Chimeric molecules and uses thereof
A chimeric polypeptide and fusion protein technology, applied in the field of treatment or prevention of enveloped virus infection, heterologous structure stabilization part oligomerization of heterologous target molecules, chimeric polypeptide complex
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
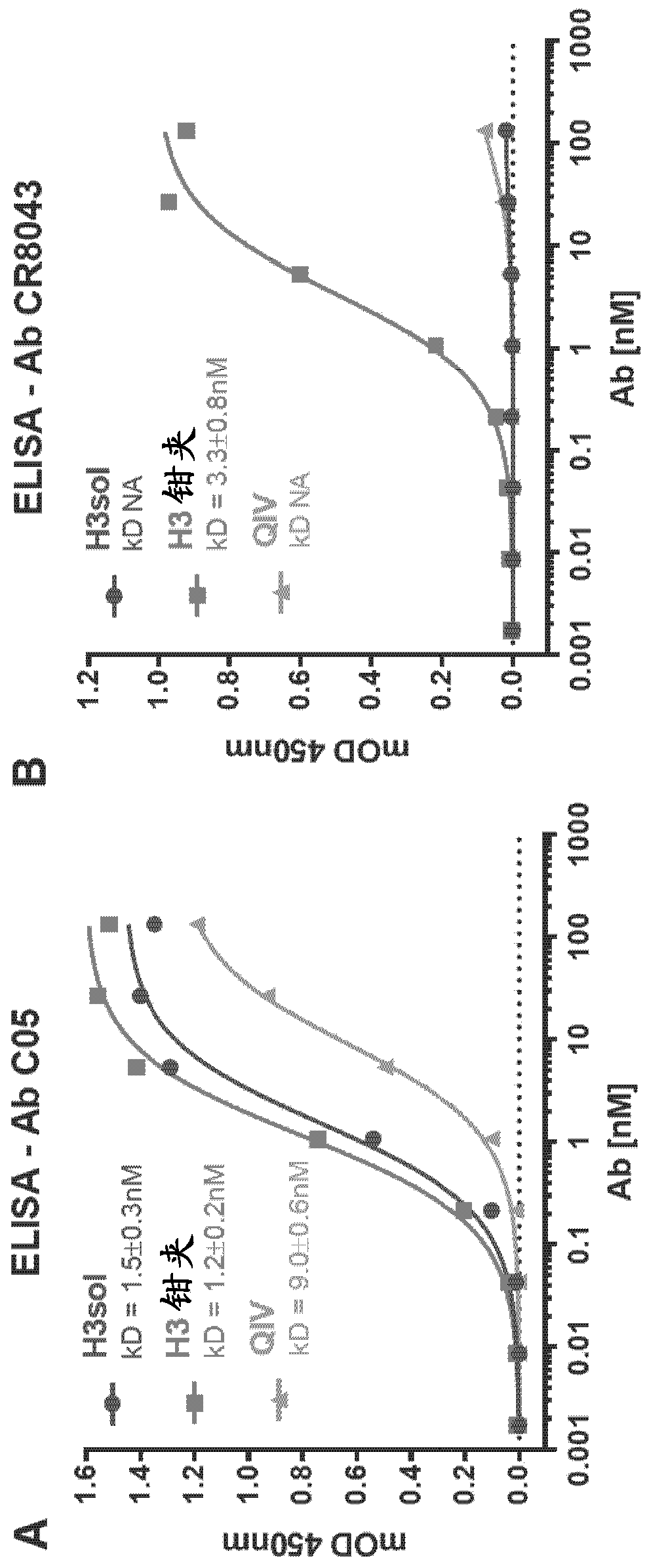
Image
Examples
Embodiment 1
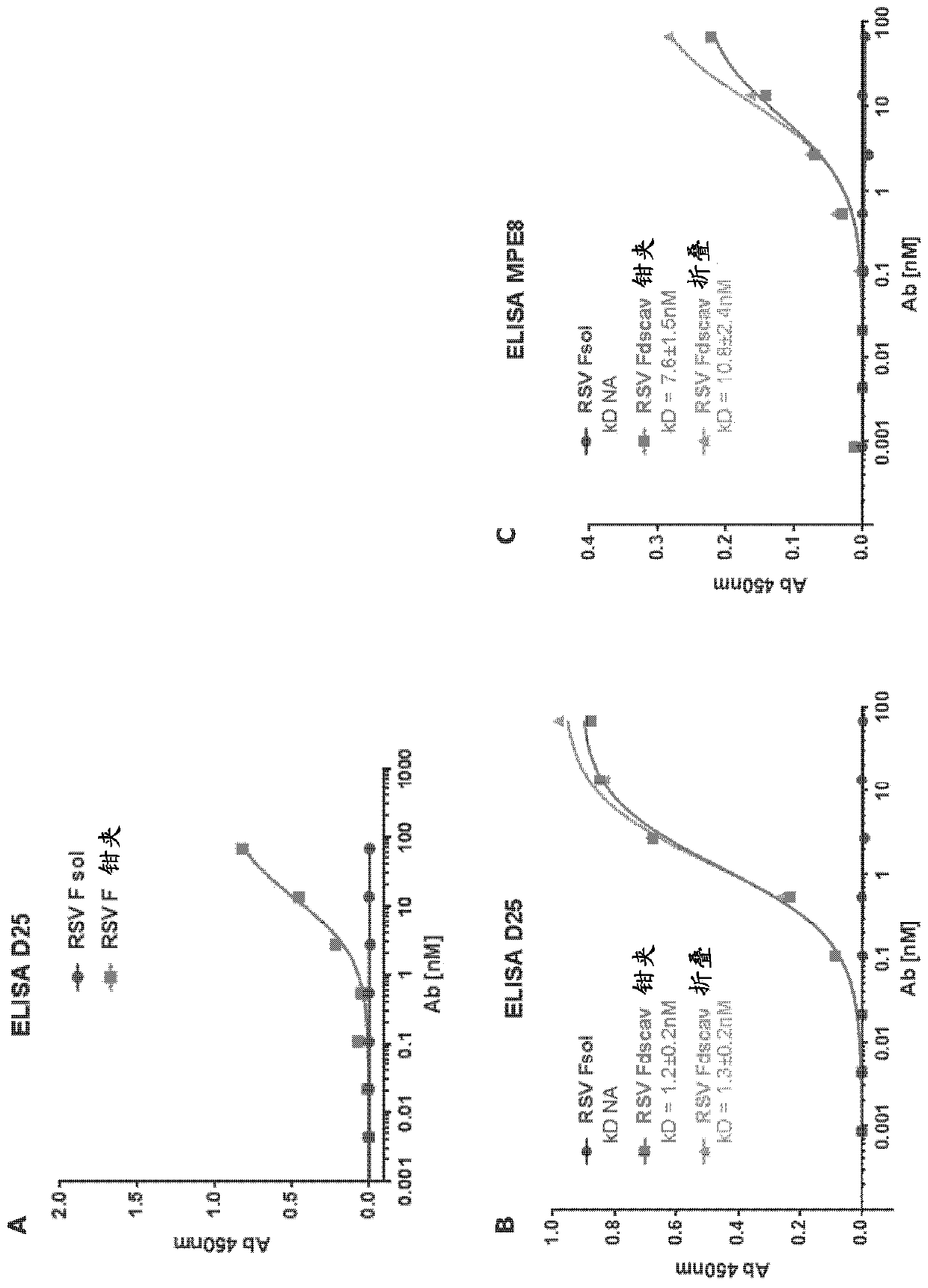
[0638] As an example of the ability of the invention to generate chimeric polypeptides comprising the extracellular domain of a viral fusion protein constrained in a prefusion conformation, representative evidence for respiratory syncytial virus fusion proteins is presented.
[0639] Materials and methods
[0640] Chimeric peptide design:
[0641] The extracellular domain of RSV F and RSV-F extracellular domain mutations containing mutations at four sites (S155C, S290C, S190F, and V207L) according to McLellan et al. (Science, 2013.342(6158):592-8) Each body (RSV F dscav) is operatively linked to a downstream heterologous structural stabilizing moiety (SSM) comprising a pair of complementary heptad repeat regions derived from HIV-1 GP160. A control ectodomain construct lacking this SSM and a positive control construct (RSV F ds cav fold) comprising RSV F ds cav operably linked to a folded SSM (foldon SSM) were also generated. The amino acid sequence of the associated protei...
Embodiment 2
[0657] As another example of the ability of the present invention to generate chimeric polypeptides comprising the extracellular domain of a viral fusion protein constrained in a prefusion conformation, the Influenza A virus (INFA) hemagglutinin (HA) protein is provided representative evidence. In addition, representative evidence was provided for the INFA HA protein that a chimeric protein constrained in a prefusion conformation by means of the aforementioned methods was able to induce an improved neutralizing immune response when administered to mice.
[0658] Materials and methods
[0659] Chimeric peptide design:
[0660] The extracellular domain of INFA HA is operably linked to a downstream heterologous structural stabilizing portion comprising a pair of complementary heptad repeat regions derived from HIV-1 GP160. The amino acid sequences of the resulting chimeric proteins and their controls are presented below.
[0661] Extracellular domain of INFA HA (1-529):
...
Embodiment 3
[0677] As yet another example of the ability of the invention to generate chimeric polypeptides comprising the extracellular domain of a viral fusion protein constrained in a prefusion conformation, representative evidence for the Spike protein of the Middle East respiratory syndrome (MERS) virus is provided .
[0678] Materials and methods
[0679] Chimeric peptide design:
[0680] The extracellular domain of the MERS spike protein is operatively linked to a downstream heterologous structural stabilizing portion comprising a pair of complementary heptad repeat regions derived from HIV-1 GP160. The amino acid sequence of the resulting chimeric protein is presented below.
[0681] Extracellular domain of the MERS spike protein (1-1296) – HIV GP160-based SSM:
[0682]MIHSVFLLMFLLTPTESYVDVGPDSVKSACIEVDIQQTFFDKTWPRPIDVSKADGIIYPQGRTYSNITITYQGLFPYQGDHGDMYVYSAGHATGTTPQKLFVANYSQDVKQFANGFVVRIGAAANSTGTVIISPSTSATIRKIYPAFMLGSSVGNFSDGKMGRFFNHTLVLLPDGCGTLLRAFYCILEPRSGNHCPAGNSYTSFATYH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com