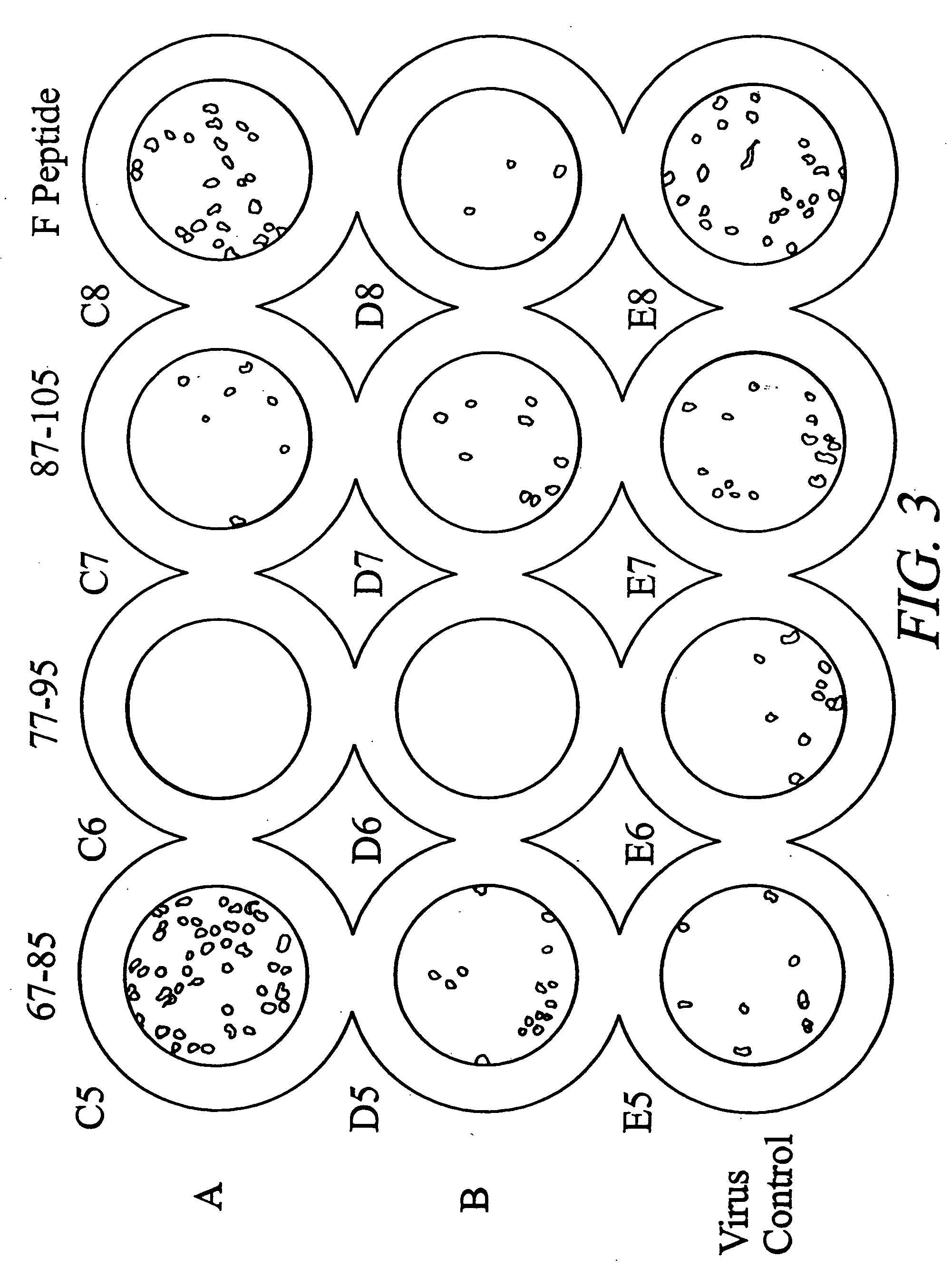
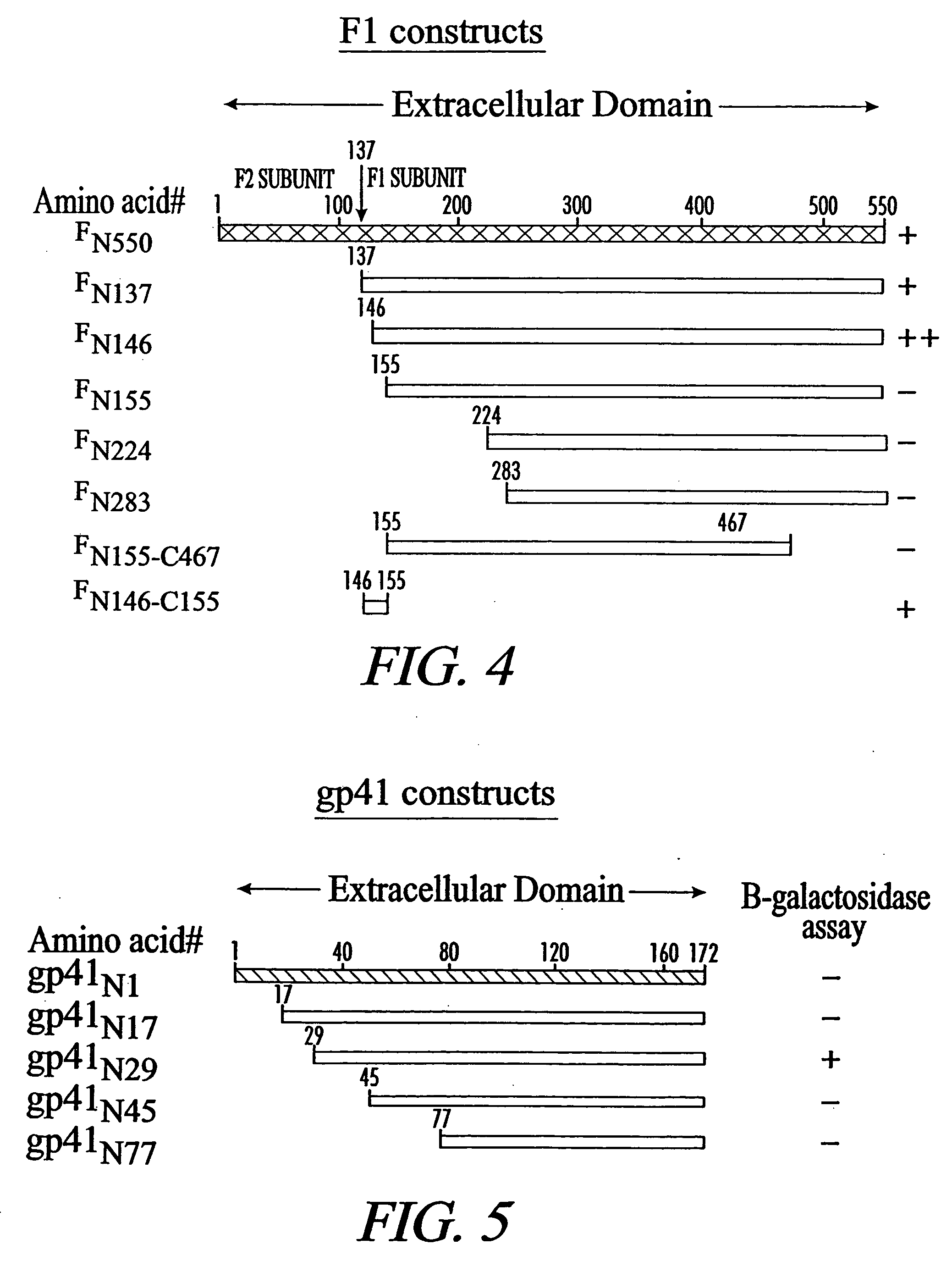
Inhibition of viral infection and spread with viral and RhoA-derived peptides
a technology of rsv and peptides, which is applied in the direction of peptides, antibody medical ingredients, peptide sources, etc., can solve the problems of rsv not preventing illness, recurrent wheezing, and severity decline, and achieve rapid screening and screening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Viruses and Cells
[0118] The A2 strain of RSV was provided by Dr. R. Chanock, National Institutes of Health, Bethesda, Maryland. Virus stocks were prepared as described by Graham et al. HEp-2 cells were maintained in Eagle's minimal essential media (MEM) supplemented with glutamine, gentamicin, penicillin G, and 10% fetal bovine serum.
[0119] The MT-2 cell line is a fusion susceptible cell line used as the target cell for the HIV assays. It is a CD4+ T-lymphoblastoid cell line derived from HTLV-1 transformed cord blood lymphocytes (Miyoshi et al.). The subclone of MT-2 cells used for the experiments was a gift from Doug Richman, San Diego Veterans Administration Hospital. MT-2 cells were maintained in RPMI-1640 supplemented with 12% fetal bovine serum (FBC) and 50 mg gentamicin / ml.
[0120] The DNA sequences encoding the respiratory syncytial virus F glycoprotein with and without the transmembrane and cytoplasmic domains were PCR amplified and cloned into Eco...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| v/v | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com