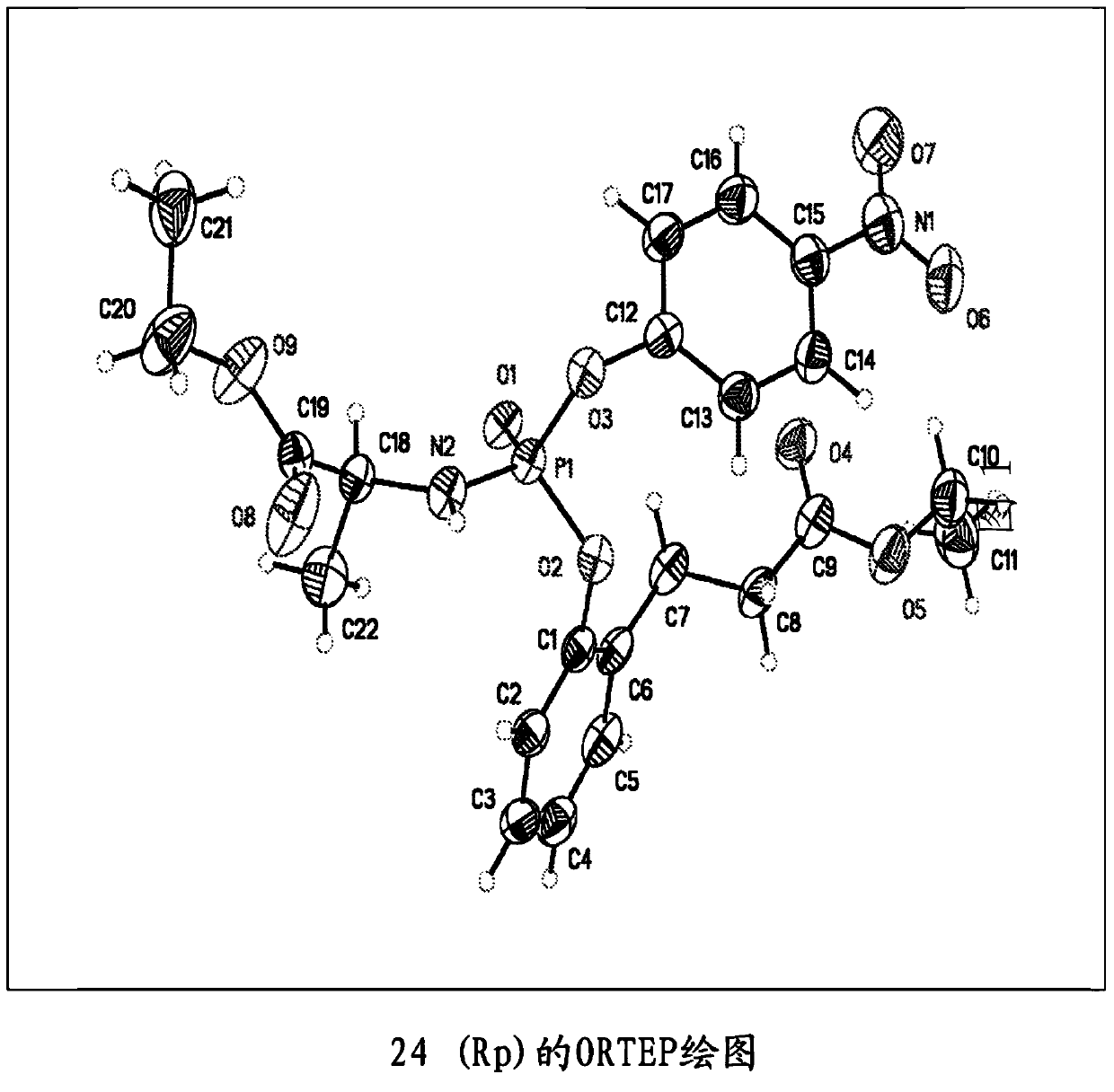
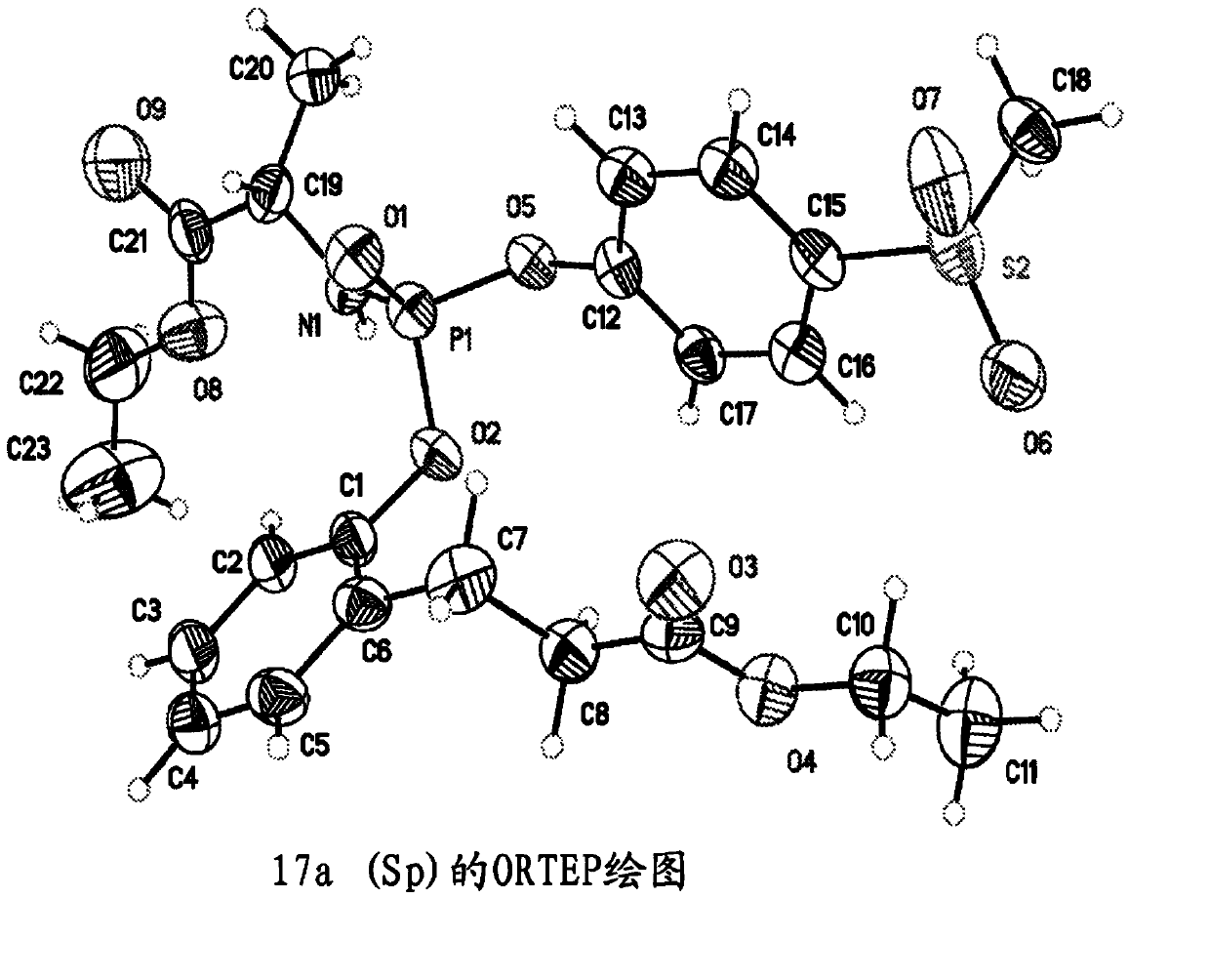
Purine monophosphate prodrugs for treatment of viral infections
A prodrug and chiral technology, applied in antiviral agents, pharmaceutical formulations, organic active ingredients, etc., can solve problems such as limiting the diversity of 6-substituted purine nucleoside triphosphates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0203]The preparation of the compound of formula A and B adopts (a) Rajagopalan, P.; Boudinot, F.D; Chu, C.K.; Tennant, B.C.; Baldwin, B.H.; .b)Recent Advances in Nucleosides:Chemistry and Chemotherapy:Chu,C.K.;Eds.Elsevier:2002.c)Frontiers in Nucleosides&Nucleic Acids,2004,Eds.R.F.Schinazi&D.C.Liotta,IHL Press,Tucker,GA,USA,pp : 319-37d) Handbook of Nucleoside Synthesis: Vorbruggen H. & Ruh-Pohlenz C. John Wiley & sons 2001) and using the general scheme 1-2 by first preparing nucleoside 1 to complete, the preparation of nucleoside 1 can be done by ordinary people in the art Technicians complete. Specifically, nucleoside 1 can be prepared by coupling sugar 2 with a protected silylated or free purine base in the presence of a Lewis acid such as TMSOTf. Deprotection of the 3' and 5' hydroxyls affords nucleoside 1.
[0204]
[0205] Scheme 1 Synthetic method of nucleoside 1. (The base is 2,6-diaminopurine or can be converted to 2,6-diaminopurine (such as 2-NH 2 , 6-Cl puri...
specific Embodiment
[0246] Specific compounds representative of the invention are prepared according to the following examples and reaction sequences; the examples and diagrams describing the reaction sequences are provided by way of illustration to aid in the understanding of the invention and should not be construed as limiting in any way the hereinafter appended The invention described in the claims. The present compounds can also be used as intermediates in subsequent examples to generate additional compounds of the invention. did not try Figure 1 Determine the yields obtained by optimizing any reaction. Those skilled in the art will know how to increase this yield through routine changes in reaction times, temperatures, solvents and / or reagents.
[0247] Anhydrous solvents were purchased from Aldrich Chemical Company, Inc. (Milwaukee). Reagents were purchased from commercial sources. Unless otherwise indicated, materials used in the examples were obtained from readily available commerci...
Embodiment 1
[0248] Example 1: Synthesis of 2,6-diamino 2'-C-methyl monophosphate prodrugs 8a and 8b
[0249]
[0250] (2R,3R,4R,5R)-5-((benzoyl)methyl)-2-(2,6-diamino-9H-purin-9-yl)-3-methyltetrahydrofuran-3,4 -Diyldiphenyl potassium ester 3
[0251] To (3R,4S,5R)-5-((benzoyl)methyl)-3-methyltetrahydrofuran-2,3,4-triyltriphenyl potassium ester 1 (2.9g, 5mmol) at -78°C To a stirred suspension of 2,6-diaminopurine 2 (830 mg, 5.5 mmol) in anhydrous acetonitrile was added DBU (2.3 mL, 15.0 mmol) followed by slow addition of TMSOTf (3.8 mL, 20.0 mmol). The reaction mixture was stirred at -78°C for 20 minutes and then warmed to 0°C. After stirring at 0 °C for 30 min, the reaction mixture was gradually heated to 65 °C and stirred overnight. The reaction mixture was washed with CH 2 Cl 2 (200mL) and dilute with saturated NaHCO 3 washing. The layers were separated and the resulting aqueous layer was washed with CH 2 Cl 2 (2x20 mL) extraction. The combined organic layers were washed wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
| optical density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com