic effect
Self-propagating, fusogenic blebs are produced from cells infected with a population of Venezuelan Equine Encephalitis virus replicon particles (VRP). The self-propagating, fusogenic nature of the blebs is derived from expression of heterologous genes encoding viral fusion proteins that are incorporated into the replication defective replicon particles. The resulting blebs can be harvested from supernatants of cells displaying severe cytopathic effects. The blebs are used to make immunogenic compositions and devise methods of immunizing mammals against paramyxoviruses such as parainfluenza virus type 3.
Owner:WYETH HOLDINGS CORP
Novel Anti-Viral Method
ActiveUS20120039978A1Reduce infectivitySsRNA viruses negative-sensePowder deliveryLipid formationBiological activation
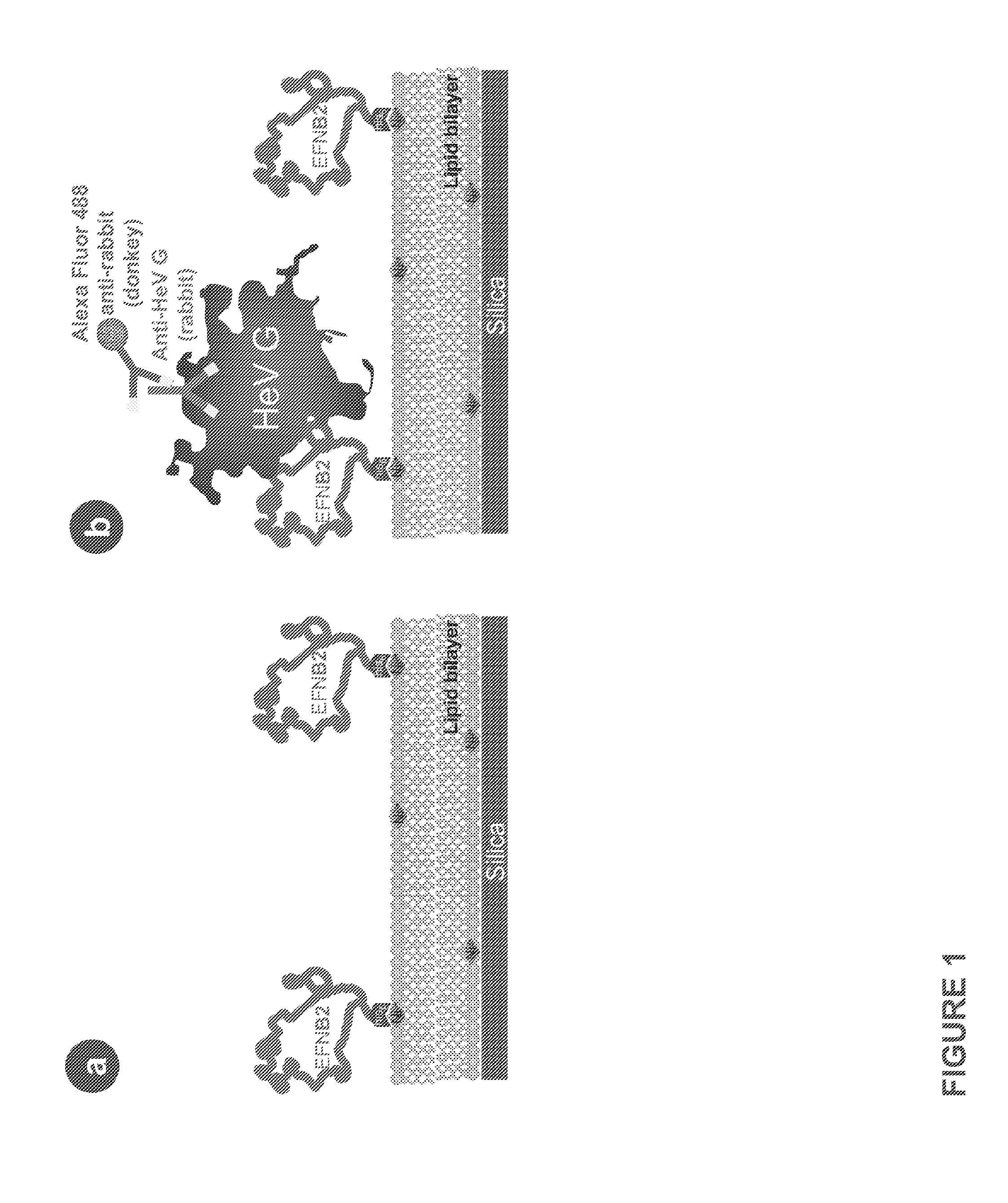
The invention provides a method of reducing viral infectivity in a sample compnsing contacting the sample with a earner matrix wherein lipids are attached to the carrier matrix, and wherein at least one virus-specific agent is attached to the lipids The virus-specific agent is capable of binding to at least one viral component The virus specific agent may be a receptor The earner matrix may be a silica particle, which may be coated with a lipid bilayer The invention further provides a method of inactivating a virus comprising contacting the virus with a earner matrix wherein lipids are attached to the earner matrix, and wherein at least one receptor is attached to the lipids, binding the receptor to at least one viral receptor-binding protein, and activating at least one viral fusion protein, wherein activation inactivates the virus, and releases the virus from the earner matrix
Owner:CORNELL UNIVERSITY +1
Self-assembled nanoparticle vaccines
InactiveUS20150110825A1Effective vaccinePrevent stericSsRNA viruses negative-sensePowder deliveryF proteinNanoparticle
The present invention provides nanoparticles and compositions of various constructs that combine meta-stable viral proteins (e.g., RSV F protein) and self-assembling molecules (e.g., ferritin, HSPs) such that the pre-fusion conformational state of these key viral proteins is preserved (and locked) along with the protein self-assembling into a polyhedral shape, thereby creating nanoparticles that are effective vaccine agents. The invention also provides nanoparticles comprising a viral fusion protein, or fragment or variant thereof, and a self-assembling molecule, and immunogenic and vaccine compositions including the same.
Owner:MASSACHUSETTS INST OF TECH
Aslv vector system
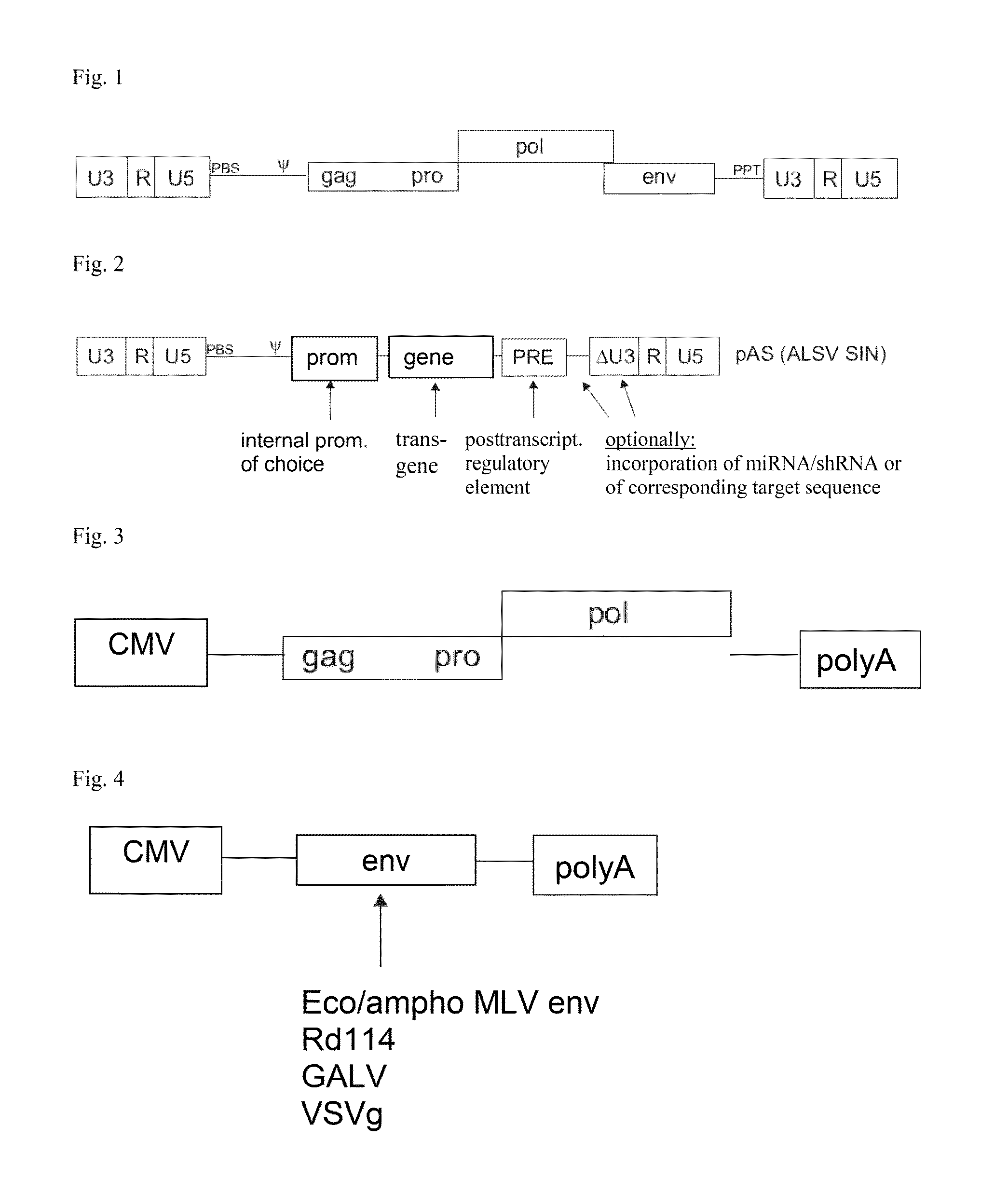
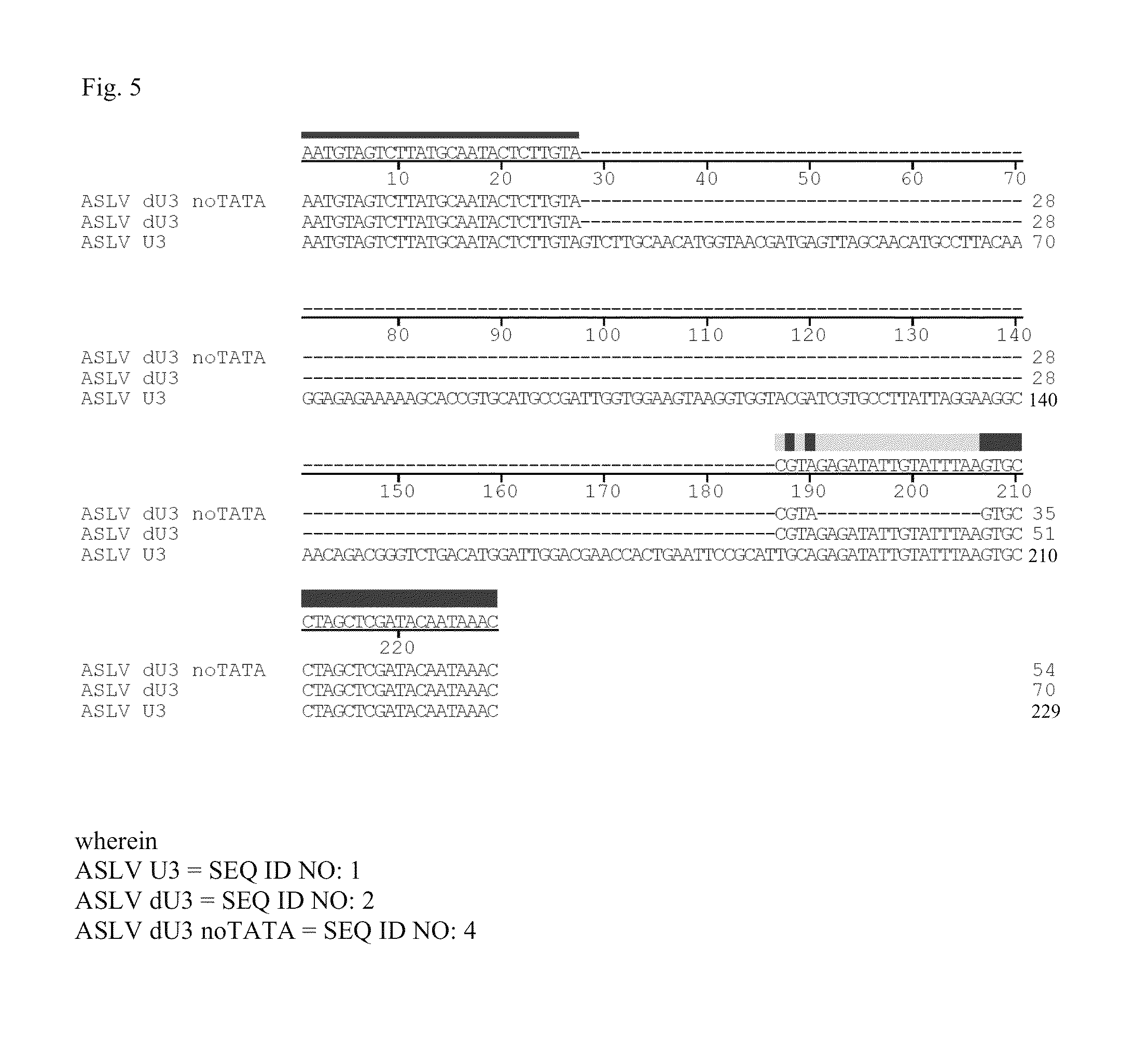
ActiveUS20120172418A1Efficiently translatedRisk minimizationSugar derivativesGenetic material ingredientsVector systemAvian leukosis viruses
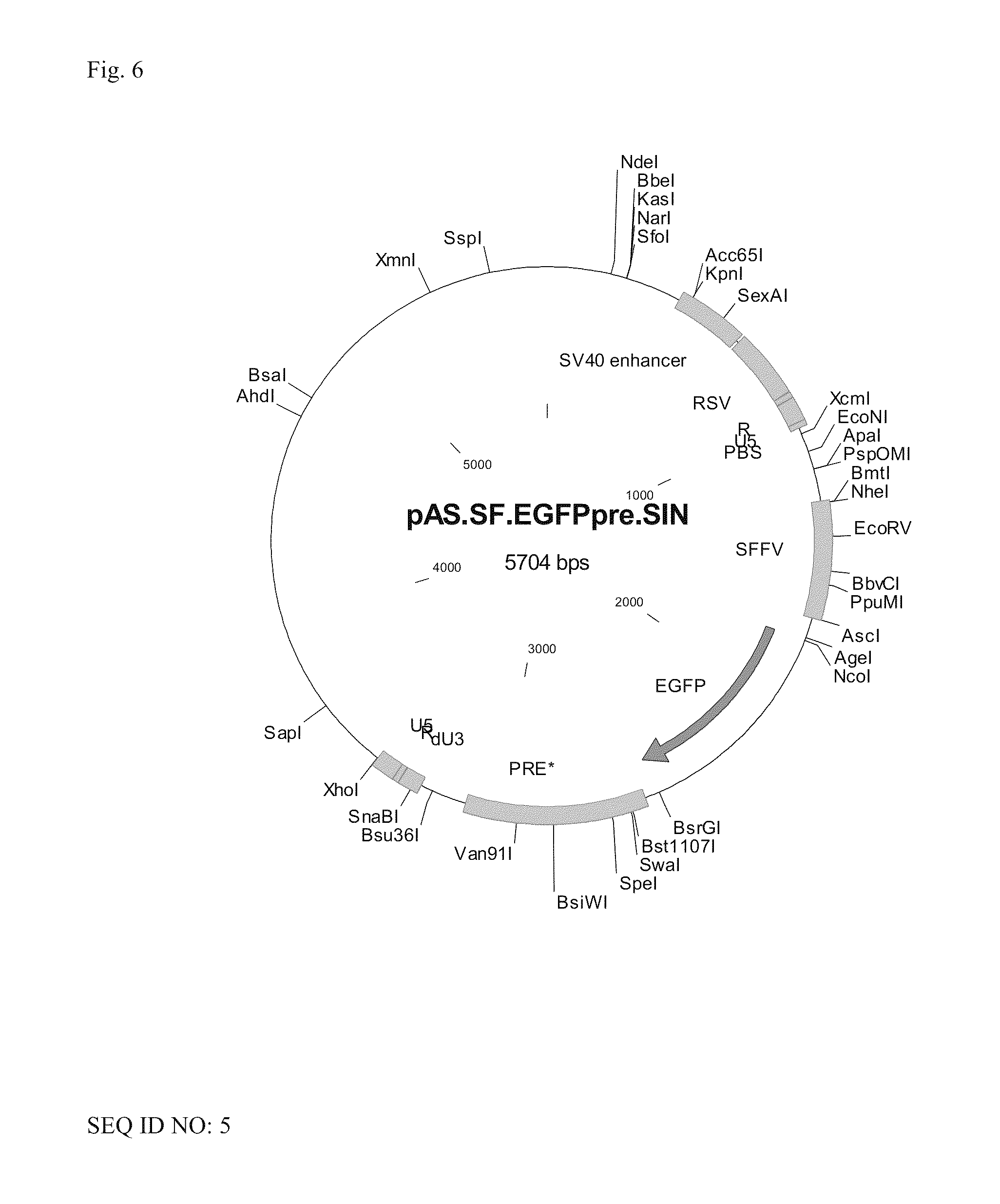
The invention provides a viral self-inactivating (SIN) vector on the basis of the avian sarcoma leukosis virus (ASLV) as well as a split-packaging system comprising in addition to the SIN vector a first helper plasmid serving for the expression of the viral fusion protein gag-pol and a second helper plasmid serving for the expression of the retroviral envelope protein (env). The first and second helper plasmid, for example contained in a packaging cell line or transiently transfected, serve for the generation of non-replicating (RCR-incompetent) viral particles containing RNA having a SIN LTR according to the invention at the 3′ terminus, wherein the RNA can have a therapeutically effective section which e.g. is denoted a transgene. This 3′ SIN LTR contains an extensive deletion of the U3 region which in the course of the reverse transcription is copied into the 5′ LTR. In addition, in the SIN vector all coding regions of ASLV as well as the retroviral splice donor site are removed.
Owner:MEDIZINISCHE HOCHSCHULE HANNOVER
Immunogenic compositions comprising venezuelan equine encephalitis virus replicon vectors and paramyxovirus protein antigens
InactiveCN1798760ASsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousCytopathic effect
Self-propagating, fusogenic blebs are produced from cells infected with a population of Venezuelan Equine Encephalitis virus replicon particles (VRP). The self-propagating, fusogenic nature of the blebs is derived from expression of heterologous genes encoding viral fusion proteins that are incorporated into the replication defective replicon particles. The resulting blebs can be harvested from supernatants of cells displaying severe cytopathic effects. The blebs are used to make immunogenic compositions and devise methods of immunizing mammals against paramyxoviruses such as parainfluenza virus type 3.
Owner:WYETH HOLDINGS CORP
ASLV vector system
ActiveUS8642570B2Risk minimizationEfficiently translatedSugar derivativesGenetic material ingredientsVector systemAvian leukosis viruses
Owner:MEDIZINISCHE HOCHSCHULE HANNOVER
Chimeric molecules and uses thereof
ActiveUS20200040042A1Improve purification effectRegulate immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousViral Membrane Fusion
Disclosed are chimeric polypeptides based on viral membrane fusion proteins. More particularly, the present invention discloses chimeric polypeptides that comprise a virion surface exposed portion of a viral fusion protein and a heterologous structure-stabilizing moiety, and to complexes of those chimeric polypeptides. The present invention also discloses the use of these complexes in compositions and methods for eliciting an immune response to a fusion protein of an enveloped virus, or complex of the fusion protein, and / or for treating or preventing an enveloped virus infection. The present invention further discloses the use of the heterologous structure-stabilizing moiety for oligomerizing heterologous molecules of interest.
Owner:THE UNIV OF QUEENSLAND
Inhibition of viral infection and spread with viral and RhoA-derived peptides
InactiveUS20050226889A1Quick screeningQuick filterSsRNA viruses negative-senseCell receptors/surface-antigens/surface-determinantsLentivirusImmunodeficiency virus
Isolated peptides, peptidomimetics, and antibodies which bind to the viral fusion protein binding domain of the RhoA protein or the RhoA binding domain of a viral fusion protein are useful for inhibiting infection in susceptible cells, in vitro and in vivo. Among these viruses are the Paramyxovirus respiratory syncytial virus (RSV) and the Lentivirus human immunodeficiency virus (HIV).
Owner:VANDERBILT UNIV
Functionallly reconstituted viral membranes containing adjuvant
ActiveUS20060193873A1Convenient treatmentImprove recognitionSsRNA viruses negative-senseBiocideLipid formationAntigen
The present invention relates to vaccines directed against antigens such as membrane proteins from pathogens or tumor cells. The invention further relates to methods of forming reconstituted viral membranes, with membrane fusion activity, which are lipid bilayer membranes preferably containing natural lipids of a virus, a viral fusion protein, one or more optional further antigens as well as amphiphilic adjuvants. Pharmaceutical compositions comprising such reconstituted viral membranes are also part of the invention.
Owner:BESTEWIL HOLDING BV
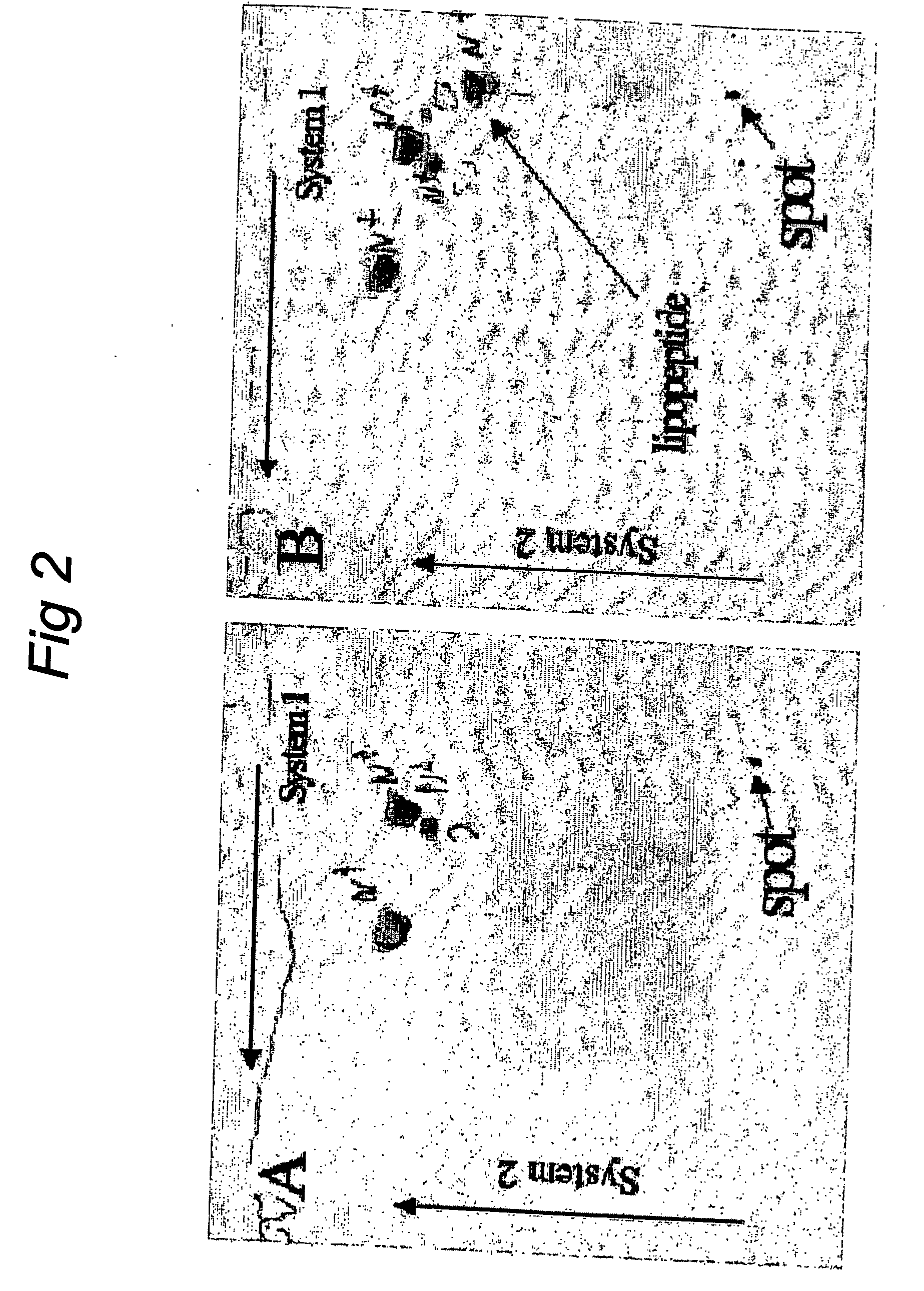
Identification and Attenuation of the Immunosuppressive Domains in Fusion Proteins of Enveloped RNA Viruses
InactiveUS20140335117A1Reduce immunosuppressive propertyEasy to optimizeSsRNA viruses negative-senseSsRNA viruses positive-senseUltrasound attenuationViral envelope
The present invention relates to enveloped RNA viruses. The invention in particular relates to the generation of superior antigens for mounting an immune response by first identifying then mutating the immunosuppressive domains in fusion proteins of enveloped RNA viruses resulting in decreased immunosuppressive properties of viral envelope proteins from the viruses.
Owner:SKAU
Chimeric molecules and uses thereof
ActiveCN110506060ASsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousViral Membrane Fusion
The present invention discloses chimeric polypeptides based on viral membrane fusion proteins. More particularly, the present invention discloses chimeric polypeptides that comprise a virion surface exposed portion of a viral fusion protein and a heterologous structure- stabilizing moiety, and to complexes of those chimeric polypeptides. The present invention also discloses the use of these complexes in compositions and methods for eliciting an immune response to a fusion protein of an enveloped virus, or complex of the fusion protein, and / or for treating or preventing an enveloped virus infection. The present invention further discloses the use of the heterologous structure-stabilizing moiety for oligomerizing heterologous molecules of interest.
Owner:THE UNIV OF QUEENSLAND
APMV (avian paramyxovirus) fusion protein, preparation method and application thereof and APMV vaccine for pigeons
ActiveCN109593136AGood antigenicityHigh expressionSsRNA viruses negative-senseAntibody mimetics/scaffoldsAvian paramyxovirusAmino acid
The invention provides APMV (avian paramyxovirus) fusion protein, a preparation method and an application thereof and an APMV vaccine for pigeons, and relates to the technical field of biology. The APMV fusion protein is obtained by serial connection of an HN gene of PPMV-1 (pigeon paramyxovirus-1) and an HN gene of APMV-7, and comprises an NDV-HN segment and an AMPV-7-HN segment, wherein the NDV-HN segment comprises the amino acid sequence shown in SEQ ID NO.1, and the AMPV-7-HN segment comprises the amino acid sequence shown in SEQ ID NO.2. The APMV fusion protein has the advantages of goodantigenicity, high expression quantity and wide application.
Owner:天康生物制药有限公司
Recombined foot and mouth disease virus amalgamation protein vaccine
The invention provides a restructured foot-mouth disease virus fusion protein vaccine designed according to Asian I-type foot-mouth disease virus, experiments proves: the vaccine of the invention produced by gene engineering technique has good immune effect and foot-mouth disease infection prevention. The invention provides the restructured foot-mouth disease virus protein vaccine amino acid sequence, and the research about the nucleotide sequence and foot-mouth disease infection prevention.
Owner:BEIJING HYDVAX BIOTECH +1
Inhibition of membrane fusion proteins
Methods of inhibiting viral infection of a eukaryotic cell by a target virus having a class II virus fusion protein are provided. Also provided are methods of screening a test compound for the ability to inhibit infection by a virus having a class II viral fusion protein. Additionally provided herewith are aqueous-soluble proteins comprising a portion of a class II viral fusion protein comprising a Domain III of the viral fusion protein.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
Respiratory syncytial virus recombinant f protein and vaccine composition containing same
PendingCN113227123AImprove expression rateExcellent neutralizing antibody titerSsRNA viruses negative-senseViral antigen ingredientsForeign proteinF protein
The present invention provides a respiratory syncytial virus (RSV) recombinant fusion protein (F protein) in which a polymerization domain derived from a foreign protein is bound to the C terminal of a fusion protein (F protein) lacking a transmembrane domain of a wild-type respiratory syncytial virus (RSV) fusion protein (F protein). The recombinant fusion protein of the present invention is soluble and can retain an F protein trimer. Excellent immune-inducing effects can be expected from the recombinant fusion protein of the present invention, and the vaccine composition containing the same.
Owner:SK BIOSCI CO LTD
Recombinant drosophila cell line for expressing porcine atypical pestivirus fusion protein as well as preparation method and application of recombinant drosophila cell line
PendingCN113736825AContinuously efficient and stable expressionImprove secretory expression abilitySsRNA viruses positive-senseViral antigen ingredientsDrosophila ornatifronsSpecific immunity
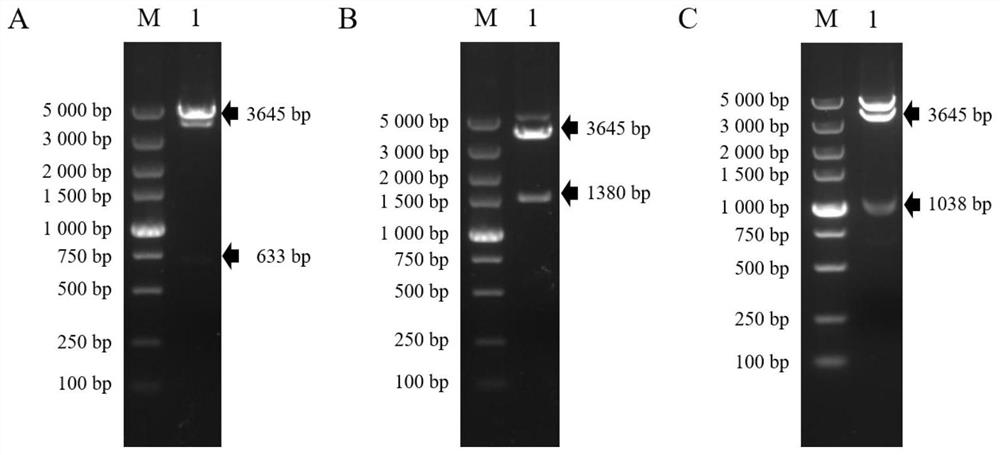
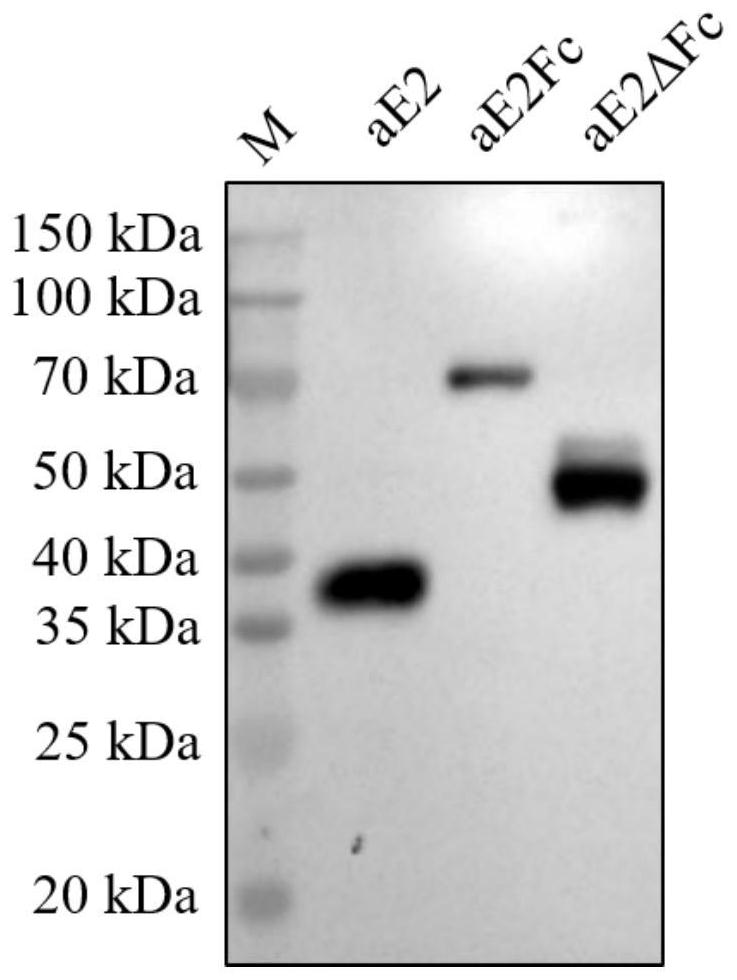
The invention provides a recombinant drosophila cell line for expressing porcine atypical pestivirus fusion protein as well as a preparation method and application of the recombinant drosophila cell line, and belongs to the technical field of vaccines. The recombinant drosophila cell line S2-aE2Fc or S2-aE2deltaFc for expressing the fusion protein of the atypical porcine pestivirus E2Fc and E2deltaFc provided by the invention can be used for preparing subunit vaccines, and after piglets are immunized, relatively strong specific immune response aiming at the atypical porcine pestivirus can be generated. The recombinant drosophila melanogaster cell lines S2-aE2Fc and S2-aE2deltaFc can be used for obtaining aE2 protein antigens in a dimer form, a prepared subunit vaccine can stimulate an organism to generate stronger humoral immunity and cellular immunity responses, and the recombinant drosophila melanogaster cell lines can be used as a promising candidate genetic engineering vaccine for preventing piglet congenital tremor caused by atypical porcine pestivirus.
Owner:HUAZHONG AGRI UNIV
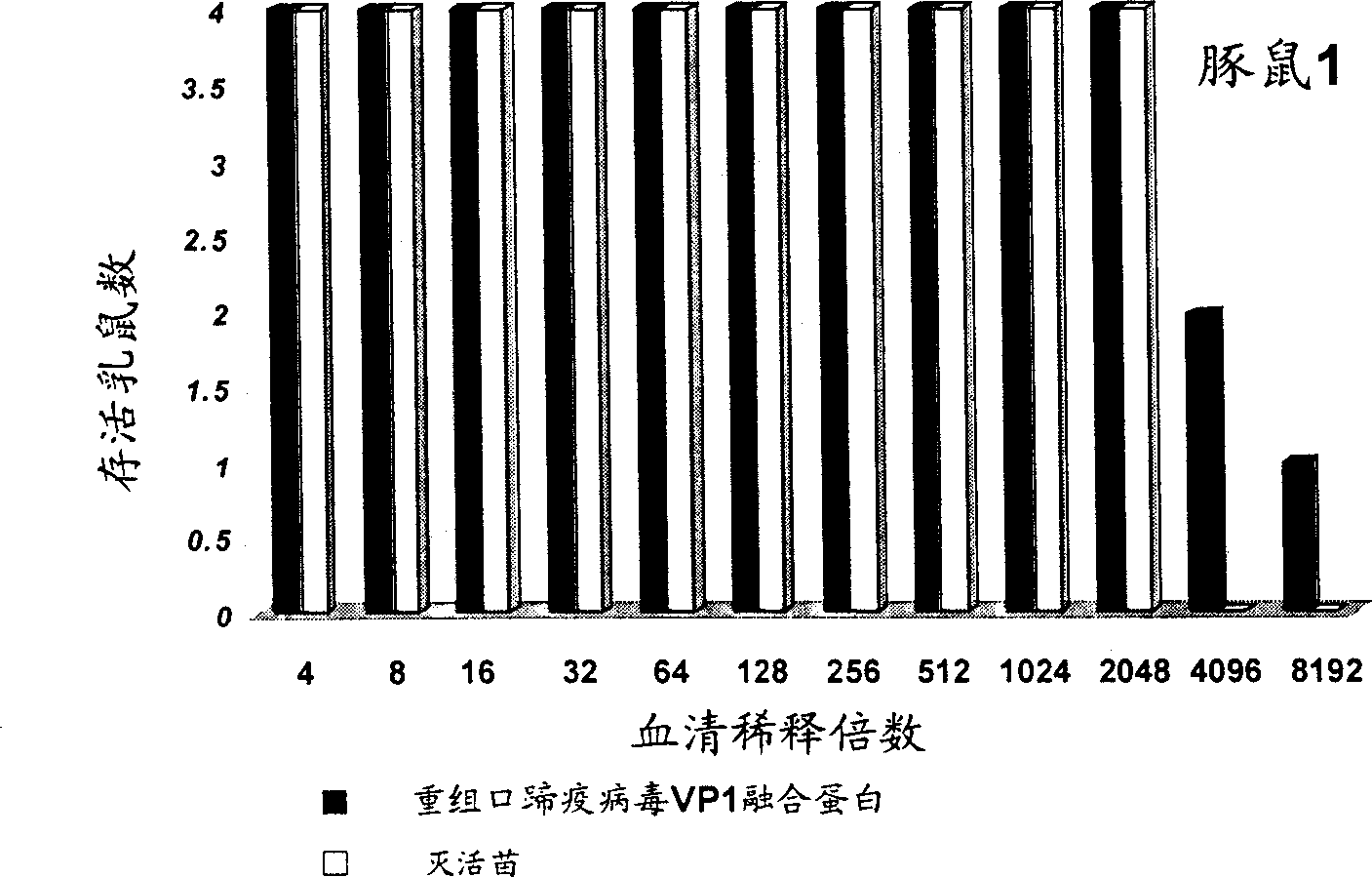
Recombinant foot-and-mouth disease virus VP1 confluent protein vaccine
Owner:BEIJING HYDVAX BIOTECH +1
Alphabodies specifically binding to class-i viral fusion proteins and methods for producing the same
Single-chain Alphabodies that comprise an alpha-helical binding region which mediates binding to a first fusion-driving region of a class-1 viral fusion protein and which structurally mimics a second fusion-driving region of said class-1 viral fusion protein, wherein said first and second fusion-driving regions of said class-1 viral fusion protein are regions which interact to drive the fusion between a virus displaying said class-1 viral fusion protein and a target cell.
Owner:COMPLIX SA
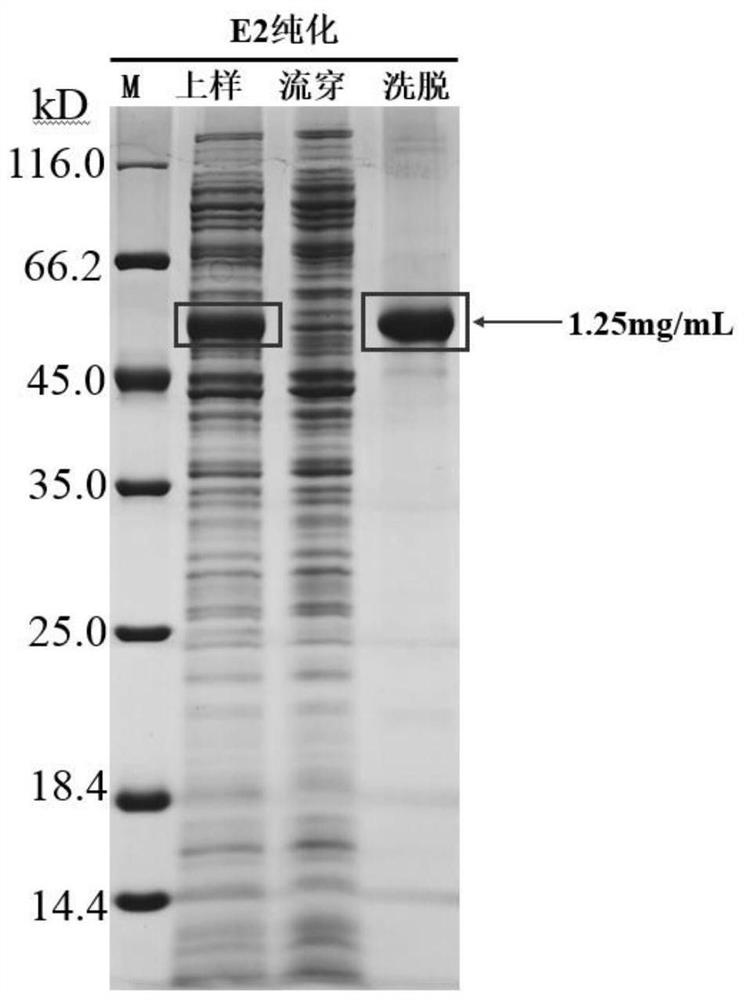
Classical swine fever virus E2-E0 fusion protein as well as preparation method and application thereof
PendingCN114805609AImproving immunogenicityImmunogenicity does not affectSsRNA viruses positive-senseAntibody mimetics/scaffoldsClassical swine fever virus CSFVGlycine
The invention belongs to the technical field of biology, and particularly relates to hog cholera virus E2-E0 fusion protein, a preparation method and application. The invention firstly provides the E2-E0 fusion protein of the classical swine fever virus E0 truncated protein and the classical swine fever virus E2 protein, which not only can promote the expression of the E0 protein, but also does not influence the immunogenicity of the E0 protein and the E2 protein, and can be used for preparing a classical swine fever subunit vaccine; on the basis of the classical swine fever virus E0 truncated protein, it is found that the 160th, 162th, 165 and 166th amino acids of the classical swine fever virus E0 truncated protein are mutated into glycine G, the expression quantity of the E2-E0 fusion protein can be further increased by 20% or above, and the immunogenicity of the E2-E0 fusion protein is not affected.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Soluble Expression Method of Recombinant Peste des Petits Ruminants Virus h-f Fusion Protein
ActiveCN107236047BImprove expression levelEasy to monitorSsRNA viruses negative-senseAntibody mimetics/scaffoldsMicroorganismBioinformatics
The invention discloses a soluble expression method of a recombinantpeste des petits ruminant virus H-F fusion protein. The solubleexpressionmethod comprises a step of expressing an encoding gene of a protein in a living thing, thereby obtaining the protein, wherein the living thing is a microorganism, a plant or a non-human animal; the protein is a protein of a) or b), namely a) a protein consisting of amino acid sequences of SEQ ID No.2, and b) a soluble protein generated by substituting and / or deleting and / or adding one or more amino acid residues into an amino acid sequence of SEQ ID No.2. The recombinantpeste des petits ruminant virus H-F fusion protein treated with the solubleexpression method of the recombinantpeste des petits ruminant virus H-F fusion protein disclosed by the invention is high in expression level and low in production cost, and a basis is made for furtherdevelopment of commercial kits.
Owner:CHINA ANIMAL DISEASE CONTROL CENT
Peste des petits ruminants virus h-f fusion protein and its related biological materials and applications
The invention discloses a peste des petits ruminants virus H-F fusion protein, a biological material related to the same and application of the peste des petits ruminants virus H-F fusion protein. The peste des petits ruminants virus H-F fusion protein is a protein a) or a protein b). The protein a) comprises amino acid sequences shown as SEQ ID No.2; the protein b) is a soluble protein, and each amino acid sequence shown as SEQ ID No.2 is substituted and / or deleted and / or added by an amino acid residue or a plurality of amino acid residues to obtain the soluble protein. The peste des petits ruminants virus H-F fusion protein, the biological material and the application have the advantages that indirect ELISA (enzyme-linked immunosorbent assay) detection methods for peste des petits ruminants virus antibodies are created by the aid of the peste des petits ruminants virus H-F fusion protein which is used as an envelope antigen, are high in specificity and sensitivity and good in accuracy and can be quickly, easily and conveniently implemented, and accordingly the peste des petits ruminants virus H-F fusion protein, the biological material and the application are favorable for clinically monitoring peste des petits ruminants.
Owner:CHINA ANIMAL DISEASE CONTROL CENT
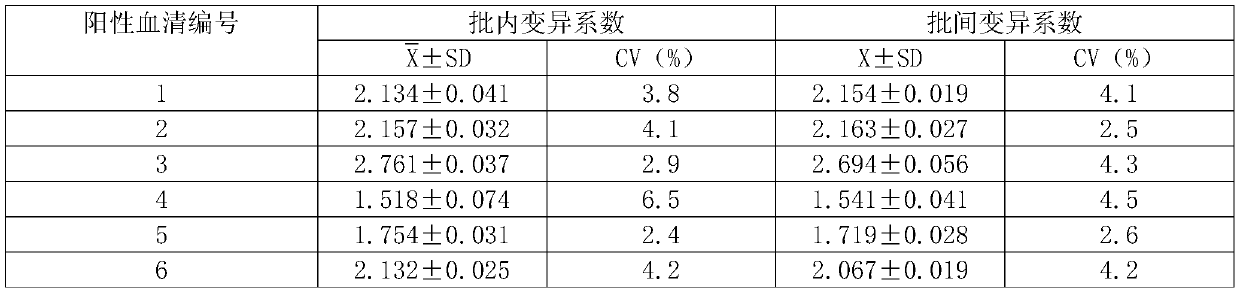
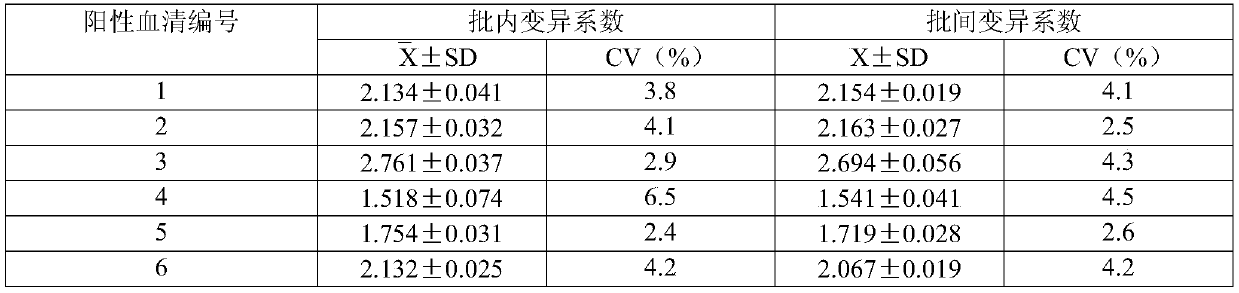
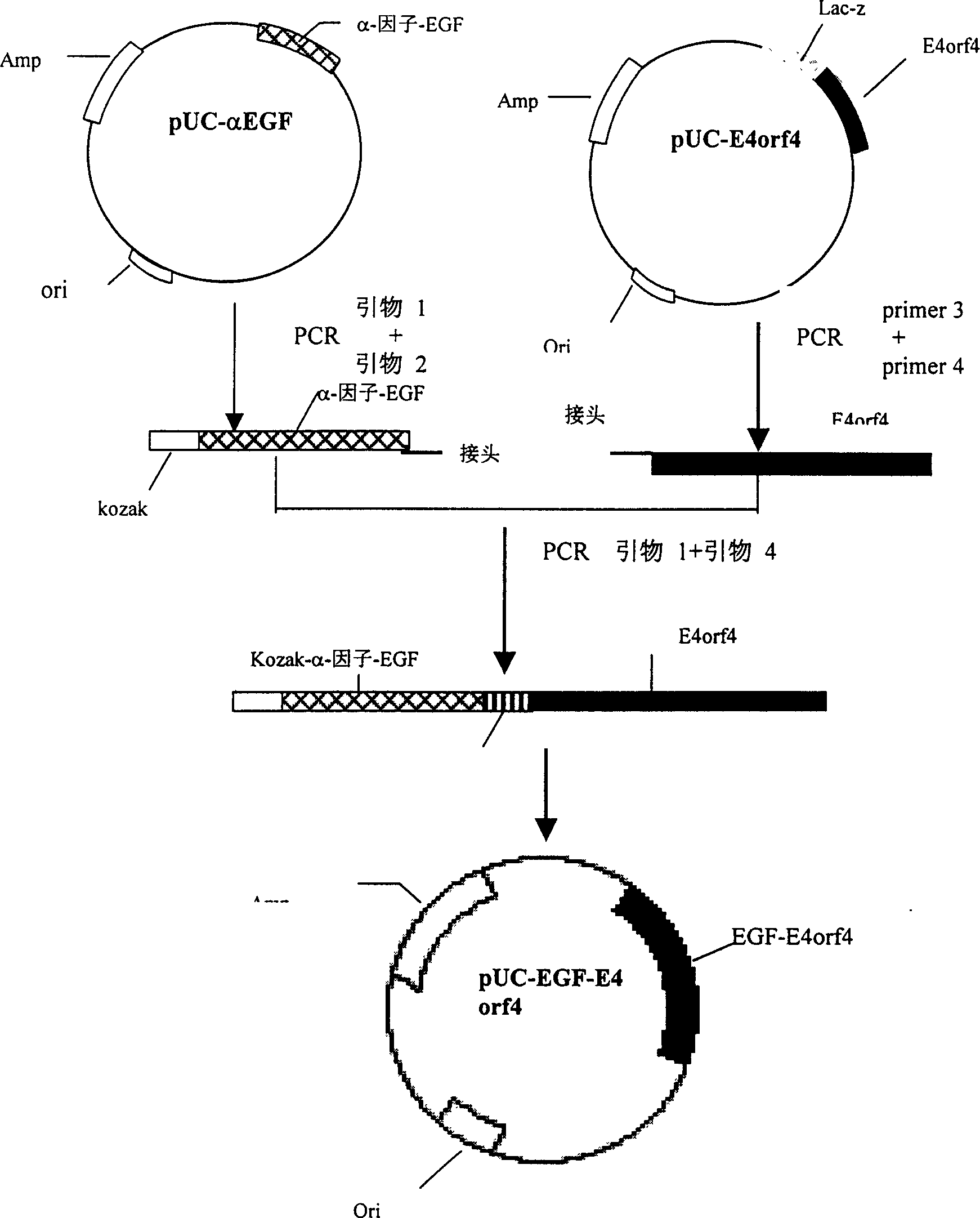
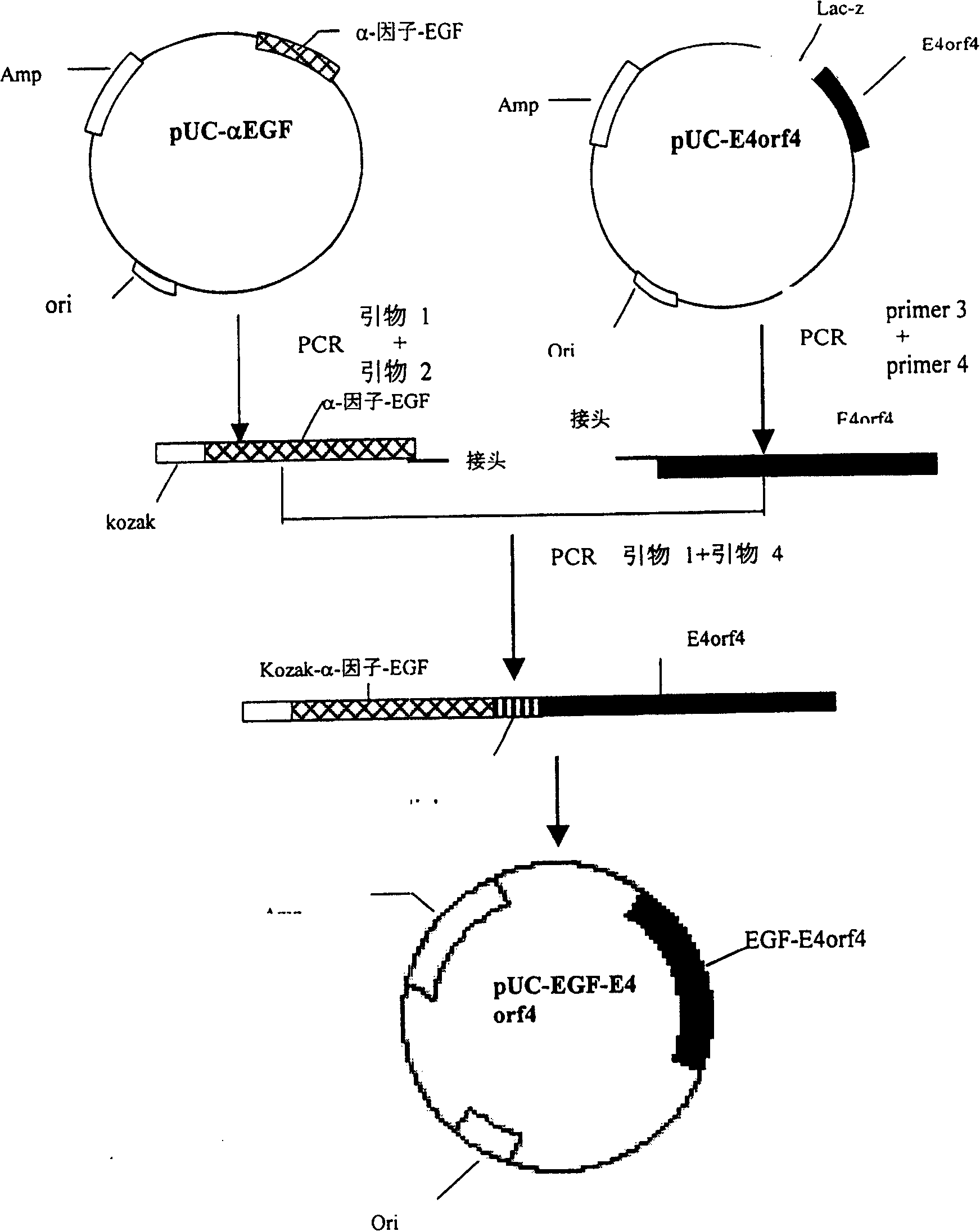
Targeted anti-tumour fused protein containing adenovirus E40rf4 protein
The invention relates to recombination human epidermis growth factor - gland virus E4orf4 fusion protein, fusion gene which codes the fusion protein, the fusion gene contained express carrier, using methyl nutritional yeast to make secretary express method of this recombination fusion protein, and the protein contained medicine combination and its treating application.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Viral fusion protein treatment for CCR8 mediated diseases
InactiveUS8987416B2Powder deliveryElectrotherapyAcquired immunodeficiencyMolluscum contagiosum virus
Compositions, methods, and kits are provided for treating CCR8 mediated diseases with applicability to atopic dermatitis and potential applicability to asthma, prurigo nodularis, nummular dermatitis, neurodermatitis, and lichen simplex chronicus and some lymphomas, multiple sclerosis, acquired immunodeficiency disease, peritoneal adhesions, Kaposi's sarcoma and atherogenesis—the expression of all of which, at least in part, is mediated by cells expressing the chemokine receptor CCR8. The compositions include proteins and fusion proteins from Molluscum contagiosum Virus (MCV) or variants, analogs and derivatives thereof which exhibit inhibitory activity. Examples of such MCV proteins are MC148 fusion protein (MC148fp) identified as MC148P-TAT-6×His (“6×His” disclosed as SEQ ID NO: 11), and its variants, fragments, analogs and derivatives which possess inhibitory activity. The variants, fragments, analogs and derivatives of MC148p and of MC148fp may be less than 100% homologous to MCV proteins as long as they are sufficiently homologous that inhibitory activity is preserved.
Owner:LARRK BIO INC
Targeted anti-tumour fused protein containing adenovirus E40rf4 protein
The invention relates to recombination human epidermis growth factor - gland virus E4orf4 fusion protein, fusion gene which codes the fusion protein, the fusion gene contained express carrier, using methyl nutritional yeast to make secretary express method of this recombination fusion protein, and the protein contained medicine combination and its treating application.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Molluscum contagiosum viral fusion protein treatment for atopic dermatitis diseases
InactiveUS20150044273A1Powder deliveryElectrotherapyImmunodeficiency diseaseMolluscum contagiosum virus
Compositions, methods, and kits are provided for treating CCR8 mediated diseases with applicability to atopic dermatitis and potential applicability to asthma, prurigo nodularis, nummular dermatitis, neurodermatitis, and lichen simplex chronicus and some lymphomas, multiple sclerosis, acquired immunodeficiency disease, peritoneal adhesions, Kaposi's sarcoma and atherogenesis—the expression of all of which, at least in part, is mediated by cells expressing the chemokine receptor CCR8. The compositions include proteins and fusion proteins from Molluscum contagiosum Virus (MCV) or variants, analogs and derivatives thereof which exhibit inhibitory activity. Examples of such MCV proteins are MC148 fusion protein (MC148fp) identified as MC148P-TAT-6xHis (“6xHis” disclosed as SEQ ID NO: 11), and its variants, fragments, analogs and derivatives which possess inhibitory activity. The variants, fragments, analogs and derivatives of MC148p and of MC148fp may be less than 100% homologous to MCV proteins as long as they are sufficiently homologous that inhibitory activity is preserved.
Owner:LARRK BIO INC
Functionally reconstituted viral membranes containing adjuvant
ActiveUS7618641B2Convenient treatmentImprove recognitionSsRNA viruses negative-senseBiocideAntigenLipid formation
Vaccines directed against antigens such as membrane proteins from pathogens or tumor cells are disclosed. Also described are methods of forming reconstituted viral membranes, with membrane fusion activity, which are lipid bilayer membranes preferably containing natural lipids of a virus, a viral fusion protein, one or more optional further antigens as well as amphiphilic adjuvants. Pharmaceutical compositions including such reconstituted viral membranes are also described.
Owner:BESTEWIL HOLDING BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com